- Internal Medicine, Qujing Third People’s Hospital, Qujing, Yunnan, China
Background: This meta-analysis was conducted to evaluate potential differences in symptoms between PD patients with or without RBD.
Methods: A systematic search was conducted in PubMed, Cochrane, Embase, and Web of Science databases (as of August 16, 2023), to identify relevant studies on PD and RBD. Statistical analysis was performed using Stata 15.0. Continuous variables were analyzed using the standardized mean difference (SMD) and 95% confidence interval (95% CI), while count data were assessed using the odds ratio (OR) and 95% CI as statistical effect sizes. Heterogeneity among all included studies was tested; for studies with low heterogeneity (I2 < 50%), a fixed-effects model was used to calculate statistical results. For studies with relatively high heterogeneity (I2 > 50%), a random-effects model was applied, followed by sensitivity and subgroup analyses to identify sources of heterogeneity.
Results: A total of 5,672 subjects were involved in this study. Compared to the NRBD group, the UPDRS-III score in the RBD group was significantly higher (SMD = 0.20, 95% CI: [0.11, 0.29], P < 0.001), and the Hoehn-Yahr score in the RBD group was also significantly higher (SMD = 0.29, 95% CI: [0.03, 0.55], P < 0.001). Patients with PD in the RBD group had more severe cognitive impairments than those in the NRBD group (SMD = −0.30, 95% CI: [−0.48, −0.11], P < 0.001). The incidence of hallucination in PD patients in the RBD group was 3.0 times that of the NRBD group (OR = 3.0, 95% CI: [2.15, 4.20], P = 0.110). PD patients in the RBD group also experienced more severe anxiety symptoms (SMD = 0.13, 95% CI: [−0.26, 0.51], P < 0.001), had higher scores in depression scales (SMD = 0.22, 95% CI: [0.02, 0.43], P < 0.001), and higher scores in sleep disorder scales than those in NRBD group (SMD = 0.10, 95% CI: [−0.11, 0.31], P < 0.001).
Conclusion: Results show PD patients with co-occurring RBD have more severe motor and non-motor symptoms likely due to overlapping affected regions in RBD and PD-related pathology, plus broader neurodegeneration seen in PD patients with RBD.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/#searchadvanced, identifier CRD42023476331.
1 Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder that predominantly affects the elderly, characterized by both motor and non-motor symptoms (Cabreira and Massano, 2019). The motor symptoms include resting tremors, rigidity, and bradykinesia, while the non-motor symptoms mainly manifest as cognitive impairments, sleep disorders, and anxiety and depression disorders (Hayes, 2019). The global prevalence of PD is reported to be 1/1000, and the incidence increases with age (Tysnes and Storstein, 2017). The pathogenesis of PD primarily involves the degeneration of the nigrostriatal pathway, leading to a reduction in the dopamine level and a relative excess of cholinergic activity (Raza et al., 2019).
Sleep disorder is among the most common non-motor symptoms of PD, with rapid eye movement sleep behavior disorder (RBD) being a prevalent abnormal sleep behavior. According to the 3rd International Classification of Sleep Disorders (ICSD-3), diagnosis of RBD is based on the presence of repeated episodes of vocalization or complex motor behaviors during REM sleep in polysomnography (PSG) and on polysomnographic recording of REM sleep without atonia (RSWA). RBD is commonly observed in neurodegenerative diseases such as multiple system atrophy (MSA), Lewy body dementia (LBD), and PD (Pilotto et al., 2019). Previous studies have identified that RBD may appear as early as the early stages of PD, which often presents approximately 10 years before the motor symptoms of PD patients and could be a precursor symptom of PD. According to relevant statistics, the prevalence of RBD in PD patients ranges from 25 to 60% (Arnulf, 2012). Recent evidence suggests that the presence of RBD is associated with differences in the clinical symptom spectrum, natural history, and prognosis of PD. These differences are likely indicative of underlying differences in the pathophysiology among PD patients with and without RBD.
Currently, existing studies have analyzed and compared the clinical characteristics of PD patients with and without RBD. The results have shown that PD patients with RBD may have an increased risk of non-motor symptoms such as anxiety, depression, sleep disorder, hallucination, and cognitive impairments. Furthermore, patients with RBD often exhibit more severe motor symptoms, including abnormalities in posture and gait, language expression difficulty, and increased resting tremor duration. However, these articles are somewhat outdated. In recent years, new studies on PD patients and RBD have emerged, yielding inconsistent results that have sparked our interest. Previous meta-analyses on PD and RBD were constrained by the number of original articles and varying research focuses, lacking comprehensive and meticulous analysis of RBD’s impact on both motor and non-motor symptoms in PD patients, including sleep conditions and anxiety levels. Moreover, these studies lacked thorough subgroup analyses, potentially introducing bias into their findings. Therefore, we conducted a systematic search and collected literature to integrate all relevant studies using meta-analysis. Through the expansion of sample size, we systematically analyzed the progression of PD with and without RBD and their clinical outcomes, so as to provide effective evidence-based medical insights to clarify the precise relationship between RBD and PD.
2 Method
This study strictly followed the PRISMA guidelines (Page et al., 2021). Besides, Its protocol was registered in the PROSPERO (registration ID: CRD42023476331).
2.1 Search strategy
Databases including PubMed, the Cochrane Library, Embase, and Web of Science were searched and records were limited to the English literature published before August 2023. The search strategy was designed by two researchers (Wentao Zheng and Yungui Yang) independently using the combination of Medical Subject Heading (MeSH) terms and their free words (i.e., “REM sleep behavior disorder” OR “rapid eye movement behavi*” OR “rapid eye movement sleep behavi*” OR “rapid eye movement sleep behavior disorder” OR“REM behavi*” OR “REM sleep behavi*” and “Parkinson disease” OR “Parkinson’s disease” OR “Primary Parkinsonism”). The full search history is presented in Supplementary Table 1. Due to the small number of the recently updated literature, we did not update the data analysis further. Any disagreement arising during the study shall be decided by arbitration by a third researcher (Yang Pan).
2.2 Inclusion and exclusion criteria
The included studies must meet the following criteria: (1) The study population should be patients with primary PD, who must be diagnosed according to the U.K. Parkinson’s Disease Society Brain Bank (PDSBB) criteria (Gelb et al., 1999) and other published criteria (Postuma and Berg, 2017; Cabreira and Massano, 2019); (2) Studies should focus on the clinical features of RBD-related PD, such as the study by Lee (Lee et al., 2010), which compared differences in the Hoehn and Yahr stage and Unified Parkinson’s Disease Rating Scale (UPDRS) scores between PD patients with and without RBD. The study also assessed common non-motor symptoms and cognitive functions in PD patients to explore the correlation between RBD and the clinical severity of PD; (3) The diagnosis of RBD must meet the diagnostic criteria of the International Classification of Sleep Disorders. Patients diagnosed with polysomnography (PSG) are considered to have confirmed RBD (cRBD), while those diagnosed based on interviews or questionnaires are considered to have confirmed probable RBD (pRBD) (St Louis and Boeve, 2017); (4) Studies must be published in English to be included.
Exclusion criteria for the study are: (1) Original research articles not covering the clinical features of RBD-related PD; (2) Meta-analyses/reviews, conference abstracts, guidelines, letters, responses, editorial materials, case reports, and animal experiments; (3) Duplicates, articles only featuring RBD characteristics, and literature on the pathogenesis of PD with RBD; (4) Articles with missing data or inaccessible data; (5) Studies with non-standard PD and RBD diagnostic criteria or poor research quality; (6) Studies reported in languages other than English.
2.3 Study selection
Based on the previously established eligible criteria, two researchers (Wentao Zheng and Yungui Yang) independently conducted the study selection. First, all potentially relevant articles retrieved from databases were imported into EndNote v9.0 for deduplication. Next, articles that did not match the research content were excluded based on their titles and abstracts. Finally, the full texts were further screened, with any disagreements resolved through discussion or consultation with a third researcher.
2.4 Data extraction
Two researchers (Wentao Zheng and Yungui Yang) independently collected relevant literature and extracted the required research information using a pre-designed datasheet. The extracted information included the first author, year of publication, country of publication, study type, diagnosis criteria, sample size, sex, age, and outcome indicators. Outcome indicators for motor symptoms included Hoehn and Yahr score and UPDRS-III score and for non-motor symptoms included mental health score (anxiety, depression), cognitive function score, sleep score, and the incidence of visual hallucinations. Any disputes during the extraction process were resolved by discussion with a third researcher.
2.5 Quality assessment
To assess the bias and quality of the included studies, the two researchers used the Newcastle-Ottawa Scale (NOS) (Wells et al., 2014) for quality evaluation, employing a semi-quantitative star rating system with a maximum score of 10 stars. Studies were categorized into very high risk of bias (0–3 stars), high risk of bias (4–6 stars), and low risk of bias (7–9 stars), with studies scoring ≥5 stars considered of high quality and eligible for inclusion in this meta-analysis. Any disagreements were resolved through discussion or negotiation with a third researcher.
2.6 Statistical analysis
All data were statistically analyzed by the professional meta-analysis software Stata 15.0. Continuous variables were analyzed using the standardized mean difference (SMD) and 95% confidence interval (95% CI), while count data were assessed using the odds ratio (OR) and 95% CI as statistical effect sizes. Heterogeneity among all included studies was tested; for studies with low heterogeneity (I2 < 50%), a fixed-effects model was used to calculate statistical results; for studies with significant heterogeneity (I2 > 50%), a random-effects model was applied, followed by sensitivity and subgroup analyses to identify sources of heterogeneity. To explore potential publication bias, if the number of included clinical study articles met the analysis criteria (≥8 studies included), funnel plot analysis would be conducted along with Begg’s test for statistical testing (Egger et al., 1997), to determine the presence of publication bias. If the funnel plot appeared symmetrical and the Begg’s test yielded a P value > 0.05, it would be considered that there was no significant publication bias.
