
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 23 February 2023
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1117802
This article is part of the Research TopicInsights in Parkinson’s Disease and Aging-related Movement Disorders: 2022View all 12 articles
 Ali Nouriani1
Ali Nouriani1 Alec Jonason2
Alec Jonason2 Luke T. Sabal2
Luke T. Sabal2 Jacob T. Hanson3
Jacob T. Hanson3 James N. Jean2
James N. Jean2 Thomas Lisko2
Thomas Lisko2 Emma Reid2
Emma Reid2 Yeng Moua2
Yeng Moua2 Shane Rozeboom2
Shane Rozeboom2 Kaiser Neverman2
Kaiser Neverman2 Casey Stowe2
Casey Stowe2 Rajesh Rajamani1
Rajesh Rajamani1 Robert A. McGovern2,4*
Robert A. McGovern2,4*The use of wearable sensors in movement disorder patients such as Parkinson’s disease (PD) and normal pressure hydrocephalus (NPH) is becoming more widespread, but most studies are limited to characterizing general aspects of mobility using smartphones. There is a need to accurately identify specific activities at home in order to properly evaluate gait and balance at home, where most falls occur. We developed an activity recognition algorithm to classify multiple daily living activities including high fall risk activities such as sit to stand transfers, turns and near-falls using data from 5 inertial sensors placed on the chest, upper-legs and lower-legs of the subjects. The algorithm is then verified with ground truth by collecting video footage of our patients wearing the sensors at home. Our activity recognition algorithm showed >95% sensitivity in detection of activities. Extracted features from our home monitoring system showed significantly better correlation (~69%) with prospectively measured fall frequency of our subjects compared to the standard clinical tests (~30%) or other quantitative gait metrics used in past studies when attempting to predict future falls over 1 year of prospective follow-up. Although detecting near-falls at home is difficult, our proposed model suggests that near-fall frequency is the most predictive criterion in fall detection through correlation analysis and fitting regression models.
Postural instability is both a cardinal symptom of movement disorders like Parkinson’s disease (PD) and a major cause of falls in these patients (Palakurthi and Burugupally, 2019). Injurious falls and hip fractures occur at higher rates in PD patients, with approximately 75% of international hospitalizations in patients with PD occurring due to fractures or falls (Chou et al., 2011). Therefore, if subtle balance dysfunction could be properly identified and characterized, this information could be used to initiate falls preventions and physical therapy programs, improve fall prediction algorithms, and monitor or evaluate new treatments.
The Movement Disorder Society-Unified Parkinson’s Disease rating scale (MDS-UPDRS) (Goetz et al., 2008) analyzes all motor symptoms using a semi-quantitative scale. Its validity and reliability are well recognized and it is the clinical gold-standard in terms of monitoring symptoms related to PD (Ramaker et al., 2002). However, these assessments are subject to inter-rater variability, and the unavailability of continuous monitoring limits these methods. The score of the evaluation depends on the patient’s current status, which may fluctuate day-to-day and depending on the time since the last dose of medication was taken. On the other hand, traditional lab-based assessments using infrared cameras or quantitative analysis to characterize postural instability in patients with movement disorders are costly, not portable, and are unable to track long-term movement data from these patients in their day-to-day lives when most falls occur. Therefore, there is a serious need for long-term, real-time, and objective characterization of movement as a complement to clinical and lab-based assessments (Ramaker et al., 2002).
A few methods of characterizing mobility in patients with movement disorders have been proposed, including home movement diaries (Hauser et al., 2006) and characterizing mobility using smartphone applications (Lorenzi et al., 2016; Zhan et al., 2018). Patient diaries and questionnaires at home are frequently used in clinical routine to study motor stages and fluctuations in late-stage PD (Papapetropoulos, 2012). However, diaries are subject to fatigue, errors, and bias which impacts the quality and credibility of the data, particularly in patients with cognitive dysfunction (Papapetropoulos, 2012). Some methods of tracking participants at home may involve using mobile phone-based systems that gather data using inertial sensors that are built into smartphones (Motolese et al., 2020). This yields data that allows for general tracking of activities such as walking, sitting and sleeping, but does not provide quantitative insights into participants’ postural responses when experiencing a fall or near-fall. Additionally, relying on data from a device that is not fixed to the patient’s body may introduce error or leave long gaps in data. More elaborate systems using multiple cameras throughout a person’s home in order to track their movements may also be used, but this may not be feasible on a wide scale due to its cost, complexity, and privacy concerns (Rougier et al., 2011). In past studies, IMUs have shown to be both accurate and repeatable for measuring gait parameters in healthy young adults (Washabaugh et al., 2017). Additionally, publicly available datasets collected from patients with movement disorders using IMUs have been utilized to analyze gait parameters and freezing of gait episodes (O’Day et al., 2022).
To address some of the problems with characterizing mobility in patients with movement disorders, we have developed an algorithm that can both accurately measure gait parameters and enable real-time detection of high-risk activities in the patient’s home environment using inexpensive and widely available wearable technology. The merging of cost-effective technology with deep learning techniques yields significant promise in the field of wearable sensor technology (Ramanujam et al., 2021). The goal of this study was to create a video-validated dataset of movement disorder patients and healthy controls engaged in daily living activities in their homes, develop an algorithm for automatic recognition of near-falls/high fall risk activities and subsequently quantitatively characterize the patient’s response to these events in order to predict future fall risk. Our collected dataset includes 29 participants in total, comprised of 11 participants with Parkinson’s disease (PD), eight participants with Normal Pressure Hydrocephalus (NPH) and 10 Healthy Controls (C). Finally, we developed novel behavioral biomarkers based on this data to assess their relationship to patients’ prospective fall risk over 1 year of follow-up.
Nineteen movement disorder patients who were being clinically evaluated and/or treated for either normal pressure hydrocephalus or Parkinson’s disease and 10 age-matched healthy control participants were enrolled over a period of 2 years from the Minneapolis VA Health Care System (MVAHCS) and University of Minnesota (UMN). Enrollment was designed to enroll a variety of types of movement disorder patients with varying gait dysfunction and postural instability ranging from normal gait and balance (MDS-UPDRS gait and pull test item scores of 0) to moderate dysfunction (MDS-UPDRS scores of 3). Patient participants were excluded if they were non-ambulatory or if they were unable to give consent. Control participants were excluded if they had any movement, gait, or balance disorders. Demographic information was collected (Table 1). This study was approved by the MVAHCS and UMN Institutional Review Boards, and all participants provided informed consent for participation according to the Declaration of Helsinki.
