
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 28 August 2023
Sec. Cellular and Molecular Mechanisms of Brain-aging
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1170879
 Olga M. Klimecki1*†
Olga M. Klimecki1*† Maxie Liebscher1†
Maxie Liebscher1† Malo Gaubert1,2
Malo Gaubert1,2 Dayana Hayek3
Dayana Hayek3 Alexis Zarucha1
Alexis Zarucha1 Martin Dyrba4
Martin Dyrba4 Claudia Bartels5
Claudia Bartels5 Katharina Buerger6,7
Katharina Buerger6,7 Michaela Butryn3
Michaela Butryn3 Peter Dechent8
Peter Dechent8 Laura Dobisch3
Laura Dobisch3 Michael Ewers6,7
Michael Ewers6,7 Klaus Fliessbach9,10
Klaus Fliessbach9,10 Silka Dawn Freiesleben11,12
Silka Dawn Freiesleben11,12 Wenzel Glanz3
Wenzel Glanz3 Stefan Hetzer13
Stefan Hetzer13 Daniel Janowitz7
Daniel Janowitz7 Ingo Kilimann4,14
Ingo Kilimann4,14 Luca Kleineidam9,10
Luca Kleineidam9,10 Christoph Laske15,16
Christoph Laske15,16 Franziska Maier17
Franziska Maier17 Matthias H. Munk15,18
Matthias H. Munk15,18 Robert Perneczky6,19,20,21
Robert Perneczky6,19,20,21 Oliver Peters11,12
Oliver Peters11,12 Josef Priller12,22,23,24
Josef Priller12,22,23,24 Boris-Stephan Rauchmann19
Boris-Stephan Rauchmann19 Nina Roy9
Nina Roy9 Klaus Scheffler25
Klaus Scheffler25 Anja Schneider9,10
Anja Schneider9,10 Eike Jakob Spruth11,12
Eike Jakob Spruth11,12 Annika Spottke9,26
Annika Spottke9,26 Stefan J. Teipel4,14
Stefan J. Teipel4,14 Jens Wiltfang5,27,28
Jens Wiltfang5,27,28 Steffen Wolfsgruber9,10
Steffen Wolfsgruber9,10 Renat Yakupov3
Renat Yakupov3 Emrah Düzel3,29
Emrah Düzel3,29 Frank Jessen9,17,30
Frank Jessen9,17,30 Michael Wagner9,10
Michael Wagner9,10 Sandra Roeske9‡
Sandra Roeske9‡ Miranka Wirth1*‡ and the DELCODE study group
Miranka Wirth1*‡ and the DELCODE study groupBackground: Sustained environmental enrichment (EE) through a variety of leisure activities may decrease the risk of developing Alzheimer’s disease. This cross-sectional cohort study investigated the association between long-term EE in young adulthood through middle life and microstructure of fiber tracts associated with the memory system in older adults.
Methods: N = 201 cognitively unimpaired participants (≥ 60 years of age) from the DZNE-Longitudinal Cognitive Impairment and Dementia Study (DELCODE) baseline cohort were included. Two groups of participants with higher (n = 104) or lower (n = 97) long-term EE were identified, using the self-reported frequency of diverse physical, intellectual, and social leisure activities between the ages 13 to 65. White matter (WM) microstructure was measured by fractional anisotropy (FA) and mean diffusivity (MD) in the fornix, uncinate fasciculus, and parahippocampal cingulum using diffusion tensor imaging. Long-term EE groups (lower/higher) were compared with adjustment for potential confounders, such as education, crystallized intelligence, and socio-economic status.
Results: Reported participation in higher long-term EE was associated with greater fornix microstructure, as indicated by higher FA (standardized β = 0.117, p = 0.033) and lower MD (β = −0.147, p = 0.015). Greater fornix microstructure was indirectly associated (FA: unstandardized B = 0.619, p = 0.038; MD: B = −0.035, p = 0.026) with better memory function through higher long-term EE. No significant effects were found for the other WM tracts.
Conclusion: Our findings suggest that sustained participation in a greater variety of leisure activities relates to preserved WM microstructure in the memory system in older adults. This could be facilitated by the multimodal stimulation associated with the engagement in a physically, intellectually, and socially enriched lifestyle. Longitudinal studies will be needed to support this assumption.
The worldwide prevalence of AD is expected to reach 153 million cases by 2050 (Nichols et al., 2022). Progressive deterioration of the memory system and associated function is a core symptom of Alzheimer’s disease (AD) (e.g., Sperling et al., 2011). AD-related memory loss and clinical progression are closely linked to accelerated brain atrophy in medio-temporal brain structures and their white matter (WM) connections, including the fornix tract as the major pathway of the hippocampus (Mielke et al., 2012). In the absence of a cure for AD, tremendous effort has been placed on developing treatment strategies focusing on the prevention of AD through protective lifestyle factors comprising physical, intellectual and social activities (Livingston et al., 2020). Participation in a greater variety of enriching leisure activities may have a particularly positive impact on brain health, including the WM microstructure of the memory system; however, these neurophysiological relations remain to be determined in older adults (OA).
Existing studies have indicated that sustained multimodal environmental enrichment (EE), combining motor, cognitive, sensory, and social stimulation, has far-reaching benefits for the brain. In animal models, groups of rats or mice exposed to EE show greater benefits compared to groups of animals with low EE (i.e., standard housing), particularly in the memory system including hippocampal structure and function (Fabel et al., 2009; Kempermann et al., 2010; Kempermann, 2019; Manno et al., 2022). In humans, multimodal activities such as regular musical instrument playing or dancing have been associated with a reduced risk of developing dementia (Verghese et al., 2003). Likewise, participating in a diversity of enriching leisure activities, including physical and cognitive activities, could be beneficial in preserving or enhancing the brain and cognition. This has been suggested by various cross-sectional (Wirth et al., 2014; Porat et al., 2016; Chan et al., 2018), longitudinal (Karp et al., 2006), and interventional (Theill et al., 2013; Ngandu et al., 2015; Köbe et al., 2016) studies as well as systematic reviews and meta-analyses (Lauenroth et al., 2016; Gavelin et al., 2021; Torre and Temprado, 2022). A study of particular interest conducted in OA over 80 years showed that 3 year changes in WM microstructure in the corticospinal tract accounted for the association between changes in self-reported leisure activities and changes in perceptual speed (Köhncke et al., 2016).
Despite these insights, the potential brain correlates associated with sustained or long-term EE in the memory system of OA remain unclear. Age-related alterations in WM microstructure, as defined by lower fractional anisotropy (FA) and higher mean diffusivity (MD) measured using diffusion-weighted imaging (DWI), begin and are most severe in the fornix, the uncinate fasciculus, and the parahippocampal cingulum (Teipel et al., 2016; Pichet Binette et al., 2021). Disruption of these critical fiber tracts has been associated with lower episodic memory functioning (Huang et al., 2007; Fellgiebel et al., 2008; Sexton et al., 2010; Metzler-Baddeley et al., 2011; Hayek et al., 2020) and cognitive worsening over time (Mielke et al., 2012; Zhuang et al., 2012; Fletcher et al., 2013) in OA. Conversely, successful preservation or adaptation of WM microstructure (as indicated by higher FA and lower MD) through enriching leisure activities could help protect the memory system in late life and strengthen brain reserve (Teipel et al., 2016; Xu et al., 2019; Luo et al., 2020). Evidence syntheses regarding lifestyle activities, such as physical or cognitive activities, and their association with WM microstructure in OA have provided inconclusive results (Sexton et al., 2016; Anatürk et al., 2018). However, participation in multimodal activities, including a 6-months piano training and a 6-months dance training, has been associated with a positive impact on fornix microstructure in OA (Burzynska et al., 2017; Jünemann et al., 2022).
Here, we investigated the cross-sectional association between sustained EE in young and middle adulthood and WM microstructure of the memory system in OA from the DZNE-Longitudinal Cognitive Impairment and Dementia (DELCODE) study (Jessen et al., 2018). Long-term EE was measured by the self-reported frequency of participation in a variety of enriching leisure activities, including physical, intellectual and social activities, between the ages 13 and 65. We hypothesized that participants with higher long-term EE would have more favorable microstructure in three WM tracts, namely the fornix, uncinate fasciculus, and parahippocampal cingulum, compared to participants with lower long-term EE. We carefully controlled for other known reserve proxies, such as education, crystallized intelligence and socio-economic status (SES) (Stern, 2009), as done in our previous study in the DELCODE cohort (Böttcher et al., 2022). Lastly, we explored neuroprotective path models (Wirth et al., 2014) conceptualized as brain maintenance and brain reserve models to better understand the interplay among long-term EE, WM microstructure and memory function in the OA.