3 Results
3.1 Retrieval results and study characteristics
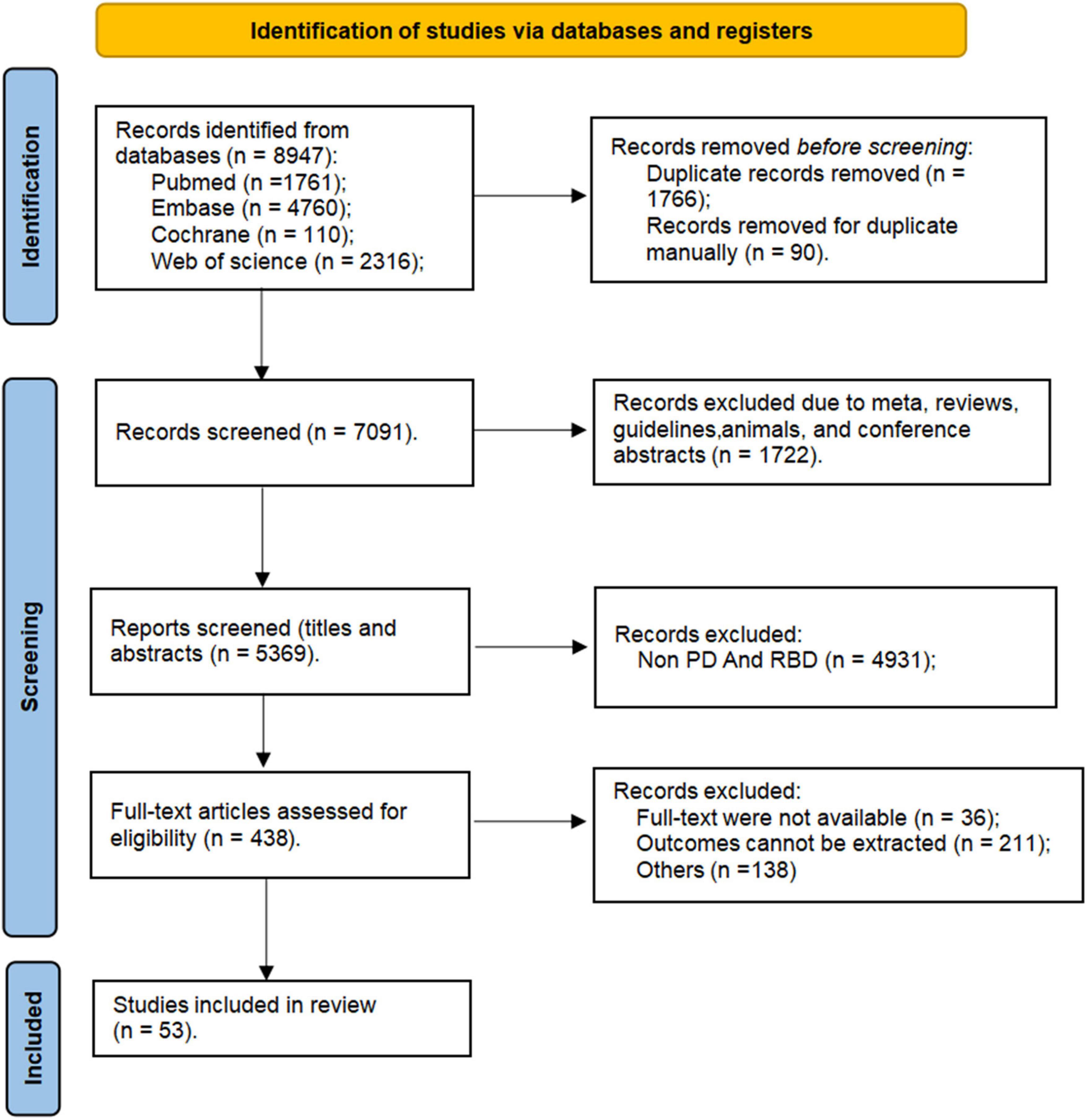
In this study, a search of relevant databases initially identified a total of 8,947 articles (Figure 1) from PubMed (n = 1761), Embase (n = 4760), Cochrane (n = 110), and Web of Science (n = 2316). Initially, 1,856 duplicates were removed automatically and manually using EndNote v9.0, followed by the exclusion of 1,722 articles of types such as meta-analyses, reviews, guidelines, animals, and conference abstracts. Through reviewing titles and abstracts, 4931 articles were excluded as they did not simultaneously include features related to both PD and RBD. Articles were further eliminated for reasons such as full-text were not available (n = 36), outcomes cannot be extracted (n = 211), and non-compliance with inclusion criteria (n = 138), leaving a total of 53 articles included in our study.
In the 53 studies included in this study, the RBD group comprised a total of 3,084 patients, with 1,265 patients diagnosed with cRBD (average age: 57.3–76.5 years; 777 males and 488 females) and 1,819 diagnosed with pRBD (average age: 60.38–73.1 years; 1,124 males and 695 females). The NRBD group included a total of 4,362 patients (average age: 57.1–74.5 years; 2,422 males and 1,940 females). The diagnosis of PD in the 53 studies was primarily based on the PDSBB criteria (Hughes et al., 1992). The diagnostic criteria for cRBD mainly followed the International Classification of Sleep Disorders and PSG (St Louis and Boeve, 2017), while pRBD diagnosis was chiefly based on the REM Sleep Behavior Disorder Screening Questionnaire (Stiasny-Kolster et al., 2007). Specific diagnostic criteria are listed in Table 1. Geographically, 14 studies originated from Europe (Sinforiani et al., 2006; Benninger et al., 2010; Lavault et al., 2010; Bugalho et al., 2011; Bugalho and Viana-Baptista, 2013; Ferri et al., 2014; Rolinski et al., 2014; Mariotti et al., 2015; Arnaldi et al., 2016; Leclair-Visonneau et al., 2017; Bargiotas et al., 2019; Figorilli et al., 2020; Assogna et al., 2021; Oltra et al., 2021), 23 from Asia (Ozekmekçi et al., 2005; Meral et al., 2007; Yoritaka et al., 2009; Lee et al., 2010; Vibha et al., 2011; Aygün et al., 2012; Nihei et al., 2012; Nomura et al., 2013, 2016, 2017; 2020; Suzuki et al., 2013; Gong et al., 2014; Kim et al., 2014; Hu et al., 2015; Kang et al., 2016; Kamble et al., 2019; Liu et al., 2019; Yan et al., 2019; Cao et al., 2020; Ashraf-Ganjouei et al., 2021; Zhu et al., 2021; Fujita et al., 2022), 15 from North America (Gagnon et al., 2004, 2009; Postuma et al., 2008, 2009, 2011; 2012; Gaudreault et al., 2013; Neikrug et al., 2014; Chahine et al., 2016; Pagano et al., 2018; Sobreira-Neto et al., 2018; Duarte Folle et al., 2019; Trout et al., 2019; Mahmood et al., 2020; Yoon and Monchi, 2021), and 1 from Oceania (Ford et al., 2013). Among the included studies, 49 reported outcome measures related to motor symptoms [e.g., UPDRS-III: Paulo Bugalho (Bugalho and Viana-Baptista, 2013); Hoehn and Yahr stage: Raffaele Ferri (Ferri et al., 2014)], while 39 reported outcome measures related to non-motor symptoms such as anxiety (Paolo Mariotti) (Mariotti et al., 2015), depression (L.M. Chahine) (Chahine et al., 2016), sleep disorder (Gennaro Pagano) (Pagano et al., 2018), hallucinations (L.M. Chahine) (Chahine et al., 2016), and Mini-Mental State Examination (MMSE) (Jun Zhu) (Zhu et al., 2021).
3.2 Quality assessment
Two researchers conducted a quality assessment of the included articles using the NOS (Wells et al., 2000), which evaluates three aspects: (1) the selection methods of cases and controls, (2) the comparability between case and control groups, (3) the methods of assessing exposure. The scores from these three evaluations are summed, with a higher total score indicating better quality, and the maximum possible score being 10. In this study, the NOS scores ranged from a high of 10 (Sinforiani et al., 2006; Postuma et al., 2009; Liu et al., 2019) to a low of 6 (Gagnon et al., 2004; Ozekmekçi et al., 2005; Lee et al., 2010; Bugalho et al., 2011; Vibha et al., 2011; Nomura et al., 2013; Suzuki et al., 2013; Kim et al., 2014; Neikrug et al., 2014; Chahine et al., 2016; Bargiotas et al., 2019; Duarte Folle et al., 2019; Yan et al., 2019; Zhu et al., 2021), indicating generally high quality of the included studies.
3.3 Differences in motor symptoms in PD patients with and without RBD
3.3.1 Analysis of UPDRS-III scores
UPDRS-III was used in a total of 41 articles (Sinforiani et al., 2006; Meral et al., 2007; Postuma et al., 2008, 2009, 2012; Gagnon et al., 2009; Benninger et al., 2010; Vibha et al., 2011; Aygün et al., 2012; Bugalho and Viana-Baptista, 2013; Neikrug et al., 2014; Mariotti et al., 2015; Leclair-Visonneau et al., 2017; Sobreira-Neto et al., 2018; Snyder et al., 2021; Zhu et al., 2021) involving 5,976 PD patients to assess the severity of motor symptoms (cRBD group: 2,392 patients; NRBD group: 3,584 patients). Due to high heterogeneity among the included studies (I2 = 53.8%), a random-effects model was used for analysis. The results showed that the UPDRS-III scores were significantly higher in the PD with RBD group compared to the PD without RBD group (SMD = 0.20, 95% CI: [0.11, 0.29], P < 0.001), (Figure 2) indicating more severe motor symptoms in PD patients with RBD. Subgroup analyses were conducted based on RBD diagnosis, geographic regions, and RBD types. According to the results, by RBD types, the heterogeneity I2 was 47.7% for the cRBD group and 53.8% for the pRBD group; by geographic regions, I2 was 35.7% for Europeans, 55.4% for Asians, and 56.1% for Americans; by RBD diagnosis, I2 was 0.0% for ICSD, while I2> 50% for other diagnostic tools like PSG and RBDSQ, indicating that RBD diagnosis, geographic regions, and RBD types might be the main sources of heterogeneity in UPDRS-III score analysis. Additionally, subgroup analysis results (Supplementary Table 2) showed that UPDRS-III scores were higher in PD patients with cRBD or pRBD compared to the control group of PD patients (PD with RBD, SMD = 0.24; PD with pRBD, SMD = 0.20). The funnel plot (Supplementary Figure 1) and the Begg’s test (P = 0.181) indicated no significant publication bias.
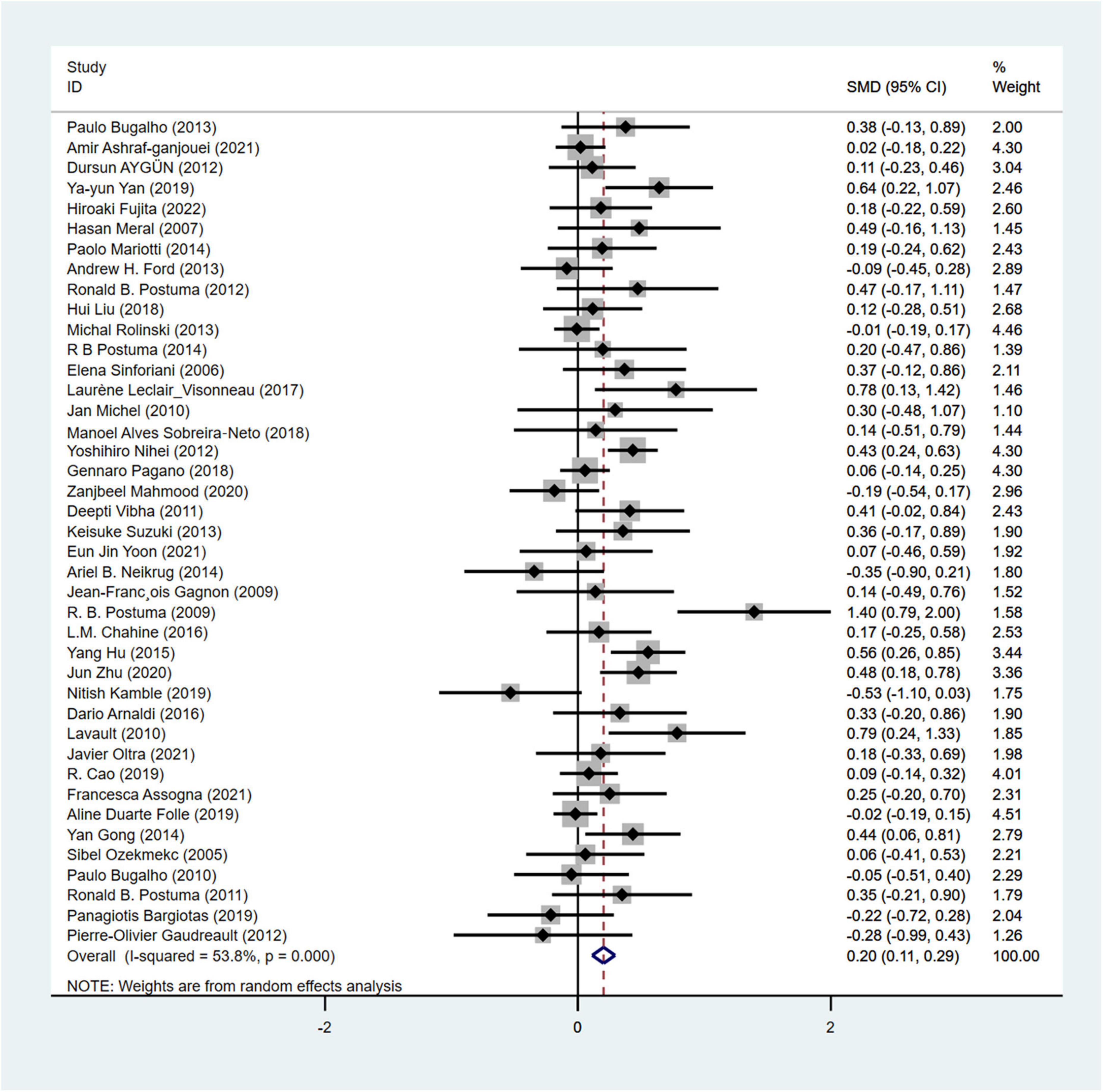
Figure 2. Forest plot based on difference in the UPDRS-III score among Parkinson’s disease patients with or without rapid eye movement sleep behavior disorder (RBD). SMD, Standardized Mean Difference; CI, confidence intervals.
