
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Aging Neurosci. , 30 June 2022
Sec. Neurocognitive Aging and Behavior
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.939848
This article is part of the Research Topic Anesthetic-Induced Neurotoxicity and Neurocognitive Impairment of Vulnerable Brains View all 23 articles
 Xiaoyu Xie1,2†
Xiaoyu Xie1,2† Rui Gao1,2†
Rui Gao1,2† Hai Chen3,4
Hai Chen3,4 Xueying Zhang1,2
Xueying Zhang1,2 Xingwei Cai1,2
Xingwei Cai1,2 Changteng Zhang1,2
Changteng Zhang1,2 Changliang Liu1,2
Changliang Liu1,2 Tao Zhu1,2*
Tao Zhu1,2* Chan Chen1,2*
Chan Chen1,2*Background: Postoperative neurocognitive disorders (PNDs) is common among surgical patients, however, the effect of glucocorticoids for preventing PNDs is not clear. This review aims to evaluate the effect of glucocorticoids on the incidence of PNDs in adult patients undergoing surgery.
Methods: The databases of PubMed/Medline, Embase, the Cochrane Library, and Web of science were searched for all available randomized controlled trials (RCTs) from inception to April 30, 2022. RCTs comparing the effect of glucocorticoids with placebo on the incidence of PNDs in adult surgical patients (≥18 years old) were eligible. Subgroup analyses and meta-regressions were performed to evaluate sources of clinical heterogeneity. The level of certainty for main outcomes were assessed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology.
Results: Eleven trials with a total of 10,703 patients were identified. Compared with the control group, glucocorticoids did not reduce the incidence of PNDs (RR: 0.84, 95% CI: 0.67 to 1.06, P = 0.13, GRADE = moderate). Secondary analyses for primary outcome did not change the result. In addition, the length of ICU stay was decreased in glucocorticoids group (RR: −13.58, 95% CI: −26.37 to −0.80, P = 0.04, GRADE = low). However, there were no significant differences between groups with regards to the incidence of postoperative infection (RR: 0.94, 95% CI: 0.84 to 1.06, P = 0.30, GRADE = moderate), blood glucose level (RR: 1.05, 95% CI: −0.09 to 2.19, P = 0.07, GRADE = low), duration of mechanical ventilation (RR: −2.44, 95% CI: −5.47 to 0.59, P = 0.14, GRADE = low), length of hospital stay (RR: −0.09, 95% CI: −0.27 to 0.09, P = 0.33, GRADE = moderate) and 30-day mortality (RR: 0.86, 95% CI: 0.70 to 1.06, P = 0.16, GRADE = moderate).
Conclusions: This meta-analysis suggests that perioperative administration of glucocorticoids may not reduce the incidence of PNDs after surgery. The effect of glucocorticoids on decreased length of ICU stay needs further researches. Future high-quality trials using acknowledged criteria and validated diagnostic tools are needed to determine the influence of glucocorticoids on long-term PNDs.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022302262, identifier: CRD42022302262.
Postoperative neurocognitive disorders (PNDs) is an overarching term that includes postoperative delirium and postoperative cognitive dysfunction (POCD) (Vacas et al., 2021). According to the Perioperative Cognition Nomenclature Working Group in 2018 (Evered et al., 2018), postoperative delirium is an acute state of cognitive impairment occurring within days after surgery and up to 1 week or until discharge, while POCD is a prolonged cognitive decline usually detected between 30 days and 12 months postoperatively. It has been reported that postoperative delirium occurred in 10–60% of elderly surgical patients, varying by surgical procedures (American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults, 2015), and the incidence of POCD is approximately 25–40% (Wei et al., 2019). Old age, low educational levels, poor preoperative cognitive function, perioperative pain and complicated surgery process are thought to be risk factors of PNDs (Xie and Shen, 2018; Evered et al., 2020; O'Gara et al., 2021). PNDs are the very common and severe postoperative neurological complications with poor outcomes, including increasing the length of hospital stay, mortality, and the risk of long-term cognitive impairment. These would cause significant clinical, social, and financial burdens on the patients and their communities (Monk et al., 2008; Inouye et al., 2014; Boone et al., 2020).
Improving cognitive outcome after surgery, therefore, is an important objective for anesthesiologists and surgeons. To date, there have been no compelling pharmacologic interventions to limit the incidence or severity of PNDs (Mahanna-Gabrielli et al., 2019; Deemer et al., 2020). Dexmedetomidine, an anesthetic agent with neural anti-inflammatory effects, has been found to show promise for PNDs prevention (Lee et al., 2018; Likhvantsev et al., 2021). However, it has common side effects such as bradycardia and hypotension (Wu et al., 2018; Shi et al., 2020; Zhao et al., 2020), and the evidence to support this effect is limited (Sanders et al., 2021). For non-pharmacologic approaches, cognitive prehabilitation, physical activity, and management of hypertension and diabetes seem to be effective to improve cognitive function (Wang et al., 2020; Humeidan et al., 2021), but there is still a gap in their integration into pathways of care for patients (Vlisides et al., 2019; Deiner et al., 2020).
Proposed potential mechanisms for PNDs, including mitochondrial dysfunction, oxidative stress (Netto et al., 2018), synaptic damage (Xiao et al., 2018), and neurotrophic support impairment (Fan et al., 2016) are speculative, among which neuroinflammation is the most significantly concerned (Luo et al., 2019). It has been reported that surgery and anesthesia could lead the peripheral immune system to produce pro-inflammatory signals (Balusu et al., 2016; Noll et al., 2017). These inflammatory mediators could transfer into the brain through paraventricular areas of the blood-brain barrier (BBB) and stimulate microglia to produce proinflammatory factors, destroying synapses and neurons, thus causing neurotoxic symptoms and cognitive disorders (Lim et al., 2013; Liu and Yin, 2018).