The measurement sensors were customized and reprogrammed inertial measurement units (IMU; SparkFun, Inc. Boulder, CO, United States). The board was equipped with a high-performance ARM Cortex-M4 processor powered by 500 mAh high-capacity Lithium battery (InvenSense. ICM-20948 - SparkFun Electronics, 2017). The measurement Integrated circuit (IC) was an ICM-20948 (InvenSense, San Jose, CA, United States) which can log nine degrees of freedom (accelerometer, gyroscope, magnetometer) at nearly 250 Hz (ICM-20948 Datasheet|TDK, 2021). The data from the IMU was sampled with a 100 Hz frequency and stored on a flash memory though it can be streamed wirelessly through Bluetooth connectivity to a smartphone or computer (Figure 1).
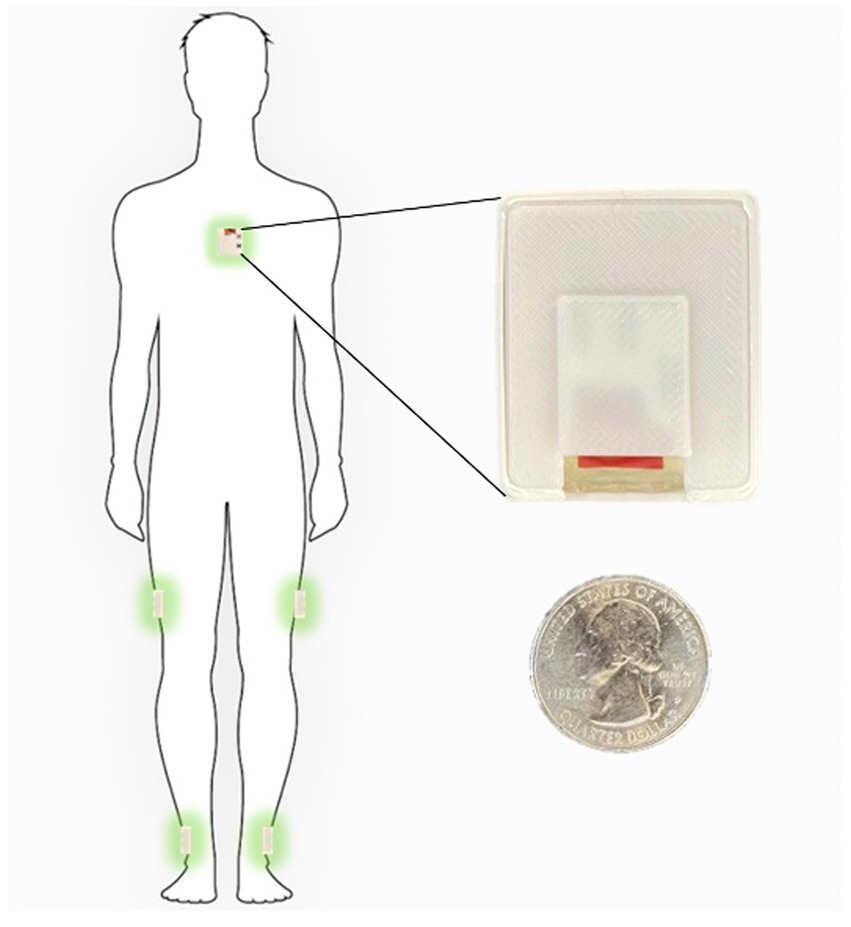
Figure 1. Inertial sensors placement on the body in 5-sensor configuration, one on the chest and one on each lower leg and on each upper leg.
The sensor configuration is one IMU sensor on each shank (just above the ankle), one IMU on each thigh, and one IMU sensor on the chest. This five sensor configuration uses an angle-based method taking advantage of the geometry of human in-plane walking. Each IMU sensor measures the acceleration and angular rate of movement of different body segments. An LMI-based non-linear sensor fusion algorithm is designed to estimate the limb segment orientations by taking advantage of the acceleration for lower frequencies and gyro in higher frequencies. The details of our estimation algorithm and the accuracy of a variety of kinematic variables which can be calculated using this configuration compared to a gold standard infrared camera measurements has been previously described (Nouriani et al., 2021).
Each participant was shown how to properly place the IMUs (Figure 1) in the clinic at their baseline visit. They were then sent home for 1 week and were instructed to wear the IMUs during all waking hours. The IMUs were charged overnight. The entirety of the dataset was able to be stored on the available flash memory on the sensor board and therefore participants did not need to upload data or stream any data to an app. They simply wore the sensors during the day and charged them at night. Each patient is provided with a custom charger connected to a Raspberry Pi Zero board (Raspberry Pi Foundation, Cambridge, England) which is programmed to synchronize the sensors together using threading with an extremely accurate real-time clock module (DS3231 RTC, Adafruit, New York, NY, United States) every time the sensors are connected to the charger (Extremely Accurate I2C-Integrated DS3231 RTC Datasheet, 2015). During the week of wearable sensor use, a research coordinator contacted the participants daily to troubleshoot any technical problems and check in. Participants were then prospectively followed for 1 year and asked to complete fall diaries according to accepted fall data formatting. To supplement the fall diaries, a research coordinator called the participants weekly for the follow-up year to inquire about any falls occurring during the past week.
In order to properly identify home activities, we first defined a variety of activities using the IMU data (Supplementary Table S1). The algorithm used to identify each home activity is a deep learning-based activity recognition architecture using a convolutional neural network with long short term memory cells (CNN-LSTM). The CNN-LSTM network implements a nonlinear observer for the estimation of the tilt angles of the human body limb segments as the input of the CNN layers followed by LSTM layers and finally fully connected layers with Softmax activation which we have detailed previously (Nouriani et al., 2022). We also used three other commonly used classifiers (logistic regression, support vector machine, decision tree) to compare their performance to our CNN-LSTM. The details of these algorithms can be found in the Supplementary methods. In order to validate the algorithm-defined activities, we asked a subset of participants (n = 10) to wear a small video camera (Runcam, Aberdeen, Hong Kong Island, Hong Kong; Runcam 5 Datasheet, 2020) with a necklace to wear at home. Each patient was asked to record for 45–60 min each day, ideally while ambulating or performing some type of algorithm-detectable activity. The videos were then manually annotated using a video-defined equivalent of each IMU-defined home activity (Supplementary Table S1) and synchronized with the sensors using the camera timestamps. Examples of the video footage captured by the patients are provided in Supplementary materials.
