- 1Center for Neurodegenerative Diseases and the Aging Brain, University of Bari Aldo Moro at Pia Fondazione “Card. G. Panico,” Tricase, Italy
- 2Department of Neurosciences, King’s College London, Institute of Psychiatry, Psychology and Neuroscience, De Crespigny Park, London, United Kingdom
- 3Neurology Unit, San Paolo Hospital, Azienda Sanitaria Locale (ASL) Bari, Bari, Italy
- 4Department of Translational Biomedicine and Neurosciences (DiBraiN), University of Bari Aldo Moro, Bari, Italy
Amyotrophic lateral sclerosis (ALS) is a devastating neurodegenerative disease that affects both motor and non-motor functions, including sleep regulation. Emerging evidence suggests that the hypothalamus, a brain region that plays a critical role in sleep-wake regulation, may be involved in the pathogenesis of ALS-related sleep disturbances. In this review, we have summarized results of studies on sleep disorders in ALS published between 2000 and 2023. Thereafter, we examined possible mechanisms by which hypothalamic dysfunctions may contribute to ALS-related sleep disturbances. Achieving a deeper understanding of the relationship between hypothalamic dysfunction and sleep disturbances in ALS can help improve the overall management of ALS and reduce the burden on patients and their families.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by the progressive degeneration of upper (UMN) and lower motor neurons (LMN) (Kiernan et al., 2011).
Amyotrophic lateral sclerosis is traditionally considered to selectively present with motor symptoms. However, growing evidence has shown that patients experience a spectrum of non-motor symptoms, ranging from cognitive to autonomic, metabolic, and endocrine dysfunctions (Günther et al., 2016; Urso et al., 2022). ALS is indeed currently considered a multiple-system disease (Ludolph, 2017; Verde et al., 2017; Chiò et al., 2021; Logroscino et al., 2022). Among non-motor symptoms, sleep disorders and metabolic alterations (weight loss and hypermetabolism) are highly prevalent and negatively impact the prognosis and patients’ quality of life (Beswick et al., 2022). The hypothalamus is a central structure of the brain that represents a critical hub between central and peripheral signals and has a major role in the regulation of sleep/wakefulness and endocrine system (Berthoud, 2002; Morton et al., 2014; Hiller and Ishii, 2018). Hypothalamic alterations have been documented in several neurodegenerative disorders, including ALS (Vercruysse et al., 2018). Nonetheless, the relationship between hypothalamic dysfunction and sleep disorders in ALS has yet to be elucidated. This review aims to summarize studies on sleep disorders in ALS and discuss the potential role of hypothalamic dysfunction in sleep disorders in ALS.
Materials and methods
A search of electronic databases to identify studies published in peer-reviewed journals starting from 1st January 2000 to 1st January 2023 was conducted. The following databases have been used to search for relevant keywords: PubMed, Web of Science and Scopus. Search terms used were amyotrophic lateral sclerosis, sleep, sleep disorders, rest-activity rhythm, insomnia, parasomnia, excessive daytime sleepiness, periodic leg movements and rapid eye movement sleep behavior disorder. As studies on sleep-related breathing disorders in ALS have been recently reviewed (Boentert, 2019), we decided to not systematically review these studies in the present review. Twenty-two studies fulfilled the criteria for full-text review, detailed information for each study is reported in Table 1.
Sleep disorders in ALS
Sleep-related breathing disorders are the most common sleep disorders experienced by patients with ALS and have been extensively documented (Boentert, 2019). Changes in breathing during sleep may precede wake respiratory symptoms and occur in patients with normal respiratory function. However, as the disease evolves, the progressive respiratory and upper airway muscles weakness, as well as the diaphragmatic weakness, lead to nocturnal hypoxia and hypoventilation (Ahmed et al., 2016; D’Cruz et al., 2018). In this regard, Arnulf et al. (2000) showed that patients with diaphragmatic dysfunction had reduced or absent REM sleep, which might represent a possible protective mechanism against hypoventilation. Conversely, Atalaia et al. (2007) showed that sleep-related breathing disorders and reduction/absence of REM sleep may be present also in patients with unimpaired diaphragmatic function, which suggest a central drive dysfunction in ALS. Moreover, a longitudinal study has shown that over a 26-months follow-up period, half of the patients in the first 12 months and 70% of patients over the entire follow-up, develop chronic hypoventilation (Lo Coco et al., 2006).
Sleep disorders other than sleep-related breathing disorders have been less studied in ALS.
The majority of studies assessed sleep disturbances through self-report measures, namely the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS) and questionnaire on quality of life. This trend has been highlighted in a recent review of ALS clinical trials which showed that sleep disturbances were assessed in only 12 studies (among 237) and exclusively through self-report measures (Beswick et al., 2022).
The study by Diaz-Abad et al. (2018) used the PSQI to assess habitual sleep quality and duration in ALS. The authors showed that 63% of ALS patients report poor sleep quality (i.e., PSQI > 5), compared to 37% of controls and that sleep quality is associated with the severity of depressive symptoms (Diaz-Abad et al., 2018). Panda et al. (2018) showed that sleep quality in ALS patients is significantly impaired in all components of PSQI and that 50% of patients report poor sleep quality. Similar results emerged in the study by Devenney et al. (2021) which used the Cambridge behavioral inventory (CBI). The authors reported a high prevalence of sleep disturbances across all ALS phenotypes. Notably, sleep disturbances were present in 99% of ALS patients with both cognitive and behavioral impairment, 80% of patients with exclusively behavioral impairment, 69.5% of patients with exclusively cognitive impairment and 70% of patients without cognitive or behavioral impairment.
However, in a subsequent study, Gabery et al. (2021) found no difference between ALS patients and controls in sleep disturbances assessed through the CBI. Sun et al. (2021) evaluated sleep quality in genetic and sporadic ALS. Genetic ALS patients presented higher PSQI score than sporadic patients with both groups presenting higher scores than controls. Finally, Wei et al. (2021) evaluated the influence of sleep quality on patients’ quality of life. The authors reported a high prevalence of poor sleep quality (57.19%) and showed that ALS patients with poor sleep quality had lower healthy utility scores.
Excessive daytime sleepiness is also highly prevalent in patients with ALS.
