
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci. , 16 March 2023
Sec. Alzheimer's Disease and Related Dementias
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1104269
This article is part of the Research Topic Applications of Herbal Medicine in Metabolic and Degenerative Diseases of Aging View all 7 articles
 Ai Shi1,2†
Ai Shi1,2† Yu Long1,2†
Yu Long1,2† Yin Ma1,2
Yin Ma1,2 Shuang Yu1,2
Shuang Yu1,2 Dan Li1,2
Dan Li1,2 Jie Deng1,2
Jie Deng1,2 Jing Wen1,2
Jing Wen1,2 Xiaoqiu Li1,2
Xiaoqiu Li1,2 Yuanyuan Wu1,2
Yuanyuan Wu1,2 Xiaofang He1,2
Xiaofang He1,2 Yue Hu1,2
Yue Hu1,2 Nan Li1,2*
Nan Li1,2* Yuan Hu1,2*
Yuan Hu1,2*Cognitive impairment (CI), mainly Alzheimer’s disease (AD), continues to increase in prevalence and is emerging as one of the major health problems in society. However, until now, there are no first-line therapeutic agents for the allopathic treatment or reversal of the disease course. Therefore, the development of therapeutic modalities or drugs that are effective, easy to use, and suitable for long-term administration is important for the treatment of CI such as AD. Essential oils (EOs) extracted from natural herbs have a wide range of pharmacological components, low toxicity, and wide sources, In this review, we list the history of using volatile oils against cognitive disorders in several countries, summarize EOs and monomeric components with cognitive improvement effects, and find that they mainly act by attenuating the neurotoxicity of amyloid beta, anti-oxidative stress, modulating the central cholinergic system, and improving microglia-mediated neuroinflammation. And combined with aromatherapy, the unique advantages and potential of natural EOs in the treatment of AD and other disorders were discussed. This review hopes to provide scientific basis and new ideas for the development and application of natural medicine EOs in the treatment of CI.
Population aging has become a serious problem in many parts of the world, and with the advent of population aging, CI, which is closely related to age, has shown a significant increase in recent years. Cognition is an indispensable ability in everyday life that enables people to live easily, solve problems and situations, and continue to learn and correctly process information from the environment for subsequent retrieval and use (Gutiérrez Rodríguez and Guzmán Gutiérrez, 2017). CI is impairment of one or more aspects of cognitive processes, including reduced efficiency or impaired functioning of processes in memory, computation, orientation, structural ability, executive ability, language comprehension and expression, and application, and can range from mild CI to dementia (China expert consensus group on prevention and treatment of cognitive dysfunction, 2006). Neurocognitive disorders, especially major neurocognitive disorders (Disorders and Specialized Committee on Cognitive Disorders, N.B., Chinese Medical Association, 2018), have serious consequences for individuals and families, health care systems, and the economy. From 1990 to 2016, the number of people suffering from dementia has more than doubled worldwide (Hugo and Ganguli, 2014), and in 2019, the number of people with dementia worldwide is estimated to be 57.4 million, which is expected to increase to 83.2 million cases by 2030, and this number is expected to reach 152.8 million in 2050 (GBD 2019 Dementia Forecasting Collaborators, 2022). The World Health Organization released the Global Status Report on Addressing Dementia in Public Health stating that the global cost of dementia is approximately $1.3 trillion in 2019 and is expected to increase to $1.7 trillion by 2030. The prevalence of dementia increases exponentially with age, doubling every 5 years after age 65, with a prevalence of 5 to 10% in high-income countries for people aged 65 and older, and is typically higher in women than in men, in large part because women live longer than men (Jia et al., 2020). At the same time, as people’s lifestyles change and living standards continue to improve, common risk factors such as hypertension, diabetes, atrial fibrillation, stroke, and smoking are increasing, and these risk factors lead to progressive damage to large, medium, and small cerebrovascular arteries, which subsequently cause neurodegenerative pathologies and cognitive dysfunction. As a result, the incidence of CI is expected to continue to increase continuously, it is posing an increasingly serious threat to human health, and is emerging as one of the major health problems of society. However, as of today, only 13 of the 193 member countries of the WTO have national dementia control, and there are no first-line therapeutic drugs for allopathic treatment or reversal of the disease course, which can only alleviate CI in early stage patients and provide moderate symptom improvement, but cannot stop the progression of the disease. Thus, the development of therapeutic modalities and drugs that are effective, easy to use, and suitable for long-term administration is of great importance for the treatment of neurological disorders such as CI.
Volatile oils of natural drugs come from nature and are also known as EOs, which are volatile oily liquids, most of which have aromatic odors and are inexpensive and easily available. In basic and clinical studies, volatile aromatic natural medicines or EOs have been found to relieve tension and anxiety, improve depression, improve cognition, and enhance sleep quality (Guo et al., 2020; Cheong et al., 2021; Dos Reis Lucena et al., 2021; Wang et al., 2021a). AD is a major form of dementia characterized pathologically by the abnormal deposition of β-amyloid (Aβ) outside brain cells leading to senile plaques (SPs) and the hyperphosphorylation of Tau proteins within brain cells leading to neuronal fibrillary tangles (NFTs; Durairajan et al., 2022). Aβ self-aggregation and abnormal aggregation of Tau proteins form SPs and NFTs, which in turn disrupt the structure and function of neuronal cells, impairing the antioxidant balance of cells, causing oxidative stress, inflammatory immune response, and mitochondrial energy disorders, further promoting Aβ deposition and exacerbating cognitive dysfunction, while pro-inflammatory factors released by glial cells exacerbate the inflammatory response, leading to severe loss, degeneration and functional deficits of cholinergic neuronal sites, damaging acetylcholinergic nerves, and leading to impaired learning and memory and cognitive impairment. The therapeutic effects of volatile oils on CI such as AD have been reported in several papers. This paper reviews and summarizes the plant volatile oils and monomeric compounds with cognitive improvement effects, as shown in Tables 1 and 2. The results indicate that plant volatile oils mainly exert their effects on controlling AD and improving cognition through their compounds such as monoterpenes, sesquiterpenes and phenylpropanoids by inhibiting the deposition of Aβ, hyperphosphorylation of Tau, and regulating the central cholinergic system, thereby exerting a response state against inflammation and oxidative stress. Thus the natural drug volatile oil may be a potential novel drug for the treatment of cognitive impairment, and aromatic substances sniffing may be a new way to prevent or delay cognitive impairment at an early stage, which has a promising future in the field of cognitive dysfunction treatment.
In this review, we summarize the available drugs for the treatment of cognitive disorders or anti-dementia, review the history of the use of plant volatile oils to combat cognitive disorders in some countries, and then summarize the material basis of natural plant volatile oils to improve cognition, and explain the mechanism of their cognitive improvement effect, hoping to provide a theoretical basis for future researchers to explore more suitable routes of administration and forms of formulation for the development of potential anti-cognitive drugs.
At present, the treatment of cognitive disorders such as dementia is mainly symptomatic and there is no exact and effective treatment. According to multinational guidelines (O’Brien et al., 2017; Disorders and Specialized Committee on Cognitive Disorders, N.B., Chinese Medical Association, 2018; Kandiah et al., 2019; Ismail et al., 2020) the listed anti-dementia drugs are mainly classified as cholinesterase inhibitor (ChEI), such as donepezil, carboplatin, galantamine, Huperzine A; N-methyl-D-aspartate receptor antagonists, such as meperidine hydrochloride; antioxidants, such as Ginkgo biloba extract; pro-intellectual drugs, such as olacetam, piracetam, aniracetam; ergot alkaloids, such as dihydroergotoxine mesylate, nicergoline and other drugs. The main risk factors for CI such as AD, VCI, and diabetes-related cognitive impairment are summarized in Table 3, A summary of current clinical medications for the treatment of cognitive disorders was made and is shown in Table 4.
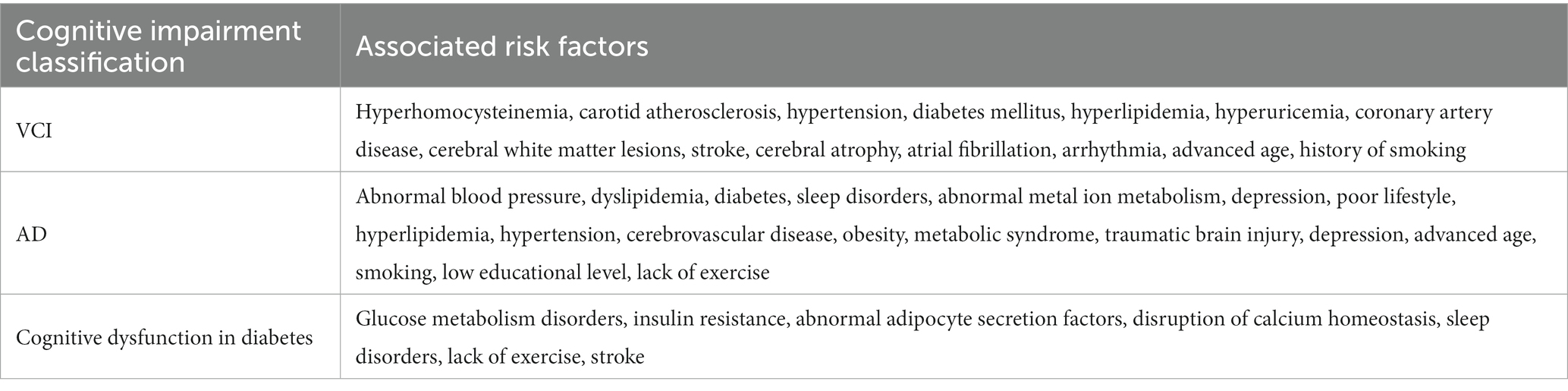
Table 3. Risk factors related to cognitive impairment (Li et al., 2014; Gottesman et al., 2017; Walker et al., 2017; Zheng and Chen, 2018; Gannon et al., 2019; Silva et al., 2019; Duan and Wen, 2020; Lyu et al., 2020).
The use of aromatic herbs and plants to treat various disorders of the mind and body has been documented long ago, and certain medicinal herbs are the source of the aromatic oils refined today. Volatile oils have been used in many countries and nations to exert a waking effect and improve cognition, as shown in Table 5. In classical Chinese medical texts, the use of intranasal administration of aromatic substances for the treatment of confusion is well documented, for example, in the “Li Yue Pian Wen,” it is written that people who suffer from unconsciousness during a stroke can use Croton tiglium oil and soapberry powder to smoke their noses to wake them up, while in India, Iran, and other countries, there are also applications of natural medicines such as volatile oils to improve cognition, and these long-term uses have provided a strong basis for modern research on volatile oils For example, modern pharmacological studies have shown that Kaixin Powder can improve psycho-behavioral symptoms of AD by adjusting transmitter homeostasis, inhibiting inflammation, protecting mitochondria, and reducing neuronal damage; Yuanzhi Powder can improve psycho-behavioral symptoms of AD by inhibiting oxidative damage, reducing Tau protein phosphorylation, and It can exert puzzling effects by inhibiting oxidative damage, reducing Tau protein phosphorylation, and regulating cholinergic effects. Studies on the action of these formulas and the volatile oils in them, and modern mechanisms we have also summarized in Table 5.
SP caused by Aβ self-aggregation, NFTs formed by abnormal tau protein aggregation and the chronic inflammation and oxidative stress they all can lead to neuronal degeneration, neuronal apoptosis, and CI. In addition, severe deletion, degeneration and functional defects of cholinergic neuronal sites, which damage acetylcholinergic nerves, also lead to learning memory impairment and CI. It can be seen that neurotoxicity of Aβ, NFTs, cholinergic system dysfunction, oxidative stress, and chronic inflammation are the main causes of cognitive dysfunction (Figure 1), and natural plant volatile oil treatment improves CI mainly through these pathways (Figures 2, 3).
