
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 22 November 2024
Sec. Neurocognitive Aging and Behavior
Volume 16 - 2024 | https://doi.org/10.3389/fnagi.2024.1463065
 Zhuo Chen1
Zhuo Chen1 Xin Yi1
Xin Yi1 Wei Fu1
Wei Fu1 Yong Wu1
Yong Wu1 Xingju Zhong1
Xingju Zhong1 Chaoli Fan1
Chaoli Fan1 Yu Jiang1
Yu Jiang1 Qi Zhou1
Qi Zhou1 Jie Peng1
Jie Peng1 Jieyu Liao1
Jieyu Liao1 Zhike You1
Zhike You1 Jingyu Tan2*
Jingyu Tan2*Background and purpose: Triggering receptor expressed on myeloid cells-1 (TREM-1) was reported to be critical for mediating the neurological function after stroke, while the impact of soluble TREM-1 (sTREM-1) on cognitive impairment after ischemic stroke is unclear. We aimed to explore the association between sTREM-1 and post-stroke cognitive impairment (PSCI).
Methods: We prospectively recruited consecutive ischemic stroke patients who admitted hospital within 7 days of onset. Serum sTREM-1 concentrations were measured after admission. Cognitive function was assessed at 90 days follow-up using the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA). PSCI was defined as a MMSE score of <27 or a MoCA score < 26.
Results: A total of 291 patients (mean age, 66.6 years; 46.0% female) were enrolled for this study. Among these participants, the median sTREM-1 concentrations were 289.4 pg/mL. According to the MoCA score, 153 (52.6%) patients experienced PSCI at 3 months. After adjustment for confounding risk factors by multivariate regression analysis, patients with sTREM-1 levels in the fourth quartile were more likely to have increased risk 3-month PSCI (as compared with the first quartile, odds ratio 12.22, 95% confidence intervals, 5.20–28.71, P = 0.001). Restricted cubic spline further confirmed a dose-dependent relationship between sTREM-1 levels and PSCI (P = 0.003 for linearity). Similar significant findings were observed when the cognitive impairment was diagnosed according to the MMSE criterion.
Conclusion: Our study revealed that higher serum sTREM-1 levels at admission were associated with increased risk of 3-month PSCI.
Ischemic stroke is a prevalent cerebrovascular disease and a major cause of mortality and long-term morbidity throughout the world (Feigin et al., 2014; Giroud et al., 2014). Post-stroke cognitive impairment (PSCI) is recognized as one of the most common complications after stroke, occurring in one half of stroke survivors (Barbay et al., 2018a; Barbay et al., 2018b). There is evidence that PSCI is an independent predictor of functional disability, as well as higher mortality and recurrent stroke risk (Melkas et al., 2009; Kjörk et al., 2016; Yaghi et al., 2020). Early identification of biomarkers for predicting PSCI may have clinical implications for better prevention, and treatment of the disease.
The triggering receptor expressed on myeloid cells-1 (TREM-1) is an immune receptor initially known to be expressed on neutrophils and monocytes (Bouchon et al., 2000). It is involved in the amplification of the innate immune response through synergizing with toll-like receptor in infectious and non-infectious diseases (Bouchon et al., 2001; Colonna and Facchetti, 2003). In recent studies, it has been shown that circulating soluble TREM-1 (sTREM-1) plays a critical role in cerebrovascular diseases, such as subarachnoid hemorrhage, in-stent restenosis, and cardiovascular events (Sun et al., 2017; Wang et al., 2017; Wang et al., 2018). Experimental data showed that LP17 targeting TREM-1 may attenuate cerebral ischemia-induced neuronal damage by inhibiting oxidative stress and pyroptosis (Liang et al., 2020). Furthermore, blockade of TREM-1 can improve long-term functional outcomes in the hippocampus by alleviating cellular proliferation and synaptic plasticity (Xu et al., 2019). Considering that TREM-1 exerts a detrimental effect on neurological function after ischemic stroke, there might be a potential correlation between circulating sTREM-1 levels and PSCI. Therefore, our study prospectively investigated whether serum sTREM-1 concentrations in acute phase were associated with cognitive impairment at 3 months after ischemic stroke in a cohort of Chinese patients.
In the present study, first-time ischemic stroke patients within 7 days of the onset of symptoms were consecutively screened for eligibility at Mianzhu People’s Hospital between January 2023 and August 2023. The exclusion criteria were as follows: (1) age ≤ 18 years old; (2) patients with pre-existing cognitive impairment, such as Alzheimer’s disease, Parkinson’s disease, and other neurodegenerative diseases; (3) patients with severe neurological deficits, which impeded the neuropsychological testing; (4) patients with any history of central nervous system disease, severe hepatic or renal disease, autoimmune disease, or thyroid disorders. We also excluded the patients with a life expectancy < 3 months. The study was approved by the ethics committee of the Mianzhu Hospital and written informed consent was obtained from each patient.
