- 1Department of Outpatient, Shandong Mental Health Center, Jinan, China
- 2Department of Geriatrics, Shandong Mental Health Center, Jinan, China
- 3Department of Lab Medicine, Shandong Public Health Clinical Center, Shandong University, Jinan, China
- 4Shandong Key Laboratory of Infectious Respiratory Disease, Jinan, China
- 5Department of Cardiovascular Surgery, Shandong Public Health Clinical Center, Shandong University, Jinan, China
Background: Previous cohort studies have found an association between Bacillus Calmette–Guérin (BCG) administration and incident dementia. In the systematic review and meta-analysis, we aimed to summarize the current evidence of the effect of BCG use on the risk of developing dementia.
Methods: We searched six databases until 20 May 2023 for studies investigating the risk of dementia and BCG administration. Hazard ratios (HRs) and 95% confidence intervals (95% CIs) were pooled in the meta-analysis. Meta-regression, subgroup, and sensitivity analysis were conducted as well.
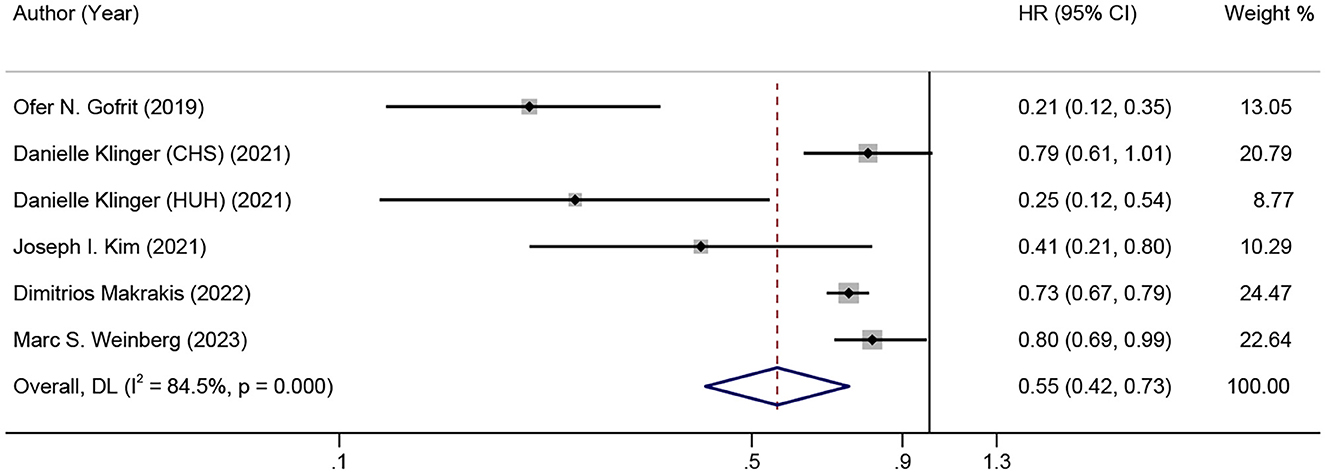
Results: Of the 4,043 records initially evaluated, five articles were included for final analysis, with a total of 45,407 bladder cancer (BC) patients. All five studies were evaluated and rated as with high quality, and a low possibility of publication bias was indicated. A significant association between BCG and the incidence of dementia in BC patients was found in all five studies. Although a high heterogeneity (I2 = 84.5%, p < 0.001) was observed, the pooled HR was 0.55 (0.42–0.73), indicating that BCG exposure or treatment reduced the risk of incident dementia by 45%. Moreover, the sensitivity analysis showed good robustness of the overall effect with no serious publication bias.
Conclusion: BCG administration is associated with a significantly lower risk of developing dementia. However, an epidemiological cohort is needed to establish a relationship between BCG use and incident dementia in the normal population. Once the relationship is confirmed, more people may benefit from the association.
Systematic review registration: identifier: CRD42023428317.
Introduction
Alzheimer's disease (AD) is the most common form of dementia, accounting for two-thirds of all dementia diagnoses (Jia et al., 2020). As an early stage of dementia, AD is known as a neurological brain disorder that could develop into progressive memory loss, loss of independence, significant comorbidities, and even death (Gaugler et al., 2022). Although a recent meta-analysis demonstrated that dementia incidence showed a decreasing trend, AD incidence did not decline (Gao et al., 2019). The US estimated healthcare costs related to AD in 2020 are estimated to be USD$ 305 billion, with the cost expected to increase to more than USD$ 1 trillion as the population ages (Wong, 2020). Fortunately, many novel mechanisms [such as vital transcription factors (Rai et al., 2021) and circular RNAs (Awasthi et al., 2018)] were revealed, and therapeutic agents [Berberine (Singh et al., 2019b, 2021b), phytoconstituents (Singh et al., 2021a)] were found, and these agents would be helpful to improve the outcome of such patients.
The pathogenesis of AD includes two key features: accumulation of amyloid β (Aβ) plaques and neurofibrillary tangles (hyperphosphorylated tau protein) (Kinney et al., 2018). Aβ and tau species could activate astrocytes and the brain's resident macrophages (microglia), which release many pro-inflammatory cytokines, such as tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), and oxidative stress biomarkers (Singh et al., 2019a; Novoa et al., 2022; Wiatrak et al., 2022). Then, neuroinflammation is triggered. However, this inflammatory response can lead to Aβ and tau overproduction and induce neurodegeneration, synapse damage, and neuronal death (Novoa et al., 2022; Wiatrak et al., 2022). In general, accumulating evidence supports that the development of AD is associated with the immune system. To date, several inflammatory mediators have been proposed as AD markers, such as TNF-α, IL-1β, ionized calcium-binding adapter molecule-1 (Iba-1), glial fibrillary acidic protein (GFAP), and nuclear factor kappa B (NF-κB) (Novoa et al., 2022). Moreover, a boost in peripheral immune cells can improve neurodegeneration by restoring the balance between the immune system and the brain (Baruch et al., 2015).
Bacillus Calmette–Guérin (BCG) is a live attenuated form of Mycobacterium bovis that was designed as a vaccine against tuberculosis. In practice, intravesical BCG is administered for preventing the recurrence of non-muscle invasive bladder cancer (NMIBC). Numerous meta-analyses have proved its efficacy (Chen et al., 2018). Although the immune effects of BCG have been recognized, the exact mechanism of anticancer activity is still unclear and may be explained as follows: it binds fibronectin in the bladder wall and stimulates Th1 cells to secrete multiple cytokines, such as interferon-γ, TNF-α, and ILs. Then, these cytokines induce cell-mediated cytotoxic activity in cancer cells (Stassar et al., 1994; Taniguchi et al., 1999).
