- 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2School of Clinical Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Background: Insulin regulates many aspects of brain function related to mild cognitive impairment (MCI) or dementia, which can be delivered to the brain center via intranasal (IN) devices. Some small, single-site studies indicated that intranasal insulin can enhance memory in patients with MCI or dementia. The pathophysiology of Alzheimer's disease (AD) and diabetes mellitus (DM) overlap, making insulin an attractive therapy for people suffering from MCI or dementia.
Objective: The goal of the study is to evaluate the effectiveness of IN insulin on cognition in patients with MCI or dementia.
Methods: We searched the electronic database for randomized controlled trials (RCTs) that verified the effects of insulin on patients with MCI or dementia.16 studies (899 patients) were identified.
Results: The pooled standard mean difference (SMD) showed no significant difference between IN insulin and placebo groups; however, statistical results suggested a difference between study groups in the effects of ADCS-ADL; AD patients with APOE4 (-) also showed improved performance in verbal memory; other cognitions did not improve significantly.
Conclusion: In view of IN insulin's promising potential, more researches should be conducted at a larger dose after proper selection of insulin types and patients.
Systematic review registration: http://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022353546.
Introduction
In recent years, a large number of researches has found that Type 2 diabetes mellitus (T2DM) may increase the incidence rate of mild cognitive impairment (MCI) and dementia (Biessels and Despa, 2018). Following 705 participants for 4.6 years, Callisaya et al. indicated that the domains of verbal fluency, verbal memory, and working memory had a greater decline in T2DM patients. Besides, they indicated that T2DM patients had both worse ventricular and brain volume at baseline (Callisaya et al., 2019). T2DM has been confirmed to double the risk of cognitive disorders and dementia, such as in Alzheimer disease (AD) and Vascular dementia (VaD) (Biessels et al., 2006; Kopf and Frölich, 2009). Patients with Parkinson's disease (PD) have nearly six times more chance of developing dementia than their counterparts without PD, according to a study (Aarsland et al., 2017). Additionally, elderly and long-term Parkinson's disease patients are more likely to develop dementia (Hely et al., 2008). Insulin, as an important neurohormone, plays a critical role in brain energy metabolism, cognitive function, axonal migration, and neurogenesis (Chen et al., 2014). Insulin resistance (IR) has been proved to be a pathogenic mechanism of cognitive disorders (Sasaoka et al., 2014). More and more evidence showed that disordered cerebral insulin signaling enhances the development and progression of AD, prompting clinicians to target the loop. Although traditional and contemporary anti-diabetes drugs have shown hope in the fight against insulin resistance (IR), IN insulin seems to be the most effective method to improve brain insulin. IN insulin was more effective than subcutaneous insulin in lowering fasting blood glucose concentrations and in counteracting rises in blood glucose concentrations (Pontiroli et al., 1982). Because of a unique characteristic, insulin can affect the central nervous system (CNS) without passing through the blood-brain barrier. This has been demonstrated to contribute to enhancing the cognitive performance of diabetics, particularly those who have Alzheimer's disease or MCI, as it has been demonstrated that decreasing insulin levels in the brain have a detrimental effect on cognitive function. This helps to reduce the peripheral side effects of insulin in addition to reducing them (Gaddam et al., 2021). Yet, significant open questions remain about the safety, efficacy, and potential of insulin as an adjunct or monotherapy (Chapman et al., 2018). Therefore, this review aims to critically assess the available evidence and future potential of IN insulin as a meaningful treatment for AD and dementia. T2DM has been verified to increase the risk of cognitive disorders and dementia, such as AD and VaD. Besides, there may be a subgroup of dementia related to specific DM-associated metabolic abnormalities (Hanyu, 2019). T2DM is a recognized risk factor for dementia. T2DM and Dementia have some common underlying pathophysiologies, which makes people interested in the reuse of therapeutic drugs for type 2 diabetes, which is beneficial to brain health (Moran et al., 2019). In dementia, there is a progressive deterioration of functional and cognitive abilities, eventually causing a heavy burden on health and social services. The global prevalence of dementia reached 35.6 million in 2010. And the number is expected to double every 20 years (Prince et al., 2013). Insulin increases serum glucose uptake in the peripheral and central nervous system. Abnormal insulin regulation can take place in the early stage of T2DM and dementia, which is a promising therapeutic target to reduce the risk of cognitive disorders (Cha et al., 2016; Arnold et al., 2018). Considering the high concentration of insulin receptors in multiple brain regions associated with dementia, IN insulin inhalation as a treatment for dementia is of great significance, even for people without T2DM (Zhao and Townsend, 2009; Craft, 2012b). In AD patients with or without T2DM, intravenous insulin and glucose normalization can improve memory scores, but peripheral hypoglycemia remains a risk. Contrary to this, IN insulin administration increased brain insulin levels without having a peripheral effect (Craft et al., 1996).
Materials and methods
Study registration
This study was registered in PROSPERO (CRD42022353546). This study was constructed according to the guidelines for preferred reporting items for systematic reviews and meta-analyses (PRISMA 2020) (Page et al., 2021).
Qualification criteria
Qualification criteria for inclusion in the study were as below: Types of studies: Only RCTs were included. Participants: Studies of human participants with MCI or dementia eligible for inclusion, all subjects were free from psychiatric disorders, alcoholism, severe head trauma, hypoxia, neurological disorders other than MCI or dementia, renal or hepatic disease, diabetes, chronic obstructive pulmonary disease, congestive heart failure, or unstable cardiac disease. Interventions: Studies that include receiving any dose of intranasal insulin at any time. Comparator: Any study containing a control group receiving placebo treatment is eligible for inclusion. Outcomes: Studies investigating the efficacy or progression of cognitive disorders or performance (the study reported specific cognitive scores at baseline and endpoints) and dementia (including subtypes of dementia, such as AD or VaD) were eligible for inclusion.
Information sources
PubMed, Web of Science, Embase, Cochrane Library, Clinical Trials (ClinicalTrials.gov), Chinese Biomedical Literature Database, Chinese National Knowledge Infrastructure (CNKI), Wanfang database were searched for related published studies as of December 20, 2021.
Search strategy
In the above databases we used the following search query: “(Intranasal insulin OR nasal insulin) AND (Dementia OR neurodegenerative disease OR Cognitive Dysfunction OR cognitive impairment OR neuroprotective OR memory OR cognition).”
