
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 21 February 2024
Sec. Neurocognitive Aging and Behavior
Volume 16 - 2024 | https://doi.org/10.3389/fnagi.2024.1356791
Introduction: Estradiol is a sex steroid hormone, which has been implicated in the pathogenesis of Alzheimer’s disease and cognitive impairment. This cross-sectional study aimed to examine the relationship between serum estradiol levels and cognitive performance in older American women.
Methods: Data were obtained from the National Health and Nutrition Examination Survey 2013–2014. A total of 731 women aged ≥60 years who met the inclusion criteria were included in this study. Serum estradiol levels were measured using the isotope dilution liquid chromatography tandem mass spectrometry (ID-LC–MS/MS) method developed by the Centers for Disease Control and Prevention for routine analysis. All measured serum levels were further divided into three parts: T1, <3.68 pg./mL; T2, 3.68–7.49 pg./mL; T3, >7.49 pg./mL, and analyzed. Participants’ cognitive abilities were tested using the Vocabulary Learning Subtest (CERAD), Animal Fluency Test (AFS), and digital symbol substitution test (DSST). Scores for each test were calculated based on the sample mean and standard deviation (SD). To examine the relationship between serum estradiol level tertiles and cognitive scores, multiple linear regression models were developed, controlling for race/ethnicity, education level, hypertension, diabetes, and insomnia.
Results: The mean age of the participants was 69.57 ± 6.68 years. The non-Hispanic whites were 78.95%, and those who had completed at least some college-level education were 60.62%. The mean BMI of the participants was 29.30 ± 6.79, and 10.85% had a history of smoking. Further, 73.41% did not have a history of alcohol consumption, and 63.03% had hypertension (63.03%). In addition, 81.81 and 88.3% did not have a history of diabetes mellitus and did not have sleep disorders, respectively. The mean serum estradiol level was 8.48 ± 0.77 pg./mL. Multivariate linear regression of the reference group consisting of participants in tertiles of serum estradiol levels revealed that one unit increase in serum estradiol levels increased DSST scores by 0.61 (0.87, 6.34) in the T3 group. However, no significant correlation was found in the CERAD and AFS tests.
Conclusion: Participants with higher estradiol levels had higher DSST scores and better processing speed, sustained attention, and working memory, suggesting that serum estradiol may serve as a biomarker for cognitive decline in older women.
Cognitive impairment is the most common neurodegenerative alteration in older adults (Islam, 2017). Its prominent clinical manifestation is a decline in memory, attention, language, and visuospatial abilities. Currently, >50 million people worldwide suffer from cognitive impairment, the majority of whom are older adults (Feigin et al., 2019). Age-related cognitive decline can serve as an early indicator of dementia, a condition that affects 5.1 million individuals in the United States. Notably, the prevalence of dementia is projected to increase two-fold by 2050 (Hebert et al., 2013). Further, the worldwide economic burden of dementia is expected to increase to USD 2.54 trillion by 2030 and approximately USD 1 trillion by 2050, which was USD 957.56 billion in 2015 (Jia et al., 2018). Cognitive impairment is linked to hereditary factors, dietary issues, mitochondrial malfunction, oxidative stress, and aging (Tobore, 2019). Cognitive impairment significantly diminishes the overall quality of life and imposes a substantial burden on society. Consequently, investigating the underlying variables that contribute to dementia is crucial to developing effective strategies for its prevention and treatment.