Data collection was conducted using a standardized case report form after admission. For each patient we recorded: demographic data (age, gender, and education); vascular risk factors (hypertension, diabetes, smoking, dyslipidemia, and coronary artery disease); clinical data (medication history, blood pressure, stroke severity, and stroke etiology); laboratory data (lipid profile, fasting blood-glucose, high-sensitivity C-reactive protein and sTREM-1 levels). Baseline stroke severity was assessed by certified neurologist using National Institutes of Health Stroke Scale (NIHSS) (Goldstein and Samsa, 1997). Stroke subtype was classified basing on the criteria of Trial of Org 10172 in Acute Stroke Treatment (Adams et al., 1993). The infarction volume was assessed by the semiquantitative DWI-Alberta Stroke Program Early CT Score (DWI-ASPECTS), which is increasingly used in clinical settings (Lassalle et al., 2016).
The blood samples were analyzed a laboratory technician who blinded to the clinical data. Blood samples were obtained from each subject within 24 h after admission. The specimens were centrifuged at 2500 g for 15 min and the isolated serum frozen at −80°C for further analysis. sTREM-1 concentrations were measured by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN). The operation was carried out according to the specification.
A cognitive function evaluation was performed by neurologists blinded to clinical and laboratory data at 3-months after stroke onset, using the Mini-Mental State Examination (MMSE) and Montreal Cognitive Assessment (MoCA). 77 tool for assessing cognitive impairment in Chinese population. In this study, PSCI was defined as a MMSE score of <27 (Webb et al., 2014; Zhong et al., 2018) or a MoCA score < 26 (Campbell et al., 2016; Geng et al., 2017). Considering the influence of education, 1 point was added for patients with education < 12 years on the total MoCA score (Nasreddine et al., 2005).
Data normality was determined using the Kolmogorov-Smirnov test. Normally distributed continuous variables are presented as means and were compared using Student’s t-test and one-way analysis of variance. Not normally distributed variables were presented as median (interquartile range) and were compared using Mann–Whitney U test and Kruskal-Wallis test. Categorical variables are expressed as percentage and were compared using χ2 test and Fisher exact test. Multiple logistic regression analysis was used to evaluate whether increased sTREM-1 levels were associated with the presence of PSCI. Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex and the variables with a P-value < 0.1 in the univariate analysis. Odds ratios (OR) and 95% confidence intervals (CI) were calculated.
Restricted cubic spline was utilized to detect the possible linear dependency of relationship between the risk of PSCI and sTREM-1 levels, using 4 knots chosen at the 5th, 35th, 65th, and 95th percentiles.
Furthermore, receiver operating characteristic (ROC) curves were applied to investigating the accuracy of different models in predicting PSCI. The Z test was used to compare the area under the curve (AUC) of different models. A P-value < 0.05 at two-tailed was considered statistically significant. All statistical analyses were performed on SPSS for Windows, version 24.0 (SPSS Inc., Chicago, IL, USA) and R 3.6.0.
We included a total of 291 stroke patients (mean age, 66.6 ± 9.2 years), which consisted of 157 males (54.0%) and 134 females (46.0%). Their median levels of sTREM-1 were 289.4 pg/mL. We divided all patients into 4 groups according to the quartiles of sTREM-1 levels: first quartile (<224.2 pg/mL); second quartile (224.2–287.4 pg/mL); third quartile (287.5–388.7 pg/mL); and fourth quartile (>388.7 pg/mL). Table 1 demonstrated the demographic characteristics, clinical data and laboratory data according to the quartiles of sTREM-1 levels. Age, hypertension, total cholesterol levels and high-sensitivity C-reactive protein levels differed significantly with increasing quartiles of sTREM-1.
Results of univariate analysis between patients with and without PSCI were showed in Table 2. According to MoCA category, 153 patients (52.6%) were diagnosed as PSCI. Univariate analysis showed that participants with PSCI were older, had higher baseline NIHSS score and fasting blood glucose levels, and were more likely to have hypertension, diabetes mellitus, white matter lesions and education < 12 years. According to the MMSE category, 140 patients (48.1%) experienced PSCI at 3 months. Patients with PSCI were older, had higher high-sensitivity C-reactive protein levels, and were more likely to have hypertension, diabetes mellitus and education < 12 years. After adjustment for age, sex, education years, and variables with P-value < 0.1 in univariate analysis, multivariate regression model demonstrated that patients with sTREM-1 levels in the fourth quartile were more likely to have increased risk 3-month PSCI (OR 12.22, 95% CI, 5.20–28.71, P = 0.001 for MoCA category; OR 6.47, 95% CI, 2.91–13.79, P = 0.001 for MMSE category), as compared with the first quartile (Figure 1). Restricted cubic spline further confirmed a dose-dependent relationship between sTREM-1 levels and PSCI (P = 0.003 for linearity for MoCA category; P = 0.001 for linearity for MMSE category; Figure 2). We also confirmed a negative association of sTREM-1 levels with MMSE score (as continuous variable, Spearman’s Rho coefficient = −0.346, P = 0.001) and MoCA score (as continuous variable, Spearman’s Rho coefficient = −0.335, P = 0.001).