Assessment of methodological quality and data extraction
Critical appraisals were conducted by independent reviewers using the risk of bias (RoB) assessment tool of the Cochrane Collaboration Network assessment checklist (Higgins et al., 2011) for experimental, case-control, cohort, and cross-sectional studies. The consensus was reached through discussion between reviewers. Researches that met >50% of the quality criteria were eligible for selection. Templates used for data extraction, including research method field, study design, data source, inclusion criteria, exposure, country, sample size/event, follow-up, results, result data, and statistical adjustment. When additional data were needed, attempts to contact the corresponding author by e-mail were unsuccessful: in these cases, findings have been summarized narratively.
Statistical analysis
The combined cognitive performance score change was calculated by the inverse variance method (random effect model) as the standardized mean difference (SMD). Some of the data of these articles (Reger et al., 2006, 2008a,b; Claxton et al., 2013, 2015; Rosenbloom et al., 2014) were obtained from the following article (Lu and Xu, 2019). X2 test was used for statistical heterogeneity. The Comprehensive Meta-analysis software (version 3.3) and Review Manager (Revman, version 5.3) were used for data analysis.
Results
Search results
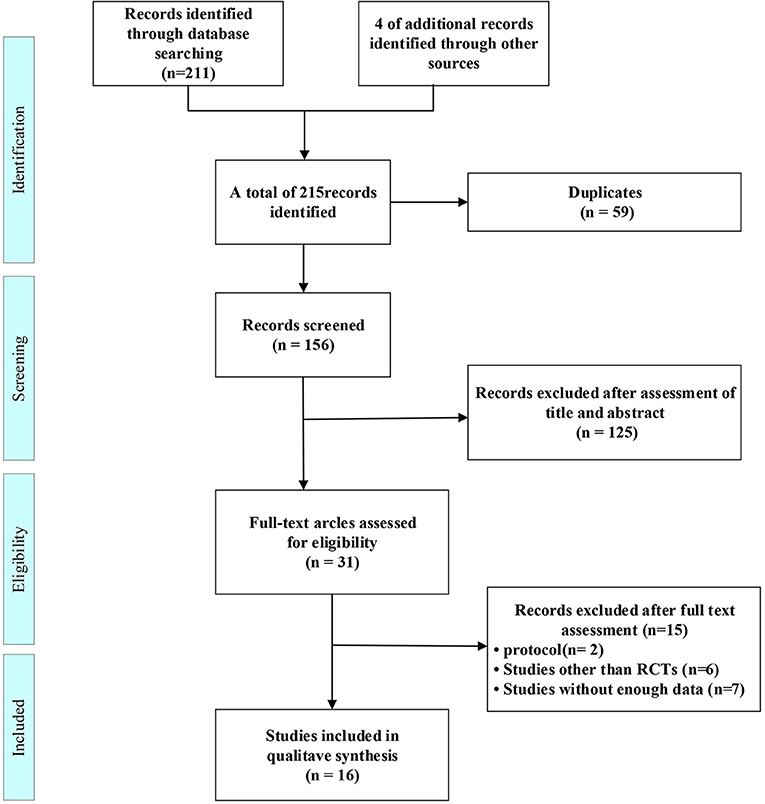
215 citations were found in the original search, which were reduced to 156 after duplicates were eliminated. 125 studies were identified after titles and abstracts were reviewed. The full-text search and 31 studies were qualified. a further 15 studies were excluded for protocol (Nct, 2021; Nitchingham et al., 2021), studies other than RCTs (Bayés et al., 2006; Reger and Craft, 2006; Xiaojiu and Xuan, 2015; Yanfang, 2016; Deng Yun, 2020; Tashima, 2020), or without enough data (Nct, 2007, 2012, 2019; Anonymous, 2008; Galindo-Mendez et al., 2020; Gwizdala et al., 2021; Roque et al., 2021) leaving 16 included studies (Figure 1).
Studies' and patients' characteristics
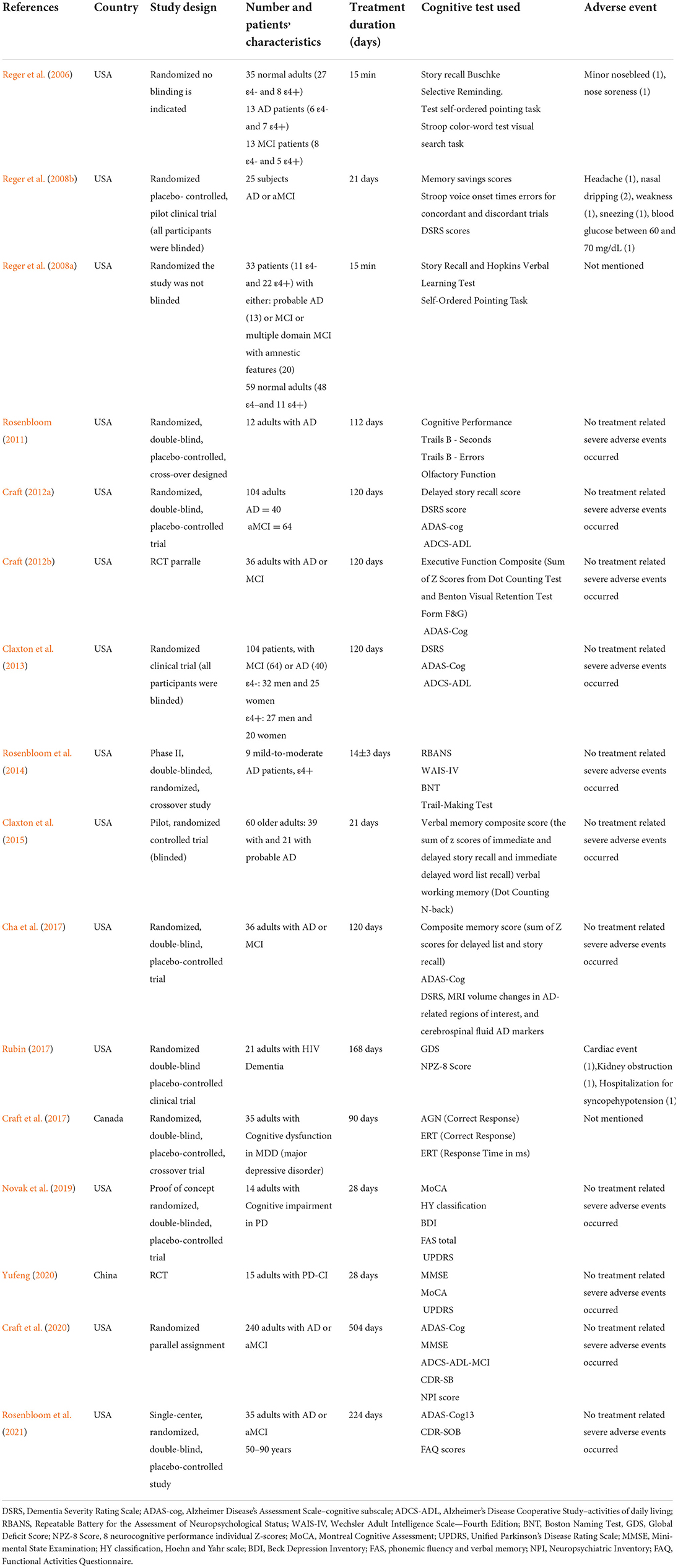
