- 1Department of Neurology, Center for Movement Disorders, Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 2China National Clinical Research Center for Neurological Diseases, Beijing, China
- 3Department of Neurology, Beijing Chaoyang Hospital, Capital Medical University, Beijing, China
Background: Lower urinary tract symptoms (LUTS) are common non-motor symptoms but are often overlooked in Parkinson's disease (PD). The prevalence of LUTS in PD is inconsistent among different studies.
Objective: To estimate the prevalence of LUTS, urinary incontinence, and urinary retention in PD patients, then, investigate potential sources of inconsistency in prevalence estimation.
Methods: We searched PubMed, EMBASE, and Web of Science databases from inception to May 2022. Studies reporting the prevalence of LUTS or LUTS subtypes in PD were included. Pooled prevalence of LUTS, LUTS subtypes, urinary incontinence, and urinary retention was calculated via random-effects models. Meta-regression and subgroup analyses were performed.
Results: Of 7,358 studies after duplicate removal, a total of 73 studies comprising 14,937 PD patients were included. The pooled prevalence of LUTS was 61% (95% CI 53–69; 27 studies; n = 5,179), while the pooled prevalence of storage symptoms and voiding symptoms was 59% (44–73; 9 studies; n = 798) and 24% (14–33; 11 studies; n = 886), respectively. The pooled prevalence of urinary incontinence, retention and post-void residual (PVR) volume ≥ 100 ml were 30% (95% CI 22–39; 21 studies; n = 6,054), 27% (17–37; 14 studies; n = 1,991), and 4% (1–7; 5 studies; n = 439), respectively. The prevalence of LUTS, urinary incontinence, or urinary retention was significantly associated with diagnostic methods.
Conclusion: LUTS and its subtypes present in a significant proportion of PD patients. It is necessary to use standardized and validated methods to detect and screen LUTS and its subtypes.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022311233, Identifier: CRD42022311233.
Introduction
Parkinson's disease (PD) is the second most prevalent neurodegenerative disease characterized by both motor and non-motor symptoms. Lower urinary tract symptoms (LUTS) are common non-motor symptoms of PD. However, they are frequently neglected (Chaudhuri et al., 2006). LUTS are group of symptoms related to the lower urinary tract. Typical LUTS include storage symptoms (including urinary incontinence) and voiding symptoms (including urinary retention), which often emerge 5–6 years after the onset of motor symptoms in PD (Bonnet et al., 1997). The mechanism of LUTS may be related to PD neuropathology, especially the disruption of the dopamine D1-GABAergic direct and bypass pathway (Sakakibara et al., 2016). α-synuclein, the pathological hallmark of PD, has been found in the pontine, sacral spinal cord, pelvic plexus, and genitourinary tract of PD patients (Wakabayashi and Takahashi, 1997; Beach et al., 2010). The structures responsible for normal bladder control, including pre-ganglionic, post-ganglionic sympathetic neurons, sacral parasympathetic nuclei, and frontal cortex were even proven to have PD neuropathology (Oyanagi et al., 1990; Braak et al., 2007; Tkaczynska et al., 2017).
LUTS exert immense impact on patients' life. The quality of life was physically and psychologically limited for PD patients with LUTS. The main effects were decline in self-esteem and social communication, in addition to depression, anxiety, deterioration of sexual life, and a decrease in physical activity (Farage et al., 2008). PD patients with LUTS showed elevated all-cause mortality and were more likely to develop severe complications including falls, disabling motor symptoms, cognitive dysfunction, and other non-motor dysfunction (Vaughan et al., 2013; Rana et al., 2015; Zhang and Zhang, 2015; Sakushima et al., 2016; Lee et al., 2018). Moreover, LUTS were correlated with increasing health-related costs. These can lead to serious burdens for PD patients, caregivers, and society (Mohammed and Ragab, 2010). This urges the need for an up-to-date estimation of the prevalence of LUTS. Establishing the prevalence of LUTS may not only raise awareness of early interventions but also help refine diagnostic criteria and differential diagnosis of PD and other parkinsonian syndromes. This may also guide effective planning of medical services.
However, there was considerable heterogeneity in the prevalence of LUTS in PD, ranging from 27 to 85% as shown in previous reports (Winge et al., 2006; Sakakibara et al., 2008; Jain, 2011; Martinez-Ramirez et al., 2020). Previous studies reporting the prevalence of LUTS were predominantly cross-sectional, with limited focus on the nature of LUTS. Furthermore, methods of determining the presence of LUTS are diverse, potentially leading to different reported rates. Urinary incontinence and retention are crucial symptoms of LUTS. PD patients were generally considered less likely to develop those two symptoms, and the presence of unexplained voiding difficulties with elevated post-void residual (PVR) volume ≥ 100 ml or unexplained urinary urge incontinence was often considered to support the diagnosis of multiple system atrophy (MSA) (Yamamoto et al., 2016; Wenning et al., 2022). However, urinary incontinence and retention are not rare, even PVR volume ≥ 100 ml can also occur in PD (Irene, 2019; Tateno et al., 2021). For example, one study with large sample size found that the prevalence of urinary incontinence was 43% in PD (Wüllner et al., 2007). Utilizing the Danish Prostate Symptom Score (Dan-PSS), the prevalence of urge incontinence in PD was up to 65.8% (Akkoç et al., 2017). Another study found the prevalence of incomplete bladder emptying was 75.5% in PD (Irene, 2019). Until now, no meta-analysis has been carried out to estimate the overall prevalence of LUTS, urinary incontinence, and retention in PD.
We aimed to conduct a systematic review and meta-analysis to determine the prevalence of the overall LUTS and its subtypes including urinary incontinence and retention in PD patients, as well as to explore potential sources of heterogeneity across prevalence estimates.
Materials and methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Barendregt et al., 2013) (Supplementary Table 1). The protocol has been registered on PROSPERP (registration number: CRD42022311233).
Date source and strategy
We screened PubMed, EMBASE, and Web of Science databases to identify relevant research from the outset until May 2022 using the following MeSH (Medical Subject Heading) terms and keyword variations: (“urination disorders” OR “urinary bladder, neurogenic” OR “urinary retention” OR “overactive bladder symptom” OR “nocturia” OR “urinary bladder, overactive” OR “urinary incontinence” OR “lower urinary tract symptoms” OR “urinary dysfunction” OR “dysuria”) AND (“Parkinson's disease” OR “Parkinsonism” OR “Parkinsonian” OR “Parkinson's disease”). Afterwards, endnote was utilized to integrate the citations from each database and additional eligible publications.
Eligibility criteria and study selection
Studies were eligible if they met the following criteria: (1) published in peer-reviewed English journals; (2) participants were diagnosed according to UK Parkinson's Disease Society Brain Bank Diagnostic Criteria (Hughes et al., 1992) or MDS clinical diagnostic criteria for Parkinson's disease (Postuma et al., 2015); (3) reporting the prevalence of LUTS or LUTS subtypes; (4) LUTS or LUTS subtypes assessed by validated scales administered by experienced clinicians, self-report questionnaire, or published criteria from classification codes/definition; (5) prospective cohort study or cross-sectional study.
The studies were excluded if they: (1) did not provide full text; (2) included insufficient or unclear fragmented data for analysis; (3) enrolled patients who have been diagnosed with prostate carcinoma, uncontrolled diabetes, as well as any other diseases that cause urinary problems, or taken drugs such as diuretics; (4) with small sample size (n < 20). (5) duplicated publications; (6) systematic reviews, meta-analyses, letters, protocols. When results on the same dataset were reported in several publications, only the most complete publication was included in the analysis. Two independent observers (FFL, YSC) evaluated the results and resolved any disagreement by discussion or with recourse to a third arbitrator (TF).
Quality assessment
Two authors (FFL, YSC) independently assessed study quality and risk of bias using the Newcastle-Ottawa Scale (NOS) (Stang, 2010) for cohort study and the Agency for Healthcare Research and Quality (AHRQ) (Williams et al., 2010) for cross-sectional study in Table 1. The highest score was 9 points for the cohort and case-control studies: low quality = 0–4 points; moderate quality = 5–7 points; high quality = 8–9 points. The highest score was 11 points for cross-sectional studies: low quality = 0–3 points; moderate quality = 4–7 points; high quality = 8–11 points. Higher score indicates better quality.
