- 1Department of Exercise Physiology, Faculty of Physical Education and Sports Science, Kharazmi University, Tehran, Iran
- 2Department of Neurology, Roozbeh Hospital, Tehran University of Medical Science, Tehran, Iran
- 3Faculty of Sport Sciences, Waseda University, Tokorozawa, Japan
Introduction: This study aimed to investigate the effects of combined remote music and exercise training on the cognitive, psychological, and physical function of patients with Alzheimer’s disease (AD).
Methods: Forty-one AD patients were randomly allocated to three groups, including control (C), training (T), and training with music (TM) groups. Participants were evaluated by cognitive and performance test batteries before and after the interventions. Both experimental groups performed 36 remote workouts in 3 months online via WhatsApp video call individually with the trainer. Training included simple and varied movements of all physical indicators. The number of sets began with two sets and progressively increased to one set every month, 5–10 repetitions per set. The overload was applied by reducing the break between sets every week. The TM group performed the same exercises while listening to Mozart and traditional Iranian songs.
Results: We observed a significant main, group, time, and interaction effect on Romberg (ηp2:0.72), 30 s chair sit and stand (ηp2:0.75), and walking on steppe test (ηp2:0.63). Furthermore, there was a significant main time and interaction effect on push-ups (ηp2:0.43), sit and reach (ηp2:0.64), and MMSE (ηp2:0.76). In all variables, two experimental groups demonstrated substantial improvements than the C group (p < 0.01). In addition, the TM group (27.8%) showed a significant improvement compared to the C group (−6.4%) and the T group (12.2%) in MMSE.
Conclusion: Combined remote training with listening to music as adjuvant treatment is an appropriate item to improve the cognitive and physical performance of Alzheimer’s patients, especially during the COVID-19 pandemic.
Introduction
Alzheimer’s disease (AD) is the most common type of dementia; the primary symptoms include impairments in cognitive function such as recent memory, attention, language, visuospatial, and praxis. The patients also have difficulties in physical function and sleep quality. Although medications have limited these effects, they also have side effects, including nausea, anorexia, diarrhea, vomiting, and weight loss (DeLaGarza, 2003). Hence, non-pharmacological treatments such as exercise, social activity, mental challenges, and a healthy diet as prevention strategies with fewer side effects are recommended (Mendiola-Precoma et al., 2016; Nemoto and Suzuki, 2018).
Research has shown that exercise training effectively reduces the complications of all types of dementia (Santana-Sosa et al., 2008). In this regard, the positive benefits of resistance training on physical performance and limiting AD progression due to increased strength and muscle mass among old adults have been reported (Tracy et al., 1999; Bamman et al., 2003). On the other hand, aerobic training significantly improves activities of daily living, cardiorespiratory fitness, and a slower decline in cognitive function in patients with AD (Venturelli et al., 2011; Sobol et al., 2016). In general, a combination of strength and endurance training, combined training, is recommended for Alzheimer’s patients to get the maximum benefits (Parvin et al., 2020). However, due to the low effectiveness of physical activity on cognitive function, adding a mental task to exercise training, known as dual-task training, has more significant effects on the mind and physical fitness of Alzheimer’s patients (Dennis et al., 2009; Parvin et al., 2020). Researchers have shown that dual-task training has more positive effects, such as improving attention and accuracy, on Alzheimer’s patients rather than the exercise alone (Åhman et al., 2019; Nam and Kim, 2021).
Music therapy is an important, low-cost, and effective way to maintain and improve cognitive function and social behavior in Alzheimer’s patients (Raglio et al., 2014). Music therapy has no side effects, so patients and their caregivers can easily use it. Many studies have shown that music therapy can improve various cognitive and psychological aspects such as attention, memory, orientation, depression, and anxiety (Bruer et al., 2007; Ozdemir and Akdemir, 2009; Särkämö et al., 2014; Satoh et al., 2015). Individuals can exercise while music is playing or perform each one separately. A few research studies have been done on the combined effects of music therapy and exercise so far; in these studies, cognitive and exercise stimuli were not applied simultaneously. Recently, Higuti et al. (2021) reported listening to music and practicing physical exercise for 12 weeks; one session per week (25–30 min) did not significantly change functional or cognitive performance in institutionalized older adults with dementia (Higuti et al., 2021). In contrast, some studies that have used more frequency (twice a week) and duration (one hour) of exercise and music sessions reported improvements in cognitive, psychological, and motor abilities in patients with mild to moderate dementia (Satoh et al., 2014, 2017; Prinz et al., 2021). Therefore, a combination of music therapy and exercise as dual-task training can be an excellent option to improve AD patients’ physical and cognitive fitness.
Listening to music in healthy human can modulate many physiological responses even during exercise (i.e., heart rate, catecholamines, muscle activation), which leads to improved performance (Ballmann, 2021). Furthermore, listening to music during exercise has positive impact on psychological (i.e., mood, motivation) and psychophysiological (i.e., rate of perceived exertion, arousal) status, which may have ergogenic potential. Moreover, research showed that music increases activity in some portions of the brain such as left inferior frontal gyrus and insular cortex activation that are important for physiological arousal, emotion, and perception (Bigliassi et al., 2018). So, listening to music during exercise could activate these brain regions and lead to an increase in cognitive processing speed and organization of movement (Bacon et al., 2012). Therefore, it seems that simultaneous exercise and music through neural activation and arousal have augmentation physiological and psychological effects to improve cognitive and physical performance.
Given that the physical effects of exercise training and the mental function of music therapy are more pronounced, we hypothesized that the combination of these two stimuli has a synergic impact on improving AD patients’ physical and mental function. Due to the pandemic of COVID-19 infection, we conducted the study online with a video call for the safety of patients and their caregivers. Therefore, the present study aimed to investigate the effects of adding music to exercise training on the mental and physical fitness of Alzheimer’s patients.