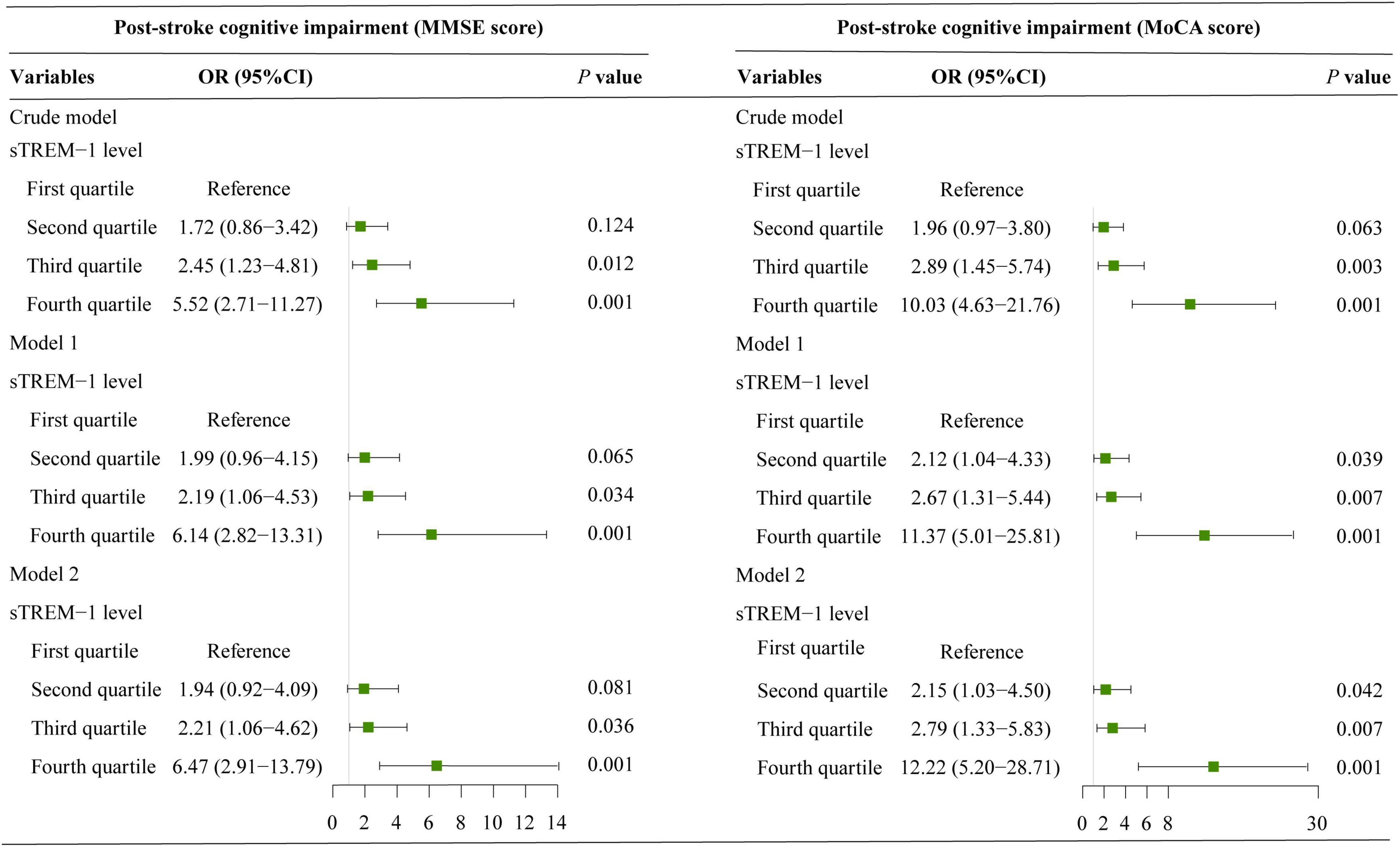
Figure 1. Binary logistic regression models explore the association between serum sTREM-1 levels and risk of PSCI. Model 1 adjusted for age and sex; model 2 adjusted age, sex and variables with a P-value < 0.1 in univariate analysis.
