
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Aging Neurosci. , 26 January 2023
Sec. Neuroinflammation and Neuropathy
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1126183
This article is part of the Research Topic Neuroinflammation in the Interaction Between Aging and Chronic Brain Injury View all 5 articles
Background: Diabetes mellitus, or hyperglycemia, is an independent risk factor for cognitive impairment. Here we systematically analyzed whether glycemic control could improve cognitive impairment in patients with diabetes mellitus (DM), hyperglycemia, or insulin resistance.
Methods: Three databases (PubMed, EMBASE, and Cochrane Library) and ClinicalTrials.gov were searched for randomized controlled trials analyzing the relationship between glycemic control and cognitive function assessments, published from database inception to June 2022. Patients in experimental groups were treated with antidiabetic drugs, while control groups were treated with a placebo or alternative antidiabetic drugs. Data analysis was conducted using RevMan 5.3 and StataSE-64, and standardized mean difference (SMD) and 95% confidence intervals (CIs) were calculated.
Results: Thirteen studies comprising 19,314 participants were included. Analysis revealed that glycemic control significantly attenuated the degree of decline in cognitive function assessment scores (SMD = 0.15; 95% CI 0.05, 0.26; p < 0.00001), and funnel plots confirmed no publication bias. Seven studies used Mini-Mental State Examination as the primary cognitive function assessment, showing that glycemic control significantly delayed the degree of decline in cognitive function assessment scores (SMD = 0.18; 95% CI 0.03, 0.34; p = 0.02). Similar results were seen in two studies using the Montreal Cognitive Assessment scale, but without significant difference (SMD = 0.05; 95% CI-0.10, 0.21; p = 0.51). One study using Auditory Word Learning Test (AVLT) showed that glycemic control significantly delayed the decline in cognitive function assessment scores (SMD = 0.52; 95% CI 0.11,0.93; p = 0.01), and another used Wechsler Memory Scale Revised, showing similar results (SMD = 1.45; 95% CI 0.86, 2.04; p < 0.00001). Likewise, a study that used Modified Mini-Mental State scale showed that glycemic control significantly delayed the decline in cognitive function assessment scores (SMD = -0.10; 95% CI-0.16, −0.03; p = 0.005). Lastly, one study used AVLT subtests to show that glycemic control delayed the decline in cognitive function assessment scores, although not statistically significant (SMD = 0.09; 95% CI-0.53, 0.71; p = 0.78).
Conclusion: Glycemic control through antidiabetic treatment correlates with the improvement of cognitive impairment in patients with DM, hyperglycemia or insulin resistance. However, further studies are needed to validate the results of this study.
Systematic Review Registration: PROSPERO, identifier CRD42022342260.
Hyperglycemic conditions, particularly diabetes mellitus (DM), are strongly associated with the incidence of cognitive impairment, including both mild cognitive impairment and dementia (Biessels and Despa, 2018; van Sloten et al., 2020). Chronic peripheral hyperinsulinemia and insulin resistance are the main features of DM, but hyperglycemia is increasingly thought to be the cause of cognitive impairment in elderly patients with DM (Umegaki et al., 2017; Tahmi et al., 2021). Several studies have shown that patients with Alzheimer’s disease (AD) have desensitized insulin signals in their brains, even in the absence of DM (Jash et al., 2020). Extensive abnormalities in insulin and insulin-like growth factor type I and II (IGF-I and IGF-II) signaling pathways in the brains of patients with AD suggest that AD may partially share characteristics with a neuroendocrine disease similar to DM (Xu et al., 2015). Chronic peripheral hyperinsulinemia can cause brain insulin resistance and defective insulin receptor activity by impairing the blood–brain barrier and insulin transport to the brain (He et al., 2020; Milstein and Ferris, 2021). Therefore, impaired brain insulin signaling may be one of the mechanisms underlying neurodegenerative disease that causes progressive impairment of learning, memory, and cognitive functions.
A previous randomized controlled trial has reported that patients with diabetes have worse cognitive performance than patients without diabetes; however, whether the incidence of dementia or cognitive impairment in patients with DM could benefit from glycemic control remains controversial (Moore et al., 2013; Biessels et al., 2021). The aim of this meta-analysis was therefore to investigate whether glycemic control in patients with DM or hyperglycemia can delay the degree of decline according to cognitive function assessment scores.
