- 1Department of Radiology, Qilu Hospital of Shandong University, Jinan, China
- 2Department of Radiology, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, China
- 3Department of Neurology, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, China
- 4Department of Medicine Experimental Center, Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University, Qingdao, China
- 5Department of Research and Development, Shanghai United Imaging Intelligence Co., Ltd., Shanghai, China
Objectives: To explore the relationship between glymphatic dysfunction and cognitive impairment in unilateral temporal lobe epilepsy (TLE).
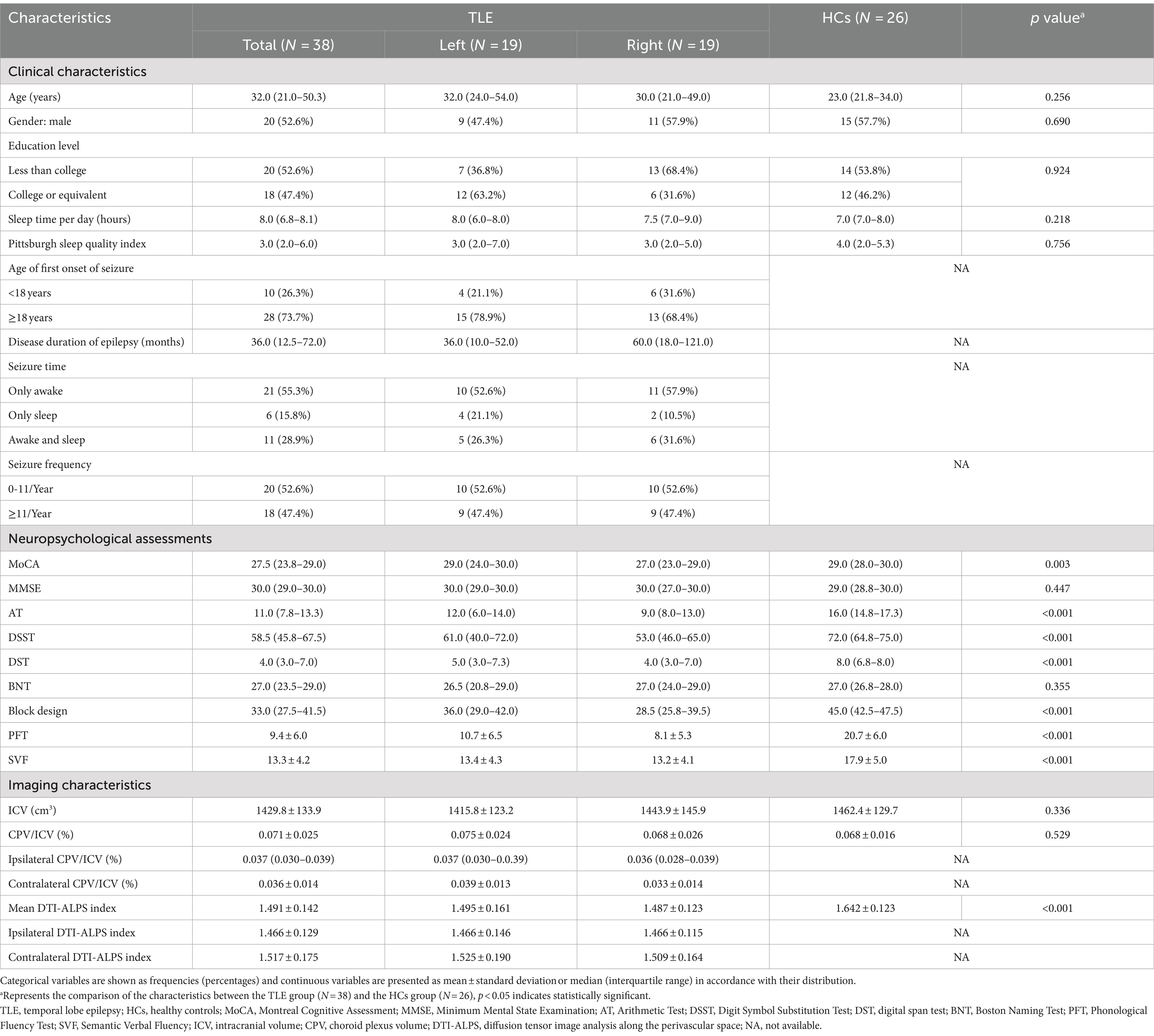
Methods: This study retrospectively included 38 patients with unilateral TLE and 26 age- and gender-matched healthy controls (HCs). The diffusion tensor image analysis along the perivascular space (DTI-ALPS) index, choroid plexus volume (CPV), and cognitive assessment were obtained for each participant. Neuropsychological test batteries included Montreal Cognitive Assessment (MoCA), Minimum Mental State Examination, Arithmetic Test (AT), Digit Symbol Substitution Test (DSST), Digit Span Test (DST), Boston Naming Test, Block design, Phonological Fluency Test (PFT), and Semantic Verbal Fluency (SVF).
Results: Compared to HCs, TLE patients had lower scores of MoCA, AT, DSST, DST, Block design, PFT and SVF (all p < 0.05) and lower values of mean DTI-ALPS index (1.491 ± 0.142 vs. 1.642 ± 0.123, p < 0.001). Significantly lower DTI-ALPS index values were observed in the ipsilateral hemisphere than in the contralateral hemisphere (1.466 ± 0.129 vs. 1.517 ± 0.175, p = 0.013) for patients with unilateral TLE. Correlation analyses found that SVF performance was significantly or borderline significantly associated with glymphatic function (FDR-corrected p < 0.05 for all DTI-ALPS index and FDR-corrected p = 0.057 for CPV) in TLE patients. Linear regression analyses showed that increased CPV and decreased DTI-ALPS index were independent risk factors for semantic fluency impairment (all p < 0.05). Furthermore, mediation analyses found the mediator role of the mean DTI-ALPS index in the relationship between choroid plexus enlargement and semantic fluency impairment (indirect effect: β = −0.182, 95%CI = −0.486 to −0.037).
Conclusion: These findings reveal the important role of the DTI-ALPS index and CPV in SVF performance in unilateral TLE. Decreased DTI-ALPS index and increased CPV are the independent risk factors for semantic fluency impairment. The DTI-ALPS index may fully mediate the relationship between CP enlargement and SVF performance. These insights provide a radiological foundation for further investigations into the mechanism of the glymphatic system in TLE pathophysiology.
1 Introduction
Over 70 million individuals worldwide suffer from epilepsy, a serious neurological condition of which temporal lobe epilepsy (TLE) accounts for 30–35% (Hauser et al., 1996; Thijs et al., 2019; Beghi, 2020). Approximately 30–40% of adult patients with epilepsy suffer from cognitive problems, with language, verbal memory, executive function, and attention being the most vulnerable domains (Wang et al., 2020), which has an important influence on quality of life and cognitive rehabilitation may help improve quality of life (Meneses et al., 2009).
The glymphatic system (GS) is a vital way to remove metabolites and misfolded peptides/proteins from the brain (Iliff et al., 2012). A novel non-invasive technique named diffusion tensor image analysis along the perivascular space (DTI-ALPS) has proven useful for evaluating glymphatic function in epilepsy (Lee D. A. et al., 2022), a condition often links to GS dysfunction (Zhang et al., 2023; Zhao et al., 2023; Hlauschek et al., 2024). Several studies have demonstrated the potential of the DTI-ALPS in localizing epileptogenic foci, evaluating surgical outcomes in unilateral TLE (Zhang et al., 2023), and providing insights into the diagnosis of childhood absence epilepsy (Pu et al., 2023). Despite some controversies surrounding this index raised in recent years (Piantino et al., 2023; Ringstad, 2024), previous studies have demonstrated that the DTI-ALPS index is consistent with other GS evaluation methods, such as glymphatic MRI, which is considered the gold standard for MRI assessment of GS using gadolinium-based contrast agents as intrathecal tracers (Zhang W. et al., 2021). Therefore, we have reason to believe that this index may serve as a potential imaging biomarker reflecting GS function. Additionally, enlarged choroid plexus (CP), an important GS component, has been associated with impaired glymphatic clearance according to a recent glymphatic MRI study (Li et al., 2023). Unlike the DTI-ALPS index, less attention has been paid to CP, even though it is one of the important components of GS (Christensen et al., 2022).
During normal aging, GS plays a protective role in cognitive decline (Wang et al., 2023). The relationship between GS function and cognitive performance has been reported across various central nervous system disorders (Taoka et al., 2017; Chen et al., 2021; Zhang Y. et al., 2021). For instance, lower DTI-ALPS index has been correlated with worser performance on tests like the Boston Naming Test (BNT), Trail Making Test A, and Digit Span Test (DST) in Alzheimer’s disease (AD) (Zhang et al., 2024). Mediation analysis further suggested that cognitive impairment in AD may be significantly mediated by GS dysfunction (Hsu et al., 2023). Similar associations between global cognition and regional glymphatic function have been observed in behavioral variant frontotemporal dementia (Jiang et al., 2023a). However, the relationship between glymphatic function and cognitive performance in unilateral TLE patients remains an understudied area.
Thus, in this study for patients with unilateral TLE, we mainly aimed to investigate (1) whether the DTI-ALPS index, in the ipsilateral hemisphere is reduced compared to the contralateral hemisphere; (2) the association between the ALPS index, CPV and performance on a battery of neuropsychological tests; and (3) whether the DTI-ALPS index mediates the relationship between CP enlargement and specific neuropsychological performance.
2 Materials and methods
This study followed the ethical guidelines expressed in the Declaration of Helsinki and the study protocol was approved by the ethics committee of Qilu Hospital of Shandong University (Qingdao) (KYLL-qdql2020070). Informed consent was exempt due to the retrospective nature of our study.
