- 1Department of Tuina, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2School of Acupuncture and Tuina, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3Department of Galactophore, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 4Department of Emergency, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
With the development trend of an aging society, Alzheimer’s disease (AD) has become an urgent problem in the field of medicine worldwide. Cognitive impairment in AD patients leads to a decline in the ability to perform daily living and abnormalities in behavior and personality, causing abnormal psychiatric symptoms, which seriously affect the daily life of patients. Currently, mainly drug therapy is used for AD patients in the clinic, but a large proportion of patients will experience drug efficacy not working, and even some drugs bring severe sleep disorders. Acupuncture, with its unique concept and treatment method, has been validated through a large number of experiments and proved its reliability of acupuncture in the treatment of AD. Many advances have been made in the study of the neurobiological mechanisms of acupuncture in the treatment of AD, further demonstrating the good efficacy and unique advantages of acupuncture in the treatment of AD. This review first summarizes the pathogenesis of AD and then illustrates the research progress of acupuncture in the treatment of AD, which includes the effect of acupuncture on the changes of biochemical indicators in AD in vivo and the specific mechanism of action to exert the therapeutic effect. Changes in relevant indicators of AD similarly further validate the effectiveness of acupuncture treatment. The clinical and mechanistic studies of acupuncture in the treatment of AD are intensified to fit the need for social development. It is believed that acupuncture will achieve new achievements in the treatment of AD as research progresses.
Introduction
Alzheimer’s disease (AD) is a chronic brain degenerative disease characterized by progressive distant and near memory impairment, decreased ability of analysis and judgment, emotional changes, behavioral disorders, and even disturbance of consciousness. it is one of the major diseases seriously endangering the health of the elderly (Pfundstein et al., 2022; Rodini et al., 2022). The etiology of AD is related to many factors. The typical pathological changes are a large number of senile plaques (SP) between nerve cells, neurofibrillary tangles (NFTs) in nerve cells, and neuronal loss. Among all dementia patients, AD patients are the most common and account for the vast majority (about 60–70% of the total number of dementia). Autopsy studies of dementia show that 50 of 70% of the patients are AD (Aborode et al., 2022; Flores et al., 2022). Recent reports have shown that there are about 46 million AD patients worldwide, which will double every 20 years in the future. AD poses a serious threat to the health and safety of the elderly. Thirty years later, it is estimated that the global number of AD patients will reach an alarming 131.5 million. The incidence of AD was positively correlated with age, and there were more females than males. Since the 1980s, the epidemiological survey data on the prevalence of AD in various countries around the world are relatively similar, and the prevalence of dementia in people over 65 years old is about 5% (Eid et al., 2022; Kishino et al., 2022). AD is present at a higher prevalence of 20% in individuals older than 80 years, and the prevalence increases with age. The highest prevalence of AD in people aged 60 and over in Europe and North America was 5.4 and 6.4%, respectively; 4.9% in Latin America; 3.8 and 3.9% in Eastern Europe; China 4%.
At present, for this intractable type of disease, there are no effective drugs and technical means for clinical treatment given at home and abroad (Martersteck et al., 2022; Ruengchaijatuporn et al., 2022). There are currently several claims in the clinic to the related pathogenesis of Alzheimer’s disease, namely the cholinergic doctrine, the tau protein hypothesis, the neurovascular doctrine, the oxidative stress doctrine, the β- Amyloid theory, the brain-gut axis theory, etc., whether brain extracellular amyloid peptide exists β (Aβ) Deposition and intracellular tau protein (Tau) hyperphosphorylation while neurofibrillary tangles are the pathological diagnostic criteria of the disease, but the exact etiology of the AD is not well understood, and an effective cure for the AD is lacking to date. AD is a multi-factor and multi-mechanism disease, which has posed a serious threat to human health. As an important part of traditional medicine, acupuncture has the characteristics of multi-target, multi-way, and multi-level functions. Similarly, there are many clinical studies on acupuncture for the treatment of AD. Li R. et al. (2020) randomized 60 AD patients into treatment and control groups, 30 each. Patients in both groups were treated with oral donepezil hydrochloride and conventional therapy. The control group was given conventional acupuncture. In the treatment group, “Fengchi,” “Tianzhu,” “Wangu,” “Panfeng,” “Fengfu,” and “Zhongwan” acupoints were sampled. In the control group, they were treated with common acupuncture, including “Baihui,” “Sishencong,” “Intang,” “Shenting,” “Taixi,” and “Xuanzhong” acupoints. The overall response rate was 82.1% in the treatment group and 72.4% in the control group. Moreover, cognition and memory were significantly improved after treatment.
This article reviews the recent studies on acupuncture and moxibustion in the treatment of AD at home and abroad. It is found that acupuncture can regulate the overall regulation of AD by regulating abnormal protein expression in the brain, regulating the physiological and pathological state of microglia, regulating mitochondrial autophagy, regulating epigenetic modification, giving full play to neuron protection, improving synaptic plasticity, regulating oxidative stress and regulating energy metabolism. In-depth analysis of the effect mechanism of acupuncture and moxibustion in the treatment of AD, and reveal its deep action principle, to provide a scientific and reasonable theoretical basis for clinical diagnosis and treatment of AD.
Research status of action mechanism
Pathways and effects of acupuncture in the treatment of AD
From the perspective of traditional Chinese medicine, neurodegenerative diseases are diseases of the brain. At present, numerous studies have shown that acupuncture at the “Baihui” acupoint can promote the treatment of encephalopathy. The acupoint selection criteria for the treatment of memory disorders, except the “Baihui” acupoint, are based on the symptoms of the brain disease itself and associated underlying diseases (de Pins et al., 2019; Li G. et al., 2019).
Acupuncture stimulation intensity and frequency are also important for the treatment of diseases. Found that high-intensity acupuncture at “Baihui,” and “Dazhui” acupoints improved the learning and memory function of AD rats better than low-intensity acupuncture. Wang et al. (2020c) studied the therapeutic effects of acupuncture “Baihui” and “Shenshu” at different frequencies (50, 30, and 2 Hz) on AD rats and the underlying mechanisms. The results showed that acupuncture downregulated GSK-3β levels in the hippocampus of AD Rats, upregulated GAP-43 levels, and 50 Hz acupuncture improved learning and memory function and repaired synaptic damage in AD rats better than 30 and 2 Hz (Lin et al., 2018).
Combination drugs have also tried to improve the effect of acupuncture on memory impairment. Yang et al. (2021) explored the efficacy and mechanism of a combination of electroacupuncture at “Baihui,” and “Yintang” acupoints and donepezil in the treatment of AD. The results showed that acupuncture enhanced the effect of donepezil on improving learning and memory function in AD rats and facilitated the transport of donepezil through the blood-brain barrier by regulating the expression of matrix metalloproteinase nine, low-density lipoprotein receptor-related protein one and PGP Aβ (Wegmann et al., 2018).
Acupuncture at the acupoints “Baihui,” “Dazhui,” and “Zusanli” combined with gastrodin in the treatment of learning and memory impairment in AD rats. Acupuncture or gastrodin both improved cognitive function and upregulated SIRT1, Bcl2, and PGC-1α in AD rats expression, inhibiting the expression of Bax, and protecting hippocampal neurons, but the effect of combined acupuncture and gastrodin was better than that of acupuncture or gastrodin alone. Interestingly, laser acupuncture improved cognitive impairment induced by cerebral ischemia and modulated the expression of CREB, BDNF, Bcl2, and Bax genes, exerting neuroprotective effects. In addition, some physical therapies, such as transcranial magnetic stimulation, moxibustion, massage, and rehabilitation therapy, can also improve the effect of acupuncture, which needs attention (Jiang et al., 2017; Guo et al., 2020; Hang et al., 2022).
As shown in Figure 1, the mechanism of action of acupuncture is closely related to the repair of synaptic plasticity in the hippocampus. This review focuses on the pathogenesis of AD (synaptic proteins, ad signature proteins, gut flora, neuroinflammation, neuronal apoptosis, and changes in energy metabolism) and the role of acupuncture in the treatment of AD. It is also worth highlighting that synaptic plasticity in the hippocampus may be a central and common link to the above mechanisms.
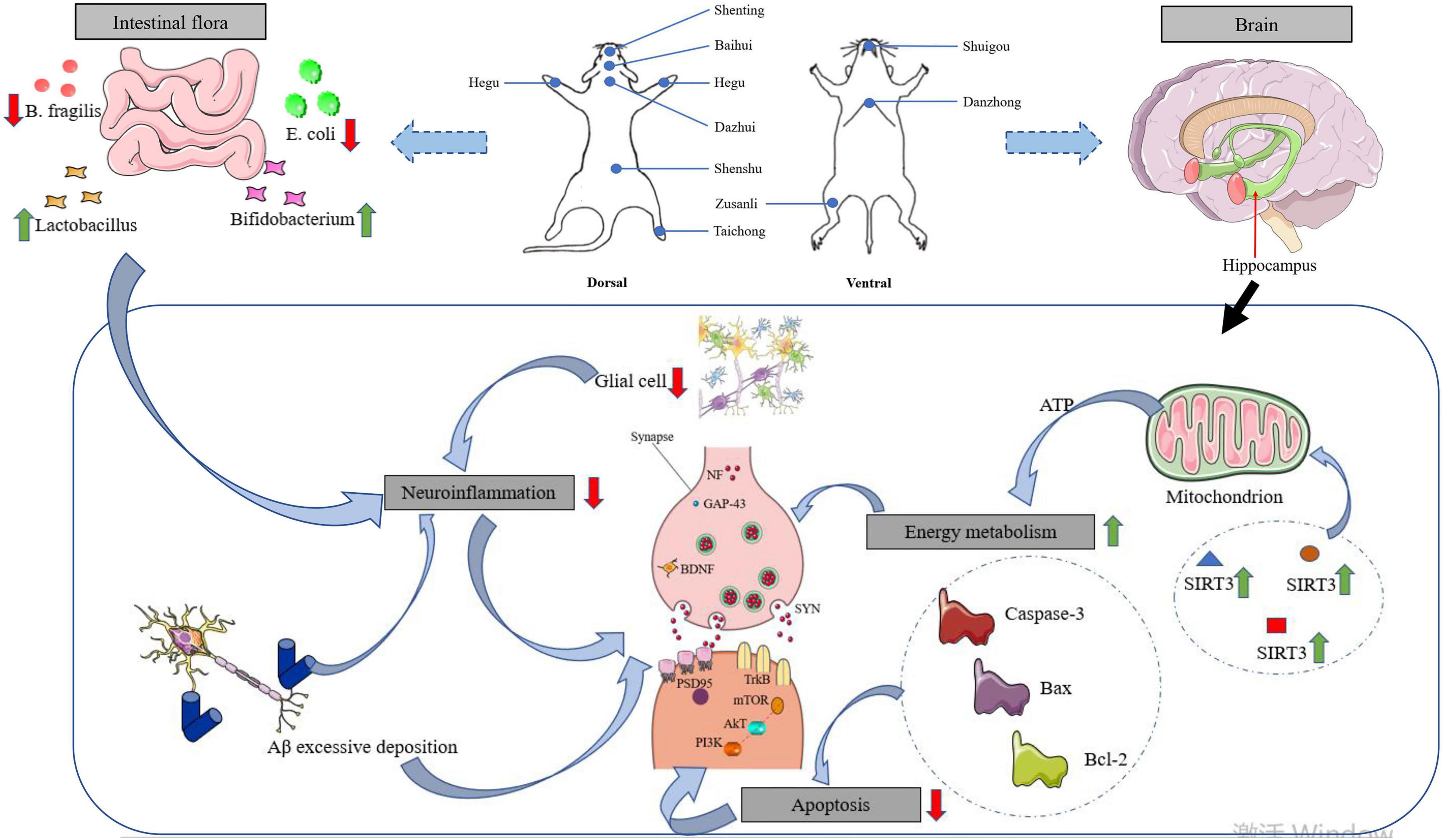
Figure 1. Effects of acupuncture intervention on neuroplasticity in AD pathogenesis. With the focus on synaptic plasticity in the hippocampus, the mechanisms by which acupuncture affects memory impairment are reviewed in terms of synaptic proteins, ad signature proteins, gut microflora, neuroinflammation, and energy metabolism.
Acupuncture intervention modulates central neurotransmitter release
Neurobiochemistry reveals that amino acid neurotransmitters are hardly related to learning and memory (Al-Nasser et al., 2022). Glutamate (Glu) is the capital excitatory neurotransmitter of pyramidal neurons, which acts an essential role in learning and memory, synaptic plasticity in development, neuronal survival, and dendritic growth and degeneration. Its function is regulated by N-methyl-D-aspartate (NMDA) receptor. Long-term synaptic enhancement (LTP) is considered to be a physiological mechanism of information storage and memory formation in the brain (Khavinson et al., 2021). DMNA receptors are considered to be closely related to learning and memory due to the existence of LTP. The relationship between Glu and LTP can be summarized as follows: stimulation-> postsynaptic NMDA receptor activation-> channel opening-> Ca2+ influx-> cell membrane depolarization-> LTP (Catarzi et al., 2007). There are excitatory amino acids and inhibitory amino acids in the brain, which play the role of neurotransmitters and act a crucial role in the process of learning and memory (Noda, 2016; Du et al., 2020).