This study was based on cross-sectional baseline data that were obtained from the ongoing multicenter observational DZNE-DELCODE study (Jessen et al., 2018). The DELCODE study was designed according to the ethical principles of the Declaration of Helsinki. Local ethical committees at each participating study site approved the study protocol. All participants gave written informed consent. The DELCODE study was registered at the German Clinical Trials Register (DRKS00007966; date: 2015/05/04).
Based on our research questions, participants in the cognitively normal range were included from the baseline DELCODE database. Specifically, our sample included older adults, participants with a family history of AD, and participants with subjective cognitive decline to increase sample size and statistical power. Briefly, all participants had fluent German skills and were 60 years of age or older. All participants included in this study had normal cognitive performance, as determined using the Consortium to Establish a Registry of Alzheimer’s Disease (CERAD) battery (Morris et al., 1989). Past or present major psychiatric, medical, or neurological disorders were set as exclusion criteria. In total, the current sample included N = 201 participants. The selection flow chart is provided in the Supplementary material (Supplementary Figure 1).
Long-term EE was operationalized using items from a German version (LEQ-D, Roeske et al., 2018) of the Lifetime of Experiences Questionnaire (LEQ; Valenzuela and Sachdev, 2007). The LEQ is an established questionnaire that assesses, among others, a wide range of leisure activities. The LEQ has been translated into multiple languages (Ourry et al., 2021) and has been implemented in previous studies on cognitive reserve (Chan et al., 2018; Collins et al., 2021).
To measure EE, we used items of the non-specific subscore of LEQ. The non-specific LEQ items are a validated measure of cognitive, artistic and social engagement across life (Valenzuela and Sachdev, 2007). Indeed, a factor analysis of the original LEQ (Valenzuela and Sachdev, 2007) indicated two main factors: factor one that captures education and occupation (the specific LEQ factor) and factor two that captures social, artistic, and musical activities (the non-specific LEQ). This factor structure was recently replicated in a cross-country validation of the LEQ in four European countries (Ourry et al., 2021). This study confirmed the essential factor structure for the specific and non-specific parts of the LEQ across the sample and for each country (Ourry et al., 2021). As the non-specific LEQ factor is closest to the concept of environmental enrichment from animal research, we use specific items of interest from the non-specific LEQ that capture environmental enrichment it in the current study. In this way, our approach is similar to previous studies (Oltmanns et al., 2017).
More specifically, we selected unspecific items of the LEQ-D questionnaire measuring the frequency of participation in 6 leisure activities on a 6-point Likert scale ranging from 0 (never), 1 (less than 1 time per month), 2 (1 time per month), 3 (2 times per month), 4 (weekly), to 5 (daily). These items are related to (1) social activity (seeing family members), (2) musical activity (playing a musical instrument) (3) artistic activity (e.g., drawing), (4) physical activity, (5) reading (as a proxy for cognitive activity), and (6) speaking an additional language. Participation in these leisure activities was rated retrospectively for three life periods, i.e., young adulthood (13–30 years), mid-life (30–65 years), and late-life (65 years and older). Only items concerning young adulthood and mid-life were included in this study, as some participants were below 65 years of age.
There are several ways to define and analyze long-term EE. From a conceptual point of view, we aimed to compare the “effects” of long-term EE between groups of OA with lower or higher frequency of participation in enriching leisure activities. This group-based comparison (lower vs. higher EE) was inspired by the classical enriched environment paradigm, as established in animal (rat and mice) models (Kempermann, 2019). In the present (human) data, we used a data-driven approach and evaluated long-term EE as a binary group variable defined by median split with lower and higher frequency of participation in the given leisure activities. Similar binary evaluations of leisure activities have been reported by previous studies (Böttcher et al., 2022; Duffner et al., 2022). Briefly, we summed the scores across the 6 leisure activities as assessed by the LEQ per life period with a maximum total score of 30 per life period. The median score of the summed leisure activities was 18 for younger adulthood and 16 for mid-life. Participants with higher EE across both life periods (median and higher) were included in the higher EE group (n = 104), while participants with lower EE across both life periods (below median) were included in the lower EE group (n = 97).
The magnetic resonance imaging (MRI) data were collected in nine participating sites, each equipped with a Siemens 3.0 Tesla MRI scanner (one Prisma, one Skyra, three TimTrio, and four Verio systems). Scanning instructions were standardized across sites and identical MRI protocol acquisition parameters were used. Details on sequence parameters are provided in the Supplementary material.
Diffusion-weighted MRI data were preprocessed with the FMRIB software library (FSL, Version 6.0.4. Jenkinson et al., 2012). The brain extraction tool (bet) was used for skull stripping. Images were corrected for distortions with a gradient-echo field map using the fsl_prepare_fieldmap and epi_reg commands. Then, images were corrected for eddy currents using the command eddy_correct and the first b0 image as a reference volume. After, bvecs were rotated to preserve correct orientation information with the command fsl_rotate_bvecs (see Leemans and Jones, 2009). The command dtifit was used to fit a diffusion tensor model at each voxel and to create the FA and MD maps. For all participants, head movement, defined by the Euclidian distance in millimeters of how much the head was moved, was calculated using the eddy_correct log file (Tromp, 2016).
To extract FA and MD values from the pre-selected fiber tracts (i.e., fornix, the uncinate fasciculus, and the parahippocampal cingulum), a tract-of-interest (TOI) based approach was chosen, following the procedure described by Antonenko et al. (2016). All TOI masks were extracted based on the 2 mm isotropic resolution Montreal Neurological Institute (MNI) template provided by FSL. Masks for the fornix and uncinate fasciculus were extracted from the probabilistic Juelich histological atlas (Bürgel et al., 2006). The mask for the parahippocampal cingulum was extracted from the non-probabilistic JHU ICBM-DTI-81 atlas (Oishi et al., 2008). Based on previous research (Antonenko et al., 2016) and visual inspection, voxels of probabilistic masks of the fornix were thresholded at 50% probability for inclusion in the mask, the uncinate fasciculus mask was thresholded at 20%. The mask for the parahippocampal cingulum was not probabilistic and, therefore, directly used as provided.
As already provided in the description of section “2.2.2. MRI acquisition and pre-processing,” we transformed the atlas-based tracts-of interest (TOI) from Montreal Neurological Institute and Hospital (MNI) reference space to individual subject native space. The TOI masks were binarized and transformed from standard space into native space to extract diffusion metrics using the statistical parametric mapping toolbox (SPM12, version 7487, Wellcome Trust Centre for Neuroimaging, London, UK)1 with the segmentation algorithm of the computational anatomy toolbox (CAT12, version 12.6, rl1450)2 implemented in Matlab 2018a (Mathworks, Natwick). The computational anatomy toolbox (CAT12) provides a one-click procedure to perform image segmentation [gray matter, white matter, cerebrospinal fluid (CSF)] and estimation of deformation fields for warping the native space t1-weighted MRI scans to MNI space. The inverse deformation field can be used to warp the images back from MNI space to native subject space. As the t1-weighted scans and diffusion scans were initially coregistered to each other, we could directly apply the deformations to the MNI TOI masks to bring the masks in the respective subject space. Subsequently, the mean FA and MD values per TOI were extracted for each participant. As the fornix tract borders the lateral ventricles, it is important to correct for CSF contamination (Hayek et al., 2020). An FA value below 0.1 and an MD value above 2.5e-03 is likely to reflect CSF rather than brain tissue (Hasan et al., 2014). Therefore, these thresholds were used for all voxels of individually extracted fornix FA and MD masks.
Co-variates of no interest considered in the present study were age, gender, education, crystalized intelligence, socio-economic status (SES), diagnostic category, and scanner site. Most of these measures are provided by the baseline database of the DELCODE study and were described in our previous study (Böttcher et al., 2022). In brief, educational attainment was assessed in years of education. Vocabulary was used as a proxy for crystallized intelligence, estimated by the total score of the German Mehrfachwahl-Wortschatz-Intelligenztest (MWT, English: Multiple-Choice Vocabulary Intelligence Test, Lehrl, 2005). MWT scores range from 0 to 37 with higher scores corresponding to higher crystallized intelligence. The SES was estimated by the international socio-economic index of occupational information (ISEI) (Ganzeboom et al., 1992). The measure was calculated using the self-reported occupational history assessed by the LEQ-D and established crosswalk procedures. ISEI scores range from 16 to 90 with higher scores corresponding to higher SES. The scanner site was included to account for scanner-related variance in the diffusion tensor imaging (DTI) measures (Brueggen et al., 2019; Finsterwalder et al., 2020).
