
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Aging Neurosci. , 08 September 2022
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.969875
 Valeria Isella1,2*†
Valeria Isella1,2*† Daniele Licciardo2,3†
Daniele Licciardo2,3† Francesca Ferri2,3
Francesca Ferri2,3 Cinzia Crivellaro4
Cinzia Crivellaro4 Sabrina Morzenti5
Sabrina Morzenti5 Ildebrando Appollonio1,2,3
Ildebrando Appollonio1,2,3 Carlo Ferrarese1,2,3
Carlo Ferrarese1,2,3Background: Reduced phonemic fluency is extremely frequent in progressive supranuclear palsy (PSP), but its neural correlate is yet to be defined.
Objective: We explored the hypothesis that poor fluency in PSP might be due to neurodegeneration within a dominant frontal circuit known to be involved in speech fluency, including the opercular area, the superior frontal cortex (BA6), and the frontal aslant tract connecting these two regions.
Methods: We correlated performance on a letter fluency task (F, A, and S, 60 s for each letter) with brain metabolism as measured with Fluoro-deoxy-glucose Positron Emission Tomography, using Statistical Parametric Mapping, in 31 patients with PSP.
Results: Reduced letter fluency was associated with significant hypometabolism at the level of left BA6.
Conclusion: Our finding is the first evidence that in PSP, as in other neurogical disorders, poor self-initiated, effortful verbal retrieval appears to be linked to dysfunction of the dominant opercular-aslant-BA6 circuit.
The ability to retrieve words upon a phonemic cue is a cognitively complex task that relies mostly on executive functions (Lezak et al., 2012; Shao et al., 2014) and depends predominantly on the integrity of the frontal lobes. Studies and meta-analyses carried out in healthy subjects with structural and functional Magnetic Resonance Imaging (MRI) or neurophysiological techniques have in fact highlighted the prominent involvement of the left inferior/middle frontal gyrus and insula and of bilateral anterior cingulate in phonemic fluency (PF) tasks (Devlin et al., 2003; Shibahara, 2004; Tremblay et al., 2004; Iyer et al., 2005; Costafreda et al., 2006; Heim et al., 2008; Meinzer et al., 2009, 2012; Cattaneo et al., 2011; Katzev et al., 2013; Wagner et al., 2014; Yuan and Raz, 2014; Smirni et al., 2017; Ghanavati et al., 2019; Vonk et al., 2019; Oswald et al., 2022). Neuroimaging studies in patients with degenerative cognitive or movement disorders such as Alzheimer’s disease (Melrose et al., 2009; Clark et al., 2014; Rodríguez-Aranda et al., 2016; Jones et al., 2019), corticobasal syndrome (Parmera et al., 2021), and frontotemporal dementia and primary progressive aphasias (Robinson, 2006; Crescentini et al., 2008; Laisney et al., 2009; Cook et al., 2014; Robinson et al., 2015; Suppa et al., 2020; Riello et al., 2022) have generally confirmed that impairment of PF is associated with left inferior frontal dysfunction, but have also indicated the involvement of other left cortical and subcortical structures like the temporal lobe (Laisney et al., 2009; Libon et al., 2009; Cook et al., 2014; Rodríguez-Aranda et al., 2016; Parmera et al., 2021; Riello et al., 2022), the superior medial frontal cortex (Robinson, 2006; Robinson et al., 2015) and the basal ganglia (Robinson, 2006; Crescentini et al., 2008; Melrose et al., 2009; Clark et al., 2014), and of right, mainly frontal, regions (Libon et al., 2009; Melrose et al., 2009; Clark et al., 2014; Riello et al., 2022).
Among all these neural loci, a complex of left frontal areas has consistently shown a relationship with PF in both healthy individuals and neurological, not only degenerative, patients (Robinson et al., 2012; Catani et al., 2013; Chapados and Petrides, 2013; Kinoshita et al., 2015; Rodríguez-Aranda et al., 2016; Li et al., 2017; Miró-Padilla et al., 2017; Blecher et al., 2019; Dick et al., 2019; Jones et al., 2019; Vonk et al., 2019; Keser et al., 2020; Suppa et al., 2020; La Corte et al., 2021; Parmera et al., 2021; Pinson et al., 2022), which includes the inferior gyrus—pars opercularis—and insula, the superior gyrus corresponding to BA6 and comprising the supplementary motor area (SMA), and the white matter tract that connects these two inferior and superior frontal regions, i.e., the frontal aslant tract (FAT) (Catani et al., 2012).
Reduced PF is one of the cognitive hallmarks of progressive supranuclear palsy (PSP). It is present in up to 85% of PSP patients irrespective of the specific phenotype, often from a very early disease stage (Kertesz and McMonagle, 2010; Burrell et al., 2014; Gerstenecker, 2017; Pellicano et al., 2017; Peterson et al., 2021), and appears to be also quite specific: most of the studies that compared PF performance in patients with PSP, Alzheimer’s disease or extrapyramidal disorders like Parkinson’s disease, corticobasal syndrome and multiple system atrophy have shown more frequent and severe impairment in PSP (Cordato et al., 2006; Rittman et al., 2013; Lee et al., 2016; Gerstenecker, 2017; Pellicano et al., 2017; Peterson et al., 2021).
Since the frontal lobes are one of the main loci of neurodegeneration in PSP (Stamelou et al., 2021), it is not surprising that a primarily frontal ability like PF is often impaired in this disorder. Nevertheless, not many studies have up to now investigated the specific neural correlate of Letter fluency in individuals with PSP. The current study was aimed at investigating the hypothesis that neurodegeneration within the left frontal opercular-aslant-BA6 complex underlying speech fluency could account for reduced PF in these patients, using 18-Fluoro-Deoxy-Glucose Positron Emission Tomography (FDG-PET). A hint that this might be the case has come from a study that found a relationship between poor Letter fluency and abundant tau deposits in the superior frontal cortex in the brains of 11 subjects with PSP-RS (Schofield et al., 2012). Another study that investigated the neural substrate of poor fluency in this disease found a relation between PF and midbrain atrophy on MRI, but did not explore the frontal lobes (Luca et al., 2021). In our PSP sample we expected to find reduced FDG uptake in regions typically affected by degenerative processes in this disorder (the frontal cortex, basal ganglia, and midbrain), and lower left dorsal inferior and superior frontal metabolism for lower fluency scores.