Estradiol, an estrogen synthesized by the ovaries and adrenal glands, plays a crucial role in the development of female sexual characteristics and fertility. Additionally, it can penetrate the blood–brain barrier and influence the brain (Brann et al., 2022). Estrogen is a steroid hormone and exists in three forms: estrone (E1), estradiol (E2), and estriol (E3). Among these, estradiol is the most prevalent in the human body. Estradiol plays a crucial role in controlling various physiological and pathological processes, including reproduction, sexual development, cancer pathogenesis, cognitive function, and neuroprotection (Azcoitia et al., 2018; Brocca and Garcia-Segura, 2019; Sahab-Negah et al., 2020; Brann et al., 2022). Although fluctuations in E2 levels during the normal menstrual cycle have no effect on women’s overall cognitive performance, the types of cognitive performance that women specialize in vary at different times during the menstrual cycle. During the pre-follicular phase (low estradiol levels), women have relatively enhanced spatial abilities, whereas women in the late follicular or mid-luteal phases (high estradiol levels) have relatively strong verbal fluency and memory abilities (Sundström Poromaa and Gingnell, 2014). Estradiol levels decline substantially during menopause and continue to decline annually with age (Rannevik et al., 1995). Decreased estradiol levels in older women affect memory, with significant reductions in both word recall and fluency (Weber et al., 2014), as well as in the ability to attend to objects and working memory (Schaafsma et al., 2010; Weber et al., 2014). As estrogen levels decline dramatically after menopause, women are more likely to develop Alzheimer’s disease and experience cognitive decline (Ryan et al., 2014; Pike, 2017; Boyle et al., 2021). Estrogen supplementation improves dementia symptoms (Wu et al., 2020). Nevertheless, some studies have demonstrated inconclusive findings, as changes in estrogen levels do not exhibit a significant correlation with immediate memory, delayed recall, language acquisition, or verbal fluency (Mihalj et al., 2014). Owing to the conflicting findings, we conducted an in-depth investigation into the precise impact of estradiol on cognitive performance in older women. We hypothesized that higher estradiol levels would be beneficial for cognitive functioning.
We examined a sample of older women, specifically those aged ≥60 years, representative of the entire nation. This study was conducted as part of the National Health and Nutrition Examination Survey (NHANES) to explore the correlation between serum estradiol levels and cognitive function.
We retrieved publicly available data on female participants aged ≥60 years from a survey cycle of the 2013–2014 NHANES, conducted by the Centers for Disease Control and Prevention (CDC) and the National Center for Health Statistics (NCHS), to monitor the health status of the population in the United States. The NHANES protocol was approved by the NCHS Research Ethics Review Board, and all participants provided written informed consent. A cutoff age of ≥60 years was set based on the availability of serum estradiol concentration data. The exclusion criteria were male participants, participants aged <60 years, and those with missing estradiol data.
A total of 10,175 individuals were included in the study, and we limited our analysis to 967 females aged ≥60 years. Further, we excluded participants with true estradiol levels and incomplete cognitive assessment data (n = 236). Finally, 731 females were included in the study (Figure 1).
The 2013–2014 NHANES Cognitive Function Data File contains three specific tests: the Alzheimer’s Disease Registry Association Vocabulary Learning Subtest (CERAD), Animal Fluency Test (AFS), and Digit Symbol Substitution Test (DSST). Although cognitive tests cannot replace diagnoses made through clinical examinations, they are employed in large-scale screening and epidemiological investigations (Fillenbaum et al., 2008; Gao et al., 2009).
The CERAD test assesses immediate and delayed learning of linguistic information (memory subdomains) (Morris et al., 1994), with word recall delays following the completion of the other two cognitive exercises (AFS and DSST) (approximately 8–10 min from the beginning of the word learning trials). Scores after recall delays were used as the results of the CERAD test in this study.
AFS assesses explicit language category fluency, which is a constituent of executive function (Strauss et al., 2006), along with other functions, including semantic memory and processing speed (Clark et al., 2009). Participants were asked to name as many animals as possible in 1 min and assign each animal a score.
The DSST is a component of the Wechsler Adult Intelligence Scale (WAIS III), which assesses processing speed, sustained attention, and working memory (Wechsler, 1997). Higher scores on each examination indicate a higher level of cognitive functionality in individuals. This examination was conducted using a physical document comprising a set of nine numbers accompanied by distinct symbols positioned at the uppermost section of the form. Participants were asked to replicate the relevant symbols found in the 133 boxes located next to the numbers in 2 min.
