
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 01 February 2023
Sec. Alzheimer's Disease and Related Dementias
Volume 15 - 2023 | https://doi.org/10.3389/fnagi.2023.1067196
This article is part of the Research Topic To Know or Not to Know: Causes and Evolution of Lack of Awareness of Cognitive Decline in Neurodegenerative Diseases, Volume II View all 9 articles
 Liat Ben-Ami1,2
Liat Ben-Ami1,2 Ramit Ravona-Springer3,4,5
Ramit Ravona-Springer3,4,5 Galia Tsarfaty1,5
Galia Tsarfaty1,5 Reut Raizman1
Reut Raizman1 Aleeza Shumacher1
Aleeza Shumacher1 Inbal Sharvit-Ginon3,6
Inbal Sharvit-Ginon3,6 Lior Greenbaum3,5,7
Lior Greenbaum3,5,7 Barbara B. Bendlin8
Barbara B. Bendlin8 Eitan Okun2,9,10
Eitan Okun2,9,10 Anthony Heymann5,11
Anthony Heymann5,11 Michal Schnaider Beeri3,12
Michal Schnaider Beeri3,12 Abigail Livny1,3,5,13*
Abigail Livny1,3,5,13*Introduction: Recently, interest has emerged in subjective cognitive decline (SCD) as a potential precursor to Alzheimer’s disease (AD) dementia. Whether individuals with SCD harbor brain alterations in midlife, when AD-related pathology begins, is yet to be elucidated. Furthermore, the role of apolipoprotein ε4 (APOE ε4) allele, a robust AD risk factor, in the relationship between SCD and brain alterations is unknown. We examined whether APOE genotype modulates the association of SCD with brain measures in individuals at high AD risk.
Methods: Middle-aged adults with parental history of AD dementia underwent magnetic resonance imaging (MRI) and the Memory Functioning Questionnaire. Regression analysis tested the extent to which SCD was associated with activation during an functional MRI (fMRI) working-memory task, and white-matter microstructure. APOE ε4 genotype was tested as a moderator.
Results: Among APOE ε4 carriers, but not among non-carriers, SCD was associated with higher activation in the anterior cingulate (p = 0.003), inferior, middle, and superior frontal cortices (p = 0.041, p = 0.048, p = 0.037, respectively); and with lower fractional anisotropy in the uncinate fasciculus (p = 0.002), adjusting for age, sex, and education.
Conclusion: In middle aged, cognitively normal individuals at high AD risk, higher SCD was associated with greater brain alterations possibly reflecting incipient AD pathology. When accompanied by a family history of AD and an APOE ε4 allele, SCD may have important clinical value, allowing a window for early intervention and for participants’ stratification in AD prevention clinical trials.
Alzheimer’s disease (AD), the leading cause of dementia, accounts for over 50% of all dementia cases worldwide. Due to the expected substantial increase in dementia prevalence in the years to come (Alzheimer’s Association, 2022) and with the recent trial reporting less clinical decline using a novel therapeutic agent (van Dyck et al., 2022), extensive scientific effort has and should be directed toward detection of early biomarkers and dementia prevention (Smith and Yaffe, 2014). This is especially relevant in the context of midlife, when the neuropathology of AD begins to aggregate (Racine et al., 2016). In order to implement such intervention strategies effectively, it is crucial to characterize the potential impact of parental family history of AD (FH) and carrying the apolipoprotein ε4 allele (APOE ε4), two major risk factors for AD which are associated with more rapid cognitive decline and earlier age of onset of AD (Martinez et al., 1998), on brain pathology.
In recent years, an increasing interest has emerged in subjective cognitive decline (SCD) leading to a conceptual framework, by the working group of the Subjective Cognitive Decline Initiative (SCD-I), aimed to increase comparability of research on SCD across settings and studies (Jessen et al., 2014).
Subjective cognitive decline represents an individual’s subjective perception of decline from previous levels of cognitive functioning, while these subtle and early changes cannot be detected on objective neuropsychological tests (Burmester et al., 2016). SCD has been associated with objective memory deficits (Amariglio et al., 2011), future cognitive decline (Jorm et al., 2001), increased risk of clinical conversion to mild cognitive impairment (MCI), dementia and AD (Jungwirth et al., 2008; Neto and Nitrini, 2016). Some studies did not find such associations (Jungwirth et al., 2004; Cargin et al., 2008). If SCD indeed indicates an early disease process, early neuropathological correlates could include alterations to the neural architecture supporting cognitive function, including changes in white matter integrity or in neural function.
As the conceptual SCD framework requires additional validation to serve as a standardized indicator for biomarker-based preclinical AD (Jessen et al., 2014), we propose a neuroimaging perspective of SCD, while applying the framework’s criteria in our study.
Neuroimaging methods have great potential for identifying the underlying pathological processes associated with SCD. Overall, the literature reveals that individuals with SCD show vulnerability of AD related regions. Nonetheless, literature exploring brain activation using task-based functional magnetic resonance imaging (fMRI), and white-matter integrity with diffusion tensor imaging in individuals with SCD reported inconsistent findings (Wang et al., 2020). Older adults with SCD showed increased brain activation relative to controls in frontal, temporal, and parietal regions (Rodda et al., 2009; Dumas et al., 2013; Hu et al., 2017) and decreased brain activation in temporal regions (Erk et al., 2011; Hayes et al., 2017) while engaged in various cognitive tasks. Participants with SCD also exhibited lower white matter integrity in temporal structures (Li et al., 2016; Ryu et al., 2017). Moreover, the integrity of some white matter tracts, among people with SCD were similar to those of patients with MCI (Hong et al., 2015). Other studies did not report findings in diffusion metrics among these participants (Kiuchi et al., 2014; Viviano et al., 2019).
Only few studies have examined the neural correlates of SCD in individuals at high AD risk due to FH and APOE ε4. In cognitively unimpaired individuals with FH, SCD was associated with a brain connectivity pattern that is similar to that of early AD (Verfaillie et al., 2018). APOE ε4 carriers with SCD also show higher tau and higher amyloid deposition, defining features of AD (Risacher et al., 2016); reduced gray matter volume in temporal, parietal, and frontal areas (Lee et al., 2016); and reduced whole-brain white matter volume (Dauphinot et al., 2020). In a previous study conducted by our group, SCD was correlated with significant cortical thinning in temporal, paralimbic, and parietal regions, even after adjusting for FH and APOE ε4 (Schultz et al., 2015).
To date, associations of SCD with brain structure and function have mostly focused on old age rather than in midlife, a critical period for potential interventions. Thus, whether SCD in midlife is related to neurobiological changes, remains to be elucidated, especially among high-risk individuals for whom SCD may reflect the initial process of neurodegeneration. To determine the role of SCD in high AD risk middle-aged individuals, we tested whether APOE genotype modulates the associations of SCD with structural and functional brain measures related to cognitive decline as indexed by brain activation during an fMRI working memory task and white matter integrity, respectively. We hypothesized that associations between SCD and brain indices will be stronger in APOE ε4 carriers compared to APOE ε4 non-carriers.
Participants were recruited from the Israel Registry for Alzheimer’s Prevention (IRAP), a collaboration between the Sheba Medical Center and Maccabi Health Services (MHS) examining the effect of sociodemographic, cognitive, health-related, lifestyle, laboratory, and genetic factors on cognitive functioning and decline along with neural characteristics in middle-aged offspring of AD patients. The IRAP study design has been previously described in detail (Ravona-Springer et al., 2020). In short, participants’ inclusion criteria was MHS membership, age at enrollment 40–65 years, fluency in Hebrew, with parental history of AD. Medical records of parents of individuals who approach the study team are provided to the study team and a Dementia Questionnaire is administered to potential participants. All the medical history and diagnostic workup available is reviewed together with the Dementia Questionnaire in order to reach a probable AD diagnosis (according to NINCDS-ADRDA criteria). Offspring of probands with partial information about dementia type or with dementia other than AD, are excluded from the study. In addition to a parental family history of AD (an inclusion criterion for the IRAP study), eligibility criteria for this analysis included: (1) available MRI scan; (2) completion of the Memory Functioning Questionnaire (MFQ); (3) available APOE genotype.
We have excluded participants with a life threatening disease such as advanced cancer or severe psychiatric disorders such as schizophrenia. Participants were recruited through an advertisement posted on the home-page of the MHS website and through participants’ word of mouth. Briefly, participants completed blood pressure measurements, neuropsychological testing, laboratory testing, APOE genotyping, history and current health assessment, lifestyle, dietary habits, medications, depression, anxiety, and a questionnaire on subjective cognitive complaints.
