
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 21 September 2022
Sec. Neurocognitive Aging and Behavior
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.963421
 Xin Ma1,2†
Xin Ma1,2† Xinchun Mei1,2†
Xinchun Mei1,2† Tianyi Tang1,2
Tianyi Tang1,2 Meijuan Wang1
Meijuan Wang1 Xiaoyi Wei1,2
Xiaoyi Wei1,2 Hailin Zheng1,2
Hailin Zheng1,2 Jing Cao2
Jing Cao2 Hui Zheng3
Hui Zheng3 Kathryn Cody4
Kathryn Cody4 Lize Xiong5
Lize Xiong5 Edward R. Marcantonio6
Edward R. Marcantonio6 Zhongcong Xie7*‡
Zhongcong Xie7*‡ Yuan Shen2,8*‡
Yuan Shen2,8*‡Background: Homocysteine and C-reactive protein (CRP) may serve as biomarkers of postoperative delirium. We set out to compare the role of blood concentration of homocysteine versus CRP in predicting postoperative delirium in patients.
Materials and methods: In this prospective observational cohort study, the plasma concentration of preoperative homocysteine and postoperative CRP was measured. Delirium incidence and severity within 3 days postoperatively were determined using the Confusion Assessment Method and Confusion Assessment Method-Severity algorithm.
Results: Of 143 participants [69% female, median (interquartile range, 25th–75th) age of 71 (67–76) years] who had knee or hip surgery under general anesthesia, 44 (31%) participants developed postoperative delirium. Postoperative plasma concentration of CRP was associated with postoperative delirium incidence [adjusted odds ratio (OR) per one standard deviation change in CRP: 1.51; 95% Confidence Interval (CI): 1.05, 2.16; P = 0.026], and severity [in which each one standard deviation increase in postoperative CRP was associated with a 0.47 point (95% CI: 0.18–0.76) increase in the severity of delirium, P = 0.002] after adjusting age, sex, preoperative Mini-Mental State Examination score and the days when postoperative CRP was measured. A statistically significant interaction (adjusted P = 0.044) was also observed, in which the association between postoperative plasma concentration of CRP and postoperative delirium incidence was stronger in the participants with lower preoperative plasma concentrations of homocysteine compared to those with higher preoperative levels.
Conclusion: Pending validation studies, these data suggest that preoperative plasma concentration of homocysteine modifies the established association between postoperative plasma concentration of CRP and postoperative delirium incidence.
Postoperative delirium, an acute confusion status after anesthesia and surgery, is associated with adverse effects with annual care costs of $ 32.9 billion (Gou et al., 2021). Population studies have demonstrated that patients with delirium may face a 12.5-fold increased incidence of newly diagnosed Alzheimer’s disease (AD) (Fong et al., 2009, 2015; Witlox et al., 2010; Ags/Nia Delirium Conference Writing Group and Faculty, 2015).
Inflammation is emerging as a candidate process propagating postoperative delirium, mainly via increased production of pro-inflammatory cytokines in the blood and brain (Wilson et al., 2002; Kalman et al., 2006; Ramlawi et al., 2006a,b; Rudolph et al., 2008). Almost every patient has postoperative inflammation, but not every patient develops postoperative delirium. Thus, inflammation alone may be insufficient to explain postoperative delirium. Instead, patients who develop postoperative delirium may have other changes (predisposing factors) that exacerbate the postoperative inflammation (precipitating factor), leading to postoperative delirium. Therefore, it is crucial to study the contributions of both predisposing and precipitating factors—and their interaction—to the development of postoperative delirium.
Hyperhomocysteine (elevated plasma homocysteine concentration) is a potential predisposing factor owing to its involvement in cardiovascular disease (Refsum et al., 1998) and AD (Morris, 2003). Preoperative plasma hyperhomocysteine concentration is associated with postoperative delirium (Kim et al., 2015; Weerink et al., 2018), but conflicting results exist (Vahdat Shariatpanahi et al., 2019; Guo et al., 2020). Moreover, hyperhomocysteine increases blood-brain barrier (BBB) permeability (Kamath et al., 2006; Beard et al., 2011) and promotes neuroinflammation, which can lead to cognitive dysfunction (Adamis et al., 2009; Varatharaj and Galea, 2017). C-reactive protein (CRP) is associated with postoperative delirium (Dillon et al., 2017; Vasunilashorn et al., 2017). However, it remains largely unknown whether the established association between the postoperative blood concentration of CRP (a precipitating factor) and postoperative delirium depends on preoperative predisposing factors such as preoperative blood homocysteine concentration.
A recent study demonstrated the interaction between gene Apolipoprotein E (a predisposing factor) and postoperative CRP (a protein and precipitating factor) on postoperative delirium (Vasunilashorn et al., 2020). However, the protein-protein interaction of predisposing factors and precipitating factors on the incidence and severity of postoperative delirium has not been investigated.
Therefore, we set out to determine the effects of preoperative plasma concentration of homocysteine, the postoperative plasma concentration of CRP, and their interactions on the incidence and severity of postoperative delirium in patients. It was hypothesized that preoperative plasma concentrations of homocysteine would modify the established association between postoperative plasma concentration of CRP and postoperative delirium in patients.
Preoperative homocysteine and postoperative CRP were evaluated in this study because postoperative CRP is an established blood biomarker of postoperative delirium (Dillon et al., 2017; Vasunilashorn et al., 2017; Slor et al., 2019). However, the role of preoperative homocysteine in postoperative delirium has not been fully revealed (Kim et al., 2015; Weerink et al., 2018; Vahdat Shariatpanahi et al., 2019; Guo et al., 2020). Moreover, we chose homocysteine and CRP, but not other inflammatory factors, e.g., S100β and interleukin 6, in the present study because we aimed to use preoperative homocysteine and postoperative CRP to specifically investigate the effects of interaction between the predisposing factor (preoperative homocysteine) and the precipitating factor (postoperative CRP) on the postoperative delirium.
We performed a prospective observational cohort study from June 22, 2016 to May 5, 2017, at Shanghai Tenth People’s Hospital, a university-affiliated hospital in Shanghai, P. R. China. The study protocol was approved by the hospital’s Human Research Ethics Committee (SHSY-IEC-3.0/15-78/01) on May 12, 2016.
All participants provided written informed consent for the study before initiating any study procedures. This study is being reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) criteria.
We screened patients scheduled for knee/hip replacement surgeries. Participants were included if they: (1) were 60 years old or older; (2) spoke Mandarin Chinese; (3) had general anesthesia, and (4) were able to provide informed consent. Patients were excluded if they had any of the following: (1) pre-existing delirium assessed according to the Confusion Assessment Method (CAM) algorithm (Inouye et al., 1990); (2) prior neurologic diseases (e.g., dementia, Parkinson’s disease, multiple sclerosis or stroke) according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision (ICD-10-version, 2016); (3) a history of mental disorders (e.g., major depressive disorder and schizophrenia) according to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association, 1997); (4) had abnormal cognitive function at the time of enrollment, evidenced by a Mini-Mental State Examination (MMSE) score above an education-adjusted threshold (18 for individuals with no school education, 20 for 1–6 years of education, or 24 for 7 or more years of education (Li et al., 2016), (5) pre-existing fever/infection, (6) significant past medical history, or (7) unwillingness to comply with the assessments.
