
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 23 August 2024
Sec. Neurocognitive Aging and Behavior
Volume 16 - 2024 | https://doi.org/10.3389/fnagi.2024.1404836
This article is part of the Research TopicSmall Vessel Disease: Cause of Cerebral Infarction without Large Vessel OcclusionView all 4 articles
Background: Lacunes, a characteristic feature of cerebral small vessel disease (CSVD), are critical public health concerns, especially in the aging population. Traditional neuroimaging techniques often fall short in early lacune detection, prompting the need for more precise predictive models.
Methods: In this retrospective study, 587 patients from the Neurology Department of the Affiliated Hospital of Hebei University who underwent cranial MRI were assessed. A nomogram for predicting lacune incidence was developed using LASSO regression and binary logistic regression analysis for variable selection. The nomogram’s performance was quantitatively assessed using AUC-ROC, calibration plots, and decision curve analysis (DCA) in both training (n = 412) and testing (n = 175) cohorts.
Results: Independent predictors identified included age, gender, history of stroke, carotid atherosclerosis, hypertension, creatinine, and homocysteine levels. The nomogram showed an AUC-ROC of 0.814 (95% CI: 0.791–0.870) for the training set and 0.805 (95% CI: 0.782–0.843) for the testing set. Calibration and DCA corroborated the model’s clinical value.
Conclusion: This study introduces a clinically useful nomogram, derived from binary logistic regression, that significantly enhances the prediction of lacunes in patients undergoing brain MRI for various indications, potentially advancing early diagnosis and intervention. While promising, its retrospective design and single-center context are limitations that warrant further research, including multi-center validation.
Cerebral small vessel disease (CSVD) represents a significant neurological condition that poses a substantial public health challenge globally, particularly prevalent among the elderly population (Wardlaw et al., 2019). It is closely associated with a spectrum of severe disease outcomes, including stroke and cognitive decline (Vermeer et al., 2003; Debette et al., 2019). Among various radiological manifestations of CSVD, lacunes hold particular clinical significance, typically resulting from occlusive ischemic injuries to small vessels (Wardlaw et al., 2013; Duering et al., 2023). These lacunes are not only markers of underlying vascular pathology but also predictors of future cerebrovascular events and cognitive impairments.
In the realm of neuroimaging, Magnetic Resonance Imaging (MRI) plays a pivotal role in identifying lacunes (Wardlaw et al., 2013; Duering et al., 2023). However, the detection capability of MRI is limited to the presence of established structural brain damage, implying that lacunes might not be accurately identified in the early stages of the lesion or when significant structural damage has not yet formed. Consequently, reliance solely on radiological findings could lead to missed or delayed detection, especially since lacunes are often asymptomatic in their early stages and can be easily overlooked (Mok and Kim, 2015). This underscores the need for a more comprehensive approach that integrates clinical history and laboratory findings to predict the presence of lacunes.
Our study addresses this challenge by developing a predictive model based on the analysis of a wide range of clinical and laboratory parameters from 587 individuals. The model aims to predict the presence of lacunes in cranial MRI, thereby facilitating early identification and intervention. Incorporating variables such as age, gender, history of hypertension, diabetes, stroke, along with blood test indicators, the model provides clinicians with a nuanced tool for risk assessment.
This predictive model represents a significant advancement in the early diagnosis of lacunes and offers a platform for understanding the complex interplay of various risk factors leading to the development of lacunes. The integration of clinical and laboratory data in predicting radiological outcomes signifies a major stride in personalized medicine and targeted interventions in the field of neurology.
Furthermore, the model’s ability to stratify patients based on their risk of developing lacunes has profound implications for preventive strategies against lacune. It enables healthcare providers to identify individuals at high risk and accordingly tailor management plans, potentially reducing the incidence of stroke and cognitive disorders associated with lacune.
This retrospective study was conducted at the Neurology Department of the Affiliated Hospital of Hebei University, spanning from January 2020 to June 2022. We systematically collected and analyzed data from existing patient records, focusing on the period mentioned. The study’s retrospective nature allowed for the utilization of a large dataset, enabling a comprehensive analysis of pre-established data. Inclusion criteria were defined to ensure a representative sample from the patient population. These criteria included patients aged over 55 years and those with complete cranial magnetic resonance imaging (MRI) sequences, comprising T1-weighted axial, T2-weighted axial, FLAIR, and axial susceptibility-weighted images. Our study population was not limited to patients with specific diseases; any patient who had undergone a brain MRI for any reason, including those with normal MRI findings, was considered a potential participant. Conversely, exclusion criteria aimed to eliminate cases that could potentially confound the study outcomes, such as patients with poor-quality MRI images, significant stroke history, or severe comorbid conditions including but not limited to advanced cardiac, respiratory, renal, or hepatic diseases, and tumors. Cases suggestive of non-vascular origins of CSVD, like multiple sclerosis or central nervous system demyelinating diseases, were also excluded, alongside those with insufficient clinical or laboratory data. To ensure the confidentiality and privacy of patient data, all records were anonymized before analysis, with all personal identifiers removed. The data handling process was conducted in strict compliance with data protection regulations. The study protocol was rigorously reviewed and approved by the Institutional Review Board (IRB) of the Affiliated Hospital of Hebei University under Approval Number HDFYLL-KY-2023-060. This study was conducted in strict accordance with the ethical standards set forth in the 1964 Declaration of Helsinki and its later amendments. Ethical approval for this research involving human participants was obtained from the appropriate ethics committee. We hereby confirm that all national laws and regulations concerning ethical standards in research were observed, ensuring that the conduct of the research complied with both international and national ethical standards.
