- 1Department of Neurology, Jingzhou Hospital Affiliated to Yangtze University, Jingzhou, China
- 2Hubei Provincial Clinical Research Center for Parkinson’s Disease, Xiangyang, China
Background: Ferroptosis is a crucial pathogenic mechanism in Parkinson’s disease, offering significant potential for pharmacological intervention. Despite its importance, the number of bibliometric analyses examining the relationship between ferroptosis and Parkinson’s disease remains limited. This study aims to elucidate the knowledge structure and primary research focuses within this field using various bibliometric tools search.
Materials and methods: We conducted a comprehensive literature son ferroptosis in Parkinson’s disease using the Web of Science Core Collection database. Bibliometric analyses and visualizations were performed with VOSviewer, examining the geographical and institutional distribution of publications, journal interconnections, and keyword prevalence. Furthermore, CiteSpace was used to visually explore and analyze journal interactions and citation dynamics. The bibliometrix R package facilitated the delineation of collaborative networks across different countries and the construction of visual network representations illustrating relationships among authors, keywords, and journals. Data visualization was further enhanced with Microsoft Office Excel 2021.
Results: Recently, there has been a significant increase in publications on ferroptosis, with China emerging as a leading contributor in this research area. Keyword analysis highlights the critical role of ferroptosis in the pathogenesis of Parkinson’s disease, identifying GPX4 as a key enzyme mitigating lipid peroxidation. This study also elucidates the connections and distinctions between ferroptosis and other cell death processes such as apoptosis, autophagy, and pyroptosis. Current research primarily focuses on immunotherapy, prognosis, oxidative stress, lipid peroxidation, and the tumor microenvironment.
Conclusion: This study provides a comprehensive initial analysis of the research landscape, identifying current focal points and potential future directions for ferroptosis research in Parkinson’s disease. The findings leverage a variety of bibliometric methodologies to offer valuable insights into this emerging field.
1 Introduction
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disorder globally, after Alzheimer’s disease. The incidence of PD is rising, driven by an aging population, particularly in China, and a concerning trend toward earlier onset in younger individuals (Dorsey et al., 2018; Pringsheim et al., 2014). Consequently, research focused on the prevention and management of PD has become increasingly vital. Despite significant advances, the etiology of PD remains largely unclear, with current treatments predominantly addressing symptoms rather than underlying causes (Armstrong and Okun, 2020; Bloem et al., 2021). Recent studies have highlighted ferroptosis as a key pathogenic mechanism in PD, positioning it as a promising target for therapeutic interventions (Do Van et al., 2016; Guiney et al., 2017; Jiang et al., 2023).
Ferroptosis, identified by Dixon et al. (2012), is a distinct form of cell death that differs from apoptosis, necrosis, and autophagy in its morphology, mechanism, and molecular pathways. It is characterized by iron-dependent lipid peroxidation, which damages the cell membrane and leads to cell death (Mortensen et al., 2023). This process can be mitigated by iron chelators or antioxidants like glutathione peroxidase 4 (GPX4). The regulation of ferroptosis involves a complex interplay of mechanisms, including iron chelation, antioxidant defense, and lipid repair (Stockwell, 2022). Ferroptosis is involved in various diseases, including neurodegenerative disorders, cancer, and ischemia-reperfusion injuries (Jiang et al., 2021; Stockwell, 2022; Stockwell et al., 2017). Thus, targeting the ferroptosis pathway presents a promising approach for therapeutic development and disease management.
Bibliometrics is a systematic methodology for evaluating and analyzing literature through quantitative measures, using mathematical and statistical techniques (Liu et al., 2024; Sun et al., 2023; Zhang et al., 2022). This approach provides insights into publication volumes, author and institution productivity, citation patterns, and the interdisciplinary evolution of research fields. Advanced visual analytics tools facilitate the identification of leading journals, research entities, influential authors, and emerging research trends (Donthu et al., 2021; Pan et al., 2018). Knowledge graphs, generated using software such as VOSviewer (Thomas and Lawrence, 2022; van Eck and Waltman, 2010), CiteSpace (Chen et al., 2012), and the R package bibliometrix (Kemeç and Altınay, 2023), offer a detailed overview of research landscapes. Databases like Web of Science, Scopus, and Google Scholar provide comprehensive bibliometric analysis capabilities, supporting thorough and nuanced research investigations (McNicholas et al., 2022).
2 Materials and methods
2.1 Data sources and retrieval strategies
On March 22, 2024, we conducted a systematic literature search using the Web of Science Core Collection (WoSCC) database. Renowned for its comprehensive and high-quality collection of scholarly literature, WoSCC is considered the gold standard for bibliometric analysis (Ding and Yang, 2020; Ma et al., 2021; Merigó and Yang, 2017). Our search utilized the terms “ferroptosis” and “Parkinson’s disease,” structured as TS = (ferroptosis OR ferroptotic OR “iron death”) AND TS = (Parkinson* OR PD). Since “ferroptosis” was first introduced in 2012, the search was limited to the period from January 1, 2012, to December 31, 2023. This search strategy identified 510 documents. After filtering to include only “articles” and “review articles” in English, the dataset was refined to 495 documents, comprising 347 articles and 148 review articles.
2.2 Data analysis
To perform the bibliometric analysis, we utilized VOSviewer, CiteSpace, and bibliometrix R package three widely recognized tools for quantitative literature assessment. VOSviewer version 1.6.19.0 was used to analyze the distribution of publications by country, institution, and journal, as well as to assess co-citation patterns and keyword frequencies. The VOSviewer analysis parameters included: counting method (full counting), visualization weights for countries, institutions, journals, and author keywords (documents), visualization weights of co-cited journals (citations), visualization weights of author keywords (occurrences), normalization method (association strength), clustering resolution (1.00), minimum cluster size (1), minimum strength (0), and maximum lines (1,000).
CiteSpace version 6.1.R6, developed in Java, facilitated the visualization and analysis of journal and reference data. Following the import of the literature into CiteSpace, the data were formatted according to the software’s requirements. The analysis parameters set included: time span (January 2012–December 2023), time slicing (1 year), g-index (k = 25), node types (Reference), top N (50), and clustering method (LLR), with other settings at default. This setup enabled the generation of visualizations for journal and reference networks.
The bibliometrix R package 4.1.3, used with RStudio 2023.03.1 and R version 4.3.1, was utilized for mapping collaborative networks between countries and constructing visual network views for authors, keywords, and the interconnections among authors, keywords, and journals. For analyzing trending topics in authors’ keywords, the parameters included: time span (2013–2023), word minimum frequency (5), number of words per year (3), with other settings at default. For general bibliometric data—such as number of publications (NPs), countries, institutions, journals, authors, and citation frequency—graphs were created using Microsoft Office Excel version 2021.
3 Results
3.1 Basic quantitative information
The defined search strategy yielded 495 documents authored by 3,269 individuals from 693 institutions across 47 countries. These documents were published in 243 journals, accumulating a total of 30,206 citations from 3,253 distinct journals.
3.2 Quantitative analysis of publications
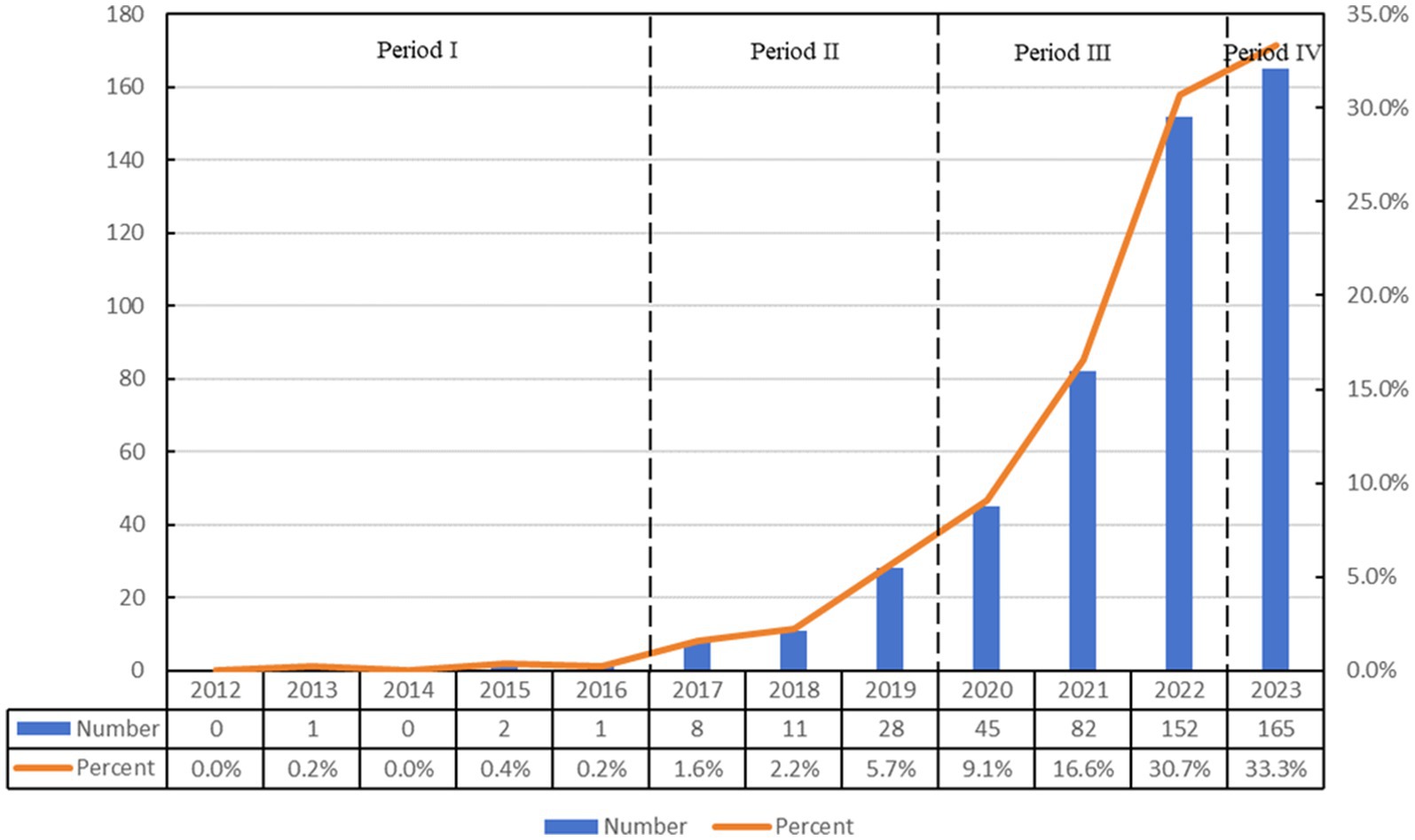
Over the past 11 years (2012–2023), our search identified 495 articles from the Web of Science database that examine the relationship between ferroptosis and Parkinson’s disease. This collection comprises 347 research articles and 148 review articles. The temporal distribution of these publications, as illustrated in Figure 1, demonstrates a consistent increase in research activity, with a significant surge starting in 2017.