Recently, some trials suggested that, in bladder cancer (BC) patients, BCG users have a decreased incidence of AD. However, these studies have some limitations, such as different diagnostic codes (Weinberg et al., 2023), selection bias (Gofrit et al., 2019a), different BCG strains (Klinger et al., 2021), and study design (Gofrit et al., 2019b; Kim et al., 2021; Makrakis et al., 2022). Hence, a systematic review is required to be performed to avoid such disadvantages. In the present report, we reviewed the most up-to-date evidence about the association between BCG administration and the risk of AD and other dementia in BC patients. Then, we tested whether intravesical BCG vaccine therapy for patients with BC is associated with a decreased risk of developing dementia. Subsequently, we further combined estimated hazard ratios (HRs) of incident dementia associated with BCG use.
Methods
Search strategy
The protocol for this study has been registered in the PROSPERO database (CRD42023428317). This meta-analysis was reported following the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines (Stroup et al., 2000).
Databases including PubMed, Embase, Scopus, Web of Science, CINAHL, and Cochrane Library were searched for relevant studies published up to 20 May 2023. The search terms included but were not limited to the following: BCG, vaccine, AD, and dementia. The search strategy for each database was detailed in the Supplementary material.
Study eligibility
Studies that evaluated the role of BCG administration on the risk of incident dementia were included. No specific criteria for the study design were given. The exclusion criteria of the study were as follows: duplicate, non-English document type (such as conference paper, review, book chapter, protocol, or comments), insufficient data, unavailable full text, animal studies, and bench studies.
Duplicates were removed by automated processing in EndNote. Then, two authors (CH and YL-C) screened titles and abstracts independently to identify relevant studies. Finally, the available full texts were systematically examined to evaluate compliance with our inclusion and exclusion criteria (CH and YL-C). When there were disagreements, a third reviewer (JW) made the final decision.
Study selection and data extraction
Data were extracted from eligible studies by two independent investigators (CH and YL-C). Any disagreements were resolved through consultation with a third investigator (JW), if necessary. The following information was extracted from the included studies: first author, year of publication, study design, study period, region, sample size, gender, age, follow-up period, BCG interventions, dementia subjects, effect size (such as HR), and corresponding 95% confidence intervals (CIs).
Quality assessment
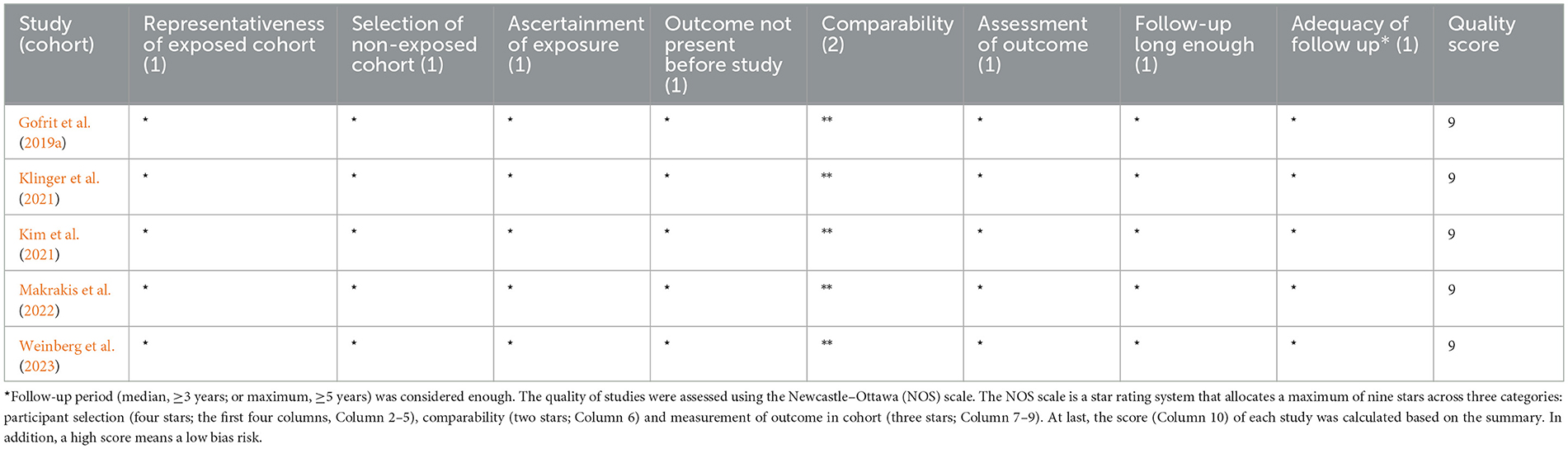
The quality of the included studies was assessed using the Newcastle–Ottawa Scale (NOS) by two independent authors (CH and YL-C), and disagreements were resolved through a third reviewer (JW). The NOS assigns a maximum of 9 points based on the following items: selection, comparability, and outcome. Subsequently, the bias risk of each study was rated as high (≤4), medium (5–6), and low (7–9), respectively.
Statistical analysis
We calculated the pooled effect size (HR) and the corresponding 95% CIs for incident dementia in the BCG group compared with the non-BCG group. The heterogeneity was quantified using the Q test and I2. If the heterogeneity is considered significant (I2 > 50% or P < 0.05), a random effects model would be employed. Otherwise, the fixed-effect model would be adopted. Subgroup analyses stratified by region, age, gender, race/ethnicity, dose, follow-up period, and outcome were performed to examine subgroup differences and potential heterogeneity sources. Sensitivity analyses were performed to assess the robustness of the primary results. Moreover, the results of the fixed effects and random effects models were compared to check the stability of the pooled results. The Harbord test and Egger's test were used to evaluate potential publication bias. All statistical analysis was performed using STATA (Version, 15.0; Stata Corporation, College Station, TX). A p < 0.05 was considered to be statistically significant.
Results
Literature selection
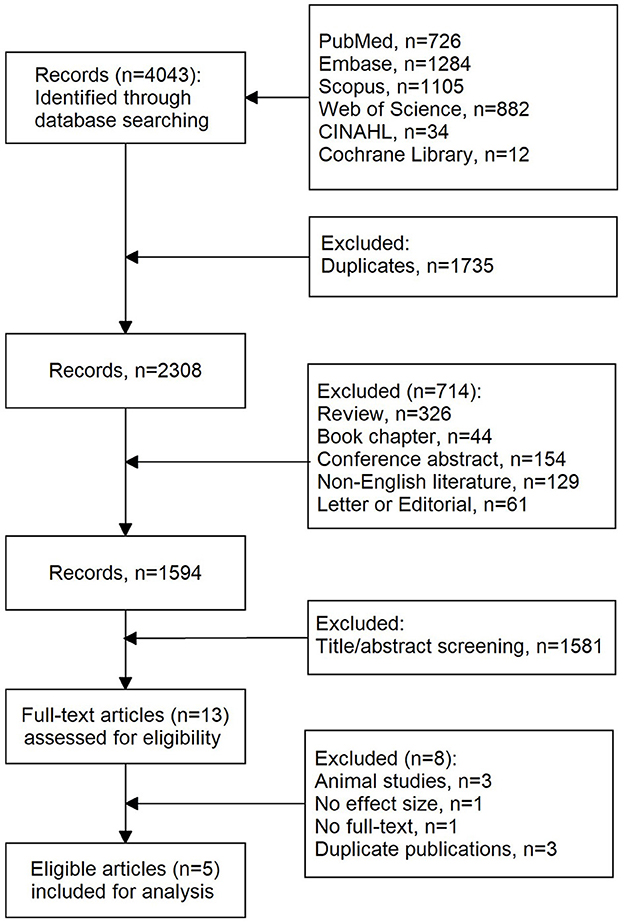
A total of 4,043 records were initially retrieved using our search strategy. As shown in Figure 1, after the removal of duplications (n = 1,735), 2,308 records were left. Of them, 714 records were further excluded due to reviews (n = 326), book chapters (n = 44), conference abstracts (n = 154), non-English literature (n = 129), and letters or editorials (n = 61). Then, 1,583 records were excluded after screening titles and abstracts. Eight studies were further excluded because of the following reasons: animal studies (n = 3), no effect size (n = 1), no full text (n = 1), and duplicates (n = 3). Finally, five eligible articles were included in this meta-analysis.