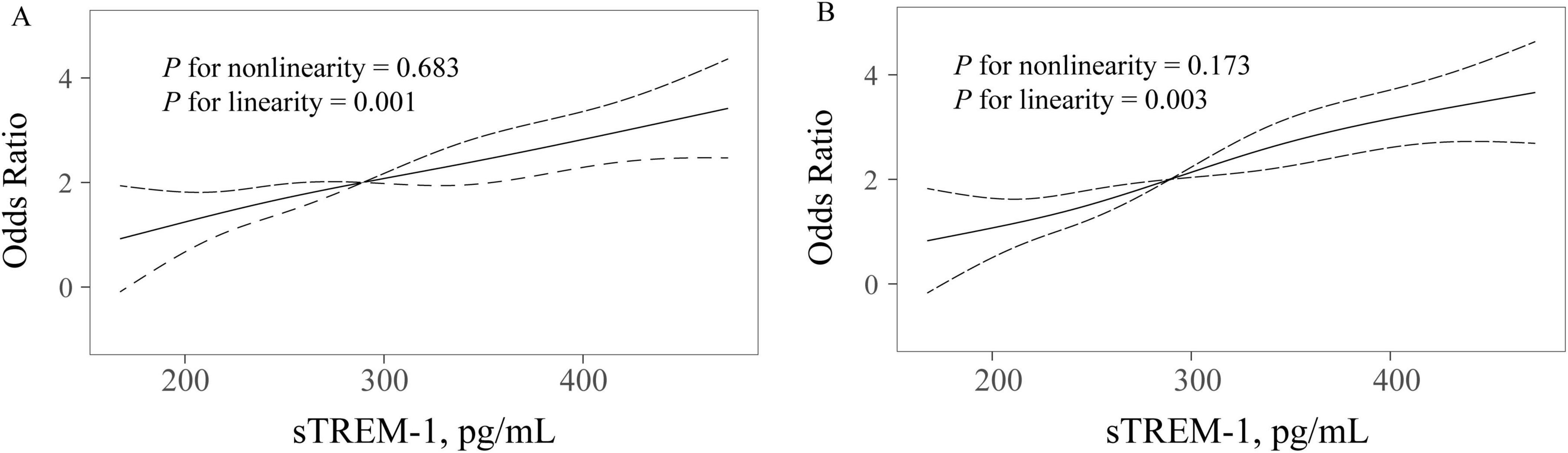
Figure 2. Restricted cubic spline detects the possible linear dependency of relationship between sTREM-1 levels and risk of PSCI (A for MMSE category; B for MoCA category). Restricted cubic spline of odds ratios and 95% confidence intervals with knots located at the 5th, 35th, 65th and 95th percentiles of the distribution of sTREM-1. The midpoint of sTREM-1 serves as the reference point. Odds ratios were adjusted for the same covariates in model 2.
We further investigated whether adding serum sTREM-1 levels to the conventional risk factors could improve the risk prediction of PSCI. As shown in Figure 3, the AUC for predicting PSCI was increased (from 0.696 to 0.779, P = 0.001 for MoCA category; from 0.703 to 0.753, P = 0.002 for MMSE category) when sTREM-1 was put into the model. Similar results were found when sTREM-1 was analyzed as a categorical variable.
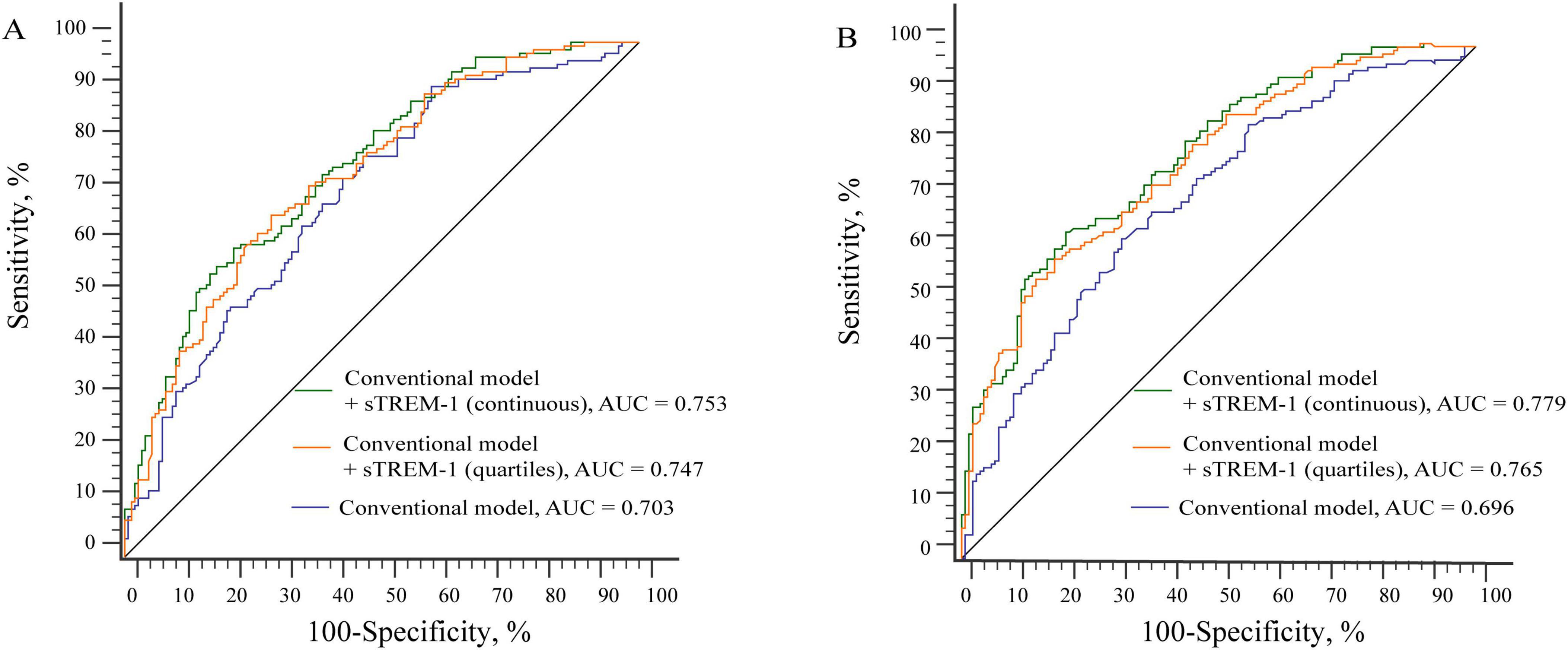
Figure 3. Receiver operating characteristic compares the potential of different models in predicting PSCI (A for MMSE category; B for MoCA category). (A) The AUC was increased from 0.703 to 0.753 when sTREM-1 was put into the conventional model. Conventional model included age, education, hypertension, diabetes mellitus and high-sensitivity C-reactive protein levels. (B) The AUC was increased from 0.696 to 0.779 when sTREM-1 was put into the conventional model. Conventional model included age, education, hypertension, diabetes mellitus, white matter lesions, baseline NIHSS score and fasting blood glucose levels.