Glucocorticoids are commonly used in the perioperative period to attenuate the inflammatory response (Holte and Kehlet, 2002; Lunn and Kehlet, 2013). And they can alleviate the inflammation by inhibiting prostaglandin production (Rhen and Cidlowski, 2005), activating endothelial nitric oxide synthetase (Hafezi-Moghadam et al., 2002), and decreasing the stability of mRNA for genes for inflammatory proteins (Gille et al., 2001; Lasa et al., 2002; Saklatvala et al., 2003). Evaluating whether the perioperative administration of glucocorticoids is helpful in preventing cognitive decline could promote targeted preventive and therapeutic interventions. Therefore, in recent years several studies have investigated the efficacy of glucocorticoids on cognitive disorders after anesthesia and surgery. However, their conclusions have been inconsistent. Qiao et al. (2015) and Valentin et al. (2016) investigated the effect of glucocorticoids on PNDs in elderly patients undergoing non-cardiac surgery. They found that the preventive administration of glucocorticoids could effectively reduce POCD. In contrast, Sauër et al. (2014) demonstrated the opposite result, showing that intraoperative administration of glucocorticoids did not reduce the incidence of delirium after cardiac surgery. Besides, Fang et al. (2014) studied the effect of glucocorticoids in patients suffering from facial spasms requiring microvascular decompression. They found that administering a higher dose of glucocorticoids increased the incidence of POCD in the early postoperative period. Therefore, we applied a systematic review and meta-analysis to explore the effect of perioperative glucocorticoids administration on the incidence of PNDs.
This meta-analysis was conducted following the recommendations of Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al., 2021). This study protocol was registered in PROSPERO database (CRD42022302262).
The databases of Pubmed/Medline, Embase, the Cochrane Library/Central, and Web of science were systematically searched for all relevant studies from inception to April 30, 2022. The references of included researches were also examined. According to the search strategy, both MeSH terms and free terms were used. The following keyword search terms were used: glucocorticoids, cognitive disorders, delirium, and surgery. The search strategy was given in the Supplementary Material.
Studies restricted to randomized controlled trials (RCTs) in adult surgical patients (≥18 years old). All published full-article RCTs compared the effect of glucocorticoids with placebo or equal volume of normal saline (NS) on the incidence of PNDs were eligible for inclusion. Language restriction was not applied.
Pediatric surgery, non-intravenous administration of glucocorticoids, no available assessment tools, and animal experiments were excluded from this meta-analysis.
Data extraction and quality assessment were completed by two authors (XX and RG) independently. One author (XX) entered the information into the table and checked for consistency and completeness. Disagreements on data extraction and quality assessment were handled by discussion or reviewed by the third author (CC). The extracted data and information were as follows: first author, year of publication, surgery type, patient age, glucocorticoids type, timing and dose, control group, PNDs type, and cognitive assessment timing and methods. In addition, the following adverse events were extracted as well, including PNDs, infection, blood glucose level, duration of mechanical ventilation, length of ICU and hospital stay, and 30-day mortality.
Quality assessment of included RCTs was performed according to the second version of the Cochrane risk-of-bias tool for RCTs (RoB 2.0) (Sterne et al., 2019). There are seven sections of this assessment, random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Each section was classified into the low, high, or unclear risk of bias.
The primary endpoint of this meta-analysis was the incidence of PNDs. The secondary outcomes were the incidence of postoperative infection, blood glucose level, duration of mechanical ventilation, the length of ICU and hospital stay, and postoperative 30-day mortality.
For dichotomous data (incidence of PNDs, postoperative infection, and 30-day mortality), the Mantel-Haenszel method was used to combine outcomes and risk ratio (RR) with 95% confidence intervals (CI) were calculated. Concerning continuous variables (blood glucose level, duration of mechanical ventilation, and the length of ICU and hospital stay), the Inverse-Variance method was used, and mean difference (MD) or standardized mean difference (SMD) with 95% CI were calculated. The I2 statistics used to evaluate heterogeneity were divided into the following three levels (Melsen et al., 2014): low (I2 < 50%), moderate (I2 = 50–75%) and high (I2 > 75%). When the heterogeneity was low, we used fixed effects model to pooled the data; otherwise, we chose random effects model.
To find sources of heterogeneity, subgroup analyses were conducted according to the type of PNDs (postoperative delirium and POCD), the type of glucocorticoids, surgery, dose, and age. In subgroup analysis for the dose of glucocorticoids, the trials were stratified into three broad dose groups: low dose group if the total dose of glucocorticoid used was ≤ 30 mg prednisolone or equivalent, medium dose group if the total dose used was between 30 and 100 mg prednisolone or equivalent, and high dose group if the total dose used was >100 mg prednisolone or equivalent. These cut points were chosen according to clinical practice (Czock et al., 2005). For studies that used dexamethasone, methylprednisolone, or hydrocortisone, the total dose of glucocorticoid used was converted to an equivalent dose of prednisolone with similar glucocorticoid effect. The dose conversion factors for dexamethasone, methylprednisolone, and hydrocortisone to prednisolone were 6, 1.25, and 0.25, respectively (https://clincalc.com/corticosteroids/). In subgroup analysis for patient age, we calculated the mean age of the study population as the basis of classification. Besides, Hartung-Knapp adjusted meta-regressions were performed to find interactions between variables if subgroup analyses could not explain sources of heterogeneity.