The pathogenesis of AD associated with changes in excitatory amino acid content in brain tissue has long been demonstrated. Tang et al. (2005) used alginic acid injection to make a rat dementia model, acupuncture “Dazhui,” “Shenshu,” “Taixi,” and “Housanli” acupoints to observe the function of acupuncture on the content of Glu and aspartic acid (Asp) in the brain of senile dementia rats. It was detected by high-performance liquid chromatography and ultraviolet spectrophotometer colorimetry. The results showed that the level of Glu and Asp in the brain tissue of the model group were significantly lower than those of the sham operation group (P < 0.01), suggesting that the changes in Glu and Asp contents were closely related to the pathogenesis of AD, which was consistent with the related experiment outcome (Hynd et al., 2004; Natale et al., 2006). After acupuncture and dihydroergotamine methanesulfonamide (Hydergine) treatment, the level of Glu and ASP in the brain of model rats decreased significantly (P < 0.05), suggesting that increasing the content of excitatory amino acids in the brain of senile dementia rats may be one of the effective mechanisms of acupuncture in the treatment of senile dementia. According to AD’s glutamatergic hypothesis, the increase of synthesis and release of excitatory amino acids (EAAS), especially Glu, leads to excitatory neurotoxicity, which leads to neuronal degeneration and death is an important mechanism of AD brain degeneration (O’Neill et al., 2004). Shi and Zhuang (2020) observed the synthesis and release of EAAS in different brain regions by acupuncture at “Shuigou” and “Neiguan” acupoints of SAM-P/8 mice, the results point out that acupuncture could decrease the abnormally increased content of Glu, Asp, and glutamine, suggesting that the regulating effect of acupuncture on EAAS metabolism may be one of the important mechanisms in the treatment of AD.
Acupuncture intervention modulates the activity of acetylcholine (ACh), acetylcholine transferase (ChAT), and acetylcholinesterase (AChE)
It has been found that the cholinergic projection system from the basal forebrain nucleus to the cerebral cortex and hippocampus is called the forebrain cholinergic system, which is closely related to advanced neural activities such as learning and memory (Zhang et al., 2022). The most obvious changes in the central cholinergic system of AD are the basal forebrain cholinergic system, including the basal nucleus of Meynert and the medial septal nucleus, which project to an extensive region of the hippocampus and cerebral cortex. About 90% of cholinergic neurons are lost in the basal forebrain of patients with AD (Li, 2022). Neurobiochemical studies have shown that Ach synthesis and ChaT activity are decreased in patients with AD, which is inextricably linked to the progression of dementia.
The degeneration of the cholinergic system in the basal forebrain may be the major cause of cognitive impairment in patients with AD (Gu and Wang, 2021; Letsinger et al., 2022). Wang and Zhou (2009) established the AD model by microinjection of Aβ1-40 into bilateral Meynert basal nuclei of rats to study the effect of acupuncture on the contents of Ach, ChAT, and AchE in the brain of AD model rats. After acupuncture at “Baihui,” “Zusanli,” and “Shenshu” acupoints for 1 month, to test the function of acupuncture on the synthesis and decomposition of ACh in the hippocampus and cortex, it is important to detect the activity and content of ache, ACh, and chat It was found that the activity of ChAT and the content of Ach in the cortex and hippocampus of AD rats induced by Aβ1-40 decreased significantly, while the activity of AchE increased significantly, and the rate of AchE and Ach in brain tissue increased significantly, which was consistent with AD’s cholinergic theory (Vecchio et al., 2021). The outcome points out that the level of activities of Ach, ChAT, and AchE in the cortex and hippocampus in the acupuncture group were different from those in the model group (P < 0.01). It has been proved that acupuncture can increase the activity of ChAT, inhibits the activity of AchE, promote the synthesis of Ach, inhibit the decomposition of Ach, increases the content of Ach in brain tissue, and reverse memory loss (Reale and Costantini, 2021). Yu et al. (2021) used scopolamine injection to make a senile dementia model in rats and mice, and acupuncture at the “Yongquan” acupoint was carried out alternately for 30 days. The activity of AchE in the brain of senile dementia mice and the hippocampus of senile dementia rats were detected, respectively. The experimental demonstration that the activity of AchE in the brain of mice in the scopolamine model group was significantly lower than that in the control group (P < 0.05), and the brain AchE in the model group was significantly higher than that in the model group after acupuncture (P < 0.05). The hippocampal AchE of senile dementia model rats was significantly lower than that of the control group, and acupuncture had a significant effect on hippocampal AchE response of senile dementia model rats, which was significantly different from that of the model group. In a word, it is suggested that acupuncture has a significant function on the activity of acetylcholinesterase in the brain of senile dementia mice and the hippocampus of senile dementia rats (Sutalangka et al., 2013; Yang Q. et al., 2019; Zhang et al., 2019).
Acupuncture modulates monoamine neurotransmitter release
Monoamine neurotransmitters in brain tissue include: 5-HT, NA, and DA, 5-HT are important neurotransmitters for maintaining normal intelligence. The brain system of patients with AD was severely damaged, with an average reduction of 61% of the concentration of 5-HT in the frontal lobe (Plini et al., 2021; Tripathi and Mazumder, 2021). NA is widely projected to the entire central nervous system through axonal connections, participates in the regulation of the excited state of the entire cerebral cortex, and has a wide range of effects on arousal, sensory, emotional, and higher cognitive functions.
The activity of NA in the cerebral cortex of AD decreased significantly, and the loss of NA neurons and the change in NA activity were related to the severity of AD. Ma et al. (2020) made an AD model by microinjection of 6-hydroxydopamine into the ascending dorsal tract of NA in the rat brain, resulting in the decrease of learning and memory function in rats, which proved that the levels of central NA and DA were closely related to learning and memory. Bao and Lv (2003) used Alcl3 to replicate the senile dementia model of chronic aluminum poisoning, acupuncture at “Fengfu” acupoint for 14 days, and drug control treatment with nimodipine tablet (NM), intragastric administration once a day for 14 days (Kaur et al., 2021). The contents of monoamine neurotransmitters 5-HT, NA, and DA in brain tissue were determined, and the behavior test was carried out by a Y-type electric maze. The results showed that acupuncture at the “Fengfu” acupoint could significantly improve the memory impairment of dementia-like mice, and significantly increase the low contents of 5-HT, NA, and DA in brain tissue (P < 0.05). It is suggested that the mechanism of acupuncture at “Fengfu” acupoint in the treatment of senile dementia may be to increase the content of monoamine neurotransmitters in brain tissue (Von Linstow et al., 2017; Wang et al., 2018; Babić Leko et al., 2021). Zhao et al. (1999) the aged SD rats were stochastically divided into the aged group and the aged acupuncture group, the young SD rats were the young control group, and the elderly acupuncture group was treated with acupuncture at “Yongquan” acupoints of both hindlimbs alternately every other day for 30 days (Esteban et al., 2017). The results prove that the level of monoamine neurotransmitters in the brain of aged acupuncture rats and aged rats were significantly lower than that of young rats (P < 0.05). The content of monoamine neurotransmitters in the brain of aged acupuncture rats was significantly higher than that of aged rats (P < 0.05). It shows that the excitability of central nervous system activity which is closely related to learning and memory ability decreases after aging, and acupuncture can reduce the extent of its decline (Nazarali and Reynolds, 1992; Storga et al., 1996; Trabace et al., 2007; Dekker et al., 2015; Gruden et al., 2016; Vermeiren et al., 2016).
Mechanisms of inflammatory action in the brain of patients with AD and acupuncture inhibition of inflammatory responses in brain tissue
A large number of research have demonstrated that the inflammatory mechanism acts a vital role in the pathogenesis of AD (Cheng et al., 2022; Sun et al., 2022). The neuroinflammatory mechanism of AD has been studied for more than 20 years, but it is still not fully understood. A large number of genetic and immunological analyses show that there is a significant link between inflammation and AD pathology, as shown in Figure 2 (Wang et al., 2022). Modern medical studies have shown that the core pathological mechanism of AD is that Aβ deposition activates microglia to cause inflammation in neuroinflammatory plaques. In the early stage of inflammation, microglia express phagocytic receptors to clear Aβ (An et al., 2022). With the increase of Aβ, Aβ blocks phagocytosis receptors and makes microglia lose phagocytosis. On the contrary, microglia are activated to release inflammatory cytokines, such as IL-1β, IL-6, TNF-α, and so on. These inflammatory factors in turn activate microglia and astrocytes to produce APP and Aβ, forming a malignant positive feedback loop, resulting in a sharp increase in the amount of APP and Aβ (Yousaf et al., 2022). Recent studies have shown that cytokines IL-1 and IL-6 are not only important immune regulatory factors but also have a wide range of central regulatory effects. IL-1 is a kind of cytokine with an immunomodulatory function.
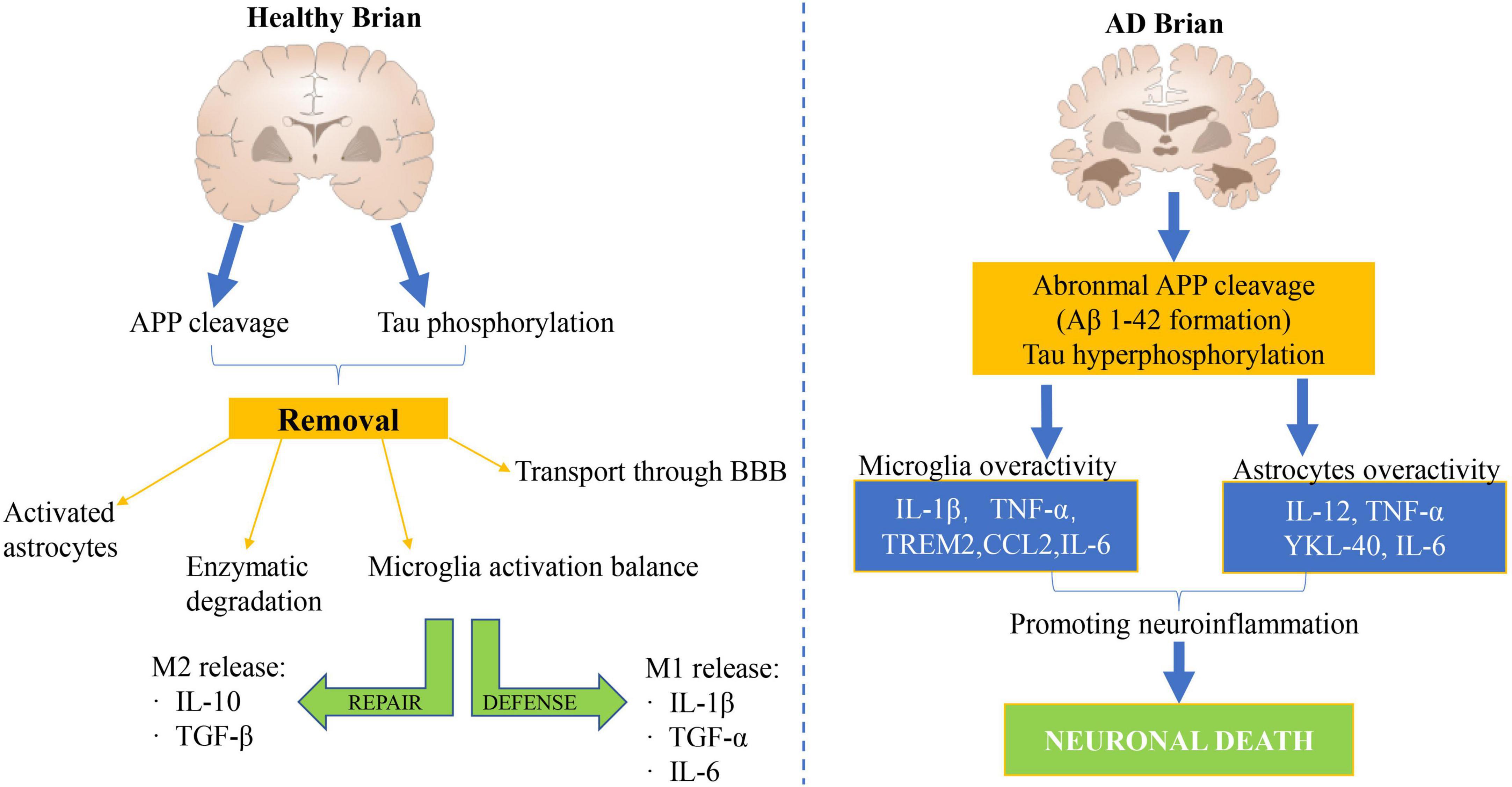
Figure 2. Mechanism of cerebral nerve inflammation in healthy people and patients with AD. A large number of genetic and immunological analyses have demonstrated a significant link between inflammation and AD pathology.