This systematic review was registered on the PROSPERO International prospective register of systematic reviews (CRD42022342260).
We searched four medical databases, PubMed, EMBASE, Cochrane Library, and the clinical registry ClinicalTrials.gov, for studies published from database inception to June 2022. Terms used as subject headings in the search strategy included cognitive impairment, dementia, blood glucose, hyperglycemia, antidiabetic drugs, insulin resistance, and randomized controlled trials. Please see the supplemental information for the complete search strategy. There were no restrictions on the language or country of publication.
Randomized controlled trials assessing changes in cognitive function in patients with DM, hyperglycemia, or insulin resistance treated with controls or antidiabetic drugs, and who underwent follow-up for at least 3 months with reported cognition scores were screened and finally enrolled. The experimental group was treated with antidiabetic drugs while the control group was treated with placebo or another active antidiabetic drug (Table 1).
Studies with incomplete information or where the full text was not available were excluded. For duplicate studies, the most recent publications were selected. We further excluded reviews, retrospective studies, case reports, animal studies, and unrelated studies.
The primary outcome indicators for the cognitive function assessment were the Mini-Mental State Examination (MMSE) scale, Montreal Cognitive Assessment (MoCA) scale, Modified Mini-Mental State (3MS) scale, Wechsler Memory Scale Revised (WMS-R), and Auditory Word Learning Test (AVLT). In addition, the digit symbol substitution test (DSST) was selected as a secondary outcome indicator.
The Endnote X9 software was used for literature management. Two researchers (Yufeng Lin and Kaiyuan Wang) searched and downloaded literature according to the search strategy, and deleted any duplicates. Any disagreements were resolved by discussion with a third researcher (Zhongying Gong). Two researchers (Yufeng Lin and Chunchao Ma) independently screened the articles while referencing the inclusion criteria, and a third researcher (Kaiyuan Wang) helped resolve any disagreement. Through reading of the study titles, abstracts, and full texts, the final selected literature was identified and the reasons for exclusion of other studies were recorded. Details such as the first author, study type, year of publication, sample size, sex, age, intervention, follow-up time, and cognitive function assessment method used were recorded for each study according to a pre-designed standardized information extraction form.
The methodological quality of the included literature was evaluated by two researchers (Yufeng Lin and Zhongying Gong) using the Revised Cochrane Risk of Bias tool (RoB 2.0; Lester-Coll et al., 2006). Specific evaluation components included randomization process, deviation from intended interventions, missing outcome data, measurement of outcomes, and selective reporting of outcomes. By reading the full text, the risk of bias for each domain was judged as high, low, or unclear. If all domains were of low risk, the overall risk of bias was considered low, if at least one domain was of high risk, the overall risk of bias was considered to be high, and if any domain showed unclear risk and there were no high risks present in any domain, the overall risk of bias was determined to be unclear. A third researcher (Chunchao Ma) convened discussions to resolve any disagreement that arose between the two reviewers.
The RevMan v5.3 software provided by the Cochrane Collaboration was used to perform statistical analysis of the extracted data. For continuous data, the analysis applied the mean difference (MD) or standardized mean difference (SMD), calculated with 95% confidence intervals (CIs). Cochrane’s X2 and I2 tests were used to assess heterogeneity. Considering that the different methods of cognitive function assessments used might impact the study results, we conducted subgroup analyzes based on the scoring methods and applied SMD and random effects models for the analysis. To ensure study integrity, we further used the STATA-64 software for sensitivity analysis, and funnel plot analysis was used to detect publication bias.
A total of 850 studies were retrieved using the search strategy, and 361 duplicate studies were excluded. After screening the retrieved titles and abstracts, 329 irrelevant studies, 89 review studies, 15 clinical study protocols, and 23 congress abstracts were excluded. The remaining 33 full-text studies were retained and evaluated for eligibility. Ten studies that did not meet the inclusion criteria, three studies with incomplete data, and seven studies that did not meet the outcome criteria were excluded. Finally, 13 relevant studies were included (Hanyu et al., 2009; Plastino et al., 2010; Sato et al., 2011; Cukierman-Yaffe et al., 2014, 2020; Guo et al., 2014; Isik et al., 2017; Köbe et al., 2017; Furie et al., 2018; Lin et al., 2018; Biessels et al., 2021; Cummings et al., 2021; Li et al., 2021). The specific literature screening process is shown in Figure 1.