The full IRAP study included 408 participants with a parental family history of AD, of whom 298 underwent an MRI scan. Figure 1 provides a flow chart leading to the final Ns of this study. The MRI cohort included 216 participants with valid fMRI data, and 208 with valid DTI data.
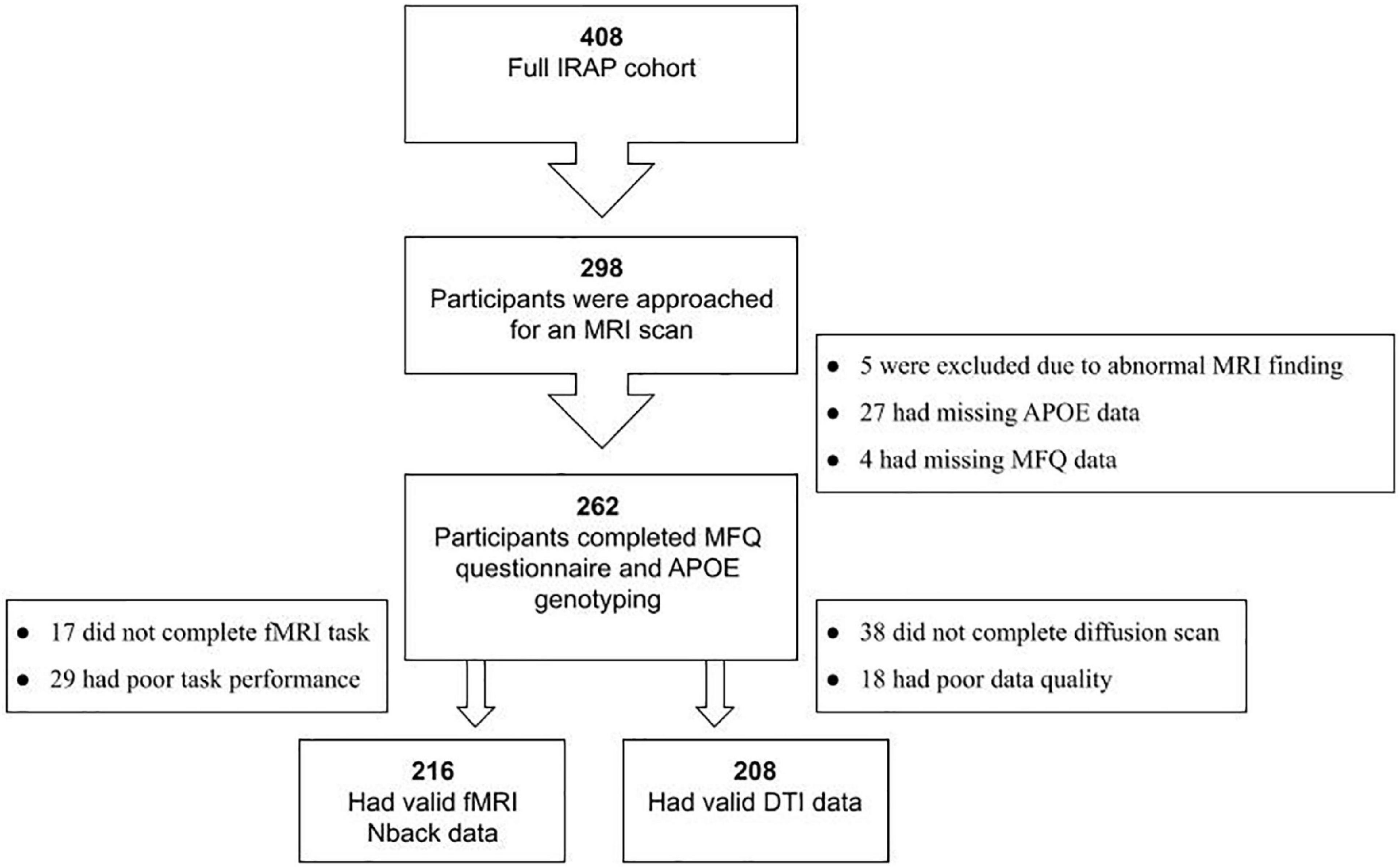
Figure 1. Flowchart of the study cohort. IRAP, Israel Registry for Alzheimer’s Prevention; FH, parental family history of AD; MRI, magnetic resonance imaging; MFQ, Memory Functioning Questionnaire; APOE, apolipoprotein E gene; fMRI, functional MRI; DTI, diffusion tensor imaging.
Subjective cognitive decline was assessed using the MFQ, a well-validated self-reported measure with four subscales identified by factor analysis (Gilewski et al., 1990). For this study, the 18-item frequency of forgetting (FF) subscale was used as the measure of SCD, as it has been shown to correspond to memory performance (Zelinski et al., 1990), and has been linked to amyloid burden and other significant memory decline measures (Merrill et al., 2012; Snitz et al., 2015; Innes et al., 2017; Sharma et al., 2021) and is known as the most reliable subscale of the MFQ (Amariglio et al., 2012). In this subscale, participants are asked to rate “How often do the following aspects of memory present a problem for you…” on a 7-point Likert scale (1 = “Always,” 7 = “Never”) for different aspects of memory (e.g., memory for faces, names, appointments, etc.). Ratings were summed across 18 items comprising the total FF score. In order to simplify the interpretation of the results, for each participant, the FF scale has been transposed to the opposite direction by subtracting the FF score from the maximal score, so that higher scores correspond to a greater frequency of memory complaints.
Blood samples were taken for DNA. DNA was extracted and frozen in −80°C and so far has been used for APOE genotyping. APOE status is determined based on rs429358 and rs7412 SNPs genotypes (Lim et al., 2019) at LGC company1 using Kompetitive allele specific PCR (KASP) technology (Cannon-Albright et al., 2019). Participants who exhibited at least one allele of APOE ε4 (APOE ε34 or APOE ε44) were categorized as APOE ε4 carriers, while those who exhibit no allele of APOE ε4 (APOE ε22, ε23 and ε33) where categorized as APOE ε4 non-carriers (Ravona-Springer et al., 2020).
Participants completed a comprehensive neuropsychological battery, which was summarized into four domains of episodic memory, executive functions, language, and working memory (for detailed descriptions refer to Ravona-Springer et al., 2020). The episodic memory factor was calculated as the sum of the z-scores of the Rey Auditory Verbal Learning Test immediate recall, delayed recall, and recognition scores; and was used as a covariate in secondary analyses.
Depressive symptomatology was assessed using the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977). The scale consists of 20 Likert-scale questions (scored 0–3) ranging from “rarely/none of the time” to “most/all of the time” about the frequency of depressive symptoms over the past week. The scale of four questions (good, future, happy, and enjoy) was reversed, and all answers were summed across the 20 items to get a total depression score.
Magnetic resonance imaging scans were acquired at the diagnostic imaging division, Sheba Medical Center, on a 3 Tesla whole body MRI scanner (GE Signa HDxt). For registration purposes high-resolution images (1 mm3) were acquired, using a standard 3D inversion recovery prepared fast gradient echo pulse (FSPGR) T1-weighted sequence: TR = 8.85 s, TE = 3.5 s, flip angle = 12°, TI = 450 ms, matrix 256 × 256, field of view (FOV) 25.6 × 25.6 cm. Spin-echo diffusion weighted echo-planar imaging (EPI) sequence was performed as well: 39 axial slices, 1 × 1 × 4 mm resolution, TR = 10 s, TE = 91.5 ms, matrix 128 × 128. We acquired 25 diffusion weighted images in isotropically distributed directions, with b = 1,000 s/mm2 (Δ/δ = 33/26 ms) and an additional non-DWI image (b0). A T2*-weighted gradient-echo EPI sequence was also acquired: TR = 3 s, TE = 32 ms, flip angle = 90°, matrix 64 × 64, FOV 22 × 22 cm, 40 contiguous oblique axial slices, slice width 3 and 0.4 mm gap.
The Nback task is a widely used task in fMRI studies for evaluating working memory (Owen et al., 2005). In this task, for each stimulus in a continuous series, participants indicate whether the item matches a stimulus presented “n” stimuli previously. Our task used letter stimuli presented at two different memory load conditions (1-, 2-back; for details see Livny et al., 2018). The Nback paradigm was presented using E-prime 2.0 software (Psychology Software Tools, Inc.), back-projected by a radio-frequency shielded projector system and viewed through a mirror device. Responses were recorded using a Lumina response box (Lumina, Cedrus Corporation, CA, USA). Participants were considered to have reached inclusion criterion if their performance was at least 65% correct responses.
