
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 06 March 2023
Sec. Alzheimer's Disease and Related Dementias
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1137197
Background: Recent studies on renin-angiotensin system (RAS) inhibitors have reported a reduced risk of Alzheimer’s disease (AD). Nevertheless, the effect of RAS inhibitor type and blood–brain barrier (BBB) permeability on the risk of AD is still unknown.
Objectives: To assess the effects of RAS inhibitors on the risk of AD based on the type and BBB permeability and investigate the cumulative duration-response relationship.
Methods: This was a population-based retrospective cohort study using the Korean Health Insurance Review and Assessment database records from 2008 to 2019. The data of patients diagnosed with ischemic heart disease between January 2009 and June 2009 were identified for inclusion in the analyses. Propensity score matching was used to balance RAS inhibitor users with non-users. The association between the use of RAS inhibitors and incident AD was evaluated using a multivariate Cox proportional hazard regression model. The results are presented in adjusted hazard ratios (aHRs) and 95% confidence intervals (CIs).
Results: Among the 57,420 matched individuals, 7,303 developed AD within the follow-up period. While the use of angiotensin-converting enzyme inhibitors (ACEIs) was not significantly associated with AD risk, the use of angiotensin II receptor blockers (ARBs) showed a significant association with reduced risk of incident AD (aHR = 0.94; 95% CI = 0.90–0.99). Furthermore, the use of BBB-crossing ARBs was associated with a lower risk of AD (aHR = 0.83; 95% CI = 0.78–0.88) with a cumulative duration-response relationship. A higher cumulative dose or duration of BBB-crossing ARBs was associated with a gradual decrease in AD risk (P for trend < 0.001). No significant association between the use of ACEIs and the risk of AD was observed regardless of BBB permeability.
Conclusion: Long-term use of BBB-crossing ARBs significantly reduced the risk of AD development. The finding may provide valuable insight into disease-modifying drug options for preventing AD in patients with cardiovascular diseases.
During the past few decades, Alzheimer’s disease (AD) has emerged as a leading global health concern (Alzheimer’s Disease International, 2019). Despite constant efforts to develop new drugs, the complexity of pathologies has left no promising treatments for AD (Cummings et al., 2014; Bachurin et al., 2017). Owing to the limitations and uncertainty of current treatments, targeting modifiable risk factors for preventing AD incidence and delaying progression has gained importance in recent years. Management of hypertension with antihypertensive drugs has been recommended for the primary prevention of AD (Crous-Bou et al., 2017; Livingston et al., 2020; Hefner et al., 2021). The experimental results, however, remain controversial, and further analyzes are required to reach a general consensus on the use of antihypertensive drugs (Yasar et al., 2013; Chuang et al., 2014; Walker et al., 2020). Among the antihypertensive agent classes, renin-angiotensin system (RAS) inhibitors, particularly angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II type 1 receptor blockers (commonly used as angiotensin II receptor blockers, ARBs), have been reported to have potential benefits on reducing the risk of incident AD (Li et al., 2010; Davies et al., 2011; Barthold et al., 2018).
RAS is a hormonal system responsible for regulating blood pressure (BP), fluid balance, electrolyte homeostasis, and vascular resistance. This system is mediated by angiotensin (Ang) ligands that interact with various receptors, including angiotensin II type 1 receptor (AT1R), angiotensin II type 2 receptor (AT2R), angiotensin IV receptor (AT4R), and Mas receptor (MASR; Gebre et al., 2018). In addition to the peripheral RAS, the receptors in the central nervous system (CNS) are involved in oxidative stress, neuroinflammation, and neuronal apoptosis, causing neurodegeneration (Abiodun and Ola, 2020; Xu et al., 2021). Accordingly, blood–brain barrier (BBB)-crossing RAS inhibitors that block neurodegenerative pathways may provide neuroprotective effects in brain disorders including AD.
A few clinical studies have reported that CNS penetration of RAS inhibitors was associated with reduced cognitive decline and a lower conversion rate from mild cognitive impairment (MCI) to AD (Ohrui et al., 2004; Sink et al., 2009; O’Caoimh et al., 2014; Wharton et al., 2015). However, other studies have shown unclear relationship between BBB permeability and increased neuroprotection by RAS inhibitors (Hebert et al., 2013; Fazal et al., 2017). Considering that most studies focused on ACEIs and the results were inconclusive, further studies are essential in clarifying the effect of BBB-crossing RAS inhibitors, independent from blood pressure lowering effect, on the risk of AD incidence.
This study aimed to assess the effects of RAS inhibitors on the risk of incident AD by using a longitudinal national health insurance database in Korea. In addition to comparing the effect of RAS inhibitors on AD based on class and BBB permeability, we further investigated the cumulative duration-response relationship.
This population-based retrospective cohort study was conducted using Health Insurance Review and Assessment Service (HIRA) data collected for reimbursing healthcare providers between 2008 and 2019. In 2000, South Korea implemented a centralized single-insurer system, achieving healthcare coverage for almost the entire Korean population (Kwon, 2009). Universal coverage of health insurance allowed HIRA to develop a database containing socio-economic information and clinical details, including healthcare services, diagnoses, and prescriptions of 50 million beneficiaries (Kim et al., 2017). Restricted access to encrypted datasets is granted to generate public statistics and clinical research.
This study was approved by the Institutional Review Board (IRB) of Yonsei University (IRB number: 7001988-202,004-HR-846-01E). The requirement for informed consent was waived owing to the anonymity of the data and the retrospective design of the study.
The study population consisted of Korean National Health Insurance beneficiaries aged 60 years or older who were diagnosed with ischemic heart disease (IHD) during the identification period (January 1, 2009, to June 30, 2009). The diagnosis of IHD was defined as the use of diagnostic codes for angina or myocardial infarction (MI) according to the International Classification of Diseases 10th Revision (ICD-10): I20.0-I22.0. Study participants were followed from the index date (July 1, 2009) to the occurrence of the outcome, the date of death, or the end of the claims record (December 31, 2019), whichever occurred first.
Users of RAS inhibitors were defined as patients with the first prescription record of RAS inhibitors during the identification period. Patients who had never been prescribed RAS inhibitors during the study period were classified as non-users. Patients were excluded from the study based on the following criteria: (1) RAS inhibitor use prior to the identification period; (2) first use of RAS inhibitors after the identification period; (3) death record or last claims record prior to follow-up; and (4) diagnosis of dementia (F00-F03, G30-G31), MCI (F06.7, R41), or Parkinson’s disease (G20) before the follow-up. The outcome variable was assessed with a 1 year lag time to control and minimize reverse causality (Rea et al., 2005).
The RAS inhibitors included in this study were ACEIs and ARBs, based on the Anatomic Therapeutic Chemical (ATC) classification system provided by the World Health Organization (WHO) Collaborating Center for Drug Statistics Methodology (WHO Collaborating Centre for Drug Statistics Methodology, 2021). RAS inhibitor users were further categorized into the following four subgroups according to the type of RAS inhibitors and BBB permeability: poor BBB-crossing ACEIs (alacepril, benazepril, cilazapril, enalapril, imidapril, moexipril, and quinapril), BBB-crossing ACEIs (captopril, delapril, fosinopril, lisinopril, perindopril, ramipril, temocapril, trandolapril, and zofenopril), poor BBB-crossing ARBs (eprosartan, irbesartan, losartan, and olmesartan), and BBB-crossing ARBs (azilsartan, candesartan, fimasartan, telmisartan, and valsartan). Categorization was based on available evidence from previous analysis and review research. Drugs were considered poor BBB-crossing if the BBB permeability were inconclusive and/or if they showed relatively low lipophilicity (Oka et al., 1988; Takai et al., 2004; Sink et al., 2009; Wharton et al., 2012; Michel et al., 2013; Yagi et al., 2013; Kim et al., 2015; Alzahrani et al., 2020; Ho et al., 2021; Ouk et al., 2021; Jo et al., 2022).
The dosage, frequency, and prescription days of RAS inhibitors during the identification and follow-up periods were multiplied and used as the cumulative dose. The cumulative doses were then converted into the cumulative defined daily dose (DDD) using the ATC-DDD toolkit provided by the WHO (WHO Collaborating Centre for Drug Statistics Methodology, 2021), as the claims database does not provide information regarding individual body weight. DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults. The cumulative exposure duration and daily equivalent dose of RAS inhibitors were defined as the sum of the prescription days and ratio of cumulative DDD to cumulative exposure duration, respectively.