For more information on scoring, see the 1999–2000 NHANES CFQ Questionnaire Data File document at https://wwwn.cdc.gov/Nchs/Nhanes/1999-2000/CFQ.htm.
Every participant underwent venipuncture, which involved drawing blood in the morning after an overnight fast. Serum samples were processed, stored, and sent to the National Center for Environmental Health, CDC, Division of Laboratory Sciences in Atlanta, Georgia, for examination. An isotope dilution liquid chromatography tandem mass spectrometry (ID-LC–MS/MS) method developed by the CDC was used to perform routine serum estradiol assays. Thereafter, the serum levels were divided into three equal halves: T1, estradiol level < 3.68 pg./mL; T2, estradiol level 3.68–7.49 pg./mL; T3: estradiol level > 7.49 pg./mL, and evaluated.
Data on covariates pertaining to estradiol and cognitive performance were collected, encompassing demographic factors including age, race, and education level. The questionnaire results provided a comprehensive range of demographic information that was subsequently transformed into relevant categorical variables. Smoking, alcohol consumption, BMI, diabetes, hypertension, and sleep disorders were identified as potential confounding factors.
The surveys relied on accurate weights for intricate surveys directly supplied by the NHANES. Continuous variables were presented as the mean ± standard deviation (SD), whereas categorical variables were presented as totals and percentages (%). The chi-square test was used to analyze categorical variables. Initially, the normality of continuous variables was assessed. A one-way analysis of variance (ANOVA) was performed for normally distributed data, whereas the Kruskal–Wallis test (a nonparametric ANOVA test) was performed for a non-normal distribution. This study evaluated the relationship between estradiol levels and cognitive function using both unadjusted and multifactor-adjusted models through generalized logistic regression. To investigate and comprehend the intricate correlation between estradiol and cognitive function, the continuous variables were transformed into categorical variables: estradiol levels <3.68 pg./mL, 3.68–7.49 pg./mL, and > 7.49 pg./mL. Multivariate models were then adjusted to account for age, ethnicity, education, BMI, smoking status, alcohol consumption, and comorbidities such as hypertension and diabetes. Statistical significance was set at p < 0.05. All statistical analyses were performed using the R statistical package (version 3.5.3) and EmpowerStats.
The study comprised 731 females aged ≥60 years, with a mean age of 69.57 ± 6.68. We observed a negative correlation between age and estradiol levels, indicating that estradiol levels decrease with age. This information is presented in Table 1. Non-Hispanic whites comprised the highest proportion of participants with varying estradiol levels. Further, serum estrogen levels exhibited an inverse correlation with BMI. A strong positive correlation was observed between blood estradiol levels and the number of patients with diabetes. Nevertheless, no notable differences were observed for education level, smoking, alcohol consumption, hypertension, or sleep disorders. In the population baseline table, no significant difference was observed (p > 0.05) between the CERAD and AFS tests and serum estradiol concentrations. However, a significant difference was observed between DSST scores and serum estradiol concentrations. The higher the estradiol concentration, the higher the DSST score (p < 0.05).
Table 2 shows the outcomes of the multivariate regression analysis of serum estradiol levels and cognitive performance. We employed three multivariate logistic regression models to demonstrate the correlation between serum estradiol levels and cognitive performance. Model 1 is the unadjusted model; that is, it does not consider any covariates. Model 2 is adjusted for age and ethnicity. Model 3 is adjusted for age, ethnicity, educational level, BMI, hypertension, diabetes mellitus, alcohol consumption, cigarette smoking, and sleep disorders. We found no significant correlation between estradiol levels and cognitive function tests for the CERAD and AFS in older women. However, a substantial positive association was observed between estradiol levels and DSST scores. After controlling for several factors, such as age, race, sex, education level, hypertension, body mass index, smoking, stroke, alcohol consumption, and diabetes, the observed correlation remained stable across all multivariate logistic regression models. In the DSST, a significant increase was observed in the DSST score of 0.61 (0.87, 6.34), p = 0.0099, for every incremental unit of serum estradiol (pg/mL) in the T3 group compared with the T1 group, after adjusting for confounding factors.