3.3.2 Analysis of Hoehn and Yahr stage
Hoehn and Yahr stage was used in a total of 23 articles involving 3,557 PD patients (RBD group: 1,630 patients, NRBD group: 1,927 patients) to assess the severity of PD. The heterogeneity test showed relatively high variability among the studies (I2 = 89.4%), thus a random-effects model was employed for the analysis. The results indicated that the Hoehn-Yahr score was higher in the PD with RBD group compared to the PD without RBD group (SMD = 0.29, 95% CI: [0.03, 0.55], P < 0.001) (Figure 3). Sensitivity analysis demonstrated the stability of the studies included in this meta-analysis with no identifiable source of heterogeneity. Subgroup analysis by geographic regions (Supplementary Table 2) showed that the geographic origin of the population might be a source of heterogeneity in the analysis of the Hoehn and Yahr stage (Americans: I2 = 0.0%, Europeans: I2 = 56.7%, Asians: I2 = 94.3%). The funnel plot (Supplementary Figure 1) and Begg’s test (P = 0.612) indicated no publication bias.
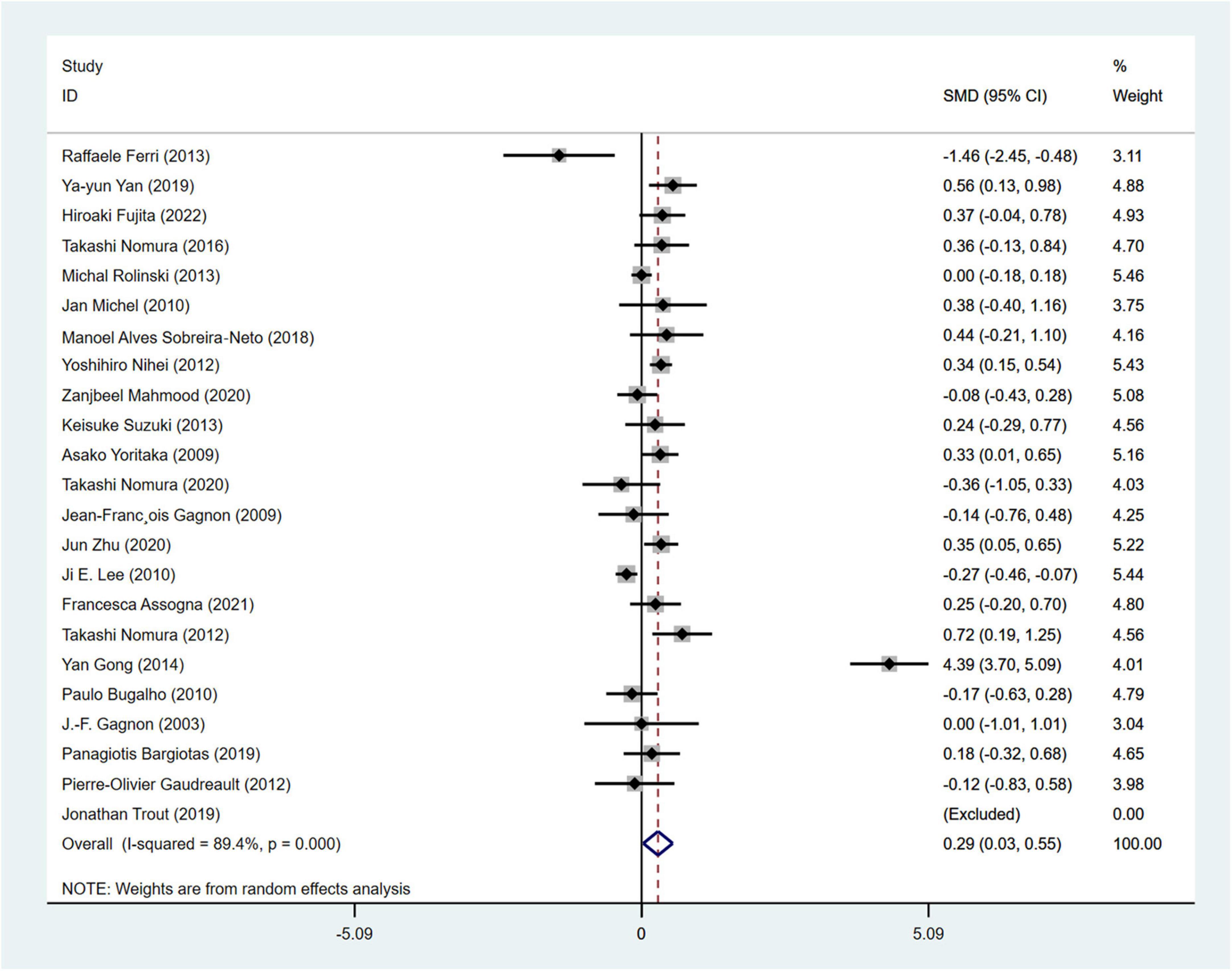
Figure 3. Forest plot based on the difference in Hoehn and Yahr stage among Parkinson’s disease patients with or without rapid eye movement sleep behavior disorder (RBD). SMD, Standardized Mean Difference; CI, confidence intervals.
3.4 Differences in non-motor symptoms in PD patients with and without RBD
3.4.1 Analysis of cognitive function performance
MMSE (Snyder et al., 2021) was used in a total of 28 studies (Sinforiani et al., 2006; Lee et al., 2010; Vibha et al., 2011; Ferri et al., 2014; Gong et al., 2014; Kim et al., 2014; Bargiotas et al., 2019; Kamble et al., 2019; Nomura et al., 2020; Assogna et al., 2021; Zhu et al., 2021) involving 2,946 PD patients (RBD group: 1,293 patients, NRBD group: 1,653 patients) to evaluate cognitive impairments. The heterogeneity among the studies was high (I2 = 84.4%), and a random-effects model was employed for the analysis. PD patients in the RBD group exhibited more severe cognitive impairments than those in the NRBD group (SMD = −0.30, 95% CI: [−0.48, −0.11], P < 0.001) (Figure 4). Subgroup analysis (Supplementary Table 3) suggested that heterogeneity might stem from geographic regions, with I2 being 47.7% for Europeans, 87.1% for Asians, and 89.0% for North Americans. The funnel plot (Supplementary Figure 2) and Begg’s test (P = 0.228) indicated no publication bias, and sensitivity analysis suggested good stability across the studies.
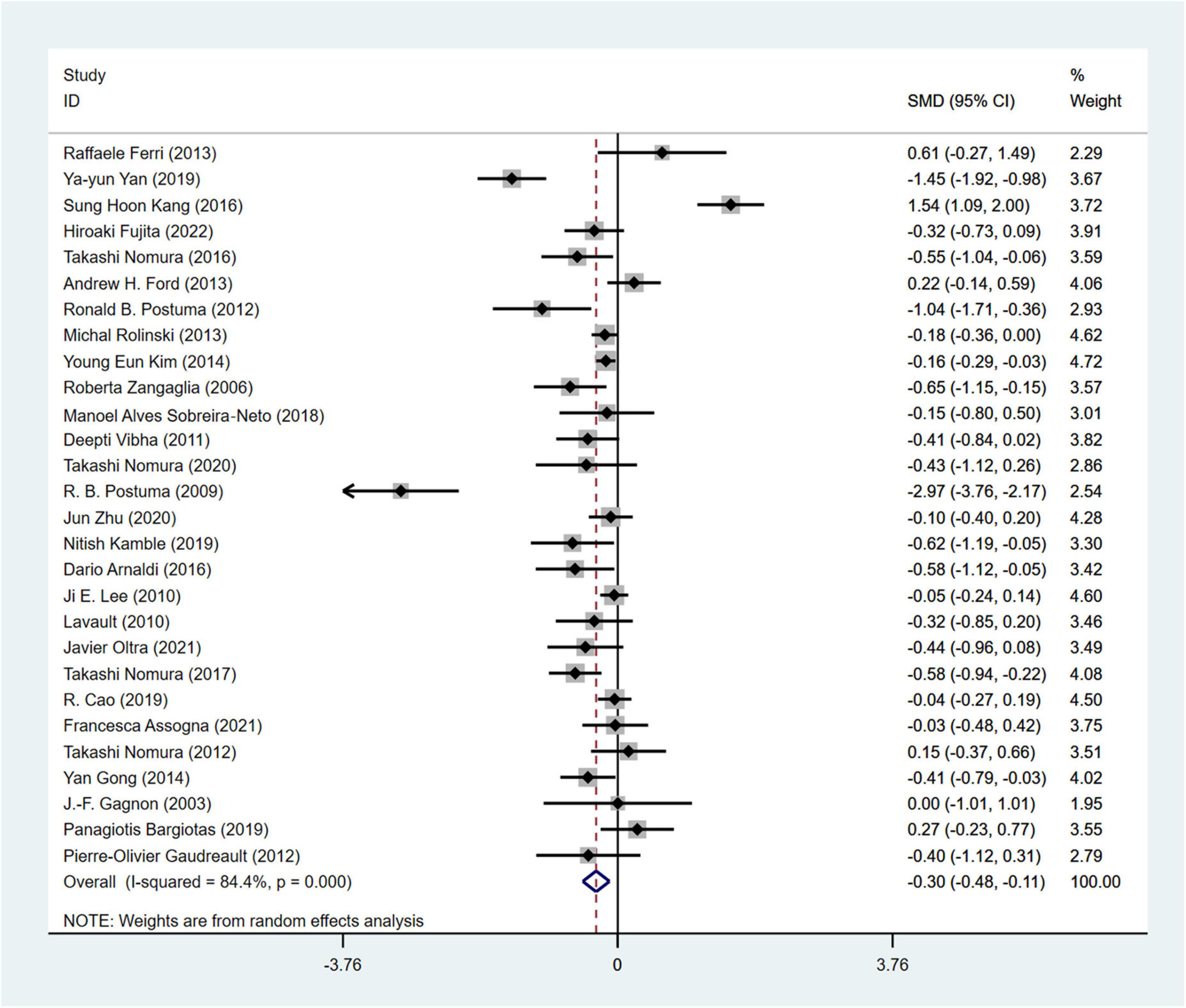
Figure 4. Forest plot based on the difference in MMSE score among Parkinson’s disease patients with or without rapid eye movement sleep behavior disorder (RBD). SMD, Standardized Mean Difference; CI, confidence intervals.
3.4.2 Analysis of hallucination incidence
A total of 10 studies (Meral et al., 2007; Yoritaka et al., 2009; Benninger et al., 2010; Lavault et al., 2010; Vibha et al., 2011; Postuma et al., 2012; Nomura et al., 2013, 2017; Gong et al., 2014; Chahine et al., 2016) involving 1,168 PD patients were included in the meta-analysis for the incidence of hallucinations (RBD group: 439 patients, NRBD group: 729 patients). The heterogeneity among the studies was relatively low (I2 = 37.4%), so a fixed-effects model was employed for integration. The results indicated that the incidence of hallucinations in PD patients in the RBD group was 3.0 times greater than that in the NRBD group (95% CI: [2.15, 4.20], P = 0.110) (Figure 5). The funnel plot (Supplementary Figure 2) yielded symmetrical patterns among the studies, and Begg’s test (P = 0.858) suggested no publication bias among them.
