
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 24 May 2023
Sec. Neurocognitive Aging and Behavior
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1168576
This article is part of the Research TopicInsights in Neurocognitive Aging and Behavior: 2022View all 33 articles
Objectives: In healthy aging, the way people cope differently with cognitive and neural decline is influenced by exposure to cognitively enriching life-experiences. Education is one of them, so that in general, the higher the education, the better the expected cognitive performance in aging. At the neural level, it is not clear yet how education can differentiate resting state functional connectivity profiles and their cognitive underpinnings. Thus, with this study, we aimed to investigate whether the variable education allowed for a finer description of age-related differences in cognition and resting state FC.
Methods: We analyzed in 197 healthy individuals (137 young adults aged 20–35 and 60 older adults aged 55–80 from the publicly available LEMON database), a pool of cognitive and neural variables, derived from magnetic resonance imaging, in relation to education. Firstly, we assessed age-related differences, by comparing young and older adults. Then, we investigated the possible role of education in outlining such differences, by splitting the group of older adults based on their education.
Results: In terms of cognitive performance, older adults with higher education and young adults were comparable in language and executive functions. Interestingly, they had a wider vocabulary compared to young adults and older adults with lower education. Concerning functional connectivity, the results showed significant age- and education-related differences within three networks: the Visual-Medial, the Dorsal Attentional, and the Default Mode network (DMN). For the DMN, we also found a relationship with memory performance, which strengthen the evidence that this network has a specific role in linking cognitive maintenance and FC at rest in healthy aging.
Discussion: Our study revealed that education contributes to differentiating cognitive and neural profiles in healthy older adults. Also, the DMN could be a key network in this context, as it may reflect some compensatory mechanisms relative to memory capacities in older adults with higher education.
Age-related changes on cognitive function may influence the quality of life (Harada et al., 2013; Murman, 2015). For example, memory performance worsens with age and well-maintained memory capacities could be considered as a “tract” of a preserved cognitive function in healthy aging (Harada et al., 2013; Nyberg and Pudas, 2019). Conversely, some cognitive abilities reflecting “crystallized” capacities, i.e., accumulated throughout the life-course, like vocabulary size, are resistant to aging (as compared to abilities that are considered “fluid” like processing speed) (Verhaeghen, 2003), and they may even improve across life (Murman, 2015). In this context, the concept of cognitive reserve (Lojo-Seoane et al., 2018) may help to explain differences in the way people get older and cope with different cognitive demands, which depend on the cognitive “resources” accrued during life (Barulli and Stern, 2013). Interestingly, although many other proxies like occupational attainment or IQ may determine CR (Lojo-Seoane et al., 2018; Montemurro et al., 2022), the quantity and type of achieved education is one of the most used. In this context, previous research has suggested that people with higher education tend to perform better in older age than those with lower education (Lovden et al., 2020). This means that older people with higher education would experience a larger age-related decline to achieve the same level of cognitive impairment as their peers with lower education. Indeed, education influences late-life cognitive function by contributing to individual differences in cognitive skills (Lovden et al., 2020); the more years of education, the better the expected cognitive performance in aging (Elkins et al., 2006; Montemurro et al., 2019a,b). This is also in line with recent research from our group reporting that education represents a protective factor against age-related cognitive decline (Mondini et al., 2022). In addition, education is one of the most accessible and suitable CR proxies (e.g., easy to collect) for aging populations (Chapko et al., 2018; Montemurro et al., 2021), besides being one of the most commonly used in clinical and experimental contexts (Meng and D’Arcy, 2012; Anaturk et al., 2021).
Whilst education has been so far mainly used to explain cognitive decline in aging as a confound (i.e., nuisance) or a predictor (i.e., proxy), a full comprehension of its role in outlining brain functioning and its relationship with cognitive function still remains challenging. Brain aging has been investigated through magnetic resonance imaging (MRI) in terms of structural changes (e.g., atrophy of cortical gray matter or increased cerebrospinal fluid volume, (Reuter-Lorenz, 2002; Raz et al., 2005; Fjell et al., 2009; Park and Reuter-Lorenz, 2009; Salthouse, 2011; Orellana et al., 2016; Oschwald et al., 2019), and functional changes (Vidal-Pineiro et al., 2014; Yoshimura et al., 2020), showing that some brain networks, like the Default Mode Network (DMN), are affected by aging. Functional connectivity, measured with functional MRI (fMRI), allows to investigate the relationship between fluctuations among different brain areas (Sporns, 2013). This technique has been largely used to study brain networks in aging (Vidal-Pineiro et al., 2014; Yoshimura et al., 2020) and their behavioral correlates (Farras-Permanyer et al., 2019; Varangis et al., 2019). For example, it has been shown that brain network integrity provides a helpful outlook on the preservation of cognitive functioning (Geerligs et al., 2015; Jockwitz and Caspers, 2021), and that such integrity is mainly investigated by resting state networks (RSNs), whose topology recalls the ones of brain networks emerging during active tasks (Smith et al., 2009). Previous studies have primarily shown that age-related decline affects DMN connectivity (Andrews-Hanna et al., 2007; Ferreira and Busatto, 2013; Dennis and Thompson, 2014; Hirsiger et al., 2016), however, more recent research has revealed that other “higher-order cognitive” networks, including the Dorsal Attention Network (DAN), are vulnerable to aging (Geerligs et al., 2015; Siman-Tov et al., 2016; Hausman et al., 2020). However, while changes in RSNs have been linked to cognitive decline in aging, a high heterogeneity has limited the understanding of the impact of aging on brain function.
In this context, CR has gained relevance and has been integrated in neuroimaging research (Bastin et al., 2012; Menardi et al., 2018). CR should be ideally helpful for a better comprehension of individual differences in aging, by using proxies that allow to estimate the individuals’ potential resilience. However, this field of research is still presenting challenges. Several theories have been already proposed to explain functional changes in aging, i.e., the Hemispheric Asymmetry Reduction in Older Adults model (HAROLD, (Cabeza, 2002), the Posterior-Anterior Shift in Aging theory (PASA, (Davis et al., 2008) the Compensation-Related Utilization of Neural Circuits Hypothesis CRUNCH, (Reuter-Lorenz and Cappell, 2008), and the Scaffolding Theory of Aging and Cognition (STAC, (Park and Reuter-Lorenz, 2009). Among them, the STAC, lately revised in the STAC-r (Reuter-Lorenz and Park, 2014), introduced education (and other CR proxies) as a crucial variable to interpret the mechanisms of aging (Nyberg et al., 2012). In STAC-r, a high level of education is conceived as being associated with an enhanced neurocognitive scaffolding, so that, despite neural deterioration, cognitive function should be maintained in older adults (Reuter-Lorenz and Park, 2014). Nevertheless, the role of education in determining cognitive and brain characteristics in healthy aging populations has also displayed some diverging findings (Nyberg et al., 2012), and although education has been much discussed such a factor that may better explain inter-individual differences in aging, it is still unclear how neurocognitive profiles could be characterized based on differences in such an important variable.
In the present study, starting from the premise that, in healthy aging, cognitive performance is better maintained with a higher education, we tested whether education could differentiate healthy older adults in terms of not only cognitive performance but also fMRI connectivity, especially for RSNs involved in high-level cognitive functions. To this end, we first compared older adults to a more preserved population, i.e., younger adults, to identify age-related differences. Then, by splitting the older adult group in two subgroups based on education (higher and lower), we specified the effect of education in healthy aging. Accordingly, we expected that cognitive and neural variables would show age-related differences and more importantly, at the basis of this study, that education would provide definite information about age-related differences in resting state fMRI connectivity and its relationship with cognition.