Preoperative assessments were performed one day before the scheduled surgery by well-trained researchers following a standard protocol. Participant characteristics were collected, including age, sex, education, body mass index (BMI), and Charlson Comorbidity Index (CCI) (Charlson et al., 1994). Preoperative cognitive function was assessed using the MMSE (Chinese version) (Li et al., 2016). The CAM algorithm (Inouye et al., 1990) was also performed preoperatively to exclude patients with pre-existing delirium. The Visual Analog Scale (VAS, ranging from 0 to 10) was used to assess subjective pain the day before surgery.
All participants underwent a hip or knee replacement under general anesthesia. The preoperative fasting, anesthetics used, airway management, opioid and neuromuscular blocking agent (and the reversal) usage, intravenous fluid administration, and mechanical ventilation were performed per the hospital policy and at the anesthesiologist’s discretion. The participants generally received 1–2 mg midazolam preoperatively. Anesthesia was induced with propofol (2 mg/kg), sufentanil (0.5–1 μg/kg), and cisatracurium (0.5 mg/kg) and was maintained with anesthetic sevoflurane or propofol. We obtained information regarding the American Society of Anesthesiologists (ASA) Physical Status Classification System (ASA Physical Status Classification System, 2014), surgery types, length of anesthesia duration, and length of surgery duration by reviewing the anesthesia records of the participants. All the patients received the standard postoperative analgesia. Moreover, the VAS was used to assess subjective pain on postoperative days 1, 2, and 3.
We collected 4 ml of venous blood during the insertion of intravenous catheters before the anesthesia and surgery in all participants to measure preoperative plasma concentrations of homocysteine. The blood sample was collected in anticoagulant tubes and was immediately centrifuged to collect the supernatant plasma. The plasma was stored in a −80°C freezer until measurement. Preoperative plasma concentration of homocysteine was determined by using the Roche Cobas 8000 system (Roche Diagnostic, Rotkreuz, Switzerland) with the enzyme cycling method.
Postoperative measurement of blood CRP was part of the clinical care of the patients. Thus, we obtained the postoperative blood CRP concentrations by checking the participants’ medical records. If participants had several postoperative CRP measurements, the concentration of the first postoperative measurement of plasm CRP was used for the final data analysis in the present study. Since the postoperative CRP measurement was part of the routine postoperative clinical care, the time of the postoperative blood collection (i.e., postoperative day) was not fixed on a particular day. Notably, in the clinical laboratory, the postoperative plasma concentrations of CRP were measured using an immunonephelometric method on a Nephelometer BNII (Siemens Healthcare, Germany), measuring a range from 3–200 mg/L.
The primary outcome was the presence of delirium at any postoperative assessments performed in the first three days postoperatively. Postoperative delirium was determined by daily interviews on postoperative days 1, 2, and 3 using the CAM (Inouye et al., 1990). Each participant was assessed with CAM twice daily between 8–10 am and 4–6 pm. Patients were considered delirious if delirium was present on any of these assessments. Patients discharged prior to day three were excluded from the analysis. Participants were included in the data analysis as long as they were assessed for delirium at least once per day following surgery. We did not use CAM-ICU in the present study.
The severity of delirium was assessed as a secondary outcome. The severity of postoperative delirium was quantified using the CAM-Severity (CAM-S) long-form (Inouye et al., 2014), comprising 10 items capturing delirium features (range from 0 to 19). The Chinese version of CAM-S has demonstrated good reliability and validity among Chinese older adults (Mei et al., 2019). The peak (worst) CAM-S scores across all postoperative days were used to assess delirium severity for the data analysis (Vasunilashorn et al., 2020).
We estimated that 140 participants would provide 90% power to detect the potential difference in postoperative plasma concentration of CRP between the participants with and without postoperative delirium at a 5% significance level. This estimation was based on a previous study of elderly patients aged 65 years old. The patients who developed postoperative delirium had higher postoperative CRP concentrations than those who did not: 10.26 ± 5.81 mg/dL versus 6.96 ± 4.89 mg/dL (Lee et al., 2011).
According to our previous study, the estimated incidence of postoperative delirium was 25.6% (Shi et al., 2014), so we set the ratio of the participants who developed postoperative delirium to normal participants as 1 to 3. We set an estimated drop-out rate of 15% during postoperative assessment (Sakpal, 2010). Thus, we determined that we should enroll 165 participants in the study to have 140 participants for the final data analysis.
The study included the data analysis of plasma concentrations of preoperative homocysteine, postoperative CRP, and the incidence and severity of postoperative delirium. Notably, all postoperative CRP measurements were performed as part of routine clinical care, mostly occurring within 1–3 days after surgery. The statistical analysis plan was finalized before analyzing the data.
Normally distributed continuous variables (e.g., BMI) and non-normally distributed continuous variables (e.g., the plasma concentration of homocysteine and CRP) are presented as the mean ± standard deviation (SD) and median [interquartile range (IQR), 25th–75th], respectively. As appropriate, the differences between patients who did and did not develop delirium were assessed with an independent samples t-test or Mann–Whitney U test. Categorical variables (e.g., sex, ASA, and CCI) are presented as frequencies and proportions and assessed with chi-square or Fisher’s exact test.
Preoperative homocysteine and postoperative CRP were scaled using z-scores in all models for analysis, which were calculated by subtracting the mean from an individual raw score and then dividing the difference by the standard deviation. The association between preoperative plasma concentration of homocysteine, the postoperative plasma concentration of CRP, and postoperative delirium incidence were assessed using logistic regression. Results are presented as odds ratio (OR) per one standard deviation change in the biomarker and their associated 95% confidence intervals (CI). The association between the biomarkers and the worst delirium severity was estimated using linear regression, with results reported as a mean difference [beta coefficient (β)] and it’s associated 95% CI. Model fit assuming a Gaussian distribution was evaluated by examining the residuals and model calibration.