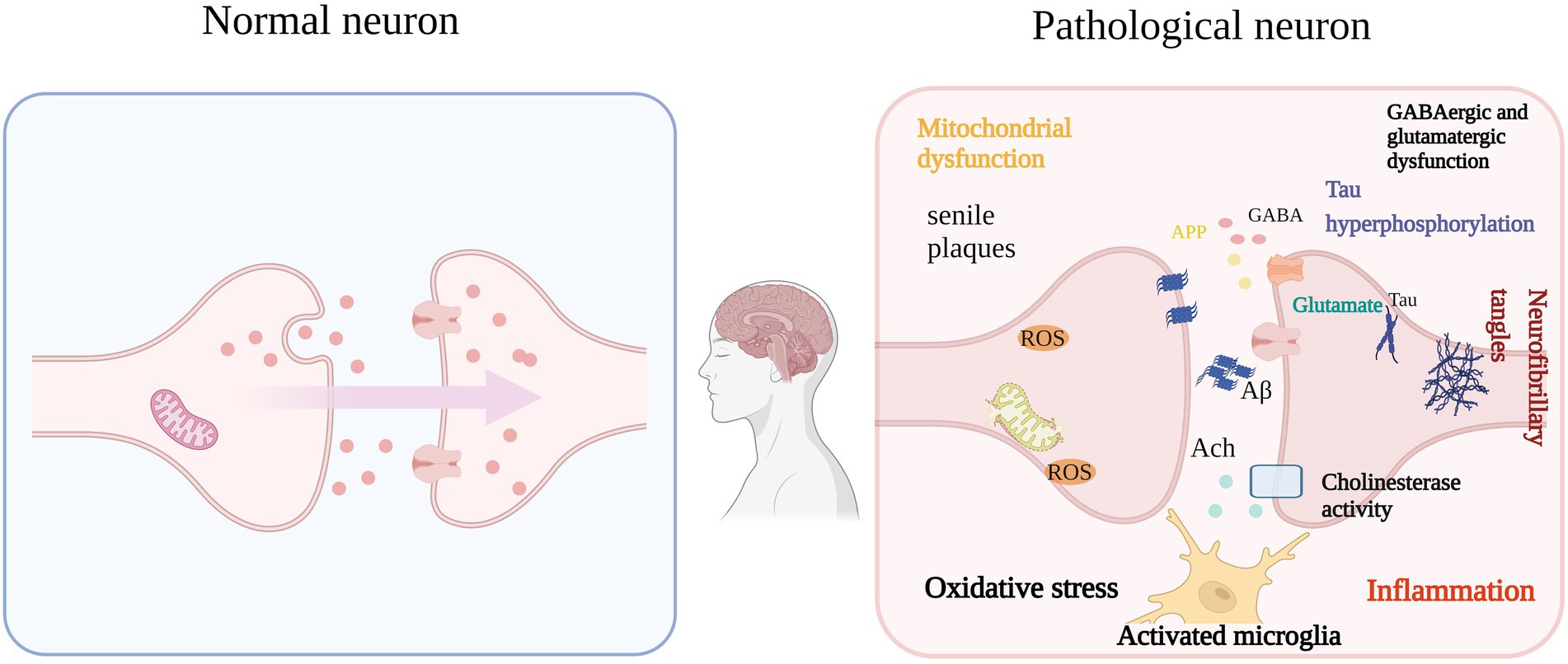
Figure 1. Aβ, NFTs, OS, etc. are the main causes of IC (e.g.: AD). The self-aggregation of Aβ and NFTs formed by abnormal aggregation of tau protein in the brain, functional degradation of central cholinergic neurons, and their resulting OS, microglial activation and proinflammatory cytokine release. The vicious circle eventually leads to cognitive impairment.
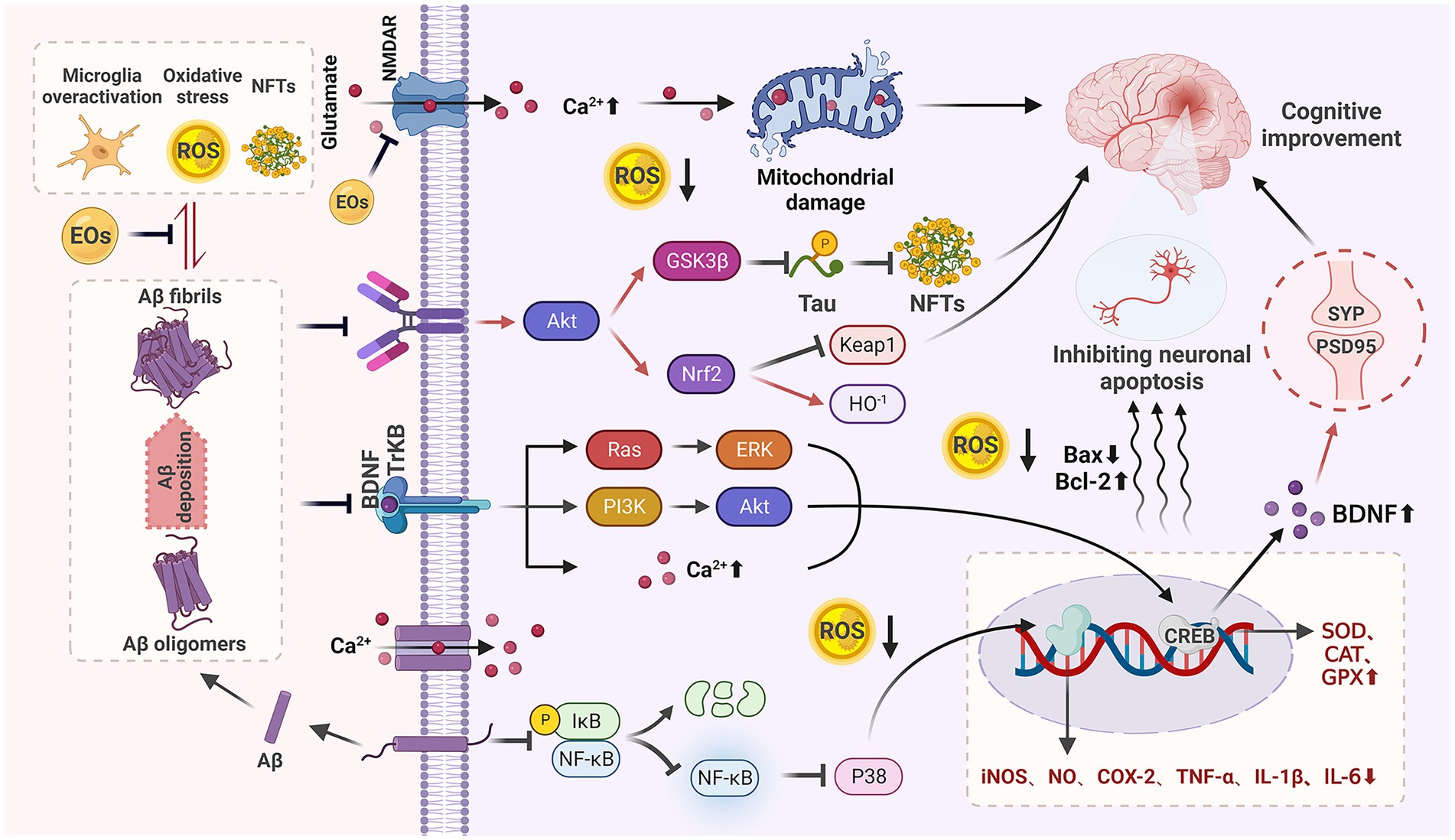
Figure 2. Anti-CI mechanism of natural EOs. Natural EOs play a role by reducing Aβ neurotoxicity, anti-oxidative stress, improving microglia-mediated neuroinflammation, regulating BDNF, and inhibiting neuronal apoptosis.
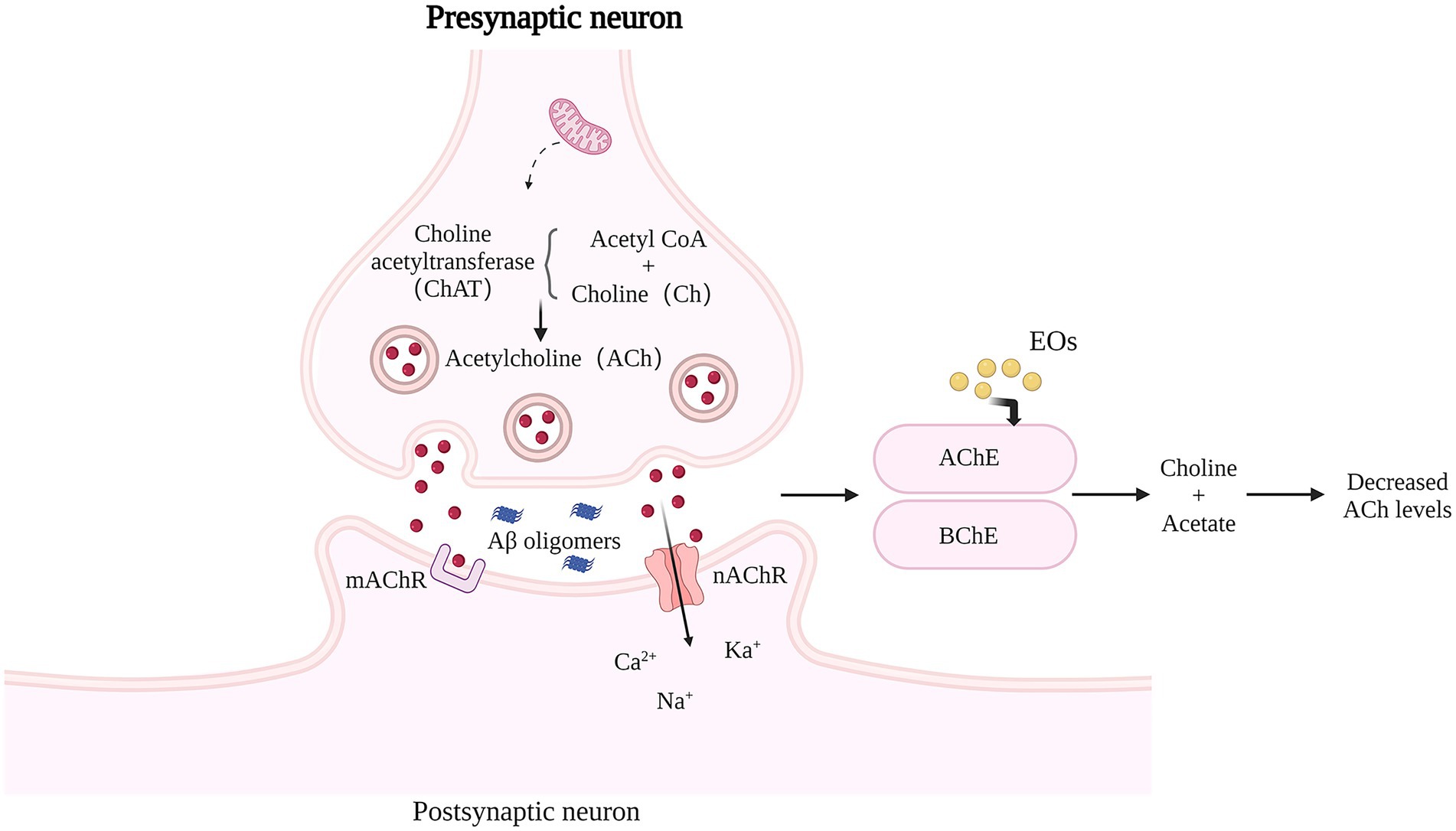
Figure 3. Effects of Natural EOs on brain cholinesterase activity. AChE or BChE decomposes ACh into choline and acetate. ACh level in AD brain is low, and cholinergic nerve transmission is impaired. Natural EOs significantly enhanced the specific activity of AChE, and increased the amount of ACh that remained in the synaptic space and interacted with postsynaptic receptors.