The evolution of publication volume over this period can be divided into four separate phases: the initial phase (2012–2016), the early development phase (2017–2019), the rapid growth phase (2020–2022), and the current phase (2023 to present). During the initial phase, there were only four publications, reflecting nascent interest in the relationship between ferroptosis and Parkinson’s disease. After 2016, the annual research output began to increase. The early development phase is marked by an average annual output of approximately 15 publications, indicating a preliminary exploration stage. The rapid growth phase is characterized by a substantial increase in publications. For example, in 2022, the publication count reached 152 articles, which was 1.85 times the output of the preceding year, accounting for a 56.4% growth rate during this stage. Although the current phase continues to show an upward trend, the rate of increase has started to stabilize, with 165 publications recorded in 2023, representing 33.3% of the overall publication count. This trajectory highlights the increasing prominence of the relationship between ferroptosis and Parkinson’s disease as a focal point of future research endeavors.
3.3 National and institutional analysis
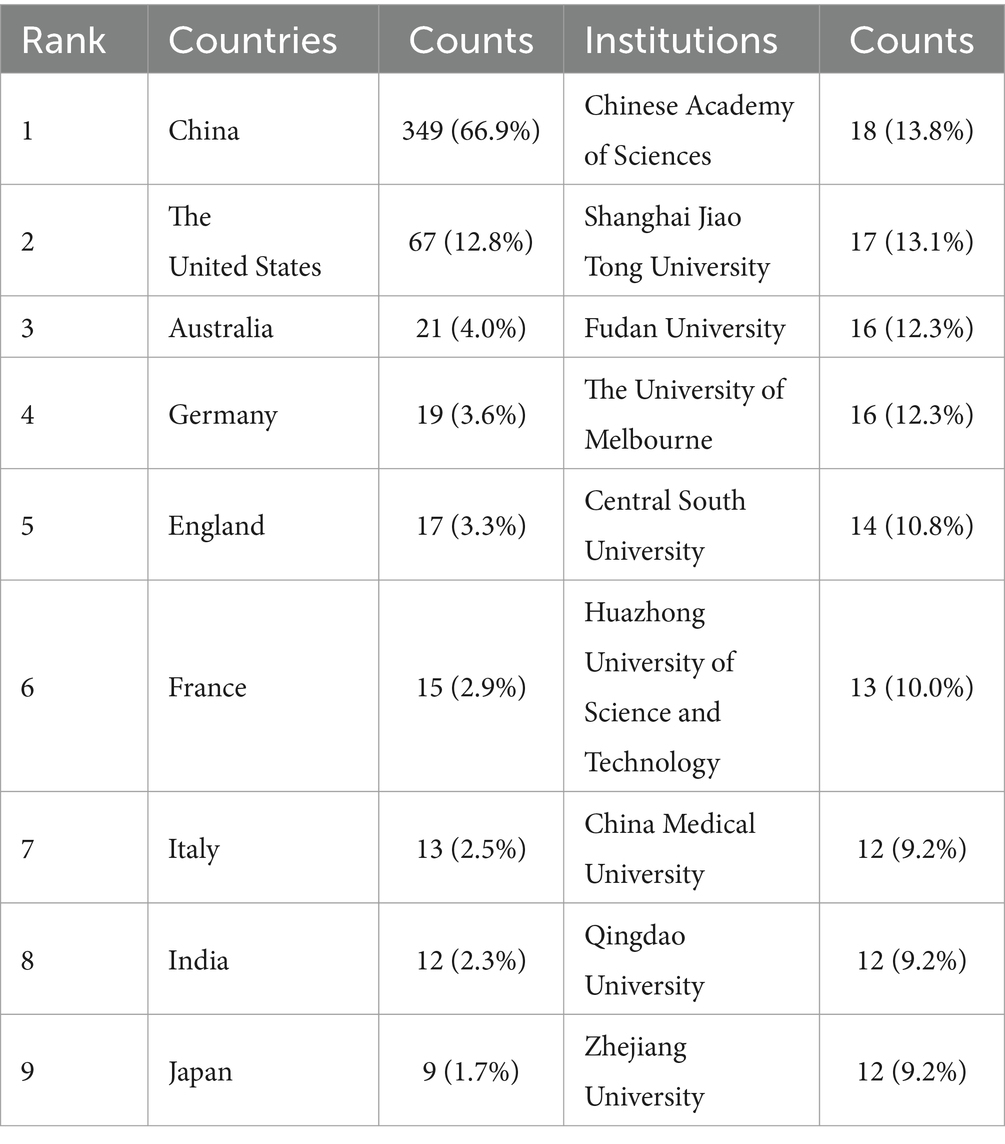
The analyzed publications span 47 countries and 693 distinct institutions, with the top nine countries representing a global distribution across Asia, North America, Europe, and Oceania. Notably, Europe and Asia dominate the publication landscape, as detailed in Table 1. This distribution reflects global patterns of economic, social, and cultural development. Among these nations, China stands out as the leading contributor, with a substantial lead in publication volume (n = 349, 66.9%), followed by the United States (n = 67, 12.8%), Australia (n = 21, 4.0%), and Germany (n = 19, 3.6%). These four countries thus form the cornerstone of research into ferroptosis in the context of Parkinson’s disease. For further analysis, we focused on the 47 countries with at least one publication. A collaborative network graph was constructed to illustrate publication output and collaboration dynamics among these nations (Figure 2). In this graph, the thickness of the matrix in the labeled view represents the volume of literature published by each country in this field, while the lines between nodes indicate the strength of collaboration. Thicker lines denote more frequent collaborative efforts. The color gradient from cold to warm represents the chronological proximity of the collaboration, with warmer colors indicating more recent interactions. For example, China has close collaborations with the United States, Australia, Germany, and the United Kingdom, while the United States also actively collaborates with China, Germany, and the United Kingdom.
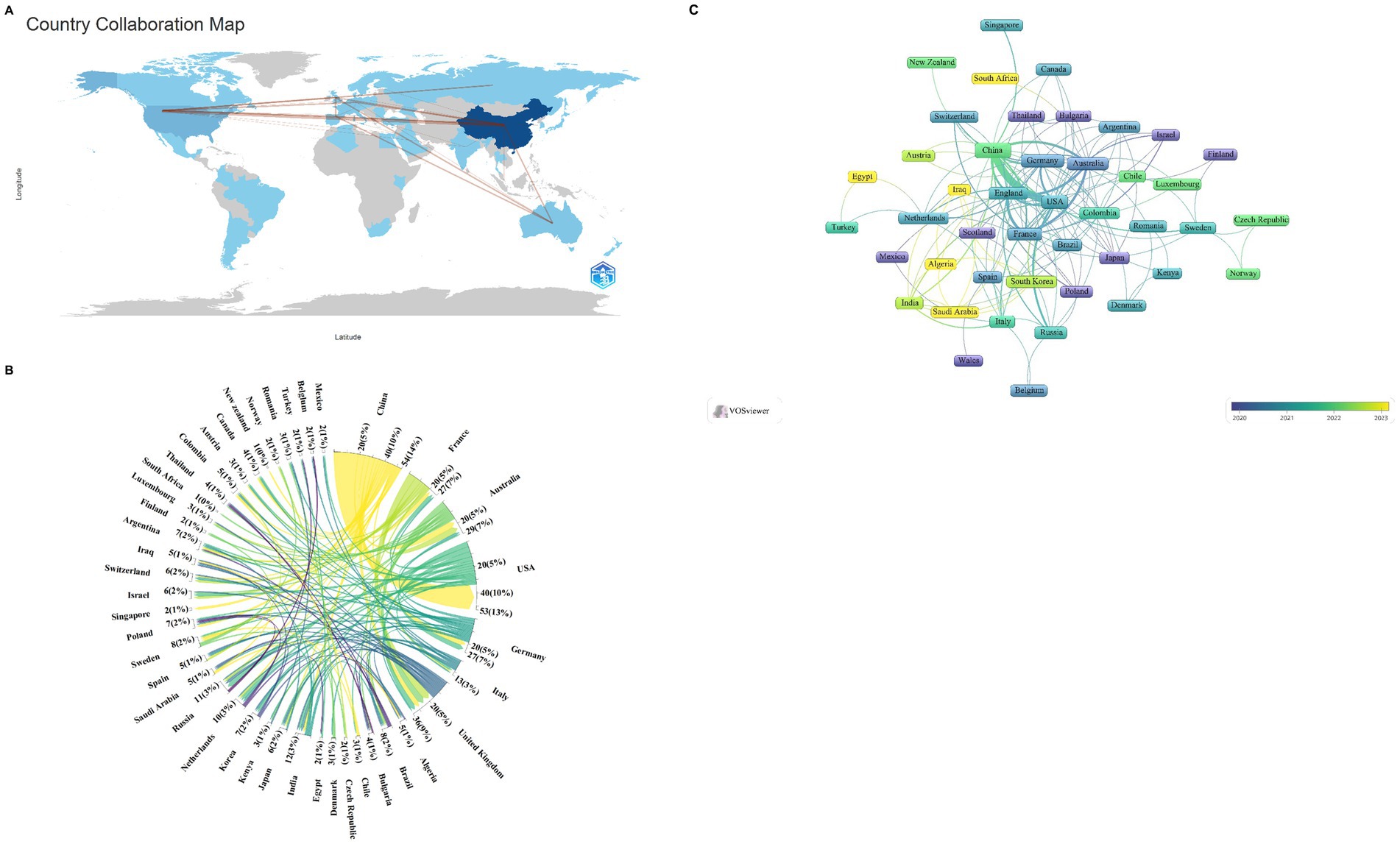
Figure 2. Geographical distribution (A), chord diagram of network relations between countries (B), and national visualization analysis (C) of ferroptosis in Parkinson’s disease.