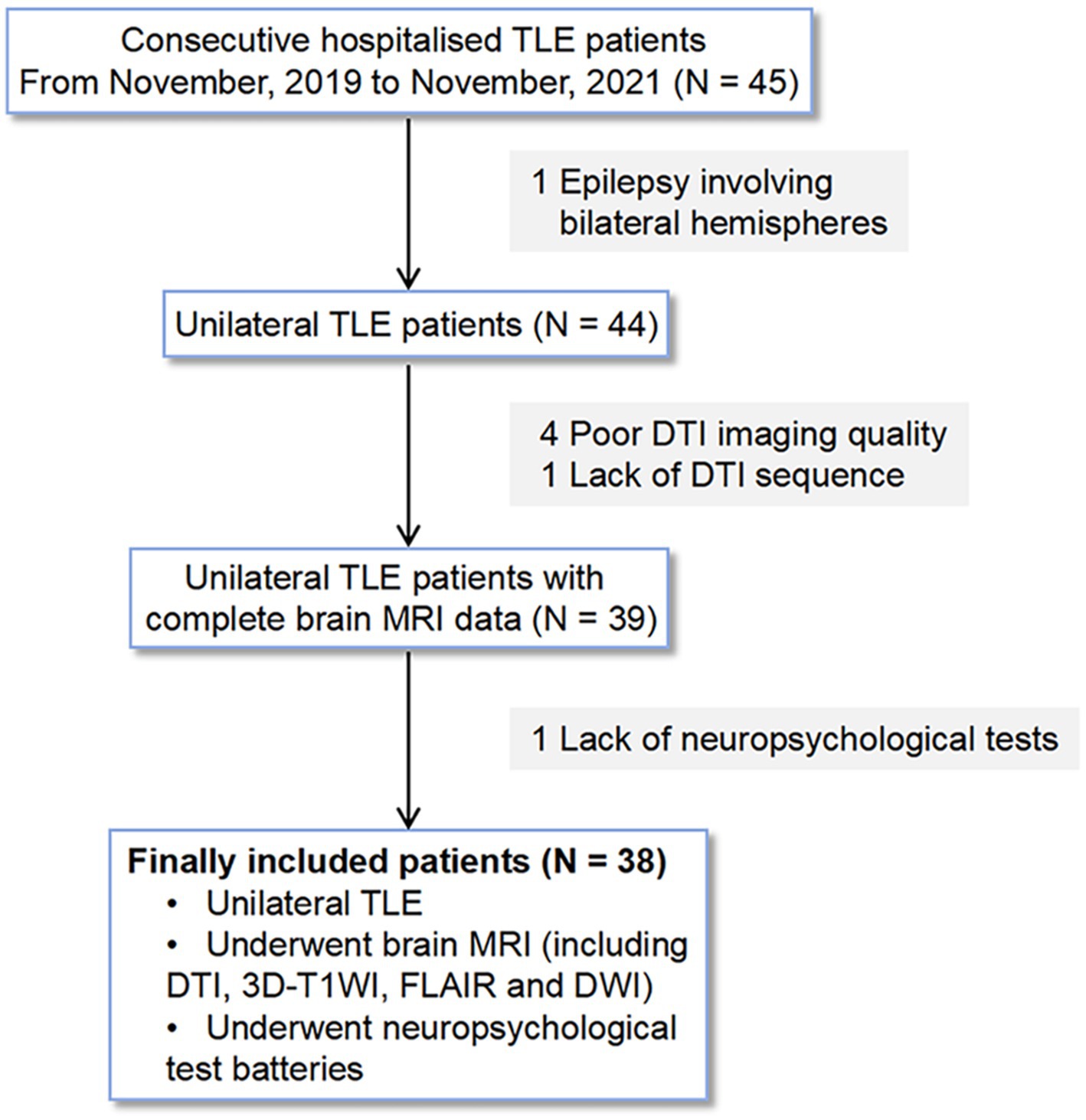
2.1 Participants
We analyzed consecutive hospitalized patients with unilateral TLE in the Neurology Department at our hospital from November, 2019 to November, 2021 based on the following inclusion criteria: (1) clinical diagnosis of unilateral TLE based on video-electroencephalography telemetry, seizure semiology, and neuroimaging by epileptologists (Stasenko et al., 2023); (2) brain MRI scans were performed, including diffusion tensor imaging (DTI), three-dimensional T1-weighted imaging (3D-T1WI), fluid-attenuated inversion recovery (FLAIR), and diffusion weighted imaging (DWI); (3) underwent a battery of neuropsychological tests within 1 week before the MRI examination. Patients were excluded if they met the following criteria: (1) brain parenchymal lesions such as tumors and vascular malformations confirmed by MRI; (2) sleep deprivation in the week before the MRI scans; (3) poor DTI image quality for analyses of glymphatic function; (4) a history of neuropsychiatric disorders; (5) a history of neurodegenerative diseases, such as AD and Parkinson’s disease; (6) epilepsy involving bilateral hemispheres. Of the 45 patients with TLE, four patients with poor DTI image quality, one without DTI data, one with epilepsy involving bilateral hemispheres, and one without neuropsychological data were excluded. Thus 38 patients with unilateral TLE were retrospectively selected in this study and divided into left TLE or right TLE groups according to their clinical symptoms, video electroencephalography, and neuroimaging findings. The detailed selection process is shown in Figure 1. Inclusion criteria for healthy controls (HCs) were as follows: (1) brain MRI scans including DTI, 3D-T1WI, FLAIR and DWI; (2) underwent a battery of neuropsychological tests within 1 week before the MRI examination. And the exclusion criteria were as follows: (1) brain parenchymal lesions; (2) sleep deprivation in the week before the MRI scans; (3) a history of neuropsychiatric disorders; (4) a history of neurodegenerative diseases, such as AD and Parkinson’s disease; (5) a history of epilepsy. Ultimately, 26 age- and gender-matched HCs were included in the study.
2.2 Clinical assessments
The following basic clinical information was collected: age, gender, education level, age of first onset of seizure, disease duration of epilepsy, seizure time, seizure frequency, sleep time per day, and Pittsburgh Sleep Quality Index. Additionally, neuropsychological assessments were performed including the Montreal Cognitive Assessment (MoCA), Minimum Mental State Examination (MMSE), Arithmetic Test (AT), Digit Symbol Substitution Test (DSST), DST, BNT, Block design, Phonological Fluency Test (PFT), and Semantic Verbal Fluency (SVF).
2.3 Image acquisition
All participants underwent MRI scans using the same protocol on a single 3 T MRI scanner (Ingenia, Philips Medical Systems, Netherlands) equipped with an eight-channel brain phased-array coil. No software or hardware updates were made to the MRI scanner during the entire scan period for the participants to ensure the consistency of the collected image data. The parameters of the MR sequences were as follows:
1. 3D-T1WI was acquired using a fast spoiled gradient echo sequence: repetition time (TR) = 6.7 msec, echo time (TE) = 3.0 msec, flip angle (FA) = 8°, field of view (FOV) = 240 mm × 240 mm, data matrix = 240 × 240, slice thickness (ST) = 1 mm, gap = 0 mm, 170 slices, number of signals averaged (NSA) = 1, orientation: sagittal.
2. T2-FLAIR: TR = 7,000 msec, TE = 125 msec, FA = 90°, FOV = 230 mm × 230 mm, data matrix = 288 × 163, ST = 6 mm, gap = 1 mm, 18 slices, NSA = 1, orientation: transverse.
3. DWI: TR = 2,235 msec, TE = 76 msec, FA = 90°, FOV = 230 mm × 230 mm, data matrix = 176 × 134, ST = 6 mm, gap = 1 mm, 18 slices, NSA = 1, orientation: transverse.
4. DTI was acquired using echo planar imaging with a total of 32 different diffusion directions with b = 1,000 s/mm2 and 1 non-diffusion-weighted (b = 0) image: TR = 4,900 msec, TE = 95 msec, FA = 90°, FOV = 224 mm × 224 mm, data matrix = 112 × 110, ST =2 mm, gap = 0 mm, 70 slices, NSA = 2, orientation: transverse.
2.4 Image preprocessing for brain volume
With 3D-T1WI, an image analysis tool named uRP, developed by Shanghai United Imaging Intelligence Co. Ltd., was used for brain segmentation and volume acquisition (Wu et al., 2023). The uRP facilitated a comprehensive preprocessing pipeline that included skull stripping, bias field correction, and image resampling to attain an isotropic resolution of 1 × 1 × 1 mm3. Subsequently, bilateral choroid plexus volume (CPV) of the lateral ventricles and total intracranial volume (ICV) were quantified according to the Desikan-Killiany atlas using a pre-trained cascaded VB-Nets model integrated into the uRP tool. The VB-Nets model is capable of segmenting the Desikan-Killiany atlas with an average Dice similarity coefficient of 91.06% between the automatic segmentation and Freesurfer-based segmentation (Ge et al., 2022). The CPV for each participant was calculated by summing the values of the left and right CPV. For unilateral TLE patients, CPV of both the ipsilateral and contralateral hemispheres were also used in the analyses. Examples of CP segmentation are shown in Supplementary Figure S1. To account for individual heterogeneity in ICV, CPV was presented as a percentage of ICV (CPV/ICV) as previously proposed (Choi et al., 2022).
2.5 Preprocessing of DTI data
DSI Studio software (version 2023 Aug) was used to preprocess the DTI data. The key steps were: (i) converted the original Digital Imaging and Communications in Medicine data into the SRC format; (ii) performed quality control to identify and address issues like eddy current artifacts or head motion (Yeh et al., 2019); (iii) preprocessing including eddy current correction, motion correction, and skull stripping; (iv) reconstruction using the DTI method (Yeh et al., 2013; Tax et al., 2022).