Liu et al. (2018) showed that excessive daytime sleepiness (EDS) was significantly more frequent in patients with ALS than in controls (26.4 vs. 8.3%). Patients with ALS with EDS had higher ALSFRS-R global scores and lower global score and delayed memory score at MMSE and MoCA than patients with ALS without EDS (Liu et al., 2018). Similarly, Lo Coco and La Bella (2012) reported that 24.2% of ALS patients present EDS. Colville et al. (2007) showed that 42% of patients with ALS report EDS although only 7% report severe sleepiness.
Conversely, the study by Diaz-Abad et al. showed that although ALS patients had higher ESS score than controls, only 14% of patients present EDS (ESS > 9) (Diaz-Abad et al., 2018). Noteworthy, ESS was not associated with sleep quality but with respiratory muscle weakness. On the contrary, in the study by Lo Coco et al. (2011) ALS patients who reported poor sleep quality showed higher ESS score. Desai (2014) showed that daytime sleepiness was not related to the degree of AHI or sleep-disordered breathing and hypothesized a dysfunction of central mechanisms. Sun et al. (2021) showed that EDS is more prevalent in genetic than in sporadic ALS and that both patient groups have more severe sleepiness than controls. Finally, two studies did not find any difference in daytime sleepiness between ALS patients and controls (Ramirez et al., 2008; Panda et al., 2018). Concerning primary sleep disorders, insomnia has been the most extensively studied and is a frequent complaint of patients with ALS (Lo Coco and La Bella, 2012; Boentert, 2019). The prevalence of insomnia in ALS may range depending on the study population and the subtypes of insomnia.
Lo Coco et al. (2011) showed that 48% of patients report middle and terminal insomnia and 32% initial insomnia, while general non-restorative sleep symptoms were reported by 29% of ALS patients. Panda et al. (2018) showed that insomnia is reported by 65% of ALS patients, specifically, 61% present difficulties initiating sleep, 88.5% difficulties in maintaining sleep, and 38.5% early morning awakenings. Finally, Choquer et al. (2021) showed that 69% of the ASL patients report insomnia, 55% of which report poor sleep quality suggesting a strong association between insomnia and poor sleep quality and that insomnia may be independent from respiratory disorders. Several factors may contribute to the development of insomnia in ALS including the features of motor neuron disease (immobilization, cramps and difficulty in turning in bed), disease-related psychological distress and medications (Dauvilliers, 2007; Lo Coco et al., 2011; Panda et al., 2018; Boentert, 2019). Objective features of disrupted sleep quality have also been documented in polysomnographic studies (PSG). PSG studies in ALS documented impaired sleep continuity and alterations of sleep macrostructure (increased N1 sleep, awakenings, wake after sleep onset and arousal index and reduced slow wave sleep, REM sleep and sleep efficiency) (Atalaia et al., 2007; Lo Coco et al., 2011, 2017; Puligheddu et al., 2016; Congiu et al., 2019; Malekshahi et al., 2019). Few studies characterized specific sleep disorders in ALS. The prevalence of restless legs syndrome (RLS) in ALS has been assessed with questionnaires and ranges from 15 to 25% (compared to 1–8% in healthy controls) (Limousin et al., 2011; Lo Coco and La Bella, 2012; Liu et al., 2018; Sun et al., 2021).
Conversely, a handful of studies used PSG to identify other movement disorders in sleep, namely Periodic Limb Movement Disorder and REM sleep behavior disorder (RBD).
Moszczynski et al. (2012) showed that patients with ALS have higher periodic limb movement index (PLMS) than controls (23.55/h vs. 3.28/h), with 54% of patients presenting PLMS > 5/h. Moreover, mortality was higher in ALS patients with PLMS > 5/h than in patients with PLMS < 5/h. By contrast, Puligheddu et al. (2016) found no difference between ALS patients and controls in different PLMS parameters.
Only a few cases of REM sleep behavior disorder have been reported in ALS. Lo Coco et al. (2017) showed that 4.9% of patients with ALS have RBD and REM sleep without atonia. In the study by Puligheddu et al. none of ALS patients had a clinical diagnosis of RBD but REM atonia index was significantly decreased compared to controls and associated with ALSFRS (i.e., REM atonia was higher in ALS patients with more preserved function) (Puligheddu et al., 2016). Finally, circadian sleep/wake rhythm alterations have received little attention (Huang et al., 2018; Dedeene et al., 2019). Only the study by Malekshahi et al. (2019) assessed circadian rhythms in ALS patients by investigating variations of EEG activity. The authors demonstrated the presence of circadian variation in EEG in ALS patients in completely locked-in state suggesting a preserved circadian sleep-wake pattern (Malekshahi et al., 2019).