Participants underwent brain MRI using a 1.5 T MRI scanner (Siemens, Munich, Germany). Standardized MRI sequences included axial T1-weighted, sagittal T2-weighted fluid-attenuated inversion recovery (FLAIR), and axial susceptibility-weighted images. The imaging parameters were as follows: slice thickness set at 5 mm with a 1-mm interslice gap; for T1-weighted spin echo, repetition time (TR)/echo time (TE) were 700/11 ms; for T2-weighted fast spin echo, TR/TE were 5200/120 ms; and for FLAIR, TR/TE and inversion time were 8500/127 ms and 2,300 ms, respectively. This protocol aimed to identify the radiological manifestations of lacunes. The evaluation was carried out according to the guidelines for reporting vascular changes in neuroimaging (STRIVE) (Wardlaw et al., 2013; Duering et al., 2023) Lacunes were identified as round or ovoid subcortical cavities, ranging from 3 to 15 mm in diameter, resembling cerebrospinal fluid (CSF) in signal characteristics. On fluid-attenuated inversion recovery (FLAIR) imaging, these vascular-origin lacunes typically exhibit a central CSF-like hypointense core surrounded by a hyperintense rim. The presence and location of lacunes were assessed by two experienced neurologists, Y. Jiang and L. Ling, who were blinded to the clinical data of the participants. This approach aimed to minimize assessment bias. To ensure reliability of the assessments, the interrater agreement was quantified using the intraclass correlation coefficient (ICC), with a substantial agreement indicated by an ICC of 0.85.
In the retrospective analysis of our patient cohort, we meticulously compiled a comprehensive dataset encompassing a broad spectrum of clinical parameters. This dataset included an extensive range of blood biochemistry markers, such as complete blood count, renal function indicators, electrolyte levels, coagulation profiles, both random and fasting blood glucose measurements, liver function tests, lipid profiles, cardiac enzyme panels, thyroid function assessments, and homocysteine levels. Altogether, the study incorporated 81 distinct laboratory parameters, covering vital aspects of patient health and providing a thorough clinical picture for each enrolled individual.
In our study, a thorough clinical evaluation was performed for each participant, which included the collection of demographic information such as age and sex, along with a comprehensive medical history. In the medical history assessment, particular emphasis was placed on key health conditions including hypertension, diabetes, and hypercholesterolemia, as well as on the history of carotid artery atherosclerosis and stroke. The diagnostic criteria for these conditions were rigorously adhered to as follows: Hypertension was defined as having a systolic blood pressure of 140 mmHg or higher, a diastolic blood pressure of 90 mmHg or higher, or being under current treatment with antihypertensive medications. Diabetes was established based on fasting blood glucose levels of 7.0 mmol/L or above, 2-h post-oral glucose tolerance test (OGTT2h) readings exceeding 11.1 mmol/L, or ongoing treatment with hypoglycemic agents. Hypercholesterolemia was identified if total cholesterol levels exceeded 5.2 mmol/L (200 mg/dL) or low-density lipoprotein (LDL) cholesterol levels surpassed 3.4 mmol/L (130 mg/dL). Carotid atherosclerosis is defined as a history of carotid atherosclerosis or evidence of increased intima-media thickness or the presence of plaques in the carotid arteries, as indicated by carotid ultrasound examination performed during hospitalization.
A cohort comprising 587 patients underwent random allocation into two distinct sets: the training dataset, consisting of 412 individuals, and the validation dataset, consisting of 175 individuals. This allocation adhered to a predetermined ratio of 7:3. In the development of the model, the conversion of continuous variables into categorical ones was adopted, a strategy commonly employed in the literature for risk prediction models to facilitate ease of interpretation and to bolster generalizability. This approach is widely recognized for its clinical applicability and broad acceptance (Bennette and Vickers, 2012; Barrio et al., 2017). The determination of cutoff values for these continuous variables was guided by using R software, In cases where explicit cutoffs were not readily available, parameters were systematically categorized into binary or tertiary groups. Categorical variables were presented as frequencies with corresponding percentages (%). To compare the baseline characteristics between lacune group and non-lacune group, we employed statistical tests appropriate for the data types. Specifically, we used the χ2 test or Fisher’s exact test for categorical variables. For variable selection in the training dataset, we employed the Least Absolute Shrinkage and Selection Operator (LASSO) regression, guided by cross-validation. To strike a balance between model complexity and predictive accuracy, the regularization parameter (lambda) was chosen based on the lambda.1se criterion, which corresponds to the value within one standard error of the minimum mean cross-validated error. This conservative approach favors a simpler model with fewer predictors, thereby reducing the risk of overfitting while maintaining sufficient predictive power. In our analysis, variables that showed significant associations in the LASSO regression (with non-zero coefficients) were selected for further exploration. These variables were then integrated into a binary logistic regression to pinpoint independent predictors of lacunes. Based on the identified predictors, we developed a nomogram to facilitate the visual interpretation of risk factors. The performance of the nomogram was evaluated through the Receiver Operating Characteristic Curve (ROC) analysis, specifically examining the Area Under the Curve (AUC) to gauge its predictive strength. Additionally, calibration plots were generated to juxtapose the predicted outcomes against actual probabilities, offering insights into the model’s accuracy. The clinical usefulness of the predictive model was further assessed using Decision Curve Analysis (DCA), which helped in evaluating the net benefits. All statistical procedures were executed using R software (version 4.3.0). For inferential statistics, a threshold of p < 0.05 (two-tailed) was set to determine statistical significance.
From January 2020 to June 2022, our study initially enrolled 683 patients who satisfied the inclusion criteria. However, upon further evaluation, 96 patients were identified as meeting the exclusion criteria and were thus removed from the study. This led to a final cohort of 587 patients who were deemed eligible for data analysis, as depicted in Figure 1. The study was structured with a training set comprising 412 individuals, while the remaining 175 formed the validation set. Table 1 presents the baseline characteristics of the patients, distinguishing between those with lacunar and non-lacunar conditions.