Data extraction
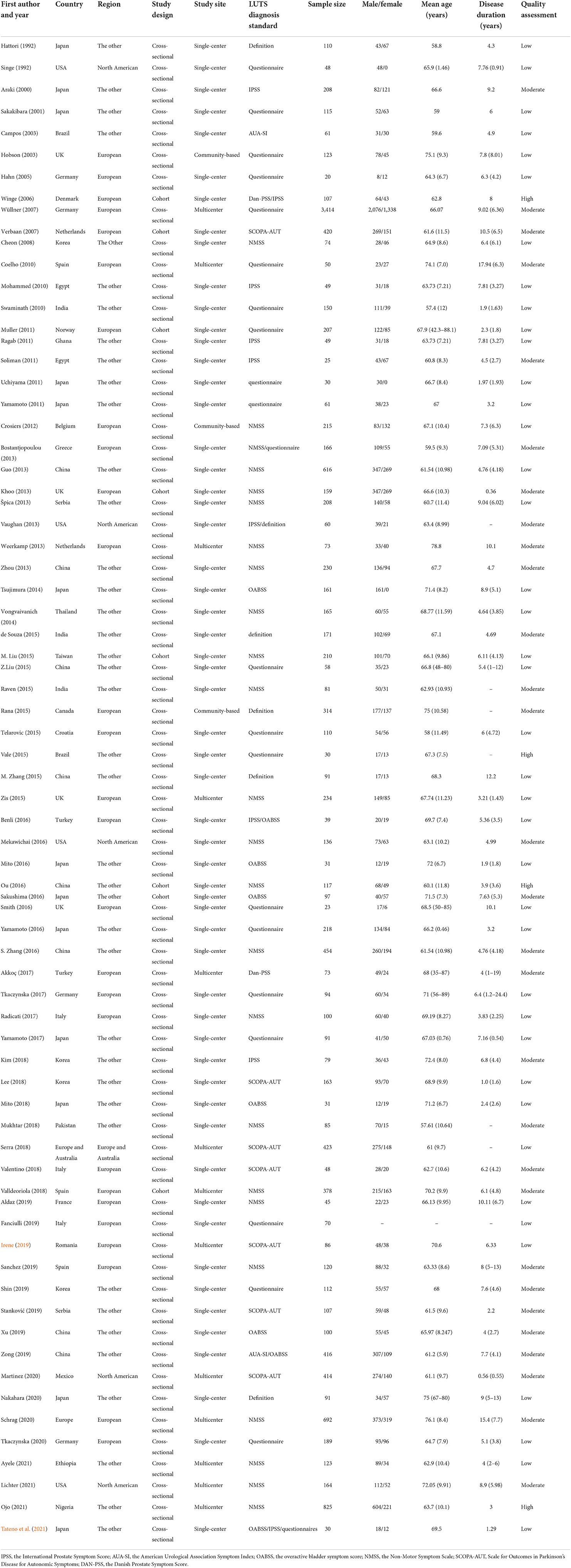
Data extraction was performed independently by two authors (FFL, YSC), using a standardized data collection spreadsheet in EXCEL. The following items were extracted: study characteristics (first author, publication year, country/region, study design, source), participant characteristics (age, disease duration, sample size, number of patients with LUTS or LUTS subtypes, gender of participants and H&Y stage), and methods used to evaluate LUTS or its subtypes (definitions, urinary dysfunction questionnaires or clinical scales).
LUTS subtypes include urinary storage symptoms [urgency, Overactive bladder (OAB) syndrome, pollakiuria, frequency, nocturia, incontinence], urinary voiding symptoms (dysuria, hesitancy, prolongation, intermittency, weak stream, retention, straining to void), and post-voiding symptoms according to the International Continence Society (ICS) report (D'Ancona et al., 2019). The clinical scales contain the overactive bladder symptom score (OAB-SS) (Homma et al., 2006), the International Prostate Symptom Score (IPSS) (Araki and Kuno, 2000), the Non-Motor Symptom Scale (NMSS) (Koh et al., 2012), Scale for Outcomes in Parkinson's Disease for Autonomic Symptoms (SCOPA-AUT) (Kim et al., 2017), the Danish Prostate Symptom Score (Dan-PSS) (Akkoç et al., 2017), and the American Urological Association Symptom Index (AUA-SI) (Barry et al., 1992). If disagreements could not be resolved through careful discussion by two investigators (FFL, YSC), a consensus was achieved by the involvement of a third investigator (TF) when necessary.
Statistical analysis
The meta-analysis was performed with Rx64 4.1.0 and RStudio using the “Matrix”, “Meta,” and “metaphor” packages to analyze pooled prevalence of LUTS or its subtypes with 95% confidence intervals (CIs). Heterogeneity for the pooled estimate of prevalence were quantified using the I2 statistic, and its significance was measured using the Q test p-value. The random-effects model was adopted if significant heterogeneity were detected (I2 > 50% or p > 0.1). Otherwise, the fixed-effects model was used. Meta-regression was performed to assess the effects of following variables: age, disease duration, regions of the participant, quality of studies and diagnostic method. We conducted subgroup analyses using diagnosis criteria (Scales, questionnaire, and definition), gender (proportion of female), H&Y stage (<3 and ≥3), study site (single-center, multicenter, and community-based), study design (cross-sectional and cohort), mean age (<65 and ≥65) of participants, region (North American, European, and the other) and quality of studies (Low, moderate and high). Sensitivity analyses were performed to evaluated the robust of the synthesis results by leave-one-out method. Publication bias was checked by funnel plot and Egger's test. The trim-and-fill computation was used to estimate the effect of publication bias on the interpretation of the results. Statistical significance was set at two-tailed p < 0.05.
Results
Characteristics of the included studies
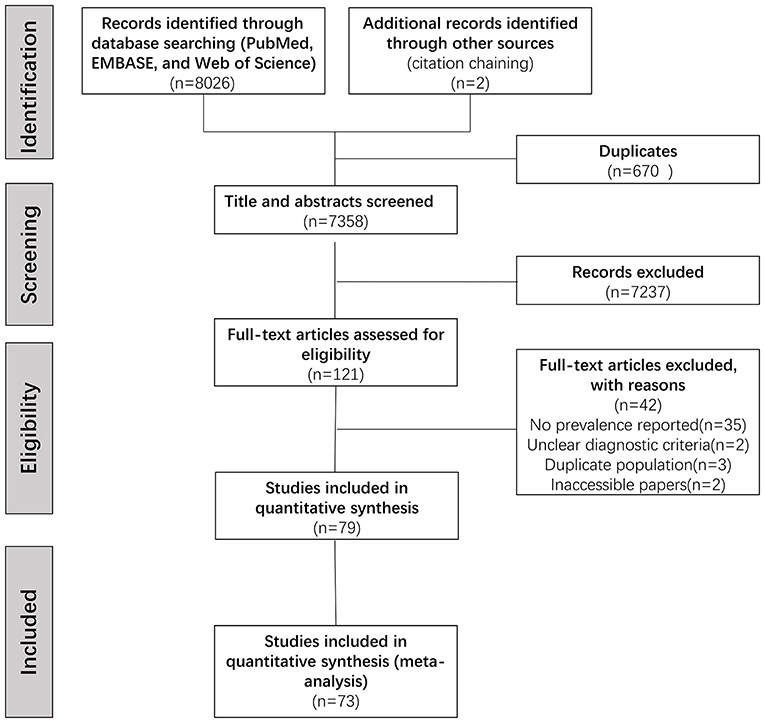
After duplicating removal, we identified 7,358 articles through the database searching. Screening titles and abstracts led to the elimination of 7,237 irrelevant articles, full-text versions of the remaining 121 potentially eligible articles were assessed. Of those, 79 articles were included in the qualitative synthesis. Overall, 73 studies comprising 14,937 PD patients were identified eligible for the meta-analysis (Supplementary Table 2). The procedure was shown flow chart in Figure 1.
Sample size ranged from 20 to 3,414. Average age of participants ranged from 57.4 to 76.1 years. The average disease duration of PD ranged from 0.36 to 17.94 years. Forty-seven studies used the scales, 19 used questionnaire, 4 used definitions, and 3 applied mixed methods. We classified 4 studies as high quality, 29 studies as moderate, and 40 studies as low. The results indicated that the overall quality of the included articles was relatively low. There were 65 cross-sectional studies and 8 cohort studies in the included research. The studies were conducted in 29 countries, 27 performed in Europe, 5 in North American, while others were undertaken in the other regions (n = 40). Table 1 shows the characteristics of included study.
Prevalence of LUTS in PD
The pooled prevalence of LUTS was 61% (95% CI 53–69; I2 = 99%; 27 studies; n = 5,179; Figures 2A, 3).