Hypothalamic dysfunction and its involvement in ALS-related sleep disorders
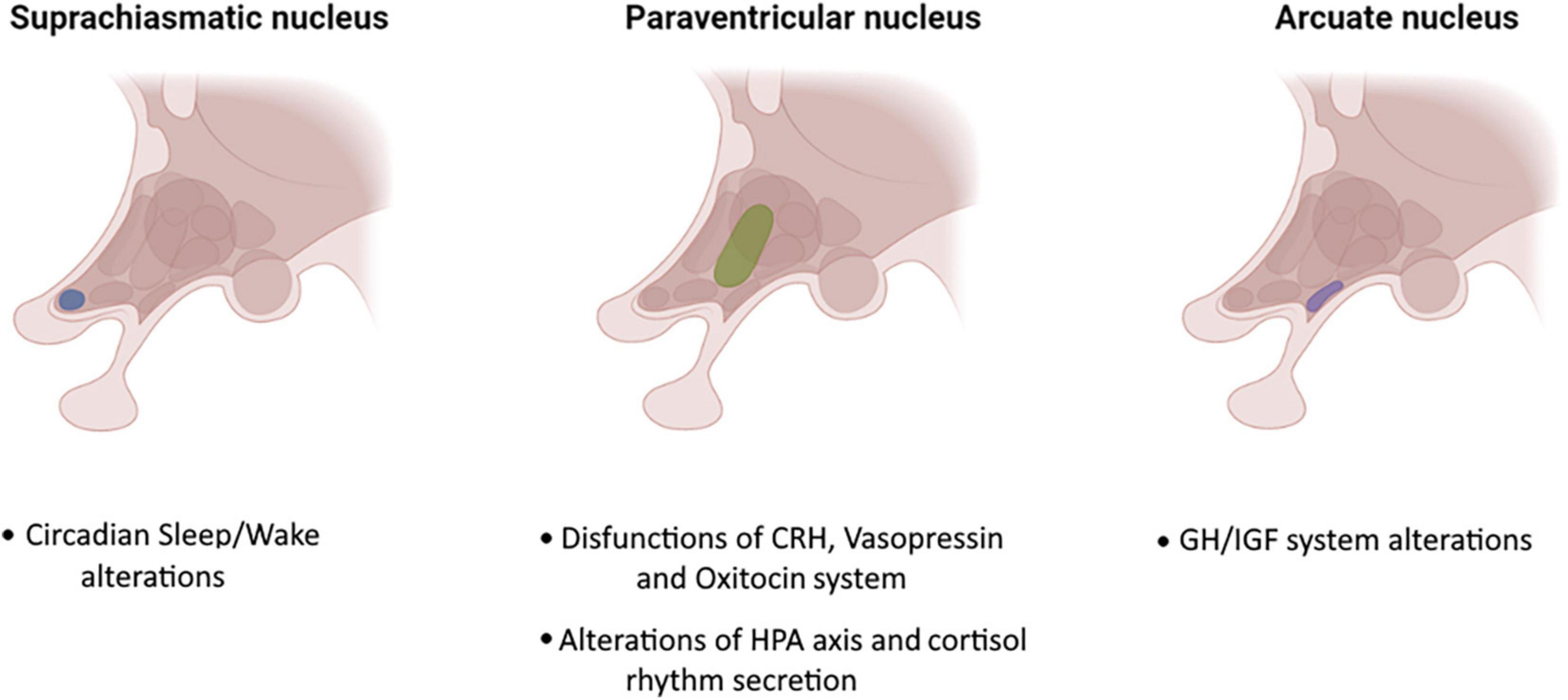
Converging evidence has shown a hypothalamic involvement in ALS. Indeed, both histopathologic and volume changes in the hypothalamus of patients with ASL has been documented (Liu et al., 2022). Hypothalamic atrophy affects both the anterior and posterior regions (Liu et al., 2022) and has been documented in both sporadic and familial ALS cases as well as in pre-symptomatic mutation carriers (Gorges et al., 2017). Furthermore, hypothalamic atrophy is not associated with whole-brain atrophy which indicates a region-selective degeneration in ALS. In addition to volumetric changes the hypothalamus of ALS patients also displays phosphorylated 43-kDa TAR DNA-binding protein (pTDP-43) aggregates.
Brettschneider et al. (2013) found pTDP-43 aggregates in 33% to 100% of ALS cases, especially in the lateral hypothalamic region. Similarly, Gabery et al. (2021) found TDP-43 inclusions in the hypothalamus of all ALS patients examined.
The hyperexpression of TDP-43 was also confirmed in mThy1-hTDP-43 transgenic mouse (Scherz et al., 2018). Phosphorylated TDP-43 is closely related to BMI, reflecting its critical role in metabolism, feeding, autonomic, sleep-wake cycle and behavior regulation (Cykowski et al., 2014).
A large amount of evidence on hypothalamic dysfunctions in ALS arises from studies on the GH/IGF-1 system (Ozdinler and Macklis, 2006; Chung et al., 2015).
The GH/IGF-1 system includes GH, Insulin-like growth factor-I (IGF-I) and -II which play a key role in brain growth, development, and metabolism (Gasperi and Castellano, 2010).
Both in vivo and animal studies demonstrated GH/IGF system alterations in ALS which may reflect glutamate-induced excitotoxicity (Pellecchia et al., 2010; Chung et al., 2015).
GH deficiency has been documented in a significant portion of ALS patients and resulted predominantly associated with upper motor neuron sign (Morselli et al., 2006; Steyn et al., 2013). Cerebrospinal fluid insulin and IGF-I are also significantly lower in ALS patients compared to controls (Bilic et al., 2006). Conversely, serum IGF-I has been shown to be slightly higher in ALS cases than in controls and very high values IGF-I were associated with a better prognosis (Nagel et al., 2020). More recently, Chung et al. (2015) showed that SOD1G93A transgenic mice exhibit a different pattern of GH secretion during the disease with unchanged GH levels before the onset, higher levels at onset, and lower levels in the late-stage of the disease suggesting that GH can have a protective effect in mutant SOD-1-expressing motor neuronal death.
Other evidence of hypothalamic involvement in ALS comes from clinical trials and mice models on melanocortin (Gasperi and Castellano, 2010; Vercruysse et al., 2016). Indeed, dysfunction of the melanocortin system may lead to peripheral (glucose intolerance) and central alterations (autonomic impairment) (Morselli et al., 2006; Vercruysse et al., 2016).
The hypothalamic-pituitary-adrenal (HPA) axis is another critical system which has been found to be dysfunctional in ALS. Patients with ALS show altered levels of cortisol, which indicates a dysregulation of circadian rhythm of cortisol secretion (Monachelli et al., 2011; Spataro et al., 2015). Cortisol levels are higher in patients than in controls, especially in the morning, in newly diagnosed, spinal-onset and rapid or intermediate progressive phenotypes (Monachelli et al., 2011; Spataro et al., 2015). Some studies hypothesized that the steroid levels could represent a marker of prognosis or susceptibility, as female patients with ALS also show higher levels of testosterone, that did not decrease with age, as in healthy controls (Gargiulo-Monachelli et al., 2014). Steroid levels are positively correlated with respiratory dysfunction in ALS (Gargiulo-Monachelli et al., 2014). In addition, the HPA axis dysfunction is also reflected by the increased levels of progesterone, which correlated negatively with age and positively with survival, time to diagnosis, spinal onset and slow disease progression (Monachelli et al., 2011). A complex interplay between sleep mechanisms and the hypothalamus is increasingly recognized as it is the relation between several features of sleep and the HPA (Lo Martire et al., 2020; Nollet et al., 2020). This association was first reported in Weitzman et al. (1983) who demonstrated that slow wave sleep has an inhibitory effect on the HPA axis and cortisol secretion.
