
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 26 September 2024
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 16 - 2024 | https://doi.org/10.3389/fnagi.2024.1449276
This article is part of the Research Topic Spotlight on the Relationship between Stress and Ageing in Brain and Heart View all articles
 Rong-Pei Liu1†
Rong-Pei Liu1† Guo-Liang Lin1†
Guo-Liang Lin1† Lu-Lu Ma1†
Lu-Lu Ma1† Shi-Shi Huang1
Shi-Shi Huang1 Cheng-Xiang Yuan1
Cheng-Xiang Yuan1 Shi-Guo Zhu1
Shi-Guo Zhu1 Mei-Ling Sheng1
Mei-Ling Sheng1 Ming Zou1
Ming Zou1 Jian-Hong Zhu2*
Jian-Hong Zhu2* Xiong Zhang1*
Xiong Zhang1* Jian-Yong Wang1*
Jian-Yong Wang1*Background: Cognitive impairment (CI) is common in Parkinson’s disease (PD). Multiple brain regions and their interactions are involved in PD associated CI. Magnetic resonance imaging (MRI) technology is a non-invasive method in investigating brain structure and inter-regional connections. In this study, by comparing cortical thickness, subcortical volume, and brain network topology properties in PD patients with and without CI, we aimed to understand the changes of brain structure and structural covariance network properties in PD associated CI.
Methods: A total of 18 PD patients with CI and 33 PD patients without CI were recruited. Movement Disorder Society Unified Parkinson’s Disease Rating Scale, Hoehn and Yahr stage, Mini Mental State Examination Scale, Non-motor Symptom Rating Scale, Hamilton Anxiety Scale, and Hamilton Depression Scale were assessed. All participants underwent structural 3T MRI. Cortical thickness, subcortical volume, global and nodal network topology properties were measured.
Results: Compared with PD patients without CI, the volumes of white matter, thalamus and hippocampus were lower in PD patients with CI. And decreased whole-brain local efficiency is associated with CI in PD patients. While the cortical thickness and nodal network topology properties were comparable between PD patients with and without CI.
Conclusion: Our findings support the alterations of brain structure and disruption of structural covariance network are involved in PD associated CI, providing a new insight into the association between graph properties and PD associated CI.
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by motor symptoms bradykinesia, rest tremor, rigidity and postural instability (Postuma et al., 2015). With the progression of the disease, the prevalence of cognitive impairment (CI) in PD patients can reach as high as 83%, which greatly affects the quality of life of PD patients (Hely et al., 2008).
Different from Alzheimer’s disease (AD), CI in PD patients mainly involves executive and visuospatial functions (Aarsland et al., 2021). The mechanisms underlying CI in PD remain largely unclear, neurotransmitters disturbance in multiple brain regions, as well as abnormal deposition of substances such as α-synuclein and β-amyloid are believed to be involved (Aarsland et al., 2021). Difficulties in obtaining brain tissue from patients are a major obstacle to the progress of related research.
As a non-invasive method, magnetic resonance imaging (MRI) technology is widely used to explore changes in brain structure and function. In addition to conventional MRI, more advanced methods such as resting state functional MRI (rs-fMRI) and diffusion tensor imaging (DTI) adept at exploring the structural and functional connections between brain regions, and have revealed relevant characteristic changes in a variety of neurodegenerative diseases (Filippi et al., 2021; Firbank et al., 2016; Dopper et al., 2014). However, we have to admit that individual functional magnetic characteristics vary in different cognitive activity states (Cole et al., 2021).
Some analysis techniques based on brain MRI morphology, which are less sensitive to brain activity state (Bernhardt et al., 2010), complement rs-fMRI and DTI in investigating brain structure and inter-regional connections. These morphological features include cortical thickness, subcortical volume, whole-brain and nodal networks. Some previous studies using voxel-based morphometry method have explored gray matter (GM) changes in PD patients with CI. For instance, a study compared GM volumes of 12 PD patients with dementia and 12 PD patients without dementia, and found that patients with PD dementia had decreased bilateral superior temporal gyrus, bilateral posterior cingulate, left cingulate gyrus, right parahippocampal gyrus, right hippocampus, right precuneus, right cuneus, left inferior frontal gyrus and left insular lobe (Xia et al., 2013). Another study including 25 PD patients with mild CI (MCI) and 65 PD patients without CI found that PD with MCI is associated reduced thalamus and nucleus accumbens volumes (Mak et al., 2014). Their results were inconclusive, and they did not simultaneously calculate the white matter volume and network properties changes.
In this study, we measured cortical thickness, subcortical volumes, and brain network topology properties in a cohort of PD patients with and without CI, and aimed to understand the changes in brain structure and structural covariance network properties associated with CI in PD patients.
A total of 51 PD patients were recruited from Department of Neurology, the Second Affiliated Hospital and Yuying Children’s Hospital from July 2019 to October 2021. All patients were diagnosed independently by two movement disorder neurologists according to the movement disorder society clinical diagnostic criteria for PD (Postuma et al., 2015). Exclusion criteria were (1) with history of other neurological or psychiatric disorders besides PD; (2) with history of alcohol or drug abuse; (3) with family history of PD or dementia; (4) with intracranial lesions detected by MRI. This study was approved by the ethics committee of the Second Affiliated Hospital and Yuying Children’s Hospital, Wenzhou Medical University. All participants signed written informed consents.