Information on the authors, time of publication, country, trial design, sample size, age, intervention modality, cognitive function assessment and scores at enrollment and follow-up are summarized and presented in Table 1.
In terms of risk of bias of individual study, seven studies were classified as low risk (Sato et al., 2011; Cukierman-Yaffe et al., 2014; Isik et al., 2017; Furie et al., 2018; Biessels et al., 2021; Cummings et al., 2021; Li et al., 2021), two studies were moderate risk (Hanyu et al., 2009; Köbe et al., 2017), and four studies were high risk (Plastino et al., 2010; Guo et al., 2014; Lin et al., 2018; Cukierman-Yaffe et al., 2020). Two of the studies (Guo et al., 2014; Lin et al., 2018) did not provide the complete method of allocation concealment (Figure 2A). In terms of the overall risk of bias, there was a low risk of other biases; unclear risks for random sequence generation, incomplete outcome data, and selective reporting; and high risks for allocation concealment, binding of participants and personnel, and binding of outcome assessment (Figure 2B).
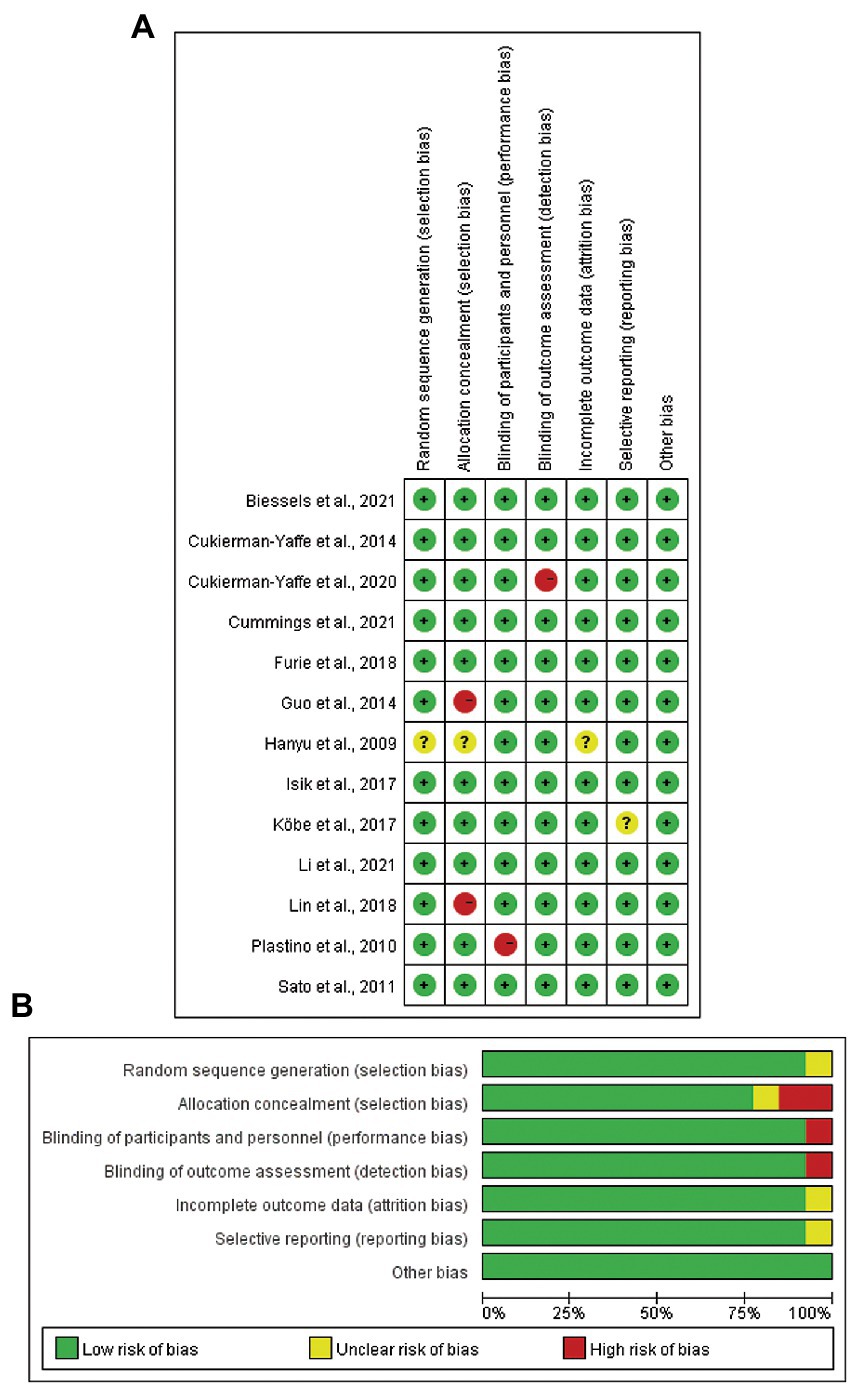
Figure 2. Risk of bias assessment of included studies in the meta-analysis. (A) Risk of bias for individual study. (B) Overall risk of bias for the 13 included studies.
