
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Aging Neurosci. , 08 January 2024
Sec. Neuroinflammation and Neuropathy
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1240509
This article is part of the Research Topic Effects of Vascular Function and Aging on Brain Circulation and Neurodegeneration View all 6 articles
Background: White matter hyperintensities (WMHs) are key neuroimaging markers of cerebral small vessel diseases. This study aimed to investigate whether intracranial and extracranial atherosclerotic stenosis is associated with WMHs.
Methods: Following a previously registered protocol (PROSPERO protocol: CRD42023407465), PubMed, Web of Science, and Embase were systematically searched for relevant literature published until March 2023. Cross-sectional studies examining the association between intracranial and extracranial atherosclerotic stenosis and WMHs were included. Random effects models were used to calculate the pooled estimates.
Results: Twenty-one eligible studies, including 10,841 participants, were identified. Intracranial and extracranial atherosclerotic stenosis was associated with an increased risk of WMHs (OR 1.80, 95% CI 1.25–2.57, I2 = 75%) and increased WMH volumes (SMD 0.40, 95% CI 0.18–0.63, I2 = 63%). Heterogeneity resulted from the WMHs rating method and the location. Extracranial atherosclerotic stenosis (ECAS) was significantly associated with WMHs (OR 2.10, 95% CI 1.22–3.62, I2 = 71%), but intracranial atherosclerotic stenosis (ICAS) was insignificantly associated with WMHs (OR 1.75, 95% CI 0.97–3.15, I2 = 84%). The association was stable in the subgroup analysis based on WMHs location, which included deep WMHs and periventricular WMHs.
Conclusion: Intracranial and extracranial atherosclerotic stenosis is associated with WMHs. This association is significant in ECAS, but attenuated in ICAS.
White matter hyperintensities (WMHs) of presumed vascular origin are ischemic manifestations of cerebral small vessel disease that can be detected through neuroimaging (Wardlaw et al., 2013). The prevalence of WMHs increases significantly with age, ranging from approximately 5% in individuals aged 40 years to nearly 100% in those aged 80 years (Zhuang et al., 2018). Severe WMHs have been linked to various negative outcomes, including cognitive impairment (Debette et al., 2019), neuropsychiatric symptoms (Clancy et al., 2021), gait dysfunction (Kim et al., 2016), increased stroke risk (Debette et al., 2019), and poorer stroke recovery (Cheng et al., 2022). However, the underlying mechanisms responsible for the development of WMHs remain incompletely understood, with current research highlighting dysfunctions in cerebral blood flow and the blood–brain barrier as crucial factors (Wardlaw et al., 2019). Previous studies have established an association between intracranial and extracranial atherosclerotic stenosis and white matter hyperintensities (WMHs), although the results of these studies are not consistent (Pu et al., 2009; Lee et al., 2011; Schulz et al., 2013; Park et al., 2015; Duan et al., 2018; Huang et al., 2022).
Atherosclerosis is the primary cause of luminal stenosis in both intracranial and extracranial arteries. Approximately 50% of Asian patients with acute ischemic stroke suffer from intracranial and extracranial atherosclerotic stenosis (Wang et al., 2014). Although WMHs are the primary imaging marker representing damage to the brain’s small vessels, some studies have reported that WMHs were more common in patients with intracranial and extracranial atherosclerotic stenosis (Lee et al., 2011; Park et al., 2015; Duan et al., 2018). A previous meta-analysis demonstrated that carotid artery stenosis was associated with total WMHs, deep WMHs (DWMHs), and periventricular WMHs (PVWMHs) (Ye et al., 2018). However, it is important to note that this meta-analysis only included a limited number of studies (n = 8). Furthermore, the study conducted by Ye et al. specifically focused on extracranial internal carotid artery stenosis, and there has been no comprehensive summary of research findings regarding intracranial atherosclerotic stenosis (ICAS) and WMHs. The pathogenic mechanisms and hemodynamic changes of ICAS and extracranial atherosclerotic stenosis (ECAS) are not entirely consistent (Kim et al., 2018). Therefore, it is necessary to determine whether ICAS, similar to ECAS, is related to WMHs. To date, a considerable number of recent studies have been published investigating the correlation between intracranial and extracranial atherosclerotic stenosis and white matter hyperintensities (WMHs) (Duan et al., 2018; Ye et al., 2019; Del Brutto et al., 2020; Fang et al., 2020; Benli et al., 2021; Yin et al., 2021; Choi et al., 2022; Ghaznawi et al., 2022; Huang et al., 2022; Wang et al., 2022). In light of this, we conducted an updated systematic review and meta-analysis to examine the potential association between ICAS or ECAS and WMHs. Additionally, the associations between intracranial and extracranial atherosclerotic stenosis and WMHs in different study designs, severity of atherosclerotic stenosis, WMHs rating method, and WMHs location were further analyzed.
This systematic review was carried out based on a predefined protocol (PROSPERO registration number: CRD42023407465), adhering to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Page et al., 2021).
The PubMed, Web of Science, and Embase databases were systematically searched from their inception to February 2023. The detailed search formula was as follows: (“white matter hyperintensit*” OR “white matter lesion*” OR “white matter change*” OR “white matter disease*” OR “white matter damage*” OR “leukoaraiosis” OR “leukoencephalopath*” OR “Binswanger’s disease”) AND (“intracranial atherosclero*” OR “cerebral atherosclero*” OR “extracranial atherosclero*” OR “arter* stenosis” OR “intracranial stenosis” OR “extracranial stenosis” OR “carotid stenosis”). The references of the included articles and relevant reviews were manually searched to identify potential studies missed during the initial literature search. Two independent investigators (ZZ and FF) performed a literature, and differences were resolved by a third investigator (WZ) joining the discussion.
This meta-analysis included studies that reported an association between ICAS or ECAS and WMHs in human. The inclusion criteria were as follows: (i) the set criteria for ICAS or ECAS severity was ≥50%; (ii) WMHs were assessed by a quantitative or semiquantitative method based on magnetic resonance imaging (MRI); (iii) the available data for meta-analysis (effect estimates or mean and standard deviation) were reported; and (iv) the article was published in full text in English. The following studies were excluded: (1) studies with unclear criteria for ICAS or ECAS severity; (2) studies with WMHs assessment based on computer tomography (CT); (3) meeting abstracts; and (4) systematic reviews. In the case of duplicated published data, we included the study with the greatest number of participants.
ICAS was defined as stenosis in intracranial internal carotid artery, middle cerebral artery, anterior cerebral artery, posterior cerebral artery, intracranial vertebral artery, and basilar artery. ECAS was defined as stenosis in extracranial internal carotid artery, extracranial vertebral artery, external carotid artery, common carotid artery, the proximal portion of subclavian artery, and aortic arch. Imaging examinations to determine the degree of vascular stenosis included Doppler ultrasonography (DUS), CT angiography (CTA), magnetic resonance angiography (MRA), and digital subtraction angiography (DSA). WMHs were defined as hyperintense in the subcortical white matter displayed on T2-weighted sequences (Figure 1) and could be divided into DWMHs and PVWMHs according to their anatomical locations. WMHs within 13 mm from ventricular surface were classified as DWMHs, and WMHs 13 mm or further from the ventricular surface were classified as PVWMHs (Kim et al., 2008). The measurement of WMHs involved quantification methods and semi-quantitative visual rating scales, such as Fazekas Scale (Fazekas et al., 1987), Scheltens Scale (Scheltens et al., 1993), and Age-Related White Matter Changes (ARWMC) Scale (Wahlund et al., 2001).