The fMRI data was processed using Statistical Parametric Mapping software (SPM12, Wellcome Trust Centre for Neuroimaging, London, UK) implemented in MATLAB (The MathWorks Inc., MA, USA). Preprocessing of the functional images included common pipeline preprocessing steps. The pre-processing of functional images included reorienting to the anterior commissure, realignment to the first image using affine transformation, co-registration to the individual’s 3D T1 images, normalization to the stereotactic space of the MNI using a 12-parameter linear affine transformation and smoothing with a Gaussian kernel of 8 × 8 × 8 mm in order to minimize anatomical differences and increase signal to noise ratio (Ashburner, 2009). The preprocessed data was then analyzed individually for each subject using the general linear model (GLM) convolved with the hemodynamic response function.
We produced a map of high working memory load constructed as the contrast of 2-back minus 0-back conditions.
Diffusion-weighted images were processed and analyzed using ExploreDTI v4.8.6 software implemented in MATLAB (The MathWorks Inc., MA, USA). Analysis steps included: regularization, brain extraction, eddy current, and motion correction. The diffusion tensors were then calculated using a non-linear regression procedure. Fractional anisotropy (FA) and mean diffusivity (MD) maps were computed, normalized to an MNI space FA template, and smoothed with a full-width half-maximum Gaussian kernel of 5 × 5 × 5 mm, using SPM12 (Wellcome Trust Centre for Neuroimaging, London, UK).
For both structural and functional imaging modalities analyses, a region of interest approach was adopted, allowing us to focus on a priori brain regions known to be affected in AD (Yetkin et al., 2006). For fMRI, six cortical regions of interest (ROIs) were extracted from the high working memory load contrast maps: inferior, middle, and superior frontal gyri, anterior cingulate, inferior parietal lobule, and medial temporal lobe. ROIs were identified with the Human Automated Anatomical Labeling (AAL) atlas (Tzourio-Mazoyer et al., 2002) within the WFU Pickatlas tool (V1.2), and signal intensity of the high working memory load contrast was extracted using the MarsBaR toolbox, implemented in SPM8 on MATLAB (The MathWorks Inc., MA, USA). For DTI, data from five white matter ROIs, known to be affected in AD (Alves et al., 2012) were extracted in MNI space, using an in-house matlab script, from the FA and MD maps: genu of the corpus callosum, fornix, the cingulum bundles adjacent to the cingulate cortex, superior longitudinal fasciculi and the uncinate fasciculi. White matter tract ROIs were derived from the JHU ICBM-DTI-81 WM Atlas (Mori et al., 2008), after examining registration between the FA ROIs and white matter tracts. Values at each voxel within an ROI were averaged to obtain a mean value for each ROI. fMRI raw signal was extracted from the left and right homologous regions and was averaged across both sides. DTI signal was thresholded for above 0.2. Finally, subsequent ROI data was transferred to SPSS for further statistical analysis.
For all analyses, the normality of the GLM was verified by inspection of whether the residuals of the regression were normally distributed around zero and had a fixed standard deviation. All analyses were found valid in terms of model normality. Sociodemographic characteristics, depressive symptoms, objective cognitive performance, and SCD score of the two APOE genotype groups were compared using Student’s t-test.
Associations between our SCD indicator (measured by the FF scale) and sociodemographic characteristics, depression score, and objective cognitive performance, were calculated by Pearson’s correlations test.
The relationship between SCD and brain measurements (fMRI brain activation or FA/MD values) was assessed using a multiple linear regression analysis. The interaction of APOE genotype with SCD on brain measurements was examined. To describe the significant interactions, the SCD and brain measurement values were then entered into a partial correlation analysis stratified by APOE genotype (APOE ε4 allele carriers and non-carriers). All analyses were controlled for age, sex, and years of education.
We performed a series of additional secondary analyses. First, we examined the interaction of APOE genotype with SCD and brain measurements using the total score of the MFQ. In addition, it has been previously argued that SCD is linked to depression (Jorm et al., 2004). Thus, to ensure that our initial findings were not driven by elevated depressive symptoms, we additionally adjusted for the CES-D score.
Third, to assure that our significant results were not driven by variability of objective memory performance, we repeated the GLM interaction analyses, including episodic memory as an additional covariate in the model. Fourth, we have also adjusted for executive function as one of the brain measures in the model, i.e., the Nback task measures working memory and executive function. In addition, our sample included 15 sibling pairs. We thus repeated the GLM interaction analyses again after excluding one random sibling from each pair. Finally, our sample included seven participants with APOE e2/e4 alleles. We thus repeated all analyses again after excluding these participants as well, since the APOE ε2 allele is considered low risk for AD (Strittmatter and Roses, 1996).
A p-value of 0.05 was used to determine statistical significance. Statistical analyses were calculated using IBM SPSS Statistics for Windows (version 19; IBM, Armonk, NY, USA).
Participants (n = 262) had a mean age of 54.30 (SD = 6.77) years, range 40–65 years. They were relatively educated with 16.58 (2.94) years of education, all participants were cognitively normal based on cognitive norms for age, sex, and education and had normal episodic memory performance [mean (SD) = 0.039 (0.855)]. APOE ε4 (33.21%) carriers did not differ from non-carriers (66.79%) on age, sex, years of education, depression score, overall cognition, working memory, episodic memory, executive motor, and SCD measures (Table 1).
No significant associations were found between SCD and the demographic and cognitive measures, except for the depression score, where we found that higher scores in SCD were associated with greater depression symptoms (r = 0.259, p = 0.001; Table 2), as was previously described in the literature (Jorm et al., 2004; Montejo et al., 2011).
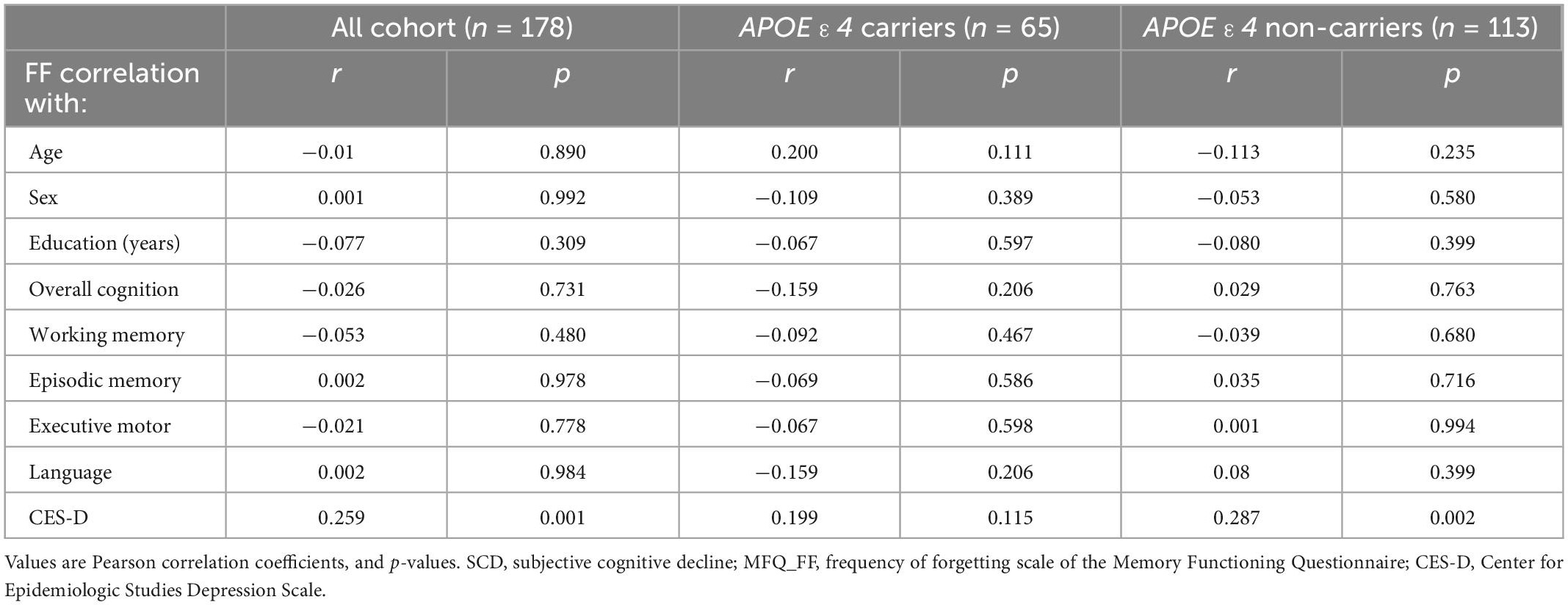
Table 2. Association between SCD (MFQ_FF) and objective cognitive performance and demographic characteristics.