Publication bias was assessed through visual inspection of funnel plots and Egger's test to evaluate the small-study effects. The influence of a potential publication bias on findings was explored by using the Duval and Tweedie trim-and-fill procedure. Sensitivity analyses were performed by excluding high-risk studies evaluated by RoB 2.0 or omitting one study each time to detect robustness of the pooled results. Finally, the level of certainty for main outcomes were assessed by the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (Langendam et al., 2013). P < 0.05 was considered statistically significant for all tests. All data analyses were performed by Revman 5.3. and Stata 16.
According to the search strategy, a total of 466 trials were identified. Among them, 128 studies were removed due to duplication, and the other 327 studies were excluded based on inclusion and exclusion criteria. Ultimately, 11 RCTs, including 10,703 patients, were included in this meta-analysis. The selection process flow chart was shown in Figure 1, and methodological quality assessment was conducted according to the RoB 2.0, and the result was summarized in Figure 2. The major characters of these eligible studies were extracted and presented in Table 1. Seven trials were cardiac surgery (Hauer et al., 2012; Mardani and Bigdelian, 2013; Ottens et al., 2014; Sauër et al., 2014; Whitlock et al., 2015; Glumac et al., 2017; Royse et al., 2017), one trial was neurologic surgery (Fang et al., 2014), and three trials were non-cardiac, non-neurologic surgery, including one laparoscopic gastrointestinal surgery (Xiang et al., 2022) and two hip fracture surgery (Clemmesen et al., 2018; Kluger et al., 2021). Furthermore, six studies used dexamethasone as intervention (Mardani and Bigdelian, 2013; Fang et al., 2014; Ottens et al., 2014; Sauër et al., 2014; Glumac et al., 2017; Kluger et al., 2021), four studies used methylprednisolone (Whitlock et al., 2015; Royse et al., 2017; Clemmesen et al., 2018; Xiang et al., 2022), and one study used hydrocortisone (Hauer et al., 2012). All of the included studies used normal saline as a placebo. Besides, eight studies investigated postoperative delirium (Hauer et al., 2012; Mardani and Bigdelian, 2013; Sauër et al., 2014; Whitlock et al., 2015; Royse et al., 2017; Clemmesen et al., 2018; Kluger et al., 2021; Xiang et al., 2022) while the other three trials investigated POCD (Fang et al., 2014; Ottens et al., 2014; Glumac et al., 2017). The timing and dose of glucocorticoids administration and cognitive assessment methods were varied between included studies.
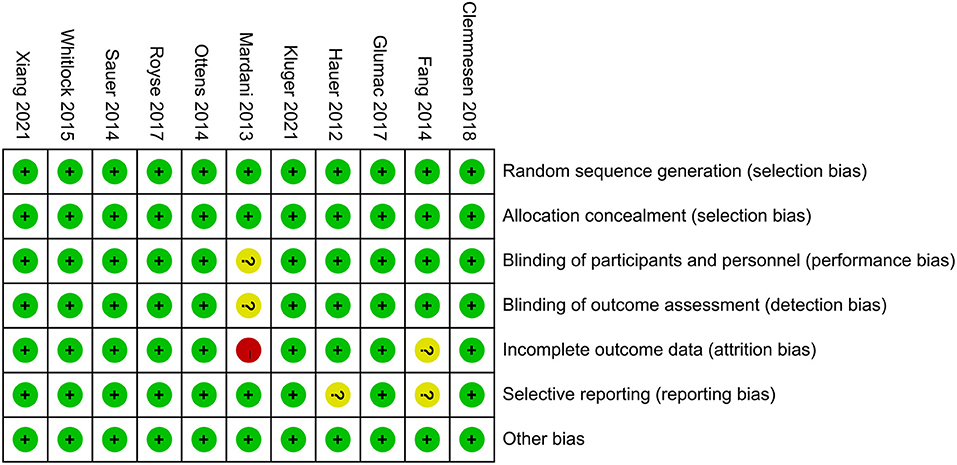
Figure 2. Risk of bias assessment was undertaken for each included trial according to the Cochrane Risk of Bias Methods.
The overall pooled result showed that glucocorticoids did not decrease the incidence of PNDs compared to the controls (RR: 0.84, 95% CI: 0.67 to 1.06, P = 0.13, I2 = 57%) (Figure 3). Sensitivity analyses were performed by excluding the high-risk study (Mardani and Bigdelian, 2013) or omitting one study each time from included studies, and the pooled result was still robust (Supplementary Figure 1). Meanwhile, no significant publication bias was evidenced by visual inspection of funnel plot (Figure 4) and Egger's test (Supplementary Table 1) for the effect of glucocorticoid administration on PNDs.
Similarly, the finding was consistent in subgroup analyses between postoperative delirium (RR: 0.78, 95% CI: 0.61 to 1.01, P = 0.05, I2 = 44%) and POCD (RR: 1.00, 95% CI: 0.51 to 1.96, P = 1.00, I2 = 77%) (Figure 5), between dexamethasone (RR: 0.87, 95% CI: 0.59 to 1.27, P = 0.46, I2 = 65%), methylprednisolone (RR: 0.72, 95% CI: 0.47 to 1.09, P = 0.12, I2 = 65%) and hydrocortisone (Figure 6). However, subgroup analyses for glucocorticoids dose, surgery type and patient age showed the inconsistent results. There were significant differences in medium dose group (RR: 0.49, 95% CI: 0.33 to 0.73, P = 0.0005, I2 = 0%) (Supplementary Figure 2), non-cardiac, non-neurologic surgery group (RR: 0.52, 95% CI: 0.33 to 0.80, P = 0.003, I2 = 0%) (Supplementary Figure 3) and mean age ≥70 years group (RR: 0.60, 95% CI: 0.43 to 0.85, P = 0.004, I2 = 0%) (Supplementary Figure 4). However, further meta-regressions showed that when glucocorticoids type, surgery type, patient age, and their interactions were entered as covariates in models, there were no significant differences between glucocorticoid group and placebo group on the incidence of PNDs (Supplementary Table 2).