2.6 Calculation of the DTI-ALPS index
A 4 × 4 × 4 mm3 region of interest (ROI) was placed on the projection and association fibers at the level of the lateral ventricle body in each hemisphere, respectively. The diffusivity along the x-, y- and z-axis was obtained at the voxel level of ROI. The DTI-ALPS index was subsequently calculated using the formula (Lee H. J. et al., 2022):
Where Dxxproj and Dxxassoc are the diffusivity along the x-axis in the projection fiber and association fiber, respectively. Dyyproj means the diffusivity along the y-axis in the projection fiber, and Dzzassoc means the diffusivity along the z-axis in the association fiber. This was done for all participants by an experienced neuroradiologist (XSM, with more than 20 years of experience) blinded to clinical data. In a subset of 30 participants, another neuroradiologist (XNX, with 10 years of experience) repositioned the ROIs and then calculated the DTI-ALPS index to evaluate the inter-rater reliability. The mean DTI-ALPS index for each participant was the average of bilateral values. For TLE patients, the DTI-ALPS index obtained from both the ipsilateral hemisphere and contralateral hemisphere was used in our analyses.
2.7 Statistical analyses
Categorical data are shown as frequencies (percentages) and continuous data are presented as mean ± standard deviation or median (interquartile range) based on distribution. The intraclass correlation coefficient (two-way mixed model, single measure, absolute agreement) was used to assess the inter-rater reliability of the DTI-ALPS index. Baseline differences between groups (TLE vs. HCs, left TLE vs. right TLE) were analyzed using two-sample t-test or Mann–Whitney test for continuous variables, and chi-square test or Fisher’s exact test for categorical variables when appropriate.
Paired t-test was used to investigate whether the ipsilateral DTI-ALPS index or CPV/ICV differed from the contralateral side. Pearson’s correlation or Spearman’s rank correlation analyses were performed to test associations, and correlations were expressed as Pearson’s correlation coefficient (r) or Spearman’s correlation coefficient (ρ). To address the issue of multiple comparisons, the Benjamini-Hochberg false discovery rate (FDR) correction was applied to p-values. FDR-corrected p < 0.05 is considered statistically significant. To identify independent risk or protective factors for neuropsychological performance, the multivariable stepwise linear regression analysis with backward elimination method (p ≥ 0.10 for exclusion) was constructed using baseline variables that were clinically relevant or showed a univariate association (p ≤ 0.10) with neuropsychological scores. The significance threshold was set at p < 0.05.
Exploratory analyses were conducted to investigate whether the DTI-ALPS index mediated the relationship between CP enlargement and neuropsychological performance. The “mediation” and “BruceR” packages (Tingley et al., 2014; Bao, 2023) were used to evaluate the direct and indirect (mediation) effects. The 95% confidence intervals (CI) and standard errors for the total/direct/indirect effects were acquired by random sampling (set to 1,000) with replacement, a reliable non-parametric technique for CI construction called bootstrapping (Alfons et al., 2022). We primarily investigated whether the mean DTI-ALPS index mediates the relationship between CPV and SVF performance. Besides, we also explored the mediation role of ipsilateral (or contralateral) DTI-ALPS index on the relationship between ipsilateral (or contralateral) CPV and SVF performance. A significant effect is indicated if the bootstrapped 95% CI does not include zero (Osborne et al., 2023).
The mediation analyses were performed using the R statistical program (version 4.2.3), while all other statistical analyses were conducted using SPSS (version 26).
3 Results
3.1 Clinical, neuropsychological, and imaging characteristics
Table 1 summarizes the clinical, neuropsychological, and imaging characteristics of all participants. The intraclass correlation coefficient of the DTI-ALPS index was 0.879 (95% CI: 0.805–0.926), which showed the excellent repeatability between two observers. The HCs were age- and gender-matched with unilateral TLE patients (p = 0.256 and 0.690), and no significant differences in ICV or CPV/ICV were found between the two groups. Compared to HCs, TLE patients had lower scores of MoCA, AT, DSST, DST, Block design, PFT and SVF (all p < 0.05) and lower values of mean DTI-ALPS index (1.491 ± 0.142 vs. 1.642 ± 0.123, p < 0.001). Paired t-test in all unilateral TLE patients found that the ipsilateral DTI-ALPS index was significantly lower than that in the contralateral side (1.466 ± 0.129 vs. 1.517 ± 0.175, p = 0.013) (Figure 2A), but CPV/ICV was not (0.035 ± 0.013 vs. 0.036 ± 0.014, p = 0.577). In addition, there were no significant differences in any of the characteristics between patients with left TLE and right TLE.
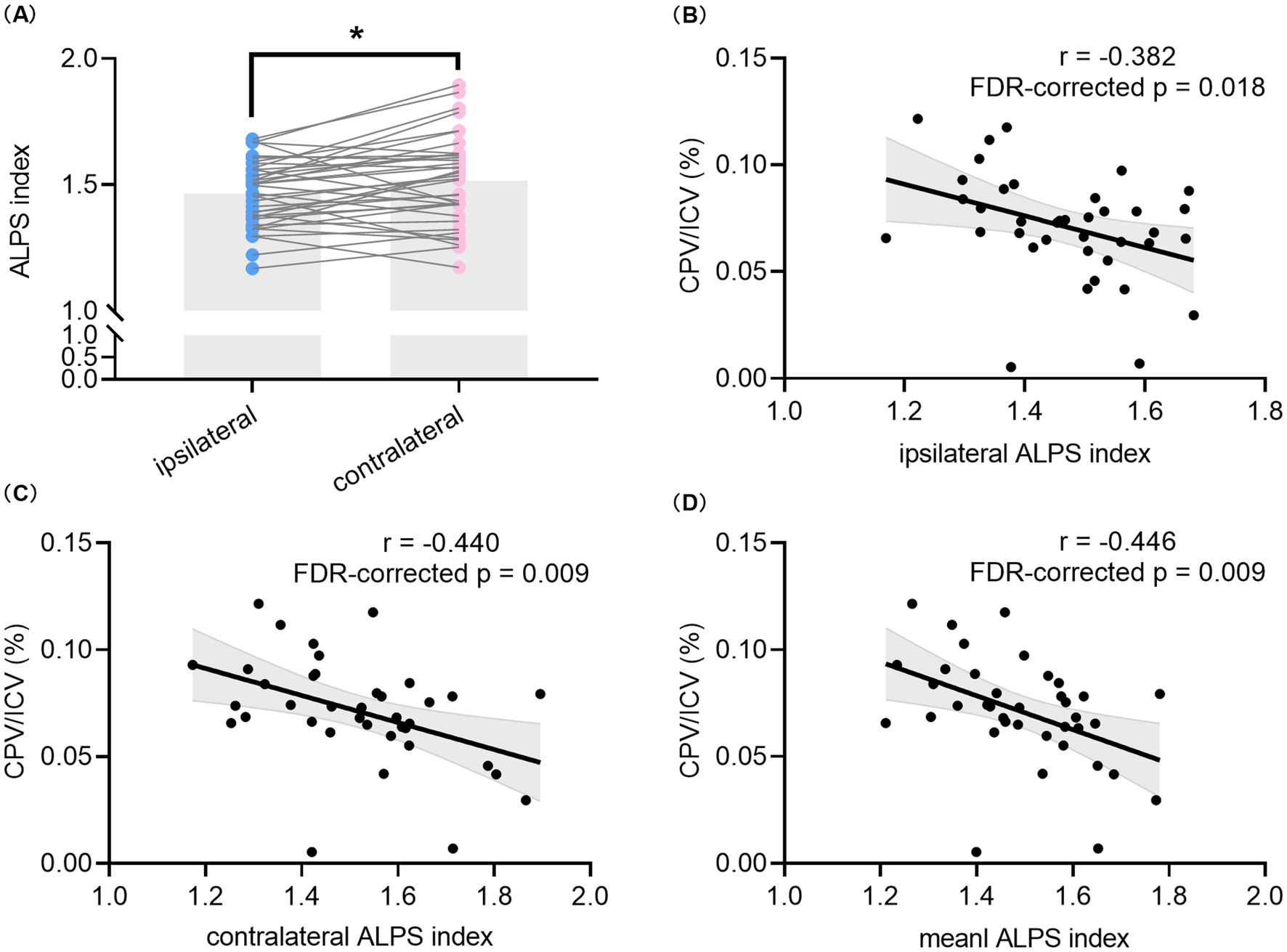
Figure 2. Comparison of the DTI-ALPS index between hemispheres and correlation analyses between the DTI-ALPS index and CPV in all TLE patients. Comparison between the ipsilateral DTI-ALPS index and contralateral DTI-ALPS index in patients with unilateral TLE (A). Correlation between CPV/ICV and the ipsilateral DTI-ALPS index (B) as well as contralateral DTI-ALPS index (C). Correlation between the mean DTI-ALPS index of bilateral hemispheres and CPV/ICV (D). * indicates p < 0.05. The p values with FDR correction of correlation analyses are shown in the figure. TLE, temporal lobe epilepsy; DTI, diffusion tensor imaging; ALPS, analysis along the perivascular space; CPV, choroid plexus volume; ICV, intracranial volume; FDR, false discovery rate.
3.2 Correlation analyses between the DTI-ALPS index and CPV
As shown in Figures 2B–D, significant negative correlations were found between CPV/ICV and the ipsilateral DTI-ALPS index (r = −0.382, p = 0.018, FDR-corrected p = 0.018), the contralateral DTI-ALPS index (r = −0.440, p = 0.006, FDR-corrected p = 0.009), and the mean DTI-ALPS index (r = −0.446, p = 0.005, FDR-corrected p = 0.009) in all TLE patients. Furthermore, CPV was always correlated with the corresponding DTI-ALPS index in all TLE patients, regardless of whether it was contralateral or ipsilateral, as shown in Supplementary Figure S2. However, no significant correlation was found between the mean DTI-ALPS index and CPV/ICV in the HCs group (data not shown).