A significant interaction of APOE genotype with SCD on brain activation was found in the following ROIs: anterior cingulate [F(1,209) = 4.527, p = 0.003], inferior frontal [F(1,209) = 4.231, p = 0.041], middle frontal [F(1,209) = 3,959, p = 0.048], and superior frontal gyri [F(1,209) = 4.414, p = 0.037].
To identify the source of the interactions, we examined the partial correlations between brain activation and SCD, stratified by APOE genotype, adjusting for the same covariates. For all significant interactions, among APOE ε4 carriers, greater SCD was associated with more brain activation during a working memory task, while no association was found among APOE ε4 non-carriers. There were no interactions of SCD with APOE on brain activation in the inferior parietal and middle temporal lobe. For all statistics values refer to Figure 2 and Table 3.
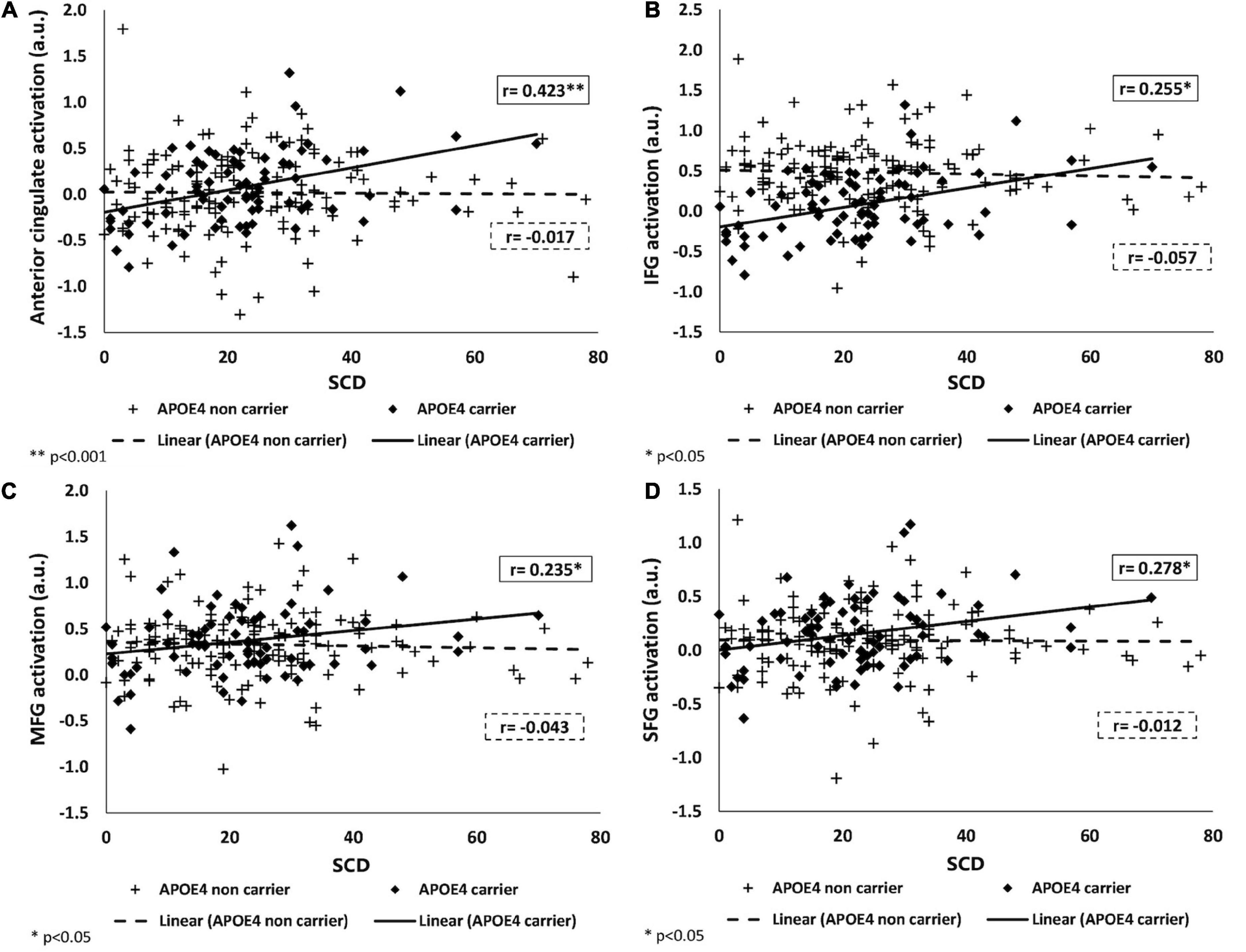
Figure 2. Associations of SCD with brain activation in APOE ε4 carriers and non-carriers. Positive association between BOLD signal intensity during a working memory task and SCD among APOE ε4 carriers (shown as black) and no association among APOE ε4 non-carriers (shown as dashed-line) in the anterior cingulate (A), IFG (B), MFG (C), and SFG (D). SCD, subjective cognitive decline; IFG, MFG, SFG, inferior, middle, and superior frontal gyri; APOE ε4, apolipoprotein E, alleles ε34 or ε44.
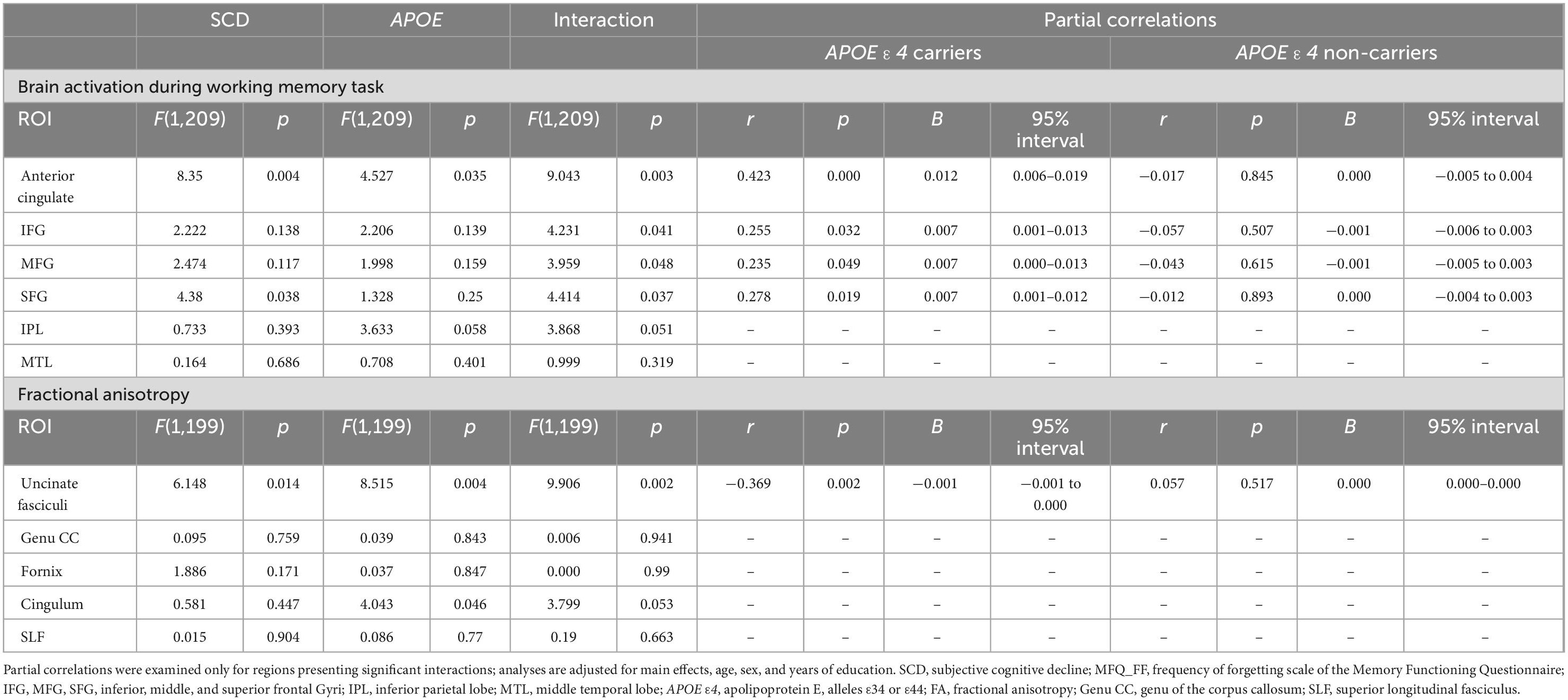
Table 3. Linear regression of APOE ε4 genotype with SCD (MFQ_FF) on brain activation during a working memory task and on fractional anisotropy in the different brain regions adjusting for age, sex, and years of education.