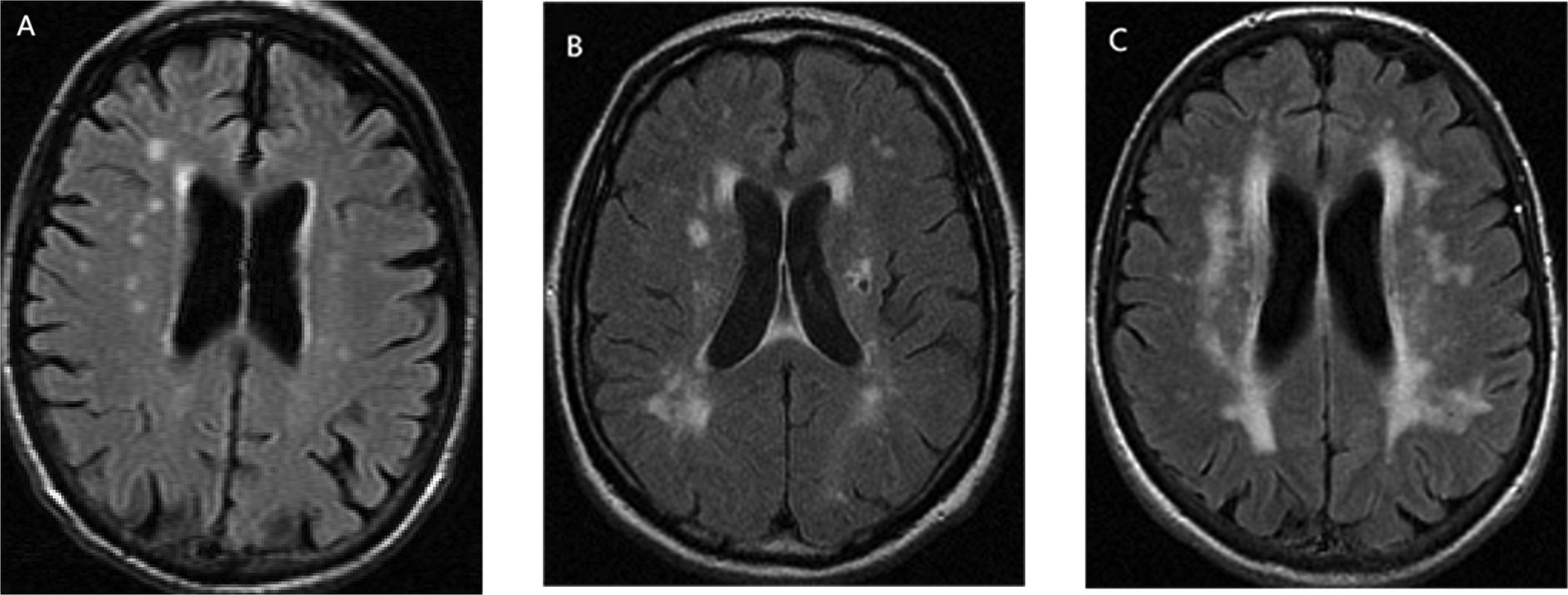
Figure 1. The form of white matter hyperintensities (WMHs), taking Fazekas Scale as an example: (A) mild, (B) moderate, and (C) severe.
Two independent reviewers (ZZ and FF) used a prespecified template to extract information on study characteristics (first author, publication year, country, study design), participant details (sample size, mean age, sex ratio), atherosclerotic stenosis assessment (location, severity, vascular image), WMHs assessment (rating method, location), and statistical analyses (effect estimate and corresponding 95% confidence interval [CI], mean and standard deviation). Relevant missing data were requested by emailing the corresponding authors when possible. Otherwise, the study was not included in the subgroup analysis grouped by missing variables. Effect estimates (usually odds ratios [ORs]) were extracted from the most fully adjusted models. When adjusted effect estimates were not directly provided, we chose unadjusted effect estimates or calculated the ORs using 2 × 2 tables.
Two independent reviewers (ZZ and FF) assessed the risk of bias in the study design using the Newcastle-Ottawa scale (Stang, 2010). The checklist consists of three domains (selection, comparability, and exposure) with a total quality score ranging from 0 to 9 points. A score equal to or exceeding 7 indicated a high-quality study. Any disagreements were resolved by a third reviewer (WZ).
Summary measures, including OR and standardized mean difference (SMD), were applied to studies reporting WMHs in the form of categorical and continuous variables, respectively. A random-effects meta-analysis model (DerSimonian Laird method) was used to calculate the pooled OR and SMD (Dersimonian and Laird, 1986). When DWMHs and PVWMHs were reported instead of total WMHs in the included studies, DWMH data was selected for the meta-analysis.
Heterogeneity was assessed using the Cochran Q statistic and was quantified using the I2 metric. I2 > 50% was considered statistically significant heterogeneity (Higgins et al., 2003). The source of heterogeneity was investigated via meta-regression (if n ≥ 10) and subgroup analyses stratified by multiple different variables. Sensitivity analyses were conducted by excluding one study at a time to examine the robustness of the synthesized results. Publication bias (if n ≥ 10) was assessed using funnel plot and Egger’s test (Egger et al., 1997). All statistical tests were two-tailed, and statistical significance was set at p < 0.05. Statistical analyses were performed using the R version 4.2.1 software (R Foundation for Statistical Computing, Vienna, Austria).
Figure 2 shows the screening and selection processes of the study. The systematic database search yielded 477, 452, and 578 records from the PubMed, Web of Science, and Embase databases, respectively. After excluding duplicates and reviewing titles and abstracts, 73 articles were considered as potential studies on the association between WMHs and ICAS or ECAS. Following a review of the full texts and a manual search, 21 studies were included in the meta-analyses. Of these, 14 studies were pooled to calculate the OR (Pu et al., 2009; Romero et al., 2009; Muñoz-Cortés et al., 2013; Schulz et al., 2013; Park et al., 2015; Duan et al., 2018; Ye et al., 2019; Del Brutto et al., 2020; Fang et al., 2020; Benli et al., 2021; Yin et al., 2021; Choi et al., 2022; Huang et al., 2022; Wang et al., 2022), and seven studies were pooled to calculate the SMD (Patankar et al., 2006; Chuang et al., 2011; Lee et al., 2011; Cheng et al., 2012; Scherr et al., 2012; Sahin et al., 2015; Ghaznawi et al., 2022).
The characteristics of the included studies are summarized in Table 1. Nine studies were conducted in Europe and America, and 12 studies were conducted in East Asia. Five studies compared WMHs in the ipsilateral and contralateral hemispheres of stenotic vessels in patients with ICAS or ECAS, and 16 studies compared WMHs in patients with ICAS or ECAS and controls without ICAS and ECAS. Overall, 21 studies included 10,841 participants (median sample size, n = 212: minimum, n = 29; maximum, n = 2,420). The average age of the participants in the included studies ranged between 51.6 and 72.7 years, and the proportion of females ranged from 8.7 to 81.0%. ICAS were investigated in 11 studies, and ECAS was investigated in 16 studies. The internal carotid artery was the artery of greatest concern (n = 20). The criterion for atherosclerotic stenosis was set as greater than 50% in 13 studies, greater than 60% in one study, and greater than 70% in seven studies. Quantitative assessment was conducted in two studies, semi-quantitative assessment in 18 studies, and qualitative assessment in one study. The commonly used scales were Fazekas Scale (n = seven), Scheltens Scale (n = four), and ARWMC Scale (n = three). Total WMHs were investigated in 14 studies, and DWMHs and PVWMHs were investigated in nine studies. According to the quality assessment, approximately half of the studies were of high quality (Supplementary Table S1).