Clinical data including age, gender, disease duration, years of education, Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), Hoehn and Yahr stage, Mini Mental State Examination Scale (MMSE), Non-motor Symptom Rating Scale (NMSS), Hamilton Anxiety Scale (HAMA), and Hamilton Depression Scale (HAMD) were assessed by face-to-face interview and physical examinations during the “OFF” state. All the patients were divided into PD with CI (including MCI and dementia) and PD without CI subtypes based on the MMSE score and their education level. In brief, subjects with MMSE score ≤ 19 for illiterate, ≤ 22 for elementary education and ≤ 26 for secondary education were considered with CI as previously described (Maimaitituerxun et al., 2023).
All participants were scanned on a 3 Tesla GE-Discovery 750 scanner, and T1-anatomical brain images were acquired with the parameters described before (Yang et al., 2021). After converting to NIFTI format, images were analyzed using FreeSurfer version 7.3.11 (Dale et al., 1999; Fischl et al., 1999). The size of a Gaussian smoothing kernel was 10 mm, which was adopted in previous studies (Gerrits et al., 2016; Tessitore et al., 2016). FreeSurfer generated a surface model for the cortex, and automatically parcellated the cortex into 31 regions-of-interest (ROIs) per hemisphere, according to the Desikan-Killiany-Tourville protocol. For each of the 62 ROIs, FreeSurfer calculated the average cortical thickness. To ensure the quality of T1 images, FreeView visualization tool was utilized for visual inspection.
Subcortical volumes including thalamus, caudate, putamen, pallidum, hippocampus, amygdala, accumbens-area, total white matter (WM), total GM, white matter hyperintensity (WMH), third ventricle (3rd-Ventricle), fourth ventricle (4th-Ventricle), and lateral ventricle were calculated automatically with FreeSurfer’s procedure for volumetric measures, including skull stripping, affine registration with MNI305 space, volumetric labeling, variation correction and visualizing volume (Fischl et al., 2002). During the processing pipeline, FreeSurfer checked the quality of segmentations and generated a quality report, which ensured the validity of the data.
The 76 morphological features (62 cortical thicknesses and 14 subcortical GM volumes) were adjusted with age, gender, and estimated total intracranial volume (eTIV), as previous described (Yun et al., 2020). The resulting residuals were z-score transformed by using the mean and standard deviation values of each ROIs calculated from PD without CI group. The similarity between the ROIs represented the edge of the network, which was calculated using the following formula: 1/e[(zscoreofxthROI-zscoreofythROI)2] (Yun et al., 2020). The networks were thresholded (using a network density of 0.01 to 0.5, with interval of 0.01) and binarized. According to the following criteria: (1) more than 80% of are connected to other nodes; (2) modularity > 0.3; (3) small-world > 1, the network density range was finally determined as 0.1–0.34, with an interval of 0.01.
From these thresholded and binarized networks, six global metrics were determined: small-world index (σ), normalized characteristic path length (λ), normalized clustering coefficient (γ), assortativity, global efficiency (Eglob), and local efficiency (Eloc). The nodal network topology properties include γ, Eloc, degree centrality, and betweenness centrality (Watts and Strogatz, 1998). Estimation of the global and nodal network topology properties was done using the Graph Theoretical Network Analysis (GRETNA)2 package in MATLAB R2022a.
The data were analyzed using Rstudio 2022.12.0. The normal distribution of the data was assessed by Shapiro–Wilk test. Differences in MDS-UPDRS Total, MDS-UPDRS I, MMSE and eTIV between PD with CI and PD without CI groups were analyzed by unpaired two-tailed t-test. Differences in age, disease duration, years of education, MDS-UPDRS II, MDS-UPDRS III, Hoehn and Yahr stage, NMSS, HAMA and HAMD were analyzed by Mann–Whitney test. Difference in gender distribution was assessed by Chi square test. Multivariate analysis was performed by binary logistic regression with age, gender and disease duration as covariates. The differences in cortical thickness were analyzed in FreeSurfer’s statistical program QDEC 1.5 (Gerrits et al., 2016). Global and nodal network topology properties were compared using GRETNA, and false discovery rate (FDR) was used to correct the multiple comparisons. A two-tailed P < 0.05 was considered statistically significant.
There were 18 cases in PD with CI group and 33 cases in PD without CI group. The demographic and clinical characteristics were summarized in Table 1. Age, gender, disease duration, years of education, MDS-UPDRS score, MDS-UPDRS I score, MDS-UPDRS II score, MDS-UPDRS III score, Hoehn and Yahr stage, NMSS score, HAMA score, HAMD score and eTIV were comparable between the two groups (P > 0.05; Table 1). While MMSE score was significantly lower in PD with CI group (P < 0.001; Table 1).