We prospectively followed all patient participants for 1 year with fall diaries and weekly individual participant contact to document the presence of any falls and the total number of falls over the entire year. From this data, we calculated the fall frequency as #falls/week. Using fall frequency as our outcome, we then examined the correlation between multiple computed features and fall frequency. These features ranged from standard demographic characteristics such as age, height, and weight, to clinical measurements such as the MDS-UPDRS pull test score, and also included a number of quantitative features from home measurements (denoted with an “_h”) that have been used in prior studies such as the total ambulatory time each day and number of ambulatory bouts each day (Supplementary Table S2). We also computed several novel features based on our previously video-validated activities described above. These include the frequency of near falls, turns and bends among others (events defined in Supplementary Table S1, all features used for correlation analysis are seen in Supplementary Table S2). We then created correlation confusion matrices to examine correlation of the previously mentioned data features with fall frequency. Using the fall diary data above, we also examined the time to first fall within the first year.
From the 29 participants, we have collected fall diaries and have survival data for 17 subjects. We did not collect the fall diaries from the control subjects and hence excluded them for the fall prediction model. From the remaining subjects, nine are censored since they are either new patients, or their home data is missing, or stopped sending their fall diaries to us before week 52.
Eight patients with NPH and 11 with PD were enrolled for a total of 19 patient participants. Ten healthy, age-matched control participants were also enrolled. Demographic characteristics are demonstrated in Table 1. There were no significant differences in age, sex, height or weight between controls and patient participants. As expected, patient participants had significantly worse gait and postural stability MDS-UPDRS scores as compared to healthy, age-matched control participants.
Ten of our patients generated more than 40 h of video footage which was manually annotated (see Supplementary Table S1 for definitions). Figure 2 demonstrates an example of 1 day of recorded data using our sensors compared to the video footage obtained from a patient at home. This patient generated approximately 90 min of video footage during which he was ambulatory for approximately 45 min punctuated in the middle by 45 min where he was sitting at rest. The algorithm-predicted activity (blue) overlies the actual video-annotated activity (orange) for the vast majority of the time, with an example of one misclassified activity (~8:58 am, standing misclassified as walking, seen in the Figure 2 inset).
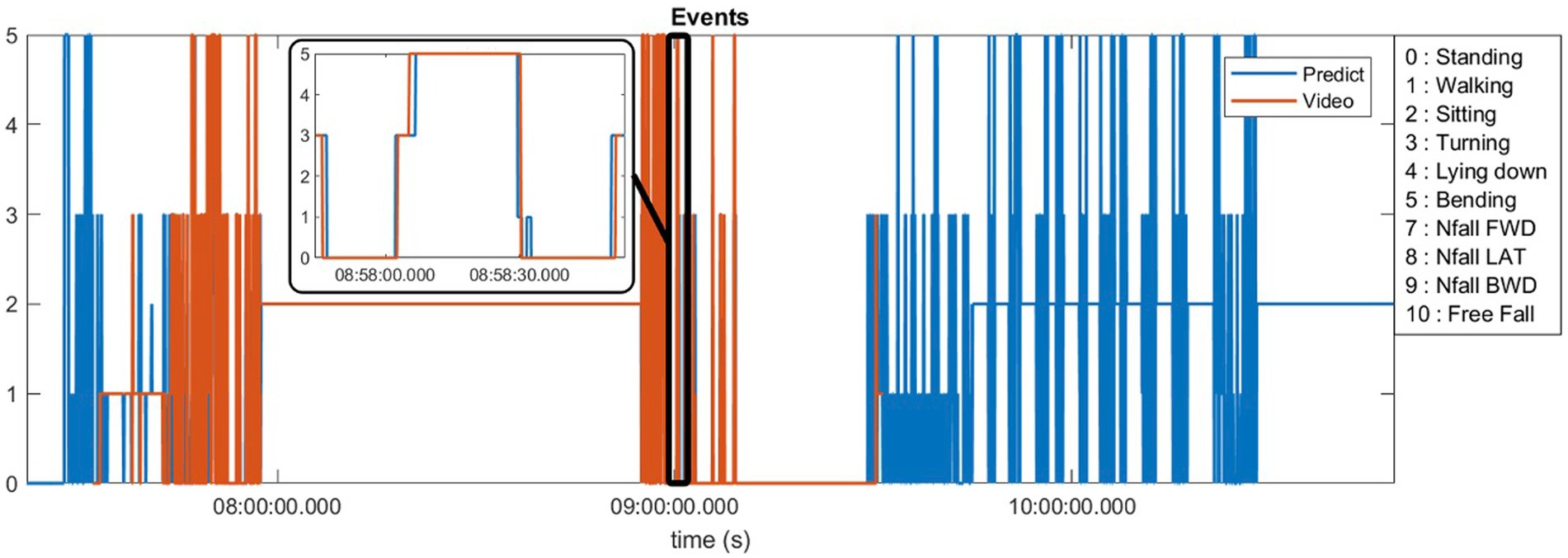
Figure 2. Predicted activities compared to the video annotations obtained from a patient at home environment.
The sum total of video footage in the entire subset of patients resulted in more than 14,000 total events which were used for algorithm predictions. Figure 3 demonstrates the home activities predicted by our activity recognition algorithm in comparison to the video-annotated data collected on the subset of patients with video recorded events. Events which were common and straightforward to both define and detect such as walking, standing and turning demonstrated the highest accuracy (>99%). Because these three events were the most common overall, they also represented most of the false positive and false negative errors for all events. Bending, sitting and transitions from sit to stand or stand to sit were significantly less common and slightly less accurately predicted (91–94%). Near falls in any direction were among the least common events and were less accurately predicted (~80%).