The primary outcome was the incidence of AD during the follow-up period. To enhance the accuracy of AD outcome measurement, new onset AD was defined as the presence of two or more prescription records of any AD treatment drug with an AD diagnostic code (ICD-10 F00, G30) generated from neurology or psychiatry department. Diagnostic codes obtained without the restriction on departments were included in the sensitivity analysis. The AD treatment drugs included donepezil, galantamine, rivastigmine, and memantine, which have been approved by the Food and Drug Administration for AD. Aducanumab was excluded because it was newly approved in June 2021. The outcome date was defined as the first occurrence of an AD diagnostic code within the follow-up period.
Sociodemographic data, including patients’ age, sex, and type of insurance (health insurance and medical aid), were collected during the identification period. Comorbid diseases and concomitant medications were recorded up to the date of outcome, death, or last claims record. Patients were defined as having comorbid diseases or concurrent medications when diagnostic or drug codes appeared annually during the follow-up period.
In this study, comorbid diseases reported to be potential risk factors of AD included atrial fibrillation (AF), atherosclerosis, bipolar disorder, cerebrovascular disease (hemorrhagic infarction, ischemic cortical infarction, and vasculopathy), depression, diabetes mellitus (DM), dyslipidemia, hypertension, Parkinson’s disease (PD), schizophrenia, sleep disorder, traumatic brain injury (TBI), and vascular dementia (VD; Profenno et al., 2010; Ballard et al., 2011; Xu et al., 2015). A list of ICD-10 codes for comorbid diseases is presented in Supplementary Table S1. Concomitant medications reported as potential protective or risk factors of AD included antidepressants, antiepileptics, antihistamines, antiparkinsonian agents, antipsychotics, antispasmodics, beta-blockers, benzodiazepines, bladder antimuscarinics, dihydropyridine calcium channel blockers (CCB-D), non-dihydropyridine calcium channel blockers (CCB-ND), 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, skeletal muscle relaxants, and zolpidem (Carnahan et al., 2006; Risacher et al., 2016; American Geriatrics Society Beers Criteria® Update Expert Panel, 2019). Diseases or medications with a frequency of less than 30 in the population were excluded from the covariates (Yu et al., 2017). A detailed list of the concomitant medications is provided in Supplementary Table S2.
We adopted propensity score matching (PSM) to control covariate imbalance and minimize treatment assignment bias with a multivariate logistic regression model. RAS inhibitor users were matched to non-users in a 1:1 ratio with no replacement, using the greedy matching method. The caliper width was set to 0.2 of the pooled standard deviation of the logit of the propensity score (Austin, 2011). The matching variables included age, sex, type of insurance, follow-up duration, comorbid diseases, and concurrent medications. PSM was validated by performing balance diagnostics using standardized mean difference (SMD). The absolute value of the SMD less than 0.1 was considered well balanced. Graphical distributions of propensity scores in cohorts before and after matching are presented in Supplementary Figure S1.
Demographics and clinical characteristics of RAS inhibitor users and non-users were compared using descriptive statistics. Categorical variables were presented as frequencies with percentages using Pearson’s chi-squared test. Continuous variables were described as mean ± standard deviation (SD) using the Student’s t-test. The incidence of AD per 1,000 person-years was calculated by dividing the number of incident AD cases by the total follow-up person-years and multiplying the rate by 1,000.
The proportional hazard assumption for the BBB-crossing ARBs was graphically validated using the log minus log plot of the Kaplan–Meier estimation (Supplementary Figure S2). The hazard ratio (HR) and 95% confidence interval (CI) of the AD incidence were estimated using multivariate Cox proportional hazard regression models adjusted for sex, age, insurance type, follow-up period, comorbid diseases, and concurrent medications. The adjusted hazard ratio (aHR) between RAS inhibitor use and incident AD was computed based on RAS type and BBB permeability. Further analyzes of cumulative dose and duration-dependent responses were performed according to the cumulative DDD, cumulative exposure duration, and daily equivalent dose with a trend test using the Cox model. The cumulative hazard of AD is also graphically shown using Kaplan–Meier curves. Subgroup analyzes to identify the effect of RAS inhibitors on AD based on the type and BBB permeability within the sex were conducted.
Sensitivity analyzes were conducted with four different designs for the index date, lag time, outcome definition, and exclusion criteria. First, the index date was shifted to July 1, 2010, and July 1, 2011. Second, the lag time of the outcome was extended to 3 and 5 years. Incidents during the lag time were excluded. Third, the AD incidence was re-defined with the following definitions: (1) AD diagnostic code (no restriction on departments) with at least two or more AD treatment prescriptions; and (2) AD diagnostic code with a neurology or psychiatry department subject code. Finally, the exclusion criteria were expanded with the following conditions for the entire study period 2008–2019: (1) presence of PD diagnostic code, and (2) concurrent use of ACEI and ARB. The study population was re-matched, and the outcome, comorbidity, and exposure to medications were re-assessed based on the new four designs of sensitivity analysis. Statistical analyzes were performed using the SAS software (version 9.4; SAS Institute, Cary, NC, United States). Pooled estimation with a value of p < 0.05 was considered significant.
The cohort of patients diagnosed with IHD between January 2009 and June 2009 consisted of 537,116 participants. After the eligibility assessment and PSM, 57,420 patients with 490,384 person-years were identified for the analysis (Figure 1). The demographic and clinical characteristics of the study population are summarized in Table 1. The mean ± SD age was 69.6 ± 6.8 years and 69.9 ± 6.8 for the matched RAS inhibitor users and non-users, respectively; the proportion of the male participants was 45.0 and 46.2% in RAS inhibitor users and non-users, respectively. The mean ± SD follow-up duration was 8.6 ± 2.9 years for RAS inhibitors users and 8.5 ± 2.9 years for non-users. Among the comorbid diseases, hypertension and dyslipidemia had a high prevalence of 61.0 and 48.0%, respectively.
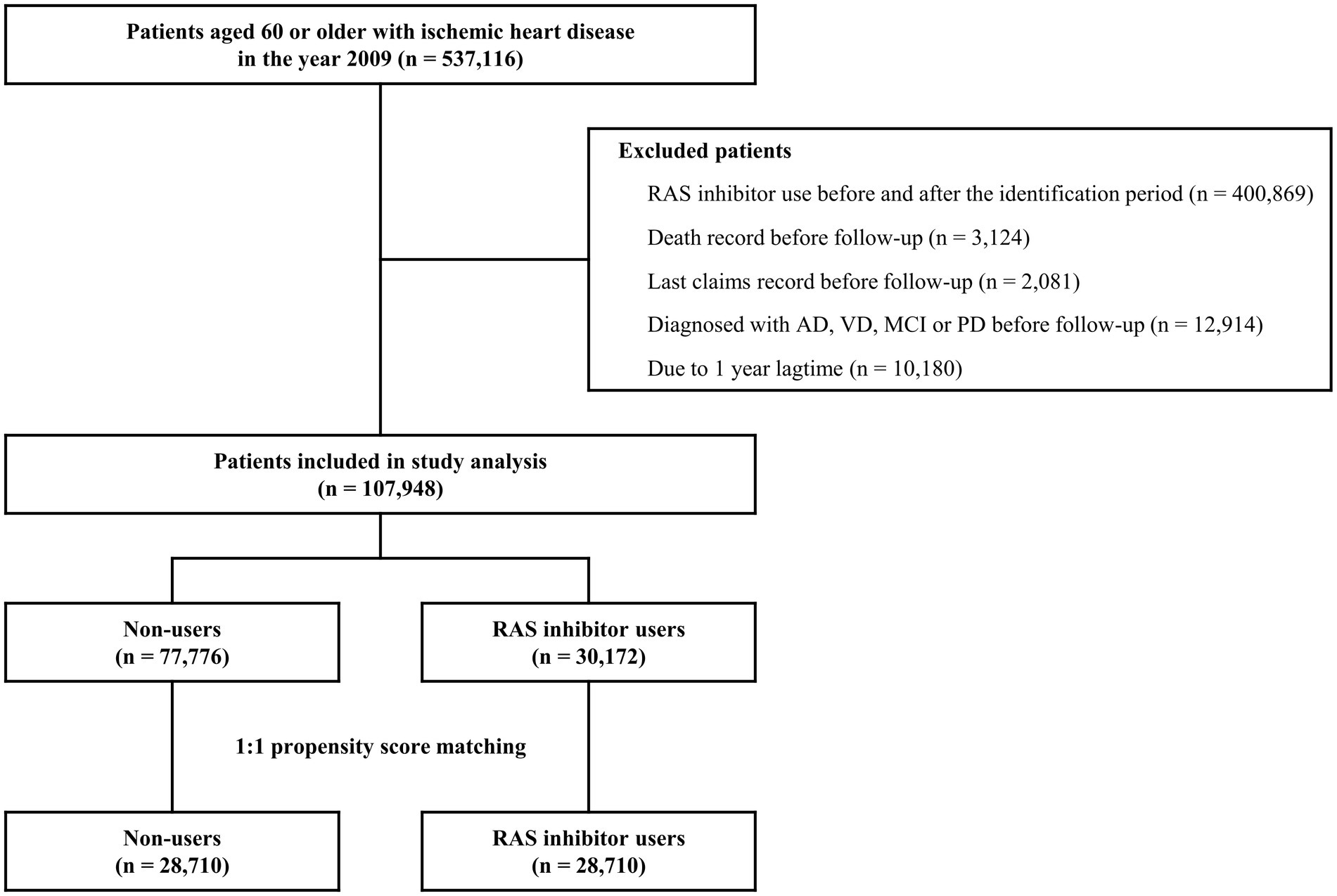
Figure 1. Flow chart of study population inclusion. AD, Alzheimer’s disease; MCI, mild cognitive impairment; PD, Parkinson’s disease; RAS, renin-angiotensin system; VD, vascular dementia.
