- Department of Neurology, University Medical Center, Georg August University of Göttingen, Göttingen, Germany
Introduction: With aging, dual task (DT) ability declines and is more cognitively demanding than single tasks. Rapidly declining DT performance is regarded as a predictor of neurodegenerative disease. Task training and non-invasive transcranial electrical stimulation (tES) are methods applied to optimize the DT ability of the elderly.
Methods: A systematic search was carried out in the PUBMED, TDCS (transcranial direct current stimulation) databases, as well as Web of Science, and a qualitative analysis was conducted in 56 included studies. Aiming to summarize the results of studies that implemented tES, task training, or the combination for improving DT ability and related performance changes in healthy elderly and geriatric patients. For different approaches, the training procedures, parameters, as well as outcomes were discussed.
Results: Task training, particularly cognitive-motor DT training, has more notable effects on improving DT performance in the elderly when compared to the neuromodulation method.
Discussion: Anodal transcranial direct current stimulation (tDCS) over the left dorsolateral prefrontal cortex (L-DLPFC), or its combination with task training could be promising tools. However, additional evidence is required from aged healthy people and patients, as well as further exploration of electrode montage.
Introduction
Walking while answering the phone, talking while preparing a meal, or texting on the bus while maintaining a standing balance, dual task (DT) happens frequently in day-to-day life. Compared with performing one task in isolation, carrying out concurrently two tasks can deteriorate mutual performance, as the cognitive system has a limited capacity for attending to several attention-demanding channels simultaneously (Navon and Gopher, 1979).
Potential factors affecting DT capacity in old adults were discussed in previous studies. It has been identified that attention as well as execution factors are the most critical predictors of DT performance, and aging-induced decline in these factors is closely related to the increased risk of falls and impaired cognition in the elderly (Holtzer et al., 2005; Ali et al., 2013; Harada et al., 2013; Sugata et al., 2018). Besides, previous study reported larger impairment in DT performance was observed in dementia patients when compared to single task (ST) performance, suggesting that DT deficit is a highly specific and sensitive indicator of cognitive decline, and can be regarded as a significant predictor of neurodegenerative diseases including Parkinson’s disease (PD) and Alzheimer’s disease (Sala and Logie, 2001; Schwenk et al., 2010; Montero-Odasso et al., 2017; Raichlen et al., 2020). The prefrontal cortex, which is associated with cognitive and motor function, has demonstrated greater activation during DT gait/balance in the healthy elderly when compared with healthy young adults (Kahya et al., 2019). The dorsolateral prefrontal cortex (DLPFC) plays a key role in cognition and executive control functions, e.g., working memory, inhibition, and task switching (Badre and Wagner, 2004; Brzezicka et al., 2019; Hertrich et al., 2021).
To optimize the DT capacity, different approaches were proposed, which can be summarized into two categories: (1) neuromodulation by applying low-intensity transcranial electrical stimulation (tES) and (2) skills acquisition based on task training.
TES is a safe neurophysiological method, which can regulate cortical excitability by altering the membrane potential and neural synchronization in a non-invasive manner (Bikson et al., 2016; Miyaguchi et al., 2020). It mainly includes transcranial direct current stimulation (tDCS) and transcranial alternating current stimulation (tACS). The former can modulate the membrane potential of neurons and therefore alter the spontaneous firing rates of neurons (Antal and Paulus, 2008). Anodal tDCS over the primary motor cortex (M1) tends to increase cortical excitability, while cathodal tDCS over M1 tends to decrease the excitability (Nitsche and Paulus, 2000). However, the net consequences of the stimulation depend on multiple factors including the state of the brain, the number of stimulation sessions, the stimulation duration, and the stimulation intensity (Bikson et al., 2019). Previous studies demonstrated that 20 min of anodal tDCS over the M1 at 2 mA reduced lower back pain (Jiang et al., 2020), while tDCS with the same parameters over the left DLPFC (L-DLPFC) enhanced working memory in old adults (Satorres et al., 2022). On the other hand, TACS modulates brain oscillations and is primarily used for improving cognitive function, and the effect is mainly affected by the frequency (Antal et al., 2008; Antal and Paulus, 2013). TACS over the DLPFC delivered at a gamma frequency has been shown to enhances working memory in healthy adults (Hoy et al., 2015) and executive function in mild cognitive impairment (MCI) patients (Kim et al., 2021).
The effectiveness of physical training in improving walking ability and balance has also been demonstrated in the elderly (Varela-Vasquez et al., 2020). A longitudinal neuroimaging study with a mean follow-up of 4 years found a close relationship between the volume loss of brain and memory, verbal fluency, visuospatial, as well as attention decline (Armstrong et al., 2020). On the other side, it has been reported that after a 6-month aerobic training, an increase in brain volume, gray matter, and white matter was observed in healthy elderly participants (Colcombe et al., 2006), whereas MCI patients showed improved cognitive function after a 12-week training (Alfini et al., 2019). Another study implementing a 3-month dual aerobic-cognitive training in healthy elderly adults found improved executive function and working memory when compared with the performance of pure cognitive training and pure aerobic training groups. The study conducted by Nouchi et al. (2021) demonstrated that attention, working memory, and processing speed can be facilitated by cognitive training. Faraza et al. (2021) observed that improved cognitive ability after a 4-week neuropsychological training could be attributed to higher functional connectivity within the DLPFC. Such evidence provides a basis for the argument that task training, including both ST and DT training, can be used for promoting DT performance (De Freitas et al., 2020; Chiaramonte et al., 2022). However, only few reviews discussed its effects in old participants (Gallou-Guyot et al., 2020; Khan et al., 2022). Besides, it has been hypothesized that the combination of these two methods may provide greater efficacy and feasibility for DT improvement in the elderly.
To our knowledge, this review is the first one to assess the relationship between potential therapeutic approaches such as tES and task training and DT performance in the elderly. We aimed to provide an overview of the previously implemented methods for enhancing DT performance in the healthy elderly and geriatric patients. Our objective was also to provide a reference for future home-based training as well as clinical rehabilitation of DT performance in the elderly. For studies that applied tES, the intensity, electrode montage, and stimulation duration were discussed, while for the training studies, we focused on the training tasks, training procedures, and the number of training sessions. Moreover, this review aimed to summarize the results of tES and training, when delivered either in combination or on its own.
Methods
This systematic review was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Page et al., 2021).
Databases and keyword searches
A literature database search for published studies including tES and training, both alone and in combination was conducted in PubMed (U.S. National Library of Medicine), tDCS database (Grossman et al., 2018) and Web of Science from their inception until December 2023. The database search was first done in PubMed, followed by the tDCS database and Web of Science. We screened all abstracts for relevance. For the tES-related articles, the search phrase was: [(tDCS) OR (Transcranial direct current stimulation) OR (tACS) OR (Transcranial alternating current stimulation) OR (noninvasive) OR (brain stimulation)] AND [(dual task) OR (multitasking) OR (dual cost)]. For the training-related articles, the search phrase was as follows: (training) AND [(dual task) OR (cognitive-motor) OR (simultaneous) OR (cognitive-physical) OR (multitasking)]. The same keywords were used for both databases.
Selection criteria
Studies that met the following criteria were included: (1) healthy elderly, or geriatric patients diagnosed with neurological conditions such as stroke and dementia or musculoskeletal diseases such as osteoarthritis, (2) tES or single/dual task training applied either on their own or in combination, (3) single or multiple intervention sessions were performed, and (4) DT performance was assessed before and after the intervention. Studies were excluded if they were published in languages other than English. Studies were excluded if they: (1) were protocol papers, (2) were letters, (3) participants were children or the mean age <60 years old, (4) participants were individuals with partial loss of limbs, (5) primary outcomes were not related to DT ability, (6) were review or meta-analysis papers, (7) were case reports, (8) were commentary papers, and (9) were conference presentations.
Risk of bias assessment
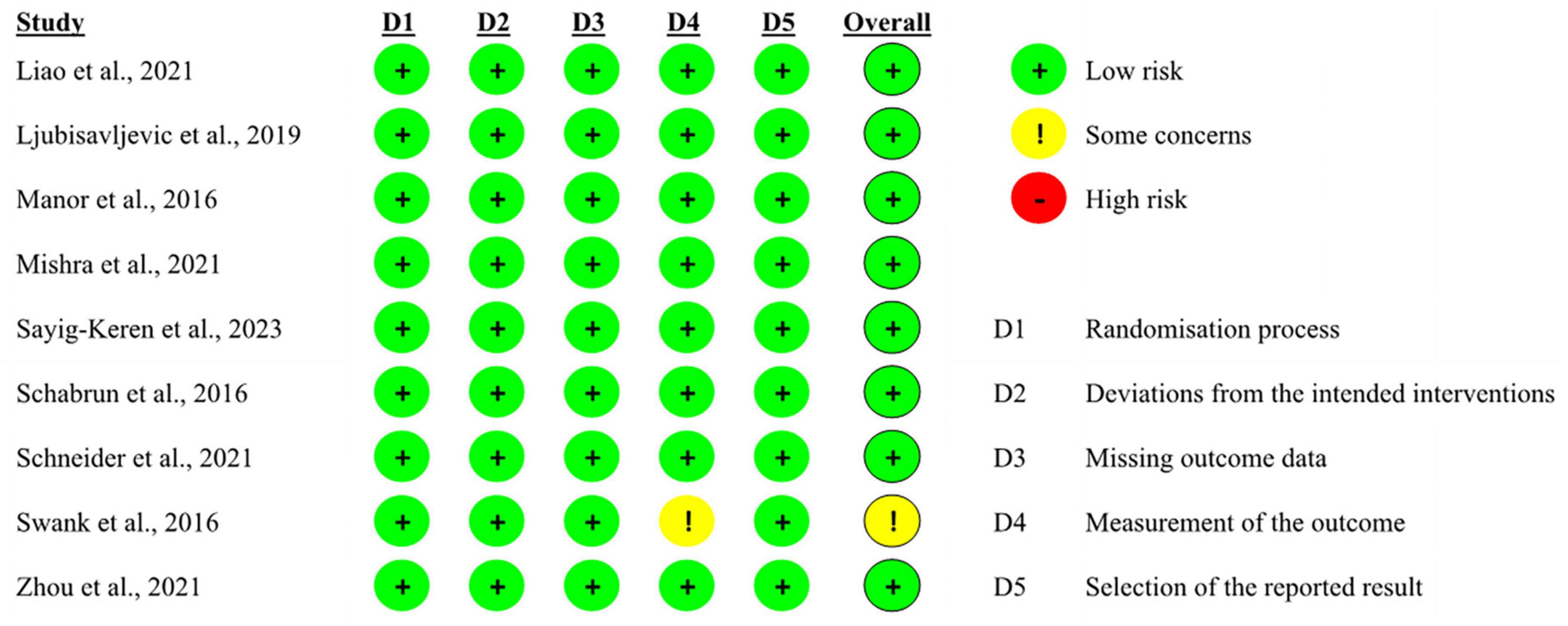
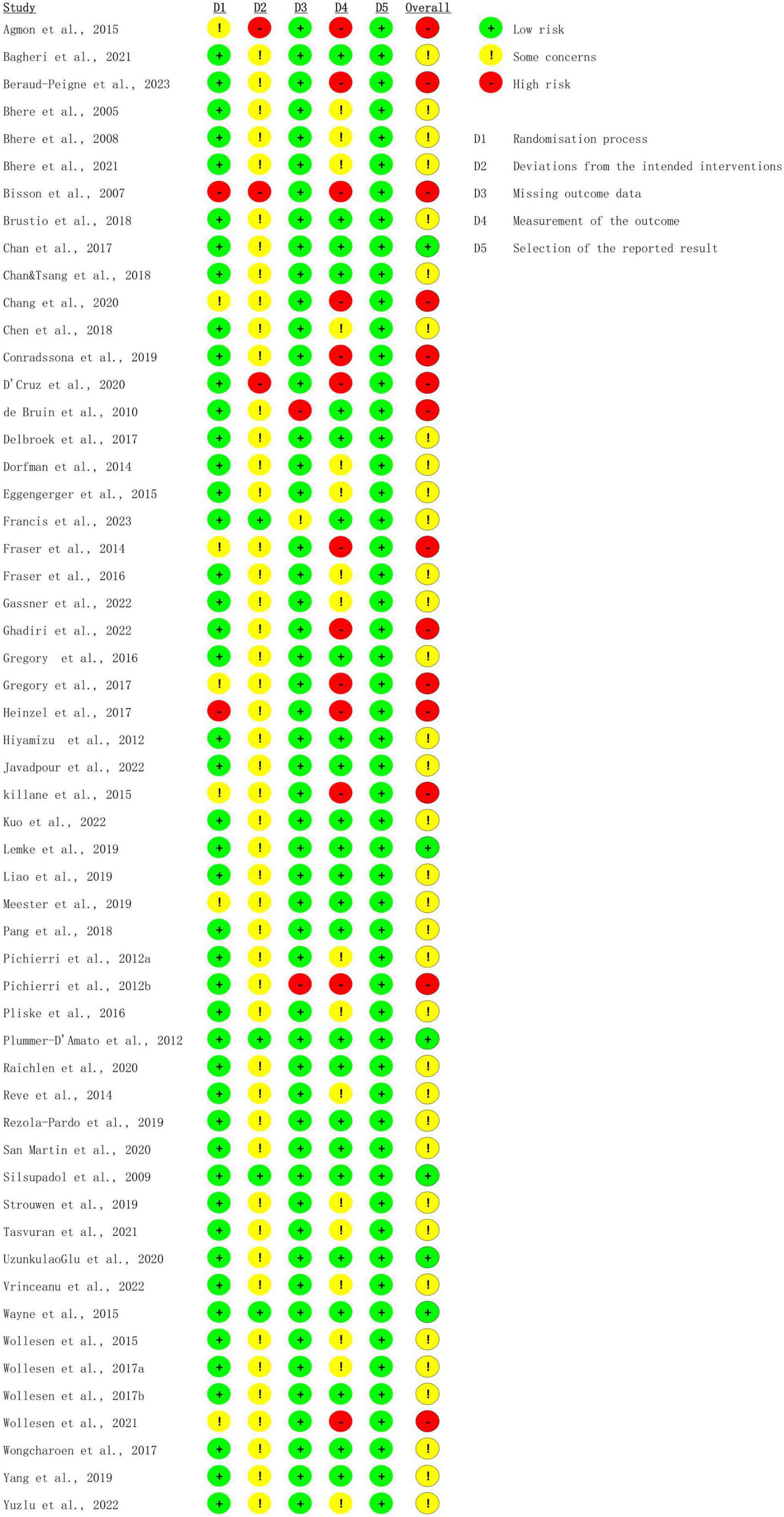
The authors (YJ and PR) assessed the risk of bias via the bias assessment tool—Cochrane risk-of-bias tool, independently. Three grades were assigned to each study individually: Low risk of bias, Some concerns, or High risk of bias. “Low risk of bias” was given when the study effectively addressed risks and elucidated its study design. Conversely, the “Some concerns” classification was given if a study failed to adequately specify details, leaving uncertainties about potential risks. A “high risk” was assigned if a study exhibited serious risks that could significantly impact outcomes due to a biased study design.