Aβ is a 36–43 amino acid polypeptide produced by β-secretase (also known as β-site amyloid cleavage enzyme, BACE) and γ-secretase mediated cleavage of amyloid precursor protein (APP; Chen and Yan, 2010), The most abundant forms of Aβ are the residue 40 and 42 peptide variants Aβ1-40 and Aβ1-42, with Aβ 42 being more hydrophobic at the C-terminus and more likely to accumulate in brain tissue and cause disease. Soluble Aβ aggregates are usually classified as oligomers and protofibrillar proteins (Tiwari et al., 2019). The main component of amyloid plaques in the brain is the larger amyloid fibrils aggregated by Aβ monomers. It has been shown that Aβ monomers do not directly affect neuronal function, but the soluble oligomers produced after monomer hydrolysis are the key factors affecting cognitive function in AD patients (Tu et al., 2014). For example, Aβ oligomers can bind to N-methyl-D-aspartic acid receptor (NMDAR) to increase Ca2+ concentration in neuronal cells, which leads to increased intracellular oxidative stress and dendritic spine loss, resulting in neuronal cell death (Birnbaum et al., 2015; Huang et al., 2015) and it has also been shown that Aβ-formed amyloid fibrils can enter the hydrophobic layer of the cell membrane and cause cell membrane damage by inducing cytoskeletal protein cross-linking (Sasahara et al., 2014). The accumulation of fibrillar amyloid in the brain of AD patients can cause permanent destruction of synapses, leading to cognitive and memory loss (Forny-Germano et al., 2014).
In addition, there is growing evidence that the imbalance of Aβ production and clearance in the brain of AD patients is a central issue contributing to the development of AD. Under physiological conditions, Aβ produced by neurons has multiple degradation pathways, including clearance by glial cells, degradation by proteases, transport by LRP-11 mediated by vascular endothelial cells, or eliminated via perivascular drainage pathways. APOE is a plasma protein involved in cholesterol transport, synthesized mainly by the brain and liver, involved in the regulation of Aβ production and influencing the clearance of Aβ by neurons and astrocytes (Liu and Cao, 2020). When aging and multiple risk factors reduce or disrupt the body’s ability to clear Aβ, resulting in the untimely deposition of Aβ produced by neurons in different parts of the brain, the abnormal accumulation of Aβ in the brain can damage the structure and function of neuronal cells, participate in oxidative damage, accelerate cellular aging, and ultimately lead to cognitive decline (Cheng et al., 2020; Wang et al., 2020). Aβ can also promote other pathophysiologies, such as increasing tau protein phosphorylation and thus promote neurogenic fiber tangling process, induce apoptosis of neuronal cells, cause cholinergic neurological damage, induce oxidative stress to increase reactive oxygen species (ROS) production, damage biomolecules, promote mitochondrial energy disorders also stimulate microglia and astrocytes to release large amounts of pro-inflammatory cytokines, turning an acute response under normal conditions into chronic inflammatory damage, etc.
lemon EO of citrus origin (Liu et al., 2020) was able to inhibit the accumulation of amyloid and reduce neuronal loss, while improving learning and memory after neurodegeneration in APP/PS1 mice. REO (Zhu et al., 2017) significantly suppressed Aβ deposits and reduced the Aβ oligomers to alleviate the toxicity induced by Aβ overexpression, Further, REO markedly activated the expression of GST-4 gene, which supported that REO reduced Aβ oligomers to treat AD worms through SKN-1 signaling pathway. It has been shown that a certain concentration of Acorus tatarinowii Schott EO (Ma et al., 2007) can effectively convert Aβ 25–35 from α-helix to β-fold, affecting its secondary structure and thus preventing Aβ aggregation and fibril formation. Intervention of APP/PS1 mice with the active ingredient of Acorus tatarinowii Schott, β-Asarone, revealed that the expression of p-mTOR and p62 was reduced in the treated group and the expression of p-Akt, Beclin-1, and LC3B was reduced in the treated group compared to the blank group, indicating that β-Asarone could inhibit Beclin-1 and LC3B by upregulating the PI3K/Akt/mTOR signaling pathway to inhibit Beclin-1-dependent autophagy to attenuate Aβ1-42-induced neuronal toxicity and improve cognitive performance in AD mice (Xue et al., 2014; Deng et al., 2016). β-Asarone can also reduce Aβ production by inhibiting the level of APP expression in the hippocampus and cortical layer of the brain in demented mice, exerting a protective and restorative effect on learning-related synapses in the hippocampus (Zhang et al., 2014). In addition, Oxyphylla A (from Alpinia oxyphylla Miq. plant EO; Bian et al., 2021) was able to reduce APP and Aβ protein expression levels and improve cognition by exerting antioxidant effects through the Akt-GSK3β and Nrf2-Keap1-HO-1 pathways, and Limonene (Piccialli et al., 2021) counteracts the increase in ROS production triggered by Aβ1-42 oligomers, thus preventing the upregulation of KV3.4 activity and cell death in primary cortical neurons, exerting a neuroprotective effect. Linalool (from Lavandula angustifolia EO, Melissa officinalis EO, Rosmarinus officinalis EO, Cymbopogon citratus EO; Sabogal-Guáqueta et al., 2016) delayed cerebral amyloidosis, including amyloid deposits and β-amyloid peptide abundance, reduces intracellular ROS production, significantly decreases the levels of IL-1β, iNOS, COX-2, p38 MAPK, and exerts anti-inflammatory and antioxidant beneficial effects on AD. These EOs and their components exert neuroprotective effects against Aβ-induced toxicity through anti-amyloid, anti-inflammatory and antioxidant effects.
Regarding the pathogenesis of CI, many studies have confirmed the association with oxidative stress damage. Oxidative stress is not only involved in the initial stages of AD, but also influences disease progression by activating various cellular signaling pathways that lead to the formation of toxic substances (Veurink et al., 2020). OS response refers to a state of imbalance between oxidation and antioxidant in the body that can lead to the accumulation of ROS, and with a large accumulation of ROS, oxidative damage reactions can occur to macromolecules, which in turn can cause damage or loss of tissue and organ function. The brain usually requires higher levels of oxygen to perform its extensive synaptic functions and is highly susceptible to oxidative stress, especially in hippocampal and cortical regions (Kamat et al., 2016). Under normal physiological conditions, antioxidant enzymes are able to overcome oxidative stress generated in vivo (Beckhauser et al., 2016), but in AD patients and aging brains, the accumulation of Aβ, mitochondrial dysfunction, etc. leads to elevated ROS levels, which further promote the aggregation of Aβ due to oxidative damage severely affecting the function of various proteins, enzymes, lipids and ion channels thus causing neurotoxicity decreasing hippocampal plasticity and directly participating in the pathogenesis of AD (Wang et al., 2023). In addition, oxidative stress can release a variety of cytotoxic substances that activate microglia and directly trigger neuronal damage and death (Knezevic and Mizrahi, 2018), and with the activation of apoptosis and the reduction of antioxidant enzymes, the accumulation of ROS/RNS has a catastrophic effect on cholinergic areas involved in cognitive performance, ultimately leading to the development of cognitive dysfunction. There are many endogenous antioxidant enzymes, HO-1 being the most potent one, and Nrf2, an upstream transcription factor that regulates HO-1 expression, and antioxidant therapies based on Nrf2 and HO-1 targets may be useful in CI prevention and treatment (Ali et al., 2018; Osama et al., 2020). Free radicals generated during oxidation are neutralized to non-free radical forms by antioxidant enzymes such as CAT, SOD, GPX. However, when free radical production is abnormally high or the immune system is depleted, free radical scavengers need to be given externally. Many plant EOs have good antioxidant, free radical scavenging effects. Chimonanthus nitens Oliv. EO (Wang et al., 2021c), Schisandra chinensis Baill. EO (Yang et al., 2018), Tetraclinis articulata EO (Sadiki et al., 2019), Pinus halepensis EO (Postu et al., 2019) and other EOs of various plants, etc. all showed strong antioxidant effects in CI, increased the activity levels of SOD, CAT and GPX in hippocampal tissues, and significantly improved the Aβ1-42-induced decrease in GSH levels, increase in protein carbonyl and MDA, thus reducing oxidative stress and improving Aβ1-42-induced memory impairment in rat hippocampus. In addition, the terpenoids such as α-Cyperone (Huang et al., 2018), 1,8-cineole (Khan et al., 2014), Linalool (Yuan et al., 2021), Thymoquinone (Abulfadl et al., 2018), Terpinolen (Bahareh et al., 2020), oxyphylla A (Bian et al., 2021) etc. in EOs were able to increase antioxidant enzyme activity in vivo and improve cognition by ameliorating oxidative stress. Studies have shown that α-Cyperone and oxyphylla A antioxidant mechanisms are associated with increased expression of Nrf2 and its downstream genes HO-1 and NQO1 in the brain and inhibition of Nrf2 regulatory protein Keap1 expression, which exert antioxidant effects through the Akt/Nrf2-Keap1-HO-1 pathway to improve cognition.
The central cholinergic nervous system (CNS), plays an important regulatory role in cognitive functions such as learning and memory, and some studies have shown that reduced neuronal activity due to degeneration of cholinergic neurons is one of the pathological factors for the appearance of symptoms in patients with cognitive dysfunction (Hampel et al., 2018). Intracerebral acetylcholine (ACh) is present in the vesicles of cholinergic neurons and is the neurotransmitter most closely related to learning and memory identified so far (Mesulam, 2013), it conducts signals related to cognition, learning and memory, and its metabolic processes are closely related to AD. A decrease in the number of central cholinergic neurons, a decrease in ACh synthesis, and a decrease in ACh receptors may lead to learning memory impairment. ACh is synthesized from choline and acetyl coenzyme A catalyzed by Recombinant Choline Acetyltransferase (Hampel et al.) and is rapidly hydrolyzed by acetylcholinesterase (Doody et al., 2007) once released from the vesicles. ChAT and AchE work together to maintain the dynamic balance of ACh. Clinical studies have found that the degree of CI in VD patients is associated with a decrease in ACh synthesis and a relative increase in AChE activity, and in particular, a sustained decrease in hippocampal ACh content may be an important factor in the development of VD (Watanabe et al., 2008). The increased central AchE content and the disruption of the body’s antioxidant enzyme system severely affect the cognitive pathways in MCI patients. In the neocortex and hippocampus, ACh is not only involved in the activity of a large number of neurons, but also regulates synaptic plasticity.
Partial ChEI increase the availability of acetylcholine at brain synapses and are one of the few pharmacological therapies clinically proven to treat CI. Capatina et al. (2020) studied a zebrafish (Danio rerio) model of memory impairment induced by scopolamine (Sco) and found a significant increase in AChE-specific activity in sco-treated zebrafish compared to controls, While TEO treatment resulted in a significant decrease in AChE-specific activity. Aazza et al. (2011) demonstrated that TEO exhibited anti-AChE activity mainly due to the presence of the phenolic monoterpenes thymol and carvacrol. In addition, EOCO (Bae et al., 2012), AOEO (Ma et al., 2018), Zataria multiflora Boiss. EO (Majlessi et al., 2012), all significantly inhibited AchE activity and increased Ach levels in the brain and reduced neuronal apoptosis. Rosmarinus officinalis EO (EORO) produced a significant improvement in the rate of spontaneous alternation behavior, improving cognitive function by activating the central nervous system. Bergapten (Kowalczyk et al., 2020), β-asarone (Saki et al., 2020), Thymoquinone (Abulfadl et al., 2018) and other active components of essential oils were also able to inhibit AChE activity in the brain. Boiangiu et al. (2020) made a blend of essential oils (MO) with limonene (91.11%) as the main chemical component and studied its cognitive facilitation effect on scopolamine-induced amnesia in rats. It was shown that MO inhibited the oxidative stress state and the activity of AChE and BChE in the brain of model mice and improved the memory impairment induced by Sco by restoring the activity of the cholinergic system and the antioxidant status of the brain.