In patients with AD, IL-1 is overexpressed in the receptive area of the cerebral cortex, and the concentration in the tissue increases accordingly (Yue et al., 2022). Tang et al. (2005) used the method of chemical damage to establish the AD rat model, and selected acupoints: “Dazhui,” “Shenshu,” “Taixi,” and “Housanli” acupoints (Wang et al., 2020c). A total of 30 days was a course of treatment. The level of IL-1 and IL-6 in rat brain tissue were tested by radioimmunoassay. The experimental results indicated that the levels of IL-1 and IL-6 in the sham operation group were significantly lower than those in the model group (P < 0.01), while the content of IL-1 and IL-6 in the acupuncture group and Hydergine group were significantly lower than those in the model group (P < 0.05), but significantly higher than those in the sham operation group (P < 0.05) (Cai et al., 2019). The results suggest that the increase of IL-1 and IL-6 levels in brain tissue is closely related to the pathogenesis of AD, which is consistent with the conclusions of previous studies. And acupuncture can significantly reduce the levels of IL-1 and IL-6 in the brain tissue of AD model rats, its effect is similar to that of Hydergine (Jiang et al., 2021; Liao et al., 2022). It is suggested that acupuncture can reduce the levels of IL-1 and IL-6 in the brain tissue of AD rats, which may be one of the effective mechanisms of acupuncture in the treatment of AD. Huang et al. (1998) observe the effect of acupuncture on the inflammatory reaction of AD. RT-PCR (reverse transcriptase polymerase chain reaction) was used to detect the expression of IL-1β and IL-6 in the brain of AD model rats induced by bacterial lipopolysaccharide (Li Y. et al., 2020; Wang et al., 2020b,c; Xie et al., 2021). Acupuncture can reduce the expression of IL-1β and IL-6, suggesting that acupuncture can play a therapeutic role by inhibiting the specific inflammatory reaction in the brain of AD (Fang et al., 2013; He et al., 2017; Cai et al., 2019; Wang et al., 2020a; Yang and Dong, 2020).
Acupuncture ameliorates neuronal antioxidant damage as well as the free radical scavenging effect
Many pieces of evidence suggest that mitochondrial abnormalities are closely related to the disease. However, the significant increase in oxidative damage of neurons is limited to the cytoplasm of susceptible neurons (Aborode et al., 2022; Beura et al., 2022; Estévez-Silva et al., 2022; Maina et al., 2022). We believe that abnormal mitochondria in susceptible neurons play a source role by providing diffusible hydrogen peroxide on the membrane to the surrounding cytoplasm, as shown in Figure 3. The cytoplasm is more vulnerable to hydrogen peroxide because: (1) The cytoplasm is less protected than mitochondria (Kaur et al., 2022); (2) the catalase / SOD ratio decreased in patients with AD, thus reducing the ability to effectively scavenge hydrogen peroxide (Mesa-Herrera et al., 2022; Yoshida et al., 2022); (3) abundant oxidizing metal ions catalyze the Fenton reaction to produce highly active hydroxyl radicals. Therefore, by releasing excess hydrogen peroxide, abnormal mitochondria transmit a series of events involving rich metal ions and cause damage in the cytoplasm (Abu-Elfotuh et al., 2022).
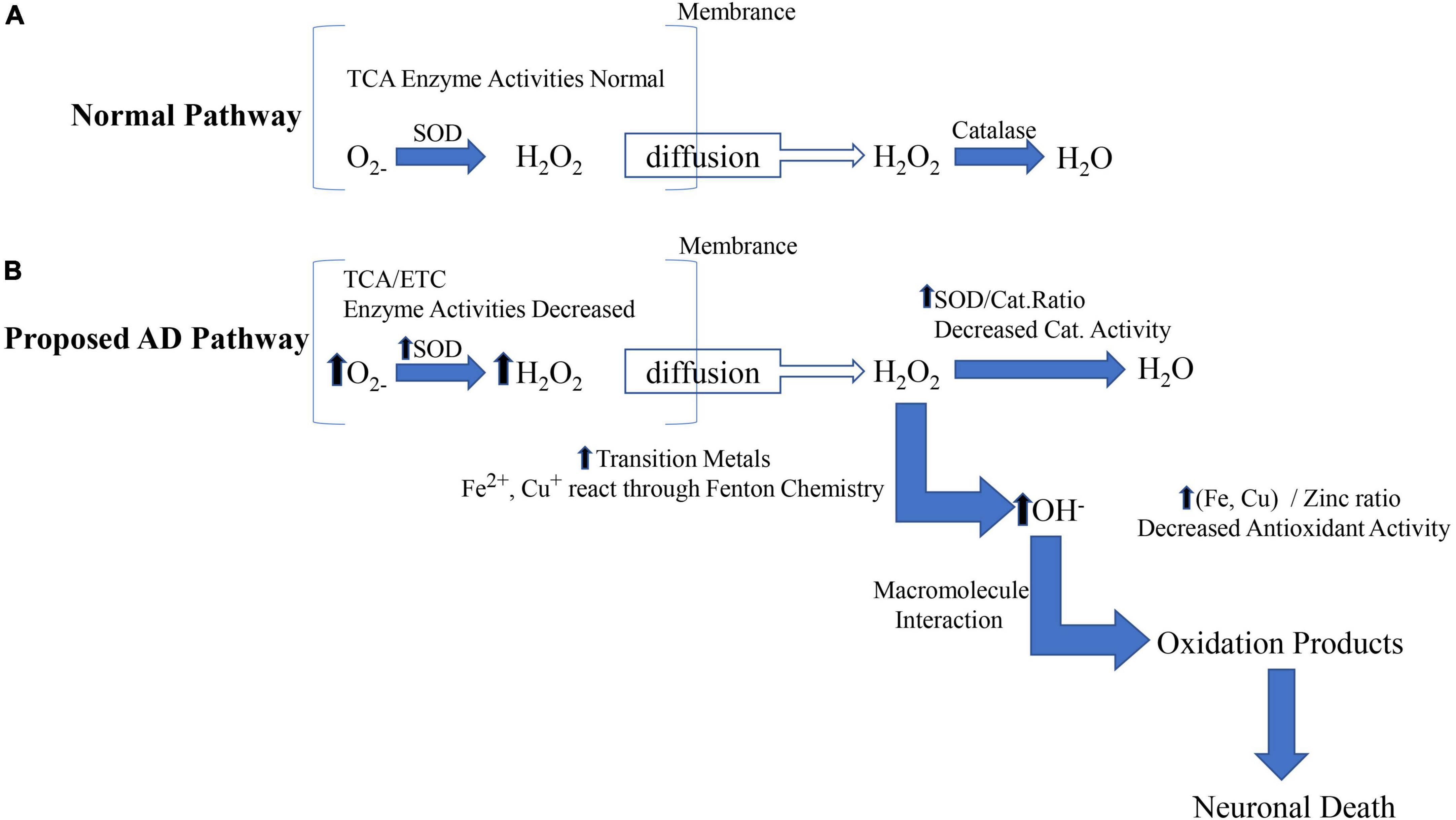
Figure 3. The source and mechanism of cytoplasmic oxidative damage are involved in AD. The mitochondrial (intrinsic) apoptotic pathway includes the release of proapoptotic factors located in the mitochondrial intermembrane space via the mitochondrial permeability transition (MPT). Once in the cytoplasm, mitochondrial proteins such as cytC, Smac/Diablo, and Omi/HtrA2 mediate caspase-dependent, whereas endog and AIF induce caspase-independent apoptosis.
While the current study showed that acupuncture largely improved the symptoms of AD by inhibiting oxidative stress in AD (Wu et al., 2017). As shown in Figure 4, it is clear to us that acupuncture specifically ameliorates oxidative stress in AD by which pathways and ways: (1) It further alleviates oxidative stress by increasing the synthesis of antioxidant components in the body, thereby reducing the generation of ROS. (2) Acupuncture exerts its effects against oxidative stress by regulating ROS-related signaling pathways and the generation of downstream proteins, thereby reducing apoptosis. (3) Acupuncture directly affects Aβ Protein generation and packing. (4) Acupuncture repairs proteins, lipids, and DNA that are directly damaged by ROS. (5) Acupuncture similarly inhibits oxidative stress by ameliorating neuroinflammation (Tain and Hsu, 2017). In addition to the specific action pathways of acupuncture described above (Nrf2/ARE related signaling pathway), acupuncture can reduce oxidative stress and reduce neuroinflammation through multiple signal transduction pathways, Aβ Production, aggregation, and phosphorylation of tau. Acupuncture can activate Nrf2 and PGC-1α, Inhibit NOx, thereby reducing ROS production. In addition, acupuncture can reverse mitochondrial dysfunction and further decrease the phosphorylation of Tau (Chang et al., 2019).
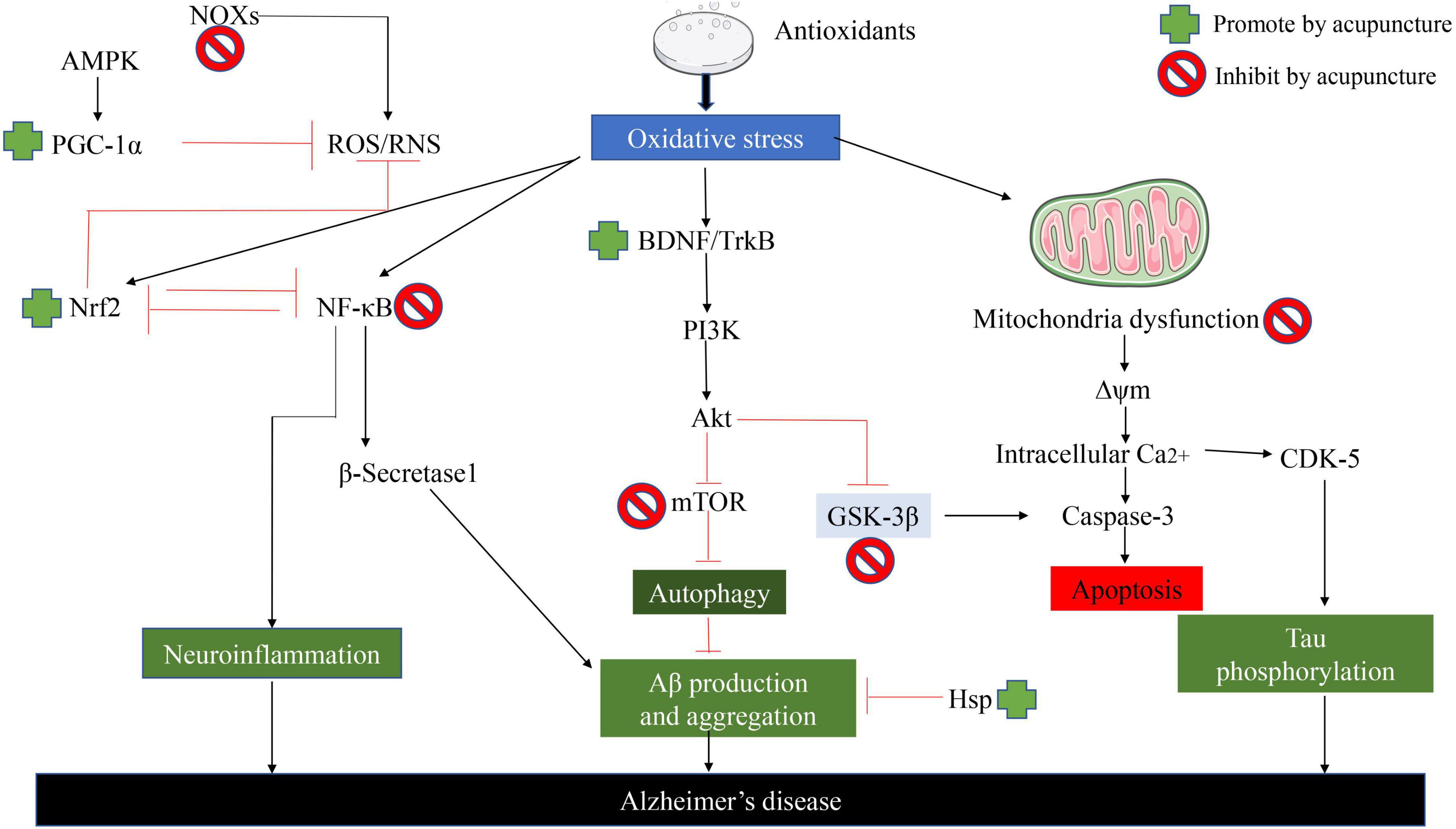
Figure 4. Acupuncture ameliorates AD by suppressing the effects of oxidative stress. Acupuncture can reduce oxidative stress and reduce neuroinflammation through multiple signal transduction pathways, Aβ Production, aggregation, and phosphorylation of Tau.