Thirteen studies comprising 19,314 participants were included. Analysis revealed that glycemic control significantly attenuated the degree of decline in cognitive function assessment scores (SMD = 0.15; 95% CI 0.05, 0.26; p < 0.00001; Figure 3).
Seven studies that included a total of 6,985 participants (Hanyu et al., 2009; Plastino et al., 2010; Sato et al., 2011; Cukierman-Yaffe et al., 2014; Isik et al., 2017; Biessels et al., 2021; Li et al., 2021) used MMSE to assess cognitive function. Meta-analysis of these studies was performed using a random effects model, which showed that glycemic control had a significant effect on cognitive function improvement (SMD = 0.18; 95% CI 0.03, 0.34; p = 0.02); however, within-group heterogeneity was significant (p = 0.0002, I2 = 77%; Figure 3). The ReVman software was subsequently used to further examine each study, and the Stata software was used to perform sensitivity analysis (Figure 4). The results indicated that the source of heterogeneity originated from mainly two studies (Cukierman-Yaffe et al., 2014; Biessels et al., 2021). After removing the two, meta-analysis was performed using a fixed response model with the five remaining studies comprising 430 participants, showing that glycemic control remained significant in improving cognitive function (SMD = 0.41; 95% CI 0.15, 0.67; p = 0.002). Within-group heterogeneity was within the normal limits (p = 0.19, I2 = 35%; Figure 5).
Two studies (Cukierman-Yaffe et al., 2020; Cummings et al., 2021) with 8,740 participants used MoCA to assess cognitive function. A meta-analysis of these studies was performed using a random effects model, which showed that glycemic control improved cognitive function, but the results were not significant (SMD = 0.05; 95% CI-0.10, 0.21; p = 0.51). The within-group heterogeneity was within the normal range (p = 0.22, I2 = 33%; Figure 5).
One study (Lin et al., 2018) which included 94 participants used AVLT for cognitive function assessment. Meta-analysis using a random effects model showed a significant improvement in cognitive function by controlling blood glucose (SMD = 0.52; 95% CI 0.11, 0.93; p = 0.01; Figure 6). Meta-analysis using a random effects model of another study (Köbe et al., 2017) with 40 participants used AVLT subtests for cognitive function assessment and showed an improvement in cognitive function by controlling blood glucose, but without statistical significance (SMD = 0.09; 95% CI-0.53, 0.71, p = 0.78; Figure 5).
One study (Guo et al., 2014) included 57 participants and used WMS-R for cognitive function assessment. Meta-analysis using a random effects model showed a significant improvement in cognitive function by controlling blood glucose (SMD = 1.45; 95% CI 0.86, 2.04; p < 0.00001; Figure 5).
One study (Furie et al., 2018) with 3,398 participants used 3MS for cognitive function assessment. Meta-analysis of this study using a random-effects model showed a significant effect of controlling blood glucose on improvement in cognitive function (SMD = -0.10; 95% CI-0.16, −0.03; p = 0.005; Figure 5).
Two studies (Cukierman-Yaffe et al., 2014, 2020) including 11,966 participants used DSST for secondary assessment of cognitive function. Meta-analysis performed on these studies using a fixed effects model showed that glycemic control had a significant effect on increasing DSST scores (SMD = -0.80; 95% CI 0.77, 0.83, p < 0.00001; Figure 6).