Study characteristics
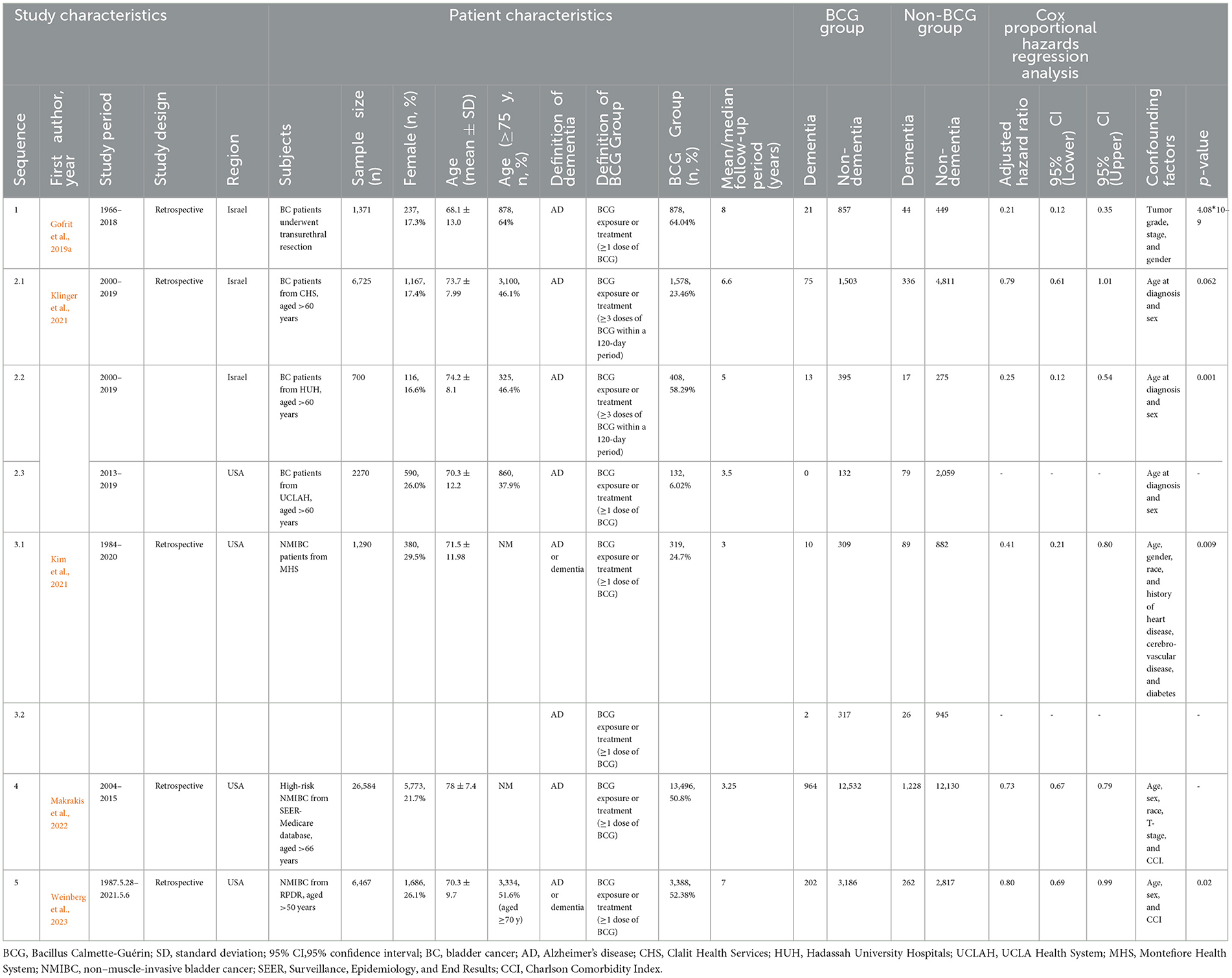
The characteristics of the included studies (n = 5) are summarized in Table 1. All studies had a retrospective nature, with a total number of 45,407 participants (sample sizes ranging from 700 to 26,584), and were published between 2019 and 2023. The studies were conducted in Israel (n = 2) or the USA (n = 3), respectively. The study period (duration from study entry date to study end) of each study ranged from 6 to 52 years, and the mean/median follow-up period varied from 3 to 8 years. All participants (experimental group) in these studies were BC patients receiving BCG induction or maintenance, while AD or other dementia patients were defined according to diagnostic codes and/or drugs prescribed. Among them, three studies considered incident AD as the outcome, and another two studies considered dementia (including AD) as the outcome. All studies were rated with the maximum score according to the NOS, indicating a high quality (Table 2).