Participants were recruited from the memory clinic and movement disorders clinic of San Gerardo Hospital, Monza. Inclusion criteria were a diagnosis of PSP according to standardized criteria (Höglinger et al., 2017) and Italian as native language. We excluded patients with a primary progressive aphasia presentation, in order to avoid the confounding effect of language impairment on the fluency task. Other exclusion criteria were moderate-to-severe vascular burden on brain imaging, and history of other neurological disorders, major psychiatric diseases, brain injury, substance abuse, developmental intellectual, or cognitive disorders.
All participants signed an informed consent before taking part in the study. The study was conducted according to the guidelines of the World Medical Association Declaration of Helsinki, and approved by our institution’s ethics committee, Comitato Etico Brianza.
Patients underwent a general neuropsychological battery that included the MiniMental State Examination [MMSE (Folstein et al., 1975; Measso et al., 1993)], and tests of selective attention [Attentional Matrices (Spinnler and Tognoni, 1987)], short-term memory [Digit span (Monaco et al., 2013)], verbal long term memory [Rey Auditory Verbal Learning Test, RAVLT (Carlesimo et al., 1996)], language production [Category fluency (Zarino et al., 2014)], visuo-constuctional abilities [copy of Rey-Osterrieth Complex Figure, ROCF (Caffarra et al., 2002)], limb apraxia [De Renzi test of Ideomotor Apraxia, IMA (De Renzi et al., 1980)], and executive functions [Frontal Assessment Battery (Appollonio et al., 2005), Raven Colored Progressive Matrices (Basso et al., 1987)]. Mood and behavior were assessed with the Neuropsychiatric Inventory (Cummings, 1997).
The Letter fluency test (Costa et al., 2014) was administered as part of the general neuropsychological battery. In this test Patients are instructed to produce words starting with letters F, A, and S, avoiding proper nouns like names of people or places, and numbers, in three 60-s trials. Repetitions, perseverations and series of the same word with a different suffix are considered errors. The test score is the sum of all correct words produced for all three letters.
18-Fluoro-Deoxy-Glucose Positron Emission Tomography scans were performed on a General Electric Discovery LS PET/CT scanner on average 2.9 ± 3.6 months within cognitive testing. After acquisition of CT images for attenuation correction, PET images were acquired for 15 min, with a thickness of 3.27 mm and a matrix of 128 × 128 pixels, and reconstructed following an ordered subset expectation maximization algorithm.
Processing of images was performed with Statistical Parametric Mapping (SPM) 8 (Wellcome Department of Imaging Neuroscience, London, United Kingdom)1 running on MATLAB R2015a (MathWorks Inc., Sherborn, MA, United States). Images were reoriented along the anterior–posterior commissure, spatially normalized to the Montreal Neurological Institute (MNI) reference space using an FDG-PET dementia-specific template (Della Rosa et al., 2014), and smoothed with an isotropic 3D Gaussian kernel of 16 mm FWHM.
Progressive supranuclear palsy patients’ scans were compared with scans from 30 neurologically healthy controls (disease-free oncologic patients undergoing PET for disease staging, 14 women, with a mean age of 71.3 years ± 7.7 and a mean MMSE score of 28.9 ± 1.2), using 2-sample t-test, including age and sex as covariates.
Association between brain metabolism and score on the letter fluency task and on copy of ROCF was assessed only within the group of PSP patients using “Multiple regression” in SPM, including age and sex as covariates of no interest. In order to control for the risk of a non-causal relationship, linked to the fact that cognition and brain metabolism are both declining in neurodegenerative patients, we also correlated FDG uptake with a neuropsychological task with no presumed relationship with the dominant frontal lobe, i.e., copy of ROCF.
Significance threshold was set at p < 0.05 Family Wise Error (FWE)-corrected or p < 0.001 uncorrected, and minimum cluster size was set at 100 voxels. Anatomical labeling of significant clusters was performed with Talairach and Automatic Labeling atlases integrated in SPM8 toolbox WFU_PickAtlas.
The study sample was composed by 31 PSP patients, whose socio-demographic and neurological features are shown in Table 1. The most prevalent phenotype was PSP with predominant frontal presentation (n. 12/31, 38.7%), followed by Richardson’s Syndrome (n. 10, 32.3%), PSP with predominant parkinsonism (n. 8, 25.8%) and PSP with predominant postural instability (n. 1, 3.2%). Nearly all patients showed cognitive/behavioral frontal symptoms and/or signs of parkinsonism, and approximately 70% showed postural instability and/or ocular motor dysfunction, while language deficits and apraxia were less frequent. Biomarkers for amyloid status were available in a minority of cases [n. 9, 29.0%, cerebrospinal fluid (CSF) in eight, PET with amyloid-tracer in one], and were all indicative of non-amyloid pathophysiology.

Table 1. Sociodemographic and clinical characteristics of the progressive supranuclear palsy (PSP) sample.
The group’s general neuropsychological profile and performance on the Letter fluency test are reported in Table 2. MMSE scores indicated that most patients had mild global cognitive impairment; the largest number of abnormal scores, based on published norms, was observed for copy of ROCF and executive and attentional tasks. More than 70% of patients showed an abnormal performance on Letter fluency.
We found no significant difference in sex distribution (x2 = 0.015, p = 1.000) and age (t = 1.547, p = 0.127) between the PSP group and the neuroimaging control group. In comparison with controls, patients with PSP showed extensive bilateral hypometabolism in the dorsolateral and mesial prefrontal cortex, inferior frontal and fronto-temporal regions, basal ganglia, and midbrain, at p < 0.05 FWE-corrected (Figure 1; see also Supplementary Table 1 for peak coordinates, clusters extent, and t and p values resulting from SPM analysis).