Numerous studies have shown that inflammation has an important role in the pathogenesis of AD, inflammatory processes may promote neuronal loss and cognitive decline (Hayes et al., 2004; Cunningham et al., 2005; Kim and Joh, 2006), brain inflammation appears to play a neuroprotective role in the acute phase response but becomes detrimental in the chronic response to toxic injury (Kim and Joh, 2006). Inflammatory inducers, such as LPS, activate microglia to promote the degradation of Aβ. Impaired microglia function promotes the progression of AD and leads to increased Aβ accumulation in the brain (Choi et al., 2009; Xiao et al., 2011). In addition to providing beneficial effects to the host, Aβ or APP-activated microglia release a variety of pro-inflammatory and toxic products, including ROS, nitric oxide (NO), and cytokines such as interleukin 1β (IL-1β), interleukin 6 (IL-6), and tumor necrosis factor α (TNF-α), ultimately leading to an increased inflammatory response and severe neuronal loss (Sajja et al., 2016). In turn, elevated IL-1β levels exacerbate the accumulation of Aβ (Goldgaber et al., 1989; Heneka et al., 2015) and increase the production of other cytokines (e.g., IL-6) thereby activating CDK-5 kinase and leading to tau hyperphosphorylation (Quintanilla et al., 2004). This means that microglia have the same dual function in the protection of cognition. Some evidence suggests that long-term use of NSAIDs reduces the risk of AD and delays disease progression, possibly through the inhibition of cyclooxygenase (COX) and activation of peroxisome proliferator-activated receptor gamma (PPARγ; Gasparini et al., 2004), thereby decreasing prostaglandin synthesis and reducing cytokine secretion.
Mohamed et al. (2021) investigated the AD-related anti-inflammatory activity of SO, and found that SO significantly improved AlCl3-induced learning and memory impairment in mice, decreased AChE and Aβ levels, down-regulated TNF-α and IL-1β, decreased NF-κB and p38MAPK expression levels, and increased BDNF and PPAR-γ expression, It was shown that SO attenuated neuroinflammation and promoted cognitive recovery by regulating NF-κB/p38MAPK/BDNF/PPAR-γ signaling pathway. Ligusticum chuanxiong hort EO (Zhou et al., 2019), reduced the level of inflammatory factors TNF-α and NO, and its mechanism of action to improve CI in VCI mice may be related to inhibition of brain inflammatory response and reduction of neuronal damage. Phenylallyl compounds in Cinnamomum cassia Presl significantly inhibited the increase in COX activity and prostaglandin E2 release caused by IL-1 stimulation of brain microvascular endothelial cells, which in turn improved the memory function of APP transgenic AD mice (Ran et al., 2017). In addition, Schisandra chinensis (Turcz.) Baill. EO (Xu et al., 2019) was able to reduce the phosphorylation of p-38, attenuate the release of pro-inflammatory cytokines IL-1β, 1L-6, and TNF-α, and ameliorate microglia hyperactivation by inhibiting the activation of NF-κB/MAPK pathway. 1,8-cineole (Khan et al., 2014), Linalool (Yuan et al., 2021), and β-caryophyllene (Sudeep et al., 2021) in the essential oils of the plant were able to reduce the levels of the pro-inflammatory markers IL-1β, iNOS, COX-2, and p38 MAPK. α-Cyperone (Huang et al., 2018) from Cyperus rotundus L. EO was able to upregulate Nrf2, HO-1, p-Akt, and downregulate p-NF-κB, p65, TNF-α, IL-6, IL-1β. It inhibited inflammatory cytokine production in BV-2 cells by activating Akt/Nrf2/HO-1 and inhibiting NF-κB pathway and thus exerted neuroprotective effects.
Hyperphosphorylated Tau-rich neurofibrillary tangles (NFTs) are another neuropathological hallmark of AD. Under physiological conditions, Tau is the most abundant microtubule-related protein in neurons, mainly concentrated in neurons in the frontal, temporal, hippocampal and entorhinal regions of the brain, as well as in the axons of peripheral nerves (Gao and Kong, 2016). Its role in initiation and stabilization in the assembly of microtubules. This is critical for axonal transport and neuronal function (Wang and Tian, 2012). While the hyperphosphorylation of Tau protein accelerates its accumulation in the brain and cerebrospinal fluid and directly promotes the formation of NFTs, the hyperphosphorylated Tau protein competes with microtubule proteins such as MAP1 and MAP2 to bind microtubules leading to microtubule depolymerization and hindering axoplasmic transport, resulting in reduced binding of Tau protein to microtubule proteins, and the Tau proteins shed from microtubule proteins aggregate with each other to form the fibrillar material with neurotoxic properties, NFTs (Horvath et al., 2014). NFTs can reduce cis-axonal transport without altering microtubule integrity, which in turn induces neuronal degeneration and ultimately leads to cognitive decline and dementia. Once NFTs are formed, they can spread to other areas of the brain. Moreover, the abnormal secretion and accumulation of Aβ in neuronal cells can overactivate Tau protein kinase and promote Tau protein phosphorylation, which in turn triggers a chronic inflammatory response, activates apoptosis, generates incomplete metabolized free radicals, and causes an imbalance between intracellular oxidative and antioxidant effects in neurons, resulting in the death of a large number of neurons and glial cells (Nussbaum et al., 2012). Thus, a therapeutic approach to the clearance of Tau aggregates appears to be a viable way to reduce their pathology (Selvarasu et al., 2022; Sreenivasmurthy et al., 2022), and one study found that Tetrandrine, a medicinal natural product derived from Stephania tetrandra S. Moore, enhances autophagy-lysosomal pathway (Panara et al., 2020) function and reduces pathological Tau by Enhancement of glial cell clearance and reduction of pathological Tau transmission (Tong et al., 2022). It has been found that various plant EOs can act as a strategy to improve CI by preventing Aβ deposition and Tau hyperphosphorylation. Jeon et al. (2011) found that early inhalation of SHXW EO improved CI caused by Aβ (1–42) and inhibited Aβ-induced Tau phosphorylation by suppressing the activation of JNK and p38 in the brain. Lee et al. (2021) fed AD mice Induced by Aβ1-40 with the same essential oil content of Litsea cubeba Persoon Powder, and showed that it reduced P-Tau content and Aβ plaques, resulting in about 3–8% reduction in brain atrophy.
Several studies have shown that Aβ downregulates response element binding protein (CREB)-mediated transcription (España et al., 2010; Pugazhenthi et al., 2011), that CREB-mediated gene expression is impaired in AD brains, and that CREB-regulated BDNF levels are reduced.BDNF is the most widely distributed neurotrophic growth factor in the CNS and plays an important role in brain regions involved in learning, memory, and higher cognitive functions (Bekinschtein et al., 2008). activation of CREB promotes transcription of key proteins of activity-dependent plasticity, particularly BDNF. Decreased levels of BDNF may contribute to the degeneration of specific neuronal populations and the progressive atrophy of neurons in AD-affected brains (Cowansage et al., 2010). Altered BDNF expression levels or disruption of the BDNF–TrkB signaling pathway may lead to synaptic loss and cognitive dysfunction (Song et al., 2015). Studies have shown that even after clinical onset of AD, increasing the expression of BDNF by modulating the mRNA level of CREB may spare patients from memory deficits and cognitive dysfunction (Rosa and Fahnestock, 2015). Increasing the mRNA expression of BDNF and its receptor tyrosine kinase-coupled receptor (TrkB) in the hippocampus also improves cognition to some extent (Amidfar et al., 2018). Ma et al. studied the improvement effect of Oil extract from Alpinia Oxyphylla Miq.fruit (Ma, 2019; AOFOE) on scopolamine-induced learning and memory impairment in mice. The results showed that AOFOE could significantly increase the mRNA levels of BDNF and CREB in the hippocampus of learning and memory impaired mice. By regulating BDNF, CREB is further activated, thereby exerting neuroprotective effects. Lemon EO of citrus origin (Liu et al., 2020) is able to enhance memory by enhancing synaptic plasticity by increasing BDNF, PSD95 and synaptophysin. In addition, the sesquiterpene β-caryophyllene decreased the proBDNF/mBDNF ratio and was able to increase the expression of TrkB. Kim et al. (2018) found that 6-Gingerol can increase the protein expression of BDNF by activating the Akt-CREB signaling pathway.
In addition, Neuronal apoptosis is one of the important mechanisms by which many neurodegenerative diseases arise. Among the major genes regulating neuronal apoptosis, the interaction between the pro-apoptotic Bax protein and the anti-apoptotic Bcl-2 protein maintains a dynamic balance between neuronal proliferation and apoptosis. Angelica sinensis (Olive.) Diels EO (Cui, 2015), Lavandula angustifolia Mill. EO (Xu et al., 2016), AOFOE (Ma, 2019), SHXW EO (Jeon et al., 2011) and other plant essential oils can promote the expression of Bcl-2 protein with neuroprotective effect in brain tissue, reduce the expression of apoptosis Bax protein, thereby inhibiting nerve cells Apoptosis, accelerates the recovery of neural function, and together play a role in brain protection and improve cognition. Changes in central amino acid levels, especially the imbalance of Glu and γ-aminobutyric acid (GABA) levels, are key factors contributing to neuronal damage. The balance of glutamate Glu and GABA levels is important for maintaining cognitive function in the hippocampus (Sartorius et al., 2007). Studies have shown that eucalyptus oil increases brain GABA levels (Yadav et al., 2019). α-Asarone (Li et al., 2019) significantly improves behavioral performance in an ethanol-induced memory impairment model in mice by controlling calcium overload, decreasing synaptophysin I (SYN I) activity, reducing Glu release, and normalizing Glu transport function by decreasing Glu concentration and The underlying mechanism is to regulate the calcium signaling cascade to correct the function of related proteins by reducing Glu concentration and regulating the level of phosphorylated calcium/calmodulin-dependent protein kinase II (pCaMKII) to reduce the overactivity of Glu receptors AMPA and NMDA to maintain the Glu and GABA levels.
CI has many pathogenic factors, a long course, and complex pathological mechanisms that are not fully understood. Currently, the treatment for improving cognitive function in patients with MCI, VCI, diabetes-related CI, depression-related CI, and AD is based on symptomatic treatment to deal with associated risk factors and antidementia therapy. As of today, there are no medications available to slow the progression of AD (Grossberg et al., 2019), commercially available ChEI and NMDA (memantine) only provide symptomatic relief, their clinical effectiveness remains controversial, and the results of meta-analyses of their effects on behavioral outcomes are inconsistent (Doody et al., 2007; Hansen et al., 2008; Tan et al., 2014; Matsunaga et al., 2015). And ChEI often lead to adverse effects of gastrointestinal disturbances, significantly increasing the risk of dizziness, nausea, anorexia, vomiting and diarrhea, memantine is usually well tolerated but also causes adverse effects such as constipation, dizziness, headache, hypertension and drowsiness. Thus, the search and discovery of drugs and methods to improve CI remains urgent at the present time.