The association between preoperative homocysteine and postoperative plasma concentration of CRP was assessed for both the primary (delirium incidence) and secondary (delirium severity) outcomes. Both predictors were evaluated in separate univariate models with each of the main effects, one model including postoperative CRP and preoperative homocysteine, and the final model including the main effects and the inclusion of an interaction term between preoperative homocysteine and postoperative CRP together. In order to visualize the interaction between postoperative CRP and preoperative homocysteine, the relationship between postoperative CRP and the predicted probability of postoperative delirium was reported for different preoperative plasma concentrations of homocysteine, namely the 10th, 25th, 50th, 75th, and 90th percentiles. Multivariable models were then created to adjust the associations between the biomarkers and outcomes for age, sex, preoperative MMSE, and day of postoperative CRP measurement for both the primary and secondary outcomes. These variables were selected for the adjustment based on previous studies as deemed clinically relevant while still considering model parsimony. Pearson Correlation was used to determine the relationship between pre-operative homocysteine and postoperative CRP. SPSS version 22.0 (SPSS Inc., Chicago, IL, United States) and R (version 4.0.5, Vienna, Austria) were used to analyze the data. Two-sided p-values less than 0.05 were considered statistically significant for all analyses.
A total of 258 patients were screened for inclusion in the study. Among them, 90 participants were excluded because they declined the required preoperative assessment of delirium (N = 65), had abnormal cognitive function (N = 6), did not plan to have general anesthesia or changed anesthesia (N = 12), had prior neurologic diseases (N = 3), had an infection/fever (N = 1), and had a significant past medical history (N = 3). Thus, 168 participants were enrolled in the study. After obtaining the consent, 25 participants were excluded because they were not interested in further participation in the study (N = 18), or canceled surgery (N = 7). Participants in the present study did not include patients who took psychoactive drugs to treat mental disorders or neurological diseases. No major complications occurred during the immediate postoperative period. There were no missing data for variables of interest in the current study. The final data analysis included 143 participants [69% female, median age 71 (IQR: 67–76) years] who underwent knee (88.0%) or hip (12.0%) surgery (Figure 1).
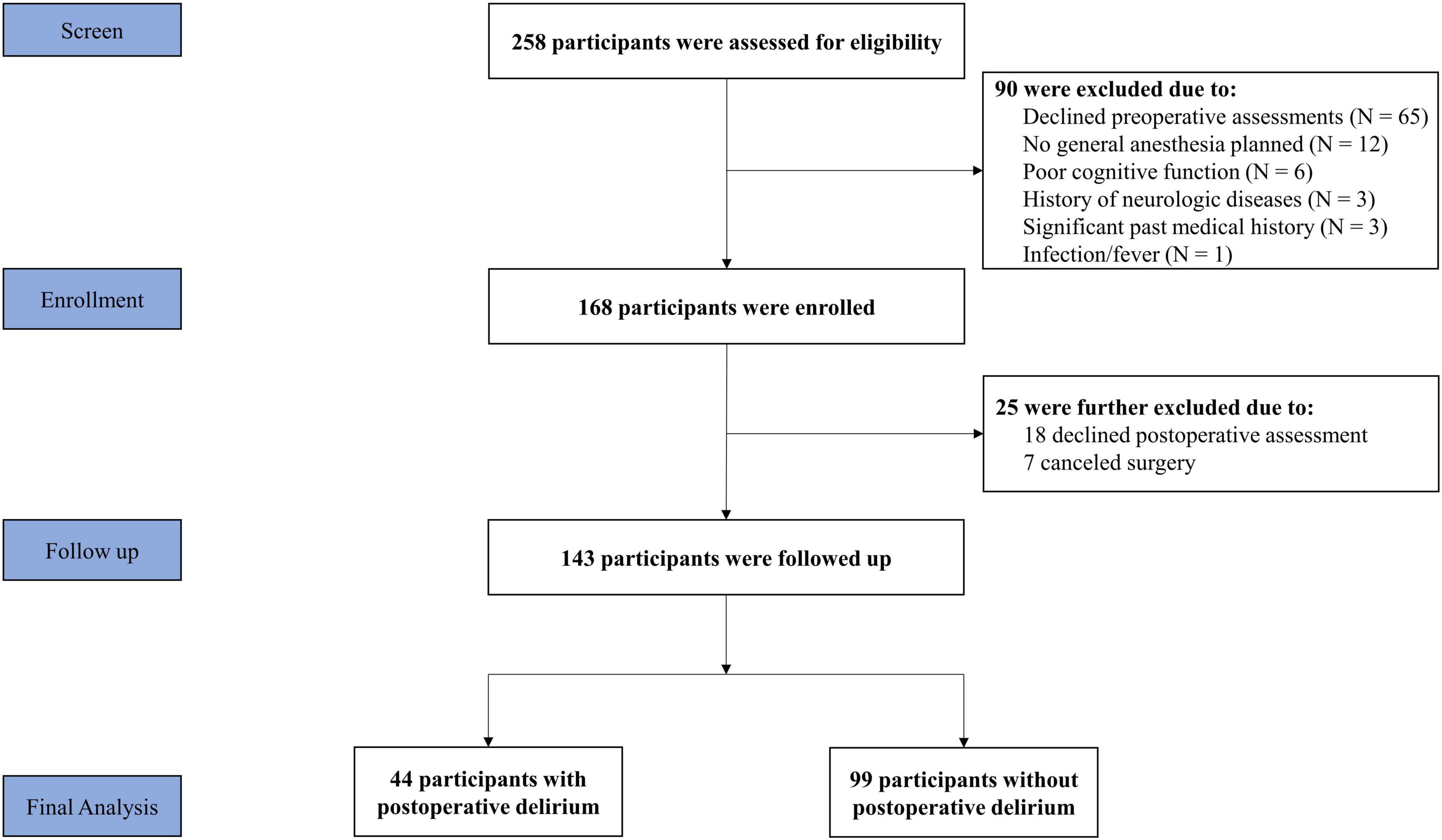
Figure 1. Flow Diagram. Two hundred and fifty-eight participants were initially screened for the study. Ninety participants were excluded, resulting in 168 participants included at the enrolment of the study. During the follow-up assessment, another 25 participants were lost. Thus, a total of 143 participants were included in the final data analysis.
Notably, postoperative CRP was measured on different days. However, there was no statistically significant difference in the percentage of participants on each of the postoperative days when the postoperative plasma concentrations of CRP were measured between the cohort with postoperative delirium and the cohort without postoperative delirium (Supplementary Figure 1).
Delirium was assessed twice daily up to postoperative day three. Among the 143 included participants, 135 (94.4%) patients received all six assessments, seven (4.9%) participants received five assessments, and one (0.7%) participant received four assessments. Forty-four of the 143 participants (31%) developed postoperative delirium. There were no statistically significant differences in demographic, clinical characteristics, type of surgery, anesthesia, and other perioperative factors between participants with (N = 44) and without (N = 99) postoperative delirium, except for the length of surgery duration, the preoperative and postoperative day 2 VAS scores (Table 1).
Participants who developed postoperative delirium had higher median preoperative plasma concentration of homocysteine [15.43 (12.23, 21.26) mmol/L versus 12.51 (10.42, 16.32) mmol/L, P = 0.036] and postoperative plasma concentration of CRP [61.60 (40.48, 106.31) mg/L versus 46.50 (27.60, 78.20), mg/L, P < 0.001] than the participants without postoperative delirium (Table 1).
