
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 02 March 2023
Sec. Alzheimer's Disease and Related Dementias
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1117851
This article is part of the Research Topic Pathological Implications of Metabolic and Cerebrovascular diseases in Neurocognitive Disorders View all 10 articles
 Shuhei Ikeda1
Shuhei Ikeda1 Yusuke Yakushiji1,2*
Yusuke Yakushiji1,2* Jun Tanaka1
Jun Tanaka1 Masashi Nishihara3
Masashi Nishihara3 Atsushi Ogata4
Atsushi Ogata4 Makoto Eriguchi1
Makoto Eriguchi1 Shohei Ono2
Shohei Ono2 Masafumi Kosugi1
Masafumi Kosugi1 Kohei Suzuyama1
Kohei Suzuyama1 Megumi Mizoguchi1
Megumi Mizoguchi1 Chika Shichijo1
Chika Shichijo1 Toshihiro Ide1
Toshihiro Ide1 Yukiko Nagaishi1
Yukiko Nagaishi1 Hodo Mori1
Hodo Mori1 Natsuki Ono1
Natsuki Ono1 Masaaki Yoshikawa1
Masaaki Yoshikawa1 Kiku Ide1
Kiku Ide1 Hiromu Minagawa1
Hiromu Minagawa1 Kotaro Iida1
Kotaro Iida1 Kazuhiro Kawamoto1
Kazuhiro Kawamoto1 Yoshiko Katsuki1
Yoshiko Katsuki1 Hiroyuki Irie3
Hiroyuki Irie3 Tatsuya Abe4
Tatsuya Abe4 Hideo Hara1
Hideo Hara1Introduction: Cerebral small vessel disease (SVD) is one of the leading causes of stroke; each neuroimaging marker of SVD is correlated with vascular risk factors and associated with poor prognosis after stroke. However, longitudinal studies investigating the association between comprehensive SVD burden scoring system, “total SVD score” – which encompasses the established neuroimaging markers of lacunae, cerebral microbleeds (CMBs), white matter hyperintensities (WMH) including periventricular hyperintensities, and perivascular spaces in basal ganglia– and clinical outcomes are limited. The aim of this study is to determine the association between SVD burden and long-term prognosis in patients with ischemic stroke.
Methods and design: This prospective, single-center, observational study enrolled patients with acute ischemic stroke, including cerebral infarction and transient ischemic attack. Magnetic resonance imaging scans were performed, and then total SVD score (range, 0–4) was calculated. We recorded baseline characteristics and evaluated the relationships of long-term outcomes to SVD neuroimaging markers and total SVD score. Stroke recurrence was thought as primary outcome. Hazard ratios (HRs) of events during follow-up were calculated using Cox proportional hazards modeling with adjustments for age, sex, hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation, and smoking. Cumulative event rates were estimated using the Kaplan–Meier method.
Results: Consecutive 564 acute ischemic stroke patients were enrolled according to inclusion and exclusion criteria. A total of 467 participants with first-ever ischemic stroke were analyzed (median age 75.0 [interquartile range, 64.0–83.0] years, 59.3% male). Total SVD score was 0 point in 47 individuals (12.0%), 1 point in 83 (21.2%), 2 points in 103 (26.3%), 3 points in 85 (21.7%), and 4 points in 73 (18.7%). Twenty-eight recurrent stroke events were identified during follow-up. Total SVD score ≥ 2, presence of CMBs, and moderate-to-severe WMH were associated with increased risk of recurrent stroke events (HR 9.31, 95% confidence interval [CI] 2.33–64.23; HR 2.81, 95% CI 1.08–7.30; HR 2.90, 95% CI 1.22–6.88, respectively).
Conclusion: The accumulation of SVD biomarkers as determined by total SVD score offered a reliable predictor of stroke recurrence. This study established a firm understanding of SVD prognosis in clinical settings.
Cerebral small vessel disease (SVD) refers to various pathological, clinical, and neuroimaging changes caused by damage to the perforating cerebral arterioles, capillaries, and venules. Neuroimaging markers of SVD are commonly seen in stroke patients and are considered to be associated with poor prognosis after stroke, including post-stroke dementia, recurrent stroke, and mortality (Greenberg et al., 2004; Best et al., 2021; Pasi et al., 2021). Effective treatment and prevention of stroke recurrence requires a better understanding of the mechanisms and underlying spectrum of SVD. Among the proposed scoring systems for comprehensive SVD burden, “total SVD score” – which encompasses the four established neuroimaging markers of lacunae, cerebral microbleeds (CMBs), white matter hyperintensities (WMH) including periventricular hyperintensities (PVH), and perivascular spaces in basal ganglia (BG-PVS) – has consistently displayed strong associations with traditional vascular risk factors.