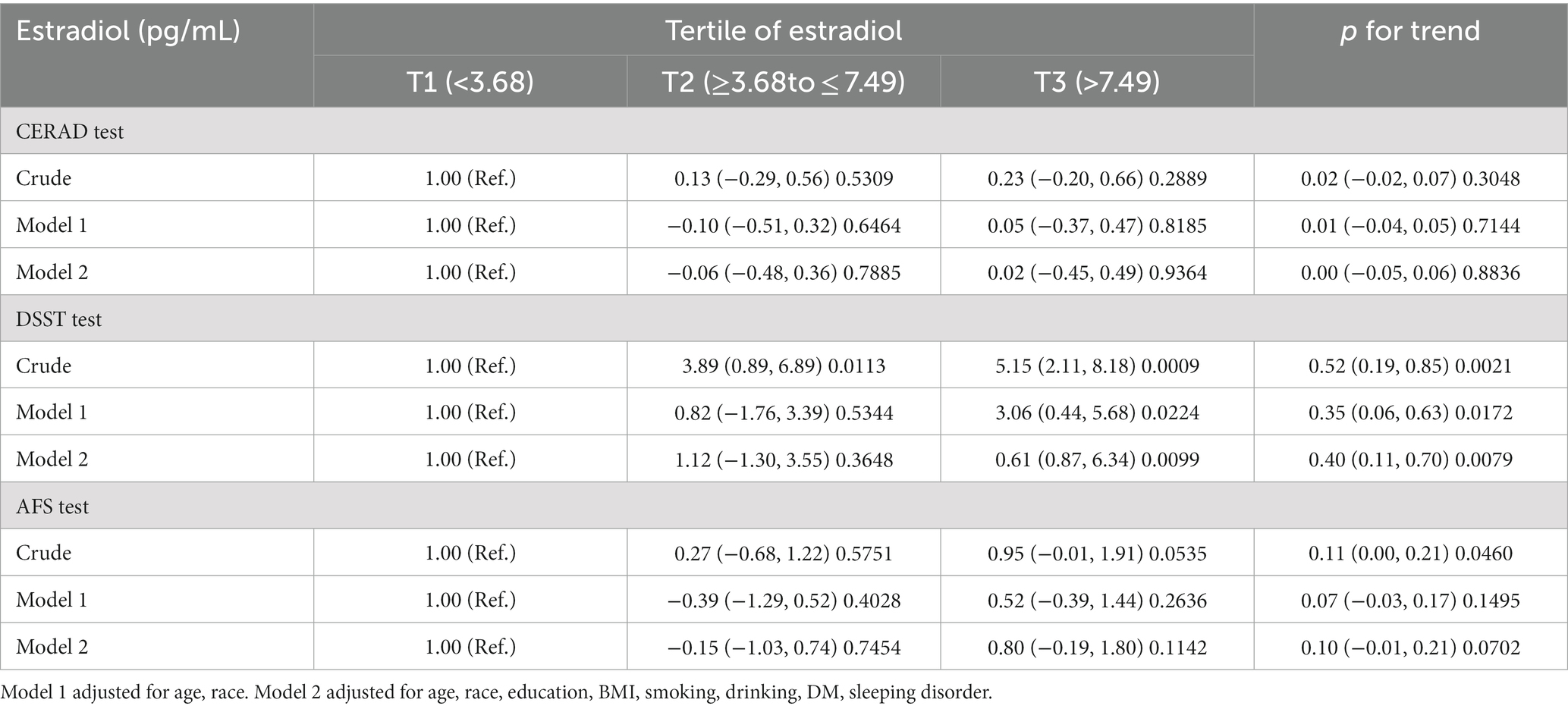
Table 2. Associations between tertile of estradiol (reference: <3.68 pg./mL) and CREAD/DSST/AFS test score, β (95%CI) p-value.