The postoperative plasma concentration of CRP was associated with the incidence of postoperative delirium before and after adjusting for age, sex, preoperative MMSE, and day of postoperative CRP measurement (adjusted OR per one standard deviation change in CRP: 1.51; 95% CI: 1.05–2.16; P = 0.026; Table 2). However, the preoperative plasma concentration of homocysteine was not associated with the incidence of postoperative delirium (Table 2).
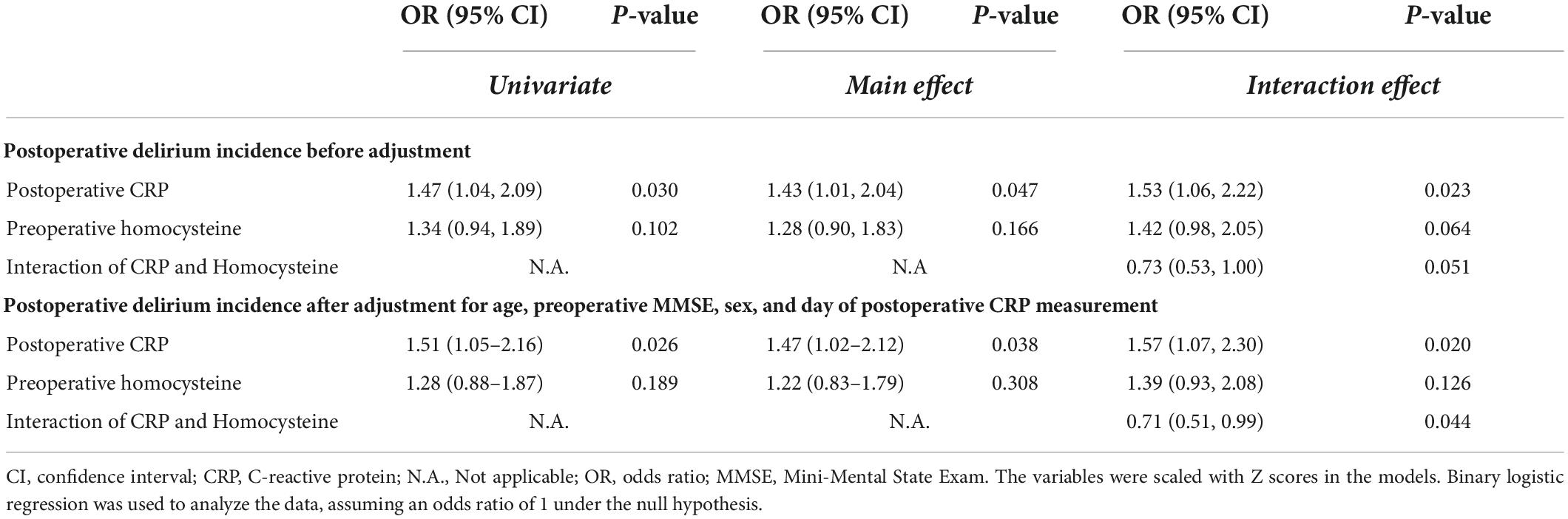
Table 2. The association between the incidence of postoperative delirium and the preoperative homocysteine, postoperative C-reactive protein, or their interaction.
Of note, a statistically significant interaction was observed between preoperative plasma concentration of homocysteine and postoperative plasma concentration of CRP on the incidence of postoperative delirium (P = 0.044, Table 2). Specifically, participants with lower preoperative homocysteine demonstrated a stronger association between postoperative CRP and postoperative delirium incidence than participants with higher levels of preoperative homocysteine. This is demonstrated in Figure 2, in which participants with 10th (9.23 mmol/L), 25th (10.81 mmol/L), or 50th percentile (13.16 mmol/L) of preoperative homocysteine plasma concentration demonstrated a stronger association between the postoperative plasma concentration of CRP and the postoperative delirium incidence when compared with participants in the 75th percentile (18.79 mmol/L). Further, in the participants with the 90th percentile (24.46 mmol/L) of preoperative homocysteine, the association between postoperative plasma concentration of CRP and postoperative delirium incidence disappeared. These data suggest that the association between the postoperative plasma concentration of CRP and the postoperative delirium incidence can be modified by the preoperative plasma concentrations of homocysteine.
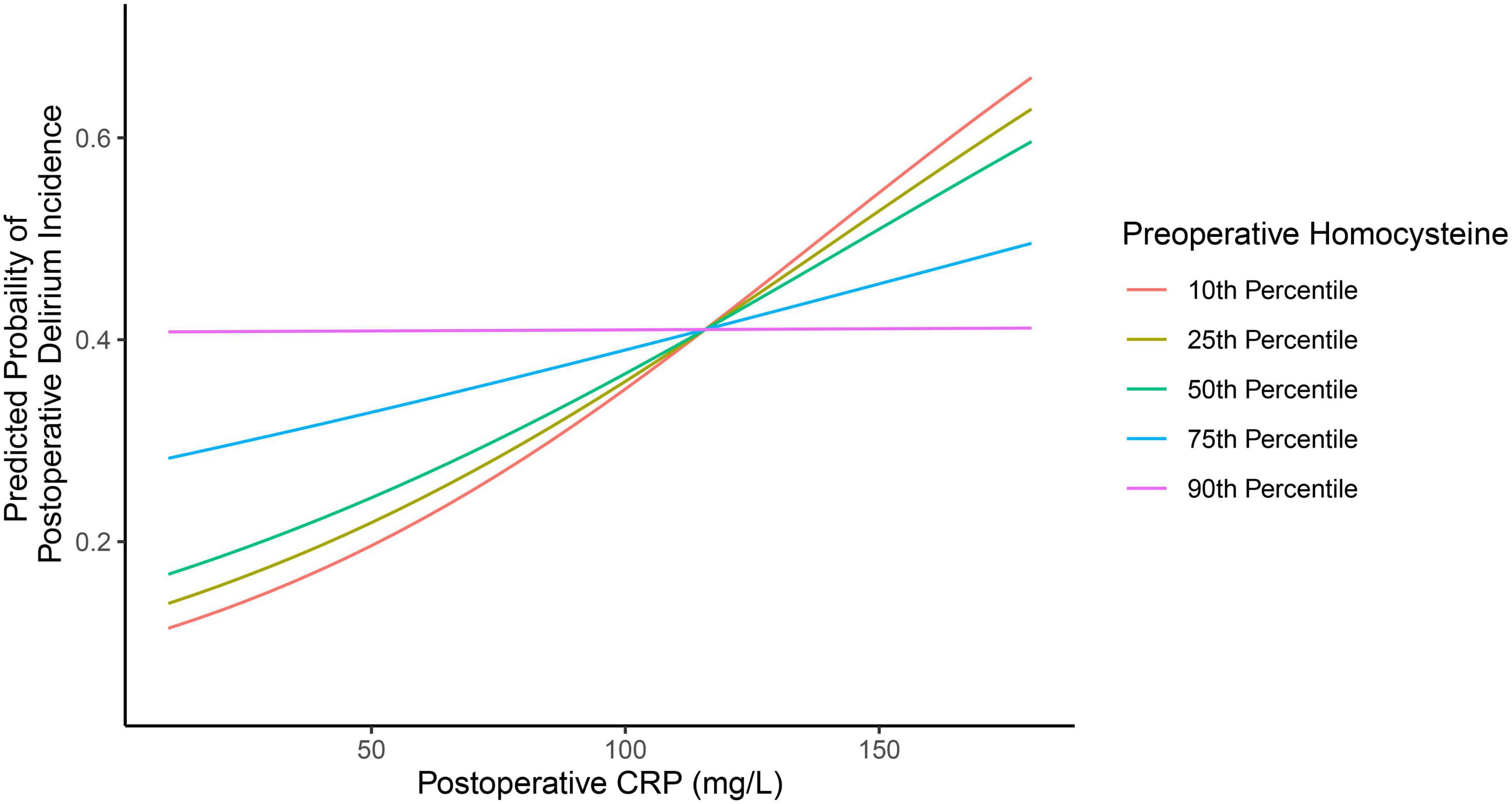
Figure 2. Different preoperative plasma concentrations of homocysteine lead to different associations between postoperative plasma concentrations of CRP and postoperative delirium incidence. There are different associations between the postoperative plasma concentration of CRP and the postoperative delirium incidence in the participants, with the preoperative plasma concentration of homocysteine at the 10th percentile, 25th percentile, 50th percentile, 75th percentile, and 90th percentile. The data suggest that the preoperative plasma concentration of homocysteine can modify the association between the postoperative plasma concentration of CRP and the postoperative delirium incidence. CRP, C-reactive protein.