Materials and methods
Study design
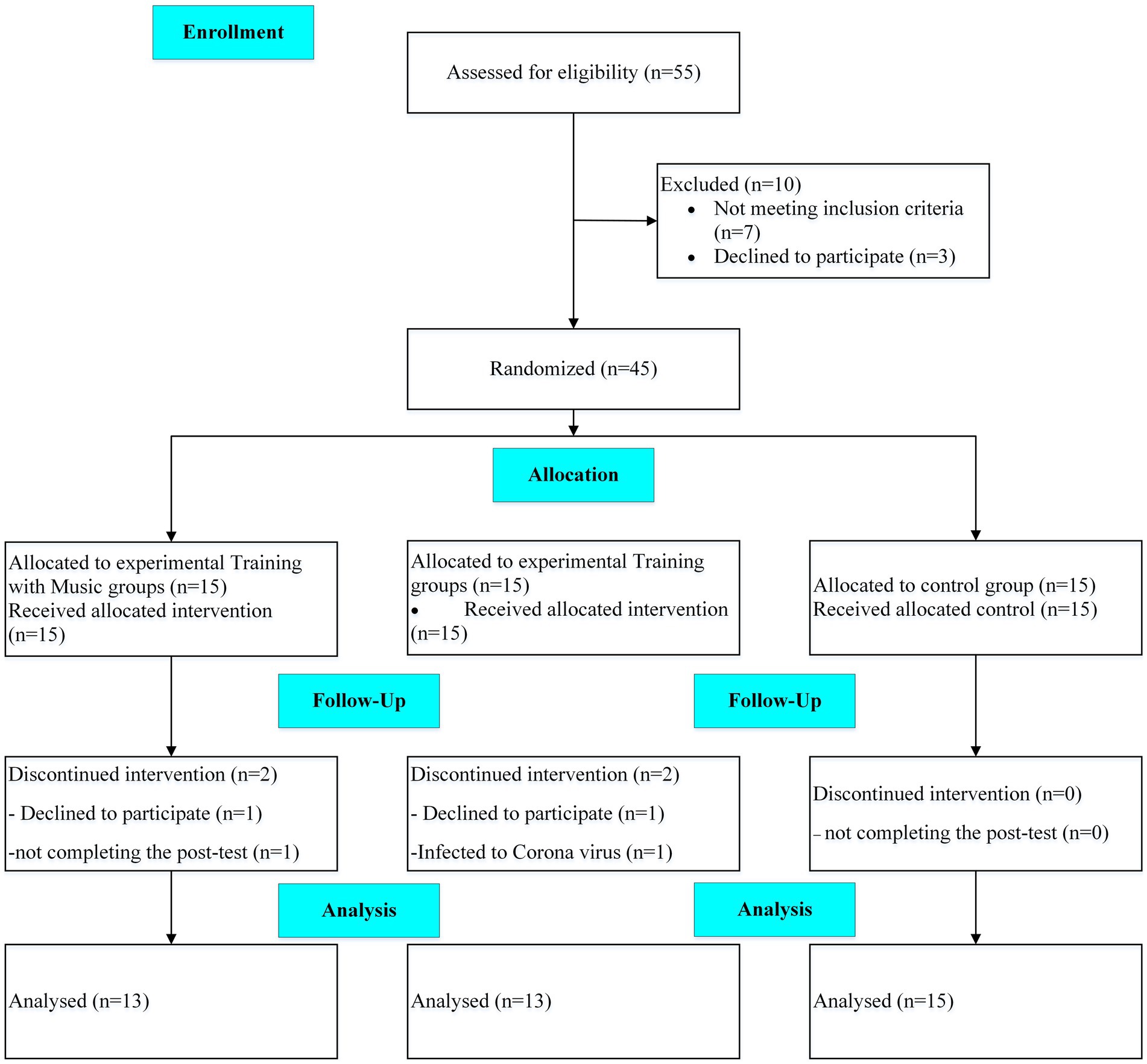
A randomized clinical trial, with control and two parallel experimental groups and a single-blind design, was conducted on AD patients from May 2021 until August 2021. This study aimed to investigate the effects of 12 weeks of adding music therapy to exercise training remotely on the physical and cognitive performance of patients with AD. The participants and their caregivers attended a familiarization session 1 week before the study. They were informed about the benefits and potential risks of the study, signed a consent form and cognitive and performance tests were taken. The block randomization method (size 6) was applied, and the participants were assigned to three groups. Because of the COVID-19 pandemic, the training sessions were executed online. A neurologist performed the cognitive test, and the trainer took the performance tests. A CONSORT flow diagram of the present study is shown in Figure 1.
Participants
Patients with AD, who were eligible to participate in this Clinical Trials study, were recruited from the memory clinic of Roozbeh Hospital in Tehran, Iran. The inclusion criteria were AD patients aged 50–75 years with mild to moderate dementia and the ability to walk and perform exercise independently. A neurologist affirmed the diagnosis of dementia based on the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) criteria, low to moderate stages of dementia based on the FAST scale (3–6), and laboratory tests. The patients’ medications were checked before beginning the study and were not changed in type or dosage during the intervention. In addition, patients with cardiac diseases (e.g., unstable angina and recent myocardial infarct) were not recruited. The exclusion criteria were the absence in more than three consecutive sessions, the unwillingness to continue training, the inability to perform exercises, not take post-tests, and the physician’s decision to exclude the patients. A sample size calculation was conducted using G*Power Software version 3.1.9.6 (Faul et al., 2007) for repeated measure ANOVA, using a rejection criterion of 0.05 and 0.8 (1-beta) power, and large effect (f = 0.5), a minimum of 13 participants needed to each group.
Forty-five eligible patients volunteered to attend the study, but the data of 41 patients (age: 67.5 ± 8.2 years; height: 169.4 ± 6.8 cm, body mass: 75.8 ± 7.7 kg) was analyzed finally. A third person who was not in the research team, based on the cognitive test scores, randomly assigned participants into three groups (n = 15), including training (T), training with music (TM), and the control group (C). Two participants from each experimental group were either not interested in continuing the intervention, infected with the coronavirus, or did not complete the post-test.