3.3 Correlation analyses between the DTI-ALPS index, CPV and neuropsychological performance
Among neuropsychological tests, the ipsilateral DTI-ALPS index showed a significantly moderate positive correlation with SVF scores (r = 0.477, uncorrected p = 0.0049 and FDR-corrected p = 0.044) in patients with TLE. Positive correlations were also discovered between SVF scores and the contralateral DTI-ALPS index (r = 0.482, uncorrected p = 0.002, and FDR-corrected p = 0.018) as well as the mean DTI-ALPS index (r = 0.502, uncorrected p = 0.001, FDR-corrected p = 0.009) for TLE patients. A borderline significant correlation was found between CP enlargement and AT, DSST and SVF scores (r = −0.400, −0.380, −0.393, uncorrected p = 0.013, 0.019, 0.015, FDR-corrected p = 0.057, 0.057, 0.057, respectively) in the TLE group. Interestingly, SVF was negatively correlated with ipsilateral CPV/ICV (r = −0.501, uncorrected p = 0.001 and FDR-corrected p = 0.009) but not with contralateral CPV/ICV (r = −0.362, uncorrected p = 0.026 and FDR-corrected p = 0.131) in patients with TLE. Details for correlation analyses for patients with TLE are shown in Figure 3 and Supplementary Table S1. Additional correlation analyses in the HCs group showed that CPV/ICV was only negatively correlated with MMSE scores (Supplementary Table S2).
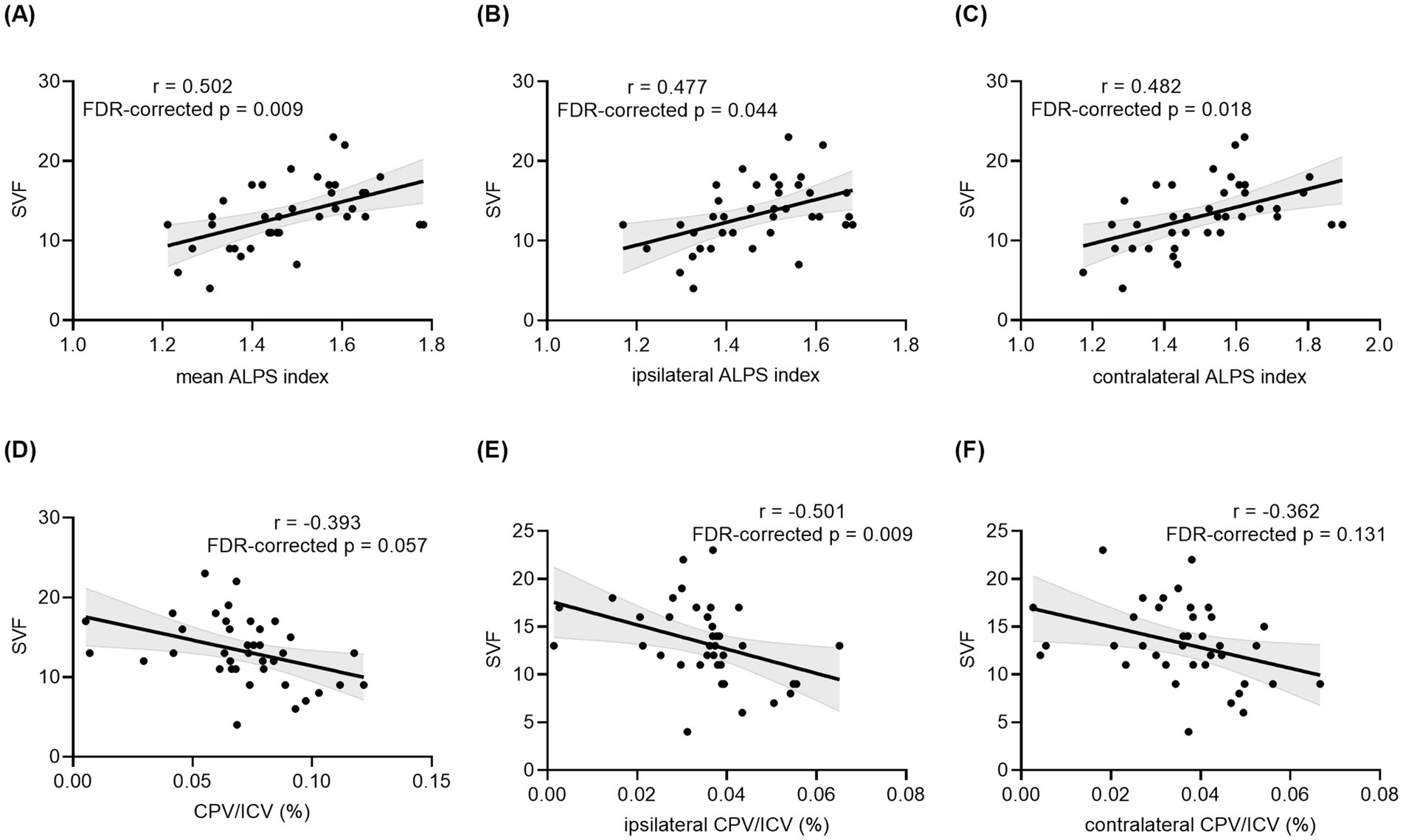
Figure 3. Correlation analyses between the DTI-ALPS index, CPV and SVF performance in all TLE patients. Correlation between SVF performance and the mean ALPS index of bilateral hemispheres (A), ipsilateral ALPS index (B), contralateral ALPS index (C), CPV/ICV (D), ipsilateral CPV/ICV (E), and contralateral CPV/ICV (F) in patients with unilateral TLE. The p values with FDR correction are shown in the figure. TLE, temporal lobe epilepsy; DTI, diffusion tensor imaging; ALPS, analysis along the perivascular space; CPV, choroid plexus volume; ICV, intracranial volume; SVF, Semantic Verbal Fluency; FDR, false discovery rate.
3.4 Linear regression analyses to explore the role of the DTI-ALPS index and CPV in semantic fluency performance in the TLE group
Multivariable stepwise linear regression analyses showed that the ipsilateral DTI-ALPS index (β = 0.393, p = 0.008) was a significant independent protective factor for SVF scores, after adjusting for the potential confounder in Model 1, as shown in Table 2. The contralateral DTI-ALPS index and mean DTI-ALPS index were also significant independent predictors for SVF scores (β = 0.503, 0.487 and p = 0.0004, 0.001 in Model 2, Model 3, respectively). Conversely, CP enlargement was identified as an independent risk factor for SVF performance (β = −0.330, p = 0.03 in Model 4), which was likewise found in both contralateral and ipsilateral CPV (Supplementary Table S3).
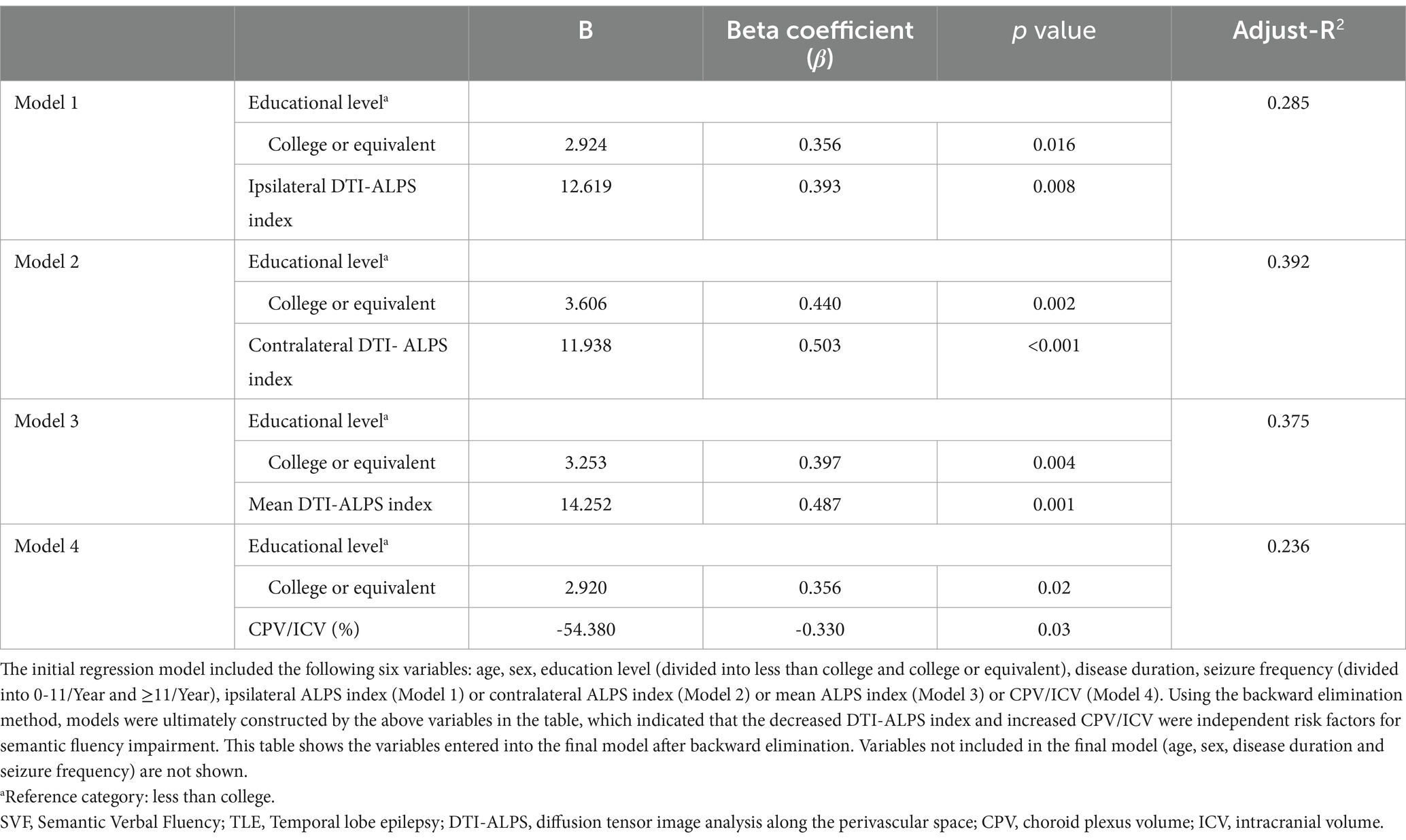
Table 2. Final results of multivariable linear regression (backward stepwise) to explore the role of the DTI-ALPS index and CPV in semantic fluency performance in the TLE group
3.5 Exploratory mediation analyses to investigate the role of the DTI-ALPS index in semantic fluency performance in the TLE group
Mediation analyses demonstrated that the indirect effect of CPV on SVF performance through the mean DTI-ALPS index was statistically significant (β = −0.182, 95%CI = −0.486 to −0.037), whereas the direct effect was not (β = −0.212, 95%CI = −0.540 to 0.153). Likewise, the indirect effects of contralateral CPV on SVF performance through the contralateral DTI-ALPS index (β = −0.179, 95%CI = −0.461 to −0.031), as well as those of ipsilateral CPV on SVF performance through the ipsilateral DTI-ALPS index (β = −0.120, 95%CI = −0.354 to −0.006), were also found to be significant. Details are shown in Table 3.
