- 1Department of Nutrition,Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China
- 2Peking University Sixth Hospital, Peking University Institute of Mental Health, NHC Key Laboratory of Mental Health (Peking University), National Clinical Research Center for Mental Disorders (Peking University Sixth Hospital), Chinese Academy of Medical Sciences Research Unit (No. 2018RU006), Peking University, Beijing, China
- 3Peking-Tsinghua Center for Life Sciences and PKU-IDG/McGovern Institute for Brain Research, Peking University, Beijing, China
- 4Department of Psychiatry, Renmin Hospital of Wuhan University, Wuhan, China
- 5Department of Psychiatry, Henan Mental Hospital, The Second Affiliated Hospital of Xinxiang Medical University, Xinxiang, China
- 6Henan Collaborative Innovation Center of Prevention and Treatment of Mental Disorder, Xinxiang, China
- 7Henan Key Lab of Biological Psychiatry, International Joint Research Laboratory for Psychiatry and Neuroscience of Henan, Xinxiang Medical University, Xinxiang, China
- 8Key Laboratory of Brain Health Intelligent Evaluation and Intervention, Ministry of Education (Beijing Institute of Technology), Beijing, China
Background: Posttraumatic stress disorder (PTSD) is associated with the development of dementia. However, the link between PTSD and preclinical Alzheimer’s disease pathology (amyloid β [Aβ]) remains controversial. Moreover, the correlation between the severity of PTSD with Aβ levels remains unknown.
Methods: This cross-sectional study sought to investigate the associations of PTSD symptoms with global and regional brain Aβ burden. To this end, data were obtained from participants in the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s Disease (A4) Study. In addition, we explored the association between the severity of PTSD symptoms and Aβ levels.
Results: A total of 4,228 participants aged 65 to 85 years were included in the final analysis. The results showed that PTSD symptoms were significantly associated with higher global Aβ levels (1.15 ± 0.20 vs. 1.09 ± 0.19; β = 0.056; p < 0.001), after adjusting for covariates. The association between PTSD symptoms and Aβ levels was not affected by sex, age, ApoE genotype, or psychiatric diseases. Similarly, PTSD symptoms were significantly associated with Aβ levels in all subregions, including the anterior cingulate, posterior cingulate, parietal cortex, precuneus, temporal cortex, and frontal cortex. In addition, the group with severe PTSD symptoms (1.22 ± 0.24) exhibited higher global Aβ levels than the groups with moderate (1.14 ± 0.19) or mild (1.12 ± 0.20) symptoms or the control (1.08 ± 0.18), with p < 0.001.
Conclusion: The findings imply a close relationship between PTSD and brain Aβ levels, irrespective of sex, age, ApoE genotype, or psychiatric diseases. More well-designed studies are needed to further explore the relationship and mechanism underlying the association between PTSD and Aβ burden.
Introduction
Posttraumatic stress disorder (PTSD) is one of the most common psychopathological consequences of exposure to trauma such as physical and sexual assaults, fatal accidents, catastrophic pandemics, or other extremely stressful events (Shalev et al., 2017; Yuan et al., 2021). Symptoms of PTSD may include flashbacks, nightmares, and severe anxiety, as well as uncontrollable thoughts about the event (Brewin et al., 2017). The lifetime prevalence of PTSD varies according to social background and country of residence, ranging from 1.3 to 12.2%, and the 1-year prevalence is 0.2 to 3.8% (Karam et al., 2014). In addition, PTSD imposes a great burden on individuals and society, and studies suggest that PTSD is associated with multiple negative health outcomes, including disability, chronic illnesses, and even suicide (Rajkumar, 2023).
In addition to the above negative health outcomes, suggestive effects have been demonstrated for an association between PTSD and the development of later-life negative cognitive outcomes, including worse-than-normal changes with aging and Alzheimer’s disease (Greenberg et al., 2014). A longitudinal cohort study of approximately 500,000 civilians found that PTSD was associated with an increased risk of dementia over an average of 8 years of follow-up (Flatt et al., 2018). In addition, increasing evidence has identified PTSD as a possible risk factor for dementia in military veterans (Rafferty et al., 2018). In general, veterans with PTSD were found to exhibit an approximately 2-fold increased risk of dementia compared to those without PTSD (Mawanda et al., 2017; Qureshi et al., 2010; Yaffe et al., 2010). These epidemiological studies imply that PTSD is associated with a high risk of dementia (Flatt et al., 2018; Rafferty et al., 2018) or AD (Greenberg et al., 2014).
Because there are no available treatments for dementia and AD, the identification of potential risk factors may reduce the risk of developing dementia and help ease the personal and economic burden caused by dementia (Livingston et al., 2024; Zheng et al., 2021). As a biomarker for dementia, detection of the amyloid burden in cognitively normal older adults has clinical implications for the prevention and treatment of cognitive decline and dementia in their early stages, and it is of primary significance to make efforts to avoid the accumulation of Aβ in the brain (Raza et al., 2021; Zheng et al., 2020, 2022). Despite multiple epidemiological studies finding that PTSD is associated with a high risk of dementia or AD, the majority of research focused on PSTD and Aβ has shown no significant association between these two factors (Elias et al., 2020; Weiner et al., 2022; Weiner et al., 2017). For example, a study found that PTSD did not significantly increase the risk for AD, as measured by amyloid PET, in veterans using the Alzheimer’s Disease Neuroimaging Initiative (Weiner et al., 2017). A cross-sectional study also indicated that there was no association between AD pathology, including Aβ and tau, and PTSD of up to 50 years duration (Elias et al., 2020). Similarly, a null association between PTSD and Aβ or tau accumulation was validated in veterans without dementia (Weiner et al., 2022). These inconsistent findings suggest the necessity for further investigation of the link between PSTD symptoms and Aβ levels.
The association between PTSD symptoms and regional Aβ levels, as well as the relationship between the severity of PTSD symptoms and amyloid burden, has also raised concerns in recent years. A study pointed out that there was no association of PTSD symptoms with Aβ levels in the subregions of the brain, including the frontal, cingulate, parietal, and temporal regions in veterans (Marcolini et al., 2023). With regard to the relationship between the severity of PTSD symptoms and Aβ burden, Caprioglio et al. found that in participants with subjective cognitive decline, amyloid positivity was associated with a higher risk of PTSD symptoms scores but no probable presence of severe PTSD (Caprioglio et al., 2023). This limited evidence further indicates the importance of investigating the relationship between PTSD symptoms and regional Aβ burden as well as between the severity of PTSD symptoms and Aβ burden. Furthermore, the findings on the association between PTSD and Aβ burden are inconclusive, and evidence on the link between PTSD and Aβ burden in cognitively normal older adults is lacking.
Based on these considerations, we aimed to identify a clear association of PTSD symptoms with global and regional Aβ burden; moreover, the link between the severity of PTSD symptoms and Aβ burden was also investigated. In this study, we analyzed a sample of 4,228 cognitively unimpaired older adults with self-reported PTSD symptoms, health information, and amyloid PET imaging. We hypothesized that (1) PTSD symptoms are associated with increased Aβ deposition in the whole-brain and regional brain areas, including frontal, temporal, parietal, precuneus, anterior cingulate, and posterior cingulate and (2) the severity of PSTD symptoms is associated with increased Aβ levels.