Table 1 shows that BMI and diabetes were significantly associated with serum estradiol levels. We further analyzed the hierarchical relationship between serum estradiol levels and cognitive function for different BMI levels and diabetes history subgroups (Tables 3, 4). Hierarchical analysis showed that participants with no history of diabetes scored higher in the cognitive function tests (CERAD, AFS, and DSST) than those with diabetes when one unit of serum estradiol was added. We divided the participants into non-overweight and overweight groups based on their BMI. Compared with overweight participants, non-overweight participants had higher DSST scores for every one-unit increase in serum estradiol levels, but this was not observed in the CERAD and AFS tests. Further, no statistically significant differences were observed in the interaction tests for these hierarchical relationships (p > 0.05).
Our findings reveal that participants with elevated estradiol levels demonstrated superior performance in the DSST, as well as enhanced processing speed, sustained attention, and working memory. However, no notable differences were observed in the AFS and CERAD test results. These findings indicate that serum estradiol levels may serve as a biomarker of cognitive decline in older individuals.
As estradiol affects women’s cognitive function at different stages of the menstrual cycle, higher estradiol levels can enhance memory in women (Sundström Poromaa and Gingnell, 2014). Notably, the DSST mainly involves replicating the symbols adjacent to numbers, thereby assessing the memory of an individual. Nevertheless, no notable differences in the AFS and CERAD tests may be attributed to the small sample size, and larger sample surveys in the future may have different outcomes.
The precise molecular mechanisms underlying the association between estradiol levels and cognitive function are currently being studied. Estradiol enhances neurotransmitter metabolism, production of neurotrophins, and formation of synapses in the hippocampus and prefrontal cortex. These brain regions are closely associated with memory consolidation and enhanced cognitive function (Luine, 2014). Cognitive dysfunction may occur due to reduced acetylcholine and its receptor levels, whereas estrogen enhances acetylcholine synthesis and mitigates cholinergic neuronal damage (Rabbani et al., 1997). Estrogens exert neuroprotective effects by mitigating oxidative stress, scavenging oxygen-free radicals, and diminishing and postponing neuronal aging (Chakrabarti et al., 2016). miR-125b, a highly prevalent miRNA in the brain, provides protection against cortical neuronal toxicity caused by Aβ (β-amyloid). Additionally, estradiol enhances miR-125b expression, which in turn inhibits genes that promote cell death, thereby reducing brain neuronal toxicity (Micheli et al., 2016; Amakiri et al., 2019). Notably, estradiol plays a crucial role in controlling the growth and survival of cells in the hippocampus, which is responsible for memory and learning (Duarte-Guterman et al., 2015). The hippocampus is more sensitive to changes in estradiol levels than other brain regions (Pletzer et al., 2018). Fluctuations in E2 levels during the female sexual cycle cause dynamic changes in hippocampal volume and dendritic spine density (Protopopescu et al., 2008; Pletzer et al., 2018). However, whether these changes are beneficial for DSST remains unclear and warrants further investigation.
In addition to estrogen, other sex hormones can also affect cognitive function. A population-based study conducted on androgens suggested that older men with higher testosterone levels demonstrated better performance in multiple cognitive function tests (Hsu et al., 2015). Further, no significant association was observed between changes in androgen levels and cognitive function in a 23-year-long prospective cohort study of 3,044 women (Koyama et al., 2016), and no significant correlation was observed between total testosterone levels and cognitive performance in later life. Longitudinal data from 4,110 study participants (Kische et al., 2017) showed that serum testosterone levels were not associated with cognitive function in older women. Overall, testosterone levels in women are much lower than those in men, and even during menopause, fluctuations in testosterone levels are relatively small, which may explain the above findings. Notably, hormone levels greatly fluctuate in perimenopausal women, and memory problems are also one of the most common complaints among menopausal women. Early supplementation of progesterone after menopause can improve speech memory and other functions in women (Alhola et al., 2010); however, the impact of progesterone on cognitive function in women >60 years of age remains unclear.