All participants included in this study were taken from the publicly available database “Leipzig Study for Mind-Body-Emotion Interactions” (LEMON) (Babayan et al., 2019). Data collection was performed in accordance with the Declaration of Helsinki, approved by the local ethics committee and all participants provided written informed consent prior to data acquisition for the study (including agreement to their data being shared anonymously). Following the application of exclusion criteria, which are listed in detail in Babayan et al. (2019), the total sample included 227 participants. Due to missing data of specific cognitive variables of interest, 30 participants were excluded from the analysis, thus the total number of participants was n = 197. In the LEMON database, the subjects were separated into two groups based on their age, resulting in one group of younger adults (young, Y, n = 137, range 20–35 years) and one of older adults (old, O, n = 60, range 55–80 years). Also, the old group was further divided into two subgroups based on their education that was collected and reported as a dichotomous variable as: higher education (old-high, OH, n = 30), i.e., lyceum/gymnasium (12 years), and lower education (old-low, OL, n = 30), i.e., technical high school/realschule (10 years). Notably, all younger adults had higher education, as the old-high group. Five cognitive tests addressing memory, language, and vocabulary were used. The behavioral tasks included: a) Short- and b) Long-term Memory tests (California Verbal Learning Task, CVLT, (Niemann et al., 2008), used to assess verbal learning and memory capacity, and to provide information about different learning strategies by testing Immediate Memory Recall and Delayed Memory Recall; c) Vocabulary test (Wortschatztest, WST, (Schmidt and Metzler, 1992), used to measure verbal intelligence, and to assess language comprehension; d) Phonemic and e) Semantic Fluency tests (Regensburger Wortflüssigkeitstest, RWT, (Aschenbrenner et al., 2000), used to assess verbal fluency. Notably, higher scores correspond to a better cognitive performance in all cognitive tests. A full description about the cognitive assessment can be found in Babayan et al. (2019). Demographic variables, such as sex and Body Mass Index (BMI) were also assessed.
MRI scans were performed on a Siemens 3 Tesla (3T) MAGNETOM Verio MR scanner (Siemens Healthcare GmbH, Erlangen, Germany) equipped with a 32-channel receiver head coil. Briefly, the data used in this study included anatomical and resting state fMRI (rs-fMRI) scans. The anatomical scans were acquired using a 3D T1-weighted (T1w) Magnetization-Prepared 2 Rapid Acquisition Gradient Echoes sequence (MP2RAGE) (Marques et al., 2010), with a MP2RAGE block time = 5,000 ms, a repetition time (TR) = 6.9 ms, an echo time (TE) = 2.92 ms, inversion time (TI1) = 700 ms, TI2 = 2,500 ms, flip angle (FA1) = 4°, FA2 = 5°, voxel dimension = 1 mm isotropic, acquisition time = 8 min 22 s. The rs-fMRI scans were acquired using a T2*-weighted echo planar imaging (EPI) sequence with a multiband factor MB = 4, TR = 1,400 ms, TE = 30 ms, FA = 69°, voxel dimension = 2.3 mm isotropic, number of volumes (N) = 657 volumes, acquisition time = 15 min 30 s. The two images acquired at TI1 and TI2 are fused into a unique anatomical image with enhanced T1 contrast and reduced bias field.
During the rs-fMRI run, participants were instructed to remain awake and lie down with their eyes open while looking at a low-contrast fixation cross. Spin echo EPI with reversed phase encoding were also acquired and used for rs-fMRI distortion correction. These scans were acquired with TR = 2,200 ms, TE = 50 ms, FA = 90°, voxel dimension = 2.3 mm isotropic, phase encoding = anterior to posterior (AP) and posterior to anterior (PA), acquisition time = 29 s each. Further details about the MRI protocol can be found in Babayan et al. (2019). To quantify eventual head motion artifacts, we calculated the framewise displacement [FD (Power et al., 2012)] computed as the sum of the absolute values of the derivatives of the translational and rotational realignment estimates at every timepoint, for which we reported values below 0.5 for all groups (FDHC = 0.18 ± 0.06, FDOH = 0.22 ± 0.14, FDOL = 0.23 ± 0.09).
Anatomical scans: Voxel-Based-Morphometry (VBM) was used to study GM differences between young and older adult groups. Preprocessing was carried out using FSL1 and included: brain extraction and brain tissues segmentation (using FAST), which allows extracting measures of the total gray matter (GM), the white matter (WM) and the cerebrospinal fluid (CSF). Brain structural imaging features, including total brain volume (TBV) and segmented GM, WM, and CSF, were computed for each participant. Whole-brain analysis was carried out with FSL-VBM (Douaud et al., 2007), using default settings. In brief, brain extraction and tissue-type segmentation were performed and resulting GM partial volume images were aligned to the standard space using FMRIB’s Linear Image Registration Tool (FLIRT) and then non-linear (FNIRT) registration tools. The resulting images were averaged, modulated, and smoothed with an isotropic Gaussian kernel of 3 mm to create a study-specific template. Finally, voxel-wise GLM was applied using permutation non-parametric testing (5,000 permutations), correcting for multiple comparisons across space.
rs-fMRI scans: Motion correction was performed using MCFLIRT, included in the fMRI Expert Analysis Tool (FEAT) toolbox (Woolrich et al., 2001). Then, distortion correction was performed using the fMRI datasets acquired with AP and PA phase-encoding directions. These were used to estimate a susceptibility induced off-resonance distortion field (Smith et al., 2004). The computed field was used to correct the, previously motion-corrected, time-averaged fMRI dataset. Following this, rigid registration of the motion and distortion corrected time-averaged fMRI data onto the individual structural T1w data was performed. After linear registration to the T1w image was performed to register the rs-fMRI data from the individual space to the Montreal Neurological Institute (MNI) standard space, a non-linear transformation was computed by processing the anatomical T1w image alone. In particular, the T1w image was registered to the MNI space in two sequential steps. First, an affine transformation (12 degrees of freedom) was computed. Then, a further non-rigid transformation was employed to reach fine-grained alignment. Note that, in this context, the affine transformation was employed as seed for the minimization of the non-linear registration step. After all these steps were performed, the off-resonance distortion field, the linear transformation mapping the fMRI data onto the T1w image, and the non-linear transformation mapping the T1w data onto the MNI space, were combined into an unique warping field, which was applied at once to the motion and distortion corrected fMRI time-series. After alignment was achieved, the fMRI data on the MNI space was finally spatially smoothed using a Gaussian kernel of full-width at half-maximum (FWHM) of 5 mm, and a high-pass temporal filtering equivalent to 100 s applied. fMRI connectivity analysis at rest was carried out using the Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC) (Beckmann and Smith, 2004). Preprocessed functional data containing N = 657 volumes for each subject were temporally concatenated across subjects to create a single 4D dataset. The between-subject analysis of the rs-fMRI data was carried out using the “dual regression” approach (Filippini et al., 2009) that allows for voxel-wise comparisons of resting state functional connectivity. In this analysis, the dataset was decomposed into 25 components, in which the model order was estimated using the Laplace approximation to the Bayesian evidence for a probabilistic principal component model (Beckmann and Smith, 2004; Filippini et al., 2009). The resulting group-ICA (Independent Component Analysis) components were visually inspected and then labelled as resting state networks (RSNs) based on the topology of their thresholded spatial maps. Thirty subjects for each of the three groups were considered to create the group-components. The mean connectivity values associated with each subject RSN maps were extracted and used as a measure of connectivity strength, referring to the spontaneous and synchronous activity within a given network for a specific subject. Statistical analysis, described in more detail in the following section (2.3.2), consisted of an Analysis of Covariance (ANCOVA), including TBV, BMI and sex as nuisances, which was run to test for differences in neural variables between the different groups.