Other studies have shown that insomnia, a frequent sleep disorder in ALS, may be related to increased levels of adrenocorticotropic hormone (Vgontzas and Chrousos, 2002). Recently, a growing body of scientific studies have documented alterations in circadian rhythm of cortisol secretion controlled by the HPA axis (Csernansky et al., 2006; Ouanes and Popp, 2019). Higher levels of morning cortisol as well as a higher levels of CSF cortisol have been found in AD and MCI (Csernansky et al., 2006; Popp et al., 2009). Elevated levels of cortisol have been also associated with more rapid cognitive decline in MCI due to AD (Ouanes and Popp, 2019). Furthermore, MCI patients with insomnia had higher cortisol levels and it has been suggested that improving insomnia and consequently cortisol levels may delay further cognitive decline in MCI (Basta et al., 2022). Circadian rhythm dysfunction may also contribute to the progression of neurodegenerative diseases through the modification of molecular, cellular, or physiologic functions (Lauretti et al., 2017). Unfortunately, only few studies investigated the association between pinealocytes/suprachiasmatic neuron loss and sleep alterations in ALS (Huang et al., 2018; Zhang et al., 2018; Dedeene et al., 2019). Huang et al. (2018) performed behavioral and physiological tests on SOD1G93A ALS model mice with and without artificially induced circadian rhythm dysfunction. Mice with circadian dysfunction had earlier symptoms onset, more rapid weight loss and shorter lifespan than mice without circadian dysfunction. Dedeene et al. (2019) performed histological examination on circadian sleep/wake-associated cells (pineal gland and suprachiasmatic nucleus-related hypothalamic neurons) in patients with ALS and/or FTLD-TDP with and without the C9orf72 repeat expansion assessing presence of ALS- and FTLD-TDP-related pathological protein inclusions (DPRs and phosphorylated TDP-43). The authors found an abundance of DPR pathology in the pineal gland of all C9orf72 cases and Poly (GA) inclusions were also identified in SCN-related neurons of C9orf72 cases (Dedeene et al., 2019). Zhang et al. (2018) introduced a point mutation equivalent to the human pathogenic mutation R521C in the rat endogenous Fus gene using CRISPR/Cas9 genome editing. The authors showed that pathogenic mutation R521C in Fus is associated with sleep-wake alterations and that sleep/wake and circadian abnormalities were the first symptoms presented by gene knock-in mice. Finally, Gabery et al. (2021) documented a selective loss of hypothalamic oxytocin- and orexin-producing neurons in ALS and showed that hypothalamic atrophy and loss of orexin neurons was associated with sleep and eating behavior alterations. A graphical representation of hypothalamic involvement in ALS is shown in Figure 1.
Discussion
Our analysis of literature data confirms that sleep disorders are common in ALS and have a relevant impact on the prognosis and quality of life of patients. Moreover, a growing body of scientific literature have consistently documented hypothalamic dysfunction in ALS.
Although the number of studies is too limited to draw definitive conclusions, based on the evidence reviewed it is reasonable to hypothesize that hypothalamic dysfunctions may play a key role in the sleep disturbances exhibited by ALS patients.
Sleep abnormalities and circadian rhythm disruption are common in neurodegenerative diseases, (Musiek and Holtzman, 2016; Brzecka et al., 2018; Fifel and Videnovic, 2020). Notably in certain cases sleep and circadian rhythm alterations are considered risk factors for the development of neurodegenerative disorders and may also represent a prodromal marker of neurodegeneration (Musiek and Holtzman, 2016; Tekriwal et al., 2017). However, while sleep and circadian rhythm disruption have been extensively studied in Alzheimer’s, Parkinson’s and Huntington’s disease few studies are available in ALS (Videnovic et al., 2014). The genesis of sleep disorders in ALS seems to be particularly complex, as some sleep disturbances might be secondary to disease-related features while others might be ascribable to hypothalamic dysfunction. The hypothalamus with its nuclei and its connections represents a high-level region of integration of sensory and motor inputs and outputs and it is responsible for the maintenance of energetic homeostasis (Timper and Brüning, 2017). The hypothalamic suprachiasmatic nucleus (SCN) contains the master circadian pacemaker in mammals commonly considered to be the main source of Process C (Stephan and Zucker, 1972; Mouret et al., 1978). Thus, the hypothalamus, regulating all the endogenous rhythms is the controller of the cyclic alternation between sleep and wake and contributes to the alternation between different stages of sleep (NREM and REM sleep) (Stephan and Zucker, 1972; Hiller and Ishii, 2018). Beyond this system, dysfunction of other hypothalamic systems, such as orexin should also be considered (Chieffi et al., 2017; Gabery et al., 2021). Orexin neurons receive input signals from regions related to sleep-wake states, motivation, and visceral cues and in turn, send output signals to a variety of brain regions involved in maintaining wakefulness, regulating REM and NREM as well as to regions involved in responses to rewards, cognition, learning, locomotion and autonomic/sympathetic tone (Chieffi et al., 2017). The dysfunction of the SCN and of the orexin system may be responsible for sleep disorders in ALS including the alteration of the sleep-wake states, disruption of the macrostructure of sleep and EDS (Chieffi et al., 2017).
Interestingly, a possible involvement of orexin in respiratory regulation has also been suggested (Kuwaki, 2010). Studies on orexin-deficient mice have shown frequent sleep apneas and loss of repetitive intermittent hypoxia-induced ventilatory and phrenic long-term facilitation (Kuwaki, 2010). Furthermore, it has been shown that other hypothalamic nuclei are actively involved in respiratory control which are interconnected with respiratory nuclei located in the midbrain, pons, medulla and spinal cord (Fukushi et al., 2019). Even though sleep-related breathing disorders in ALS mainly result from muscle weakness in the pharyngeal muscles, diaphragm, external intercostal and accessory respiratory muscles, the role of the hypothalamus needs to be further investigated. Finally, a series of studies suggested that inflammation and particularly neuroinflammation may be involved in the pathogenesis of sleep disorders in ALS (Liu and Wang, 2017). Altered levels of pro-inflammatory interleukins IL-6 and IL-1β and tumor necrosis factor (TNF) have been described in ALS cases (Hu et al., 2017; Tortelli et al., 2020). Notably, IL-6 and TNF are key molecules in the interactions between sleep and neuroinflammation and have been found to be elevated also in several sleep disorders (Vgontzas and Chrousos, 2002). The complex interplay between neuroinflammation, hypothalamic dysfunction, and sleep disturbances in ALS is intriguing and further research, including preclinical and clinical studies, is needed to gain deeper insights into the underlying mechanisms.