In this study using a prospective cohort, we examined the association between serum sTREM-1 levels on admission and 3-month PSCI. Our results indicated that higher serum sTREM-1 levels were independently associated with cognitive impairment following acute ischemic stroke, regardless of age, gender, degree of education or other known risk factors. There is a wide range of cognitive impairment after stroke, ranging from 20 to 80% (Sun et al., 2014). The variation in prevalence is mainly due to the difference in definition of PSCI, the interval since stroke onset, study populations, and study methods. Using the MoCA category, 52.6% of stroke patients presented with PSCI in this study, which is similar to previous meta-analysis (Barbay et al., 2018a).
According to our results, PSCI patients at 3 months had a significantly higher NIHSS score than patients without PSCI, in line with previous studies (Melkas et al., 2009; Geng et al., 2017). The patients from PSCI group were also more likely to have diabetes mellitus. There are several mechanisms by which hyperglycemia can impair cognitive function, including advanced glycation end-products, inflammation, and microvascular disease (Yaffe et al., 2012). Furthermore, PSCI was also more prevalent in patients with white matter lesions, which was also consistent with previous study (Zhang et al., 2017; Khan et al., 2019). The reason for this is likely to be caused by loss of microstructural integrity in white matter tracts, which prevents structural reorganization after a stroke and reduces functional compensation through remote areas of the brain (Della Nave et al., 2007; Pantoni, 2010; Grefkes and Fink, 2014). It has been reported that proinflammatory factors play an important role in PSCI in previous studies (Narasimhalu et al., 2015). However, there were no significant differences in levels of high-sensitivity C-reactive protein between PSCI and non-PSCI groups, which was potentially due to the different definitions of PSCI.