Training protocol
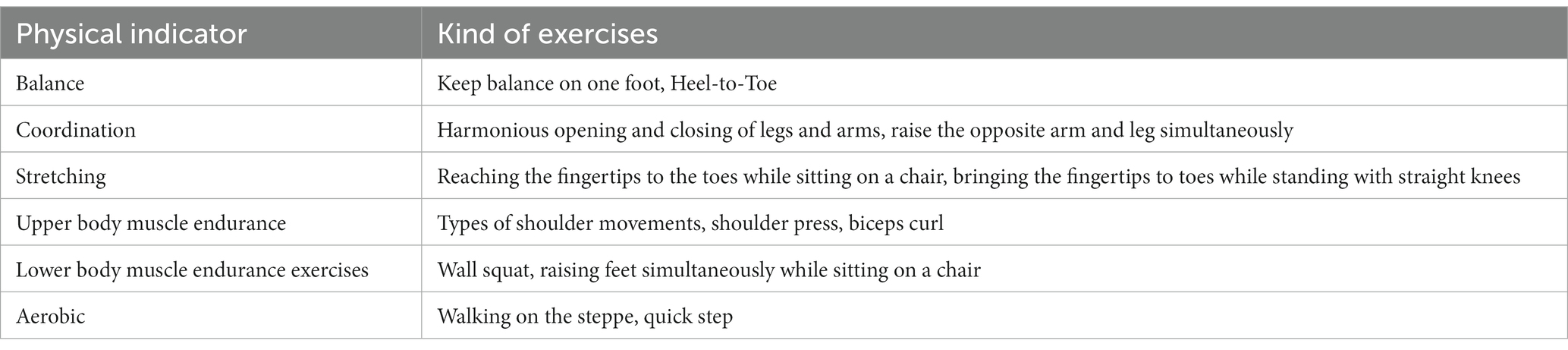
The intervention lasted 3 months, 3 days per week (36 workouts). Each session was around 35–45 min, including 10 min of warm-up with dynamic movements, 20–35 min of main exercises, and 5 minutes of cool down with stretching movements. Because of the Coronavirus pandemic, all workouts were conducted online via WhatsApp video call individually with the trainer (researcher). The presence of a caregiver in the training session was mandatory. The training group performed a combination of sitting and standing movements. The movements involved the whole body and large muscle groups. The main exercises are presented in Table 1. In each session, exercises were selected from all indicators of physical fitness. The break between sets started at 70 s, and gradually it reduced 10 s every two weeks until the tenth week eventually reduced by 5 s to reach 25 s. The number of sets began with two sets and progressively increased to one set every month, 5–10 repetitions per set depending on the difficulty of the movement and the patient’s fitness. The trainer explained and demonstrated the activities to patients clearly. The TM group performed the same exercises while listening to the mix of Mozart’s sonatas and traditional and instrumental Persian music (Johnson et al., 1998; Li et al., 2015). We used the examiner-choose music playlist based on expert cognitive neurologist opinion, including some sort of Mozart music Symphonia, and also traditional Iranian music by the patient’s selection. Since familiarity with the music playlist could have positive effects on the patient’s cognitive function and probably mood improvements, we preferred to utilize the mix of classical Mozart music with established impact on cognitive function and a more familiar music playlist with possible familiarity evidence on cognition function. The session started with Mozart’s sonatas for 10–15 min, continued with Persian music, and then during the cool down, Mozart’s sonatas were played. The patient’s caregivers played music because of the greater clarity of the sound. Music was played throughout the exercise session. The overload was applied by reducing the break between sets every week (every three sessions). The training intensity was monitored using the Borg scale, a 10-point scale, in which we prescribed exercises in intensities of 4 to 6 on this scale. The C group did not receive any physical or cognitive intervention and continued their routine life; they participated in the pre and post-tests.
Measurements
All the following tests were taken before the implementation of the research protocol and 48 h after the last training session. Initially, anthropometric measurements were taken, followed by cognitive tests, and finally performance tests.
Anthropometric indices
Height and body mass were measured using a standard stadiometer (Seca 213, Germany) and a calibrated digital scale (Seca 769, Germany).
Cognitive tests
Cognitive functions were assessed by the Mini-Mental State Examination (MMSE). The MMSE is a short exam to evaluate the quality of consciousness and has high sensitivity and specificity in detecting dementia across different age groups. This questionnaire included six subsets: orientation, registration, attention, recall, language, and constructional praxis, with a total score of 30 points. A score below 23 indicates the possibility of the disorder (Hobson, 2015).
Performance tests
Physical performance tests were conducted in a written order with a 5 min interval. Before starting the testing, the participants had a 10 min warm-up, which included walking in place, chair sit-ups, wall push-ups, and light stretching. The Romberg, modified push-up, chair stand, sit and reach, and step tests were taken, respectively, to assess the patient’s physical performance.
The Romberg test for assessing a standing balance or participant’s ability to stand unassisted was performed on a firm and compliant support surface. Participants stood with both feet together without shoes, held their arms next to the body, or crossed in front of the body. Then, they were asked to close their eyes and keep their static balance. The time that the participants could stand with their eyes closed was recorded. The test was finished if participants opened their eyes, lost their balance by moving arms or feet, swayed their body, or began to fall and needed the examiner’s intervention to maintain balance (Forbes and Cronovich, 2021). Participants performed this test twice, and the best time was recorded.
The modified push-up is a test for the assessment of the patient’s shoulder endurance. In this test, the patients were asked to get push-ups positioned on their knees and do the push-up on their knees for 1 minute. The number of complete movements during 1 minute was counted.
We used the 30 s chair stand test to measure the subject’s lower body muscle endurance. The test needs participants to sit on a chair, put their hand on the opposite shoulder, and cross at the wrists; then, from the sitting position, subjects stand completely up and back down as many times as possible within 30 s. Count the total number of complete chair stands. This test was taken twice, and the best result was recorded for each patient (Rikli and Jones, 2013).
The sit and reach test measured the lower back and hamstring’s flexibility. The participants were asked to sit on the floor with their legs stretched out. The soles are placed flat against the box. Knees must be locked and pressed flat to the floor. With the palms facing downwards and the hands-on top of each other, participants reached forward and tried to touch or pass their toes. The participants reach out and hold that position for at least one-two seconds while the distance is recorded. The level of the feet was considered as recording zero so that any measure that does not reach the toes is negative and any reach past the toes is positive.
A step test measured the patient’s aerobic fitness. The participants stepped on and off the box (15–17 cm high) for two minutes, which was recorded by a chronometer. If patients start with the right or left foot, they should go down with the same leg with the “up,” “up,” “down,” and “down” rhythm. The number of complete steps on and off the box was counted for statistical analysis.
Ethical aspect
All research procedures conducted in studies involving human participants were in accordance with the declaration of Helsinki. The study was approved by the Ethics Committee for Sport Sciences Research Institute of Iran (approval number: IR.SSRI.REC.1400.070). In addition, this research was registered in the Iranian Registry of Clinical Trials (IRCT) with registration number: IRCT20210726051993N1.