In conclusion, several studies suggest that the hypothalamus represents a pivotal area of interest in the pathogenesis of ALS. Understanding its role in sleep disturbances in ALS may be crucial for the development of new therapeutic opportunities and strategies aimed at improving sleep quality and the quality of life of patients with ALS.
Author contributions
VG, SZ, and GL: conceptualization. VG, SZ, AG, DU, and GL: methodology. VG, SZ, AG, LT, and MF: data curation. VG, SZ, AG, and DU: writing–original draft preparation. VG, SZ, AG, DU, LT, MF, and GL: writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by the founding of Regione Puglia and CNR for Tecnopolo per la Medicina di Precisione. D.G.R. no. 2117 of 21.11.2018 (CUPB84I1 8000540002)–C.I.R.E.M.I.C (Research Center of Excellence for Neurodegenerative Diseases and Brain Aging)–University of Bari “Aldo Moro.”
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, R. M., Newcombe, R. E. A., Piper, A. J., Lewis, S. J., Yee, B. J., Kiernan, M. C., et al. (2016). Sleep disorders and respiratory function in amyotrophic lateral sclerosis. Sleep Med. Rev. 26, 33–42. doi: 10.1016/j.smrv.2015.05.007
Arnulf, I., Similowski, T., Salachas, F., Garma, L., Mehiri, S., Attali, V., et al. (2000). Sleep disorders and diaphragmatic function in patients with amyotrophic lateral sclerosis. Am. J. Respir Crit. Care Med. 161, 849–856. doi: 10.1164/ajrccm.161.3.9805008
Atalaia, A., De Carvalho, M., Evangelista, T., and Pinto, A. (2007). Sleep characteristics of amyotrophic lateral sclerosis in patients with preserved diaphragmatic function. Amyotroph. Lateral Scler. 8, 101–105. doi: 10.1080/17482960601029883
Basta, M., Vgontzas, A. N., Fernandez-Mendoza, J., Antypa, D., Li, Y., Zaganas, I., et al. (2022). Basal cortisol levels are increased in patients with mild cognitive impairment: role of insomnia and short sleep duration. J. Alzheimers Dis. 87, 933–944. doi: 10.3233/JAD-215523
Berthoud, H.-R. (2002). Multiple neural systems controlling food intake and body weight. Neurosci. Biobehav. Rev. 26, 393–428. doi: 10.1016/s0149-7634(02)00014-3
Beswick, E., Forbes, D., Hassan, Z., Wong, C., Newton, J., Carson, A., et al. (2022). A systematic review of non-motor symptom evaluation in clinical trials for amyotrophic lateral sclerosis. J. Neurol. 269, 411–426. doi: 10.1007/s00415-021-10651-1
Bilic, E., Bilic, E., Rudan, I., Kusec, V., Zurak, N., Delimar, D., et al. (2006). Comparison of the growth hormone, IGF-1 and insulin in cerebrospinal fluid and serum between patients with motor neuron disease and healthy controls. Eur. J. Neurol. 13, 1340–1345. doi: 10.1111/j.1468-1331.2006.01503.x
Boentert, M. (2019). Sleep disturbances in patients with amyotrophic lateral sclerosis: current perspectives. Nat. Sci. Sleep 11, 97–111. doi: 10.2147/NSS.S183504
Brettschneider, J., Del Tredici, K., Toledo, J. B., Robinson, J. L., Irwin, D. J., Grossman, M., et al. (2013). Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann. Neurol. 74, 20–38. doi: 10.1002/ana.23937
Brzecka, A., Leszek, J., Ashraf, G. M., Ejma, M., Ávila-Rodriguez, M. F., Yarla, N. S., et al. (2018). Sleep disorders associated with Alzheimer’s disease: a perspective. Front. Neurosci. 12:330. doi: 10.3389/fnins.2018.00330
Chieffi, S., Carotenuto, M., Monda, V., Valenzano, A., Villano, I., Precenzano, F., et al. (2017). Orexin system: the key for a healthy life. Front. Physiol. 8:357. doi: 10.3389/fphys.2017.00357
Chiò, A., Canosa, A., Calvo, A., Moglia, C., Cicolin, A., and Mora, G. (2021). Developments in the assessment of non-motor disease progression in amyotrophic lateral sclerosis. Expert Rev. Neurother. 21, 1419–1440. doi: 10.1080/14737175.2021.1984883
Choquer, M., Blasco, H., Plantier, L., Beltran, S., Bakkouche, S. E., Corcia, P., et al. (2021). Insomnia is frequent in amyotrophic lateral sclerosis at the time of diagnosis. Sleep Biol. Rhythms 19, 121–126. doi: 10.1007/s41105-020-00296-4
Chung, J.-Y., Kim, H.-J., and Kim, M. (2015). The protective effect of growth hormone on Cu/Zn superoxide dismutase-mutant motor neurons. BMC Neurosci. 16:1. doi: 10.1186/s12868-015-0140-z
Colville, S., Swingler, R. J., Grant, I. S., and Williams, F. L. R. (2007). A population based study of respiratory function in motor neuron disease patients living in Tayside and North East Fife. Scotland. J. Neurol. 254, 453–458. doi: 10.1007/s00415-006-0389-3
Congiu, P., Mariani, S., Milioli, G., Parrino, L., Tamburrino, L., Borghero, G., et al. (2019). Sleep cardiac dysautonomia and EEG oscillations in amyotrophic lateral sclerosis. Sleep 42:zsz164. doi: 10.1093/sleep/zsz164
Csernansky, J. G., Dong, H., Fagan, A. M., Wang, L., Xiong, C., Holtzman, D. M., et al. (2006). Plasma cortisol and progression of dementia in subjects with Alzheimer-type dementia. Am. J. Psychiatry 163, 2164–2169. doi: 10.1176/ajp.2006.163.12.2164
Cykowski, M. D., Takei, H., Schulz, P. E., Appel, S. H., and Powell, S. Z. (2014). TDP-43 pathology in the basal forebrain and hypothalamus of patients with amyotrophic lateral sclerosis. Acta Neuropathol. Commun. 2:171. doi: 10.1186/s40478-014-0171-1
Dauvilliers, Y. (2007). Insomnia in patients with neurodegenerative conditions. Sleep Med. 8(Suppl. 4), S27–S34. doi: 10.1016/S1389-9457(08)70006-6
D’Cruz, R. F., Murphy, P. B., and Kaltsakas, G. (2018). Sleep disordered breathing in motor neurone disease. J. Thorac. Dis. 10, S86–S93. doi: 10.21037/jtd.2017.12.19
Dedeene, L., Van Schoor, E., Vandenberghe, R., Van Damme, P., Poesen, K., and Thal, D. R. (2019). Circadian sleep/wake-associated cells show dipeptide repeat protein aggregates in C9orf72-related ALS and FTLD cases. Acta Neuropathol. Commun. 7:189. doi: 10.1186/s40478-019-0845-9
Desai, U. (2014). Excessive daytime sleepiness does not predict the degree of sleep disordered breathing in ALS: an autonomic dysfunction matrix (p4.089). Neurology 82.