BCG administration and incident dementia
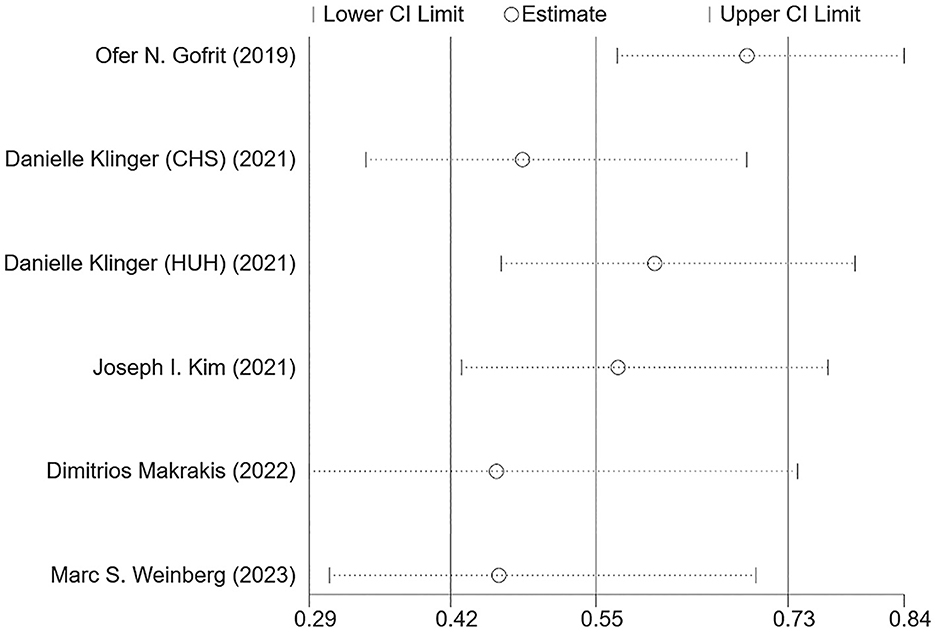
Figure 2 shows the combined effect estimate (HR) for the incidence of dementia in relation to BCG. A high heterogeneity (I2 = 84.5%, p < 0.001) was observed, so the random effects model was used. All studies showed a significant association between BCG and the incidence of dementia in BC patients, and the pooled HR was 0.55 [95% CI: 0.42–0.73, p (overall effect) < 0.001], indicating that BCG exposure or treatment reduced the risk of incident dementia by 45%.
Subgroup analyses
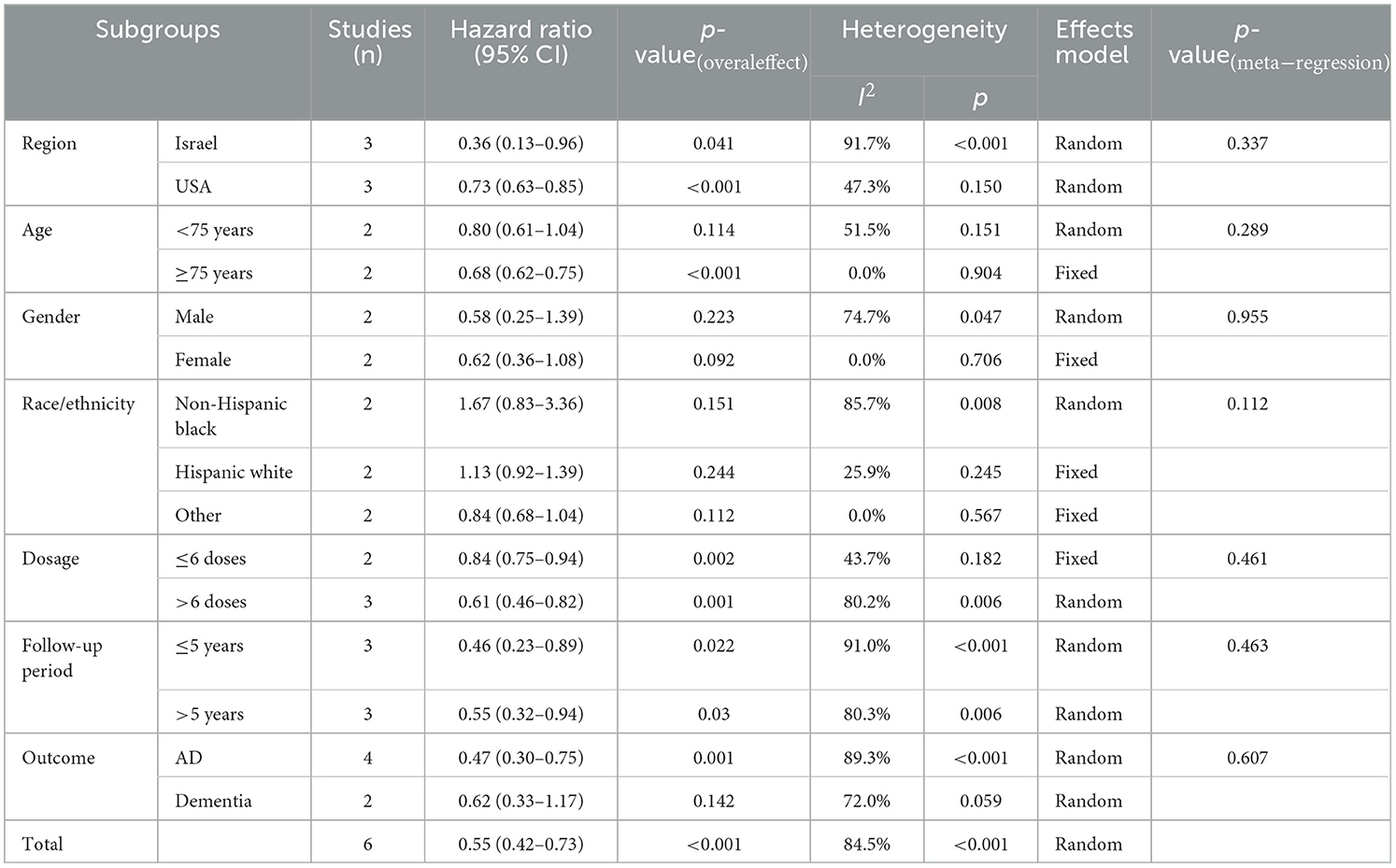
Subgroup analyses were performed stratified by region, age, gender, race/ethnicity, dose, follow-up period, and outcome (Table 3). The study heterogeneity was associated with Israel (HR = 0.36, 95% CI: 0.13–0.96; p = 0.041), the USA (HR = 0.73, 95% CI: 0.63–0.85; p < 0.001), age (≥75 years: HR = 0.68, 95% CI: 0.62–0.75; p < 0.001), dose (≤6 doses: HR = 0.84, 95% CI: 0.75–0.94, p = 0.002; >6 doses: HR = 0.61, 95% CI: 0.46–0.82, p = 0.001), follow-up period (≤5 years: HR = 0.46, 95% CI: 0.23–0.89, p = 0.022; >5 years: HR = 0.55, 95% CI: 0.32–0.94, p = 0.03), and AD (HR = 0.47, 95% CI: 0.30–0.75, p = 0.001). Therefore, BC patients with such characteristics (from Israel, old age, high dosage, short follow-up period, and AD as outcome) may have a low risk of incident dementia.