The TREM-1 immune receptor amplifies the innate immune response by expressing itself on myeloid cells (Bouchon et al., 2001; Colonna and Facchetti, 2003). The circulating form of TREM-1 arises from spliced TREM-1 on neutrophils, macrophages, and mature monocyte membranes. Experimental data have demonstrated that the upregulation of neutrophil and monocyte membrane TREM-1 during endotoxemia is associated with an elevated release of sTREM-1 in the blood (Gibot et al., 2004). This process also occurs in various cerebrovascular diseases including subarachnoid hemorrhage, in-stent restenosis and cardiovascular events (Sun et al., 2017; Wang et al., 2017; Wang et al., 2018). Patients with early post-stroke depressive symptoms also showed a change in sTREM-1 levels (Pedroso et al., 2020). Our present study demonstrated that increased serum sTREM-1 concentrations were associated with a higher risk of PSCI. There are several possible mechanisms explaining the relationship between sTREM-1 levels and cognitive impairment after an ischemic stroke. First, Xu et al. (2019) found that microglial TREM-1 expression was upregulated following cerebral ischemic injury. By inhibiting TREM-1 with synthetic peptide LP17, neuronal injury may be alleviated and synaptic plasticity may be improved in the hippocampus (Xu et al., 2019). Second, oxidative stress was confirmed to be one of the pathophysiological mechanisms of cognitive impairment after ischemic cerebrovascular disease (Jurcau and Simion, 2020). Studies in both vivo and in vitro showed that inhibiting TREM-1 could reduce ROS accumulation and increase superoxide dismutase activity (Liang et al., 2020). Additionally, inhibiting TREM-1 might reduce myeloid cell infiltration and matrix metalloproteinase-9 expression (Boufenzer et al., 2015). Matrix metalloproteinases, whose major source was neutrophils, were associated with the disruption of the blood-brain barrier and cognitive impairment (Lassalle et al., 2016; Sarvari et al., 2020). All of these points strongly suggest that TREM-1 mediates PSCI development through its anti-inflammatory and antioxidative properties.
The advantages of our study include sufficient sample size, prospective cohort study nature, and detailed assessment of cognitive function, all of which made it possible to investigate the association between sTREM-1 concentrations and risk of PSCI. However, some limitations of our study should also be acknowledged. First, since the study was conducted in only one stroke center, our results may not be generalizable to other Chinese patients with ischemic strokes. Second, the subjects with serious illnesses or those with aphasia or dementia were excluded from this study, so a selection bias might be inevitable. This could lead to an underestimation of PCI prevalence. Third, as the study was observational, it was not possible to establish a causal link between STREM-1 levels and PSCI. Finally, serum sTREM-1 concentrations were assessed only once post-admission, restricting our ability to investigate the temporal association between sTREM-1 changes and PSCI following stroke.
In conclusion, higher circulating sTRME-1 levels were independently associated with increased risk of PSCI. Our results provide evidence supporting that sTREM-1 plays a vital role in the PSCI prediction. In addition, further studies with large sample sizes are required to evaluate these associations comprehensively.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of the Mianzhu Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
ZC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Visualization, Writing – original draft. XY: Conceptualization, Data curation, Methodology, Project administration, Validation, Writing – original draft. WF: Data curation, Formal analysis, Methodology, Writing – review and editing. YW: Data curation, Investigation, Writing – review and editing. XZ: Formal analysis, Methodology, Software, Supervision, Validation, Writing – review and editing. CF: Formal analysis, Investigation, Methodology, Writing – review and editing. YJ: Data curation, Formal analysis, Funding acquisition, Investigation, Project administration, Writing – review and editing. QZ: Formal analysis, Methodology, Resources, Validation, Writing – review and editing. JP: Formal analysis, Investigation, Methodology, Project administration, Resources, Validation, Writing – review and editing. JL: Investigation, Methodology, Supervision, Validation, Writing – review and editing. ZY: Writing – review and editing, Methodology, Validation. JT: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Supervision, Validation, Writing – original draft, Writing – review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adams, H., Bendixen, B., Kappelle, L., Biller, J., Love, B., and Gordon, D. (1993). Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 24, 35–41. doi: 10.1161/01.str.24.1.35
Barbay, M., Diouf, M., Roussel, M., and Godefroy, O. (2018a). Systematic review and meta-analysis of prevalence in post-stroke neurocognitive disorders in hospital-based studies. Dement. Geriatr. Cogn. Disord. 46, 322–334.