Subcortical volume including WM, GM, WMH, 3rd-ventricle, 4th-ventricle and lateral-ventricle were calculated and their ratios to eTIV were compared between PD patients with and without CI. Results showed that WM/eTIV and GM/eTIV were significantly lower in PD with CI group than in PD without CI group (P = 0.012 and P = 0.026, respectively; Figures 1A, B). No significant difference was found in WMH/eTIV, 3rd-ventricle/eTIV, 4th-ventricle/eTIV and lateral-ventricle/eTIV between the two groups (Figures 1C–F). Further binary logistic regression analysis adjusted for age, gender, and disease duration confirmed a decrease in WM/eTIV in PD patients with CI (P = 0.012, OR 0.72, 95% confidence interval 0.50–0.95; Table 2).
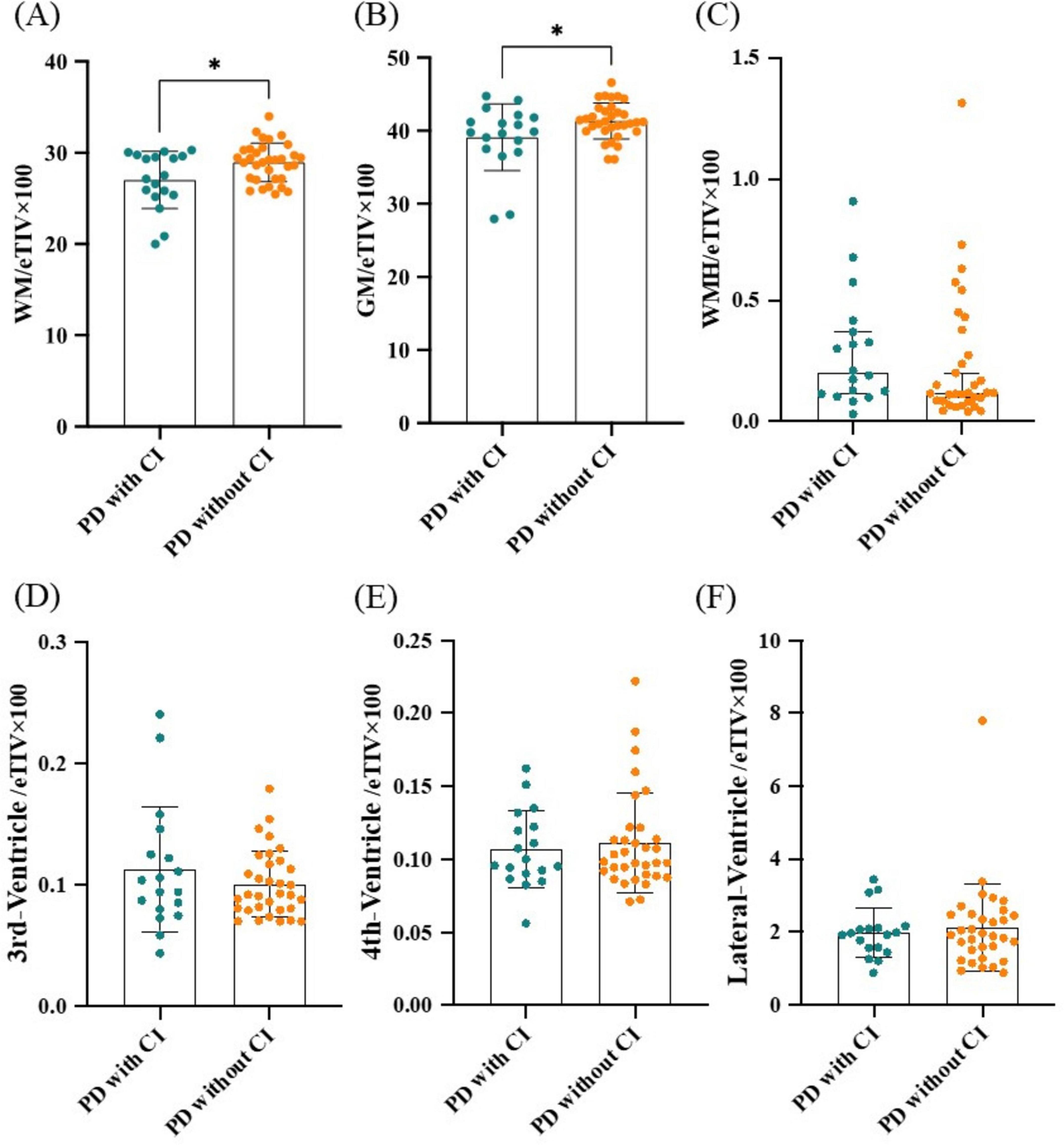
Figure 1. Comparison of subcortical volumes between PD patients with and without CI. (A) WM/eTIV. (B) GM/eTIV. (C) WMH/eTIV. (D) 3rd-Ventricle/eTIV. (E) 4th-Ventricle/eTIV. (F) Lateral-Ventricle/eTIV. n = 18 for PD patients with CI; n = 33 for PD patients without CI. Values are expressed as means ± standard error. *P < 0.05. CI, Cognitive impairment; eTIV, estimated total intracranial volume; GM, Gray matter; PD, Parkinson’s disease; WM, White matter; WMH, White matter hyperintensity.