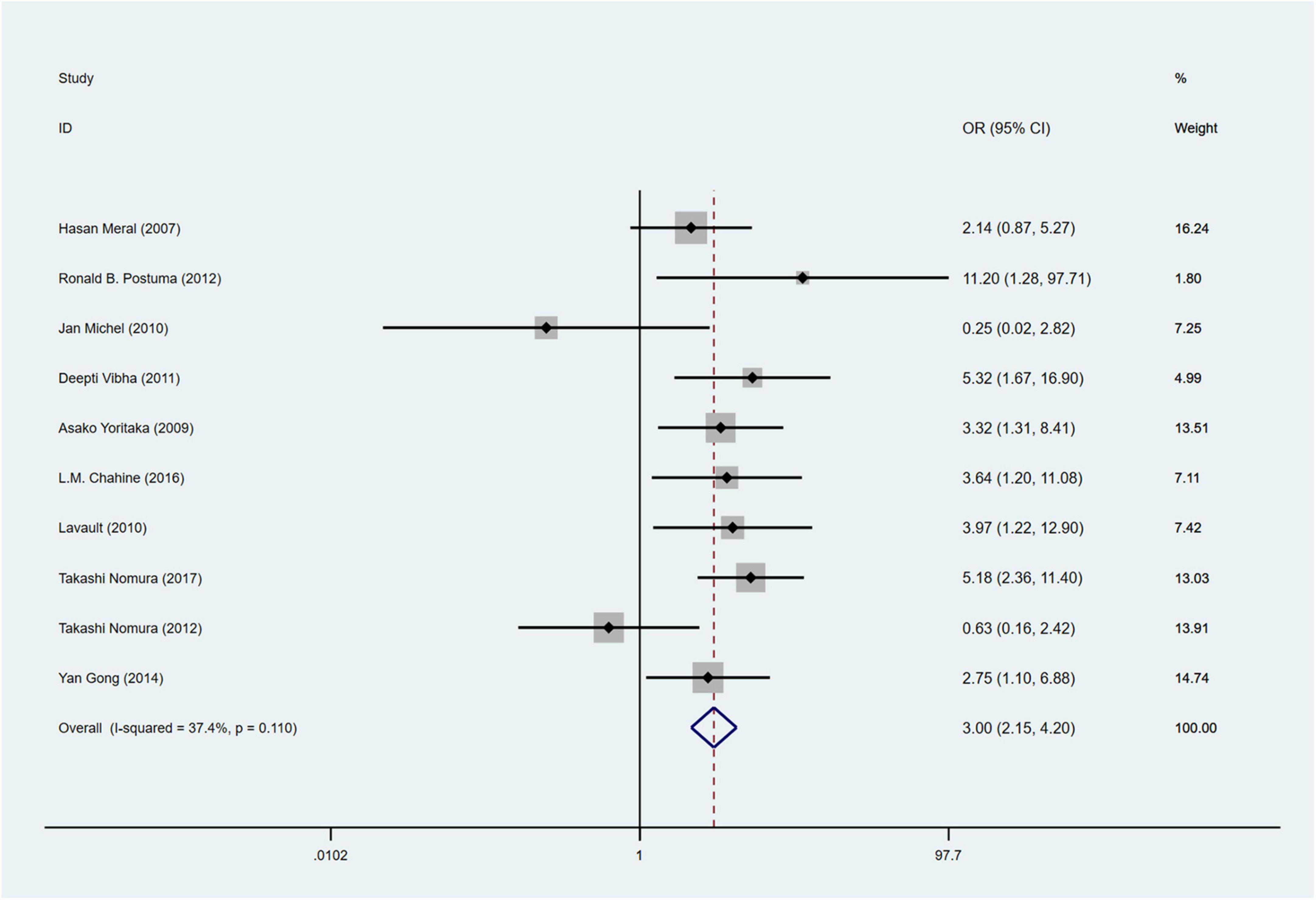
Figure 5. Forest plot based on the odds ratio (OR) for incidence of hallucinations in Parkinson’s disease patients with or without rapid eye movement sleep behavior disorder (RBD). CI, confidence intervals.
3.4.3 Analysis of anxiety
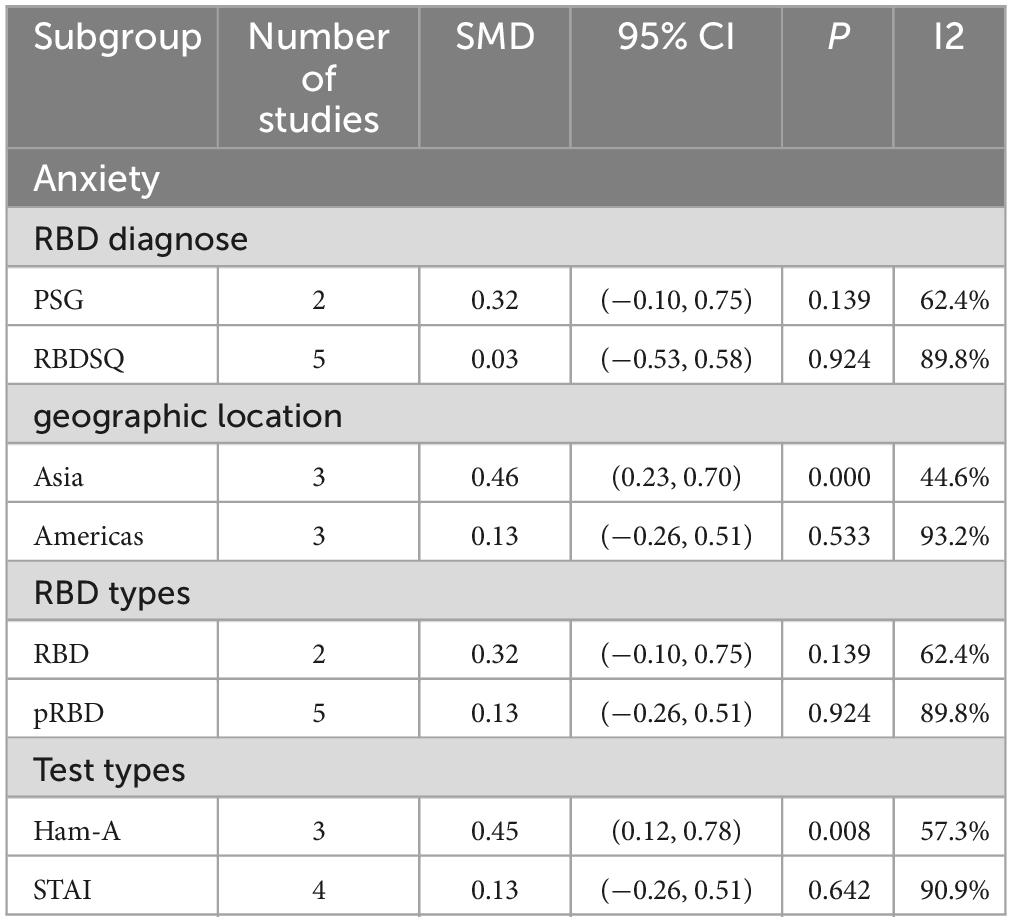
A total of 7 studies18, 29, 48, 57, 58, 61, 66 involving 989 PD patients (RBD group: 468 patients, NRBD group: 521 patients) were included in the meta-analysis of anxiety. In this study, Hamilton Anxiety Scale (Ham-A) and the State-Trait Anxiety Inventory (STAI) were adopted to assess anxiety. Due to the variety of tools used to assess anxiety, subgroup analyses were conducted based on the assessment tools. The heterogeneity among the studies was high (I2 = 85.9%), therefore, a random-effects model was employed for integration. The results showed that PD patients in the RBD group had more severe anxiety symptoms than those in the NRBD group (SMD = 0.13, 95% CI: [−0.26, 0.51], P < 0.001) (Figure 6). Further subgroup analyses (Table 2) based on RBD type, RBD diagnostic tools, geographic regions, and the type of anxiety rating scales all indicated that the study by Jonathan Trout57 was a source of heterogeneity. After excluding this study, the homogeneity of the remaining studies improved (I2 = 21%), and a fixed-effects model was used for re-analysis, showing that the RBD group had more severe anxiety symptoms compared to the NRBD group (SMD = 0.36, 95% CI: [0.20, 0.52], P = 0.276).
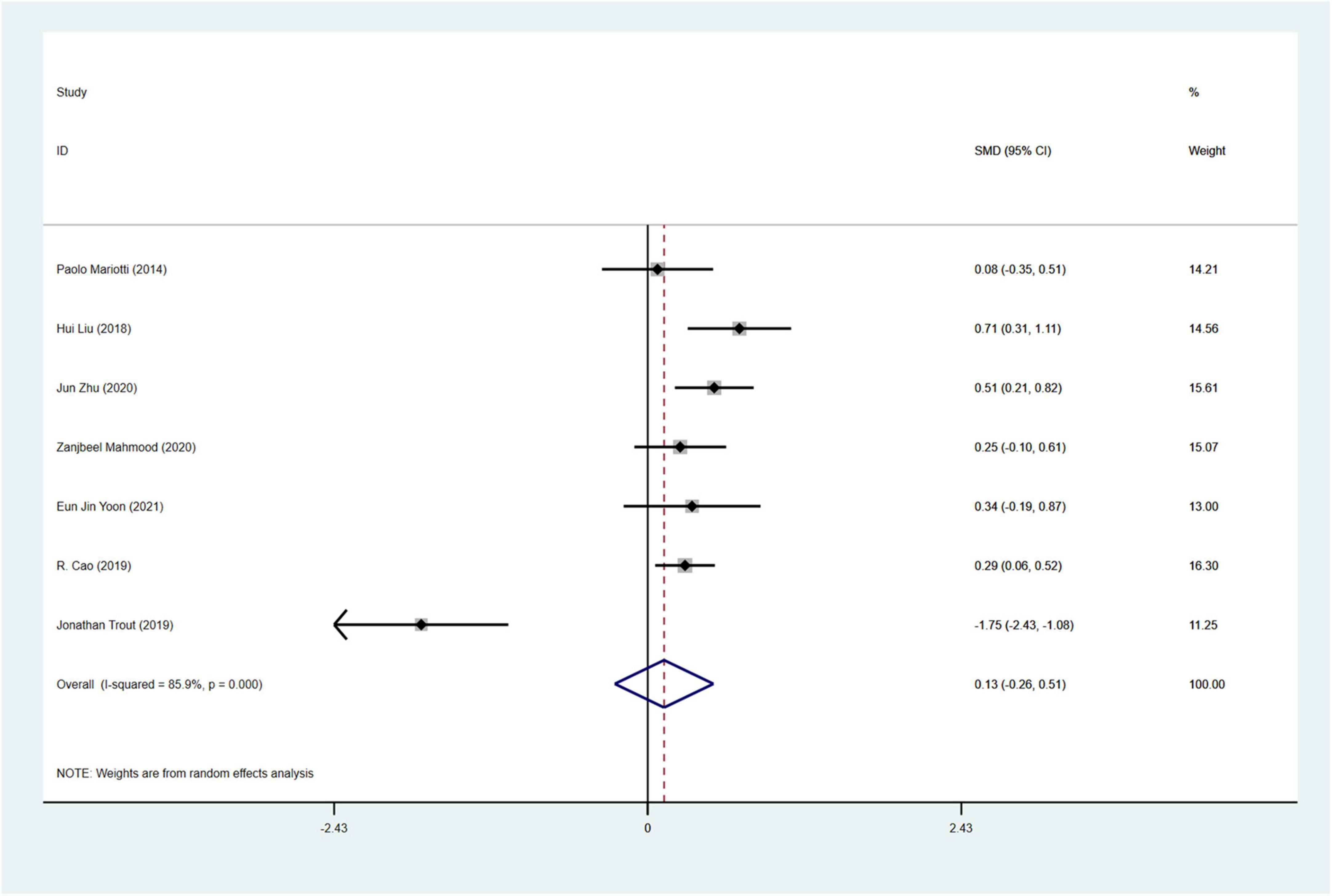
Figure 6. Forest plot based on the difference in the anxious severity among Parkinson’s disease patients with or without rapid eye movement sleep behavior disorder (RBD). SMD, Standardized Mean Difference; CI, confidence intervals.