Barbay, M., Taillia, H., Nédélec-Ciceri, C., Bompaire, F., Bonnin, C., Varvat, J., et al. (2018b). Prevalence of poststroke neurocognitive disorders using national institute of neurological disorders and stroke-canadian stroke network, VASCOG criteria (Vascular Behavioral and Cognitive Disorders), and optimized criteria of cognitive deficit. Stroke 49, 1141–1147. doi: 10.1161/STROKEAHA.117.018889
Bouchon, A., Dietrich, J., and Colonna, M. (2000). Cutting edge: Inflammatory responses can be triggered by TREM-1, a novel receptor expressed on neutrophils and monocytes. J. Immunol. 164, 4991–4995. doi: 10.4049/jimmunol.164.10.4991
Bouchon, A., Facchetti, F., Weigand, M., and Colonna, M. (2001). TREM-1 amplifies inflammation and is a crucial mediator of septic shock. Nature 410, 1103–1107. doi: 10.1038/35074114
Boufenzer, A., Lemarié, J., Simon, T., Derive, M., Bouazza, Y., Tran, N., et al. (2015). TREM-1 mediates inflammatory injury and cardiac remodeling following myocardial infarction. Circ. Res. 116, 1772–1782. doi: 10.1161/CIRCRESAHA.116.305628
Campbell, N., Rice, D., Friedman, L., Speechley, M., and Teasell, R. (2016). Screening and facilitating further assessment for cognitive impairment after stroke: Application of a shortened Montreal Cognitive Assessment (miniMoCA). Disabil. Rehabil. 38, 601–604. doi: 10.3109/09638288.2015.1047968
Colonna, M., and Facchetti, F. (2003). TREM-1 (triggering receptor expressed on myeloid cells): A new player in acute inflammatory responses. J. Infect. Dis. 187(Suppl. 2), S397–S401.
Della Nave, R., Foresti, S., Pratesi, A., Ginestroni, A., Inzitari, M., Salvadori, E., et al. (2007). Whole-brain histogram and voxel-based analyses of diffusion tensor imaging in patients with leukoaraiosis: Correlation with motor and cognitive impairment. Am. J. Neuroradiol. 28, 1313–1319. doi: 10.3174/ajnr.A0555
Feigin, V., Forouzanfar, M., Krishnamurthi, R., Mensah, G., Connor, M., Bennett, D., et al. (2014). Global and regional burden of stroke during 1990-2010: Findings from the Global Burden of Disease Study 2010. Lancet 383, 245–254.
Geng, S., Liu, N., Meng, P., Ji, N., Sun, Y., Xu, Y., et al. (2017). Midterm blood pressure variability is associated with poststroke cognitive impairment: A Prospective Cohort study. Front. Neurol. 8:365. doi: 10.3389/fneur.2017.00365
Gibot, S., Kolopp-Sarda, M., Béné, M., Bollaert, P., Lozniewski, A., Mory, F., et al. (2004). A soluble form of the triggering receptor expressed on myeloid cells-1 modulates the inflammatory response in murine sepsis. J. Exp. Med. 200, 1419–1426.
Giroud, M., Jacquin, A., and Béjot, Y. (2014). The worldwide landscape of stroke in the 21st century. Lancet 383, 195–197. doi: 10.1016/s0140-6736(13)62077-2
Goldstein, L., and Samsa, G. (1997). Reliability of the National Institutes of Health Stroke Scale. Extension to non-neurologists in the context of a clinical trial. Stroke 28, 307–310. doi: 10.1161/01.str.28.2.307
Grefkes, C., and Fink, G. (2014). Connectivity-based approaches in stroke and recovery of function. Lancet Neurol. 13, 206–216.
Jurcau, A., and Simion, A. (2020). Oxidative stress in the pathogenesis of Alzheimer’s disease and cerebrovascular disease with therapeutic implications. CNS Neurol. Disord. Drug Targets 19, 94–108.
Khan, M., Heiser, H., Bernicchi, N., Packard, L., Parker, J., Edwardson, M., et al. (2019). Leukoaraiosis predicts short-term cognitive but not motor recovery in ischemic stroke patients during rehabilitation. J. Stroke Cerebrovasc. Dis. 28, 1597–1603.
Kjörk, E., Blomstrand, C., Carlsson, G., Lundgren-Nilsson, Å, and Gustafsson, C. (2016). Daily life consequences, cognitive impairment, and fatigue after transient ischemic attack. Acta Neurol. Scand. 133, 103–110.
Lassalle, L., Turc, G., Tisserand, M., Charron, S., Roca, P., Lion, S., et al. (2016). ASPECTS (Alberta Stroke Program Early CT Score) assessment of the perfusion-diffusion mismatch. Stroke 47, 2553–2558.
Liang, Y., Song, P., Zhu, Y., Xu, J., Zhu, P., Liu, R., et al. (2020). TREM-1-targeting LP17 attenuates cerebral ischemia-induced neuronal injury by inhibiting oxidative stress and pyroptosis. Biochem. Biophys. Res. Commun. 529, 554–561.
Melkas, S., Oksala, N., Jokinen, H., Pohjasvaara, T., Vataja, R., Oksala, A., et al. (2009). Poststroke dementia predicts poor survival in long-term follow-up: Influence of prestroke cognitive decline and previous stroke. J. Neurol. Neurosurg. Psychiatry. 80, 865–870. doi: 10.1136/jnnp.2008.166603
Narasimhalu, K., Lee, J., Leong, Y., Ma, L., Silva, D., Wong, M., et al. (2015). Inflammatory markers and their association with post stroke cognitive decline. Int. J. Stroke 10, 513–518.