Three crossover studies (Rosenbloom, 2011; Rosenbloom et al., 2014; Cha et al., 2017) out of the 16 papers we analyzed were included in our meta-analysis, Two studies used a parallel design (Craft, 2012a; Craft et al., 2020). The follow-up period is from 1 day to 72 weeks. The sample size ranged from 9 to 240. One study was HIV Dementia (Rubin, 2017), one was diagnosed with MDD(major depressive disorder) (Cha et al., 2017), and two studies enrolled patients with PDD (Parkinson's disease Dementia) (Novak et al., 2019; Yufeng, 2020), the others were about AD or MCI (Reger et al., 2006, 2008a,b; Rosenbloom, 2011; Craft, 2012a,b; Claxton et al., 2013, 2015; Rosenbloom et al., 2014, 2021; Craft et al., 2017; Yufeng, 2020). These studies were conducted in the following regions: Canada (n = 1), China (n = 1), America (n = 14). The publication year of the included articles ranged from 2006 to 2021. The mean age of patients ranged from 18 to 90 years. The baseline characteristics of the eligible studies are shown in Table 1.
Risk of bias
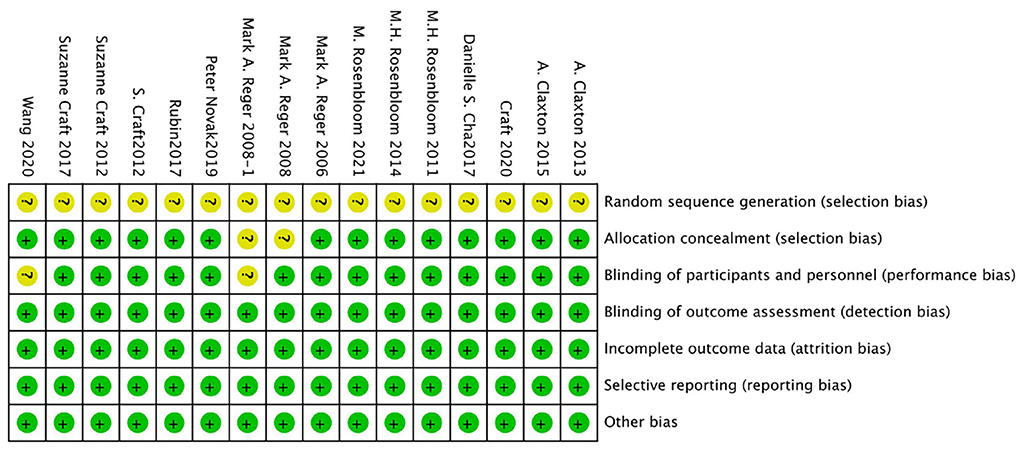
The bias risk of the randomized controlled trial was independently assessed by two reviewers (Yunjiao Yang and Tongyi Li) using the bias risk assessment tool of the Cochrane Collaboration Network (RoB). All of the researches were deemed to be low risk in the evaluation. For all studies, the risk of “random sequence generation” is unclear, and the RoB is unclear; The RoBs of the four studies were not clear because the risks of “blinding of participants” and/or “allocation concealment” were not clear (Reger et al., 2006, 2008a,b; Novak et al., 2019). See Figures 2, 3 for RoB evaluation details. A cross-over study of Rosenbloom et al. was identified as low risk due to the design of the study (AD is a disease with a persistent course), randomization of the treatment sequence resulted in no subsequent treatment effect.
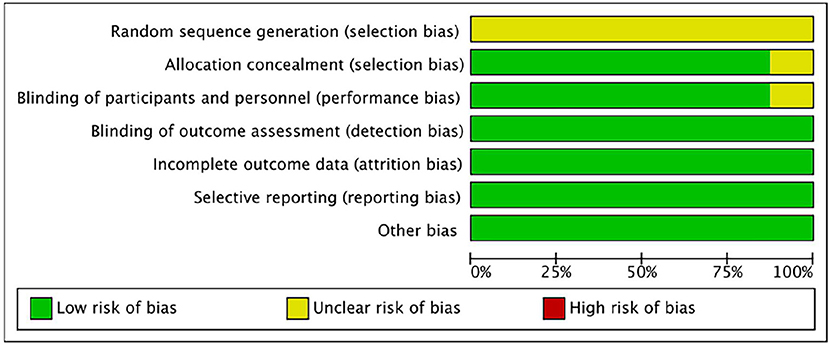
Figure 3. Risk of bias graph authors' judgments about each risk of bias item, presented as a percentage across all induded studies.
Publication bias
We used The Comprehensive Meta-analysis software (version 3.3) to analyze and make a funnel plot, as shown in Figure 4. In order to further test publication bias, we further used Egger' test. The results of Egger's tests indicates that there is no publication bias. In this case the intercept (B0) is 0.28494, 95% confidence interval (−0.76470, 1.33458), with t = 0.58223, df = 14. The 1-tailed p-value (recommended) is 0.28483, and the 2-tailed p-value is 0.56967.