We also found an interaction of APOE genotype with SCD on FA values, in the uncinate fasciculi [F(1,199) = 9.906, p = 0.002].
Similarly to the brain activation results, in the stratified analysis, among APOE ε4 carriers, higher SCD was associated with lower FA, whereas, no association was found in the APOE ε4 non-carriers (see Figure 3 and Table 3). No significant interactions were found between SCD and APOE on the FA values of the genu of the corpus callosum, fornix, cingulum, superior longitudinal fasciculus, or on the MD values of all selected ROIs (Table 3 and Supplementary Table 1).
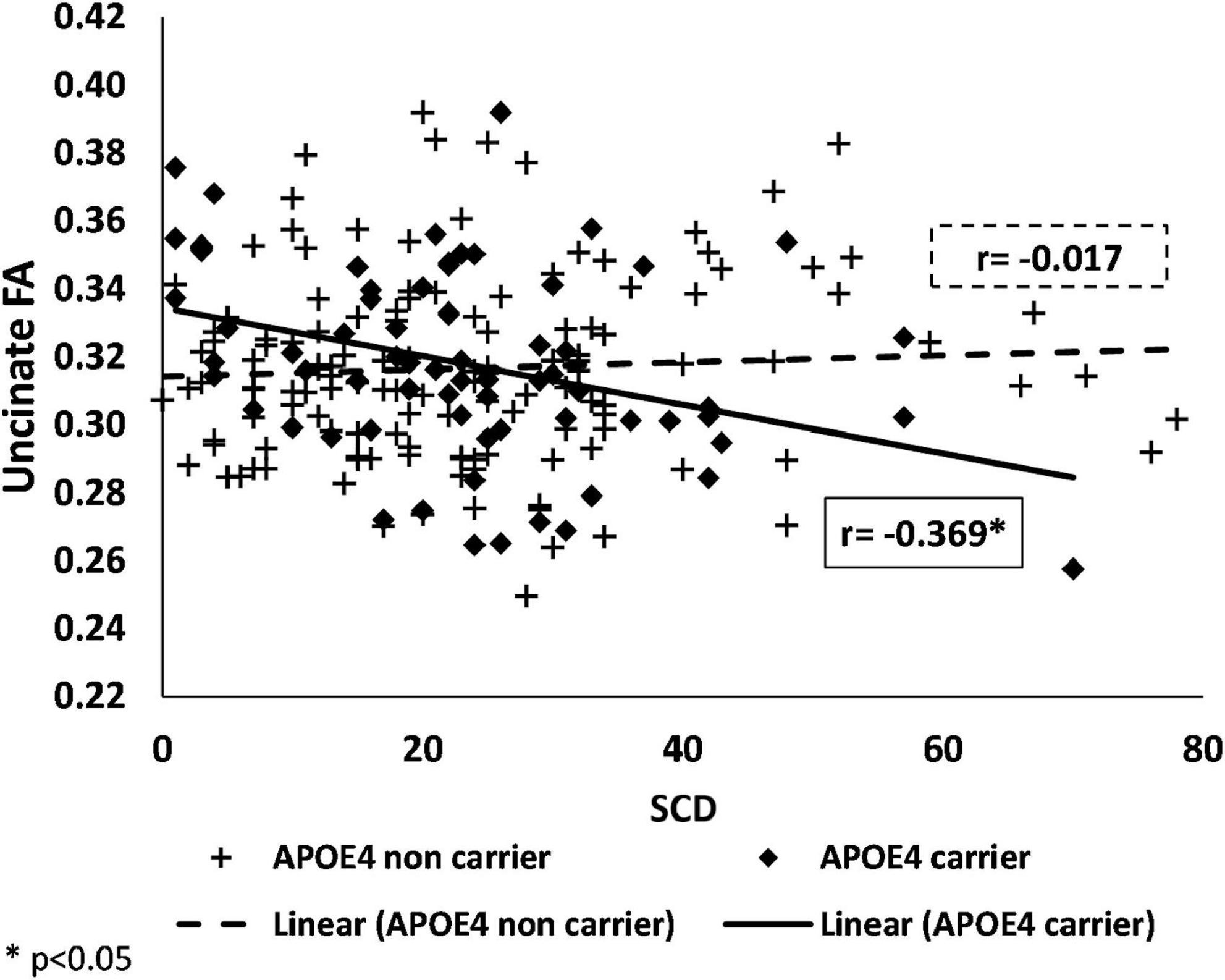
Figure 3. Associations of SCD with fractional anisotropy in APOE ε4 carriers and non-carriers. Negative association between FA and SCD among APOE ε4 carriers (shown as black) and no association among APOE ε4 non-carriers (shown as a dashed-line) in the uncinate fasciculi. SCD, subjective cognitive decline; APOE ε4, apolipoprotein E, alleles ε34 or ε44; FA, fractional anisotropy.
When using the total score of the MFQ as the SCD measure, only the uncinate displayed a significant interaction of APOE genotype with matter integrity [F(1,198) = 5.649, p = 0.018, see Supplementary Table 2].
Results remained essentially unchanged after adding depression scores, episodic memory, and executive function to the GLM models (see Supplementary Table 3).
In addition, after excluding one random sibling from each of the 15 pairs of siblings, results remained significant in all ROIs, except for the interactions of SCD with APOE on the Nback activation in the superior and middle frontal gyri, and middle temporal lobe which were attenuated {[F(1,194) = 3.827, p = 0.052], [F(1,194) = 3.380, p = 0.068], [F(1,194) = 0.916, p = 0.340] respectively, see Supplementary Table 4}.
Finally, after excluding participants with APOE e2/e4 alleles results remained significant in all ROIs, except the interactions of SCD with APOE on the Nback activation in the middle temporal lobe [F(1,202) = 0.632, p = 0.428, see Supplementary Table 5]. All secondary analyses results can be found in Supplementary Tables 1–5.
In this study, we used a comprehensive approach to examine the impact of SCD and moderation by APOE on structural and functional neural correlates that may underlie early memory dysfunction. We found significant interactions between SCD and APOE on both brain activation and white matter integrity. Specifically, the interaction of APOE genotype with SCD on brain activation was found in the anterior cingulate cortex and in the inferior, middle and superior frontal cortices, such that higher SCD was associated with increased brain activation in APOE ε4 carriers. We also found a significant interaction of APOE genotype with SCD on white matter integrity in the uncinate fasciculi, where higher SCD was associated with lower FA specifically in APOE ε4 carriers. Our results suggest that among individuals who are enriched for AD risk, APOE genotype modulates the association between SCD and brain alterations, possibly reflecting a preclinical disease process that precedes overt clinically significant cognitive dysfunction. Our study innovates by identifying APOE ε4 as a crucial modifying factor of structural and functional brain integrity in high risk individuals due to SCD and a parental history of AD.
Moreover, our results lend new evidence to the field by shedding light on potential abnormalities in neural activity during working memory observed in SCD. Working memory and other executive functions decline in individuals has been shown in SCD (Viviano et al., 2019) and characterizes cognitive functioning of AD patients (Kirova et al., 2015). Prior studies have largely focused on episodic memory tasks, but SCD may represent abnormalities in cognitive functions apart from episodic memory. Indeed, we found widespread differences in functional activation among brain regions that subserve working memory, a fundamental cognitive function underlying higher-order cognitive abilities, and which deteriorates early in the progression of AD (Baudic et al., 2006; Kirova et al., 2015).