Of the 11 studies included in this meta-analysis, four studies (Hauer et al., 2012; Mardani and Bigdelian, 2013; Whitlock et al., 2015; Glumac et al., 2017) compared the length of ICU stay between groups and the glucocorticoid group significantly reduced the length stay in ICU (RR: −13.58, 95% CI: −26.37 to −0.80, P = 0.04, I2 = 86%) (Figure 7). However, there were no significant differences in postoperative infection [five trials (Mardani and Bigdelian, 2013; Whitlock et al., 2015; Clemmesen et al., 2018; Kluger et al., 2021; Xiang et al., 2022); RR: 0.94, 95% CI: 0.84 to 1.06, P = 0.30, I2 = 26%] (Figure 8), blood glucose level [two trials (Mardani and Bigdelian, 2013; Whitlock et al., 2015); RR: 1.05, 95% CI: −0.09 to 2.19, P = 0.07, I2 = 61%] (Figure 9), duration of mechanical ventilation [two trials (Hauer et al., 2012; Glumac et al., 2017); RR: −2.44, 95% CI: −5.47 to 0.59, P = 0.14, I2 = 0%] (Figure 10), length of hospital stay [six trials (Mardani and Bigdelian, 2013; Whitlock et al., 2015; Glumac et al., 2017; Clemmesen et al., 2018; Kluger et al., 2021; Xiang et al., 2022); RR: −0.09, 95% CI: −0.27 to 0.09, P = 0.33, I2 = 11%] (Figure 11) and 30-day mortality [four trials (Whitlock et al., 2015; Clemmesen et al., 2018; Kluger et al., 2021; Xiang et al., 2022); RR: 0.86, 95% CI: 0.70 to 1.06, P = 0.16, I2 = 0%] (Figure 12).
Basing on GRADE framework, we evaluated the level of certainty for our main outcomes. The quality of these outcomes varied from low to moderate and the detailed information were shown in Table 2.
This meta-analysis suggests that perioperative glucocorticoids administration does not reduce the incidence of PNDs. Subgroup analyses and meta-regressions considering potential variables such as PNDs type, glucocorticoids type, dose, surgery type and patient age remained no difference in the outcome. Besides, glucocorticoids infusion was associated with a shorter length of ICU stay, while the incidence of postoperative infection, blood glucose level, duration of mechanical ventilation, length of hospital stay, and 30-day mortality did not differ significantly between groups.
Neuroinflammation has become a key hallmark of neurological complications including PNDs (Subramaniyan and Terrando, 2019). Perioperative glucocorticoid administration has been used in different surgical settings to counter the detrimental effect of inflammation induced by surgery and anesthesia (Awada et al., 2022). Several studies have shown that the preoperative administration of glucocorticoids reduces peripheral inflammatory markers in hepatic surgery (Orci et al., 2013; Richardson et al., 2014). However, the effect of glucocorticoids on PNDs was not observed despite pooling data for more than 10,000 randomized participants, with overall low risk of bias across studies in our review. Several reasons may account for this result. First, although excessive neuroinflammation leads to injury and death of neural elements, a large body of literatures have demonstrated that proper neuroinflammatory response can benefit outcomes of central nerve system (Yong et al., 2019). For example, neuroinflammation can promote neurogenesis (Ziv et al., 2006), facilitate axonal regeneration (David et al., 1990), and is critical for remyelination (Goldstein et al., 2016). It's crucial to suppress the excessive inflammation that mediates damage without inhibiting the repairment effect from preventing PNDs. Second, the genesis of PNDs is multifactorial that other factors except for neuroinflammation may play great roles in the development of neurocognitive deficit (Siddiqi et al., 2016). Third, prolonged exposure to high concentrations of glucocorticoids can be toxic to neural structures, especially the glucocorticoid receptor–rich hippocampus (Sapolsky, 2000).
To explore sources of heterogeneity, we performed subgroup analyses based on PNDs type and glucocorticoids type, and the outcome remained no difference. Although subgroup analyses about glucocorticoids dose, surgery type and patient age seemed significantly different, meta-regressions were further conducted to understand the interactions between these variables on our outcomes and the results changed to no difference. Here what should be noted was that the subgroup of age was classified according to the mean age values in studies, which might induce some misclassification of accurate age. In general, these secondary analyses suggest that the genesis of PNDs is multifactorial that only administration of glucocorticoids may not significantly reduce the incidence of neurocognitive disorders after surgery. Besides, the impact of patient, surgery, or other variables, both measured and unmeasured, on the PNDs development likely far outweighs the impact of glucocorticoids.
PNDs is a summarized term encompassing postoperative delirium, a most pronounced and acute postoperative form, and POCD which is described as a long-term neurocognitive impairment (Evered et al., 2018). Delirium and POCD previously were considered distinct entities, but recent data has suggested an underlying relationship between them (Olotu, 2020). Several risk factors are common to both postoperative delirium and POCD, and animal studies raise the possibility that neuroinflammation may play a role in both of these states (Berger et al., 2018; Devinney et al., 2018; Daiello et al., 2019). Therefore, in this meta-analysis we choose PNDs as the endpoint which in other words, combining postoperative delirium and POCD in the composite outcome, is acceptable from perspective of pathogenesis.