Bias assessment and sensitivity analysis
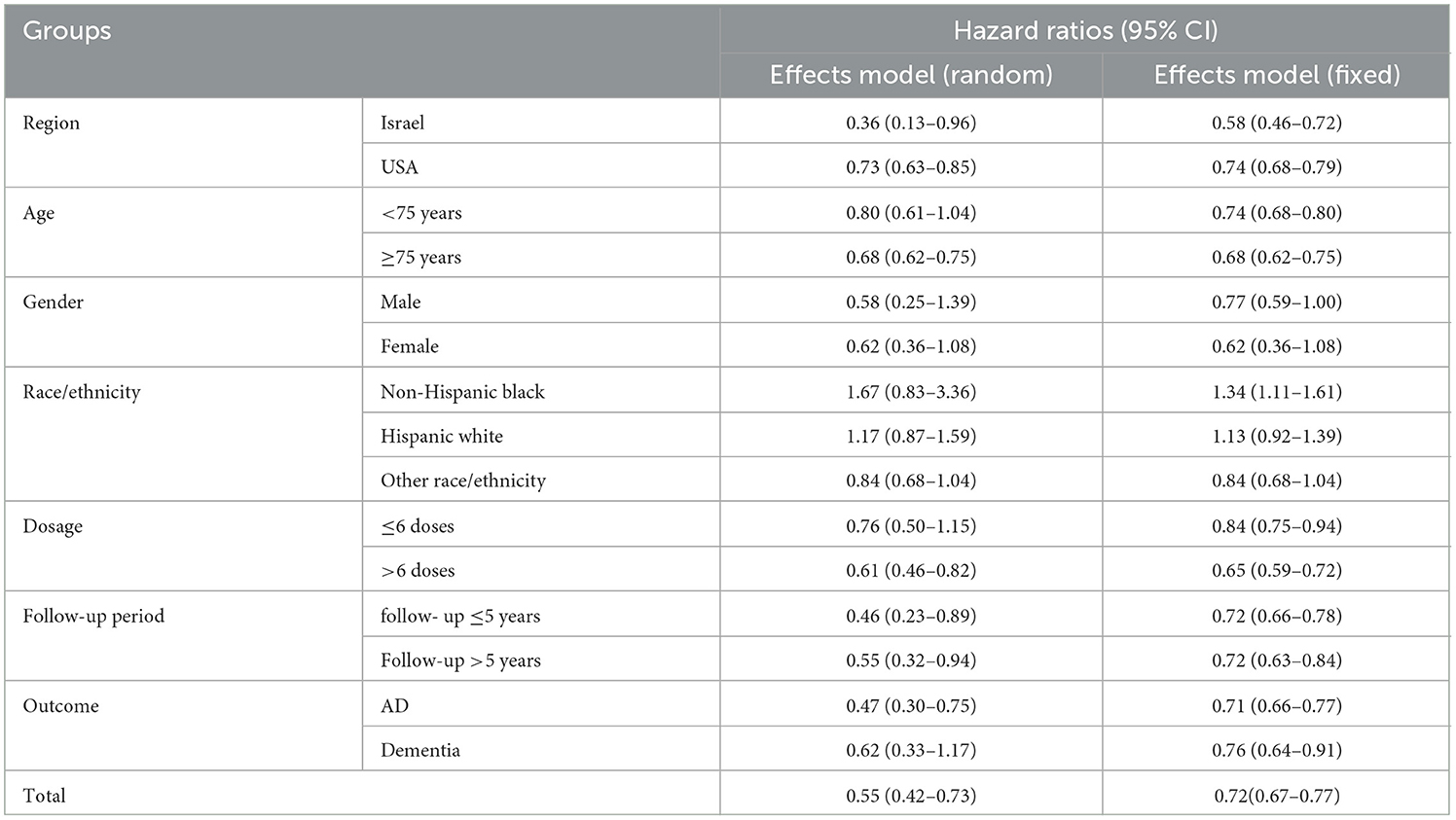
Egger's (Figure 3A) and Harbor's tests (Figure 3B) were performed for the assessment of publication bias, and the corresponding p-values were 0.108 and 0.052, respectively. The results indicated a low possibility of publication bias. Then, the stability of the meta-analysis results was assessed using the sensitivity analysis (leave-one-out method). When excluding any single study, there was no significant change in the pooled HR, indicating a robust result (Figure 4). Moreover, we further tested the stability of the results by comparing the results of the fixed and random effects models. The results remained stable in all groups, except in groups involving patients aged <75 years, male individuals, non-Hispanic black individuals, and patients with dementia, where their results shifted from a non-significant stage to a significant stage (Table 4).
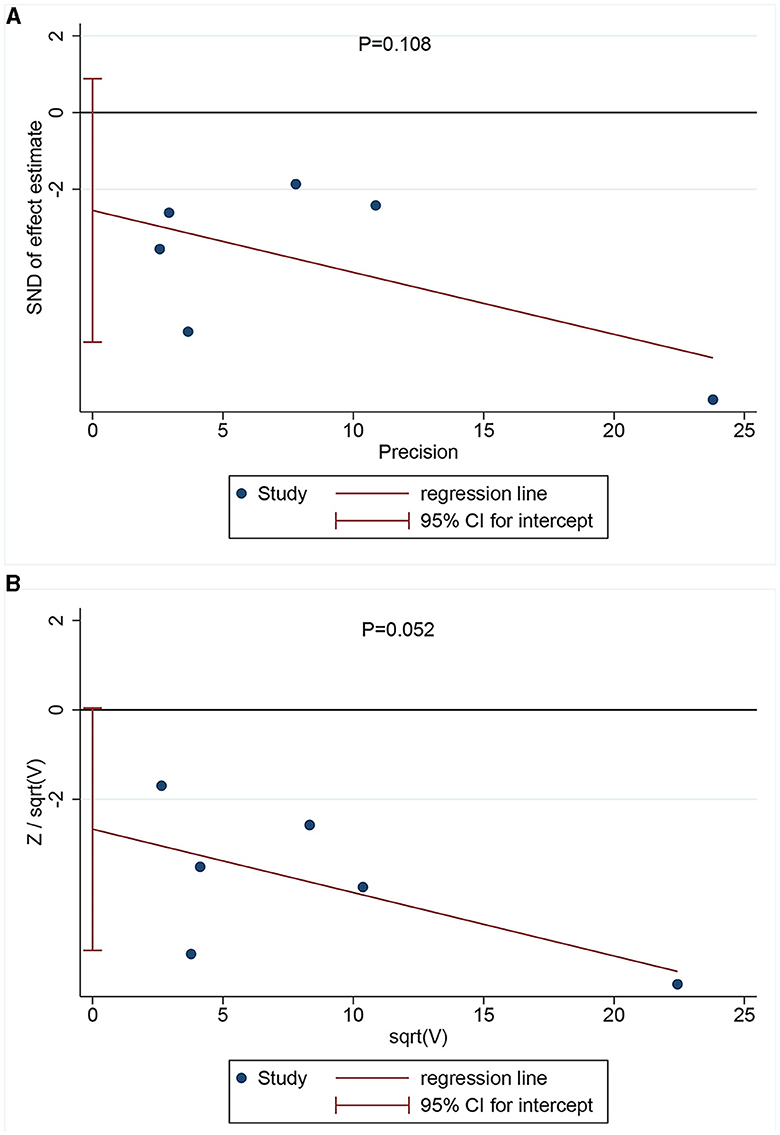
Figure 3. Publication bias assessment via Egger's (A) (P = 0.108) and Harbor's tests (B) (P = 0.052).