Oxidative stress acts an increasingly crucial role in the pathogenesis of AD. Lipid peroxidation is not only the inducement of cell membrane aging but also the result of cell aging (Wei et al., 2022; Xin et al., 2022). Lipid peroxidation is induced by oxygen free radicals, and the cytotoxicity of lipid peroxidation products such as MDA plays a vital role in neuronal degeneration and necrosis, leading to the occurrence and development of AD. Wang et al. (2004) used free radical theory and cholinergic theory, to explore the effects of acupuncture on antioxidation and cholinergic system function of the hippocampus and cerebral cortex of pseudo-AD rats (Chang et al., 2019). It was found that the level of MDA in the cerebral cortex of the acupuncture group was significantly lower than that of the model group (P < 0.01), while the activity of SOD was significantly increased (P < 0.01). In the determination of AchE, the activity of AchE in the model group was significantly lower than that in the acupuncture group (P < 0.01) (Yang J. et al., 2019; Zhang et al., 2019). The experimental results show that acupuncture treatment of AD may be achieved through antioxidation. Liu and Fu (2014) made an AD rat model by intraperitoneal injection of D-galactose. The effects of acupuncture at “Baihui,” “Fengfu,” “Shenshu,” and “Xuanzhong” acupoints on the behavior and the contents of serum MDA and T-AOC in AD rats were observed. It turned out that the acupuncture group and acupuncture combined with the medicine group could prolong the latency of the step-down test, reduce the number of errors (P < 0.05), decrease the level of serum MDA and increase the content of T-AOC (P < 0.05) (Wu et al., 2017). To summarize the above results, acupuncture can improve the level of serum T-AOC, enhance the ability of antioxidation, and reduce the damage of free radicals in AD rats. Guan et al. (2001) in the observation of the effects of acupuncture at “Baihui,” “Dazhui,” and “Mingmen” acupoints on the level of nitric oxide (NO), MDA, and the activity of SOD in the brain tissue of subacute aging mice induced by D-galactose, the experiments proved that the level of NO and MDA in the brain tissue of subacute aging mice increased significantly, while the activity of SOD decreased significantly, which could reverse the above indexes after acupuncture, suggesting that acupuncture at “Baihui,” “Dazhui,” and “Mingmen” acupoints has the effect of anti-brain aging (Ye et al., 2017). Its mechanism may be related to its ability to inhibit the free radical reaction and increase the activity of antioxidant enzymes.
Inhibition of neuronal apoptosis
Long-term experimental results proved that mitochondria are the critical factors in the early induction and regulation of apoptosis (Li Y. et al., 2022; Weber Boutros et al., 2022). Apoptotic stimuli such as DNA damage, ROS, or Fas signals mediate the death of mitochondrial cells by causing the release of small pro-apoptotic proteins generally located in the intermembrane space of the mitochondria, as shown in Figure 5 (Xiong et al., 2022). Once in the cytoplasm, pro-apoptotic proteins such as Cytochrome c (Cyt c), mitochondrial-derived second caspase activator/low-PI direct IAP binding protein (Smac/Diablo), AIF, and endonuclease G trigger caspase-dependent or independent apoptotic death pathways. In the caspase-dependent mechanism, Cyt c binds to a junction molecule, apoptotic protein activator-1 (APAF-1), to form an apoptotic body. In the presence of ATP or dATP, Caspase 9 is recruited and activated. Caspase 9 further cleaves and activates effector Caspase 3 and/or 7, which treats substrates such as caspase-activated DNA enzyme (ICAD) or PARP, and leads to DNA fragmentation. AIF translocates to the nucleus in a caspase-independent manner, where it induces DNA fragmentation and chromatin agglutination, while ending induces internucleosomal DNA fragmentation.
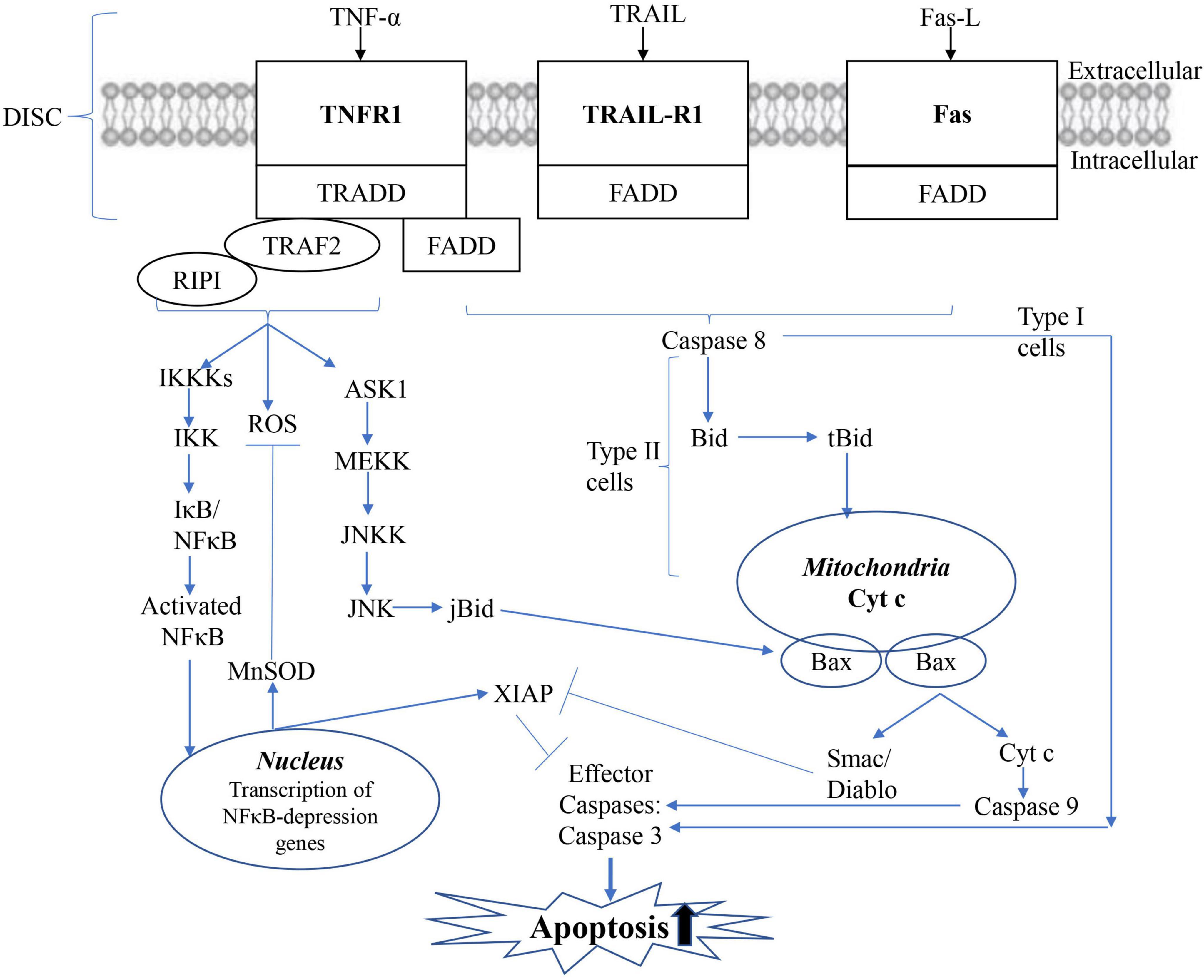
Figure 5. Mitochondrial apoptotic pathway. The mitochondrial (intrinsic) apoptotic pathway includes the release of proapoptotic factors located in the mitochondrial intermembrane space via the mitochondrial permeability transition (MPT). Once in the cytoplasm, mitochondrial proteins such as cytC, Smac/Diablo, and Omi/HtrA2 mediate caspase-dependent, whereas endog and AIF induce caspase-independent apoptosis.
It is well known that acupuncture can improve the symptoms of AD patients by inhibiting the apoptosis of nerve cells in a variety of ways and pathways, as shown in Figure 6. (1) On the one hand, increasing the synthesis of antioxidants, and on the other hand, reducing the generation of oxidative stress products, thereby exerting a protective effect on nerve cells. (2) Exerts anti-apoptotic effects on nerve cells by regulating ROS-related signaling pathways and the expression of downstream proteins. (3) Acupuncture inhibition α-Synuclein production as well as accelerating its clearance. The effect of acupuncture on improving neuronal apoptosis in AD via the Nrf2/ARE-related pathway is shown in Figure 6 (Ebrahimi-Fakhari et al., 2016). On the other hand, we can also clearly see that acupuncture inhibits GSK-3β by upregulating the expression of BDNF, PI3K/Akt, and Protein Kinase C pathway, further increasing the nuclear translocation, accumulation, and transactivation of Nrf2. In addition, acupuncture can also exert a protective effect on nerve cells by affecting the nuclear translocation of NFkB and thereby downregulating the expression of proinflammatory genes. In addition, acupuncture also promotes mitochondrial biogenesis. Interestingly, the effect of acupuncture on the p38 MAPK pathway has a duality (Gao et al., 2021).
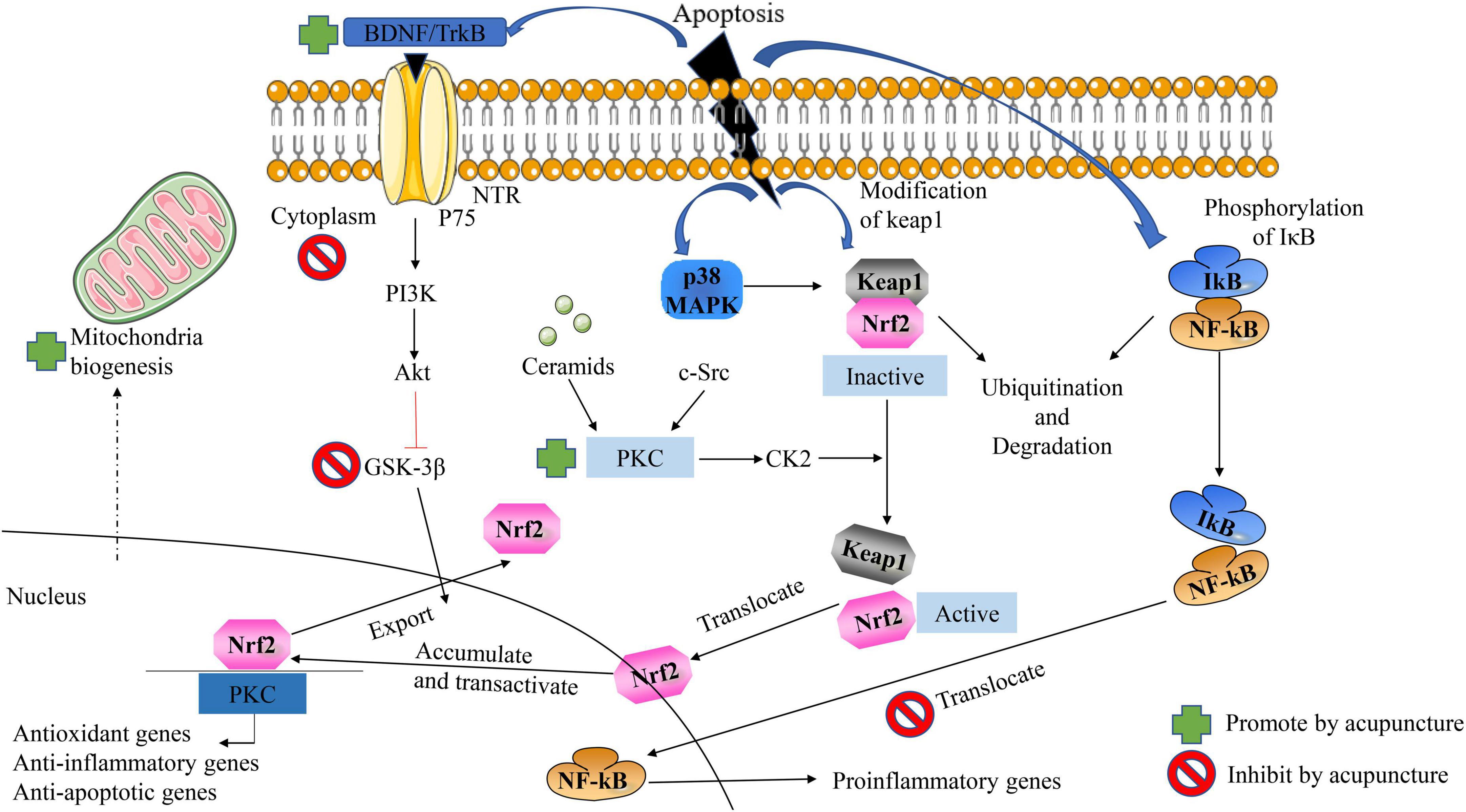
Figure 6. Acupuncture ameliorates neuronal apoptosis in AD by regulating the Nrf2/ARE-related pathway in vivo. NFκB the antioxidant effect of Nrf2 can be inhibited by blocking the area region and thereby preventing gene transcription.