However, longitudinal studies systematically investigating associations between scoring and clinical outcomes have been limited (Lau et al., 2017). We therefore initiated a prospective observational study named HAGAKURE (Hypertension, cerebral Amyloid, aGe Associated Known neuroimaging markers of cerebral small vessel disease Undertaken with stroke REgistry) to explore longitudinal associations between SVD and prognosis with recurrent stroke using a stroke cohort. In this article, we detail the protocol for the ischemic stroke (IS) cohort of the HAGAKURE study. Moreover, we present baseline data and associations of neuroimaging markers for SVD with long-term prognosis among patients with IS.
The HAGAKURE study is a Japanese prospective single-center hospital-based observational study investigating the prognosis of SVD at 3, 12, and 24 months after stroke onset. The study is being performed and reported with reference to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines (von Elm et al., 2007).
The study protocol was approved by the ethics committee at our hospital (approval nos. 24-26, 27-45, and 29-55) and is registered with the University Hospital Medical Information Network clinical trial registry in Japan (UMIN 000037894). The investigators obtained written informed consent from patients or their family members before registration. The study is being performed in accordance with the principles of the Declaration of Helsinki 59th WMA General Assembly, Seoul, (October 2008). SI and YY had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Our dataset can be shared on reasonable request after appropriate approval.
Table 1 shows the inclusion and exclusion criteria for the IS cohort of this study. Patients with IS (i.e., cerebral infarction or transient ischemic attack [TIA]) admitted to our division within a week after onset were considered eligible for the study. Exclusion criteria were: (1) duplicated registration; or (2) unavailability of brain magnetic resonance imaging (MRI) data. Eligible individuals are shown in Figure 1.
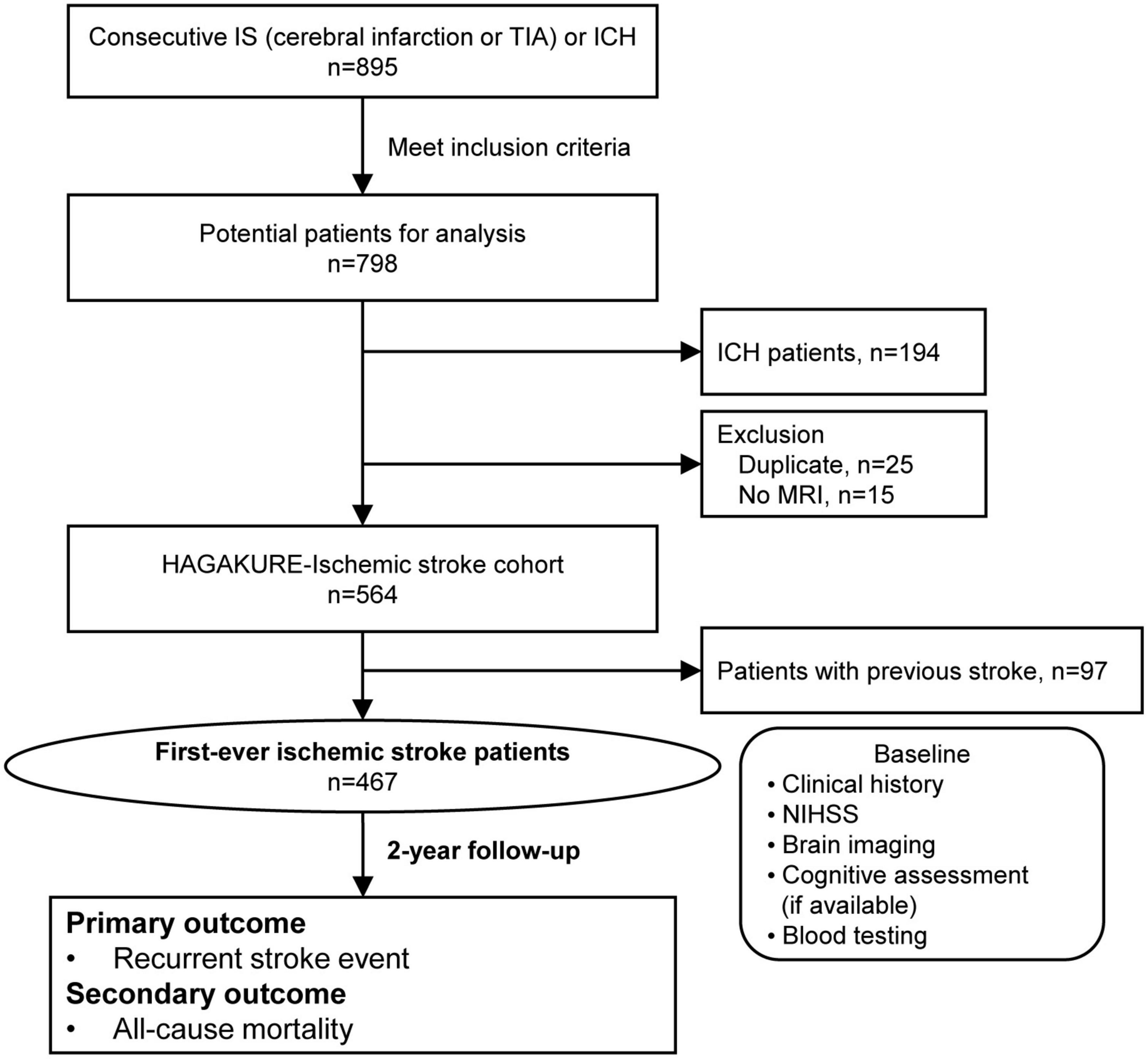
Figure 1. Flow diagram of patient selection for the HAGAKURE study. The HAGAKURE study is a single-center, prospective, longitudinal, observational study investigating the association between cerebral small vessel disease burden in patients with stroke (cerebral infarction, TIA, or ICH) and their associations (e.g., outcomes). ICH, intracerebral hemorrhage; IS, ischemic stroke; MRI, magnetic resonance imaging; TIA, transient ischemic attack.
Using a standardized case record form, data on demographics, medical history, medication use, physical examination, lifestyle, and pre-stroke functional status were collected. The details of these variables are described in the supplemental methods. We did not obtain ethnicity data directly from the patient or their family. Instead, we retrospectively checked the medical records (place of birth, name, family structure, facial photograph), and judged that all patients were East Asian. At baseline, we recorded stroke severity using the National Institutes of Health Stroke Scale score. These data were entered into the database by a single research nurse (YK) and double-checked by the co-authors (YY, JT, ME, MK, KS, MM, CS, YN, or NO).