We found intracranial and extracranial atherosclerotic stenosis to be associated with an increased risk of WMHs (OR 1.80, 95% CI 1.25–2.57, I2 = 75%, 14 studies; Figure 3). Sensitivity analyses further revealed a stable association between intracranial and extracranial atherosclerotic stenosis and WMHs (Supplementary Figure S1). Meta-regression analysis revealed that the source of heterogeneity originated from the WMHs rating method and location (both p < 0.05). Subgroup analyses showed that race, age, and sex did not influence this association (Figure 4). Not only patients with intracranial and extracranial atherosclerotic stenosis had increased odds of WMHs compared to controls without intracranial and extracranial atherosclerotic stenosis (OR 1.64, 95% CI 1.07–2.49, I2 = 77%, 11 studies), but also patients with intracranial and extracranial atherosclerotic stenosis had increased odds of WMHs in the ipsilateral hemisphere of the stenotic vessel compared to the contralateral side (OR 2.66, 95% CI 1.65–4.31, I2 = 0%, three studies). Intracranial and extracranial atherosclerotic stenosis with a threshold of 50% stenosis was associated with WMHs (OR 1.94, 95% CI 1.31–2.88, I2 = 76%, 11 studies). According to the location of atherosclerotic stenosis, ECAS (OR 2.10, 95% CI 1.22–3.62, I2 = 71%, six studies) but not ICAS (OR 1.75, 95% CI 0.97–3.15, I2 = 84%, seven studies) was associated with WMHs. Both DWMHs (OR 2.97, 95% CI 2.20–4.02, I2 = 0%, five studies), and PVWMHs (OR 1.81, 95% CI 1.01–3.26, I2 = 65%, five studies) were associated with intracranial and extracranial atherosclerotic stenosis. WMHs assessed by ARWMC Scale were not associated with intracranial and extracranial atherosclerotic stenosis. The funnel plot (Supplementary Figure S2) for the studies investigating the association between intracranial and extracranial atherosclerotic stenosis and WMHs showed mild asymmetry. However, Egger’s tests (p = 0.17) indicated no significant publication bias in this meta-analysis.
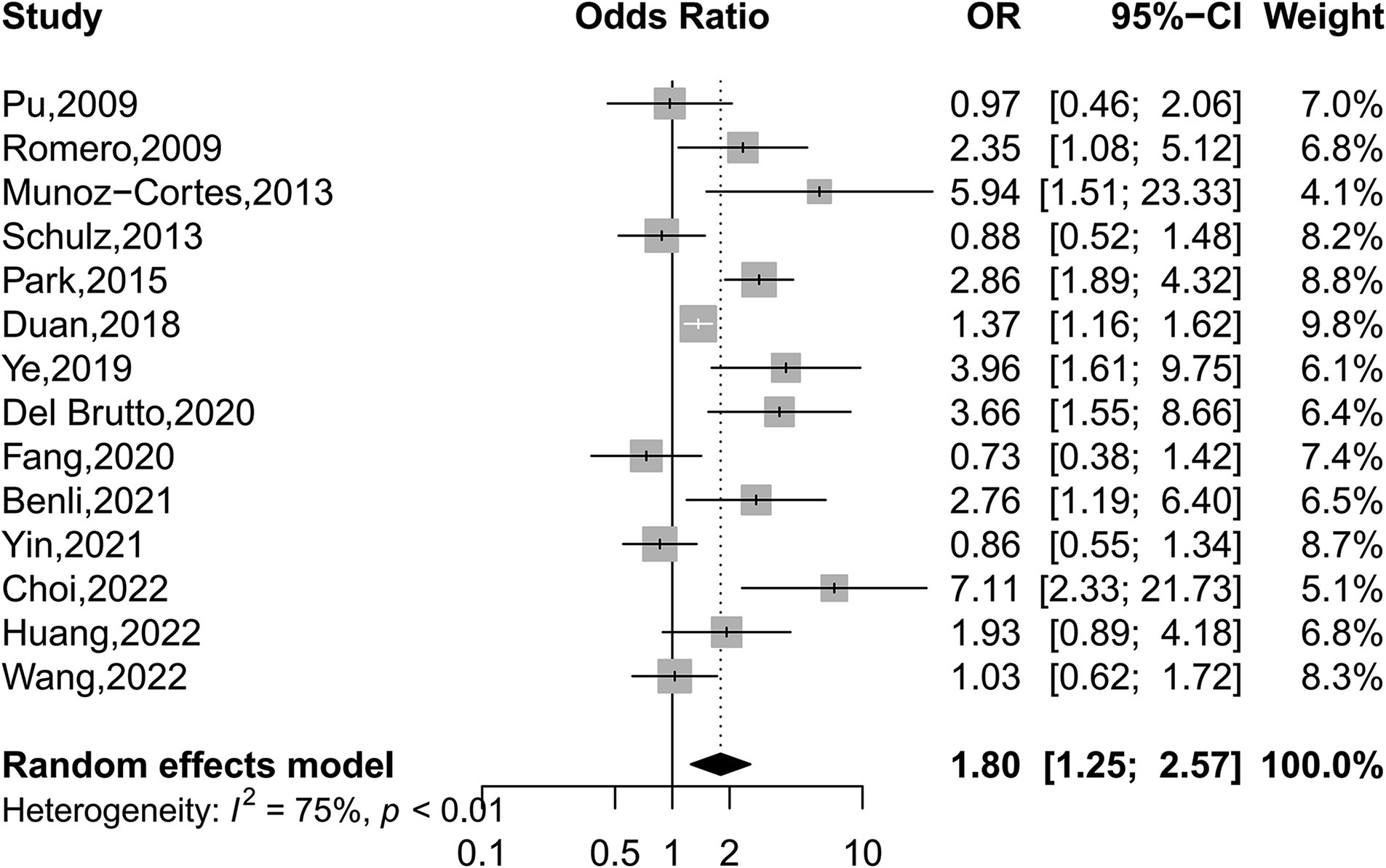
Figure 3. Forest plot depicting intracranial and extracranial atherosclerotic stenosis and the risk of WMHs.