Materials and methods
This cross-sectional study was conducted between April 2014 and December 2017. Participants screened for inclusion in the A4 Study were included in this study if they had undergone an 18F-florbetapir PET scan, had ApoE genotype information, completed a battery of neuropsychological testing, scored between 25 and 30 on the Mini-Mental State Examination (MMSE), had a Clinical Dementia Rating of 0, and were aged between 65 and 85 years. Exclusion criteria for the A4 Study have been described previously (Sperling et al., 2014; Zheng et al., 2023). Briefly, participants were excluded from the A4 Study if they were taking a prescription Alzheimer’s medication or had a current serious or unstable illness that could interfere with the study. This study was approved by the institutional review boards of all participating institutions, and written informed consent was obtained from all participants. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines (Insel et al., 2021). The study also received approval from the Institutional Review Board of the Peking University Sixth Hospital for the use of A4 data.
PTSD symptoms assessment
The Impact of Event Scale (IES) is widely used to assess PTSD, with satisfactory test–retest reliability (r = 0.79 to 0.89) and internal consistency (Cronbach’s α = 0.78 to 0.82) (Horowitz et al., 1979; Joseph, 2000). The IES had 15 items and took 5–10 min to complete. Respondents were asked to rate the frequency, on a 4-point scale, with which each symptom has occurred over the last week. The 4 points were: 0 (not at all), 1 (rarely), 3 (sometimes), and 5 (often). The total scores ranged from 0 to 75, and the interpretation of the total score was based on the following dimensions of posttraumatic stress symptoms: 0 to 8 as subclinical range, 9 to 25 as mild range, 26 to 43 as moderate range, and 44+ as severe range. Participants were considered to have PTSD symptoms if the score exceeded the cutoff point of 26, based on the established literature (Horowitz et al., 1979; Sterling, 2008).
18F-Florbetapir PET imaging
Florbetapir PET scan images were acquired in accordance with previous studies (Clark et al., 2012; Joshi et al., 2015). A 10-min PET acquisition was performed approximately 50 min after administration of 370 MBq (10 mCi) florbetapir. Images were acquired with a 128 × 128 matrix and reconstructed with iterative or row action maximization likelihood algorithms with a post-reconstruction Gaussian filter. Images for each subject were converted to Montreal Neurologic Institute (MNI) standard stereotactic brain atlas space using a specialized F 18 Florbetapir PET template (Joshi et al., 2015). The resulting transformation matrix was then used to spatially normalize all PET image frames for each subject to the MNI space. The SUVR values for all scans were calculated using the whole cerebellum as a reference. Aβ positivity was defined as an 18F-florbetapir PET SUVR ≥1.10, while Aβ negativity was defined as an SUVR <1.10, as previously described (Clark et al., 2012; Joshi et al., 2012).
Semiautomated quantitative analysis was performed using the mean signal of six predefined anatomically relevant cortical regions of interest (frontal, temporal, parietal, precuneus, anterior cingulate, and posterior cingulate), with the whole cerebellum as a reference region (Clark et al., 2012).
Covariates
Covariates included demographic information (age, sex, body mass index [BMI], marital status, and educational attainment); habits (sleep duration, alcohol consumption, and cigarette consumption); ApoE genotypes; and comorbidities (psychiatric, neurological, cardiovascular, and endocrinological diseases). A summary of the covariates is provided in Supplementary Table S1. There were six ApoE genotypes: ε22, ε23, ε24, ε33, ε34, and ε44. The most common ApoE allele, ApoE-ε3, was associated with an average risk of AD, whereas the ApoE-ε4 and ApoE-ε2 alleles were linked with higher and lower AD risks, respectively (Liu et al., 2013; Shen and Jia, 2016).
Statistical analysis
Descriptive statistics were used to present the demographic and clinical characteristics of participants according to their PTSD status. Independent sample t-tests and χ2 tests were used to compare continuous and categorical variables, respectively. Multivariate linear regression analyses were performed to explore the association between PTSD symptoms and Aβ in whole brain and subregions, with the covariates mentioned above included. Moreover, to investigate the association between the severity of PTSD symptoms (including control, mild, moderate, and severe PTSD) and Aβ levels, an analysis of variance was performed and LSD correction was carried out to adjust for multiple comparisons. Multivariate linear regression analyses were also performed to validate the association between the severity of PTSD symptoms (mild, moderate, and severe symptoms were coded as dummy variables) and Aβ levels. The statistical analyses were performed using SPSS statistical software version 22 (IBM Corp), and the level of significance was set to a p-value of <0.05.
Sensitivity analysis
To further validate the robustness of the association of PTSD symptoms with Aβ burden, logistic regression analysis was performed to explore the association between PTSD symptoms and Aβ positivity. Furthermore, the association between the severity of PTSD symptoms and Aβ positivity was explored using logistic regression analysis. In addition, to explore the effect of the sex, age, ApoE genotypes, and comorbidity of psychiatric diseases on PTSD and amyloid burden, the interactions sex × PTSD, age × PTSD, ApoE genotypes × PTSD, and psychiatric diseases × PTSD were added to the multilinear regression analysis. Pearson’s correlation analyses were used to validate the correlation between PTSD scores and regional Aβ levels, and related correlation coefficients were also calculated. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for all regression analyses, and the level of significance was set to a p-value of <0.05.
Results
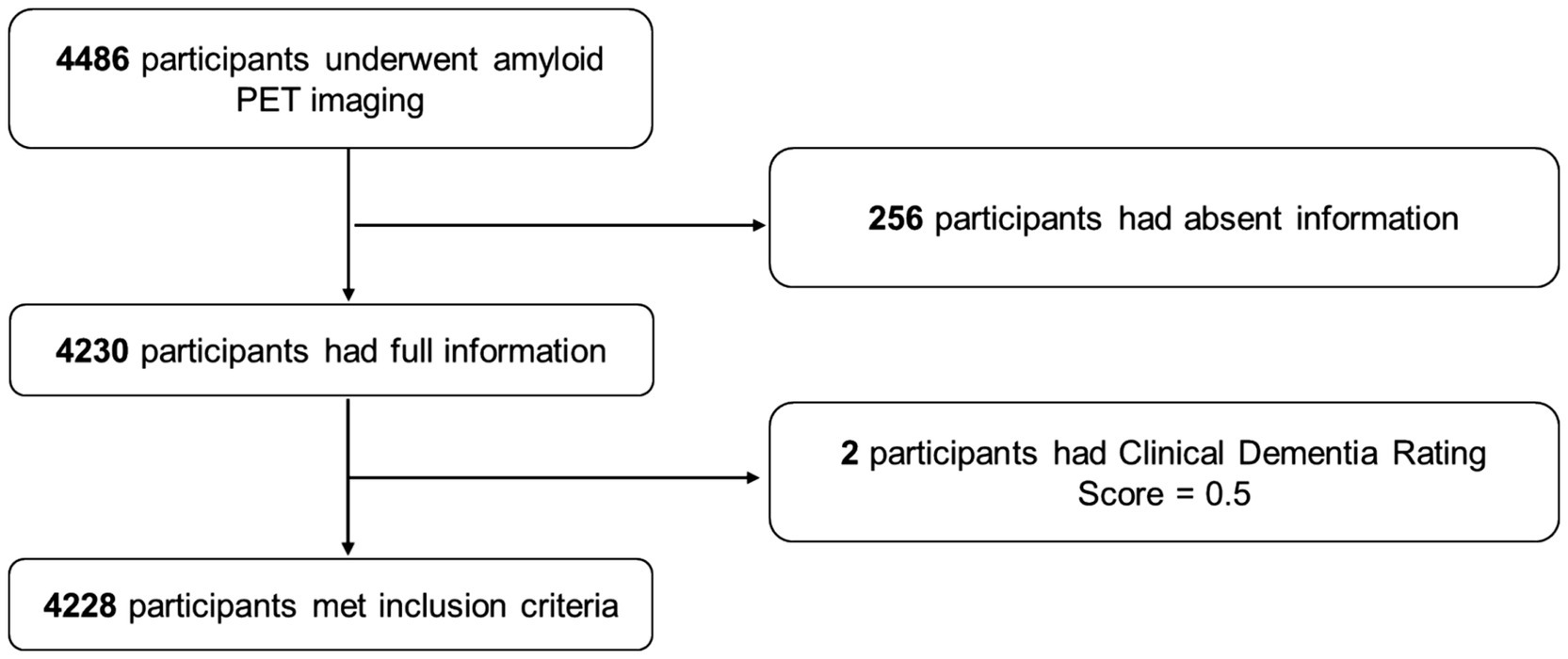
A total of 4,486 participants underwent PET; of this sample, 4,230 had available information on caffeine consumption, demographic information, habits, ApoE genotypes, and comorbidities. Two participants were further excluded for preclinical dementia with a CDR of 0.5, and finally, 4,228 participants were included in the present analysis. The detailed process of sample inclusion is presented in Figure 1.