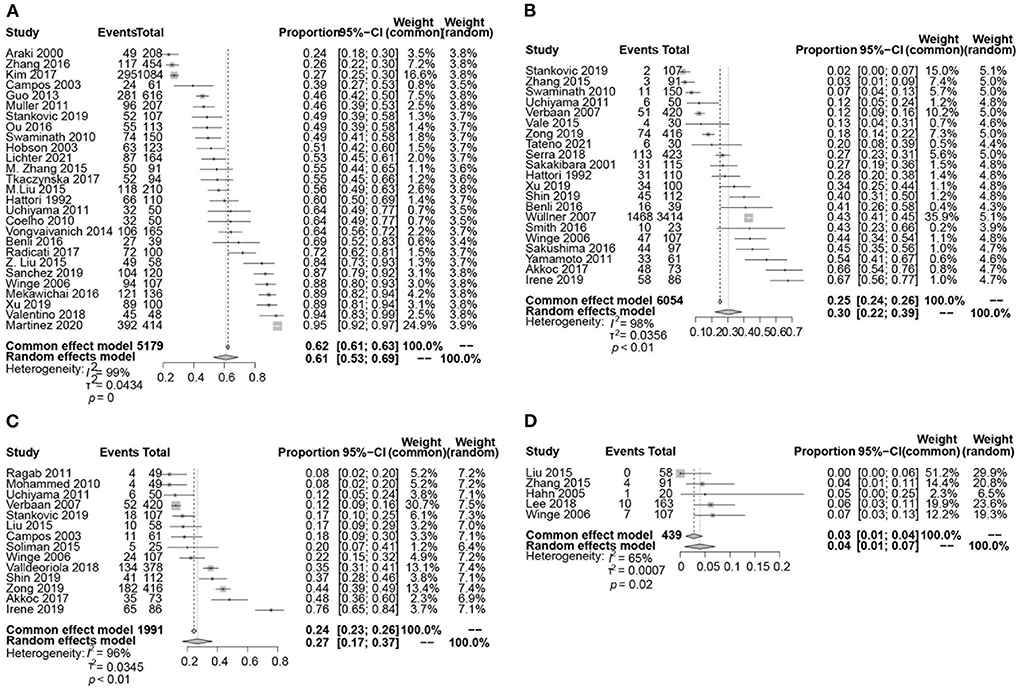
Figure 2. Forest plot showing the prevalence of LUTS (A), urinary incontinence (B), urinary retention (C), and post-void residual (PVR) volume ≥ 100 ml (D) in PD patients.
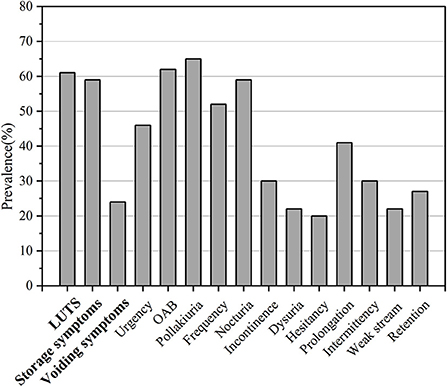
Figure 3. Frequency of LUTS or its subtypes in PD patients. The x-axis shows different kinds of LUTS while the y-axis shows the percentage.
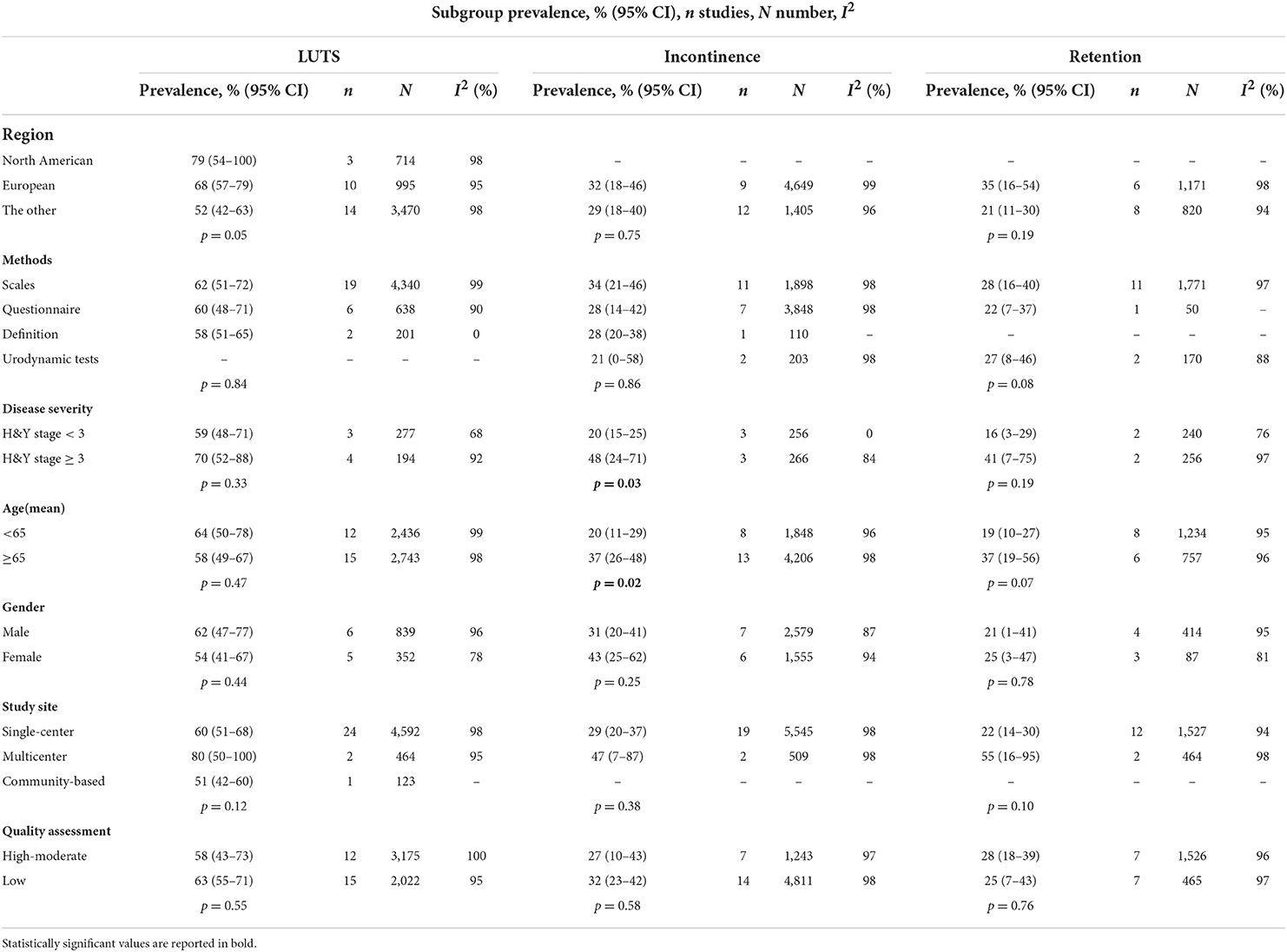
For subgroup analyses, we found that H&Y stage, gender, and different diagnostic tools may be cause of heterogeneity related to the prevalence of LUTS (Table 2). The pooled prevalence of LUTS was 59% (95% CI 48–71; I2 = 68%; 3 studies; n = 277) in PD with H&Y stage <3, whereas 70% (95% CI 52–88; I2 = 92%; 4 studies; n = 194) in PD with H&Y stage ≥ 3. The pooled prevalence of LUTS was 62% (95% CI 47–77; I2 = 96%; 6 studies; n = 839) in male PD patients, whereas 54% (95% CI 41–67; I2 = 78%; 5 studies; n = 352) in female PD patients.
Different diagnostic tools also influenced prevalence of LUTS. The pooled prevalence of LUTS based on definitions was 58% (95% CI 51–65; 2 studies; n = 201) with considerable heterogeneity (I2 = 0%; p = 0.47). Using NMSS, the pooled prevalence of LUTS was 60% (95% CI 48–72; 10 studies; n = 2,172), also with considerable heterogeneity (I2 = 98%; p < 0.01). Using IPSS, the pooled prevalence of LUTS was 39% (95% CI 11–67) in 1,331 PD patients reported by 3 studies, with considerable heterogeneity (I2 = 94%; p < 0.01). Using SCOPA-AUT, the pooled prevalence of LUTS was 79% (95% CI 50– 100) estimated from 569 PD patients, with considerable heterogeneity of these 3 studies (I2 = 98%; p < 0.01). Using other questionnaires of LUTS diagnostic tools, the pooled prevalence of LUTS was 60% (95% CI 48–71) of 638 PD reported by 6 studies, with considerable heterogeneity (I2 = 90%; p < 0.01). The prevalence of LUTS was 89% using OABSS (n = 100), 39% using AUA-SI (n = 61), and 88% using Dan-PSS (n = 107), respectively, all from one study (Supplementary Figure S1; Supplementary Table 3).
By meta-regression analyses, region (p = 0.016) and number of PD patients (p = 0.031) were causes of heterogeneity related to the prevalence of LUTS, while the study site, quality assessment, age, and disease duration were not.
No significant publication bias was found by Begg's funnel plot (p = 0.695).