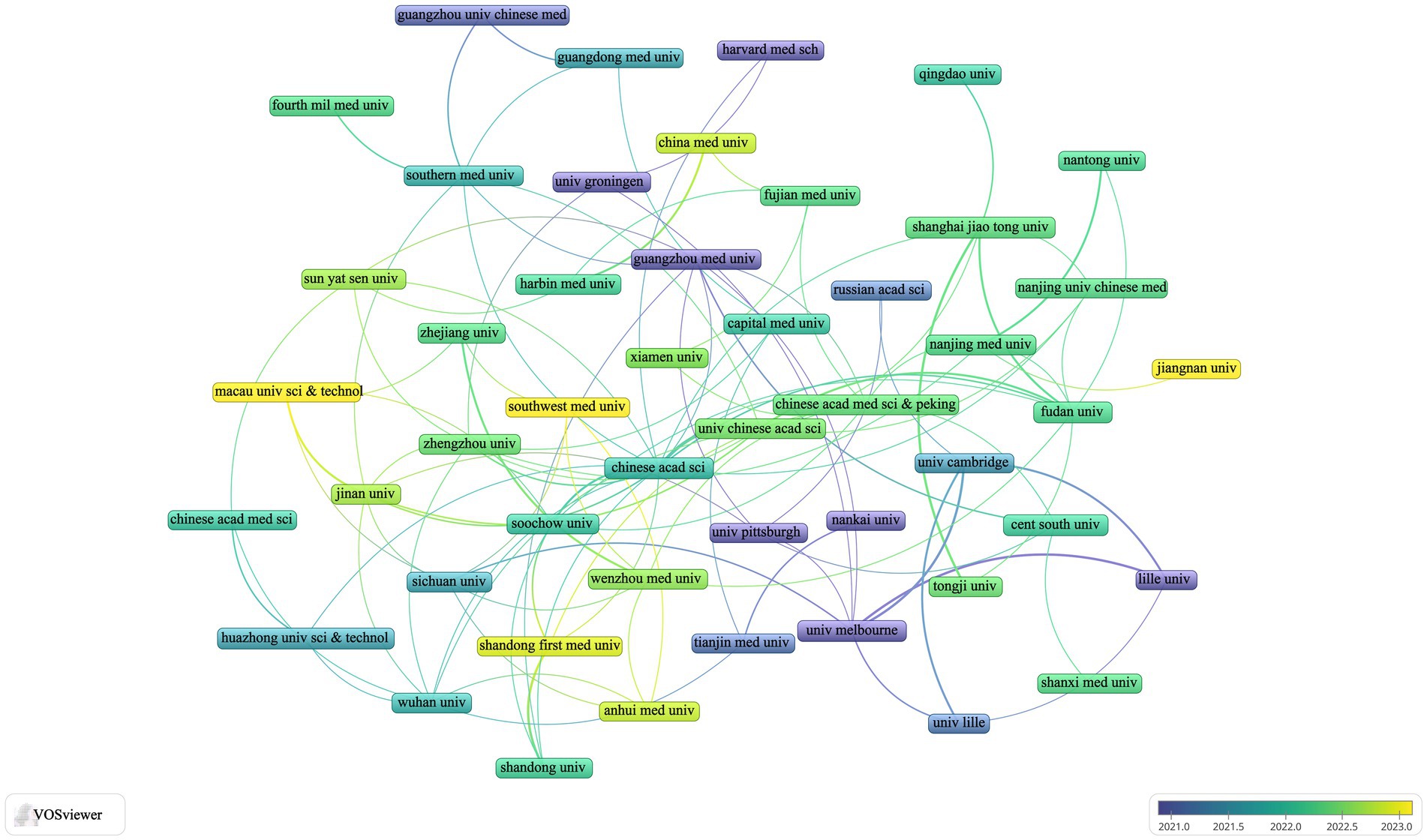
Among the top nine institutions engaged in this research area, eight are located in China. The leading institutions in terms of publication volume included the Chinese Academy of Sciences (n = 18, 13.8%), Shanghai Jiao Tong University (n = 17, 13.1%), Fudan University (n = 16, 12.3%), and the University of Melbourne (n = 16, 12.3%) (Table 1). For further analysis, we selected 82 of the 693 institutions with a publication volume of at least three articles. A collaborative network view was constructed based on each institution’s publication volume and their interrelationships (Figure 3). In this figure, the thickness of the matrix indicates the volume of publications by each institution, highlighting the Chinese Academy of Sciences as the most prolific entity. The thickness of the lines represents the intensity of cooperation between institutions, with thicker lines indicating a greater number of co-authored papers. For instance, the Chinese Academy of Sciences has close collaborations with the University of Chinese Academy of Sciences, Soochow University, and the First Medical University of Shandong Province. Meanwhile, the University of Melbourne has established significant collaborations with the University of Lille, the University of Cambridge, and Sichuan University. The color gradient from cold to warm in Figure 3 visualizes the temporal proximity of these collaborations, highlighting the core research institutions in this field.
3.4 Journals and co-cited journals
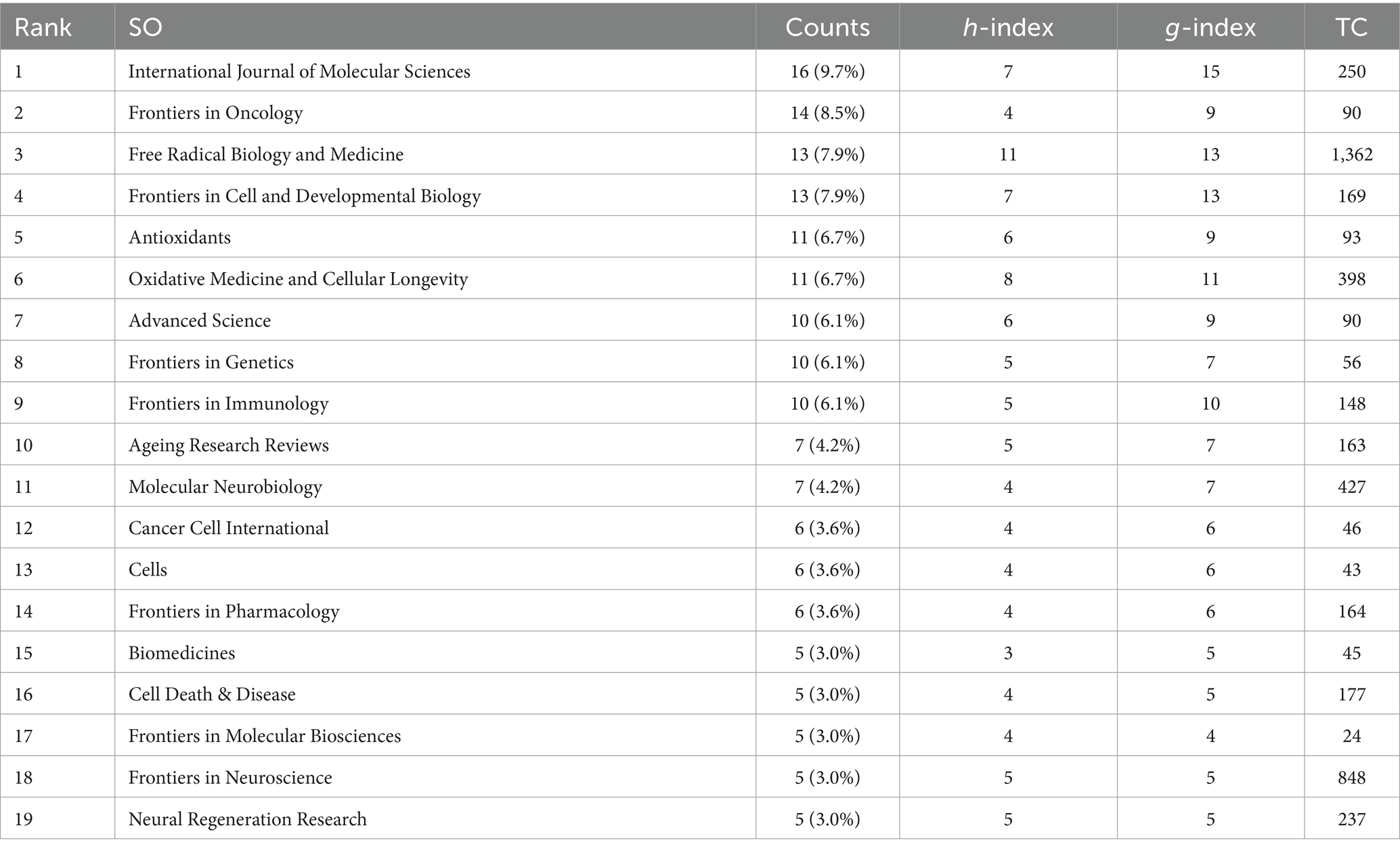
The articles analyzed were published across 243 journals focusing on ferroptosis in Parkinson’s disease. Based on Bradford’s law, we identified 19 core journals. Among these, the International Journal of Molecular Sciences published the most articles (n = 16, 9.7%), followed by Frontiers in Oncology (n = 14, 8.5%), Free Radical Biology and Medicine (n = 13, 7.9%), and Frontiers in Cell and Developmental Biology (n = 13, 7.9%). The h-index, introduced by Hirsch (2005), evaluates a journal’s productivity and citation impact. The h-index determines the number of articles (h) that have been cited at least h times each, reflecting the journal’s influence in the field (Hou et al., 2022). As detailed in Table 2, the journal with the highest h-index is Free Radical Biology and Medicine (h-index = 11), followed by Oxidative Medicine and Cellular Longevity (h-index = 8). Additionally, the g-index, proposed by Egghe (2006) and Woeginger (2008), measures both the impact of each paper and the overall impact of the journal’s output. According to Table 2, the highest g-index is found in the International Journal of Molecular Sciences (g-index = 15), followed by Free Radical Biology and Medicine, Frontiers in Cell and Developmental Biology, and Oxidative Medicine and Cellular Longevity. Among these journals, Free Radical Biology and Medicine leads in total citations (TC = 1,362), followed by Frontiers in Neuroscience (TC = 848) and Molecular Neurobiology (TC = 427).
For a more detailed analysis, we screened 44 journals with three or more relevant publications and created a network view of these journals (Figure 4A). This figure illustrates that the International Journal of Molecular Sciences has strong collaborations with Frontiers in Neuroscience, Molecular Neurobiology, Free Radical Biology and Medicine, and Neurobiology of Disease. Additionally, Free Radical Biology and Medicine maintains close relationships with journals such as Ageing Research Reviews, International Journal of Molecular Sciences, Molecular Neurobiology, and Frontiers in Aging Neuroscience.