Discussion
In this systematic review and meta-analysis, the association between BCG use and incident dementia was investigated. Since only BC patients were included, the study overcame the health patient bias, which is a serious concern for most studies of vaccination and dementia risk. Our data demonstrated that patients not treated with BCG had a significantly higher risk of developing dementia. Although a significant heterogeneity (I2 = 84.5%, p < 0.001) was observed, a pooled HR was estimated at 0.55 (0.42–0.73), which indicated that BCG use could reduce the risk of incident dementia in BC patients. Moreover, according to meta-regression results, patients have the following characteristics, such as from Israel, old age, high dosage, short follow-up period, and AD as outcomes appear to have a reduced incidence of dementia after BCG vaccine treatment. Regarding the management of dementia, a dose-response relationship should be considered in practice, and BCG treatment may be more effective for AD than other types of dementia.
Due to the long-term non-specific immune effects of BCG exposure, a hypothesis that BCG could decrease the prevalence of AD in the elderly population was proposed (Gofrit et al., 2019a), which was investigated subsequently, and several claims were made previously. First, previous studies supported that BCG treatment is associated with a reduced risk of developing AD or other dementias (Gofrit et al., 2019b; Kim et al., 2021; Klinger et al., 2021; Makrakis et al., 2022; Weinberg et al., 2023). Second, old age was usually known as a risk factor for developing AD (Makrakis et al., 2022). Fortunately, BCG was effective for BC patients at any age to reduce the risk of incident AD (Gofrit et al., 2019a), especially in the elderly population (Klinger et al., 2021; Weinberg et al., 2023). Our study supports the idea that an older age appears to have a high non-statistical risk of incident AD and other types of dementia. Third, although a previous study found that sex distribution is associated with the prevalence of incident AD (Kim et al., 2021), our data do not support it. Although no significant variable was found in our meta-regression, patients with female sex and old age appear to have a lower incidence of dementia. Fourth, patients with high BCG exposure had a further lowered incidence of AD or another type of dementia than patients who had low BCG exposure (Kim et al., 2021; Makrakis et al., 2022), which was dose-dependent. Fifth, the role of BCG exposure in the prevalence of Parkinson's disease remains controversial due to a paradoxical response (Gofrit et al., 2019b; Klinger et al., 2021).
To date, the mechanism of association between BCG and dementia remains unclear and requires further investigation. Several potential mechanisms have been discussed previously: (1) intravesical BCG instillation may increase the immunosuppressive Treg population by IL-2 cytokines (Baek et al., 2016; Li et al., 2018), which could slow the development of clinical definite syndrome as in the case of multiple sclerosis (Sethi et al., 2014); (2) the BCG vaccination of adults could increase the glucose utilization (Kuhtreiber et al., 2018), which reverses cognitive aging (Minhas et al., 2021); and (3) the neuroinflammation of AD is primarily driven by the microglia (Gate et al., 2020). Neuroinflammation could be alleviated by recruiting inflammation-resolving monocytes to the brain, which was confirmed by the animal AD model (Zuo et al., 2017). In general, more evidence is required to clarify the precise mechanisms underlying the effects of BCG on neuroinflammation.
Recently, similar studies were evaluated for other vaccinations [such as influenza vaccination (Wiemken et al., 2021), herpes zoster vaccination (Scherrer et al., 2021b, 2022), and tetanus, diphtheria, and pertussis vaccination (Scherrer et al., 2021a)]. In a recent meta-analysis, all vaccinations (such as rabies, tetanus, diphtheria, and pertussis, herpes zoster, influenza, hepatitis A, typhoid, and hepatitis B) were associated with a trend toward reduced dementia risk, and an overall 35% lower dementia risk was reported (Itzhaki et al., 2020). Moreover, full vaccination types have a lower risk of developing dementia (Itzhaki et al., 2020; Wiemken et al., 2022). The role of influenza vaccination is also confirmed in another two meta-analyses, which suggests that it could reduce dementia risk significantly (Veronese et al., 2022; Sun et al., 2023). The vaccination against influenza may be helpful for the prevention of dementia, and subjects may benefit from more annual influenza vaccinations (Itzhaki et al., 2020). Interestingly, a paradoxical finding was observed for infections and a recent cohort (Douros et al., 2023). Infections, such as Chlamydia pneumoniae, human herpesvirus-6, Epstein-Barr virus, Herpes simplex virus-1, and the Herpesviridae family were found to be associated with a higher risk of AD (Ou et al., 2020). The association between infections and AD failed to gain substantial traction and was largely debated by the AD research community for many years (Itzhaki et al., 2020; Rai et al., 2022). More evidence for and against the infectious theory of AD should be weighed up, which may facilitate future research and drug development. Douros A et al. found that, although a decreased risk of dementia was observed for individual vaccines (shingles and diphtheria vaccines), all vaccines (such as influenza, pneumococcal, shingle, diphtheria, tetanus, and pertussis vaccines) were found with an increased risk of dementia [OR, 1.38 (95% CI, 1.36–1.40)], compared with no exposure (Douros et al., 2023). A further systematic review may be required to investigate the association between dementia risk and all vaccination.
The interpretation of this systematic review and meta-analysis must be considered within its limitations. First, all studies have a retrospective nature. Therefore, selection bias cannot be ruled out. Second, BC patients are indicated for BCG administration, and most BC patients have a good prognosis and long-term follow-up. The two key elements support testing the hypothesis in BC patients. However, the role of BCG administration remains unclear in the normal population, and further cohort studies are required. Third, although meta-regression results support the dose-dependent relationship between BCG use and the risk of dementia, the relationship was not evaluated in the study. Moreover, there was a lack of competing risk assessments. These were due to the limited availability of data from included studies. Finally, caution is required for the interpretation since a high heterogeneity exists in the analysis.
In conclusion, the present systematic review and meta-analysis indicates that BCG administration is associated with a decreased risk of dementia in BC patients. The findings suggest that the BCG administration may also have a preventive role in dementia. However, more epidemiological studies are needed to clarify the association between BCG use and decreased dementia risk in the normal population. In addition, further bench studies are needed to better understand the mechanisms linking BCG and incident dementia.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
M-SW and Y-AZ designed the study and drafted the initial manuscript. CH and Y-LC performed the screening and collected data. JW and C-PG supervised data collection. All authors approved the final version of the report.
Funding
This project was supported by the Taishan Scholar Project of Shandong Province (no. tsqn202211358).