In older adults, SCD has been found to be correlated with a decrease in overall gray matter volume (Saykin et al., 2006), in temporal areas known to be affected in AD (Schultz et al., 2015; Ryu et al., 2017). Regarding brain function, altered task-related brain activations have been associated with SCD (Rodda et al., 2009; Dumas et al., 2013). Similarly to our finding of higher activation in the frontal and anterior cingulate among APOE ε4 carriers with SCD, increased activation in the prefrontal cortex among people with SCD has been found in previous studies (Rodda et al., 2009). Dumas et al. (2013) reported greater activation in a broad network of areas involved in a working memory task including the middle frontal gyrus, the precuneus and the cingulate gyrus in middle-aged adults reporting SCD (Dumas et al., 2013). Similarly, MCI and AD patients showed more activation in anterior cingulate, fusiform, and frontal and middle temporal areas while performing the working memory task (Yetkin et al., 2006).
The prefrontal cortex and anterior cingulate are well-known regions recruited during working memory tasks, and are also known to show greater activation as cognitive effort increases (Owen et al., 2005).
The APOE ε4 allele has been associated with higher activation in several brain regions (Bookheimer et al., 2000) relative to non-carriers. Congruent with these findings linked to APOE ε4, we found increased brain activity in two of the areas that have previously presented reduced gray matter volume in patients with APOE ε4 (Lee et al., 2016), the anterior cingulum and middle frontal gyrus. Thus, we speculate that the higher activation observed in APOE ε4 carriers may represent a compensatory process, by which participants who report SCD require the use of additional neural resources to achieve cognitive performance at normal levels (Bondi et al., 2005). This is consistent with “brain or cognitive reserve theory” defined as the ability to optimize performance through differential recruitment of brain networks (Stern, 2002) and is consistent with increased activation as a compensatory mechanism found in other conditions such as MCI (Solé-Padullés et al., 2009) and multiple sclerosis (Forn et al., 2007).
We have found that in APOE ε4 carriers, SCD is associated with lower white matter integrity of the uncinate fasciculi. Reduced white matter integrity, is believed to reflect disruption of axonal structure or myelin loss (Basser, 1995), and has been previously described in MCI and AD (Alves et al., 2012), and among individuals at risk for AD due to APOE ε4 genotype (Lee et al., 2016).
Supporting our findings of an association between SCD and white matter integrity, older adults with SCD have previously been observed to exhibit lower FA in the hippocampal body and entorhinal white matter (Ryu et al., 2017). Higher SCD has also been previously associated with lower white matter volume among APOE ε4 carriers only (Dauphinot et al., 2020).
Impairment of FA in the uncinate fasciculi in high AD risk individuals is reinforced by another study from our group showing that FH participants who were also APOE ε4 carriers, had the lowest FA compared to other groups (Bendlin et al., 2010). The uncinate fasciculus, a cortico-cortical association tract, supports decision-making, emotion, and episodic memory, and exhibits changes during the pathophysiology of AD (Kier et al., 2004). There is evidence that a disruption of the uncinate fasciculus fibers can cause severe memory impairment (Levine et al., 1998). We suggest that high SCD accompanied by low white matter integrity in this tract may represent an early indicator of incipient cognitive impairment, though further longitudinal studies should be conducted.
While the structural and functional data in this study were assessed in independent models, the use of the two imaging modalities may shed additional light on structure and function relationship. According to disconnection theories, reduced white matter integrity can be related to changes in cortical activation mediating cognitive function (Bartzokis et al., 2004). Given that the white matter integrity of the uncinate fasciculi, a fronto-temporal association tract, was found to be impaired in people with higher SCD, it is plausible that frontal areas which are connected through this tract exhibit higher activation as a compensatory response.
Study strengths include the relatively large cohort of middle-aged participants enriched for AD risk due to a parental family history who had both structural and functional MRI; the use of a well-validated questionnaire assessing broad aspects of SCD rather than the often used 1-question for SCD (Hong et al., 2015; Schultz et al., 2015) as well as comprehensive neuroimaging to facilitate testing the association between SCD and both structural and functional brain measures, as well as moderating impacts of genotype. Our study is limited by its cross sectional design. The IRAP study longitudinal component is ongoing and examination of the inter-relationship of SCD with APOE ε4 on objectively measured cognitive decline is warranted. The IRAP study is based on a community sample of individuals with FH, and results may not generalize to individuals without a FH. The choice of ROIs in our study was done a priori and therefore all primary analyses were not adjusted for multiple comparisons. The interaction between APOE genotype and brain activation in the anterior cingulate and white matter integrity in the uncinate remained significant after applying Bonferroni correction of 0.05/6 = 0.008 for the fMRI analysis and 0.05/5 = 0.01 for the DTI analysis. Finally, our white-matter integrity methods are limited by a relatively small number of directions (32 directions) and due to the fact that FA and MD values were extracted from ROIs rather than from probabilistic tractography.
We conclude that APOE ε4 allele moderates the association between SCD and neural correlates supporting memory function. Our results indicate that among cognitively normal, middle-aged individuals due to a family history of AD and the APOE ε4 allele, SCD is associated with brain alteration including disrupted white matter integrity and abnormal brain activity during a working memory task. It is possible that these neural alterations reflect incipient AD pathology in a phase where objective cognitive impairment is yet undetectable. AD offspring with APOE ε4 presenting SCD may represent an enriched population and serve as potential candidates for future clinical trials of interventions for prevention of AD. In addition, our results suggest that clinicians may need to follow particularly closely those asymptomatic individuals who are enriched for AD risk and are reporting SCD, as it may represent a window for intervention before frank AD dementia develops.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Sheba Medical Center Institutional Review Board (IRB). The patients/participants provided their written informed consent to participate in this study.
LB-A researched the data and wrote the manuscript. RR-S and MS researched the data, reviewed the manuscript, and contributed to the discussion. GT, RR, and LG researched the data and reviewed the manuscript. AS and IS-G researched the data. BB, EO, and AH reviewed the manuscript and contributed to the discussion. AL researched the data and wrote and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
This study was funded by the NIH (P01-AG02219/AG/NIA NIH HHS, R01 AG034087/AG/NIA NIH, HHS, AG051545/AG/NIA NIH HHS, AG053446/AG/NIA NIH HHS, and AG061093/AG/NIA NIH HHS).
We thank the LeRoy Schecter Foundation and Marina Nissim for their kind gifts which supported this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2023.1067196/full#supplementary-material
AD, Alzheimer’s dementia; SCD, subjective cognitive decline; APOE ε4, apolipoprotein E e4 allele; FH, parental family history of AD; fMRI, functional MRI; DTI, diffusion tensor imaging; FA, fractional anisotropy; MD, mean diffusivity; TR, repetition time; ROIs, region of interests; WM, white matter.
Alves, G. S., O’Dwyer, L., Jurcoane, A., Oertel-Knöchel, V., Knöchel, C., Prvulovic, D., et al. (2012). Different patterns of white matter degeneration using multiple diffusion indices and volumetric data in mild cognitive impairment and Alzheimer patients. PLoS One 7:e52859. doi: 10.1371/journal.pone.0052859
Alzheimer’s Association (2022). Alzheimer’s facts and figures. Available online at: https://www.alz.org/alzheimers-dementia/facts-figures (accessed January 10, 2023).
Amariglio, R. E., Becker, J. A., Carmasin, J., Wadsworth, L. P., Lorius, N., Sullivan, C., et al. (2012). Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 50, 2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011
Amariglio, R. E., Townsend, M. K., Grodstein, F., Sperling, R. A., and Rentz, D. M. (2011). Specific subjective memory complaints in older persons may indicate poor cognitive function. J. Am. Geriatr. Soc. 59, 1612–1617. doi: 10.1111/j.1532-5415.2011.03543.x
Ashburner, J. (2009). Preparing fMRI data for statistical analysis. Neuromethods 41, 151–178. doi: 10.1007/978-1-60327-919-2_6/COVER
Bartzokis, G., Sultzer, D., Lu, P. H., Nuechterlein, K. H., Mintz, J., and Cummings, J. L. (2004). Heterogeneous age-related breakdown of white matter structural integrity: Implications for cortical “disconnection” in aging and Alzheimer’s disease. Neurobiol. Aging 25, 843–851. doi: 10.1016/j.neurobiolaging.2003.09.005
Basser, P. J. (1995). Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed. 8, 333–344. doi: 10.1002/nbm.1940080707
Baudic, S., Barba, G. D., Thibaudet, M. C., Smagghe, A., Remy, P., and Traykov, L. (2006). Executive function deficits in early Alzheimer’s disease and their relations with episodic memory. Arch. Clin. Neuropsychol. 21, 15–21. doi: 10.1016/j.acn.2005.07.002
Bendlin, B. B., Ries, M. L., Canu, E., Sodhi, A., Lazar, M., Alexander, A. L., et al. (2010). White matter is altered with parental family history of Alzheimer’s disease. Alzheimers Dement. 6, 394–403. doi: 10.1016/j.jalz.2009.11.003
Bondi, M., Houston, W., Eyler, L., and Brown, G. (2005). fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology 64, 501–508.