Results
Study characteristics
Out of 682 articles screened, 64 studies published between 2005 and 2023 were included in this review (Figure 1). Study characteristics are shown in Figure 2.
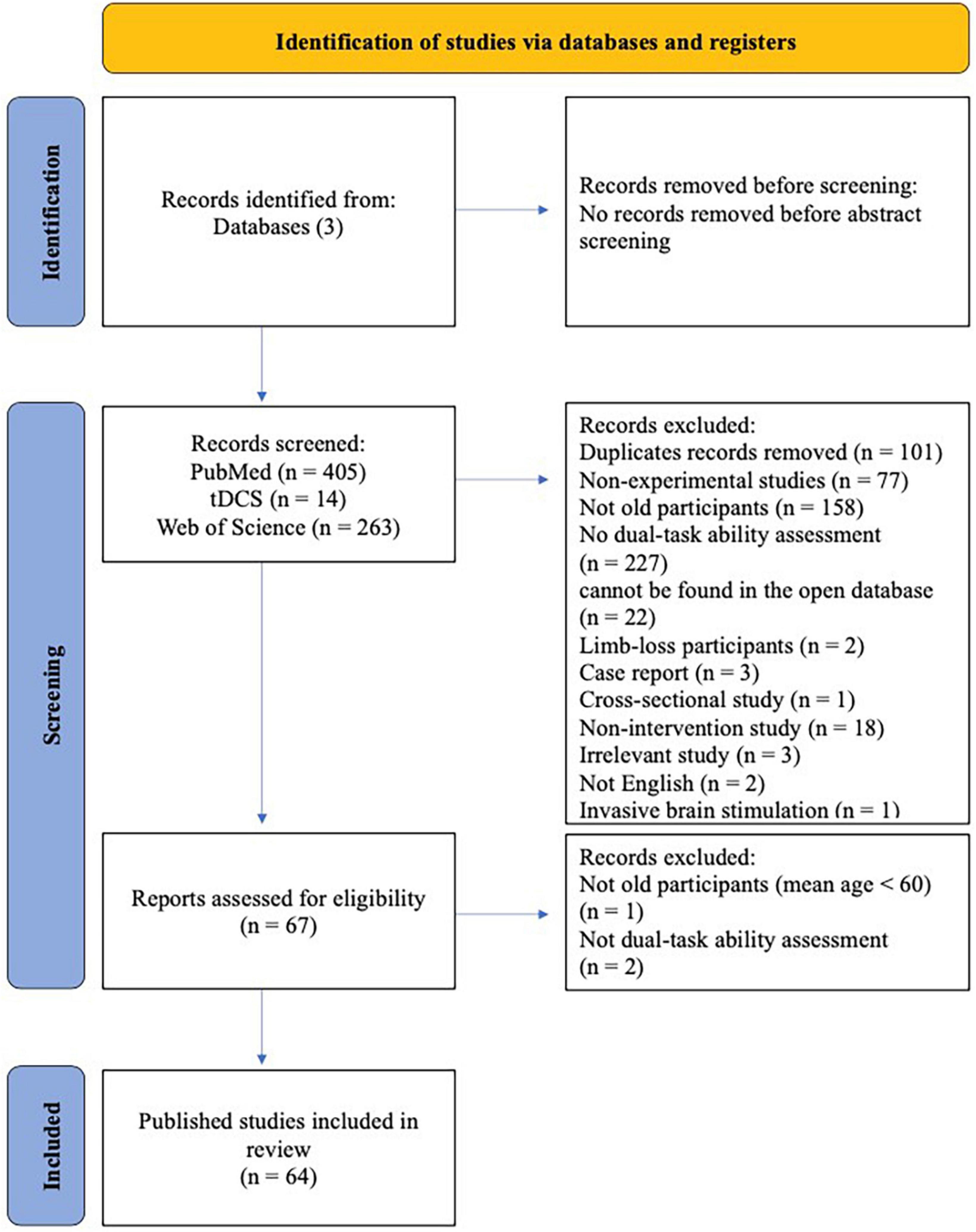
Figure 1. PRISMA flow diagram illustrating identification, screening, and inclusion strategies for the selection of articles.
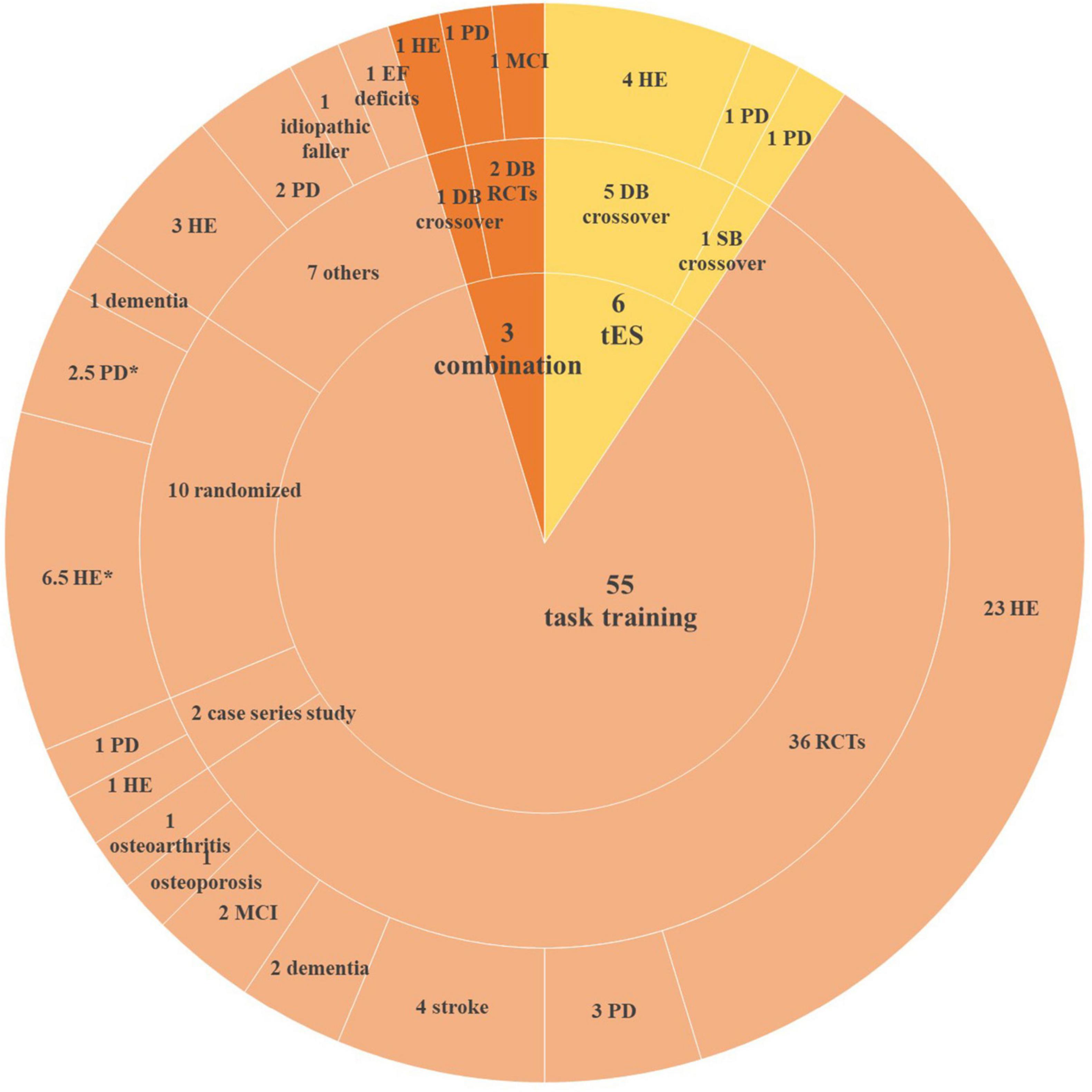
Figure 2. Characteristics of included studies. HE, healthy elderly; PD, Parkinson’s disease; EF, executive function; DB, double-blinded; SB, single-blinded; RCT, randomized clinical trial; MCI, mild cognitive impairment. *One study not only recruited healthy participants and PD patients but also assigned them to the same group.
Our review included six tES studies (five applied tDCS and one applied both tDCS and tACS), of which five were double-blinded randomized controlled trials (RCTs) and one was a single-blinded RCT which recruited PD patients. Out of these five RCTs, four recruited healthy participants and one recruited PD patients.
Fifty-five studies applied only task training. Thirty-six studies were RCTs, of which 24 studies recruited healthy participants and 12 studies recruited geriatric patients, including PD, stroke, dementia, MCI, as well as osteoporosis and osteoarthritis patients. Two studies were case series studies, recruited healthy participants and PD patients separately. Ten studies were randomized studies (participants were assigned to different groups randomly), of which six studies allocated healthy elderly, two studies recruited PD patients, one with dementia patients, and the other one recruited both healthy and PD participants. Seven studies did not mention their designs.
Three studies combined anodal tDCS with task training. All of them were double-blinded RCTs, recruited healthy participants, MCI patients, and PD patients, respectively.
Risk of bias assessment
The overall bias in tES-related studies was low, only one study had a concerned risk in measurement (Figure 3). Thirteen training studies had high risk in measurement and 48 studies had concerned risk in intended intervention (Figure 4), as the training conductor might also participated in the data analysis in these studies (Figure 5). Nevertheless, this phenomenon is explicable and understandable. Compared with tES-related studies, which employ shorter durations (20–30 min) and fewer sessions (mostly only 1), the majority of training studies implemented an intervention of 60 min with more than 12 sessions.