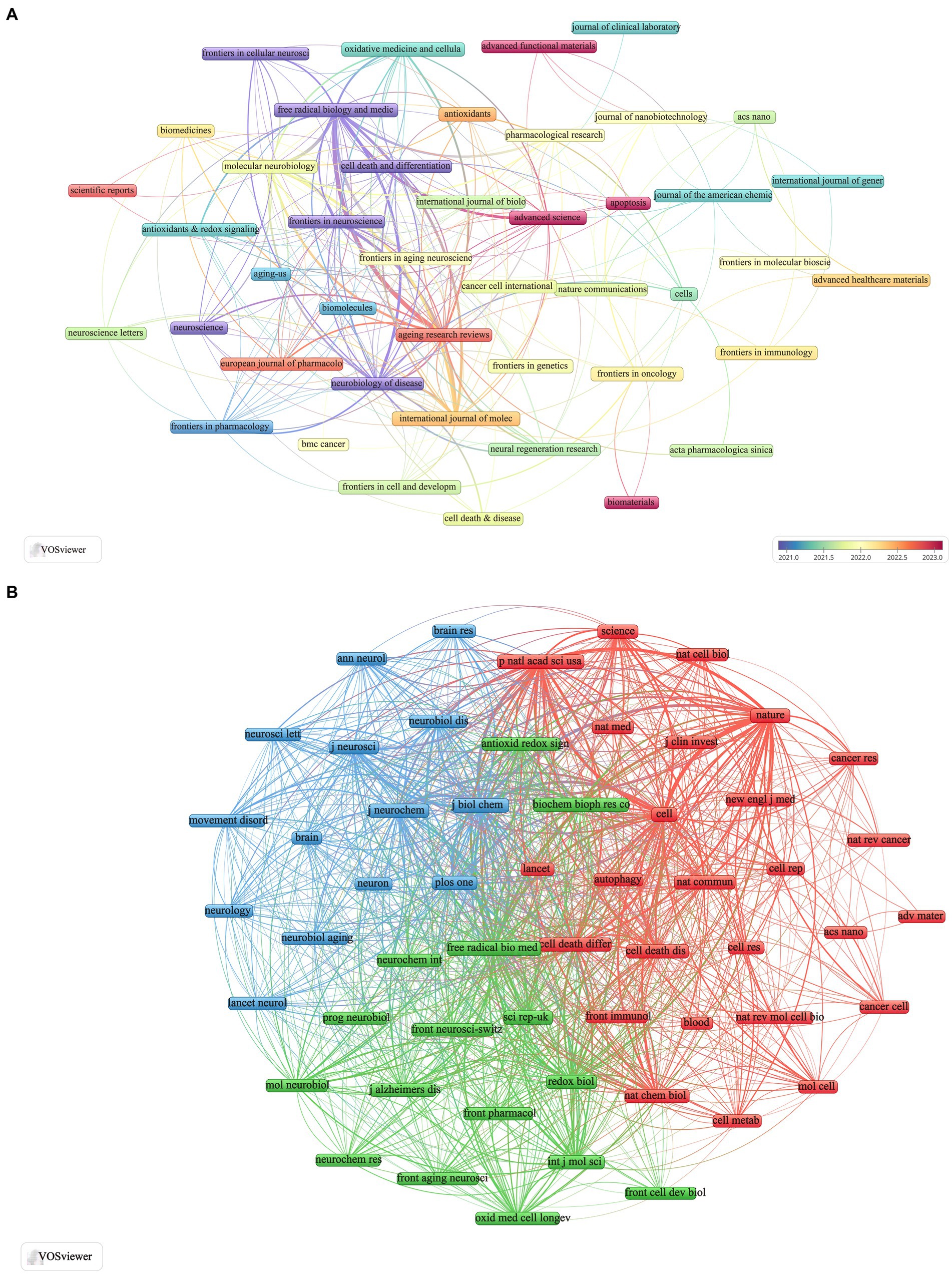
Figure 4. Visualization of ferroptosis in Parkinson’s disease in journals (A) and co-cited journals (B).
Table 3 lists 11 journals that have each accrued more than 500 TCs. Notably, Cell and Nature lead with co-citations totaling 1,269 and 1,200, respectively. Nature has the highest impact factor (2022–2023) at 64.8, closely followed by Cell with an impact factor of 64.5. From an initial pool of 3,253 journals, we selected 56 with 167 or more co-citations and visualized their network (Figure 4B). The clustering view in Figure 4B reveals strong relationships between Nature, Cell, Proceedings of the National Academy of Sciences of the United States of America, Journal of Biological Chemistry, and Free Radical Biology and Medicine. This inter-journal co-citation highlights the central role these publications play in disseminating influential research findings on ferroptosis in Parkinson’s disease, underscoring their authoritative position in the field.
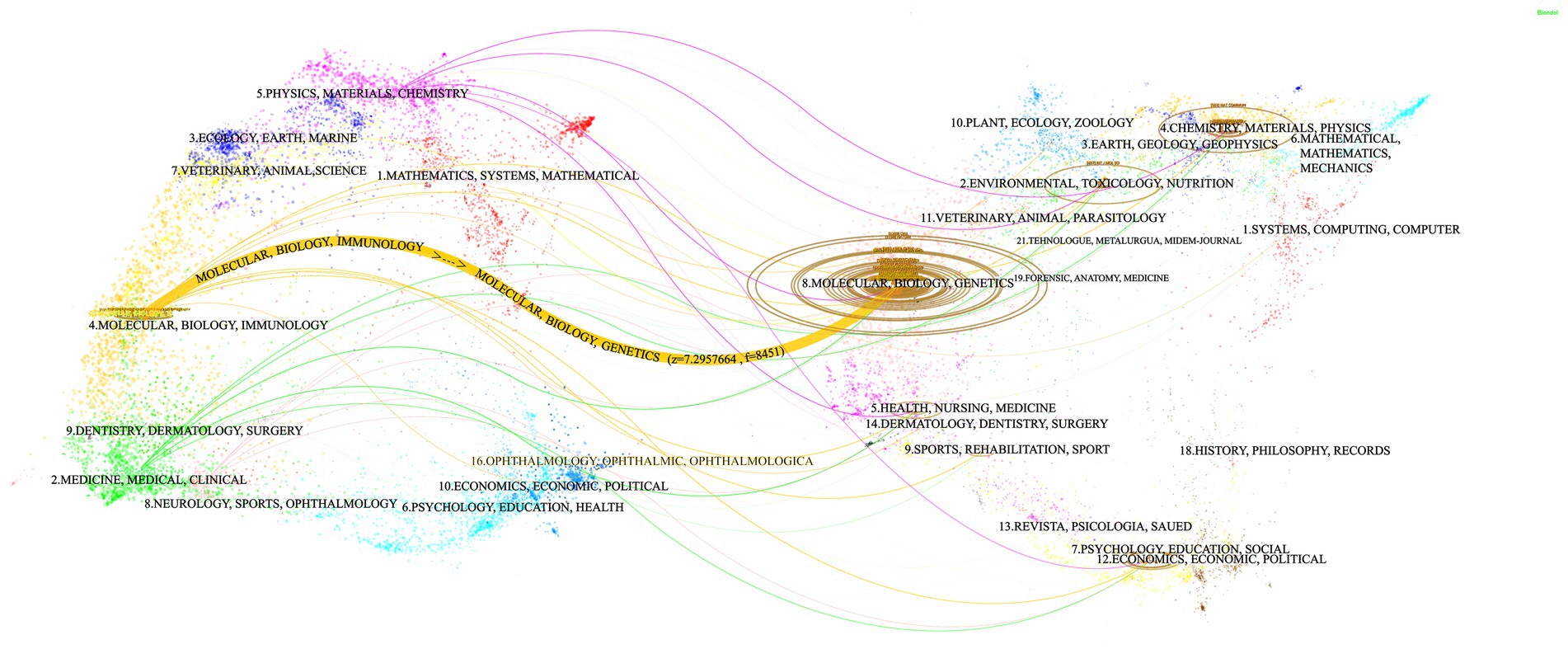
The double graph overlay illustrates the citation relationships between journals and co-cited journals, with the citing journals clustered on the left and the co-cited journals clustered on the right. The curves represent citation paths, while the size of the ellipses reflects the NPs (Li et al., 2022). Using the Z-scores function in CiteSpace, a dual overlay visualization of the interaction between journals and their cited counterparts is created. Figure 5 reveals a prominent orange trajectory, indicating the primary citation pathway. This trajectory suggests that research in this domain is predominantly concentrated in molecular/biology/immunology journals, with citations largely originating from molecular/biology/genetics journals. This pattern underscores the interdisciplinary nature of research in ferroptosis and Parkinson’s disease, highlighting the engagement of experts from multiple disciplines. It also emphasizes the critical role of interdisciplinary communication and collaboration for advancing future research.
3.5 Authors and co-cited authors
The study of ferroptosis in Parkinson’s disease involves contributions from 3,269 authors. The top 10 authors with the highest publication output are listed in Figure 6A, including their NPs, h-index, g-index, TCs, and local citations. Li Y. leads with the highest output (NP = 12, h-index = 6, g-index = 12), followed by Zhang Y. (NP = 10, h-index = 6, g-index = 10). Both authors have at least 10 publications in the field, but Li Y.’s higher g-index indicates a more substantial impact. Figure 6B illustrates the temporal evolution of publications and citations for these top 10 authors, showing a relationship between publication volume and citation frequency. Notably, Devos D. was an early contributor, publishing in 2016 with a paper titled “Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC” (TC = 443). Li Y.’s 2019 publication, “Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion,” received the highest citation count (TC = 470). In 2022, Chen X. had the highest annual publication count (NP = 6).
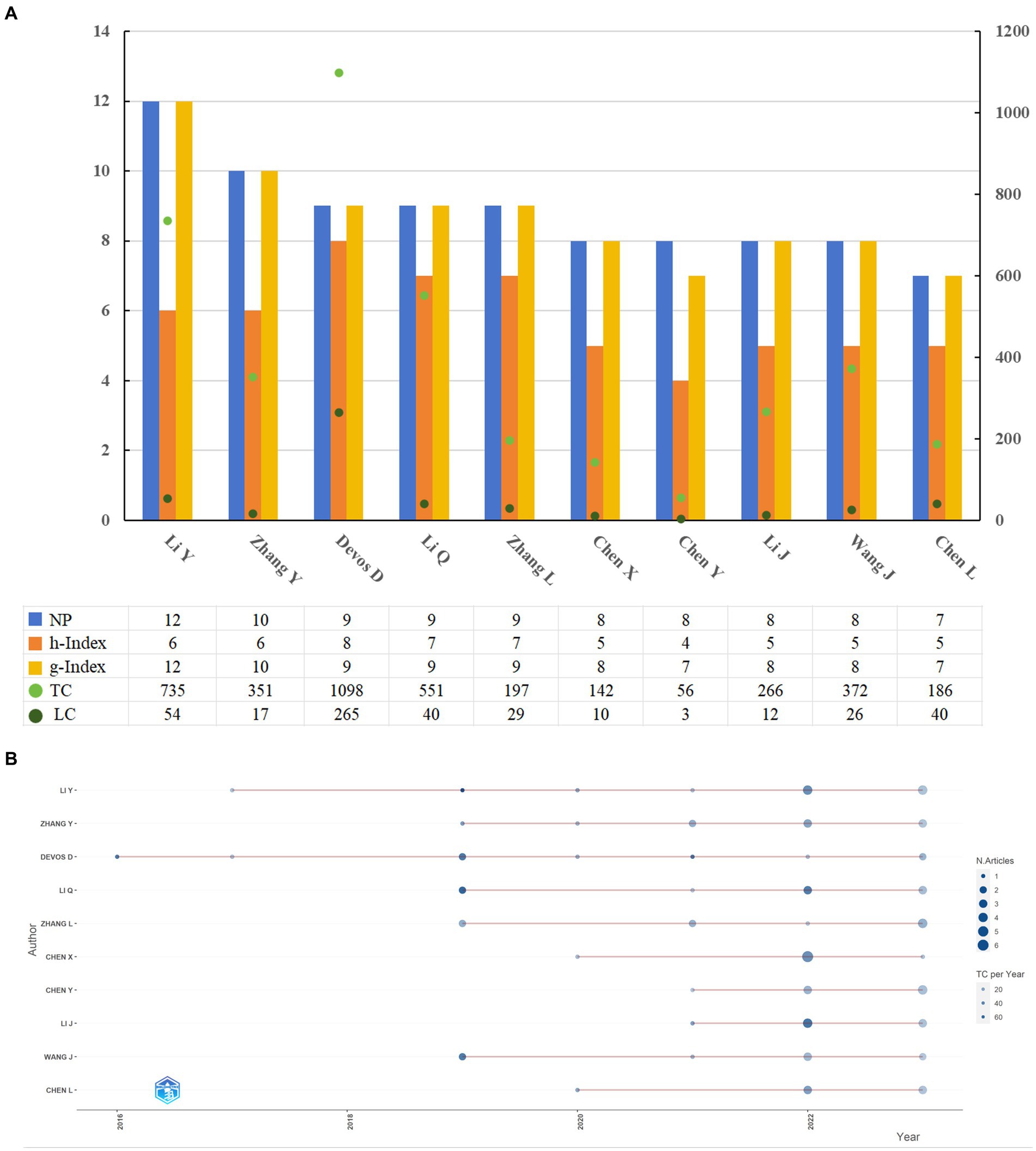
Figure 6. Relevant information displaying the 10 most productive authors (A); the productivity of the 10 most productive authors over time (B). NP, number of publications; TC, total citation; LC, local citation.