Statistical analyses on continuous variables (i.e., socio-demographic, cognitive scores and brain measures) and dichotomic variables (i.e., sex) were carried out using SPSS software (SPSS, Inc.). A t-test was used to compare those variables between younger and older participants, whereas the Analysis of Variance (ANOVA) was used to compare socio-demographic variables and structural brain measures between the young, old-high, and old-low groups. The ANCOVA was then used to compare cognitive scores and RSN measures among all groups. The ANCOVA analysis included TBV, BMI and sex as nuisances (Table 1). Both ANOVA and ANCOVA post-hoc analyses were Bonferroni-corrected. Pearson’s correlation coefficients between cognitive scores and connectivity strength of RSNs that revealed significant group differences were calculated through a partial correlation analysis including TBV, BMI and sex as nuisances. The Fisher’s-r-to-Z transformation of the Pearson’s correlation was used to compare the partial correlation coefficients. Notably, in this study, TBV, BMI and sex were used as nuisances as, on the one hand, they presented significant differences between the younger and older groups, and, on the other hand, they are commonly used variables in neuroimaging studies (Hyatt et al., 2020).
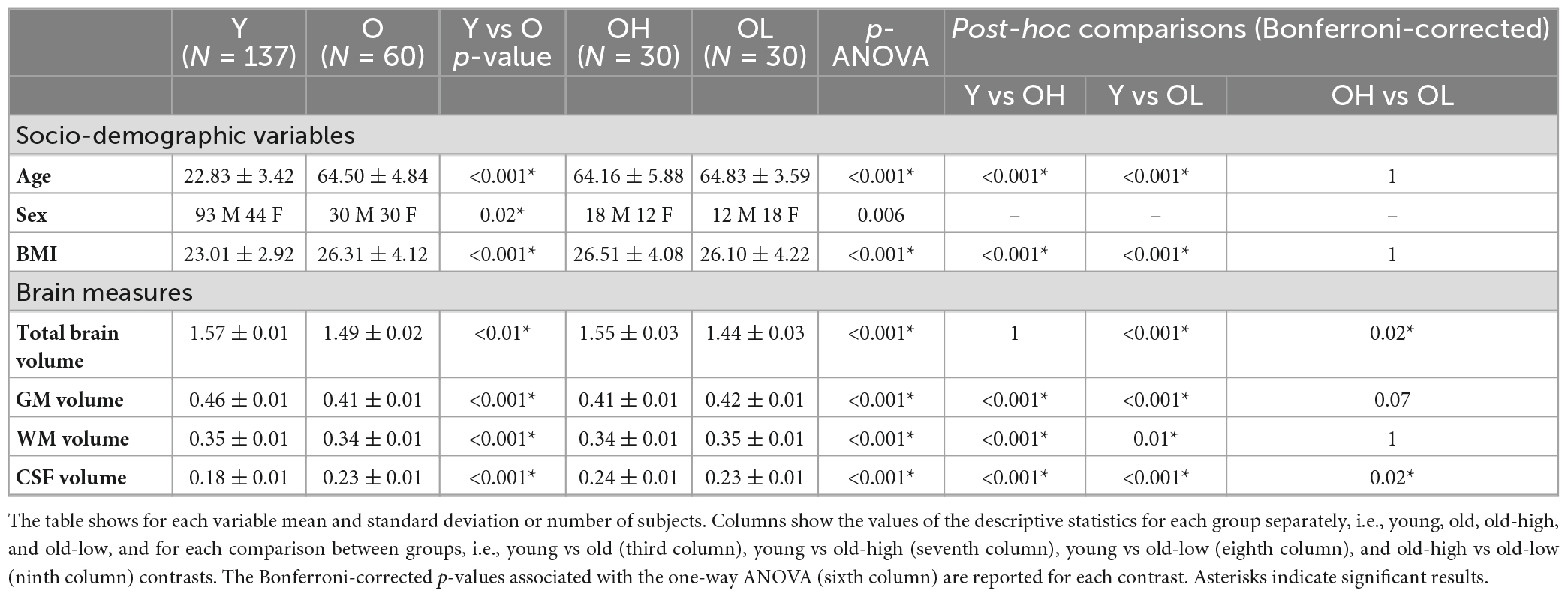
Table 1. Descriptive analyses and Analysis of Variance (ANOVA) of socio-demographic variables, and brain measures for all the groups, i.e., younger (Y), older (O), older with higher level of education (OH), and older with lower level of education (OL).
The analysis of socio-demographic variables revealed that the young group differed from the old group in terms of BMI and sex (Table 1). VBM analysis showed widespread reduction in GM volume in the old group compared to the young group. Brain regions with group differences included the anterior cingulate, precuneus, superior, middle and inferior frontal gyri, supramarginal gyrus, insular cortex, temporal, parietal and lateral occipital regions bilaterally (Supplementary Figure 1). These results are in line with previously reported findings for this type of comparison (Filippini et al., 2012). No voxel-wise structural differences were observed between old-high and old-low participants. For completeness and descriptive purposes, we presented further structural MRI data analysis showing that the young group had higher TBV, GM, and WM, and lower CSF values, compared to the old group (Table 1). Differences were found between the old-high and the old-low groups in terms of TBV and CSF.
The analysis of cognitive scores showed that the young group performed better than the old group in all the cognitive tests, apart from vocabulary (Table 2). For this variable, the old group did not differ from the young group. However, when splitting the older participants according to their educational level, the old-high, who had both higher word knowledge and higher education, showed the largest vocabulary size (p < 0.01). Overall, the old-high group showed lower scores on cognitive tests as compared to the young group, but higher scores as compared to the old-low group, see Table 2. Moreover, the old-high participants did not differ from the young participants in two cognitive tests requiring the use of high-level cognitive function (i.e., verbal fluency), see Table 2. The rs-fMRI analysis revealed both age- and education-related differences in functional connectivity values. From the derived 25 components representing temporally correlated fMRI signals in different brain regions, we were able to identify eight spatial maps showing topological patterns associated with well-known RSNs (Figure 1). The RSNs included Somatomotor network (SMN), Visual-Medial network (VMN), Visual-Peripheral network (VPN), Salience network (SN), Dorsal Attention network (DAN), Default Mode network (DMN), right Fronto-Parietal network (rFPN), and left Fronto-Parietal network (lFPN).