Statistical analysis was conducted in IBM SPSS 23 and RStudio Version 1.4.1103 (R Core Team, 2020). The mediation package (version 4.5.0) was used to construct path models (Tingley et al., 2014). An alpha value of 0.05 was considered statistically significant. As the TOIs were considered as independent from each other, tests were not corrected for multiple comparisons. Statistical assumptions for each analysis were checked following guidelines provided by Field (2013). Plots presented in the results section were plotted in R with the package ggplot2 (version 3.3.5) (Wickham, 2016).
Long-term EE groups (lower/higher) were first compared on baseline demographic, behavioral, neuropsychological, and neuroimaging variables. Statistical comparisons were conducted with independent samples t-tests for continuous variables and chi-squared (χ2) tests for categorical variables.
To assess our main hypothesis for WM microstructure, multiple linear regression models were computed with FA or MD of each TOI (fornix, uncinate fasciculus, and parahippocampal cingulum) as dependent variables and long-term EE (lower/higher) as an independent variable. In all regression models, we accounted for age, gender, education, crystallized intelligence, SES, and diagnostic category. Furthermore, we tested for multicollinearity among all covariates using simple Pearson correlation and VIF (variance inflation factor). In the Pearson correlation, the highest correlation was between the covariates education and SES (r = 0.59). According to Field et al. (2012), multicollinearity is only present when the correlation coefficient reaches 0.9 or −0.9. In line with this result, the VIF method also indicated that there is no multicollinearity among the covariates, as the highest values were observed for education (1.67) and SES (1.74), and according to Kutner et al. (2005), VIFs exceeding 10 may indicate multicollinearity. To minimize site-effects, regression models for TOIs included scanner site as a categorical covariate. All categorical covariates were included as dummy coded variables in all models specified above, granted they had more than two levels. For all regression models, the lower long-term EE group was the reference category for long-term EE, female was the reference category for gender, and OA was the reference category for the diagnostic category.
We conducted exploratory path analyses using the mediation package v.4.5.0 (Tingley et al., 2014) in RStudio version 1.4.1103 (R Core Team, 2020) and adjusted for co-variates of no interest, i.e., age, gender, education, crystallized intelligence, SES, diagnostic category, and scanner location, in a methodological approach similar to Wirth et al. (2014). Path models were computed for fornix FA and MD similar to a previous study (Hayek et al., 2020). One path model, here conceptualized as brain maintenance model, assessed long-term EE as independent variable, fornix FA/MD as the mediator, and memory function as dependent variable. The second (reverse) path model, here conceptualized as brain reserve model, assessed fornix FA/MD as independent variable, long-term EE as mediator, and memory function as dependent variable. We examined the contribution of the indirect (mediator) effect as the outcome of interest, computed by the product of path coefficients, similar to our preceding study (Wirth et al., 2014). Indirect effects were evaluated using bias-corrected 95% confidence intervals (CI) established with 5,000 bootstrap samples. The indirect effect was considered significant if the CI did not include the value zero.
In both path models, we evaluated memory function by the neuropsychological learning and memory (MEM) factor score, in detail described elsewhere (Wolfsgruber et al., 2020). The incorporated neuropsychological tests were provided by Wolfsgruber et al. (2020). In brief, they consist of the following tests: performance in learning and memory (MEM) was assessed using a latent factor score that was previously constructed based on the extensive neuropsychological test battery of the DELCODE cohort (Wolfsgruber et al., 2020). The MEM factor score included the Free and Cued Selective Reminding Test (FCSRT, Grober et al., 2009), the Logical Memory Test of the Wechsler Memory Scale (WMS-IV, Lepach and Petermann, 2012), the incidental learning scale of the Symbol Digit Modalities Test (Smith, 1982), the computerized Face Name Associative Recognition Test (Polcher et al., 2017), the word list learning, recall, and recognition of the ADAS-Cog 13 (Mohs et al., 1997) and a recall task of previously copied figures (analogous to the CERAD battery, Thalmann et al., 2000). For the present purpose, the MEM factor score was z-transformed using the mean and standard deviation of the present sample (N = 201).
A post hoc comparison was performed to explore relative differences in the frequency of participation across the 6 different leisure activities (as assessed by the LEQ-D) between higher and lower long-term EE groups. We conducted simple regression models with long-term EE (lower/higher) as the independent variable and the respective leisure activity (language, artistic, musical, physical, reading, or social) as the dependent variable. For the present purpose, the effect size measure (standardized regression coefficient) was evaluated, classified as small (0.2–0.5), medium (0.5–0.8) or large (0.8 and larger) effects (Ferguson, 2009).
This study included a total of N = 201 participants from the DELCODE study. Of those, n = 97 were in the lower long-term EE group and n = 104 participants were in the higher long-term EE group. A summary of the sample characteristics at baseline including demographics, behavioral and biological measures is given in Table 1. Participants with higher long-term EE had significantly higher years of education, crystallized intelligence, and SES compared to participants with lower EE. The two groups were comparable in the other baseline characteristics.
The association between long-term EE and WM microstructure, measured by FA and MD, in the pre-selected TOIs is shown in Table 2. As the selected LEQ items that were our measure of EE correlated with years of education (r = 0.34), crystallized intelligence (r = 0.22), and SES (r = 0.29), we corrected for these collinear influences in the statistical analysis procedure. Even after controlling for these co-variates in the main and exploratory analyses, EE still explained unique variance in the reported analyses, thus demonstrating the importance of EE. We found significant group differences for fornix FA (β = 0.117, p = 0.033) and fornix MD (β = −0.147, p = 0.015), after adjusting for co-variates that normally contribute to cognitive reserve and resilience and were correlated with EE in our sample. More specifically, participants who reported participating in higher long-term EE had significantly greater WM microstructure in the fornix, as indicated by higher FA and lower MD, compared to participants with lower long-term EE (Figure 1). There were no significant group differences in the other TOIs (all p’s > 0.1).
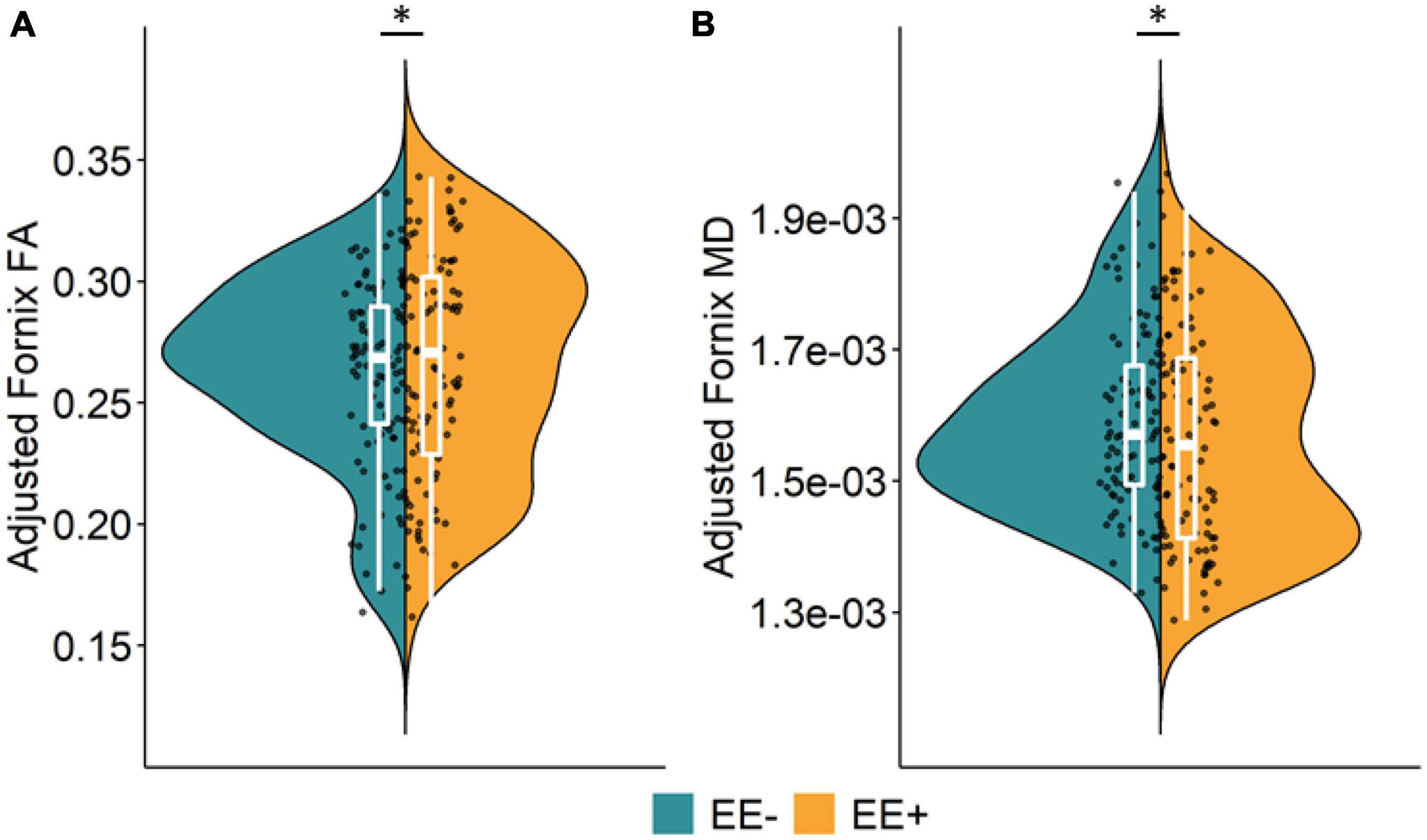
Figure 1. Main effect of long-term EE on fornix microstructure. Significant group differences were found for fornix fractional anisotropy, FA (A) and fornix mean diffusivity, MD (B). (A,B) Significantly better white matter microstructure (higher FA, lower MD) in the fornix was detected for participants with higher long-term EE (EE+, orange) compared to participants with lower long-term EE (EE–, green). Split violin plots display data adjusted for covariates (age, gender, education, crystallized intelligence, socioeconomic status, diagnostic category, and scanner site for fornix microstructure). Boxplots display the median and interquartile range between the 1st and 3rd quartile. Individual data points display data adjusted for covariates. *p < 0.05. Key: EE, environmental enrichment; EE–, lower long-term EE; EE+, higher long-term EE; FA, fractional anisotropy; MD, mean diffusivity.