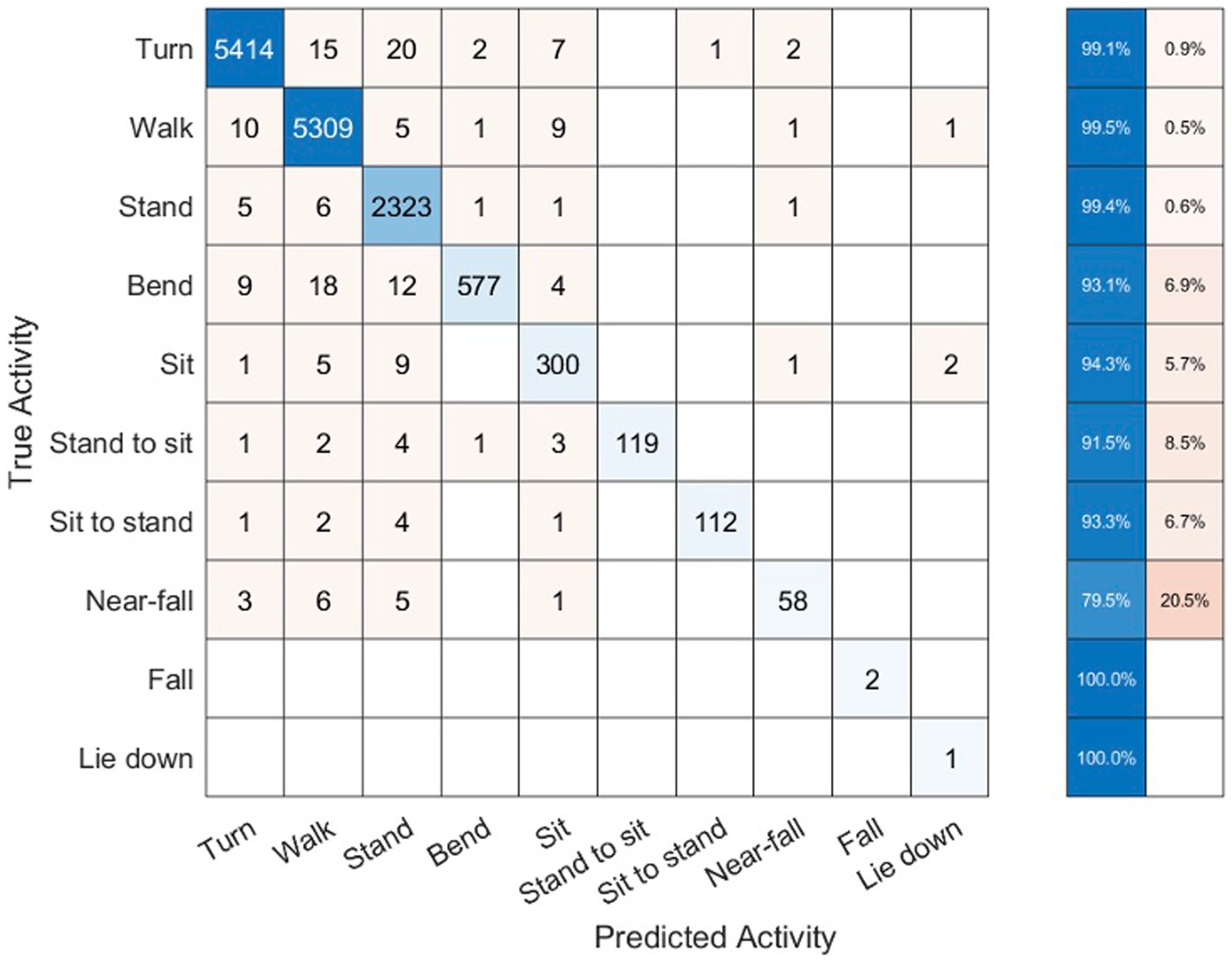
Figure 3. Confusion matrix of activity recognition algorithm result compared to the annotated videos for six subjects.
Table 2 shows the number of true positive (TP), false positive (FP), true negative (TN), and false negative (FN) samples from our activity recognition algorithm compared to the ground truth from video annotations. We can use these values to calculate the sensitivity (true positive rate or TPR), specificity (true negative rate or TNR), positive predictive value (PPV), negative predictive value (NPV), and accuracy (ACC). Because the number of total events is quite high, the specificity and overall accuracy of all the events are high, particularly for the low likelihood events such as sit to stand transitions, near-falls and falls. Nonetheless, even the low likelihood events had sensitivities >95% with the exception of near-falls which was 80%.
To compare our LSTM algorithm to other standard classifiers commonly used to make predictions on large datasets, we have created receiver operating characteristic (ROC) curves for 6 activities of standing, walking, sit-stand transitions, turning, bending and near-falls. Figure 4 shows the ROC curves for four binary classifiers of Logistic Regression (LOG), Support Vector Machines (SVM), Decision Tree (DT), and our Long-Short-Term-Memory cells (LSTM) for each activity (Nouriani et al., 2022). The area under the curve for each plot is summarized in Table 3. The performance of the LSTM classifier is superior in all activities with AUCs ranging from 0.982 to 0.999 for all activities while DT performs next best with slightly worse results. The SVM and LOG methods are significantly less accurate than LSTM or DT but still show acceptable results for a diagnostic classifier with AUCs ranging from 0.65 to 0.97. All classifiers worked well for relatively easily classified activities such as standing, walking or bending, but more difficult activities to classify such as near falls required the more sophisticated CNN-LSTM algorithm. There were no differences in activity classification between groups such that the CNN-LSTM algorithm was able to accurately classify activities in controls, NPH and PD patients equally well (Supplementary Figure S2). The overall performance (in terms of area under the curve) of each classifier is very similar across different patient groups.