Next, subcortical GM volume/eTIV including thalamus/eTIV, caudate/eTIV, putamen/eTIV, pallidum/eTIV, hippocampus/eTIV, amygdala/eTIV and accumbens-area/eTIV were compared between PD with and without CI groups. Results showed that thalamus/eTIV, hippocampus/eTIV and amygdala/eTIV were significantly lower in PD patients with CI (P = 0.013, P = 0.016, and P = 0.025; Figures 2A, E, F). The decline in thalamus/eTIV and hippocampus/eTIV was further confirmed by binary logistic regression analysis adjusted for age, gender, and disease duration (P = 0.046, OR 1.10 × 10–4, 95% confidence interval 3.03 × 10–9 to 3.08 × 10–1 and P = 0.044, OR 4.30 × 10–6, 95% confidence interval 5.69 × 10–12 to 2.81 × 10–1, respectively; Table 3). No significant difference was found in caudate/eTIV, putamen/eTIV, pallidum/eTIV and accumbens-area/eTIV between the two groups (Figure 2 and Table 3).
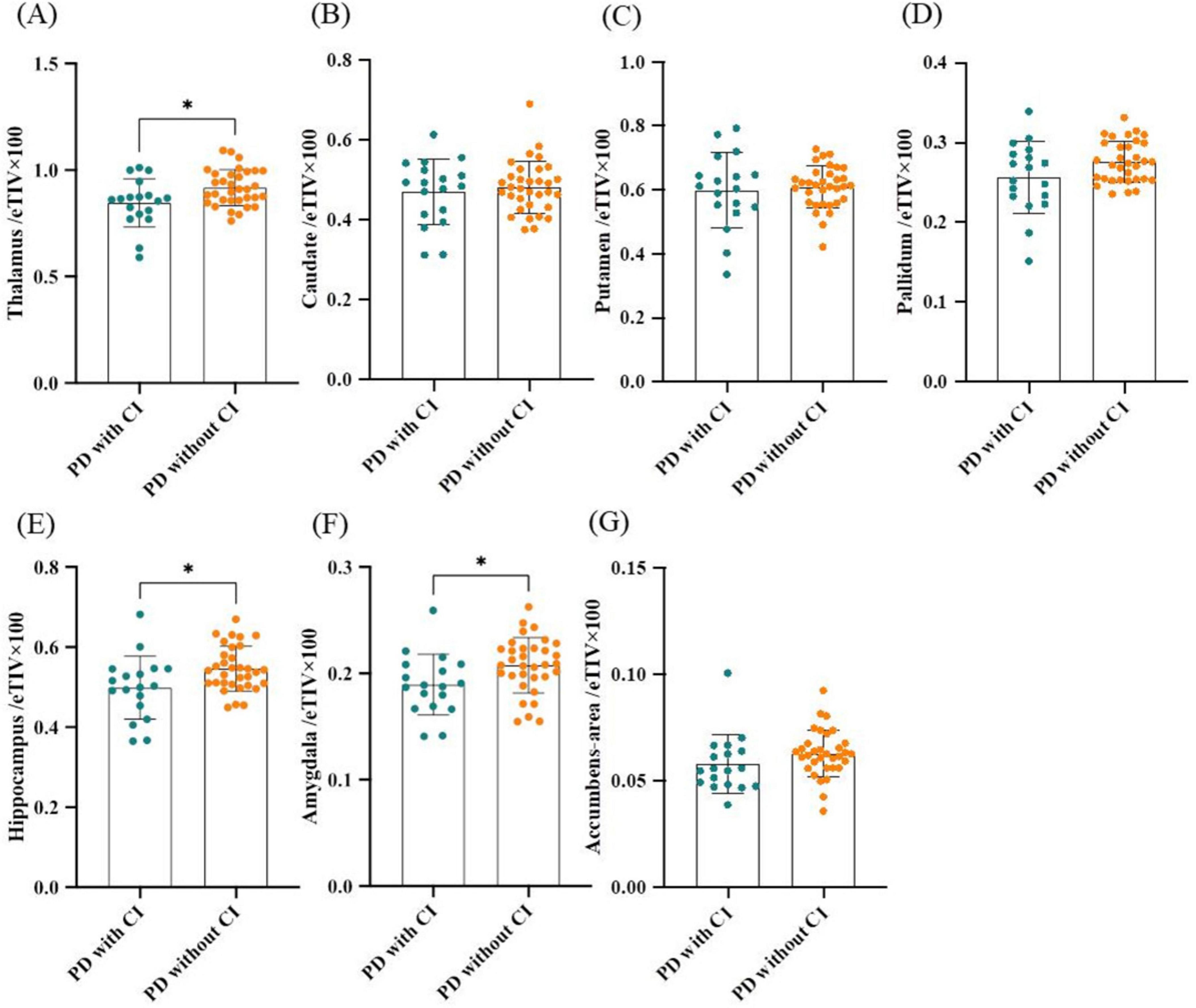
Figure 2. Comparison of subcortical gray matter volumes between PD patients with and without CI. (A) Thalamus/eTIV. (B) Caudate/eTIV. (C) Putamen/eTIV. (D) Pallidum/eTIV. (E) Hippocampus/eTIV. (F) Amygdala/eTIV. (G) Accumbens-area/eTIV. n = 18 for PD patients with CI; n = 33 for PD patients without CI. Values are expressed as means ± standard error. *P < 0.05. CI, Cognitive impairment; eTIV, estimated total intracranial volume; PD, Parkinson’s disease.