In our study, we compared two groups: the lacune group (n = 184)and the non-lacune group (n = 403). We found significant differences (p < 0.05) in 27 out of the 88 analyzed variable between these two groups, while the remaining 61 biomarkers showed no significant differences. The 27 biomarkers with significant differences included: Gender, Age, History of Stroke, Carotid atherosclerosis, Hypertension, Diabetes, Homocysteine, White Blood Cell Count, Monocyte count, Basophil Count, Urea, Uric Acid, Creatinine, Carbon Dioxide Content in Plasma, Fibrinogen, Globulin, Albumin to Globulin Ratio, Free Thyroxine, Total Cholesterol, High-Density Lipoprotein, Low-Density Lipoprotein, Lipoprotein (a), Apolipoprotein A1, Apolipoprotein E, Monocyte-to-HDL Ratio, Neutrophil-to-HDL Ratio, Lymphocyte-to-Monocyte Ratio (Table 1). The other 61 biomarkers showed no significant differences between the two groups including: Hyperlipidemia, Red Blood Cell Count, Hemoglobin, Hematocrit, Mean Corpuscular Volume, Mean Corpuscular Hemoglobin, Mean Corpuscular Hemoglobin Concentration, Platelet Count, Platelet Distribution Width, Mean Platelet Volume, Plateletcrit, Neutrophil Percentage, Lymphocyte Percentage, Monocyte Percentage, Eosinophil Percentage, Basophil Percentage, Neutrophil Count, Lymphocyte Count, Eosinophil Count, Platelet Large Cell Ratio, Random Glucose, Potassium, Sodium, Chloride, Calcium, Magnesium, Serum Phosphate, Prothrombin Time, Prothrombin Time Ratio, International Normalized Ratio, Activated Partial Thromboplastin Time, Thrombin Time, Fasting Glucose, Hemoglobin A1c, Triglycerides, Very Low-Density Lipoprotein, Apolipoprotein B100, ApoA1 vs. ApoB100 Ratio, Creatine Kinase, Creatine Kinase-MB, Alpha-Hydroxybutyrate Dehydrogenase, Alanine Aminotransferase, Aspartate Aminotransferase, Lactate Dehydrogenase, Alkaline Phosphatase, Gamma-Glutamyl Transferase, Total Protein, Albumin, Total Bilirubin, Direct Bilirubin, Unconjugated Bilirubin, Bile Acid, Triiodothyronine, Thyroxine, Free Triiodothyronine, Thyroid-Stimulating Hormone, Systemic Immune-Inflammatory Index, Systemic Inflammation Response Index, Neutrophil-to-Lymphocyte Ratio, Neutrophil-to-Monocyte Ratio, Lymphocyte-to-HDL Ratio.
We collected data covering a total of 88 variables, including age, gender, medical history, and laboratory tests. In our study, the LASSO regression was implemented using the “glmnet package” in R with a 10-fold cross-validation approach to optimize the regularization parameter λ. We selected the value of λ based on the 1SE (one standard error) criterion, which aims at choosing a simpler model with a performance within one standard error of the minimum cross-validation error. This approach helped in striking a balance between model complexity and prediction accuracy, enabling us to identify the most relevant predictors for our model while controlling for overfitting (Figures 2A,B). Through LASSO regression, we selected 9 indicators: Gender, Age, History of Stroke, Carotid Atherosclerosis, Hypertension, Creatinine, High-Density Lipoprotein, Homocysteine, Monocyte-to-HDL Ratio (Table 2). The variables with non-zero coefficients in the LASSO regression model were considered to be related to lacune.
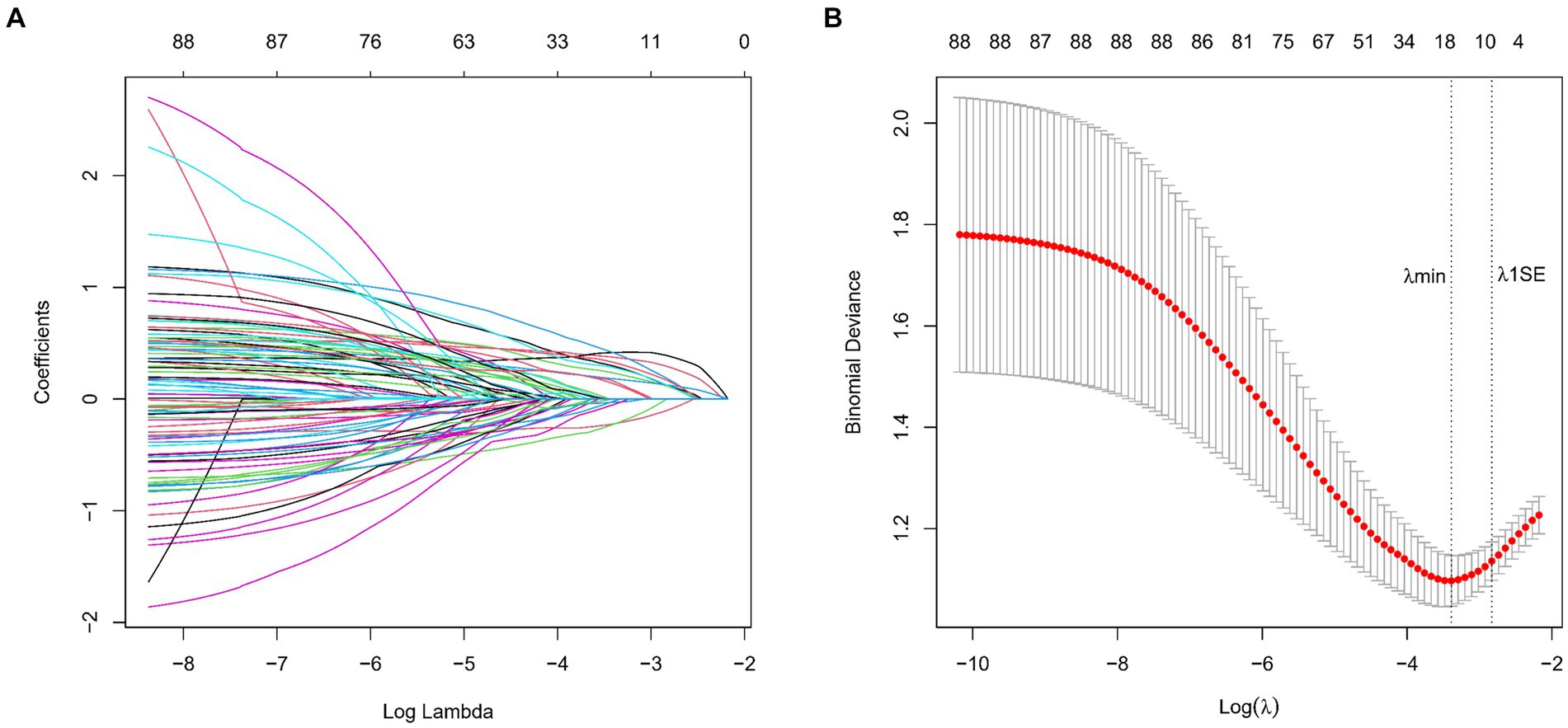
Figure 2. (A) LASSO Coefficient Profiles for lacune Clinical Predictors. This figure presents the coefficient profiles of 88 features considered in the LASSO model for predicting the risk of lacune. The graph displays how each feature’s coefficient varies with the log of lambda (log(lambda)), demonstrating the shrinkage effect of the LASSO technique. (B) LASSO Regression Cross-Validation Results. This figure illustrates the evaluation of model performance under various regularization parameters λ through cross-validation in LASSO regression. A vertical dashed line on the left side represents λmin, which corresponds to the model with the best performance. On the right side, another vertical dashed line denotes λ1SE, representing a slightly sparser model. The numbers of selected variables are annotated above each line.
