
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 15 December 2023
Sec. Neurocognitive Aging and Behavior
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1267061
 Einat K. Brenner1*
Einat K. Brenner1* Katherine J. Bangen1,2
Katherine J. Bangen1,2 Alexandra L. Clark3
Alexandra L. Clark3 Lisa Delano-Wood1,2
Lisa Delano-Wood1,2 Nicole D. Evangelista4
Nicole D. Evangelista4 Lauren Edwards5
Lauren Edwards5 Scott F. Sorg6
Scott F. Sorg6 Amy J. Jak1,2
Amy J. Jak1,2 Mark W. Bondi1,2
Mark W. Bondi1,2 Sean C. L. Deoni7
Sean C. L. Deoni7 Melissa Lamar8,9
Melissa Lamar8,9Background: Decreasing white matter integrity in limbic pathways including the fornix and cingulum have been reported in Alzheimer’s disease (AD), although underlying mechanisms and potential sex differences remain understudied. We therefore sought to explore sex as a moderator of the effect of age on myelin water fraction (MWF), a measure of myelin content, in older adults without dementia (N = 52).
Methods: Participants underwent neuropsychological evaluation and 3 T MRI at two research sites. Multicomponent driven equilibrium single pulse observation of T1 and T2 (mcDESPOT) quantified MWF in 3 a priori regions including the fornix, hippocampal cingulum (CgH), and cingulate cingulum (CgC). The California Verbal Learning Test-Second Edition assessed learning and delayed recall. Multiple linear regressions assessed for (1) interactions between age and sex on regional MWF and (2) associations of regional MWF and memory.
Results: (1) There was a significant age by sex interaction on MWF of the fornix (p = 0.002) and CgC (p = 0.005), but not the CgH (p = 0.192); as age increased, MWF decreased in women but not men. (2) Fornix MWF was associated with both learning and recall (ps < 0.01), but MWF of the two cingulum regions were not (p > 0.05). Results were unchanged when adjusting for hippocampal volume.
Conclusion: The current work adds to the literature by illuminating sex differences in age-related myelin decline using a measure sensitive to myelin and may help facilitate detection of AD risk for women.
The investigation of white matter demyelination and degeneration in the brain has helped further elucidate the pathophysiological mechanism of both normal aging and neurodegenerative diseases such as Alzheimer’s disease (AD). Previously, many neuroimaging studies of AD have focused on gray matter atrophy thought to be reflective of neurodegeneration more broadly, including beta amyloid (Aβ) and tau tangle accumulation. However, alterations in white matter network topology have been observed in preclinical AD (Fischer et al., 2015), and age-related differences in white matter microstructure that predate gray matter atrophy have been demonstrated using multiple MRI methods (Arshad et al., 2016; Bangen et al., 2021). Also, cognitive decline in normal aging and AD has been associated with changes in several widely distributed cerebral white matter tracts (Delano-Wood et al., 2012; Amlien and Fjell, 2014; Bangen et al., 2021).
Microstructural changes of limbic system regions such as the fornix and cingulum, which have been associated with memory function (Delano-Wood et al., 2012; Van Der Holst et al., 2013; Bangen et al., 2021) remain less understood. Compared to healthy controls, patients with AD show both macrostructural and microstructural white matter alterations across several regions, including the thalamus, thalamic radiation, cingulum, splenium of corpus callosum, and the fornix (Serra et al., 2010). Lower fornix myelin water fraction (MWF)—a measure of myelin content—has been associated with worse memory performance, even after adjusting for hippocampal volume (Metzler-Baddeley et al., 2019; Bangen et al., 2021), and age has been shown to be associated with white matter decline in the fornix, but not the cingulum in healthy older adults (Stadlbauer et al., 2008). A different group observed these same patterns in the fornix and in the inferior, but not superior cingulate bundle (Sullivan et al., 2010). Furthermore, damage to fornix white matter glia contributes to hippocampal gray matter damage in age-dependent limbic decline (Metzler-Baddeley et al., 2019).
Most studies have used diffusion tensor imaging (DTI) to examine microstructural white matter changes. However, this methodology is not specific to myelin and may be associated with a variety of neuropathological metrics such as axonal size, density, and configuration (Beaulieu, 2002). Critically, DTI and myelin water fraction parameters may each offer unique data, and there is therefore increased focus on studying myelin more specifically since its repair and loss has direct implications for the development of white matter abnormalities and associations with Aβ toxicity (Bartzokis, 2011; McAleese et al., 2017). A mutation in oligodendrocytes, which generate myelin, has been associated with white matter damage in AD (Pak et al., 2003), and age-related changes in white matter were also found to be more strongly associated with myelin sheath degeneration than axonal degeneration (Inano et al., 2011). Myelin water fraction has also been shown to be sensitive to age-related changes in white matter, and it increases specificity of measuring myelin content (Faizy et al., 2020).