Before matching, significant differences in baseline characteristics were observed for the type of insurance, hypertension, DM, AF, and CCB-ND. Post-PSM results showed that the absolute SMD values for all variables were remodeled below 0.1. This indicated that the differences between the covariates were statistically well balanced.
A total of 7,303 new AD cases were observed, with an overall incidence of 14.9 per 1,000 person-years. The median time of censoring was 10.5 years (interquartile range, 8.3–10.5 years) in patients with AD.
The use of RAS inhibitors was not significantly associated with AD risk. Females showed an increased risk of AD. The population aged 65 years or older had an increased risk of AD compared to those aged 60–64 years (aHR = 2.61; 95% CI = 2.41–2.83), and the risk was even greater in those aged 80 years or older (aHR = 5.71; 95% CI = 5.16–6.31). Comorbid diseases including atherosclerosis, AF, bipolar disease, cerebrovascular disease, depression, DM, dyslipidemia, hypertension, PD, schizophrenia, sleep disorder, TBI, and VD were all significant risk factors for AD incidence. Prescriptions of antidepressants, antiepileptics, antihistamines, antiparkinsonian agents, antipsychotics, antispasmodics, benzodiazepines, beta-blockers, benzodiazepines, bladder antimuscarinics, dihydropyridine CCB-D, CCB-ND, skeletal muscle relaxants, and zolpidem were also associated with an increased risk of AD. HMG-CoA reductase inhibitors were observed to be significant protective factors against the incidence of AD (Table 2).
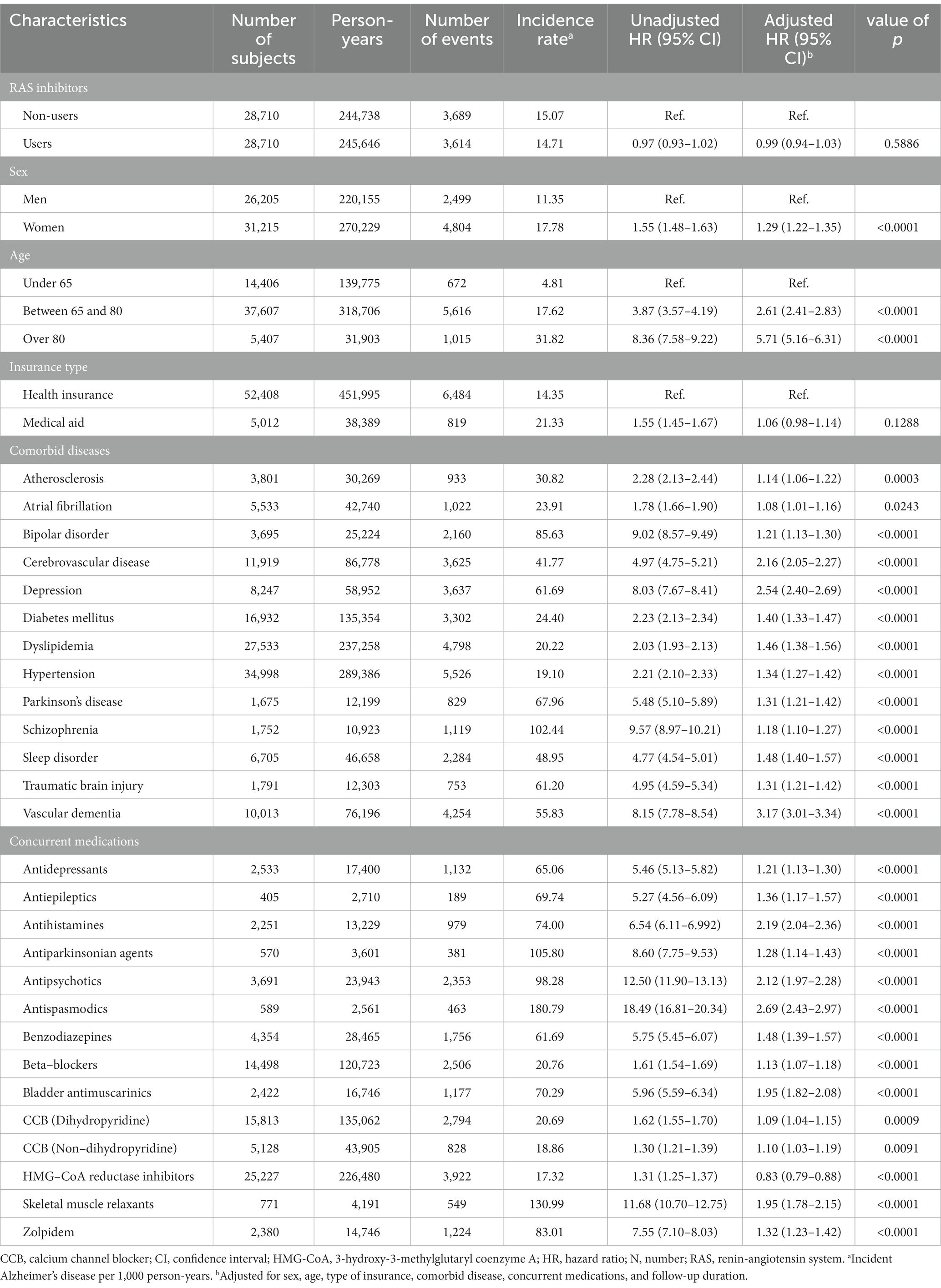
Table 2. Cox regression analysis of the association between incident Alzheimer’s disease and renin-angiotensin system inhibitors and confounding factors (N = 57,420).
A significant reduction in the risk of AD was observed with ARB use (aHR = 0.94; 95% CI = 0.90–0.99). ACEIs did not show significant association with the AD incidence (aHR = 1.03; 95% CI = 0.97–1.10). Regarding the risk of AD based on the type of RAS inhibitor and BBB permeability, only the use of BBB-crossing ARBs demonstrated a significant protective effect (aHR = 0.83; 95% CI = 0.78–0.88; Table 3). BBB-crossing ARBs were subdivided for additional analyzes based on cumulative DDD, cumulative exposure duration, and daily equivalent dose. Responses depending on cumulative DDD, and duration were observed at 1-year intervals (Supplementary Table S3). Longer exposure to BBB-crossing ARBs was significantly associated with a gradual reduction in AD risk in the trend analysis (p < 0.001). The Kaplan–Meier curves of the cumulative hazard according to the cumulative DDD of BBB-crossing ARBs with a 2-year interval are shown in Figure 2. Both ≥4 years of cumulative DDD and ≥ 4 years of cumulative exposure duration showed significantly reduced AD incidence, regardless of daily equivalent dose (Figure 3). The subgroup analysis showed that ARBs were superior to ACEIs in AD risk both in men and women, and there was no difference in the protective effect of BBB-crossing ARBs between men and women (Supplementary Table S4).