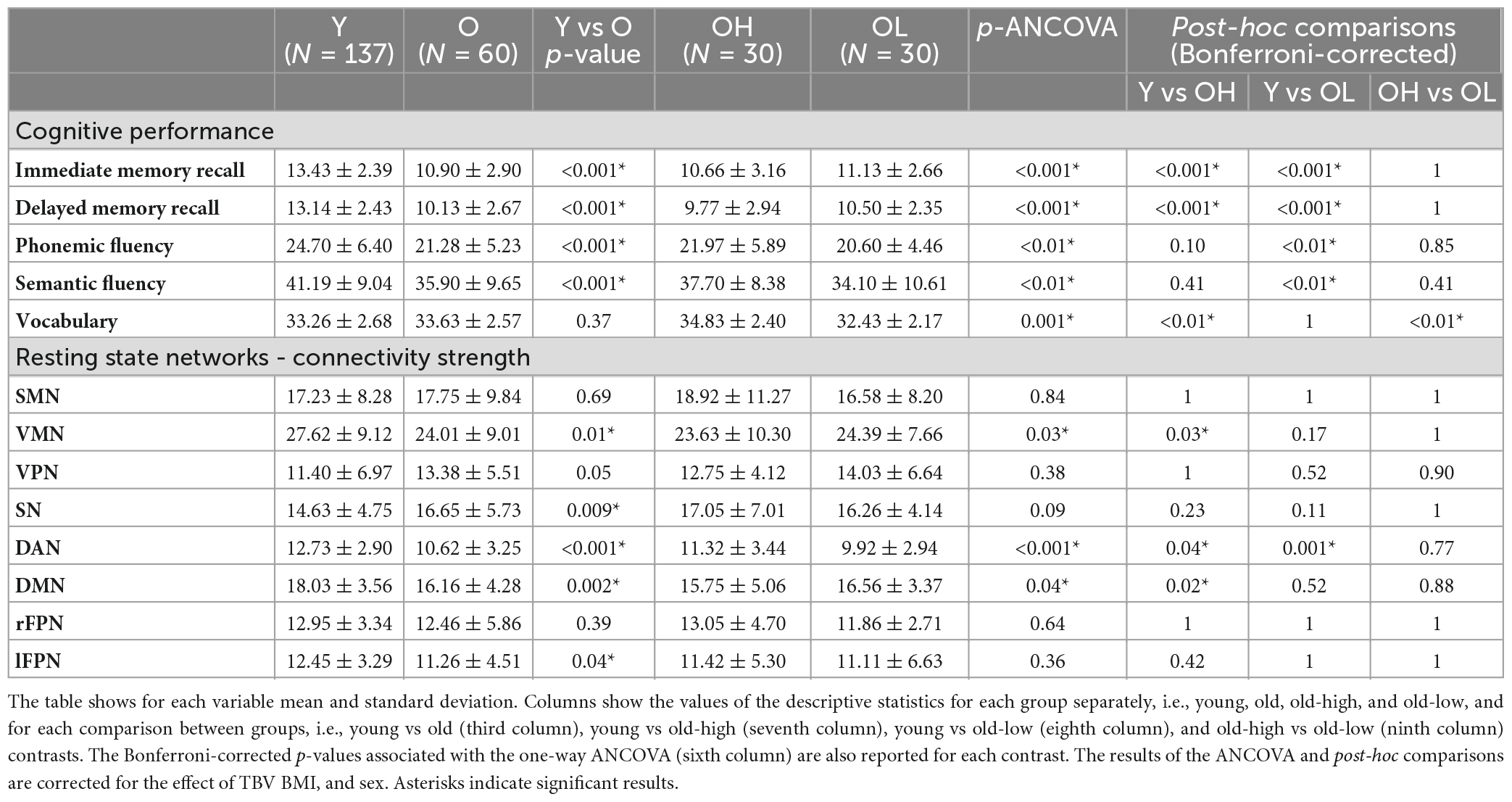
Table 2. Descriptive analyses and Analysis of Covariance (ANCOVA) of cognitive performance and resting state networks connectivity strength for all the groups, i.e., younger (Y), older (O), older with higher level of education (OH), and older with lower level of education (OL).
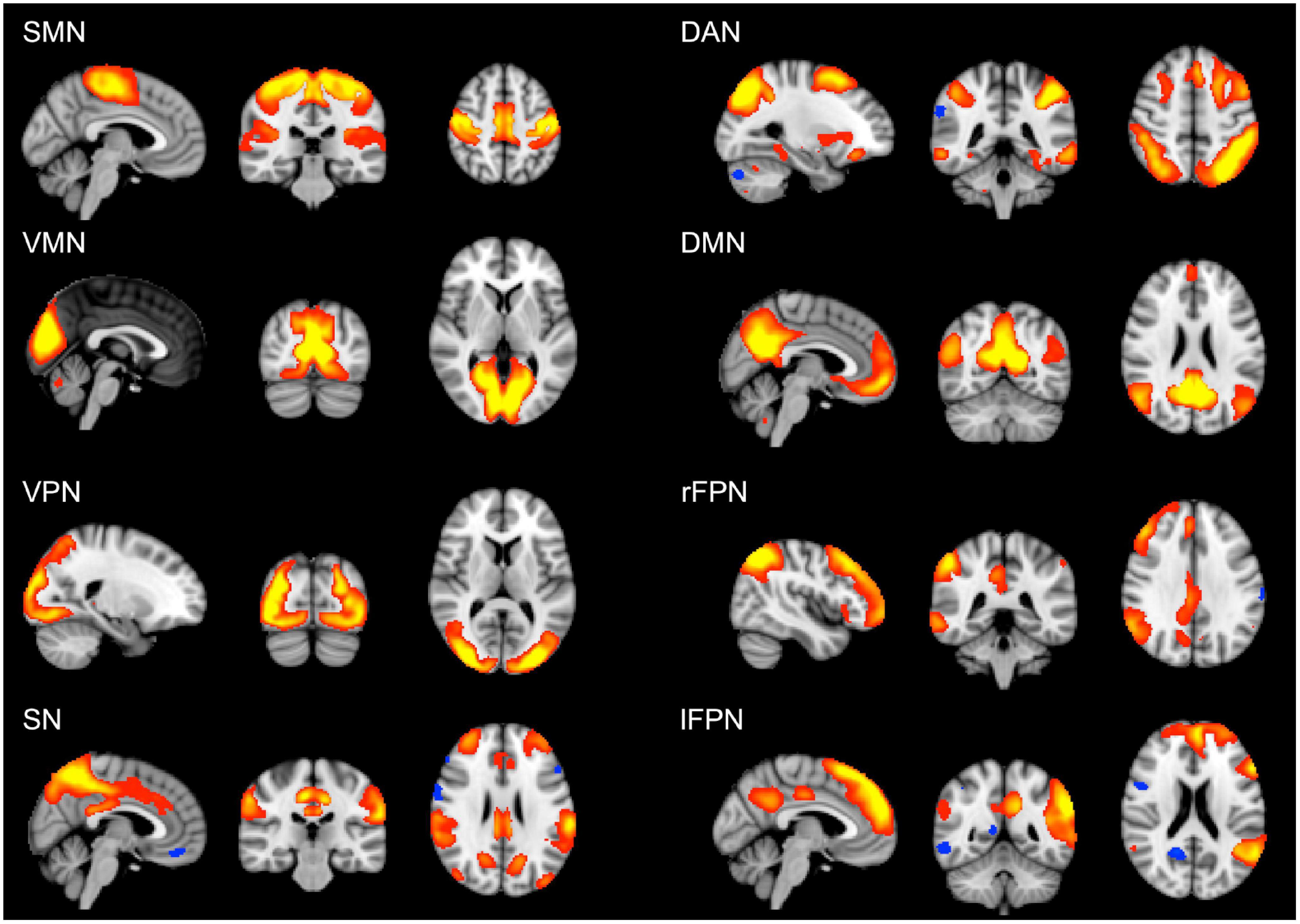
Figure 1. Large-scale brain networks reconstructed using rs-fMRI data considering all the subjects. RSNs were selected and labeled following visual check as: SMN, somatomotor network; VMN, visual-medial network; VPN, visual-peripheral network; SN, salience network; DAN, dorsal attention network; DMN, default mode network; rFPN, right fronto-parietal network; lFPN, left fronto-parietal network. Group-level RSN maps (N = 197) are thresholded at z = 3. Red to yellow colors represent z-scores > 3.