Statistical analysis
The Statistical Package of Social Sciences (SPSS, IBM, v19) was used to analyze row data. Data presented in mean ± standard deviation (SD). The normality distribution of the variables was done using the Shapiro–Wilk test. A repeated measure analysis of variance ANOVA with the time (T1 vs. T2) and group (C, MT, T) was performed to analyze the data. We calculated the effect size (ES) by the change score divided by the SD of the change score to examine the magnitude of differences while controlling for the influence of the sample size (Dankel and Loenneke, 2018), with 0.2 considered as a small ES, 0.5 as a moderate ES and >0.8 as a large ES. The significance level was considered at p ≤ 0.05 for all statistical analyses.
Results
Cognitive performance
There was no significant difference between groups at the MMSE test at baseline (p > 0.05). The statistical analysis demonstrated there was no significant main group effect for MMSE (F2,24 = 3.50 p = 0.054, ηp2:0.23), through a significant time (F1,12 = 74.13 p = 0.001, ηp2:0.86) and interaction effect (group × time) (F2,24 = 38.71 p = 0.001, ηp2:0.76) observed. The TM group (27.8%) demonstrated a significant improvement compared to the C group (−6.4%) (p = 0.001), and the T group (12.2%) (p = 0.002). In addition, there was a significant difference between the T group and the C group (p = 0.001). In details, we observed significant differences at orientation (F2,24 = 9.74 p = 0.002, ηp2: 0.45), language (F2,24 = 3.52 p = 0.047, ηp2: 0.23), memory (F2,24 = 3.76 p = 0.042, ηp2:0.24) and delay recall (F2,24 = 5.59 p = 0.017, ηp2:0.32) between groups. However, there were not any significant differences at registration (F2,24 = 1.45 p = 0.257, ηp2: 0.11), visual-spatial (F2,24 = 1.32 p = 0.287, ηp2:0.10), and comprehension (F2,24 = 0.79 p = 0.412, ηp2: 0.06) following interventions between groups. For orientation and delay recall, there was a significant difference between the TM group and the other two groups (p < 0.05). Additionally, the scores obtained in the T group were significantly higher than in the C group in orientation (p = 0.013). The scores obtained in the TM group for language (p = 0.014) and memory (p = 0.035) were significantly higher than in the C group. Figure 2 displays the scores obtained from the MMSE test pre- and post-intervention.
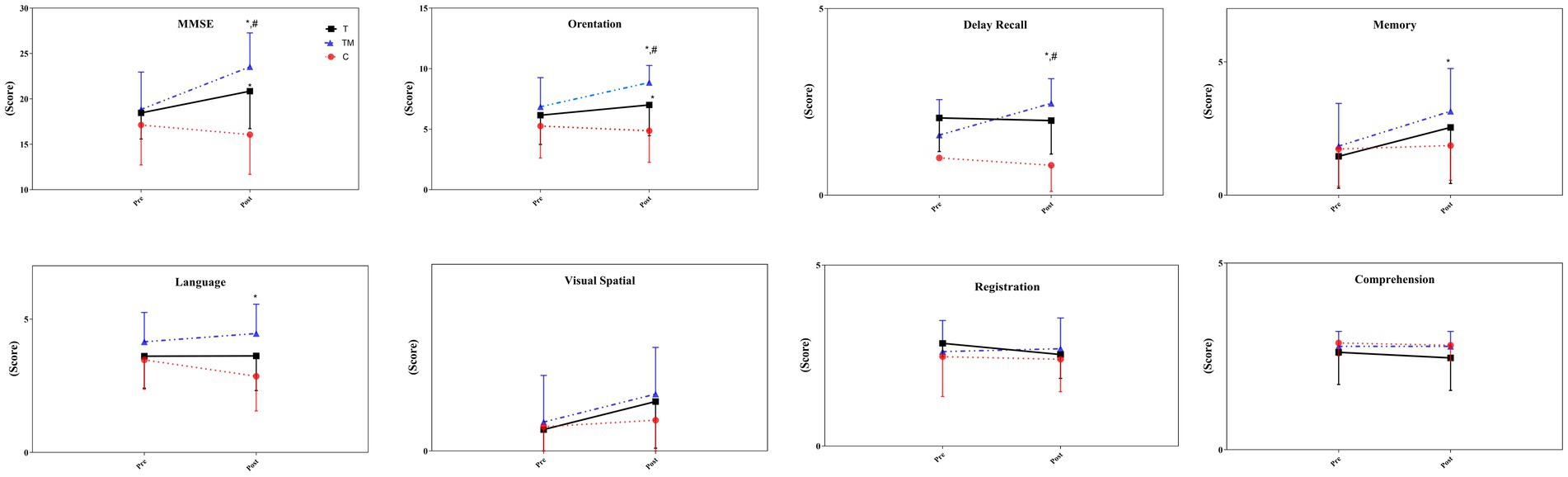
Figure 2. The scores of the MMSE test following the intervention. C, control group; T, training group; TM, training with the music group. *: significant difference with the C group; #: significant difference with the T group.