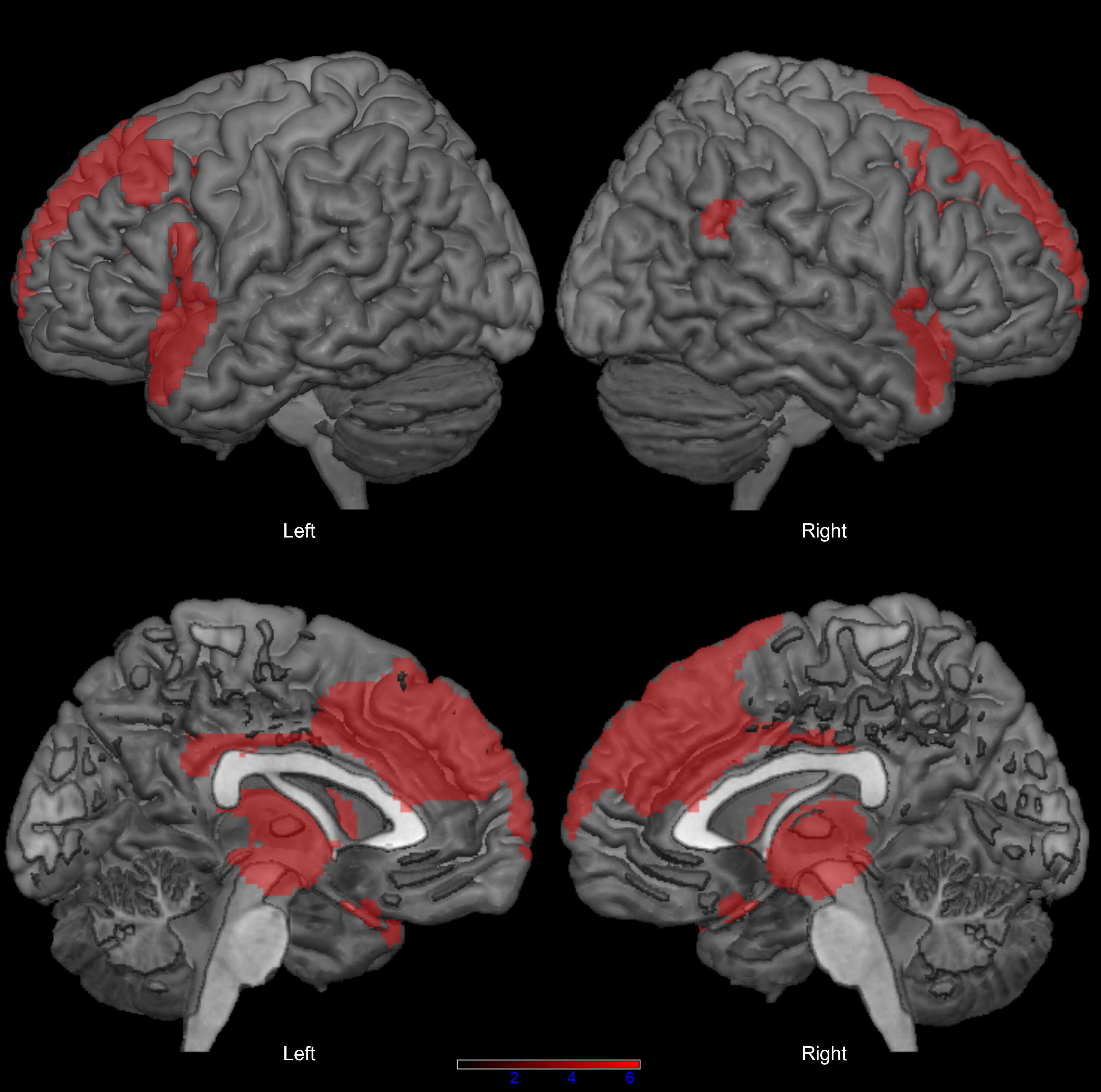
Figure 1. Distribution of hypometabolism in patients with progressive supranuclear palsy (PSP) compared with healthy controls (p < 0.05 family-wise-error corrected, minimum cluster size: 100 voxels).
Figure 2 shows the clusters of lower brain metabolism associated with lower scores on PF (in red) and copy of ROCF (in blue), both significant at p < 0.001 uncorrected. The neurometabolic correlate of PF performance was a 1,544 voxel cluster lateralized to the left hemisphere, and involving mainly the SMA (BA6) and the lateral surface of the superior-middle frontal gyri. The neurometabolic correlate of ROCF performance was a 1,910 voxel cluster encompassing the superior parietal cortex bilaterally. Supplementary Table 2 reports details for both SPM analyses.