In the binary logistic regression analysis, we included the 9 variables identified from the LASSO regression. The analysis revealed that seven of these variables—Gender, Age, History of Stroke, Carotid Atherosclerosis, Hypertension, Creatinine, and Homocysteine—were significantly associated with the risk of lacune (p < 0.05), as detailed in Table 3. These results indicate that these seven variables are independent clinical predictors of lacune. The remaining two variables, High-Density Lipoprotein (HDL) and Monocyte-to-HDL Ratio, were included in the model but did not show a statistically significant association in this analysis.
In this study, binary logistic regression analysis was employed to identify key variables associated with the risk of lacunes. Seven variables were selected based on their statistical significance: Gender, Age, History of Stroke, Carotid Atherosclerosis, Hypertension, Creatinine, and Homocysteine. These variables were then used to construct a nomogram, as depicted in Figure 3. The nomogram operates by assigning point values to each variable based on their calculated beta coefficients, reflecting their proportional prognostic impact. The total points accrued from each variable are then used to estimate the patient’s probability of developing lacunes. This probability is derived from the total point’s projection on the probability scale provided at the bottom of the nomogram. The inclusion of diverse variables, ranging from biochemical markers like Creatinine and Homocysteine to clinical features such as the presence of Carotid Atherosclerosis, allows for a comprehensive assessment of lacunes risk. This approach enables healthcare providers to make more informed decisions regarding patient care and risk management.
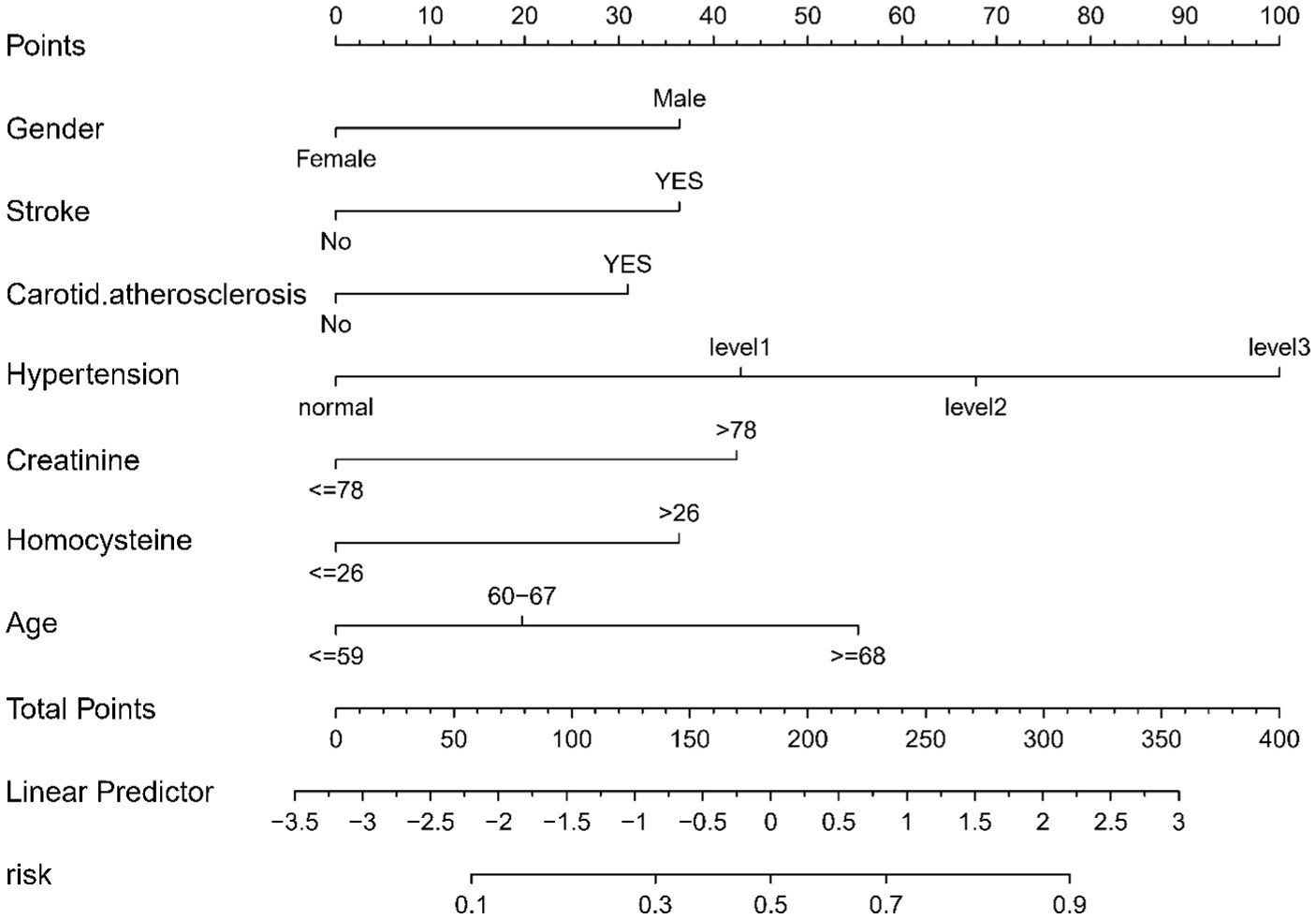
Figure 3. Nomogram for Predicting the Risk of lacune. For all patients, points are calculated based on seven indicators by aligning them with corresponding point scales. The cumulative sum of points is then located on the “Total Points” axis. Subsequently, the risk of lacune, as determined by the nomogram, corresponds to the probability indicated on the “lacune” scale corresponding to the “Total Points”.