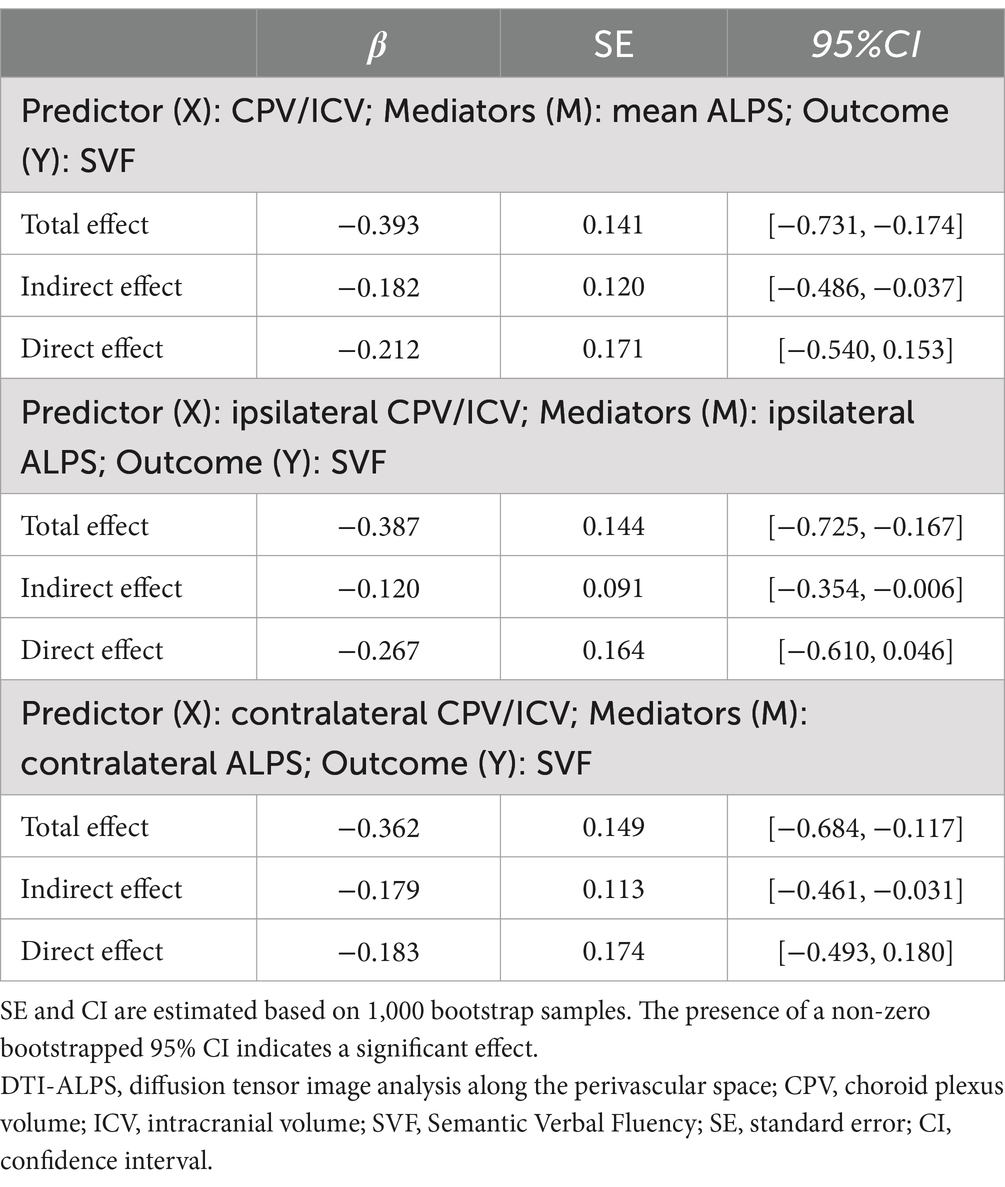
Table 3. Simple mediation analyses of the DTI-ALPS index in the relationship between CP enlargement and SVF performance in all TLE patients.
4 Discussion
This study represents the first attempt to investigate the role of glymphatic function on SVF performance in unilateral TLE patients using non-invasive 3D-TIWI and DTI methods. The main findings of the present study in patients with unilateral TLE were as follows: (1) the ipsilateral side exhibited decreased glymphatic clearance function compared to the contralateral side, as indicated by the DTI-ALPS index; (2) both the increased CPV/ICV and decreased DTI-ALPS index, which significantly correlated with each other, were identified as independent risk factors for semantic fluency impairment; and (3) the DTI-ALPS index may totally mediate the relationship between CP enlargement and semantic fluency impairment.
GS plays a crucial role in removing metabolites and misfolded proteins from the brain by facilitating the exchange of para-arterial cerebrospinal fluid (CSF) and paravenous interstitial fluid (ISF) through aquaporin-4 located at astrocytic end feet (Iliff et al., 2012; Christensen et al., 2022). While the gold standard for evaluating GS function involves invasive intrathecal contrast agent injection and repeated MRI scans (Eide and Ringstad, 2015), a novel non-invasive method, DTI-ALPS, has been developed and demonstrated to be closely correlate with the gold standard (Zhang W. et al., 2021), where decreased DTI-ALPS index indicates impaired GS function (Taoka et al., 2017; McKnight et al., 2021). In this research, we found that the ipsilateral DTI-ALPS index was lower than the contralateral side, consistent with previous studies in TLE (Zhang et al., 2023; Zhao et al., 2023), which seems to indicate the worse glymphatic clearance function in the affected hemisphere. The mechanism may be that excessive oxidative stress, glutamic acid, and pro-inflammatory mediators produced by seizures can induce disruption of the blood–brain barrier and may lead to abnormalities in the CSF-ISF circulation, thereby leading to GS dysfunction (Gorter et al., 2015; Liu et al., 2020). The potential vulnerability of the ipsilateral hemisphere of patients with unilateral focal epilepsy (Sidhu et al., 2018) and the independent blood supply system of the bilateral hemispheres (Zhang et al., 2023) may contribute to asynchronous GS function changes.
However, some controversies regarding the interpretation of the DTI-ALPS method have recently been raised (Agarwal et al., 2024; Ringstad, 2024). The main arguments are as follows: (1) Due to the partial volume effect and the limited imaging resolution, the ROI may contain information from the nonperivascular space in addition to the perivascular space (PVS) (Ringstad, 2024). (2) Based on prior findings that blood vessels are more abundant in the cortex than in the subcortical white matter, along with the sparse tracer enhancement observed in deep white matter areas, Ringstad (2024) supposed that the GS plays a minor role in brain clearance within the deep white matter. The above experimental results only indicate that the number of PVS in deep white matter is less than that in the cortex, but cannot infer that the role of GS in clearing metabolic waste in deep white matter is smaller and Ringstad’s speculation is lack of pathophysiological confirmation. In 2021, Zhang et al. placed ROIs in six brain areas (lateral ventricle, third ventricle, fourth ventricle, precentral gyrus, frontal horn, and inferior frontal gyrus) on 3D-T1WI at baseline and 39 h after intrathecal gadolinium injection to obtain glymphatic clearance function (Zhang W. et al., 2021). They found that the DTI-ALPS index was always correlated with glymphatic clearance function calculated by the gold standard in all six brain regions and thus concluded that the DTI-ALPS index might represent glymphatic clearance function. Most studies have found that the DTI-ALPS index is associated with other GS evaluation parameters, such as PVS volume and CPV (Tu et al., 2023; Pang et al., 2024). Regardless of the specific mechanism underlying this index, we believe that the correlation between the decrease in its value and the decline in GS function does exist. Therefore, we propose that the DTI-ALPS index can serve as an indirect imaging marker of GS function.