Prevalence of LUTS subtypes in PD
Storage symptoms
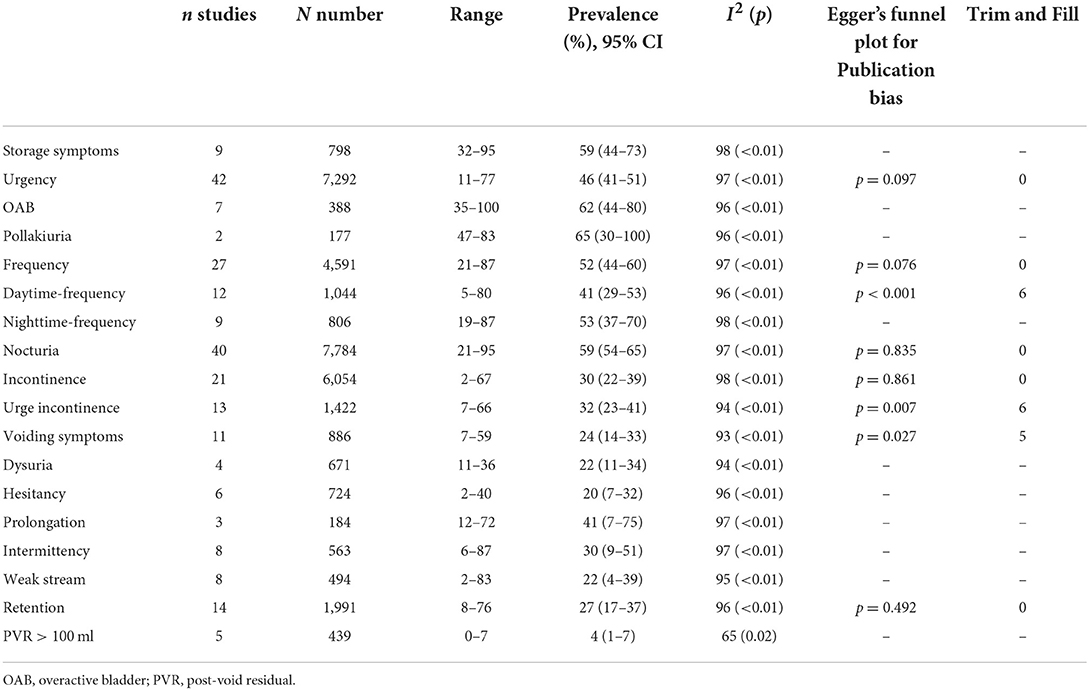
The pooled prevalence of storage symptoms was 59% (95% CI 44–73; 9 studies; 798 PD; I2 = 98%; p < 0.01; Table 3; Figure 3; Supplementary Figure S2).
Incontinence
The pooled prevalence of urinary incontinence was 30% (95% CI 22–39; I2 = 98%; p < 0.01; 21 studies; n = 6,054; Figure 2B). The pooled prevalence of urge incontinence was 32% (95% CI 23–41; I2 = 94%; p < 0.01; 13 studies; n = 1,422).
Using urodynamic tests, the pooled prevalence of urinary incontinence was 21% (95% CI 0–58; 2 studies; n = 203), with significant heterogeneity (I2 = 98%; p < 0.01). Using clinical scales, the pooled prevalence of urinary incontinence was 34% (95% CI 21–46; 11 studies; n = 1,898), with significant heterogeneity (I2 = 98%; p < 0.01); The pooled prevalence of urinary incontinence was 28% (95% CI 14–42; I2 = 98%; p < 0.01; 7 studies; n = 3,843) using questionnaires and 28% using definition in one study.
The pooled prevalence of urinary incontinence was 20% (95% CI 15–25; I2 = 0%; 3 studies; n = 256) in PD with H&Y stage <3, whereas 48% (95% CI 24–71; I2 = 84%; 3 studies; n = 266) in PD with H&Y stage ≥ 3. The pooled prevalence of urinary incontinence was 20% (95% CI 11–29; I2 = 96%; 8 studies; n = 1,848) in PD with age <65 years, whereas 37% (95% CI 26–48; I2 = 98%; 13 studies; n = 4,206) in PD with age ≥ 65 years (Table 2).
By meta-regression analyses, age (p = 0.017) was a source of heterogeneity related to the prevalence of urinary incontinence, while the study site, quality assessment, region, number of PD patients, and disease duration were not.
OAB The pooled prevalence of OAB was 62% (95% CI 44–80; I2 = 96%; p < 0.01; 7 studies; n = 388) (Supplementary Figure S3).
Urinary urgency
The pooled prevalence of urgency was 46% (95% CI 41–51; I2 = 97%; p < 0.01; 46 studies; n = 7,292) (Supplementary Figure S4).
By subgroup analysis, we found that H&Y stage, sex, and different diagnostic tools may be factors affecting the prevalence of urgency. By meta-regression analyses, region (p = 0.046) was a source of heterogeneity, while the study site, quality assessment, age, number of PD patients, and disease duration were not.
Urinary frequency
The pooled prevalence of frequency was 52% (95% CI 44–60; I2 = 97%; p < 0.01; 27 studies; n = 4,591). The pooled prevalence of daytime frequency was 41% (95% CI 29–53; I2 = 96%; p < 0.01; 12 studies; n = 1,044). The pooled prevalence of nighttime frequency was 53% (95% CI 37–70; I2 = 98%; p < 0.01; 9 studies; n = 806) (Supplementary Figure S5).
By meta-regression analyses, age (p = 0.028) was a cause of heterogeneity related to the prevalence of frequency, while study site, quality assessment, region, number of PD patients, and disease duration were not.
Nocturia
The pooled prevalence of nocturia was 59% (95% CI 54–65; I2 = 97%; p < 0.01; 40 studies; n = 7,784) (Supplementary Figure S6).
By subgroup analysis, we found that H&Y stage and different diagnostic tools may be factors affecting the prevalence of nocturia.
By meta-regression analyses, age (p = 0.026) was a cause of heterogeneity related to the prevalence of nocturia, while the study site, quality assessment, region, number of PD patients, and disease duration were not.
Pollakiuria
The pooled prevalence of pollakiuria was 65% (95% CI 30–100; I2 = 96%; p < 0.01; 2 studies; n = 177) (Supplementary Figure S7).
Voiding symptoms
The pooled prevalence of voiding symptoms was 24% (95% CI 14–33; 11 studies; n = 886; Figure 3). There was significant heterogeneity across these studies (I2 = 93%; p < 0.01) (Supplementary Figure S8). We did not find the factors such as study site, region, quality assessment, age, disease duration, number had effect on the heterogeneity of the prevalence of voiding symptoms by meta-regression.
Retention
A total of 14 studies investigated the prevalence of retention in 1,991 patients with PD ranging from 8 to 76% and yielding a pooled prevalence of 27% (95% CI 17–37; I2 = 96%; p < 0.01; Figure 2C). The pooled prevalence of PVR volume ≥ 100 ml was 4% (95% CI 1–7; I2 = 65%; p = 0.02; 5 studies; n = 439; Figure 2D).
Using the subgroup analysis, the pooled prevalence of urinary retention was 16% (95% CI 3–29; I2 = 76%; 2 studies; n = 240) in PD with H&Y stage <3, whereas 41% (95% CI 7–75; I2 = 97%; 2 studies; n = 256) in PD with H&Y stage ≥ 3. The pooled prevalence of urinary retention was 19% (95% CI 10–27; I2 = 95%; 8 studies; n = 1,234) in PD with age <65 years, whereas 37% (95% CI 19–56; I2 = 96%; 6 studies; n = 757) in PD with age ≥ 65 years (Table 2).
Using urodynamic tests, the pooled prevalence of retention was 27% (95% CI 8–46) estimated from 170 PD patients, 2 studies, with considerable heterogeneity (I2 = 88%; p < 0.01). Using clinical scales, the pooled prevalence of urinary incontinence was 28% (95% CI 16–40) estimated from 1,771 PD patients, 11 studies, with significant heterogeneity (I2 = 97%; p < 0.01); The pooled prevalence of urinary retention was 12% (95% CI 5–24; 1 study; n = 50) using questionnaires.
Further subgroup analysis showed that using urodynamic tests, the pooled prevalence of retention was 27% (95% CI 8–46) estimated from 170 PD patients, 2 studies, with considerable heterogeneity (I2 = 88%; p < 0.01). I prevalence of retention in PD was 12% (95% CI 5–24) using questionnaire and 35% (95% CI 31–41) using NMSS, respectively, all from one study. Utilizing IPSS, the estimated pooled prevalence of urinary retention was 21% (95% CI 7–35), the heterogeneity across these 5 studies in 303 PD patients was considerable (I2 = 90%; p < 0.01). Using SCOPA-AUT, the pooled prevalence of urinary retention was 35% (95% CI 0–75; I2 = 99%; p < 0.01; 3 studies; n = 613). Using AUA, the pooled prevalence of retention was 31% (95% CI 6–56) estimated from 477 PD patients, 2 studies, with considerable heterogeneity (I2 = 95%; p < 0.01) (Supplementary Figure S9; Supplementary Table 3).
Age was a source of heterogeneity related to the prevalence of retention (p = 0.024) by meta-regression.