As a multi-causal and heterogeneous disease, it is difficult to achieve the desired effect with single drug and single target treatment for dementia, and synergistic treatment with multiple links and targets may be the future trend in drug treatment and research and development. Chinese medicine is expected to be a new source of drugs as it contains multiple active ingredients and can act on multiple targets at the same time, which is in line with the multi-factorial and multi-pathological pathogenesis of the disease. In addition, Chinese medicines have relatively low toxic side effects and are safe, and can be used in combination with Western medicines to achieve good therapeutic results. Chinese volatile oils are the most representative active components of aromatic Chinese medicine, with the main active ingredients being aldehydes and esters, monoterpenes and sesquiterpenes, and other substances that have been used for generations to alleviate the symptoms of AD and other dementias. These small molecule active ingredients are highly lipid soluble and can cross cell membranes and the BBB (Agatonovic-Kustrin et al., 2019), compensating for the low permeability and pharmacokinetic problems of some drugs. The preparation of essential oils extracted from natural plants into appropriate dosage forms and their entry into the body to exert therapeutic effects through inhalation, massage, intestinal and oral routes is known as aromatherapy. Aromatherapy is a branch of Western complementary and alternative medicine that is highly effective in improving cognition and is convenient and inexpensive, with few side effects. Aromatherapy has been tested in animals, cellular models, and clinical trials in subjects with CI to explore the underlying pathological mechanisms that reduce symptoms or affect the disease in patients with dementia (Forrester et al., 2014), and the results showed that a variety of plant essential oils showed cognitive improvement anti-dementia-related activities in vitro and in vivo, through anti-amyloid, anti-acetylcholinesterase, antioxidant, anti-inflammatory, anti-apoptotic, modulation of cell plasticity and exert neuroprotective and memory enhancing effects.
In addition, aromatic inhalation and olfaction therapy can exert its unique therapeutic advantages in improving cognition, reducing dementia agonistic behavior, and promoting memory. It has been found that olfactory function is closely related to memory, and olfactory impairment may be used as an early diagnosis of neurodegenerative diseases (Doty, 2009). The olfactory receptors on the olfactory epithelium of the nasal mucosa, after sensing aromatic molecules, produce olfactory signals that project through the olfactory bulb to the primary olfactory cortex, and then from the fibers on the primary olfactory cortical centers to the neocortex, hypothalamus, hippocampus and other olfactory secondary cortical centers to regulate central and somatic functions. Whereas the hippocampus is closely related to learning memory, olfactory impulses afferent to the hippocampus can generate olfactory memory, trigger the remodeling of neurosynapses, and directly participate in the process of organizing, recognizing, encoding, and storing learning memory as a way to improve memory and cognition. Moreover, the olfactory pathway is not affected by peripheral metabolism and BBB, and the drug is delivered to the brain non-invasively through the olfactory nerve and trigeminal nerve bypassing the BBB and entering the central nervous system directly after inhalation through the nasal cavity (Crowe et al., 2018). In a clinical study, Jimbo et al. (2009) used aromatherapy to treat 28 patients with dementia with rose-lemon EO in the morning and lavender-Orange peel EO in the evening and found that the essential oil group had a better sense of cognitively relevant spatial orientation compared to the control group. Wang (2014) observed the intervention effect of rosemary inhalation and sniffing method in patients with CI after cerebral infarction, and the results showed that conventional rehabilitation therapy combined with rosemary inhalation and sniffing had the immediate efficacy of improving cognitive function and activities of daily living in patients with CI after cerebral infarction and improving the effectiveness of rehabilitation therapy.
In summary, the natural drug volatile oil may play a role in improving cognition by reducing the neurotoxicity of Aβ, anti-oxidative stress, regulating the central cholinergic system, anti-acetylcholinesterase activity, improving microglia-mediated neuroinflammation, reducing inflammatory factor levels, inhibiting neuronal apoptosis, and regulating the balance of central amino acid levels. Compared to existing drugs, volatile oils from natural medicines are a unique health service resource because they are convenient and inexpensive, have fewer side effects and easily cross the blood–brain barrier. The combination of aromatic inhalation therapy, based on the olfactory pathway, can better reflect the characteristics of volatile oils, which are not affected by peripheral metabolism and BBB to deliver drugs into the brain to improve cognition, and can be used as a treatment or as an adjunctive therapy to improve CI, with clear efficacy and simple safety. However, we also realize that, at present, the research on volatile oils for AD and other cognitive disorders is still mainly preclinical studies, most of which focus on behavioral observations and related biochemical indicators, while clinical studies on aromatherapy interventions for dementia are still relatively few, and there is no unified standardized protocol for aromatherapy interventions for patients with cognitive disorders, and the intervention protocols are mostly developed by researchers themselves. Therefore, there is still a need for in vivo, in vitro experiments and clinical research to deeply explore the substance basis, action targets and mechanisms of natural plant volatile oils for the treatment of AD and other cognitive disorders, and it is believed that with the deepening of basic research and the rapid promotion of translational medicine, more effective and safe aromatic formulations will be used for the improvement and prevention of cognitive disorders. At the same time, we should also be aware that the causes of CI are diverse, with a variety of diseases such as hypertension, diabetes and depression as their risk factors. The causes and mechanisms of AD are still unclear, involving multiple signalling pathways, and there are often interconnections between different pathogenic mechanisms. Thus, the reuse or combination of existing drugs using artificial intelligence technology and network pharmacology may be a potential treatment option for AD clinical trials.
NL conceived the work. YuaH reviewed the manuscript and provided corrections. AS, YL, YM, SY, DL, and JD co-wrote the paper. JW and XL prepared the figures. XH, YueH, and YW commented and corrected the paper. All authors contributed to the article and approved the submitted version.
This work was supported by Chengdu University of Traditional Chinese Medicine Youth Foundation Advanced Talents Project (Number: QJJJ2022014), Chengdu University of Traditional Chinese Medicine Rural Revitalization Project (Number: XCZX2022007), and Sichuan Natural Science Foundation (Number: 2022NSFSC1406).
The author would like to thank Biorender for creating custom scientific figures (https://biorender.com/).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aazza, S., Lyoussi, B., and Miguel, M. G. (2011). Antioxidant and antiacetylcholinesterase activities of some commercial essential oils and their major compounds. Molecules 16, 7672–7690. doi: 10.3390/molecules16097672
Abulfadl, Y. S., El-Maraghy, N. N., Ahmed, A. A. E., Nofal, S., and Badary, O. A. (2018). Protective effects of thymoquinone on D-galactose and aluminum chloride induced neurotoxicity in rats: biochemical, histological and behavioral changes. Neurol. Res. 40, 324–333. doi: 10.1080/01616412.2018.1441776
Agatonovic-Kustrin, S., Kustrin, E., and Morton, D. W. (2019). Essential oils and functional herbs for healthy aging. Neural Regen. Res. 14, 441–445. doi: 10.4103/1673-5374.245467
Ali, T., Kim, T., Rehman, S. U., Khan, M. S., Amin, F. U., Khan, M., et al. (2018). Natural dietary supplementation of anthocyanins via PI3K/Akt/Nrf2/HO-1 pathways mitigate oxidative stress, neurodegeneration, and memory impairment in a mouse model of Alzheimer’s disease. Mol. Neurobiol. 55, 6076–6093. doi: 10.1007/s12035-017-0798-6
Amidfar, M., Kim, Y.-K., and Wiborg, O. (2018). Effectiveness of memantine on depression-like behavior, memory deficits and brain mRNA levels of BDNF and TrkB in rats subjected to repeated unpredictable stress. Pharm. Reports 70, 600–606. doi: 10.1016/j.pharep.2017.12.007
Bae, D., Seol, H., Yoon, H.-G., Na, J.-R., Oh, K., Choi, C. Y., et al. (2012). Inhaled essential oil from Chamaecyparis obtuse ameliorates the impairments of cognitive function induced by injection of β-amyloid in rats. Pharm. Biol. 50, 900–910. doi: 10.3109/13880209.2011.642886
Bahareh, S. N., Shahin, A., Eh, A., and Maryam, G. (2020). Effects of terpinolene and physical activity on memory and learning in a model of Alzheimer’s disease among rats. Antioxidants 14, 25–33. doi: 10.29252/qums.14.10.25
Beckhauser, T. F., Francis-Oliveira, J., and De Pasquale, R. (2016). Reactive oxygen species: physiological and physiopathological effects on synaptic plasticity. J. Exp. Neurosci. 10s1, JEN.S39887–JEN.S39848. doi: 10.4137/JEN.S39887
Bekinschtein, P., Cammarota, M., Katche, C., Slipczuk, L., Rossato, J. I., Goldin, A., et al. (2008). BDNF is essential to promote persistence of long-term memory storage. Proc. Natl. Acad. Sci. U. S. A. 105, 2711–2716. doi: 10.1073/pnas.0711863105
Bian, Y., Chen, Y., Wang, X., Cui, G., Ung, C. O. L., Lu, J.-H., et al. (2021). Oxyphylla a ameliorates cognitive deficits and alleviates neuropathology via the Akt-GSK3β and Nrf2-Keap1-HO-1 pathways in and in murine models of Alzheimer’s disease. J. Adv. Res. 34, 1–12. doi: 10.1016/j.jare.2021.09.002
Binyamin, O., Nitzan, K., Frid, K., Ungar, Y., Rosenmann, H., and Gabizon, R. (2019). Brain targeting of 9c,11t-conjugated linoleic acid, a natural calpain inhibitor, preserves memory and reduces Aβ and P25 accumulation in 5XFAD mice. Sci. Rep. 9:18437. doi: 10.1038/s41598-019-54971-9
Birnbaum, J. H., Bali, J., Rajendran, L., Nitsch, R. M., and Tackenberg, C. (2015). Calcium flux-independent NMDA receptor activity is required for Aβ oligomer-induced synaptic loss. Cell Death Dis. 6:e1791. doi: 10.1038/cddis.2015.160
Boiangiu, R. S., Brinza, I., Hancianu, M., Erdogan Orhan, I., Eren, G., Gündüz, E., et al. (2020). Cognitive facilitation and antioxidant effects of an essential oil mix on scopolamine-induced amnesia in rats: molecular modeling of in vitro and in vivo approaches. Molecules 25:1519. doi: 10.3390/molecules25071519
Capatina, L., Todirascu-Ciornea, E., Napoli, E. M., Ruberto, G., Hritcu, L., and Dumitru, G. (2020). Thymus vulgaris essential oil protects Zebrafish against cognitive dysfunction by regulating cholinergic and antioxidants systems. Antioxidants 9:1083. doi: 10.3390/antiox9111083
Caputo, L., Piccialli, I., Ciccone, R., de Caprariis, P., Massa, A., De Feo, V., et al. (2021). Lavender and coriander essential oils and their main component linalool exert a protective effect against amyloid-β neurotoxicity. Phytother. Res. 35, 486–493. doi: 10.1002/ptr.6827
Chen, X.-Y., Liao, D.-C., Sun, M.-L., Cui, X.-H., and Wang, H.-B. (2020). Essential oil of Acorus tatarinowii Schott ameliorates -induced toxicity in through an autophagy pathway. Oxidative Med. Cell. Longev. 2020:3515609. doi: 10.1155/2020/3515609
Chen, J. X., and Yan, S. S. (2010). Role of mitochondrial amyloid-beta in Alzheimer’s disease. J. Alzheimer’s Dis. 20, S569–S578. doi: 10.3233/JAD-2010-100357
Cheng, Y., Tian, D.-Y., and Wang, Y.-J. (2020). Peripheral clearance of brain-derived Aβ in Alzheimer’s disease: pathophysiology and therapeutic perspectives. Transl. Neuro. 9:16. doi: 10.1186/s40035-020-00195-1
Cheong, M. J., Kim, S., Kim, J. S., Lee, H., Lyu, Y.-S., Lee, Y. R., et al. (2021). A systematic literature review and meta-analysis of the clinical effects of aroma inhalation therapy on sleep problems. Medicine 100:e24652. doi: 10.1097/MD.0000000000024652
China expert consensus group on prevention and treatment of cognitive dysfunction (2006). China expert consensus on prevention and treatment of cognitive dysfunction, Chin. J. Intern. Med., 171–173.