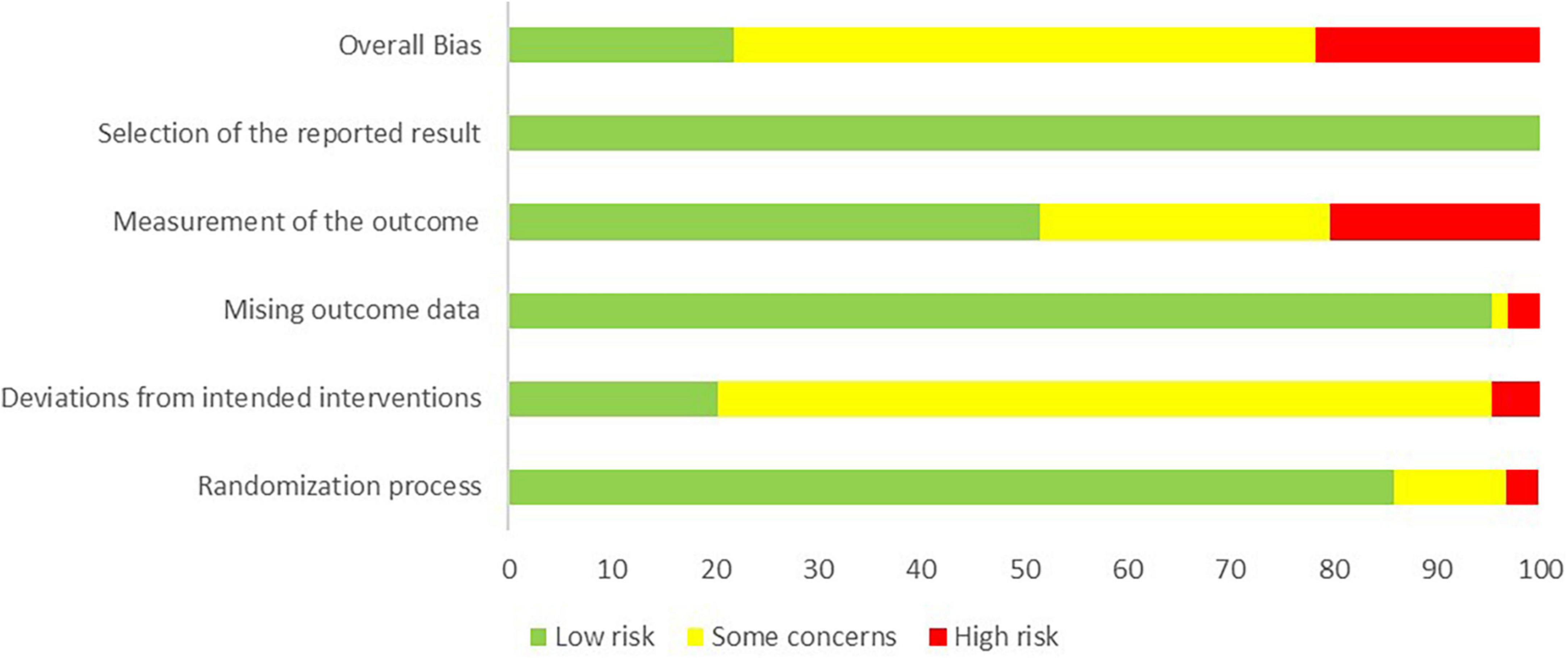
Figure 5. Risk of bias assessment based on subscales for all included studies based on authors’ judgment.
Intervention characteristics
tES only intervention
All six studies applied a crossover design, whereby all participants received both anodal and sham stimulation with a different washout period, ranging from 3 days to 2 weeks. Four of them applied a single session of classical anodal tDCS intervention at a 1.5–2 mA over the L-DLPFC for a duration ranging from 20 to 30 min (Table 1), while one study applied a single session of 4 mA, 20-min high-definition tDCS (HD-tDCS) (Zhou et al., 2021). The other one applied both anodal-tDCS and tACS over L-DLPFC and the left frontoparietal network (L-FNP) separately for 20 min with the current at 1.5 mA (Sayig-Keren et al., 2023).
Training-only intervention
Fifty-five studies implemented only task training, with a maximum of 78 sessions. The intervention programs included: (1) single motor task training (balance standing, walking, or aerobic training), (2) single cognitive task training (visual or auditory working memory task training), (3) dual cognitive task training (visual and auditory working memory task training), (4) dual motor task training (walking while bouncing a basketball), and (5) cognitive-motor dual task training (walking while performing visual recognition task) (Tables 2, 3). Participants in the control group were normally asked to keep their daily activities or perform simple ST, for example, walking at a self-suitable speed.
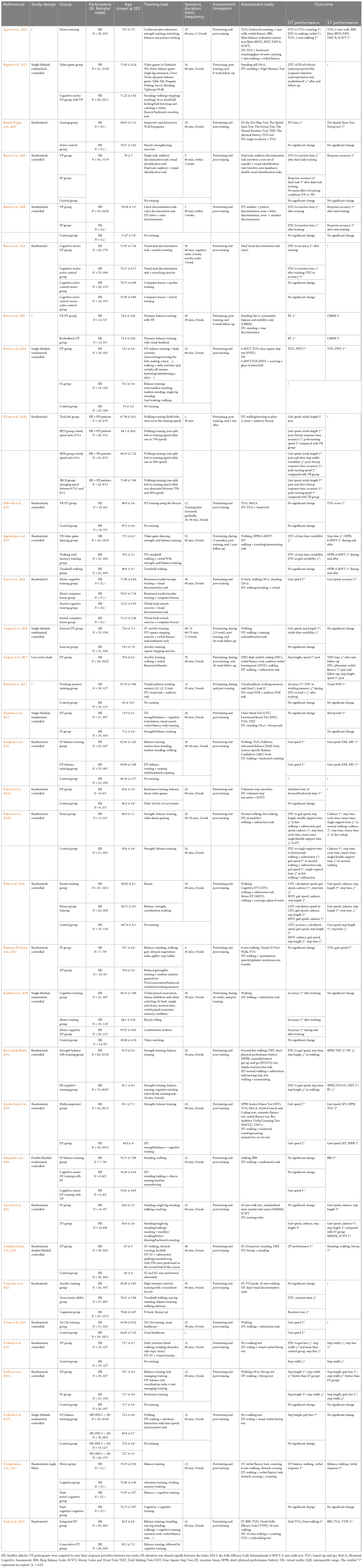
Table 2. Summary of the training procedures used to enhance DT in healthy elderly and task performance.
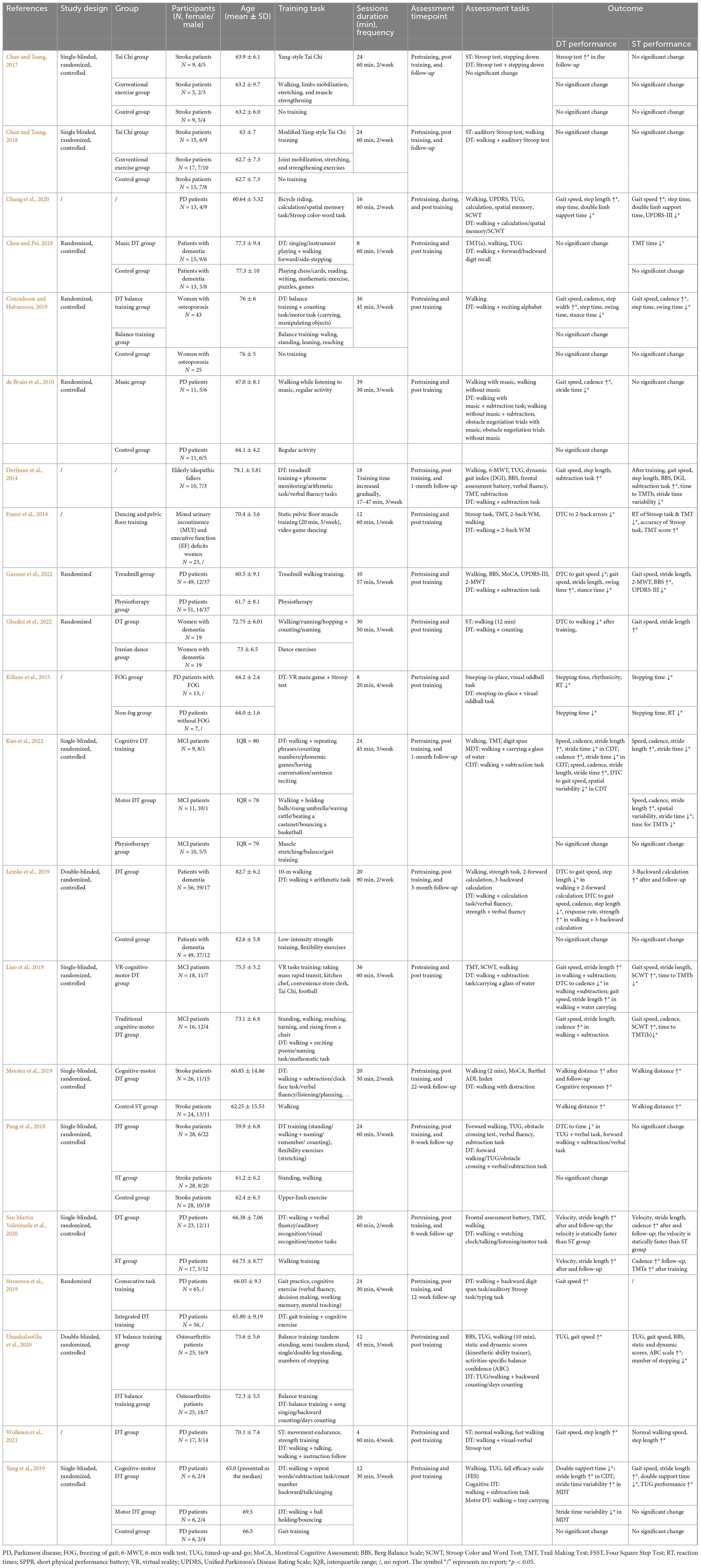
Table 3. Summary of the training procedures used to enhance DT in geriatric patients and task performance.
Combined tDCS and training intervention
Transcranial direct current stimulation (TDCS) was delivered during task training, and different quantities of training sessions (1 vs. 9 vs. 36) were applied in these combined methods’ studies. Particularly, one study applied the same duration of exercise training and tDCS (Schneider et al., 2021), while longer exercise durations were applied and tDCSs were delivered for the first 20 min in the other studies (Schabrun et al., 2016; Liao et al., 2021) (Table 4).