Although women are at higher risk of developing AD, findings from studies investigating the effect of sex on white matter integrity have been mixed. Some researchers have found minimal or no sex effects in the relationship between age and white matter metrics (Salat et al., 2009; Inano et al., 2011; Faizy et al., 2018). Others have observed differences in the corpus callosum and left-hemisphere regions, where men show greater myelin content than women (Westerhausen, 2003; Liu et al., 2010; Canales-Rodríguez et al., 2021). Sullivan and colleagues noted that men and women showed similar age-related increases in fornix fiber diffusivity, but men showed a significant decline in corpus callosum genu fractional anisotropy compared to women (Sullivan et al., 2010). Most of these studies included young and middle-aged adults. Further research is needed to understand the effect sex may play in the relationship between age and myelin content, particularly in older adults and those at risk for AD.
Decreasing white matter integrity in limbic pathways including the fornix and cingulum have been reported in AD, although underlying mechanisms and potential sex differences remain understudied. Given this, we examined sex as a moderator of the effect of age on MWF, a measure of myelin content, in older adults.
Fifty-two older adults without dementia were recruited from ongoing aging studies at the University of California, San Diego (UCSD) and University of Illinois Chicago (UIC). Participants were excluded if they had a history of dementia, clinical stroke, neurologic disease (e.g., Parkinson’s disease, multiple sclerosis), head injury with residual cognitive sequelae, or major psychiatric disorder.
Participants underwent clinical interview, brachial artery blood pressure measurement, neuropsychological assessment, and MR exams. Participants at UIC also underwent fasting blood draws. Arterial stiffening was indexed by pulse pressure, which was quantified as systolic minus diastolic pressure. Participants were classified as having diabetes based on self-report, hemoglobin A1c values ≥6.5%, and/or use of an anti-diabetic medication. Self-reported medical history was used to obtain current cigarette smoking, history of cardiovascular disease, history of atrial fibrillation, and current antihypertensive medication use in order to calculate Framingham Stroke Risk Profile (FSRP) score, an index of stroke risk. The updated FSRP provides sex-corrected scores based on age, systolic blood pressure, diabetes, cigarette smoking, cardiovascular disease, atrial fibrillation, and antihypertensive medication use (Dufouil et al., 2017). The study protocol was approved by Institutional Review Board at each institution, and all participants provided informed consent.
Episodic memory was assessed by the California Verbal Learning Test – Second Edition (CVLT-II) (Delis et al., 2000). Two metrics were assessed: (1) learning – total learning trials 1–5 and (2) delayed recall – long delay free recall. Standardized scores were adjusted for age and sex (Delis et al., 2001).
MRI data were acquired on one of two GE 3 T scanners (one at UCSD, the other at UIC). Harmonized MRI acquisition across both scanner sites and analysis methods for this study have been previously described (Bangen et al., 2021). T1-weighted high-resolution anatomical scans were collected at UCSD using a Fast Spoiled Gradient Recall acquisition (172 1 mm × 0.977 mm × 0.977 mm contiguous sagittal slices, field of view [FOV] = 25 cm, repetition time [TR] = 8 ms, echo time [TE] = 3.1 ms, flip angle = 12, inversion time [T1] = 600 ms, 256 × 192 matrix, Bandwidth = 31.25 kHz, frequency direction = S-I, NEX = 1). T1-weighted high-resolution anatomical scans were collected at UIC using Brain Volume (BRAVO) imaging sequence (120 interleaved axial slices, FOV = 22 mm2; TR/TE = 1,200 ms/5.3 ms). The T1-weighted scans collected at UCSD had dimensions of 1.0 mm x 0.977 mm x 0.977 mm. The T1-weighted scans collected at UIC had dimensions of 1.5 mm x 0.43 mm x 0.43 mm. T1-weighted images at both sites were processed with FreeSurfer 6.0 (Dale et al., 1999; Fischl et al., 2002). Volumetric data, including hippocampal volume, was derived using FreeSurfer 6.0 and visually inspected. Normalized hippocampal volume was calculated by dividing by total intracranial volume.
For the mcDESPOT sequence, a series of spoiled gradient recalled echo were acquired (SPGR; TR = 5.3 ms, TE = Min Full, flip angle = 18, FOV =24.0) and T2/T1-weighted balanced steady-state free precession (SSFP) data over a range of flip angles (Deoni, 2011). To correct for B1 inhomogeneities, we collected an inversion-recovery prepared SPGR (IR-SPGR) scan (TR = 5.3 ms, TE = Min Full, flip angle = 60, field of view = 24.0) and SSFP phase 0 (TE = Min Full, flip angle = 60, FOV = 24.0) with two phase-cycling patterns to correct for main magnetic field (B0) off-resonance effects. Following acquisition, the SPGR and SSFP images comprising each participant’s dataset were linearly coregistered to account for subtle intrasession head movement (Jenkinson et al., 2002). We obtained MWF maps (Figure 1) by fitting SPGR and bSSFP data to a three-pool model that included two exchanging water pools (myelin water and water both inside and outside the axon) as well as a third non-exchanging free water pool (Deoni et al., 2012). MWF map voxel dimensions were approximately 1.7 mm3 isotropic.