Choi, S.-H., Aid, S., and Bosetti, F. (2009). The distinct roles of cyclooxygenase-1 and -2 in neuroinflammation: implications for translational research. Trends Pharmacol. Sci. 30, 174–181. doi: 10.1016/j.tips.2009.01.002
Cioanca, O., Hritcu, L., Mihasan, M., and Hancianu, M. (2013). Cognitive-enhancing and antioxidant activities of inhaled coriander volatile oil in amyloid β(1-42) rat model of Alzheimer’s disease. Physiol. Behav. 120, 193–202. doi: 10.1016/j.physbeh.2013.08.006
Cioanca, O., Hritcu, L., Mihasan, M., Trifan, A., and Hancianu, M. (2014). Inhalation of coriander volatile oil increased anxiolytic-antidepressant-like behaviors and decreased oxidative status in beta-amyloid (1-42) rat model of Alzheimer’s disease. Physiol. Behav. 131, 68–74. doi: 10.1016/j.physbeh.2014.04.021
Cowansage, K. K., LeDoux, J. E., and Monfils, M.-H. (2010). Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr. Mol. Pharmacol. 3, 12–29. doi: 10.2174/1874467211003010012
Crowe, T. P., Greenlee, M. H. W., Kanthasamy, A. G., and Hsu, W. H. (2018). Mechanism of intranasal drug delivery directly to the brain. Life Sci. 195, 44–52. doi: 10.1016/j.lfs.2017.12.025
Cui, X. (2015). Min Angelia sinensis on vascular cognitive function impairment in model rats apotosis of neurocyte Gansu University of Traditional Chinese Medicine.
Cunningham, C., Wilcockson, D. C., Campion, S., Lunnon, K., and Perry, V. H. (2005). Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J. Neurosci. 25, 9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005
Deng, M., Huang, L., Ning, B., Wang, N., Zhang, Q., Zhu, C., et al. (2016). β-Asarone improves learning and memory and reduces acetyl cholinesterase and Beta-amyloid 42 levels in APP/PS1 transgenic mice by regulating Beclin-1-dependent autophagy. Brain Res. 1652, 188–194. doi: 10.1016/j.brainres.2016.10.008
Deng, M., Huang, L., and Zhong, X. (2020). β-Asarone modulates Beclin-1, LC3 and p62 expression to attenuate Aβ40 and Aβ42 levels in APP/PS1 transgenic mice with Alzheimer’s disease. Mol. Med. Rep. 21, 2095–2102. doi: 10.3892/mmr.2020.11026
Deng, M., Ning, B., Wang, N., Zhang, Q., Zhu, C., and Huang, L. (2019). Effects of volatile oil of shichangpu combined with ginsenoside saponins on choline acetyl transferace, glial fibrillary acidic protein and Histopathological morphology of APP/PS1 double transgenic rats. Acta Chin. Med. 34, 80–85. doi: 10.16368/j.issn.1674-8999.2019.01.019
Disorders and Specialized Committee on Cognitive Disorders, N.B., Chinese Medical Association (2018). China dementia and cognitive impairment diagnosis and treatment guidelines (II): Alzheimer’s disease diagnosis and treatment guidelines. Chin. Med. J. 98, 971–977.
Doody, R. S., Tariot, P. N., Pfeiffer, E., Olin, J. T., and Graham, S. M. (2007). Meta-analysis of six-month memantine trials in Alzheimer’s disease. Alzheimers Dement. 3, 7–17. doi: 10.1016/j.jalz.2006.10.004
Dos Reis Lucena, L., Dos Santos-Junior, J. G., Tufik, S., and Hachul, H. (2021). Lavender essential oil on postmenopausal women with insomnia: double-blind randomized trial. Complement. Ther. Med. 59:102726. doi: 10.1016/j.ctim.2021.102726
Doty, R. L. (2009). The olfactory system and its disorders. Semin. Neurol. 29, 074–081. doi: 10.1055/s-0028-1124025
Duan, Y.-M., and Wen, G.-Q. (2020). Research progress on risk factors and prevention strategies for Alzheimer’s disease. Hainan Med. J. 31, 224–227.
Durairajan, S. S. K., Selvarasu, K., Bera, M. R., Rajaram, K., Iyaswamy, A., and Li, M. (2022). Alzheimer’s disease and other Tauopathies: exploring efficacy of medicinal plant-derived compounds in alleviating tau-mediated neurodegeneration. Curr. Mol. Pharmacol. 15, 361–379. doi: 10.2174/1874467214666210906125318
España, J., Valero, J., Miñano-Molina, A. J., Masgrau, R., Martín, E., Guardia-Laguarta, C., et al. (2010). Beta-amyloid disrupts activity-dependent gene transcription required for memory through the CREB coactivator CRTC1. J. Neurosci. 30, 9402–9410. doi: 10.1523/JNEUROSCI.2154-10.2010
Forny-Germano, L., Lyra E Silva, N. M., Batista, A. F., Brito-Moreira, J., Gralle, M., Boehnke, S. E., et al. (2014). Alzheimer’s disease-like pathology induced by amyloid-β oligomers in nonhuman primates. J. Neurosci. 34, 13629–13643. doi: 10.1523/JNEUROSCI.1353-14.2014
Forrester, L. T., Maayan, N., Orrell, M., Spector, A. E., Buchan, L. D., and Soares-Weiser, K. (2014). Aromatherapy for dementia. Coch. Database Syst. Rev. 25:CD003150. doi: 10.1002/14651858.CD003150.pub2
Gannon, O. J., Robison, L. S., Custozzo, A. J., and Zuloaga, K. L. (2019). Sex differences in risk factors for vascular contributions to cognitive impairment & dementia. Neurochem. Int. 127, 38–55. doi: 10.1016/j.neuint.2018.11.014
Gao, S., and Kong, L.-H. (2016). Mechanism of tau protein hyperphosphorylation and its role in Alzheimer’s disease. Acta Med. Univ. Sci. Technol. Huazhong 45, 711–715.
Gasparini, L., Ongini, E., and Wenk, G. (2004). Non-steroidal anti-inflammatory drugs (NSAIDs) in Alzheimer’s disease: old and new mechanisms of action. J. Neurochem. 91, 521–536. doi: 10.1111/j.1471-4159.2004.02743.x
GBD 2019 Dementia Forecasting Collaborators (2022). Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health 7, e105–e125. doi: 10.1016/S2468-2667(21)00249-8
Goldgaber, D., Harris, H. W., Hla, T., Maciag, T., Donnelly, R. J., Jacobsen, J. S., et al. (1989). Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 86, 7606–7610. doi: 10.1073/pnas.86.19.7606
Gottesman, R. F., Albert, M. S., Alonso, A., Coker, L. H., Coresh, J., Davis, S. M., et al. (2017). Associations between midlife vascular risk factors and 25-year incident dementia in the atherosclerosis risk in communities (Bahareh SN) cohort. JAMA Neurol. 74, 1246–1254. doi: 10.1001/jamaneurol.2017.1658
Grossberg, G. T., Tong, G., Burke, A. D., and Tariot, P. N. (2019). Present algorithms and future treatments for Alzheimer’s disease. J. Alzheimer’s Dis. 67, 1157–1171. doi: 10.3233/JAD-180903
Guo, J., Li, B., Wang, Z.-C., Wu, Y.-S., Yang, Q., and And Chen, X., (2019). Effects of yuanzhi powder on learning and memory ability and oxidative stress level of D–Galactose induced aging mice. Chinese Archives of Traditional Chinese Medicine. 37, 2144–2147+2314. doi: 10.13193/j.issn.1673-7717.2019.09.023
Guo, P., Li, P., Zhang, X., Liu, N., Wang, J., Yang, S., et al. (2020). The effectiveness of aromatherapy on preoperative anxiety in adults: a systematic review and meta-analysis of randomized controlled trials. Int. J. Nurs. Stud. 111:103747. doi: 10.1016/j.ijnurstu.2020.103747
Gutiérrez Rodríguez, J., and Guzmán Gutiérrez, G. (2017). Definition and prevalence of mild cognitive impairment. Rev. Espanola Geriatria Gerontol. 52, 3–6. doi: 10.1016/S0211-139X(18)30072-6
Hampel, H., Mesulam, M. M., Cuello, A. C., Farlow, M. R., Giacobini, E., Grossberg, G. T., et al. (2018). The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain J. Neurol. 141, 1917–1933. doi: 10.1093/brain/awy132
Hansen, R. A., Gartlehner, G., Webb, A. P., Morgan, L. C., Moore, C. G., and Jonas, D. E. (2008). Efficacy and safety of donepezil, galantamine, and rivastigmine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. Clin. Interv. Aging 3, 211–225.
Hayes, A., Green, E. K., Pritchard, A., Harris, J. M., Zhang, Y., Lambert, J. C., et al. (2004). A polymorphic variation in the interleukin 1A gene increases brain microglial cell activity in Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 75, 1475–1477. doi: 10.1136/jnnp.2003.030866
He, L.-M. (2016). The observation of neural behavior and Magnolia volatile oil via the olfactory pathway to improve learning and memory ability in Kunming mice with autism Anhui Medical University.
He, Z.-F., Yang, Q.-Q., Hou, M.-M., Li, Y., and Li, X.-Y. (2020). Progress on the mechanism of action of Jiaweibuwang powder and its active ingredients in the prevention and treatment of Alzheimer’s disease. Chin. J. Integr. Med. Cardio. Cerebrovascular Dis. 18, 3588–3591.