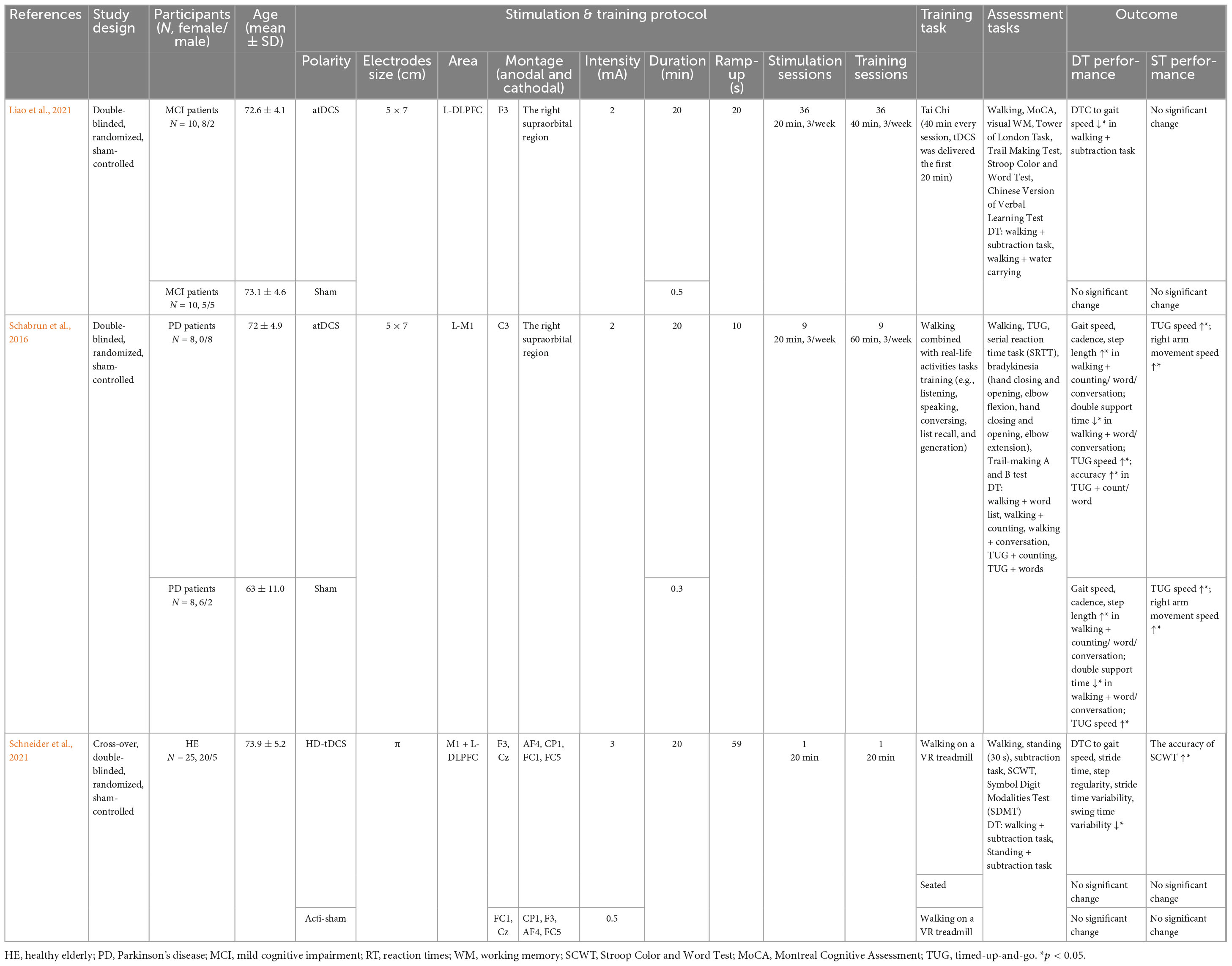
Table 4. Summary of the tDCS and training parameters and procedure used in combination and task performance.
Study outcomes
In the included studies, the primary outcomes were dual task cost (DTC) and gait-related parameters in ST and DT conditions, such as gait speed, cadence, and step length. DTC refers to the percentage change in performance markers from single to dual task (Manor et al., 2016), the performance outcome could be time, dimensions, speed, and accuracy among others.
The secondary outcomes were spatial-temporal parameters. Single motor tasks were assessed by time, speed, cadence, step length, center of pressure, or sway area. Single cognitive tasks were assessed via accuracy, error rate, reaction time, or scores.
Single session tES on dual-task performance
In healthy elderly
Ljubisavljevic et al. (2019) reported that there was no significant change in the DT walking performance after one session of 2 mA anodal tDCS over the L-DLPFC in the healthy elderly. Manor et al. (2016), nevertheless, reported a significantly lower DTC to standing, walking, and a lower error rate in the healthy elderly following anodal tDCS over the L-DLPFC compared to sham stimulation. Findings from Sayig-Keren et al. (2023) also supported that DTC to walking was decreased after one session either anodal tDCS over L-DLPFC or 6 HZ tACS over L-FPN. Significant decreased DTC during standing, assessed by sway speed and sway area following the intervention targeting only L-DLPFC and both L-DLPFC and the primary somatosensory cortex (SM1), was also observed (Zhou et al., 2021). But this was not observed for the acti-sham and SM1 only interventions. In addition, a similar observation was made for DTC during walking, as measured through speed (Table 1). A noteworthy observation for the studies that found enhanced DT performance is a simultaneous absence of alterations in ST performance following the intervention.
In geriatric patients
Only two studies explored the effects of anodal tDCS over the left DLPFC on DT performance in old PD patients. Swank et al. (2016) reported there was no significant enhancement in DT standing after one 20-min tDCS intervention with F3-F4 montage. However, Mishra and Thrasher, 2021 reported increased dual gait speed in the real tDCS condition while using anodal tDCS with the F3-FP2 montage but the stimulation was delivered for 30 min.
Task training on dual-task performance
In healthy elderly
A few studies reported limited effects of task training on DT performance in the healthy elderly. In a study conducted by Hiyamizu et al. (2012), participants were randomly allocated into a balance group and a balance-cognitive group which involved calculation, visual search, and verbal fluency tasks training. After 24 × 60-min training sessions over 12 weeks, no significant changes in DT performance during concurrent balance standing and Stroop task were observed in either group. A similar finding was reported when 12 sessions (gradually increased from 18 to 30 min in 5 weeks) of virtual reality (VR) force plate-based DT training (Delbroek et al., 2017), or 4 × 45-min balance/gait training sessions combined with working memory/calculation task over 4 weeks were applied in the healthy elderly (Plummer-D’Amato et al., 2012).
However, increased number of studies reported significantly improved DT performance in healthy old adults after motor-cognitive DT training was applied (Bherer et al., 2005, 2008, 2021; Fraser et al., 2016; Gregory et al., 2016; Heinzel et al., 2017; Brustio et al., 2018; Bagheri et al., 2021; Tasvuran et al., 2021), such as faster gait speed (Rezola-Pardo et al., 2019) and faster initiation time of steps (Pichierri et al., 2012a) after 24 × 60-min concurrent balance and cognitive training sessions over 12 weeks, as well as lower step time variability after 52 × 60-min concurrent walking and memory training sessions over 26 weeks (Eggenberger et al., 2015). Wollesen et al. (2017b) applied both single walking and DT walking (concurrent walking and attention distraction task/visio-spatial executive task) for 12 × 60-min over 12 weeks in the healthy old adults. Compared to baseline, increased step length was observed after training, which was in line with their previous study (Wollesen et al., 2015).
The effects of ST training on DT performance were also explored. Nevertheless, it is contentious whether ST training has the same role in improving DT performance in the healthy elderly. Silsupadol et al. (2009) divided the participants into balance training group and DT training group, in which old participants were asked to perform standing/walking and objects naming or remembering tasks simultaneously. It was found that after 12 × 45-min training sessions over 4 weeks, balance performance and DT gait speed in the DT training group increased significantly, while no significant change was observed in the balance group. Recent studies reported that standing/walking training for 12 × 60-min sessions over 6 weeks (Tasvuran et al., 2021) did not alter DT performance in the healthy elderly. A similar finding was observed after healthy participants received strength/balance training for 24 × 30-min sessions over 12 weeks (Hiyamizu et al., 2012) or 4 × 45-min sessions over 4 weeks (Plummer-D’Amato et al., 2012). Nevertheless, some studies reported that fitness training for 18 × 60-min sessions over 3 weeks (Agmon et al., 2015), balance training for 24 × 30-min sessions over 12 weeks (Reve and de Bruin, 2014) or 18 × 60-min over 6 weeks (Javadpour et al., 2022), 36 × 30-min bicycle riding sessions over 12 weeks (Raichlen et al., 2020), 20 × 60-min Karate training sessions over 10 weeks (Pliske et al., 2016), or 52 × 30-min Tai Chi training sessions over 26 weeks (Wayne et al., 2015), contributed to enhancing DT ability in healthy old subjects (Table 2).
In geriatric patients
Based on the data so far, both ST and DT Training have been shown to have noticeable effects in improving DT ability in old PD patients (Chang et al., 2020; D’Cruz et al., 2020; Gassner et al., 2022; Table 3).
In the first study using ST training to improve DT ability in geriatric patients (de Bruin et al., 2010), PD patients in the training group were asked to perform single tasks, consisting of walking and regular activity, whereas participants in the control group conducted only regular activity. After 39 × 30-min training sessions over 13 weeks, patients in the training group showed increased gait speed, cadence, and lower stride time during walking combined with performing subtraction tasks, while no significant change was observed in the control group. Strouwen et al. (2019) applied gait training and cognitive auditory training in isolation in PD patients for 24 × 30-min training sessions over 6 weeks, significant faster gait speed during concurrent walking and cognitive tasks (digit span task, auditory Stroop task, and typing task) were observed. UzunkulaoGlu et al. (2020) reported that after 12 × 45-min training sessions over 4 weeks, gait speed under DT conditions improved in osteoarthritis patients participating in both the single-balance training group (e.g., single/double leg standing) and the dual-balance training group (single balance combined with counting/singing). This finding was supported by Conradsson and Halvarsson (2019). A similar single-balance and dual-balance training protocol was implemented in female participants with osteoporosis, but for 36 × 45-min training sessions over 12 weeks. In both groups, gait speed, cadence, and step width improved under DT conditions.
The effects of DT training on PD patients have been further explored. Yang et al. (2019) randomly allocated PD patients into (1) cognitive-motor DT group, in which participants were asked to conduct walking and a subtraction task simultaneously, or (2) motor DT group, where participants were asked to walk while holding a ball, or (3) control group, where patients only performed gait training. Shorter double support time and longer stride length were observed in the motor-cognitive DT group after 12 × 30-min sessions over 4 weeks, while the motor DT group showed significantly reduced stride time variability during DT walking. No improvement was observed in the control group. A study conducted by Killane et al. (2015) applied concurrent VR maze game and Stroop test in PD patients with freezing of gait (FOG) and without FOG separately. After 8 × 20-min sessions over 2 weeks, significantly shorter stepping time in concurrent steeping and visual oddball tasks has been observed in both groups. San Martin Valenzuela et al. (2020) allocated 40 PD patients into a cognitive-motor DT group and a walking group. Participants in the DT group were asked to walk while carrying out one cognitive task such as verbal fluency, or auditory recognition task while those in the ST group performed only walking training. After 20 × 60-min sessions over 10 weeks, the DT gait speed and stride length of patients increased significantly in both groups, notably, the DT group exhibited an even greater increase in gait speed compared to the single walking group.
Many clinical studies support the efficiency of task training on improving DT ability in elderly patients including stroke (Pang et al., 2018; Meester et al., 2019), MCI (Liao et al., 2019; Kuo et al., 2022), osteoporosis (Conradsson and Halvarsson, 2019), osteoarthritis (UzunkulaoGlu et al., 2020), idiopathic fallers (Dorfman et al., 2014), and executive functioning disorders (Fraser et al., 2014). However, two included studies which allocated dementia patients and inconsistent results were reported. Chen and Pei (2018) applied music DT training, where patients were asked to perform concurrent singing/instrument playing and walking tasks for 8 × 60-min training sessions over 8 weeks. No enhancement was observed within concurrent walking and forward/backward digit recall tasks but better cognitive function, assessed by a single trail making test. Another study reported enhanced cognitive function and better performance under concurrent walking and calculation task after concurrent walking and arithmetic training were applied for 20 × 90-min training sessions over 10 weeks (Lemke et al., 2019).
Combined tDCS with task training
As previous studies reported positive results for tDCS and task training in improving DT capacity, only several studies combined these two approaches and explored its effectiveness on DT performance in older adults (Table 4).
In healthy elderly
To our knowledge, only one study explored its effectiveness in healthy elderly. In this study, participants received either real HD-tDCS at 3 mA for 20 min, or sham stimulation (0.5 mA, 20 min, near zero normal electric fields) targeting the left M1 and L-DLPFC while walking on a VR treadmill, or real HD-tDCS while sitting (Schneider et al., 2021). Compared to the sham condition, lower DTC during concurrent walking/standing and subtraction tasks was observed after real stimulation.
In geriatric patients
In a study conducted by Schabrun et al. (2016), 16 PD patients were recruited and received 9 sessions of either 20-min anodal tDCS over left M1 at 2 mA or sham tDCS paired with 60-min motor-cognitive DT training, which entailed walking combined with real-life activities such as talking. The sessions took place 3 times per week and tDCS was delivered for the first 20 min of each session. Following the intervention, both groups showed a significant enhancement in DT walking, including increased gait speed, cadence, step length, and reduced double support time under walking while conversing/performing a word-list task. Moreover, significantly increased gait speed and lower error rate under dual Time-Up-and-Go test were observed.
Recently, Liao et al. (2021) applied 36 sessions of combined either 20 min 2 mA anodal tDCS over the L-DLPFC or sham stimulation with 40 min Tai-Chi training over 12 weeks in old MCI patients. TDCS was delivered for the first 20 min. Compared to sham stimulation, the anodal tDCS group showed significantly improved walking performance during concurrent walking and subtraction/water carrying tasks after intervention.