This study has several strengths. The study population consisted of older individuals who are representative of the entire nation, thereby increasing the applicability of our results. Furthermore, this research targeted a specific population—older women—with heightened susceptibility to cognitive decline. Nevertheless, this study also has some limitations. First, the cross-sectional design of this study is the primary limitation. Consequently, we could not establish a cause-and-effect relationship or observe any variation in estradiol levels or cognitive performance in the older women throughout the study period. Second, confounding factors, such as depressive symptoms, which may have influenced our study results, were not included in the analyses. Notably, depressive symptoms were not documented in the 2013–2014 NHANES. Third, as these data were gathered during 2013–2014, they may no longer be relevant and may not accurately represent the current estradiol levels and cognitive performance in older individuals. Finally, the NHANES survey may lack a comprehensive representation of specific categories, including rural communities, homeless individuals, and non-native English speakers. Subsequent investigations must incorporate a longitudinal framework to scrutinize the correlation between estradiol levels and cognitive function in older women, particularly in non-Western nations.
In summary, our results revealed a significant correlation between estradiol levels and cognitive function in older women. Our study indicates that estradiol levels could potentially be used as a biomarker to measure cognitive function deterioration in older individuals.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the NHANES has been approved by the National Center for Health Statistics Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
QX: Formal analysis, Investigation, Writing – original draft, Writing – review & editing. MJ: Conceptualization, Investigation, Methodology, Software, Writing – original draft. SH: Data curation, Formal analysis, Validation, Writing – review & editing. WG: Data curation, Project administration, Supervision, Validation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alhola, P., Tuomisto, H., Saarinen, R., Portin, R., Kalleinen, N., and Polo-Kantola, P. (2010). Estrogen + progestin therapy and cognition: a randomized placebo-controlled double-blind study. J. Obstet. Gynaecol. Res. 36, 796–802. doi: 10.1111/j.1447-0756.2010.01214.x
Amakiri, N., Kubosumi, A., Tran, J., and Reddy, P. H. (2019). Amyloid Beta and MicroRNAs in Alzheimer’s disease. Front. Neurosci. 13:430. doi: 10.3389/fnins.2019.00430
Azcoitia, I., Arevalo, M. A., and Garcia-Segura, L. M. (2018). Neural-derived estradiol regulates brain plasticity. J. Chem. Neuroanat. 89, 53–59. doi: 10.1016/j.jchemneu.2017.04.004
Boyle, C. P., Raji, C. A., Erickson, K. I., Lopez, O. L., Becker, J. T., Gach, H. M., et al. (2021). Estrogen, brain structure, and cognition in p OSTMENOPAUSAL women. Hum. Brain Mapp. 42, 24–35. doi: 10.1002/hbm.25200
Brann, D. W., Lu, Y., Wang, J., Zhang, Q., Thakkar, R., Sareddy, G. R., et al. (2022). Brain-derived estrogen and neural function. Neurosci. Biobehav. Rev. 132, 793–817. doi: 10.1016/j.neubiorev.2021.11.014
Brocca, M. E., and Garcia-Segura, L. M. (2019). Non-reproductive functions of aromatase in the central nervous system under physiological and pathological conditions. Cell. Mol. Neurobiol. 39, 473–481. doi: 10.1007/s10571-018-0607-4