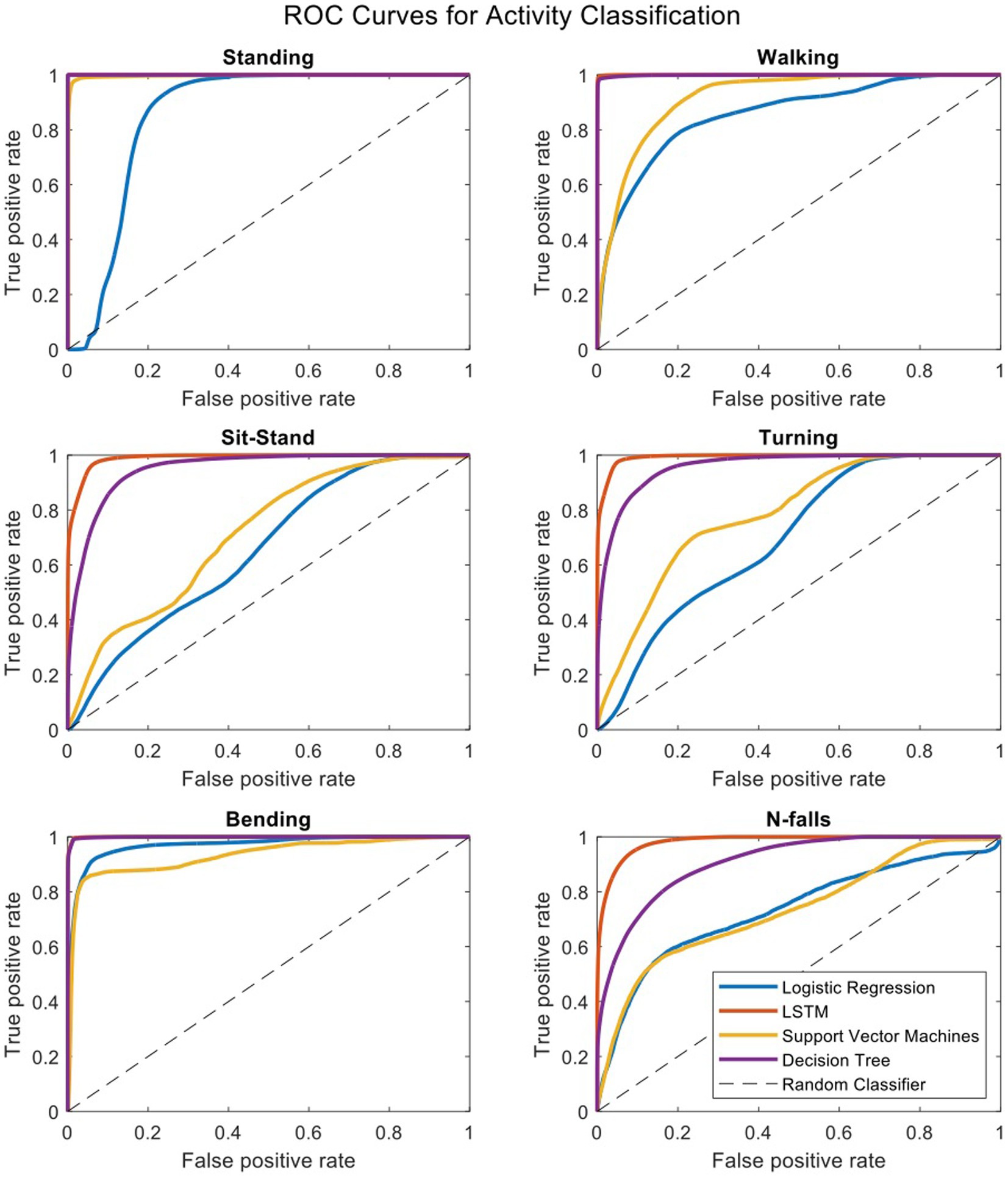
Figure 4. Receiver operating characteristic curves for four binary classifiers plotted separately for each activity.
Figure 5 demonstrates a correlation matrix examining the top 10 features correlated with prospectively observed fall frequency over the subsequent year of follow-up. Two of our novel metrics: the total number and frequency of near falls detected by the home monitoring setup showed the highest correlation with patient fall frequency (0.69 and 0.67, respectively). Supplementary Figure S1 shows the median of number of near-falls per week (Nfalls_h) for the three groups of our subjects. As expected, control subjects had significantly lower number of near-falls than our PD and NPH patients. PD patients showed a slightly higher median compared to NPH patients.
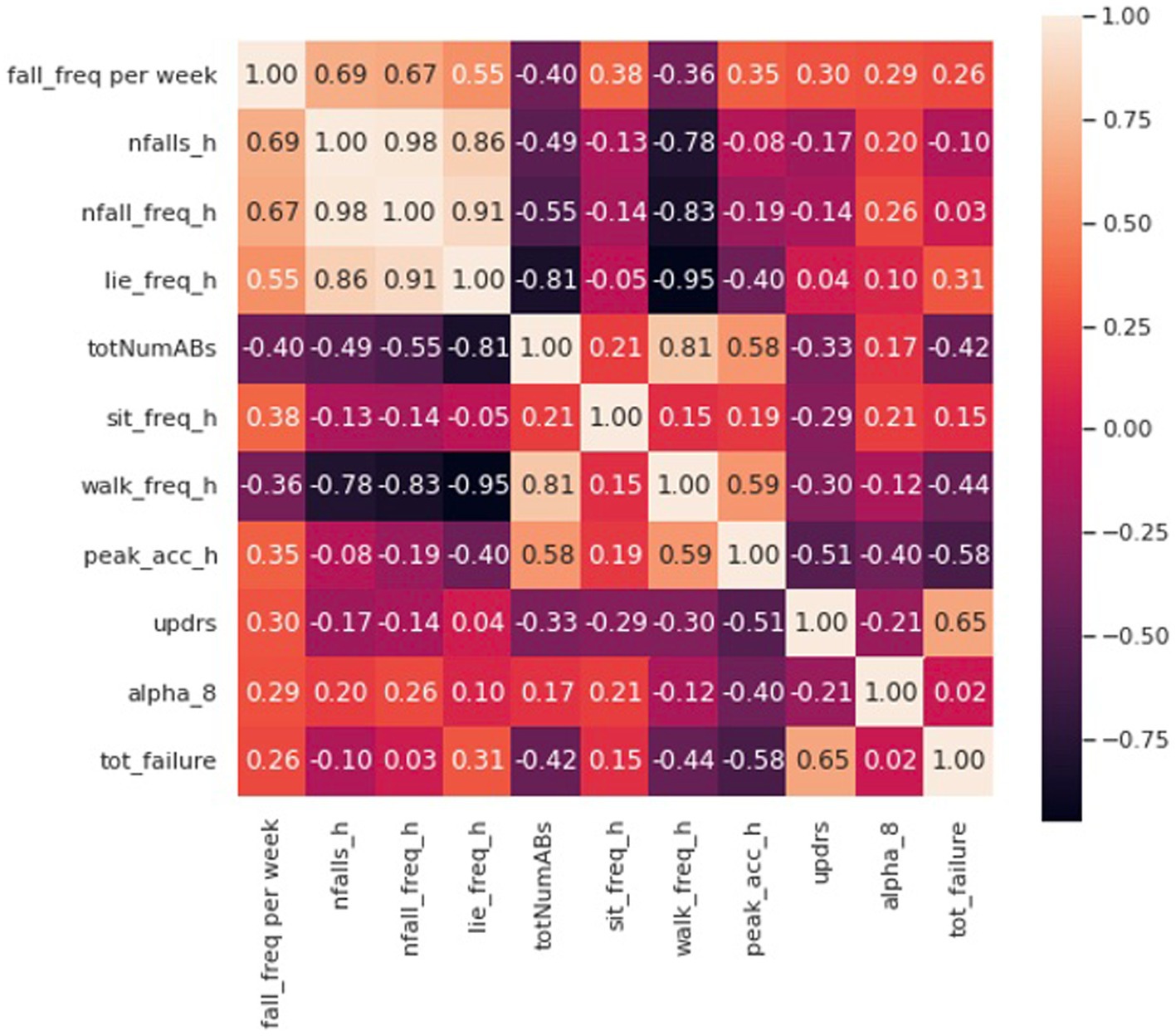
Figure 5. Correlation matrix including the first 10 features with the most correlation with the fall frequency of patients at home.