For the seven studies in which MMSE was the primary assessment method of cognitive function, we performed publication bias analysis and subsequently created funnel plots. As shown in Figure 7, the left and right scatter points within the plot were largely symmetrical, and Egger’s test further confirmed no publication bias (p = 0.076; see Figure 8).
The incidence of hyperglycemia or DM and cognitive impairment both increase progressively with age. In a 10-year population-based cohort study of individuals aged 65 years and older, a modest degree of hyperglycemia was proven to independently predispose to faster cognitive decline, and glucose and hemoglobin A1c (HbA1c) were proposed as more sensitive markers of glycemia (Ganguli et al., 2020). Other studies have shown that the risk of developing cognitive decline or dementia in patients with type 2 DM is 1.25 to 2 times higher than that in patients without diabetes (Gudala et al., 2013; Xue et al., 2019). Morris et al. (2016) used the hyperinsulinemic-euglycemic clamp technique to detect systemic insulin resistance in patients with mild cognitive impairment (MCI) and AD as compared to normal controls, observing increased insulin resistance in 15 patients with cognitive impairment. Even in children with newly diagnosed type 1 DM, a single DKA episode was found to be associated with cognitive decline, particularly in subtle memory function (Ghetti et al., 2020). Although severe hypoglycemia may also lead to poor global cognition in older adults (Lacy et al., 2020), mounting clinical evidence has shown that cognitive impairment is exacerbated by hyperglycemia or DM in large populations.
The pathophysiological process of cognitive decline in patients with hyperglycemia or DM is complex and may involve common features with the pathogenesis of AD and vascular dementia (Gerstein et al., 2020), although the molecular interactions between the two diseases are not fully understood. The physio-pathological mechanisms that characterize AD, including molecular, biochemical, and signaling abnormalities, are known to be similar to those of patients with diabetes. In addition, reduced insulin signaling in the brain due to insulin dysfunction may be the primary mechanism shared by both diseases (Duarte et al., 2012). The concept of “insulin-resistant brain state (IRBS)” has thus been proposed to better describe the nature of AD (de la Monte and Wands, 2008). Insulin resistance is associated with reduced cortical insulin receptor activation, impaired clearance of amyloid-β (Aβ) oligomers, increased cerebral abnormal neurotic plaque burden, and the cerebral microvascular dysfunction which is associated with memory loss or decline of cognition (Umegaki et al., 2017; van Sloten et al., 2020). Glucotoxicity from the accumulation of advanced glycation end products (AGEs) and their precursor methylglyoxal (MGO) could induce dopaminergic dysfunction, thereby playing a role in DM-associated cognitive impairment (Pignalosa et al., 2021).
In the present study, we reviewed and evaluated the potential protective effect of blood glucose control therapy on cognitive function in patients with DM, hyperglycemia, or insulin resistance. Thirteen trials with 19,134 participants were enrolled for preliminary outcome analysis. The MMSE, MoCA, AVLT, WMS-R, 3MS, and AVLT were used as primary cognitive function assessment methods. Overall analysis showed that glycemic control significantly attenuated cognitive decline. Several recent reviews and meta-analyzes have also investigated the relationship between antidiabetic therapy and cognitive status, with inconsistent primary findings (Areosa Sastre et al., 2017; Cao et al., 2018; McMillan et al., 2018). These inconsistencies are mainly due to differences in the focus and detailed design of the studies. For example, Areosa Sastre’s review only enrolled patients diagnosed with type 2 DM, while Cao’s study enrolled patients diagnosed with Alzheimer’s disease, but was not restricted to those with DM. In McMillan’s review, only the incidence of dementia was analyzed, and the change in cognitive score which may compromise the potential cerebral protection of blood glucose control therapy was not evaluated (McMillan et al., 2018).
The present review and analysis have several limitations which should be noted. First, the enrolled studies applied different cognitive function assessment methods, resulting in heterogeneity between groups. Second, the studies had various follow-up times, and longer follow-up periods would have allowed for more accurate detection of changes in cognitive function. Third, the optimal glycemic range for the prevention of cognitive decline could not be determined in this study, and thus further exploration through high-quality clinical trials is required.