Physical performance
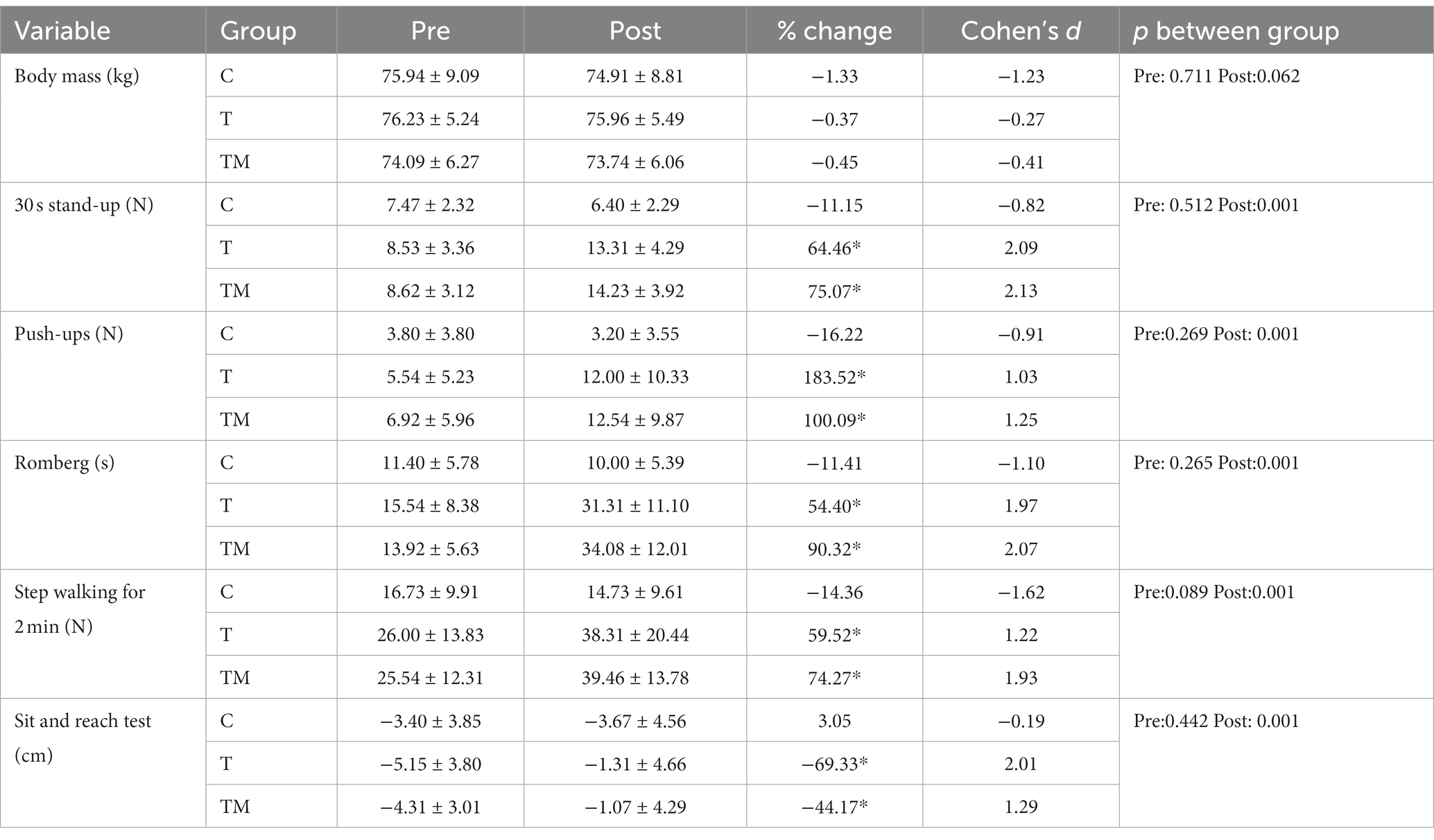
There were no significant differences between groups at physical performance tests at baseline (p > 0.05). Table 2 presents the descriptive statistics of body mass and performance parameters, pre- and post-intervention. There was a significant main time effect (F1,12 = 0.17.55, p = 0.001, ηp2:0.59), but no main group (F2.24 = 0.25 p = 0.779, ηp2:0.02), and interaction effect (F2.24 = 3.33 p = 0.053, ηp2:0.22) for body mass following intervention.
Overall, compared to the C group, both experimental groups demonstrated substantial improvements in all performance indices following 12 weeks of training protocols. We found no significant main group effect for push-ups (F2,24 = 2.66 p = 0.091, ηp2:0.18), through a significant time (F1,12 = 32.56 p = 0.001, ηp2:0.73) and interaction effect observed (F2.24 = 9.20 p = 0.001, ηp2:0.43). The Bonferroni post hoc test showed the difference between the C group and both trained groups (p < 0.01). We observed a significant main group (F2,24 = 9.99 p = 0.001, ηp2:0.45), time (F1,12 = 103.55 p = 0.001, ηp2:0.90), and interaction effect (F2.24 = 30.41 p = 0.001, ηp2:0.72) for Romberg test. Two experimental groups demonstrated significant improvement than the C group (p < 0.01). For 30 s chair sit and stand, there was a significant main group (F2,24 = 6.18 p = 0.007, ηp2:0.34), time (F1,12 = 79.86 p = 0.001, ηp2:0.87), and interaction effect (F2.24 = 36.48 p = 0.001, ηp2:0.75). The difference was between the trained groups with the C group (p < 0.01). We found a significant main group (F2,24 = 4.2 p = 0.027, ηp2:0.26), time (F1,12 = 46.46 p = 0.001, ηp2:0.79), and interaction effect (F2.24 = 20.35 p = 0.001, ηp2:0.63) for walking on steppe test. The Bonferroni post hoc test displayed two trained groups performed far better than the C group (p < 0.01). There was no significant main group effect for sit and reach test (F2,24 = 0.06 p = 0.094, ηp2:0.01), through a significant time (F1,12 = 33.24 p = 0.001, ηp2:0.74) and interaction effect observed (F2.24 = 21.06 p = 0.001, ηp2:0.64). The Bonferroni post hoc test showed both experimental groups demonstrated further stretch than the C group (p < 0.001).
Discussion
The primary aim of this study was to examine the effectiveness of adding music as a cognitive activity to exercise at home on the cognitive and physical performance of Alzheimer’s patients. The present study’s findings indicated both trained groups demonstrated considerable improvements in all performance indicators and cognitive function than in the C group. However, the TM group showed much more significant improvement in cognitive function compared to the T group alone. The TM group demonstrated substantial improvements in orientation, delay recall, memory, and language. Therefore, the research findings show that adding cognitive stimuli such as music during exercise training has synergistic effects potentially through neural activation and arousal the on improving mental and physical performance to gain further benefits from physical training.