Among the included participants, 238 had PTSD symptoms and 3,990 had no PTSD symptoms. The following factors were found to be significantly different between the two groups of participants: gender, age, ApoE genotype, and comorbidity with psychiatric diseases. The detailed demographic and clinical characteristics of the participants, grouped by PTSD symptoms, are shown in Table 1.
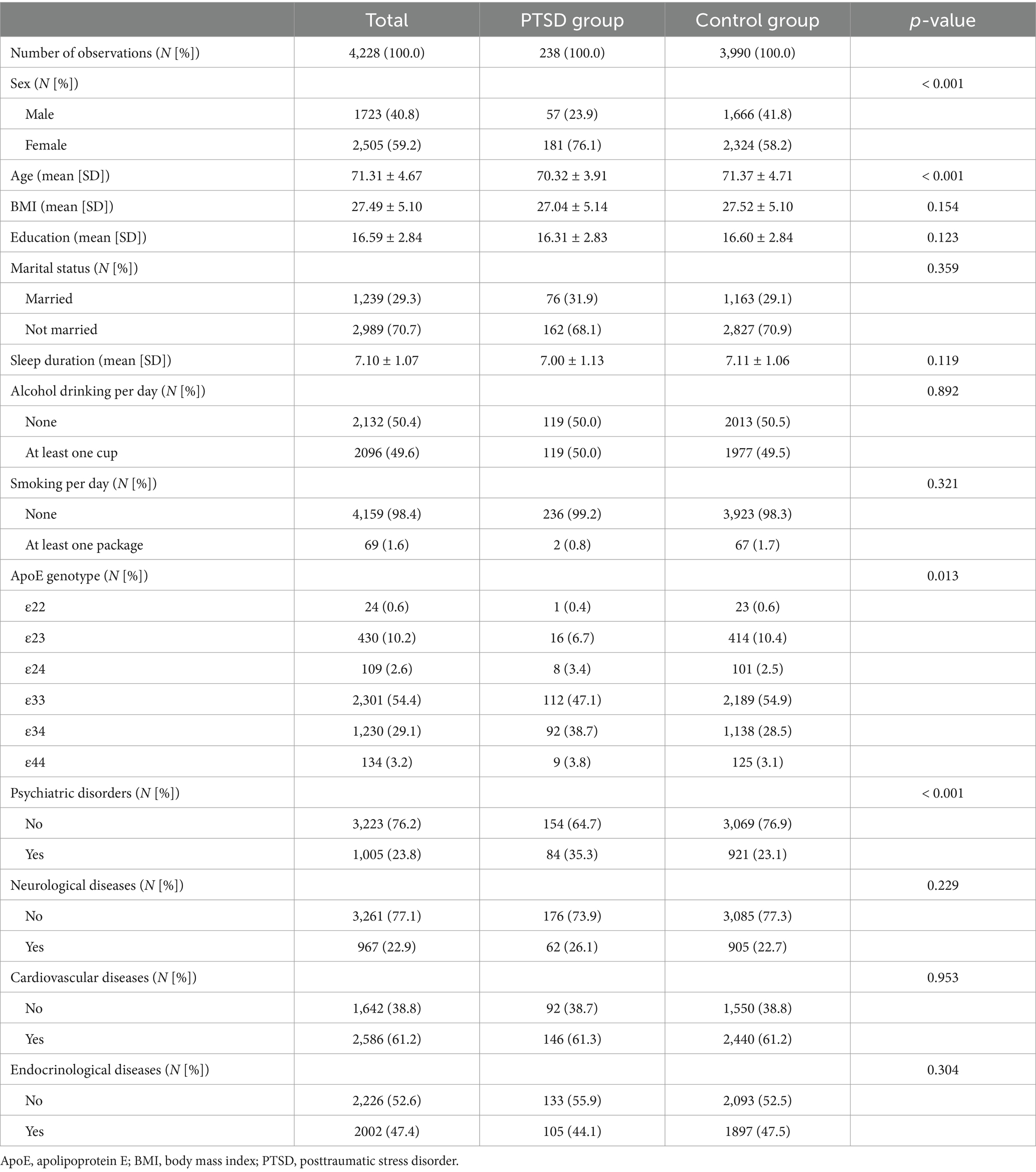
Table 1. Demographic and clinical characteristics of the participants by posttraumatic stress disorder symptoms.
Relationship between PTSD symptoms and global Aβ burden
Multilinear linear regression showed that PTSD symptoms were significantly associated with higher whole-brain Aβ levels (1.15 ± 0.20 vs. 1.09 ± 0.19; β = 0.056; p < 0.001) when adjusted for covariates (Figure 2 and Table 2). Logistic regression analysis showed that PTSD symptoms were significantly associated with Aβ positivity (OR = 2.130, 95% CI = 1.601–2.834) compared to the controls (Supplementary Table S2).
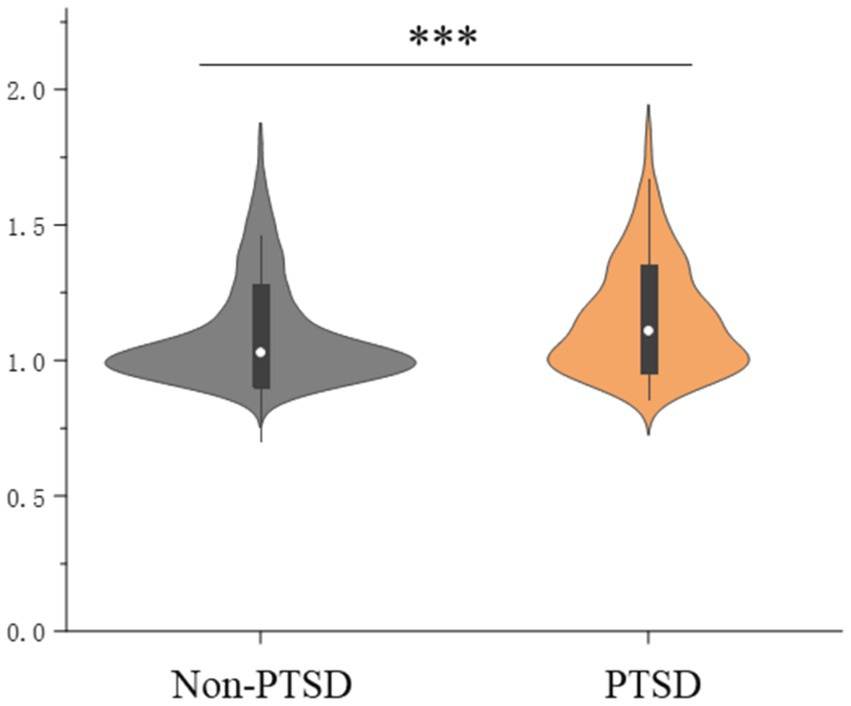
Figure 2. Posttraumatic stress disorder symptoms and global brain amyloid burden; ***indicates p < 0.001.