The lacunar infarction risk prediction nomogram underwent thorough validation using Receiver Operating Characteristic (ROC) curves, calibration plots, and Decision Curve Analysis (DCA) across both the training and validation cohorts. In the ROC curve analysis, the training set demonstrated an AUC of 0.779 (95% CI: 0.731–0.826), and the validation set an AUC of 0.764 (95% CI: 0.691–0.838), indicating the nomogram’s consistent ability to differentiate risk levels of developing lacunar infarctions. Specifically, in the training set, the sensitivity was 67.0%, and the specificity was 76.6%. In the validation set, the sensitivity further improved to87.8%, while the specificity was53.3% (Figures 4A,B). Calibration analysis revealed an alignment between the predicted and observed outcomes in both cohorts, with intercepts and slopes at 0.000 and 1.000, respectively (Figures 5A,B). The Brier scores were 0.167 for the training set and 0.178 for the validation set, reflecting the model’s accuracy in each dataset. The Decision Curve Analysis (Figures 6A,B) showed the model’s curve notably deviating from the extremes, suggesting its clinical benefit compared to baseline strategies. These results collectively demonstrate the nomogram’s accuracy and reliability in predicting lacunar infarction risk, affirming its potential utility in clinical settings.
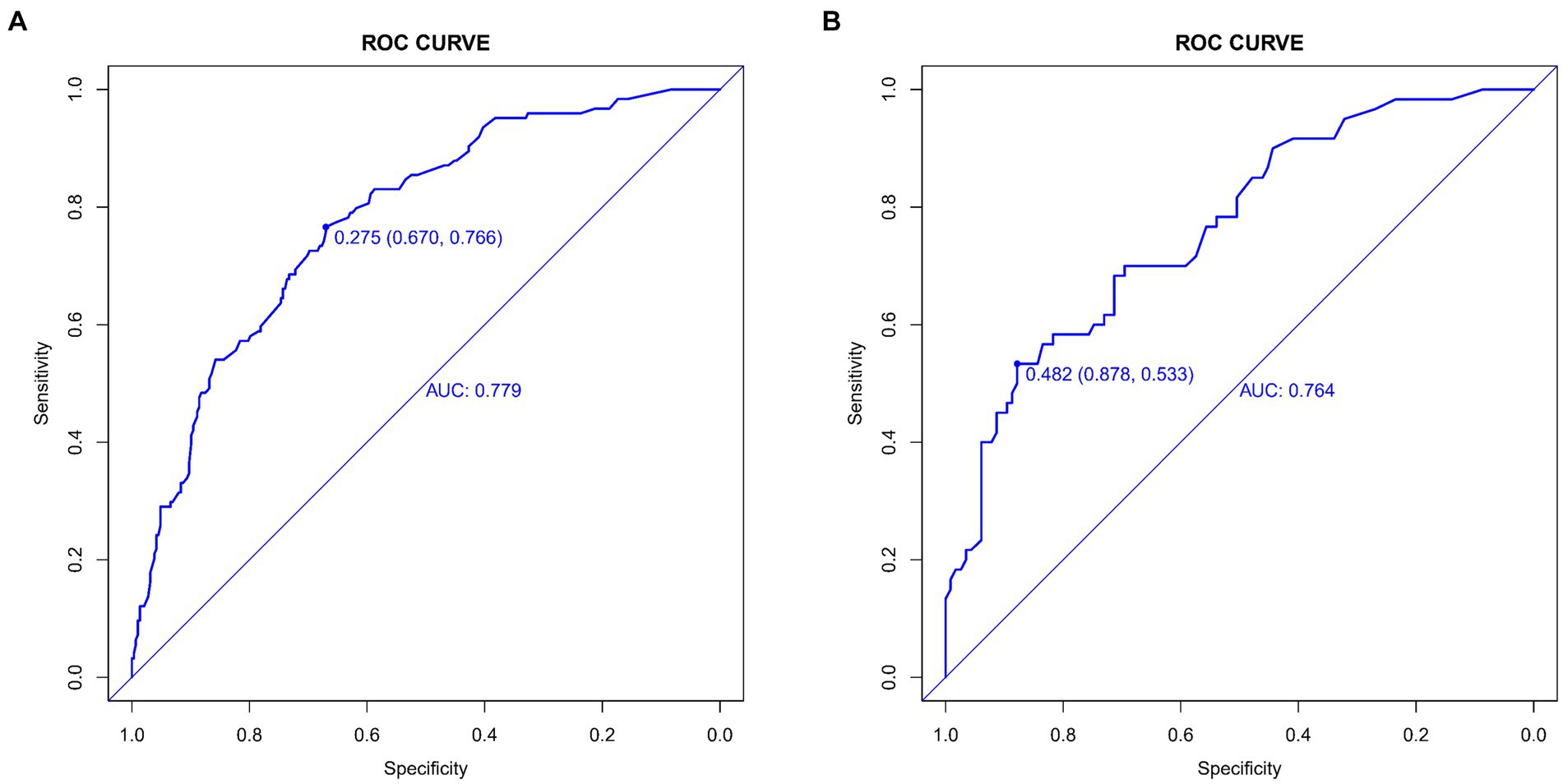
Figure 4. Receiver operating characteristic (ROC) curves for lacune Predictive Model in Training Set (A) and Validation Set (B). The ROC curves plot the sensitivity against the specificity for various threshold levels.