The cortical thickness was comparable between PD patients with and without CI (Supplementary Table 1).
Whole-brain network topology properties including σ, λ, γ, assortativity, Eglob, Eloc, and nodal network topology properties including γ, Eloc, degree centrality, betweenness centrality were calculated and analyzed in PD patients with and without CI. Results showed that whole-brain Eloc in PD with CI was significantly decreased (Table 4). No significant differences were found in the remaining whole-brain topology properties between the two groups (Table 4), none was found in nodal network topology properties (Supplementary Tables 2–5).
CI is one of the most common non-motor symptoms of PD patients, and it is believed to be associated with abnormalities in the structural and functional connectivity of multiple brain regions. In the current study, we analyzed cortical thickness, subcortical volume, and brain network topology properties in 18 PD patients with CI and 33 PD patients without CI. Our results demonstrate that WM, thalamus and hippocampus volumes are lower in PD patients with CI than in those without CI. And decreased whole-brain Eloc is associated with CI in PD patients. We did not find differences in cortical thickness and nodal network topology properties between PD patients with and without CI.
In recent years, the non-motor symptoms of PD have received increasing attention (Kalia and Lang, 2015). As the disease progresses, CI in PD patients seems inevitable, which greatly affects their quality of life (Hely et al., 2008; Aarsland et al., 2005). It is believed that PD associated CI involves disturbances in multiple brain regions and neurotransmitter systems (Aarsland et al., 2021).
Some neurocognitive disorders, such as AD and frontotemporal dementia, are more likely to involve the cerebral cortex and cause corresponding symptoms. Therefore, in this study, we first measured the cortical thickness. However, we did not find any difference in cortical thickness between PD patients with CI and those without CI. Similarly, a previous study compared cortical thickness between 29 PD dementia patients and 76 cognitively normal controls, and found that regional cortical thickness was indistinguishable between the two groups (Colloby et al., 2020). However, some other studies have reported that PD associated CI is associated with cortical atrophy in frontal lobe (Gao et al., 2017), left precuneus (Pereira et al., 2014), parietotemporal lobe (Segura et al., 2014), right temporal lobe, right insula, right inferior frontal gyrus and right supplementary motor area (Hanganu et al., 2014). The diversity of results may arise from differences in study designs, cognitive status, ethnicity, and disease duration. For example, only one study was a cohort study that focused on the rate of cortical thinning in different groups (Hanganu et al., 2014), while several other cross-sectional studies focused on baseline differences in cortical thickness between groups (Colloby et al., 2020; Gao et al., 2017; Pereira et al., 2014; Segura et al., 2014). On the other hand, one study compared the differences in cortical thickness between patients with PD dementia and healthy controls using samples from different studies, whereas other studies included comparisons between PD-MCI and PD without MCI groups (Gao et al., 2017; Pereira et al., 2014; Segura et al., 2014; Hanganu et al., 2014). What’s more, the populations in these different studies across China (Gao et al., 2017), Spain (Segura et al., 2014), Canada (Hanganu et al., 2014) and Parkinson’s Progression Markers Initiative (Pereira et al., 2014). In one study, there was a significant difference in disease duration between PD-MCI and PD without MCI groups, which may also interfere with the analysis of results (Segura et al., 2014). In order to understand the relationship between cortical thickness and CI in PD more accurately, future investigations are warranted in larger populations.
Next, subcortical volumes including WM, GM, WMH and ventricles were compared between PD patients with and without CI. Our results showed that WM volume in PD patients with CI is lower than in PD patients without CI. WM is composed of nerve fibers and plays a major role in coordinating communication between brain regions, the dysfunction of which is associated with cognitive slowing, executive dysfunction and memory retrieval deficit (Fields, 2008; Filley and Fields, 2016). It has been noted that changes in WM microstructure precede those in GM in PD patients (Andica et al., 2019). Previous studies have linked WM lesions and hyperintensities to PD associated CI (Melzer et al., 2013; Hattori et al., 2012; Carvalho de Abreu et al., 2023). Our study further indicates that the reduction of WM volume is associated with CI in PD patients. Based on this finding, we suggest that sufficient attention should be paid to WM-related memory and executive functions in PD patients, so that CI can be detected and intervened early.
Subcortical GM is a group of scattered GM islets beneath cerebral cortex. They have their own unique functions and also play a role in integrating nerve impulses from different brain regions. In this study, we found that PD patients with CI had smaller thalamus and hippocampus volumes compared with PD patients without CI. Several previous studies have explored the relationship between subcortical GM change and PD associated CI. Interestingly, reduced hippocampus (Chung et al., 2017; Foo et al., 2017; Schneider et al., 2017) and thalamus (Mak et al., 2014; Chung et al., 2017; Foo et al., 2017) volumes in PD patients with CI seem to be a consistent finding across studies. In addition, right caudate (Chung et al., 2017), caudate, presubiculum, cornu ammonis (Foo et al., 2017), and nucleus accumbens (Mak et al., 2014) have also been reported to be associated with CI in PD. However, they have not yet been replicated in different studies. Therefore, we believe that learning, memory, and sensory integration mediated by the hippocampus and thalamus are worthy of attention when studying PD associated CI.