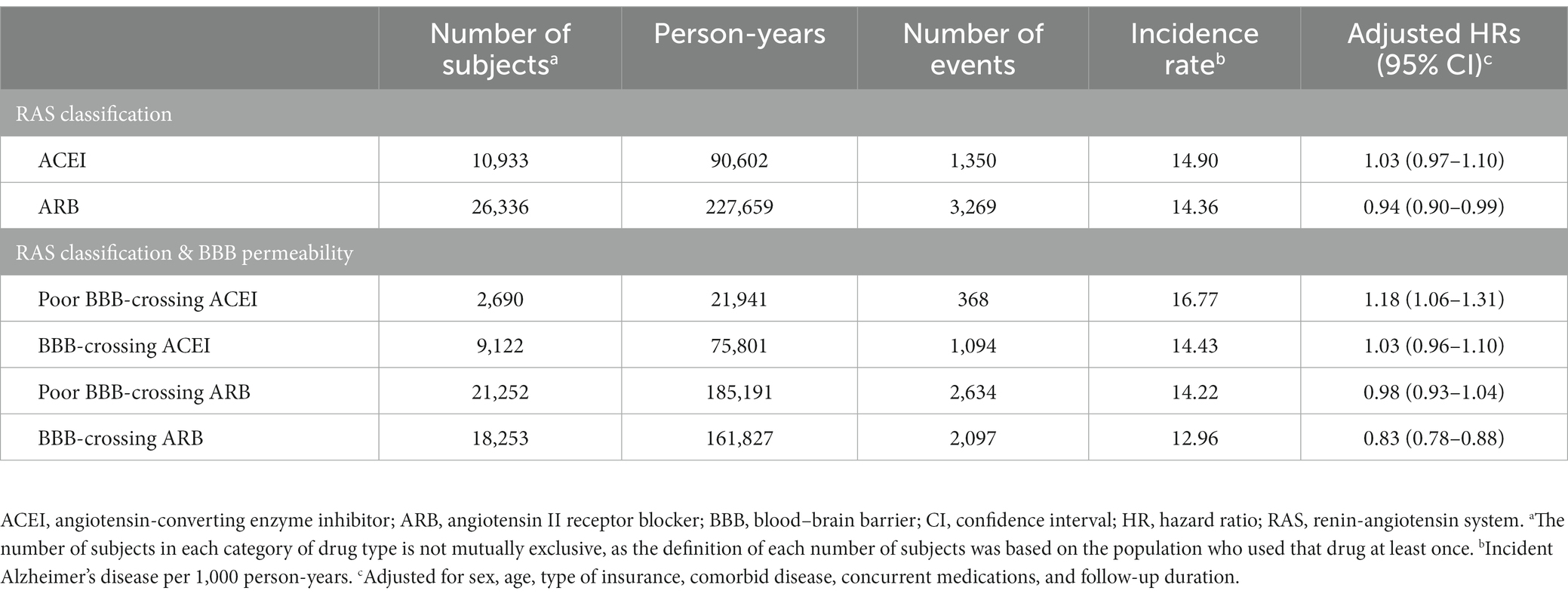
Table 3. Risk of Alzheimer’s disease by renin-angiotensin system inhibitor type and blood–brain barrier permeability (N = 57,420).

Figure 2. Kaplan–Meier curves for the cumulative hazard of Alzheimer’s disease with a 2 defined daily dose (DDD)-year interval by blood–brain barrier (BBB)-crossing angiotensin II receptor blockers (ARBs).
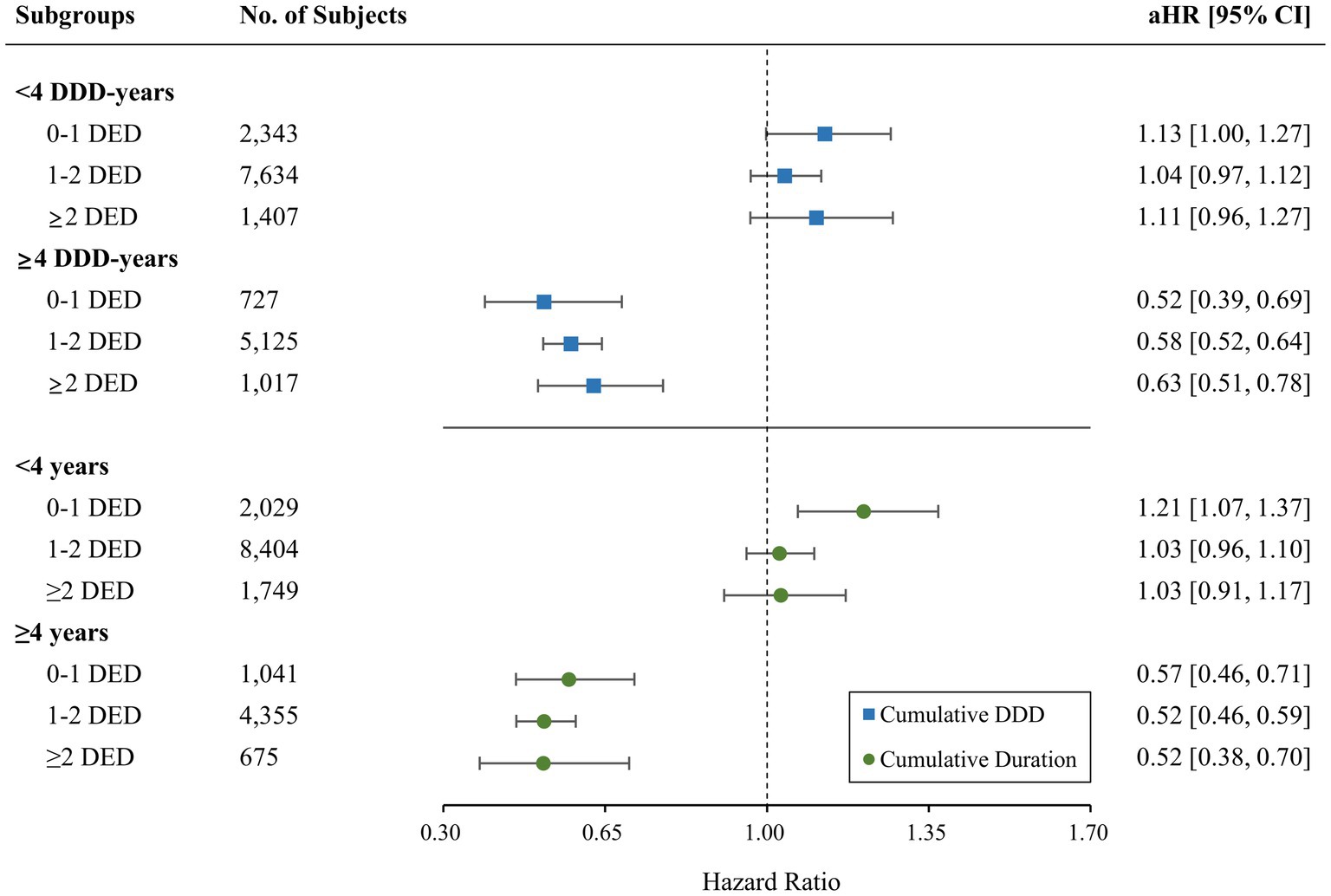
Figure 3. Comparison of the risk of incident Alzheimer’s disease for blood–brain barrier (BBB)-crossing angiotensin II receptor blockers (ARBs) according to the daily equivalent dose (DED) by cumulative daily defined dose (DDD) and duration. aHR, adjusted hazard ratio; CI, confidence interval.
Sensitivity analyzes for the AD risk of BBB-crossing ARB use for ≥4 DDD years are presented in Table 4. In all sensitivity analyzes with index date shift, lag time extension, outcome definition change, and exclusion criteria expansion, the aHRs of incident AD in users of BBB-crossing ARBs ≥4 DDD-years remained significantly lower than those in RAS inhibitor non-users. Sensitivity analyzes for AD risk of BBB-crossing ARB use <4 DDD-years are shown in Supplementary Table S5.