Overall, t-tests showed significant differences in connectivity strength, between the young and the old group for the VMN, the SN, the DAN, the DMN, and the lFPN (Table 2). By splitting the old group based on education, in ANCOVA, we identified some specific findings. In particular, the VMN showed that the old-high group had significantly lower network connectivity strength compared to the young group (p = 0.03), which was not reported in the case of the old-low group. Similarly, in the DMN, the old-high group showed significantly lower connectivity strength compared to the young group (p = 0.02), which was not reported in the case of the old-low group. Differently from the above reported RSNs showing significant differences between the young and the old group, the DAN showed significant differences for both the old-high and the old-low groups compared to the young group (old-high vs young p = 0.04; old-low vs young p = 0.001) also in the post-hoc comparisons.
An exploratory correlation analysis between network connectivity strength and cognitive scores was performed for those RSNs revealing significant differences between groups, i.e., the VMN, the DAN, and the DMN (Table 2). The correlation analysis showed a significant negative relationship between the DMN connectivity strength and the memory scores (Supplementary Table 1). The Fisher’s-r-to-Z transformation of the Pearson’s correlation relative to the DMN-memory showed that the correlation slopes of the young and the old groups were significantly different between each other, in both Immediate Memory (two-tailed p = 0.02) and Delayed Memory recall (two-tailed p = 0.02). Such differences were substantially carried out by the old-high group, which showed preserved significance (two-tailed p = 0.04) for the Delayed Memory recall and approached significance (two-tailed p = 0.06) for the Immediate Memory. The relationship between both memory scores and the DMN connectivity strength were clearly not significant in the old-low group Indeed, the differences between correlation slopes approached a significant result in the comparison between the old-high and the young groups (two-tailed p = 0.05), while they were clearly non-significant in the comparison between the old-high and the old-low groups (two-tailed p = 0.20), see Figure 2.

Figure 2. Correlation analysis (Pearson’s correlation) between DMN connectivity strength and delayed memory recall. The panel on the left-hand side shows the slopes, the residuals and the Pearson’s r associated with the partial correlation analyses between DMN connectivity strength and the memory score, in the young and the old groups. The panel on the right-hand side shows the slopes, the residuals and the Pearson’s r associated with the partial correlation analyses between DMN connectivity strength and the memory score in the young, the old-high, and the old-low groups. The asterisk indicates the result of significant two-tailed differences between slopes.
In this study, we investigated whether education differentiates cognitive performance and resting state fMRI connectivity in healthy aging. To this end, we analyzed cognitive, structural and functional brain imaging variables and we considered education like a crucial socio-demographic aspect. Older adults typically show cognitive, structural, and functional age-related differences compared to younger adults (e.g., lower accuracy and slower response times in cognitive tasks, as also cortical thinning and brain atrophy) as the possible result of a reduced neural activity [see in Festini et al. (2018)]. However, this is not always the case, as brain overactivation has also been reported in healthy aging, in response to a compensatory necessity (e.g., Cabeza, 2002). Accordingly, age-related differences at the cognitive level are not always associated with the differences at brain structural and functional level. These differences might also be linked to education, which has been used in previous literature as a predictor of cognitive performance in healthy aging (Montemurro et al., 2021). Notably, education helps to estimate resources possibly accumulated from early life, that may support cognition also in later life (Mondini et al., 2022), although some studies have reported different findings (Nyberg et al., 2012). For this reason, we wanted to analyze whether cognitive and neural variables in aging could be further specified whereby a difference at the level of education is taken int account. In this study, the variable education did not vary within the young adults, who were all highly educated (i.e., gymnasium), but varied within the older adults, who were divided in two subgroups based on their level of education (i.e., gymnasium and realschule for old-high and old-low groups, respectively). With this procedure, we first examined whether there were any age-related differences between young adults and older adults. In this context, we used the group of young adults as a sort of “control condition” which was characterized by a homogenous level of education (i.e., higher), to provide a measure of efficient cognitive performance, as it is expected in young age stages. Then, we investigated whether education-specific features were identified in the old-high and the old-low groups, which is the core novelty of this study.
In line with previous literature, younger adults performed better than older adults in all cognitive tests, except for the vocabulary in which older adults performed better than younger adults (Bettio et al., 2017; Nyberg and Pudas, 2019). Importantly, the young and the old-high groups did not differ between each other in terms of language performance (i.e., verbal fluency tests) as a possible effect of education (Barulli and Stern, 2013) but the old-high group had a significantly higher vocabulary score compared to the other two groups. Overall, these results confirmed that higher education may provide for a more “youth-like” performance on language tasks requiring flexibility and cognitive control. In addition, vocabulary may have increased both along the lifespan and through the education level, which may have played as a sort of reserve/storage of verbal capacities too [see also the DACHA theoretical model in Verhaeghen (2003), Spreng and Turner (2019)].
We found between-group differences in terms of brain structure, with the older adults showing signs of deterioration, i.e., reduced gray and white matter, increased CSF, compared to young adults (Fjell et al., 2009; Salthouse, 2011; Orellana et al., 2016; Oschwald et al., 2019), regardless the level of their education. From a functional point of view, the derived RSNs (i.e., SMN, VMN, VPN, SN, DAN, DMN, rFPN, and lFPN) presented a more heterogeneous scenario. A significantly higher RSN connectivity strength was found in the young group compared to the old group in the VMN, the DAN, and the DMN, which notably may be affected by aging (Onoda et al., 2012; Jockwitz et al., 2017; Farras-Permanyer et al., 2019), while the SN showed an opposite trend (higher connectivity strength for the older adults, especially for those with a high level of education). When accounting for education, the results significantly differed in the RSN connectivity strength for the VMN, the DAN, and the DMN (Table 2). In the DAN, the highest level of network connectivity strength was found for the young group, followed by the old-high, and then the by the old-low group. Interestingly, in the DAN, both the old-high and the old-low groups significantly differed from the young group, which suggested a clear age-related effect for this network (Jockwitz and Caspers, 2021). For both the VMN and the DMN, the old-high group had the lowest network connectivity strength in line with previous research that showed an age-related decreased connectivity of these RSNs (Farras-Permanyer et al., 2019; Jockwitz and Caspers, 2021) which in turn display the potential compensatory effects carried out by education. These findings are also in line with the STAC-r model (Reuter-Lorenz and Park, 2014), which highlighted education for explaining compensation processes in aging. Interestingly, despite the high similarities between the two older groups, we revealed that subtle differences in education (i.e., 10 years versus 12, and technical high school versus lyceum, for the lower versus higher education groups, respectively) are associated with dissimilarities in both cognitive and neuroimaging variables. More specifically, despite subtle differences in education, results showed significant differences between the two older populations in the performance of vocabulary, which notably is considered a crystallized ability, but not in the performance of memory, for example, which is considered a fluid ability. This suggests that some variables more than others, such as vocabulary, for cognition, and DMN connectivity, for neuroimaging variables, might allow to focusing, depending on education, on specific features that could allow the detection at an earlier stage of a possible cognitive decline.