Eight of the most correlated features with fall frequency come from values calculated solely using the IMUs while the patient moves around their home and surrounding environment. For example, time spent lying down per day (lie down frequency, i.e., lying duration/total time), the total number of ambulatory bouts at home (totNumABs), the frequency of sitting at home (sit_freq_h, i.e., sitting duration/total time), walking frequency at home (walk_freq_h), the peak acceleration of the chest at home (peak_acc_h, which represents the strongest perturbation the subject experiences at home as measured by the chest accelerometer in each day) and the distribution of ambulatory bouts in time [alpha parameter for ABs of more than 8 s (alpha_8) (Nouriani et al., 2022)] at home are all within the 10 features with the highest correlation with fall frequency. It should be noted that some features were inversely correlated with fall frequency such that increased number of ambulatory bouts and increased walking frequency at home were associated with fewer falls (−0.40 and –0.36, respectively). Furthermore, Figure 5 also shows how correlated some of these features are with each other. For example, the total number of ABs are highly correlated with walking frequency (0.81), and the total number and frequency of near falls are almost perfectly correlated (0.98). We constructed a linear regression model on 5 features which are not highly correlated with each other. The details of the regression model are presented in Tables 4, 5. The model shows that near-fall frequency (nfall_freq), alpha parameter for ABs of more than 8 s (alpha_8) and UPDRS score (updrs) were the most significant predictors, respectively. The regression model parameters are summarized in Table 5. The only non-quantitative features included in the 10 most correlated features were the MDS-UPDRS pull test item score measured in clinic, and the total number of failures (needing to be caught by examiner) in clinical pull tests (tot_failures). These were among the most weakly correlated features (0.30 and 0.26, respectively) overall and were not significantly associated with fall frequency in the multivariable linear regression model.
Using a combination of domain specific knowledge and machine learning techniques, we developed an automatic algorithm for detection and characterization of near-falls and high fall risk activities of the patients. We created a validated, video annotated and quantitative dataset of movement disorder subjects wearing inertial sensors at their home environment. The statistical analysis of our algorithm shows >95% sensitivity in detection of activities apart from near-falls, which showed 80% sensitivity. The correlation analysis of the computed features in our dataset showed that our novel metrics based on near-falls are superior in terms of the highest correlation with patient fall frequency over an entire year of follow-up while clinic-based features were either not correlated or were among the most weakly correlated features.
Using video to validate algorithm predictions based on IMU data is a necessary component to reliably interpret any wearable dataset, but is frequently lacking in studies employing wearable sensors (Del Din et al., 2019). Studies that do employ some measure of validation typically do so in the clinic or laboratory setting, rather than “in the wild” as we have done in this study (Nouredanesh et al., 2021). There are essentially no studies that have attempted to capture and validate near falls or “stumbles” in the wild. Nor are there any widely accepted definitions or datasets that contain or even attempt to define these events. For example, one study asked participants in a survey whether they had near falls which they defined as “a fall initiated but arrested by support from the wall, railing or another person.” This study used purely clinical data to predict falls and near falls. No quantitative data was included (Lindholm et al., 2015). Another study artificially induced “missteps” in the laboratory and then developed an algorithm to detect such “missteps” during 3 days of home wearable use. There were no video recordings to validate these missteps and as such, they acknowledge there is no way to know if any of their detected missteps was in fact a misstep. Additionally, anything that did not look like a misstep in the lab would, by definition, be missing from their dataset (Iluz et al., 2014).
As such, our video-validated home data represents an ecologically valid quantitative dataset that can then be leveraged to understand the factors relevant in producing falls with far more detail compared to the current standard solution which involves qualitative clinical examination or laboratory-based task assessments that may or not be related to real world balance perturbations. Since most falls occur at home and surrounding environment, a wearable dataset that is “validated” in the clinic or lab may not be ecologically valid for fall prediction or other uses. On the other hand, some activities such as walking, standing and bending may be able to be easily validated in the clinic setting and so datasets validated with video “in the wild” should be compared to datasets using clinic-based assessments in order to better understand what can accurately assessed in the clinic vs. which assessments need to occur at home.
Our activity recognition algorithm uses a nonlinear switched-gain observer based on measurements from IMUs worn on leg segments and the chest in order to estimate body segment orientation. The observer estimates the tilt angles and measurement bias is estimated and removed. This has been measured in prior studies using infrared-based motion capture systems to ensure its accuracy (Nouriani et al., 2021). These estimates are then used to train the LSTM deep learning algorithm on all of the activities. Since many of the activity definitions are based on the tilt angles (Supplementary Table S1), this may be one reason that our LSTM activity recognition method showed superior accuracy. In addition, our deep learning network demonstrated lower computation cost compared to the other methods as it reduces the number of raw IMU signals necessary for activity recognition. Future studies should investigate how to reduce both the number of worn IMUs and number of recorded events without affecting diagnostic accuracy in order to minimize the burden on patients wearing the IMUs.
We have developed prospective, predictive falls risk metrics that integrate the patient’s postural response along with data that reflects the patient’s home environment based on near-falls detection. We have included all known gait parameters used in prior studies and current clinical standards in the study, however our proposed metrics showed superior performance in predicting falls in these patients. While there are simple measures which may be more easily measured with a smartphone or smartwatch (e.g., frequency of lying down, number of ambulatory bouts), our study suggests these are inferior to the number or frequency of near-falls. Similarly, clinical tests are typically inadequate in describing the likelihood of the subject falling and in characterizing the extent of their postural instability (Ramaker et al., 2002). Inter-and intra-rater variability in the execution and interpretation of clinical testing likely is responsible for some of their poor predictive power. Furthermore, incidents that trigger stumbles and falls at home are almost certainly different from testing conducted by clinicians or researchers in an artificial environment. Unfortunately, there are few studies on postural instability in home environments and these studies typically do not provide enough validation for their results in real-life situations at home or at least the demonstration of generalizability to the home environment (Silva de Lima et al., 2020). While there may be overlap in the postural response to balance testing in the clinic or lab and that at home, datasets such as the one described in our study should be used to investigate similarities and differences between these two settings in the future. This could lead to better fall prediction algorithms and improved diagnostic monitoring and treatment evaluations in the clinic, lab and at home in the future.