Dysuria
The pooled prevalence of dysuria was 22% in PD (95% CI 11–34; I2 = 94%; p < 0.01; 4 studies; n = 671) (Supplementary Figure S10).
Hesitancy
The pooled prevalence of hesitancy was 20% in PD (95% CI 7–32; I2 = 96%; p < 0.01; 6 studies; n = 724) (Supplementary Figure S11).
Slow urinary stream/prolongation
The pooled prevalence of slow urinary stream was 41% in PD (95% CI 7–75; I2 = 97%; p < 0.01; 3 studies; n = 184) (Supplementary Figure S12).
Intermittency
The pooled prevalence of intermittency was 30% in PD (95% CI 9–51; I2 = 97%; p < 0.01; 8 studies; n = 563) (Supplementary Figure S13).
Spraying of urinary stream/weak stream of urine
The pooled prevalence of weak stream of urine was 22% in PD (95% CI 4–39; I2 = 95%; p < 0.01; 8 studies; n = 494) (Supplementary Figure S14).
Sensitivity analysis
This analysis validated the result's stability.
Discussion
This meta-analysis study indicates that LUTS and its subtypes present in a significant proportion of PD patients, occurring at rates much higher than those found in the general population. The pooled prevalence was 61% (95% CI 53–69) for LUTS, 59% (44–73) for storage symptoms, and 24% (14–33) for voiding symptoms. We found that urinary incontinence and retention have higher prevalence in PD than previously assumed. The pooled prevalence was 30% (95% CI 22–39) for urinary incontinence, 27% (17–37) for retention and 4% (1–7) for post-void residual (PVR) volume ≥100 ml, respectively. Overall, we found considerable heterogeneity among studies. The wide variety of methods were used to assess LUTS and its subtypes. The prevalence of LUTS, urinary incontinence, and urinary retention was significantly associated with diagnostic methods.
LUTS in PD
LUTS were found in more than half of PD patients in this meta-analysis. Previous studies showed that LUTS was age-associated (Lee et al., 1998; Takahashi et al., 2022). However, it was found that patients with PD experienced significantly more LUTS than age-matched controls (10.8%) (Campos-Sousa et al., 2003). The prevalence of urinary incontinence (5.5%) in community-based elder people was lower than PD of the same age (Takahashi et al., 2022). Moreover, the prevalence of urinary retention (20.6%) in community-based men were also lower than PD in our meta-analysis (Lee et al., 1998).
However, the estimated prevalence rate of LUTS in PD was much lower than other neurodegenerative diseases, such as MSA (96%) (Sakakibara et al., 2000), progressive supranuclear palsy (PSP) (80%) (Xie et al., 2015), and dementia with Lewy bodies (91%) (Tateno et al., 2015). Previous study showed PD patients with LUTS demonstrated significantly lower dopamine transporter uptake in the striatum than patients without LUTS, according to single-photon emission computerized tomography (SPECT) imaging. LUTS were demonstrated to be correlated with putamen/caudate ratio and the degeneration of nigrostriatal dopaminergic neurons in PD patients with severe LUTS, suggesting that LUTS was associated with the degeneration of the caudate nucleus in patients with severe bladder dysfunction (Winge et al., 2005). Besides, there were potential associations between frontal executive dysfunction and LUTS or its subtypes in PD (Tkaczynska et al., 2017, 2020; Xu et al., 2019). PD dementia (PDD) and dementia with Lewy bodies were diseases with high prevalence of LUTS and characterized by cognitive impairment. Cortical alpha synuclein deposition and dysfunction of frontal cortex-basal ganglia dopaminergic circuit were hallmarks for those diseases. This further illustrated the role of frontal cortex in cognitive impairment and LUTS (Sakakibara et al., 2014). Unlike motor symptoms, LUTS were levodopa refractory symptoms, which suggest complex pathophysiological mechanisms beyond the dopaminergic system.
There were significant variations among the 27 studies evaluating LUTS prevalence in this meta-analysis. The pooled prevalence of LUTS was 59% in PD patients with mild H&Y score, whereas result for patients with severe H&Y score was 70% in this meta-analysis. This was in line with previous studies. However, the prevalence of LUTS did not differ significantly by gender, age, study site, and quality assessment of articles by subgroup analyses.
This discrepancy could be attributed to the following reasons. Firstly, the inclusion criteria of LUTS were variable among prevalence studies. LUTS can be divided into three categories including urinary storage symptoms, urinary voiding symptoms and post-voiding symptoms according to the international continence society (ICS) report (D'Ancona et al., 2019). Some studies assessing prevalence of LUTS included both storage and voiding symptoms, whereas others did not. Second, considerable heterogeneity mainly originated from the diagnostic methodological difference of LUTS. Studies assessed the prevalence of LUTS by definitions, urinary dysfunction questionnaires, clinical scales and urodynamic tests. The current meta revealed those validated ways, particularly those based on Dan-PSS and SCOPA-AUT, had higher prevalence estimates than non-validated methods, suggesting that such an approach had advantages of LUTS screening. Prevalence of LUTS assessed by IPSS scale was relatively low, suggesting that the prevalence of LUTS may be underestimated. However, some of clinical scales such as OAB-SS and AUA-SI were not validated in PD domain. OAB-SS only evaluated storage symptoms, such as urgency, frequency, nocturia and urge incontinence. NMSS only assessed urgency, frequency and nocturia, suggesting inaccuracy in diagnosing LUTS. It is quite necessary to unify the research methods. Thirdly, the heterogeneity may equally have arisen from confounders within gender, age, and disease severity. Sex and H&Y stage has been shown to be potential modifiers in our study, while age, study site and quality assessment were not.
Storage symptoms in PD
The pooled prevalence of storage symptoms was 59%. Polyuria was the most prevalent type of LUTS in the patients with PD (65%) followed by OAB (62%), nocturia (59%), frequency (52%), urgency (46%), and incontinence (30%). The results were almost the same with those reported previously (Sakakibara et al., 2001; Tateno et al., 2021). OAB was the second most prevalent storage symptom. It was urinary urgency, usually accompanied by increased daytime frequency and/or nocturia, with urinary incontinence or without according to the International Continence Society (ICS) report (D'Ancona et al., 2019).
Storage symptoms were frequent in PD because degeneration of dopaminergic neurons might lead to urination irritative symptoms in PD (Mito et al., 2018). Striatum functionally controlled the urine storage. Disruption of D1-GABAergic direct striatal output pathway may cause bladder hyperactivity in PD which had been validated in SPECT and animal studies (Sakakibara et al., 2002; Yamamoto et al., 2005; Tateno et al., 2021). Besides, objective assessment with urodynamics showed that detrusor overactivity (DO) occur in 45–93% of PD patients, which was the most prevalent cause to OAB in PD (Sakakibara et al., 2001).
This meta-analysis revealed that the pooled prevalence of urinary incontinence was 30% in PD. Severe urinary incontinence in the first 5 year of disease is a red flag according to the MDS clinical diagnostic criteria for PD. However, a postmortem study identifying 11 cases confirmed the prevalence of urinary incontinence in PD was 82%, similar to that of MSA (87%) (Wenning et al., 1999). Although the sample size was relatively small in this study, the high prevalence rate challenged the prevailing view that urinary incontinence tended to be an exclusion criteria for PD diagnosis. However, the mean disease duration of collected cases was 16.58 years, much longer than the “5 year” indicated in the MDS diagnostic criteria. The finding suggested the possibility that disease duration might influence prevalence rate of urinary incontinence. Indeed, converging evidence pointed that urinary incontinence mainly occurs in the advanced stage of PD, ~12–15.5 years after the onset of motor symptoms (Wenning et al., 1999; Uchiyama et al., 2011; Khoo et al., 2013), because urethral sphincter function was preserved in the early stages of PD (Stocchi et al., 1997). With the increasing age and disease duration, frontal lobe damage worsened accompanied with increase of alpha synuclein deposition, leading to cognitive dysfunction and LUTS (Tkaczynska et al., 2017). Among all the studies reporting urinary incontinence, the mean disease duration ranged from 1.29 to 12.2 years. One study showed that the frequency of urinary incontinence increased in the 1st (4.1%), 2nd (12%) and 3rd (15.1%) year of follow-up with mean 2.2 years of disease duration (Stanković et al., 2019). Another study showed that the prevalence of incontinence was 8.3, 30, 43.8, and 50% in PD patients with disease duration <2 years, 2–5 years, 5–10 years, and >10 years, respectively (Liu et al., 2015). The study with the largest sample size in this meta reported that incontinence prevalence was 43% in 3,414 PD patients with mean disease duration for 9 years (Wüllner et al., 2007). All of these suggested PD patients with longer disease duration may experience more urinary incontinence. H&Y score serves as a proxy for disease severity, which was closely correlated with disease duration. The pooled prevalence of urinary incontinence was 20% in PD patients with mild H&Y score, whereas 48% in patients with severe H&Y score in this meta-analysis. The consistent results indicate the severity of the disease should also be considered as a factor affecting prevalence in addition to the course of the disease.