Bookheimer, S. Y., Strojwas, M. H., Cohen, M. S., Saunders, A. M., Pericak-Vance, M. A., Mazziotta, J. C., et al. (2000). Patterns of brain activation in people at risk for Alzheimer’s disease. N. Engl. J. Med. 343, 450–456. doi: 10.1056/NEJM200008173430701
Burmester, B., Leathem, J., and Merrick, P. (2016). Subjective cognitive complaints and objective cognitive function in aging: A systematic review and meta-analysis of recent cross-sectional findings. Neuropsychol. Rev. 26, 376–393. doi: 10.1007/s11065-016-9332-2
Cannon-Albright, L. A., Foster, N. L., Schliep, K., Farnham, J. M., Teerlink, C. C., Kaddas, H., et al. (2019). Relative risk for Alzheimer disease based on complete family history. Neurology 92, e1745–e1753. doi: 10.1212/WNL.0000000000007231
Cargin, J. W., Collie, A., Masters, C., and Maruff, P. (2008). The nature of cognitive complaints in healthy older adults with and without objective memory decline. J. Clin. Exp. Neuropsychol. 30, 245–257. doi: 10.1080/13803390701377829
Dauphinot, V., Bouteloup, V., Mangin, J., Vellas, B., Pasquier, F., Blanc, F., et al. (2020). Subjective cognitive and non-cognitive complaints and brain MRI biomarkers in the MEMENTO cohort. Alzheimers Dement. Diagnosis Assess. Dis. Monit. 12, 1–15. doi: 10.1002/dad2.12051
Dumas, J. A., Kutz, A. M., Mcdonald, B. C., Naylor, M. R., Pfaff, C., Saykin, A. J., et al. (2013). Increased working memory-related brain activity in middle- aged women with cognitive complaints. Neurobiol. Aging 34, 1145–1147. doi: 10.1016/j.neurobiolaging.2012.08.013.Increased
Erk, S., Spottke, A., Meisen, A., Wagner, M., Walter, H., and Jessen, F. (2011). Evidence of neuronal compensation during episodic memory in subjective memory impairment. Arch. Gen. Psychiatry 68, 845–852. doi: 10.1001/archgenpsychiatry.2011.80
Forn, C., Barros-Loscertales, A., Escudero, J., Benlloch, V., Campos, S., Parcet, M. A., et al. (2007). Compensatory activations in patients with multiple sclerosis during preserved performance on the auditory N-back task. Hum. Brain Mapp. 28, 424–430. doi: 10.1002/HBM.20284
Gilewski, M. J., Zelinski, E. M., and Schaie, K. W. (1990). The memory functioning questionnaire for assessment of memory complaints in adulthood and old age. Psychol. Aging 5, 482–490. doi: 10.1037/0882-7974.5.4.482
Hayes, J. M., Tang, L., Viviano, R. P., van Rooden, S., Ofen, N., and Damoiseaux, J. S. (2017). Subjective memory complaints are associated with brain activation supporting successful memory encoding. Neurobiol. Aging 60, 71–80. doi: 10.1016/j.neurobiolaging.2017.08.015
Hong, Y. J., Yoon, B., Shim, Y. S., Ahn, K. J., Yang, D. W., and Lee, J. H. (2015). Gray and white matter degenerations in subjective memory impairment: Comparisons with normal controls and mild cognitive impairment. J. Korean Med. Sci. 30, 1652–1658. doi: 10.3346/jkms.2015.30.11.1652
Hu, X., Uhle, F., Fliessbach, K., Wagner, M., Han, Y., Weber, B., et al. (2017). Reduced future-oriented decision making in individuals with subjective cognitive decline: A functional MRI study. Alzheimers Dement. Diagnosis Assess. Dis. Monit. 6:222. doi: 10.1016/J.DADM.2017.02.005
Innes, K. E., Selfe, T. K., Khalsa, D. S., and Kandati, S. (2017). Meditation and music improve memory and cognitive function in adults with subjective cognitive decline: A pilot randomized controlled trial. J. Alzheimers. Dis. 56:899. doi: 10.3233/JAD-160867
Jessen, F., Amariglio, R. E., van Boxtel, M., Breteler, M., Ceccaldi, M., Chételat, G., et al. (2014). A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 10, 844–852. doi: 10.1016/j.jalz.2014.01.001
Jorm, A., Butterworth, P., Anstey, K. J., Christensen, H., Easteal, S., Maller, J., et al. (2004). Memory complaints in a community sample aged 60-64 years: Associations with cognitive functioning, psychiatric symptoms, medical conditions, APOE genotype, hippocampus and amygdala volumes, and white-matter hyperintensities. Psychol. Med. 34, 1495–1506. doi: 10.1017/S0033291704003162
Jorm, A., Christensen, H., Korten, A., Jacomb, P., and Henderson, A. (2001). Memory complaints as a precursor of memory impairment in older people: A longitudinal analysis over 7-8 years. Psychol. Med. 31, 441–449.
Jungwirth, S., Fischer, P., Weissgram, S., Kirchmeyr, W., Bauer, P., and Tragl, K. H. (2004). Subjective Memory Complaints and Objective Memory Impairment in the Vienna-Transdanube aging community. J. Am. Geriatr. Soc. 52, 263–268. doi: 10.1111/j.1532-5415.2004.52066.x
Jungwirth, S., Zehetmayer, S., Weissgram, S., Weber, G., Tragl, K. H., and Fischer, P. (2008). Do subjective memory complaints predict senile Alzheimer dementia? Wien. Med. Wochenschr. 158, 71–77. doi: 10.1007/s10354-007-0446-2
Kier, E. L., Staib, L. H., Davis, L. M., and Bronen, R. A. (2004). MR imaging of the temporal stem: Anatomic dissection tractography of the uncinate fasciculus, inferior occipitofrontal fasciculus, and Meyer’s loop of the optic radiation. Am. J. Neuroradiol. 25, 677–691.
Kirova, A. M., Bays, R. B., and Lagalwar, S. (2015). Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed. Res. Int. 2015:748212. doi: 10.1155/2015/748212
Kiuchi, K., Kitamura, S., Taoka, T., Yasuno, F., Tanimura, M., Matsuoka, K., et al. (2014). Gray and white matter changes in subjective cognitive impairment, amnestic mild cognitive impairment and Alzheimer’s disease: A voxel-based analysis study. PLoS One 9:e104007. doi: 10.1371/JOURNAL.PONE.0104007
Lee, Y. M., Ha, J. K., Park, J. M., Lee, B. D., Moon, E., Chung, Y. I., et al. (2016). Impact of apolipoprotein E4 polymorphism on the gray matter volume and the white matter integrity in subjective memory impairment without white matter hyperintensities: Voxel-based morphometry and tract-based spatial statistics study under 3-Tesla MRI. J. Neuroimaging 26, 144–149. doi: 10.1111/jon.12207
Levine, B., Black, S. E., Cabeza, R., Sinden, M., Mcintosh, A. R., Toth, J. P., et al. (1998). Episodic memory and the self in a case of isolated retrograde amnesia. Brain 121(Pt 1), 1951–1973. doi: 10.1093/brain/121.10.1951
Li, X. Y., Tang, Z. C., Sun, Y., Tian, J., Liu, Z. Y., and Han, Y. (2016). White matter degeneration in subjective cognitive decline: A diffusion tensor imaging study. Oncotarget 7, 54405–54414. doi: 10.18632/oncotarget.10091
Lim, Y. Y., Yassi, N., Bransby, L., Properzi, M., and Buckley, R. (2019). The healthy brain project: An online platform for the recruitment, assessment, and monitoring of middle-aged adults at risk of developing Alzheimer’s disease. J. Alzheimers. Dis. 68, 1211–1228. doi: 10.3233/JAD-181139
Livny, A., Cohen, K., Tik, N., Tsarfaty, G., Rosca, P., and Weinstein, A. (2018). The effects of synthetic cannabinoids (SCs) on brain structure and function. Eur. Neuropsychopharmacol. 28, 1047–1057. doi: 10.1016/j.euroneuro.2018.07.095
Martinez, M., Campion, D., Brice, A., Hannequin, D., and Dubois, B. (1998). Apolipoprotein E 4 allele and familial aggregation of Alzheimer disease. Arch. Neurol. 55, 810–816.