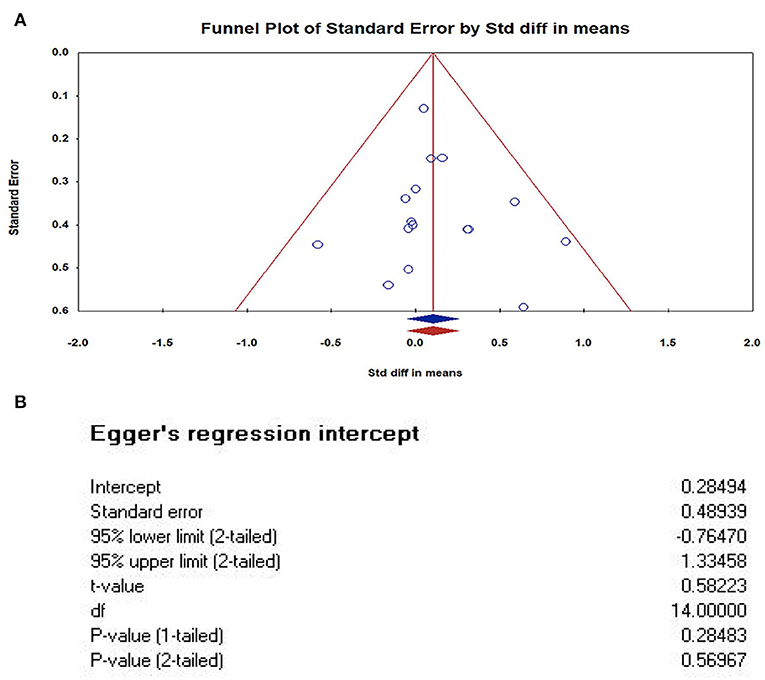
Figure 4. Publication bias of studies. (A) Funnel Plot of Standard Error by Std diff in means (Plot observed and imputed); (B) The results of Egger's tests. In this case the intercept (BO) is 0.28494, 95% confidence interval (−0.76470, 1.33458), with t = 0.58223, df = 14. The 1-tailed p-value (recommended) is 0.28483, and the 2-tailed p-value is 0.56967. The results of Egger's tests indicates that there is no publication bias.
Cognitive performance
Combined cognitive performance outcome
The pooled SMD was 0.103 [16 studies; 95% confidence interval (CI), −0.05 to 0.25; P = 0.18], showing no significant difference between IN insulin and placebo groups (see Figure 5).
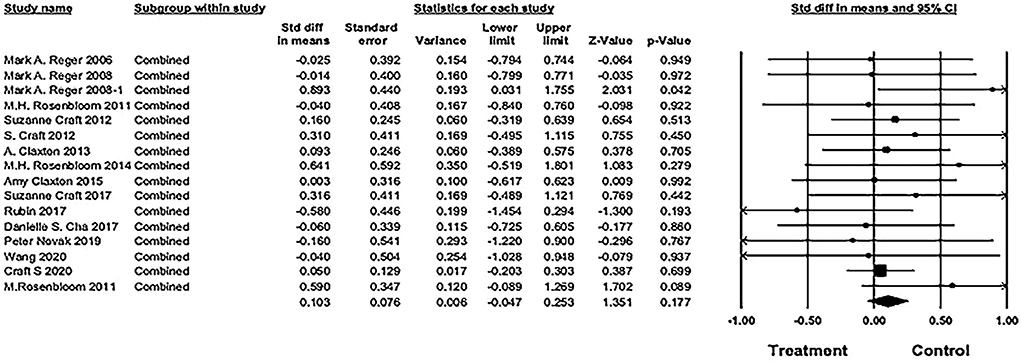
Figure 5. Forest plot of pooled standard mean difference (SMD) for combined cognitive score. The pooled SMD was 0.103 [16 studies; 95% confidence interval (CI), −0.05 to 0.25; P = 0.18), showing no significant difference between IN insulin and placebo groups (weights and heterogeneity test are from random-effects model).
Effects of ADAS-cog
There was no difference between study groups: (5 studies: SMD = 0.15, 95% CI= −0.04 to 0.34; P = 0.12; see Figure 6).
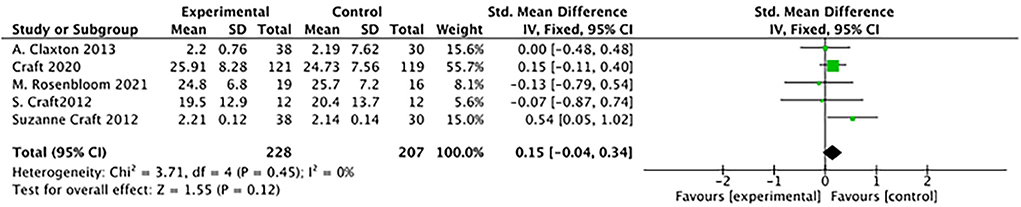
Figure 6. Forest plot for the Effects of ADAS-cog (Alzheimer's Disease's Assessment Scale–cognitive subscale). There was no difference between study groups: SMD = 0.15, 95% CI = −0.04 to 0.34; P = 0.12).
Effects of ADCS-ADL, MMSE, MoCA, UPDRS
Statistical results suggested a difference of ADCS-ADL between study groups: (3 studies): SMD = 0.26, 95% CI = 0.05–0.47; P = 0.01) (see Figure 7A); SMD favored insulin but not statistically different of the effects of MMSE (2 studies): SMD = 0.07, 95% CI = −0.50 to 0.64; P = 0.81; see Figure 7B); No differences between study groups of the effects of MoCA (2 studies): SMD= −0.01, 95% CI = −0.81 to 0.79; P = 0.98; see Figure 7C); There was no difference between study groups of the effects of UPDRS (2 studies: SMD = −0.41, 95% CI = −1.00 to 0.18; P = 0.17; see Figure 7D).