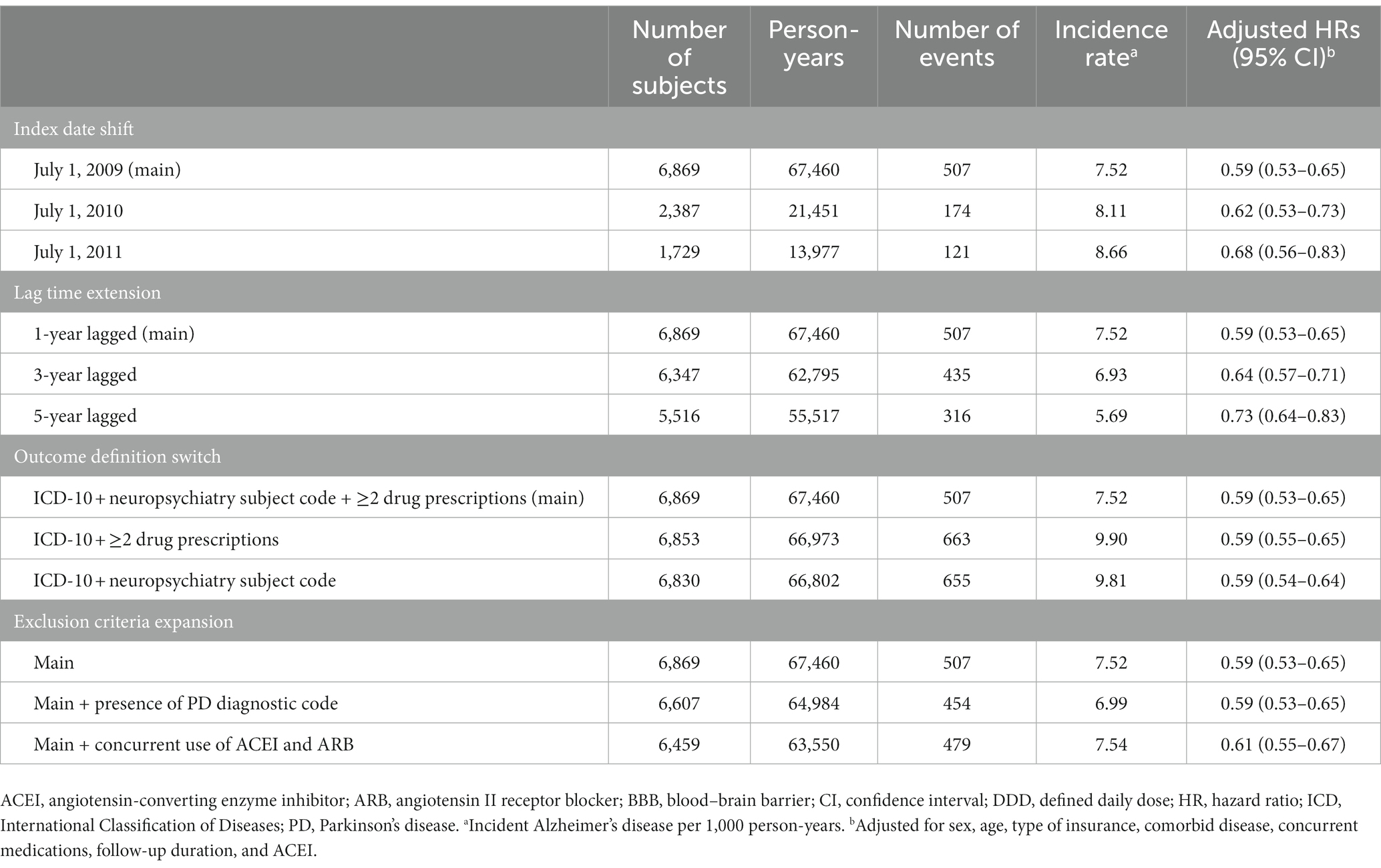
Table 4. Sensitivity analyzes for the risk of Alzheimer’s disease in ≥4 DDD-years of BBB-crossing ARB users.
As the protective effect of antihypertensive agents on cognitive decline beyond their blood pressure-lowering effects has emerged, the potential effect of reducing the risk of AD via the renin-angiotensin system has been demonstrated in animal and human studies (Li et al., 2010; Davies et al., 2011; Barthold et al., 2018; Abiodun and Ola, 2020). However, the neuroprotective effects of RAS inhibitors reported in previous studies have been conflicting (Ohrui et al., 2004; Sink et al., 2009; Hebert et al., 2013; Hsu et al., 2013; O’Caoimh et al., 2014; Qiu et al., 2014; Wharton et al., 2015). In this nationwide population-based cohort study, patients with IHD who used BBB-crossing ARBs had a lower risk of incident AD than those who did not use RAS inhibitors. Notably, our study showed a significant reduction in the risk of incident AD in patients who used BBB-permeable ARBs at higher cumulative doses. While previous studies have focused on comparing the effects of ARBs and ACEIs (Marcum et al., 2022) or BBB permeability within RAS inhibitors (Hebert et al., 2013; Qiu et al., 2014; Ho et al., 2021), to the best of our knowledge, this is the first study to simultaneously assess risk reduction considering both BBB permeability and cumulative doses. Our results were robust owing to a valid study design with a long-term follow-up based on nationwide study samples with appropriate comparisons and sensitivity analyzes.
In this study, we revealed that the use of ARBs, but not ACEIs, was associated with a reduced risk of AD. This result is consistent with some clinical studies that have shown the advantageous effect of ARBs over ACEIs in reducing AD risk (Li et al., 2010; Davies et al., 2011; Barthold et al., 2018; Marcum et al., 2022). Additionally, a number of animal studies have supported this difference in protective effect by suggesting potential underlying mechanisms. ACEIs target the angiotensin-converting enzyme (ACE), which is responsible for converting Ang I to Ang II, thus attenuating AT1R and AT2R activation, whereas ARBs selectively block the Ang II/AT1R axis (Gebre et al., 2018). AT1R activation induces oxidative stress, neuroinflammation, and apoptosis, whereas AT2R counteracts AT1R-mediated neurodegeneration by various mechanisms (Lanz et al., 2010; Faraco et al., 2016; Abiodun and Ola, 2020). Hence, the blockade of AT1R by ARBs may induce indirect activation of the Ang II/AT2R axis to provide neuroprotection (Mogi and Horiuchi, 2013). Enhancement of cognitive function by direct stimulation of AT2R was also demonstrated in the animal study (Jing et al., 2012). Moreover, the benefits of ARBs can be attributed to the conversion of Ang II into Ang IV and Ang (1–7), which are selective for AT4R and MASR, respectively. AT4R has been suggested to have a positive effect on cerebral blood flow, memory, and neuroprotection (Näveri et al., 1994; Royea and Hamel, 2020), whereas enhancement of the Ang (1–7)/MASR axis has been reported to have a potential anti-inflammatory effect and facilitate hippocampal long-term potentiation (Hellner et al., 2005; Wright and Harding, 2019). Several studies have also found that the expression of ACE and ACE2 is related to a decreased amyloid-beta (Aβ) load (Hemming and Selkoe, 2005; Zou et al., 2007; Kehoe et al., 2016). However, further translational investigation is essential to confirm the association between the neuroprotective effects of ARBs and Aβ pathology (Loera-Valencia et al., 2021).
Our analyzes indicated that the use of BBB-crossing ARBs was associated with a reduced risk of AD. The finding was especially significant as CI did not overlap with those of the other three types of RAS inhibitors. Additional benefit of using BBB-crossing RAS inhibitors has been demonstrated in previous studies assessing the effect on cognitive function and MCI to AD conversion (Wharton et al., 2015; Ouk et al., 2021). Another longitudinal study, which investigated the effect of ACEIs depending on central exposure, reported no significant association between BBB-crossing ACEIs and AD and a risk of incident AD with poor BBB-crossing ACEIs (Sink et al., 2009). However, a previous meta-analysis (Ho et al., 2021) assessing the effect of BBB-crossing RAS inhibitors on seven cognitive domains reported that poor BBB-crossing RAS inhibitors demonstrated a better effect in the attention domain compared to that of BBB-crossing RAS inhibitors. As previous studies on cognitive decline and incident AD by BBB permeability of RAS inhibitors largely focused on ACEIs (Ohrui et al., 2004; Sink et al., 2009; Hebert et al., 2013; O’Caoimh et al., 2014; Ouk et al., 2021), our results on BBB-crossing ARBs are noteworthy, but the benefit of using BBB-crossing ARBs as potential drugs for preventing AD should be carefully interpreted. In addition to BBB-crossing effects, some in vitro/vivo and animal studies have reported that some BBB-crossing ARBs, such as telmisartan, showed partial peroxisome proliferator-activated receptors (PPAR) gamma activation effects that have beneficial effects on cognitive functions (Mogi et al., 2008; Pang et al., 2012; Garg et al., 2021). However, the PPAR-gamma activation effect of ARBs is still controversial, with limited evidence for the attenuation of cognitive functions in only some ARBs (Benson et al., 2004; Erbe et al., 2006; Kajiya et al., 2011). Therefore, further comprehensive studies that have considered the PPAR-gamma binding affinities on ARBs, as well as the BBB-crossing characteristics, are needed.