Despite being the most relevant feature of the dataset for fall prediction, near-falls were the most difficult activity to accurately detect with a sensitivity of 80%. This was for several reasons. First, near-falls look similar to other activities (like sit-stand transitions and bending) when examining inertial sensor data. Second, some of the near-falls were so subtle that they could not even be detected in videos. Finally, the natural occurrence of near-falls is relatively rare and obtaining video-validated samples is difficult since most of the patients who are at risk of falling are usually less active or use a walking aid to avoid falling. As such, they were also among the least common events and our algorithm, like all machine learning algorithms, performs better with more samples. Continued data collection with more validated events will likely help increase the accuracy of the algorithm over time.
We have collected our dataset using an inexpensive wearable system based on inertial sensors to provide kinematic data of PD and NPH patients at home. Typical uses of IMUs worn by movement disorder patients at home are detailed gait analysis and metrics on mobility/ambulation which can be used for a wide variety of purposes such as disease stage assessment, fall prediction, and treatment evaluation, among others (Lee et al., 2014; Mohammadian Rad et al., 2018; Pang et al., 2019; Pardoel et al., 2019). An advantage of our system is that it contains data from sensors on the chest and both feet that can be used to give detailed information on the postural response to near falls that occur in a natural setting in addition to all of those typical uses described above. Given the contribution of postural instability to falls in these patients, characterizing postural instability at home could potentially be very useful in their monitoring and treatment evaluation, particularly as their disease progresses and their likelihood of falling increases. Most studies of postural instability are still based on questionnaires or short-term simulations of near-falls in a clinical or lab setup (Ramaker et al., 2002). In addition, while wearable studies are becoming more common, clinical fall risk assessment is usually performed using diaries and questionnaires or one-time evaluations of gait and balance factors of the patients in a clinical trial (Ramaker et al., 2002; Hauser et al., 2006; Papapetropoulos, 2012). These methods are questionable in their quality and credibility due to their short-term and subjective assessment of the patients’ response (Ramaker et al., 2002). Thus, there is a crucial need for a long-term, easily obtained, and objective characterization of gait and postural instability in the home setting as a complement to clinical assessments. We would argue that a dataset such as the one described in this manuscript would represent the first step toward that goal.
One limitation of this dataset is its practicality as the current setup with five sensors might not be practical for everyday patient use. Future research should develop algorithms to use as few sensors as possible in optimal locations on the body. Because we were interested mainly in postural instability and falls, we did not include IMUs on the upper limbs and so our dataset does not include hand or arm movements. Given the frequent presence of upper extremity tremor in PD, this is particularly relevant for these patients in their diagnosis, monitoring and treatment evaluation. In addition, many activities of daily living can likely be classified with an upper extremity IMU. Further research should plan to integrate IMU/smartwatch-based data to obtain the widest variety of activities with the best diagnostic accuracy. Wearable usage should also be tailored to the specific usage desired by the clinician and patient. Finally, we plan to further develop activity recognition algorithms using unsupervised and semi-supervised learning methods to increase their accuracy or discover new activities which might have been missed by the current methods.
Even though near-fall detection is difficult to recognize and our algorithm shows 80% sensitivity, near-fall frequency at home was still the most predictive criterion in the linear regression model compared to any other metric. Our results showed that the detection of near-falls is a far more powerful way to examine home monitoring data compared to current methods and should be incorporated into fall prediction algorithms. This validated dataset of movement disorder patients engaged in daily living activities in their homes can serve as a valuable resource for researchers to provide a ground truth for IMU algorithm comparison that include the natural responses of patients at home.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation upon request.
The studies involving human participants were reviewed and approved by University of Minnesota IRB. The patients/participants provided their written informed consent to participate in this study.
RM conceived the original idea. AN developed the algorithms, designed the sensors and measurement setup with the help and supervision of RR. AN, AJ, JH, LS, JJ, TL, ER, YM, SR, KN, and CS helped with the data collection and annotations. AN analyzed and interpreted the results with the help and supervision of RM. AN and RM wrote the manuscript with the help of AJ and LS. RM and RR supervised the project. All authors contributed to the article and approved the submitted version.
This work was funded in part by a grant from the University of Minnesota MnDRIVE Neuromodulation Program and Institute for Engineering in Medicine (IEM) doctoral fellowship.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1117802/full#supplementary-material
Chou, K. L., Zamudio, J., Schmidt, P., Price, C. C., Parashos, S. A., Bloem, B. R., et al. (2011). Hospitalization in Parkinson disease: a survey of national Parkinson foundation centers. Parkinsonism Relat. Disord. 17, 440–445. doi: 10.1016/j.parkreldis.2011.03.002
Del Din, S., Galna, B., Godfrey, A., Bekkers, E. M., Pelosin, E., Nieuwhof, F., et al. (2019). Analysis of free-living gait in older adults with and without Parkinson’s disease and with and without a history of falls: identifying generic and disease-specific characteristics. J. Gerontol. Series A 74, 500–506. doi: 10.1093/gerona/glx254
Extremely Accurate I2C-Integrated DS3231 RTC Datasheet (2015). Extremely Accurate I2C-Integrated DS3231 RTC Datasheet. Available at: https://datasheets.maximintegrated.com/en/ds/DS3231.pdf ().
Goetz, C. G., Tilley, B. C., Shaftman, S. R., Stebbins, G. T., Fahn, S., Martinez-Martin, P., et al. (2008). Movement Disorder Society-sponsored revision of the unified Parkinson's disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Movement Disord. 23, 2129–2170. doi: 10.1002/mds.22340
Hauser, R. A., Russ, H., Haeger, D. A., Bruguiere-Fontenille, M., Müller, T., and Wenning, G. K. (2006). Patient evaluation of a home diary to assess duration and severity of dyskinesia in Parkinson disease. Clin. Neuropharmacol. 29, 322–330. doi: 10.1097/01.WNF.0000229546.81245.7F
ICM-20948 Datasheet|TDK (2021). ICM-20948 Datasheet|TDK. Available at: https://invensense.tdk.com/download-pdf/icm-20948-datasheet/ (Accessed December 19, 2021).