Discussion
Does single session tDCS influence DT ability in the elderly?
In the above-mentioned studies, L-DLPFC was the main target area as it is highly related to executive function (Zhou et al., 2014). A combined tDCS and functional near-infrared spectroscopy study revealed lower oxygenated hemoglobin response in the left prefrontal cortex within DT condition after 2 mA, 20 min tDCS over L-DLPFC was applied, suggesting that L-DLPFC tDCS modulates the prefrontal recruiting, and the reduction of DTC may be due to the reduced oxygen consumption (Jor’dan et al., 2022) as neuroimaging study which mentioned above reported a higher activation in the healthy elderly during DT condition. Compared to tDCS over the right DLPFC or sham stimulation, anodal tDCS over the L-DLPFC can significantly enhance executive functions within conflict-related tasks (Dubreuil-Vall et al., 2019). Patel et al. (2019) noted that the improvement in cognitive and behavioral skills induced after tDCS over M1 could be attributed to cortical plasticity, potentially triggered by a decrease in gamma-aminobutyric acid concentration, which has an important role during motor learning. The higher performance after tDCS over DLPFC may be due to the increased cortical excitability in the executive control and ventral attention networks within the brain (Soleimani et al., 2022).
Nevertheless, the low number of studies and inconsistent findings within these studies make the ability of a single tDCS session to adequately enhance DT performance in the healthy elderly or geriatric patients inconclusive. As shown in Table 1, Manor et al. (2016) reported significantly lower DTC to standing and walking in DT condition after 2 mA anodal tDCS with F3-FP2 montage for 20 min. However, results from Ljubisavljevic et al. (2019) suggested that 30 min anodal tDCS with F3-FP2 montage at 1.5 mA had no effects on DT ability. This inconsistence is less likely due to the study design as most studies applied double-blinded, crossover design. However, multiple other factors may contribute to this discrepancy, such as sample size, stimulation parameters (intensity, duration, electrodes sizes), as well as the participants’ characteristics [exercise frequency, or the proficiency in skills (Furuya et al., 2014)]. Therefore, more studies are needed to explore the effects of tES on DT performance in the healthy elderly.
Similarly, there is insufficient evidence to suggest that tDCS has a facilitative effect on DT performance in PD patients. Mishra and Thrasher (2021) reported enhanced DT walking after 1.5 mA, 20 min anodal tDCS over L-DLPFC, while Swank et al. (2016) reported no significant changes but a trend of reduced DTC on walking was observed after anodal tDCS via two electrodes of size π (3.14 cm2). The result can be attributed to various factors, e.g., the disease stages of patients. Mishra recruited participants with mild-moderate severity (stage I–III, assessed by the Hoehn and Yahr scale), while Swank included patients with stage II. In addition, stimulation parameters also contributed to the inconsistent results. For instance, a 30-min stimulation duration was utilized in the study conducted by Mishra, whereas Swank applied tDCS for 20 min. Mishra and Thrasher (2021) positioned the anodal electrode over F3, with the cathode electrode placed over the right supraorbital region, while a F3-F4 montage was applied by Swank et al. (2016). The inconsistent size of the electrodes (35 cm2 vs. 3.14 cm2) also contributed, as Hashemirad et al. (2017) reported that tDCS with small electrodes (3 cm2) over left M1 or L-DLPFC did not affect cognitive functions.
Interestingly, only one study reported improved ST (component tasks) performance after intervention (Mishra and Thrasher, 2021; Table 1). Out of these six studies, most reported enhanced DT performance without altering single cognitive or motor task performance. For instance, Manor et al. (2016) reported notable DT standing/walking performance after a single session of tDCS, assessed by a slower sway velocity/increased gait speed and subtraction error rate within DT condition, but no significant improvements in walking, standing, or subtraction task was observed when these tasks were performed in isolation after intervention. Ceiling effects such as low error rate for cognitive tasks and relatively high motor performance in the baseline within ST condition may be an underlying confounding factor since healthy old participants were recruited, or the functional integrity of the underlying network was at its limits and could not be further improved by the stimulation (Ljubisavljevic et al., 2019; Zhou et al., 2021). This could be explored further in the following studies.
Based on the limited research, it is insufficient to demonstrate the effectiveness of HD-tDCS with multi-area stimulation in improving DT performance in the elderly. Therefore, to explore further the possibilities of utilizing anodal tDCS and HD-tDCS of the L-DLPFC in promoting DT capacity in the elderly, more studies should be conducted in the future.
Does task training influence DT ability in the elderly?
The existing evidence suggests that the DT capacity of the elderly can be enhanced by training, especially, cognitive-motor DT training. In comparison to tDCS studies, almost all training studies applied multiple sessions. Recent research reported enhanced DT gait speed and DT cognitive performance after one walking training session with the split-belt treadmill in both PD patients and healthy old adults, with greater improvements obtained while two belts were set at different speeds (D’Cruz et al., 2020).
The underlying mechanism between improved DT performance and task training has not been addressed, but a few hypotheses have been proposed. Some theorists suggested that DTC might originate from two separate sources: (1) incomplete conversion of verbal descriptions to procedural memory (i.e., muscular memory) and (2) conservative execution control with postponing certain stages of a task while another task is in progress due to the response-selection bottleneck (Pashler, 1994; Schumacher et al., 2001).
In the former context, DT ability can be regarded as one particular skill. According to the skilled performance model, every single skill is the programmatic knowledge in the form of condition-action rules. This programmatic knowledge can be converted from declarative knowledge through practice, and once the conversion is completed, performing skill/actions in an easy way (performing DT at a lower cost) could be possible (Schumacher et al., 2001). Schumacher et al. (2001) applied concurrent cognitive-demanding tasks and visual-auditory tasks in young adults, participants were asked to respond to the visual stimulus (e.g., circle) by pressing keyboards with one hand, and report numbers (e.g., 1, 2, and 3) orally for the auditory stimulus simultaneously. The training lasted for 8 sessions, participants performed single tasks in session 1, while both STs and DT were performed from sessions 2 to 8. Results have shown that the DTC to reaction times was significantly lower after practice, and even a “perfect time-sharing” was observed between two tasks within DT condition in session 8. That could partially explain why significant improvements were observed in both ST and DT conditions in studies which utilized identical single and dual tasks during training and assessment phases. For instance, Heinzel et al. (2017) combined visual working memory (WM) task training and auditory WM task training simultaneously for 12 × 45-min sessions over 4 weeks in the healthy elderly, participants showed notable lower DTC to WM and a significantly higher accuracy within the same DT condition, as well as better single WM performance during assessment phase. Gregory et al. (2017) applied a concurrent walking and arithmetic task in the training phase for 78 × 40-min sessions over 24 weeks, increased gait speed and step length were observed in both DT walking (concurrent walking and calculation task) and single walking condition.
The second theory holds that the response-selection stage of the second component task is processed only when the stage of the first task has been completed (Strobach et al., 2013). This delay is considered to be the source of DTC. Evidence provided by Strobach et al. (2013) suggested that the reason for higher DT performance after training/practice might come from speed-up central response-selection stages of both tasks. In this study, eight training sessions similar to Schumacher et al. (2001) were applied but followed up by two more sessions. The old visual stimulus was intermixed with novel visual stimulus (different shape, e.g., triangle), and participants were instructed to respond to the stimulus with the unpracticed hand in session 10. Results revealed a great reduction of reaction times in both component tasks during the ST condition, and significantly reduced reaction times within the DT condition, which was maintained in the last two sessions, suggesting that practice can facilitate such response-selection processing as well. Besides, this gained facilitation can be still observed in the new DT condition.
To address why practice effects were maintained in new conditions, “transfer effects” and “task coordination skills” were cited. The acquisition of the task coordination skill is related to the optimization of executive function within DT condition through training, and this optimization is associated with the enhancement of DT, as DT is regarded as a particular part of the execution function (Kramer et al., 1995; Strobach et al., 2014). A previous study showed that task coordination skills can be acquired only in DT conditions and the acquired task coordination skills are independent of the training settings and tasks (Kramer et al., 1995). Namely, these skills are transferable and can benefit new, unpracticed conditions or DTs, which have (1) identical or similar structures, such as motor-cognitive DT, motor-motor DT, or cognitive-cognitive DT, or (2) contain the same component task, for instance, practiced walking task and a changed subtraction task, practiced subtraction task and a different walking task, or (3) contain the same/similar input or output, for example, practiced/similar auditory/visual input, practiced/similar keyboard response. As a consequence, even though the DTC cannot be eliminated, extensive practice-gained task coordination skills induce an optimization of executive function, which enables efficient processing of two concurrent task streams and reduces the cost of performing dual tasks. Moreover, the “transfer effects” partially explained why significant improvements can still be observed after training in studies that applied different DT conditions in the training phase and assessment phase, e.g., whole body stretch exercise combined with visual discrimination task for training but concurrent walking and n-back task for assessment (Fraser et al., 2016), or concurrent balance and cognitive task for training but simultaneous walking and backward counting task for assessment (Rezola-Pardo et al., 2019).
However, improved DT performance was observed in studies, that applied STs for training in the healthy elderly (Wollesen et al., 2017a; Raichlen et al., 2020), PD patients (Chang et al., 2020; Gassner et al., 2022), stroke patients (Meester et al., 2019), and women with osteoporosis (Conradsson and Halvarsson, 2019). Pashler and Baylis (1991) suggested that the practice effects produced by ST training might shorten the response selection stage during the DT condition because, as discussed above, the enhancement of DT performance after ST training is not related to task coordination skills. Strobach et al. (2013) found that ST training might shorten other processing stages, such as the initial perception stage, or final motor stage since the ST used for training in these studies was one component of DT used for assessment (Strobach et al., 2013). Namely, the transfer effects might be observed when the same task exists both in the training phase and as one component of the DT during the assessment phase, e.g., calculation task and aerobic task were used for training in isolation but performed concurrent walking and calculation task in DT assessment (Chang et al., 2020), or walking task and standing task were practiced in isolation and combined walking and backward counting task was applied during DT assessment (Javadpour et al., 2022), or balance and postural training were applied in the training phase and concurrent standing and tone discrimination task was applied for the assessment (Agmon et al., 2015).
One interesting observation was that DT performance also improved in studies that did not have the same component task during the training and evaluation phases. For instance, Raichlen et al. (2020) applied only bicycle riding training for 36 × 30-min sessions over 4 weeks in healthy old adults, an increased DT cognitive function, assessed by significantly higher accuracy during a concurrent walking and subtraction task, was observed after training. Reve and de Bruin (2014) applied single strength training, balance training, as well as cognitive training for 24 × 30-min sessions over 12 weeks in the healthy elderly, lower DTC to walking was observed in a concurrent walking and object naming task. Furthermore, a greater improvement in cognitive function was also observed within ST condition after training, assessed by enhanced performance on the trail-making test and simple reaction time task. In these cases, it seems that the boosted DT performance was not related to the transfer effects, task coordination skills, or shortened certain stages in DT condition.
A few studies applied VR-based training (Delbroek et al., 2017) or game-based training (Pichierri et al., 2012b; Fraser et al., 2014), and positive effects were observed in old patients (Killane et al., 2015) as well as healthy elderly (Bisson et al., 2007; Eggenberger et al., 2015; Bagheri et al., 2021). Game-based training is widely used nowadays and its effects on brain plasticity were explored, such as improving cognitive function in old adults (Maillot et al., 2012), and decreasing depression in young individuals (Li et al., 2022). In the future, these methods could be popular technologies in improving DT capacity.
Does the combined approach influence DT ability in the elderly?
Transcranial direct current stimulation combined with proper task training can be a promising tool for improving DT capacity in the elderly. However, further studies are required to validate its effectiveness.
The study conducted by Liao et al. (2021) emphasized the role of tDCS, as DT performance was significantly improved in patients with MCI only after combined Tai Chi training with anodal tDCS, but not sham, over DLPFC.
Schabrun et al. (2016) demonstrated that training played a key role in the improvement of DT performance as both DT training conjunct with 2 mA 20 min anodal tDCS over M1 and sham showed significantly improved DT performance.