Nasreddine, Z., Phillips, N., Bédirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Pantoni, L. (2010). Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701.
Pedroso, V. S., Vieira, ÉL., de Miranda, A. S., Venna, V. R., McCullough, L. D., and Teixeira, A. L. (2020). Early post-stroke depressive symptoms are associated with low peripheral levels of Soluble Triggering Receptor Expressed on Myeloid Cells-1 (sTREM-1) and Glial Cell-derived Neurotrophic Factor (GDNF). Curr. Neurovasc. Res. 17, 495–501. doi: 10.2174/1567202617999200819155636
Sarvari, S., Moakedi, F., Hone, E., Simpkins, J., and Ren, X. (2020). Mechanisms in blood-brain barrier opening and metabolism-challenged cerebrovascular ischemia with emphasis on ischemic stroke. Metab. Brain Dis. 35, 851–868. doi: 10.1007/s11011-020-00573-8
Sun, J., Tan, L., and Yu, J. (2014). Post-stroke cognitive impairment: Epidemiology, mechanisms and management. Ann. Transl. Med. 2:80.
Sun, X., Ma, Q., Jing, G., Wang, L., Hao, X., and Wang, G. (2017). Early elevated levels of soluble triggering receptor expressed on myeloid cells-1 in subarachnoid hemorrhage patients. Neurol. Sci. 38, 873–877. doi: 10.1007/s10072-017-2853-5
Wang, F., Li, C., Ding, F., Shen, Y., Gao, J., Liu, Z., et al. (2017). Increased serum TREM-1 level is associated with in-stent restenosis, and activation of TREM-1 promotes inflammation, proliferation and migration in vascular smooth muscle cells. Atherosclerosis 267, 10–18.
Wang, Y., Tang, J., Shen, Y., Hu, B., Zhang, C., Li, M., et al. (2018). Prognostic utility of soluble TREM-1 in predicting mortality and cardiovascular events in patients with acute myocardial infarction. J. Am. Heart Assoc. 7:e008985. doi: 10.1161/JAHA.118.008985
Webb, A., Pendlebury, S., and Li, L. (2014). Validation of the Montreal cognitive assessment versus mini-mental state examination against hypertension and hypertensive arteriopathy after transient ischemic attack or minor stroke. Stroke 45, 3337–3342.
Xu, P., Zhang, X., Liu, Q., Xie, Y., Shi, X., Chen, J., et al. (2019). Microglial TREM-1 receptor mediates neuroinflammatory injury via interaction with SYK in experimental ischemic stroke. Cell Death Dis. 10:555. doi: 10.1038/s41419-019-1777-9
Yaffe, K., Falvey, C., Hamilton, N., Schwartz, A., Simonsick, E., Satterfield, S., et al. (2012). Diabetes, glucose control, and 9-year cognitive decline among older adults without dementia. Arch. Neurol. 69, 1170–1175. doi: 10.1001/archneurol.2012.1117
Yaghi, S., Cotsonis, G., de Havenon, A., Prahbakaran, S., Romano, J., Lazar, R., et al. (2020). Poststroke montreal cognitive assessment and recurrent stroke in patients with symptomatic intracranial atherosclerosis. J. Stroke Cerebrovasc. Dis. 29:104663. doi: 10.1016/j.jstrokecerebrovasdis.2020.104663
Zhang, Z., Ren, W., Shao, B., Xu, H., Cheng, J., Wang, Q., et al. (2017). Leukoaraiosis is associated with worse short-term functional and cognitive recovery after minor stroke. Neurol. Med. Chir. (Tokyo) 57, 136–143. doi: 10.2176/nmc.oa.2016-0188
Keywords: biomarker, prediction, triggering receptor expressed on myeloid cells-1, stroke, cognitive impairment
Citation: Chen Z, Yi X, Fu W, Wu Y, Zhong X, Fan C, Jiang Y, Zhou Q, Peng J, Liao J, You Z and Tan J (2024) Higher soluble TREM-1 levels are associated with cognitive impairment after acute ischemic stroke. Front. Aging Neurosci. 16:1463065. doi: 10.3389/fnagi.2024.1463065
Received: 15 July 2024; Accepted: 11 November 2024;
Published: 22 November 2024.
Edited by:
Stephen D. Ginsberg, Nathan S. Kline Institute for Psychiatric Research, United StatesReviewed by:
Yi Xie, Nanjing University, ChinaCopyright © 2024 Chen, Yi, Fu, Wu, Zhong, Fan, Jiang, Zhou, Peng, Liao, You and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingyu Tan, dGp5aGVyZTEwNkAxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.