3.6 Co-occurrence of keywords
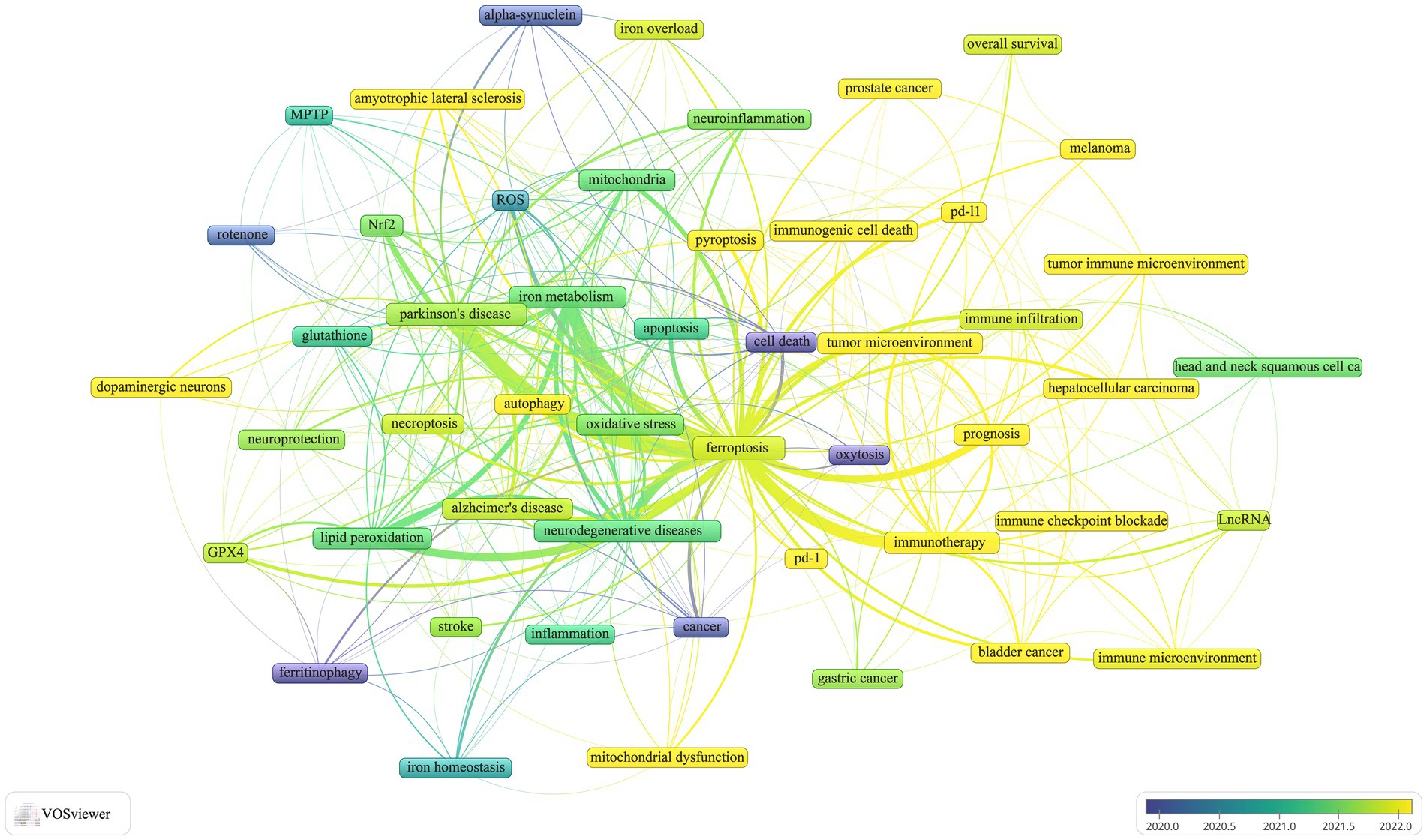
Keyword co-occurrence analysis is a powerful tool for identifying research hotspots and emerging trends. In this study, we analyzed a dataset of 1,053 author keywords using a threshold of five or more occurrences. This analysis, conducted with VOSviewer, resulted in 52 distinct keywords after consolidating synonyms. Figure 7 presents the visual analysis, where the thickness of the matrix lines denotes keyword frequency, and lines connecting nodes represent the strength of relationship between keywords. Thicker lines indicate more frequent co-occurrences within the same documents. Node colors differentiate clusters, with lighter shades highlighting dominant research directions. The analysis identified five main clusters, each representing a distinct research trajectory:
• Cluster 1: Keywords included ferroptosis, apoptosis, autophagy, necroptosis, pyroptosis, immunotherapy, immunogenic cell death (ICD), immune checkpoint blockade, gastric cancer, hepatocellular carcinoma, melanoma, tumor microenvironment, overall survival, PD-1, PD-L1, and prognosis.
• Cluster 2: Keywords included Parkinson’s disease, neurodegenerative diseases, alpha-synuclein, rotenone, MPTP, neuroprotection, neuroinflammation, inflammation, mitochondrial dysfunction, mitochondria, iron, Nrf2, oxeiptosis, and oxidative stress.
• Cluster 3: Keywords included Alzheimer’s disease, amyotrophic lateral sclerosis, cell death, lipid peroxidation, reactive oxygen species, glutathione, GPX4, iron metabolism, and iron overload.
• Cluster 4: Keywords included tumor immune microenvironment, immune microenvironment, immune infiltration, long non-coding RNAs (lncRNAs), bladder cancer, head and neck squamous cell carcinoma, and prostate cancer.
• Cluster 5: Keywords included cancer, dopaminergic neurons, stroke, ferrinophagy, and iron homeostasis.
Frequency analysis reveals that “immunotherapy,” “prognosis,” “tumor microenvironment,” and “mitochondrial dysfunction” are the current focal points of research. Among these, “immunotherapy” appears most frequently (n = 53), followed by “iron” (n = 48) and “neurodegenerative diseases” (n = 47). This indicates a significant interdisciplinary interest and convergence of research efforts on these themes.
Additionally, Figure 8 illustrates keyword trends from 2012 to 2023, filtering for terms with a frequency of five or more occurrences. The primary keywords during this period, besides “ferroptosis” and “Parkinson’s disease,” include “immunotherapy,” “prognosis,” “neuroprotection,” “pyroptosis,” and “autophagy.” These terms highlight the current main research directions, focusing on mechanisms of disease progression and potential therapeutic strategies (see Table 4).
3.7 Three-field plot of author-keyword-journal
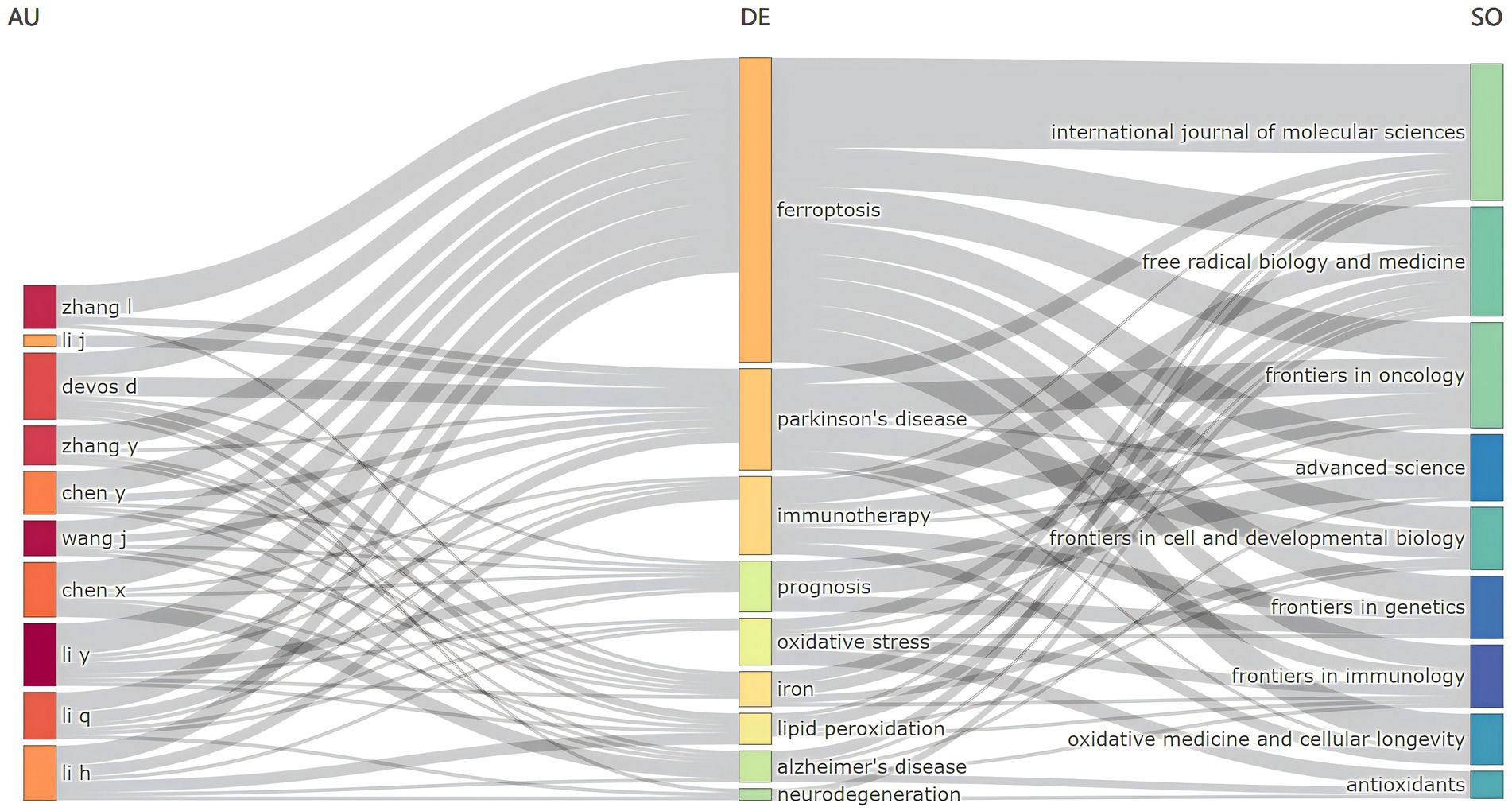
The three-field plot methodology enables a comprehensive synthesis and analysis of the relationships among various bibliometric indicators by constructing an intricate network map that links these indicators. Using the three-field plot approach within bibliometrix, we delineated three distinct domains: “author” on the left, “keyword” in the center, and “journal” on the right, resulting in the creation of an “author-keyword-journal” network, as shown in Figure 9. This figure highlights that, in addition to the central keywords “ferroptosis” and “Parkinson’s disease,” key research trajectories include “immunotherapy,” “prognosis,” “oxidative stress,” “lipid peroxidation,” and “tumor microenvironment.” A notable co-occurrence relationship is observed between authors and specific keywords. For example, Chen X. is strongly associated with “prognosis,” Li Y. with “prognosis,” and both Devedjian J. C. and Devos D. with “lipid peroxidation.” Furthermore, significant co-occurrence relationships are evident between keywords and specific journals: “ferroptosis” frequently appears in the International Journal of Molecular Sciences, “Parkinson’s disease” in Free Radical Biology and Medicine, “immunotherapy” in both Frontiers in Cell and Developmental Biology and Frontiers in Oncology, and “prognosis” in Advanced Science and Frontiers in Genetics. These findings highlight the influential authors and seminal contributions in the field and identify emerging research hotspots, underscoring the pivotal role of journals like the International Journal of Molecular Sciences, Frontiers in Cell and Developmental Biology, and Free Radical Biology and Medicine in shaping the research landscape.