The diagnosis of PNDs, especially POCD, is complex requiring neuropsychological tests which are varied in studies. A recent systematic review noted that in 274 existing studies of POCD, diagnosis was based on 259 different cognitive assessment tools (Borchers et al., 2021). Moreover, neuropsychological tests have been undertaken at variable time intervals after anesthesia and surgery. In 2018, Evered et al. (2018) clarified that postoperative delirium was defined as occurring in hospital and up to 1 week post-procedure or until discharge, while POCD persisted for more than 30 days but <12 months following anesthesia and surgery. However, in trials included in this meta-analysis, Fang et al. (2014) and Glumac et al. (2017) examined POCD in postoperative day 5 and 6, respectively, in which delirium is usually to be assessed; while only Ottens et al. (2014) examined POCD at 1 month after surgery. In summary, heterogeneities of assessment tools, diagnostic criteria, and follow-up time limit the interpretation of existing data surrounding PNDs. Besides, there were only three studies (Fang et al., 2014; Ottens et al., 2014; Glumac et al., 2017) examining POCD in our meta-analysis, and two of them (Fang et al., 2014; Glumac et al., 2017) assessing this entity in the early postoperative day. Thus, the effect of glucocorticoids on long-term PNDs is still unclear. PNDs is associated with long-term sequelae including ongoing impaired cognition, increased risk of dementia, increased mortality, and premature retirement from work (Steinmetz et al., 2009). Interventions to mitigate these sequelae may therefore provide clinical and economic benefit in the long run. Further trials are needed to assess POCD using uniform criteria and validated diagnostic tools and then evaluate the effects of glucocorticoids on the incidence of long-term PNDs.
In our meta-analysis, glucocorticoids significantly reduced the length of ICU stay. However, there were only four studies examining this outcome with high heterogeneity. Besides, it is still obscure whether administration of glucocorticoids could impact the risk of postoperative infection, blood glucose level, length of hospital stay, duration of mechanical ventilation, and 30-day mortality. In the Dexamethasone for Cardiac Surgery (DECS) trial, Dieleman et al. (2012) reported that intraoperative administration of dexamethasone for cardiac surgery was associated with higher postoperative glucose level, lower infection rate, decreased duration of mechanical ventilation, and reduced length of hospital stay. Because of high heterogeneity and limited studies in this meta-analysis, future high-quality researches are still needed to confirm these outcomes.
To our knowledge, this is the first systematic review and meta-analysis to comprehensively evaluate effects of glucocorticoids on PNDs. A meta-analysis by Li et al. (2019) studied effects of dexamethasone on postoperative cognitive dysfunction and delirium in adults following general anesthesia, which did not take other types of glucocorticoids into consideration. Another systematic review and meta-analysis performed by Liu et al. (2021) was about effects of glucocorticoids on postoperative delirium in adult patients undergoing cardiac surgery. Similarly, POCD, another form of PNDs mentioned above, and non-cardiac surgery patients were not included in this study. In our review, we thought through PNDs type, glucocorticoids type, and surgery type to make conclusions as rigorous as possible.
There are still several potential limitations in this meta-analysis. First, we studied three types of glucocorticoids and two types of PNDs in cardiac, neurologic, and non-cardiac non-neurologic surgery, so there was potential heterogeneity such as methods of diagnosis and dosages of glucocorticoids, which may affect the precision and reliability of the results. Second, most of these included studies excluded patients with pre-existing cognitive impairment, and children were also excluded from this meta-analysis. Therefore, the extrapolation of this meta-analysis was limited. Third, some studies that contained our interested second outcomes, but not PNDs data, were excluded, thus influencing this meta-analysis's completeness of secondary outcomes. Further, more structured and standardized perioperative glucocorticoids protocols and uniform definition and assessment tools of PNDs may be necessary to accurately evaluate the effect of glucocorticoids on PNDs.
In summary, our findings suggest that perioperative administration of glucocorticoids does not reduce the incidence of PNDs, regardless of PNDs type, glucocorticoids type, dose, surgery type and patient age. The effect of glucocorticoids on decreased length of ICU stay needs further researches. Future high-quality trials using acknowledged criteria and validated diagnostic tools are needed to determine the influence of glucocorticoids on long-term PNDs.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
XX and RG wrote the manuscript and collected the data. XX, RG, TZ, and CC chose the topic. HC, XZ, and XC contributed to the conception. CZ and CL searched the literature. XX, RG, HC, XZ, and XC analyzed the data. TZ and CC made final decisions. All authors have read and approved the final manuscript.
This work was supported by the National Natural Science Foundation of China (Nos. 82171185 and 81870858 to CC); The National Key R&D Program of China (No. 2018YFC2001800 to TZ) and the National Natural Science Foundation of China (No. 81671062 to TZ); China Postdoctoral Science Foundation (Grant No. 2020M673234 to RG), Post-doctoral Research Project, West China Hospital, Sichuan University (Grant No. 2020HXBH022 to RG).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank the authors of the primary studies for providing their data and other critical information. Additionally, we thank all participants for their valuable contributions to this article.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.939848/full#supplementary-material
Supplementary Material. Search strategy.
Supplementary Figure 1. Sensitivity analysis of the incidence of PNDs by excluding each study individually.
Supplementary Figure 2. Subgroup analysis of the incidence of PNDs for the dose of glucocorticoids.
Supplementary Figure 3. Subgroup analysis of the incidence of PNDs for the type of surgery.