Moreover, preoperative plasma homocysteine was not associated with postoperative plasma CRP before and after adjusting for age and sex (before adjustment: R = 0.125, P = 0.138; after adjustment: R = 0.126, P = 0.135; Pearson Correlation).
We demonstrated that the participants with higher postoperative CRP concentrations were more likely to have higher CAM-S peak scores before and after adjusting age, sex, preoperative MMSE score, and day of postoperative CRP measurement (mean difference [β] in delirium severity per one standard deviation increase in CRP: 0.47 points; 95% CI: 0.18–0.76, P = 0.002, Table 3). However, there was no univariate, main, or interaction effect of preoperative plasma concentration of homocysteine on predicting the severity of postoperative delirium, suggesting that preoperative plasma concentration of homocysteine did not modify the association between postoperative plasma concentration of CRP and the severity of postoperative delirium.
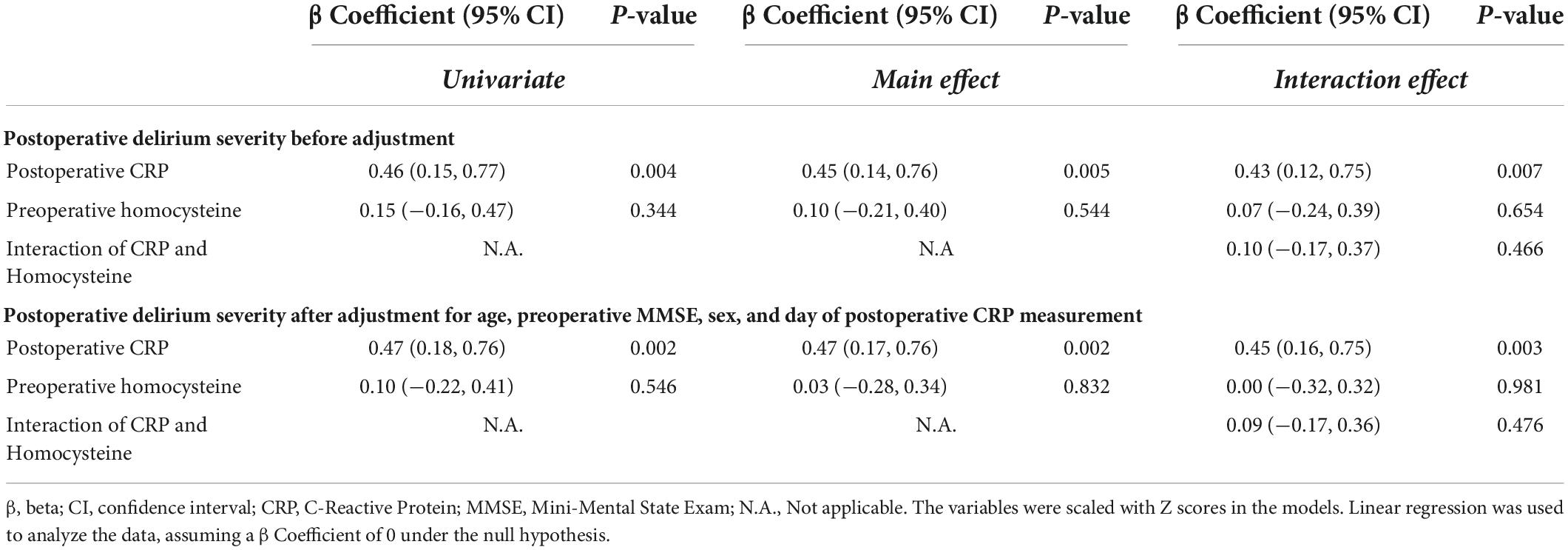
Table 3. The association between the severity of postoperative delirium and the preoperative homocysteine, postoperative C-reactive protein, or their interaction.
The data demonstrated an interaction of preoperative plasma homocysteine and postoperative plasma CRP on the incidence, but not severity, of postoperative delirium in patients. As a predisposing factor, the preoperative plasma homocysteine modified the established association between the postoperative plasma CRP, a precipitating factor, and the incidence of postoperative delirium. Specifically, the postoperative plasma concentration of CRP was associated with the postoperative delirium incidence in the patients with lower, but not higher, preoperative plasma concentrations of homocysteine. Pending further investigation, these data raise the possibility that preoperative blood homocysteine concentration may modify the relationship between postoperative blood CRP concentration and the incidence of postoperative delirium, suggesting that postoperative blood CRP amount may only predict postoperative delirium in specific, but not all, conditions.
We did not find an interaction between pre-operative plasma homocysteine and postoperative CRP on the severity of postoperative delirium. The reasons for such findings are not known at present. However, similar disassociation between incidence and severity of postoperative delirium has also been observed in previous studies (Larsen et al., 2010; Van Norden et al., 2021). Van Norden, J. et al. found that the peri-operative administration of dexmedetomidine was associated with a lower incidence, but not severity, of postoperative delirium (Van Norden et al., 2021). Larsen, K. A. et al. found that preoperative administration of olanzapine was associated with a significantly lower incidence of delirium but a longer duration and greater severity of postoperative delirium (Larsen et al., 2010).