The IS cohort comprised patients with cerebral infarction or TIA. Cerebral infarction was defined as sudden neurological dysfunction with evidence of acute infarction on brain imaging, regardless of symptom duration. TIA was defined as a transient episode of neurological dysfunction caused by focal brain ischemia without evidence of acute infarction on brain imaging (Easton et al., 2009). Subtypes of IS were classified according to the classification of Trial of Org 10172 in Acute Stroke Treatment (Adams et al., 1993).
All patients were evaluated by brain MRI at baseline. Sequences of axial brain MRI and the associated parameters are described in Supplementary Table 1. Digital Imaging and Communications in Medicine (DICOM)® data from MRI for use in rating were selected according to priori-defined criteria as described in Supplementary Table 2. DICOM data were anonymized and uploaded by a single co-author (S.O.) to a central imaging lab, and viewed on OsiriX medical imaging software (OsiriX MD; Pixmeo, Geneva, Switzerland) using a dedicated monitor (EIZO MX270W; EIZO Corporation, Ishikawa, Japan).
The definitions and classifications of neuroimaging markers, including lacunae (of presumed vascular origin), CMBs, WMH (of presumed vascular origin), and BG-PVS were classified according to recent consensus criteria and validated scales (Pasquier et al., 1996; Gregoire et al., 2009; Wardlaw et al., 2013; Charidimou et al., 2015), as described in Supplementary Table 3. Our main interest in this study was in identifying the total SVD score. According to a recently developed scoring system (Staals et al., 2014), total SVD scores were assigned as follows: presence of lacunae or CMBs was defined as the presence of one or more foci (1 point each if present); moderate-to-severe WMH including PVH was defined as either (early) confluent deep WMH (Fazekas score 2 or 3) and/or irregular PVH extending into deep white matter (Fazekas score 3) (1 point if present); and presence of moderate-to-severe BG-PVS (i.e., ≥ 11 BG-PVS on one side) (1 point if present). Total SVD score thus ranged from 0 to 4.
Neuroimaging markers on MRI were assessed by three raters, comprising a single board-certified neuroradiologist (MN) and two neurologists (YY and JT). All raters were blinded to all clinical information. Inter-rater reliability for these findings was calculated by comparing the certified neuroradiologist and neurologists using 40 MRI scans that were randomly selected. Intra-rater reliability for these tests was scored twice with an interval of ≥ 4 weeks, with raters blinded to the initial ratings.