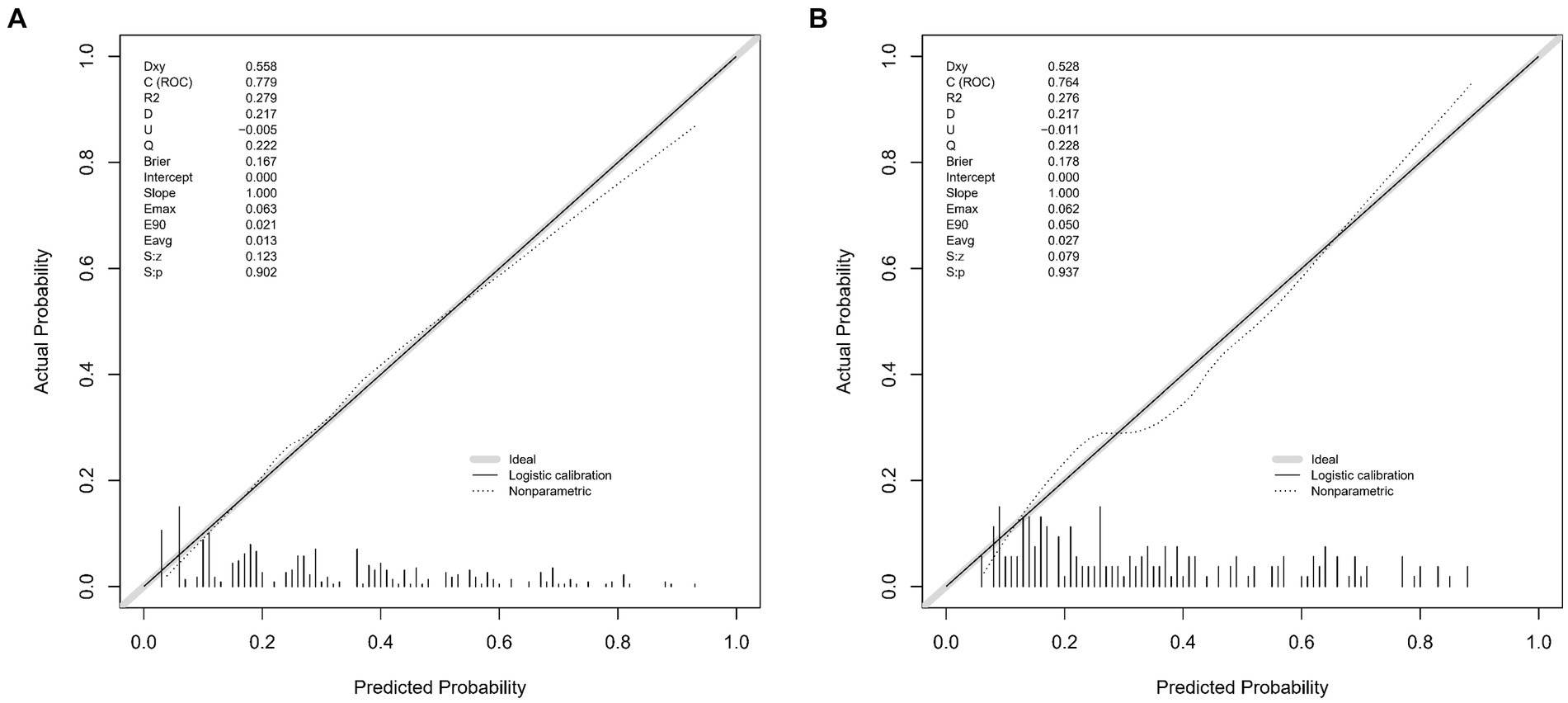
Figure 5. Calibration plots for the lacune predictive model in the training set (A) and validation set (B). The x-axis represents the predicted probability of lacune occurrence, while the y-axis represents the observed frequency of lacunes. The diagonal 45-degree line indicates perfect calibration, where predicted probabilities exactly match the observed outcomes. The dashed line represents the performance of our nomogram, with deviations from the diagonal line illustrating the degree of miscalibration. These plots demonstrate the accuracy and reliability of our predictive model in both the training and validation cohorts, with the calibration curve closer to the diagonal indicating better predictive accuracy.
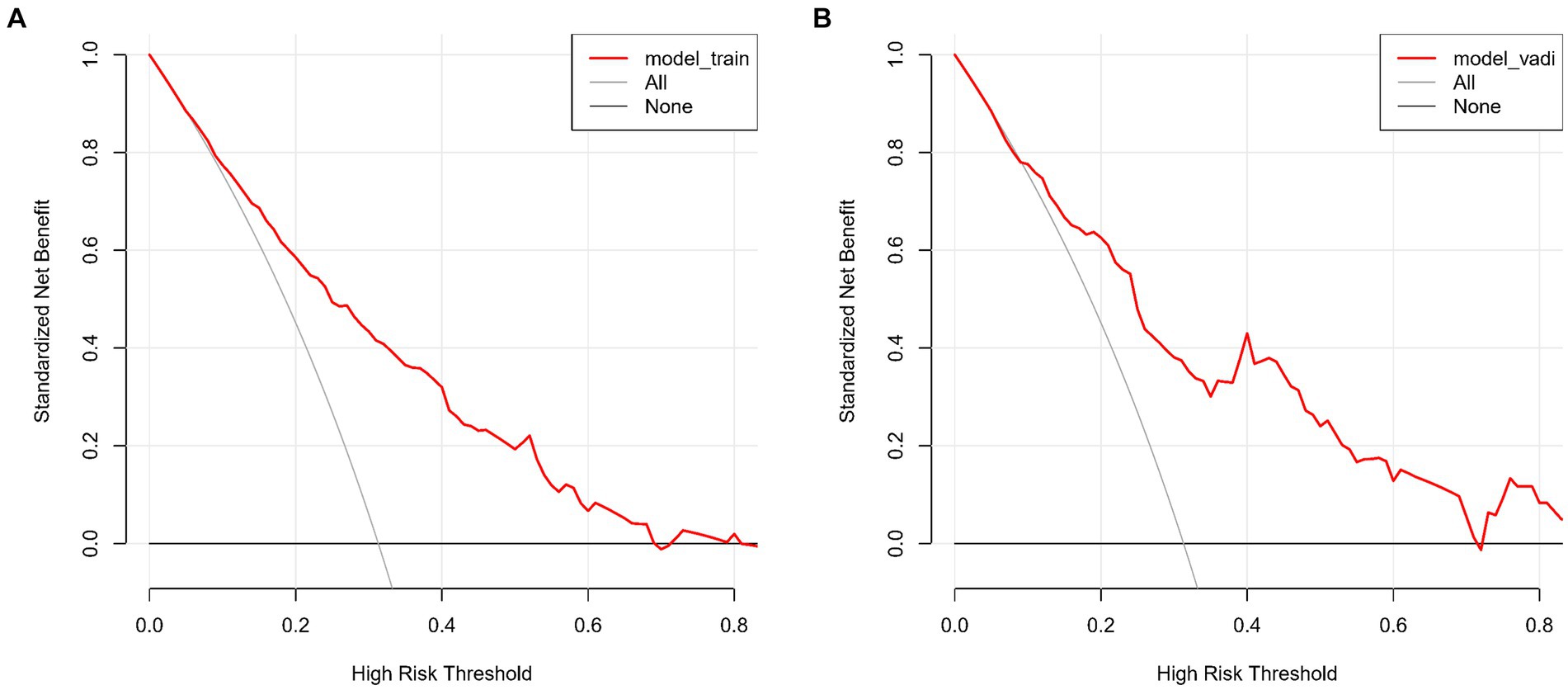
Figure 6. (A,B) show the Decision Curve Analysis (DCA) for our lacune predictive model on both the training (A) and validation sets (B). The graphs illustrate the net benefit across various threshold probabilities of using our model versus either treating all or no patients. The model’s curves demonstrate its clinical value by offering a balanced approach to making treatment decisions, deviating from the extremes represented by the horizontal (no treatment) and oblique (universal treatment) lines.