A previous study showed that the participants with plasma concentrations of CRP greater than 235.73 mg/mL on postoperative day two were 1.5 times more likely to develop postoperative delirium with more severe symptoms (Vasunilashorn et al., 2017). Patients with postoperative delirium had a higher plasma concentration of CRP from postoperative days 2–5 (Slor et al., 2019). Consistently, we confirmed that the high postoperative plasma concentration of CRP was associated with postoperative delirium incidence and severity. Moreover, we further demonstrated that the established association between the postoperative plasma concentration of CRP and the postoperative delirium incidence could be modified by the preoperative plasma concentrations of homocysteine.
Preoperative plasma homocysteine is associated with postoperative delirium in patients after cancer surgery (Weerink et al., 2018) or hemiarthroplasty (Guo et al., 2020). However, another study showed no association between preoperative plasma homocysteine and the incidence of postoperative delirium in patients with cardiac surgery (Vahdat Shariatpanahi et al., 2019). Here, we showed that participants with postoperative delirium had higher median preoperative plasma concentrations of homocysteine than participants without postoperative delirium after hip or knee surgery. However, the preoperative plasma concentrations of homocysteine were not associated with the incidence or severity of postoperative delirium. Nevertheless, the present study showed that the preoperative plasma concentrations of homocysteine modified the established association between postoperative plasma concentration of CRP and postoperative delirium incidence.
The association between the postoperative plasma concentration of CRP and the incidence, duration, and severity of postoperative delirium can be modified by the apolipoprotein E (APOE) 4 genotype (Vasunilashorn et al., 2020) and the catechol-O-methyltransferase (COMT) genotype (Vasunilashorn et al., 2019). Specifically, the association between postoperative blood CRP concentration and postoperative delirium occurred in patients with APOE4, but not non-APOE4, carriers (Vasunilashorn et al., 2020), suggesting the contribution of gene-protein interaction to the development of postoperative delirium. In the present study, the association between the postoperative blood CRP concentration and postoperative delirium only occurred in patients with lower, but not higher, the preoperative blood concentration of homocysteine, suggesting the contribution of protein-protein interaction to the development of postoperative delirium.
Moreover, given that genotype is innate and cannot be modulated by intervention, our findings suggest a potential practical pathway for the intervention of postoperative delirium. We previously showed that preoperative dietary supplementation of VitB12 and folic acid could reduce cognitive impairment in aged mice by lowering homocysteine concentrations (Zhao et al., 2019). Thus, future studies could include exploring the possibility of reducing homocysteine concentrations as a potential targeted intervention for postoperative delirium.
Hyperhomocysteine causes BBB impairment (Tyagi et al., 2005; Kamath et al., 2006; Beard et al., 2011). CRP, produced by hepatocytes and then released into the blood (Sproston and Ashworth, 2018), can induce neuroinflammation and neurotoxicity (Song et al., 2015). Participants with hyperhomocysteine may have increased BBB permeability. Thus, mild inflammation, represented by a slight increase of postoperative plasma CRP, can promote postoperative delirium. On the other hand, participants without hyperhomocysteine may not have increased BBB permeability. Thus, greater postoperative concentrations of CRP are needed to develop postoperative delirium in the patients. More studies to test this hypothesis are warranted in the future.
There were several limitations of the present studies. First, the postoperative plasma concentrations of CRP were measured on different days postoperatively. However, there was no statistically significant difference in the percentage of participants whose postoperative plasma concentrations of CRP were measured on each of the postoperative days between the participants with postoperative delirium and the participants without postoperative delirium (Supplementary Figure 1). More importantly, the preoperative plasma concentration of homocysteine still modified the association between the postoperative plasma concentration of CRP and the incidence of postoperative delirium after adjusting the days when postoperative CRP amounts were measured (Table 2). Therefore, the conclusion that preoperative plasma concentration of homocysteine modified the association between the postoperative plasma concentrations of CRP and postoperative delirium incidence was not changed by the fact that postoperative plasma concentrations of CRP were measured on different days after the anesthesia/surgery. Second, 69% of the participants in the present study were female, and most had knee surgery. Thus, the present study’s findings need to be validated for broader applicability to male patients and other types of surgery. Third, it should be noted that the patients with postoperative delirium had worse pre- and postoperative pain scores than those without postoperative delirium. These data suggest pain can contribute to postoperative delirium, consistent with the results from the previous studies (Vaurio et al., 2006; Ma et al., 2022). Finally, the patients who developed postoperative delirium had a longer duration of surgery but not a longer duration of general anesthesia. These findings suggest that surgery contributes more to the development of postoperative delirium, consistent with the results from the recent studies that there was no significant difference in the incidence of postoperative delirium between the patients who underwent surgery with general anesthesia and the patients who underwent surgery with regional anesthesia (Neuman et al., 2021; Li et al., 2022).
In conclusion, the postoperative plasma concentration of CRP, but not the preoperative plasma concentration of homocysteine, was associated with the incidence and severity of postoperative delirium. However, the preoperative plasma concentration of homocysteine could modify the established association between the postoperative plasma concentration of CRP and the incidence of postoperative delirium. Pending further confirmative investigations, these findings suggest the regulation effects of predisposing factors on the established association of precipitating factors and postoperative delirium, promoting more research to reveal the pathogenesis, biomarkers, and targeted interventions of postoperative delirium in patients.
The data are available from the corresponding authors on reasonable request.
The studies involving human participants were reviewed and approved by the Human Research Ethics Committee of Shanghai Tenth People’s Hospital (SHSY-IEC-3.0/15-78/01) on May 12, 2016. The patients/participants provided their written informed consent to participate in this study.
YS and ZX: study concept and design. XM, XCM, TT, MW, XW, HLZ, and JC: acquisition of data. HZ, KC, EM, ZX, and YS: analysis and interpretation of data. ZX, XM, and YS: drafting of the manuscript. KC, LX, and EM: critical manuscript revision for important intellectual content. XCM, LX, and YS: obtained funding. YS and XM: administrative, technical, and material support. YS: study supervision. All authors contributed to the article and approved the submitted version.
This study was supported by grants No. 81720108012 and No. 81571034 from China’s National Natural Science Foundation (YS). EM was partly funded by a Mid-Career Investigator Award (K24AG035075) from the National Institute on Aging. ZX was supported by Henry K. Beecher Professorship from Harvard University. LX was supported by grants from the State Key Program of the National Natural Science Foundation of China (No. 81730032). XCM was funded by grant from China’s National Natural Science Foundation (No. 82101252).
The Confusion Assessment Method (CAM) algorithm used in the current study was adapted from Inouye SK et al. Ann Intern Med.1990; 113: 941–948. Confusion Assessment Method: Copyright 2003, Hospital Elder Life Program, LLC.