In conclusion, the current study provides evidence that glycemic control could improve the cognitive impairment through cognitive function assessment scores in patients with DM, hyperglycemia or insulin resistance.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
YL, KW, and ZW designed and carried out the study. ZG and CM participated in analyzing and interpretation of the results. YL and KW wrote the manuscript with other authors’ inputs. ZW revised the manuscript. All authors contributed to the article and approved the submitted version.
This research was partially funded by grants from Tianjin “project + team” key cultivation program (No. XC202034) and Tianjin Key Medical Discipline (Specialty) Construction Project (No.TJYXZDXK-009A).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor XG declared a shared parent affiliation with the author KW at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1126183/full#supplementary-material
Areosa Sastre, A., Vernooij, R. W., González-Colaço Harmand, M., and Martínez, G. (2017). Effect of the treatment of type 2 diabetes mellitus on the development of cognitive impairment and dementia. Cochrane Database Syst. Rev. 2017:Cd003804. doi: 10.1002/14651858.CD003804.pub2
Biessels, G. J., and Despa, F. (2018). Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat. Rev. Endocrinol. 14, 591–604. doi: 10.1038/s41574-018-0048-7
Biessels, G. J., Verhagen, C., Janssen, J., van den Berg, E., Wallenstein, G., Zinman, B., et al. (2021). Effects of linagliptin vs glimepiride on cognitive performance in type 2 diabetes: results of the randomised double-blind, active-controlled Carolina-Cognition study. Diabetologia 64, 1235–1245. doi: 10.1007/s00125-021-05393-8
Cao, B., Rosenblat, J. D., Brietzke, E., Park, C., Lee, Y., Musial, N., et al. (2018). Comparative efficacy and acceptability of antidiabetic agents for Alzheimer's disease and mild cognitive impairment: a systematic review and network meta-analysis. Diabetes Obes. Metab. 20, 2467–2471. doi: 10.1111/dom.13373
Cukierman-Yaffe, T., Bosch, J., Diaz, R., Dyal, L., Hancu, N., Hildebrandt, P., et al. (2014). Effects of basal insulin glargine and omega-3 fatty acid on cognitive decline and probable cognitive impairment in people with dysglycaemia: a substudy of the ORIGIN trial. Lancet Diabetes Endocrinol. 2, 562–572. doi: 10.1016/s2213-8587(14)70062-2
Cukierman-Yaffe, T., Gerstein, H. C., Colhoun, H. M., Diaz, R., García-Pérez, L. E., Lakshmanan, M., et al. (2020). Effect of dulaglutide on cognitive impairment in type 2 diabetes: an exploratory analysis of the REWIND trial. Lancet Neurol. 19, 582–590. doi: 10.1016/s1474-4422(20)30173-3
Cummings, J., Schwartz, G. G., Nicholls, S. J., Khan, A., Halliday, C., Toth, P. P., et al. (2021). Cognitive effects of the BET protein inhibitor Apabetalone: a Prespecified Montreal cognitive assessment analysis nested in the BETonMACE randomized controlled trial. J. Alzheimers Dis. 83, 1703–1715. doi: 10.3233/jad-210570
de la Monte, S. M., and Wands, J. R. (2008). Alzheimer's disease is type 3 diabetes-evidence reviewed. J. Diabetes Sci. Technol. 2, 1101–1113. doi: 10.1177/193229680800200619
Duarte, A. I., Moreira, P. I., and Oliveira, C. R. (2012). Insulin in central nervous system: more than just a peripheral hormone. J. Aging Res. 2012:384017. doi: 10.1155/2012/384017
Furie, K. L., Viscoli, C. M., Gorman, M., Ford, G. A., Young, L. H., Inzucchi, S. E., et al. (2018). Effects of pioglitazone on cognitive function in patients with a recent ischaemic stroke or TIA: a report from the IRIS trial. J. Neurol. Neurosurg. Psychiatry 89, 21–27. doi: 10.1136/jnnp-2017-316361