Apoptosis refers to the orderly, cell-autonomous death of cells controlled by genes that maintain homeostasis (Ynag et al., 2021). The apoptosis of brain neurons in patients with AD is 30–50 times higher than that in normal subjects, resulting in a decrease in neurons in the hippocampus, basal forebrain, and neocortex, so apoptosis is one of the important reasons for the decrease in the number of neurons in AD brain tissue (Song et al., 2020; Zhang et al., 2020). It has been found that the changes in morphological structure and function of mitochondria are the key links leading to the disturbance of energy metabolism in the brain, and the disturbance of energy metabolism is closely related to the loss of AD neurons, the formation of senile plaque, nerve fiber tangles and so on. It is well known that apoptosis refers to the programmed death of cells, and as the key molecule “Bcl” of the apoptosis family, one, in turn, divides it into two categories, Bcl2 and Bax, according to the role it plays. Dong et al., found that acupuncture at the “Baihui” and “Shenting” acupoints could directly inhibit apoptosis of cells and thus ameliorate symptoms (improve memory impairment) in MCAO rats by regulating the interaction of Bax and BCL2. Huang et al. (1998) found that by acupuncture model rats (Aβ-140 stimuli) of “Baihui,” “Dazhui,” and “Zusanli” acupoints, which can significantly downregulate Bax protein expression in the rat hippocampus, while upregulating Bcl2 protein expression, thereby inhibiting hippocampal neuronal apoptosis (Yang et al., 2021). Zhang et al. (2017) found that acupuncture at “Baihui,” “Fengfu,” and “Renyu” acupoints significantly decreased the expression levels of Caspase 3 and Bax proteins in the hippocampus of model mice (APP / PS1), which exert protective effects on nerve cells, further improve the symptoms of mice (improved learning and memory ability). In addition to this, numerous studies directly point out that acupuncture exerts anti-apoptotic effects by inhibiting C-Jun amino-terminal protein kinase signaling pathway and thereby inhibiting hippocampal neuronal apoptosis in AD model mice (Huang et al., 2019).
Activation of hippocampal protein kinase
Protein kinase (PKC) takes part in physiological processes related to cognitive ability (Yang S. et al., 2022). To some degree, PKC is included in so-called cognitive kinases (Lu et al., 2022). It regulates synaptic transmission, and several of its substrates, including Marcks, GAP-43, and NMDA receptors, take part in information processing and storage (Wu et al., 2022). For GAP-43 at least, PKC phosphorylation sites act a crucial role in regulating memory-related tasks, as shown in Figure 7 (Yamahashi et al., 2022).
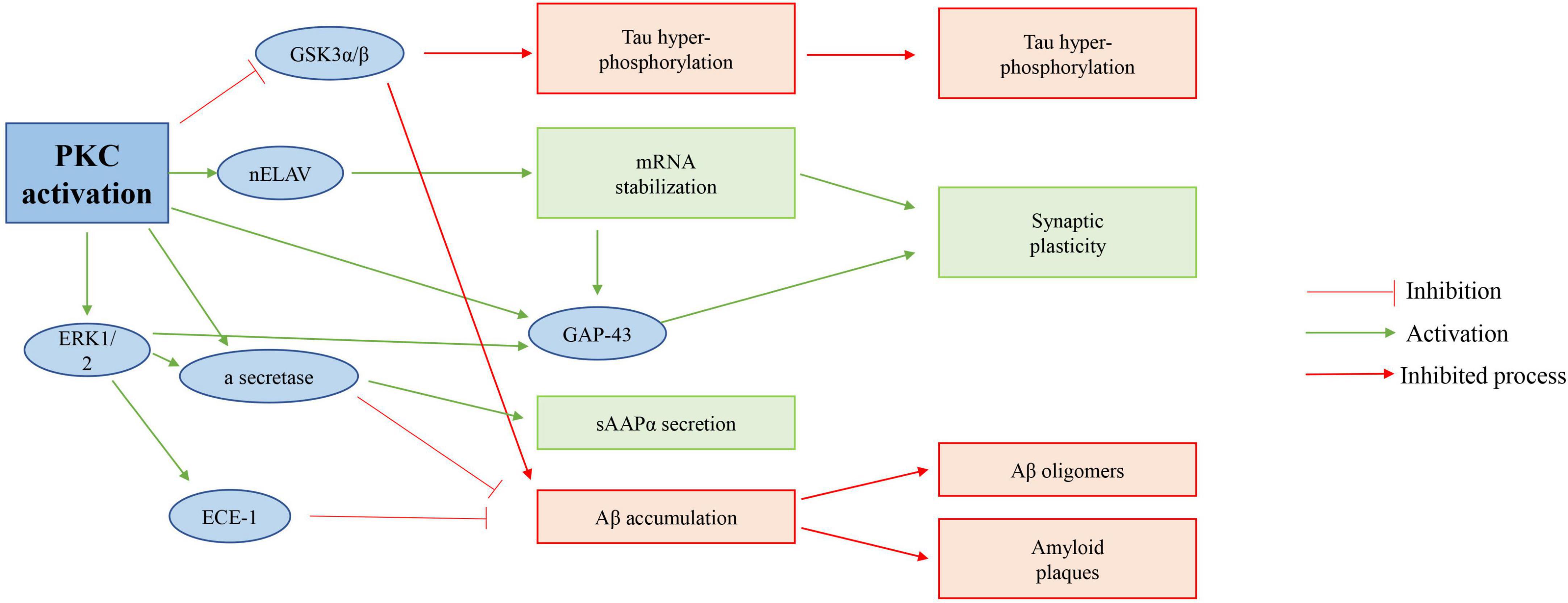
Figure 7. Possible role of PKC activation in AD. Activation of PKC directly inhibits GSK 3β of activity, increases APP protein processing, and in turn inhibits Tau hyperphosphorylation; And reduces Aβ accumulation of proteins.
In recent years, it has been found that the dysfunction of the signal pathway in the brain of senile dementia is closely related to the decrease in the activity of protein kinase. the imbalance between the activity of protein kinase and phosphatase will lead to the hyperphosphorylation of the tau protein, which aggravates the pathological changes of senile dementia (Ortiz-Sanz et al., 2022). Traditional Chinese medicine and acupuncture have certain advantages in the treatment of senile dementia, and whether their mechanism is related to the activation of the suppressed protein kinase signal pathway in the hippocampus is worthy of in-depth study (Zou et al., 2022). Tang et al. (2005) cut off the fimbria-fornix to establish the model of senile dementia. “Baihui,” “Yongquan,” “Taixi,” and “Xuehai” acupoints were selected in the acupuncture group. After 20 times of treatments, the contents of membrane PKC and tyrosine-protein kinase (PTK) in hippocampal tissue cells of each group were measured by radioimmunoassay (Wang et al., 2021). The results show that acupuncture may activate membrane receptors to activate protein kinases such as PKC through corresponding signal transduction pathways and participate in cell growth factor signal transduction: One may be by activating the intracellular PTK connected to it, which can take the receptor itself as the substrate and transduce the signal of tyrosine phosphorylation by PTK, which converts the growth factor signal into an intracellular signal; and maintains the normal metabolism, proliferation, and growth of cells; promote the regeneration of neurons (Li G. et al., 2019; Zheng et al., 2020). The second is that acupuncture promotes the synthesis of nerve growth factor (NGF) in the hippocampus of senile dementia rats. Together with activated PTK, activating PKC; PKC through PLC or IP3-Ca2+ pathway can further promote protein synthesis or related gene expression necessary for the formation of learning and memory, to improve the learning and memory impairment of senile dementia (Lin et al., 2015; Zhang et al., 2017).
Inhibition of microtubule-associated protein expression
The Aβ cascade hypothesis suggests that the treatment of AD can start with the restoration of the Aβ balance in the brain (Li Q. et al., 2019; Wang et al., 2021). Based on this, there are mainly four ways to reduce the formation of Aβ, that is, to prevent or reduce the formation of Aβ by targeting amyloid precursor secretase, to clear the deposition of amyloid in the brain by active or passive immunity, to prevent or reduce the accumulation of Aβ and to enhance the clearance of Aβ, as shown in Figure 8 (Zhang et al., 2017).
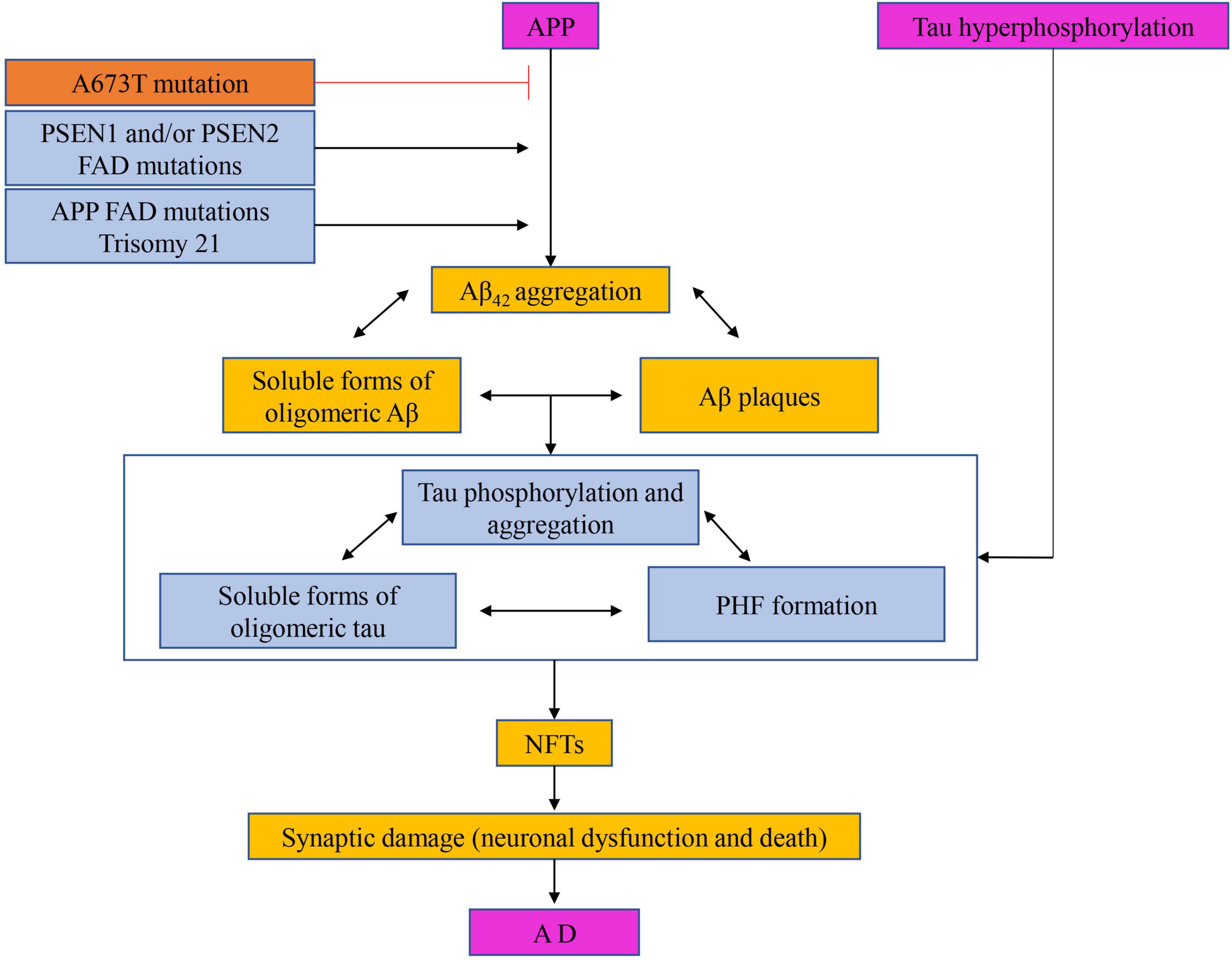
Figure 8. The hypothesis of amyloid cascade with the participation of tau protein. Targeting amyloid precursor secretase thereby prevents or reduces Aβ formation, clearance of amyloid deposits from the brain by active or passive immunization, and prevention or reduction of Aβ aggregation and enhancement of Aβ of clearance.