Other evaluations and methodologies at baseline (i.e., cognitive function, blood tests, and physiological and imaging evaluations) are described in the supplemental methods.
The primary outcome was defined as recurrent stroke (i.e., cerebral infarction, TIA, intracerebral hemorrhage [ICH], or subarachnoid hemorrhage treated with admission). The secondary outcome was defined as all-cause mortality. To obtain information about the events, we sent periodic (3, 12, and 24 months after stroke onet) questionnaires to all participants (or conducted telephone surveys for patients who did not reply). When cerebrovascular events were reported, the authors checked the medical records and images of brain computed tomography and/or MRI. If respondents were lost to follow-up, data obtained from the latest questionnaire were used as the final records. Participants with incomplete information for follow-up questionnaires or with follow-up duration < 3 month were excluded. The HAGAKURE study commenced in September 2012 and final follow-up finished in March 2019.
In a previous longitudinal follow-up study in Japan (Hata et al., 2005), 10% of participants experienced a subsequent cerebrovascular event within 2 years of follow-up. Based on this, to perform multivariate analysis with about six variables, 60 participants with recurrent stroke within the first 2 years after stroke were required. We aimed to recruit up to 600 IS patients and 200 ICH patients, to allow for 25% loss to follow-up.
We analyzed associations between baseline characteristics and recurrent stroke events or all-cause mortality using Student’s t test for continuous variables and the χ2 test for categorical variables. These outcomes were evaluated using Kaplan–Meier curves. We determined both unadjusted and adjusted (for age, sex, and vascular risk factors) risks of recurrent stroke and all-cause mortality in patients with: (1) total SVD score ≥ 3; (2) total SVD score ≥ 2; and (3) increasing burden of each neuroimaging marker through Cox regression analysis. Covariates including MRI results and clinical biomarkers were selected according to previous studies. Two-sided testing was performed with a significance level of 5%. The 95% confidence intervals (CI) were two-sided. All analyses were performed using SPSS version 23.0 software (IBM Corporation, Armonk, NY) and JMP version 15.0 software (SAS Institute, Cary, NC).
The HAGAKURE was organized by a central coordinating center located at Saga University Hospital and an associated center at Kansai Medical University, with funding support by a Grant-in-Aid for Scientific Research (C), JSPS KAKENHI (grant numbers 15K10364 and 21K10510).
A flow diagram of patient selection for the IS cohort of the HAGAKURE study is shown in Figure 1. A total of 895 consecutive patients were admitted to our division with acute cerebral infarction, TIA, and ICH. Of those, 604 patients met the inclusion criteria for the IS cohort, then another 40 patients were excluded: 25 due to duplications; 15 due to lack of MRI data. A final total of 564 patients were included in this study, then 467 patients were diagnosed with first-ever IS. Baseline characteristics and imaging findings are shown in Table 2. The most common subtype of IS was cardioembolism (31.1%), followed by undetermined cause (21.6%), large-artery atherosclerosis (21.2%), small vessel occlusion (15.6%), and other cause (10.5%). Median duration from admission to brain MRI for the evaluation of SVD markers was 8 days (interquartile range [IQR] 5–10 days). Inter- and intra-rater reliabilities of SVD markers on MRI are shown in Supplementary Table 4. Proportions of each total SVD score were as follows: 0 points, 12.0%; 1 point, 21.2%; 2 points, 26.3%; 3 points, 21.7%; and 4 points, 18.7%. Main baseline laboratory data are shown in Supplementary Table 5. During the study periods, 36 patients were excluded from the analysis of recurrent stroke events, and 8 patients were excluded from the analysis of all-cause mortality due to inadequate duration of follow-up. A total of 28 recurrent stroke events (mean follow-up, 27 ± 12 months) and 104 cases of all-cause mortality (mean follow-up, 26 ± 13 months) occurred. Univariate analyses for prognosis are shown in Table 3.
Hazard ratios (HRs) for recurrent stroke and all-cause mortality based on total SVD score and individual markers of SVD are shown in Table 4. Multivariate Cox proportional-hazards regression analysis adjusting for age, sex, hypertension, dyslipidemia, diabetes mellitus, atrial fibrillation, and smoking showed that total SVD score ≥ 2 was associated with an increased risk of recurrent stroke events (HR 9.31, 95% CI 2.33–64.23). For individual SVD markers, presence of CMBs and moderate-to-severe WMH were more common in cases with recurrent stroke events (HR 2.90, 95% CI 1.22–6.88; HR 2.81, 95% CI 1.08–7.30, respectively). In a similar analysis for all-cause mortality, neither total SVD score nor any individual neuroimaging marker were associated with increased risk of all-cause mortality. Kaplan–Meier curves for the recurrent stroke event rate of total SVD score ≥ 2, presence of CMBs, and moderate-to-severe WMH are shown in Figure 2.