Although both the T (12.2%) and TM (27.8%) groups showed improvements in cognitive function, measured by the MMSE test, a significant improvement was observed in the TM group. It seems that performing exercises simultaneously with music has caused a further improvement in cognitive function by activating brain cells in remembering the music and remote memories. Given that there is an overlap between the areas of musical memory and the areas spared in AD, it was shown that listening to music can preserve these areas. In this regard, minimal cortical atrophy and minimally impaired glucose metabolism were observed despite a non-significant change in Aβ deposition (Jacobsen et al., 2015). In addition, music activates a broad network in the brain: bilateral temporal lobes, superior temporal regions, parahippocampal gyri, caudal anterior cingulate cortex, and ventral pre-supplementary motor areas (Satoh et al., 2006; Jacobsen et al., 2015). Moreover, the positive effect of music on cognition could be interpreted based on impacts on arousal and evoking autobiographical memory, which was demonstrated in previous studies. That could improve self-consciousness, global cognitive function, and especially behavioral symptoms of dementia. In detail, the TM group showed significant improvement in orientation, delay recall, memory, and language compared to the C group. Therefore, simultaneously performing these two effective interventions by further perfusion and activating some areas of the mentioned brain could be effective in remembering events and maintaining cognitive performance. In contrast, Higuti et al. did not find any improvement in the cognitive function of patients after 12 weeks of exercise with music (Higuti et al., 2021). The methodological differences between our research and the former study may explain this contradiction. Our research was a dual-task in which music and exercise were performed simultaneously, but in Higuti’s research, they were conducted separately. Overall, performing exercise and listening to music simultaneously by activating the brain and maintaining muscle fitness effectively improves cognitive function and quality of life in Alzheimer’s patients.
Loss of muscle fitness due to muscle atrophy is a complication of AD that can lead to the inability to perform daily tasks, a poor psychological state, and ultimately a decline in quality of life (Burns et al., 2010; Lane et al., 2018). Our findings indicated that upper and lower body muscle endurance improved in both TM and T groups. Our training protocol involved many muscle endurance exercises for the upper and lower body, which were performed in several sets in all workouts; thus, expecting an improvement in muscle fitness was especially obvious in these patients whose physical fitness had considerably declined. Our finding has been supported by previous studies showing that a period of combined training, including resistance and aerobic exercises, significantly improved muscle strength and endurance in patients with cognitive impairment (Santana-Sosa et al., 2008; Langoni et al., 2019; Parvin et al., 2020). As there is a strong relationship between muscle atrophy and declined cognitive function (Burns et al., 2010), it was recommended that muscle maintenance exercises be included in AD patients’ training programs. Therefore, combined training with enhancing the strength and muscular endurance of AD patients can promote patients’ independence in performing routine activities and, to some extent, be effective in improving patients’ mood and quality of life.
AD is also associated with reduced cardiovascular fitness (Lane et al., 2018) due to a sedentary lifestyle. Our findings show that both TM and T groups demonstrated an improvement in aerobic fitness, which was measured by walking on the steppe. Activities such as walking steps, quick going back and forth, and repetition of movements improve aerobic fitness. In this regard, Parvin et al. (2020) showed that a 12 week combined training period significantly improves aerobic fitness (Parvin et al., 2020). Interestingly, increased cardiorespiratory fitness is associated with reduced brain atrophy in AD patients (Burns et al., 2008). Thus, aerobic exercise is as essential as resistance training for Alzheimer’s patients. The TM group showed a slightly better aerobic performance than the T group. Studies reported that the synchronization of music and exercise can result in improved running economy, efficiency, oxygen consumption and performance (Bacon et al., 2012; Terry et al., 2012). Therefore, exercising while listening to music, cardiovascular fitness can be improved through running economy.
One of the problems that Alzheimer’s patients encounter is an imbalance resulting from loss of muscle strength and destruction of nerve cells. Both experimental groups enhanced their balance following the research protocol. Participants performed many balance exercises during the intervention; thus, continuous activation of the proprioceptive sense caused the patients’ balance to develop. A previous study also confirmed that combined training had positive effects on the balance of Alzheimer’s patients and reduced the risk of falls (Santana-Sosa et al., 2008). The most non-significant difference was seen in balance between two training groups. It’s clear that cognitive processes, particularly attention are crucial when performing a balancing movement (Woollacott and Shumway-Cook, 2002). Hence, listening to music during exercise can activate the brain regions, involved in cognitive processing and organization of movement (Bacon et al., 2012); thus, this intervention can lead to the improvement of balance movements. In this regards, it has shown, listening to music improved postural balance in visually impaired adolescents (Maatoug et al., 2023).
Changes in body mass and composition need long-term manipulation of intake and expended calories. Although the current intervention lasted 3 months and is a good opportunity for changes in body weight, no significant change was observed following the interventions in all groups. In the present study, the amount of calories consumed was not calculated; however, it is evident that the calories expended in the exercise groups increased. Research has shown that Alzheimer’s patients progressively lost their appetite due to hypo-perfusion to the right and left anterior cingulate and orbitofrontal cortices, ultimately leading to weight loss (Ismail et al., 2008). In the C group, we observed slightly more weight loss than in the two experimental groups, which indicates that exercise training has effectively restored patients’ appetite.
We acknowledged that there were some limitations in our study. Firstly, we had limitations in using laboratory tools due to the coronavirus pandemic; we did not measure patients’ body composition, which can be used to interpret the findings. Secondly, we also did not assess the changes in brain imaging after intervention due to the pandemic crisis. Hence, we propose evaluating the correlation between cognitive changes and alteration in brain structure by utilizing brain MRI and functional brain imaging such as brain Single Photon Emission Computed Tomography (SPECT) and Positron emission tomography (PET) scan and measuring body composition by dual energy X-ray (DEXA) in future studies. Additionally, we suggest that future studies evaluate the effects of the dual-task intervention on behavioral and psychological symptoms of dementia, sleep problems, and eventually the quality of life in dementia patients. In addition, given that familiar and unfamiliar music may have different effects on cognitive and performance indicators, it is recommended that researchers investigate this issue in a research. At last, in follow-up studies after 3–6 months could help us determine the constant effect of dual-task training on cognition.
Conclusion
In conclusion, our findings showed a combining remote training with music as appropriate non-pharmacological treatment is useful for improving the physical and cognitive function of Alzheimer’s patients and could ultimately decrease the AD burden on society. Thus, we recommend that neurologists include dual-task training (training with music) in the adjunctive treatment of Alzheimer’s patients, along with pharmacological therapies.
Data availability statement
The datasets presented in this article are not readily available because the datasets generated and analysed during the current study are available from the corresponding author on reasonable request. Requests to access the datasets should be directed to YW1hbmlfc2FkZWdoQGtodS5hYy5pcg==.