Devenney, E. M., McErlean, K., Tse, N. Y., Caga, J., Dharmadasa, T., Huynh, W., et al. (2021). Factors that influence non-motor impairment across the ALS-FTD spectrum: impact of phenotype, sex, age, onset and disease stage. Front. Neurol. 12:743688. doi: 10.3389/fneur.2021.743688
Diaz-Abad, M., Buczyner, J. R., Venza, B. R., Scharf, S. M., Kwan, J. Y., Lubinski, B., et al. (2018). Poor sleep quality in patients with amyotrophic lateral sclerosis at the time of diagnosis. J. Clin. Neuromuscul. Dis. 20, 60–68. doi: 10.1097/CND.0000000000000234
Fifel, K., and Videnovic, A. (2020). Circadian and sleep dysfunctions in neurodegenerative disorders-an update. Front. Neurosci. 14:627330. doi: 10.3389/fnins.2020.627330
Fukushi, I., Yokota, S., and Okada, Y. (2019). The role of the hypothalamus in modulation of respiration. Respir. Physiol. Neurobiol. 265, 172–179. doi: 10.1016/j.resp.2018.07.003
Gabery, S., Ahmed, R. M., Caga, J., Kiernan, M. C., Halliday, G. M., and Petersén, Å (2021). Loss of the metabolism and sleep regulating neuronal populations expressing orexin and oxytocin in the hypothalamus in amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 47, 979–989. doi: 10.1111/nan.12709
Gargiulo-Monachelli, G. M., Sivori, M., Meyer, M., Sica, R. E. P., De Nicola, A. F., and Gonzalez-Deniselle, M. C. (2014). Circulating gonadal and adrenal steroids in amyotrophic lateral sclerosis: possible markers of susceptibility and outcome. Horm. Metab. Res. 46, 433–439. doi: 10.1055/s-0034-1371891
Gasperi, M., and Castellano, A. E. (2010). Growth hormone/insulin-like growth factor I axis in neurodegenerative diseases. J. Endocrinol. Invest. 33, 587–591. doi: 10.1007/BF03346653
Gorges, M., Vercruysse, P., Müller, H.-P., Huppertz, H.-J., Rosenbohm, A., Nagel, G., et al. (2017). Hypothalamic atrophy is related to body mass index and age at onset in amyotrophic lateral sclerosis. J. Neurol Neurosurg. Psychiatry 88, 1033–1041. doi: 10.1136/jnnp-2017-315795
Günther, R., Richter, N., Sauerbier, A., Chaudhuri, K. R., Martinez-Martin, P., Storch, A., et al. (2016). Non-motor symptoms in patients suffering from motor neuron diseases. Front. Neurol. 7:117. doi: 10.3389/fneur.2016.00117
Hiller, A. J., and Ishii, M. (2018). Disorders of Body weight, sleep and circadian rhythm as manifestations of hypothalamic dysfunction in Alzheimer’s disease. Front. Cell Neurosci. 12:471. doi: 10.3389/fncel.2018.00471
Hu, Y., Cao, C., Qin, X.-Y., Yu, Y., Yuan, J., Zhao, Y., et al. (2017). Increased peripheral blood inflammatory cytokine levels in amyotrophic lateral sclerosis: a meta-analysis study. Sci. Rep. 7:9094. doi: 10.1038/s41598-017-09097-1
Huang, Z., Liu, Q., Peng, Y., Dai, J., Xie, Y., Chen, W., et al. (2018). Circadian rhythm dysfunction accelerates disease progression in a mouse model with amyotrophic lateral sclerosis. Front. Neurol. 9:218. doi: 10.3389/fneur.2018.00218
Kiernan, M. C., Vucic, S., Cheah, B. C., Turner, M. R., Eisen, A., Hardiman, O., et al. (2011). Amyotrophic lateral sclerosis. Lancet 377, 942–955. doi: 10.1016/S0140-6736(10)61156-7
Kuwaki, T. (2010). Hypothalamic modulation of breathing. Adv. Exp. Med. Biol. 669, 243–247. doi: 10.1007/978-1-4419-5692-7_49
Lauretti, E., Di Meco, A., Merali, S., and Praticò, D. (2017). Circadian rhythm dysfunction: a novel environmental risk factor for Parkinson’s disease. Mol. Psychiatry 22, 280–286. doi: 10.1038/mp.2016.47
Limousin, N., Blasco, H., Corcia, P., Arnulf, I., and Praline, J. (2011). The high frequency of restless legs syndrome in patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler 12, 303–306. doi: 10.3109/17482968.2011.557736
Liu, J., and Wang, F. (2017). Role of neuroinflammation in amyotrophic lateral sclerosis: cellular mechanisms and therapeutic implications. Front. Immunol. 8:1005. doi: 10.3389/fimmu.2017.01005
Liu, S., Huang, Y., Tai, H., Zhang, K., Wang, Z., Shen, D., et al. (2018). Excessive daytime sleepiness in Chinese patients with sporadic amyotrophic lateral sclerosis and its association with cognitive and behavioural impairments. J. Neurol. Neurosurg. Psychiatry 89, 1038–1043. doi: 10.1136/jnnp-2018-318810
Liu, S., Ren, Q., Gong, G., Sun, Y., Zhao, B., Ma, X., et al. (2022). Hypothalamic subregion abnormalities are related to body mass index in patients with sporadic amyotrophic lateral sclerosis. J. Neurol. 269, 2980–2988. doi: 10.1007/s00415-021-10900-3
Lo Coco, D., and La Bella, V. (2012). Fatigue, sleep, and nocturnal complaints in patients with amyotrophic lateral sclerosis. Eur. J. Neurol. 19, 760–763. doi: 10.1111/j.1468-1331.2011.03637.x
Lo Coco, D., Marchese, S., Corrao, S., Cettina Pesco, M., La Bella, V., Piccoli, F., et al. (2006). Development of chronic hypoventilation in amyotrophic lateral sclerosis patients. Respir. Med. 100, 1028–1036. doi: 10.1016/j.rmed.2005.09.035