Acknowledgments
This study is marked as the second Shandong Tuberculosis Elimination Project (STEP-2). The authors thank Prof. Mark Nicol (University of Western Australia) and Dr. VN Dahl (Aarhus University) for their kind advice and insightful questions, which helped shape the project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1243588/full#supplementary-material
References
Awasthi, R., Singh, A. K., Mishra, G., Maurya, A., Chellappan, D. K., Gupta, G., et al. (2018). An overview of circular RNAs. Adv. Exp. Med. Biol. 1087, 1–15. doi: 10.1007/978-981-13-1426-1_1
Baek, H., Ye, M., Kang, G. H., Lee, C., Lee, G., Choi, D. B., et al. (2016). Neuroprotective effects of CD4+CD25+Foxp3+ regulatory T cells in a 3xTg-AD Alzheimer's disease model. Oncotarget 7, 69347–57. doi: 10.18632/oncotarget.12469
Baruch, K., Rosenzweig, N., Kertser, A., Deczkowska, A., Sharif, A. M., Spinrad, A., et al. (2015). Breaking immune tolerance by targeting Foxp3(+) regulatory T cells mitigates Alzheimer's disease pathology. Nat. Commun. 6, 7967. doi: 10.1038/ncomms8967
Chen, S., Zhang, N., Shao, J., and Wang, X. (2018). Maintenance versus non-maintenance intravesical Bacillus Calmette-Guerin instillation for non-muscle invasive bladder cancer: a systematic review and meta-analysis of randomized clinical trials. Int. J. Surg. 52, 248–57. doi: 10.1016/j.ijsu.2018.02.045
Douros, A., Ante, Z., Suissa, S., and Brassard, P. (2023). Common vaccines and the risk of incident dementia: a population-based cohort study. J. Infect. Dis. 227, 1227–36. doi: 10.1093/infdis/jiac484
Gao, S., Burney, H. N., Callahan, C. M., Purnell, C. E., and Hendrie, H. C. (2019). Incidence of dementia and alzheimer disease over time: a meta-analysis. J.. Am.. Geriatr.. Soc. 67, 1361–9. doi: 10.1111/jgs.16027
Gate, D., Saligrama, N., Leventhal, O., Yang, A. C., Unger, M. S., Middeldorp, J., et al. (2020). Clonally expanded CD8 T cells patrol the cerebrospinal fluid in Alzheimer's disease. Nature 577, 399–404. doi: 10.1038/s41586-019-1895-7
Gaugler, J., James, B., Johnson, T., Reimer, J., Solis, M., Weuve, J. (2022). 2022 Alzheimer's disease facts and figures. Alzheimers Dement. 18, 700–789. doi: 10.1002/alz.12638
Gofrit, O. N., Bercovier, H., Klein, B. Y., Cohen, I. R., Ben-Hur, T., Greenblatt, C. L., et al. (2019a). Can immunization with bacillus calmette-guerin (BCG) protect against Alzheimer's disease? Med. Hypotheses 123, 95–7. doi: 10.1016/j.mehy.2019.01.007
Gofrit, O. N., Klein, B. Y., Cohen, I. R., Ben-Hur, T., Greenblatt, C. L., Bercovier, H., et al. (2019b). Bacillus calmette-guerin (BCG) therapy lowers the incidence of Alzheimer's disease in bladder cancer patients. PLoS ONE 14, e0224433. doi: 10.1371/journal.pone.0224433
Itzhaki, R. F., Golde, T. E., Heneka, M. T., and Readhead, B. (2020). Do infections have a role in the pathogenesis of Alzheimer disease? Nat. Rev. Neurol. 16, 193–7. doi: 10.1038/s41582-020-0323-9
Jia, L., Du, Y., Chu, L., Zhang, Z., Li, F., Lyu, D., et al. (2020). Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health 5, e661–71. doi: 10.1016/S2468-2667(20)30185-7
Kim, J. I., Zhu, D., Barry, E., Kovac, E., Aboumohamed, A., Agalliu, I., et al. (2021). Intravesical bacillus calmette-guerin treatment is inversely associated with the risk of developing alzheimer disease or other dementia among patients with non-muscle-invasive bladder cancer. Clin. Genitourin. Cancer 19, e409–16. doi: 10.1016/j.clgc.2021.05.001
Kinney, J. W., Bemiller, S. M., Murtishaw, A. S., Leisgang, A. M., Salazar, A. M., Lamb, B. T., et al. (2018). Inflammation as a central mechanism in Alzheimer's disease. Alzheimers Dement. 4, 575–90. doi: 10.1016/j.trci.2018.06.014
Klinger, D., Hill, B. L., Barda, N., Halperin, E., Gofrit, O. N., Greenblatt, C. L., et al. (2021). Bladder cancer immunotherapy by bcg is associated with a significantly reduced risk of Alzheimer's disease and Parkinson's disease. Vaccines 9, 491. doi: 10.1101/2021.01.30.21250811
Kuhtreiber, W. M., Tran, L., Kim, T., Dybala, M., Nguyen, B., Plager, S., et al. (2018). Long-term reduction in hyperglycemia in advanced type 1 diabetes: the value of induced aerobic glycolysis with BCG vaccinations. NPJ Vaccines 3, 23. doi: 10.1038/s41541-018-0062-8
Li, Y., Liu, X., Wang, W., Wang, S., Zhang, J., Jiang, S., et al. (2018). Low-dose IL-2 expands CD4(+) regulatory T cells with a suppressive function in vitro via the STAT5-dependent pathway in patients with chronic kidney diseases. Ren. Fail. 40, 280–8. doi: 10.1080/0886022X.2018.1456462
Makrakis, D., Holt, S. K., Bernick, C., Grivas, P., Gore, J. L., Wright, J. L., et al. (2022). Intravesical BCG and incidence of Alzheimer disease in patients with bladder cancer: results from an administrative dataset. Alzheimer Dis. Assoc. Disord. 36, 307–11. doi: 10.1097/WAD.0000000000000530
Minhas, P. S., Latif-Hernandez, A., McReynolds, M. R., Durairaj, A. S., Wang, Q., Rubin, A., et al. (2021). Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature 590, 122–8. doi: 10.1038/s41586-020-03160-0
Novoa, C., Salazar, P., Cisternas, P., Gherardelli, C., Vera-Salazar, R., Zolezzi, J. M., et al. (2022). Inflammation context in Alzheimer's disease, a relationship intricate to define. Biol. Res. 55, 39. doi: 10.1186/s40659-022-00404-3
Ou, Y. N., Zhu, J. X., Hou, X. H., Shen, X. N., Xu, W., Dong, Q., et al. (2020). Associations of infectious agents with Alzheimer's disease: a systematic review and meta-analysis. J. Alzheimers Dis. 75, 299–309. doi: 10.3233/JAD-191337
Rai, S. N., Tiwari, N., Singh, P., Mishra, D., Vamanu, E., Singh, M. P., et al. (2021). Therapeutic potential of vital transcription factors in Alzheimer's and Parkinson's disease with particular emphasis on transcription factor EB mediated autophagy. Front. Neurosci. 15, 777347. doi: 10.3389/fnins.2021.777347