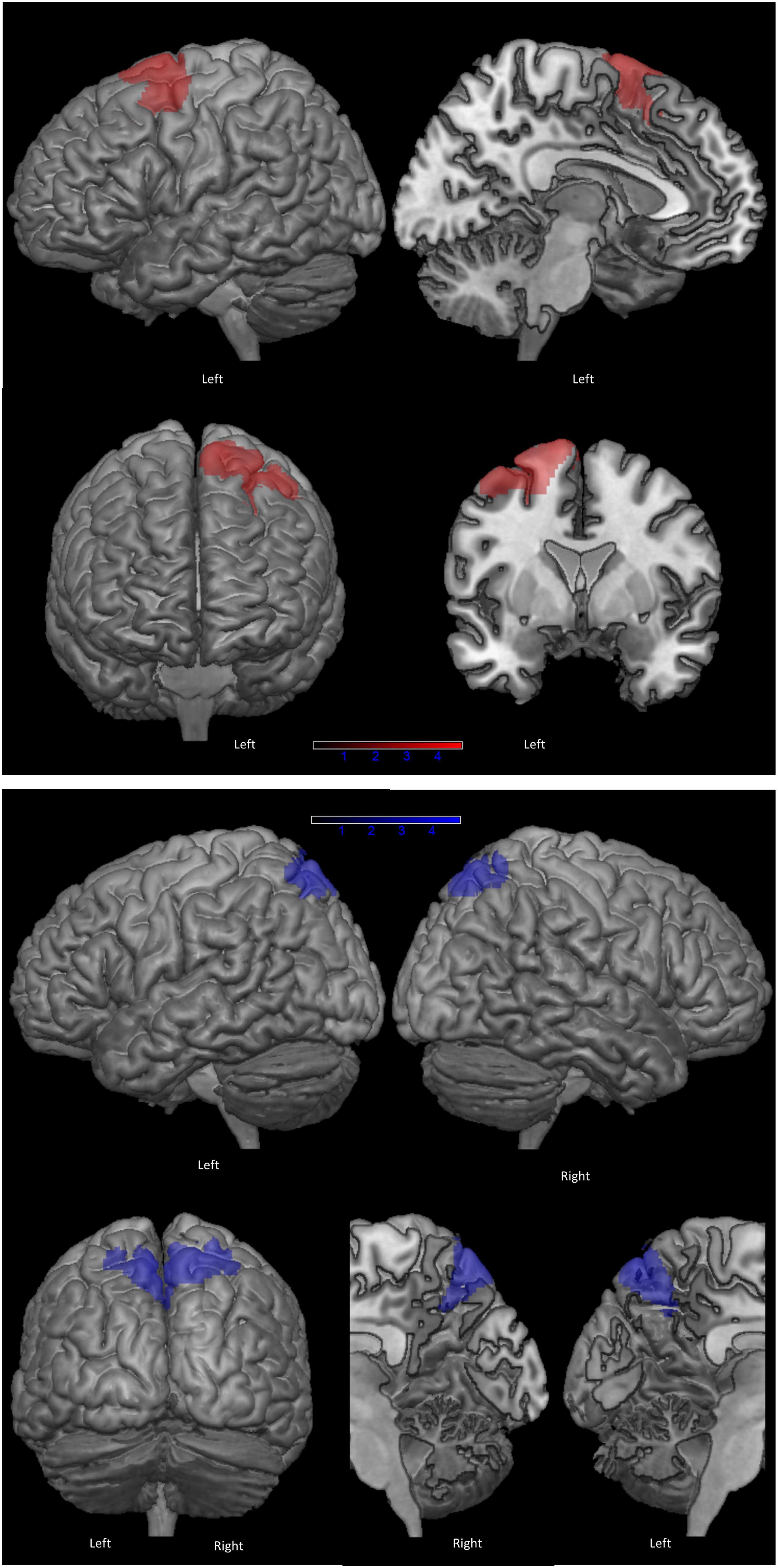
Figure 2. Clusters of hypometabolism correlated with phonemic fluency (in red) and with copy of Rey-Osterrieth Complex Figure (ROCF), as control task (in blue) (p < 0.001 uncorrected, minimum cluster size: 100 voxels).
Impairment of PF is extremely common and a very early sign of cognitive dysfunction in PSP. Although more frequent in Richardson’s Syndrome (Burrell et al., 2014; Peterson et al., 2021), and exacerbated by speech deficits in PSP-related progressive aphasia, it is present in most PSP phenotypes (Kertesz and McMonagle, 2010; Burrell et al., 2014; Gerstenecker, 2017; Peterson et al., 2021). Indeed the Movement Disorders Society criteria have cataloged poor letter fluency among the core frontal cognitive features of the disease (Höglinger et al., 2017). Neuroimaging studies in patients with focal brain damage or neurodegenerative disorders and in young and old healthy individuals have consistently shown a relationship between poor PF and left frontal dysfunction, in particular within two sets of regions: (i) the inferior gyrus (BAs) (Kertesz and McMonagle, 2010; Gerstenecker, 2017; Peterson et al., 2021) and insula, and (ii) the middle gyrus (BA) (Burrell et al., 2014), lateral and mesial superior gyrus including the SMA (BA6), and anterior cingulate (BA9) (Chapados and Petrides, 2013; Miró-Padilla et al., 2017; Vonk et al., 2019; Pinson et al., 2022). Importantly, these opercularis and superior frontal regions are interconnected through the FAT (Catani et al., 2012), whose damage has been shown to account for decreased verbal fluency in various neurological disorders (Catani et al., 2013; Chapados and Petrides, 2013; Kinoshita et al., 2015; Li et al., 2017; Blecher et al., 2019; Dick et al., 2019; Keser et al., 2020; La Corte et al., 2021; Pinson et al., 2022).
Results of the current study suggest that dysfunction within this left opercular-aslant-BA6 complex accounts for impairment of PF also in PSP. In agreement with the autopsy study mentioned in the Introduction, which showed a relationship between a higher degree of tau deposits in the left superior frontal cortex and worse fluency (Schofield et al., 2012), our correlation analysis between Letter fluency and brain metabolism in 31 patients with PSP identified a very definite locus of dysfunction centered around left BA6, and including both the SMA and the lateral superior frontal cortex. The same analysis on copy of ROCF yielded a different correlate [placed in the superior parietal cortex, known to be involved in visuoconstructional abilities (Raimo et al., 2021)], ruling out the possibility that the association between PF and BA6 was coincidental, i.e., due to a parallel, but causally unrelated, progression of cognitive impairment and hypometabolism.
The dominant mesial and lateral superior frontal cortex, and the SMA in particular, is thought to modulate the activity of the pars opercularis of the inferior frontal gyrus, via the FAT, during speech production tasks involving self-initiation, effortful retrieval, competition resolution, and monitoring (Alario et al., 2006; Catani et al., 2013; Kinoshita et al., 2015; Dick et al., 2019; Ardila, 2020). All functions engaged in Letter fluency, which relies on response initiation and inhibition, development and implementation of a lexical retrieval strategy, shifting, and output supervision (Lezak et al., 2012; Shao et al., 2014).