The results of this study suggest that higher education makes older individuals possibly more cognitively specialized in terms of verbal abilities, which is shown by the highest vocabulary skills in the old-high group compared to the other two groups, and by the non-significant differences between the old-high and the young group on verbal fluency (which also requires executive control). This suggests that a higher education defines specific neural patterns in the brain at rest. Critically, the old-high group not only displayed the lowest connectivity strength in the DMN, but, in this group, the DMN was the only network showing a selective, negative, association with memory capacities (Supplementary Table 1). Although the correlation analyses were only observational and exploratory, we may explain these results by underlining that the DMN, which is known to “deactivate” proportionally with the increasing cognitive demand (Anticevic et al., 2012), may reflect a peculiar compensatory effect carried out by education at the level of memory capacities, which are typically vulnerable to aging (Farras-Permanyer et al., 2019). Age-related dysregulation in the connectivity of the DMN has been previously found in association with compensatory mechanisms, expressed in terms of recruitment of alternative brain areas (Park and Reuter-Lorenz, 2009; Reuter-Lorenz and Park, 2010). Moreover, our findings fit with the DECHA theoretical model (i.e., Default-Executive Coupling Hypothesis of Aging, (Spreng and Turner, 2019), which has more recently been developed for investigating complex compensatory mechanisms in aging. According to DECHA, education might help in the shift toward a more “semanticized” cognition (Spreng and Turner, 2019), which in turn would explain how the old-high group in this study have also better coped with cognitive demands in the semantic verbal fluency tasks compared with the old-low group. The DECHA model has been developed to describe the shift, or the inversion, of some cognitive capacities, due to aging. Fluid abilities (which require goal-directed, or controlled, processing) and crystallized abilities (which require the accumulation of semantic knowledge about ourselves and the world), are indeed known to age differently. More specifically, along with age, fluid abilities are expected to decline, and crystalized ones are expected to enhance. The DECHA model intriguingly describes aging as a process in which older adults show both losses and gains. Individuals in fact, across their lifespan, are observed in their “cognitive semanticization” which reflects the natural shift from exploration and novelty seeking at young age, to greater exploitation of existing knowledge stores in later life (Spreng and Turner, 2019). In this study, education seems to represent a crucial variable for a deeper understanding of the DECHA model. The results of this study in fact indicate that education may “boost” cognitive semanticization. However, future research, with a broader range of cognitive reserve proxies and methods of investigation, is necessary to comprehend the potential effect of compensatory indices in the DECHA theoretical model.
The cross-sectional nature of this dataset represents a limitation in this study. Longitudinal studies would be warranted to properly study age-related differences by detecting changes in brain structure and function over time (Damoiseaux, 2017). Furthermore, criteria used to define the level of education might be considered to some extent suboptimal, as we could not use the continuous education values but two broad classes. Indeed, by considering a continuous measure of education, further insights into the relationship between education and cognitive performance in healthy aging might have been unveiled. Further research could expand the present results, accounting for other variables that could affect cognition, such as occupational complexity, leisure activities and life-style, as also with socio-economic status. However, such limitations were not specifically linked to the experimental choices of this study, but continuous values for education were not available in the database (LEMON).
An important aspect to consider is that the level of education of the sample is high, as compared with low-and-middle income countries in other parts of the world. It is possible that larger differences would be observed in cognitive and neural indices, with larger ranges in socio-economic variables. This will further open to advances in research, not only considering participants with heterogeneous socio-economic status, but also considering cross-cultural investigations.
Education maintains its modulatory role in neurocognitive functioning. It is able to differentiate cognitive and neural profiles of healthy older adults, based on their high-order verbal proficiency and resting-state connectivity of neural networks typically employed in high-order cognitive functioning. A key network in this context is the DMN as it might reflect memory compensatory mechanisms in older adults with higher education, who may have, at the cognitive level, a higher potential amount of resources to employ in their every-day lives.
Publicly available datasets were analyzed in this study. This data can be found here: https://ftp.gwdg.de/pub/misc/MPI-Leipzig_Mind-Brain-Body-LEMON.
The studies involving human participants were reviewed and approved by the Medical Faculty of the University of Leipzig (reference number: 154/13-ff). The patients/participants provided their written informed consent to participate in this study.
SM: conceptualization, data curation, formal analysis, and writing – original draft. NF: conceptualization, formal analysis, and writing – review and editing. GF: data curation, formal analysis, and writing – review and editing. DM: funding acquisition, and writing – review and editing. GA: conceptualization, funding acquisition, and writing – review and editing. MM: conceptualization, data curation, formal analysis, funding acquisition, and writing – original draft. All authors contributed to the article and approved the submitted version.
MM was funded by the Research Foundation Flanders (FWO) (postdoctoral fellowship 1211820N).
A special thanks to the Day Clinic for Cognitive Neurology of the University Clinic Leipzig and the Max Planck Institute for Human Cognitive and Brain Sciences (MPI CBS) in Leipzig, Germany.
GF was employed by Philips Healthcare.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1168576/full#supplementary-material
Anaturk, M., Kaufmann, T., Cole, J. H., Suri, S., Griffanti, L., Zsoldos, E., et al. (2021). Prediction of brain age and cognitive age: Quantifying brain and cognitive maintenance in aging. Hum. Brain Mapp. 42, 1626–1640. doi: 10.1002/hbm.25316
Andrews-Hanna, J. R., Snyder, A. Z., Vincent, J. L., Lustig, C., Head, D., Raichle, M. E., et al. (2007). Disruption of large-scale brain systems in advanced aging. Neuron 56, 924–935. doi: 10.1016/j.neuron.2007.10.038
Anticevic, A., Cole, M. W., Murray, J. D., Corlett, P. R., Wang, X. J., and Krystal, J. H. (2012). The role of default network deactivation in cognition and disease. Trends Cogn. Sci. 16, 584–592. doi: 10.1016/j.tics.2012.10.008
Aschenbrenner, S., Tucha, O., and Lange, K. W. (2000). Regensburger Wortflüssigkeits-Test: RWT. Hogrefe: Verlag für Psychologie.