Supplementary Figure 4. Subgroup analysis of the incidence of PNDs based on age classification.
Supplementary Table 1. Tests for publication bias.
Supplementary Table 2. Meta-regressions for the incidence of PNDs.
American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults (2015). Postoperative delirium in older adults: best practice statement from the American Geriatrics Society. J. Am. Coll. Surg. 220, 136–148.e131. doi: 10.1016/j.jamcollsurg.2014.10.019
Awada, H. N., Steinthorsdottir, K. J., Schultz, N. A., Hillings,ø, J. G., Larsen, P. N., Jans, Ø., et al. (2022). High-dose preoperative glucocorticoid for prevention of emergence and postoperative delirium in liver resection: a double-blinded randomized clinical trial substudy. Acta Anaesthesiol. Scand. doi: 10.1111/aas.14057. [Epub ahead of print].
Balusu, S., Van Wonterghem, E., De Rycke, R., Raemdonck, K., Stremersch, S., Gevaert, K., et al. (2016). Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol. Med. 8, 1162–1183. doi: 10.15252/emmm.201606271
Berger, M., Terrando, N., Smith, S. K., Browndyke, J. N., Newman, M. F., and Mathew, J. P. (2018). Neurocognitive function after cardiac surgery: from phenotypes to mechanisms. Anesthesiology 129, 829–851. doi: 10.1097/aln.0000000000002194
Boone, M. D., Sites, B., von Recklinghausen, F. M., Mueller, A., Taenzer, A. H., and Shaefi, S. (2020). Economic burden of postoperative neurocognitive disorders among US medicare patients. JAMA Netw. Open 3, e208931. doi: 10.1001/jamanetworkopen.2020.8931
Borchers, F., Spies, C. D., Feinkohl, I., Brockhaus, W. R., Kraft, A., Kozma, P., et al. (2021). Methodology of measuring postoperative cognitive dysfunction: a systematic review. Br. J. Anaesth. 126, 1119–1127. doi: 10.1016/j.bja.2021.01.035
Clemmesen, C. G., Lunn, T. H., Kristensen, M. T., Palm, H., and Foss, N. B. (2018). Effect of a single pre-operative 125 mg dose of methylprednisolone on postoperative delirium in hip fracture patients; a randomised, double-blind, placebo-controlled trial. Anaesthesia 73, 1353–1360. doi: 10.1111/anae.14406
Czock, D., Keller, F., Rasche, F. M., and Häussler, U. (2005). Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin. Pharmacokinet. 44, 61–98. doi: 10.2165/00003088-200544010-00003
Daiello, L. A., Racine, A. M., Yun Gou, R., Marcantonio, E. R., Xie, Z., Kunze, L. J., et al. (2019). Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology 131, 477–491. doi: 10.1097/aln.0000000000002729
David, S., Bouchard, C., Tsatas, O., and Giftochristos, N. (1990). Macrophages can modify the nonpermissive nature of the adult mammalian central nervous system. Neuron 5, 463–469. doi: 10.1016/0896-6273(90)90085-t
Deemer, K., Zjadewicz, K., Fiest, K., Oviatt, S., Parsons, M., Myhre, B., et al. (2020). Effect of early cognitive interventions on delirium in critically ill patients: a systematic review. Can. J. Anaesth. 67, 1016–1034. doi: 10.1007/s12630-020-01670-z
Deiner, S., Fleisher, L. A., Leung, J. M., Peden, C., Miller, T., and Neuman, M. D. (2020). Adherence to recommended practices for perioperative anesthesia care for older adults among US anesthesiologists: results from the ASA Committee on Geriatric Anesthesia-Perioperative Brain Health Initiative ASA member survey. Perioper. Med. 9, 6. doi: 10.1186/s13741-020-0136-9
Devinney, M. J., Mathew, J. P., and Berger, M. (2018). Postoperative delirium and postoperative cognitive dysfunction: two sides of the same coin? Anesthesiology 129, 389–391. doi: 10.1097/aln.0000000000002338
Dieleman, J. M., Nierich, A. P., Rosseel, P. M., van der Maaten, J. M., Hofland, J., Diephuis, J. C., et al. (2012). Intraoperative high-dose dexamethasone for cardiac surgery: a randomized controlled trial. JAMA 308, 1761–1767. doi: 10.1001/jama.2012.14144
Evered, L., Silbert, B., Knopman, D. S., Scott, D. A., DeKosky, S. T., Rasmussen, L. S., et al. (2018). Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br. J. Anaesth. 121, 1005–1012. doi: 10.1016/j.bja.2017.11.087
Evered, L. A., Vitug, S., Scott, D. A., and Silbert, B. (2020). Preoperative frailty predicts postoperative neurocognitive disorders after total hip joint replacement surgery. Anesth. Analg. 131, 1582–1588. doi: 10.1213/ane.0000000000004893
Fan, D., Li, J., Zheng, B., Hua, L., and Zuo, Z. (2016). Enriched environment attenuates surgery-induced impairment of learning, memory, and neurogenesis possibly by preserving BDNF expression. Mol. Neurobiol. 53, 344–354. doi: 10.1007/s12035-014-9013-1
Fang, Q., Qian, X., An, J., Wen, H., Cope, D. K., and Williams, J. P. (2014). Higher dose dexamethasone increases early postoperative cognitive dysfunction. J. Neurosurg. Anesthesiol. 26, 220–225. doi: 10.1097/ana.0000000000000024
Gille, J., Reisinger, K., Westphal-Varghese, B., and Kaufmann, R. (2001). Decreased mRNA stability as a mechanism of glucocorticoid-mediated inhibition of vascular endothelial growth factor gene expression by cultured keratinocytes. J. Invest. Dermatol. 117, 1581–1587. doi: 10.1046/j.0022-202x.2001.01573.x