Results of the exploratory path models are shown in Figure 2. The first path model (brain maintenance model) assessed long-term EE (lower/higher) as independent variable, fornix FA as mediator, and memory function as dependent variable. There was no indication that long-term EE was indirectly associated with memory function through fornix FA (unstandardized B = −0.020, SE = 0.007, bootstrapped bias-corrected 95% CI: −0.076, 0.010). A similar non-significant result was obtained for fornix MD (unstandardized B = −0.020, SE = 0.008, bootstrapped bias-corrected 95% CI: −0.081, 0.010, Supplementary Figure 2). The second alternative path model (brain reserve model) assessed fornix FA as independent variable, long-term EE (lower/higher) as mediator, and memory function as dependent variable. In this model, greater fornix FA was associated with better memory function through an indirect (mediation) effect of long-term EE (unstandardized B = 0.619, SE = 0.102, bootstrapped bias-corrected 95% CI: 0.089, 1.710), even after controlling for co-variates. A similar significant indirect effect was observed for the corresponding path model with fornix MD (unstandardized B = −0.035, SE = 0.005, bootstrapped bias-corrected 95% CI: −0.090, −0.010, Supplementary Figure 2).
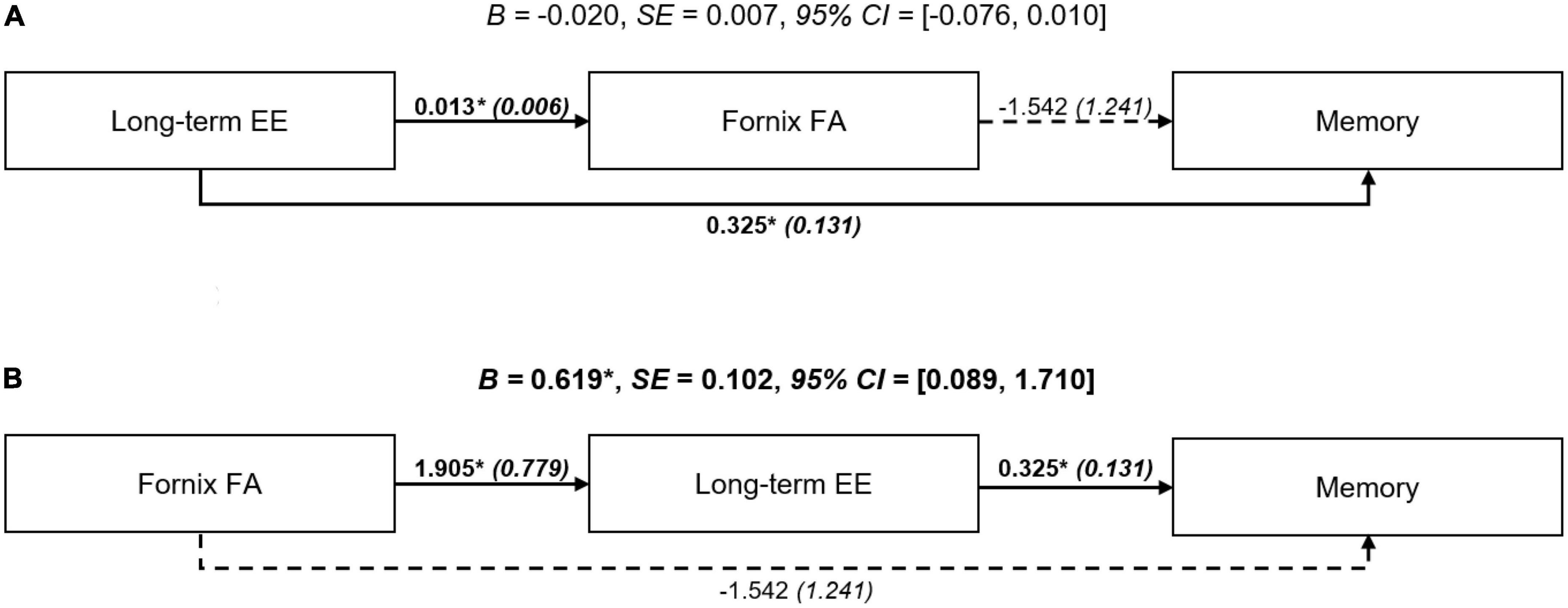
Figure 2. Path models investigating the association among long-term EE, fornix FA, and memory function. (A) Brain maintenance model: There was no statistical indication for a significant indirect (mediation) effect of the fornix FA. (B) Brain reserve model: Greater FA of the fornix was indirectly associated with better memory function through long-term EE. Path diagrams were adjusted for age, gender, education, crystallized intelligence, socioeconomic status, diagnostic category, and scanner site. Terms show unstandardized beta (B) coefficients and standardized errors (SE). Significant terms are indicated with bold font. Significant paths are indicated with continuous lines. Indirect effects with 95% CI are provided. *p < 0.05. Key: CI, confidence interval; EE, environmental enrichment; FA, fractional anisotropy.
We compared the two groups (lower/higher EE) to explore the relative differences in the frequency of participation across the 6 leisure activities (i.e., social, musical, artistic, physical, reading and additional language activities) to better characterize long-term EE. Compared to the lower EE group, participants with higher EE reported a greater frequency of participation in socio-cultural activities, resulting in large effect sizes of long-term EE (lower/higher) in musical (standardized regression coefficient: β = 0.538), artistic (β = 0.670), and additional language (β = 0.754) activities. In contrast, both EE groups (lower/higher) reported regular frequency of participation in the remaining leisure activities with small effect sizes of long-term EE in physical (β = 0.394), reading (β = 0.245), and social (β = 0.079) activities (Supplementary Table 1).
This study investigated the cross-sectional association between EE during life and WM microstructure of fiber tracts associated with the memory system in cognitively unimpaired OA of the DELCODE study. We compared two groups of OA that reported sustained higher or lower participation (between 13 and 65 years of age) in a variety of enriching leisure activities, adjusting for other known reserve proxies. We show that participants with higher long-term EE had better microstructure in the fornix tract (higher FA and lower MD) compared to participants with lower long-term EE. A similar effect was not seen in the other WM tracts. Follow-up exploratory path models suggested that greater fornix microstructure (higher FA and lower MD) was indirectly associated with better memory function through higher long-term EE. Our findings imply that sustained participation in a greater diversity of leisure activities may help preserve WM microstructure in the memory system of OA.