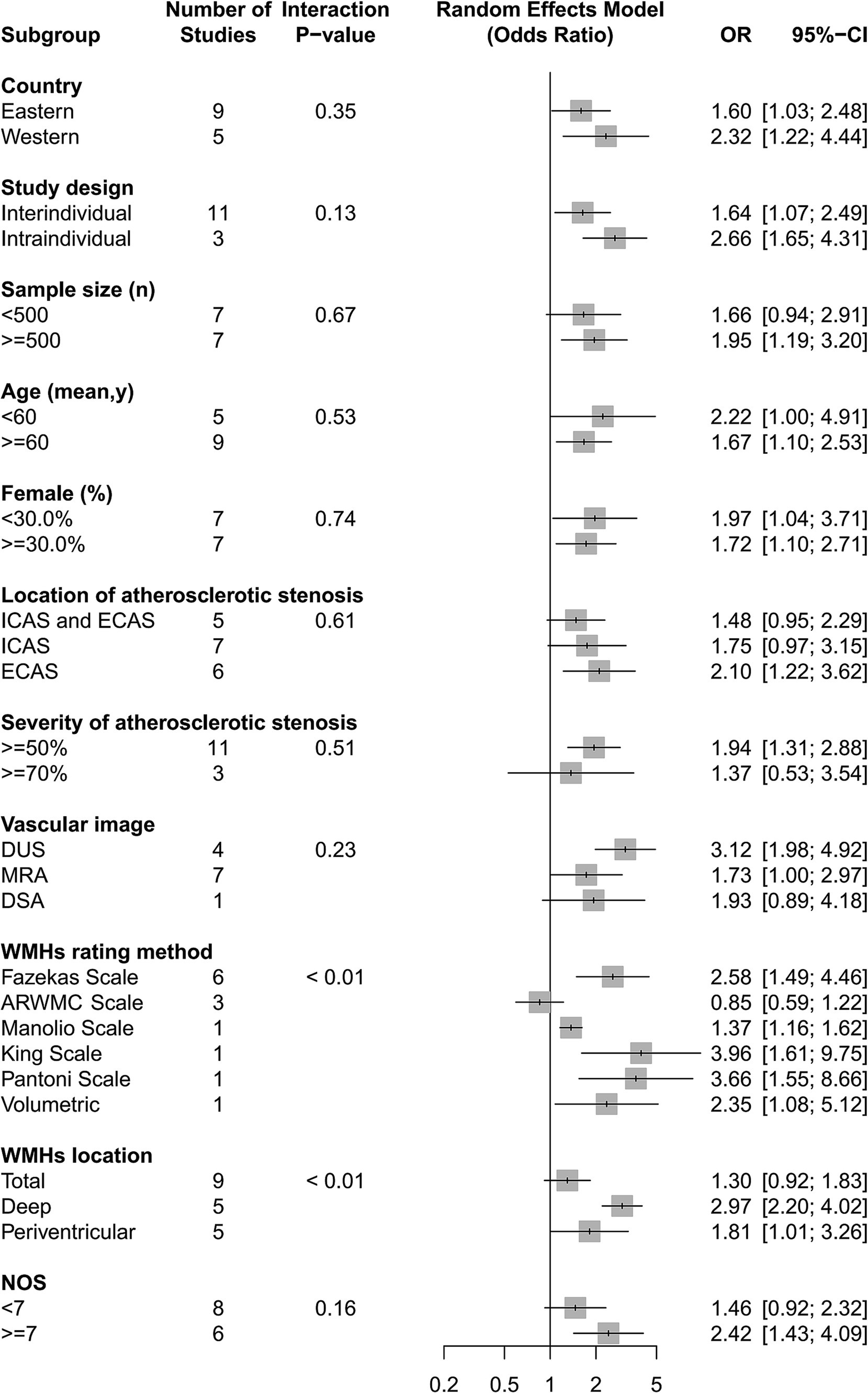
Figure 4. The association of intracranial and extracranial atherosclerotic stenosis with the risk of WMHs in subgroup analyses.
Our analyses found that intracranial and extracranial atherosclerotic stenosis to be associated with increased volume of WMHs (SMD 0.40, 95% CI 0.18–0.63, I2 = 63%, seven studies; Figure 5). The result remained stable in sensitivity analyses, excluding one study each time (Supplementary Figure S3). Subgroup analyses showed that race and sex did not influence this association (Figure 6). The results were similar between comparison the volume of WMHs in patients with intracranial and extracranial atherosclerotic stenosis and controls without intracranial and extracranial atherosclerotic stenosis (SMD 0.42, 95% CI 0.09–0.75, I2 = 75%, five studies) and comparison the volume of WMHs in the ipsilateral hemisphere of the stenotic vessel and contralateral side in patients with intracranial and extracranial atherosclerotic stenosis (SMD 0.40, 95% CI 0.16–0.64, I2 = 0%, two studies). According to the location of atherosclerotic stenosis, both ICAS (SMD 0.52, 95% CI 0.25–0.79, one study) and ECAS (SMD 0.34, 95% CI 0.07–0.62, I2 = 70%, seven studies) were associated with WMHs. Intracranial and extracranial atherosclerotic stenosis was associated with increased volume of DWMHs (SMD 0.54, 95% CI 0.22–0.86, I2 = 65%, four studies) and PVWMHs (SMD 0.41, 95% CI 0.25–0.57, I2 = 0%, four studies). No significant differences in this association were observed between the different semi-quantitative assessment scales.
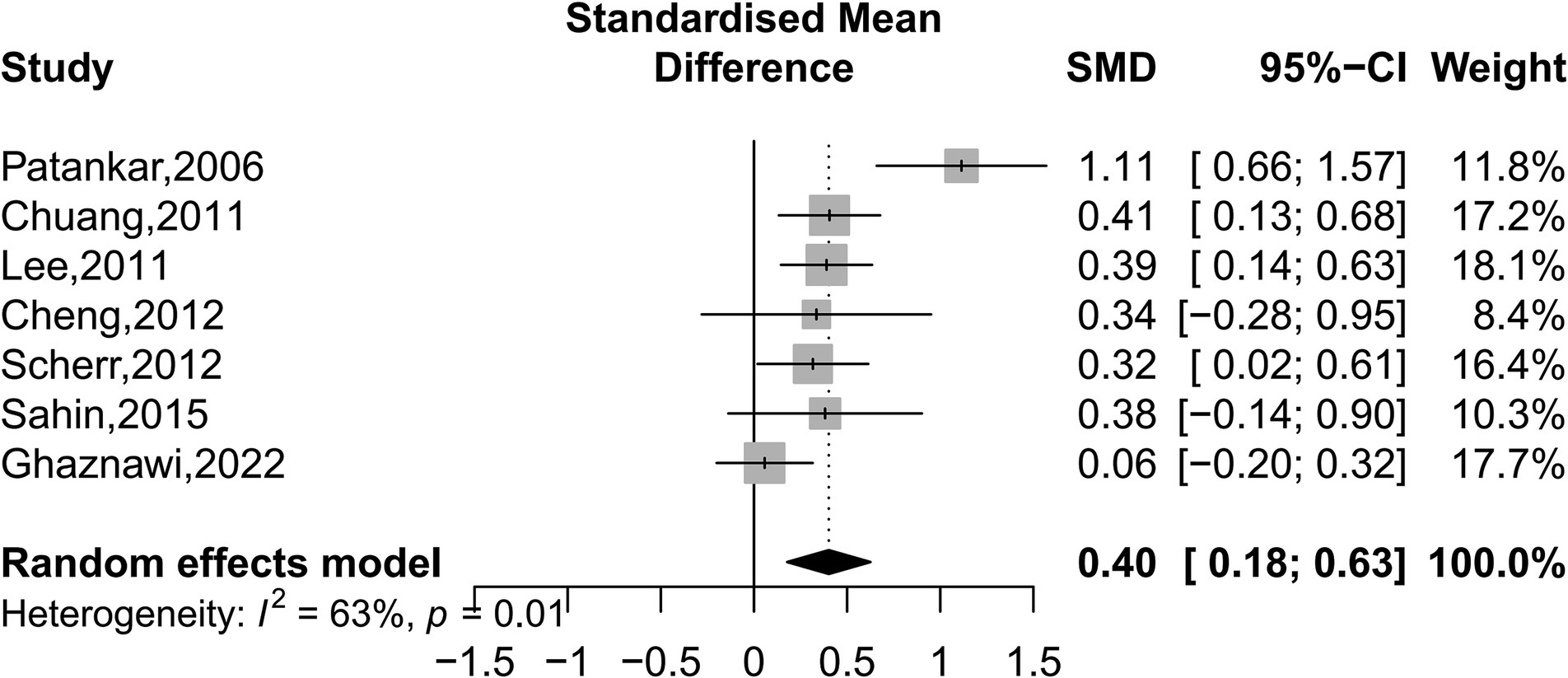
Figure 5. Forest plot depicting intracranial and extracranial atherosclerotic stenosis and the volume of WMHs.