Rai, S. N., Tiwari, N., Singh, P., Singh, A. K., Mishra, D., Imran, M., et al. (2022). Exploring the paradox of COVID-19 in neurological complications with emphasis on parkinson's and Alzheimer's disease. Oxid. Med. Cell. Longev. 2022, 3012778. doi: 10.1155/2022/3012778
Scherrer, J., Salas, J., Jacobs, C., and Wiemken, T. (2022). Lower dementia risk in patients vaccinated against herpes zoster. Ann. Fam. Med. 9, 2590. doi: 10.1370/afm.20.s1.2590
Scherrer, J. F., Salas, J., Wiemken, T. L., Hoft, D. F., Jacobs, C., Morley, J. E., et al. (2021a). Impact of herpes zoster vaccination on incident dementia: a retrospective study in two patient cohorts. PLoS ONE 16, e0257405. doi: 10.1371/journal.pone.0257405
Scherrer, J. F., Salas, J., Wiemken, T. L., Jacobs, C., Morley, J. E., Hoft, D. F., et al. (2021b). Lower risk for dementia following adult tetanus, diphtheria, and pertussis (Tdap) vaccination. J. Gerontol. Biol. Sci. Med. Sci. 76, 1436–43. doi: 10.1093/gerona/glab115
Sethi, N. K., Ristori, G., Romano, S., Coarelli, G., Buscarinu, M. C., Salvetti, M., et al. (2014). Effects of bacille calmette-guerin after the first demyelinating event in the CNS. Neurology. 83, 293. doi: 10.1212/01.wnl.0000452303.37990.ff
Singh, A. K., Mishra, G., Maurya, A., Awasthi, R., Kumari, K., Thakur, A., et al. (2019a). Role of TREM2 in Alzheimer's disease and its consequences on β- amyloid, tau and neurofibrillary tangles. Curr. Alzheimer Res. 16, 1216–29. doi: 10.2174/1567205016666190903102822
Singh, A. K., Rai, S. N., Maurya, A., Mishra, G., Awasthi, R., Shakya, A., et al. (2021a). Therapeutic potential of phytoconstituents in management OF Alzheimer's disease. Evid. Based. Complement. Alternat. Med. 2021, 5578574. doi: 10.1155/2021/5578574
Singh, A. K., Singh, S. K., Nandi, M. K., Mishra, G., Maurya, A., Rai, A., et al. (2019b). Berberine: a plant-derived alkaloid with therapeutic potential to combat Alzheimer's disease. Cent. Nerv. Syst. Agents. Med. Chem. 19, 154–70. doi: 10.2174/1871524919666190820160053
Singh, A. K., Singh, S. S., Rathore, A. S., Singh, S. P., Mishra, G., Awasthi, R., et al. (2021b). Lipid-Coated MCM-41 mesoporous silica nanoparticles loaded with berberine improved inhibition of acetylcholine esterase and amyloid formation. ACS. Biomater. Sci. Eng. 7, 3737–53. doi: 10.1021/acsbiomaterials.1c00514
Stassar, M. J., Vegt, P. D., Steerenberg, P. A., van der Meijden, A. P., Meiring, H. D., Dessens-Kroon, M., et al. (1994). Effects of isoniazid (INH) on the BCG-induced local immune response after intravesical BCG therapy for superficial bladder cancer. Urol. Res. 22, 177–84. doi: 10.1007/BF00571847
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2000). Meta-analysis of observational studies in epidemiology: a proposal for reporting. MOOSE Group JAMA 283, 2008–12. doi: 10.1001/jama.283.15.2008
Sun, H., Liu, M., and Liu, J. (2023). Association of influenza vaccination and dementia risk: a meta-analysis of cohort studies. J. Alzheimers Dis. 92, 667–78. doi: 10.3233/JAD-221036
Taniguchi, K., Koga, S., Nishikido, M., Yamashita, S., Sakuragi, T., Kanetake, H., et al. (1999). Systemic immune response after intravesical instillation of bacille Calmette-Guerin (BCG) for superficial bladder cancer. Clin. Exp. Immunol. 115, 131–5. doi: 10.1046/j.1365-2249.1999.00756.x
Veronese, N., Demurtas, J., Smith, L., Michel, J. P., Barbagallo, M., Bolzetta, F., et al. (2022). Influenza vaccination reduces dementia risk: a systematic review and meta-analysis. Ageing Res. Rev. 73, 101534. doi: 10.1016/j.arr.2021.101534
Weinberg, M. S., Zafar, A., Magdamo, C., Chung, S. Y., Chou, W. H., Nayan, M., et al. (2023). Association of BCG vaccine treatment with death and dementia in patients with non-muscle-invasive bladder cancer. JAMA. Netw. Open. 6, e2314336. doi: 10.1001/jamanetworkopen.2023.14336
Wiatrak, B., Jawien, P., Szelag, A., and Jeskowiak-Kossakowska, I. (2022). Does inflammation play a major role in the pathogenesis of Alzheimer's disease? Neuromolecular Med. 7, 1–6. doi: 10.1007/s12017-023-08741-6
Wiemken, T. L., Salas, J., Hoft, D. F., Jacobs, C., Morley, J. E., Scherrer, J. F., et al. (2021). Dementia risk following influenza vaccination in a large veteran cohort. Vaccine. 39, 5524–31. doi: 10.1016/j.vaccine.2021.08.046
Wiemken, T. L., Salas, J., Morley, J. E., Hoft, D. F., Jacobs, C., Scherrer, J. F., et al. (2022). Comparison of rates of dementia among older adult recipients of two, one, or no vaccinations. J. Am. Geriatr. Soc. 70, 1157–68. doi: 10.1111/jgs.17606
Wong, W. (2020). Economic burden of Alzheimer disease and managed care considerations. Am. J. Manag. Care. 26, S177–83. doi: 10.37765/ajmc.2020.88482
Zuo, Z., Qi, F., Yang, J., Wang, X., Wu, Y., Wen, Y., et al. (2017). Immunization with Bacillus Calmette-Guerin (BCG) alleviates neuroinflammation and cognitive deficits in APP/PS1 mice via the recruitment of inflammation-resolving monocytes to the brain. Neurobiol. Dis. 101, 27–39. doi: 10.1016/j.nbd.2017.02.001
Keywords: Bacillus Calmette-Guérin, Alzheimer's disease, dementia, risk factor, hazard ratio
Citation: Han C, Wang J, Chen Y-L, Guan C-P, Zhang Y-A and Wang M-S (2023) The role of Bacillus Calmette-Guérin administration on the risk of dementia in bladder cancer patients: a systematic review and meta-analysis. Front. Aging Neurosci. 15:1243588. doi: 10.3389/fnagi.2023.1243588
Received: 21 June 2023; Accepted: 28 July 2023;
Published: 24 August 2023.
Edited by:
Richard Lathe, University of Edinburgh, United KingdomReviewed by:
Anurag Kumar Singh, Alabama State University, United StatesJeffrey Scherrer, Saint Louis University, United States
Copyright © 2023 Han, Wang, Chen, Guan, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan-An Zhang, emhhbmd0Ymdyb3VwQDE2My5jb20=; Mao-Shui Wang, d2FuZ21hb3NodWlAZ21haWwuY29t
†ORCID: Mao-Shui Wang orcid.org/0000-0001-6046-3953
 Chao Han1
Chao Han1 Ya-Li Chen
Ya-Li Chen Mao-Shui Wang
Mao-Shui Wang