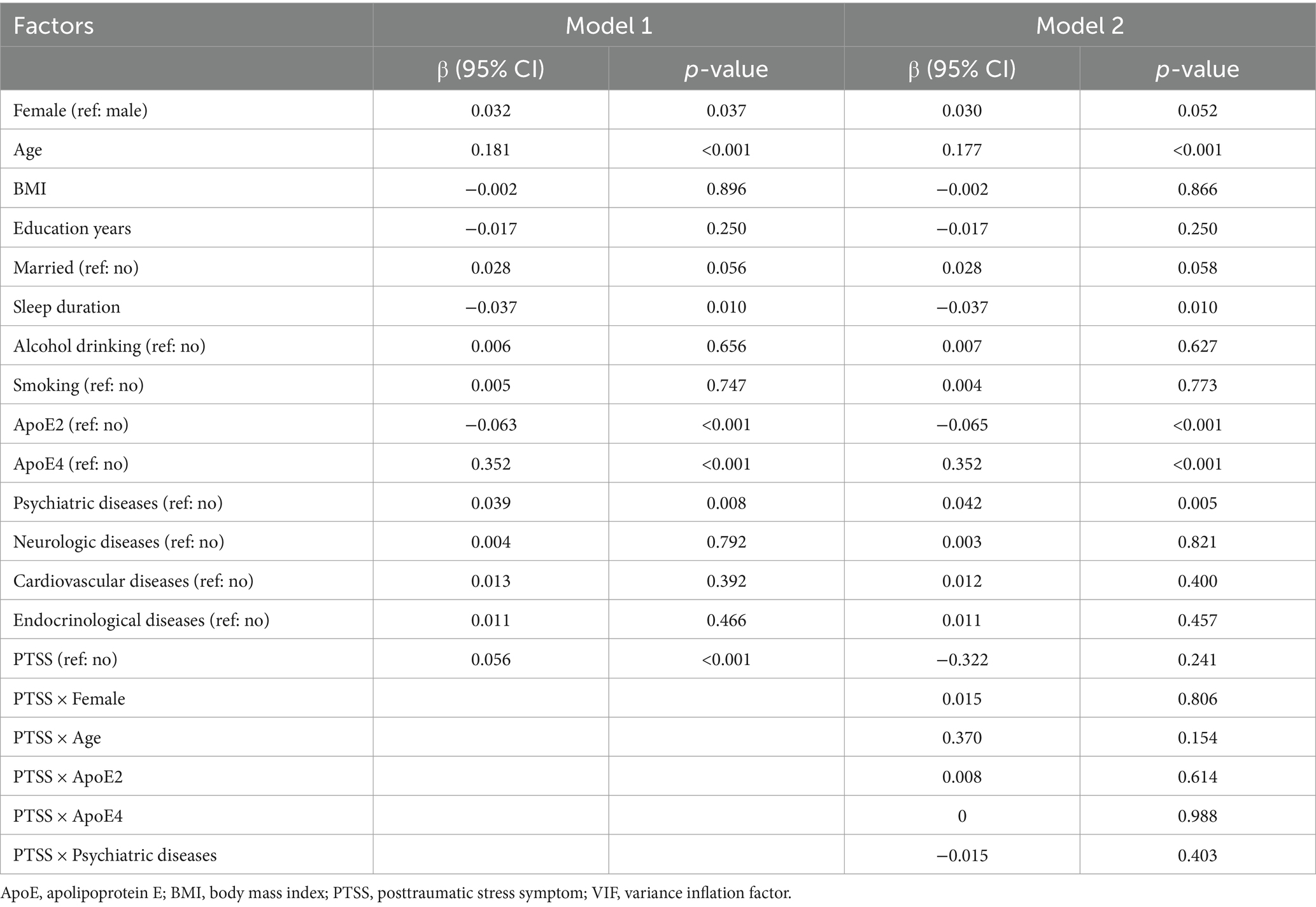
Interactions, namely sex × PTSD, age × PTSD, APOE genotypes × PTSD, and psychiatric diseases × PTSD, were added to the multivariable logistic regression analysis to explore the effect of PTSD symptoms on the amyloid burden. The results did not indicate statistical significance (Table 2). These findings indicate that PTSD was significantly associated with global Aβ burden in the studied population and was influenced by sex, age, APOE genotype, or the presence of comorbid psychiatric diseases.
Association of PTSD symptoms with regional Aβ burden
In addition, significant associations were found between PTSD symptoms and regional Aβ levels (anterior cingulate: 1.21 ± 0.25 vs. 1.14 ± 0.23; β = 0.048; p = 0.001; posterior cingulate: 1.16 ± 0.22 vs. 1.10 ± 0.22; β = 0.040; p = 0.006; parietal cortex: 1.109 ± 0.21 vs. 1.04 ± 0.20; β = 0.056; p < 0.001; precuneus: 1.24 ± 0.25 vs. 1.18 ± 0.25; β = 0.047; p = 0.001; temporal cortex: 1.19 ± 0.20 vs. 1.13 ± 0.18; β = 0.061; p < 0.001; and frontal cortex: 1.02 ± 0.20 vs. 0.96 ± 0.18; β = 0.055; p < 0.001). The results are presented in Figure 3 and Supplementary Table S3. Moreover, correlation analyses further validated that PTSD symptoms were significantly correlated with regional Aβ levels (anterior cingulate: r = 0.124, p < 0.001; posterior cingulate: r = 0.119, p < 0.001; parietal cortex: r = 0.106, p < 0.001; precuneus: r = 0.123, p < 0.001; temporal cortex: r = 0.132, p < 0.001; and frontal cortex: r = 0.148, p < 0.001); the results are presented in Supplementary Figure S1.