3.4.4 Analysis of depression
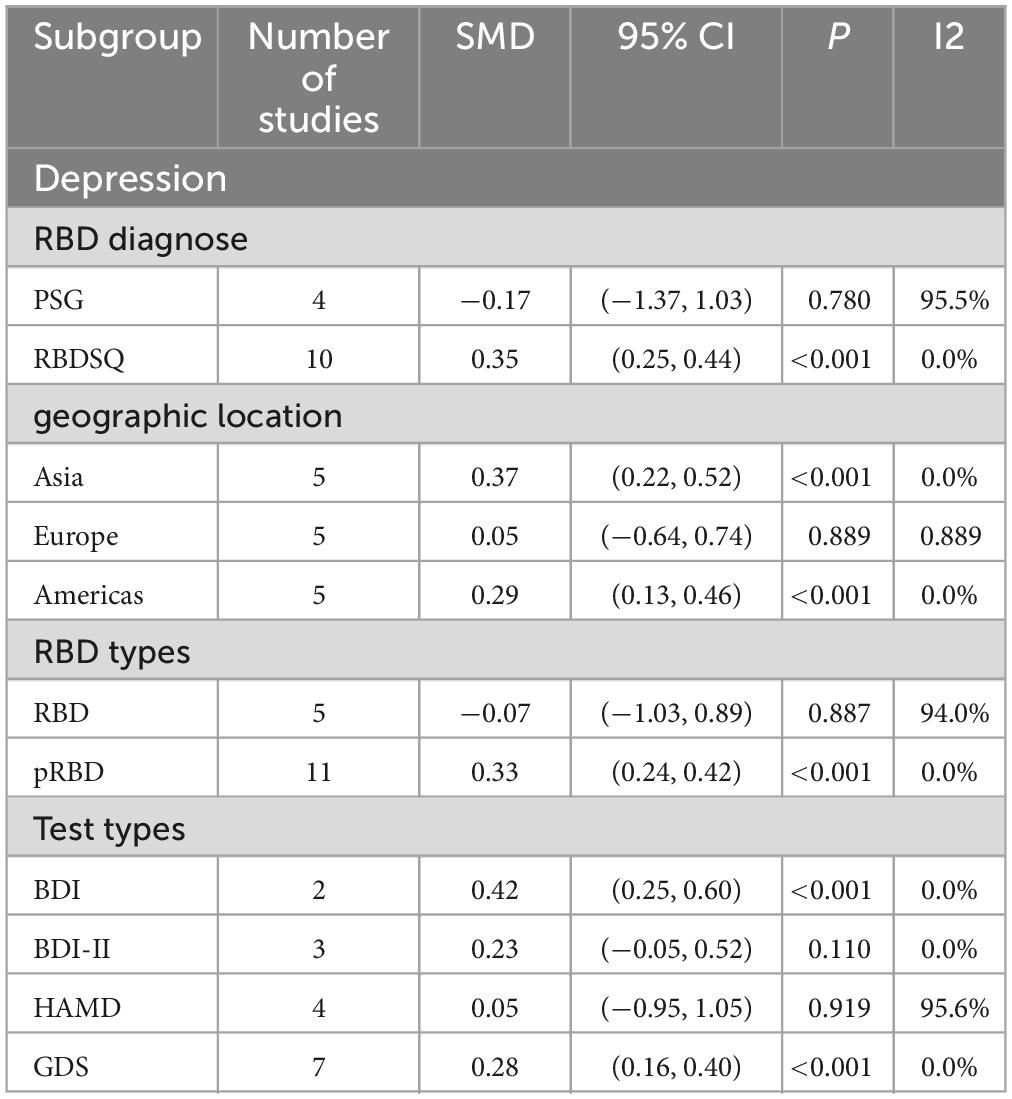
A total of 16 studies (Gagnon et al., 2004, 2009; Lavault et al., 2010; Ford et al., 2013; Suzuki et al., 2013; Rolinski et al., 2014; Mariotti et al., 2015; Chahine et al., 2016; Pagano et al., 2018; Bargiotas et al., 2019; Liu et al., 2019; Cao et al., 2020; Mahmood et al., 2020; Yoon and Monchi, 2021; Zhu et al., 2021; Fujita et al., 2022) involving 2,511 PD patients (RBD group: 1,030; NRBD group: 1,481) were included in the meta-analysis of depression. In this study, Beck Depression Inventory (BDI), Beck Depression Inventory-II (BDI-II), Hamilton Depression Scale (HAMD), and Geriatric Depression Scale (GDS) were used to assess depression. Due to the variety of tools used to assess depression, subgroup analyses were conducted based on the assessment tools. The heterogeneity among the studies was relatively high (I2 = 79.9%), therefore, a random-effects model was employed for integration. The results indicated that the RBD group had higher depression scores compared to the NRBD group (SMD = 0.22, 95% CI: [0.02, 0.43], P < 0.001) (Figure 7). The funnel plot (Supplementary Figure 2) and Begg’s test (P = 0.260) suggested no publication bias; sensitivity analysis did not identify a source of heterogeneity. Subgroup analyses (Table 3) indicated that the RBD type, the diagnostic tools for RBD, the type of depression rating scales, and the geographic origin of the study population might be sources of heterogeneity in the analysis of depression symptoms.
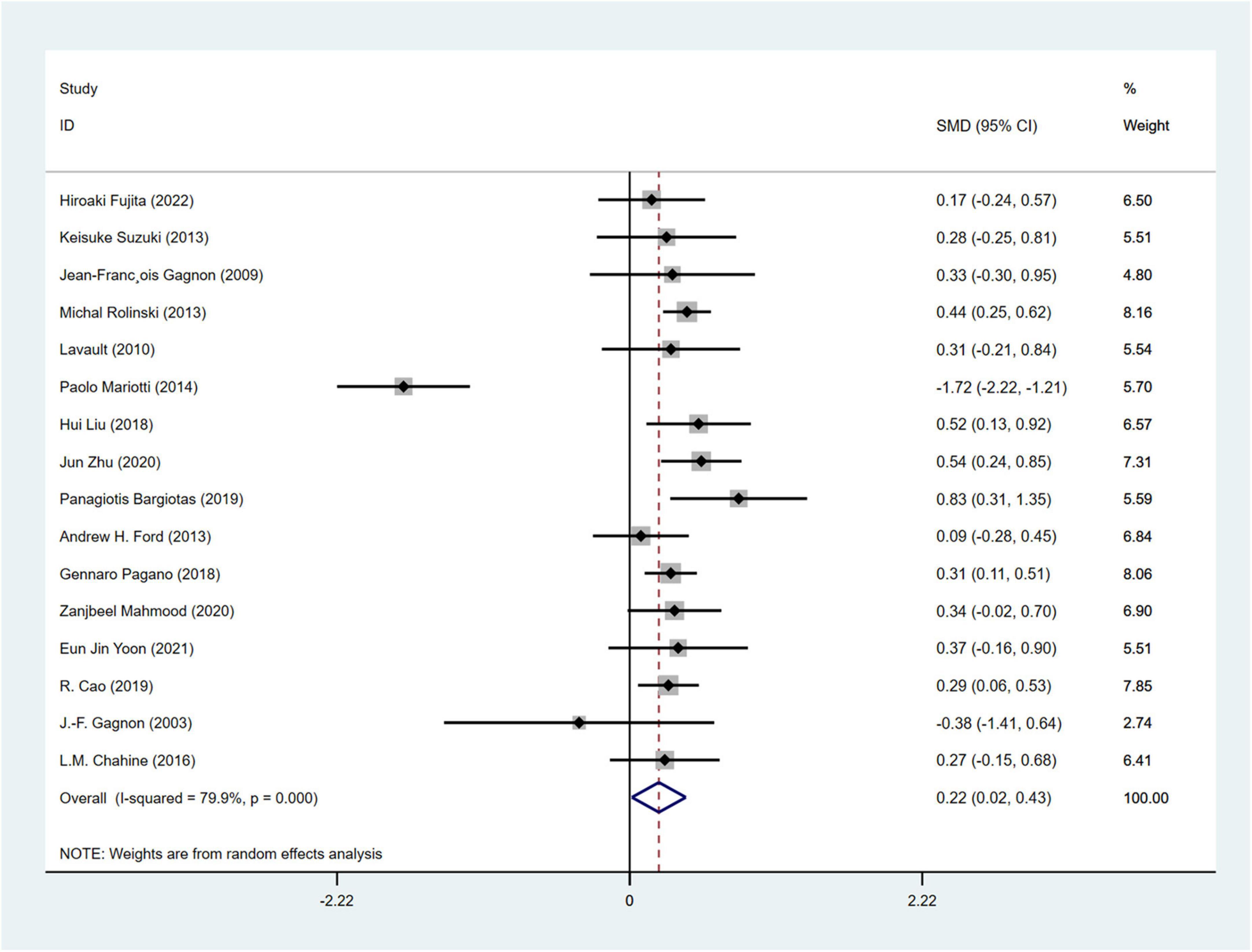
Figure 7. Forest plot based on difference in the depression severity among Parkinson’s disease patients with or without rapid eye movement sleep behavior disorder (RBD). SMD, Standardized Mean Difference; CI, confidence intervals.