This study successfully established a clinical and laboratory parameter-based model for predicting lacune, demonstrating robust predictive capabilities across two independent cohorts. By integrating variables such as gender, age, history of stroke, carotid atherosclerosis, hypertension, creatinine, and homocysteine, our model provides a comprehensive risk assessment for the occurrence of lacune. We emphasize the importance of a comprehensive assessment in predicting CSVD, extending beyond the traditionally focused radiological features to include clinical and biochemical markers. Our model underscores the correlation between these parameters and lacune development, offering new insights into the pathophysiology of CSVD. The identification of age and hypertension as predictors corroborates findings by scholars like Cannistraro et al. (2019) and Joutel (2020), who noted hypertension’s significant role in CSVD pathogenesis. Similarly, Pantoni (2010) and Wardlaw et al. (2013) have highlighted the influence of age in CSVD, reinforcing the relevance of these factors in lacune prediction and clinical management.
Elevated creatinine levels were identified as predictors of lacune, aligning with studies by Akoudad et al. (2015) and Xiao et al. (2015), which observed associations between renal impairment and CSVD or lacunar stroke. This suggests the indirect influence of renal function on lacune risk via cerebral vasculature, highlighting the importance of monitoring creatinine levels in lacune risk assessment. Our model also recognizes male gender as a significant predictor, resonating with Jiménez-Sánchez et al. (2021)'s findings on sex differences in CSVD. This suggests a possibly higher risk or distinct pathophysiological responses in males leading to lacune development, indicating the need for gender-specific approaches in lacune management and further investigation into underlying mechanisms. Furthermore, the role of homocysteine as a predictive factor for lacune aligns with the research by Nam et al. (2019), Rutten-Jacobs et al. (2016), and Kloppenborg et al. (2014), reinforcing its potential as a biomarker and its interaction with genetic factors in CSVD and lacune formation. Lastly, our study highlights prior stroke history and carotid atherosclerosis as key predictors for lacunar infarctions, suggesting a link between previous cerebrovascular events and the development of small vessel disease. This highlights the need for comprehensive management of carotid artery disease and vigilant monitoring in patients with a history of stroke, providing novel strategies for lacune prevention.
Our model represents a significant advance in the field, particularly due to the scarcity of similar studies. It enhances predictive accuracy and raises the likelihood of early detection of lacune through integrated analysis, crucial for early intervention and prevention of cerebral small vessel disease progression. However, our model has limitations. The retrospective nature of the study may introduce selection bias. Additionally, being a single-center study might affect the model’s generalizability. Furthermore, the impact of certain variables, such as lifestyle factors and genetic background, may not have been fully captured. Additionally, our nomogram has not yet been validated using other comparable external data sources. This limitation should be addressed in future research by validating the model with multicenter data to ensure its broader applicability. Future research should consider expanding the sample size and incorporating multi-center data to validate the model’s wider applicability. Exploring additional potential predictors, including lifestyle and genetic factors, and the model’s applicability across different ethnic and age groups, is also imperative. Our model can serve as a powerful tool for clinicians in assessing patients’ risk of lacune, especially for those whose radiological findings are inconclusive but clinical and biochemical markers indicate high risk. It could aid in more precise risk assessment and early intervention.
In summary, our predictive model for lacunes, integrating multiple clinical and laboratory parameters, demonstrates notable predictive capabilities. However, it is important to note that the model’s applicability is specific to predicting lacunes in patients undergoing brain MRI for various indications, and not broader manifestations of CSVD. Despite its limitations, such as the retrospective nature and single-center data source, and the lack of validation using external data sources, the model offers a novel perspective in predicting lacunes, contributing valuable insights for future research and clinical practice. Future endeavors should focus on expanding sample sizes, incorporating multi-center studies, and exploring additional influencing factors, to further validate and enhance the model’s utility. This work lays the groundwork for advancing personalized treatment strategies in neurology, particularly for patients at high risk of lacunes whose radiological findings may be inconclusive but clinical and biochemical markers indicate elevated risk.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Ethics Committee of the Affiliated Hospital of Hebei University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
NL: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Y-DH: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. YJ: Writing – review & editing, Writing – original draft, Software, Resources, Investigation, Formal analysis, Data curation, Conceptualization. LL: Writing – original draft. C-HW: Writing – review & editing, Validation, Software, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. J-MS: Writing – review & editing, Supervision, Software, Resources, Methodology, Investigation, Formal analysis, Data curation, Conceptualization. S-BL: Data curation, Conceptualization, Writing – review & editing, Software, Project administration, Methodology, Investigation, Formal Analysis. W-YD: Writing – review & editing, Writing – original draft, Visualization, Validation, Supervision, Software, Resources, Project administration, Formal analysis, Data curation, Conceptualization.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Hospital fund of Affiliated Hospital of Hebei University, Grant/Award Number: 2022QC43; Hebei Medical Science Research Project, Grant/Award Number: 20230200.