Although severe urinary incontinence in the first 5 years of disease is a red flag of PD diagnosis, long-standing or small amount stress incontinence in women should be excluded. Unexplained urge incontinence was a supportive diagnostic criterion of MSA according to the newest MSA diagnostic (Wenning et al., 2022). These criteria suggest simply functional incontinence alone is not enough to distinguish Parkinson's disease from multisystem atrophy. Of the 21 included studies, there were 13 studies involving urge incontinence. The pooled prevalence of urge incontinence was 32% in PD patients in this meta-analysis. Few studies reported the types of urinary incontinence in PD. Therefore, we should pay attention to the types of urinary incontinence in the analysis of urinary incontinence.
The current study also demonstrated age and gender difference in prevalence of urinary in continence. The pooled prevalence of urinary incontinence was 20% in PD patients with age <65 years, whereas 37% in PD with age ≥ 65 years in this meta-analysis. We found that the mean age was the source of heterogeneity related to the prevalence of urinary incontinence by meta-regression. The average age of 6,054 PD patients in the 21 studies varied from 57.4 to 75 years. In the study with youngest mean age, the prevalence of urinary incontinence was only 7% (Swaminath et al., 2010). These results suggested that age of PD patients contributed to the prevalence variance of urinary incontinence.
We found that the prevalence of urinary incontinence was 31% in the male PD patients, whereas 43% in female patients with PD. These results suggest that both age and gender influence the outcome of estimated prevalence.
The heterogeneity was equally possible to generate from other confounders. In the study of urinary incontinence, the diagnostic methods were not uniform, and the objective indicators are seldom applied. Using urodynamic tests, the pooled prevalence of urinary incontinence was 21%, which was less than that using scales, questionnaires, and definition. It implied that application of scales, questionnaires, and definition may have overestimated the prevalence of urinary incontinence. However, there are only two studies combining urodynamic tests. No studies in this meta-analysis reporting the urinary incontinence of pathological confirmed PD which indicated the scarcity of research in this meta. No significant publication bias of urinary incontinence was found.
The majority of studies were rated as low quality because of small sample sizes and other reason, although the results were robust in sensitivity analysis. Accounting for aforementioned factors, the prevalence results of urinary incontinence should be interpreted with caution.
Voiding symptoms in PD
The pooled prevalence of voiding symptoms was 24%. Prolongation was the most prevalent type of voiding symptoms in PD (41%) followed by the intermittency (30%), urinary retention (27%), dysuria (22%), weak stream (22%), and hesitancy (20%). Some studies found that subclinical detrusor weakness during voiding may also occur in PD (Terayama et al., 2012; Sakakibara et al., 2016; Ogawa et al., 2017). These findings revealed that PD patients had both subclinical and clinical voiding symptoms. There was substantial heterogeneity in prevalence of voiding symptoms and its subtypes (intermittency, dysuria, weak stream, and hesitancy) among studies. The source of heterogeneity could be the limited number of articles, diverse diagnostic tools and different characteristic of participants.
Urinary retention is the most disabling voiding symptoms and often used to differentiate Parkinson's disease from multisystem atrophy. Previous studies have shown that the prevalence of urinary retention and large PVR was 43 and 14% in MSA (Ito et al., 2006; Lee et al., 2018) and PVR volume ≥ 100 ml might be an effective indicator to differentiate PD from MSA (Yamamoto et al., 2017; Lee et al., 2018; Wenning et al., 2022). Unexplained voiding difficulties with PVR volume ≥ 100 ml was one of the core clinical features to identify the clinically established MSA (Wenning et al., 2022). Furthermore, severe urinary retention in the first 5 year of disease is a red flag according to MDS clinical diagnostic criteria for PD.
Our study showed that the prevalence of retention was not low (27%) and the prevalence of large PVR (≥100 ml) was even higher than 4% in PD. Using urodynamic tests, the pooled prevalence of retention was 27% (95% CI 8–46%). Of the overall 14 studies involved urinary retention, the mean disease duration ranged from 1.97 to 12.2 years. One study revealed that the duration of disease in patients without residual urine was lower than PD patients with residual urine (4.6 vs. 7.1 years) (Zhang and Zhang, 2015). Another finding of our study was that the pooled prevalence of urinary retention was 16% in PD with H&Y stage <3, whereas 41% in PD with H&Y stage ≥ 3. The pooled prevalence of urinary retention was 19% in PD patients with age <65 years, whereas 37% in PD by age ≥ 65 years. Furthermore, we found that age was the source of heterogeneity related to the prevalence of urinary retention by meta-regression. Of 1,991 PD in the 14 studies, the median age of participants varied from 59.6 to 70.6 years. One study revealed that the patients without residual urine were younger than patients with residual urine (67.5 vs. 70.1 years) (Zhang and Zhang, 2015). A higher weighted prevalence was calculated in older people than younger people (76 vs. 18%) (Campos-Sousa et al., 2003; Irene, 2019), suggesting PD patients with older age might be more likely to experience urinary retention. Overall, when distinguishing PD from MSA with urinary retention symptoms, we should be more cautious and consider relevant factors such as age, disease duration, and disease severity.
Diagnostic tools influenced prevalence results. The prevalence of urinary retention diagnosed using questionnaire was lower than that using clinical scales and urodynamic tests., There was difference in the prevalence identified by different scales. The pooled prevalence of urinary retention was 21% (using IPSS), 31% (using AUA) and 35% (using SCOPA-AUT), which was similar to 27% (using urodynamic tests).
No significant publication bias of urinary retention was found. The majority of articles evaluated with low-moderate quality were included due to the small number of research, but there was no significant difference between high-moderate and low quality of articles. Therefore, urinary retention requires attention and investigation in PD patients. Further research on mechanisms of urinary retention is also necessary.
Limitations
There were some limitations of the current study constituting an overall weaker level of evidence. First, present meta-analysis might be misleading given the variations in diagnostic tools of LUTS and its subtypes between studies. The heterogeneity of diagnostic tools limited the generalizability of the results. Second, only a small number of studies investigating urinary incontinence or retention were enrolled, which also indicated the scarcity of research on this area. Third, given the effect of geographical location, risk of bias, and population, high heterogeneity was found among the included studies. LUTS is associated with cognitive impairment. However, many articles did not distinguish subjects according to cognitive function. We could not conclude the definitive prevalence of LUTS and its subtypes in patients with PD. Fourth, the variables associate with LUTS, such as the UPDRS scores, laboratory indicators and related treatment methods were not included in most studies. Fifth, there was no studies reporting the prevalence of LUTS in pathological confirmed PD patients. Finally, the majority of studies were rated as low quality, although the results were robust in sensitivity analysis.