Heneka, M. T., Carson, M. J., El Khoury, J., Landreth, G. E., Brosseron, F., Feinstein, D. L., et al. (2015). Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 14, 388–405. doi: 10.1016/S1474-4422(15)70016-5
Horvath, J., Burkhard, P. R., Herrmann, F. R., Bouras, C., and Kövari, E. (2014). Neuropathology of parkinsonism in patients with pure Alzheimer’s disease. J. Alzheimer’s Dis. 39, 115–120. doi: 10.3233/JAD-131289
Huang, H.-C., Chang, P., Lu, S.-Y., Zheng, B.-W., and Jiang, Z.-F. (2015). Protection of curcumin against amyloid-β-induced cell damage and death involves the prevention from NMDA receptor-mediated intracellular Ca2+ elevation. J. Recept. Signal Transduct. Res. 35, 450–457. doi: 10.3109/10799893.2015.1006331
Huang, B., He, D., Chen, G., Ran, X., Guo, W., Kan, X., et al. (2018). α-Cyperone inhibits LPS-induced inflammation in BV-2 cells through activation of Akt/Nrf2/HO-1 and suppression of the NF-κB pathway. Food Funct. 9, 2735–2743. doi: 10.1039/c8fo00057c
Huang, J., Yang, J., Zou, X., Zuo, S., Wang, J., Cheng, J., et al. (2021). Ginkgolide B promotes oligodendrocyte precursor cell differentiation and survival via Akt/CREB/bcl-2 signaling pathway after white matter lesion. Exp. Biol. Med. 246, 1198–1209. doi: 10.1177/1535370221989955
Hugo, J., and Ganguli, M. (2014). Dementia and cognitive impairment: epidemiology, diagnosis, and treatment. Clin. Geriatr. Med. 30, 421–442. doi: 10.1016/j.cger.2014.04.001
Ismail, Z., Black, S. E., Camicioli, R., Chertkow, H., Herrmann, N., Laforce, R., et al. (2020). Recommendations of the 5th Canadian consensus conference on the diagnosis and treatment of dementia. Alzheimers Dement. 16, 1182–1195. doi: 10.1002/alz.12105
Jeon, S., Hur, J., Jeong, H. J., Koo, B.-S., and Pak, S. C. (2011). SuHeXiang wan essential oil alleviates amyloid beta induced memory impairment through inhibition of tau protein phosphorylation in mice. Am. J. Chin. Med. 39, 917–932. doi: 10.1142/S0192415X11009305
Jia, L., Du, Y., Chu, L., Zhang, Z., Li, F., Lyu, D., et al. (2020). Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health 5, e661–e671. doi: 10.1016/S2468-2667(20)30185-7
Jimbo, D., Kimura, Y., Taniguchi, M., Inoue, M., and Urakami, K. (2009). Effect of aromatherapy on patients with Alzheimer’s disease. Psychogeriatrics 9, 173–179. doi: 10.1111/j.1479-8301.2009.00299.x
Kamat, P. K., Kalani, A., Rai, S., Swarnkar, S., Tota, S., Nath, C., et al. (2016). Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: understanding the therapeutics strategies. Mol. Neurobiol. 53, 648–661. doi: 10.1007/s12035-014-9053-6
Kandiah, N., Ong, P. A., Yuda, T., Ng, L.-L., Mamun, K., Merchant, R. A., et al. (2019). Treatment of dementia and mild cognitive impairment with or without cerebrovascular disease: expert consensus on the use of Ginkgo biloba extract, EGb 761. CNS Neurosci. Ther. 25, 288–298. doi: 10.1111/cns.13095
Karimzadeh, F., Hosseini, M., Mangeng, D., Alavi, H., Hassanzadeh, G. R., Bayat, M., et al. (2012). Anticonvulsant and neuroprotective effects of Pimpinella anisum in rat brain. BMC Complement. Altern. Med. 12:76. doi: 10.1186/1472-6882-12-76
Khan, A., Vaibhav, K., Javed, H., Tabassum, R., Ahmed, M. E., Khan, M. M., et al. (2014). 1,8-cineole (eucalyptol) mitigates inflammation in amyloid Beta toxicated PC12 cells: relevance to Alzheimer’s disease. Neurochem. Res. 39, 344–352. doi: 10.1007/s11064-013-1231-9
Kim, Y. S., and Joh, T. H. (2006). Microglia, major player in the brain inflammation: their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 38, 333–347. doi: 10.1038/emm.2006.40
Kim, C.-Y., Seo, Y., Lee, C., Park, G. H., and Jang, J.-H. (2018). Neuroprotective effect and molecular mechanism of [6]-Gingerol against scopolamine-induced amnesia in C57BL/6 mice. ECAM 2018, 8941511–8941564. doi: 10.1155/2018/8941564
Knezevic, D., and Mizrahi, R. (2018). Molecular imaging of neuroinflammation in Alzheimer’s disease and mild cognitive impairment. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 80, 123–131. doi: 10.1016/j.pnpbp.2017.05.007
Kowalczyk, J., Kurach, Ł., Boguszewska-Czubara, A., Skalicka-Woźniak, K., Kruk-Słomka, M., Kurzepa, J., et al. (2020). Bergapten improves scopolamine-induced memory impairment in mice cholinergic and antioxidative mechanisms. Front. Neurosci. 14:730. doi: 10.3389/fnins.2020.00730
Lee, K.-T., Chu, C.-Y., and Chiang, S.-S. (2021). Ameliorating effect on Aβ-induced Alzheimer’s mice by Persoon powder. Molecules 26:5709. doi: 10.3390/molecules26185709
Li, Z., Ma, J., Kuang, Z., and Jiang, Y. (2021). β-Asarone attenuates Aβ-induced neuronal damage in PC12 cells overexpressing APPswe by restoring autophagic flux. Front. Pharmacol. 12:701635. doi: 10.3389/fphar.2021.701635
Li, B., Sun, Z.-L., Chen, G.-R., Zheng, Y.-Q., He, X.-J., Li, G.-M., et al. (2017). Effects of Yuanzhi san on ethology and cerebral acetylcholinesterase activity of memory disorder mouse model induced by scopolamine. J. Guangzhou Univ. Trad. Chin. Med. 34, 733–736. doi: 10.13359/j.cnki.gzxbtcm.2017.05.024
Li, Q., Xia, M.-W., and Lei, Y.-B. (2014). New clinical research on factors influencing vascular cognitive dysfunction. Health Prot. Promotion. 234, 57–58.
Li, Q., Xu, F., Zhang, Q., Li, X., Guo, M., Zhang, Y., et al. (2019). Effect of α-asarone on ethanol-induced learning and memory impairment in mice and its underlying mechanism. Life Sci. 238:116898. doi: 10.1016/j.lfs.2019.116898
Liu, J., and Cao, Z.-S. (2020). The role of beta amyloid in the pathogenesis of cerebral amyloid angiopathy and Alzheimer’s disease should be clarified. Nat. Med. J. Chin. 100, 3385–3387.
Liu, B., Kou, J., Li, F., Huo, D., Xu, J., Zhou, X., et al. (2020). Lemon essential oil ameliorates age-associated cognitive dysfunction via modulating hippocampal synaptic density and inhibiting acetylcholinesterase. Aging 12, 8622–8639. doi: 10.18632/aging.103179
Lv, X., Feng, Y., Ma, R., Tang, Y., Li, Y., Cui, D., et al. (2022). Effects of peppermint essential oil on learning and memory ability in APP/PS1 transgenic mice. Molecules 27:2051. doi: 10.3390/molecules27072051
Lyu, F., Wu, D., Wei, C., and Wu, A. (2020). Vascular cognitive impairment and dementia in type 2 diabetes mellitus: an overview. Life Sci. 254:117771. doi: 10.1016/j.lfs.2020.117771
Ma, J.-Q. (2019). The effect and mechanism of oil extract from Alpinia Oxyphylla Miq. Fruit on learning and memory impairment in mice Hubei University of Traditional Chinese Medicine.
Ma, Y.-X., Jiao, J.-L., Liu, C.-Y., and Dong, J. (2007). Effect of rhizoma acori graminei on the secondary structure of amyloid beta-protein 25-35. Chin. J. Pathophysiol. 2, 352–355.
Ma, J.-Q., Wu, Y., Zhou, J.-X., Qiu, Z.-P., Zhang, B.-H., Hu, J.-J., et al. (2018). Study on improvement effects of volatile oil from the fruit of Alpinia oxyphylla on scopolamine-induced learning and memory impairment in mice. Chin. Pharm. 29, 3074–3078.
Majlessi, N., Choopani, S., Kamalinejad, M., and Azizi, Z. (2012). Amelioration of amyloid β-induced cognitive deficits by Zataria multiflora Boiss. Essential oil in a rat model of Alzheimer’s disease. CNS Neurosci. Ther. 18, 295–301. doi: 10.1111/j.1755-5949.2011.00237.x
Matsunaga, S., Kishi, T., and Iwata, N. (2015). Memantine monotherapy for Alzheimer’s disease: a systematic review and meta-analysis. PLoS One 10:e0123289. doi: 10.1371/journal.pone.0123289
Mesulam, M. M. (2013). Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. J. Comp. Neurol. 521, 4124–4144. doi: 10.1002/cne.23415
Mohamed, E. A., Ahmed, H. I., Zaky, H. S., and Badr, A. M. (2021). Sesame oil mitigates memory impairment, oxidative stress, and neurodegeneration in a rat model of Alzheimer’s disease. A pivotal role of NF-κB/p38MAPK/BDNF/PPAR-γ pathways. J. Ethnopharmacol. 267:113468. doi: 10.1016/j.jep.2020.113468
Nussbaum, J. M., Schilling, S., Cynis, H., Silva, A., Swanson, E., Wangsanut, T., et al. (2012). Prion-like behaviour and tau-dependent cytotoxicity of pyroglutamylated amyloid-β. Nature 485, 651–655. doi: 10.1038/nature11060
O’Brien, J. T., Holmes, C., Jones, M., Jones, R., Livingston, G., McKeith, I., et al. (2017). Clinical practice with anti-dementia drugs: a revised (third) consensus statement from the British Association for Psychopharmacology. J. Psychopharmacol. 31, 147–168. doi: 10.1177/0269881116680924
Omari, Z., Kazunori, S., Sabti, M., Bejaoui, M., Hafidi, A., Gadhi, C., et al. (2021). Dietary administration of cumin-derived cuminaldehyde induce neuroprotective and learning and memory enhancement effects to aging mice. Aging 13, 1671–1685. doi: 10.18632/aging.202516
Osama, A., Zhang, J., Yao, J., Yao, X., and Fang, J. (2020). Nrf2: a dark horse in Alzheimer’s disease treatment. Ageing Res. Rev. 64:101206. doi: 10.1016/j.arr.2020.101206
Panara, K., Nariya, M., and Karra, N. (2020). Central nervous system depressant activity of (DC.) rhizome. Ayu 41, 250–254. doi: 10.4103/ayu.AYU_251_20
Peng, D., Qiao, H.-Z., Tan, H.-Y., Wang, Y.-X., Luo, D., Qiao, L.-J., et al. (2022a). Ligustilide ameliorates cognitive impairment via AMPK/SIRT1 pathway in vascular dementia rat. Metab. Brain Dis. 37, 1401–1414. doi: 10.1007/s11011-022-00947-0
Peng, D., Wang, Y.-X., Huang, T.-H., Luo, D., Qiao, L.-J., Wang, Q., et al. (2022b, 2022). Ligustilide improves cognitive impairment via regulating the SIRT1/IRE1/XBP1s/CHOP pathway in vascular dementia rats. Oxidative Med. Cell. Longev. 2022:6664990. doi: 10.1155/2022/6664990
Piccialli, I., Tedeschi, V., Caputo, L., Amato, G., De Martino, L., De Feo, V., et al. (2021). The antioxidant activity of limonene counteracts neurotoxicity triggered byAβ oligomers in primary cortical neurons. Antioxidants 10:937. doi: 10.3390/antiox10060937
Postu, P. A., Sadiki, F. Z., El Idrissi, M., Cioanca, O., Trifan, A., Hancianu, M., et al. (2019). Pinus halepensis essential oil attenuates the toxic Alzheimer’s amyloid beta (1-42)-induced memory impairment and oxidative stress in the rat hippocampus. Biomed. Pharmaco. 112:108673. doi: 10.1016/j.biopha.2019.108673
Pugazhenthi, S., Wang, M., Pham, S., Sze, C.-I., and Eckman, C. B. (2011). Downregulation of CREB expression in Alzheimer’s brain and in Aβ-treated rat hippocampal neurons. Mol. Neurodegener. 6:60. doi: 10.1186/1750-1326-6-60
Quintanilla, R. A., Orellana, D. I., González-Billault, C., and Maccioni, R. B. (2004). Interleukin-6 induces Alzheimer-type phosphorylation of tau protein by deregulating the cdk5/p35 pathway. Exp. Cell Res. 295, 245–257. doi: 10.1016/j.yexcr.2004.01.002
Ran, Q.-S., Zhan, X.-L., Li, X.-D., Gu, L.-W., Li, L.-F., Guo, S.-Y., et al. (2017). Analysis and adscription of volatiles fromGuizhi Tang using gas chromatography-mass spectroscopy and improvement of the learning and memory in mice. Int. J. Trad. Chin. Med. 39, 435–441.