3.8 Reference citation burst analysis
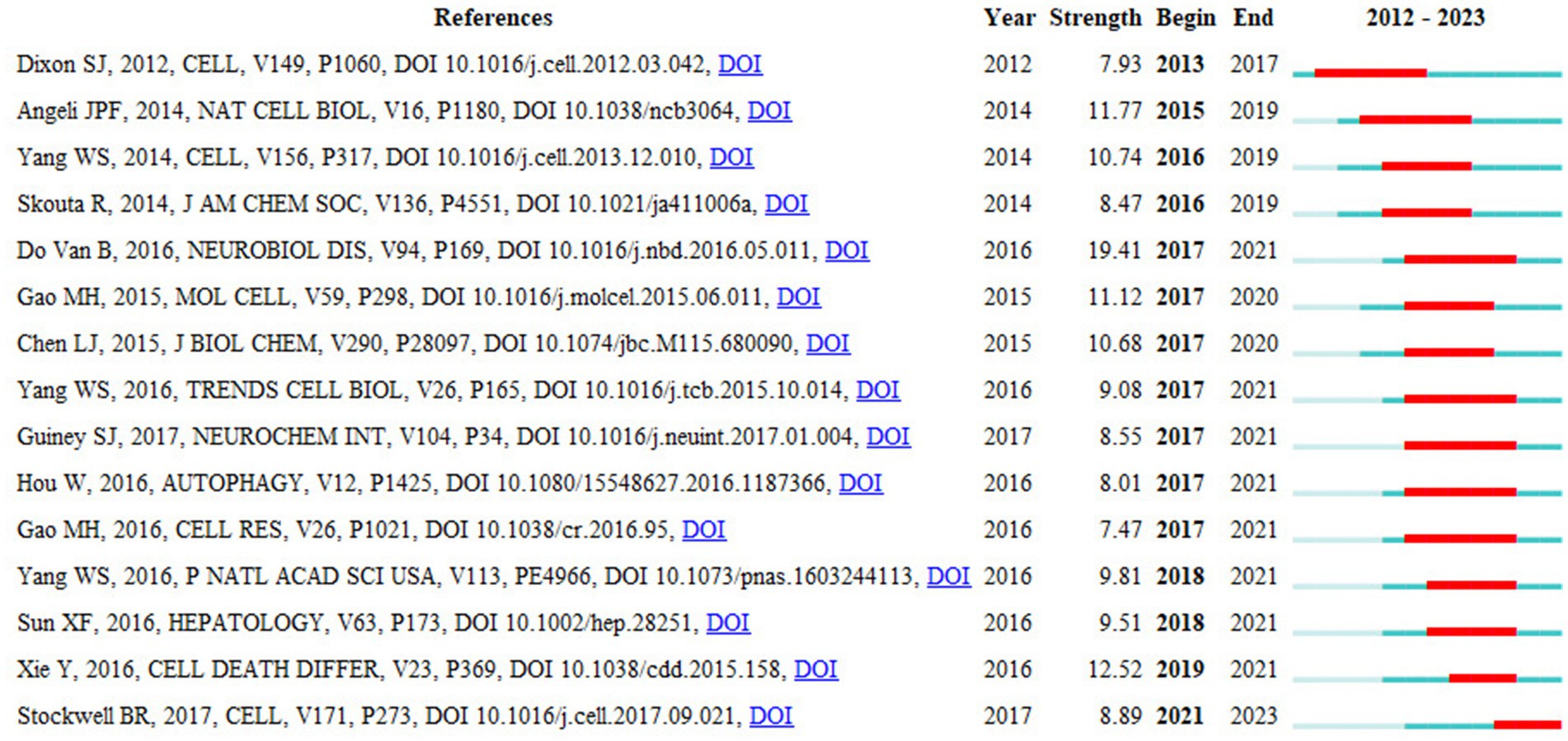
Reference citation burst analysis identifies literature that experiences a surge in citations within a specific period, reflecting research hotspots and trends. The blue line in Figure 10 indicates the time interval, while the red columns on the blue line represent burst periods. The strength of the burst reflects the importance of the literature in the research field, with “year” indicating the publication year, and “begin” and “end” marking the start and end of the citation burst. Using CiteSpace with parameters set to number of states = 2, γ[0, 1] = 1.0, and minimum duration = 2, we analyzed a total of 81 references. Among these, the reference with the strongest burst intensity was “Ferroptosis is a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC by Do Van’s et al. (2016) study, indicating it is the most frequently cited. The earliest significant study in this field was “Ferroptosis: an iron-dependent form of nonapoptotic cell death” by Dixon et al. (2012). Additionally, “Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease” by Stockwell’s et al. (2017) is still experiencing a citation burst.
4 Discussion
4.1 Research status
Since the introduction of ferroptosis, research has increasingly highlighted its critical connection to a range of diseases, including neurodegenerative disorders, cancer, and ischemia-reperfusion injuries (Lee et al., 2020; Li et al., 2019). Despite this growing interest, bibliometric analyses specific to this field are still limited. This study provides a comprehensive bibliometric analysis of the relationship between ferroptosis and Parkinson’s disease, utilizing various bibliometric software tools. Our findings indicate that from 2012 to 2016, there were few publications addressing ferroptosis in Parkinson’s disease, reflecting an initial scarcity of research in this area. However, since 2017, there has been a notable increase in literature, with publications in this domain representing 58.09% of the total articles over the past 2 years. The volume of publications in 2023 was 1.7 times higher than that in 2022, underscoring ferroptosis in Parkinson’s disease as a rapidly growing research focus. This study aims to provide a reference for scholars entering this expanding field through bibliometric analysis and visual presentation.
Our analysis also reveals that China is at the forefront of publications in this field, with two-thirds of the top 12 research institutions based there. However, there is a significant lack of collaborative efforts both among Chinese institutions and with international partners. This limited collaboration could hinder the sustainable progress of research. To address this, enhancing academic exchanges and fostering international cooperation are crucial for advancing collective research on ferroptosis and Parkinson’s disease.
The journal analysis, based on Bradford’s law, identified 19 core journals within this field, collectively publishing 165 articles. Notably, nine journals from the Frontiers series accounted for 38.2% of these publications, spanning diverse areas such as neurology, oncology, genetics, and molecular biology. Among these, the International Journal of Molecular Sciences emerged as the leading venue, predominantly featuring articles on biosynthetic agents related to ferroptosis, including both inducers and inhibitors (Du and Guo, 2022). This journal extensively covers neurodegenerative disorders such as Parkinson’s disease, Alzheimer’s disease, and Huntington’s disease, focusing on the role of ferroptosis in disease mechanisms. This includes initiating cell death through lipid peroxidation, glutathione depletion, and iron accumulation, which can lead to inflammatory responses, myelin degeneration, astrocyte dysfunction, and cognitive impairments (Reichert et al., 2020). Additionally, the journal explores potential therapeutic targets for ferroptosis in neurological diseases (Peeples and Genaro-Mattos, 2022; Tang et al., 2023). Free Radical Biology and Medicine, known for its high citation count and h-index, specializes in examining oxidative reactions’ roles in diseases, including the mechanisms of ferroptosis and associated treatment strategies. Research published in this journal has significantly advanced the understanding of ferroptosis and its relationship with Parkinson’s disease (Anandhan et al., 2022; Xia et al., 2024; Zhang et al., 2020).
Most publications in this research area are concentrated in molecular biology, immunology, and genetics, with comparatively fewer articles in clinically oriented journals. This suggests that the application of ferroptosis research to clinical practice for Parkinson’s disease remains underdeveloped. There is a critical need for increased interdisciplinary collaboration to bridge the gap between fundamental research and clinical applications.
A visual analysis of author publication outputs revealed that 90% of the top 10 authors in this field are affiliated with institutions in China. Conrad M is noted for having the highest citation count for published articles, followed by Bush A. I., Friedmann Angeli J. P., Bayir H., and Kagan V. E. This trend highlights the significant contributions of Chinese scholars to the field. However, there is a need to enhance the quality of publications and to promote more rigorous academic discourse. Fostering collaboration among researchers will be essential for advancing more profound and nuanced investigations in this domain.