Merrill, D. A., Siddarth, P., Saito, N. Y., Ercoli, L. M., Burggren, A. C., Kepe, V., et al. (2012). Self-reported memory impairment and brain PET of amyloid and tau in middle-aged and older adults without dementia. Int. Psychogeriatr. 24, 1076–1084. doi: 10.1017/S1041610212000051.SELF-REPORTED
Montejo, P., Montenegro, M., Fernández, M. A., and Maestú, F. (2011). Subjective memory complaints in the elderly: Prevalence and influence of temporal orientation, depression and quality of life in a population-based study in the city of Madrid. Aging Ment. Heal. 15, 85–96. doi: 10.1080/13607863.2010.501062
Mori, S., Oishi, K., Jiang, H., Jiang, L., Li, X., Akhter, K., et al. (2008). Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage 40, 570–582. doi: 10.1016/j.neuroimage.2007.12.035
Neto, A. S., and Nitrini, R. (2016). Subjective cognitive decline: The first clinical manifestation of Alzheimer’s disease? Dement. Neuropsychol. 10, 170–177. doi: 10.1590/s1980-5764-2016dn1003002
Owen, A. M., McMillan, K. M., Laird, A. R., and Bullmore, E. (2005). N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 25, 46–59. doi: 10.1002/hbm.20131
Racine, A. M., Koscik, R. L., Berman, S. E., Nicholas, C. R., Clark, L. R., Okonkwo, O. C., et al. (2016). Biomarker clusters are differentially associated with longitudinal cognitive decline in late midlife. Brain 139, 2261–2274. doi: 10.1093/brain/aww142
Radloff, L. S. (1977). The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1, 385–401. doi: 10.1177/014662167700100306
Ravona-Springer, R., Sharvit-Ginon, I., Ganmore, I., Greenbaum, L., Bendlin, B. B., Sternberg, S. A., et al. (2020). The israel registry for Alzheimer’s prevention (IRAP) study: Design and baseline characteristics. J. Alzheimers Dis. 78, 777–788. doi: 10.3233/JAD-200623
Risacher, S. L., Kim, S., Nho, K., Foroud, T., Shen, L., and Petersen, R. C. Jr., et al. (2016). APOE effect on Alzheimer’s disease biomarkers in older adults with significant memory concerns. Alzheimers Dement. 11, 1417–1429. doi: 10.1016/j.jalz.2015.03.003.APOE
Rodda, J., Dannhauser, T. M., Cutinha, D. J., Shergill, S. S., and Walker, Z. (2009). Subjective cognitive impairment: Increased prefrontal cortex activation compared to controls during an encoding task. Int. J. Geriatr. Psychiatry 24, 865–874. doi: 10.1002/gps.2207
Ryu, S. Y., Lim, E. Y., Na, S., Shim, Y. S., Cho, J. H., Yoon, B., et al. (2017). Hippocampal and entorhinal structures in subjective memory impairment: A combined MRI volumetric and DTI study. Int. Psychogeriatrics 29, 785–792. doi: 10.1017/S1041610216002349
Saykin, A. J., Wishart, H. A., Rabin, L. A., Santulli, R. B., Flashman, L. A., West, J. D., et al. (2006). Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 67, 834–842. doi: 10.1212/01.wnl.0000234032.77541.a2
Schultz, S. A., Oh, J. M., Koscik, R. L., Dowling, N. M., Gallagher, C. L., Carlsson, C. M., et al. (2015). Subjective memory complaints, cortical thinning, and cognitive dysfunction in middle-age adults at risk of AD. Alzheimers Dement. Diagnosis Assess. Dis. Monit. 1, 33–40. doi: 10.1016/j.dadm.2014.11.010
Sharma, N., Murari, G., Vandermorris, S., Verhoeff, N. P. L. G., Herrmann, N., Chen, J. J., et al. (2021). Functional connectivity between the posterior default mode network and parahippocampal gyrus is disrupted in older adults with subjective cognitive decline and correlates with subjective memory ability. J. Alzheimers. Dis. 82, 435–445. doi: 10.3233/JAD-201579
Smith, A. D., and Yaffe, K. (2014). Dementia (including Alzheimer’s disease) can be prevented: Statement supported by international experts. J. Alzheimers Dis. 38, 699–703. doi: 10.3233/JAD-132372
Snitz, B. E., Weissfeld, L. A., Cohen, A. D., Lopez, O. L., Nebes, R. D., Aizenstein, H. J., et al. (2015). Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am. J. Geriatr. Psychiatry 23:985. doi: 10.1016/J.JAGP.2015.01.008
Solé-Padullés, C., Bartrés-Faz, D., Junqué, C., Vendrell, P., Rami, L., Clemente, I. C., et al. (2009). Brain structure and function related to cognitive reserve variables in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol. Aging 30, 1114–1124. doi: 10.1016/J.NEUROBIOLAGING.2007.10.008
Stern, Y. (2002). What is cognitive reserve? Theory and research application of the reserve concept. J. Int. Neuropsychol. Soc. 8, 448–460. doi: 10.1017/S1355617702813248
Strittmatter, W. J., and Roses, A. D. (1996). Apolipoprotein E and Alzheimer’s disease. Annu. Rev. Neurosci. 19, 53–77. doi: 10.1146/annurev.ne.19.030196.000413
Tzourio-Mazoyer, N., Landeau, B., Papathanassiou, D., Crivello, F., Etard, O., Delcroix, N., et al. (2002). Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. doi: 10.1006/nimg.2001.0978
van Dyck, C. H., Swanson, C. J., Aisen, P., Bateman, R. J., Chen, C., Gee, M., et al. (2022). Lecanemab in early Alzheimer’s disease. N. Engl. J. Med. 388, 9–21. doi: 10.1056/NEJMoa2212948
Verfaillie, S. C. J., Pichet Binette, A., Vachon-Presseau, E., Tabrizi, S., Savard, M., Bellec, P., et al. (2018). Subjective cognitive decline is associated with altered default mode network connectivity in individuals with a family history of Alzheimer’s disease. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 463–472. doi: 10.1016/j.bpsc.2017.11.012
Viviano, R. P., Hayes, J. M., Pruitt, P. J., Fernandez, Z. J., van Rooden, S., van der Grond, J., et al. (2019). Aberrant memory system connectivity and working memory performance in subjective cognitive decline. Neuroimage 185, 556–564. doi: 10.1016/J.NEUROIMAGE.2018.10.015
Wang, X., Huang, W., Su, L., Xing, Y., Jessen, F., Sun, Y., et al. (2020). Neuroimaging advances regarding subjective cognitive decline in preclinical Alzheimer’s disease. Mol. Neurodegener. 15, 1–27. doi: 10.1186/S13024-020-00395-3/FIGURES/2
Yetkin, F. Z., Rosenberg, R. N., Weiner, M. F., Purdy, P. D., and Cullum, C. M. (2006). FMRI of working memory in patients with mild cognitive impairment and probable Alzheimer’s disease. Eur. Radiol. 16, 193–206. doi: 10.1007/s00330-005-2794-x
Keywords: subjective cognitive decline (SCD), Alzheimer’s dementia (AD), neuroimaging, fMRI, working memory, diffusion tensor imaging, family history (FH), APOE
Citation: Ben-Ami L, Ravona-Springer R, Tsarfaty G, Raizman R, Shumacher A, Sharvit-Ginon I, Greenbaum L, Bendlin BB, Okun E, Heymann A, Schnaider Beeri M and Livny A (2023) Neural correlates of subjective cognitive decline in adults at high risk for Alzheimer’s disease. Front. Aging Neurosci. 15:1067196. doi: 10.3389/fnagi.2023.1067196
Received: 11 October 2022; Accepted: 16 January 2023;
Published: 01 February 2023.
Edited by:
Boon-Seng Wong, Singapore Institute of Technology, SingaporeReviewed by:
Laura Korthauer, Brown University, United StatesCopyright © 2023 Ben-Ami, Ravona-Springer, Tsarfaty, Raizman, Shumacher, Sharvit-Ginon, Greenbaum, Bendlin, Okun, Heymann, Schnaider Beeri and Livny. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abigail Livny,  YWJpZ2FpbC5saXZueWV6ZXJAZ21haWwuY29t
YWJpZ2FpbC5saXZueWV6ZXJAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.