Iluz, T., Gazit, E., Herman, T., Sprecher, E., Brozgol, M., Giladi, N., et al. (2014). Automated detection of missteps during community ambulation in patients with Parkinson’s disease: a new approach for quantifying fall risk in the community setting. J. Neuroeng. Rehabil. 11, 1–9. doi: 10.1186/1743-0003-11-48
InvenSense. ICM-20948 - SparkFun Electronics. (2017) Available at: https://cdn.sparkfun.com/assets/7/f/e/c/d/DS-000189-ICM-20948-v1.3.pdf (Accessed December 19, 2021).
Lee, J. K., Robinovitch, S. N., and Park, E. J. (2014). Inertial sensing-based pre-impact detection of falls involving near-fall scenarios. IEEE Trans. Neural Syst. Rehabil. Eng. 23, 258–266. doi: 10.1109/TNSRE.2014.2357806
Lindholm, B., Hagell, P., Hansson, O., and Nilsson, M. H. (2015). Prediction of falls and/or near falls in people with mild Parkinson’s disease. PLoS One 10:e0117018. doi: 10.1371/journal.pone.0117018
Lorenzi, P., Rao, R., Romano, G., Kita, A., and Irrera, F. (2016). Mobile devices for the real-time detection of specific human motion disorders. IEEE Sensors J. 16, 1–7. doi: 10.1109/JSEN.2016.2530944
Mohammadian Rad, N., Van Laarhoven, T., Furlanello, C., and Marchiori, E. (2018). Novelty detection using deep normative modeling for IMU-based abnormal movement monitoring in Parkinson’s disease and autism spectrum disorders. Sensors 18:3533. doi: 10.3390/s18103533
Motolese, F., Magliozzi, A., Puttini, F., Rossi, M., Capone, F., Karlinski, K., et al. (2020). Parkinson's disease remote patient monitoring during the COVID-19 lockdown. Front. Neurol. 11:567413. doi: 10.3389/fneur.2020.567413
Nouredanesh, M., Godfrey, A., Howcroft, J., Lemaire, E. D., and Tung, J. (2021). Fall risk assessment in the wild: a critical examination of wearable sensor use in free-living conditions. Gait Posture 85, 178–190. doi: 10.1016/j.gaitpost.2020.04.010
Nouriani, A., McGovern, R. A., and Rajamani, R. (2021). Step length estimation with wearable sensors using a switched-gain nonlinear observer. Biomed. Signal Process. Control 69:102822. doi: 10.1016/j.bspc.2021.102822
Nouriani, A., McGovern, R. A., and Rajamani, R. (2022). Deep-learning-based human activity recognition using wearable sensors. IFAC-PapersOnLine 55, 1–6. doi: 10.1016/j.ifacol.2022.11.152
O’Day, J., Lee, M., Seagers, K., Hoffman, S., Jih-Schiff, A., Kidziński, Ł., et al. (2022). Assessing inertial measurement unit locations for freezing of gait detection and patient preference. J. Neuroeng. Rehabil. 19:20. doi: 10.1186/s12984-022-00992-x
Palakurthi, B., and Burugupally, S. P. (2019). Postural instability in Parkinson's disease: a review. Brain Sci. 9:239. Published 2019 Sep 18. doi: 10.3390/brainsci9090239
Pang, I., Okubo, Y., Sturnieks, D., Lord, S. R., and Brodie, M. A. (2019). Detection of near falls using wearable devices: a systematic review. J. Geriatr. Phys. Ther. 42, 48–56. doi: 10.1519/JPT.0000000000000181
Papapetropoulos, S. (2012). Patient diaries as a clinical endpoint in Parkinson's disease clinical trials. CNS Neurosci. Ther. 18, 380–387. doi: 10.1111/J.1755-5949.2011.00253.X
Pardoel, S., Kofman, J., Nantel, J., and Lemaire, E. D. (2019). Wearable-sensor-based detection and prediction of freezing of gait in Parkinson’s disease: a review. Sensors 19:5141. doi: 10.3390/s19235141
Ramaker, C., Marinus, J., Stiggelbout, A. M., and Van Hilten, B. J. (2002). Systematic evaluation of rating scales for impairment and disability in Parkinson's disease. Mov. Disord. 17, 867–876. doi: 10.1002/mds.10248
Ramanujam, E., Perumal, T., and Padmavathi, S. (2021). Human activity recognition with smartphone and wearable sensors using deep learning techniques: a review. IEEE Sensors J. 21, 13029–13040. doi: 10.1109/JSEN.2021.3069927
Rougier, C., Meunier, J., St-Arnaud, A., and Rousseau, J. (2011). Robust video surveillance for fall detection based on human shape deformation. IEEE Trans. Circuits Syst. Video Technol. 21, 611–622. doi: 10.1109/TCSVT.2011.2129370
Runcam 5 Datasheet (2020). Runcam 5 Datasheet. Available at: https://www.runcam.com/download/runcam5/RunCam5-Manual-EN.pdf (Accessed December 19, 2021).
Silva de Lima, A. L., Smits, T., Darweesh, S. K., Valenti, G., Milosevic, M., Pijl, M., et al. (2020). Home-based monitoring of falls using wearable sensors in Parkinson's disease. Mov. Disord. 35, 109–115. doi: 10.1002/mds.27830
Washabaugh, E. P., Kalyanaraman, T., Adamczyk, P. G., Claflin, E. S., and Krishnan, C. (2017). Validity and repeatability of inertial measurement units for measuring gait parameters. Gait Posture 55, 87–93. doi: 10.1016/j.gaitpost.2017.04.013
Keywords: Parkinson’s disease, postural instability, gait, wearable sensors, falls, near-falls
Citation: Nouriani A, Jonason A, Sabal LT, Hanson JT, Jean JN, Lisko T, Reid E, Moua Y, Rozeboom S, Neverman K, Stowe C, Rajamani R and McGovern RA (2023) Real world validation of activity recognition algorithm and development of novel behavioral biomarkers of falls in aged control and movement disorder patients. Front. Aging Neurosci. 15:1117802. doi: 10.3389/fnagi.2023.1117802
Received: 06 December 2022; Accepted: 31 January 2023;
Published: 23 February 2023.
Edited by:
Muthuraman Muthuraman, Johannes Gutenberg University Mainz, GermanyReviewed by:
Abdulrahman Aloyayri, University of Birmingham, United KingdomCopyright © 2023 Nouriani, Jonason, Sabal, Hanson, Jean, Lisko, Reid, Moua, Rozeboom, Neverman, Stowe, Rajamani and McGovern. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert A. McGovern, ✉ cm1jZ292ZXJAdW1uLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.