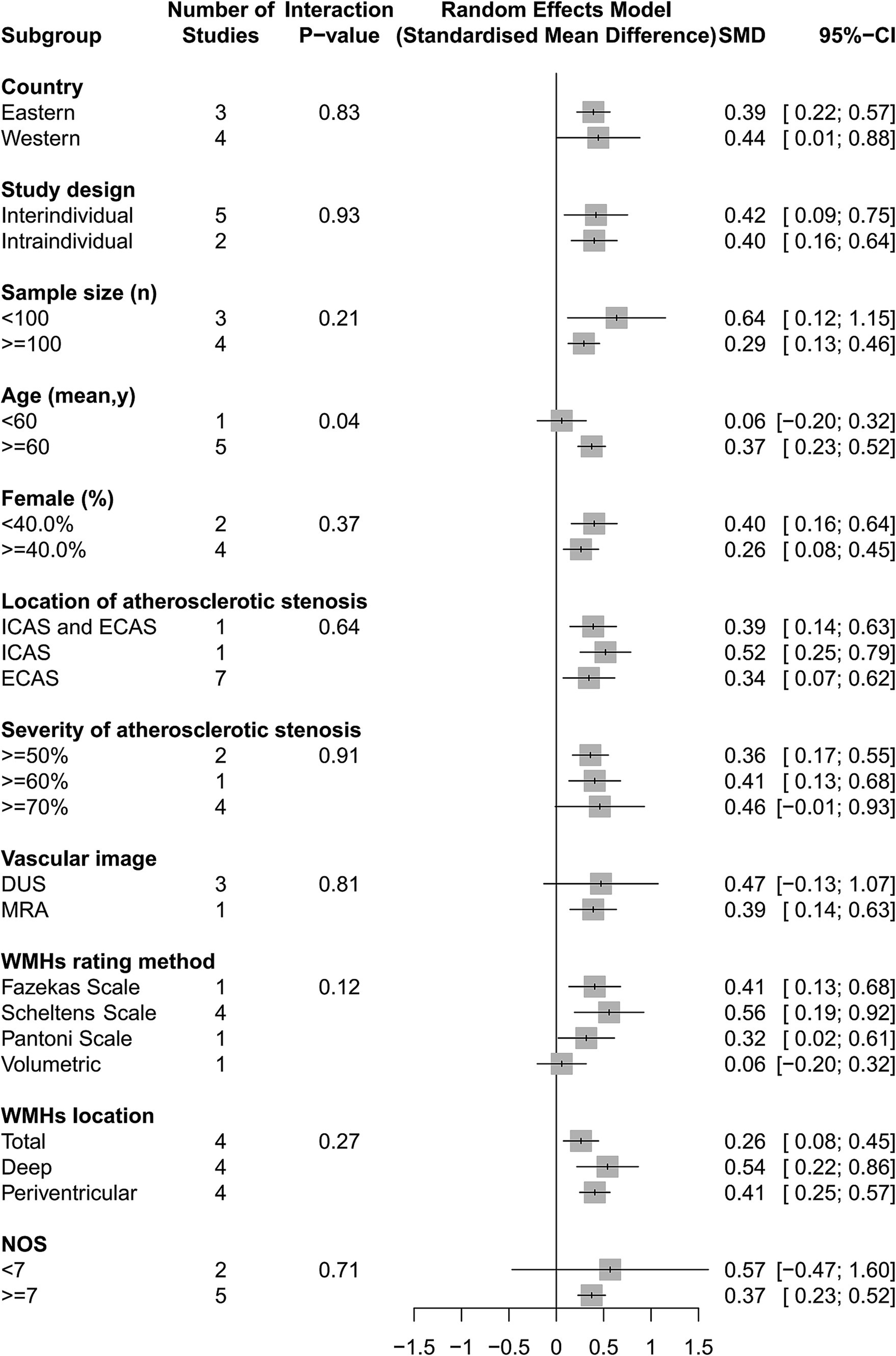
Figure 6. The association of intracranial and extracranial atherosclerotic stenosis with the volume of WMHs in subgroup analyses.
This systematic review and meta-analysis showed that intracranial and extracranial atherosclerotic stenosis ≥50% was associated with WMHs in deep and periventricular regions. The association was validated in studies designed for both inter-and intra-individual comparisons. Our study showed that ECAS was significantly associated with WMHs, whereas ICAS was only marginally associated with WMHs.
The correlation between ECAS and WMHs confirmed the findings of a previous meta-analysis by Ye et al. (2018). Consistent with previous meta-analysis, the extracranial artery of interest in the included studies was the carotid artery, particularly the extracranial internal carotid artery, which is the main blood vessel supplying the supratentorial white matter. Compared to the study by Ye et al., which only provided SMD values, our study also provided OR values. Our study was conducted to verify the correlation between ICAS and WMHs; however, the results were borderline. Several studies on ICAS and WMHs were excluded because of the lack of data available for meta-analysis, which reported contradictory results, including positive and null associations (Chutinet et al., 2012; Li et al., 2014; Nam et al., 2017; Zhai et al., 2018; Zhao et al., 2019; Ren et al., 2021; Feng et al., 2023). The inconsistencies in the results might be due to the differences in study subjects, sample size, ICAS assessment method, WMHs rating method and statistical analysis between the studies. Overall, several studies with a large sample size (n > 1,000) reported similar results, indicating that ICAS was associated with WMHs (Nam et al., 2017; Duan et al., 2018; Zhai et al., 2018; Choi et al., 2022). Another strength of our study is to explore the source of heterogeneity using meta-regression and subgroup analyses. The WMHs rating method was an important source of heterogeneity. Fazekas Scale was suitable for the analysis of WMHs in the form of categorical variable, and Scheltens Scale was suitable for the analysis of WMHs in the form of continuous variable.