As the frontal lobes are the main cortical locus of neurodegeneration in PSP (Williams et al., 2007; Dickson et al., 2010), the dysfunction of the opercular-aslant-BA6 complex is not surprising, and probably has a multifactorial origin: tau deposits, neuronal and axonal loss, and also glial pathology are all typical findings in PSP brains (Zhukareva et al., 2006; Williams et al., 2007; Dickson et al., 2010). With the present study we have demonstrated the presence of synaptic dysfunction at the level of the superior frontal cortex related to poor PF. In the past, structural imaging studies showed diffuse white matter abnormalities in PSP (Agosta et al., 2010; Sajjadi et al., 2013), but the direct involvement of the FAT is yet to be investigated.
Future research will also have to overcome some of the limitations of our study. First of all, our sample was large enough for detecting an extensive area of significantly reduced metabolism associated with poor PF, but these results will have to be validated in a more numerous population, ideally including neuropathology-confirmed cases, or at least a higher number of biomarker-confirmed diagnoses. Our sample’s neurological and neuropsychological profile and FDG-PET pattern were very typical for PSP (and in all patients with a biomarker results ruled out amyloid), but pathology would guarantee the highest degree of diagnostic certainty (Höglinger et al., 2017).
In addition to contributing to knowledge about the source of reduced fluency in PSP, we believe that our findings also have potentially useful implications for the clinical management of the disease. Firstly, we have provided evidence in support of the specificity of Letter fluency as a tool for the assessment of frontal executive dysfunction in this disorder. Secondly, we have better defined the anatomical target for transcranial stimulation techniques, which are starting to show promise for treating fluency deficits in PSP (Madden et al., 2019; Valero-Cabré et al., 2019), a disease that is still completely lacking effective pharmacotherapy.
Study data are available from the corresponding author upon reasonable request. Requests to access the datasets should be directed to VI, dmFsZXJpYS5pc2VsbGFAdW5pbWliLml0.
The studies involving human participants were reviewed and approved by Comitato Etico Brianza. The patients/participants provided their written informed consent to participate in this study.
VI conceived and organized the research project, designed and executed the statistical analysis, and wrote the manuscript draft. DL organized and executed the research project, executed the statistical analysis, and wrote the manuscript draft. FF and SM executed the research project and reviewed the draft. CC organized the research project and reviewed the draft. IA and CF conceived and organized the research project and reviewed the draft. All authors contributed to the manuscript and approved the final version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.969875/full#supplementary-material
Agosta, F., Kostić, V. S., Galantucci, S., Mesaroš, Š, Svetel, M., Pagani, E., et al. (2010). The in vivo distribution of brain tissue loss in Richardson’s syndrome and PSP-parkinsonism: A VBM-DARTEL study. Eur. J. Neurosci. 32, 640–647. doi: 10.1111/j.1460-9568.2010.07304.x
Alario, F. X., Chainay, H., Lehericy, S., and Cohen, L. (2006). The role of the supplementary motor area (SMA) in word production. Brain Res. 1076, 129–143. doi: 10.1016/j.brainres.2005.11.104
Appollonio, I., Leone, M., Isella, V., Piamarta, F., Consoli, T., Villa, M. L., et al. (2005). The frontal assessment battery (FAB): Normative values in an Italian population sample. Neurol. Sci. 26, 108–116. doi: 10.1007/s10072-005-0443-4
Ardila, A. (2020). Supplementary motor area aphasia revisited. J. Neuroling. 54:100888. doi: 10.1016/j.jneuroling.2020.100888
Basso, A., Capitani, E., and Laiacona, M. (1987). Raven’s coloured progressive matrices: Normative values on 305 adult normal controls. Funct. Neurol. 2, 189–194.
Blecher, T., Miron, S., Schneider, G. G., Achiron, A., and Ben-Shachar, M. (2019). Association between white matter microstructure and verbal fluency in patients with multiple sclerosis. Front. Psychol. 10:1607. doi: 10.3389/fpsyg.2019.01607
Burrell, J. R., Hodges, J. R., and Rowe, J. B. (2014). Cognition in corticobasal syndrome and progressive supranuclear palsy: A review. Mov. Disord. 29, 684–693. doi: 10.1002/mds.25872
Caffarra, P., Vezzadini, G., Dieci, F., Zonato, F., and Venneri, A. (2002). Rey-Osterrieth complex figure: Normative values in an Italian population sample. Neurol. Sci. 22, 443–447. doi: 10.1007/s100720200003
Carlesimo, G. A., Caltagirone, C., Gainotti, G., Facida, L., Gallassi, R., Lorusso, S., et al. (1996). The mental deterioration battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. Eur. Neurol. 36, 378–384. doi: 10.1159/000117297
Catani, M., Dell’Acqua, F., Vergani, F., Malik, F., Hodge, H., Roy, P., et al. (2012). Short frontal lobe connections of the human brain. Cortex 48, 273–291. doi: 10.1016/j.cortex.2011.12.001
Catani, M., Mesulam, M. M., Jakobsen, E., Malik, F., Martersteck, A., Wieneke, C., et al. (2013). A novel frontal pathway underlies verbal fluency in primary progressive aphasia. Brain 136, 2619–2628. doi: 10.1093/brain/awt163
Cattaneo, Z., Pisoni, A., and Papagno, C. (2011). Transcranial direct current stimulation over Broca’s region improves phonemic and semantic fluency in healthy individuals. Neuroscience 183, 64–70. doi: 10.1016/j.neuroscience.2011.03.058
Chapados, C., and Petrides, M. (2013). Impairment only on the fluency subtest of the frontal assessment battery after prefrontal lesions. Brain 136, 2966–2978. doi: 10.1093/brain/awt228
Clark, D. G., Wadley, V. G., Kapur, P., DeRamus, T. P., Singletary, B., Nicholas, A. P., et al. (2014). Lexical factors and cerebral regions influencing verbal fluency performance in MCI. Neuropsychologia 54, 98–111. doi: 10.1016/j.neuropsychologia.2013.12.010