Ethics statement
The studies involving humans were approved by Sport Sciences Research Institute of Iran. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GS: Data curation, Methodology, Writing – original draft. FM: Conceptualization, Supervision, Writing – review & editing. MN: Conceptualization, Writing – review & editing, Formal analysis. SA-S: Conceptualization, Formal analysis, Writing – review & editing, Supervision. KS: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to acknowledge the memory clinic of Roozbeh Hospital, as well as the patients and caregivers who collaborated with us.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AD, Alzheimer’s disease; T, Training; TM, Training with music; DSM-5, Diagnostic and Statistical Manual of Mental Disorders; MMSE, Mini-Mental State Examination; ES, Effect size; SD, Standard deviation; SPSS, Statistical Package of Social Sciences; DEXA, Dual-energy X-ray; SPECT, Brain Single Photon Emission Computed Tomography; PET, Positron emission tomography.
References
Åhman, H. B., Giedraitis, V., Cedervall, Y., Lennhed, B., Berglund, L., and McKee, K. (2019). Dual-task performance and neurodegeneration: correlations between timed up-and-go dual-task test outcomes and Alzheimer’s disease cerebrospinal fluid biomarkers. J. Alzheimers Dis. 71, S75–s83. doi: 10.3233/jad-181265
Bacon, C. J., Myers, T. R., and Karageorghis, C. I. (2012). Effect of music-movement synchrony on exercise oxygen consumption. J. Sports Med. Phys. Fitness 52, 359–365.
Ballmann, C. G. (2021). The influence of music preference on exercise responses and performance: a review. J. Funct. Morphol. Kinesiol. 6:2033. doi: 10.3390/jfmk6020033
Bamman, M. M., Hill, V. J., Adams, G. R., Haddad, F., and Wetzstein, C. J. (2003). Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J. Gerontol. A Biol. Sci. Med. Sci. 58, B108–B116. doi: 10.1093/gerona/58.2.b108
Bigliassi, M., Karageorghis, C. I., Bishop, D. T., Nowicky, A. V., and Wright, M. J. (2018). Cerebral effects of music during isometric exercise: an fMRI study. Int. J. Psychophysiol. 133, 131–139. doi: 10.1016/j.ijpsycho.2018.07.475
Bruer, R. A., Spitznagel, E., and Cloninger, C. R. (2007). The temporal limits of cognitive change from music therapy in elderly persons with dementia or dementia-like cognitive impairment: a randomized controlled trial. J. Music. Ther. 44, 308–328. doi: 10.1093/jmt/44.4.308
Burns, J. M., Cronk, B. B., Anderson, H. S., Donnelly, J. E., and Thomas, G. P. (2008). Cardiorespiratory fitness and brain atrophy in early Alzheimer disease. Neurology 71, 210–216. doi: 10.1212/01.wnl.0000317094.86209.cb
Burns, J. M., Johnson, D. K., Watts, A., Swerdlow, R. H., and Brooks, W. M. (2010). Reduced lean mass in early Alzheimer disease and its association with brain atrophy. Arch. Neurol. 67, 428–433. doi: 10.1001/archneurol.2010.38
Dankel, S. J., and Loenneke, J. P. (2018). Effect sizes for paired data should use the change score variability rather than the pre-test variability. J. Strength. Cond. Res. 35, 1773–1778. doi: 10.1519/jsc.0000000000002946
DeLaGarza, V. W. (2003). Pharmacologic treatment of Alzheimer’s disease: an update. Am. Fam. Physician 68, 1365–1372.
Dennis, A., Dawes, H., Elsworth, C., Collett, J., Howells, K., and Wade, D. T. (2009). Fast walking under cognitive-motor interference conditions in chronic stroke. Brain Res. 1287, 104–110. doi: 10.1016/j.brainres.2009.06.023
Faul, F., Erdfelder, E., Lang, A. G., and Buchner, A. (2007). G*power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39, 175–191. doi: 10.3758/bf03193146
Forbes, J., and Cronovich, H.. (2021). Romberg test. StatPearls. Treasure Island, FL: StatPearls Publishing
Higuti, A. Y., Barbosa, S. R. M., Corrêa, L. M., Izzo, T. F., and Ansai, J. H. (2021). Effects of listening to music and practicing physical exercise on functional and cognitive aspects in institutionalized older adults with dementia: pilot study. Explore (N.Y.) 17, 292–296. doi: 10.1016/j.explore.2020.07.006
Hobson, J. (2015). The Montreal cognitive assessment (MoCA). Occup. Med. 65, 764–765. doi: 10.1093/occmed/kqv078
Ismail, Z., Herrmann, N., Rothenburg, L. S., Cotter, A., Leibovitch, F. S., and Rafi-Tari, S. (2008). A functional neuroimaging study of appetite loss in Alzheimer’s disease. J. Neurol. Sci. 271, 97–103. doi: 10.1016/j.jns.2008.03.023
Jacobsen, J. H., Stelzer, J., Fritz, T. H., Chételat, G., La Joie, R., and Turner, R. (2015). Why musical memory can be preserved in advanced Alzheimer’s disease. Brain 138, 2438–2450. doi: 10.1093/brain/awv135
Johnson, J. K., Cotman, C. W., Tasaki, C. S., and Shaw, G. L. (1998). Enhancement of spatial-temporal reasoning after a Mozart listening condition in Alzheimer’s disease: a case study. Neurol. Res. 20, 666–672. doi: 10.1080/01616412.1998.11740582
Lane, C. A., Hardy, J., and Schott, J. M. (2018). Alzheimer’s disease. Eur. J. Neurol. 25, 59–70. doi: 10.1111/ene.13439
Langoni, C. D. S., Resende, T. L., Barcellos, A. B., Cecchele, B., Knob, M. S., and Silva, T. D. N. (2019). Effect of exercise on cognition, conditioning, muscle endurance, and balance in older adults with mild cognitive impairment: a randomized controlled trial. J. Geriatr. Phys. Ther. 42, E15–E22. doi: 10.1519/jpt.0000000000000191
Li, C. H., Liu, C. K., Yang, Y. H., Chou, M. C., Chen, C. H., and Lai, C. L. (2015). Adjunct effect of music therapy on cognition in Alzheimer’s disease in Taiwan: a pilot study. Neuropsychiatr. Dis. Treat. 11, 291–296. doi: 10.2147/ndt.S73928