The application of brain network topological properties is based on the assumption that neurons in interconnected regions share similar developmental and maturational influences, so they have covariant morphological properties (Alexander-Bloch et al., 2013). In the current study, we found whole-brain Eloc is decreased in PD patients with CI. A previous study analyzed the global and regional network topology properties of 33 PD patients with mild CI (MCI) and 90 cognitively normal PD patients from Parkinson’s Progression Markers Initiative, and found that PD patients with MCI showed reduced global average correlation strength and lower regional efficiency in frontal and parietal regions compared with cognitively normal PD patients (Pereira et al., 2015). It is worth noting that unlike our study, which constructed a network for each individual, this study constructed a network for each group. Here, we insist that PD patients with CI are disrupted in the coordination of the brain network.
The interpretation of topology properties changes is difficult, because the precise biological interpretation of each topology property remains unclear. Considering a topological property as a characteristic signature of any specific disease at a single time point may lead to ambiguous conclusions (Douw et al., 2019). However, previous study has shown that topological properties can monitor disease progression from more perspectives (Tijms et al., 2013). Longitudinal studies are needed to further validate the value of whole-brain Eloc in monitoring the progression of PD associated CI.
Meanwhile, we have to acknowledge certain limitations. Due to the limited number of cases, we did not stratify patients according to different cognitive levels. Meanwhile, our study only included a global efficiency scale and no tool to measure daily life functioning. Therefore, the division between patients with and without CI didn’t follow the published guidelines for PD-MCI (Litvan et al., 2012) and PD dementia (Emre et al., 2007). In addition, our study lacks longitudinal observation, the presence of which would make the conclusions more accurate and valuable.
In summary, our study demonstrates a reduction of WM, thalamus and hippocampus volumes and a decrease of whole-brain Eloc in PD patients with CI. Our findings support the alteration of brain structure and disruption of structural covariance network are involved in PD associated CI.
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Ethics committee of the Second Affiliated Hospital and Yuying Children’s Hospital, Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
R-PL: Writing – review & editing, Investigation, Conceptualization. G-LL: Writing – review & editing, Investigation, Conceptualization. L-LM: Writing – original draft, Methodology, Investigation, Conceptualization. S-SH: Writing – review & editing, Methodology, Conceptualization. C-XY: Writing – review & editing, Investigation, Data curation. S-GZ: Writing – review & editing, Investigation, Data curation. M-LS: Writing – review & editing, Investigation. MZ: Writing – review & editing, Funding acquisition, Data curation. J-HZ: Writing – review & editing, Supervision. XZ: Writing – review & editing, Supervision, Conceptualization. J-YW: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition, Data curation, Conceptualization.
The authors declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported in part by funding from Zhejiang Provincial Medical Technology Program (2023RC215) and Wenzhou Municipal Science and Technology Bureau (Y2023125).
We are thankful to all of the subjects who participated in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2024.1449276/full#supplementary-material
Aarsland, D., Batzu, L., Halliday, G. M., Geurtsen, G. J., Ballard, C., Ray Chaudhuri, K., et al. (2021). Parkinson disease-associated cognitive impairment. Nat. Rev. Dis. Prim. 7:47. doi: 10.1038/s41572-021-00280-3
Aarsland, D., Zaccai, J., and Brayne, C. (2005). A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov. Disord. 20, 1255–1263. doi: 10.1002/mds.20527
Alexander-Bloch, A., Giedd, J. N., and Bullmore, E. (2013). Imaging structural co-variance between human brain regions. Nat. Rev. Neurosci. 14, 322–336. doi: 10.1038/nrn3465
Andica, C., Kamagata, K., Hatano, T., Saito, A., Uchida, W., Ogawa, T., et al. (2019). Free-water imaging in white and gray matter in Parkinson’s disease. Cells 8:839. doi: 10.3390/cells8080839