The pathophysiology linking intracranial and extracranial atherosclerotic stenosis to WMHs remains unclear. The shared risk factors (e.g., advanced age, hypertension, and diabetes) of intracranial and extracranial atherosclerotic stenosis and cerebral small vessel disease may play a mediating role. However, the intra-individual comparison design which eliminates the influence of vascular risk factors still showed more severe WMHs in the ipsilateral hemisphere of intracranial and extracranial atherosclerotic stenosis than that in the contralateral hemisphere. This indicates that other important mechanisms link intracranial and extracranial atherosclerotic stenosis with WMHs. Cerebral blood flow and cerebrovascular reactivity are impaired in patients with ICAS (Liu and Li, 2016; Yang et al., 2017) or ECAS (Hartkamp et al., 2018), especially in the ipsilateral hemisphere of the stenotic vessel. Endothelial dysfunction (including cerebral blood flow, cerebrovascular reactivity, intracranial pulsatility) is a pivotal pathogenesis of cerebral small vessel disease (Wardlaw et al., 2019). Three studies reported that WMHs were not directly related to arterial stenosis but were independently associated with the stenosis-induced cerebral hemodynamic changes in patients with ICAS (Fang et al., 2020; Ren et al., 2021; Feng et al., 2023). Intracranial and extracranial atherosclerotic stenosis does not necessarily cause decreased cerebral blood flow (Shakur et al., 2014) as it also depends on collateral blood flow, which may result in a null association between intracranial and extracranial atherosclerotic stenosis and WMHs in some previous studies. Recently, high intracranial vascular pulsatility has been linked to the formation of WMHs in individuals with asymptomatic ICAS (Zhao et al., 2022).
Our results showed that the association between intracranial and extracranial atherosclerotic stenosis and WMHs was stronger in the deep white matter than in the periventricular white matter. Neuropathological examination reveals that the main pathological changes are demyelination in DWMHs and the main changes are interstitial edema in PVWMHs (Haller et al., 2013). A cross-sectional study of arterial spin-labeling images showed that ischemia-hypoperfusion is the pathogenesis of DWMHs rather than PVWMHs (Cai et al., 2022). This difference in underlying mechanisms explains the differences in the strength of the association between intracranial and extracranial atherosclerotic stenosis and WMHs at different sites.
Our meta-analysis was based on cross-sectional studies; therefore, causality between intracranial and extracranial atherosclerotic stenosis and WMHs could not be established. A 1 year follow-up study reported no association between 50–69% carotid artery stenosis and ipsilateral WMHs progression (Kwee et al., 2011). Another 7 years follow-up study also found that carotid artery stenosis ≥50% was not associated with WMHs progression, as assessed using the Fazekas Scale (Ihle-Hansen et al., 2021). The only positive findings were observed in a retrospective longitudinal study that reported that ICAS ≥20% was associated with WMHs progression assessed using the modified Rotterdam Progression scale after a 3 years interval (Zhong et al., 2022). A drawback of these studies is that cerebral perfusion was not considered. Reduced cerebral blood flow has been demonstrated to predict WMHs progression in longitudinal studies (Ten Dam et al., 2007; Bernbaum et al., 2015; Promjunyakul et al., 2018). Because cerebral blood flow can be normal in patients with carotid artery stenosis, it is necessary to conduct follow-up studies on specific patients with carotid artery stenosis who have a decrease in cerebral blood flow.
Our study has some limitations. First, gray literature such as meeting abstracts were not included in our meta-analysis. Second, a significant number of studies were excluded due to insufficient data availability, thereby impacting the comprehensiveness of our analysis on the association between ICAS and WMHs. Consequently, further high-quality studies are necessary to conduct a more robust meta-analysis. Third, substantial heterogeneity was observed in the meta-analyses. To address this, we analyzed the data using a random-effect model and explored the heterogeneity using meta-regression. Fourth, some included studies provided univariate analysis results on ICAS or ECAS and WMHs. Thus, the association may be biased due to confounding factors. Finally, a limitation of this study is that it only provides information that intracranial and extracranial atherosclerotic stenosis is associated with WMHs. Further studies are warranted to determine whether improving intracranial and extracranial atherosclerotic stenosis (e.g., stenting) can prevent WMHs progression.
In summary, despite the considerable heterogeneity and the cross-sectional nature of the included studies, this meta-analysis showed that intracranial and extracranial atherosclerotic stenosis was related to WMHs severity. Notably, this association was found to be significant in ECAS, but less pronounced in ICAS. Future studies should obtain longitudinal data on intracranial and extracranial atherosclerotic stenosis and WMHs progression.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
WZ conceived and designed the study and wrote the manuscript. WZ, ZZ, and FF performed the literature search and data extraction. FF assisted in data analysis. All authors contributed to the article and approved the submitted version.
This work was supported by the Medical Science and Technology Project of Zhejiang Province of China (No. 2021KY797).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1240509/full#supplementary-material
Benli, M. D., Güven, B., Güven, H., and Conkbayır, I. (2021). Silent brain infarcts and white matter lesions in patients with asymptomatic carotid stenosis. Acta Neurol. Belg. 121, 983–991. doi: 10.1007/s13760-020-01517-w
Bernbaum, M., Menon, B. K., Fick, G., Smith, E. E., Goyal, M., Frayne, R., et al. (2015). Reduced blood flow in normal white matter predicts development of leukoaraiosis. J. Cereb. Blood Flow Metab. 35, 1610–1615. doi: 10.1038/jcbfm.2015.92
Cai, J., Sun, J., Chen, H., Chen, Y., Zhou, Y., Lou, M., et al. (2022). Different mechanisms in periventricular and deep white matter hyperintensities in old subjects. Front. Aging Neurosci. 14:940538. doi: 10.3389/fnagi.2022.940538
Cheng, H. L., Lin, C. J., Soong, B. W., Wang, P. N., Chang, F. C., et al. (2012). Impairments in cognitive function and brain connectivity in severe asymptomatic carotid stenosis. Stroke 43, 2567–2573. doi: 10.1161/STROKEAHA.111.645614
Cheng, Z., Zhang, W., Zhan, Z., Xia, L., and Han, Z. (2022). Cerebral small vessel disease and prognosis in intracerebral haemorrhage: a systematic review and meta-analysis of cohort studies. Eur. J. Neurol. 29, 2511–2525. doi: 10.1111/ene.15363
Choi, J., Kim, J. Y., Kwon, H. J., Choi, H. J., Kim, S. H., Kim, S., et al. (2022). Association of cerebral white matter hyperintensities with coronary artery calcium in a healthy population: a cross-sectional study. Sci. Rep. 12:21562. doi: 10.1038/s41598-022-25654-9
Chuang, Y. M., Huang, K. L., Chang, Y. J., Chang, C. H., Chang, T. Y., et al. (2011). Associations between circle of Willis morphology and white matter lesion load in subjects with carotid artery stenosis. Eur. Neurol. 66, 136–144. doi: 10.1159/000329274
Chutinet, A., Biffi, A., Kanakis, A., Fitzpatrick, K. M., Furie, K. L., et al. (2012). Severity of leukoaraiosis in large vessel atherosclerotic disease. AJNR Am. J. Neuroradiol. 33, 1591–1595. doi: 10.3174/ajnr.A3015
Clancy, U., Gilmartin, D., Jochems, A. C. C., Knox, L., Doubal, F. N., et al. (2021). Neuropsychiatric symptoms associated with cerebral small vessel disease: a systematic review and meta-analysis. Lancet Psychiatry 8, 225–236. doi: 10.1016/S2215-0366(20)30431-4
Debette, S., Schilling, S., Duperron, M. G., Larsson, S. C., and Markus, H. S. (2019). Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol. 76, 81–94. doi: 10.1001/jamaneurol.2018.3122
Del Brutto, O. H., Del Brutto, V. J., Mera, R. M., Pérez, P., Recalde, B. Y., et al. (2020). Prevalence and correlates of intracranial atherosclerotic disease among community-dwelling older adults of Amerindian ancestry. The three villages study. J. Stroke Cerebrovasc. Dis. 29:105135. doi: 10.1016/j.jstrokecerebrovasdis.2020.105135
Dersimonian, R., and Laird, N. (1986). Meta-analysis in clinical trials. Control. Clin. Trials 7, 177–188. doi: 10.1016/0197-2456(86)90046-2
Duan, W., Pu, Y., Liu, H., Jing, J., Pan, Y., Zou, X., et al. (2018). Association between Leukoaraiosis and symptomatic intracranial large artery Stenoses and occlusions: the Chinese intracranial atherosclerosis (CICAS) study. Aging Dis. 9, 1074–1083. doi: 10.14336/AD.2018.0118
Egger, M., Davey Smith, G., Schneider, M., and Minder, C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634. doi: 10.1136/bmj.315.7109.629
Fang, H., Leng, X., Pu, Y., Zou, X., Pan, Y., Song, B., et al. (2020). Hemodynamic significance of middle cerebral artery stenosis associated with the severity of ipsilateral white matter changes. Front. Neurol. 11:214. doi: 10.3389/fneur.2020.00214
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., and Zimmerman, R. A. (1987). MR signal abnormalities at 1.5-T in ALZHEIMERS dementia and normal aging. Am. J. Neuroradiol. 8, 421–426.