Post-processing of mcDESPOT data was completed using the Oxford Centre for Functional Magnetic Resonance Imaging of the Brain (FMRIB) Software Library (FSL) (Smith et al., 2004). T1-weighted images were downsampled to the 2mm3 resolution of the MNI152 template. Brain Extraction Tool (BET) was used to remove non-brain voxels from images. We then used FMRIB’s Linear Image Registration Tool (FLIRT) (Jenkinson et al., 2002) and FMRIB’s Nonlinear Image Registration Tool (FNIRT) to linearly and then non-linearly register T1-weighted images and mcDESPOT myelin volume fraction images to the MNI152 T1 2mm resolution brain template. FSL’s Automated Segmentation Tool (FAST) was applied to segment T1-weighted images into white matter, gray matter, and cerebrospinal fluid components. The resulting FNIRT transforms were then applied to the myelin volume fraction masks. We multiplied segmented white matter masks by ROI masks to ensure inclusion of exclusively white matter voxels prior to extracting regional MWF values. The ROIs applied to the mcDESPOT data were selected using the ICBM-DTI-81 stereotaxic white matter parcellation map and included the fornix, cingulate cingulum (CgC), and hippocampal cingulum (CgH). Visual inspection of each participant’s T1-weighted image, MWF map, and the MNI152 template showed good alignment.
Prior to analyses, data were examined for violations of assumptions of the statistical procedures employed including posterior predictive check, linearity, homogeneity of variance, influential observations, collinearity, and normality of residuals. There were no outliers greater or less than three standard deviations away from the mean in any of the independent and dependent variables.
First, we used linear regression models to examine two-way interactions between age and sex on MWF of 3 a priori regions (fornix, CgC, and CgH) as the dependent variable (Table 1). Each region was examined in a separate model. These models included covariates of site and pulse pressure, main effects of age and sex, and the two-way interaction of age and sex. To interpret significant interactions, we then stratified by sex and ran linear regression models examining the associations between age and regional MWF (adjusting for site and pulse pressure) separately for women and men.
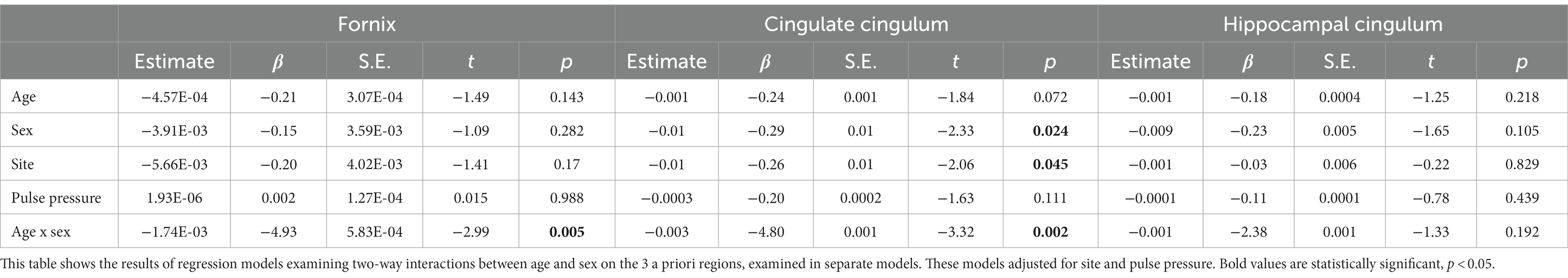
Table 1. Regression models examining interactive effects of age and sex on regional myelin water fraction.
Second, we examined associations between MWF in the a priori regions of interest and each memory measure (i.e., learning and recall) as dependent variables in separate models adjusting for collection site, pulse pressure, age, and sex. We first ran the models across the entire sample, and then these models were stratified by sex for further interpretation.
For all models, we ran secondary analyses additionally adjusting for normalized hippocampal volume. We also ran all primary analyses not adjusting for pulse pressure, and the results remained the same. The only result that changed was that, additionally adjusting for normalized hippocampal volume, there was a significant association between age and MWF on fornix in men. All analyses were conducted using R Statistical Software (R Core Team, 2023) with significance set at p < 0.05.