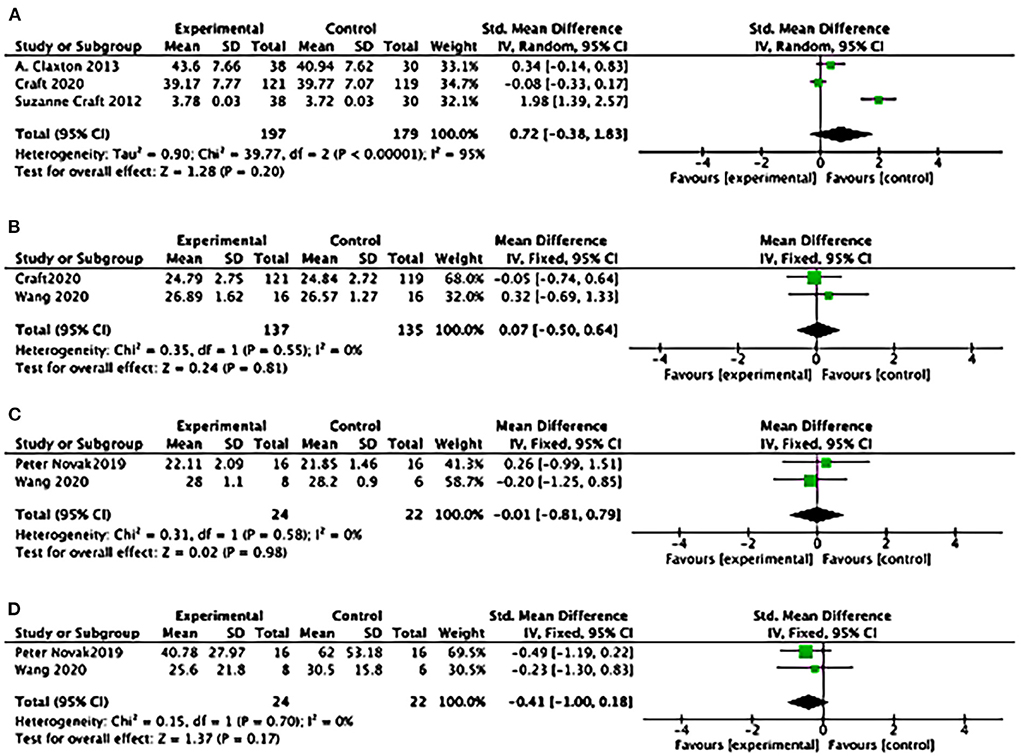
Figure 7. Forest plot for the effects of cognitive performance. (A) Forest plot for the effects of the Alzheimer's Disease Cooperative Study–activities of daily living Statistical (ADCS-ADL): results suggested a difference between study groups: (3 studies): SMD = 0.26, 95% CI = 0.05 to 0.47; P = 0.01); (B) Forest plot for the effects of Mini-mental Atate Examination (MMSE): SMD favoured Insulin but not statistically different of the effects (2 studies): SMD = 0.07, 95% CI = −0.50 to 0.64; P = 0.81; (C) Forest plot for the effects of Montreal Cognitive Assessment (MoCA): No differences between study groups of the effects (2 studies): SMD = −0.01, 95% CI = −0.81 to 0.79; P = 0.98; (D) Forest plot for the effects of Unified Parkinson's Disease Rating Scale (UPDRS): There was no difference between study groups (2 studies): SMD = −0.41, 95% CI = −1.00 to 0.18; P = 0.17).
Effects modulation by APOE genotype
In the study (Reger et al., 2006) insulin treatment facilitated recall on two measures of verbal memory in memory-impaired ε4– adults. Findings in this study (Reger et al., 2008a) suggests that IN insulin administration dose-dependently modulates verbal memory, the acute clinical benefits of treatment were greatest at 20 IU.
Effects of Aβ42 and Aβ40
In two studies, IN insulin was found to affect plasma amyloid levels Aβ42 and Aβ40 (Reger et al., 2008a,b). Another two studies also cited the effects on CerebroSpinal Fluid(CSF) Aβ42 and Aβ40 levels (Craft, 2012b; Craft et al., 2017). Rather contradictory results emerged.
Metabolic data
In four studies, IN insulin therapy was shown to affect plasma glucose and insulin levels (Reger et al., 2006, 2008a,b; Rosenbloom et al., 2014). Blood glucose and insulin levels in three of these studies did not change significantly after treatment (Reger et al., 2006, 2008a; Rosenbloom et al., 2014). After 21 days of treatment, fasting glucose or insulin levels did not change in Rosenbloom et al.'s study. In spite of this, postprandial plasma insulin levels decreased in the treatment group when compared to the placebo group [F(1, 20) = 4.43, P = 0.0481)] (Rosenbloom et al., 2014).
Discussion
Main findings and conclusions
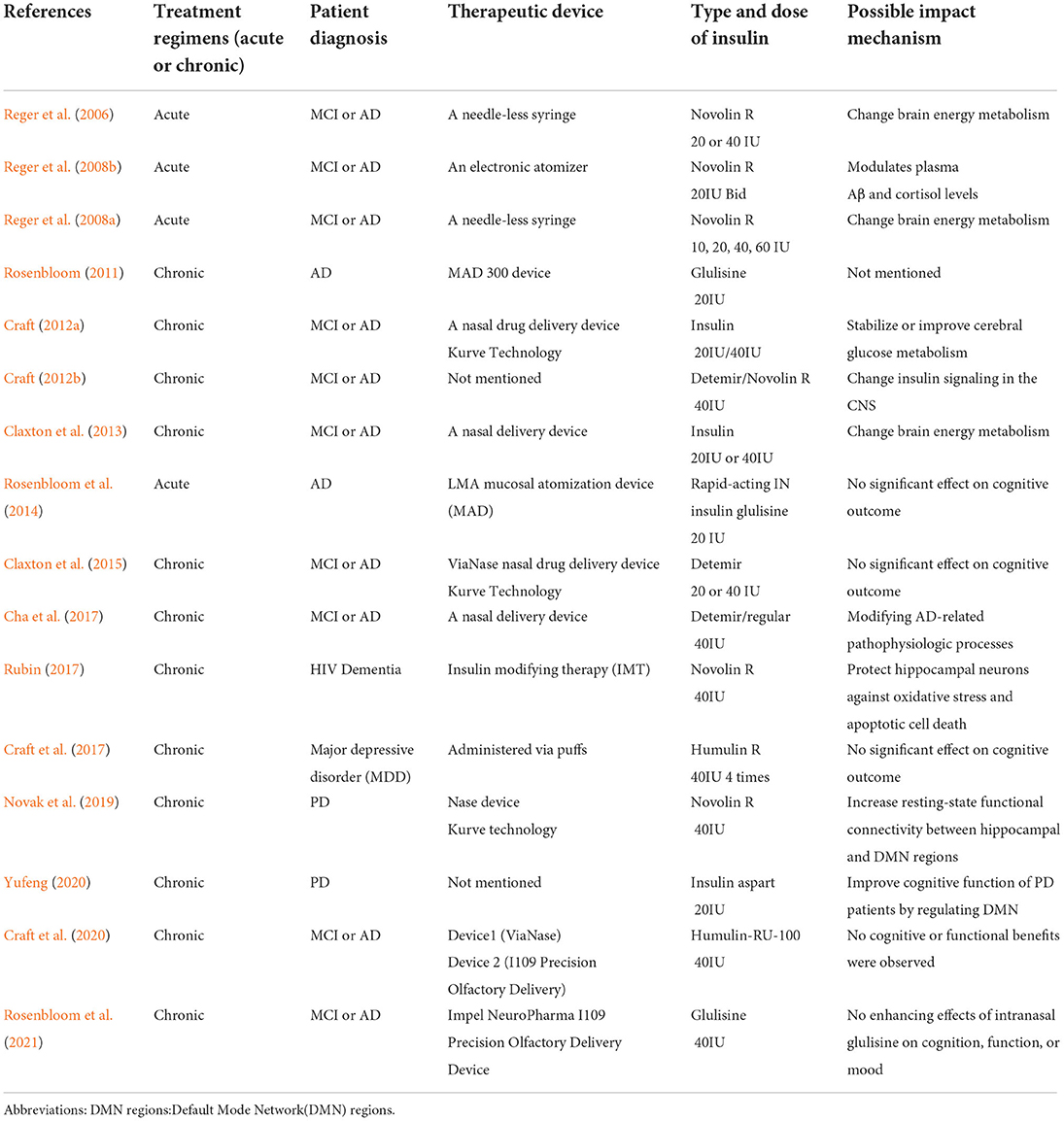
Based on our current study, there was no significant effects of intranasal insulin on the improvement of cognitive function in patients with MCI/dementia. However, Even though the results were not statistically significant, this study illustrates the potential of IN insulin efficacy. According to Mark A Reger's work, insulin dose-response curves vary by APOE genotype and IN insulin treatment may offer therapeutic benefits without the danger of peripheral hypoglycemia, the effect of APOE genotype on cognitive and metabolic responses to insulin may reflect a specific pattern of abnormal insulin metabolism among ε4– subjects, differential cognitive responses to insulin treatment by APOE genotype may result from differences in insulin sensitivity (Reger et al., 2006, 2008a). There is growing evidence that persons with AD who do not carry ApoE-ε4 may experience IR more frequently. Higher doses of insulin are needed to elicit biological reactions that are typically seen at lower insulin doses in healthy persons with IR, which is a condition in which muscle, fat, and hepatic cell responses to insulin are impaired. But evidence is still lacking to explain the mechanism by which the ApoE4 genotype attenuates the cognitive response to IN insulin. Statistical results also revealed a difference between study groups in the effects of ADCS-ADL. We also find some subtle but meaningful patterns of improvement in cognitive function with intranasal insulin. For example, the type of intranasal insulin, the treatment course