The study which was conducted by Schneider et al. (2021) highlighted the function of combination. In this study, improved DT walking performance and cognitive ST performance were observed after participants received concurrent HD-tDCS over M1 and DLPFC and walking training. However, neither the HD-tDCS condition nor concurrent walking and sham stimulation condition showed improved ST or DT performance in healthy elderly after one session intervention. Contrarily, a study by D’Cruz et al. (2020) reported the effectiveness of single-session split-belt treadmill walking training in DT performance enhancement. This may indicate that more complicated training tasks can yield the same or more pronounced training effects with less training effort than training with simple tasks. In this context, split-belt training trained the motor coordination of the lower and upper limbs in addition to walking training when compared with conventional treadmill walking training.
We suggest the combined approach can be a valid tool to improve DT performance, however, the effects of independent components need to be further explored. For example, the role of training and tES in the enhancement of DT performance if exists, and whether tDCS accelerates the training process when compared with pure training. Furthermore, more evidence is required from aged healthy people and geriatric patients.
Limitations and future direction
This review is a traditional, systematic, qualitative clinical review of studies involving some form of tDCS and treatment techniques. It must be pointed out that some training-only studies were not RCTs. Further limitations were the variability in the number of sessions and type of tasks for the different studies involving task training as well as the low sample size of multiple studies.
In the future, a more in-depth exploration of the montage of tES intervention should be conducted, despite specific positions or cortices, brain networks such as the frontoparietal network can also be targeted. Additionally, it is important to highlight that all included tES-only studies in this review utilized only a single intervention session, further investigation through multi-session interventions is recommended as it has been reported that repeated tES can induce long-term potential effects and boost cognitive enhancement and lasting up to 1 month (Korai et al., 2021; Antonenko et al., 2023). In the realm of training studies or clinical rehabilitations, virtual reality techniques could be an optimized tool moving forward. Besides, the understanding of the underlying mechanisms behind the improved DT performance can be facilitated while a comprehensive, multimodal method is adopted in the assessment, for example, EEG-fMRI.
Conclusion
This review is the first article to discuss the relationship between different potentially therapeutic approaches – tES, training, and DT performance in old adults. Sixty-four studies including tDCS only, task training only, and the combination of both to improve DT capacity in both healthy elderly and old patients were discussed in this study. This study provides an overview that task training, particularly cognitive-motor DT training, can be a validated method for enhancing DT performance in the elderly. The effectiveness and potential mechanisms of task training in improving DT abilities in older adults were also further addressed. However, the possibility of tDCS-only intervention in improving DT capability in older adults requires further exploration. The potential of tACS, the combination of tES and training in the enhancement of DT performance in the elderly deserves further investigation.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YJ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft. PR: Validation, Visualization, Writing – review & editing. AA: Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
We acknowledge the support by the Open Access Publication Funds of the Göttingen University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Agmon, M., Kelly, V. E., Logsdon, R. G., Nguyen, H., and Belza, B. (2015). The Effects of EnhanceFitness (EF) training on dual-task walking in older adults. J. Appl. Gerontol. 34, N128–N142. doi: 10.1177/0733464812465921
Alfini, A. J., Weiss, L. R., Nielson, K. A., Verber, M. D., and Smith, J. C. (2019). Resting Cerebral blood flow after exercise training in mild cognitive impairment. J. Alzheimers Dis. 67, 671–684. doi: 10.3233/JAD-180728
Ali, M. M., Sellers, K. K., and Frohlich, F. (2013). Transcranial alternating current stimulation modulates large-scale cortical network activity by network resonance. J. Neurosci. 33, 11262–11275. doi: 10.1523/JNEUROSCI.5867-12.2013
Antal, A., Boros, K., Poreisz, C., Chaieb, L., Terney, D., and Paulus, W. (2008). Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 1, 97–105. doi: 10.1016/j.brs.2007.10.001
Antal, A., and Paulus, W. (2008). Transcranial direct current stimulation and visual perception. Perception 37, 367–374. doi: 10.1068/p5872
Antal, A., and Paulus, W. (2013). Transcranial alternating current stimulation (tACS). Front. Hum. Neurosci. 7:317. doi: 10.3389/fnhum.2013.00317
Antonenko, D., Fromm, A. E., Thams, F., Grittner, U., Meinzer, M., and Flöel, A. (2023). Microstructural and functional plasticity following repeated brain stimulation during cognitive training in older adults. Nat. Commun. 14:3184. doi: 10.1038/s41467-023-38910-x
Armstrong, N. M., An, Y., Shin, J. J., Williams, O. A., Doshi, J., Erus, G., et al. (2020). Associations between cognitive and brain volume changes in cognitively normal older adults. Neuroimage 223:117289. doi: 10.1016/j.neuroimage.2020.117289
Badre, D., and Wagner, A. D. (2004). Selection, integration, and conflict monitoring; assessing the nature and generality of prefrontal cognitive control mechanisms. Neuron 41, 473–487. doi: 10.1016/s0896-6273(03)00851-1
Bagheri, H., Khanmohammadi, R., Olyaei, G., Talebian, S., Reza Hadian, M., and Najafi, M. (2021). Video game and motor-cognitive dual-task training could be suitable treatments to improve dual-task interference in older adults. Neurosci. Lett. 760:136099. doi: 10.1016/j.neulet.2021.136099
Beraud-Peigne, N., Maillot, P., and Perrot, A. (2023). The effects of a new immersive multidomain training on cognitive, dual-task and physical functions in older adults. Geroscience 46, 1825–1841. doi: 10.1007/s11357-023-00952-w
Bherer, L., Gagnon, C., Langeard, A., Lussier, M., Desjardins-Crepeau, L., Berryman, N., et al. (2021). Synergistic effects of cognitive training and physical exercise on dual-task performance in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 76, 1533–1541. doi: 10.1093/geronb/gbaa124
Bherer, L., Kramer, A. F., Peterson, M. S., Colcombe, S., Erickson, K., and Becic, E. (2005). Training effects on dual-task performance: Are there age-related differences in plasticity of attentional control? Psychol. Aging 20, 695–709. doi: 10.1037/0882-7974.20.4.695
Bherer, L., Kramer, A. F., Peterson, M. S., Colcombe, S., Erickson, K., and Becic, E. (2008). Transfer effects in task-set cost and dual-task cost after dual-task training in older and younger adults: Further evidence for cognitive plasticity in attentional control in late adulthood. Exp. Aging Res. 34, 188–219. doi: 10.1080/03610730802070068
Bikson, M., Esmaeilpour, Z., Adair, D., Kronberg, G., Tyler, W. J., Antal, A., et al. (2019). Transcranial electrical stimulation nomenclature. Brain Stimul. 12, 1349–1366. doi: 10.1016/j.brs.2019.07.010
Bikson, M., Grossman, P., Thomas, C., Zannou, A. L., Jiang, J., Adnan, T., et al. (2016). Safety of transcranial direct current stimulation: Evidence based update 2016. Brain Stimul. 9, 641–661. doi: 10.1016/j.brs.2016.06.004
Bisson, E., Contant, B., Sveistrup, H., and Lajoie, Y. (2007). Functional balance and dual-task reaction times in older adults are improved by virtual reality and biofeedback training. Cyberpsychol. Behav. 10, 16–23. doi: 10.1089/cpb.2006.9997
Brustio, P. R., Rabaglietti, E., Formica, S., and Liubicich, M. E. (2018). Dual-task training in older adults: The effect of additional motor tasks on mobility performance. Arch. Gerontol. Geriatr. 75, 119–124. doi: 10.1016/j.archger.2017.12.003
Brzezicka, A., Kamiński, J., Reed, C. M., Chung, J. M., Mamelak, A. N., and Rutishauser, U. (2019). Working memory load-related theta power decreases in dorsolateral prefrontal cortex predict individual differences in performance. J. Cogn. Neurosci. 31, 1290–1307. doi: 10.1162/jocn_a_01417
Chan, W. N., and Tsang, W. W. (2017). Effect of Tai Chi training on dual-tasking performance that involves stepping down among stroke survivors: A pilot study. Evid. Based Complement. Alternat. Med. 2017:9134173. doi: 10.1155/2017/9134173
Chan, W. N., and Tsang, W. W. (2018). The effect of Tai Chi training on the dual-tasking performance of stroke survivors: A randomized controlled trial. Clin. Rehabil. 32, 1076–1085. doi: 10.1177/0269215518777872
Chang, H. C., Chen, C. C., Weng, Y. H., Chiou, W. D., Chang, Y. J., and Lu, C. S. (2020). The efficacy of cognitive-cycling dual-task training in patients with early-stage Parkinson’s disease: A pilot study. NeuroRehabilitation 47, 415–426. doi: 10.3233/NRE-203090
Chen, Y. L., and Pei, Y. C. (2018). Musical dual-task training in patients with mild-to-moderate dementia: A randomized controlled trial. Neuropsychiatr. Dis. Treat. 14, 1381–1393. doi: 10.2147/NDT.S159174
Chiaramonte, R., Bonfiglio, M., Leonforte, P., Coltraro, G. L., Guerrera, C. S., and Vecchio, M. (2022). Proprioceptive and dual-task training: The key of stroke rehabilitation, a systematic review. J. Funct. Morphol. Kinesiol. 7:53. doi: 10.3390/jfmk7030053
Colcombe, S. J., Erickson, K. I., Scalf, P. E., Kim, J. S., Prakash, R., McAuley, E., et al. (2006). Aerobic exercise training increases brain volume in aging humans. J. Gerontol. A Biol. Sci. Med. Sci. 61, 1166–1170. doi: 10.1093/gerona/61.11.1166
Conradsson, D., and Halvarsson, A. (2019). The effects of dual-task balance training on gait in older women with osteoporosis: A randomized controlled trial. Gait Posture 68, 562–568. doi: 10.1016/j.gaitpost.2019.01.005
D’Cruz, N., Seuthe, J., Ginis, P., Hulzinga, F., Schlenstedt, C., and Nieuwboer, A. (2020). Short-term effects of single-session split-belt treadmill training on dual-task performance in Parkinson’s disease and healthy elderly. Front. Neurol. 11:560084. doi: 10.3389/fneur.2020.560084
de Bruin, N., Doan, J. B., Turnbull, G., Suchowersky, O., Bonfield, S., Hu, B., et al. (2010). Walking with music is a safe and viable tool for gait training in Parkinson’s disease: The effect of a 13-week feasibility study on single and dual task walking. Parkinsons Dis. 2010:483530. doi: 10.4061/2010/483530
De Freitas, T., Leite, P., Doná, F., Pompeu, J., Swarowsky, A., and Torriani-Pasin, C. (2020). The effects of dual task gait and balance training in Parkinson’s disease: A systematic review. Physiother. Theory Pract. 36, 1088–1096. doi: 10.1080/09593985.2018.1551455
Delbroek, T., Vermeylen, W., and SpilDooren, J. (2017). The effect of cognitive-motor dual task training with the biorescue force platform on cognition, balance and dual task performance in institutionalized older adults: A randomized controlled trial. J. Phys. Ther. Sci. 29:8.