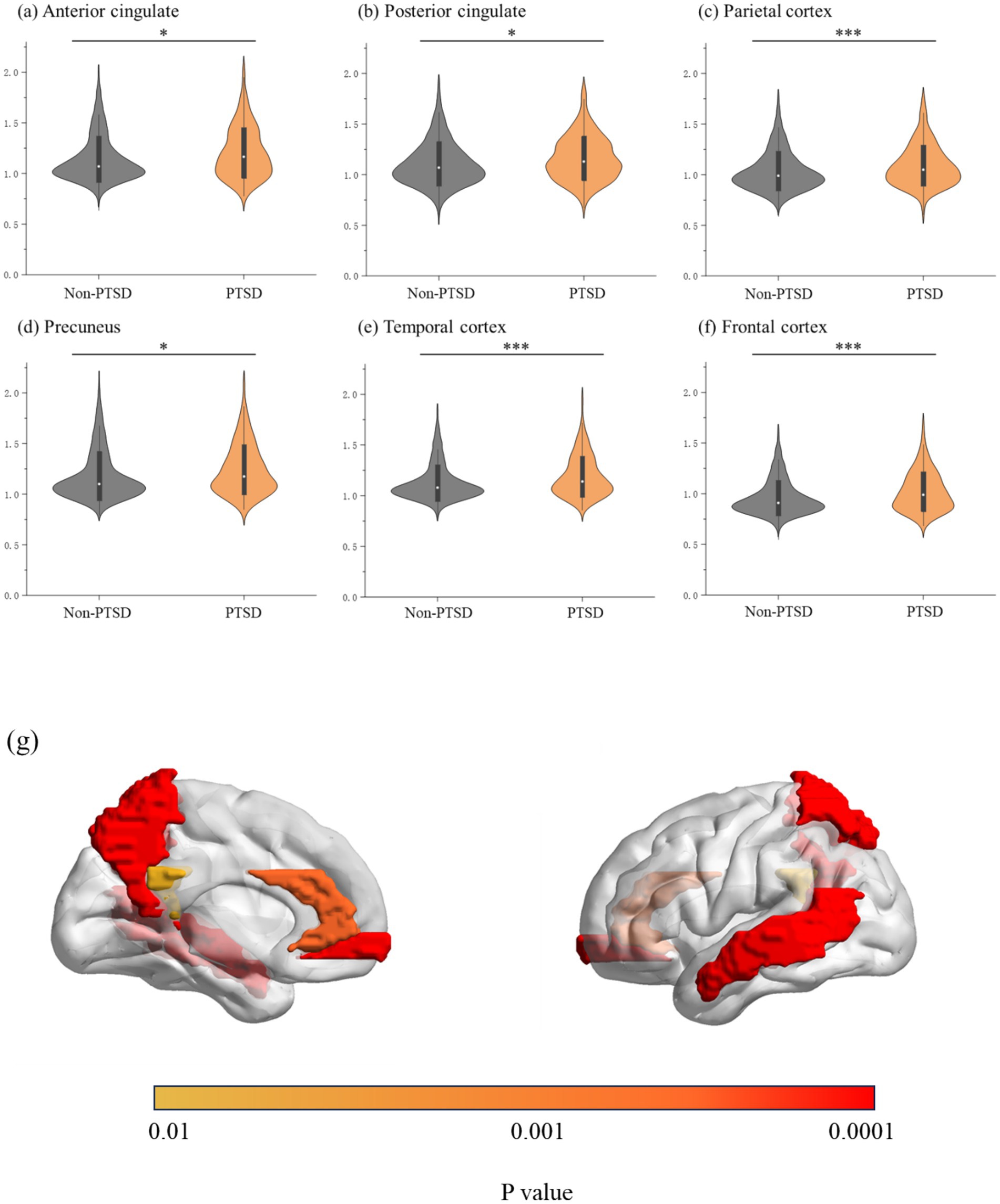
Figure 3. (A–E) Posttraumatic stress disorder symptoms and regional brain amyloid burden; (G) brain regions with increased amyloid burden in participants with posttraumatic stress disorder symptoms; *indicates 0.001 ≤ p < 0.05, and ***indicates p < 0.001.
Association between severity of PTSD symptoms and global Aβ burden
The results for the analysis of the association between the severity of PTSD symptoms and Aβ burden are presented in Figure 4 and Table 3. The Aβ burden in groups with no, mild, moderate, and severe PTSD symptoms were 1.08 ± 0.18, 1.12 ± 0.20, 1.14 ± 0.19, and 1.22 ± 0.24, respectively (p < 0.001). The post-hoc analysis showed that the group with severe PTSD symptoms had a higher global Aβ burden than the other groups, and the control group had a lower global Aβ burden than the other groups. In addition, compared to the control group, individuals with mild (OR = 1.543, 95% CI = 1.322–1.800), moderate (OR = 2.315, 95% CI = 1.700–3.153), and severe PTSD symptoms (OR = 3.709, 95% CI = 1.703–8.077) exhibited higher ORs for amyloid positivity (Supplementary Table S4).
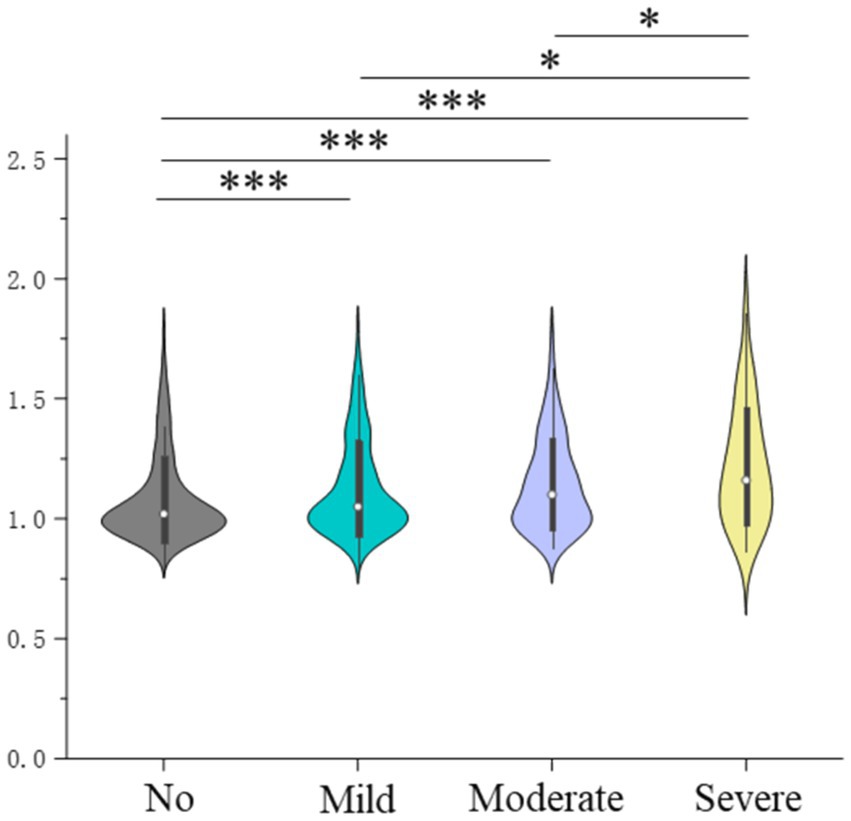
Figure 4. Severity of posttraumatic stress disorder symptoms and global brain amyloid burden; *indicates 0.001 ≤ p < 0.05, and ***indicates p < 0.001.