Conclusion
This meta-analysis showed that urinary incontinence and retention are not uncommon in PD. The high prevalence of LUTS confirmed the importance of LUTS as a notable non-motor characteristic of PD. LUTS were found in more than one-half of PD patients. It was evident that patients with PD experience significantly more urinary symptoms than age-matched controls. Overall, when distinguishing PD from MSA with urinary incontinence or retention, we should be more cautious and consider relevant factors such as age, disease duration, cognitive function and disease severity. The heterogeneity of diagnostic tools of LUTS and its subtypes limited the generalizability of the results. Therefore, future studies are warranted to address this issue by developing standardized diagnostic tools of LUTS, and the utility of which needs to be validated in larger prospective trials.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
TF conceived and designed the study. F-FL performed the meta analysis and wrote the manuscript. F-FL and Y-SC prepared the draft and figures. All authors have read, revised, and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant Number: 82071422, 81771367, 81901151, 82020108012) and Beijing Municipal Natural Science Foundation (Grand Number: 7212031).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.977572/full#supplementary-material
References
Akkoç, Y., Gök, H., Karapolat, H., Ersöz, M., Sungur, U., Köklü, K., et al. (2017). Assessment of voiding dysfunction in Parkinson's disease: reliability and validity of the Turkish version of the Danish Prostate Symptom Score. Neurourol. Urodyn. 36, 1903–1909. doi: 10.1002/nau.23208
Araki, I., and Kuno, S. (2000). Assessment of voiding dysfunction in Parkinson's disease by the international prostate symptom score. J. Neurol. Neurosurg. Psychiatry. 68, 429–433. doi: 10.1136/jnnp.68.4.429
Barendregt, J. J., Doi, S. A., Lee, Y. Y., Norman, R. E., and Vos, T. (2013). Meta-analysis of prevalence. J. Epidemiol. Community Health. 67, 974–978. doi: 10.1136/jech-2013-203104
Barry, M. J., Fowler, F. Jr., O' Leary, M. P., Bruskewitz, R. C., Holtgrewe, H. L., Mebust, W. K., et al. (1992). The American Urological Association symptom index for benign prostatic hyperplasia. The Measurement Committee of the American Urological Association. J. Urol. 148, 1549–1557. doi: 10.1016/S0022-5347(17)36966-5
Beach, T. G., Adler, C. H., Sue, L. I., Vedders, L., Lue, L., White Iii, C. L., et al. (2010). Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 119, 689–702. doi: 10.1007/s00401-010-0664-3
Bonnet, A. M., Pichon, J., Vidailhet, M., Gouider-Khouja, N., Robain, G., Perrigot, M., et al. (1997). Urinary disturbances in striatonigral degeneration and Parkinson's disease: clinical and urodynamic aspects. Mov. Disord. 12, 509–513. doi: 10.1002/mds.870120406
Braak, H., Sastre, M., Bohl, J. R., de Vos, R. A., and Del Tredici, K. (2007). Parkinson's disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol. 113, 421–429. doi: 10.1007/s00401-007-0193-x
Campos-Sousa, R. N., Quagliato, E., da Silva, B. B., de Carvalho, R. M. Jr., Ribeiro, S. C., and de Carvalho, D. F. (2003). Urinary symptoms in Parkinson's disease: prevalence and associated factors. Arq. Neuropsiquiatr. 61, 359–363. doi: 10.1590/S0004-282X2003000300007
Chaudhuri, K. R., Healy, D. G., and Schapira, A. H. (2006). Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol. 5, 235–245. doi: 10.1016/S1474-4422(06)70373-8
D'Ancona, C., Haylen, B., Oelke, M., Abranches-Monteiro, L., Arnold, E., Goldman, H., et al. (2019). The International Continence Society (ICS) report on the terminology for adult male. Neurourol. Urodyn. 38, 433–477. doi: 10.1002/nau.23897
Farage, M. A., Miller, K. W., Berardesca, E., and Maibach, H. I. (2008). Psychosocial and societal burden of incontinence in the aged population: a review. Arch. Gynecol. Obstet. 277, 285–290. doi: 10.1007/s00404-007-0505-3
Homma, Y., Yoshida, M., Seki, N., Yokoyama, O., Kakizaki, H., Gotoh, M., et al. (2006). Symptom assessment tool for overactive bladder syndrome–overactive bladder symptom score. Urology 68, 318–323. doi: 10.1016/j.urology.2006.02.042
Hughes, A. J., Daniel, S. E., Kilford, L., and Lees, A. J. (1992). Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatry 55, 181–184. doi: 10.1136/jnnp.55.3.181
Irene, R. (2019). Genitourinary dysfunction prevalence in parkinson disease patients. ARS Medica Tomitana 25, 6–10. doi: 10.2478/arsm-2019-0002
Ito, T., Sakakibara, R., Yasuda, K., Yamamoto, T., Uchiyama, T., Liu, Z., et al. (2006). Incomplete emptying and urinary retention in multiple-system atrophy: when does it occur and how do we manage it? Mov. Disord. 21, 816–823. doi: 10.1002/mds.20815
Jain, S. (2011). Multi-organ autonomic dysfunction in Parkinson disease. Parkinsonism Relat. Disord. 17, 77–83. doi: 10.1016/j.parkreldis.2010.08.022
Khoo, T. K., Yarnall, A. J., Duncan, G. W., Coleman, S., O'Brien, J. T., Brooks, D. J., et al. (2013). The spectrum of nonmotor symptoms in early Parkinson disease. Neurology 80, 276–281. doi: 10.1212/WNL.0b013e31827deb74
Kim, J. Y., Song, I. U., Koh, S. B., Ahn, T. B., Kim, S. J., Cheon, S. M., et al. (2017). Validation of the Korean Version of the Scale for Outcomes in Parkinson's Disease-Autonomic. J. Mov. Disord. 10, 29–34. doi: 10.14802/jmd.16057
Koh, S. B., Kim, J. W., Ma, H. I., Ahn, T. B., Cho, J. W., Lee, P. H., et al. (2012). Validation of the korean-version of the nonmotor symptoms scale for Parkinson's disease. J. Clin. Neurol. 8, 276–283. doi: 10.3988/jcn.2012.8.4.276
Lee, E., Yoo, K. Y., Kim, Y., Shin, Y., and Lee, C. (1998). Prevalence of lower urinary tract symptoms in Korean men in a community-based study. Eur. Urol. 33, 17–21. doi: 10.1159/000019529
Lee, Y. H., Lee, J. E., Ryu, D. W., Oh, Y. S., Lee, K. S., Hong, S. H., et al. (2018). Urinary dysfunctions and post-void residual urine in typical and atypical Parkinson diseases. J. Parkinsons Dis. 8, 145–152. doi: 10.3233/JPD-171254
Liu, Z., Uchiyama, T., Sakakibara, R., and Yamamoto, T. (2015). Underactive and overactive bladders are related to motor function and quality of life in Parkinson's disease. Int. Urol. Nephrol. 47, 751–757. doi: 10.1007/s11255-015-0951-y
Martinez-Ramirez, D., Velazquez-Avila, E. S., Almaraz-Espinoza, A., Gonzalez-Cant,ú, A., Vazquez-Elizondo, G., Overa-Posada, D., et al. (2020). Lower urinary tract and gastrointestinal dysfunction are common in early parkinson's disease. Parkinsons Dis. 2020, 1694547. doi: 10.1155/2020/1694547
Mito, Y., Yabe, I., Yaguchi, H., Takei, T., Terae, S., and Tajima, Y. (2018). Relation of overactive bladder with motor symptoms and dopamine transporter imaging in drug-naïve Parkinson's disease. Parkinsonism Relat. Disord. 50, 37–41. doi: 10.1016/j.parkreldis.2018.02.017
Mohammed, E. S., and Ragab, M. M. (2010). Idiopathic Parkinson's disease: lower urinary tract dysfunctions and urodynamic abnormalities. Egypt J. Neurol. Psychiatr. Neurosurg. 47, 381–386. Available online at: https://www.researchgate.net/publication/332544887_Idiopathic_Parkinson%27s_Disease_Lower_Urinary_Tract_Dysfunctions_and_Urodynamic_Abnormalities#fullTextFileContent
Ogawa, T., Sakakibara, R., Kuno, S., Ishizuka, O., Kitta, T., and Yoshimura, N. (2017). Prevalence and treatment of LUTS in patients with Parkinson disease or multiple system atrophy. Nat. Rev. Urol. 14, 79–89. doi: 10.1038/nrurol.2016.254
Oyanagi, K., Wakabayashi, K., Ohama, E., Takeda, S., Horikawa, Y., Morita, T., et al. (1990). Lewy bodies in the lower sacral parasympathetic neurons of a patient with Parkinson's disease. Acta Neuropathol. 80, 558–559. doi: 10.1007/BF00294619
Postuma, R. B., Berg, D., Stern, M., Poewe, W., Olanow, C. W., Oertel, W., et al. (2015). MDS clinical diagnostic criteria for Parkinson's disease. Mov. Disord. 30, 1591–1601. doi: 10.1002/mds.26424
Rana, A. Q., Paul, D. A., Qureshi, A. M., Ghazi, A., Alenezi, S., Rana, M. A., et al. (2015). Association between nocturia and anxiety in Parkinson's disease. Neurol. Res. 37, 563–567. doi: 10.1179/1743132815Y.0000000010
Sakakibara, R., Hattori, T., Uchiyama, T., Kita, K., Asahina, M., Suzuki, A., et al. (2000). Urinary dysfunction and orthostatic hypotension in multiple system atrophy: which is the more common and earlier manifestation? J. Neurol. Neurosurg. Psychiatry. 68, 65–69. doi: 10.1136/jnnp.68.1.65
Sakakibara, R., Nakazawa, K., Uchiyama, T., Yoshiyama, M., Yamanishi, T., and Hattori, T. (2002). Micturition-related electrophysiological properties in the substantia nigra pars compacta and the ventral tegmental area in cats. Auton. Neurosci. 102, 30–38. doi: 10.1016/S1566-0702(02)00180-7
Sakakibara, R., Panicker, J., Finazzi-Agro, E., Iacovelli, V., and Bruschini, H. (2016). A guideline for the management of bladder dysfunction in Parkinson's disease and. Neurourol. Urodyn. 35, 551–563. doi: 10.1002/nau.22764
Sakakibara, R., Shinotoh, H., Uchiyama, T., Sakuma, M., Kashiwado, M., Yoshiyama, M., et al. (2001). Questionnaire-based assessment of pelvic organ dysfunction in Parkinson's disease. Auton. Neurosci. 92, 76–85. doi: 10.1016/S1566-0702(01)00295-8
Sakakibara, R., Tateno, F., Nagao, T., Yamamoto, T., Uchiyama, T., Yamanishi, T., et al. (2014). Bladder function of patients with Parkinson's disease. Int. J. Urol. 21, 638–646. doi: 10.1111/iju.12421
Sakakibara, R., Uchiyama, T., Yamanishi, T., Shirai, K., and Hattori, T. (2008). Bladder and bowel dysfunction in Parkinson's disease. J. Neural. Transm. 115, 443–460. doi: 10.1007/s00702-007-0855-9
Sakushima, K., Yamazaki, S., Fukuma, S., Hayashino, Y., Yabe, I., Fukuhara, S., et al. (2016). Influence of urinary urgency and other urinary disturbances on falls in Parkinson's disease. J. Neurol. Sci. 360, 153–157. doi: 10.1016/j.jns.2015.11.055
Stang, A. (2010). Critical evaluation of the Newcastle-Ottawa scale for the assessment of the. Eur. J. Epidemiol. 25, 603–605. doi: 10.1007/s10654-010-9491-z
Stanković, I., Petrović, I., Pekmezović, T., Marković, V., Stojković, T., Dragašević-Mišković, N., et al. (2019). Longitudinal assessment of autonomic dysfunction in early Parkinson's disease. Parkinsonism Relat. Disord. 66, 74–79. doi: 10.1016/j.parkreldis.2019.07.008
Stocchi, F., Carbone, A., Inghilleri, M., Monge, A., Ruggieri, S., Berardelli, A., et al. (1997). Urodynamic and neurophysiological evaluation in Parkinson's disease and multiple system atrophy. J. Neurol. Neurosurg. Psychiatry 62, 507–511. doi: 10.1136/jnnp.62.5.507
Swaminath, P. V., Ragothaman, M., Koshy, S., Sarangmath, N., Adhyam, M., Subbakrishna, D. K., et al. (2010). Urogenital symptoms in Parkinson's disease and multiple system atrophy-Parkinsonism: at onset and later. J. Assoc. Physicians India 58, 86–90.