No significant difference was observed between ipsilateral and contralateral CPV in unilateral TLE patients in our study. This lack of difference may be attributed to the communication of CSF between the lateral ventricles through the interventricular foramen, which exposes the CP on both sides to the same fluid pressure within the lateral ventricles (Lehtinen et al., 2013). Currently, most previous studies have investigated the role of total CPV rather than the unilateral CPV (Choi et al., 2022; Jeong et al., 2023; Jiang et al., 2023b). In a physiologically normal state, CP, a highly vascularized tissue, plays a crucial role in regulating CSF production (Lindvall et al., 1978; Moskowitz et al., 1979; Lindvall and Owman, 1981). CP regulates GS function through several possible mechanisms. First, CP may respond to increased metabolic waste by promoting CSF production, thereby facilitating the removal of waste products in the brain (McKnight et al., 2020). Second, the clearance function of GS is associated with the sleep–wake cycle, with GS function being more active at night (Xie et al., 2013). The synchronization of CSF production and drainage improves the effectiveness of waste elimination, suggesting that clearance efficiency is optimized when circadian rhythms of CSF production align with GS activity (Myung et al., 2018). Enlarged CP, associated with factors such as increased blood-CSF barrier permeability and oxidative stress, has been confirmed to correlate with impaired glymphatic clearance function (Li et al., 2023). In this study, both ipsilateral and contralateral DTI-ALPS index, as well as the mean DTI-ALPS index, exhibited a moderately negative correlation with CP enlargement, probably indicating an association between enlarged CP and impaired glymphatic clearance function in TLE patients. The CP-associated GS dysfunction could be attributed to the impaired astrocyte-dependent CSF-ISF exchange caused by inflammatory chemicals in the CSF (Xie et al., 2024). In addition, our correlation analyses in HCs revealed that CPV was negatively correlated with the MMSE score, a finding also observed in the Alzheimer’s continuum (Jiang et al., 2024), cerebral small vessel disease (Zhang W. et al., 2021) and older adults with objectively normal cognition (Park et al., 2023). To confirm this conclusion, however, future studies with large, healthy populations are needed.
The SVF test is a widely used cognitive screening tool that assesses language generation speed, naming ability, memory, executive function, semantic organization, and extraction tactics (Lezak et al., 2012). Impairment in semantic memory can also affect other cognitive functions (Mardh et al., 2013). In this study, we assessed two aspects of verbal fluency, phonological fluency and semantic fluency. Our findings indicated that GS function correlated with SVF but not with PFT. This observation is partially supported by previous research showing that patients with unilateral temporal lobe lesions had impaired semantic fluency with maintaining intact phonological fluency (Troyer et al., 1998). These findings can be explained by the anatomical and functional characteristics of the temporal lobe. Anatomically, the bilateral anterior temporal lobe is identified as the semantic hub, an area affected in cases of semantic dementia (Hodges and Patterson, 2007; Patterson et al., 2007; Landin-Romero et al., 2016). In neurologically normal participants, the left temporal lobe density exhibited a stronger correlation with SVF than with PFT (Grogan et al., 2009). Functionally, the left temporal cortex demonstrated greater activation during semantic fluency than phonological fluency (Gourovitch et al., 2000).
Recent research has demonstrated semantic memory impairment in TLE patients (Giovagnoli et al., 2005; Messas et al., 2008; Lomlomdjian et al., 2011), which was also observed in our study. Our findings of associations between glymphatic function and semantic fluency performance are partially supported by a study that found enlarged perivascular space (EPVS) burden was associated with worse semantic memory performance (Javierre-Petit et al., 2020). EPVS is considered a potential indicator of decreased glymphatic clearance (Braffman et al., 1988; Wardlaw et al., 2013), which could lead to the accumulation of neurotoxic metabolites (Hlauschek et al., 2024). Previous research in early dementia mice has shown that unimpeded GS function plays an important role in maintaining memory functions (Lee et al., 2020). To the best of our knowledge, this study is the first to demonstrate that GS dysfunction exacerbates the semantic fluency impairment in TLE patients, but further research is needed to corroborate this finding by integrating various MRI methods of evaluating human GS function, such as free water analysis and PVS volume, to have a more comprehensive understanding of the role of GS in semantic fluency performance (Taoka et al., 2024).
To examine the clinical relevance of CP enlargement, we analyzed the association between CP enlargement and semantic fluency performance. An indirect association totally mediated by the mean DTI-ALPS index of bilateral hemispheres was identified. Additionally, the relationship between CP enlargement in the ipsilateral (or contralateral) hemisphere and semantic fluency performance was found to be mediated by the DTI-ALPS index in the ipsilateral (or contralateral) hemisphere. As far as we are aware, this is the first research demonstrating the mediating role of the DTI-ALPS index in the relationship between CP enlargement and semantic fluency performance. Previous studies in multiple sclerosis, where the incidence of epilepsy is 2.5 times than that of normal people (Burman and Zelano, 2017), have reported that the relationship between CP enlargement and deep gray matter atrophy was partially mediated by the DTI-ALPS index (Xie et al., 2024), and that deep gray matter volume was positively correlated with semantic fluency (Kania et al., 2024). We hypothesize that a similar mediating path occurs in TLE. Specifically, aberrant glymphatic drainage caused by abnormal CSF production could lead to changes in deep grey matter volume, which may further contribute to impaired semantic fluency performance. The underlying mechanism could be either that the rhythm of CP production is impacted by epileptic seizure and the nonsynchronous state between the circadian rhythm of CP production and GS activity results in poor GS function, or that the reduced capacity to produce more CSF in response to increased metabolic waste affects fluid flow within the GS, thereby reducing its clearance. The accumulation of metabolic waste in the brain caused by the deterioration of GS function may be the cause of the neuronal damage that may lead to semantic fluency impairment. However, this needs confirmation in future studies with large sample sizes and complex experimental designs where deep grey matter volume is available. Our mediation analyses suggest that the impaired clearance function indicated by the DTI-ALPS index is more closely related to SVF performance than the CSF production function represented by CP. Future insights into how CP function and SVF relate to each other as well as which part of GS function is more strongly correlated with semantic fluency performance are needed. To improve semantic fluency in TLE patients, promoting CSF drainage rather than production may be a preferable therapeutic target.
Our study has several limitations. First, the generalizability of our findings is constrained by this being a single-center study with a small sample size. Second, the relationship between glymphatic measures and neuropsychological performance did not account for potential confounders, such as medication use. Third, we did not explore the role of the burden of EPVS, a known marker of dementia and cognitive deterioration (Yang et al., 2023), in neuropsychological performance. Fourth, as the participants exhibited relatively high cognitive function, it remains unknown whether GS involvement in semantic fluency is present in epilepsy patients with cognitive impairment or dementia. Finally, the causal relationship between CP enlargement and decreased DTI-ALPS index cannot be fully deduced from this cross-sectional study. Large-scale longitudinal studies are needed to validate this proposed mediation relationship.
5 Conclusion
Our study provides evidence of decreased DTI-ALPS index in the ipsilateral hemisphere compared to the contralateral side in unilateral TLE. Decreased DTI-ALPS index and increased CPV may be the independent risk factor for semantic fluency impairment. The DTI-ALPS index may fully mediate the relationship between CP enlargement and semantic fluency performance. These findings establish a radiological basis for future investigations into the role of the GS in the pathophysiology of TLE.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the ethics committee of Qilu Hospital of Shandong University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
JW: Writing – original draft, Methodology, Conceptualization. XX: Writing – review & editing, Conceptualization, Methodology. BZ: Writing – review & editing, Resources. XMa: Writing – review & editing, Methodology, Formal analysis. FS: Writing – review & editing, Software, Methodology. YW: Writing – review & editing, Software, Methodology. LL: Writing – review & editing, Resources. XMe: Writing – review & editing, Supervision, Methodology.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was supported by the Qingdao Natural Science Foundation (23-2-1-201-zyyd-jch), Qingdao Clinical Research Center for Rare Diseases of Nervous System (22-3-7-lczx-3-nsh), and Qingdao Key Health Discipline Development Fund (QDZDZK-2022-097).
Conflict of interest
FS and YW were employed by Shanghai United Imaging Intelligence Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2024.1459580/full#supplementary-material
Abbreviations
TLE, temporal lobe epilepsy; GS, glymphatic system; DTI-ALPS, diffusion tensor image analysis along the perivascular space; CP, choroid plexus; BNT, Boston Naming Test; DST, Digit Span Test; AD, Alzheimer’s disease; MoCA, Montreal Cognitive Assessment; MMSE, Minimum Mental State Examination; AT, Arithmetic Test; DSST, Digit Symbol Substitution Test; PFT, Phonological Fluency Test; SVF, Semantic Verbal Fluency; DTI, diffusion tensor image; 3D-T1WI, three-dimensional T1-weighted imaging; FLAIR, fluid-attenuated inversion recovery; DWI, diffusion weighted imaging; ICV, intracranial volume; CPV, choroid plexus volume; ROI, region of interest; FDR, false discovery rate; CI, confidence interval; CSF, cerebrospinal fluid; ISF, interstitial fluid; PVS, perivascular space; EPVS, enlarged perivascular space
References
Agarwal, N., Lewis, L. D., Hirschler, L., Rivera, L. R., Naganawa, S., Levendovszky, S. R., et al. (2024). Current understanding of the anatomy, physiology, and magnetic resonance imaging of Neurofluids: update from the 2022 "ISMRM imaging Neurofluids study group" workshop in Rome. J. Magn. Reson. Imaging 59, 431–449. doi: 10.1002/jmri.28759
Alfons, A., Ates, N. Y., and Groenen, P. J. F. (2022). A robust bootstrap test for mediation analysis. Organ. Res. Methods 25, 591–617. doi: 10.1177/1094428121999096
Bao, H.-W.-S. (2023). bruceR: Broadly Useful Convenient and Efficient R Functions [Online]. Available at: https://CRAN.R-project.org/package=bruceR (accessed May 5, 2024).