Current research has demonstrated that the phosphorylation of tau protein is closely related to NFT and AD, and tau protein is the only essential component of NFT (Li R. et al., 2022; Yang B. et al., 2022). Hyperphosphorylation of Tau protein not only reduces its activity of promoting microtubule assembly but also further destroys microtubules by consuming normal tau protein, microtubule-associated protein MAP1, and MAP2, resulting in axoplasmic transport disorder, neuronal process breakage, neuronal degeneration and disintegration, neuronal degeneration and formation of NFT (Teja et al., 2021). Neuronal fiber degeneration caused by NFT act an essential role in the pathogenesis of AD and is parallel to the clinical dementia symptoms of AD patients (Wang et al., 2021). Jiang (2020) taking the AD model rats induced by intraperitoneal injection of D-galactose and intragastric feeding of AlCl3 as the research object, acupuncture at “Baihui,” “Dazhui,” “Shenshu,” “Zusanli,” and “Taixi” acupoints (Yu et al., 2020a). The number of Tau protein-positive cells in the hippocampal CA1 region was detected by immunohistochemistry and in situ hybridization. The results showed that the expression of Tau protein in the hippocampal CA1 region in the acupuncture group and western medicine group was significantly lower than that in the blank group and model control group (P < 0.01). It is suggested that acupuncture can prevent and treat AD by inhibiting the expression of the microtubule-associated protein Tau (Lee et al., 2009; Liu and Fu, 2014; Zhang et al., 2017; Huang et al., 2020; Ma et al., 2020; Yang et al., 2020; Yu et al., 2020b,2021).
Thinking and prospect
As one of the characteristic parts of nonpharmacological therapies and traditional Chinese medicine (TCM), acupuncture plays an effective defensive and therapeutic role for ad with many advantages: (1) It can obviously improve AD symptoms, green and safe with no toxic side effects; (2) Most existing studies of acupuncture have confirmed that acupuncture treatment of ad is not achieved by only one mechanism of action, but by multi-target multi-pathway intervention; (3) All derived from traditional Chinese medicine ideas, according to the characteristics of different etiological disease mechanisms combined with traditional Chinese medicine theory and acupuncture theory as a support based on acupoint selection group formula, is a new idea and method of delaying the progress of AD, and is a new way to prevent and treat AD; (4) Forming a standardized diagnosis and treatment protocol, and making clear the selection of points, operations, parameters, courses, etc., are significant for the promotion of clinical practical applications, it also needs to be continually verified and continually improved in clinical practice, aiming to enrich the means of acupuncture treatment and improve clinical efficacy.
With the development trend of social aging, AD is an urgent problem to be solved in the medical field all over the world. at present, there is no specific drug to treat this disease. Acupuncture with its unique ideas and treatment methods, through a large number of experiments, to verify the reliability of acupuncture treatment of AD. Many advances have been made in the study of the neurobiological mechanism of acupuncture in the treatment of AD, which further proves the good efficacy and unique advantages of acupuncture in the treatment of AD. Animal experiments on related indicators have been widely carried out, but there are few studies on clinical cases, which is also related to the fact that patients and their families do not pay enough attention to the disease. Strengthen the clinical and mechanism research of acupuncture and moxibustion treatment of AD to meet the needs of social development. It is believed that with the progress of research, acupuncture will make new achievements in the treatment of AD.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This work was supported in part by the Sichuan Administration of Traditional Chinese Medicine (grant no. 2020ZD003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aborode, A., Pustake, M., Awuah, W., Alwerdani, M., Shah, P., Yarlagadda, R., et al. (2022). Targeting oxidative stress mechanisms to treat Alzheimer’s and Parkinson’s disease: A critical review. Oxid. Med. Cell. Longev. 2022:7934442. doi: 10.1155/2022/7934442
Abu-Elfotuh, K., Al-Najjar, A., Mohammed, A., Aboutaleb, A., and Badawi, G. (2022). Fluoxetine ameliorates Alzheimer’s disease progression and prevents the exacerbation of cardiovascular dysfunction in socially isolated depressed rats through activation of Nrf2/HO-1 and hindering TLR4/NLRP3 inflammasome signaling pathway. Int. Immunopharmacol. 104:108488. doi: 10.1016/j.intimp.2021.108488
Al-Nasser, M., Mellor, I., and Carter, W. (2022). Is L-glutamate toxic to neurons and thereby contributes to neuronal loss and neurodegeneration? A systematic review. Brain Sci. 12:577. doi: 10.3390/brainsci12050577
An, F., Xuan, X., Liu, Z., Bian, M., Shen, Q., Quan, Z., et al. (2022). HAnti-Inflammatory activity of 4-(4-(Heptyloxy)phenyl)-2,4-dihydro-3-1,2,4-triazol-3-one via repression of MAPK/NF-κB signaling pathways in β-amyloid-induced Alzheimer’s disease models. Molecules 27:5035. doi: 10.3390/molecules27155035
Babić Leko, M., Hof, P., and Šimić, G. (2021). Alterations and interactions of subcortical modulatory systems in Alzheimer’s disease. Prog. Brain Res. 261, 379–421. doi: 10.1016/bs.pbr.2020.07.016
Bao, Y., and Lv, G. (2003). Effects of acupuncture on memory impairment and monoamine neurotransmitters in demented mice. Shang. Acupunc. J. 7, 23–25.
Beura, S., Dhapola, R., Panigrahi, A., Yadav, P., Reddy, D., and Singh, S. (2022). Redefining oxidative stress in Alzheimer’s disease: Targeting platelet reactive oxygen species for novel therapeutic options. Life Sci. 306:120855. doi: 10.1016/j.lfs.2022.120855
Cai, M., Lee, J., and Yang, E. (2019). Electroacupuncture attenuates cognition impairment via anti-neuroinflammation in an Alzheimer’s disease animal model. J. Neuroinflammation 16:264. doi: 10.1186/s12974-019-1665-3
Catarzi, D., Colotta, V., and Varano, F. (2007). Competitive AMPA receptor antagonists. Med. Res. Rev. 27, 239–278. doi: 10.1002/med.20084
Chang, S., Guo, X., Li, G., Zhang, X., Li, J., Jia, Y., et al. (2019). Acupuncture promotes expression of Hsp84/86 and delays brain aging in SAMP8 mice. Acupunct. Med. 37, 340–347. doi: 10.1136/acupmed-2017-011577
Cheng, X., Wei, Y., Qian, Z., and Han, L. (2022). Autophagy balances neuroinflammation in Alzheimer’s disease. Cell. Mol. Neurobiol. doi: 10.1007/s10571-022-01269-6 [Epub ahead of print].
de Pins, B., Cifuentes-Díaz, C., Farah, A., López-Molina, L., Montalban, E., Sancho-Balsells, A., et al. (2019). Conditional BDNF delivery from astrocytes rescues memory deficits, spine density, and synaptic properties in the 5xFAD mouse model of Alzheimer disease. J. Neurosci. 39, 2441–2458. doi: 10.1523/JNEUROSCI.2121-18.2019
Dekker, A., Coppus, A., Vermeiren, Y., Aerts, T., van Duijn, C., Kremer, B., et al. (2015). Serum MHPG strongly predicts conversion to Alzheimer’s disease in behaviorally characterized subjects with down syndrome. J. Alzheimers Dis. 43, 871–891. doi: 10.3233/JAD-140783
Du, X., Li, J., Li, M., Yang, X., Qi, Z., Xu, B., et al. (2020). Research progress on the role of type I vesicular glutamate transporter (VGLUT1) in nervous system diseases. Cell Biosci. 10:26. doi: 10.1186/s13578-020-00393-4
Ebrahimi-Fakhari, D., Saffari, A., Wahlster, L., Di Nardo, A., Turner, D., Lewis, T. L., et al. (2016). Impaired mitochondrial dynamics and mitophagy in neuronal models of tuberous sclerosis complex. Cell Rep. 17, 1053–1070. doi: 10.1016/j.celrep.2016.09.054
Eid, A., Mhatre-Winters, I., Sammoura, F., Edler, M., von Stein, R., Hossain, M., et al. (2022). Effects of DDT on amyloid precursor protein levels and amyloid beta pathology: Mechanistic links to Alzheimer’s disease risk. Environ. Health Perspect. 130:87005. doi: 10.1289/EHP10576
Esteban, G., Van Schoors, J., Sun, P., Van Eeckhaut, A., Marco-Contelles, J., Smolders, I., et al. (2017). In-vitro and in-vivo evaluation of the modulatory effects of the multitarget compound ASS234 on the monoaminergic system. J. Pharm. Pharmacol. 69, 314–324. doi: 10.1111/jphp.12697
Estévez-Silva, H., Cuesto, G., Romero, N., Brito-Armas, J., Acevedo-Arozena, A., Acebes, Á., et al. (2022). Pridopidine promotes synaptogenesis and reduces spatial memory deficits in the Alzheimer’s disease APP/PS1 mouse model. Neurotherapeutics 19, 1566–1587. doi: 10.1007/s13311-022-01280-1
Fang, J., Zhu, S., Zhang, Y., Wang, F., and Zhu, Q. (2013). Effect of electroacupuncture on expression of phosphorylated P 38 MAPK and IL-1beta in frontal lobe and hippocampus in rats with Alzheimer’s disease. Zhen Ci Yan Jiu 38, 35–39.
Flores, S., Chen, C., Su, Y., Dincer, A., Keefe, S., McKay, N., et al. (2022). Investigating tau and amyloid tracer skull binding in studies of Alzheimer disease. J. Nucl. Med. 64, 287–293. doi: 10.2967/jnumed.122.263948
Gao, S., Zhang, S., Zhou, H., Tao, X., Ni, Y., Pei, D., et al. (2021). Role of mTOR-regulated autophagy in synaptic plasticity related protei ns downregulation and the reference memory Deficits induced by anesthe sia/surgery in aged mice. Front. Aging Neurosci. 13:628541. doi: 10.3389/fnagi.2021.628541
Gruden, M., Davydova, T., Wang, C., Narkevich, V., Fomina, V., Kudrin, V., et al. (2016). The misfolded pro-inflammatory protein S100A9 disrupts memory via neurochemical remodelling instigating an Alzheimer’s disease-like cognitive deficit. Behav. Brain Res. 306, 106–116. doi: 10.1016/j.bbr.2016.03.016
Gu, X., and Wang, X. (2021). An overview of recent analysis and detection of acetylcholine. Anal. Biochem. 632:114381. doi: 10.1016/j.ab.2021.114381
Guan, C., Gao, X., and Liang, J. (2001). Effects of acupuncture on nitric oxide, malondialdehyde, and superoxide dismutase in brain tissue of subacute aging mice. Acupun. Stud. 2, 111–113.
Guo, F., Zhang, S., Chen, S., Zhang, C., Zhang, X., Gao, F., et al. (2020). Electroacupuncture improved learning-memory ability by reducing hippoc ampal apoptosis and suppressing JNK signaling in rats with vascular dementia. Zhen Ci Yan Jiu 45, 21–26.
Hang, Z., Lei, T., Zeng, Z., Cai, S., Bi, W., and Du, H. (2022). Composition of intestinal flora affects the risk relationship between Alzheimer’s disease/Parkinson’s disease and cancer. Biomed. Pharmacother. 145:112343. doi: 10.1016/j.biopha.2021.112343
He, J., Liao, T., Zhong, G., Zhang, J., Chen, Y., Wang, Q., et al. (2017). Alzheimer’s disease-like early-phase brain pathogenesis: Self-curing amelioration of neurodegeneration from pro-inflammatory ‘Wounding’ to anti-inflammatory ‘Healing’. Curr. Alzheimer Res. 14, 1123–1135.
Huang, C., Chen, H., Qin, X., Zhou, L., and Cheng, J. (1998). Acupuncture suppresses cytokine gene expression in the brain and pituitary of aged rats. Acupun. Stud. 1, 24–27.
Huang, R., Gong, X., Ni, J., Jia, Y., and Zhao, J. (2019). Effect of acupuncture plus medication on expression of Bcl-2 and Bax in the hippocampus in rats with Alzheimer’s disease. Zhongguo Zhen Jiu 39, 397–402.
Huang, X., Huang, K., Li, Z., Bai, D., Hao, Y., Wu, Q., et al. (2020). Electroacupuncture improves cognitive deficits and insulin resistance in an OLETF rat model of Al/D-gal induced aging model via the PI3K/Akt signaling pathway. Brain Res. 1740:146834. doi: 10.1016/j.brainres.2020.146834
Hynd, M., Scott, H., and Dodd, P. (2004). Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem. Int. 45, 583–595. doi: 10.1016/j.neuint.2004.03.007
Jiang, X. (2020). Effects of acupuncture on β-AP and tau protein expression in brain tissue of rats with senile dementia. Heilong. Univ. Chin. Med.
Jiang, J., Liu, H., Wang, Z., Tian, H., Wang, S., Yang, J., et al. (2021). Electroacupuncture could balance the gut microbiota and improve the learning and memory abilities of Alzheimer’s disease animal model. PLoS One 16:e0259530. doi: 10.1371/journal.pone.0259530
Jiang, L. G., Zhang, H. W., Zhang, Z., and Shao, Y. (2017). Influence of electroacupuncture stimulation with different intensities and therapeutic intervals on learning-memory ability and expression of aβ 1-40 and arginine vasopressin genes in the hippocampal CA 1 regio n in VD rats. Zhen Ci Yan Jiu 42, 20–24.