3.4.5 Analysis of sleep disorder
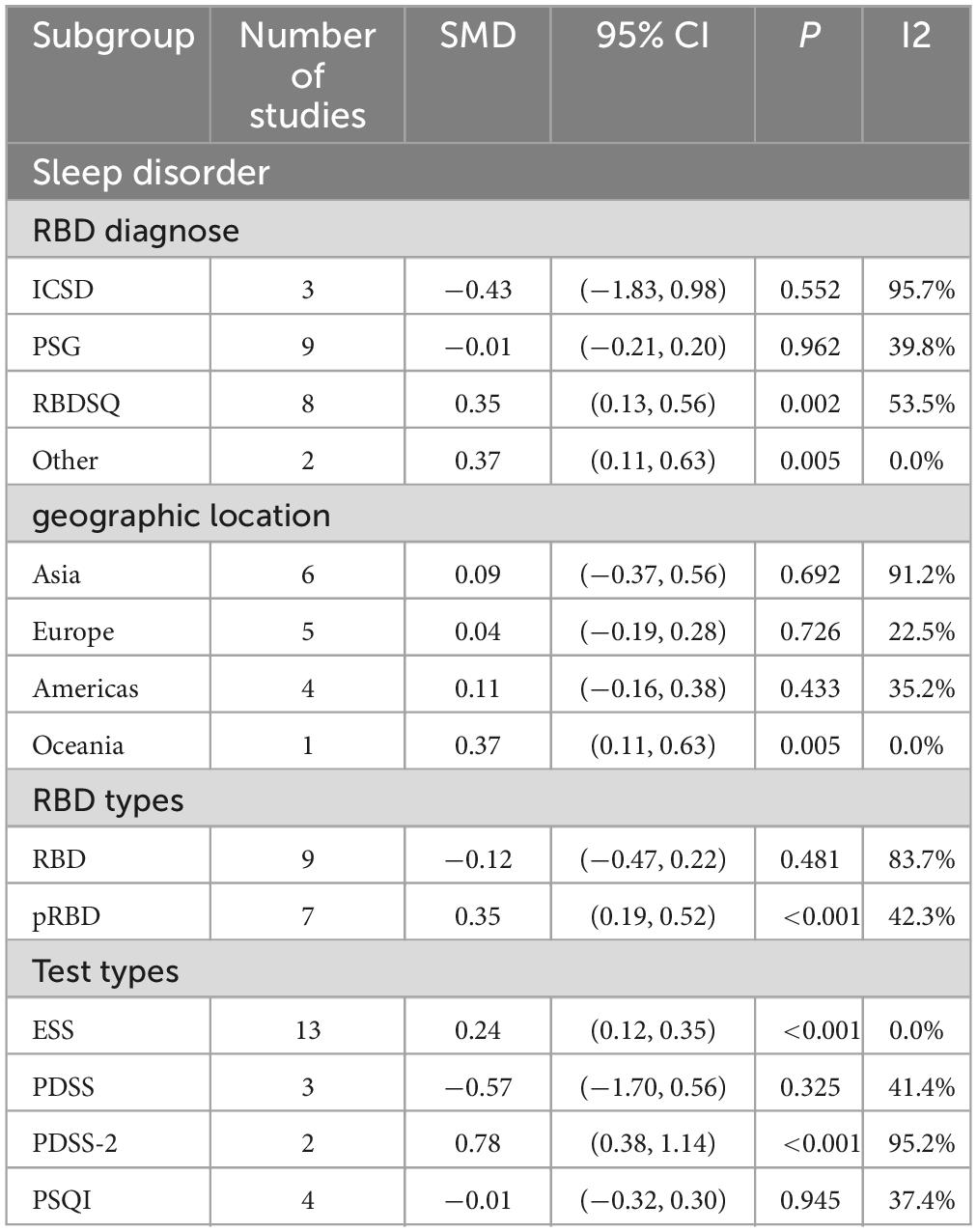
A total of 16 studies(1, 17, 20, 23, 25, 33, 35, 42, 45, 47, 54, 56, 64, 65, 67, 70) (Gagnon et al., 2009; Benninger et al., 2010; Iranzo et al., 2011; Ford et al., 2013; Suzuki et al., 2013; Ferri et al., 2014; Gong et al., 2014; Mariotti et al., 2015; Pagano et al., 2018; Sobreira-Neto et al., 2018; Bargiotas et al., 2019; Cabreira and Massano, 2019; Liu et al., 2019; Trout et al., 2019; Zhu et al., 2021; Fujita et al., 2022) with 1,509 PD patients (RBD group: 709, NRBD group: 800) were included in the meta-analysis concerning sleep conditions. The Epworth Sleepiness Score (ESS), Parkinson’s Disease Sleep Scale (PDSS), Parkinson’s Disease Sleep Scale-2 (PDSS-2), and Pittsburgh Sleep Quality Index (PSQI) were used to assess the sleep quality of PD patients in the included studies. Among them, 6 studies (Vibha et al., 2011; Ford et al., 2013; Suzuki et al., 2013; Mariotti et al., 2015; Sobreira-Neto et al., 2018; Fujita et al., 2022) utilized two different sleep assessment scales for evaluating the sleep quality of PD patients. Due to the diversity of tools used to assess sleep disorder, subgroup analyses were conducted based on the assessment tools. The analysis showed relatively high heterogeneity among the studies (I2 = 80.6%), and a random-effects model was employed for integration. The results indicated that patients in the RBD group had higher scores for sleep disorder compared to those in the NRBD group (SMD = 0.10, 95% CI: [−0.11, 0.31], P < 0.001) (Figure 8), and the funnel plot and Begg’s test (P = 0.114) suggested no publication bias among the studies. Sensitivity analysis indicated good stability across the studies. Subgroup analyses (Table 4) suggested that the geographic origin of the study population, the type of sleep disorder rating scales, the RBD type, and the diagnostic tools might be sources of heterogeneity in the analysis of sleep disorder.
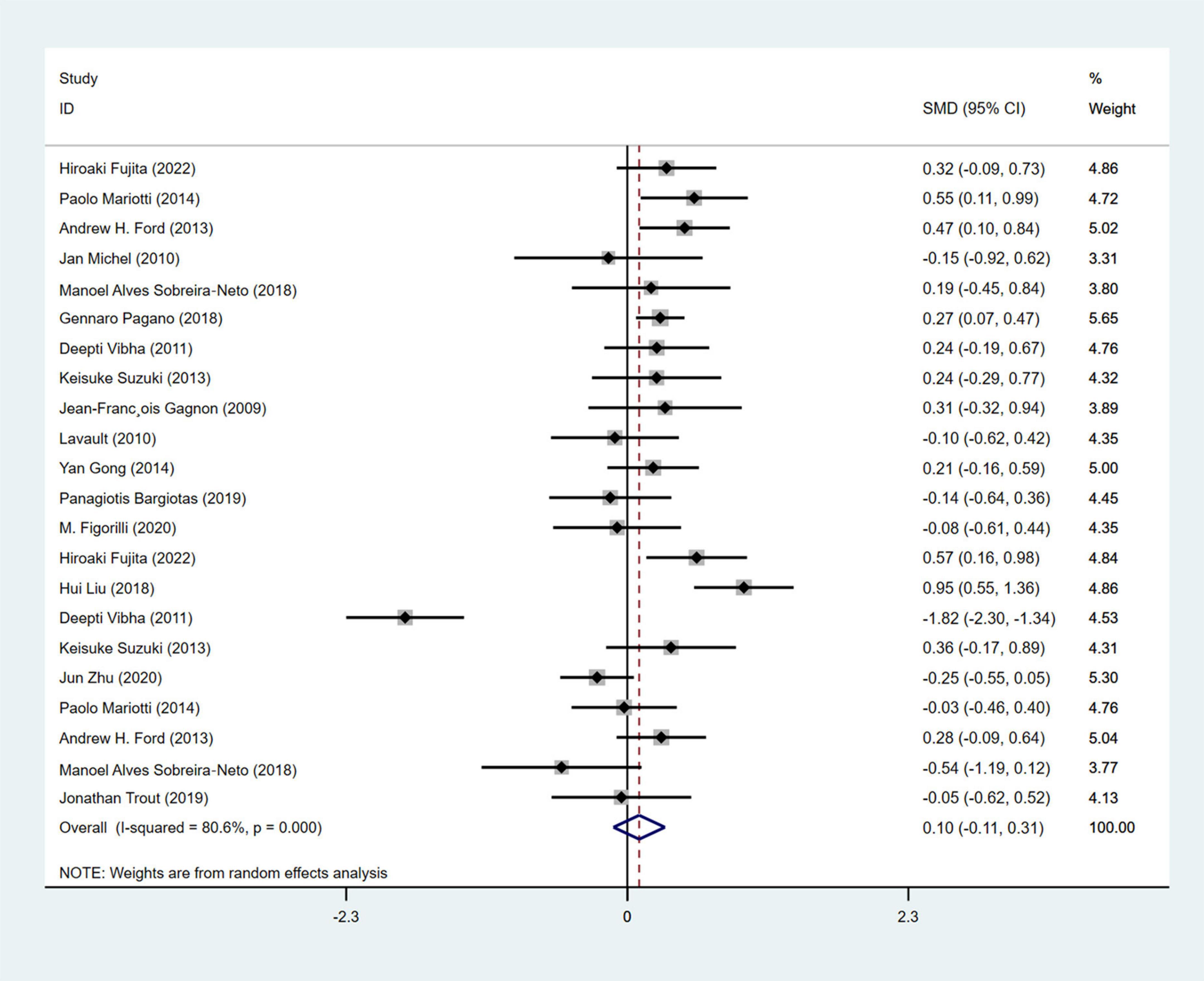
Figure 8. Forest plot based on difference in the sleep quality among Parkinson’s disease patients with or without rapid eye movement sleep behavior disorder (RBD). SMD, Standardized Mean Difference; CI, confidence intervals.
4 Discussion
To the best of our knowledge, this study is the first systematic meta-analysis comparing the differences in motor and non-motor symptoms between PD patients with and without RBD. Through the extensive database search, a total of 53 articles encompassing 3,084 PD patients with RBD were analyzed. The results revealed that PD patients in the RBD group experienced more severe motor symptoms, including higher UPDRS-III scores and Hoehn and Yahr stages, compared to the NRBD group. Additionally, RBD patients exhibited a greater range of non-motor symptoms, such as cognitive impairment, psychological health impacts, and increased incidence of sleep disorder and visual hallucinations. Sensitivity analysis confirmed the stability and reliability of these findings.
The meta-analysis found that PD patients with RBD have higher Hoehn and Yahr stages and UPDRS-III scores, indicating a more severe disease condition and faster progression compared to those without RBD. These findings are consistent with previous reports (Zhu et al., 2021). As PD progresses, the dopaminergic neurotransmission in the striatum weakens, which leads to a reduction in dopamine regulatory function, resulting in motor control impairments. PD patients with RBD often exhibit more severe and widespread neurodegeneration in the substantia nigra of the midbrain (Iranzo et al., 2011), further diminishing dopaminergic neurons and exacerbating motor symptoms. The relatively high heterogeneity in studies on PD-RBD motor symptoms could be related to the diagnosis criteria for RBD. Many patients diagnosed with cRBD were not tested with PSG (Stefani and Högl, 2020) but diagnosed through the 2005 ICSD criteria (Thorpy, 2012), potentially introducing variability due to the questionnaire’s design limitations and evaluator subjectivity, suggesting the need for more standardized diagnosis procedures in future research.
Moreover, the study found that cognitive functions in PD patients were affected by RBD, with lower MMSE scores observed in the RBD group, indicating more severe cognitive impairments, which aligns with findings from Xie et al. (2021). The mechanism behind this phenomenon may be related to the shared pathological basis of cognitive impairment in patients with both RBD and PD. The structures involved in controlling the onset of REM sleep are located in the periaqueductal gray area near the aqueduct of the midbrain and in the anterior part of the locus coeruleus, the sublaterodorsal nucleus, and dorsal raphe nucleus (Jiménez-Jiménez et al., 2021). A study by Meyer et al. (2009) found that the worsening of cognitive impairment in PD patients is associated with a reduction in acetylcholine receptors in various brain regions, including the thalamus, midbrain, pons, hippocampus, and cerebellum. Given that this study solely utilized the MMSE for cognitive assessment, it might be challenging to detect mild cognitive impairments or impairments due to other causes during the evaluation process. For future research, it’s recommended that researchers combine patient MR imaging and clinical manifestations to interpret more precise cognitive assessment results.
According to research (Meral et al., 2007), visual hallucination is a common symptom of PD, with PD patients with RBD showing a higher likelihood of experiencing visual hallucination compared to those without RBD. This finding aligns with the results of this study. Although the mechanisms behind the occurrence of visual hallucinations may be associated with the ascending cholinergic system and the serotonin release involved in the sleep-wake cycle, and abnormal discharges in the brain cortex areas processing visual information (Gelb et al., 1999), the use of certain medications such as dopamine receptor agonists and anticholinergic drugs (Sanchez-Ramos et al., 1996) may also be related to the occurrence of visual hallucination in PD patients. Therefore, the mechanism behind the occurrence of visual hallucination in PD patients with RBD remains unclear, and more basic experiments are required in future research to confirm its mechanism.
Previous research has reported an association between PD and the onset of psychiatric disorders, including anxiety and depression disorders. One mechanism is that damage to the limbic system can lead to a reduction in neurotransmitters such as dopamine and norepinephrine, which may exacerbate psychiatric symptoms. Another mechanism is that the accumulation of Lewy bodies in the midbrain raphe nuclei can lead to a decrease in the serotonin level in the body (Iranzo et al., 2011). In our study, it was observed that patients in the RBD group exhibited more severe symptoms of anxiety and depression compared to those in the NRBD group, which is consistent with previous reports (Mahmood et al., 2020). The underlying reason may be related to the anatomical overlap between the occurrence of anxiety, depression, and RBD. The onset of RBD is associated with structures such as the raphe nucleus and locus coeruleus, which are anatomically linked to the pathogenesis of anxiety and depression. In the analysis of anxiety symptoms, it was found that the study by Jonathan Trout (Trout et al., 2019) was a source of heterogeneity. After excluding this study, the homogeneity among the remaining studies improved, and the PD-RBD group exhibited markedly more severe anxiety symptoms than the NRBD group. For depression symptoms, factors such as RBD type, RBD diagnostic tools, geographic regions, and the type of depression rating scales were considered potential sources of heterogeneity among studies. Future research should employ larger sample sizes and more precise scoring criteria to assess the occurrence of psychiatric disorders in PD patients.