ZX provided consulting services to Shanghai 9th and 10th hospitals, and Baxter (invited speaker) in last 36 months.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.963421/full#supplementary-material
Adamis, D., Lunn, M., Martin, F. C., Treloar, A., Gregson, N., Hamilton, G., et al. (2009). Cytokines and Igf-I in delirious and non-delirious acutely ill older medical inpatients. Age Ageing 38, 326–332. doi: 10.1093/ageing/afp014
Ags/Nia Delirium Conference Writing Group and Faculty (2015). The American Geriatrics Society/National Institute on Aging Bedside-to-Bench Conference: Research Agenda on Delirium in Older Adults. J. Am. Geriatr. Soc. 63, 843–852. doi: 10.1111/jgs.13406
American Psychiatric Association (1997). Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: APA.
ASA Physical Status Classification System (2014). Guidelines, statements, clinical resources. Available online at: https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system
Beard, R. S. Jr., Reynolds, J. J., Bearden, S. E. J. B., and The Journal Of The American Society Of Hematology (2011). Hyperhomocysteinemia increases permeability of the blood-brain barrier by Nmda receptor-dependent regulation of adherens and tight junctions. Blood 118, 2007–2014. doi: 10.1182/blood-2011-02-338269
Charlson, M., Szatrowski, T. P., Peterson, J., and Gold, J. (1994). Validation of a combined comorbidity index. J. Clin. Epidemiol. 47, 1245–1251. doi: 10.1016/0895-4356(94)90129-5
Dillon, S. T., Vasunilashorn, S. M., Ngo, L., Otu, H. H., Inouye, S. K., Jones, R. N., et al. (2017). Higher C-Reactive Protein Levels Predict Postoperative Delirium in Older Patients Undergoing Major Elective Surgery: A Longitudinal Nested Case-Control Study. Biol. Psychiatry 81, 145–153. doi: 10.1016/j.biopsych.2016.03.2098
Fong, T. G., Davis, D., Growdon, M. E., Albuquerque, A., and Inouye, S. K. (2015). The interface between delirium and dementia in elderly adults. Lancet Neurol. 14, 823–832. doi: 10.1016/S1474-4422(15)00101-5
Fong, T. G., Jones, R. N., Shi, P., Marcantonio, E. R., Yap, L., Rudolph, J. L., et al. (2009). Delirium accelerates cognitive decline in Alzheimer disease. Neurology 72, 1570–1575. doi: 10.1212/WNL.0b013e3181a4129a
Gou, R. Y., Hshieh, T. T., Marcantonio, E. R., Cooper, Z., Jones, R. N., and Travison, T. G. (2021). One-Year Medicare Costs Associated With Delirium in Older Patients Undergoing Major Elective Surgery. JAMA Surg. 156, 430–442. doi: 10.1001/jamasurg.2020.7260
Guo, Y., Li, Y., Zhang, Y., Fang, S., Xu, X., Zhao, A., et al. (2020). Post-operative delirium associated with metabolic alterations following hemi-arthroplasty in older patients. Age Ageing 49, 88–95. doi: 10.1093/ageing/afz132
ICD-10-version (2016). International statistical classification of diseases and related health problems 10th revision. Available online at: http://apps.who.int/classifications/icd10/browse/2016/en
Inouye, S. K., Kosar, C. M., Tommet, D., Schmitt, E. M., Puelle, M. R., Saczynski, J. S., et al. (2014). The Cam-S: Development and validation of a new scoring system for delirium severity in 2 cohorts. Ann. Intern. Med. 160, 526–533. doi: 10.7326/M13-1927
Inouye, S. K., Van Dyck, C. H., Alessi, C. A., Balkin, S., Siegal, A. P., and Horwitz, R. I. (1990). Clarifying confusion: The confusion assessment method: A new method for detection of delirium. Ann. Intern. Med. 113, 941–948. doi: 10.7326/0003-4819-113-12-941
Kalman, J., Juhasz, A., Bogats, G., Babik, B., Rimanoczy, A., Janka, Z., et al. (2006). Elevated levels of inflammatory biomarkers in the cerebrospinal fluid after coronary artery bypass surgery are predictors of cognitive decline. Neurochem. Int. 48, 177–180. doi: 10.1016/j.neuint.2005.10.007
Kamath, A. F., Chauhan, A. K., Kisucka, J., Dole, V. S., Loscalzo, J., Handy, D. E., et al. (2006). Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood 107, 591–593. doi: 10.1182/blood-2005-06-2506
Kim, D. W., Kim, H. K., Bae, E. K., Park, S. H., and Kim, K. K. (2015). Clinical predictors for delirium tremens in patients with alcohol withdrawal seizures. Am. J. Emerg. Med. 33, 701–704. doi: 10.1016/j.ajem.2015.02.030
Larsen, K. A., Kelly, S. E., Stern, T. A., Bode, R. H. Jr., Price, L. L., Hunter, D. J., et al. (2010). Administration of olanzapine to prevent postoperative delirium in elderly joint-replacement patients: A randomized, controlled trial. Psychosomatics 51, 409–418. doi: 10.1176/appi.psy.51.5.409
Lee, H. J., Hwang, D. S., Wang, S. K., Chee, I. S., Baeg, S., and Kim, J. L. (2011). Early assessment of delirium in elderly patients after hip surgery. Psychiatry Investig. 8, 340–347. doi: 10.4306/pi.2011.8.4.340
Li, H., Jia, J., and Yang, Z. (2016). Mini-Mental State Examination in Elderly Chinese: A Population-Based Normative Study. J. Alzheimers Dis. 53, 487–496. doi: 10.3233/JAD-160119
Li, T., Li, J., Yuan, L., Wu, J., Jiang, C., and Daniels, J. (2022). Effect of Regional vs General Anesthesia on Incidence of Postoperative Delirium in Older Patients Undergoing Hip Fracture Surgery: The Raga Randomized Trial. JAMA 327, 50–58. doi: 10.1001/jama.2021.22647
Ma, J. H., Liu, Y. F., Hong, H., Li, C. J., Cui, F., Mu, D. L., et al. (2022). Effect of acute pain on the association between preoperative cognitive impairment and postoperative delirium: A secondary analysis of three trials. Br. J. Anaesth. [Epub ahead of print]. doi: 10.1016/j.bja.2022.06.033
Mei, X., Chen, Y., Zheng, H., Shi, Z., Marcantonio, E. R., Xie, Z., et al. (2019). The Reliability and Validity of the Chinese Version of Confusion Assessment Method Based Scoring System for Delirium Severity (Cam-S). J. Alzheimers Dis. 69, 709–716. doi: 10.3233/JAD-181288
Morris, M. S. (2003). Homocysteine and Alzheimer’s disease. Lancet Neurol. 2, 425–428. doi: 10.1016/S1474-4422(03)00438-1