Characteristics of each group can be viewed in Table 1. The men and women did not differ by average age, education, collection site, or race, p > 0.05. There were also no significant differences in sex distribution, age, fornix MWF, or CgH MWF between the collection sites, p > 0.05. T-tests showed a significant difference between CgC MWF between the collection sites (UIC M = 0.11, UCSD M = 0.09, p = 0.027).
Adjusting for collection site and pulse pressure as well as the main effects of age and sex, there was a significant interaction between age and sex on fornix MWF (t = −2.99, p = 0.005, β = −4.93; Figure 2A; Table 2) as well as on CgC MWF (t = −3.32, p = 0.002, β = −4.80; Figure 2B). The interaction between age and sex on CgH MWF was not significant (t = −1.33, p = 0.192, β = 0.08).
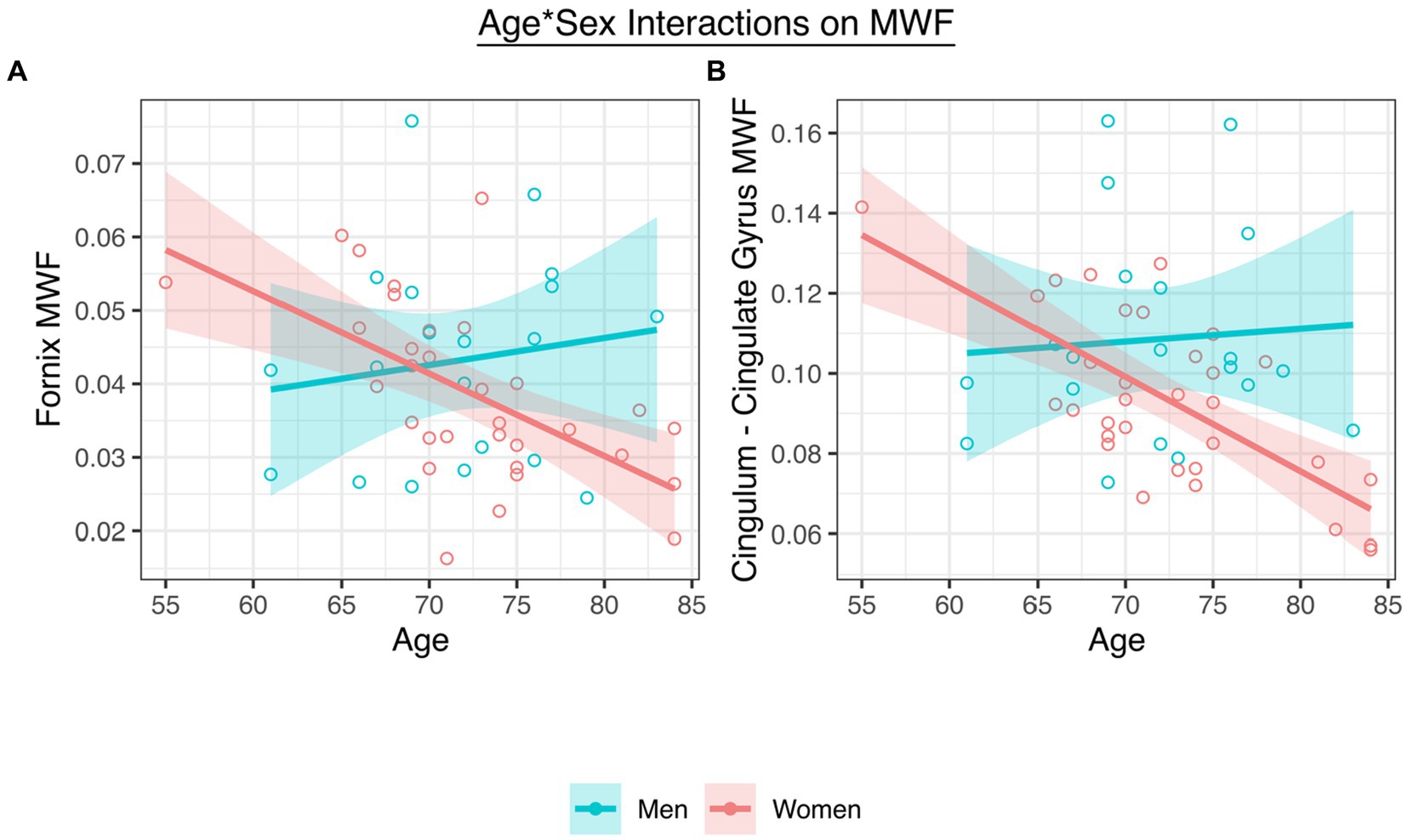
Figure 2. (A) A significant interaction between age and sex on fornix MWF. (B) A significant interaction between age and sex on CgC MWF.