Remarkably, the significantly reduced risk of AD by BBB-crossing ARBs was robust in patients with a larger cumulative dose or longer duration, regardless of the daily equivalent dose. These results implied that the cumulative exposure duration was a more crucial factor in the neuroprotective effect of ARBs than the daily exposure dose. Risk-reducing effect of ARBs on AD with larger cumulative dose and longer exposure were also demonstrated in a previous longitudinal study, supporting our findings (Chiu et al., 2014). Considering that antihypertensive drugs are generally used for an extended period and our results showed a cumulative effect of BBB-crossing ARBs on AD, they could be suggested as promising targets for drug repurposing. The current treatment for AD shows modest effects only on symptoms (Atri, 2019; Cummings et al., 2020), and the efficacy of the newly approved drug, aducanumab, is also controversial (Whitehouse et al., 2022). Moreover, midlife hypertension has been associated with an increased risk of AD, and blood pressure control is a modifiable risk factor for cognitive decline (Lennon et al., 2019). Taken together, BBB-crossing ARBs might be a promising disease-modifying drug option for reducing the risk of AD in patients with cardiovascular diseases, such as hypertension.
In the subgroup analysis, no difference in the protective effect of BBB-crossing ARBs was identified between men and women. A study by Barthold et al. reported that ARBs were superior to ACEIs in risk of AD incidence for white men and women, but no association was observed for the black and Hispanic populations (Barthold et al., 2018). Estrogen lowers AT1R expression, prevents the production and action of angiotensin II, and decreases NADPH-oxidase activity and expression of neuroinflammatory markers (De Silva and Faraci, 2012; O’Hagan et al., 2012; Rodriguez-Perez et al., 2015). Aging men with aromatization of androgens to estrogens have a higher estrogen level than that of aging women with dramatic ovarian loss of 17β-estradiol (Rosario et al., 2011). A study on the pathophysiology of sex differences in the protective effect of ARB owing to race is needed. Moreover, further studies in other Asian countries are needed to confirm the sex difference in the protective effect of ARBs in Asians.
This study has several limitations. First, this study used a secondary claims database; therefore, we could not verify detailed clinical information, including symptoms, body weight, blood pressure, smoking, alcohol intake, education level, and genetic factors, such as APOE ε4. In addition, the limitation related to the accuracy of incident AD needs to be considered because the outcome variable was identified based on ICD-10 diagnostic codes. Given that the diagnosis of AD is based on the patient’s symptoms (Atri, 2019) and the protective effects of RAS inhibitors have been reported to vary according to cognitive symptoms (Ho et al., 2021), our results should be carefully interpreted. However, we attempted to use the medication prescription claims along with the diagnostic codes for enhanced accuracy of outcome definitions. Moreover, we adopted various outcome definitions in sensitivity analyzes to confirm the robustness of the study results. Second, the possibility of a selection bias cannot be neglected, as our study population was selected based on a very short identification period of 6 months. Moreover, we could not consider active comparators and make a direct comparison of the effect of drugs with different mechanisms, such as beta-blockers, CCBs, or thiazides, because antihypertensive agents are usually used in combination. To minimize this selection bias, we balanced RAS inhibitor users and non-users by PS matching and adjusted for various confounders using rigorous definitions. Moreover, our sensitivity analysis by shifting the index date provided comparable results. Third, it is difficult to generalize the study results to the entire population, as our study population included patients with IHD, who have a high cardiovascular profile. RAS inhibitors, possessing strong vascular effects, has been used for treating various cardiovascular diseases, and conflicting results have been reported on the neuroprotective effect of ARBs, depending on the study population. Further research on the effects of RAS inhibitors on AD in patients with various cardiovascular diseases is required. Finally, the duration or cumulative doses of concomitant medications could not be considered in our study. Instead of considering the variability of confounder status by using time-varying Cox regression, this study used the precise definition of comorbidities and concurrent medications that appeared at least once every year during the follow-up period.
To the best of our knowledge, this is the first longitudinal study to demonstrate the effect of BBB-crossing ARBs on the incidence of AD with cumulative dose and duration subgroups using a population-based cohort. In this study, we highlighted the neuroprotective effect of ARBs, particularly BBB-crossing ARBs, on AD. Additionally, we present a novel finding of the protective effects against AD conferred by long-term use of BBB-crossing ARBs. In addition to existing evidence, these results are expected to provide valuable insights for AD-targeted drug development.
The datasets presented in this article are not readily available because the primary data analyzed in this study are handled and stored by the Health Insurance Review and Assessment Service. Requests to access the datasets should be directed to Health Insurance Review and Assessment Service, https://www.hira.or.kr.
The studies involving human participants were reviewed and approved by Institutional Review Board (IRB) of Yonsei University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
HWL and SK contributed for study design, data analysis, data interpretation, and writing of the manuscript. YMY contributed for study conceptualization, data interpretation, critical revision of the manuscript, and supervision of the study. YJ and YK contributed to data analysis and manuscript revision. BSY contributed to clinical interpretation of the data and critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT) of the Korea government (No. 2020R1G1A110120513).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1137197/full#supplementary-material
Abiodun, O. A., and Ola, M. S. (2020). Role of brain renin angiotensin system in neurodegeneration: an update. Saudi J. Biol. Sci. 27, 905–912. doi: 10.1016/j.sjbs.2020.01.026
Alzahrani, Y. M., Alim, A. S. M. A., Kamel, F. O., Ramadan, W. S., and Alzahrani, Y. A. (2020). Possible combined effect of perindopril and Azilsartan in an experimental model of dementia in rats. Saudi Pharm. J. 28, 574–581. doi: 10.1016/j.jsps.2020.03.009
Alzheimer’s Disease International. (2019). World Alzheimer Report 2019: Attitudes to Dementia. London: Alzheimer’s Disease International.
American Geriatrics Society Beers Criteria® Update Expert Panel (2019). American Geriatrics Society 2019 updated Ags beers criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 67, 674–694. doi: 10.1111/jgs.15767
Atri, A. (2019). The Alzheimer's disease clinical Spectrum: diagnosis and management. Med. Clin. North Am. 103, 263–293. doi: 10.1016/j.mcna.2018.10.009
Austin, P. C. (2011). Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm. Stat. 10, 150–161. doi: 10.1002/pst.433
Bachurin, S. O., Bovina, E. V., and Ustyugov, A. A. (2017). Drugs in clinical trials for Alzheimer's disease: the major trends. Med. Res. Rev. 37, 1186–1225. doi: 10.1002/med.21434
Ballard, C., Gauthier, S., Corbett, A., Brayne, C., Aarsland, D., and Jones, E. (2011). Alzheimer's disease. Lancet 377, 1019–1031. doi: 10.1016/S0140-6736(10)61349-9
Barthold, D., Joyce, G., Wharton, W., Kehoe, P., and Zissimopoulos, J. (2018). The association of multiple anti-hypertensive medication classes with Alzheimer's disease incidence across sex, race, and ethnicity. PLoS One 13:e0206705. doi: 10.1371/journal.pone.0206705
Benson, S. C., Pershadsingh, H. A., Ho, C. I., Chittiboyina, A., Desai, P., Pravenec, M., et al. (2004). Identification of Telmisartan as a unique angiotensin ii receptor antagonist with selective Ppargamma-modulating activity. Hypertension 43, 993–1002. doi: 10.1161/01.HYP.0000123072.34629.57
Carnahan, R. M., Lund, B. C., Perry, P. J., Pollock, B. G., and Culp, K. R. (2006). The anticholinergic drug scale as a measure of drug-related anticholinergic burden: associations with serum anticholinergic activity. J. Clin. Pharmacol. 46, 1481–1486. doi: 10.1177/0091270006292126