Beghi, E. (2020). The epidemiology of epilepsy. Neuroepidemiology 54, 185–191. doi: 10.1159/000503831
Braffman, B. H., Zimmerman, R. A., Trojanowski, J. Q., Gonatas, N. K., Hickey, W. F., and Schlaepfer, W. W. (1988). Brain MR: pathologic correlation with gross and histopathology. 2. Hyperintense white-matter foci in the elderly. AJR Am. J. Roentgenol. 151, 559–566. doi: 10.2214/ajr.151.3.559
Burman, J., and Zelano, J. (2017). Epilepsy in multiple sclerosis: a nationwide population-based register study. Neurology 89, 2462–2468. doi: 10.1212/WNL.0000000000004740
Chen, H. L., Chen, P. C., Lu, C. H., Tsai, N. W., Yu, C. C., Chou, K. H., et al. (2021). Associations among cognitive functions, plasma DNA, and diffusion tensor image along the perivascular space (DTI-ALPS) in patients with Parkinson's disease. Oxidative Med. Cell. Longev. 2021:4034509. doi: 10.1155/2021/4034509
Choi, J. D., Moon, Y., Kim, H. J., Yim, Y., Lee, S., and Moon, W. J. (2022). Choroid plexus volume and permeability at brain MRI within the Alzheimer disease clinical Spectrum. Radiology 304, 635–645. doi: 10.1148/radiol.212400
Christensen, J., Li, C., and Mychasiuk, R. (2022). Choroid plexus function in neurological homeostasis and disorders: the awakening of the circadian clocks and orexins. J. Cereb. Blood Flow Metab. 42, 1163–1175. doi: 10.1177/0271678X221082786
Eide, P. K., and Ringstad, G. (2015). MRI with intrathecal MRI gadolinium contrast medium administration: a possible method to assess glymphatic function in human brain. Acta Radiol. Open 4:2058460115609635. doi: 10.1177/2058460115609635
Ge, Y. A., Tang, Z. Y., Ma, L., Jiang, C. W., Shi, F., Du, S. Y., et al. (2022). "Multi-scale and focal region based deep learning network for fine brain Parcellation", in: 13th international workshop on machine learning in medical imaging (MLMI). (Singapore, Singapore: Springer International Publishing Ag)
Giovagnoli, A. R., Erbetta, A., Villani, F., and Avanzini, G. (2005). Semantic memory in partial epilepsy: verbal and non-verbal deficits and neuroanatomical relationships. Neuropsychologia 43, 1482–1492. doi: 10.1016/j.neuropsychologia.2004.12.010
Gorter, J. A., van Vliet, E. A., and Aronica, E. (2015). Status epilepticus, blood-brain barrier disruption, inflammation, and epileptogenesis. Epilepsy Behav. 49, 13–16. doi: 10.1016/j.yebeh.2015.04.047
Gourovitch, M. L., Kirkby, B. S., Goldberg, T. E., Weinberger, D. R., Gold, J. M., Esposito, G., et al. (2000). A comparison of rCBF patterns during letter and semantic fluency. Neuropsychology 14, 353–360. doi: 10.1037//0894-4105.14.3.353
Grogan, A., Green, D. W., Ali, N., Crinion, J. T., and Price, C. J. (2009). Structural correlates of semantic and phonemic fluency ability in first and second languages. Cereb. Cortex 19, 2690–2698. doi: 10.1093/cercor/bhp023
Hauser, W. A., Annegers, J. F., and Rocca, W. A. (1996). Descriptive epidemiology of epilepsy: contributions of population-based studies from Rochester, Minnesota. Mayo Clin. Proc. 71, 576–586. doi: 10.4065/71.6.576
Hlauschek, G., Nicolo, J. P., Sinclair, B., Law, M., Yasuda, C. L., Cendes, F., et al. (2024). Role of the glymphatic system and perivascular spaces as a potential biomarker for post-stroke epilepsy. Epilepsia Open 9, 60–76. doi: 10.1002/epi4.12877
Hodges, J. R., and Patterson, K. (2007). Semantic dementia: a unique clinicopathological syndrome. Lancet Neurol. 6, 1004–1014. doi: 10.1016/S1474-4422(07)70266-1
Hsu, J. L., Wei, Y. C., Toh, C. H., Hsiao, I. T., Lin, K. J., Yen, T. C., et al. (2023). Magnetic resonance images implicate that glymphatic alterations mediate cognitive dysfunction in Alzheimer disease. Ann. Neurol. 93, 164–174. doi: 10.1002/ana.26516
Iliff, J. J., Wang, M., Liao, Y., Plogg, B. A., Peng, W., Gundersen, G. A., et al. (2012). A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci. Transl. Med. 4:147ra111. doi: 10.1126/scitranslmed.3003748
Javierre-Petit, C., Schneider, J. A., Kapasi, A., Makkinejad, N., Tamhane, A. A., Leurgans, S. E., et al. (2020). Neuropathologic and cognitive correlates of enlarged perivascular spaces in a community-based cohort of older adults. Stroke 51, 2825–2833. doi: 10.1161/STROKEAHA.120.029388
Jeong, S. H., Park, C. J., Jeong, H. J., Sunwoo, M. K., Ahn, S. S., Lee, S. K., et al. (2023). Association of choroid plexus volume with motor symptoms and dopaminergic degeneration in Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 94, 1047–1055. doi: 10.1136/jnnp-2023-331170
Jiang, D., Liu, L., Kong, Y., Chen, Z., Rosa-Neto, P., Chen, K., et al. (2023a). Correction: regional glymphatic abnormality in behavioral variant frontotemporal dementia. Ann. Neurol. 95:209. doi: 10.1002/ana.26806
Jiang, D., Liu, L., Kong, Y., Chen, Z., Rosa-Neto, P., Chen, K., et al. (2023b). Regional glymphatic abnormality in behavioral variant frontotemporal dementia. Ann. Neurol. 94, 442–456. doi: 10.1002/ana.26710
Jiang, J., Zhuo, Z., Wang, A., Li, W., Jiang, S., Duan, Y., et al. (2024). Choroid plexus volume as a novel candidate neuroimaging marker of the Alzheimer's continuum. Alzheimers Res. Ther. 16:149. doi: 10.1186/s13195-024-01520-w
Kania, K., Pawlak, M. A., Forycka, M., Wiłkość-Dębczyńska, M., Michalak, S., Łukaszewska, A., et al. (2024). Predicting clinical progression and cognitive decline in patients with relapsing-remitting multiple sclerosis: a 6-year follow-up study. Neurol. Neurochir. Pol. 58, 176–184. doi: 10.5603/pjnns.97714
Landin-Romero, R., Tan, R., Hodges, J. R., and Kumfor, F. (2016). An update on semantic dementia: genetics, imaging, and pathology. Alzheimers Res. Ther. 8:52. doi: 10.1186/s13195-016-0219-5
Lee, Y., Choi, Y., Park, E. J., Kwon, S., Kim, H., Lee, J. Y., et al. (2020). Improvement of glymphatic-lymphatic drainage of beta-amyloid by focused ultrasound in Alzheimer's disease model. Sci. Rep. 10:16144. doi: 10.1038/s41598-020-73151-8
Lee, H. J., Lee, D. A., Shin, K. J., and Park, K. M. (2022). Glymphatic system dysfunction in patients with juvenile myoclonic epilepsy. J. Neurol. 269, 2133–2139. doi: 10.1007/s00415-021-10799-w
Lee, D. A., Park, B. S., Ko, J., Park, S. H., Lee, Y. J., Kim, I. H., et al. (2022). Glymphatic system dysfunction in temporal lobe epilepsy patients with hippocampal sclerosis. Epilepsia Open 7, 306–314. doi: 10.1002/epi4.12594
Lehtinen, M. K., Bjornsson, C. S., Dymecki, S. M., Gilbertson, R. J., Holtzman, D. M., and Monuki, E. S. (2013). The choroid plexus and cerebrospinal fluid: emerging roles in development, disease, and therapy. J. Neurosci. 33, 17553–17559. doi: 10.1523/JNEUROSCI.3258-13.2013
Lezak, M. D., Howieson, D. B., Bigler, E. D., and Tranel, D. (2012). Neuropsychological assessment. (5th ed.) Edn. Oxford: Oxford University Press.