Reference analysis indicates that since the introduction of the ferroptosis concept, various ferroptosis inducers (such as erastin, sorafenib, and buthionine sulfoximine) and inhibitors (such as iron chelators, Fer-1 derivatives, PKC inhibitors) have been explored (Do Van et al., 2016). There is increasing anticipation that ferroptosis inhibitors may serve as adjunctive therapies for neurodegenerative disorders, including Parkinson’s disease (Zhang et al., 2023). Research has demonstrated that iron accumulation in the brain establishes a complex relationship between ferroptosis and Parkinson’s disease (Lin et al., 2022). Additionally, GPX4, a critical antioxidant enzyme in mammals, plays a pivotal role in the ferroptosis pathway and in therapeutic interventions for the disease (Liu et al., 2023; Ursini and Maiorino, 2020).
4.2 Research hot spots and frontiers
The keyword analysis, depicted in Figure 7, highlights Cluster 1 as the predominant research focus, which includes ferroptosis, apoptosis, autophagy, necroptosis, pyroptosis, immunotherapy, tumor microenvironment, immune checkpoint inhibitors, and ICD. Emerging evidence suggests that therapies targeting non-apoptotic cell death pathways—specifically ferroptosis, pyroptosis, and autophagy—could potentially enhance the effectiveness of immunotherapy (Tang et al., 2020). Ferroptosis, necroptosis, and pyroptosis are increasingly recognized as novel forms of ICD that may influence the tumor microenvironment. Combining inducers of these cell death pathways with immune checkpoint inhibitors could amplify tumor immunotherapy outcomes (Gao et al., 2022; Niu et al., 2022).
Research also highlights that factors such as ferroptosis, apoptosis, autophagy, necrosis, oxidative stress, neuromelanin, and neuroinflammation are integral to neurodegenerative processes (Chen et al., 2020; Moreno-Garcia et al., 2021; Pomilio et al., 2023; Silva et al., 2022). Future research should focus on the sequential development of various cell death modalities in Parkinson’s disease models to identify stage-specific interventions that may slow or halt disease progression.
Furthermore, the co-occurrence analysis of keywords reveals a significant relationship between lncRNAs, ferroptosis, immunotherapy, and the tumor microenvironment. Recent studies have identified several lncRNAs related to ferroptosis that play crucial roles in disease progression. For instance, lncRNA NEAT1 regulates ferroptosis sensitivity in non-small-cell lung cancer by interacting with ACSL4 and GPX4 (Wu and Liu, 2021). Additionally, lncRNA NEAT1 impacts Parkinson’s disease pathology by sponging miR-212-3p, which targets AXIN1, while lncRNA SNHG1 exacerbates neuroinflammation in Parkinson’s disease through the miR-7/NLRP3 pathway (Cao et al., 2018; Liu et al., 2021). Given that ACSL4 and GPX4 are pivotal regulators of ferroptosis, lncRNA NEAT1 might modulate ferroptosis via these molecules, presenting a promising therapeutic approach for Parkinson’s disease (Liu et al., 2021; Zhou et al., 2023). Future studies should aim to elucidate the precise mechanisms by which NEAT1 affects ferroptosis and neurodegenerative diseases, and explore targeted therapies that modulate this lncRNA.
In addition to the aforementioned keywords, oxidative stress emerges as a critical term in this field. Oxidative stress is characterized by an imbalance between pro-oxidants (e.g., free radicals) and antioxidants, leading to excessive reactive oxygen species (Dossena and Marino, 2021; Sies, 2015). This imbalance damages cellular components, including DNA, proteins, and lipids (Martinez and Kannan, 2018). Oxidative stress is a major factor in the pathogenesis of Parkinson’s disease and other neurodegenerative disorders, and is a key feature of ferroptosis (Barnham et al., 2004; Dionisio et al., 2021; Yu et al., 2021). Therefore, future research should focus on a detailed examination of the relationship between ferroptosis and Parkinson’s disease, with a particular emphasis on the role of oxidative stress in this relationship.
4.3 Advantages and disadvantages
This study offers several distinct advantages. First, bibliometric analyses focused on the intersection of ferroptosis and Parkinson’s disease are scarce, making this investigation a pioneering effort. By using a range of bibliometric tools, this study systematically analyzes the existing body of research, providing an objective and thorough examination of the field. This approach yields valuable insights and serves as a significant reference for scholars exploring this niche area. Second, bibliometric analysis surpasses traditional research methods by offering a more expansive view of research hotspots and emerging trends, thus facilitating a deeper understanding of both current dynamics and future directions in the field.
Nevertheless, the study has some limitations too. First, it relies solely on data from the WoSCC database, which may exclude relevant studies indexed in other databases. Second, focusing exclusively on English-language publications could result in an underrepresentation of research published in other languages. Furthermore, newly published studies, especially those from the current year that have yet to achieve significant citation counts, may be underrepresented. To address this, continuous monitoring of emerging research is essential. Despite these limitations, the literature analyzed provides a comprehensive overview of the current state of research, identifying key trends and future research directions in the study of ferroptosis in Parkinson’s disease.
5 Conclusion
The volume of scholarly work exploring the relationship between ferroptosis and Parkinson’s disease has shown a notable upward trend, with China emerging as a prominent contributor to this research domain. Collaborative efforts across institutions, countries, and researchers have significantly advanced understanding in this field. The focus of research has shifted over time from foundational studies on the mechanisms of ferroptosis to more applied clinical evaluations concerning its implications for treatment and prognosis in ferroptosis-related diseases. Ferroptosis has been identified as a crucial regulatory factor in a range of diseases, with recent findings underscoring its significant role in the pathogenesis of neurodegenerative conditions, particularly Parkinson’s disease. This evolving landscape highlights ferroptosis as a critical area of study and suggests that it will continue to be a central focus for future research. Continued interdisciplinary collaboration and research are essential to further unravel the complexities of ferroptosis and its impact on disease mechanisms and treatments.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JL: Conceptualization, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. YW: Formal analysis, Investigation, Visualization, Writing – review & editing. JH: Formal analysis, Investigation, Visualization, Writing – review & editing, Data curation. DG: Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Hubei Provincial Clinical Research Center for Parkinson’s Disease (HBPDZ02). Hubei Provincial Science and Technology Plan-Key R&D Project (2023BCB140).
Acknowledgments
The authors extend their gratitude to the editors and reviewers for their insightful comments and suggestions. Such contributions have substantially enhanced the quality of the manuscript. We are grateful to the “Hubei Provincial Clinical Research Center for Parkinson’s Disease” for its strong support of our scientific work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2024.1433325/full#supplementary-material
References
Anandhan, A., Chen, W., Nguyen, N., Madhavan, L., Dodson, M., and Zhang, D. D. (2022). α-Syn overexpression, NRF2 suppression, and enhanced ferroptosis create a vicious cycle of neuronal loss in Parkinson’s disease. Free Radic. Biol. Med. 192, 130–140. doi: 10.1016/j.freeradbiomed.2022.09.015
Armstrong, M. J., and Okun, M. S. (2020). Diagnosis and treatment of Parkinson disease: a review. JAMA 323, 548–560. doi: 10.1001/jama.2019.22360
Barnham, K. J., Masters, C. L., and Bush, A. I. (2004). Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 3, 205–214. doi: 10.1038/nrd1330
Bloem, B. R., Okun, M. S., and Klein, C. (2021). Parkinson’s disease. Lancet 397, 2284–2303. doi: 10.1016/s0140-6736(21)00218-x
Cao, B. Q., Wang, T., Qu, Q. M., Kang, T., and Yang, Q. (2018). Long noncoding RNA SNHG1 promotes neuroinflammation in Parkinson’s disease via regulating miR-7/NLRP3 pathway. Neuroscience 388, 118–127. doi: 10.1016/j.neuroscience.2018.07.019
Chen, S. Y., Gao, Y., Sun, J. Y., Meng, X. L., Yang, D., Fan, L. H., et al. (2020). Traditional Chinese medicine: role in reducing beta-amyloid, apoptosis, autophagy, neuroinflammation, oxidative stress, and mitochondrial dysfunction of Alzheimer's disease. Front. Pharmacol. 11:497. doi: 10.3389/fphar.2020.00497
Chen, C. M., Hu, Z. G., Liu, S. B., and Tseng, H. (2012). Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert. Opin. Biol. Ther. 12, 593–608. doi: 10.1517/14712598.2012.674507
Ding, X., and Yang, Z. (2020). Knowledge mapping of platform research: a visual analysis using VOSviewer and CiteSpace. Electron. Commer. Res. 22, 787–809. doi: 10.1007/s10660-020-09410-7
Dionisio, P. A., Amaral, J. D., and Rodrigues, C. M. P. (2021). Oxidative stress and regulated cell death in Parkinson’s disease. Ageing Res. Rev. 67:101263. doi: 10.1016/j.arr.2021.101263
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072. doi: 10.1016/j.cell.2012.03.042
Do Van, B., Gouel, F., Jonneaux, A., Timmerman, K., Gele, P., Petrault, M., et al. (2016). Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol. Dis. 94, 169–178. doi: 10.1016/j.nbd.2016.05.011
Donthu, N., Kumar, S., Mukherjee, D., Pandey, N., and Lim, W. M. (2021). How to conduct a bibliometric analysis: an overview and guidelines. J. Bus. Res. 133, 285–296. doi: 10.1016/j.jbusres.2021.04.070
Dorsey, E., Elbaz, A., Nichols, E., Abd-Allah, F., Abdelalim, A., Adsuar, J., et al. (2018). Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17, 939–953. doi: 10.1016/S1474-4422(18)30295-3