and the device used will affect the treatment, and these studies suggest potential mechanisms of intranasal insulin in MCI or dementia treatment: change brain energy metabolism, modulates plasma Aβ and cortisol levels, stabilize or improve cerebral glucose metabolism, change insulin signal in the CNS, modifying AD-related pathophysiologic processes, protect hippocampal neurons against oxidative stress and apoptotic cell death, increase resting-state functional connectivity between hippocampal and Default Mode Network(DMN) regions (see Table 2). However, due to the lack of sample size, the amount of data for specific analysis is not enough. In the future, we will continue to pay attention in this field to see if we can dig out some specific patterns of intranasal insulin treatment.
In light of those promising results, further studies with larger sample sizes and longer duration are needed to verify these findings. The effects of IN insulin on MCI/dementia needs to highlight in future studies. With the rapid increase in the aging population, effective treatments for MCI/dementia are in short supply. Since MCI and dementia involve the imbalance of neuropeptide signals in the brain, neuropeptide in management may be a promising therapeutic target, directly aiming at the brain and restoring signals, and ultimately, cognition. In insulin is expected to be a successful treatment for MCI/dementia.
Limitations
In insulin may be a new therapy for patients with MCI or dementia, but for now, it has only been tested in a few clinical trials, particularly for the treatment of dementia. In our study, there only included 899 patients in all. No safe conclusions can be drawn from such a small sample size. Further, there are restrictions on the properties that can be attributed to the results given that most included studies were conducted in one country (the United States). The heterogeneity of the included studies is another limitation of this research. The first factor that was heterogeneous was the patients' characteristics, particularly their type of dementia, ApoE4 status, and cognitive tests (see Table 1). Moreover, different researches have used different types and doses of insulin (see Table 1). As a final point, the treatment time was uneven (the treatment days ranged between 15 min and 504 days). Therefore, it is not possible to conduct quantitative analysis (meta-analysis) of the included studies.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
CL and QC proposed the idea and designed the whole research, literature research, study selection,. and data extraction were conducted by XH, TL, YY, and QZ. The article was written and revised by CL. All authors approved the final manuscript before submission.
Funding
This study was supported by the Sichuan Science and Technology Program (2019YFS0085). The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Acknowledgments
The contents in this manuscript and the manuscript itself has never been published electronically or printed.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Aarsland, D., Creese, B., Politis, M., Chaudhuri, K. R., Ffytche, D. H., Weintraub, D., et al. (2017). Cognitive decline in Parkinson disease. Nat. Rev. Neurol. 13, 217–231. doi: 10.1038/nrneurol.2017.27
Anonymous (2008). Treatment with intranasal insulin improves memory in early Alzheimer's. disease. China Medical News 23:1
Arnold, S. E., Arvanitakis, Z., Macauley-Rambach, S. L., Koenig, A. M., Wang, H. Y., Ahima, R. S., et al. (2018). Brain insulin resistance in type 2 diabetes and Alzheimer disease: concepts and conundrums. Nat. Rev. Neurol. 14, 168–181. doi: 10.1038/nrneurol.2017.185
Bayés, M., Rabasseda, X., and Prous, J. R. (2006). Gateways to clinical trials. Methods Find. Exp. Clin. Pharmacol. 28, 719–740.