Babayan, A., Erbey, M., Kumral, D., Reinelt, J. D., Reiter, A. M. F., Robbig, J., et al. (2019). A mind-brain-body dataset of MRI, EEG, cognition, emotion, and peripheral physiology in young and old adults. Sci. Data 6:180308. doi: 10.1038/sdata.2018.308
Barulli, D., and Stern, Y. (2013). Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn. Sci. 17, 502–509. doi: 10.1016/j.tics.2013.08.012
Bastin, C., Yakushev, I., Bahri, M. A., Fellgiebel, A., Eustache, F., Landeau, B., et al. (2012). Cognitive reserve impacts on inter-individual variability in resting-state cerebral metabolism in normal aging. Neuroimage 63, 713–722. doi: 10.1016/j.neuroimage.2012.06.074
Beckmann, C. F., and Smith, S. M. (2004). Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging 23, 137–152. doi: 10.1109/TMI.2003.822821
Bettio, L. E. B., Rajendran, L., and Gil-Mohapel, J. (2017). The effects of aging in the hippocampus and cognitive decline. Neurosci. Biobehav. Rev. 79, 66–86. doi: 10.1016/j.neubiorev.2017.04.030
Cabeza, R. (2002). Hemispheric asymmetry reduction in older adults: The HAROLD model. Psychol. Aging 17, 85–100. doi: 10.1037//0882-7974.17.1.85
Chapko, D., McCormack, R., Black, C., Staff, R., and Murray, A. (2018). Life-course determinants of cognitive reserve (CR) in cognitive aging and dementia - a systematic literature review. Aging Ment. Health 22, 915–926. doi: 10.1080/13607863.2017.1348471
Damoiseaux, J. S. (2017). Effects of aging on functional and structural brain connectivity. Neuroimage 160, 32–40. doi: 10.1016/j.neuroimage.2017.01.077
Davis, S. W., Dennis, N. A., Daselaar, S. M., Fleck, M. S., and Cabeza, R. (2008). Que PASA? The posterior-anterior shift in aging. Cereb. Cortex 18, 1201–1209. doi: 10.1093/cercor/bhm155
Dennis, E. L., and Thompson, P. M. (2014). Functional brain connectivity using fMRI in aging and Alzheimer’s disease. Neuropsychol. Rev. 24, 49–62. doi: 10.1007/s11065-014-9249-6
Douaud, G., Smith, S., Jenkinson, M., Behrens, T., Johansen-Berg, H., Vickers, J., et al. (2007). Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain 130(Pt 9), 2375–2386. doi: 10.1093/brain/awm184
Elkins, J. S., Longstreth, W. T. Jr., Manolio, T. A., Newman, A. B., Bhadelia, R. A., and Johnston, S. C. (2006). Education and the cognitive decline associated with MRI-defined brain infarct. Neurology 67, 435–440. doi: 10.1212/01.wnl.0000228246.89109.98
Farras-Permanyer, L., Mancho-Fora, N., Montala-Flaquer, M., Bartres-Faz, D., Vaque-Alcazar, L., Pero-Cebollero, M., et al. (2019). Age-related changes in resting-state functional connectivity in older adults. Neural Regen. Res. 14, 1544–1555. doi: 10.4103/1673-5374.255976
Ferreira, L. K., and Busatto, G. F. (2013). Resting-state functional connectivity in normal brain aging. Neurosci. Biobehav. Rev. 37, 384–400. doi: 10.1016/j.neubiorev.2013.01.017
Festini, S. B., Zahodne, L., and Reuter-Lorenz, P. A. (2018). “Theoretical perspectives on age differences in brain activation: HAROLD, PASA, CRUNCH—How Do They STAC Up?,” in Oxford Research Encyclopedia of Psychology, ed. B. G. Knight (Oxford: Oxford University Press).
Filippini, N., MacIntosh, B. J., Hough, M. G., Goodwin, G. M., Frisoni, G. B., Smith, S. M., et al. (2009). Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. U. S. A. 106, 7209–7214. doi: 10.1073/pnas.0811879106
Filippini, N., Nickerson, L. D., Beckmann, C. F., Ebmeier, K. P., Frisoni, G. B., Matthews, P. M., et al. (2012). Age-related adaptations of brain function during a memory task are also present at rest. Neuroimage 59, 3821–3828. doi: 10.1016/j.neuroimage.2011.11.063
Fjell, A. M., Walhovd, K. B., Fennema-Notestine, C., McEvoy, L. K., Hagler, D. J., Holland, D., et al. (2009). One-year brain atrophy evident in healthy aging. J. Neurosci. 29, 15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009
Geerligs, L., Renken, R. J., Saliasi, E., Maurits, N. M., and Lorist, M. M. (2015). A brain-wide study of age-related changes in functional connectivity. Cereb. Cortex 25, 1987–1999. doi: 10.1093/cercor/bhu012
Harada, C. N., Natelson Love, M. C., and Triebel, K. L. (2013). Normal cognitive aging. Clin. Geriatr. Med. 29, 737–752. doi: 10.1016/j.cger.2013.07.002
Hausman, H. K., O’Shea, A., Kraft, J. N., Boutzoukas, E. M., Evangelista, N. D., Van Etten, E. J., et al. (2020). The role of resting-state network functional connectivity in cognitive aging. Front. Aging Neurosci. 12:177. doi: 10.3389/fnagi.2020.00177
Hirsiger, S., Koppelmans, V., Merillat, S., Liem, F., Erdeniz, B., Seidler, R. D., et al. (2016). Structural and functional connectivity in healthy aging: Associations for cognition and motor behavior. Hum. Brain Mapp. 37, 855–867. doi: 10.1002/hbm.23067
Hyatt, C. S., Owens, M. M., Crowe, M. L., Carter, N. T., Lynam, D. R., and Miller, J. D. (2020). The quandary of covarying: A brief review and empirical examination of covariate use in structural neuroimaging studies on psychological variables. Neuroimage 205:116225. doi: 10.1016/j.neuroimage.2019.116225
Jockwitz, C., and Caspers, S. (2021). Resting-state networks in the course of aging-differential insights from studies across the lifespan vs. amongst the old. Pflugers Arch. 473, 793–803. doi: 10.1007/s00424-021-02520-7
Jockwitz, C., Caspers, S., Lux, S., Eickhoff, S. B., Jutten, K., Lenzen, S., et al. (2017). Influence of age and cognitive performance on resting-state brain networks of older adults in a population-based cohort. Cortex 89, 28–44. doi: 10.1016/j.cortex.2017.01.008
Lojo-Seoane, C., Facal, D., Guardia-Olmos, J., Pereiro, A. X., and Juncos-Rabadan, O. (2018). Effects of cognitive reserve on cognitive performance in a follow-up study in older adults with subjective cognitive complaints. The role of working memory. Front. Aging Neurosci. 10:189. doi: 10.3389/fnagi.2018.00189
Lovden, M., Fratiglioni, L., Glymour, M. M., Lindenberger, U., and Tucker-Drob, E. M. (2020). Education and cognitive functioning across the life span. Psychol. Sci. Public Interest 21, 6–41. doi: 10.1177/1529100620920576
Marques, J. P., Kober, T., Krueger, G., van der Zwaag, W., Van de Moortele, P. F., and Gruetter, R. (2010). MP2RAGE, a self bias-field corrected sequence for improved segmentation and T1-mapping at high field. Neuroimage 49, 1271–1281. doi: 10.1016/j.neuroimage.2009.10.002
Menardi, A., Pascual-Leone, A., Fried, P. J., and Santarnecchi, E. (2018). The role of cognitive reserve in Alzheimer’s disease and aging: A multi-modal imaging review. J. Alzheimers Dis. 66, 1341–1362. doi: 10.3233/JAD-180549
Meng, X., and D’Arcy, C. (2012). Education and dementia in the context of the cognitive reserve hypothesis: A systematic review with meta-analyses and qualitative analyses. PLoS One 7:e38268. doi: 10.1371/journal.pone.0038268
Mondini, S., Pucci, V., Montemurro, S., and Rumiati, R. I. (2022). Protective factors for subjective cognitive decline individuals: Trajectories and changes in a longitudinal study with Italian elderly. Eur. J. Neurol 29, 691–697. doi: 10.1111/ene.15183
Montemurro, S., Daini, R., Tagliabue, C., Guzzetti, S., Gualco, G., Mondini, S., et al. (2022). Cognitive reserve estimated with a life experience questionnaire outperforms education in predicting performance on MoCA: Italian normative data. Curr. Psychol. Advance online publication. doi: 10.1007/s12144-022-03062-6
Montemurro, S., Mondini, S., and Arcara, G. (2021). Heterogeneity of effects of cognitive reserve on performance in probable Alzheimer’s disease and in subjective cognitive decline. Neuropsychology 35, 876–888. doi: 10.1037/neu0000770
Montemurro, S., Mondini, S., Crovace, C., and Jarema, G. (2019a). Cognitive reserve and its effect in older adults on retrieval of proper names, logo names and common nouns. Front. Commun. 4:14. doi: 10.3389/fcomm.2019.00014
Montemurro, S., Mondini, S., Signorini, M., Marchetto, A., Bambini, V., and Arcara, G. (2019b). Pragmatic language disorder in parkinson’s disease and the potential effect of cognitive reserve. Front. Psychol. 10:1220. doi: 10.3389/fpsyg.2019.01220
Murman, D. L. (2015). The impact of age on cognition. Semin. Hear. 36, 111–121. doi: 10.1055/s-0035-1555115
Niemann, H., Sturm, W., Thoene-Otto, A. I., and Willmes, K. (2008). Der California Verbal Learning Test CVLT. Sydney, NSW: Pearsson Assessment.