Rao, R. V., Descamps, O., John, V., and Bredesen, D. E. (2012). Ayurvedic medicinal plants for Alzheimer’s disease: a review. Alzheimers Res. Ther. 4:22. doi: 10.1186/alzrt125
Rosa, E., and Fahnestock, M. (2015). CREB expression mediates amyloid β-induced basal BDNF downregulation. Neurobiol. Aging 36, 2406–2413. doi: 10.1016/j.neurobiolaging.2015.04.014
Sabogal-Guáqueta, A. M., Osorio, E., and Cardona-Gómez, G. P. (2016). Linalool reverses neuropathological and behavioral impairments in old triple transgenic Alzheimer’s mice. Neuropharmacology 102, 111–120. doi: 10.1016/j.neuropharm.2015.11.002
Sadiki, F. Z., Idrissi, M. E., Cioanca, O., Trifan, A., Hancianu, M., Hritcu, L., et al. (2019). Tetraclinis articulata essential oil mitigates cognitive deficits and brain oxidative stress in an Alzheimer’s disease amyloidosis model. Phytomed. Int. J. Phytother. Phytopharmacol. 56, 57–63. doi: 10.1016/j.phymed.2018.10.032
Sajja, V. S. S. S., Hlavac, N., and VandeVord, P. J. (2016). Role of glia in memory deficits following traumatic brain injury: biomarkers of glia dysfunction. Front. Integr. Neurosci. 10:7. doi: 10.3389/fnint.2016.00007
Saki, G., Eidi, A., Mortazavi, P., Panahi, N., and Vahdati, A. (2020). Effect of β-asarone in normal and β-amyloid-induced Alzheimeric rats. Arch. Med. Sci. 16, 699–706. doi: 10.5114/aoms.2020.94659
Sartorius, A., Mahlstedt, M. M., Vollmayr, B., Henn, F. A., and Ende, G. (2007). Elevated spectroscopic glutamate/gamma-amino butyric acid in rats bred for learned helplessness. Neuroreport 18, 1469–1473. doi: 10.1097/WNR.0b013e3282742153
Sasahara, K., Morigaki, K., and Shinya, K. (2014). Amyloid aggregation and deposition of human islet amyloid polypeptide at membrane interfaces. FEBS J. 281, 2597–2612. doi: 10.1111/febs.12807
Satou, T., Hanashima, Y., Mizutani, I., and Koike, K. (2018). The effect of inhalation of essential oil from Rosmarinus officinalis on scopolamine-induced Alzheimer’s type dementia model mice. Flavour Fragr. J. 33, 230–234. doi: 10.1002/ffj.3435
Selvarasu, K., Singh, A. K., Iyaswamy, A., Gopalkrishnashetty Sreenivasmurthy, S., Krishnamoorthi, S., Bera, A. K., et al. (2022). Reduction of kinesin I heavy chain decreases tau hyperphosphorylation, aggregation, and memory impairment in Alzheimer’s disease and tauopathy models. Front. Mol. Biosci. 9:1050768. doi: 10.3389/fmolb.2022.1050768
Silva, M. V. F., Loures, C. D. M. G., Alves, L. C. V., De Souza, L. C., Borges, K. B. G., and Carvalho, M. D. G. (2019). Alzheimer’s disease: risk factors and potentially protective measures. J. Biomed. Sci. 26:33. doi: 10.1186/s12929-019-0524-y
Song, J.-H., Yu, J.-T., and Tan, L. (2015). Brain-derived Neurotrophic factor in Alzheimer’s disease: risk, mechanisms, and therapy. Mol. Neurobiol. 52, 1477–1493. doi: 10.1007/s12035-014-8958-4
Sreenivasmurthy, S. G., Iyaswamy, A., Krishnamoorthi, S., Reddi, R. N., Kammala, A. K., Vasudevan, K., et al. (2022). Bromo-protopine, a novel protopine derivative, alleviates tau pathology by activating chaperone-mediated autophagy for Alzheimer’s disease therapy. Front. Mol. Biosci. 9:1030534. doi: 10.3389/fmolb.2022.1030534
Sudeep, H. V., Venkatakrishna, K., Amritharaj,, Gouthamchandra, K., Reethi, B., Naveen, P., et al. (2021). A standardized black pepper seed extract containing β-caryophyllene improves cognitive function in scopolamine-induced amnesia model mice via regulation of brain-derived neurotrophic factor and MAPK proteins. J. Food Biochem. 45:e13994. doi: 10.1111/jfbc.13994
Tan, C.-C., Yu, J.-T., Wang, H.-F., Tan, M.-S., Meng, X.-F., Wang, C., et al. (2014). Efficacy and safety of donepezil, galantamine, rivastigmine, and memantine for the treatment of Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimer’s Dis. 41, 615–631. doi: 10.3233/JAD-132690
Tiwari, S., Atluri, V., Kaushik, A., Yndart, A., and Nair, M. (2019). Alzheimer’s disease: pathogenesis, diagnostics, and therapeutics. Int. J. Nanomedicine Volume 14, 5541–5554. doi: 10.2147/IJN.S200490
Tong, B. C.-K., Huang, A. S., Wu, A. J., Iyaswamy, A., Ho, O. K.-Y., Kong, A. H.-Y., et al. (2022). Tetrandrine ameliorates cognitive deficits and mitigates tau aggregation in cell and animal models of tauopathies. J. Biomed. Sci. 29:85. doi: 10.1186/s12929-022-00871-6
Tu, S., Okamoto, S.-I., Lipton, S. A., and Xu, H. (2014). Oligomeric Aβ-induced synaptic dysfunction in Alzheimer’s disease. Mol. Neurodegener. 9:48. doi: 10.1186/1750-1326-9-48
Veurink, G., Perry, G., and Singh, S. K. (2020). Role of antioxidants and a nutrient rich diet in Alzheimer’s disease. Open Biol. 10:200084. doi: 10.1098/rsob.200084
Walker, K. A., Power, M. C., and Gottesman, R. F. (2017). Defining the relationship between hypertension, cognitive decline, and dementia: a review. Curr. Hypertens. Rep. 19:24. doi: 10.1007/s11906-017-0724-3
Wang, D. (2014). Effect of rosemary sniffing on cognitive impairment after cerebral infarction Anhui Medical University.
Wang, B.-B., Fen, X.-X., Berk, E., Zhao, H., and Li, Z.-X. (2021b). Pharmacological effects of Kaixin San on neuroinflammation and Aβ deposition in APP/PS1 mice. Chin. Tradit. Herb. Drug 52, 7511–7519.
Wang, X., Iyaswamy, A., Xu, D., Krishnamoorthi, S., Sreenivasmurthy, S. G., Yang, Y., et al. (2023). Real-time detection and visualization of amyloid-β aggregates induced by hydrogen peroxide in cell and mouse models of Alzheimer’s disease. ACS Appl. Mater. Interfaces 15, 39–47. doi: 10.1021/acsami.2c07859
Wang, R.-R., Mao, Z.-N., and And Wang, R.-X. (2020). Research progress on the mechanism of herbal treatment for cognitive impairment after stroke. J. Mod. Clin. Med. 46, 146–149.
Wang, J.-Z., and Tian, Q. (2012). Melecular mechanisms underlie Alzheimer-like tau hyperphosphorylation and neurodegeneration. Prog. Biochem. Biophys. 39, 771–777. doi: 10.3724/SP.J.1206.2012.00333
Wang, Y., Wu, S.-S., Xu, T., Rao, G.-L., Liu, X.-L., Li, Y.-F., et al. (2021c). Improving effect of volatile oil from Chimonanthus nitens Oliv.Leaves on cognitive disorder in rats with vascular dementia. J. Anhui Agric. Sci. 49, 170–174.
Wang, Y.-Y., Zhao, Y., and Li, J., and Chen, H.-y., (2021a). Application of aroma inhalation therapy in neurological diseases. Chin. J. Ethnomed. Ethnopharm. 30, 69–73.
Watanabe, T., Iwasaki, K., Ishikane, S., Naitou, T., Yoshimitsu, Y., Yamagata, N., et al. (2008). Spatial memory impairment without apoptosis induced by the combination of beta-amyloid oligomers and cerebral ischemia is related to decreased acetylcholine release in rats. J. Pharmacol. Sci. 106, 84–91. doi: 10.1254/jphs.FP0071648
Xiao, X., Wu, Z.-C., and Chou, K.-C. (2011). A multi-label classifier for predicting the subcellular localization of gram-negative bacterial proteins with both single and multiple sites. PLoS One 6:e20592. doi: 10.1371/journal.pone.0020592
Xu, P., Wang, K., Lu, C., Dong, L., Gao, L., Yan, M., et al. (2016). Protective effect of lavender oil on scopolamine induced cognitive deficits in mice and HO induced cytotoxicity in PC12 cells. J. Ethnopharmacol. 193, 408–415. doi: 10.1016/j.jep.2016.08.030
Xu, M., Zhang, X., Ren, F., Yan, T., Wu, B., Bi, K., et al. (2019). Essential oil of Schisandra chinensis ameliorates cognitive decline in mice by alleviating inflammation. Food Funct. 10, 5827–5842. doi: 10.1039/c9fo00058e
Xue, Z., Guo, Y., Zhang, S., Huang, L., He, Y., Fang, R., et al. (2014). Beta-asarone attenuates amyloid beta-induced autophagy via Akt/mTOR pathway in PC12 cells. Eur. J. Pharmacol. 741, 195–204. doi: 10.1016/j.ejphar.2014.08.006
Yadav, M., Jindal, D. K., Parle, M., Kumar, A., and Dhingra, S. (2019). Targeting oxidative stress, acetylcholinesterase, proinflammatory cytokine, dopamine and GABA by eucalyptus oil (Eucalyptus globulus) to alleviate ketamine-induced psychosis in rats. Inflammopharmacology 27, 301–311. doi: 10.1007/s10787-018-0455-3
Yang, B., Liu, B., Liu, Y., Han, H., and Kuang, H. (2018). Cognitive enhancement of volatile oil from the stems of Schisandra chinensis Baill. In Alzheimer’s disease rats. Can. J. Physiol. Pharmacol. 96, 550–555. doi: 10.1139/cjpp-2016-0194
Yuan, C., Shin, M., Park, Y., Choi, B., Jang, S., Lim, C., et al. (2021). Linalool alleviates A42-induced neurodegeneration via suppressing ROS production and inflammation in Fly and rat models of Alzheimer’s disease. Oxidative Med. Cell. Longev. 2021:8887716. doi: 10.1155/2021/8887716
Zhang, C.-X., Guo, J.-H., Chen, Y.-B., Yan, R., Wang, Q., Yu, X.-H., et al. (2014). Effects of active components of Rhizoma Acori Tatarinowii on APP and Synapit ultrastructure in Tg-APPswe/PS1de9 mice. Trad. Chin. Drug Res. Clin. Pharmacol. 25, 18–23.
Zheng, J.-Y., and Chen, X.-P. (2018). Alzheimer’s disease and depression correlation. Chin. J. Gerontol. 38, 2039–2041.
Zhou, C.-M. (2021). Study on the effect and mechanism in treating MCI model mice by sniffing Bingchangsan based on pharmacodynamics, Nanjing University of Traditional Chinese Medicine.
Zhou, X., Li, X.-Q., LIU, Q., Xun, J.-Y., Xiang, J.-F., Cao, F.-Y., et al. (2019). Mechanism of essential oil of Ligusticum chuanxiong to prevent and treat vascular cognitive impairment induced by lipopolysaccharide in mice. Chin. Trad. Herbal Drugs 50, 2390–2397.
Zhu, S., Li, H., Dong, J., Yang, W., Liu, T., Wang, Y., et al. (2017). Rose essential oil delayed Alzheimer’s disease-like symptoms by SKN-1 pathway in C. elegans. J. Agric. Food Chem. 65, 8855–8865. doi: 10.1021/acs.jafc.7b03224
Zhu, W.-L., Zheng, J.-Y., Cai, W.-W., Dai, Z., Li, B.-Y., Xu, T.-T., et al. (2020). Ligustilide improves aging-induced memory deficit by regulating mitochondrial related inflammation in SAMP8 mice. Aging 12, 3175–3189. doi: 10.18632/aging.102793
Keywords: cognitive impairment, Alzheimer’s disease, natural essential oils, mechanism, advantages
Citation: Shi A, Long Y, Ma Y, Yu S, Li D, Deng J, Wen J, Li X, Wu Y, He X, Hu Y, Li N and Hu Y (2023) Natural essential oils derived from herbal medicines: A promising therapy strategy for treating cognitive impairment. Front. Aging Neurosci. 15:1104269. doi: 10.3389/fnagi.2023.1104269
Received: 21 November 2022; Accepted: 21 February 2023;
Published: 16 March 2023.
Edited by:
Lu Zhao, Zhejiang University, ChinaReviewed by:
Ashok Iyaswamy, Hong Kong Baptist University, Hong Kong SAR, ChinaCopyright © 2023 Shi, Long, Ma, Yu, Li, Deng, Wen, Li, Wu, He, Hu, Li and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nan Li, NTU3NDMxOThAcXEuY29t; Yuan Hu, Z3VvNTk2aHV5dWFuQGNkdXRjbS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.