Dorfman, M., Herman, T., Brozgol, M., Shema, S., Weiss, A., Hausdorff, J. M., et al. (2014). Dual-task training on a treadmill to improve gait and cognitive function in elderly idiopathic fallers. J. Neurol. Phys. Ther. 38, 246–253. doi: 10.1097/NPT.0000000000000057
Dubreuil-Vall, L., Chau, P., Ruffini, G., Widge, A. S., and Camprodon, J. A. (2019). tDCS to the left DLPFC modulates cognitive and physiological correlates of executive function in a state-dependent manner. Brain Stimul. 12, 1456–1463. doi: 10.1016/j.brs.2019.06.006
Eggenberger, P., Theill, N., Holenstein, S., Schumacher, V., and de Bruin, E. D. (2015). Multicomponent physical exercise with simultaneous cognitive training to enhance dual-task walking of older adults: A secondary analysis of a 6-month randomized controlled trial with 1-year follow-up. Clin. Interv. Aging 10, 1711–1732. doi: 10.2147/CIA.S91997
Faraza, S., Waldenmaier, J., Dyrba, M., Wolf, D., Fischer, F. U., Knaepen, K., et al. (2021). Dorsolateral prefrontal functional connectivity predicts working memory training gains. Front. Aging Neurosci. 13:592261. doi: 10.3389/fnagi.2021.592261
Fraser, S. A., Elliott, V., de Bruin, E. D., Bherer, L., and Dumoulin, C. (2014). The effects of combining videogame dancing and pelvic floor training to improve dual-task gait and cognition in women with mixed-urinary incontinence. Games Health J. 3, 172–178. doi: 10.1089/g4h.2013.0095
Fraser, S. A., Li, K. Z., Berryman, N., Desjardins-Crepeau, L., Lussier, M., Vadaga, K., et al. (2016). Does combined physical and cognitive training improve dual-task balance and gait outcomes in sedentary older adults? Front. Hum. Neurosci. 10:688. doi: 10.3389/fnhum.2016.00688
Furuya, S., Klaus, M., Nitsche, M. A., Paulus, W., and Altenmüller, E. (2014). Ceiling effects prevent further improvement of transcranial stimulation in skilled musicians. J. Neurosci. 34, 13834–13839. doi: 10.1523/jneurosci.1170-14.2014
Gallou-Guyot, M., Mandigout, S., Bherer, L., and Perrochon, A. (2020). Effects of exergames and cognitive-motor dual-task training on cognitive, physical and dual-task functions in cognitively healthy older adults: An overview. Ageing Res. Rev. 63:101135. doi: 10.1016/j.arr.2020.101135
Gassner, H., Trutt, E., Seifferth, S., Friedrich, J., Zucker, D., Salhani, Z., et al. (2022). Treadmill training and physiotherapy similarly improve dual task gait performance: A randomized-controlled trial in Parkinson’s disease. J. Neural Transm. 129, 1189–1200. doi: 10.1007/s00702-022-02514-4
Ghadiri, F., Bahmani, M., Paulson, S., and Sadeghi, H. (2022). Effects of fundamental movement skills based dual-task and dance training on single- and dual-task walking performance in older women with dementia. Geriatr. Nurs. 45, 85–92. doi: 10.1016/j.gerinurse.2022.03.003
Gregory, M. A., Boa Sorte Silva, N. C., Gill, D. P., McGowan, C. L., Liu-Ambrose, T., Shoemaker, J. K., et al. (2017). Combined Dual-task gait training and aerobic exercise to improve cognition, mobility, and vascular health in community-dwelling older adults at risk for future cognitive decline1. J. Alzheimers Dis. 57, 747–763. doi: 10.3233/JAD-161240
Gregory, M. A., Gill, D. P., Zou, G., Liu-Ambrose, T., Shigematsu, R., Fitzgerald, C., et al. (2016). Group-based exercise combined with dual-task training improves gait but not vascular health in active older adults without dementia. Arch. Gerontol. Geriatr. 63, 18–27. doi: 10.1016/j.archger.2015.11.008
Grossman, P., Alekseichuk, I., de Lara, G., Paneri, K., Kunz, P., Turi, Z., et al. (2018). Transcranial direct current stimulation studies open database. BioRxiv [Preprint]. doi: 10.1101/369215
Harada, C. N., Natelson Love, M. C., and Triebel, K. L. (2013). Normal cognitive aging. Clin. Geriatr. Med. 29, 737–752. doi: 10.1016/j.cger.2013.07.002
Hashemirad, F., Fitzgerald, P. B., Zoghi, M., and Jaberzadeh, S. (2017). Single-Session anodal tDCS with small-size stimulating electrodes over frontoparietal superficial sites does not affect motor sequence learning. Front. Hum. Neurosci. 11:153. doi: 10.3389/fnhum.2017.00153
Heinzel, S., Rimpel, J., Stelzel, C., and Rapp, M. A. (2017). Transfer effects to a multimodal dual-task after working memory training and associated neural correlates in older adults – a pilot study. Front. Hum. Neurosci. 11:85. doi: 10.3389/fnhum.2017.00085
Hertrich, I., Dietrich, S., Blum, C., and Ackermann, H. (2021). The role of the dorsolateral prefrontal cortex for speech and language processing. Front. Hum. Neurosci. 15:645209. doi: 10.3389/fnhum.2021.645209
Hiyamizu, M., Morioka, S., Shomoto, K., and Shimada, T. (2012). Effects of dual task balance training on dual task performance in elderly people: A randomized controlled trial. Clin. Rehabil. 26, 58–67. doi: 10.1177/0269215510394222
Holtzer, R., Stern, Y., and Rakitin, B. C. (2005). Predicting age-related dual-task effects with individual differences on neuropsychological tests. Neuropsychology 19, 18–27. doi: 10.1037/0894-4105.19.1.18
Hoy, K. E., Bailey, N., Arnold, S., Windsor, K., John, J., Daskalakis, Z. J., et al. (2015). The effect of γ-tACS on working memory performance in healthy controls. Brain Cogn. 101, 51–56. doi: 10.1016/j.bandc.2015.11.002
Javadpour, S., Sinaei, E., Salehi, R., Zahednejad, S., and Motealleh, A. (2022). Comparing the effects of single-task versus dual-task balance training on gait smoothness and functional balance in community-dwelling older adults: A randomized controlled trial. J. Aging Phys. Act. 30, 308–315. doi: 10.1123/japa.2020-0523
Jiang, N., Wei, J., Li, G., Wei, B., Zhu, F. F., and Hu, Y. (2020). Effect of dry-electrode-based transcranial direct current stimulation on chronic low back pain and low back muscle activities: A double-blind sham-controlled study. Restor. Neurol. Neurosci. 38, 41–54. doi: 10.3233/rnn-190922
Jor’dan, A. J., Bernad-Elazari, H., Mirelman, A., Gouskova, N. A., Lo, O. Y., Hausdorff, J. M., et al. (2022). Transcranial direct current stimulation may reduce prefrontal recruitment during dual task walking in functionally limited older adults – a pilot study. Front. Aging Neurosci. 14:843122. doi: 10.3389/fnagi.2022.843122
Kahya, M., Moon, S., Ranchet, M., Vukas, R. R., Lyons, K. E., Pahwa, R., et al. (2019). Brain activity during dual task gait and balance in aging and age-related neurodegenerative conditions: A systematic review. Exp. Gerontol. 128:110756. doi: 10.1016/j.exger.2019.110756
Khan, M. J., Kannan, P., Wong, T. W., Fong, K. N. K., and Winser, S. J. (2022). A systematic review exploring the theories underlying the improvement of balance and reduction in falls following dual-task training among older adults. Int. J. Environ. Res. Public Health 19:16890. doi: 10.3390/ijerph192416890
Killane, I., Fearon, C., Newman, L., McDonnell, C., Waechter, S. M., Sons, K., et al. (2015). Dual Motor-cognitive virtual reality training impacts dual-task performance in freezing of gait. IEEE J. Biomed. Health Inform. 19, 1855–1861. doi: 10.1109/JBHI.2015.2479625
Kim, J., Kim, H., Jeong, H., Roh, D., and Kim, D. H. (2021). tACS as a promising therapeutic option for improving cognitive function in mild cognitive impairment: A direct comparison between tACS and tDCS. J. Psychiatr. Res. 141, 248–256. doi: 10.1016/j.jpsychires.2021.07.012
Korai, S. A., Ranieri, F., Di Lazzaro, V., Papa, M., and Cirillo, G. (2021). Neurobiological After-effects of low intensity transcranial electric stimulation of the human nervous system: From basic mechanisms to metaplasticity. Front. Neurol. 12:587771. doi: 10.3389/fneur.2021.587771
Kramer, A. F., Larish, J. F., and Strayer, D. L. (1995). Training for attentional control in dual task settings: A comparison of young and old adults. J. Exp. Psychol. Appl. 1, 50–76.
Kuo, H. T., Yeh, N. C., Yang, Y. R., Hsu, W. C., Liao, Y. Y., and Wang, R. Y. (2022). Effects of different dual task training on dual task walking and responding brain activation in older adults with mild cognitive impairment. Sci. Rep. 12:8490. doi: 10.1038/s41598-022-11489-x
Lemke, N. C., Werner, C., Wiloth, S., Oster, P., Bauer, J. M., and Hauer, K. (2019). Transferability and Sustainability of motor-cognitive dual-task training in patients with dementia: A randomized controlled trial. Gerontology 65, 68–83. doi: 10.1159/000490852
Li, X., Zheng, M., Zhang, Y., Wang, Y., Nie, L., Yuan, Y., et al. (2022). Music-based casual video game training alleviates symptoms of subthreshold depression. Front. Public Health 10:961425. doi: 10.3389/fpubh.2022.961425
Liao, Y. Y., Chen, I. H., Lin, Y. J., Chen, Y., and Hsu, W. C. (2019). Effects of virtual reality-based physical and cognitive training on executive function and dual-task gait performance in older adults with mild cognitive impairment: A randomized control trial. Front. Aging Neurosci. 11:162. doi: 10.3389/fnagi.2019.00162
Liao, Y. Y., Liu, M. N., Wang, H. C., Walsh, V., and Lau, C. I. (2021). Combining transcranial direct current stimulation with tai chi to improve dual-task gait performance in older adults with mild cognitive impairment: A randomized controlled trial. Front. Aging Neurosci. 13:766649. doi: 10.3389/fnagi.2021.766649
Ljubisavljevic, M. R., Oommen, J., Filipovic, S., Bjekic, J., Szolics, M., and Nagelkerke, N. (2019). Effects of tDCS of dorsolateral prefrontal cortex on dual-task performance involving manual dexterity and cognitive task in healthy older adults. Front. Aging Neurosci. 11:144. doi: 10.3389/fnagi.2019.00144
Maillot, P., Perrot, A., and Hartley, A. (2012). Effects of interactive physical-activity video-game training on physical and cognitive function in older adults. Psychol. Aging 27, 589–600. doi: 10.1037/a0026268
Manor, B., Zhou, J., Jor’dan, A., Zhang, J., Fang, J., and Pascual-Leone, A. (2016). Reduction of dual-task costs by noninvasive modulation of prefrontal activity in healthy elders. J. Cogn. Neurosci. 28, 275–281. doi: 10.1162/jocn_a_00897
Meester, D., Al-Yahya, E., Dennis, A., Collett, J., Wade, D. T., Ovington, M., et al. (2019). A randomized controlled trial of a walking training with simultaneous cognitive demand (dual-task) in chronic stroke. Eur. J. Neurol. 26, 435–441. doi: 10.1111/ene.13833
Mishra, R. K., and Thrasher, A. T. (2021). Transcranial direct current stimulation of dorsolateral prefrontal cortex improves dual-task gait performance in patients with Parkinson’s disease: A double blind, sham-controlled study. Gait Posture 84, 11–16. doi: 10.1016/j.gaitpost.2020.11.012
Miyaguchi, S., Inukai, Y., Matsumoto, Y., Miyashita, M., Takahashi, R., Otsuru, N., et al. (2020). Effects on motor learning of transcranial alternating current stimulation applied over the primary motor cortex and cerebellar hemisphere. J. Clin. Neurosci. 78, 296–300. doi: 10.1016/j.jocn.2020.05.024
Montero-Odasso, M. M., Sarquis-Adamson, Y., Speechley, M., Borrie, M. J., Hachinski, V. C., Wells, J., et al. (2017). Association of dual-task gait with incident dementia in mild cognitive impairment: Results from the gait and brain study. JAMA Neurol. 74, 857–865. doi: 10.1001/jamaneurol.2017.0643
Navon, D., and Gopher, D. (1979). On the economy of the human processing system. Psychol. Rev. 86, 214–255.
Nitsche, M. A., and Paulus, W. (2000). Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 527:7.
Nouchi, R., Nouchi, H., Dinet, J., and Kawashima, R. (2021). Cognitive Training with neurofeedback using NIRS improved cognitive functions in young adults: Evidence from a randomized controlled trial. Brain Sci. 12:5. doi: 10.3390/brainsci12010005
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Rev. Esp. Cardiol. 74, 790–799. doi: 10.1016/j.rec.2021.07.010
Pang, M. Y. C., Yang, L., Ouyang, H., Lam, F. M. H., Huang, M., and Jehu, D. A. (2018). Dual-task exercise reduces cognitive-motor interference in walking and falls after stroke. Stroke 49, 2990–2998. doi: 10.1161/STROKEAHA.118.022157
Pashler, H. (1994). Dual-task interference in simple tasks: Data and theory. Psychol. Bull. 116, 220–244. doi: 10.1037/0033-2909.116.2.220
Pashler, H., and Baylis, G. (1991). Procedural learning: 1. Locus of practice effects in speeded choice tasks. J. Exp. Psychol. Learn. Mem. Cogn. 17, 20–32.