Chakrabarti, M., Das, A., Samantaray, S., Smith, J. A., Banik, N. L., Haque, A., et al. (2016). Molecular mechanisms of estrogen for neuroprotection in spinal cord injury and traumatic brain injury. Rev. Neurosci. 27, 271–281. doi: 10.1515/revneuro-2015-0032
Clark, L. J., Gatz, M., Zheng, L., Chen, Y.-L., McCleary, C., and Mack, W. J. (2009). Longitudinal verbal fluency in Normal aging, preclinical, and prevalent Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 24, 461–468. doi: 10.1177/1533317509345154
Duarte-Guterman, P., Lieblich, S. E., Chow, C., and Galea, L. A. M. (2015). Estradiol and GPER activation differentially affect cell proliferation but not GPER expression in the Hippocampus of adult female rats. PLoS One 10:e0129880. doi: 10.1371/journal.pone.0129880
Feigin, V. L., Nichols, E., Alam, T., Bannick, M. S., Beghi, E., Blake, N., et al. (2019). Global, regional, and national burden of neurological disorders, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol 18, 459–480. doi: 10.1016/S1474-4422(18)30499-X
Fillenbaum, G. G., Van Belle, G., Morris, J. C., Mohs, R. C., Mirra, S. S., Davis, P. C., et al. (2008). Consortium to establish a registry for Alzheimer’s disease (CERAD): the first twenty years. Alzheimers Dement. 4, 96–109. doi: 10.1016/j.jalz.2007.08.005
Gao, S., Jin, Y., Unverzagt, F. W., Liang, C., Hall, K. S., Ma, F., et al. (2009). Hypertension and cognitive decline in rural elderly Chinese. J. Am. Geriatr. Soc. 57, 1051–1057. doi: 10.1111/j.1532-5415.2009.02267.x
Hebert, L. E., Weuve, J., Scherr, P. A., and Evans, D. A. (2013). Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 80, 1778–1783. doi: 10.1212/WNL.0b013e31828726f5
Hsu, B., Cumming, R. G., Waite, L. M., Blyth, F. M., Naganathan, V., Le Couteur, D. G., et al. (2015). Longitudinal relationships between reproductive hormones and cognitive decline in older men: the Concord health and ageing in men project. J. Clin. Endocrinol. Metabol. 100, 2223–2230. doi: 10.1210/jc.2015-1016
Islam, M. T. (2017). Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 39, 73–82. doi: 10.1080/01616412.2016.1251711
Jia, J., Wei, C., Chen, S., Li, F., Tang, Y., Qin, W., et al. (2018). The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimers Dement. 14, 483–491. doi: 10.1016/j.jalz.2017.12.006
Kische, H., Gross, S., Wallaschofski, H., Grabe, H. J., Völzke, H., Nauck, M., et al. (2017). Associations of androgens with depressive symptoms and cognitive status in the general population. PLoS One 12:e0177272. doi: 10.1371/journal.pone.0177272
Koyama, A. K., Tworoger, S. S., Eliassen, A. H., Okereke, O. I., Weisskopf, M. G., Rosner, B., et al. (2016). Endogenous sex hormones and cognitive function in older women. Alzheimers Dement. 12, 758–765. doi: 10.1016/j.jalz.2015.12.010
Luine, V. N. (2014). Estradiol and cognitive function: past, present and future. Horm. Behav. 66, 602–618. doi: 10.1016/j.yhbeh.2014.08.011
Micheli, F., Palermo, R., Talora, C., Ferretti, E., Vacca, A., and Napolitano, M. (2016). Regulation of proapoptotic proteins Bak1 and p53 by miR-125b in an experimental model of Alzheimer’s disease: protective role of 17β-estradiol. Neurosci. Lett. 629, 234–240. doi: 10.1016/j.neulet.2016.05.049
Mihalj, M., Drenjancevic, I., Sumanovac, A. V. A., Cavka, A., and Gmajnic, V. R. (2014). Basic cognitive functions across the menstrual cycle in a con-trolled female cohort. Med. Glas. 11, 177–185.