Neuman, M. D., Feng, R., Carson, J. L., Gaskins, L. J., Dillane, D., Sessler, D. I., et al. (2021). Spinal Anesthesia or General Anesthesia for Hip Surgery in Older Adults. N. Engl. J. Med. 385, 2025–2035. doi: 10.1056/NEJMoa2113514
Ramlawi, B., Rudolph, J. L., Mieno, S., Feng, J., Boodhwani, M., Khabbaz, K., et al. (2006a). C-Reactive protein and inflammatory response associated to neurocognitive decline following cardiac surgery. Surgery 140, 221–226. doi: 10.1016/j.surg.2006.03.007
Ramlawi, B., Rudolph, J. L., Mieno, S., Khabbaz, K., Sodha, N. R., Boodhwani, M., et al. (2006b). Serologic markers of brain injury and cognitive function after cardiopulmonary bypass. Ann. Surg. 244, 593–601. doi: 10.1097/01.sla.0000239087.00826.b4
Refsum, H., Ueland, P. M., Nygård, O., and Vollset, S. E. (1998). Homocysteine and Cardiovascular Disease. Annu. Rev. Med. 49, 31–62. doi: 10.1146/annurev.med.49.1.31
Rudolph, J. L., Ramlawi, B., Kuchel, G. A., Mcelhaney, J. E., Xie, D., Sellke, F. W., et al. (2008). Chemokines are associated with delirium after cardiac surgery. J. Gerontol. A Biol. Sci. Med. Sci. 63, 184–189. doi: 10.1093/gerona/63.2.184
Shi, Z., Wu, Y., Li, C., Fu, S., Li, G., Zhu, Y., et al. (2014). Using the Chinese version of Memorial Delirium Assessment Scale to describe postoperative delirium after hip surgery. Front. Aging Neurosci. 6:297. doi: 10.3389/fnagi.2014.00297
Slor, C. J., Witlox, J., Adamis, D., Jansen, R., Houdijk, A. P. J., Van Gool, W. A., et al. (2019). The trajectory of C-reactive protein serum levels in older hip fracture patients with postoperative delirium. Int. J. Geriatr. Psychiatry 34, 1438–1446. doi: 10.1002/gps.5139
Song, I. U., Chung, S. W., Kim, Y. D., and Maeng, L. S. (2015). Relationship between the hs-Crp as non-specific biomarker and Alzheimer’s disease according to aging process. Int. J. Med. Sci. 12, 613–617. doi: 10.7150/ijms.12742
Sproston, N. R., and Ashworth, J. J. (2018). Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 9:754. doi: 10.3389/fimmu.2018.00754
Tyagi, S. C., Lominadze, D., and Roberts, A. M. (2005). Homocysteine in microvascular endothelial cell barrier permeability. Cell Biochem. Biophys. 43, 37–44. doi: 10.1385/CBB:43:1:037
Vahdat Shariatpanahi, M., Velayati, A., Jamalian, S. A., Babevaynejad, M., and Vahdat Shariatpanahi, Z. (2019). The relationship between serum cobalamin, folic acid, and homocysteine and the risk of post-cardiac surgery delirium. Neuropsychiatr. Dis. Treat. 15, 1413–1419. doi: 10.2147/NDT.S201620
Van Norden, J., Spies, C. D., Borchers, F., Mertens, M., Kurth, J., Heidgen, J., et al. (2021). The effect of peri-operative dexmedetomidine on the incidence of postoperative delirium in cardiac and non-cardiac surgical patients: A randomised, double-blind placebo-controlled trial. Anaesthesia 76, 1342–1351. doi: 10.1111/anae.15469
Varatharaj, A., and Galea, I. (2017). The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 60, 1–12. doi: 10.1016/j.bbi.2016.03.010
Vasunilashorn, S. M., Dillon, S. T., Inouye, S. K., Ngo, L. H., Fong, T. G., Jones, R. N., et al. (2017). High C-Reactive Protein Predicts Delirium Incidence, Duration, and Feature Severity After Major Noncardiac Surgery. J. Am. Geriatr. Soc. 65, e109–e116. doi: 10.1111/jgs.14913
Vasunilashorn, S. M., Ngo, L. H., Inouye, S. K., Fong, T. G., Jones, R. N., Dillon, S. T., et al. (2020). Apolipoprotein E genotype and the association between C-reactive protein and postoperative delirium: Importance of gene-protein interactions. Alzheimers Dement. 16, 572–580. doi: 10.1016/j.jalz.2019.09.080
Vasunilashorn, S. M., Ngo, L. H., Jones, R. N., Inouye, S. K., Hall, K. T., Gallagher, J., et al. (2019). The Association Between C-Reactive Protein and Postoperative Delirium Differs by Catechol-O-Methyltransferase Genotype. Am. J. Geriatr. Psychiatry 27, 1–8. doi: 10.1016/j.jagp.2018.09.007
Vaurio, L. E., Sands, L. P., Wang, Y., Mullen, E. A., and Leung, J. M. (2006). Postoperative delirium: The importance of pain and pain management. Anesth. Analg. 102, 1267–1273. doi: 10.1213/01.ane.0000199156.59226.af
Weerink, L. B. M., Van Leeuwen, B. L., Gernaat, S. A. M., Absalom, A. R., Huisman, M. G., Van Der Wal-Huisman, H., et al. (2018). Vitamin Status and the Development of Postoperative Cognitive Decline in Elderly Surgical Oncologic Patients. Ann. Surg. Oncol. 25, 231–238. doi: 10.1245/s10434-017-6118-6
Wilson, C. J., Finch, C. E., and Cohen, H. J. (2002). Cytokines and cognition–the case for a head-to-toe inflammatory paradigm. J. Am. Geriatr. Soc. 50, 2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x
Witlox, J., Eurelings, L. S., De Jonghe, J. F., Kalisvaart, K. J., Eikelenboom, P., and Van Gool, W. A. (2010). Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: A meta-analysis. JAMA 304, 443–451. doi: 10.1001/jama.2010.1013
Keywords: anesthesia, surgery, postoperative delirium, C-reactive protein, homocysteine
Citation: Ma X, Mei X, Tang T, Wang M, Wei X, Zheng HL, Cao J, Zheng H, Cody K, Xiong L, Marcantonio ER, Xie Z and Shen Y (2022) Preoperative homocysteine modifies the association between postoperative C-reactive protein and postoperative delirium. Front. Aging Neurosci. 14:963421. doi: 10.3389/fnagi.2022.963421
Received: 08 June 2022; Accepted: 02 September 2022;
Published: 21 September 2022.
Edited by:
Allison B. Reiss, New York University, United StatesReviewed by:
Mian Peng, Wuhan University, ChinaCopyright © 2022 Ma, Mei, Tang, Wang, Wei, Zheng, Cao, Zheng, Cody, Xiong, Marcantonio, Xie and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongcong Xie, enhpZUBtZ2guaGFydmFyZC5lZHU=; Yuan Shen, a21zaHlAdG9uZ2ppLmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
‡These authors share senior authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.