Biessels, G. J., and Despa, F. (2018). Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat. Rev. Endocrinol. 14, 591–604. doi: 10.1038/s41574-018-0048-7
Biessels, G. J., Staekenborg, S., Brunner, E., Brayne, C., and Scheltens, P. (2006). Risk of dementia in diabetes mellitus: a systematic review. Lancet Neurol. 5, 64–74. doi: 10.1016/S1474-4422(05)70284-2
Callisaya, M. L., Beare, R., Moran, C., Phan, T., Wang, W., and Srikanth, V. K. (2019). Type 2 diabetes mellitus, brain atrophy and cognitive decline in older people: a longitudinal study. Diabetologia 62, 448–458. doi: 10.1007/s00125-018-4778-9
Cha, D. S., Best, M. W., Bowie, C. R., Gallaugher, L. A., Woldeyohannes, H. O., Soczynska, J. K., et al. (2017). A randomized, double-blind, placebo-controlled, crossover trial evaluating the effect of intranasal insulin on cognition and mood in individuals with treatment-resistant major depressive disorder. J. Affect. Disord. 210, 57–65. doi: 10.1016/j.jad.2016.12.006
Cha, D. S., Vahtra, M., Ahmed, J., Kudlow, P. A., Mansur, R. B., Carvalho, A. F., et al. (2016). Repurposing of anti-diabetic agents for the treatment of cognitive impairment and mood disorders. Curr. Mol. Med. 16, 465–473. doi: 10.2174/1566524016666160429121737
Chapman, C. D., Schiöth, H. B., Grillo, C. A., and Benedict, C. (2018). Intranasal insulin in Alzheimer's disease: food for thought. Neuropharmacology 136, 196–201. doi: 10.1016/j.neuropharm.2017.11.037
Chen, Y., Deng, Y., Zhang, B., and Gong, C. X. (2014). Deregulation of brain insulin signaling in Alzheimer's disease. Neurosci. Bull. 30, 282–294. doi: 10.1007/s12264-013-1408-x
Claxton, A., Baker, L. D., Hanson, A., Trittschuh, E. H., Cholerton, B., Morgan, A., et al. (2015). Long-acting intranasal insulin detemir improves cognition for adults with mild cognitive impairment or early-stage Alzheimer's disease dementia. J. Alzheimers Dis. 44, 897–906. doi: 10.3233/JAD-141791
Claxton, A., Baker, L. D., Wilkinson, C. W., Trittschuh, E. H., Chapman, D., Watson, G. S., et al. (2013). Sex and ApoE genotype differences in treatment response to two doses of intranasal insulin in adults with mild cognitive impairment or Alzheimer's disease. J. Alzheimers Dis. 35, 789–797. doi: 10.3233/JAD-122308
Craft, S. (2012a). Study of Nasal Insulin to Fight Forgetfulness - Long-acting Insulin Detemir - 120 Days (SL120). Available online at: https://clinicaltrials.gov/show/NCT01595646
Craft, S. (2012b). Intranasal insulin therapy for Alzheimer disease and amnestic mild cognitive impairment: a pilot clinical trial. Arch. Neurol. 69, 29–38. doi: 10.1001/archneurol.2011.233
Craft, S., Claxton, A., Baker, L. D., Hanson, A. J., Cholerton, B., Trittschuh, E. H., et al. (2017). Effects of regular and long-acting insulin on cognition and Alzheimer's disease biomarkers: a pilot clinical trial. J. Alzheimers Dis. 57, 1325–1334. doi: 10.3233/JAD-161256
Craft, S., Newcomer, J., Kanne, S., Dagogo-Jack, S., Cryer, P., Sheline, Y., et al. (1996). Memory improvement following induced hyperinsulinemia in Alzheimer's disease. Neurobiol. Aging 17, 123–130. doi: 10.1016/0197-4580(95)02002-0
Craft, S., Raman, R., Chow, T. W., Rafii, M. S., Sun, C. K., Rissman, R. A., et al. (2020). The Study of Nasal Insulin in the Fight Against Forgetfulness (SNIFF). JAMA Neurol. 77, 1099–1109. doi: 10.1001/jamaneurol.2020.1840
Deng Yun, X. U. (2020). Potential hypoglycemic drug pathways in the treatment of Alzheimer's disease. Chinese J. Clin. Res. 33:552–555. doi: 10.13429/j.cnki.cjcr.2020.04.030
Gaddam, M., Singh, A., Jain, N., Avanthika, C., Jhaveri, S., De la Hoz, I., et al. (2021). A comprehensive review of intranasal insulin and its effect on the cognitive function of diabetics. Cureus 13, e17219. doi: 10.7759/cureus.17219
Galindo-Mendez, B., Trevino, J. A., McGlinchey, R., Fortier, C., Lioutas, V., Novak, P., et al. (2020). Memory advancement by intranasal insulin in type 2 diabetes (MemAID) randomized controlled clinical trial: design, methods and rationale. Contemp. Clin. Trials 89, 105934. doi: 10.1016/j.cct.2020.105934
Gwizdala, K. L., Ferguson, D. P., Kovan, J., Novak, V., and Pontifex, M. B. (2021). Placebo controlled phase II clinical trial: Safety and efficacy of combining intranasal insulin and acute exercise. Metab. Brain Dis. 36, 1289–1303. doi: 10.1007/s11011-021-00727-2
Hanyu, H. (2019). Diabetes-related dementia. Adv. Exp. Med. Biol. 1128, 147–160. doi: 10.1007/978-981-13-3540-2_8
Hely, M. A., Reid, W. G., Adena, M. A., Halliday, G. M., and Morris, J. G. (2008). The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov. Disord. 23, 837–844. doi: 10.1002/mds.21956
Higgins, J. P., Altman, A. D., Gotzsche, P. C., Juni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343:d5928. doi: 10.1136/bmj.d5928
Kopf, D., and Frölich, L. (2009). Risk of incident Alzheimer's disease in diabetic patients: a systematic review of prospective trials. J. Alzheimers Dis. 16, 677–685. doi: 10.3233/JAD-2009-1011
Lu, J., and Xu, Z. (2019). Efficacy of intranasal insulin in improving cognition in mild cognitive impairment and Alzheimer disease: a systematic review and meta-analysis. Am. J. Ther. 26, e756–e762. doi: 10.1097/MJT.0000000000000926
Moran, C., Callisaya, M. L., Srikanth, V., and Arvanitakis, Z. (2019). Diabetes therapies for dementia. Curr. Neurol. Neurosci. Rep. 19, 58. doi: 10.1007/s11910-019-0973-4
Nct (2007). SNIFF 120: study of Nasal Insulin to Fight Forgetfulness (120 Days). Available online at: https://clinicaltrials.gov/show/NCT00438568