Ganguli, M., Beer, J. C., Zmuda, J. M., Ryan, C. M., Sullivan, K. J., Chang, C. H., et al. (2020). Aging, diabetes, obesity, and cognitive decline: a population-based study. J. Am. Geriatr. Soc. 68, 991–998. doi: 10.1111/jgs.16321
Gerstein, H. C., Hart, R., Colhoun, H. M., Diaz, R., Lakshmanan, M., Botros, F. T., et al. (2020). The effect of dulaglutide on stroke: an exploratory analysis of the REWIND trial. Lancet Diabetes Endocrinol. 8, 106–114. doi: 10.1016/s2213-8587(19)30423-1
Ghetti, S., Kuppermann, N., Rewers, A., Myers, S. R., Schunk, J. E., Stoner, M. J., et al. (2020). Cognitive function following diabetic ketoacidosis in children with new-onset or previously diagnosed type 1 diabetes. Diabetes Care 43, 2768–2775. doi: 10.2337/dc20-0187
Gudala, K., Bansal, D., Schifano, F., and Bhansali, A. (2013). Diabetes mellitus and risk of dementia: a meta-analysis of prospective observational studies. J. Diabetes Investig. 4, 640–650. doi: 10.1111/jdi.12087
Guo, M., Mi, J., Jiang, Q. M., Xu, J. M., Tang, Y. Y., Tian, G., et al. (2014). Metformin may produce antidepressant effects through improvement of cognitive function among depressed patients with diabetes mellitus. Clin. Exp. Pharmacol. Physiol. 41, 650–656. doi: 10.1111/1440-1681.12265
Hanyu, H., Sato, T., Kiuchi, A., Sakurai, H., and Iwamoto, T. (2009). Pioglitazone improved cognition in a pilot study on patients with Alzheimer's disease and mild cognitive impairment with diabetes mellitus. J. Am. Geriatr. Soc. 57, 177–179. doi: 10.1111/j.1532-5415.2009.02067.x
He, J. T., Zhao, X., Xu, L., and Mao, C. Y. (2020). Vascular risk factors and Alzheimer's disease: blood-brain barrier disruption, metabolic syndromes, and molecular links. J. Alzheimers Dis. 73, 39–58. doi: 10.3233/jad-190764
Isik, A. T., Soysal, P., Yay, A., and Usarel, C. (2017). The effects of sitagliptin, a DPP-4 inhibitor, on cognitive functions in elderly diabetic patients with or without Alzheimer's disease. Diabetes Res. Clin. Pract. 123, 192–198. doi: 10.1016/j.diabres.2016.12.010
Jash, K., Gondaliya, P., Kirave, P., Kulkarni, B., Sunkaria, A., and Kalia, K. (2020). Cognitive dysfunction: a growing link between diabetes and Alzheimer's disease. Drug Dev. Res. 81, 144–164. doi: 10.1002/ddr.21579
Köbe, T., Witte, A. V., Schnelle, A., Tesky, V. A., Pantel, J., Schuchardt, J. P., et al. (2017). Impact of resveratrol on glucose control, hippocampal structure and connectivity, and memory performance in patients with mild cognitive impairment. Front. Neurosci. 11:105. doi: 10.3389/fnins.2017.00105
Lacy, M. E., Gilsanz, P., Eng, C., Beeri, M. S., Karter, A. J., and Whitmer, R. A. (2020). Severe hypoglycemia and cognitive function in older adults with type 1 diabetes: the study of longevity in diabetes (SOLID). Diabetes Care 43, 541–548. doi: 10.2337/dc19-0906
Lester-Coll, N., Rivera, E. J., Soscia, S. J., Doiron, K., Wands, J. R., and de la Monte, S. M. (2006). Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer's disease. J. Alzheimers Dis. 9, 13–33. doi: 10.3233/jad-2006-9102
Li, Q., Jia, M., Yan, Z., Li, Q., Sun, F., He, C., et al. (2021). Activation of glucagon-like Peptide-1 receptor ameliorates cognitive decline in type 2 diabetes mellitus through a metabolism-independent pathway. J. Am. Heart Assoc. 10:e020734. doi: 10.1161/jaha.120.020734
Lin, Y., Wang, K., Ma, C., Wang, X., Gong, Z., Zhang, R., et al. (2018). Evaluation of metformin on cognitive improvement in patients with non-dementia vascular cognitive impairment and abnormal glucose metabolism. Front. Aging Neurosci. 10:227. doi: 10.3389/fnagi.2018.00227