To interpret the significant interactions, analyses stratified by sex revealed that women (fornix t = −3.40, p = 0.002, β = −0.51; CgC t = −4.71, p < 0.001, β = −0.59), but not men (fornix t = 0.94, p = 0.423, β = 0.21; CgC t = 1.47, p = 0.160, β = 0.37), showed significant age-associated declines in MWF.
In a secondary analysis, all results were unchanged after additionally adjusting for normalized hippocampal volume. That is, there was a significant interaction between age and sex on fornix MWF (t = −3.12, p = 0.003, β = 0.43) as well as on CgC MWF (t = −3.25, p = 0.002, β = 0.34). The interaction between age and sex on CgH MWF remained non-significant (t = −1.19, p = 0.239, β = 0.14). In interpreting the significant interactions through regression models stratified by sex, the relationships between age and MWF remained significant in women (fornix t = −2.73, p = 0.011, β = −0.40; CgC t = −4.13, p < 0.001, β = −0.55) but not men (fornix t = 1.96, p = 0.069, β = 0.40; CgC t = 2.01, p = 0.063, β = 0.49).
Across all participants, adjusting for age, sex, pulse pressure, and collection site, fornix MWF was associated with measures of learning (t = 3.41, p = 0.001, β = 0.47) and recall (t = 2.88, p = 0.006, β = 0.43). Stratifying by sex, associations between fornix MWF and learning were significant in both men (t = 2.51, p = 0.024, β = 0.60) and women (t = 2.78, p = 0.010, β = 0.60), and the association between fornix MWF and recall was significant in women (t = 2.93, p = 0.007, β = 0.64), but not men (t = 1.80, p = 0.093, β = 0.51). MWF of the two cingulum regions were not associated with either learning or recall (p’s > 0.05).
In a secondary analysis additionally adjusting for normalized hippocampal volume, significant associations remained between MWF and both learning (t = 2.30, p = 0.027, β = 0.36) and recall (t = 2.06, p = 0.046, β = 0.36). Stratifying by sex, the associations between fornix MWF and both learning and recall remained significant in women (learning t = 2.30, p = 0.031, β = 0.55; recall t = 2.71, p = 0.012, β = 0.66), but not in men (learning t = 0.94, p = 0.364, β = 0.28; recall t = 0.50, p = 0.624, β = 0.18).
Our results demonstrate that sex moderates the associations between age and MWF in both the fornix and the CgC, but not the CgH. Specifically, the significant interactions showed that for women, but not men, as age increased, MWF decreased. We also examined associations between MWF in each of the three regions and both learning and delayed recall. Results showed that fornix MWF was associated with learning regardless of sex and was associated with delayed recall only in women. MWF of the two cingulum regions were not associated with either cognitive measure regardless of sex.
Our findings suggest that, in older adults, the association between age and MWF of both the fornix and CgC is dependent on sex, even after adjusting for several relevant risk factors. Notably, these interactions showed that for women, as age increased, fornix and CgC MWF decreased, but for men, the relationship between age and MWF was not significant. Age-related white matter decline in the fornix and CgC have been observed in previous studies (Stadlbauer et al., 2008; Bastin et al., 2010; Fletcher et al., 2013), but few have stratified by sex and even fewer have investigated MWF using multicomponent relaxometry techniques. Thus, while some (Bastin et al., 2010) but not all (Kodiweera et al., 2016) studies have reported sex differences in DTI-derived white matter alterations, ours is the first to examine this relationship as it relates to myelin integrity. Furthermore, although previous work has shown age-related alterations in the fornix (Bastin et al., 2010), ours is the first to carefully consider sex in tandem with age. Interactions between age and sex were not significant when examining the CgH suggesting that the fornix and CgC may be a more sensitive marker of age-related demyelination and other neurodegenerative changes in the brain (Gazes et al., 2019; Metzler-Baddeley et al., 2019). Taken together, our results suggest that women exhibit more age-related demyelination in brain regions critical for learning and memory than their male counterparts.
Results also showed significant associations between learning and fornix MWF for women and between recall and fornix MWF across both groups, and these relationships remained significant in women, but not men, even after adjusting for hippocampal volume. However, the associations between CgC or CgH MWF and learning/recall were not significant. These results are in line with research showing that fornix, but not hippocampal, integrity is associated with performance on a memory test (Gazes et al., 2019). Furthermore, volume of the fornix has been shown to be a stronger predictor of cognitive decline among the cognitively normal than hippocampal volume (Fletcher et al., 2013). Also, although decreased cingulum white matter integrity has been observed in individuals with amnestic mild cognitive impairment or AD (Gozdas et al., 2020; Luo et al., 2020; Wong et al., 2020), our sample of older adults without dementia may be too early in the aging or disease process to detect these changes. Future work using MWF should examine these associations in older adults with AD, as well as middle-aged adults, to further elucidate the timeline of its effect on cognition across the AD spectrum.