Cook, P. A., McMillan, C. T., Avants, B. B., Peelle, J. E., Gee, J. C., and Grossman, M. (2014). Relating brain anatomy and cognitive ability using a multivariate multimodal framework. Neuroimage 99, 477–486. doi: 10.1016/j.neuroimage.2014.05.008
Cordato, N. J., Halliday, G. M., Caine, D., and Morris, J. G. L. (2006). Comparison of motor, cognitive, and behavioral features in progressive supranuclear palsy and Parkinson’s disease. Mov. Disord. 21, 632–638. doi: 10.1002/mds.20779
Costa, A., Bagoj, E., Monaco, M., Zabberoni, S., De Rosa, S., Papantonio, A. M., et al. (2014). Standardization and normative data obtained in the Italian population for a new verbal fluency instrument, the phonemic/semantic alternate fluency test. Neurol. Sci. 35, 365–372. doi: 10.1007/s10072-013-1520-8
Costafreda, S. G., Fu, C. H. Y., Lee, L., Everitt, B., Brammer, M. J., and David, A. S. (2006). A systematic review and quantitative appraisal of fMRI studies of verbal fluency: Role of the left inferior frontal gyrus. Hum. Brain Mapp. 27, 799–810. doi: 10.1002/hbm.20221
Crescentini, C., Lunardelli, A., Mussoni, A., Zadini, A., and Shallice, T. (2008). A left basal ganglia case of dynamic aphasia or impairment of extra-language cognitive processes? Neurocase 14, 184–203. doi: 10.1080/13554790802108380
Cummings, J. L. (1997). The neuropsychiatric inventory: Assessing psychopathology in dementia patients. Neurology 48(5 Suppl. 6) S10–S16. doi: 10.1212/WNL.48.5_Suppl_6.10S
De Renzi, E., Motti, F., and Nichelli, P. (1980). Imitating gestures: A quantitative approach to ideomotor apraxia. Arch. Neurol. 37, 6–10. doi: 10.1001/archneur.1980.00500500036003
Della Rosa, P. A., Cerami, C., Gallivanone, F., Prestia, A., Caroli, A., Castiglioni, I., et al. (2014). A standardized [18F]-FDG-PET template for spatial normalization in statistical parametric mapping of dementia. Neuroinformatics 12, 575–593. doi: 10.1007/s12021-014-9235-4
Devlin, J. T., Matthews, P. M., and Rushworth, M. F. S. (2003). Semantic processing in the left inferior prefrontal cortex: A combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J. Cogn. Neurosci. 15, 71–84. doi: 10.1162/089892903321107837
Dick, A. S., Garic, D., Graziano, P., and Tremblay, P. (2019). The frontal aslant tract (FAT) and its role in speech, language and executive function. Cortex 111, 148–163. doi: 10.1016/j.cortex.2018.10.015
Dickson, D. W., Ahmed, Z., Algom, A. A., Tsuboi, Y., and Josephs, K. A. (2010). Neuropathology of variants of progressive supranuclear palsy. Curr. Opin. Neurol. 23, 394–400. doi: 10.1097/WCO.0b013e32833be924
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Gerstenecker, A. (2017). The neuropsychology (Broadly Conceived) of multiple system atrophy, progressive supranuclear palsy, and corticobasal degeneration. Arch. Clin. Neuropsychol. 32, 861–875. doi: 10.1093/arclin/acx093
Ghanavati, E., Salehinejad, M. A., Nejati, V., and Nitsche, M. A. (2019). Differential role of prefrontal, temporal and parietal cortices in verbal and figural fluency: Implications for the supramodal contribution of executive functions. Sci. Rep. 9:3700. doi: 10.1038/s41598-019-40273-7
Heim, S., Eickhoff, S. B., and Amunts, K. (2008). Specialisation in Broca’s region for semantic, phonological, and syntactic fluency? Neuroimage 40, 1362–1368. doi: 10.1016/j.neuroimage.2008.01.009
Höglinger, G. U., Respondek, G., Stamelou, M., Kurz, C., Josephs, K. A., Lang, A. E., et al. (2017). Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 32, 853–864. doi: 10.1002/mds.26987
Iyer, M. B., Mattu, U., Grafman, J., Lomarev, M., Sato, S., and Wassermann, E. M. (2005). Safety and cognitive effect of frontal DC brain polarization in healthy individuals. Neurology 64, 872–875. doi: 10.1212/01.WNL.0000152986.07469.E9
Jones, S. E., Idris, A., Bullen, J. A., Miller, J. B., and Banks, S. J. (2019). Relationship between cortical thickness and fluency in the memory disorders clinic population. Neuropsychologia 129, 294–301. doi: 10.1016/j.neuropsychologia.2019.03.021
Katzev, M., Tüscher, O., Hennig, J., Weiller, C., and Kaller, C. P. (2013). Revisiting the functional specialization of left inferior frontal gyrus in phonological and semantic fluency: The crucial role of task demands and individual ability. J. Neurosci. 33, 7837–7845. doi: 10.1523/JNEUROSCI.3147-12.2013
Kertesz, A., and McMonagle, P. (2010). Behavior and cognition in corticobasal degeneration and progressive supranuclear palsy. J. Neurol. Sci. 289, 138–143. doi: 10.1016/j.jns.2009.08.036
Keser, Z., Hillis, A. E., Schulz, P. E., Hasan, K. M., and Nelson, F. M. (2020). Frontal aslant tracts as correlates of lexical retrieval in MS. Neurol. Res. 42, 805–810. doi: 10.1080/01616412.2020.1781454
Kinoshita, M., de Champfleur, N. M., Deverdun, J., Moritz-Gasser, S., Herbet, G., and Duffau, H. (2015). Role of fronto-striatal tract and frontal aslant tract in movement and speech: An axonal mapping study. Brain Struct. Funct. 220, 3399–3412. doi: 10.1007/s00429-014-0863-0
La Corte, E., Eldahaby, D., Greco, E., Aquino, D., Bertolini, G., Levi, V., et al. (2021). The frontal aslant tract: A systematic review for neurosurgical applications. Front Neurol. 12:641586. doi: 10.3389/fneur.2021.641586
Laisney, M., Matuszewski, V., Mézenge, F., Belliard, S., De La Sayette, V., Eustache, F., et al. (2009). The underlying mechanisms of verbal fluency deficit in frontotemporal dementia and semantic dementia. J. Neurol. 256, 1083–1094. doi: 10.1007/s00415-009-5073-y
Lee, Y. E. C., Williams, D. R., and Anderson, J. F. I. (2016). Frontal deficits differentiate progressive supranuclear palsy from Parkinson’s disease. J. Neuropsychol. 10, 1–14. doi: 10.1111/jnp.12053
Lezak, M. D., Howieson, D. B., Bigler, E. D., and Tranel, D. (2012). Neuropsychological assessment, 5th Edn. New York, NY: Oxford University Press, 1200.