Bernhardt, B. C., Bernasconi, N., Concha, L., and Bernasconi, A. (2010). Cortical thickness analysis in temporal lobe epilepsy: Reproducibility and relation to outcome. Neurology 74, 1776–1784. doi: 10.1212/WNL.0b013e3181e0f80a
Carvalho de Abreu, D. C., Pieruccini-Faria, F., Sarquis-Adamson, Y., Black, A., and Fraser, J. (2023). White matter hyperintensity burden predicts cognitive but not motor decline in Parkinson’s disease: Results from the Ontario neurodegenerative diseases research initiative. Eur. J. Neurol. 30, 920–933. doi: 10.1111/ene.15692
Chung, S. J., Shin, J. H., Cho, K. H., Lee, Y., Sohn, Y. H., Seong, J. K., et al. (2017). Subcortical shape analysis of progressive mild cognitive impairment in Parkinson’s disease. Mov. Disord. 32, 1447–1456. doi: 10.1002/mds.27106
Cole, M. W., Ito, T., Cocuzza, C., and Sanchez-Romero, R. (2021). The functional relevance of task-state functional connectivity. J. Neurosci. 41, 2684–2702. doi: 10.1523/JNEUROSCI.1713-20.2021
Colloby, S. J., Watson, R., Blamire, A. M., O’brien, J. T., and Taylor, J. P. (2020). Cortical thinning in dementia with Lewy bodies and Parkinson disease dementia. Aust. N. Z. J. Psychiatry 54, 633–643. doi: 10.1177/0004867419885165
Dale, A. M., Fischl, B., and Sereno, M. I. (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. doi: 10.1006/nimg.1998.0395
Dopper, E. G., Rombouts, S. A., Jiskoot, L. C., Den Heijer, T., De Graaf, J. R., De Koning, I., et al. (2014). Structural and functional brain connectivity in presymptomatic familial frontotemporal dementia. Neurology 83, e19–e26. doi: 10.1212/WNL.0000000000000583
Douw, L., Van Dellen, E., Gouw, A. A., Griffa, A., De Haan, W., Van Den Heuvel, M., et al. (2019). The road ahead in clinical network neuroscience. Netw. Neurosci. 3, 969–993. doi: 10.1162/netn_a_00103
Emre, M., Aarsland, D., Brown, R., Burn, D. J., Duyckaerts, C., Mizuno, Y., et al. (2007). Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov. Disord. 22, 1689–1707; quiz1837. doi: 10.1002/mds.21507
Fields, R. D. (2008). White matter matters. Sci. Am. 298, 42–49. doi: 10.1038/scientificamerican032008-6w7I2bYHafzcEXbvQWzr4d
Filippi, M., Basaia, S., Sarasso, E., Stojkovic, T., Stankovic, I., Fontana, A., et al. (2021). Longitudinal brain connectivity changes and clinical evolution in Parkinson’s disease. Mol. Psychiatry 26, 5429–5440. doi: 10.1038/s41380-020-0770-0
Filley, C. M., and Fields, R. D. (2016). White matter and cognition: Making the connection. J. Neurophysiol. 116, 2093–2104. doi: 10.1152/jn.00221.2016
Firbank, M. J., Watson, R., Mak, E., Aribisala, B., Barber, R., Colloby, S. J., et al. (2016). Longitudinal diffusion tensor imaging in dementia with Lewy bodies and Alzheimer’s disease. Parkinsonism Relat. Disord. 24, 76–80. doi: 10.1016/j.parkreldis.2016.01.003
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. doi: 10.1016/s0896-6273(02)00569-x
Fischl, B., Sereno, M. I., and Dale, A. M. (1999). Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage 9, 195–207. doi: 10.1006/nimg.1998.0396
Foo, H., Mak, E., Yong, T. T., Wen, M. C., Chander, R. J., Au, W. L., et al. (2017). Progression of subcortical atrophy in mild Parkinson’s disease and its impact on cognition. Eur. J. Neurol. 24, 341–348. doi: 10.1111/ene.13205
Gao, Y., Nie, K., Huang, B., Mei, M., Guo, M., Xie, S., et al. (2017). Changes of brain structure in Parkinson’s disease patients with mild cognitive impairment analyzed via VBM technology. Neurosci. Lett. 658, 121–132. doi: 10.1016/j.neulet.2017.08.028
Gerrits, N. J., Van Loenhoud, A. C., Van Den Berg, S. F., Berendse, H. W., Foncke, E. M., Klein, M., et al. (2016). Cortical thickness, surface area and subcortical volume differentially contribute to cognitive heterogeneity in Parkinson’s disease. PLoS One 11:e0148852. doi: 10.1371/journal.pone.0148852
Hanganu, A., Bedetti, C., Degroot, C., Mejia-Constain, B., Lafontaine, A. L., Soland, V., et al. (2014). Mild cognitive impairment is linked with faster rate of cortical thinning in patients with Parkinson’s disease longitudinally. Brain 137, 1120–1129. doi: 10.1093/brain/awu036
Hattori, T., Orimo, S., Aoki, S., Ito, K., Abe, O., Amano, A., et al. (2012). Cognitive status correlates with white matter alteration in Parkinson’s disease. Hum. Brain Mapp. 33, 727–739. doi: 10.1002/hbm.21245
Hely, M. A., Reid, W. G., Adena, M. A., Halliday, G. M., and Morris, J. G. (2008). The Sydney multicenter study of Parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 23, 837–844. doi: 10.1002/mds.21956