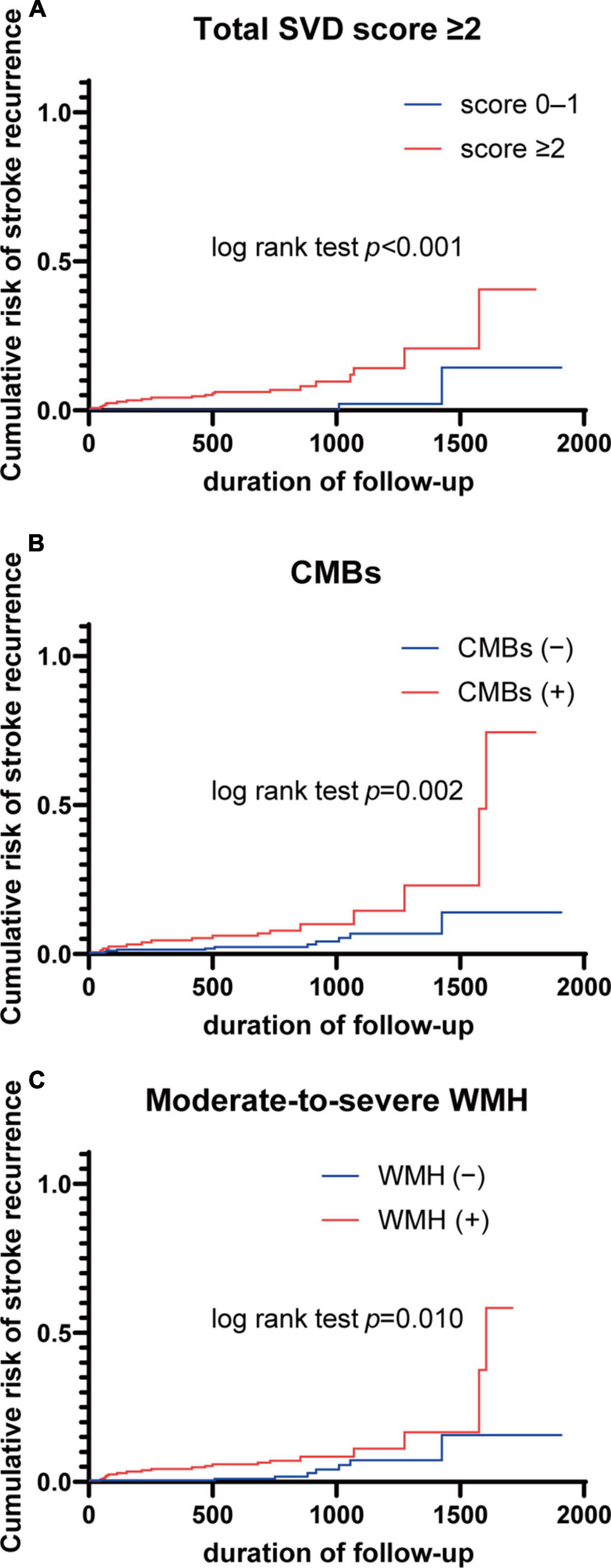
Figure 2. Kaplan–Meier curves for stroke recurrence according to total SVD score and neuroimaging markers. (A) Total SVD score ≥ 2; (B) CMBs; (C) moderate-to-severe WMH. CMBs, cerebral microbleeds; SVD, small vessel disease; WMH, white matter hyperintensity.
The purpose of this longitudinal prospective cohort study was to investigate the association between severity of SVD and long-term prognosis in an IS cohort from a single primary stroke center in Japan. This paper presented baseline data, including the distribution of neuroimaging markers, and clarified that high-burden SVD as determined by total SVD score ≥ 2 was strongly associated with the incidence of recurrent stroke. In addition, the presence of CMBs and moderate-to-severe WMH were associated with a higher incidence of recurrent stroke. The accumulation of SVD biomarkers as determined by total SVD score was found to offer a more reliable predictor of stroke recurrence than individual biomarkers alone, confirming the utility of total SVD score as a reliable visual scale for estimating comprehensive SVD burden. These findings provide important insights into the mechanisms underlying SVD and highlight the complex relationship between SVD and recurrent stroke.