Maatoug, H., Baccouch, R., Borji, R., Rebai, H., and Sahli, S. (2023). Effects of music listening on postural balance in adolescents with visual impairment. Percept. Mot. Skills 130, 112–126. doi: 10.1177/00315125221130548
Mendiola-Precoma, J., Berumen, L. C., Padilla, K., and Garcia-Alcocer, G. (2016). Therapies for prevention and treatment of Alzheimer’s disease. Biomed. Res. Int. 2016:1. doi: 10.1155/2016/2589276
Nam, S. M., and Kim, S. G. (2021). Dual-task training effect on cognitive and body function, β-amyloid levels in alzheimer’s dementia patients: a randomized controlled trial. J. Korean Phys. Ther. 33, 136–141. doi: 10.18857/jkpt.2021.33.3.136
Nemoto, Y., and Suzuki, K. (2018). Prevention of dementia onset with targeting at physical activity and social participation among Japanese community-dwelling older adults. Arch. Phys. Health Sports Med. 1, 39–43. doi: 10.22259/2639-1805.0101006
Ozdemir, L., and Akdemir, N. (2009). Effects of multisensory stimulation on cognition, depression and anxiety levels of mildly-affected Alzheimer’s patients. J. Neurol. Sci. 283, 211–213. doi: 10.1016/j.jns.2009.02.367
Parvin, E., Mohammadian, F., Amani-Shalamzari, S., Bayati, M., and Tazesh, B. (2020). Dual-task training affect cognitive and physical performances and brain oscillation ratio of patients with Alzheimer’s disease: a randomized controlled trial. Front. Aging Neurosci. 12:605317. doi: 10.3389/fnagi.2020.605317
Prinz, A., Langhans, C., Rehfeld, K., Partie, M., and Hökelmann, A. (2021). Effects of music-based physical training on selected motor and cognitive abilities in seniors with dementia-results of an intervention pilot study. J. Geriatr. Med. Gerontol. 7, 1–9. doi: 10.23937/2469-5858/1510124
Raglio, A., Filippi, S., Bellandi, D., and Stramba-Badiale, M. (2014). Global music approach to persons with dementia: evidence and practice. Clin. Interv. Aging 9, 1669–1676. doi: 10.2147/cia.S71388
Santana-Sosa, E., Barriopedro, M. I., López-Mojares, L. M., Pérez, M., and Lucia, A. (2008). Exercise training is beneficial for Alzheimer’s patients. Int. J. Sports Med. 29, 845–850. doi: 10.1055/s-2008-1038432
Särkämö, T., Tervaniemi, M., Laitinen, S., Numminen, A., Kurki, M., and Johnson, J. K. (2014). Cognitive, emotional, and social benefits of regular musical activities in early dementia: randomized controlled study. Gerontologist 54, 634–650. doi: 10.1093/geront/gnt100
Satoh, M., Ogawa, J.-i., Tokita, T., Nakaguchi, N., and Nakao, K. (2014). The effects of physical exercise with music on cognitive function of elderly people: Mihama-Kiho project. PLoS One 9, –e95230. doi: 10.1371/journal.pone.0095230
Satoh, M., Ogawa, J. I., Tokita, T., Nakaguchi, N., Nakao, K., and Kida, H. (2017). Physical exercise with music maintains activities of daily living in patients with dementia: Mihama-Kiho project part 21. J. Alzheimers Dis. 57, 85–96. doi: 10.3233/jad-161217
Satoh, M., Takeda, K., Nagata, K., Shimosegawa, E., and Kuzuhara, S. (2006). Positron-emission tomography of brain regions activated by recognition of familiar music. AJNR Am. J. Neuroradiol. 27, 1101–1106.
Satoh, M., Yuba, T., Tabei, K., Okubo, Y., Kida, H., and Sakuma, H. (2015). Music therapy using singing training improves psychomotor speed in patients with Alzheimer’s disease: a neuropsychological and fMRI study. Dement. Geriatr. Cogn. Dis. Extra 5, 296–308. doi: 10.1159/000436960
Sobol, N. A., Hoffmann, K., Frederiksen, K. S., Vogel, A., Vestergaard, K., and Brændgaard, H. (2016). Effect of aerobic exercise on physical performance in patients with Alzheimer’s disease. Alzheimers Dement. 12, 1207–1215. doi: 10.1016/j.jalz.2016.05.004
Terry, P. C., Karageorghis, C. I., Saha, A. M., and D’Auria, S. (2012). Effects of synchronous music on treadmill running among elite triathletes. J. Sci. Med. Sport 15, 52–57. doi: 10.1016/j.jsams.2011.06.003
Tracy, B. L., Ivey, F. M., Hurlbut, D., Martel, G. F., Lemmer, J. T., and Siegel, E. L. (1999). Muscle quality. II. Effects of strength training in 65- to 75-yr-old men and women. J. Appl. Physiol 86, 195–201. doi: 10.1152/jappl.1999.86.1.195
Venturelli, M., Scarsini, R., and Schena, F. (2011). Six-month walking program changes cognitive and ADL performance in patients with Alzheimer. Am. J. Alzheimers Dis. Other Dement. 26, 381–388. doi: 10.1177/1533317511418956
Keywords: music-therapy, combined training, muscle endurance, dementia, mental readiness
Citation: Shokri G, Mohammadian F, Noroozian M, Amani-Shalamzari S and Suzuki K (2024) Effects of remote combine exercise-music training on physical and cognitive performance in patients with Alzheimer’s disease: a randomized controlled trial. Front. Aging Neurosci. 15:1283927. doi: 10.3389/fnagi.2023.1283927
Edited by:
Jin-Tai Yu, Fudan University, ChinaReviewed by:
Lidia Castillo-Mariqueo, Universidad Católica de Temuco, ChileFahad Naveed Ahmad, Wilfrid Laurier University, Canada
Copyright © 2024 Shokri, Mohammadian, Noroozian, Amani-Shalamzari and Suzuki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sadegh Amani-Shalamzari, YW1hbmlfc2FkZWdoQGtodS5hYy5pcg==; Katsuhiko Suzuki, a2F0c3Uuc3V6dUB3YXNlZGEuanA=
 Ghazaleh Shokri1
Ghazaleh Shokri1 Fatemeh Mohammadian
Fatemeh Mohammadian Sadegh Amani-Shalamzari
Sadegh Amani-Shalamzari Katsuhiko Suzuki
Katsuhiko Suzuki