Lo Coco, D., Mattaliano, P., Spataro, R., Mattaliano, A., and La Bella, V. (2011). Sleep-wake disturbances in patients with amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 82, 839–842. doi: 10.1136/jnnp.2010.228007
Lo Coco, D., Puligheddu, M., Mattaliano, P., Congiu, P., Borghero, G., Fantini, M. L., et al. (2017). REM sleep behavior disorder and periodic leg movements during sleep in ALS. Acta Neurol. Scand. 135, 219–224. doi: 10.1111/ane.12593
Lo Martire, V., Caruso, D., Palagini, L., Zoccoli, G., and Bastianini, S. (2020). Stress & sleep: a relationship lasting a lifetime. Neurosci. Biobehav. Rev. 117, 65–77. doi: 10.1016/j.neubiorev.2019.08.024
Logroscino, G., Urso, D., and Tortelli, R. (2022). The challenge of amyotrophic lateral sclerosis descriptive epidemiology: to estimate low incidence rates across complex phenotypes in different geographic areas. Curr. Opin. Neurol. 35, 678–685. doi: 10.1097/WCO.0000000000001097
Ludolph, A. (2017). ALS - A multisystem degeneration. J. Neurol. Sci. 381:42. doi: 10.1016/j.jns.2017.08.173
Malekshahi, A., Chaudhary, U., Jaramillo-Gonzalez, A., Lucas Luna, A., Rana, A., Tonin, A., et al. (2019). Sleep in the completely locked-in state (CLIS) in amyotrophic lateral sclerosis. Sleep 42:zsz185. doi: 10.1093/sleep/zsz185
Monachelli, G. G., Meyer, M., Rodríguez, G., Garay, L., Sica, R. E., De Nicola, A. F., et al. (2011). Progesterone and cortisol levels in sporadic amyotrophic lateral sclerosis (sALS): correlation with prognostic factors. Horm. Mol. Biol. Clin. Investig. 6, 167–173. doi: 10.1515/HMBCI.2011.006
Morselli, L. L., Bongioanni, P., Genovesi, M., Licitra, R., Rossi, B., Murri, L., et al. (2006). Growth hormone secretion is impaired in amyotrophic lateral sclerosis. Clin. Endocrinol. 65, 385–388. doi: 10.1111/j.1365-2265.2006.02609.x
Morton, G. J., Meek, T. H., and Schwartz, M. W. (2014). Neurobiology of food intake in health and disease. Nat. Rev. Neurosci. 15, 367–378. doi: 10.1038/nrn3745
Moszczynski, A. J., Tandon, A., Coelho, F. M. S., Zinman, L., and Murray, B. (2012). Mortality associated with periodic limb movements during sleep in amyotrophic lateral sclerosis patients. Einstein 10, 428–432. doi: 10.1590/s1679-45082012000400006
Mouret, J., Coindet, J., Debilly, G., and Chouvet, G. (1978). Suprachiasmatic nuclei lesions in the rat: alterations in sleep circadian rhythms. Electroencephalogr. Clin. Neurophysiol. 45, 402–408. doi: 10.1016/0013-4694(78)90191-8
Musiek, E. S., and Holtzman, D. M. (2016). Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science 354, 1004–1008. doi: 10.1126/science.aah4968
Nagel, G., Peter, R. S., Rosenbohm, A., Koenig, W., Dupuis, L., Rothenbacher, D., et al. (2020). Association of insulin-like growth factor 1 concentrations with risk for and prognosis of amyotrophic lateral sclerosis - Results from the ALS registry swabia. Sci. Rep. 10:736. doi: 10.1038/s41598-020-57744-x
Nollet, M., Wisden, W., and Franks, N. P. (2020). Sleep deprivation and stress: a reciprocal relationship. Interface Focus 10:20190092. doi: 10.1098/rsfs.2019.0092
Ouanes, S., and Popp, J. (2019). High cortisol and the risk of dementia and Alzheimer’s disease: a review of the literature. Front. Aging Neurosci. 11:43. doi: 10.3389/fnagi.2019.00043
Ozdinler, P. H., and Macklis, J. D. (2006). IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat. Neurosci. 9, 1371–1381. doi: 10.1038/nn1789
Panda, S., Gourie-Devi, M., and Sharma, A. (2018). Sleep disorders in amyotrophic lateral sclerosis: a questionnaire-based study from India. Neurol. India 66, 700–708. doi: 10.4103/0028-3886.232327
Pellecchia, M. T., Pivonello, R., Monsurrò, M. R., Trojsi, F., Longo, K., Piccirillo, G., et al. (2010). The GH-IGF system in amyotrophic lateral sclerosis: correlations between pituitary GH secretion capacity, insulin-like growth factors and clinical features. Eur. J. Neurol. 17, 666–671. doi: 10.1111/j.1468-1331.2009.02896.x
Popp, J., Schaper, K., Kölsch, H., Cvetanovska, G., Rommel, F., Klingmüller, D., et al. (2009). CSF cortisol in Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 30, 498–500. doi: 10.1016/j.neurobiolaging.2007.07.007
Puligheddu, M., Congiu, P., Aricò, D., Rundo, F., Borghero, G., Marrosu, F., et al. (2016). Isolated rapid eye movement sleep without atonia in amyotrophic lateral sclerosis. Sleep Med. 26, 16–22. doi: 10.1016/j.sleep.2016.05.016
Ramirez, C., Piemonte, M. E. P., Callegaro, D., and Da Silva, H. C. A. (2008). Fatigue in amyotrophic lateral sclerosis: frequency and associated factors. Amyotroph. Lateral Scler. 9, 75–80. doi: 10.1080/17482960701642502