McMillan, J. M., Mele, B. S., Hogan, D. B., and Leung, A. A. (2018). Impact of pharmacological treatment of diabetes mellitus on dementia risk: systematic review and meta-analysis. BMJ Open Diabetes Res. Care 6:e000563. doi: 10.1136/bmjdrc-2018-000563
Milstein, J. L., and Ferris, H. A. (2021). The brain as an insulin-sensitive metabolic organ. Mol. Metab. 52:101234. doi: 10.1016/j.molmet.2021.101234
Moore, E. M., Mander, A. G., Ames, D., Kotowicz, M. A., Carne, R. P., Brodaty, H., et al. (2013). Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care 36, 2981–2987. doi: 10.2337/dc13-0229
Morris, J. K., Vidoni, E. D., Mahnken, J. D., Montgomery, R. N., Johnson, D. K., Thyfault, J. P., et al. (2016). Cognitively impaired elderly exhibit insulin resistance and no memory improvement with infused insulin. Neurobiol. Aging 39, 19–24. doi: 10.1016/j.neurobiolaging.2015.11.005
Pignalosa, F. C., Desiderio, A., Mirra, P., Nigro, C., Perruolo, G., Ulianich, L., et al. (2021). Diabetes and cognitive impairment: a role for Glucotoxicity and dopaminergic dysfunction. Int. J. Mol. Sci. 22:12366. doi: 10.3390/ijms222212366
Plastino, M., Fava, A., Pirritano, D., Cotronei, P., Sacco, N., Sperlì, T., et al. (2010). Effects of insulinic therapy on cognitive impairment in patients with Alzheimer disease and diabetes mellitus type-2. J. Neurol. Sci. 288, 112–116. doi: 10.1016/j.jns.2009.09.022
Sato, T., Hanyu, H., Hirao, K., Kanetaka, H., Sakurai, H., and Iwamoto, T. (2011). Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiol. Aging 32, 1626–1633. doi: 10.1016/j.neurobiolaging.2009.10.009
Tahmi, M., Palta, P., and Luchsinger, J. A. (2021). Metabolic syndrome and cognitive function. Curr. Cardiol. Rep. 23:180. doi: 10.1007/s11886-021-01615-y
Umegaki, H., Makino, T., Uemura, K., Shimada, H., Hayashi, T., Cheng, X. W., et al. (2017). The associations among insulin resistance, hyperglycemia, physical performance, diabetes mellitus, and cognitive function in relatively healthy older adults with subtle cognitive dysfunction. Front. Aging Neurosci. 9:72. doi: 10.3389/fnagi.2017.00072
van Sloten, T. T., Sedaghat, S., Carnethon, M. R., Launer, L. J., and Stehouwer, C. D. A. (2020). Cerebral microvascular complications of type 2 diabetes: stroke, cognitive dysfunction, and depression. Lancet Diabetes Endocrinol. 8, 325–336. doi: 10.1016/s2213-8587(19)30405-x
Xu, W., Yang, Y., Yuan, G., Zhu, W., Ma, D., and Hu, S. (2015). Exendin-4, a glucagon-like peptide-1 receptor agonist, reduces Alzheimer disease-associated tau hyperphosphorylation in the hippocampus of rats with type 2 diabetes. J. Investig. Med. 63, 267–272. doi: 10.1097/jim.0000000000000129
Keywords: diabetes mellitus, hyperglycemia, antidiabetic drugs, cognitive impairment, meta-analysis
Citation: Lin Y, Gong Z, Ma C, Wang Z and Wang K (2023) Relationship between glycemic control and cognitive impairment: A systematic review and meta-analysis. Front. Aging Neurosci. 15:1126183. doi: 10.3389/fnagi.2023.1126183
Received: 17 December 2022; Accepted: 11 January 2023;
Published: 26 January 2023.
Edited by:
Xintong Ge, Tianjin Medical University General Hospital, ChinaReviewed by:
Junli Zhao, Duke University, United StatesCopyright © 2023 Lin, Gong, Ma, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaiyuan Wang,  a3l3YW5nQHRtdS5lZHUuY24=; Zhiyun Wang,
a3l3YW5nQHRtdS5lZHUuY24=; Zhiyun Wang,  MTM4MjA1ODU2MjVAMTYzLmNvbQ==
MTM4MjA1ODU2MjVAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.