Patel, H. J., Romanzetti, S., Pellicano, A., Nitsche, M. A., Reetz, K., and Binkofski, F. (2019). Proton magnetic resonance spectroscopy of the motor cortex reveals long term GABA change following anodal transcranial direct current stimulation. Sci. Rep. 9:2807. doi: 10.1038/s41598-019-39262-7
Pichierri, G., Coppe, A., Lorenzetti, S., Murer, K., and de Bruin, E. D. (2012a). The effect of a cognitive-motor intervention on voluntary step execution under single and dual task conditions in older adults: A randomized controlled pilot study. Clin. Interv. Aging 7, 175–184. doi: 10.2147/CIA.S32558
Pichierri, G., Murer, K., and de Bruin, E. D. (2012b). A cognitive-motor intervention using a dance video game to enhance foot placement accuracy and gait under dual task conditions in older adults: A randomized controlled trial. BMC Geriatr. 12:74. doi: 10.1186/1471-2318-12-74
Pliske, G., Emmermacher, P., Weinbeer, V., and Witte, K. (2016). Changes in dual-task performance after 5 months of karate and fitness training for older adults to enhance fall prevention. Aging Clin. Exp. Res. 28, 1179–1186. doi: 10.1007/s40520-015-0508-z
Plummer-D’Amato, P., Cohen, Z., Daee, N. A., Lawson, S. E., Lizotte, M. R., and Padilla, A. (2012). Effects of once weekly dual-task training in older adults: A pilot randomized controlled trial. Geriatr. Gerontol. Int. 12, 622–629. doi: 10.1111/j.1447-0594.2011.00825.x
Raichlen, D. A., Bharadwaj, P. K., Nguyen, L. A., Franchetti, M. K., Zigman, E. K., Solorio, A. R., et al. (2020). Effects of simultaneous cognitive and aerobic exercise training on dual-task walking performance in healthy older adults: Results from a pilot randomized controlled trial. BMC Geriatr. 20:83. doi: 10.1186/s12877-020-1484-5
Reve, E. V., and de Bruin, E. D. (2014). Strength-balance supplemented with computerized cognitive training to improve dual task gait and divided attention in older adults: A multicenter randomized-controlled trial. BMC Geriatr. 14:134. doi: 10.1186/1471-2318-14-134
Rezola-Pardo, C., Arrieta, H., Gil, S. M., Zarrazquin, I., Yanguas, J. J., Lopez, M. A., et al. (2019). Comparison between multicomponent and simultaneous dual-task exercise interventions in long-term nursing home residents: The ageing-ONDUAL-TASK randomized controlled study. Age Ageing 48, 817–823. doi: 10.1093/ageing/afz105
Sala, S. D., and Logie, R. H. (2001). Theoretical and practical implications of dual-task performance in Alzheimer’s disease. Brain 124, 1479–1481. doi: 10.1093/brain/124.8.1479
San Martin Valenzuela, C., Moscardo, L. D., Lopez-Pascual, J., Serra-Ano, P., and Tomas, J. M. (2020). Effects of dual-task group training on gait, cognitive executive function, and quality of life in people with Parkinson disease: Results of randomized controlled DUALGAIT trial. Arch. Phys. Med. Rehabil. 184:e1841. doi: 10.1016/j.apmr.2020.07.008
Satorres, E., Melendez, J. C., Pitarque, A., Real, E., Abella, M., and Escudero, J. (2022). Enhancing Immediate memory, potential learning, and working memory with transcranial direct current stimulation in healthy older adults. Int. J. Environ. Res. Public Health 19:12716. doi: 10.3390/ijerph191912716
Sayig-Keren, R. M., Dagan, M., Cornejo Thumm, P., Brozgol, M., Gazit, E., Manor, B., et al. (2023). The potential of transcranial alternating current stimulation to alleviate dual-task gait costs in older adults: Insights from a double-blinded pilot study. Gerontology 69, 513–518. doi: 10.1159/000527171
Schabrun, S. M., Lamont, R. M., and Brauer, S. G. (2016). Transcranial direct current stimulation to enhance dual-task gait training in Parkinson’s disease: A pilot RCT. PLoS One 11:e0158497. doi: 10.1371/journal.pone.0158497
Schneider, N., Dagan, M., Katz, R., Thumm, P. C., Brozgol, M., Giladi, N., et al. (2021). Combining transcranial direct current stimulation with a motor-cognitive task: The impact on dual-task walking costs in older adults. J. Neuroeng. Rehabil. 18:23. doi: 10.1186/s12984-021-00826-2
Schumacher, E. H., Seymour, T. L., Glass, J. M., Fencsik, D. E., Lauber, E. J., Kieras, D. E., et al. (2001). Virtually perfect time sharing in dual-task performance: Uncorking the central cognitive bottleneck. Psychol. Sci. 12, 101–108. doi: 10.1111/1467-9280.00318
Schwenk, M., Zieschang, T., Oster, P., and Hauer, K. (2010). Dual-task performances can be improved in patients with dementia. Neurology 74, 1961–1968. doi: 10.1212/WNL.0b013e3181e39696
Silsupadol, P., Shumway-Cook, A., Lugade, V., van Donkelaar, P., Chou, L. S., Mayr, U., et al. (2009). Effects of single-task versus dual-task training on balance performance in older adults: A double-blind, randomized controlled trial. Arch. Phys. Med. Rehabil. 90, 381–387. doi: 10.1016/j.apmr.2008.09.559
Soleimani, G., Towhidkhah, F., Oghabian, M. A., and Ekhtiari, H. (2022). DLPFC stimulation alters large-scale brain networks connectivity during a drug cue reactivity task: A tDCS-fMRI study. Front. Syst. Neurosci. 16:956315. doi: 10.3389/fnsys.2022.956315
Strobach, T., Liepelt, R., Pashler, H., Frensch, P. A., and Schubert, T. (2013). Effects of extensive dual-task practice on processing stages in simultaneous choice tasks. Atten. Percept. Psychophys. 75, 900–920. doi: 10.3758/s13414-013-0451-z
Strobach, T., Salminen, T., Karbach, J., and Schubert, T. (2014). Practice-related optimization and transfer of executive functions: A general review and a specific realization of their mechanisms in dual tasks. Psychol. Res. 78, 836–851. doi: 10.1007/s00426-014-0563-7
Strouwen, C., Molenaar, E., Munks, L., Broeder, S., Ginis, P., Bloem, B. R., et al. (2019). Determinants of dual-task training effect size in Parkinson disease: Who will benefit most? J. Neurol. Phys. Ther. 43, 3–11. doi: 10.1097/NPT.0000000000000247
Sugata, H., Yagi, K., Yazawa, S., Nagase, Y., Tsuruta, K., Ikeda, T., et al. (2018). Modulation of motor learning capacity by transcranial alternating current stimulation. Neuroscience 391, 131–139. doi: 10.1016/j.neuroscience.2018.09.013
Swank, C., Mehta, J., and Criminger, C. (2016). Transcranial direct current stimulation lessens dual task cost in people with Parkinson’s disease. Neurosci. Lett. 626, 1–5. doi: 10.1016/j.neulet.2016.05.010
Tasvuran, H. E., Cetin, S. Y., and Erel, S. (2021). Effects of individual progressive single- and dual-task training on gait and cognition among older healthy adults: A randomized-controlled comparison study. Eur. Geriatr. Med. 12, 363–370. doi: 10.1007/s41999-020-00429-5
Trombini-Souza, F., de Moura, V. T. G., da Silva, L. W. N., Leal, I. D. S., Nascimento, C. A., Silva, P. S. T., et al. (2023). Effects of two different dual-task training protocols on gait, balance, and cognitive function in community-dwelling older adults: A 24-week randomized controlled trial. PeerJ 11:e15030. doi: 10.7717/peerj.15030
UzunkulaoGlu, A., KerIm, D., Ay, S., and ErgIn, S. (2020). Effects of single-task versus dual-task training on balance performance in elderly patients with knee osteoarthritis. Arch. Rheumatol. 35, 35–40. doi: 10.5606/ArchRheumatol.2020.7174
Varela-Vasquez, L. A., Minobes-Molina, E., and Jerez-Roig, J. (2020). Dual-task exercises in older adults: A structured review of current literature. J. Frailty Sarcopenia Falls 5, 31–37. doi: 10.22540/JFSF-05-031
Vrinceanu, T., Blanchette, C. A., Intzandt, B., Lussier, M., Pothier, K., Vu, T. T. M., et al. (2022). A comparison of the effect of physical activity and cognitive training on dual-task performance in older adults. J. Gerontol. B Psychol. Sci. Soc. Sci. 77, 1069–1079. doi: 10.1093/geronb/gbab216
Wayne, P. M., Hausdorff, J. M., Lough, M., Gow, B. J., Lipsitz, L., Novak, V., et al. (2015). Tai Chi Training may reduce dual task gait variability, a potential mediator of fall risk, in healthy older adults: Cross-sectional and randomized trial studies. Front. Hum. Neurosci. 9:332. doi: 10.3389/fnhum.2015.00332
Wollesen, B., Schulz, S., Seydell, L., and Delbaere, K. (2017b). Does dual task training improve walking performance of older adults with concern of falling? BMC Geriatr. 17:213. doi: 10.1186/s12877-017-0610-5
Wollesen, B., Mattes, K., Schulz, S., Bischoff, L. L., Seydell, L., Bell, J. W., et al. (2017a). Effects of dual-task management and resistance training on gait performance in older individuals: A randomized controlled trial. Front. Aging Neurosci. 9:415. doi: 10.3389/fnagi.2017.00415
Wollesen, B., Rudnik, S., Gulberti, A., Cordes, T., Gerloff, C., and Poetter-Nerger, M. (2021). A feasibility study of dual-task strategy training to improve gait performance in patients with Parkinson’s disease. Sci. Rep. 11:12416. doi: 10.1038/s41598-021-91858-0
Wollesen, B., Voelcker-Rehage, C., Willer, J., Zech, A., and Mattes, K. (2015). Feasibility study of dual-task-managing training to improve gait performance of older adults. Aging Clin. Exp. Res. 27, 447–455. doi: 10.1007/s40520-014-0301-4
Wongcharoen, S., Sungkarat, S., Munkhetvit, P., Lugade, V., and Silsupadol, P. (2017). Home-based interventions improve trained, but not novel, dual-task balance performance in older adults: A randomized controlled trial. Gait Posture 52, 147–152. doi: 10.1016/j.gaitpost.2016.11.036
Yang, Y. R., Cheng, S. J., Lee, Y. J., Liu, Y. C., and Wang, R. Y. (2019). Cognitive and motor dual task gait training exerted specific training effects on dual task gait performance in individuals with Parkinson’s disease: A randomized controlled pilot study. PLoS One 14:e0218180. doi: 10.1371/journal.pone.0218180
Yuzlu, V., Oguz, S., Timurtas, E., Aykutoglu, E., and Polat, M. G. (2022). The Effect of 2 different dual-task balance training methods on balance and gait in older adults: A randomized controlled trial. Phys. Ther. 102:zab298. doi: 10.1093/ptj/pzab298
Zhou, J., Hao, Y., Wang, Y., Jor’dan, A., Pascual-Leone, A., Zhang, J., et al. (2014). Transcranial direct current stimulation reduces the cost of performing a cognitive task on gait and postural control. Eur. J. Neurosci. 39, 1343–1348. doi: 10.1111/ejn.12492
Keywords: non-invasive brain stimulation, multitasking, task training, elderly, tDCS
Citation: Jiang Y, Ramasawmy P and Antal A (2024) Uncorking the limitation—improving dual tasking using transcranial electrical stimulation and task training in the elderly: a systematic review. Front. Aging Neurosci. 16:1267307. doi: 10.3389/fnagi.2024.1267307
Received: 26 July 2023; Accepted: 22 March 2024;
Published: 08 April 2024.
Edited by:
Kristy A. Nielson, Marquette University, United StatesReviewed by:
Dongning Su, Capital Medical University, ChinaHeather Brooks, University of Toronto, Canada
Sara Assecondi, University of Trento, Italy
Copyright © 2024 Jiang, Ramasawmy and Antal. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Jiang, eW9uZy5qaWFuZ0BtZWQudW5pLWdvZXR0aW5nZW4uZGU=
 Yong Jiang
Yong Jiang