In this study, we also discovered that the RBD group had more sleep issues compared to the NRDB group, consistent with previous reports. This may be attributed to multiple factors. On one hand, PD patients with RBD often experience excessive loss of dopamine neurons, leading to an imbalance in neurotransmitter projections of the ascending reticular activating system, causing disturbances in the ascending arousal system. On the other hand, PD patients with RBD often display abnormal sleep behaviors at night, such as kicking, excessive clenching of the jaw, and twitching, which often disrupt sleep and lead to daytime somnolence and nighttime insomnia, inducing rhythm sleep disorders. Moreover, the patients often have comorbid psychiatric disorders, which may further exacerbate their sleep issues (Raggi et al., 2013). In this study, the sources of heterogeneity in the analysis of sleep disorders in RBD may mainly stem from the geographic origin of the study population, the type of sleep disorder rating scales, the RBD type, and the diagnostic tools. Future research should focus on larger-scale clinical studies to elucidate the relationship between RBD and sleep disorders in patients with PD. In summary, RBD may correlate with degeneration in the locus coeruleus, subcoeruleus area, and adjacent areas at the midbrain-pontine junction, as well as the midbrain-striatal dopaminergic neuronal pathways. Anatomically, these structures are interconnected with PD, potentially exacerbating both motor and non-motor symptoms in PD patients with RBD. Clinically, it is crucial to monitor PD patients with concurrent RBD and intervene with prompt treatments, thereby enhancing the overall quality of life for these patients.
Some limitations exist in this study. Firstly, the diagnostic tools for cRBD in patients included in the PD-RBD group are diverse, and most studies did not implement PSG monitoring. Such a diagnostic approach may affect the reliability of the results of PD-related symptoms. Secondly, due to the small sample size in some studies and limitations in study design, it was not possible to include enough data samples for further analysis of more motor symptoms (such as bradykinesia, rigidity, and gait impairment) and some non-motor symptoms (like constipation, sweating, and orthostatic hypotension) in PD patients Additionally, the evaluation of mental health (anxiety and depression) and sleep disorder used various measurement scales, which could contribute to relatively high heterogeneity among studies. Ultimately, the evaluation of motor and non-motor symptoms necessitates the adoption of more uniformly standardized tools.
5 Conclusion
In conclusion, our study results indicated that PD patients with RBD can exhibit more severe motor and non-motor symptoms. Clinicians should identify the presence of RBD in PD patients early and take proactive interventions for better treatment outcomes. In future research, larger sample sizes and more uniform RBD diagnostic criteria should be adopted to convincingly demonstrate the correlation of RBD to the progression of PD and the potential pathological mechanisms involved.
Data availability statement
The original contributions presented in this study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WZ: Conceptualization, Writing – original draft, Writing – review and editing. YP: Methodology, Writing – original draft. KL: Formal analysis, Investigation, Writing – original draft. KT: Formal analysis, Investigation, Writing – original draft. QW: Resources, Writing – original draft. YY: Supervision, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2024.1418751/full#supplementary-material
Supplementary Figure 1 | Funnel plot displaying the probable publication bias for estimated UPDRS-III (A) and Hoehn and Yahr stage (B) between PD patients with and without RBD.
Supplementary Figure 2 | Funnel plot displaying the probable publication bias for estimated MMSE (A), hallucination (B), anxiety, (C), depression, and (D), sleep disorder (E) between PD patients with and without RBD.
References
Arnaldi, D., Morbelli, S., Brugnolo, A., Girtler, N., Picco, A., Ferrara, M., et al. (2016). Functional neuroimaging and clinical features of drug naive patients with de novo Parkinson’s disease and probable RBD. Parkinsonism Relat. Disord. 29, 47–53. doi: 10.1016/j.parkreldis.2016.05.031
Arnulf, I. (2012). Rem sleep behavior disorder: Motor manifestations and pathophysiology. Mov. Disord. 27, 677–689.
Ashraf-Ganjouei, A., Moradi, K., Aarabi, M., Abdolalizadeh, A., Kazemi, S. Z., Kasaeian, A., et al. (2021). The association between REM sleep behavior disorder and autonomic dysfunction in Parkinson’s disease. J. Parkinsons Dis. 11, 747–755.
Assogna, F., Liguori, C., Cravello, L., Macchiusi, L., Belli, C., Placidi, F., et al. (2021). Cognitive and neuropsychiatric profiles in idiopathic rapid eye movement sleep behavior disorder and Parkinson’s disease. J. Pers. Med. 11:51. doi: 10.3390/jpm11010051
Aygün, D., Türkel, Y., Akkurt, A., and Onar, M. K. (2012). The effect of REM sleep behaviour disorder on clinical severity in Parkinson’s disease. Turk. J. Med. Sci. 42, 1033–1038.
Bargiotas, P., Ntafouli, M., Lachenmayer, M. L., Krack, P., Schüpbach, W. M. M., and Bassetti, C. L. A. (2019). Apathy in Parkinson’s disease with REM sleep behavior disorder. J. Neurol. Sci. 399, 194–198.
Benninger, D. H., Michel, J., Waldvogel, D., Candia, V., Poryazova, R., Van Hedel, H. J., et al. (2010). REM sleep behavior disorder is not linked to postural instability and gait dysfunction in Parkinson. Mov. Disord. 25, 1597–1604.
Bugalho, P., and Viana-Baptista, M. (2013). REM sleep behavior disorder and motor dysfunction in Parkinson’s disease–a longitudinal study. Parkinsonism Relat. Disord. 19, 1084–1087.
Bugalho, P., Da Silva, J. A., and Neto, B. (2011). Clinical features associated with REM sleep behavior disorder symptoms in the early stages of Parkinson’s disease. J. Neurol. 258, 50–55.
Cabreira, V., and Massano, J. (2019). [Parkinson’s disease: Clinical review and update]. Acta Med. Port. 32, 661–670.
Cao, R., Chen, X., Xing, F., Xie, C., Hu, P., and Wang, K. (2020). Cross-sectional and longitudinal associations between probable rapid eye movement sleep behavior disorder and impulse control disorders in Parkinson’s disease. Eur. J. Neurol. 27, 757–763. doi: 10.1111/ene.14177
Chahine, L. M., Xie, S. X., Simuni, T., Tran, B., Postuma, R., Amara, A., et al. (2016). Longitudinal changes in cognition in early Parkinson’s disease patients with Rem sleep behavior disorder. Parkinsonism Relat. Disord. 27, 102–106.
Daniel, S. E., and Lees, A. J. (1993). Parkinson’s disease society brain bank, London: Overview and research. J. Neural Transm. Suppl. 39, 165–172.
Duarte Folle, A., Paul, K. C., Bronstein, J. M., Keener, A. M., and Ritz, B. (2019). Clinical progression in Parkinson’s disease with features of REM sleep behavior disorder: A population-based longitudinal study. Parkinsonism Relat. Disord. 62, 105–111. doi: 10.1016/j.parkreldis.2019.01.018
Egger, M., Smith, G. D., and Phillips, A. N. (1997). Meta-analysis: Principles and procedures. BMJ 315, 1533–1537.
Ferri, R., Cosentino, F. I., Pizza, F., Aricò, D., and Plazzi, G. (2014). The timing between REM sleep behavior disorder and Parkinson’s disease. Sleep Breath 18, 319–323.
Figorilli, M., Marques, A. R., Vidal, T., Delaby, L., Meloni, M., Pereira, B., et al. (2020). Does REM sleep behavior disorder change in the progression of Parkinson’s disease? Sleep Med. 68, 190–198.
Ford, A. H., Duncan, G. W., Firbank, M. J., Yarnall, A. J., Khoo, T. K., Burn, D. J., et al. (2013). Rapid eye movement sleep behavior disorder in Parkinson’s disease: Magnetic resonance imaging study. Mov. Disord. 28, 832–836.
Fujita, H., Shiina, T., Sakuramoto, H., Nozawa, N., Ogaki, K., and Suzuki, K. (2022). Sleep and autonomic manifestations in Parkinson’s disease complicated with probable rapid eye movement sleep behavior disorder. Front. Neurosci. 16:874349. doi: 10.3389/fnins.2022.874349
Gagnon, J. F., Fantini, M. L., Bédard, M. A., Petit, D., Carrier, J., Rompré, S., et al. (2004). Association between waking EEG slowing and REM sleep behavior disorder in Pd without dementia. Neurology 62, 401–406.
Gagnon, J. F., Vendette, M., Postuma, R. B., Desjardins, C., Massicotte-Marquez, J., Panisset, M., et al. (2009). Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson’s disease. Ann. Neurol. 66, 39–47.
Gaudreault, P. O., Gagnon, J. F., Montplaisir, J., Vendette, M., Postuma, R. B., Gagnon, K., et al. (2013). Abnormal occipital event-related potentials in Parkinson’s disease with concomitant REM sleep behavior disorder. Parkinsonism Relat. Disord. 19, 212–217. doi: 10.1016/j.parkreldis.2012.10.006
Gelb, D. J., Oliver, E., and Gilman, S. (1999). Diagnostic criteria for Parkinson disease. Arch. Neurol. 56:59.
Gong, Y., Xiong, K. P., Mao, C. J., Shen, Y., Hu, W. D., Huang, J. Y., et al. (2014). Clinical manifestations of Parkinson disease and the onset of rapid eye movement sleep behavior disorder. Sleep Med. 15, 647–653.
Hu, Y., Yu, S. Y., Zuo, L. J., Piao, Y. S., Cao, C. J., Wang, F., et al. (2015). Investigation on abnormal iron metabolism and related inflammation in Parkinson disease patients with probable RBD. PLoS One 10:e0138997. doi: 10.1371/journal.pone.0138997
Hughes, A. J., Daniel, S. E., Kilford, L., and Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184. doi: 10.1136/jnnp.55.3.181
Iranzo, A., Valldeoriola, F., LomeñA, F., Molinuevo, J. L., Serradell, M., Salamero, M., et al. (2011). Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: A prospective study. Lancet Neurol. 10, 797–805. doi: 10.1016/S1474-4422(11)70152-1
Jiménez-Jiménez, F. J., Alonso-Navarro, H., GarcíA-MartíN, E., and Agúndez, J. A. G. (2021). Neurochemical features of REM sleep behaviour disorder. J. Pers. Med. 11:880.
Kamble, N., Yadav, R., Lenka, A., Kumar, K., Nagaraju, B. C., and Pal, P. K. (2019). Impaired sleep quality and cognition in patients of Parkinson’s disease with REM sleep behavior disorder: A comparative study. Sleep Med. 62, 1–5. doi: 10.1016/j.sleep.2019.04.001
Kang, S. H., Lee, H. M., Seo, W. K., Kim, J. H., and Koh, S. B. (2016). The combined effect of REM sleep behavior disorder and hyposmia on cognition and motor phenotype in Parkinson’s disease. J. Neurol. Sci. 368, 374–378. doi: 10.1016/j.jns.2016.07.057
Kim, Y. E., Jeon, B. S., Yang, H. J., Ehm, G., Yun, J. Y., Kim, H. J., et al. (2014). REM sleep behavior disorder: Association with motor complications and impulse control disorders in Parkinson’s disease. Parkinsonism Relat. Disord. 20, 1081–1084.
Lavault, S., Leu-Semenescu, S., Tezenas, Du Montcel, S., Cochen, De Cock, V., et al. (2010). Does clinical rapid eye movement behavior disorder predict worse outcomes in Parkinson’s disease? J. Neurol. 257, 1154–1159.