Dossena, S., and Marino, A. (2021). Cellular oxidative stress. Antioxidants 10:8416763. doi: 10.3390/antiox10030399
Du, Y., and Guo, Z. (2022). Recent progress in ferroptosis: inducers and inhibitors. Cell Death Discov. 8:501. doi: 10.1038/s41420-022-01297-7
Egghe, L. (2006). Theory and practise of the g-index. Scientometrics 69, 131–152. doi: 10.1007/s11192-006-0144-7
Gao, W., Wang, X., Zhou, Y., Wang, X., and Yu, Y. (2022). Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immunotherapy. Signal Transduct. Target. Ther. 7:196. doi: 10.1038/s41392-022-01046-3
Guiney, S. J., Adlard, P. A., Bush, A. I., Finkelstein, D. I., and Ayton, S. (2017). Ferroptosis and cell death mechanisms in Parkinson’s disease. Neurochem. Int. 104, 34–48. doi: 10.1016/j.neuint.2017.01.004
Hirsch, J. E. (2005). An index to quantify an individual’s scientific research output. Proc. Natl. Acad. Sci. U.S.A. 102, 16569–16572. doi: 10.1073/pnas.0507655102
Hou, Z., Jiang, P., Su, S., and Zhou, H. (2022). Hotspots and trends in multiple myeloma bone diseases: a bibliometric visualization analysis. Front. Pharmacol. 13:1003228. doi: 10.3389/fphar.2022.1003228
Jiang, X., Stockwell, B. R., and Conrad, M. (2021). Ferroptosis: mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 22, 266–282. doi: 10.1038/s41580-020-00324-8
Jiang, X., Wu, K., Ye, X. Y., Xie, T., Zhang, P., Blass, B. E., et al. (2023). Novel druggable mechanism of Parkinson’s disease: potential therapeutics and underlying pathogenesis based on ferroptosis. Med. Res. Rev. 43, 872–896. doi: 10.1002/med.21939
Kemeç, A., and Altınay, A. T. (2023). Sustainable energy research trend: a bibliometric analysis using VOSviewer, RStudio Bibliometrix, and CiteSpace software tools. Sustainability 15:3618. doi: 10.3390/su15043618
Lee, H., Zandkarimi, F., Zhang, Y. L., Meena, J. K., Kim, J., Zhuang, L., et al. (2020). Energy-stress-mediated AMPK activation inhibits ferroptosis. Nat. Cell Biol. 22, 225–234. doi: 10.1038/s41556-020-0461-8
Li, Y., Feng, D. C., Wang, Z. Y., Zhao, Y., Sun, R. M., Tian, D. H., et al. (2019). Ischemia-induced ACSL4 activation contributes to ferroptosis-mediated tissue injury in intestinal ischemia/reperfusion. Cell Death Differ. 26, 2284–2299. doi: 10.1038/s41418-019-0299-4
Li, X., Yu, J., and Shu, C. (2022). Bibliometric analysis of global research trends on post-stroke pneumonia: current development status and research. Front. Public Health 10:950859. doi: 10.3389/fpubh.2022.950859
Lin, K. J., Chen, S. D., Lin, K. L., Liou, C. W., Lan, M. Y., Chuang, Y. C., et al. (2022). Iron brain menace: the involvement of ferroptosis in parkinson disease. Cells 11:3829. doi: 10.3390/cells11233829
Liu, Y., Li, S., Chen, L., Lin, L., Xu, C., Qiu, H., et al. (2024). Global trends in tumor microenvironment-related research on tumor vaccine: a review and bibliometric analysis. Front. Immunol. 15:1341596. doi: 10.3389/fimmu.2024.1341596
Liu, Y., Wan, Y., Jiang, Y., Zhang, L., and Cheng, W. (2023). GPX4: the hub of lipid oxidation, ferroptosis, disease and treatment. Biochim. Biophys. Acta Rev. Cancer 1878:188890. doi: 10.1016/j.bbcan.2023.188890
Liu, T., Zhang, Y., Liu, W. H., and Zhao, J. S. (2021). LncRNA NEAT1 regulates the development of Parkinson’s disease by targeting AXIN1 via sponging miR-212-3p. Neurochem. Res. 46, 230–240. doi: 10.1007/s11064-020-03157-1
Ma, D., Yang, B., Guan, B., Song, L., Liu, Q., Fan, Y., et al. (2021). A bibliometric analysis of pyroptosis from 2001 to 2021. Front. Immunol. 12:731933. doi: 10.3389/fimmu.2021.731933
Martinez, M. P., and Kannan, K. (2018). Simultaneous analysis of seven biomarkers of oxidative damage to lipids, proteins, and DNA in urine. Environ. Sci. Technol. 52, 6647–6655. doi: 10.1021/acs.est.8b00883
Mcnicholas, P. J., Floyd, R. G., Fennimore, L. E., and Fitzpatrick, S. A. (2022). Determining journal article citation classics in school psychology: an updated bibliometric analysis using Google Scholar, Scopus, and Web of Science. J. Sch. Psychol. 90, 94–113. doi: 10.1016/j.jsp.2021.11.001
Merigó, J. M., and Yang, J.-B. (2017). A bibliometric analysis of operations research and management science. Omega 73, 37–48. doi: 10.1016/j.omega.2016.12.004
Moreno-Garcia, A., Kun, A., Calero, M., and Calero, O. (2021). The neuromelanin paradox and its dual role in oxidative stress and neurodegeneration. Antioxidants 10:124. doi: 10.3390/antiox10010124
Mortensen, M. S., Ruiz, J., and Watts, J. L. (2023). Polyunsaturated fatty acids drive lipid peroxidation during ferroptosis. Cells 12:804. doi: 10.3390/cells12050804
Niu, X., Chen, L. J., Li, Y., Hu, Z. J., and He, F. (2022). Ferroptosis, necroptosis, and pyroptosis in the tumor microenvironment: perspectives for immunotherapy of SCLC. Semin. Cancer Biol. 86, 273–285. doi: 10.1016/j.semcancer.2022.03.009
Pan, X., Yan, E., Cui, M., and Hua, W. (2018). Examining the usage, citation, and diffusion patterns of bibliometric mapping software: a comparative study of three tools. J. Informetr. 12, 481–493. doi: 10.1016/j.joi.2018.03.005
Peeples, E. S., and Genaro-Mattos, T. C. (2022). Ferroptosis: a promising therapeutic target for neonatal hypoxic-ischemic brain injury. Int. J. Mol. Sci. 23:7420. doi: 10.3390/ijms23137420
Pomilio, A. B., Vitale, A. A., and Lazarowski, A. J. (2023). COVID-19 and Alzheimer’s disease: neuroinflammation, oxidative stress, ferroptosis, and mechanisms involved. Curr. Med. Chem. 30, 3993–4031. doi: 10.2174/0929867329666221003101548
Pringsheim, T., Jette, N., Frolkis, A., and Steeves, T. D. (2014). The prevalence of Parkinson’s disease: a systematic review and meta-analysis. Mov. Disord. 29, 1583–1590. doi: 10.1002/mds.25945
Reichert, C. O., De Freitas, F. A., Sampaio-Silva, J., Rokita-Rosa, L., Barros, P. D., Levy, D., et al. (2020). Ferroptosis mechanisms involved in neurodegenerative diseases. Int. J. Mol. Sci. 21:8765. doi: 10.3390/ijms21228765
Sies, H. (2015). Oxidative stress: a concept in redox biology and medicine. Redox Biol. 4, 180–183. doi: 10.1016/j.redox.2015.01.002
Silva, D. F., Empadinhas, N., Cardoso, S. M., and Esteves, A. R. (2022). Neurodegenerative microbially-shaped diseases: oxidative stress meets neuroinflammation. Antioxidants 11:2141. doi: 10.3390/antiox11112141
Stockwell, B. R. (2022). Ferroptosis turns 10: emerging mechanisms, physiological functions, and therapeutic applications. Cell 185, 2401–2421. doi: 10.1016/j.cell.2022.06.003
Stockwell, B. R., Friedmann Angeli, J. P., Bayir, H., Bush, A. I., Conrad, M., Dixon, S. J., et al. (2017). Ferroptosis: a regulated cell death Nexus linking metabolism, redox biology, and disease. Cell 171, 273–285. doi: 10.1016/j.cell.2017.09.021
Sun, N., Xing, Y., Jiang, J., Wu, P., Qing, L., and Tang, J. (2023). Knowledge mapping and emerging trends of ferroptosis in ischemia reperfusion injury research: a bibliometric analysis (2013–2022). Heliyon 9:e20363. doi: 10.1016/j.heliyon.2023.e20363
Tang, L., Liu, S., Li, S., Chen, Y., Xie, B., and Zhou, J. (2023). Induction mechanism of ferroptosis, necroptosis, and pyroptosis: a novel therapeutic target in nervous system diseases. Int. J. Mol. Sci. 24:10127. doi: 10.3390/ijms241210127
Tang, R., Xu, J., Zhang, B., Liu, J., Liang, C., Hua, J., et al. (2020). Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J. Hematol. Oncol. 13:110. doi: 10.1186/s13045-020-00946-7
Thomas, K. N., and Lawrence, A. (2022). Ab1205 medical and psychological comorbidities in fibromyalgia: a cross sectional study. Ann. Rheum. Dis. 81, 1717–1718. doi: 10.1136/annrheumdis-2022-eular.3567
Ursini, F., and Maiorino, M. (2020). Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic. Biol. Med. 152, 175–185. doi: 10.1016/j.freeradbiomed.2020.02.027
van Eck, N. J., and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. doi: 10.1007/s11192-009-0146-3
Woeginger, G. J. (2008). An axiomatic analysis of Egghe’s g-index. J. Informetr. 2, 364–368. doi: 10.1016/j.joi.2008.05.002
Wu, H. X., and Liu, A. W. (2021). Long non-coding RNA NEAT1 regulates ferroptosis sensitivity in non-small-cell lung cancer. J. Int. Med. Res. 49:030006052199618. doi: 10.1177/0300060521996183
Xia, Y., Wang, H., Xie, Z., Liu, Z. H., and Wang, H. L. (2024). Inhibition of ferroptosis underlies EGCG mediated protection against Parkinson’s disease in a Drosophila model. Free Radic. Biol. Med. 211, 63–76. doi: 10.1016/j.freeradbiomed.2023.12.005
Yu, Y., Yan, Y., Niu, F., Wang, Y., Chen, X., Su, G., et al. (2021). Ferroptosis: a cell death connecting oxidative stress, inflammation and cardiovascular diseases. Cell Death Discov. 7:193. doi: 10.1038/s41420-021-00579-w
Zhang, P., Chen, L., Zhao, Q., Du, X., Bi, M., Li, Y., et al. (2020). Ferroptosis was more initial in cell death caused by iron overload and its underlying mechanism in Parkinson’s disease. Free Radic. Biol. Med. 152, 227–234. doi: 10.1016/j.freeradbiomed.2020.03.015
Zhang, J., Song, L., Jia, J., Tian, W., Lai, R., Zhang, Z., et al. (2022). Knowledge mapping of necroptosis from 2012 to 2021: a bibliometric analysis. Front. Immunol. 13:917155. doi: 10.3389/fimmu.2022.917155
Zhang, R. F., Zeng, M., Lv, N., Wang, L. M., Yang, Q. Y., Gan, J. L., et al. (2023). Ferroptosis in neurodegenerative diseases: inhibitors as promising candidate mitigators. Eur. Rev. Med. Pharmacol. Sci. 27, 46–65. doi: 10.26355/eurrev_202301_30852
Keywords: ferroptosis, Parkinson’s disease, RStudio bibliometrix, CiteSpace, VOSviewers, bibliometrics
Citation: Li J, Wang Y, Huang J and Gong D (2024) Knowledge mapping of ferroptosis in Parkinson’s disease: a bibliometric analysis: 2012–2023. Front. Aging Neurosci. 16:1433325. doi: 10.3389/fnagi.2024.1433325
Edited by:
Shahnawaz Ali Bhat, Aligarh Muslim University, IndiaReviewed by:
Junke Song, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaEmir Malovic, University of Illinois Chicago, United States
Copyright © 2024 Li, Wang, Huang and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Daokai Gong, ZGtfZ29uZ0AxNjMuY29t
 Juanqin Li
Juanqin Li Yanli Wang
Yanli Wang Jing Huang
Jing Huang Daokai Gong
Daokai Gong