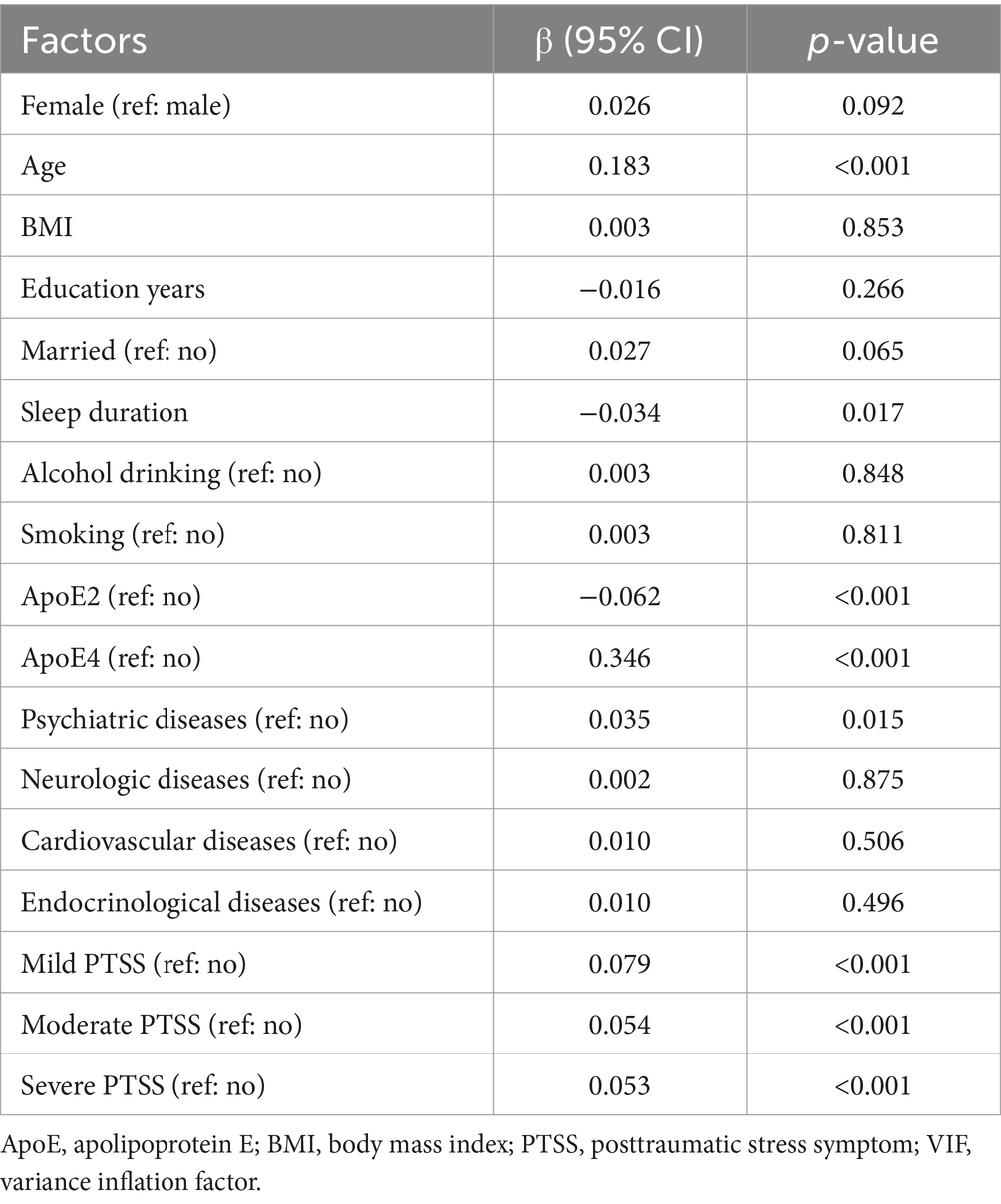
Table 3. Multilinear regression analysis of the association of severity of posttraumatic stress symptoms with whole-brain amyloid burden.
To further validate the association between the severity of PTSD symptoms and Aβ burden, we performed multivariable linear regression of associations between factors and Aβ burden in the whole brain and subregions, with mild, moderate, and severe symptoms as dumb variables. The results are summarized in Supplementary Table S4. The findings indicate that the global and regional Aβ burden levels are ranked in the order of severe, moderate, mild, and no PTSD symptoms, with statistical significance.
Discussion
The current findings imply that PTSD symptoms are associated with a higher global brain Aβ burden, irrespective of sex, age, ApoE genotype, or comorbid psychiatric diseases. Similarly, PTSD symptoms were significantly associated with Aβ levels in all subregions, including the anterior cingulate, posterior cingulate, parietal cortex, precuneus, temporal cortex, and frontal cortex. In addition, the groups with severe PTSD symptoms exhibited higher global Aβ levels than the groups with moderate, mild, or no PTSD symptoms. The findings indicate a close relationship between PTSD and brain Aβ levels, which may provide a possible explanation for the correlation of PTSD symptoms with dementia or cognitive decline. More well-designed studies are needed to further explore the relationship of PTSD with Aβ burden and the underlying mechanisms.
The present findings also provide a neuroimaging-based explanation for the negative association between PSTD symptoms and lower ORs for dementia. As indicated in extant studies (Greenberg et al., 2014; Flatt et al., 2018), PTSD is associated with an increased risk of dementia, which may be explained by increased Aβ levels. However, the current findings may not be consistent with previous studies, which report a null association between PTSD symptoms and Aβ burden (Weiner et al., 2022; Weiner et al., 2017). These inconsistent results may be partially explained by the method used for the assessment of PTSD and the demographic information of included subjects (such as age, sex, and BMI) (Bhattarai et al., 2019; Girgenti et al., 2017). More well-designed studies are needed to explore the relationship and mechanism of PTSD symptoms and amyloid in the future.
Numerous mechanisms may help explain the relationship between PTSD symptoms and Aβ burden, for example: (1) PTSD symptoms increase peripheral and central inflammation and elevate Aβ levels. It has been suggested that inflammatory biomarkers, such as interleukin-6 (IL-6), C-reactive protein (CRP), and tumor necrosis factor (TNF), are significantly elevated in those with PTSD (Hori and Kim, 2019; Mohlenhoff et al., 2017). Chronic inflammation can disrupt the normal functioning of the immune system and exacerbate Aβ deposition. (2) Repeated exposure to stress-induced hormone dysregulation may cause the misprocessing of amyloid precursor peptides. Xie et al. (2013) showed that stress and glucocorticoids promote the misprocessing of amyloid precursor peptides in the rat hippocampus, a brain region within the temporal cortex, as well as in the prefrontal cortex, resulting in the generation of Aβ. (3) Genetic variants and epigenetic alterations related to Aβ metabolism occur in individuals with PTSD. Research has shown that exposure to traumatic stress influences the expression of epigenetic genes such as FKBP5, BDNF, and REST, which may directly and indirectly influence Aβ levels (Rajkumar, 2023; Guo et al., 2024). (4) Mediating factors, such as disrupted sleep and comorbid mental health issues, may also accelerate the aggregation of Aβ. One of the main manifestations of PTSD is nightmares, which would disrupt sleep. Disturbed sleep and increased wakefulness are strongly associated with increased Aβ production and decreased Aβ clearance (Zheng et al., 2024; Xie et al., 2013). It is worthwhile to elucidate these factors and the association between PTSD and Aβ levels as clearly as possible.
In this study, we also found, for the first time, that PTSD symptoms were associated with elevated regional brain Aβ levels in cognitively normal older adults. This association was observed in several brain regions, including the anterior cingulate, posterior cingulate, parietal cortex, precuneus, temporal cortex, and frontal cortex. These findings may indicate that repeated exposure may impact the whole brain rather than specific regions. As mentioned above, PTSD symptoms may cause extensive changes to the whole brain, including increased peripheral and central inflammation, induced hormone dysregulation, as well as genetic and epigenetic alterations related to Aβ metabolism (Hori and Kim, 2019; Mohlenhoff et al., 2017; Guo et al., 2024). However, the exact mechanism by which PTSD symptoms cause whole-brain Aβ increases in cognitively normal older adults still needs to be investigated and validated.
In our study, a higher level of brain Aβ burden was associated with PTSD symptom severity, which suggests the existence of a dose–response relationship. The established literature on the association of the severity of PTSD symptoms with Aβ burden is limited, and this is the first study to show that individuals with severe PTSD symptoms have a higher global Aβ burden than those with moderate, mild, or no symptoms. The findings have significant clinical meaning, suggesting the importance of early diagnosis and treatment for individuals with PTSD symptoms (Al Jowf et al., 2023). Further results from well-designed and well-conducted experiments are needed to derive robust evidence regarding the association between the severity of PTSD symptoms and Aβ levels.