We show that OA with higher long-term EE had more favorable microstructure in the fornix, both in terms of higher FA and lower MD, than OA with lower long-term EE. The present beneficial association between enriching leisure activities and fornix microstructure was found, accounting for other reserve proxies of education, crystallized intelligence, and SES (Stern, 2009; Jones et al., 2011) that were enhanced in the higher EE group. At the same time, we observed no significant differences between lower and higher EE for the other WM tracts, namely the uncinate fasciculus and the parahippocampal cingulum.
Our results align with and extend findings from previous review and meta-analytical studies reporting small and inconsistent effects of leisure activities on WM microstructure (Sexton et al., 2016; Anatürk et al., 2018; Duffner et al., 2023). One possible explanation for the selective sensitivity of the fornix, as observed in our study, may be that this major output tract of the hippocampus appears to be highly susceptible to aging processes (Zhuang et al., 2012; Hayek et al., 2020) and environmental challenges. The latter has been demonstrated by short-term learning/cognitive training studies in younger and older adults, respectively (Hofstetter et al., 2013; Antonenko et al., 2016). Intervention studies have further shown positive effects of longer-term music and dance trainings over 6 months on WM microstructure of the fornix in OA (Burzynska et al., 2017; Jünemann et al., 2022). In addition, other WM tracts seem to be important for the relation between EE and cognitive capacities. A longitudinal study in OA over 80 years showed that three year changes in WM microstructure in the corticospinal tract accounted for the association between changes in self-reported leisure activities and changes in perceptual speed (Köhncke et al., 2016).
Together, our and previous findings may reflect a lasting capability for neuroplastic changes or adaptations of WM microstructure including the fornix tract−even in late life−in response to or in interrelation with environmental challenges and experiences (see below). In light of this, our results may suggest that a greater variety of leisure activities could help preserve WM microstructure of the memory system in older age.
Path modeling showed a significant indirect association between fornix microstructure and late-life cognition through long-term EE. The result is challenging to interpret, given the cross-sectional nature of our study, which limits possibilities for causal inference. Nevertheless, our observation may suggest that greater fornix microstructure−if conceptualized as an indicator of brain reserve−could be associated with better memory function, when sustainably challenged by participation in enriching leisure activities. This interpretation aligns with the view that complex brain-behavior dynamics are associated with EE over the life course (Richards and Deary, 2005; Olszewska et al., 2021). It has been argued that more favorable brain properties in high-reserve individuals may facilitate exposure to or engagement in complex EE during life (see Maguire et al. (2000) and Olszewska et al. (2021) for a similar discussion). Other results have shown that a greater diversity of physically, intellectually and socially enriching activities in early life (before 13 years of age) are associated with variations in brain properties in later life (Morris et al., 2021). Greater brain resources (via predisposition or early EE) could support the maintenance of an enriched lifestyle throughout life and vice versa, which might promote a self-sustaining preservation of brain reserve into older age.
We show that participants with higher long-term EE reported a more frequent participation in enriching leisure activities including music, art and language. Due to the multimodal sensory, motor, cognitive and social stimulation as inherent to socio-cultural activities (Wan and Schlaug, 2010; Herold et al., 2018), one could hence reason that participants in the higher EE group were more likely exposed to additive or synergistic effects of EE (Kempermann, 2019). Participants in the lower EE group also reported having performed enriching physical, cognitive and social leisure activities, albeit with relatively lesser frequency of participation in socio-cultural activities. The proposed benefits of leisure activities might thus be encouraged by the engagement in art, music, and other cultural activities, which have been associated with far-reaching positive effects on the brain and mental health (Fancourt and Finn, 2019). Regular participation in socio-cultural activities appears to act through broad neurobiological mechanisms to promote brain reserve and resilience in late life, which should be addressed in future studies.
Taken together, our findings propose that long-term participation in a greater variety of leisure activities during young and middle adulthood may help preserve WM microstructure in the memory system of OA. This could be facilitated by the complex stimulation and integration processes associated with a physically, intellectually, and socially enriched lifestyle. In this line, studies have shown enhanced cognitive functioning after multimodal training compared with unimodal training in younger adults (Ward et al., 2017). The multimodal combination of modifiable lifestyle activities could serve as a low-threshold health strategy to foster brain and cognitive health throughout life to an older age. Targeted interventional studies with multimodal enrichment strategies will help to systematically evaluate the proposed merits of long-term EE. In addition, the relevance of socio-cultural activities in the benefits of an enriched lifestyle warrants further investigation.
The present study indicates a positive association between self-reported participation in long-term EE during life and WM microstructure of fiber tracts associated with the memory system in a relatively large sample of clinically normal and well-characterized OA from the DZNE-DELCODE cohort (Jessen et al., 2018). To further investigate the sustained benefits of long-term EE, longitudinal studies with fine-tuned assessments of enriching leisure activities are needed to assess the progression of cognitive reserve and related brain properties over time. Several limitations need to be considered. (1) As the present results are based on cross-sectional data, caution needs to be taken concerning possible conclusions regarding the directionality of effects. Future longitudinal cohort studies could test the relation between environmental enrichment and fornix FA and MD over timespans to infer causality. (2) Self-reported retrospective measures of participation in leisure activities can be biased by the participant’s current cognitive status. However, the LEQ is considered an established instrument used in prior studies in cognitively unimpaired OA (Chan et al., 2018; Collins et al., 2021; Ourry et al., 2021) and we selected participants with a normal cognitive status from the DELCODE database. (3) The current study was translational in nature and applied group-based comparisons of lower and higher EE, to follow procedures from classical animal studies on enriched environments (Kempermann, 2019). Future studies may use other approaches to assess associations between EE, brain microstructure and memory and may additionally incorporate educational/occupational enrichment as assessed with the LEQ (Valenzuela and Sachdev, 2007). Further, it might be argued that exposure to earlier-life EE (before the age of 13 years) may facilitate later-life EE and brain functioning in older age (Morris et al., 2021). This assumption cannot be evaluated in the present study, as no information on early-life EE was available. (4) Studies with native diffusion tractography are needed to replicate the present results obtained with a probabilistic atlas-based tracts-of interest analysis. Prospective studies should explore other ways of addressing partial volume contamination from cerebrospinal fluid to see whether approaches with different methods yield robust results. It should be noted that head movements tend to increase with age, leading to more artifacts, as demonstrated in a resting-state study by Saccà et al. (2021). These head movements cannot always be entirely corrected through preprocessing and should therefore be considered as a possible limitation. (5) We did not find evidence of a direct cross-sectional association between fornix microstructure and memory performance in the present sample, although previous studies have reported such relations (Huang et al., 2007; Fellgiebel et al., 2008; Sexton et al., 2010; Hayek et al., 2020). Cross-sectional correlations between WM microstructure and cognitive function might be blurred by inter-subject heterogeneity in these measures, mixed brain pathologies and/or differences in reserve and resilience factors in our cohort.
Our results show that sustained participation in a greater diversity of leisure activities is associated with better fornix microstructure in OA. This beneficial association between long-term enrichment and brain reserve was found, accounting for other known reserve proxies such as SES, crystallized intelligence, and education. Regular engagement in multimodal physical, intellectual, and social enrichment during young and middle adulthood might represent an easily-accessible behavioral strategy to contribute to memory preservation and thus strengthen reserve mechanisms in late life.
The data analyzed in this study is subject to the following licenses/restrictions: the data of the DELCODE study are available on reasonable request (https://www.dzne.de/en/research/research-areas/clinical-research/databases-of-the-clinical-research/). Existing data analysis packages were used for statistical analyses. Respective scripts for the use of these packages are also available from the authors on reasonable request.
The general study protocol for the DELCODE study was approved by the Ethical Committees of the medical faculties of all sites, i.e., the Ethical Committees of Berlin (Charité – Universitätsmedizin), Bonn (Medical Faculty, University of Bonn), Cologne (Medical Faculty, University of Cologne), Göttingen (Universitätsmedizin Göttingen), Magdeburg (Medical Faculty, Otto-von-Guericke University, Magdeburg), Munich (Medical Faculty, Ludwig-Maximilians-Universität), Rostock (Medical Faculty, University of Rostock), and Tübingen (Medical Faculty, University of Tübingen). The process was led and coordinated by the Ethical Committee of the medical faculty of the University of Bonn under the registration number: 171/13. The patients/participants provided written informed consent to participate in the DELCODE study.