Kaur, S., Minhas, R., Mishra, S., Kaur, B., Bansal, Y., and Bansal, G. (2022). Design, synthesis and evaluation of benzimidazole hybrids to inhibit acetylcholinesterase and COX for treatment of Alzheimer’s disease. Cent. Nerv. Syst. Agents Med. Chem. 22, 68–78. doi: 10.2174/1871524922666220428134001
Kaur, S., Raj, K., Gupta, Y., and Singh, S. (2021). Allicin ameliorates aluminium- and copper-induced cognitive dysfunction in Wistar rats: Relevance to neuro-inflammation, neurotransmitters and Aβ analysis. J. Biol. Inorg. Chem. 26, 495–510. doi: 10.1007/s00775-021-01866-8
Khavinson, V., Lin’kova, N., and Umnov, R. (2021). Peptide KED: Molecular-genetic aspects of neurogenesis regulation in Alzheimer’s disease. Bull. Exp. Biol. Med. 171, 190–193. doi: 10.1007/s10517-021-05192-6
Kishino, Y., Sugimoto, T., Kimura, A., Kuroda, Y., Uchida, K., Matsumoto, N., et al. (2022). Longitudinal association between nutritional status and behavioral and psychological symptoms of dementia in older women with mild cognitive impairment and early-stage Alzheimer’s disease. Clin. Nutr. 41, 1906–1912. doi: 10.1016/j.clnu.2022.06.035
Lee, M., Shin, B., and Ernst, E. (2009). Acupuncture for Alzheimer’s disease: A systematic review. Int. J. Clin. Pract. 63, 874–879. doi: 10.1111/j.1742-1241.2009.02043.x
Letsinger, A., Gu, Z., and Yakel, J. (2022). α7 nicotinic acetylcholine receptors in the hippocampal circuit: Taming complexity. Trends Neurosci. 45, 145–157. doi: 10.1016/j.tins.2021.11.006
Li, G., Zeng, L., Cheng, H., Han, J., Zhang, X., and Xie, H. (2019). Acupuncture administration improves cognitive functions and alleviates inflammation and nuclear damage by regulating phosphatidylinositol 3 kinase (PI3K)/phosphoinositol-dependent kinase 1 (PDK1)/novel protein kinase C (nPKC)/Rac 1 signaling pathway in senescence-accelerated prone 8 (SAM-P8) mice. Med. Sci. Monit. 25, 4082–4093. doi: 10.12659/MSM.913858
Li, Q., Wu, X., Na, X., Ge, B., Wu, Q., Guo, X., et al. (2019). Impaired cognitive function and altered hippocampal synaptic plasticity in mice lacking dermatan sulfotransferase Chst14/D4st1. Front. Mol. Neurosci. 12:26. doi: 10.3389/fnmol.2019.00026
Li, R., Zhao, Y., and Cai, L. (2020). Effects of donepezil hydrochloride on cognitive function and oxidative stress levels in Alzheimer’s disease patients. China Pharm 15, 382–385. doi: 10.1007/s40261-014-0235-9
Li, R., He, J., Jiang, Y., and Jia, B. (2022). Progress of experimental researches on acupuncture intervention for Alzheimer’s disease based on SAMP8 mice model. Zhen Ci Yan Jiu 47, 466–470.
Li, Y., Wang, H., Chen, L., Wei, K., Liu, Y., Han, Y., et al. (2022). Circ_0003611 regulates apoptosis and oxidative stress injury of Alzheimer’s disease via miR-383-5p/KIF1B axis. Metab. Brain Dis. 37, 2915–2924. doi: 10.1007/s11011-022-01051-z
Li, X. T. (2022). Alzheimer’s disease therapy based on acetylcholinesterase inhibitor/blocker effects on voltage-gated potassium channels. Metab. Brain Dis. 37, 581–587. doi: 10.1007/s11011-022-00921-w
Li, Y., Jiang, J., Tang, Q., Tian, H., Wang, S., Wang, Z., et al. (2020). Microglia TREM2: A potential role in the mechanism of action of electroacupuncture in an alzheimer’s disease animal model. Neural Plast. 2020:8867547. doi: 10.1155/2020/8867547
Liao, D., Pang, F., Zhou, M., Li, Y., Yang, Y., Guo, X., et al. (2022). Effect of electroacupuncture on cognitive impairment in APP/PS1 mice based on TLR4/NF-κB/NLRP3 pathway. Zhen Ci Yan Jiu 47, 565–572.
Lin, D., Wu, Q., Lin, X., Borlongan, C., He, Z., Tan, J., et al. (2015). Brain-derived neurotrophic factor signaling pathway: Modulation by acupuncture in telomerase knockout mice. Altern. Ther. Health Med. 21, 36–46.
Lin, Y., Lee, W., Wang, S., and Fuh, J. (2018). Levels of plasma neurofilament light chain and cognitive function in patients with Alzheimer or Parkinson disease. Sci. Rep. 8:17368. doi: 10.1038/s41598-018-35766-w
Liu, Y., and Fu, Y. (2014). Progress of experimental research on treating Alzheimer’s disease by acupuncture. Zhongguo Zhong Xi Yi Jie He Za Zhi 34, 359–361.
Lu, N., Tan, G., Tan, H., Zhang, X., Lv, Y., Song, X., et al. (2022). Maackiain prevents amyloid-beta-induced cellular injury via priming PKC-Nrf2 pathway. Biomed. Res. Int. 2022:4243210.
Ma, R., Kong, L., Qi, F., He, R., Zheng, Q., Lu, W., et al. (2020). Effect of electroacupuncture on cyclin-dependent kinase 5 and Tau protein in hippocampus of SAMP8 mice. Zhen Ci Yan Jiu 45, 529–534.
Maina, M., Al-Hilaly, Y., Oakley, S., Burra, G., Khanom, T., Biasetti, L., et al. (2022). Dityrosine cross-links are present in Alzheimer’s disease-derived tau oligomers and paired helical filaments (PHF) which promotes the stability of the PHF-core tau (297-391) in vitro. J. Mol. Biol. 434:167785. doi: 10.1016/j.jmb.2022.167785
Martersteck, A., Ayala, I., Ohm, D., Spencer, C., Coventry, C., Weintraub, S., et al. (2022). Focal amyloid and asymmetric tau in an imaging-to-autopsy case of clinical primary progressive aphasia with Alzheimer disease neuropathology. Acta Neuropathol. Commun. 10:111. doi: 10.1186/s40478-022-01412-w
Mesa-Herrera, F., Marín, R., Torrealba, E., and Díaz, M. (2022). Multivariate assessment of lipoxidative metabolites, trace biometals, and antioxidant and detoxifying activities in the cerebrospinal fluid define a fingerprint of preclinical stages of Alzheimer’s disease. J. Alzheimers Dis. 86, 387–402. doi: 10.3233/JAD-215437
Natale, N., Magnusson, K., and Nelson, J. (2006). Can selective ligands for glutamate binding proteins be rationally designed? Curr. Top. Med. Chem. 6, 823–847. doi: 10.2174/156802606777057535
Nazarali, A., and Reynolds, G. (1992). Monoamine neurotransmitters and their metabolites in brain regions in Alzheimer’s disease: A postmortem study. Cell. Mol. Neurobiol. 12, 581–587. doi: 10.1007/BF00711237
Noda, M. (2016). Dysfunction of glutamate receptors in microglia may cause neurodegeneration. Curr. Alzheimer Res. 13, 381–386.
O’Neill, M., Bleakman, D., Zimmerman, D., and Nisenbaum, E. (2004). AMPA receptor potentiators for the treatment of CNS disorders. Curr. Drug Targets CNS Neurol. Disord. 3, 181–194. doi: 10.2174/1568007043337508
Ortiz-Sanz, C., Balantzategi, U., Quintela-López, T., Ruiz, A., Luchena, C., Zuazo-Ibarra, J., et al. (2022). Amyloid β / PKC-dependent alterations in NMDA receptor composition are detected in early stages of Alzheimer’s disease. Cell Death Dis. 13:253. doi: 10.1038/s41419-022-04687-y
Pfundstein, G., Nikonenko, A., and Sytnyk, V. (2022). Amyloid precursor protein (APP) and amyloid β (Aβ) interact with cell adhesion molecules: Implications in Alzheimer’s disease and normal physiology. Front. Cell Dev. Biol. 10:969547. doi: 10.3389/fcell.2022.969547
Plini, E., O’Hanlon, E., Boyle, R., Sibilia, F., Rikhye, G., Kenney, J., et al. (2021). Examining the role of the noradrenergic locus coeruleus for predicting attention and brain maintenance in healthy old age and disease: An MRI structural study for the Alzheimer’s disease neuroimaging initiative. Cells 10:1829. doi: 10.3390/cells10071829
Reale, M., and Costantini, E. (2021). Cholinergic modulation of the immune system in neuroinflammatory diseases. Diseases 9:29. doi: 10.3390/diseases9020029
Rodini, M., De Simone, M., Caltagirone, C., and Carlesimo, G. (2022). Accelerated long-term forgetting in neurodegenerative disorders: A systematic review of the literature. Neurosci. Biobehav. Rev. 141:104815. doi: 10.1016/j.neubiorev.2022.104815
Ruengchaijatuporn, N., Chatnuntawech, I., Teerapittayanon, S., Sriswasdi, S., Itthipuripat, S., and Hemrungrojn, S. (2022). An explainable self-attention deep neural network for detecting mild cognitive impairment using multi-input digital drawing tasks. Alzheimers Res. Ther. 14:111. doi: 10.1186/s13195-022-01043-2
Shi, J., and Zhuang, P. (2020). Effects and biological mechanisms of traditional Chinese medicine (TCM) modulating the brain microenvironment to ameliorate cognitive impairment triggered by Alzheimer’s disease. Tian. Trad. Chin. Med. 37, 475–480.
Song, Y., Xu, W., Zhang, X., and Ni, G. (2020). Mechanisms of electroacupuncture on Alzheimer’s disease: A review of animal studies. Chin. J. Integr. Med. 26, 473–480. doi: 10.1007/s11655-020-3092-9
Storga, D., Vrecko, K., Birkmayer, J., and Reibnegger, G. (1996). Monoaminergic neurotransmitters, their precursors and metabolites in brains of Alzheimer patients. Neurosci. Lett. 203, 29–32. doi: 10.1016/0304-3940(95)12256-7
Sun, J., Zhang, Y., Kong, Y., Ye, T., Yu, Q., Kumaran Satyanarayanan, S., et al. (2022). Microbiota-derived metabolite Indoles induced aryl hydrocarbon receptor activation and inhibited neuroinflammation in APP/PS1 mice. Brain Behav. Immun. 106, 76–88. doi: 10.1016/j.bbi.2022.08.003
Sutalangka, C., Wattanathorn, J., Muchimapura, S., Thukham-Mee, W., Wannanon, P., and Tong-un, T. (2013). Laser acupuncture improves memory impairment in an animal model of Alzheimer’s disease. J. Acupunct. Meridian Stud. 6, 247–251. doi: 10.1016/j.jams.2013.07.001
Tain, Y., and Hsu, C. (2017). Interplay between oxidative stress and nutrient sensing signaling in the developmental origins of cardiovascular disease. Int. J. Mol. Sci. 18:841. doi: 10.3390/ijms18040841
Tang, C., Lai, X., Lin, Z., Yang, J., Lin, J., Xian, Z., et al. (2005). Effects of Electroacupuncture on IL-1, IL-6 in brain tissue of rats with senile dementia. J. Basic Chin. Med. 7, 532–533.
Teja, Y., Helianthi, D., and Nareswari, I. (2021). The role of medical acupuncture therapy in Alzheimer’s disease. Med. Acupunct. 33, 396–402. doi: 10.1089/acu.2021.0014
Trabace, L., Cassano, T., Colaianna, M., Castrignanò, S., Giustino, A., Amoroso, S., et al. (2007). Neurochemical and neurobehavioral effects of ganstigmine (CHF2819), a novel acetylcholinesterase inhibitor, in rat prefrontal cortex: An in vivo study. Pharmacol. Res. 56, 288–294. doi: 10.1016/j.phrs.2007.07.006
Tripathi, S., and Mazumder, P. (2021). Neuroprotective Efficacy of apple cider vinegar on zinc-high fat diet-induced mono amine oxidase alteration in murine model of AD. J. Am. Nutr. Assoc. 41, 658–667. doi: 10.1080/07315724.2021.1948933
Vecchio, I., Sorrentino, L., Paoletti, A., Marra, R., and Arbitrio, M. (2021). The state of the art on acetylcholinesterase inhibitors in the treatment of Alzheimer’s disease. J. Cent. Nerv. Syst. Dis. 13:11795735211029113. doi: 10.1177/11795735211029113
Vermeiren, Y., Janssens, J., Aerts, T., Martin, J., Sieben, A., Van Dam, D., et al. (2016). Brain serotonergic and noradrenergic deficiencies in behavioral variant frontotemporal dementia compared to early-onset Alzheimer’s disease. J. Alzheimers Dis. 53, 1079–1096. doi: 10.3233/JAD-160320
Von Linstow, C., Severino, M., Metaxas, A., Waider, J., Babcock, A., Lesch, K., et al. (2017). Effect of aging and Alzheimer’s disease-like pathology on brain monoamines in mice. Neurochem. Int. 108, 238–245. doi: 10.1016/j.neuint.2017.04.008
Wang, L., and Zhou, L. (2009). Effects of electroacupuncture treatment on ACh, chat, and ache in Alzheimer’s disease rats. Acupunc. Clin. 25, 40–42.