Takahashi, K., Tanaka, T., Yoshizawa, Y., Fujisaki-Sueda-Sakai, M., Son, B. K., and Iijima, K. (2022). Lower urinary tract symptoms and functional ability in older adults: a community-based cross-sectional study. BMJ Open 12, e054530. doi: 10.1136/bmjopen-2021-054530
Tateno, F., Sakakibara, R., Ogata, T., Aiba, Y., Takahashi, O., and Sugiyama, M. (2021). The relationship between lower urinary tract function and (123)ioflupane scintigraphy in drug-naive Parkinson's disease. Auton. Neurosci. 233, 102813. doi: 10.1016/j.autneu.2021.102813
Tateno, F., Sakakibara, R., Ogata, T., Kishi, M., Tsuyusaki, Y., Takahashi, O., et al. (2015). Lower urinary tract function in dementia with Lewy bodies (DLB). Mov. Disord. 30, 411–415. doi: 10.1002/mds.25985
Terayama, K., Sakakibara, R., Ogawa, A., Haruta, H., Akiba, T., Nagao, T., et al. (2012). Weak detrusor contractility correlates with motor disorders in Parkinson's disease. Mov. Disord. 27, 1775–1780. doi: 10.1002/mds.25225
Tkaczynska, Z., Becker, S., Maetzler, W., Timmers, M., Van Nueten, L., Sulzer, P., et al. (2020). Executive function is related to the urinary urgency in non-demented patients with parkinson's disease. Front. Aging Neurosci. 12, 55. doi: 10.3389/fnagi.2020.00055
Tkaczynska, Z., Pilotto, A., Becker, S., Gräber-Sultan, S., Berg, D., and Liepelt-Scarfone, I. (2017). Association between cognitive impairment and urinary dysfunction in Parkinson's disease. J. Neural. Transm. 124, 543–550. doi: 10.1007/s00702-017-1690-2
Uchiyama, T., Sakakibara, R., Yamamoto, T., Ito, T., Yamaguchi, C., Awa, Y., et al. (2011). Urinary dysfunction in early and untreated Parkinson's disease. J. Neurol. Neurosurg. Psychiatry 82, 1382–1386. doi: 10.1136/jnnp.2011.241075
Vaughan, C. P., Juncos, J. L., Trotti, L. M., Johnson Ii, T. M., and Bliwise, D. L. (2013). Nocturia and overnight polysomnography in Parkinson disease. Neurourol. Urodyn. 32, 1080–1085. doi: 10.1002/nau.22365
Wakabayashi, K., and Takahashi, H. (1997). Neuropathology of autonomic nervous system in Parkinson's disease. Eur. Neurol. 38, 2–7. doi: 10.1159/000113469
Wenning, G. K., Scherfler, C., Granata, R., Bösch, S., Verny, M., Chaudhuri, K. R., et al. (1999). Time course of symptomatic orthostatic hypotension and urinary incontinence in patients with postmortem confirmed parkinsonian syndromes: A clinicopathological study. J. Neurol. Neurosurg. Psychiatry. 67, 620–623. doi: 10.1136/jnnp.67.5.620
Wenning, G. K., Stankovic, I., Vignatelli, L., Fanciulli, A., Calandra-Buonaura, G., Seppi, K., et al. (2022). The movement disorder society criteria for the diagnosis of multiple system atrophy. Mov. Disord. 37, 1131–1148. doi: 10.1002/mds.29005
Williams, J. W., Plassman, B. L., Burke, J., and Benjamin, S. (2010). Preventing Alzheimer's disease and cognitive decline. Evid. Rep. Technol. Assess. 193, 1–727. doi: 10.1037/e554772010-001
Winge, K., Friberg, L., Werdelin, L., Nielsen, K., and Stimpel, H. (2005). Relationship between nigrostriatal dopaminergic degeneration, urinary symptoms, and bladder control in Parkinson's disease. Eur. J. Neurol. 12, 842–850. doi: 10.1111/j.1468-1331.2005.01087.x
Winge, K., Skau, A. M., Stimpel, H., Nielsen, K. K., and Werdelin, L. (2006). Prevalence of bladder dysfunction in Parkinsons disease. Neurourol. Urodyn. 25, 116–122. doi: 10.1002/nau.20193
Wüllner, U., Schmitz-Hübsch, T., Antony, G., Fimmers, R., Spottke, A., Oertel, W. H., et al. (2007). Autonomic dysfunction in 3414 Parkinson's disease patients enrolled in the German Network on Parkinson's disease (KNP e.V.): the effect of ageing. Eur. J. Neurol. 14, 1405–1408. doi: 10.1111/j.1468-1331.2007.01982.x
Xie, T., Kang, U., Kuo, S., Poulopoulos, M., Greene, P., and Fahn, S. (2015). Comparison of clinical features in pathologically confirmed PSP and MSA patients. NPJ Parkinsons Dis. 21, 15007. doi: 10.1038/npjparkd.2015.7
Xu, D., Han, S., Wang, J., and Feng, J. (2019). Relationship between lower urinary tract dysfunction and clinical features in Chinese parkinson's disease patients. Parkinsons Dis. 2019, 6820937. doi: 10.1155/2019/6820937
Yamamoto, T., Asahina, M., Yamanaka, Y., Uchiyama, T., Hirano, S., Fuse, M., et al. (2017). The utility of post-void residual volume versus sphincter electromyography to distinguish between multiple system atrophy and Parkinson's disease. PLoS ONE 12, e0169405. doi: 10.1371/journal.pone.0169405
Yamamoto, T., Sakakibara, R., Hashimoto, K., Nakazawa, K., Uchiyama, T., Liu, Z., et al. (2005). Striatal dopamine level increases in the urinary storage phase in cats: an in vivo microdialysis study. Neuroscience 135, 299–303. doi: 10.1016/j.neuroscience.2005.06.007
Yamamoto, T., Tateno, F., Sakakibara, R., Furukawa, S., Asahina, M., Uchiyama, T., et al. (2016). Urinary dysfunction in progressive supranuclear palsy compared with other parkinsonian disorders. PLoS ONE 11, e0149278. doi: 10.1371/journal.pone.0149278
Keywords: Parkinson's disease, urinary incontinence, urinary retention, meta-analysis, review, prevalence, lower urinary tract symptoms (LUTS)
Citation: Li F-F, Cui Y-S, Yan R, Cao S-S and Feng T (2022) Prevalence of lower urinary tract symptoms, urinary incontinence and retention in Parkinson's disease: A systematic review and meta-analysis. Front. Aging Neurosci. 14:977572. doi: 10.3389/fnagi.2022.977572
Received: 24 June 2022; Accepted: 23 August 2022;
Published: 12 September 2022.
Edited by:
Jifeng Guo, Xiangya Hospital, Central South University, ChinaReviewed by:
Milton Cesar Biagioni, UCB Pharma, BelgiumElisabetta Dell'Anna, Consultant, Milano, Italy
Copyright © 2022 Li, Cui, Yan, Cao and Feng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Feng, Ynhia3lqc0BzaW5hLmNvbQ==
 Fang-Fei Li
Fang-Fei Li Yu-Sha Cui
Yu-Sha Cui Rui Yan
Rui Yan Shuang-Shuang Cao1,2
Shuang-Shuang Cao1,2 Tao Feng
Tao Feng