The authors thank the staff of the Department of Neurology, The Affiliated Hospital of Hebei University.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Akoudad, S., Sedaghat, S., Hofman, A., Koudstaal, P. J., van der Lugt, A., Ikram, M. A., et al. (2015). Kidney function and cerebral small vessel disease in the general population. Int. J. Stroke 10, 603–608. doi: 10.1111/ijs.12465
Barrio, I., Arostegui, I., Rodríguez-Álvarez, M.-X., and Quintana, J.-M. (2017). A new approach to categorising continuous variables in prediction models: proposal and validation. Stat. Methods Med. Res. 26, 2586–2602. doi: 10.1177/0962280215601873
Bennette, C., and Vickers, A. (2012). Against quantiles: categorization of continuous variables in epidemiologic research, and its discontents. BMC Med. Res. Methodol. 12:21. doi: 10.1186/1471-2288-12-21
Cannistraro, R. J., Badi, M., Eidelman, B. H., Dickson, D. W., Middlebrooks, E. H., and Meschia, J. F. (2019). CNS small vessel disease: a clinical review. Neurology 92, 1146–1156. doi: 10.1212/WNL.0000000000007654
Debette, S., Schilling, S., Duperron, M.-G., Larsson, S. C., and Markus, H. S. (2019). Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and Meta-analysis. JAMA Neurol. 76, 81–94. doi: 10.1001/jamaneurol.2018.3122
Duering, M., Biessels, G. J., Brodtmann, A., Chen, C., Cordonnier, C., de Leeuw, F. E., et al. (2023). Neuroimaging standards for research into small vessel disease-advances since 2013. Lancet Neurol. 22, 602–618. doi: 10.1016/S1474-4422(23)00131-X
Jiménez-Sánchez, L., Hamilton, O. K. L., Clancy, U., Backhouse, E. V., Stewart, C. R., Stringer, M. S., et al. (2021). Sex differences in cerebral small vessel disease: a systematic review and Meta-analysis. Front. Neurol. 12:756887. doi: 10.3389/fneur.2021.756887
Joutel, A. (2020). Prospects for diminishing the impact of nonamyloid small-vessel diseases of the brain. Annu. Rev. Pharmacol. Toxicol. 60, 437–456. doi: 10.1146/annurev-pharmtox-010818-021712
Kloppenborg, R. P., Geerlings, M. I., Visseren, F. L., Mali, W. P. T. M., Vermeulen, M., van der Graaf, Y., et al. (2014). Homocysteine and progression of generalized small-vessel disease: the SMART-MR study. Neurology 82, 777–783. doi: 10.1212/WNL.0000000000000168
Mok, V., and Kim, J. S. (2015). Prevention and Management of Cerebral Small Vessel Disease. J. Stroke 17, 111–122. doi: 10.5853/jos.2015.17.2.111
Nam, K. W., Kwon, H. M., Jeong, H. Y., Park, J. H., Kwon, H., and Jeong, S. M. (2019). Serum homocysteine level is related to cerebral small vessel disease in a healthy population. Neurology 92, e317–e325. doi: 10.1212/WNL.0000000000006816
Pantoni, L. (2010). Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701. doi: 10.1016/S1474-4422(10)70104-6
Rutten-Jacobs, L. C., Traylor, M., Adib-Samii, P., Vincent, T., Cathie, S., Peter, M. R., et al. (2016). Association of MTHFR C677T genotype with ischemic stroke is confined to cerebral small vessel disease subtype. Stroke 47, 646–651. doi: 10.1161/STROKEAHA.115.011545
Vermeer, S. E., Prins, N. D., den Heijer, T., Hofman, A., Koudstaal, P. J., and Breteler, M. M. B. (2003). Silent brain infarcts and the risk of dementia and cognitive decline. N. Engl. J. Med. 348, 1215–1222. doi: 10.1056/NEJMoa022066
Wardlaw, J. M., Smith, E. E., Biessels, G. J., Cordonnier, C., Fazekas, F., Frayne, R., et al. (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838. doi: 10.1016/S1474-4422(13)70124-8
Wardlaw, J. M., Smith, C., and Dichgans, M. (2013). Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 12, 483–497. doi: 10.1016/S1474-4422(13)70060-7
Wardlaw, J. M., Smith, C., and Dichgans, M. (2019). Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 18, 684–696. doi: 10.1016/S1474-4422(19)30079-1
Xiao, L., Lan, W., Sun, W., Dai, Q., Xiong, Y., Li, L., et al. (2015). Chronic kidney disease in patients with lacunar stroke: association with enlarged perivascular spaces and Total magnetic resonance imaging burden of cerebral small vessel disease. Stroke 46, 2081–2086. doi: 10.1161/STROKEAHA.114.008155
Keywords: cerebral small vessel disease (CSVD), lacunes, nomogram, predictive model, clinical and biochemical markers, ROC curve analysis
Citation: Li N, Hu Y-D, Jiang Y, Ling L, Wang C-H, Shao J-M, Li S-B and Di W-Y (2024) Integrating clinical and biochemical markers: a novel nomogram for predicting lacunes in cerebral small vessel disease. Front. Aging Neurosci. 16:1404836. doi: 10.3389/fnagi.2024.1404836
Received: 21 March 2024; Accepted: 07 August 2024;
Published: 23 August 2024.
Edited by:
Marialuisa Zedde, IRCCS Local Health Authority of Reggio Emilia, ItalyReviewed by:
Mustapha Muzaimi, Universiti Sains Malaysia Health Campus, MalaysiaCopyright © 2024 Li, Hu, Jiang, Ling, Wang, Shao, Li and Di. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Ying Di, ZGl3ZWl5aW5naGRmeUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.