AZ, MWa, SR, and MWi: conceptualization and design of the current study. MD, CB, KB, MB, PD, LD, ME, KF, SF, WG, SH, DJ, IK, LK, CL, FM, MM, RP, OP, JP, B-SR, NR, KS, ASc, ES, ASp, ST, JW, SW, RY, ED, FJ, MWa, and SR: overall design and implementation of the DELCODE study. ML, AZ, MG, DH, and MWi: methodology/statistical analysis. OK, AZ, and MWi: interpretation of data. All authors contributed to drafting and/or revision of manuscript and approved the final version.
The DELCODE study was funded by the German Center for Neurodegenerative Diseases [Deutsches Zentrum für Neurodegenerative Erkrankungen (DZNE)], reference number: BN012.
We are grateful for the tremendous efforts of all DELCODE study teams across participating DZNE sites. Further, we express our sincere gratitude to all volunteers that participate in the DELCODE study. We gratefully acknowledge all administrative and scientific staff members involved in data acquisition, data management, as well as quality control. We thank Dr. Catharina Lange (Charité–Universitätsmedizin Berlin, Berlin, Germany) for her support in mask transformation and Dr. Ben Meuleman (University of Geneva, Switzerland) for his statistical support. Furthermore, we acknowledge the DELCODE study group: https://www.dzne.de/en/research/studies/clinical-studies/delcode/.
OP received fees for consultation from Abbvie, Biogen, Eisai, Griffols, MSD Roche, and Schwabe. JP received fees for consultation, lectures, and patents from Neurimmune, Axon, Desitin, and Epomedics. JW is an advisory board member of Abbott, Biogen, Boehringer Ingelheim, Immunogenetics, Lilly, MSD Sharp & Dohme, and Roche Pharma and received honoraria for lectures from Actelion, Amgen, Beijing Yibai Science and Technology Ltd., Janssen Cilag, Med Update GmbH, Pfizer, Roche Pharma and holds the following patents: PCT/EP 2011 001724 and PCT/EP 2015 052945 and also supported by an Ilidio Pinho professorship, iBiMED (UIDB/04501/2020) at the University of Aveiro, Portugal. ED received fees for consultation from Roche, Biogen, RoxHealth and holds shares in neotiv. FJ received fees for consultation from Eli Lilly, Novartis, Roche, BioGene, MSD, Piramal, Janssen, and Lundbeck.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1170879/full#supplementary-material
Anatürk, M., Demnitz, N., Ebmeier, K. P., and Sexton, C. E. (2018). A systematic review and meta-analysis of structural magnetic resonance imaging studies investigating cognitive and social activity levels in older adults. Neurosci. Biobehav. Rev. 93, 71–84. doi: 10.1016/j.neubiorev.2018.06.012
Antonenko, D., Külzow, N., Cesarz, M. E., Schindler, K., Grittner, U., and Flöel, A. (2016). Hippocampal pathway plasticity is associated with the ability to form novel memories in older adults. Front. Aging Neurosci. 8:61. doi: 10.3389/fnagi.2016.00061
Böttcher, A., Zarucha, A., Köbe, T., Gaubert, M., Höppner, A., Altenstein, S., et al. (2022). Musical activity during life is associated with multi-domain cognitive and brain benefits in older adults. Front. Psychol. 13:945709. doi: 10.3389/fpsyg.2022.945709
Brueggen, K., Dyrba, M., Cardenas-Blanco, A., Schneider, A., Fliessbach, K., Buerger, K., et al. (2019). Structural integrity in subjective cognitive decline, mild cognitive impairment and Alzheimer’s disease based on multicenter diffusion tensor imaging. J. Neurol. 266, 2465–2474. doi: 10.1007/s00415-019-09429-3
Bürgel, U., Amunts, K., Hoemke, L., Mohlberg, H., Gilsbach, J. M., and Zilles, K. (2006). White matter fiber tracts of the human brain: Three-dimensional mapping at microscopic resolution, topography and intersubject variability. Neuroimage 29, 1092–1105. doi: 10.1016/j.neuroimage.2005.08.040
Burzynska, A. Z., Jiao, Y., Knecht, A. M., Fanning, J., Awick, E. A., Chen, T., et al. (2017). White matter integrity declined over 6-months, but dance intervention improved integrity of the fornix of older adults. Front. Aging Neurosci. 9:59. doi: 10.3389/fnagi.2017.00059
Chan, D., Shafto, M., Kievit, R., Matthews, F., Spink, M., Valenzuela, M., et al. (2018). Lifestyle activities in mid-life contribute to cognitive reserve in late-life, independent of education, occupation, and late-life activities. Neurobiol. Aging 70, 180–183. doi: 10.1016/j.neurobiolaging.2018.06.012
Collins, J. M., Hill, E., Bindoff, A., King, A. E., Alty, J., Summers, M. J., et al. (2021). Association between components of cognitive reserve and serum BDNF in healthy older adults. Front. Aging Neurosci. 13:725914. doi: 10.3389/fnagi.2021.725914
Duffner, L. A., Deckers, K., Cadar, D., Steptoe, A., de Vugt, M., and Köhler, S. (2022). The role of cognitive and social leisure activities in dementia risk: Assessing longitudinal associations of modifiable and non-modifiable risk factors. Epidemiol. Psychiatr. Sci. 31:e5. doi: 10.1017/S204579602100069X
Duffner, L. A., Dejong, N. R., Jansen, J. F. A., Backes, W. H., De Vugt, M., Deckers, K., et al. (2023). Associations between social health factors, cognitive activity and neurostructural markers for brain health – A systematic literature review and meta-analysis. Ageing Res. Rev. 89:101986.
Fabel, K., Wolf, S. A., Ehninger, D., Babu, H., Leal-Galicia, P., and Kempermann, G. (2009). Additive effects of physical exercise and environmental enrichment on adult hippocampal neurogenesis in mice. Front. Neurosci. 3:2009. doi: 10.3389/neuro.22.002.2009
Fancourt, D., and Finn, S. (2019). What is the evidence on the role of the arts in improving health and well-being? A scoping review. World Heal. Organ. 2, 77–83. doi: 10.18261/issn.2535-7913-2020-01-08
Fellgiebel, A., Schermuly, I., Gerhard, A., Keller, I., Albrecht, J., Weibrich, C., et al. (2008). Functional relevant loss of long association fibre tracts integrity in early Alzheimer’s disease. Neuropsychologia 46, 1698–1706. doi: 10.1016/j.neuropsychologia.2007.12.010
Ferguson, C. J. (2009). An effect size primer: A guide for clinicians and researchers. Prof. Psychol. Res. Pract. 40, 532–538. doi: 10.1037/a0015808
Field, A., Miles, J., and Field, Z. (2012). Discovering statistics using R. London: SAGE Publications Ltd.
Finsterwalder, S., Vlegels, N., Gesierich, B., Araque Caballero, M., Weaver, N. A., Franzmeier, N., et al. (2020). Small vessel disease more than Alzheimer’s disease determines diffusion MRI alterations in memory clinic patients. Alzheimers Dement. 16:12150. doi: 10.1002/alz.12150
Fletcher, E., Raman, M., Huebner, P., Liu, A., Mungas, D., Carmichael, O., et al. (2013). Loss of fornix white matter volume as a predictor of cognitive impairment in cognitively normal elderly individuals. JAMA Neurol. 70, 1389–1395. doi: 10.1001/jamaneurol.2013.3263
Ganzeboom, H. B. G., De Graaf, P. M., and Treiman, D. J. (1992). A standard international socio-economic index of occupational status. Soc. Sci. Res. 21, 1–56. doi: 10.1016/0049-089X(92)90017-B
Gavelin, H. M., Dong, C., Minkov, R., Bahar-Fuchs, A., Ellis, K. A., Lautenschlager, N. T., et al. (2021). Combined physical and cognitive training for older adults with and without cognitive impairment: A systematic review and network meta-analysis of randomized controlled trials. Ageing Res. Rev. 66:101232. doi: 10.1016/j.arr.2020.101232
Grober, E., Ocepek-Welikson, K., and Teresi, J. (2009). The free and cued selective reminding test: Evidence of psychometric adequacy. Psychol. Sci. Q. 51, 266–282.