There are several limitations to our study worth noting. First, our study is preliminary given its small sample size and cross-sectional design. Longitudinal studies with larger samples are needed to further clarify the role that sex plays in limbic system myelin integrity across the aging spectrum. Our sample was also mostly comprised of White and relatively highly educated individuals. The relationships between age, sex, and myelin content may be different in a more diverse sample or one with greater variability in education or socioeconomic status. Additionally, although defined as such in this study, sex is not necessarily a binary construct, and we did not evaluate intersex. We also did not have body mass index or cholesterol data for all participants, and these are factors that can impact white matter integrity. Our analyses were also limited to one shared memory measure between the two collection sites. Finally, the myelin-related signal can be influenced by physiological factors such as white matter injury and inflammation as well as data acquisition factors such as flip angle errors, which may reduce the precision of individual MWF estimates. In the present study, we visually inspected all data to ensure adequate quality data. Future large-scale studies should employ multiple measures of learning and memory in order to more comprehensively evaluate both verbal and visual memory measures as they relate to myelin metrics in at-risk older adults.
Results of our study suggest that the relationship between age and myelin content of limbic fiber pathways depends on sex, with women showing age-related decreases that are associated with poorer memory performance. We also found significant associations between learning/recall and fornix MWF in women. The current work adds to the literature by illuminating the role that sex plays in age-related myelin decline using a more sensitive measure of myelin content. Understanding sex differences in MWF may also facilitate earlier detection of AD risk for women. Future work will expand on these findings in a larger, longitudinal sample, such as whether baseline fornix MWF is differentially associated with memory decline in men versus women. Future analyses may also examine whether age interacts with sex to affect change in myelin content of the limbic fiber pathways.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by University of California San Diego IRB and the University of Illinois Chicago IRB. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
EB: Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. KB: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. AC: Writing – review & editing. LD-W: Writing – review & editing. NE: Writing – review & editing, Data curation. LE: Writing – review & editing. SS: Writing – review & editing, Data curation, Methodology. AJ: Writing – review & editing, Conceptualization. MB: Writing – review & editing. SD: Methodology, Writing – review & editing. ML: Conceptualization, Data curation, Funding acquisition, Investigation, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing, Formal analysis, Methodology.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Sources of funding for this project include VA Clinical Science Research & Development (Career Development Award-2 1IK2CX000938 to KB, Merit Award 1I01CX001842 to KB, and IK2CX001865); grants from the National Institutes of Health National Institute on Aging R01AG063782 to KB, Diversity Supplement R01AG063782A to EB, NIA R03AG070435 to K01AG040192 and R21AG048176 to ML, and National Institute on Aging R01AG049810 to MB and P30 AG062429 Research Education Component Scholars Program to EB. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
MB receives royalties from Oxford University Press.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Amlien, I. K., and Fjell, A. M. (2014). Diffusion tensor imaging of white matter degeneration in Alzheimer’s disease and mild cognitive impairment. Neuroscience 276, 206–215. doi: 10.1016/j.neuroscience.2014.02.017
Arshad, M., Stanley, J. A., and Raz, N. (2016). Adult age differences in subcortical myelin content are consistent with protracted myelination and unrelated to diffusion tensor imaging indices. NeuroImage 143, 26–39. doi: 10.1016/j.neuroimage.2016.08.047
Bangen, K. J., Delano-Wood, L., Deoni, S. C. L., Clark, A. L., Evangelista, N. D., Hoffman, S. N., et al. (2021). Decreased myelin content of the fornix predicts poorer memory performance beyond vascular risk, hippocampal volume, and fractional anisotropy in nondemented older adults. Brain Imaging Behav. 15, 2563–2571. doi: 10.1007/s11682-021-00458-z
Bartzokis, G. (2011). Alzheimer’s disease as homeostatic responses to age-related myelin breakdown. Neurobiol. Aging 32, 1341–1371. doi: 10.1016/j.neurobiolaging.2009.08.007
Bastin, M. E., Maniega, S. M., Ferguson, K. J., Brown, L. J., Wardlaw, J. M., MacLullich, A. M. J., et al. (2010). Quantifying the effects of normal ageing on white matter structure using unsupervised tract shape modelling. NeuroImage 51, 1–10. doi: 10.1016/j.neuroimage.2010.02.036
Beaulieu, C. (2002). The basis of anisotropic water diffusion in the nervous system-a technical review. NMR Biomed. 15, 435–455. doi: 10.1002/nbm.782
Canales-Rodríguez, E. J., Alonso-Lana, S., Verdolini, N., Sarró, S., Feria, I., Montoro, I., et al. (2021). Age-and gender-related differences in brain tissue microstructure revealed by multi-component T2 relaxometry. Neurobiol. Aging 106, 68–79. doi: 10.1016/j.neurobiolaging.2021.06.002
Dale, A. M., Fischl, B., and Sereno, M. I. (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9, 179–194.