Chiu, W. C., Ho, W. C., Lin, M. H., Lee, H. H., Yeh, Y. C., Wang, J. D., et al. (2014). Angiotension receptor blockers reduce the risk of dementia. J. Hypertens. 32, 938–947. doi: 10.1097/HJH.0000000000000086
Chuang, Y. F., Breitner, J. C. S., Chiu, Y. L., Khachaturian, A., Hayden, K., Corcoran, C., et al. (2014). Use of diuretics is associated with reduced risk of Alzheimer's disease: the Cache County study. Neurobiol. Aging 35, 2429–2435. doi: 10.1016/j.neurobiolaging.2014.05.002
Crous-Bou, M., Minguillõn, C., Gramunt, N., and Molinuevo, J. L. (2017). Alzheimer’s disease prevention: from risk factors to early intervention. Alzheimers Res. Ther. 9:71. doi: 10.1186/s13195-017-0297-z
Cummings, J., Lee, G., Ritter, A., Sabbagh, M., and Zhong, K. (2020). Alzheimer’s disease drug development pipeline: 2020. Alzheimers Dement. 6:E12050. doi: 10.1002/trc2.12050
Cummings, J. L., Morstorf, T., and Zhong, K. (2014). Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimers Res. Ther. 6:37. doi: 10.1186/alzrt269
Davies, N. M., Kehoe, P. G., Ben-Shlomo, Y., and Martin, R. M. (2011). Associations of anti-hypertensive treatments with Alzheimer's disease, vascular dementia, and other dementias. J. Alzheimers Dis. 26, 699–708. doi: 10.3233/JAD-2011-110347
De Silva, T. M., and Faraci, F. M. (2012). Effects of angiotensin ii on the cerebral circulation: role of oxidative stress. Front. Physiol. 3:484. doi: 10.3389/fphys.2012.00484
Erbe, D. V., Gartrell, K., Zhang, Y. L., Suri, V., Kirincich, S. J., Will, S., et al. (2006). Molecular activation of Ppargamma by angiotensin ii type 1-receptor antagonists. Vasc. Pharmacol. 45, 154–162. doi: 10.1016/j.vph.2006.05.002
Faraco, G., Sugiyama, Y., Lane, D., Garcia-Bonilla, L., Chang, H., Santisteban, M. M., et al. (2016). Perivascular macrophages mediate the neurovascular and cognitive dysfunction associated with hypertension. J. Clin. Invest. 126, 4674–4689. doi: 10.1172/JCI86950
Fazal, K., Perera, G., Khondoker, M., Howard, R., and Stewart, R. (2017). Associations of centrally acting ace inhibitors with cognitive decline and survival in Alzheimer's disease. BJPsych Open 3, 158–164. doi: 10.1192/bjpo.bp.116.004184
Garg, S., Khan, S. I., Malhotra, R. K., Sharma, M. K., Kumar, M., Kaur, P., et al. (2021). Cardioprotective effects of Azilsartan compared with that of Telmisartan on an in vivo model of myocardial ischemia-reperfusion injury. J. Biochem. Mol. Toxicol. 35:E22785. doi: 10.1002/jbt.22785
Gebre, A. K., Altaye, B. M., Atey, T. M., Tuem, K. B., and Berhe, D. F. (2018). Targeting Renin-Angiotensin System Against Alzheimer's Disease. Front. Pharmacol. 9:440. doi: 10.3389/fphar.2018.00440
Hebert, P. L., Mcbean, A. M., O'connor, H., Frank, B., Good, C., and Maciejewski, M. L. (2013). Time until incident dementia among Medicare beneficiaries using centrally acting or non-centrally acting ace inhibitors. Pharmacoepidemiol. Drug Saf. 22, 641–648. doi: 10.1002/pds.3449
Hefner, M., Baliga, V., Amphay, K., Ramos, D., and Hegde, V. (2021). Cardiometabolic modification of amyloid Beta in Alzheimer's disease pathology. Front. Aging Neurosci. 13:721858. doi: 10.3389/fnagi.2021.721858
Hellner, K., Walther, T., Schubert, M., and Albrecht, D. (2005). Angiotensin-(1-7) enhances Ltp in the hippocampus through the G-protein-coupled receptor mas. Mol. Cell. Neurosci. 29, 427–435. doi: 10.1016/j.mcn.2005.03.012
Hemming, M. L., and Selkoe, D. J. (2005). Amyloid Beta-protein is degraded by cellular angiotensin-converting enzyme (ace) and elevated by an ace inhibitor. J. Biol. Chem. 280, 37644–37650. doi: 10.1074/jbc.M508460200
Ho, J. K., Moriarty, F., Manly, J. J., Larson, E. B., Evans, D. A., Rajan, K. B., et al. (2021). Blood-brain barrier crossing renin-angiotensin drugs and cognition in the elderly: a meta-analysis. Hypertension 78, 629–643. doi: 10.1161/HYPERTENSIONAHA.121.17049
Hsu, C. Y., Huang, C. C., Chan, W. L., Huang, P. H., Chiang, C. H., Chen, T. J., et al. (2013). Angiotensin-receptor blockers and risk of Alzheimer’s disease in hypertension population. Circ. J. 77, 405–410. doi: 10.1253/circj.CJ-12-0658
Jing, F., Mogi, M., Sakata, A., Iwanami, J., Tsukuda, K., Ohshima, K., et al. (2012). Direct stimulation of angiotensin ii type 2 receptor enhances spatial memory. J. Cereb. Blood Flow Metab. 32, 248–255. doi: 10.1038/jcbfm.2011.133
Jo, Y., Kim, S., Ye, B. S., Lee, E., and Yu, Y. M. (2022). Protective effect of renin-angiotensin system inhibitors on Parkinson’s disease: a Nationwide cohort study. Front. Pharmacol. 13:837890. doi: 10.3389/fphar.2022.837890
Kajiya, T., Ho, C., Wang, J., Vilardi, R., and Kurtz, T. W. (2011). Molecular and cellular effects of Azilsartan: a new generation angiotensin ii receptor blocker. J. Hypertens. 29, 2476–2483. doi: 10.1097/HJH.0b013e32834c46fd
Kehoe, P. G., Wong, S., Al Mulhim, N., Palmer, L. E., and Miners, J. S. (2016). Angiotensin-converting enzyme 2 is reduced in Alzheimer's disease in association with increasing amyloid-Β and tau pathology. Alzheimers Res. Ther. 8:50. doi: 10.1186/s13195-016-0217-7
Kim, C. K., Yang, X. L., Kim, Y. J., Choi, I. Y., Jeong, H. G., Park, H. K., et al. (2015). Effect of long-term treatment with Fimasartan on transient focal ischemia in rat brain. Biomed. Res. Int. 2015:295925. doi: 10.1155/2015/295925
Kim, J.-A., Yoon, S., Kim, L.-Y., and Kim, D.-S. (2017). Towards actualizing the value potential of Korea health insurance review and assessment (Hira) data as a resource for Health Research: strengths, limitations, applications, and strategies for optimal use of Hira data. J. Korean Med. Sci. 32, 718–728. doi: 10.3346/jkms.2017.32.5.718
Kwon, S. (2009). Thirty years of National Health Insurance in South Korea: lessons for achieving universal health care coverage. Health Policy Plan. 24, 63–71. doi: 10.1093/heapol/czn037
Lanz, T. V., Ding, Z., Ho, P. P., Luo, J., Agrawal, A. N., Srinagesh, H., et al. (2010). Angiotensin ii sustains brain inflammation in mice via Tgf-Beta. J. Clin. Invest. 120, 2782–2794. doi: 10.1172/JCI41709
Lennon, M. J., Makkar, S. R., Crawford, J. D., and Sachdev, P. S. (2019). Midlife hypertension and Alzheimer's disease: a systematic review and meta-analysis. J. Alzheimers Dis. 71, 307–316. doi: 10.3233/JAD-190474
Li, N. C., Lee, A., Whitmer, R. A., Kivipelto, M., Lawler, E., Kazis, L. E., et al. (2010). Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ 340:b5465. doi: 10.1136/bmj.b5465
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
Loera-Valencia, R., Eroli, F., Garcia-Ptacek, S., and Maioli, S. (2021). Brain renin-angiotensin system as novel and potential therapeutic target for Alzheimer's disease. Int. J. Mol. Sci. 22:10139. doi: 10.3390/ijms221810139
Marcum, Z. A., Cohen, J. B., Zhang, C., Derington, C. G., Greene, T. H., Ghazi, L., et al. (2022). Association of Antihypertensives that stimulate vs inhibit types 2 and 4 angiotensin ii receptors with cognitive impairment. JAMA Netw. Open 5:E2145319. doi: 10.1001/jamanetworkopen.2021.45319
Michel, M. C., Foster, C., Brunner, H. R., and Liu, L. (2013). A systematic comparison of the properties of clinically used angiotensin ii type 1 receptor antagonists. Pharmacol. Rev. 65, 809–848. doi: 10.1124/pr.112.007278
Mogi, M., and Horiuchi, M. (2013). Effect of angiotensin ii type 2 receptor on stroke, cognitive impairment and neurodegenerative diseases. Geriatr Gerontol Int 13, 13–18. doi: 10.1111/j.1447-0594.2012.00900.x
Mogi, M., Li, J. M., Tsukuda, K., Iwanami, J., Min, L. J., Sakata, A., et al. (2008). Telmisartan prevented cognitive decline partly due to Ppar-gamma activation. Biochem. Biophys. Res. Commun. 375, 446–449. doi: 10.1016/j.bbrc.2008.08.032
Näveri, L., Strømberg, C., and Saavedra, J. M. (1994). Angiotensin iv reverses the acute cerebral blood flow reduction after experimental subarachnoid hemorrhage in the rat. J. Cereb. Blood Flow Metab. 14, 1096–1099. doi: 10.1038/jcbfm.1994.143
O’Caoimh, R., Healy, L., Gao, Y., Svendrovski, A., Kerins, D. M., Eustace, J., et al. (2014). Effects of centrally acting angiotensin converting enzyme inhibitors on functional decline in patients with Alzheimer's disease. J. Alzheimers Dis. 40, 595–603. doi: 10.3233/JAD-131694
O’Hagan, T. S., Wharton, W., and Kehoe, P. G. (2012). Interactions between Oestrogen and the renin angiotensin system–potential mechanisms for gender differences in Alzheimer's disease. Am. J. Neurodegener. Dis. 1, 266–279 3560469.