The strength of this study is its large population-based sample size; however, several limitations exist. First, the assessment of PTSD symptoms was based on self-reported scales, which cannot replace diagnoses by psychiatrists. Second, this study was limited to participants aged >65 years without cognitive impairment, thereby limiting the analyses to the outcome of the emerging pathology in the absence of significant cognitive dysfunction. Third, the association between specific PTSD symptoms, such as flashbacks and nightmares, and Aβ levels was not detected. Fourth, covariates, such as the classification of multiple chronic diseases, were not clear. Chronic diseases, such as cardiovascular (Stakos et al., 2020) and neurological diseases (Vaquer-Alicea and Diamond, 2019), are associated with Aβ burden. In this study, whether the individual was diagnosed with chronic disease, the number of chronic diseases, diagnosis time, and related treatment are unknown, which may bias the results. Fifth, this is a cross-sectional study. Therefore, the associations between PTSD and amyloid burden should not necessarily be considered causal relationships.
Conclusion
The present results indicate that PTSD symptoms are associated with higher global and regional brain Aβ burden in cognitively normal older adults. In addition, increased Aβ levels were associated with the severity of PTSD symptoms. Further studies are required to explore and validate the effects of PTSD on brain amyloid burden.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: The data used in this study were extracted from the Anti-Amyloid Treatment in Asymptomatic Alzheimer’s (A4)/Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) Study.
Ethics statement
The participants involved in the A4 study provided informed consent before the study. For the use of A4 data, the study was received approval from the Institutional Review Board of the Peking University Sixth Hospital.
Author contributions
LZ: Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. Y-MG: Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. S-WW: Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. P-LS: Writing – review & editing. M-ZL: Formal analysis, Writing – original draft, Writing – review & editing. XW: Visualization, Writing – original draft, Writing – review & editing. D-XW: Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. Y-BZ: Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. YH: Funding acquisition, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This study was supported by grants from Henan Province Science and Technology Projects [232102311031], the Open Project of Henan Key Lab of Biological Psychiatry [ZDSYS2021005], the Joint Co-construction Project of Henan Medical Science and Technology Research Plan [LHGJ20220637], Henan Province Science and Technology Research and Development Plan Joint Fund (industry) major project [235101610004], and the Xinjiang 13th Division Xinxing City Science and Technology Plan Project [2024B14].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2024.1422862/full#supplementary-material
References
Al Jowf, G. I., Ahmed, Z. T., Reijnders, R. A., de Nijs, L., and Eijssen, L. M. T. (2023). To predict, prevent, and manage post-traumatic stress disorder (PTSD): a review of pathophysiology, treatment, and biomarkers. Int. J. Mol. Sci. 24:5238. doi: 10.3390/ijms24065238
Bhattarai, J. J., Oehlert, M. E., Multon, K. D., and Sumerall, S. W. (2019). Dementia and cognitive impairment among U.S. veterans with a history of MDD or PTSD: a retrospective cohort study based on sex and race. J. Aging Health 31, 1398–1422. doi: 10.1177/0898264318781131
Brewin, C. R., Cloitre, M., Hyland, P., Shevlin, M., Maercker, A., Bryant, R. A., et al. (2017). A review of current evidence regarding the ICD-11 proposals for diagnosing PTSD and complex PTSD. Clin. Psychol. Rev. 58, 1–15. doi: 10.1016/j.cpr.2017.09.001
Caprioglio, C., Ribaldi, F., Visser, L. N. C., Minguillon, C., Collij, L. E., Grau-Rivera, O., et al. (2023). Analysis of psychological symptoms following disclosure of amyloid-positron emission tomography imaging results to adults with subjective cognitive decline. JAMA Netw. Open 6:e2250921. doi: 10.1001/jamanetworkopen.2022.50921
Clark, C. M., Pontecorvo, M. J., Beach, T. G., Bedell, B. J., Coleman, R. E., Doraiswamy, P. M., et al. (2012). Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 11, 669–678. doi: 10.1016/S1474-4422(12)70142-4
Elias, A., Cummins, T., Lamb, F., Tyrrell, R., Dore, V., Williams, R., et al. (2020). Amyloid-β, tau, and 18F-Fluorodeoxyglucose positron emission tomography in posttraumatic stress disorder. J. Alzheimer's Dis. 73, 163–173. doi: 10.3233/JAD-190913
Flatt, J. D., Gilsanz, P., Quesenberry, C. P. Jr., Albers, K. B., and Whitmer, R. A. (2018). Post-traumatic stress disorder and risk of dementia among members of a health care delivery system. Alzheimers Dement. 14, 28–34. doi: 10.1016/j.jalz.2017.04.014
Girgenti, M. J., Hare, B. D., Ghosal, S., and Duman, R. S. (2017). Molecular and cellular effects of traumatic stress: implications for PTSD. Curr. Psychiatry Rep. 19:85. doi: 10.1007/s11920-017-0841-3
Greenberg, M. S., Tanev, K., Marin, M. F., and Pitman, R. K. (2014). Stress, PTSD, and dementia. Alzheimers Dement. 10, S155–S165. doi: 10.1016/j.jalz.2014.04.008
Guo, J., Orgeta, V., Olivé, I., Hoff, E., Huntley, J., Olff, M., et al. (2024). Biomarkers associated with cognitive impairment in post-traumatic stress disorder: a systematic review of current evidence. Ageing Res. Rev. 95:102198. doi: 10.1016/j.arr.2024.102198
Hori, H., and Kim, Y. (2019). Inflammation and post-traumatic stress disorder. Psychiatry Clin. Neurosci. 73, 143–153. doi: 10.1111/pcn.12820
Horowitz, M., Wilner, N., and Alvarez, W. (1979). Impact of event scale: a measure of subjective stress. Psychosom. Med. 41, 209–218. doi: 10.1097/00006842-197905000-00004
Insel, P. S., Mohlenhoff, B. S., Neylan, T. C., Krystal, A. D., and Mackin, R. S. (2021). Association of sleep and β-amyloid pathology among older cognitively unimpaired adults. JAMA Netw. Open 4:e2117573. doi: 10.1001/jamanetworkopen.2021.17573
Joseph, S. (2000). Psychometric evaluation of Horowitz's impact of event scale: a review. J. Trauma. Stress. 13, 101–113. doi: 10.1023/A:1007777032063
Joshi, A. D., Pontecorvo, M. J., Clark, C. M., Carpenter, A. P., Jennings, D. L., Sadowsky, C. H., et al. (2012). Performance characteristics of amyloid PET with florbetapir F 18 in patients with alzheimer's disease and cognitively normal subjects. J. Nucl. Med. 53, 378–384. doi: 10.2967/jnumed.111.090340
Joshi, A. D., Pontecorvo, M. J., Lu, M., Skovronsky, D. M., Mintun, M. A., and Devous, M. D. Sr. (2015). A Semiautomated method for quantification of F 18 Florbetapir PET images. J. Nucl. Med. 56, 1736–1741. doi: 10.2967/jnumed.114.153494