Although prior research has investigated correlations between total SVD score and cognitive decline or mortality (Song et al., 2017; Del Brutto et al., 2021; Pasi et al., 2021), relatively few studies have examined the association between total SVD score and recurrent stroke (Lau et al., 2017; Chen et al., 2021). In a previous population-based study, we reported that total SVD score ≥ 2 was associated with increased risk of cerebro-cardiovascular events (Suzuyama et al., 2020). An earlier prospective cohort study found a correlation between higher total SVD score and incidence of stroke (Lau et al., 2017). Our findings agree with and extend these previous studies, and this was the first investigation to evaluate the effects of various SVD features among Japanese IS patients on multiple clinical outcomes.
Furthermore, our study revealed that individual neuroimaging markers were associated with an increased risk of recurrent stroke events, a conclusion supported by previous research linking SVD to recurrent stroke. For example, a large-scale, pooled analysis of 38 cohorts including 20,322 IS patients found that the presence of CMBs was associated with increased risk of recurrent stroke (Wilson et al., 2019). In addition, a literature review by Rensma et al. demonstrated that individual neuroimaging markers are associated with poor functional outcomes, cognitive decline, and increased mortality following stroke (Rensma et al., 2018). Our data, however, did not support any role for BG-PVS in increasing the risk of recurrent stroke in our IS cohort. We have previously reported BG-PVS as markers of hypertensive angiopathy (Yakushiji et al., 2014), but that significance appears limited, as comparatively few studies have established correlations with long-term prognosis (Rensma et al., 2018). BG-PVS are thought to appear from early in the course of SVD (Staals et al., 2014) and were present in the majority of patients in our stroke cohort. Such results suggest that pathological severity among neuroimaging markers is not equivalent. For example, a previous study raised the scoring cutoff for BG-PVS from > 10 to > 20 (Lau et al., 2017).
Other studies have demonstrated that total SVD score and individual neuroimaging markers correlated with all-cause mortality (Song et al., 2017; Rensma et al., 2018), but our findings appear to contradict this association. Despite higher total SVD score, our study population showed a comparable relative mortality rate when compared to the Korean population (Song et al., 2017). These discrepancies may be attributed to variations in study samples and cohort characteristics, such as age, which can significantly impact SVD and mortality. In addition, disparities in healthcare systems, including differences in post-stroke recovery care, may also play a role. Overall, the present findings imply that the impact of SVD on mortality may be limited in Japan according to underlying characteristics of the population, highlighting the need for further research to better understand the relationship between SVD and mortality.
Our findings have several important clinical implications. First, our results suggest that SVD may play an active role in recurrent stroke events, and that interventions targeting SVD may thus be effective in reducing recurrent stroke events. Second, our findings indicate that SVD may not be a significant contributor to long-term mortality after stroke, highlighting the importance of targeting other risk factors for stroke-related death. Third, our findings suggest that SVD is not only a passive outcome of vascular risk factors, but also a strong active contributor to recurrent stroke events. Although the most appropriate cutoff for total SVD score to indicate an increased risk of recurrent stroke has yet to be established, our findings suggest that low-burden SVD as determined by a total SVD score of 0–1 predicts a decreased risk of recurrent stroke.
This study had several limitations that need to be considered. First, among patients who underwent MRI, heterogeneity could conceivably exist in the assessment of SVD markers given differences in the field strengths of different scanners and variations in the sequence parameters applied. Second, our study was limited by the single-center design, and the results thus may not be generalizable to other populations. Third, we used a single time point assessment of SVD markers, and progression of SVD over time and its impacts on recurrent stroke events and all-cause mortality remain unclear. In addition, our study did not undertake a stratified analysis by subtype of IS, which would have provided valuable insights into associations between SVD markers, recurrent stroke events and all-cause mortality among different subtypes. We hypothesized that the effect is more pronounced in subtypes of small vessel occlusion and large-artery atherosclerosis, but the sample size in this study was inadequate to permit a comprehensive examination of this aspect. Finally, the proportion of patients categorized with small vessel occlusion in this study is lower than that reported in a previous study (Toyoda et al., 2022). This discrepancy may be due to the fact that a higher proportion of patients with cardioembolism, who require reperfusion therapy, are transported to our tertiary-care institution.
In conclusion, this study found that severity of SVD was associated with recurrent stroke. Compared with individual neuroimaging markers, total SVD score may provide more comprehensive information and allow improvements in personalized care for IS patients. Our findings establish a firmer understanding of SVD prognosis that will help guide the future development of strategies against SVD.