Nyberg, L., Lovden, M., Riklund, K., Lindenberger, U., and Backman, L. (2012). Memory aging and brain maintenance. Trends Cogn. Sci. 16, 292–305. doi: 10.1016/j.tics.2012.04.005
Nyberg, L., and Pudas, S. (2019). Successful memory aging. Annu. Rev. Psychol. 70, 219–243. doi: 10.1146/annurev-psych-010418-103052
Onoda, K., Ishihara, M., and Yamaguchi, S. (2012). Decreased functional connectivity by aging is associated with cognitive decline. J. Cogn. Neurosci. 24, 2186–2198. doi: 10.1162/jocn_a_00269
Orellana, C., Ferreira, D., Muehlboeck, J. S., Mecocci, P., Vellas, B., Tsolaki, M., et al. (2016). Measuring global brain atrophy with the brain volume/cerebrospinal fluid index: Normative values, cut-offs and clinical associations. Neurodegener. Dis. 16, 77–86. doi: 10.1159/000442443
Oschwald, J., Guye, S., Liem, F., Rast, P., Willis, S., Rocke, C., et al. (2019). Brain structure and cognitive ability in healthy aging: A review on longitudinal correlated change. Rev. Neurosci. 31, 1–57. doi: 10.1515/revneuro-2018-0096
Park, D. C., and Reuter-Lorenz, P. (2009). The adaptive brain: Aging and neurocognitive scaffolding. Annu. Rev. Psychol. 60, 173–196. doi: 10.1146/annurev.psych.59.103006.093656
Power, J. D., Barnes, K. A., Snyder, A. Z., Schlaggar, B. L., and Petersen, S. E. (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–2154. doi: 10.1016/j.neuroimage.2011.10.018
Raz, N., Lindenberger, U., Rodrigue, K. M., Kennedy, K. M., Head, D., Williamson, A., et al. (2005). Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb. Cortex 15, 1676–1689. doi: 10.1093/cercor/bhi044
Reuter-Lorenz, P. (2002). New visions of the aging mind and brain. Trends Cogn. Sci. 6:394. doi: 10.1016/s1364-6613(02)01957-5
Reuter-Lorenz, P. A., and Cappell, K. A. (2008). Neurocognitive aging and the compensation hypothesis. Curr. Direct. Psychol. Sci. 17, 177–182. doi: 10.1111/j.1467-8721.2008.00570.x
Reuter-Lorenz, P. A., and Park, D. C. (2010). Human neuroscience and the aging mind: A new look at old problems. J. Gerontol. B Psychol. Sci. Soc. Sci. 65, 405–415. doi: 10.1093/geronb/gbq035
Reuter-Lorenz, P. A., and Park, D. C. (2014). How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol. Rev. 24, 355–370. doi: 10.1007/s11065-014-9270-9
Salthouse, T. A. (2011). Neuroanatomical substrates of age-related cognitive decline. Psychol. Bull. 137, 753–784. doi: 10.1037/a0023262
Siman-Tov, T., Bosak, N., Sprecher, E., Paz, R., Eran, A., Aharon-Peretz, J., et al. (2016). Early age-related functional connectivity decline in high-order cognitive networks. Front. Aging Neurosci. 8:330. doi: 10.3389/fnagi.2016.00330
Smith, S. M., Fox, P. T., Miller, K. L., Glahn, D. C., Fox, P. M., Mackay, C. E., et al. (2009). Correspondence of the brain’s functional architecture during activation and rest. Proc. Natl. Acad. Sci. U. S. A. 106, 13040–13045. doi: 10.1073/pnas.0905267106
Smith, S. M., Jenkinson, M., Woolrich, M. W., Beckmann, C. F., Behrens, T. E., Johansen-Berg, H., et al. (2004). Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, (Suppl. 1), S208–S219. doi: 10.1016/j.neuroimage.2004.07.051
Sporns, O. (2013). Structure and function of complex brain networks. Dialogues Clin. Neurosci. 15, 247–262. doi: 10.31887/DCNS.2013.15.3/osporns
Spreng, R. N., and Turner, G. R. (2019). The shifting architecture of cognition and brain function in older adulthood. Perspect. Psychol. Sci. 14, 523–542. doi: 10.1177/1745691619827511
Varangis, E., Habeck, C. G., Razlighi, Q. R., and Stern, Y. (2019). The effect of aging on resting state connectivity of predefined networks in the brain. Front. Aging Neurosci. 11:234. doi: 10.3389/fnagi.2019.00234
Verhaeghen, P. (2003). Aging and vocabulary scores: A meta-analysis. Psychol. Aging 18, 332–339. doi: 10.1037/0882-7974.18.2.332
Vidal-Pineiro, D., Valls-Pedret, C., Fernandez-Cabello, S., Arenaza-Urquijo, E. M., Sala-Llonch, R., Solana, E., et al. (2014). Decreased Default Mode Network connectivity correlates with age-associated structural and cognitive changes. Front. Aging Neurosci. 6:256. doi: 10.3389/fnagi.2014.00256
Woolrich, M. W., Ripley, B. D., Brady, M., and Smith, S. M. (2001). Temporal autocorrelation in univariate linear modeling of FMRI data. Neuroimage 14, 1370–1386. doi: 10.1006/nimg.2001.0931
Keywords: education, resting state, fMRI, default mode network, memory, aging, cognitive semanticization
Citation: Montemurro S, Filippini N, Ferrazzi G, Mantini D, Arcara G and Marino M (2023) Education differentiates cognitive performance and resting state fMRI connectivity in healthy aging. Front. Aging Neurosci. 15:1168576. doi: 10.3389/fnagi.2023.1168576
Received: 17 February 2023; Accepted: 05 May 2023;
Published: 24 May 2023.
Edited by:
Rubem C. A. Guedes, Federal University of Pernambuco, BrazilReviewed by:
Andrea Mendez Colmenares, Colorado State University, United StatesCopyright © 2023 Montemurro, Filippini, Ferrazzi, Mantini, Arcara and Marino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marco Marino, bWFyY28ubWFyaW5vQGt1bGV1dmVuLmJl
†These authors share first authorship
‡These authors share last authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.