Karam, E. G., Friedman, M. J., Hill, E. D., Kessler, R. C., McLaughlin, K. A., Petukhova, M., et al. (2014). Cumulative traumas and risk thresholds: 12-month PTSD in the world mental health (WMH) surveys. Depress. Anxiety 31, 130–142. doi: 10.1002/da.22169
Liu, C. C., Liu, C. C., Kanekiyo, T., Xu, H., and Bu, G. (2013). Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat. Rev. Neurol. 9, 106–118. doi: 10.1038/nrneurol.2012.263
Livingston, G., Huntley, J., Liu, K. Y., Costafreda, S. G., Selbæk, G., Alladi, S., et al. (2024). Dementia prevention, intervention, and care: 2024 report of the lancet standing commission. Lancet (London, England) 404, 572–628. doi: 10.1016/S0140-6736(24)01296-0
Marcolini, S., Rojczyk, P., Seitz-Holland, J., Koerte, I. K., Alosco, M. L., and Bouix, S. (2023). Posttraumatic stress and traumatic brain injury: cognition, behavior, and neuroimaging markers in Vietnam veterans. J. Alzheimer's Dis. 95, 1427–1448. doi: 10.3233/JAD-221304
Mawanda, F., Wallace, R. B., McCoy, K., and Abrams, T. E. (2017). PTSD, psychotropic medication use, and the risk of dementia among US veterans: a retrospective cohort study. J. Am. Geriatr. Soc. 65, 1043–1050. doi: 10.1111/jgs.14756
Mohlenhoff, B. S., O'Donovan, A., Weiner, M. W., and Neylan, T. C. (2017). Dementia risk in posttraumatic stress disorder: the relevance of sleep-related abnormalities in brain structure, amyloid, and inflammation. Curr. Psychiatry Rep. 19:89. doi: 10.1007/s11920-017-0835-1
Qureshi, S. U., Kimbrell, T., Pyne, J. M., Magruder, K. M., Hudson, T. J., Petersen, N. J., et al. (2010). Greater prevalence and incidence of dementia in older veterans with posttraumatic stress disorder. J. Am. Geriatr. Soc. 58, 1627–1633. doi: 10.1111/j.1532-5415.2010.02977.x
Rafferty, L. A., Cawkill, P. E., Stevelink, S. A. M., Greenberg, K., and Greenberg, N. (2018). Dementia, post-traumatic stress disorder and major depressive disorder: a review of the mental health risk factors for dementia in the military veteran population. Psychol. Med. 48, 1400–1409. doi: 10.1017/S0033291717001386
Rajkumar, R. P. (2023). Biomarkers of neurodegeneration in post-traumatic stress disorder: an integrative review. Biomedicines 11:1465. doi: 10.3390/biomedicines11051465
Raza, Z., Hussain, S. F., Ftouni, S., Spitz, G., Caplin, N., Foster, R. G., et al. (2021). Dementia in military and veteran populations: a review of risk factors-traumatic brain injury, post-traumatic stress disorder, deployment, and sleep. Mil. Med. Res. 8:55. doi: 10.1186/s40779-021-00346-z
Shalev, A., Liberzon, I., and Marmar, C. (2017). Post-traumatic stress disorder. N. Engl. J. Med. 376, 2459–2469. doi: 10.1056/NEJMra1612499
Shen, L., and Jia, J. (2016). An overview of genome-wide association studies in Alzheimer's disease. Neurosci. Bull. 32, 183–190. doi: 10.1007/s12264-016-0011-3
Sperling, R. A., Rentz, D. M., Johnson, K. A., Karlawish, J., Donohue, M., Salmon, D. P., et al. (2014). The A4 study: stopping AD before symptoms begin? Sci. Transl. Med. 6:228fs13. doi: 10.1126/scitranslmed.3007941
Stakos, D. A., Stamatelopoulos, K., Bampatsias, D., Sachse, M., Zormpas, E., Vlachogiannis, N. I., et al. (2020). The Alzheimer's disease amyloid-Beta hypothesis in cardiovascular aging and disease: JACC focus seminar. J. Am. Coll. Cardiol. 75, 952–967. doi: 10.1016/j.jacc.2019.12.033
Sterling, M. (2008). The impact of event scale (IES). Aust. J. Physiother. 54:78. doi: 10.1016/S0004-9514(08)70074-6
Vaquer-Alicea, J., and Diamond, M. I. (2019). Propagation of protein aggregation in neurodegenerative diseases. Annu. Rev. Biochem. 88, 785–810. doi: 10.1146/annurev-biochem-061516-045049
Weiner, M. W., Harvey, D., Hayes, J., Landau, S. M., Aisen, P. S., Petersen, R. C., et al. (2017). Effects of traumatic brain injury and posttraumatic stress disorder on development of Alzheimer's disease in Vietnam veterans using the Alzheimer's disease neuroimaging initiative: preliminary report. Alzheimers Dement. 3, 177–188. doi: 10.1016/j.trci.2017.02.005
Weiner, M. W., Harvey, D., Landau, S. M., Veitch, D. P., Neylan, T. C., Grafman, J. H., et al. (2022). Traumatic brain injury and post‐traumatic stress disorder are not associated with Alzheimer's disease pathology measured with biomarkers. Alzheimers Dement. 19, 884–895. doi: 10.1002/alz.12712
Xie, L., Kang, H., Xu, Q., Chen, M. J., Liao, Y., Thiyagarajan, M., et al. (2013). Sleep drives metabolite clearance from the adult brain. Science 342, 373–377. doi: 10.1126/science.1241224
Yaffe, K., Vittinghoff, E., Lindquist, K., Barnes, D., Covinsky, K. E., Neylan, T., et al. (2010). Posttraumatic stress disorder and risk of dementia among US veterans. Arch. Gen. Psychiatry 67, 608–613. doi: 10.1001/archgenpsychiatry.2010.61
Yuan, K., Gong, Y. M., Liu, L., Sun, Y. K., Tian, S. S., Wang, Y. J., et al. (2021). Prevalence of posttraumatic stress disorder after infectious disease pandemics in the twenty-first century, including COVID-19: a meta-analysis and systematic review. Mol. Psychiatry 26, 4982–4998. doi: 10.1038/s41380-021-01036-x
Zheng, Y. B., Huang, Y. T., Gong, Y. M., Li, M. Z., Zeng, N., Wu, S. L., et al. (2024). Association of lifestyle with sleep health in general population in China: a cross-sectional study. Transl. Psychiatry 14. doi: 10.1038/s41398-024-03002-x
Zheng, Y. B., Shi, L., Gong, Y. M., Wang, X. X., Lu, Q. D., Que, J. Y., et al. (2020). Public awareness and knowledge of factors associated with dementia in China. BMC Public Health 20:1567. doi: 10.1186/s12889-020-09665-7
Zheng, Y. B., Shi, L., Que, J. Y., Deng, J. H., Wang, Q. W., Su, S. Z., et al. (2022). Linking knowledge with attitude: a cross-sectional study of public knowledge and attitude towards sleep disturbances and dementia. BMJ Open 12:e067055. doi: 10.1136/bmjopen-2022-067055
Zheng, Y. B., Shi, L., Zhu, X. M., Bao, Y. P., Bai, L. J., Li, J. Q., et al. (2021). Anticholinergic drugs and the risk of dementia: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 127, 296–306. doi: 10.1016/j.neubiorev.2021.04.031
Keywords: amyloid, posttraumatic stress disorder symptom, older adult, cross-sectional study, dementia
Citation: Zhang L, Gong Y-M, Wang S-W, Shi P-L, Li M-Z, Wen X, Wang D-X, Zheng Y-B and Han Y (2024) Associations of posttraumatic stress disorder symptoms with amyloid burden in cognitively normal older adults. Front. Aging Neurosci. 16:1422862. doi: 10.3389/fnagi.2024.1422862
Edited by:
Sindre Rolstad, University of Gothenburg, SwedenReviewed by:
Riccardo Manca, Brunel University London, United KingdomLisa C. Bratzke, University of Wisconsin-Madison, United States
Copyright © 2024 Zhang, Gong, Wang, Shi, Li, Wen, Wang, Zheng and Han. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Han, aHlfdmlwQDEyNi5jb20=; Yong-Bo Zheng, eW9uZ2JvemhlbmdAYmptdS5lZHUuY24=; Di-Xin Wang, d2FuZ2R4MjBAbHp1LmVkdS5jbg==
†These authors have contributed equally to this work
 Lei Zhang1†
Lei Zhang1† Di-Xin Wang
Di-Xin Wang Yong-Bo Zheng
Yong-Bo Zheng Yong Han
Yong Han