Ohrui, T., Matsui, T., Yamaya, M., Arai, H., Ebihara, S., Maruyama, M., et al. (2004). Angiotensin-converting enzyme inhibitors and incidence of Alzheimer's disease in Japan. J. Am. Geriatr. Soc. 52, 649–650. doi: 10.1111/j.1532-5415.2004.52178_7.x
Oka, Y., Nishikawa, K., Kito, G., Mayahara, H., Tanayama, S., Muto, H., et al. (1988). Delapril. Cardiovasc. Drug Rev. 6, 192–205. doi: 10.1111/j.1527-3466.1988.tb00376.x
Ouk, M., Wu, C. Y., Rabin, J. S., Jackson, A., Edwards, J. D., Ramirez, J., et al. (2021). The use of angiotensin-converting enzyme inhibitors vs. angiotensin receptor blockers and cognitive decline in Alzheimer’s disease: the importance of blood-brain barrier penetration and APOE ε4 carrier status. Alzheimers Res. Ther. 13, 13:43:43. doi: 10.1186/s13195-021-00778-8
Pang, T., Benicky, J., Wang, J., Orecna, M., Sanchez-Lemus, E., and Saavedra, J. M. (2012). Telmisartan ameliorates lipopolysaccharide-induced innate immune response through peroxisome proliferator-activated receptor-Γ activation in human monocytes. J. Hypertens. 30, 87–96. doi: 10.1097/HJH.0b013e32834dde5f
Profenno, L. A., Porsteinsson, A. P., and Faraone, S. V. (2010). Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol. Psychiatry 67, 505–512. doi: 10.1016/j.biopsych.2009.02.013
Qiu, W. W. Q., Lai, A., Mon, T., Mwamburi, M., Taylor, W., Rosenzweig, J., et al. (2014). Angiotensin converting enzyme inhibitors and Alzheimer disease in the presence of the apolipoprotein E4 allele. Am. J. Geriatr. Psychiatr. 22, 177–185. doi: 10.1016/j.jagp.2012.08.017
Rea, T. D., Breitner, J. C., Psaty, B. M., Fitzpatrick, A. L., Lopez, O. L., Newman, A. B., et al. (2005). Statin use and the risk of incident dementia: the cardiovascular health study. Arch. Neurol. 62, 1047–1051. doi: 10.1001/archneur.62.7.1047
Risacher, S. L., Mcdonald, B. C., Tallman, E. F., West, J. D., Farlow, M. R., Unverzagt, F. W., et al. (2016). Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively Normal older adults. JAMA Neurol. 73, 721–732. doi: 10.1001/jamaneurol.2016.0580
Rodriguez-Perez, A. I., Borrajo, A., Valenzuela, R., Lanciego, J. L., and Labandeira-Garcia, J. L. (2015). Critical period for dopaminergic neuroprotection by hormonal replacement in menopausal rats. Neurobiol. Aging 36, 1194–1208. doi: 10.1016/j.neurobiolaging.2014.10.028
Rosario, E. R., Chang, L., Head, E. H., Stanczyk, F. Z., and Pike, C. J. (2011). Brain levels of sex steroid hormones in men and women during Normal aging and in Alzheimer's disease. Neurobiol. Aging 32, 604–613. doi: 10.1016/j.neurobiolaging.2009.04.008
Royea, J., and Hamel, E. (2020). Brain angiotensin ii and angiotensin iv receptors as potential Alzheimer's disease therapeutic targets. Geroscience 42, 1237–1256. doi: 10.1007/s11357-020-00231-y
Sink, K. M., Leng, X., Williamson, J., Kritchevsky, S. B., Yaffe, K., Kuller, L., et al. (2009). Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the cardiovascular health study. Arch. Intern. Med. 169, 1195–1202. doi: 10.1001/archinternmed.2009.175
Takai, S., Jin, D., Sakaguchi, M., and Miyazaki, M. (2004). Significant target organs for hypertension and cardiac hypertrophy by angiotensin-converting enzyme inhibitors. Hypertens. Res. 27, 213–219. doi: 10.1291/hypres.27.213
Walker, V. M., Davies, N. M., Martin, R. M., and Kehoe, P. G. (2020). Comparison of antihypertensive drug classes for dementia prevention. Epidemiology 31, 852–859. doi: 10.1097/EDE.0000000000001245
Wharton, W., Goldstein, F. C., Zhao, L., Steenland, K., Levey, A. I., and Hajjar, I. (2015). Modulation of renin-angiotensin system may slow conversion from mild cognitive impairment to Alzheimer's disease. J. Am. Geriatr. Soc. 63, 1749–1756. doi: 10.1111/jgs.13627
Wharton, W., Stein, J. H., Korcarz, C., Sachs, J., Olson, S. R., Zetterberg, H., et al. (2012). The effects of Ramipril in individuals at risk for Alzheimer's disease: results of a pilot clinical trial. J. Alzheimers Dis. 32, 147–156. doi: 10.3233/JAD-2012-120763
Whitehouse, P., Gandy, S., Saini, V., George, D. R., Larson, E. B., Alexander, G. C., et al. (2022). Making the case for accelerated withdrawal of Aducanumab. J. Alzheimers Dis. 87, 1003–1007. doi: 10.3233/JAD-220262
WHO Collaborating Centre for Drug Statistics Methodology. (2021). Atc/Ddd index 2022. World Health Organization. Available at: Https://Www.Whocc.No/Atc_Ddd_Index/ (Accessed February 10, 2022).
Wright, J. W., and Harding, J. W. (2019). Contributions by the brain renin-angiotensin system to memory, cognition, and Alzheimer's disease. J. Alzheimers Dis. 67, 469–480. doi: 10.3233/JAD-181035
Xu, X., Du, L., Jiang, J., Yang, M., Wang, Z., Wang, Y., et al. (2021). Microglial Trem2 mitigates inflammatory responses and neuronal apoptosis in angiotensin ii-induced hypertension in middle-aged mice. Front. Aging Neurosci. 13:716917. doi: 10.3389/fnagi.2021.716917
Xu, W., Tan, L., Wang, H. F., Jiang, T., Tan, M. S., Tan, L., et al. (2015). Meta-analysis of modifiable risk factors for Alzheimer's disease. J. Neurol. Neurosurg. Psychiatry 86, 1299–1306. doi: 10.1136/jnnp-2015-310548
Yagi, S., Akaike, M., Ise, T., Ueda, Y., Iwase, T., and Sata, M. (2013). Renin-angiotensin-aldosterone system has a pivotal role in cognitive impairment. Hypertens. Res. 36, 753–758. doi: 10.1038/hr.2013.51
Yasar, S., Xia, J., Yao, W., Furberg, C. D., Xue, Q. L., Mercado, C. I., et al. (2013). Antihypertensive drugs decrease risk of Alzheimer disease: ginkgo evaluation of memory study. Neurology 81, 896–903. doi: 10.1212/WNL.0b013e3182a35228
Yu, Y. M., Lee, J. Y., and Lee, E. (2017). Access to anti-osteoporosis medication after hip fracture in Korean elderly patients. Maturitas 103, 54–59. doi: 10.1016/j.maturitas.2017.06.021
Keywords: Alzheimer’s disease, renin-angiotensin system, angiotensin II receptor blocker, blood–brain barrier, neuroprotective agents
Citation: Lee HW, Kim S, Jo Y, Kim Y, Ye BS and Yu YM (2023) Neuroprotective effect of angiotensin II receptor blockers on the risk of incident Alzheimer’s disease: A nationwide population-based cohort study. Front. Aging Neurosci. 15:1137197. doi: 10.3389/fnagi.2023.1137197
Received: 04 January 2023; Accepted: 13 February 2023;
Published: 06 March 2023.
Edited by:
Stephen D. Ginsberg, Nathan Kline Institute for Psychiatric Research, United StatesReviewed by:
Caglar Cosarderelioglu, Johns Hopkins Medicine, Johns Hopkins University, United StatesCopyright © 2023 Lee, Kim, Jo, Kim, Ye and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Mi Yu, yunmiyu@yonsei.ac.kr
†These authors have contributed equally to this work and share first authorship
‡ORCID: Yun Mi Yu https://orcid.org/0000-0003-0544-1967
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.