Scherz, B., Rabl, R., Flunkert, S., Rohler, S., Neddens, J., Taub, N., et al. (2018). mTh1 driven expression of hTDP-43 results in typical ALS/FTLD neuropathological symptoms. PLoS One 13:e0197674. doi: 10.1371/journal.pone.0197674
Spataro, R., Volanti, P., Vitale, F., Meli, F., Colletti, T., Di Natale, A., et al. (2015). Plasma cortisol level in amyotrophic lateral sclerosis. J. Neurol. Sci. 358, 282–286. doi: 10.1016/j.jns.2015.09.011
Stephan, F. K., and Zucker, I. (1972). Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc. Natl. Acad. Sci. U.S.A. 69, 1583–1586. doi: 10.1073/pnas.69.6.1583
Steyn, F. J., Lee, K., Fogarty, M. J., Veldhuis, J. D., McCombe, P. A., Bellingham, M. C., et al. (2013). Growth hormone secretion is correlated with neuromuscular innervation rather than motor neuron number in early-symptomatic male amyotrophic lateral sclerosis mice. Endocrinology 154, 4695–4706. doi: 10.1210/en.2013-1570
Sun, X., Zhao, X., Liu, Q., Liu, S., Zhang, K., Wang, Z.-L., et al. (2021). Study on sleep-wake disorders in patients with genetic and non-genetic amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 92, 96–102. doi: 10.1136/jnnp-2020-324544
Tekriwal, A., Kern, D. S., Tsai, J., Ince, N. F., Wu, J., Thompson, J. A., et al. (2017). REM sleep behaviour disorder: prodromal and mechanistic insights for Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 88, 445–451. doi: 10.1136/jnnp-2016-314471
Timper, K., and Brüning, J. C. (2017). Hypothalamic circuits regulating appetite and energy homeostasis: pathways to obesity. Dis. Model Mech. 10, 679–689. doi: 10.1242/dmm.026609
Tortelli, R., Zecca, C., Piccininni, M., Benmahamed, S., Dell’Abate, M. T., Barulli, M. R., et al. (2020). Plasma inflammatory cytokines are elevated in ALS. Front. Neurol. 11:552295. doi: 10.3389/fneur.2020.552295
Urso, D., Zoccolella, S., Gnoni, V., and Logroscino, G. (2022). Amyotrophic lateral sclerosis-the complex phenotype-from an epidemiological perspective: a focus on extrapyramidal and non-motor features. Biomedicines 10:2537. doi: 10.3390/biomedicines10102537
Vercruysse, P., Sinniger, J., El Oussini, H., Scekic-Zahirovic, J., Dieterlé, S., Dengler, R., et al. (2016). Alterations in the hypothalamic melanocortin pathway in amyotrophic lateral sclerosis. Brain 139, 1106–1122. doi: 10.1093/brain/aww004
Vercruysse, P., Vieau, D., Blum, D., Petersén, Å, and Dupuis, L. (2018). Hypothalamic alterations in neurodegenerative diseases and their relation to abnormal energy metabolism. Front. Mol. Neurosci. 11:2. doi: 10.3389/fnmol.2018.00002
Verde, F., Del Tredici, K., Braak, H., and Ludolph, A. (2017). The multisystem degeneration amyotrophic lateral sclerosis - neuropathological staging and clinical translation. Arch. Ital. Biol. 155, 118–130. doi: 10.12871/00039829201746
Vgontzas, A. N., and Chrousos, G. P. (2002). Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol. Metab. Clin. North Am. 31, 15–36. doi: 10.1016/s0889-8529(01)00005-6
Videnovic, A., Lazar, A. S., Barker, R. A., and Overeem, S. (2014). ‘The clocks that time us’–circadian rhythms in neurodegenerative disorders. Nat. Rev. Neurol. 10, 683–693. doi: 10.1038/nrneurol.2014.206
Wei, Q.-Q., Hou, Y., Chen, Y., Ou, R., Cao, B., Zhang, L., et al. (2021). Health-related quality of life in amyotrophic lateral sclerosis using EQ-5D-5L. Health Qual. Life Outcomes 19:181. doi: 10.1186/s12955-021-01822-9
Weitzman, E. D., Zimmerman, J. C., Czeisler, C. A., and Ronda, J. (1983). Cortisol secretion is inhibited during sleep in normal man. J. Clin. Endocrinol. Metab. 56, 352–358. doi: 10.1210/jcem-56-2-352
Keywords: amyotrophic lateral sclerosis, hypothalamus, sleep disorders, circadian rhythm, neurodegeneration
Citation: Gnoni V, Zoccolella S, Giugno A, Urso D, Tamburrino L, Filardi M and Logroscino G (2023) Hypothalamus and amyotrophic lateral sclerosis: potential implications in sleep disorders. Front. Aging Neurosci. 15:1193483. doi: 10.3389/fnagi.2023.1193483
Received: 24 March 2023; Accepted: 20 June 2023;
Published: 03 July 2023.
Edited by:
Rosario Vasta, University of Turin, ItalyReviewed by:
Adeel Ali Memon, West Virginia University, United StatesEdoardo Gioele Spinelli, Vita-Salute San Raffaele University, Italy
Copyright © 2023 Gnoni, Zoccolella, Giugno, Urso, Tamburrino, Filardi and Logroscino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giancarlo Logroscino, Z2lhbmNhcmxvLmxvZ3Jvc2Npbm9AdW5pYmEuaXQ=
†These authors have contributed equally to this work and share last authorship
 Valentina Gnoni
Valentina Gnoni Stefano Zoccolella
Stefano Zoccolella Alessia Giugno1
Alessia Giugno1 Daniele Urso
Daniele Urso Marco Filardi
Marco Filardi Giancarlo Logroscino
Giancarlo Logroscino