The studies involving human participants were reviewed and approved by Ethics Committee of Saga University. The patients/participants provided their written informed consent to participate in this study.
SI, YY, JT, and HH: study conception. SI, YY, JT, ME, SO, MK, KS, MM, CS, TI, YN, HMo, NO, MY, KId, HMi, KIi, KK, and YK: data acquisition. SI, YY, JT, MN, and AO: analysis and interpretation of data. SI, YY, and HH: drafting the manuscript. YY, HI, TA, and HH: supervision of the study. All authors contributed to the article and approved the submitted version.
The HAGAKURE was organized by a central coordinating centre located at Saga University Hospital and an associated centre at Kansai Medical University, with funding support by a Grant-in-Aid for Scientific Research (C), JSPS KAKENHI (Grant Nos. 15k10364 and 21K10510).
We are indebted to Ms. Yoko Wakabayashi and Miki Fuji for data management.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1117851/full#supplementary-material
Adams, H. P. Jr., Bendixen, B. H., Kappelle, L. J., Biller, J., Love, B. B., Gordon, D. L., et al. (1993). Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 24, 35–41. doi: 10.1161/01.str.24.1.35
Best, J. G., Ambler, G., Wilson, D., Lee, K. J., Lim, J. S., Shiozawa, M., et al. (2021). Development of imaging-based risk scores for prediction of intracranial haemorrhage and ischaemic stroke in patients taking antithrombotic therapy after ischaemic stroke or transient ischaemic attack: A pooled analysis of individual patient data from cohort studies. Lancet Neurol. 20, 294–303. doi: 10.1016/s1474-4422(21)00024-7
Charidimou, A., Linn, J., Vernooij, M. W., Opherk, C., Akoudad, S., Baron, J. C., et al. (2015). Cortical superficial siderosis: Detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 138(Pt 8), 2126–2139. doi: 10.1093/brain/awv162
Chen, X., Wang, L., Jiang, J., Gao, Y., Zhang, R., Zhao, X., et al. (2021). Association of neuroimaging markers of cerebral small vessel disease with short-term outcomes in patients with minor cerebrovascular events. BMC Neurol. 21:21. doi: 10.1186/s12883-021-02043-9
Del Brutto, V. J., Mera, R., Recalde, B. Y., Rumbea, D. A., Costa, A. F., and Del Brutto, O. H. (2021). Total cerebral small vessel disease score and all-cause mortality in older adults of Amerindian ancestry: The Atahualpa Project. Eur. Stroke J. 6, 412–419. doi: 10.1177/23969873211060803
Easton, J. D., Saver, J. L., Albers, G. W., Alberts, M. J., Chaturvedi, S., Feldmann, E., et al. (2009). Definition and evaluation of transient ischemic attack: A scientific statement for healthcare professionals from the American heart association/American stroke association stroke council; council on cardiovascular surgery and anesthesia; council on cardiovascular radiology and intervention; council on cardiovascular nursing; and the interdisciplinary council on peripheral vascular disease. The American academy of neurology affirms the value of this statement as an educational tool for neurologists. Stroke 40, 2276–2293. doi: 10.1161/strokeaha.108.192218
Greenberg, S. M., Eng, J. A., Ning, M., Smith, E. E., and Rosand, J. (2004). Hemorrhage burden predicts recurrent intracerebral hemorrhage after lobar hemorrhage. Stroke 35, 1415–1420. doi: 10.1161/01.STR.0000126807.69758.0e
Gregoire, S. M., Chaudhary, U. J., Brown, M. M., Yousry, T. A., Kallis, C., Jäger, H. R., et al. (2009). The microbleed anatomical rating scale (MARS): Reliability of a tool to map brain microbleeds. Neurology 73, 1759–1766. doi: 10.1212/WNL.0b013e3181c34a7d
Hata, J., Tanizaki, Y., Kiyohara, Y., Kato, I., Kubo, M., Tanaka, K., et al. (2005). Ten year recurrence after first ever stroke in a Japanese community: The Hisayama study. J. Neurol. Neurosurg. Psychiatry 76, 368–372. doi: 10.1136/jnnp.2004.038166