Kalia, L. V., and Lang, A. E. (2015). Parkinson’s disease. Lancet 386, 896–912. doi: 10.1016/S0140-6736(14)61393-3
Litvan, I., Goldman, J. G., Troster, A. I., Schmand, B. A., Weintraub, D., Petersen, R. C., et al. (2012). Diagnostic criteria for mild cognitive impairment in Parkinson’s disease: Movement Disorder Society Task Force guidelines. Mov. Disord. 27, 349–356. doi: 10.1002/mds.24893
Maimaitituerxun, R., Chen, W., Xiang, J., Xie, Y., Kaminga, A. C., Wu, X. Y., et al. (2023). The use of nomogram for detecting mild cognitive impairment in patients with type 2 diabetes mellitus. J. Diabetes 15, 448–458. doi: 10.1111/1753-0407.13384
Mak, E., Bergsland, N., Dwyer, M. G., Zivadinov, R., and Kandiah, N. (2014). Subcortical atrophy is associated with cognitive impairment in mild Parkinson disease: A combined investigation of volumetric changes, cortical thickness, and vertex-based shape analysis. AJNR Am. J. Neuroradiol. 35, 2257–2264. doi: 10.3174/ajnr.A4055
Melzer, T. R., Watts, R., Macaskill, M. R., Pitcher, T. L., Livingston, L., Keenan, R. J., et al. (2013). White matter microstructure deteriorates across cognitive stages in Parkinson disease. Neurology 80, 1841–1849. doi: 10.1212/WNL.0b013e3182929f62
Pereira, J. B., Aarsland, D., Ginestet, C. E., Lebedev, A. V., Wahlund, L. O., Simmons, A., et al. (2015). Aberrant cerebral network topology and mild cognitive impairment in early Parkinson’s disease. Hum. Brain Mapp. 36, 2980–2995. doi: 10.1002/hbm.22822
Pereira, J. B., Svenningsson, P., Weintraub, D., Bronnick, K., Lebedev, A., Westman, E., et al. (2014). Initial cognitive decline is associated with cortical thinning in early Parkinson disease. Neurology 82, 2017–2025. doi: 10.1212/WNL.0000000000000483
Postuma, R. B., Berg, D., Stern, M., Poewe, W., Olanow, C. W., Oertel, W., et al. (2015). MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 30, 1591–1601. doi: 10.1002/mds.26424
Schneider, C. B., Donix, M., Linse, K., Werner, A., Fauser, M., Klingelhoefer, L., et al. (2017). Accelerated age-dependent hippocampal volume loss in parkinson disease with mild cognitive impairment. Am. J. Alzheimers Dis. Other Dement. 32, 313–319. doi: 10.1177/1533317517698794
Segura, B., Baggio, H. C., Marti, M. J., Valldeoriola, F., Compta, Y., Garcia-Diaz, A. I., et al. (2014). Cortical thinning associated with mild cognitive impairment in Parkinson’s disease. Mov. Disord. 29, 1495–1503. doi: 10.1002/mds.25982
Tessitore, A., Santangelo, G., De Micco, R., Vitale, C., Giordano, A., Raimo, S., et al. (2016). Cortical thickness changes in patients with Parkinson’s disease and impulse control disorders. Parkinsonism Relat. Disord. 24, 119–125. doi: 10.1016/j.parkreldis.2015.10.013
Tijms, B. M., Wink, A. M., De Haan, W., Van Der Flier, W. M., Stam, C. J., Scheltens, P., et al. (2013). Alzheimer’s disease: Connecting findings from graph theoretical studies of brain networks. Neurobiol. Aging 34, 2023–2036. doi: 10.1016/j.neurobiolaging.2013.02.020
Watts, D. J., and Strogatz, S. H. (1998). Collective dynamics of ‘small-world’ networks. Nature 393, 440–442. doi: 10.1038/30918
Xia, J., Miu, J., Ding, H., Wang, X., Chen, H., Wang, J., et al. (2013). Changes of brain gray matter structure in Parkinson’s disease patients with dementia. Neural Regen. Res. 8, 1276–1285. doi: 10.3969/j.issn.1673-5374.2013.14.004
Yang, L., Wu, B., Fan, L., Huang, S., Vigotsky, A. D., Baliki, M. N., et al. (2021). Dissimilarity of functional connectivity uncovers the influence of participant’s motion in functional magnetic resonance imaging studies. Hum. Brain Mapp. 42, 713–723. doi: 10.1002/hbm.25255
Keywords: Parkinson’s disease, cognitive impairment, brain structure, network, magnetic resonance imaging
Citation: Liu R-P, Lin G-L, Ma L-L, Huang S-S, Yuan C-X, Zhu S-G, Sheng M-L, Zou M, Zhu J-H, Zhang X and Wang J-Y (2024) Changes of brain structure and structural covariance networks in Parkinson’s disease associated cognitive impairment. Front. Aging Neurosci. 16:1449276. doi: 10.3389/fnagi.2024.1449276
Received: 14 June 2024; Accepted: 11 September 2024;
Published: 26 September 2024.
Edited by:
Wen-Jun Duan, Jinan University, ChinaReviewed by:
Alberto Benelli, University of Florence, ItalyCopyright © 2024 Liu, Lin, Ma, Huang, Yuan, Zhu, Sheng, Zou, Zhu, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Yong Wang, d2FuZ2ppYW55b25nMjAyMEAxMjYuY29t; Xiong Zhang, emhhbmd4aW9uZzk4QGdtYWlsLmNvbQ==; Jian-Hong Zhu, amh6aHVAd211LmVkdS5jbg==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.