Hasan, K. M., Moeller, F. G., and Narayana, P. A. (2014). DTI-based segmentation and quantification of human brain lateral ventricular CSF volumetry and mean diffusivity: Validation, age, gender effects and biophysical implications. Magn. Reson. Imaging 32, 405–412. doi: 10.1016/j.mri.2014.01.014
Hayek, D., Thams, F., Flöel, A., and Antonenko, D. (2020). Dentate gyrus volume mediates the effect of fornix microstructure on memory formation in older adults. Front. Aging Neurosci. 12:79. doi: 10.3389/fnagi.2020.00079
Herold, F., Hamacher, D., Schega, L., and Müller, N. G. (2018). Thinking while moving or moving while thinking - concepts of motor-cognitive training for cognitive performance enhancement. Front. Aging Neurosci. 10:228. doi: 10.3389/fnagi.2018.00228
Hofstetter, S., Tavor, I., Tzur Moryosef, S., and Assaf, Y. (2013). Short-term learning induces white matter plasticity in the fornix. J. Neurosci. 33, 12844–12850. doi: 10.1523/JNEUROSCI.4520-12.2013
Huang, J., Friedland, R. P., and Auchus, A. P. (2007). Diffusion tensor imaging of normal-appearing white matter in mild cognitive impairment and early Alzheimer disease: Preliminary evidence of axonal degeneration in the temporal lobe. Am. J. Neuroradiol. 28, 1943–1948. doi: 10.3174/ajnr.A0700
Jenkinson, M., Beckmann, C. F., Behrens, T. E. J., Woolrich, M. W., and Smith, S. M. (2012). FSL. Neuroimage 62, 782–790. doi: 10.1016/j.neuroimage.2011.09.015
Jessen, F., Spottke, A., Boecker, H., Brosseron, F., Buerger, K., Catak, C., et al. (2018). Design and first baseline data of the DZNE multicenter observational study on predementia Alzheimer’s disease (DELCODE). Alzheimers Res. Ther. 10:15. doi: 10.1186/s13195-017-0314-2
Jones, R. N., Manly, J., Glymour, M. M., Rentz, D. M., Jefferson, A. L., and Stern, Y. (2011). Conceptual and measurement challenges in research on cognitive reserve. J. Int. Neuropsychol. Soc. 17, 593–601. doi: 10.1017/S1355617710001748
Jünemann, K., Marie, D., Worschech, F., Scholz, D. S., Grouiller, F., Kliegel, M., et al. (2022). Six months of piano training in healthy elderly stabilizes white matter microstructure in the fornix, compared to an active control group. Front. Aging Neurosci. 14:817889. doi: 10.3389/fnagi.2022.817889
Karp, A., Paillard-Borg, S., Wang, H. X., Silverstein, M., Winblad, B., and Fratiglioni, L. (2006). Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement. Geriatr. Cogn. Disord. 21, 65–73. doi: 10.1159/000089919
Kempermann, G. (2019). Environmental enrichment, new neurons and the neurobiology of individuality. Nat. Rev. Neurosci. 20, 235–245. doi: 10.1038/s41583-019-0120-x
Kempermann, G., Fabel, K., Ehninger, D., Babu, H., Leal-Galicia, P., Garthe, A., et al. (2010). Why and how physical activity promotes experience-induced brain plasticity. Front. Neurosci. 4:189. doi: 10.3389/FNINS.2010.00189
Köbe, T., Witte, A. V., Schnelle, A., Lesemann, A., Fabian, S., Tesky, V. A., et al. (2016). Combined omega-3 fatty acids, aerobic exercise and cognitive stimulation prevents decline in gray matter volume of the frontal, parietal and cingulate cortex in patients with mild cognitive impairment. Neuroimage 131, 226–238. doi: 10.1016/j.neuroimage.2015.09.050
Köhncke, Y., Laukka, E. J., Brehmer, Y., Kalpouzos, G., Li, T. Q., Fratiglioni, L., et al. (2016). Three-year changes in leisure activities are associated with concurrent changes in white matter microstructure and perceptual speed in individuals aged 80 years and older. Neurobiol. Aging 41, 173–186. doi: 10.1016/j.neurobiolaging.2016.02.013
Kutner, M. H., Nachtsheim, C. J., Neter, J., and Li, W. (2005). Applied linear statistical models. New York, NY: McGraw-Hill.
Lauenroth, A., Ioannidis, A. E., and Teichmann, B. (2016). Influence of combined physical and cognitive training on cognition: A systematic review. BMC Geriatr. 16:141. doi: 10.1186/s12877-016-0315-1
Leemans, A., and Jones, D. K. (2009). The B -matrix must be rotated when correcting for subject motion in DTI data. Magn. Reson. Med. 61, 1336–1349. doi: 10.1002/mrm.21890
Lepach, A. C., and Petermann, F. (2012). Memory assessment with the german wechsler memory scale - fourth edition. Zeitschrift fur Neuropsychol. 23, 123–132.
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
Luo, C., Li, M., Qin, R., Chen, H., Yang, D., Huang, L., et al. (2020). White matter microstructural damage as an early sign of subjective cognitive decline. Front. Aging Neurosci. 11:378. doi: 10.3389/fnagi.2019.00378
Maguire, E. A., Gadian, D. G., Johnsrude, I. S., Good, C. D., Ashburner, J., Frackowiak, R. S., et al. (2000). Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. U. S. A. 97, 4398–4403. doi: 10.1073/pnas.070039597
Manno, F. A. M., Kumar, R., An, Z., Khan, M. S., Su, J., Liu, J., et al. (2022). Structural and functional hippocampal correlations in environmental enrichment during the adolescent to adulthood transition in mice. Front. Syst. Neurosci. 15:807297. doi: 10.3389/fnsys.2021.807297
Metzler-Baddeley, C., Jones, D. K., Belaroussi, B., Aggleton, J. P., and O’Sullivan, M. J. (2011). Frontotemporal connections in episodic memory and aging: A diffusion MRI tractography study. J. Neurosci. 31, 13236–13245.
Mielke, M. M., Okonkwo, O. C., Oishi, K., Mori, S., Tighe, S., Miller, M. I., et al. (2012). Fornix integrity and hippocampal volume predict memory decline and progression to Alzheimer’s disease. Alzheimers Dement. 8, 105–113. doi: 10.1016/j.jalz.2011.05.2416
Mohs, R. C., Knopman, D., Petersen, R. C., Ferris, S. H., Ernesto, C., Grundman, M., et al. (1997). Development of cognitive instruments for use in clinical trials of antidementia drugs: Additions to the Alzheimer’s disease assessment scale that broaden its scope. Alzheimer Dis. Assoc. Disord. 11, 13–21. doi: 10.1097/00002093-199700112-00003
Morris, J. C., Heyman, A., Mohs, R. C., Hughes, J. P., van Belle, G., Fillenbaum, G., et al. (1989). The consortium to establish a registry for Alzheimer’s disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology 39, 1159–1165. doi: 10.1212/wnl.39.9.1159
Morris, T. P., Chaddock-Heyman, L., Ai, M., Anteraper, S. A., Castañon, A. N., Whitfield-Gabrieli, S., et al. (2021). Enriching activities during childhood are associated with variations in functional connectivity patterns later in life. Neurobiol. Aging 104, 92–101. doi: 10.1016/j.neurobiolaging.2021.04.002
Ngandu, T., Lehtisalo, J., Solomon, A., Levälahti, E., Ahtiluoto, S., Antikainen, R., et al. (2015). A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet 385, 2255–2263. doi: 10.1016/S0140-6736(15)60461-5
Nichols, E., Steinmetz, J. D., Vollset, S. E., Fukutaki, K., Chalek, J., Abd-Allah, F., et al. (2022). Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 7, e105–e125. doi: 10.1016/S2468-2667(21)00249-8
Oishi, K., Zilles, K., Amunts, K., Faria, A., Jiang, H., Li, X., et al. (2008). Human brain white matter atlas: Identification and assignment of common anatomical structures in superficial white matter. Neuroimage 43, 447–457. doi: 10.1016/j.neuroimage.2008.07.009
Olszewska, A. M., Gaca, M., Herman, A. M., Jednoróg, K., and Marchewka, A. (2021). How musical training shapes the adult brain: Predispositions and neuroplasticity. Front. Neurosci. 15:630829. doi: 10.3389/fnins.2021.630829
Oltmanns, J., Godde, B., Winneke, A. H., Richter, G., Niemann, C., Voelcker-Rehage, C., et al. (2017). Don’t lose your brain at work–The role of recurrent novelty at work in cognitive and brain aging. Front. Psychol. 8:117. doi: 10.3389/fpsyg.2017.00117
Ourry, V., Marchant, N. L., Schild, A. K., Coll-Padros, N., Klimecki, O. M., Krolak-Salmon, P., et al. (2021). Harmonisation and between- country differences of the Lifetime of Experiences Questionnaire in older adults. Front. Aging Neurosci. 13:740005. doi: 10.3389/fnagi.2021.740005
Pichet Binette, A., Theaud, G., Rheault, F., Roy, M., Collins, D. L., Levin, J., et al. (2021). Bundle-specific associations between white matter microstructure and Aβ and tau pathology in preclinical Alzheimer’s disease. eLife 10:62929. doi: 10.7554/elife.62929
Polcher, A., Frommann, I., Koppara, A., Wolfsgruber, S., Jessen, F., and Wagner, M. (2017). Face-name associative recognition deficits in cubjective cognitive decline and mild cognitive impairment. J. Alzheimers Dis. 56, 1185–1196. doi: 10.3233/JAD-160637
Porat, S., Goukasian, N., Hwang, K. S., Zanto, T., Do, T., Pierce, J., et al. (2016). Dance experience and associations with cortical gray matter thickness in the aging population. Dement. Geriatr. Cogn. Dis. Extra 6, 508–517. doi: 10.1159/000449130
Richards, M., and Deary, I. J. (2005). A life course approach to cognitive reserve: A model for cognitive aging and development? Ann. Neurol. 58, 617–622. doi: 10.1002/ana.20637
Roeske, S., Wolfsgruber, S., Kleineidam, L., Zulka, L., Buerger, K., Ewers, M., et al. (2018). P3-591: A German version of the lifetime of experiences questionnaire (LEQ) to measure cognitive reserve: Validation results from the DELCODE study. Alzheimers Dement. 14, 1352–1353. doi: 10.1016/j.jalz.2018.06.1957
Saccà, V., Sarica, A., Quattrone, A., Rocca, F., Quattrone, A., and Novellino, F. (2021). Aging effect on head motion: A machine learning study on resting state fMRI data. J. Neurosci. Methods 352:109084.