Li, M., Zhang, Y., Song, L., Huang, R., Ding, J., Fang, Y., et al. (2017). Structural connectivity subserving verbal fluency revealed by lesion-behavior mapping in stroke patients. Neuropsychologia 101, 85–96. doi: 10.1016/j.neuropsychologia.2017.05.008
Libon, D. J., McMillan, C., Gunawardena, D., Powers, C., Massimo, L., Khan, A., et al. (2009). Neurocognitive contributions to verbal fluency deficits in frontotemporal lobar degeneration. Neurology 73, 535–542. doi: 10.1212/WNL.0b013e3181b2a4f5
Luca, A., Nicoletti, A., Donzuso, G., Terravecchia, C., Cicero, C. E., D’Agate, C., et al. (2021). Phonemic verbal fluency and midbrain atrophy in progressive supranuclear palsy. J. Alzheimers Dis. 80, 1669–1674. doi: 10.3233/JAD-210023
Madden, D. L., Sale, M. V., O’Sullivan, J., and Robinson, G. A. (2019). Improved language production with transcranial direct current stimulation in progressive supranuclear palsy. Neuropsychologia 127, 148–157. doi: 10.1016/j.neuropsychologia.2019.02.022
Measso, G., Cavarzeran, F., Zappala, G., Lebowitz, B. D., Crook, T. H., Pirozzolo, F. J., et al. (1993). The mini-mental-state-examination – Normative study of an italian random sample. Dev Neuropsychol. 9, 77–85. doi: 10.1080/87565649109540545
Meinzer, M., Antonenko, D., Lindenberg, R., Hetzer, S., Ulm, L., Avirame, K., et al. (2012). Electrical brain stimulation improves cognitive performance by modulating functional connectivity and task-specific activation. J. Neurosci. 32, 1859–1866. doi: 10.1523/JNEUROSCI.4812-11.2012
Meinzer, M., Flaisch, T., Wilser, L., Eulitz, C., Rockstroh, B., Conway, T., et al. (2009). Neural signatures of semantic and phonemic fluency in young and old adults. J. Cogn. Neurosci. 21, 2007–2018. doi: 10.1162/jocn.2009.21219
Melrose, R. J., Campa, O. M., Harwood, D. G., Osato, S., Mandelkern, M. A., and Sultzer, D. L. (2009). The neural correlates of naming and fluency deficits in Alzheimer’s disease: An FDG-PET study. Int. J. Geriatr. Psychiatry 24, 885–893. doi: 10.1002/gps.2229
Miró-Padilla, A., Bueichekú, E., Ventura-Campos, N., Palomar-García, M. Á, and Ávila, C. (2017). Functional connectivity in resting state as a phonemic fluency ability measure. Neuropsychologia 97, 98–103. doi: 10.1016/j.neuropsychologia.2017.02.009
Monaco, M., Costa, A., Caltagirone, C., and Carlesimo, G. A. (2013). Forward and backward span for verbal and visuo-spatial data: Standardization and normative data from an Italian adult population. Neurol. Sci. 34, 749–754. doi: 10.1007/s10072-012-1130-x
Oswald, V., Zerouali, Y., Boulet-Craig, A., Krajinovic, M., Laverdière, C., Sinnett, D., et al. (2022). Magnetoencephalography resting-state correlates of executive and language components of verbal fluency. Sci. Rep. 12:476. doi: 10.1038/s41598-021-03829-0
Parmera, J. B., de Almeida, I. J., de Oliveira, M. C. B., Silagi, M. L., de Godoi Carneiro, C., Studart-Neto, A., et al. (2021). Metabolic and structural signatures of speech and language impairment in corticobasal syndrome: A multimodal PET/MRI study. Front. Neurol. 12:702052. doi: 10.3389/fneur.2021.702052
Pellicano, C., Assogna, F., Cellupica, N., Piras, F., Pierantozzi, M., Stefani, A., et al. (2017). Neuropsychiatric and cognitive profile of early Richardson’s syndrome, progressive supranuclear Palsy-parkinsonism and Parkinson’s disease. Park. Relat. Disord. 45, 50–56. doi: 10.1016/j.parkreldis.2017.10.002
Peterson, K. A., Patterson, K., and Rowe, J. B. (2021). Language impairment in progressive supranuclear palsy and corticobasal syndrome. J. Neurol. 268, 796–809. doi: 10.1007/s00415-019-09463-1
Pinson, H., Van Lerbeirghe, J., Vanhauwaert, D., Van Damme, O., Hallaert, G., and Kalala, J. P. (2022). The supplementary motor area syndrome: A neurosurgical review. Neurosurg. Rev. 45, 81–90. doi: 10.1007/s10143-021-01566-6
Raimo, S., Santangelo, G., and Trojano, L. (2021). The neural bases of drawing. A meta-analysis and a systematic literature review of neurofunctional studies in healthy individuals. Neuropsychol. Rev. 31, 689–702. doi: 10.1007/s11065-021-09494-4
Riello, M., Frangakis, C. E., Ficek, B., Webster, K. T., Desmond, J. E., Faria, A. V., et al. (2022). Neural correlates of letter and semantic fluency in primary progressive aphasia. Brain Sci. 12:1. doi: 10.3390/brainsci12010001
Rittman, T., Ghosh, B. C., McColgan, P., Breen, D. P., Evans, J., Williams-Gray, C. H., et al. (2013). The Addenbrooke’s cognitive examination for the differential diagnosis and longitudinal assessment of patients with parkinsonian disorders. J. Neurol. Neurosurg. Psychiatry 84, 544–551. doi: 10.1136/jnnp-2012-303618
Robinson, G. A. (2006). The contribution of the frontal lobes to propositional language. London: University of London.