Li, Y., Zhou, Y., Zhong, W., Zhu, X., Chen, Y., Zhang, K., et al. (2023). Choroid plexus enlargement exacerbates white matter hyperintensity growth through glymphatic impairment. Ann. Neurol. 94, 182–195. doi: 10.1002/ana.26648
Lindvall, M., Alumets, J., Edvinsson, L., Fahrenkrug, J., Hakanson, R., Hanko, J., et al. (1978). Peptidergic (VIP) nerves in the mammalian choroid plexus. Neurosci. Lett. 9, 77–82. doi: 10.1016/0304-3940(78)90051-4
Lindvall, M., and Owman, C. (1981). Autonomic nerves in the mammalian choroid plexus and their influence on the formation of cerebrospinal fluid. J. Cereb. Blood Flow Metab. 1, 245–266. doi: 10.1038/jcbfm.1981.30
Liu, C., Habib, T., Salimeen, M., Pradhan, A., Singh, M., Wang, M., et al. (2020). Quantification of visible Virchow-Robin spaces for detecting the functional status of the glymphatic system in children with newly diagnosed idiopathic generalized epilepsy. Seizure 78, 12–17. doi: 10.1016/j.seizure.2020.02.015
Lomlomdjian, C., Solis, P., Medel, N., and Kochen, S. (2011). A study of word finding difficulties in Spanish speakers with temporal lobe epilepsy. Epilepsy Res. 97, 37–44. doi: 10.1016/j.eplepsyres.2011.06.016
Mardh, S., Nagga, K., and Samuelsson, S. (2013). A longitudinal study of semantic memory impairment in patients with Alzheimer's disease. Cortex 49, 528–533. doi: 10.1016/j.cortex.2012.02.004
McKnight, C. D., Rouleau, R. M., Donahue, M. J., and Claassen, D. O. (2020). The regulation of cerebral spinal fluid flow and its relevance to the glymphatic system. Curr. Neurol. Neurosci. Rep. 20:58. doi: 10.1007/s11910-020-01077-9
McKnight, C. D., Trujillo, P., Lopez, A. M., Petersen, K., Considine, C., Lin, Y. C., et al. (2021). Diffusion along perivascular spaces reveals evidence supportive of glymphatic function impairment in Parkinson disease. Parkinsonism Relat. Disord. 89, 98–104. doi: 10.1016/j.parkreldis.2021.06.004
Meneses, R. F., Pais-Ribeiro, J. L., da Silva, A. M., and Giovagnoli, A. R. (2009). Neuropsychological predictors of quality of life in focal epilepsy. Seizure 18, 313–319. doi: 10.1016/j.seizure.2008.11.010
Messas, C. S., Mansur, L. L., and Castro, L. H. (2008). Semantic memory impairment in temporal lobe epilepsy associated with hippocampal sclerosis. Epilepsy Behav. 12, 311–316. doi: 10.1016/j.yebeh.2007.10.014
Moskowitz, M. A., Liebmann, J. E., Reinhard, J. F. Jr., and Schlosberg, A. (1979). Raphe origin of serotonin-containing neurons within choroid plexus of the rat. Brain Res. 169, 590–594. doi: 10.1016/0006-8993(79)90410-4
Myung, J., Wu, D., Simonneaux, V., and Lane, T. J. (2018). Strong circadian rhythms in the choroid plexus: implications for sleep-independent brain metabolite clearance. J. Exp. Neurosci. 12:1179069518783762. doi: 10.1177/1179069518783762
Osborne, E. L., Ainsworth, B., Chadwick, P., and Atkinson, M. J. (2023). The role of emotion regulation in the relationship between mindfulness and risk factors for disordered eating: a longitudinal mediation analysis. Int. J. Eat. Disord. 56, 458–463. doi: 10.1002/eat.23849
Pang, H., Wang, J., Yu, Z., Yu, H., Li, X., Bu, S., et al. (2024). Glymphatic function from diffusion-tensor MRI to predict conversion from mild cognitive impairment to dementia in Parkinson's disease. J. Neurol. 271, 5598–5609. doi: 10.1007/s00415-024-12525-8
Park, C. J., Kim, S. Y., Kim, J. H., Son, N. H., Park, J. Y., Jeong, Y. H., et al. (2023). Evaluation of glymphatic system activity using diffusion tensor image analysis along the perivascular space and amyloid PET in older adults with objectively normal cognition: a preliminary study. Front. Aging Neurosci. 15:1221667. doi: 10.3389/fnagi.2023.1221667
Patterson, K., Nestor, P. J., and Rogers, T. T. (2007). Where do you know what you know? The representation of semantic knowledge in the human brain. Nat. Rev. Neurosci. 8, 976–987. doi: 10.1038/nrn2277
Piantino, J. A., Iliff, J. J., Lim, M. M., and Levendovszky, S. R. (2023). Reader response: association of sleep, neuropsychological performance, and gray matter volume with glymphatic function in community-dwelling older adults. Neurology 100, 355–356. doi: 10.1212/WNL.0000000000206874
Pu, W., Wei, S., Qiu, M., Chen, X., Zou, W., Ge, Y., et al. (2023). Dysfunction of the glymphatic system in childhood absence epilepsy. Front. Neurosci. 17:1312676. doi: 10.3389/fnins.2023.1312676
Ringstad, G. (2024). Glymphatic imaging: a critical look at the DTI-ALPS index. Neuroradiology 66, 157–160. doi: 10.1007/s00234-023-03270-2
Sidhu, M. K., Duncan, J. S., and Sander, J. W. (2018). Neuroimaging in epilepsy. Curr. Opin. Neurol. 31, 371–378. doi: 10.1097/WCO.0000000000000568
Stasenko, A., Kaestner, E., Arienzo, D., Schadler, A., Reyes, A., Shih, J. J., et al. (2023). Bilingualism and structural network organization in temporal lobe epilepsy: resilience in neurologic disease. Neurology 100, e1887–e1899. doi: 10.1212/WNL.0000000000207087
Taoka, T., Ito, R., Nakamichi, R., Nakane, T., Kawai, H., and Naganawa, S. (2024). Diffusion tensor image analysis ALong the perivascular space (DTI-ALPS): revisiting the meaning and significance of the method. Magn. Reson. Med. Sci. 23, 268–290. doi: 10.2463/mrms.rev.2023-0175
Taoka, T., Masutani, Y., Kawai, H., Nakane, T., Matsuoka, K., Yasuno, F., et al. (2017). Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer's disease cases. Jpn. J. Radiol. 35, 172–178. doi: 10.1007/s11604-017-0617-z
Tax, C. M. W., Bastiani, M., Veraart, J., Garyfallidis, E., and Okan Irfanoglu, M. (2022). What's new and what's next in diffusion MRI preprocessing. NeuroImage 249:118830. doi: 10.1016/j.neuroimage.2021.118830
Thijs, R. D., Surges, R., O'Brien, T. J., and Sander, J. W. (2019). Epilepsy in adults. Lancet 393, 689–701. doi: 10.1016/S0140-6736(18)32596-0
Tingley, D., Yamamoto, T., Hirose, K., Keele, L., and Imai, K. (2014). Mediation: R package for causal mediation analysis. J. Stat. Softw. 59:38. doi: 10.18637/jss.v059.i05
Troyer, A. K., Moscovitch, M., Winocur, G., Alexander, M. P., and Stuss, D. (1998). Clustering and switching on verbal fluency: the effects of focal frontal- and temporal-lobe lesions. Neuropsychologia 36, 499–504. doi: 10.1016/s0028-3932(97)00152-8
Tu, Y., Li, Z., Xiong, F., and Gao, F. (2023). Decreased DTI-ALPS and choroid plexus enlargement in fibromyalgia: a preliminary multimodal MRI study. Neuroradiology 65, 1749–1755. doi: 10.1007/s00234-023-03240-8
Wang, L., Chen, S., Liu, C., Lin, W., and Huang, H. (2020). Factors for cognitive impairment in adult epileptic patients. Brain Behav. 10:e01475. doi: 10.1002/brb3.1475
Wang, J., Zhou, Y., Zhang, K., Ran, W., Zhu, X., Zhong, W., et al. (2023). Glymphatic function plays a protective role in ageing-related cognitive decline. Age Ageing 52:afad107. doi: 10.1093/ageing/afad107
Wardlaw, J. M., Smith, E. E., Biessels, G. J., Cordonnier, C., Fazekas, F., Frayne, R., et al. (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838. doi: 10.1016/S1474-4422(13)70124-8
Wu, J., Xia, Y., Wang, X., Wei, Y., Liu, A., Innanje, A., et al. (2023). uRP: an integrated research platform for one-stop analysis of medical images. Front Radiol 3:1153784. doi: 10.3389/fradi.2023.1153784
Xie, L., Kang, H., Xu, Q., Chen, M. J., Liao, Y., Thiyagarajan, M., et al. (2013). Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. doi: 10.1126/science.1241224
Xie, Y., Zhu, H., Yao, Y., Liu, C., Wu, S., Zhang, Y., et al. (2024). Enlarged choroid plexus in relapsing-remitting multiple sclerosis may lead to brain structural changes through the glymphatic impairment. Mult. Scler. Relat. Disord. 85:105550. doi: 10.1016/j.msard.2024.105550
Yang, Y., Wang, M., Luan, M., Song, X., Wang, Y., Xu, L., et al. (2023). Enlarged perivascular spaces and age-related clinical diseases. Clin. Interv. Aging 18, 855–867. doi: 10.2147/CIA.S404908
Yeh, F. C., Verstynen, T. D., Wang, Y., Fernandez-Miranda, J. C., and Tseng, W. Y. (2013). Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS One 8:e80713. doi: 10.1371/journal.pone.0080713
Yeh, F. C., Zaydan, I. M., Suski, V. R., Lacomis, D., Richardson, R. M., Maroon, J. C., et al. (2019). Differential tractography as a track-based biomarker for neuronal injury. NeuroImage 202:116131. doi: 10.1016/j.neuroimage.2019.116131
Zhang, X., Wang, Y., Jiao, B., Wang, Z., Shi, J., Zhang, Y., et al. (2024). Glymphatic system impairment in Alzheimer's disease: associations with perivascular space volume and cognitive function. Eur. Radiol. 34, 1314–1323. doi: 10.1007/s00330-023-10122-3
Zhang, C., Xu, K., Zhang, H., Sha, J., Yang, H., Zhao, H., et al. (2023). Recovery of glymphatic system function in patients with temporal lobe epilepsy after surgery. Eur. Radiol. 33, 6116–6123. doi: 10.1007/s00330-023-09588-y
Zhang, Y., Zhang, R., Ye, Y., Wang, S., Jiaerken, Y., Hong, H., et al. (2021). The influence of demographics and vascular risk factors on glymphatic function measured by diffusion along perivascular space. Front. Aging Neurosci. 13:693787. doi: 10.3389/fnagi.2021.693787
Zhang, W., Zhou, Y., Wang, J., Gong, X., Chen, Z., Zhang, X., et al. (2021). Glymphatic clearance function in patients with cerebral small vessel disease. NeuroImage 238:118257. doi: 10.1016/j.neuroimage.2021.118257
Keywords: central nervous system, temporal lobe epilepsy, diffusion tensor imaging, glymphatic system, semantic fluency, choroid plexus
Citation: Wang J, Xia X, Zhang B, Ma X, Shi F, Wei Y, Li L and Meng X (2024) Association of glymphatic system dysfunction with cognitive impairment in temporal lobe epilepsy. Front. Aging Neurosci. 16:1459580. doi: 10.3389/fnagi.2024.1459580
Edited by:
Alexandre Bejanin, Hospital de la Santa Cruz and San Pablo, SpainReviewed by:
Kang Min Park, Inje University Haeundae Paik Hospital, Republic of KoreaDongcui Wang, Central South University, China
Copyright © 2024 Wang, Xia, Zhang, Ma, Shi, Wei, Li and Meng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Li, bGlsaW5nNTMxM0BzaW5hLmNvbQ==; Xiangshui Meng, bWVuZ3hpYW5nc2h1aTIwMjFAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share last authorship
 Jiajia Wang
Jiajia Wang Xiaona Xia2†
Xiaona Xia2† Xiaotian Ma
Xiaotian Ma Feng Shi
Feng Shi Ying Wei
Ying Wei Ling Li
Ling Li Xiangshui Meng
Xiangshui Meng