Feng, F., Kan, W., Yang, H., Ding, H., Wang, X., and Dong, R. (2023). White matter hyperintensities had a correlation with the cerebral perfusion level, but no correlation with the severity of large vessel stenosis in the anterior circulation. Brain Behav. 13. doi: 10.1002/brb3.2932
Ghaznawi, R., Rissanen, I., De Bresser, J., Kuijf, H. J., Zuithoff, N. P. A., et al. (2022). Carotid artery stenosis and progression of hemispheric brain atrophy: the SMART-MR study. Cerebrovasc. Dis., 1–8.
Haller, S., Kovari, E., Herrmann, F. R., Cuvinciuc, V., Tomm, A. M., Zulian, G. B., et al. (2013). Do brain T2/FLAIR white matter hyperintensities correspond to myelin loss in normal aging? A radiologic-neuropathologic correlation study. Acta Neuropathol. Commun. 1:14. doi: 10.1186/2051-5960-1-14
Hartkamp, N. S., Petersen, E. T., Chappell, M. A., Okell, T. W., Uyttenboogaart, M., et al. (2018). Relationship between haemodynamic impairment and collateral blood flow in carotid artery disease. J. Cereb. Blood Flow Metab. 38, 2021–2032. doi: 10.1177/0271678X17724027
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring inconsistency in meta-analyses. BMJ 327, 557–560. doi: 10.1136/bmj.327.7414.557
Huang, K. L., Chang, T. Y., Wu, Y. M., Chang, Y. J., Wu, H. C., Liu, C. H., et al. (2022). Mediating roles of leukoaraiosis and infarcts in the effects of unilateral carotid artery stenosis on cognition. Front. Aging Neurosci. 14:972480. doi: 10.3389/fnagi.2022.972480
Ihle-Hansen, H., Ihle-Hansen, H., Fure, B., Thommessen, B., Helland, G. B., Oksengard, A. R., et al. (2021). Carotid atherosclerosis and longitudinal changes of MRI visual rating measures in stroke survivors: a seven-year follow-up study. J. Stroke Cerebrovasc. Dis. 30:106010. doi: 10.1016/j.jstrokecerebrovasdis.2021.106010
Kim, J. S., Kim, Y. J., Ahn, S. H., and Kim, B. J. (2018). Location of cerebral atherosclerosis: why is there a difference between east and west? Int. J. Stroke 13, 35–46. doi: 10.1177/1747493016647736
Kim, Y. J., Kwon, H. K., Lee, J. M., Cho, H., Kim, H. J., Park, H. K., et al. (2016). Gray and white matter changes linking cerebral small vessel disease to gait disturbances. Neurology 86, 1199–1207. doi: 10.1212/WNL.0000000000002516
Kim, K. W., Macfall, J. R., and Payne, M. E. (2008). Classification of white matter lesions on magnetic resonance imaging in elderly persons. Biol. Psychiatry 64, 273–280. doi: 10.1016/j.biopsych.2008.03.024
Kwee, R. M., Hofman, P. A., Gronenschild, E. H., Van Oostenbrugge, R. J., Mess, W. H., Ter Berg, J. W., et al. (2011). Association between carotid plaque characteristics and cerebral white matter lesions: one-year follow-up study by MRI. PLoS One 6:e17070. doi: 10.1371/journal.pone.0017070
Lee, S. J., Kim, J. S., Chung, S. W., Kim, B. S., Ahn, K. J., and Lee, K. S. (2011). White matter hyperintensities (WMH) are associated with intracranial atherosclerosis rather than extracranial atherosclerosis. Arch. Gerontol. Geriatr. 53, e129–e132. doi: 10.1016/j.archger.2010.07.008
Li, H., Xu, G., Xiong, Y., Zhu, W., Yin, Q., Fan, X., et al. (2014). Relationship between cerebral atherosclerosis and leukoaraiosis in aged patients: results from DSA. J. Neuroimaging 24, 338–342. doi: 10.1111/jon.12047
Liu, Z., and Li, Y. (2016). Cortical cerebral blood flow, oxygen extraction fraction, and metabolic rate in patients with middle cerebral artery stenosis or acute stroke. AJNR Am. J. Neuroradiol. 37, 607–614. doi: 10.3174/ajnr.A4624
Muñoz-Cortés, M., Cabré, C., Villa, D., Vives, J. P., Arruche, M., Soler, J., et al. (2013). Oxidative stress and other risk factors for white matter lesions in chronic hemodialysis patients. Clin. Nephrol. 80, 187–197. doi: 10.5414/CN107943
Nam, K. W., Kwon, H. M., Jeong, H. Y., Park, J. H., Kim, S. H., Jeong, S. M., et al. (2017). Cerebral white matter hyperintensity is associated with intracranial atherosclerosis in a healthy population. Atherosclerosis 265, 179–183. doi: 10.1016/j.atherosclerosis.2017.09.010
Page, M. J., Mckenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71
Park, J. H., Kwon, H. M., Lee, J., Kim, D. S., and Ovbiagele, B. (2015). Association of intracranial atherosclerotic stenosis with severity of white matter hyperintensities. Eur. J. Neurol. 22, e2–e3.