Robinson, G. A., Spooner, D., and Harrison, W. J. (2015). Frontal dynamic aphasia in progressive supranuclear palsy: Distinguishing between generation and fluent sequencing of novel thoughts. Neuropsychologia 77, 62–75. doi: 10.1016/j.neuropsychologia.2015.08.001
Robinson, G., Shallice, T., Bozzali, M., and Cipolotti, L. (2012). The differing roles of the frontal cortex in fluency tests. Brain 135, 2202–2214. doi: 10.1093/brain/aws142
Rodríguez-Aranda, C., Waterloo, K., Johnsen, S. H., Eldevik, P., Sparr, S., Wikran, G. C., et al. (2016). Neuroanatomical correlates of verbal fluency in early Alzheimer’s disease and normal aging. Brain Lang. 155–156, 24–35. doi: 10.1016/j.bandl.2016.03.001
Sajjadi, S. A., Acosta-Cabronero, J., Patterson, K., Diaz-De-Grenu, L. Z., Williams, G. B., and Nestor, P. J. (2013). Diffusion tensor magnetic resonance imaging for single subject diagnosis in neurodegenerative diseases. Brain 136, 2253–2261. doi: 10.1093/brain/awt118
Schofield, E. C., Hodges, J. R., Bak, T. H., Xuereb, J. H., and Halliday, G. M. (2012). The relationship between clinical and pathological variables in Richardson’s syndrome. J. Neurol. 259, 482–490. doi: 10.1007/s00415-011-6205-8
Shao, Z., Janse, E., Visser, K., and Meyer, A. S. (2014). What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Front. Psychol. 5:772. doi: 10.3389/fpsyg.2014.00772
Shibahara, N. (2004). Picture-name priming in the cerebral hemispheres: Evidence for phonological access in the right hemisphere. Percept. Mot. Skills 99, 305–314. doi: 10.2466/pms.99.1.305-314
Smirni, D., Turriziani, P., Mangano, G. R., Bracco, M., Oliveri, M., and Cipolotti, L. (2017). Modulating phonemic fluency performance in healthy subjects with transcranial magnetic stimulation over the left or right lateral frontal cortex. Neuropsychologia 102, 109–115. doi: 10.1016/j.neuropsychologia.2017.06.006
Spinnler, H., and Tognoni, G. (1987). Standardizzazione e taratura italiana di test neuropsicologici. Gruppo Italiano per lo Studio Neuropsicologico dell’Invecchiamento. Ital. J. Neurol. Sci. 8(Suppl.) 1–120.
Stamelou, M., Respondek, G., Giagkou, N., Whitwell, J. L., Kovacs, G. G., and Höglinger, G. U. (2021). Evolving concepts in progressive supranuclear palsy and other 4-repeat tauopathies. Nat. Rev. Neurol. 17, 601–620. doi: 10.1038/s41582-021-00541-5
Suppa, A., Fabbrini, A., Guerra, A., Petsas, N., Asci, F., Di Stasio, F., et al. (2020). Altered speech-related cortical network in frontotemporal dementia. Brain Stimul. 13, 765–773. doi: 10.1016/j.brs.2020.02.029
Tremblay, T., Monetta, L., and Joanette, Y. (2004). Phonological processing of words in right- and left-handers. Brain Cogn. 55, 427–432. doi: 10.1016/j.bandc.2004.02.068
Valero-Cabré, A., Sanches, C., Godard, J., Fracchia, O., Dubois, B., Levy, R., et al. (2019). Language boosting by transcranial stimulation in progressive supranuclear palsy. Neurology 93, E537–E547. doi: 10.1212/WNL.0000000000007893
Vonk, J. M. J., Rizvi, B., Lao, P. J., Budge, M., Manly, J. J., Mayeux, R., et al. (2019). Letter and category fluency performance correlates with distinct patterns of cortical thickness in older adults. Cereb. Cortex 29, 2694–2700. doi: 10.1093/cercor/bhy138
Wagner, S., Sebastian, A., Lieb, K., Tüscher, O., and Tadić, A. (2014). A coordinate-based ALE functional MRI meta-analysis of brain activation during verbal fluency tasks in healthy control subjects. BMC Neurosci. 15:19. doi: 10.1186/1471-2202-15-19
Williams, D. R., Holton, J. L., Strand, C., Pittman, A., De Silva, R., Lees, A. J., et al. (2007). Pathological tau burden and distribution distinguishes progressive supranuclear palsy-parkinsonism from Richardson’s syndrome. Brain 130, 1566–1576. doi: 10.1093/brain/awm104
Yuan, P., and Raz, N. (2014). Prefrontal cortex and executive functions in healthy adults: A meta-analysis of structural neuroimaging studies. Neurosci. Biobehav. Rev. 42, 180–192. doi: 10.1016/j.neubiorev.2014.02.005
Zarino, B., Crespi, M., Launi, M., and Casarotti, A. (2014). A new standardization of semantic verbal fluency test. Neurol. Sci. 35, 1405–1411. doi: 10.1007/s10072-014-1729-1
Keywords: supplementary motor area, frontal aslant tract, progressive supranuclear palsy, fluency, FDG-PET
Citation: Isella V, Licciardo D, Ferri F, Crivellaro C, Morzenti S, Appollonio I and Ferrarese C (2022) Reduced phonemic fluency in progressive supranuclear palsy is due to dysfunction of dominant BA6. Front. Aging Neurosci. 14:969875. doi: 10.3389/fnagi.2022.969875
Received: 15 June 2022; Accepted: 24 August 2022;
Published: 08 September 2022.
Edited by:
Bogdan O. Popescu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Riccardo Manca, Brunel University London, United KingdomCopyright © 2022 Isella, Licciardo, Ferri, Crivellaro, Morzenti, Appollonio and Ferrarese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valeria Isella, dmFsZXJpYS5pc2VsbGFAdW5pbWliLml0
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.