Delano-Wood, L., Stricker, N. H., Sorg, S. F., Nation, D. A., Jak, A. J., Woods, S. P., et al. (2012). Posterior cingulum white matter disruption and its associations with verbal memory and stroke risk in mild cognitive impairment. J. Alzheimers Dis. 29, 589–603. doi: 10.3233/JAD-2012-102103
Delis, D. C., Kaplan, E., and Kramer, J. H. (2001). Delis-Kaplan executive function system. APA Psyctests 20:117. doi: 10.1037/t15082-000
Delis, D. C., Kramer, J. H., Kaplan, E., and Ober, B. A., (2000). California verbal learning test--assessment. San Antonio, TX: Psychological Corporation.
Deoni, S. C. L. (2011). Correction of main and transmit magnetic field (B0 and B1) inhomogeneity effects in multicomponent-driven equilibrium single-pulse observation of T1 and T2. Magn. Reson. Med. 65, 1021–1035. doi: 10.1002/mrm.22685
Deoni, S. C. L., Dean, D. C., O’Muircheartaigh, J., Dirks, H., and Jerskey, B. A. (2012). Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. NeuroImage 63, 1038–1053. doi: 10.1016/j.neuroimage.2012.07.037
Dufouil, C., Beiser, A., McLure, L. A., Wolf, P. A., Tzourio, C., Howard, V. J., et al. (2017). Revised Framingham stroke risk profile to reflect temporal trends. Circulation 135, 1145–1159. doi: 10.1161/CIRCULATIONAHA.115.021275
Faizy, T. D., Kumar, D., Broocks, G., Thaler, C., Flottmann, F., Leischner, H., et al. (2018). Age-related measurements of the myelin water fraction derived from 3D multi-echo GRASE reflect myelin content of the cerebral white matter. Sci. Rep. 8:14991. doi: 10.1038/s41598-018-33112-8
Faizy, T. D., Thaler, C., Broocks, G., Flottmann, F., Leischner, H., Kniep, H., et al. (2020). The myelin water fraction serves as a marker for age-related myelin alterations in the cerebral white matter – a multiparametric MRI aging study. Front. Neurosci. 14:136. doi: 10.3389/fnins.2020.00136
Fischer, F. U., Wolf, D., Scheurich, A., and Fellgiebel, A. (2015). Altered whole-brain white matter networks in preclinical Alzheimer’s disease. Neuroimage Clin. 8, 660–666. doi: 10.1016/j.nicl.2015.06.007
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355.
Fletcher, E., Raman, M., Huebner, P., Liu, A., Mungas, D., Carmichael, O., et al. (2013). Loss of fornix white matter volume as a predictor of cognitive impairment in cognitively Normal elderly individuals. JAMA Neurol. 70, 1389–1395. doi: 10.1001/jamaneurol.2013.3263
Gazes, Y., Li, P., Sun, E., Razlighi, Q., and Tsapanou, A. (2019). Age specificity in fornix-to-hippocampus association. Brain Imaging Behav. 13, 1444–1452. doi: 10.1007/s11682-018-9958-1
Gozdas, E., Fingerhut, H., Chromik, L. C., O’Hara, R., Reiss, A. L., and Hosseini, S. M. H. (2020). Focal white matter disruptions along the cingulum tract explain cognitive decline in amnestic mild cognitive impairment (aMCI). Sci. Rep. 10:10213. doi: 10.1038/s41598-020-66796-y
Inano, S., Takao, H., Hayashi, N., Abe, O., and Ohtomo, K. (2011). Effects of age and gender on white matter integrity. Am. J. Neuroradiol. 32, 2103–2109. doi: 10.3174/ajnr.A2785
Jenkinson, M., Bannister, P., Brady, M., and Smith, S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17, 825–841. doi: 10.1006/nimg.2002.1132
Kodiweera, C., Alexander, A. L., Harezlak, J., McAllister, T. W., and Wu, Y.-C. (2016). Age effects and sex differences in human brain white matter of young to middle-aged adults: a DTI, NODDI, and q-space study. Neuroimage 128, 180–192. doi: 10.1016/j.neuroimage.2015.12.033
Liu, F., Vidarsson, L., Winter, J. D., Tran, H., and Kassner, A. (2010). Sex differences in the human corpus callosum microstructure: a combined T2 myelin-water and diffusion tensor magnetic resonance imaging study. Brain Res. 1343, 37–45. doi: 10.1016/j.brainres.2010.04.064
Luo, C., Li, M., Qin, R., Chen, H., Yang, D., Huang, L., et al. (2020). White matter microstructural damage as an early sign of subjective cognitive decline. Front. Aging Neurosci. 11:378. doi: 10.3389/fnagi.2019.00378