Sexton, C. E., Betts, J. F., Demnitz, N., Dawes, H., Ebmeier, K. P., and Johansen-Berg, H. (2016). A systematic review of MRI studies examining the relationship between physical fitness and activity and the white matter of the ageing brain. Neuroimage 131, 81–90. doi: 10.1016/j.neuroimage.2015.09.071
Sexton, C. E., Mackay, C. E., Lonie, J. A., Bastin, M. E., Terrière, E., O’Carroll, R. E., et al. (2010). MRI correlates of episodic memory in Alzheimer’s disease, mild cognitive impairment, and healthy aging. Psychiatry Res. Neuroimaging 184, 57–62. doi: 10.1016/j.pscychresns.2010.07.005
Smith, A. (1982). Symbol digit modalities test (SDMT) manual (revised). Los Angeles, CA: Western Psychological Services.
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., et al. (2011). Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292. doi: 10.1016/j.jalz.2011.03.003
Stern, Y. (2009). Cognitive reserve. Neuropsychologia 47, 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004
Teipel, S., Grothe, M. J., Zhou, J., Sepulcre, J., Dyrba, M., Sorg, C., et al. (2016). Measuring cortical connectivity in Alzheimer’s disease as a brain neural network pathology: Toward clinical applications. J. Int. Neuropsychol. Soc. 22, 138–163. doi: 10.1017/S1355617715000995
Thalmann, B., Urs Monsch, A., Schneitter, M., Bernasconi, F., Aebi, C., Camachova-Davet, Z., et al. (2000). The cerad neuropsychological assessment battery (Cerad-NAB)—A minimal data set as a common tool for German-speaking Europe. Neurobiol. Aging 21, 30–30. doi: 10.1016/s0197-4580(00)82810-9
Theill, N., Schumacher, V., Adelsberger, R., Martin, M., and Jäncke, L. (2013). Effects of simultaneously performed cognitive and physical training in older adults. BMC Neurosci. 14:103. doi: 10.1186/1471-2202-14-103
Tingley, D., Yamamoto, T., Hirose, K., Keele, L., and Imai, K. (2014). Mediation: R package for causal mediation analysis. J. Stat. Softw. 59, 1–38.
Torre, M. M., and Temprado, J. J. (2022). A review of combined training studies in older adults according to a new categorization of conventional interventions. Front. Aging Neurosci. 13:808539. doi: 10.3389/fnagi.2021.808539
Tromp, D. (2016). A guide to quantifying head motion in DTI studies. Winnower. 3:e146228.88496. doi: 10.15200/winn.146228.88496
Valenzuela, M. J., and Sachdev, P. (2007). Assessment of complex mental activity across the lifespan: Development of the lifetime of experiences questionnaire (LEQ). Psychol. Med. 37, 1015–1025. doi: 10.1017/S003329170600938X
Verghese, J., Lipton, R. B., Katz, M. J., Hall, C. B., Derby, C. A., Kuslansky, G., et al. (2003). Leisure activities and the risk of dementia in the elderly. N. Engl. J. Med. 348, 2508–2516. doi: 10.1056/NEJMoa022252
Wan, C. Y., and Schlaug, G. (2010). Music making as a tool for promoting brain plasticity across the life span. Neuroscientist 16, 566–577. doi: 10.1177/1073858410377805
Ward, N., Paul, E., Watson, P., Cooke, G. E., Hillman, C. H., Cohen, N. J., et al. (2017). Enhanced learning through multimodal training: Evidence from a comprehensive cognitive, physical fitness, and neuroscience intervention. Sci. Rep. 7:5808.
Wirth, M., Haase, C. M., Villeneuve, S., Vogel, J., and Jagust, W. J. (2014). Neuroprotective pathways: Lifestyle activity, brain pathology, and cognition in cognitively normal older adults. Neurobiol. Aging 35, 1873–1882. doi: 10.1016/j.neurobiolaging.2014.02.015
Wolfsgruber, S., Kleineidam, L., Guski, J., Polcher, A., Frommann, I., Roeske, S., et al. (2020). Minor neuropsychological deficits in patients with subjective cognitive decline. Neurology 95, e1134–e1143. doi: 10.1212/WNL.0000000000010142
Xu, H., Yang, R., Qi, X., Dintica, C., Song, R., Bennett, D. A., et al. (2019). Association of lifespan cognitive reserve indicator with dementia risk in the presence of brain pathologies. JAMA Neurol. 76, 1184–1191. doi: 10.1001/jamaneurol.2019.2455
Keywords: multimodal leisure activities, brain reserve, brain plasticity, memory, prevention, Alzheimer’s disease
Citation: Klimecki OM, Liebscher M, Gaubert M, Hayek D, Zarucha A, Dyrba M, Bartels C, Buerger K, Butryn M, Dechent P, Dobisch L, Ewers M, Fliessbach K, Freiesleben SD, Glanz W, Hetzer S, Janowitz D, Kilimann I, Kleineidam L, Laske C, Maier F, Munk MH, Perneczky R, Peters O, Priller J, Rauchmann B-S, Roy N, Scheffler K, Schneider A, Spruth EJ, Spottke A, Teipel SJ, Wiltfang J, Wolfsgruber S, Yakupov R, Düzel E, Jessen F, Wagner M, Roeske S, Wirth M and the DELCODE study group (2023) Long-term environmental enrichment is associated with better fornix microstructure in older adults. Front. Aging Neurosci. 15:1170879. doi: 10.3389/fnagi.2023.1170879
Received: 21 February 2023; Accepted: 04 August 2023;
Published: 28 August 2023.
Edited by:
Shaimaa Ibrahim El-Jaafary, Cairo University, EgyptReviewed by:
Qingwei Ruan, Fudan University, ChinaCopyright © 2023 Klimecki, Liebscher, Gaubert, Hayek, Zarucha, Dyrba, Bartels, Buerger, Butryn, Dechent, Dobisch, Ewers, Fliessbach, Freiesleben, Glanz, Hetzer, Janowitz, Kilimann, Kleineidam, Laske, Maier, Munk, Perneczky, Peters, Priller, Rauchmann, Roy, Scheffler, Schneider, Spruth, Spottke, Teipel, Wiltfang, Wolfsgruber, Yakupov, Düzel, Jessen, Wagner, Roeske, Wirth and the DELCODE study group. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miranka Wirth, bWlyYW5rYS53aXJ0aEBkem5lLmRl; Olga M. Klimecki, b2xnYW1hcmlhLmtsaW1lY2tpLWxlbnpAZHpuZS5kZQ==
†These authors share first authorship
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.