Leclair-Visonneau, L., Clairembault, T., Coron, E., Le Dily, S., Vavasseur, F., Dalichampt, M., et al. (2017). REM sleep behavior disorder is related to enteric neuropathology in Parkinson disease. Neurology 89, 1612–1618. doi: 10.1212/WNL.0000000000004496
Lee, J. E., Kim, K. S., Shin, H. W., and Sohn, Y. H. (2010). Factors related to clinically probable REM sleep behavior disorder in Parkinson disease. Parkinsonism Relat. Disord. 16, 105–108.
Liu, H., Ou, R., Wei, Q., Hou, Y., Cao, B., Zhao, B., et al. (2019). Rapid eye movement behavior disorder in drug-naïve patients with Parkinson’s disease. J. Clin. Neurosci. 59, 254–258. doi: 10.1016/j.jocn.2018.07.007
Mahmood, Z., Van Patten, R., Nakhla, M. Z., Twamley, E. W., Filoteo, J. V., and Schiehser, D. M. (2020). REM sleep behavior disorder in Parkinson’s disease: Effects on cognitive, psychiatric, and functional outcomes. J. Int. Neuropsychol. Soc. 26, 894–905.
Mariotti, P., Quaranta, D., Di Giacopo, R., Bentivoglio, A. R., Mazza, M., Martini, A., et al. (2015). Rapid eye movement sleep behavior disorder: A window on the emotional world of Parkinson disease. Sleep 38, 287–294. doi: 10.5665/sleep.4416
Meral, H., Aydemir, T., Ozer, F., Ozturk, O., Ozben, S., Erol, C., et al. (2007). Relationship between visual hallucinations and REM sleep behavior disorder in patients with Parkinson’s disease. Clin. Neurol. Neurosurg. 109, 862–867.
Meyer, P. M., Strecker, K., Kendziorra, K., Becker, G., Hesse, S., Woelpl, D., et al. (2009). Reduced alpha4beta2*-nicotinic acetylcholine receptor binding and its relationship to mild cognitive and depressive symptoms in Parkinson disease. Arch. Gen. Psychiatry 66, 866–877. doi: 10.1001/archgenpsychiatry.2009.106
Neikrug, A. B., Avanzino, J. A., Liu, L., Maglione, J. E., Natarajan, L., Corey-Bloom, J., et al. (2014). Parkinson’s disease and REM sleep behavior disorder result in increased non-motor symptoms. Sleep Med. 15, 959–966.
Nihei, Y., Takahashi, K., Koto, A., Mihara, B., Morita, Y., Isozumi, K., et al. (2012). REM sleep behavior disorder in Japanese patients with Parkinson’s disease: A multicenter study using the REM sleep behavior disorder screening questionnaire. J. Neurol. 259, 1606–1612.
Nomura, T., Inoue, Y., Kagimura, T., and Nakashima, K. (2013). Clinical significance of REM sleep behavior disorder in Parkinson’s disease. Sleep Med. 14, 131–135.
Nomura, T., Kishi, M., and Nakashima, K. (2017). Differences in clinical characteristics when REM sleep behavior disorder precedes or comes after the onset of Parkinson’s disease. J. Neurol. Sci. 382, 58–60.
Nomura, T., Nomura, Y., Oguri, M., Hirooka, Y., and Hanajima, R. (2020). Olfactory function deteriorates in patients with Parkinson’s disease complicated with REM sleep behavior disorder. eNeurologicalSci 20:100261.
Nomura, T., Tanaka, K., Tajiri, Y., Kishi, M., and Nakashima, K. (2016). Screening tools for clinical characteristics of probable REM sleep behavior disorder in patients with Parkinson’s disease. eNeurologicalSci 4:22–24.
Oltra, J., Campabadal, A., Segura, B., Uribe, C., Marti, M. J., Compta, Y., et al. (2021). Disrupted functional connectivity in PD with probable RBD and its cognitive correlates. Sci. Rep. 11:24351.
Ozekmekçi, S., Apaydin, H., and Kiliç, E. (2005). Clinical features of 35 patients with Parkinson’s disease displaying REM behavior disorder. Clin. Neurol. Neurosurg. 107, 306–309. doi: 10.1016/j.clineuro.2004.09.021
Pagano, G., De Micco, R., Yousaf, T., Wilson, H., Chandra, A., and Politis, M. (2018). REM behavior disorder predicts motor progression and cognitive decline in Parkinson disease. Neurology 91, e894–e905.
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372:n71.
Pilotto, A., Romagnolo, A., Tuazon, J. A., Vizcarra, J. A., Marsili, L., Zibetti, M., et al. (2019). Orthostatic hypotension and REM sleep behaviour disorder: Impact on clinical outcomes in α-synucleinopathies. J. Neurol. Neurosurg. Psychiatry 90, 1257–1263. doi: 10.1136/jnnp-2019-320846
Postuma, R. B., and Berg, D. (2017). The new diagnostic criteria for Parkinson’s disease. Int. Rev. Neurobiol. 132, 55–78.
Postuma, R. B., Bertrand, J. A., Montplaisir, J., Desjardins, C., Vendette, M., Rios Romenets, S., et al. (2012). Rapid eye movement sleep behavior disorder and risk of dementia in Parkinson’s disease: A prospective study. Mov. Disord. 27, 720–726.
Postuma, R. B., Gagnon, J. F., Vendette, M., and Montplaisir, J. Y. (2009). Markers of neurodegeneration in idiopathic rapid eye movement sleep behaviour disorder and Parkinson’s disease. Brain 132, 3298–3307.
Postuma, R. B., Gagnon, J. F., Vendette, M., Charland, K., and Montplaisir, J. (2008). REM sleep behaviour disorder in Parkinson’s disease is associated with specific motor features. J. Neurol. Neurosurg. Psychiatry 79, 1117–1121.
Postuma, R. B., Montplaisir, J., Lanfranchi, P., Blais, H., Rompré, S., Colombo, R., et al. (2011). Cardiac autonomic denervation in Parkinson’s disease is linked to REM sleep behavior disorder. Mov. Disord. 26, 1529–1533.
Raggi, A., Bella, R., Pennisi, G., Neri, W., and Ferri, R. (2013). Sleep disorders in Parkinson’s disease: A narrative review of the literature. Rev. Neurosci. 24, 279–291.
Raza, C., Anjum, R., and Shakeel, N. U. (2019). Parkinson’s disease: Mechanisms, translational models and management strategies. Life Sci. 226, 77–90.
Rolinski, M., Szewczyk-Krolikowski, K., Tomlinson, P. R., Nithi, K., Talbot, K., Ben-Shlomo, Y., et al. (2014). REM sleep behaviour disorder is associated with worse quality of life and other non-motor features in early Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 85, 560–566. doi: 10.1136/jnnp-2013-306104
Sanchez-Ramos, J. R., Ortoll, R., and Paulson, G. W. (1996). Visual hallucinations associated with Parkinson disease. Arch. Neurol. 53, 1265–1268.
Sinforiani, E., Zangaglia, R., Manni, R., Cristina, S., Marchioni, E., Nappi, G., et al. (2006). REM sleep behavior disorder, hallucinations, and cognitive impairment in Parkinson’s disease. Mov. Disord. 21, 462–466.
Snyder, A., Gruber-Baldini, A. L., Rainer Von, Coelln, F., Savitt, J. M., Reich, S. G., et al. (2021). Comparison of mini-mental state examination and montreal cognitive assessment ratings across levels of Parkinson’s disease severity. J. Parkinsons Dis. 11, 1995–2003. doi: 10.3233/JPD-212705
Sobreira-Neto, M. A., Pena-Pereira, M. A., Sobreira, E. S. T., Chagas, M. H. N., Fernandes, R. M. F., Tumas, V., et al. (2018). High frequency of sleep disorders in Parkinson’s disease and its relationship with quality of life. Eur. Neurol. 78, 330–337.
St Louis, E. K., and Boeve, B. F. (2017). REM sleep behavior disorder: Diagnosis, clinical implications, and future directions. Mayo Clin. Proc. 92, 1723–1736. doi: 10.1016/j.mayocp.2017.09.007
Stefani, A., and Högl, B. (2020). Sleep in Parkinson’s disease. Neuropsychopharmacology 45, 121–128.
Stiasny-Kolster, K., Mayer, G., Schäfer, S., Möller, J. C., Heinzel-Gutenbrunner, M., and Oertel, W. H. (2007). The REM sleep behavior disorder screening questionnaire–a new diagnostic instrument. Mov. Disord. 22, 2386–2393. doi: 10.1002/mds.21740
Suzuki, K., Miyamoto, T., Miyamoto, M., Watanabe, Y., Suzuki, S., Tatsumoto, M., et al. (2013). Probable rapid eye movement sleep behavior disorder, nocturnal disturbances and quality of life in patients with Parkinson’s disease: A case-controlled study using the rapid eye movement sleep behavior disorder screening questionnaire. BMC Neurol. 13:18. doi: 10.1186/1471-2377-13-18
Trout, J., Christiansen, T., Bulkley, M. B., Tanner, J. J., Sozda, C. N., Bowers, D., et al. (2019). Cognitive impairments and self-reported sleep in early-stage Parkinson’s disease with versus without probable rem sleep behavior disorder. Brain Sci. 10:9. doi: 10.3390/brainsci10010009
Tysnes, O. B., and Storstein, A. (2017). Epidemiology of Parkinson’s disease. J. Neural Transm. 124, 901–905.
Vibha, D., Shukla, G., Goyal, V., Singh, S., Srivastava, A. K., and Behari, M. (2011). RBD in Parkinson’s disease: A clinical case control study from North India. Clin. Neurol. Neurosurg. 113, 472–476. doi: 10.1016/j.clineuro.2011.02.007
Wells, G. A., Shea, B. J., O’connell, D., Peterson, J., and Tugwell, P. (2000). The Newcastle–Ottawa scale (NOS) for assessing the quality of non-randomized studies in meta-analysis. ScienceOpen 4, 59–63.
Wells, G., Shea, B., and O’connell, D. (2014). Newcastle-Ottawa quality assessment scale cohort studies. Ottawa, ON: University of Ottawa.
Xie, D., Shen, Q., Zhou, J., and Xu, Y. (2021). Non-motor symptoms are associated with REM sleep behavior disorder in Parkinson’s disease: A systematic review and meta-analysis. Neurol. Sci. 42, 47–60.
Yan, Y. Y., Lei, K., Li, Y. Y., Liu, X. F., and Chang, Y. (2019). The correlation between possible RBD and cognitive function in Parkinson’s disease patients in China. Ann. Clin. Transl. Neurol. 6, 848–853. doi: 10.1002/acn3.747
Yoon, E. J., and Monchi, O. (2021). Probable REM sleep behavior disorder is associated with longitudinal cortical thinning in Parkinson’s disease. NPJ Parkinsons Dis. 7:19.
Yoritaka, A., Ohizumi, H., Tanaka, S., and Hattori, N. (2009). Parkinson’s disease with and without REM sleep behaviour disorder: Are there any clinical differences? Eur. Neurol. 61, 164–170.
Keywords: Parkinson’s disease, rapid eye movement sleep behavior disorder, motor symptom, non-motor symptom, meta-analysis
Citation: Zheng W, Pan Y, Li K, Tao K, Wang Q and Yang Y (2024) The correlation between rapid eye movement sleep behavior disorder and the progress of Parkinson’s disease: a systematic review and meta-analysis. Front. Aging Neurosci. 16:1418751. doi: 10.3389/fnagi.2024.1418751
Received: 17 April 2024; Accepted: 02 July 2024;
Published: 17 July 2024.
Edited by:
Jurgen Germann, University Health Network, CanadaReviewed by:
Steven Gunzler, Case Western Reserve University, United StatesFlavia Venetucci Gouveia, University of Toronto, Canada
Copyright © 2024 Zheng, Pan, Li, Tao, Wang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yungui Yang, MTkxNzg5ODQ1NUBxcS5jb20=
 Wentao Zheng
Wentao Zheng Yungui Yang
Yungui Yang