McAleese, K. E., Walker, L., Graham, S., Moya, E. L. J., Johnson, M., Erskine, D., et al. (2017). Parietal white matter lesions in Alzheimer’s disease are associated with cortical neurodegenerative pathology, but not with small vessel disease. Acta Neuropathol. 134, 459–473. doi: 10.1007/s00401-017-1738-2
Metzler-Baddeley, C., Mole, J. P., Sims, R., Fasano, F., Evans, J., Jones, D. K., et al. (2019). Fornix white matter glia damage causes hippocampal gray matter damage during age-dependent limbic decline. Sci. Rep. 9:1060. doi: 10.1038/s41598-018-37658-5
Pak, K., Chan, S. L., and Mattson, M. P. (2003). Presenilin-1 mutation sensitizes oligodendrocytes to glutamate and amyloid toxicities, and exacerbates white matter damage and memory impairment in mice. NeuroMolecular Med. 3, 53–64. doi: 10.1385/NMM:3:1:53
R Core Team (2023). R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
Salat, D., Greve, D., Pacheco, J., Quinn, B., Helmer, K., Buckner, R., et al. (2009). Regional white matter volume differences in nondemented aging and Alzheimer’s disease. NeuroImage 44, 1247–1258. doi: 10.1016/j.neuroimage.2008.10.030
Serra, L., Cercignani, M., Lenzi, D., Perri, R., Fadda, L., Caltagirone, C., et al. (2010). Grey and white matter changes at different stages of Alzheimer’s disease. J. Alzheimers Dis. 19, 147–159. doi: 10.3233/JAD-2010-1223
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E., Johansen-Berg, H., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23, S208–S219. doi: 10.1016/j.neuroimage.2004.07.051
Stadlbauer, A., Salomonowitz, E., Strunk, G., Hammen, T., and Ganslandt, O. (2008). Quantitative diffusion tensor fiber tracking of age-related changes in the limbic system. Eur. Radiol. 18, 130–137. doi: 10.1007/s00330-007-0733-8
Sullivan, E. V., Rohlfing, T., and Pfefferbaum, A. (2010). Quantitative fiber tracking of lateral and interhemispheric white matter systems in normal aging: relations to timed performance. Neurobiol. Aging 31, 464–481. doi: 10.1016/j.neurobiolaging.2008.04.007
Van Der Holst, H. M., Tuladhar, A. M., Van Norden, A. G. W., De Laat, K. F., Van Uden, I. W. M., Van Oudheusden, L. J. B., et al. (2013). Microstructural integrity of the cingulum is related to verbal memory performance in elderly with cerebral small vessel disease. Neuroimage 65, 416–423. doi: 10.1016/j.neuroimage.2012.09.060
Westerhausen, R. (2003). The influence of handedness and gender on the microstructure of the human corpus callosum: a diffusion-tensor magnetic resonance imaging study. Neurosci. Lett. 351, 99–102. doi: 10.1016/S0304-3940(03)00946-7
Wong, D., Atiya, S., Fogarty, J., Montero-Odasso, M., Pasternak, S. H., Brymer, C., et al. (2020). Reduced hippocampal glutamate and posterior cingulate N-acetyl aspartate in mild cognitive impairment and Alzheimer’s disease is associated with episodic memory performance and white matter integrity in the cingulum: a pilot study. J. Alzheimers Dis. 73, 1385–1405. doi: 10.3233/JAD-190773
Keywords: myelin, sex, age, myelin water fraction (MWF), fornix (brain), cingulum, memory, neuroimaging
Citation: Brenner EK, Bangen KJ, Clark AL, Delano-Wood L, Evangelista ND, Edwards L, Sorg SF, Jak AJ, Bondi MW, Deoni SCL and Lamar M (2023) Sex moderates the association between age and myelin water fraction in the cingulum and fornix among older adults without dementia. Front. Aging Neurosci. 15:1267061. doi: 10.3389/fnagi.2023.1267061
Received: 25 July 2023; Accepted: 27 November 2023;
Published: 15 December 2023.
Edited by:
Philip P. Foster, Baylor College of Medicine, United StatesReviewed by:
Harald E. Möller, Max Planck Institute for Human Cognitive and Brain Sciences, GermanyCopyright © 2023 Brenner, Bangen, Clark, Delano-Wood, Evangelista, Edwards, Sorg, Jak, Bondi, Deoni and Lamar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Einat K. Brenner, ZWluYXQuay5icmVubmVyQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.