Glumac, S., Kardum, G., Sodic, L., Supe-Domic, D., and Karanovic, N. (2017). Effects of dexamethasone on early cognitive decline after cardiac surgery: a randomised controlled trial. Eur. J. Anaesthesiol. 34, 776–784. doi: 10.1097/eja.0000000000000647
Goldstein, E. Z., Church, J. S., Hesp, Z. C., Popovich, P. G., and McTigue, D. M. (2016). A silver lining of neuroinflammation: Beneficial effects on myelination. Exp. Neurol. 283(Pt B), 550–559. doi: 10.1016/j.expneurol.2016.05.001
Hafezi-Moghadam, A., Simoncini, T., Yang, Z., Limbourg, F. P., Plumier, J. C., Rebsamen, M. C., et al. (2002). Acute cardiovascular protective effects of corticosteroids are mediated by non-transcriptional activation of endothelial nitric oxide synthase. Nat. Med. 8, 473–479. doi: 10.1038/nm0502-473
Hauer, D., Weis, F., Campolongo, P., Schopp, M., Beiras-Fernandez, A., Strewe, C., et al. (2012). Glucocorticoid-endocannabinoid interaction in cardiac surgical patients: relationship to early cognitive dysfunction and late depression. Rev. Neurosci. 23, 681–690. doi: 10.1515/revneuro-2012-0058
Holte, K., and Kehlet, H. (2002). Perioperative single-dose glucocorticoid administration: pathophysiologic effects and clinical implications. J. Am. Coll. Surg. 195, 694–712. doi: 10.1016/s1072-7515(02)01491-6
Humeidan, M. L., Reyes, J. C., Mavarez-Martinez, A., Roeth, C., Nguyen, C. M., Sheridan, E., et al. (2021). Effect of cognitive prehabilitation on the incidence of postoperative delirium among older adults undergoing major noncardiac surgery: the neurobics randomized clinical trial. JAMA Surg. 156, 148–156. doi: 10.1001/jamasurg.2020.4371
Inouye, S. K., Westendorp, R. G., and Saczynski, J. S. (2014). Delirium in elderly people. Lancet 383, 911–922. doi: 10.1016/s0140-6736(13)60688-1
Kluger, M. T., Skarin, M., Collier, J., Rice, D. A., McNair, P. J., Seow, M. Y., et al. (2021). Steroids to reduce the impact on delirium (STRIDE): a double-blind, randomised, placebo-controlled feasibility trial of pre-operative dexamethasone in people with hip fracture. Anaesthesia 76, 1031–1041. doi: 10.1111/anae.15465
Langendam, M. W., Akl, E. A., Dahm, P., Glasziou, P., Guyatt, G., and Schünemann, H. J. (2013). Assessing and presenting summaries of evidence in Cochrane reviews. Syst. Rev. 2, 81. doi: 10.1186/2046-4053-2-81
Lasa, M., Abraham, S. M., Boucheron, C., Saklatvala, J., and Clark, A. R. (2002). Dexamethasone causes sustained expression of mitogen-activated protein kinase (MAPK) phosphatase 1 and phosphatase-mediated inhibition of MAPK p38. Mol. Cell. Biol. 22, 7802–7811. doi: 10.1128/mcb.22.22.7802-7811.2002
Lee, C., Lee, C. H., Lee, G., Lee, M., and Hwang, J. (2018). The effect of the timing and dose of dexmedetomidine on postoperative delirium in elderly patients after laparoscopic major non-cardiac surgery: a double blind randomized controlled study. J. Clin. Anesth. 47, 27–32. doi: 10.1016/j.jclinane.2018.03.007
Li, L. Q., Wang, C., Fang, M. D., Xu, H. Y., Lu, H. L., and Zhang, H. Z. (2019). Effects of dexamethasone on post-operative cognitive dysfunction and delirium in adults following general anaesthesia: a meta-analysis of randomised controlled trials. BMC Anesthesiol. 19:113. doi: 10.1186/s12871-019-0783-x
Likhvantsev, V. V., Landoni, G., Grebenchikov, O. A., Ovezov, A. M., Skripkin, Y. V., Lembo, R., et al. (2021). Perioperative dexmedetomidine supplement decreases delirium incidence after adult cardiac surgery: a randomized, double-blind, controlled study. J. Cardiothorac. Vasc. Anesth. 35, 449–457. doi: 10.1053/j.jvca.2020.02.035
Lim, A., Krajina, K., and Marsland, A. L. (2013). Peripheral inflammation and cognitive aging. Mod Trends Pharmacopsychiatry 28, 175–187. doi: 10.1159/000346362
Liu, W., Wang, Y., Wang, J., Shi, J., Pan, J., and Wang, D. (2021). Effects of glucocorticoids on postoperative delirium in adult patients undergoing cardiac surgery: a systematic review and meta-analysis. Clin. Ther. 43, 1608–1621. doi: 10.1016/j.clinthera.2021.07.021
Liu, Y., and Yin, Y. (2018). Emerging roles of immune cells in postoperative cognitive dysfunction. Mediators Inflamm. 2018, 6215350. doi: 10.1155/2018/6215350
Lunn, T. H., and Kehlet, H. (2013). Perioperative glucocorticoids in hip and knee surgery – benefit vs. harm? A review of randomized clinical trials. Acta Anaesthesiol. Scand. 57, 823–834. doi: 10.1111/aas.12115
Luo, A., Yan, J., Tang, X., Zhao, Y., Zhou, B., and Li, S. (2019). Postoperative cognitive dysfunction in the aged: the collision of neuroinflammaging with perioperative neuroinflammation. Inflammopharmacology 27, 27–37. doi: 10.1007/s10787-018-00559-0
Mahanna-Gabrielli, E., Schenning, K. J., Eriksson, L. I., Browndyke, J. N., Wright, C. B., Culley, D. J., et al. (2019). State of the clinical science of perioperative brain health: report from the American Society of Anesthesiologists Brain Health Initiative Summit 2018. Br. J. Anaesth. 123, 464–478. doi: 10.1016/j.bja.2019.07.004
Mardani, D., and Bigdelian, H. (2013). Prophylaxis of dexamethasone protects patients from further post-operative delirium after cardiac surgery: a randomized trial. J. Res. Med. Sci. 18, 137–143.