Wang, J., Zhu, X., Li, Y., Guo, W., and Li, M. (2022). Jiedu-yizhi formula alleviates neuroinflammation in AD rats by modulating the gut microbiota. Evid. Based Comp. Alternat. Med. 2022:4023006. doi: 10.1155/2022/4023006
Wang, S., Kang, S., and Li, A. (2004). Analysis of the correlation between the functional effects of acupuncture on the brain free radical system and the cholinergic system in rats with dementia mimicking. Acupunct. Res. 102–106.
Wang, Y., Wu, X., Tang, C., Xu, Y., Wang, J., Xu, J., et al. (2020c). Effect of electroacupuncture of different acupoint groups on learning-memory ability and expression of IL-1β and TNF-α in hippocampus and prefrontal cortex in rats with Alzheimer’s disease. Zhen Ci Yan Jiu 45, 617–622. doi: 10.13702/j.1000-0607.190887
Wang, X., Li, Z., Li, C., Wang, Y., Yu, S., and Ren, L. (2020b). Electroacupuncture with Bushen Jiannao improves cognitive deficits in senescence-accelerated mouse prone 8 mice by inhibiting neuroinflammation. J. Tradit. Chin. Med. 40, 812–819.
Wang, L., Zhao, T., Zhou, H., Zhou, Z., Huang, S., Ling, Y., et al. (2020a). Effect of electroacupuncture on recognition memory and levels of Aβ, inflammatory factor proteins and aquaporin 4 in hippocampus of APP/PS1 double transgenic mice. Zhen Ci Yan Jiu 45, 431–437.
Wang, S., Yabuki, Y., Matsuo, K., Xu, J., Izumi, H., Sakimura, K., et al. (2018). T-type calcium channel enhancer SAK3 promotes dopamine and serotonin releases in the hippocampus in naive and amyloid precursor protein knock-in mice. PLoS One 13:e0206986. doi: 10.1371/journal.pone.0206986
Wang, Y., Zhao, L., Shi, H., Jia, Y., and Kan, B. (2021). RhoA/ROCK pathway involved in effects of Sanjiao acupuncture on learning and memory and synaptic plasticity in Alzheimer’s disease mice. Zhen Ci Yan Jiu 46, 635–641.
Weber Boutros, S., Unni, V., and Raber, J. (2022). An adaptive role for DNA double-strand breaks in hippocampus-dependent learning and memory. Int. J. Mol. Sci. 23:8352. doi: 10.3390/ijms23158352
Wegmann, S., Eftekharzadeh, B., Tepper, K., Zoltowska, K., Bennett, R., Dujardin, S., et al. (2018). Tau protein liquid-liquid phase separation can initiate tau aggregation. EMBO J. 37:e98049. doi: 10.15252/embj.201798049
Wei, Y., Zhu, T., Jia, J., and Yan, X. (2022). Research progress on the mechanism of acupuncture intervention on Alzheimer’s disease. Zhen Ci Yan Jiu 47, 362–368.
Wu, G., Li, L., Li, H. M., Zeng, Y., and Wu, W. C. (2017). Electroacupuncture ameliorate spatial learning and memory impairment via attenuating NOX2-related oxidative stress in a rat model of Alzheimer’s disease induced by Aβ1-42. Cell. Mol. Biol. 63, 38–45. doi: 10.14715/cmb/2017.63.4.7
Wu, J., Yang, Y., Wan, Y., Xia, J., Xu, J., Zhang, L., et al. (2022). New insights into the role and mechanisms of ginsenoside Rg1 in the management of Alzheimer’s disease. Biomed. Pharmacother. 152:113207. doi: 10.1016/j.biopha.2022.113207
Xie, L., Liu, Y., Zhang, N., Li, C., Sandhu, A., Williams, G. III, et al. (2021). Electroacupuncture improves M2 microglia polarization and glia anti-inflammation of hippocampus in Alzheimer’s disease. Front. Neurosci. 15:689629. doi: 10.3389/fnins.2021.689629
Xin, Y., Wang, J., and Xu, A. (2022). Electroacupuncture ameliorates neuroinflammation in animal models. Acupunct. Med. 40, 474–483.
Xiong, J., Lu, D., Chen, B., Liu, T., and Wang, Z. (2022). Dimethyl itaconate reduces cognitive impairment and neuroinflammation in APPswe/PS1ΔE9 transgenic mouse model of Alzheimer’s disease. Neuromol. Med. doi: 10.1007/s12017-022-08725-y [Epub ahead of print].
Yamahashi, Y., Lin, Y., Mouri, A., Iwanaga, S., Kawashima, K., Tokumoto, Y., et al. (2022). Phosphoproteomic of the acetylcholine pathway enables discovery of the PKC-β-PIX-Rac1-PAK cascade as a stimulatory signal for aversive learning. Mol. Psychiatry 27, ages3479–ages3492. doi: 10.1038/s41380-022-01643-2
Yang, B., He, M., Chen, X., Sun, M., Pan, T., Xu, X., et al. (2022). Acupuncture Effect Assessment in APP/PS1 Transgenic Mice: On Regulating Learning-Memory Abilities, Gut Microbiota, and Microbial Metabolites. Comput. Math. Methods Med. 2022:1527159. doi: 10.1155/2022/1527159
Yang, S., Wang, L., Xie, Z., Zeng, Y., Xiong, Q., Pei, T., et al. (2022). The Combination of Salidroside and Hedysari Radix Polysaccharide Inhibits Mitochondrial Damage and Apoptosis via the PKC/ERK Pathway. Evid. Based Complement. Alternat. Med. 2022:9475703. doi: 10.1155/2022/9475703
Yang, J., Wang, X., Ma, S., Yang, N., Li, Q., and Liu, C. (2019). Acupuncture attenuates cognitive impairment, oxidative stress and NF-κB activation in cerebral multi-infarct rats. Acupunct. Med. 37, 283–291. doi: 10.1136/acupmed-2017-011491
Yang, Q., Zhu, S., Xu, J., Tang, C., Wu, K., Wu, Y., et al. (2019). Effect of the electro-acupuncture on senile plaques and its formation in APP/PS1 double transgenic mice. Genes Dis. 6, 282–289. doi: 10.1016/j.gendis.2018.06.002
Yang, K., Song, X.-G., Ruan, J.-R., Cai, S.-C., Zhu, C.-F., Qin, X.-F., et al. (2021). Effect of moxibustion on cognitive function and proteins related with apoptosis of hippocampal neurons in rats with vascular dementia. Zhong. Zhen Jiu 41, 1371–1378.
Yang, W., and Dong, W. (2020). Mechanisms of electroacupuncture for improving Alzheimer’s disease from reducing β amyloid protein level. Zhen Ci Yan Jiu 45, 426–431.
Yang, Y., Hu, S., Lin, H., He, J., and Tang, C. (2020). Electroacupuncture at GV24 and bilateral GB13 improves cognitive ability via influences the levels of Aβ, p-tau (s396) and p-tau (s404) in the hippocampus of Alzheimer’s disease model rats. Neuroreport 31, 1072–1083. doi: 10.1097/WNR.0000000000001518
Ye, Y., Zhu, W., Wang, X., Yang, J., Xiao, L., Liu, Y., et al. (2017). Mechanisms of acupuncture on vascular dementia-A review of animal studies. Neurochem. Int. 107, 204–210. doi: 10.1016/j.neuint.2016.12.001
Ynag, J., Jiang, J., Tian, H., Wang, Z., Ren, J., Liu, H., et al. (2021). Effect of electroacupuncture on learning-memory ability and expression of IL-1β, IL-6 and TNF-α in hippocampus and spleen in mice with Alzheimer’s disease. Zhen Ci Yan Jiu 46, 353–361.
Yoshida, N., Kato, Y., Takatsu, H., and Fukui, K. (2022). Relationship between cognitive dysfunction and age-related variability in oxidative markers in isolated mitochondria of Alzheimer’s disease transgenic mouse brains. Biomedicines 10:281. doi: 10.3390/biomedicines10020281
Yousaf, M., Chang, D., Liu, Y., Liu, T., and Zhou, X. (2022). Neuroprotection of cannabidiol, its synthetic derivatives and combination preparations against microglia-mediated neuroinflammation in neurological disorders. Molecules 27:4961. doi: 10.3390/molecules27154961
Yu, C., Du, Y., Wang, S., Liu, L., Shen, F., Wang, L., et al. (2020a). Experimental evidence of the benefits of acupuncture for Alzheimer’s disease: An updated review. Front. Neurosci. 14:549772. doi: 10.3389/fnins.2020.549772
Yu, C., Wang, J., Ye, S., Gao, S., Li, J., Wang, L., et al. (2020b). Preventive electroacupuncture ameliorates D-galactose-induced Alzheimer’s disease-like pathology and memory deficits probably via inhibition of GSK3/mTOR signaling pathway. Evid. Based Complement. Alternat. Med. 2020:1428752.
Yu, C., He, C., Du, Y., Gao, S., Lin, Y., Wang, S., et al. (2021). Preventive electroacupuncture reduces cognitive deficits in a rat model of D-galactose-induced aging. Neural Regen. Res. 16, 916–923. doi: 10.4103/1673-5374.297090
Yue, Q., Song, Y., Liu, Z., Zhang, L., Yang, L., and Li, J. (2022). Receptor for advanced glycation end products (RAGE): A pivotal hub in immune diseases. Molecules 27:4922. doi: 10.3390/molecules27154922
Zhang, H., Wang, Y., Wang, Y., Li, X., Wang, S., and Wang, Z. (2022). Recent advance on carbamate-based cholinesterase inhibitors as potential multifunctional agents against Alzheimer’s disease. Eur. J. Med. Chem. 240:114606. doi: 10.1016/j.ejmech.2022.114606
Zhang, J., Tang, C., Liao, W., Zhu, M., Liu, M., and Sun, N. (2019). The antiapoptotic and antioxidative stress effects of Zhisanzhen in the Alzheimer’s disease model rat. Neuroreport 30, 628–636. doi: 10.1097/WNR.0000000000001243
Zhang, M., Xv, G., Wang, W., Meng, D., and Ji, Y. (2017). Electroacupuncture improves cognitive deficits and activates PPAR-γ in a rat model of Alzheimer’s disease. Acupunct. Med. 35, 44–51. doi: 10.1136/acupmed-2015-010972
Zhang, S., Su, S., and Gao, J. (2020). Electroacupuncture improves learning-memory ability possibly by suppressing apoptosis and down-regulating expression of apoptosis-related proteins in hippocampus and cerebral cortex in immature mice with Alzheimer’s disease. Zhen Ci Yan Jiu 45, 611–616.
Zhao, J., Yu, S., Zhou, Q., Wang, Z., and Wei, J. (1999). Effects of acupuncture on central neurotransmitters in aged rats and mice. J. Cheng. Univ. Trad. Chin. Med. 3, 30–31.
Zheng, Q., Kong, L., Yu, C., He, R., Wang, X., Jiang, T., et al. (2020). [Effects of electroacupuncture on cognitive function and neuronal autophagy in rats with D-galactose induced Alzheimer’s disease]. Zhen Ci Yan Jiu 45, 689–695.
Keywords: Alzheimer’s disease, senile dementia, mechanism of action, acupuncture therapy, curative effect
Citation: Wu L, Dong Y, Zhu C and Chen Y (2023) Effect and mechanism of acupuncture on Alzheimer’s disease: A review. Front. Aging Neurosci. 15:1035376. doi: 10.3389/fnagi.2023.1035376
Received: 02 September 2022; Accepted: 13 February 2023;
Published: 03 March 2023.
Edited by:
Zhengzhe Lan, Zhejiang University, ChinaReviewed by:
Yuanxiang Zhang, Wannan Medical College, ChinaMei Wang, First Affiliated Hospital of Wannan Medical College, China
Huaping Chen, Hangzhou First People’s Hospital, China
Yuan Zhang, First Affiliated Hospital of Wannan Medical College, China
Copyright © 2023 Wu, Dong, Zhu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Chen, Y2hlbnlvbmdAY2R1dGNtLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Liu Wu
Liu Wu Yuting Dong
Yuting Dong Chengcheng Zhu3
Chengcheng Zhu3 Yong Chen
Yong Chen