Lau, K. K., Li, L., Schulz, U., Simoni, M., Chan, K. H., Ho, S. L., et al. (2017). Total small vessel disease score and risk of recurrent stroke: Validation in 2 large cohorts. Neurology 88, 2260–2267. doi: 10.1212/wnl.0000000000004042
Pasi, M., Sugita, L., Xiong, L., Charidimou, A., Boulouis, G., Pongpitakmetha, T., et al. (2021). Association of cerebral small vessel disease and cognitive decline after intracerebral hemorrhage. Neurology 96, e182–e192. doi: 10.1212/wnl.0000000000011050
Pasquier, F., Leys, D., Weerts, J. G., Mounier-Vehier, F., Barkhof, F., and Scheltens, P. (1996). Inter- and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur. Neurol. 36, 268–272. doi: 10.1159/000117270
Rensma, S. P., van Sloten, T. T., Launer, L. J., and Stehouwer, C. D. A. (2018). Cerebral small vessel disease and risk of incident stroke, dementia and depression, and all-cause mortality: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 90, 164–173. doi: 10.1016/j.neubiorev.2018.04.003
Song, T. J., Kim, J., Song, D., Yoo, J., Lee, H. S., Kim, Y. J., et al. (2017). Total cerebral small-vessel disease score is associated with mortality during follow-up after acute ischemic stroke. J. Clin. Neurol. 13, 187–195. doi: 10.3988/jcn.2017.13.2.187
Staals, J., Makin, S. D., Doubal, F. N., Dennis, M. S., and Wardlaw, J. M. (2014). Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 83, 1228–1234. doi: 10.1212/wnl.0000000000000837
Suzuyama, K., Yakushiji, Y., Ogata, A., Nishihara, M., Eriguchi, M., Kawaguchi, A., et al. (2020). Total small vessel disease score and cerebro-cardiovascular events in healthy adults: The Kashima scan study. Int. J. Stroke 15, 973–979. doi: 10.1177/1747493020908144
Toyoda, K., Yoshimura, S., Nakai, M., Koga, M., Sasahara, Y., Sonoda, K., et al. (2022). Twenty-year change in severity and outcome of ischemic and hemorrhagic strokes. JAMA Neurol. 79, 61–69. doi: 10.1001/jamaneurol.2021.4346
von Elm, E., Altman, D. G., Egger, M., Pocock, S. J., Gøtzsche, P. C., and Vandenbroucke, J. P. (2007). The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 370, 1453–1457. doi: 10.1016/s0140-6736(07)61602-x
Wardlaw, J. M., Smith, E. E., Biessels, G. J., Cordonnier, C., Fazekas, F., Frayne, R., et al. (2013). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838. doi: 10.1016/s1474-4422(13)70124-8
Wilson, D., Ambler, G., Lee, K. J., Lim, J. S., Shiozawa, M., Koga, M., et al. (2019). Cerebral microbleeds and stroke risk after ischaemic stroke or transient ischaemic attack: A pooled analysis of individual patient data from cohort studies. Lancet Neurol. 18, 653–665. doi: 10.1016/s1474-4422(19)30197-8
Keywords: small vessel disease (SVD), cerebral Amyloid aGe (CAA), magnetic resonance imaging (MRI), hypertension, ischemic stroke (IS)
Citation: Ikeda S, Yakushiji Y, Tanaka J, Nishihara M, Ogata A, Eriguchi M, Ono S, Kosugi M, Suzuyama K, Mizoguchi M, Shichijo C, Ide T, Nagaishi Y, Mori H, Ono N, Yoshikawa M, Ide K, Minagawa H, Iida K, Kawamoto K, Katsuki Y, Irie H, Abe T and Hara H (2023) Hypertension, cerebral Amyloid, aGe Associated Known neuroimaging markers of cerebral small vessel disease Undertaken with stroke REgistry (HAGAKURE) prospective cohort study: Baseline characteristics and association of cerebral small vessel disease with prognosis in an ischemic stroke cohort. Front. Aging Neurosci. 15:1117851. doi: 10.3389/fnagi.2023.1117851
Received: 06 December 2022; Accepted: 30 January 2023;
Published: 02 March 2023.
Edited by:
Satoshi Saito, National Cerebral and Cardiovascular Center, JapanReviewed by:
Kenji Sakai, Jōetsu General Hospital, JapanCopyright © 2023 Ikeda, Yakushiji, Tanaka, Nishihara, Ogata, Eriguchi, Ono, Kosugi, Suzuyama, Mizoguchi, Shichijo, Ide, Nagaishi, Mori, Ono, Yoshikawa, Ide, Minagawa, Iida, Kawamoto, Katsuki, Irie, Abe and Hara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yusuke Yakushiji, eWFrdXNoaXlAaGlyYWthdGEua211LmFjLmpw
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.