Patankar, T., Widjaja, E., Chant, H., Mccollum, C., Baldwin, R., Jeffries, S., et al. (2006). Relationship of deep white matter hyperintensities and cerebral blood flow in severe carotid artery stenosis. Eur. J. Neurol. 13, 10–16. doi: 10.1111/j.1468-1331.2006.01115.x
Promjunyakul, N. O., Dodge, H. H., Lahna, D., Boespflug, E. L., Kaye, J. A., et al. (2018). Baseline NAWM structural integrity and CBF predict periventricular WMH expansion over time. Neurology 90, e2119–e2126. doi: 10.1212/WNL.0000000000005684
Pu, Y., Liu, L., Zou, X., Chen, P., Wang, Y., Zhou, Y., et al. (2009). Relationship between leukoaraiosis and cerebral large artery stenosis. Neurol. Res. 31, 376–380. doi: 10.1179/174313209X444071
Ren, T., Sun, S., Li, B., Chen, Y., Qu, X., et al. (2021). Study on the correlation between ischemic Leukoaraiosis and cerebral large artery stenosis using the stages of the preinfarction period based on the result of computed tomography perfusion. Neurologist 27, 1–5.
Romero, J. R., Beiser, A., Seshadri, S., Benjamin, E. J., Polak, J. F., Vasan, R. S., et al. (2009). Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment: the Framingham study. Stroke 40, 1590–1596. doi: 10.1161/STROKEAHA.108.535245
Sahin, N., Solak, A., Genc, B., and Akpinar, M. B. (2015). Dilatation of the Virchow-Robin spaces as an indicator of unilateral carotid artery stenosis: correlation with white matter lesions. Acta Radiol. 56, 852–859. doi: 10.1177/0284185114544243
Scheltens, P., Barkhof, F., Leys, D., Pruvo, J. P., Nauta, J. J. P., et al. (1993). A semiquantitative rating-scale for the assessment of signal HYPERINTENSITIES on magnetic-resonance-imaging. J. Neurol. Sci. 114, 7–12. doi: 10.1016/0022-510X(93)90041-V
Scherr, M., Trinka, E., Mc Coy, M., Krenn, Y., Staffen, W., Kirschner, M., et al. (2012). Cerebral hypoperfusion during carotid artery stenosis can lead to cognitive deficits that may be independent of white matter lesion load. Curr. Neurovasc. Res. 9, 193–199. doi: 10.2174/156720212801619009
Schulz, U. G., Grüter, B. E., Briley, D., and Rothwell, P. M. (2013). Leukoaraiosis and increased cerebral susceptibility to ischemia: lack of confounding by carotid disease. J. Am. Heart Assoc. 2:e000261. doi: 10.1161/JAHA.113.000261
Shakur, S. F., Hrbac, T., Alaraj, A., Du, X., Aletich, V. A., Charbel, F. T., et al. (2014). Effects of extracranial carotid stenosis on intracranial blood flow. Stroke 45, 3427–3429. doi: 10.1161/STROKEAHA.114.006622
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 25, 603–605. doi: 10.1007/s10654-010-9491-z
Ten Dam, V. H., Van Den Heuvel, D. M., Craen AJ, D. E., Bollen, E. L., Murray, H. M., et al. (2007). Decline in total cerebral blood flow is linked with increase in periventricular but not deep white matter hyperintensities. Radiology 243, 198–203.
Wahlund, L. O., Barkhof, F., Fazekas, F., Bronge, L., Augustin, M., Sjögren, M., et al. (2001). A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 32, 1318–1322. doi: 10.1161/01.STR.32.6.1318
Wang, X. Y., Lyu, J. H., Zhang, S. H., Duan, C. H., Duan, Q., Ma, X. X., et al. (2022). Severity of intracranial large artery disease correlates with cerebral small vessel disease. J. Magn. Reson. Imaging 56, 264–272. doi: 10.1002/jmri.28004
Wang, Y., Zhao, X., Liu, L., Soo, Y. O., Pu, Y., Pan, Y., et al. (2014). Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese intracranial atherosclerosis (CICAS) study. Stroke 45, 663–669. doi: 10.1161/STROKEAHA.113.003508
Wardlaw, J. M., Smith, E. E., Biessels, G. J., Cordonnier, C., Fazekas, F., Frayne, R., et al. (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838. doi: 10.1016/S1474-4422(13)70124-8
Wardlaw, J. M., Smith, C., and Dichgans, M. (2019). Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 18, 684–696. doi: 10.1016/S1474-4422(19)30079-1
Yang, F., Shi, W., Shi, J., Zhang, Y., Yin, Y., Shi, H., et al. (2017). Assessment of cerebrovascular reserve in unilateral middle cerebral artery stenosis using perfusion CT and CO(2) inhalation tests. Int. J. Neurosci. 127, 320–325. doi: 10.1080/00207454.2016.1235044
Ye, H., Wang, Y., Qiu, J., Wu, Q., Xu, M., and Wang, J. (2018). White matter hyperintensities and their subtypes in patients with carotid artery stenosis: a systematic review and meta-analysis. BMJ Open 8:e020830. doi: 10.1136/bmjopen-2017-020830
Ye, H., Wu, X., Yan, J., Wang, J., Qiu, J., and Wang, Y. (2019). Completeness of circle of Willis and white matter hyperintensities in patients with severe internal carotid artery stenosis. Neurol. Sci. 40, 509–514. doi: 10.1007/s10072-018-3683-9
Yin, K., Liang, S., Tang, X., Li, M., Yuan, J., Wu, M., et al. (2021). The relationship between intracranial arterial dolichoectasia and intracranial atherosclerosis. Clin. Neurol. Neurosurg. 200:106408. doi: 10.1016/j.clineuro.2020.106408
Zhai, F. F., Yan, S., Li, M. L., Han, F., Wang, Q., Zhou, L. X., et al. (2018). Intracranial arterial dolichoectasia and stenosis: risk factors and relation to cerebral small vessel disease. Stroke 49, 1135–1140. doi: 10.1161/STROKEAHA.117.020130
Zhao, Y. Y., Dang, L., Tian, X., Yang, M. L., Lv, M., Sun, Q. J., et al. (2022). Association between intracranial Pulsatility and white matter Hyperintensities in asymptomatic intracranial arterial stenosis: A population-based study in Shandong, China. J. Stroke Cereb. Diseases 31:106406. doi: 10.1016/j.jstrokecerebrovasdis.2022.106406
Zhao, M., Liu, M., Nie, Z., Lu, Z., Jin, L., and Li, Y. (2019). Correlation of moderate-to-severe intracranial arterial stenosis load with low cerebral perfusion, white matter hyperintensity, and cognitive dysfunction in patients without strokes. Int. J. Clin. Exp. Med. 12, 5051–5059.
Zhong, T., Qi, Y., Li, R., Zhou, H., Ran, B., Wang, J., et al. (2022). Contribution of intracranial artery stenosis to white matter hyperintensities progression in elderly Chinese patients: A 3-year retrospective longitudinal study. Front. Neurol. 13:922320. doi: 10.3389/fneur.2022.922320
Keywords: cerebral small vessel disease, extracranial atherosclerosis, intracranial atherosclerosis, meta-analysis, white matter hyperintensities
Citation: Zhang W, Fu F and Zhan Z (2024) Association between intracranial and extracranial atherosclerosis and white matter hyperintensities: a systematic review and meta-analysis. Front. Aging Neurosci. 15:1240509. doi: 10.3389/fnagi.2023.1240509
Received: 15 June 2023; Accepted: 28 November 2023;
Published: 08 January 2024.
Edited by:
Stefano Tarantini, University of Oklahoma Health Sciences Center, United StatesReviewed by:
Ma Yuanyuan, Fudan University, ChinaCopyright © 2024 Zhang, Fu and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenyuan Zhang, emhhbmd3ZW55dWFuMTFAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.