Nct (2012). Study of Nasal Insulin to Fight Forgetfulness-Long-acting Insulin Detemir-21 Days. Available online at: https://clinicaltrials.gov/show/NCT01547169
Nct (2019). SNIFF Multi-Device Study 2. Available online at: https://clinicaltrials.gov/show/NCT04199767
Nct (2021). SNIFF - 3-Week Aptar CPS Device. Available online at: https://clinicaltrials.gov/show/NCT05006599
Nitchingham, A., Milne, A., Toson, B., Tuch, B., Agar, M., Close, J., et al. (2021). Intranasal insulin for treatment of delirium in older hospitalised patients: study protocol for a randomised controlled trial. BMJ Open 11, e050765. doi: 10.1136/bmjopen-2021-050765
Novak, P., Pimentel Maldonado, D. A., and Novak, V. (2019). Safety and preliminary efficacy of intranasal insulin for cognitive impairment in Parkinson disease and multiple system atrophy: a double-blinded placebo-controlled pilot study. PLoS ONE 14, e0214364. doi: 10.1371/journal.pone.0214364
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372, n71. doi: 10.1136/bmj.n71
Pontiroli, A. E., Alberetto, M., Secchi, A., Dossi, G., Bosi, I., and Pozza, G. (1982). Insulin given intranasally induces hypoglycaemia in normal and diabetic subjects. Br. Med. J. 284, 303–306. doi: 10.1136/bmj.284.6312.303
Prince, M., Bryce, R., Albanese, E., Wimo, A., Ribeiro, W., and Ferri, C. P. (2013). The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 9, 63–75.e62. doi: 10.1016/j.jalz.2012.11.007
Reger, M. A., and Craft, S. (2006). Intranasal insulin administration: a method for dissociating central and peripheral effects of insulin. Drugs Today 42, 729–739. doi: 10.1358/dot.2006.42.11.1007675
Reger, M. A., Watson, G. S., Frey, W. H. II., Baker, L. D., Cholerton, B., Keeling, M. L., Belongia, D. A., et al. (2006). Effects of intranasal insulin on cognition in memory-impaired older adults: modulation by APOE genotype. Neurobiol. Aging 27, 451–458. doi: 10.1016/j.neurobiolaging.2005.03.016
Reger, M. A., Watson, G. S., Green, P. S., Baker, L. D., Cholerton, B., Fishel, M. A., et al. (2008a). Intranasal insulin administration dose-dependently modulates verbal memory and plasma amyloid-beta in memory-impaired older adults. J. Alzheimers Dis. 13, 323–331. doi: 10.3233/JAD-2008-13309
Reger, M. A., Watson, G. S., Green, P. S., Wilkinson, C. W., Baker, L. D., Cholerton, B., et al. (2008b). Intranasal insulin improves cognition and modulates beta-amyloid in early AD. Neurology 70, 440–448. doi: 10.1212/01.WNL.0000265401.62434.36
Roque, P., Nakadate, Y., Sato, H., Sato, T., Wykes, L., Kawakami, A., et al. (2021). Intranasal administration of 40 and 80 units of insulin does not cause hypoglycemia during cardiac surgery: a randomized controlled trial. Can. J. Anesth. 68, 991–999. doi: 10.1007/s12630-021-01969-5
Rosenbloom, M., Barclay, T. R., Kashyap, B., Hage, L., O'Keefe, L. R., Svitak, A., et al. (2021). A phase II, single-center, randomized, double-blind, placebo-controlled study of the safety and therapeutic efficacy of intranasal glulisine in amnestic mild cognitive impairment and probable mild Alzheimer's disease. Drugs Aging 38, 407–415. doi: 10.1007/s40266-021-00845-7
Rosenbloom, M. H. (2011). Safety and effectiveness study of intranasal insulin glulisine on cognitive and memory in Mild-Mod AD patients. Available online at: https://clinicaltrials.gov/show/NCT01436045
Rosenbloom, M. H., Barclay, T. R., Pyle, M., Owens, B. L., Cagan, A. B., Anderson, C. P., et al. (2014). A single-dose pilot trial of intranasal rapid-acting insulin in apolipoprotein E4 carriers with mild-moderate Alzheimer's disease. CNS Drugs 28, 1185–1189. doi: 10.1007/s40263-014-0214-y
Rubin (2017). Intranasal Insulin for the Treatment of HAND. Available online at: https://clinicaltrials.gov/show/NCT03081117
Sasaoka, T., Wada, T., and Tsuneki, H. (2014). [Insulin resistance and cognitive function]. Nippon. Rinsho 72, 633–640.
Tashima, T. (2020). Shortcut approaches to substance delivery into the brain based on intranasal administration using nanodelivery strategies for insulin. Molecules 25, 5188. doi: 10.3390/molecules25215188
Xiaojiu, L., and Xuan, B. (2015). The effect of intranasal instillation of insulin on cognitive function in patients with Alzheimer disease. Chinese J. Postgraduates Med. 38, 25–28. doi: 10.3760/cma.j.issn.16734904.2015.01.009
Yanfang, M. (2016). The function and mechanism of intranasal insulin on Alzhemer's disease (Doctoral Dissertation, Zhejiang university).
Yufeng, W. (2020). Effects of intranasal insulin on Cognitive Function and Regulation of Brain Network in Parkinson's disease Patients (D). North Sichuan Medical College.
Keywords: dementia, MCI (mild cognitive impairment), cognitive, intranasal insulin, systematic review, meta-analysis
Citation: Long C, Han X, Yang Y, Li T, Zhou Q and Chen Q (2022) Efficacy of intranasal insulin in improving cognition in mild cognitive impairment or dementia: a systematic review and meta-analysis. Front. Aging Neurosci. 14:963933. doi: 10.3389/fnagi.2022.963933
Received: 08 June 2022; Accepted: 23 August 2022;
Published: 12 September 2022.
Edited by:
Xianliang Zhang, Shandong University, ChinaReviewed by:
Antonio E. Pontiroli, University of Milan, ItalyJim R. Fadel, University of South Carolina, United States
Fang Huang, Huazhong University of Science and Technology, China
Copyright © 2022 Long, Han, Yang, Li, Zhou and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiu Chen, Y2hlbnFpdTEwMDVAY2R1dGNtLmVkdS5jbg==
 Cong Long
Cong Long Xuke Han1,2
Xuke Han1,2 Yunjiao Yang
Yunjiao Yang Tongyi Li
Tongyi Li Qiu Chen
Qiu Chen