Morris, J. C., Clark, C. M., Kukull, W., and Heyman, A. (1994). The consortium to establish a registry for Alzheimer’s disease (CERAD): part VI. Family history assessment: a multicenter study of first-degree relatives of Alzheimer’s disease probands and nondemented spouse controls. Neurology 44:1253. doi: 10.1212/WNL.44.7.1253
Pike, C. J. (2017). Sex and the development of Alzheimer’s disease. J. Neurosci. Res. 95, 671–680. doi: 10.1002/jnr.23827
Pletzer, B., Harris, T., and Hidalgo-Lopez, E. (2018). Subcortical structural changes along the menstrual cycle: beyond the hippocampus. Sci. Rep. 8:16042. doi: 10.1038/s41598-018-34247-4
Protopopescu, X., Butler, T., Pan, H., Root, J., Altemus, M., Polanecsky, M., et al. (2008). Hippocampal structural changes across the menstrual cycle. Hippocampus 18, 985–988. doi: 10.1002/hipo.20468
Rabbani, O., Panickar, K. S., Rajakumar, G., King, M. A., Bodor, N., Meyer, E. M., et al. (1997). 17 b-estradiol attenuates Fimbrial lesion-induced decline of ChAT-Immunoreactive neurons in the rat medial septum. Exp. Neurol. 146, 179–186. doi: 10.1006/exnr.1997.6516
Rannevik, G., Jeppsson, S., Johnell, O., Bjerre, B., Laurell-Borulf, Y., and Svanberg, L. (1995). A longitudinal study of the perimenopausal transition: altered profiles of steroid and pituitary hormones, SHBG and bone mineral density. Maturitas 21, 103–113. doi: 10.1016/0378-5122(94)00869-9
Ryan, J., Carrière, I., Carcaillon, L., Dartigues, J., Auriacombe, S., Rouaud, O., et al. (2014). Estrogen receptor polymorphisms and incident dementia: the prospective 3C study. Alzheimers Dement. 10, 27–35. doi: 10.1016/j.jalz.2012.12.008
Sahab-Negah, S., Hajali, V., Moradi, H. R., and Gorji, A. (2020). The impact of estradiol on neurogenesis and cognitive functions in Alzheimer’s disease. Cell. Mol. Neurobiol. 40, 283–299. doi: 10.1007/s10571-019-00733-0
Schaafsma, M., Homewood, J., and Taylor, A. (2010). Subjective cognitive complaints at menopause associated with declines in performance of verbal memory and attentional processes. Climacteric 13, 84–98. doi: 10.3109/13697130903009187
Strauss, E., Sherman, E. M. S., and Spreen, O. (2006). A compendium of neuropsychological tests: Administration, norms and commentary. Oxford University Press. Oxford
Sundström Poromaa, I., and Gingnell, M. (2014). Menstrual cycle influence on cognitive function and emotion processing-from a reproductive perspective. Front. Neurosci. 8:380. doi: 10.3389/fnins.2014.00380
Tobore, T. O. (2019). On the Etiopathogenesis and pathophysiology of Alzheimer’s disease: a comprehensive theoretical review. J. Alzheimers Dis. 68, 417–437. doi: 10.3233/JAD-181052
Weber, M. T., Maki, P. M., and McDermott, M. P. (2014). Cognition and mood in perimenopause: a systematic review and meta-analysis. J. Steroid Biochem. Mol. Biol. 142, 90–98. doi: 10.1016/j.jsbmb.2013.06.001
Keywords: biomarker, estradiol, cognitive function, gerontology, national survey
Citation: Xu Q, Ji M, Huang S and Guo W (2024) Association between serum estradiol levels and cognitive function in older women: a cross-sectional analysis. Front. Aging Neurosci. 16:1356791. doi: 10.3389/fnagi.2024.1356791
Received: 16 December 2023; Accepted: 07 February 2024;
Published: 21 February 2024.
Edited by:
Nadine Correia Santos, University of Minho, PortugalReviewed by:
Alicia A. Walf, Rensselaer Polytechnic Institute, United StatesCopyright © 2024 Xu, Ji, Huang and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weifeng Guo, Z3dmd2ZnMjAwM0BuanVjbS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.