Melsen, W. G., Bootsma, M. C., Rovers, M. M., and Bonten, M. J. (2014). The effects of clinical and statistical heterogeneity on the predictive values of results from meta-analyses. Clin. Microbiol. Infect. 20, 123–129. doi: 10.1111/1469-0691.12494
Monk, T. G., Weldon, B. C., Garvan, C. W., Dede, D. E., van der Aa, M. T., Heilman, K. M., et al. (2008). Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 108, 18–30. doi: 10.1097/01.anes.0000296071.19434.1e
Netto, M. B., de Oliveira Junior, A. N., Goldim, M., Mathias, K., Fileti, M. E., da Rosa, N., et al. (2018). Oxidative stress and mitochondrial dysfunction contributes to postoperative cognitive dysfunction in elderly rats. Brain Behav. Immun. 73, 661–669. doi: 10.1016/j.bbi.2018.07.016
Noll, F., Behnke, J., Leiting, S., Troidl, K., Alves, G. T., Müller-Redetzky, H., et al. (2017). Self-extracellular RNA acts in synergy with exogenous danger signals to promote inflammation. PLoS ONE 12:e0190002. doi: 10.1371/journal.pone.0190002
O'Gara, B. P., Gao, L., Marcantonio, E. R., and Subramaniam, B. (2021). Sleep, pain, and cognition: modifiable targets for optimal perioperative brain health. Anesthesiology 135, 1132–1152. doi: 10.1097/aln.0000000000004046
Olotu, C. (2020). Postoperative neurocognitive disorders. Curr. Opin. Anaesthesiol. 33, 101–108. doi: 10.1097/aco.0000000000000812
Orci, L. A., Toso, C., Mentha, G., Morel, P., and Majno, P. E. (2013). Systematic review and meta-analysis of the effect of perioperative steroids on ischaemia-reperfusion injury and surgical stress response in patients undergoing liver resection. Br. J. Surg. 100, 600–609. doi: 10.1002/bjs.9035
Ottens, T. H., Dieleman, J. M., Sauër, A. M., Peelen, L. M., Nierich, A. P., de Groot, W. J., et al. (2014). Effects of dexamethasone on cognitive decline after cardiac surgery: a randomized clinical trial. Anesthesiology 121, 492–500. doi: 10.1097/aln.0000000000000336
Page, M. J., Moher, D., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ 372, n160. doi: 10.1136/bmj.n160
Qiao, Y., Feng, H., Zhao, T., Yan, H., Zhang, H., and Zhao, X. (2015). Postoperative cognitive dysfunction after inhalational anesthesia in elderly patients undergoing major surgery: the influence of anesthetic technique, cerebral injury and systemic inflammation. BMC Anesthesiol. 15:154. doi: 10.1186/s12871-015-0130-9
Rhen, T., and Cidlowski, J. A. (2005). Antiinflammatory action of glucocorticoids–new mechanisms for old drugs. N. Engl. J. Med. 353, 1711–1723. doi: 10.1056/NEJMra050541
Richardson, A. J., Laurence, J. M., and Lam, V. W. (2014). Use of pre-operative steroids in liver resection: a systematic review and meta-analysis. HPB 16, 12–19. doi: 10.1111/hpb.12066
Royse, C. F., Saager, L., Whitlock, R., Ou-Young, J., Royse, A., Vincent, J., et al. (2017). Impact of methylprednisolone on postoperative quality of recovery and delirium in the steroids in cardiac surgery trial: a randomized, double-blind, placebo-controlled substudy. Anesthesiology 126, 223–233. doi: 10.1097/aln.0000000000001433
Saklatvala, J., Dean, J., and Clark, A. (2003). Control of the expression of inflammatory response genes. Biochem. Soc. Symp. 70, 95–106. doi: 10.1042/bss0700095
Sanders, R. D., Wehrman, J., Irons, J., Dieleman, J., Scott, D., and Shehabi, Y. (2021). Meta-analysis of randomised controlled trials of perioperative dexmedetomidine to reduce delirium and mortality after cardiac surgery. Br. J. Anaesth. 127, e168–e170. doi: 10.1016/j.bja.2021.08.009
Sapolsky, R. M. (2000). Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch. Gen. Psychiatry 57, 925–935. doi: 10.1001/archpsyc.57.10.925
Sauër, A. M., Slooter, A. J., Veldhuijzen, D. S., van Eijk, M. M., Devlin, J. W., and van Dijk, D. (2014). Intraoperative dexamethasone and delirium after cardiac surgery: a randomized clinical trial. Anesth Analg 119, 1046–1052. doi: 10.1213/ane.0000000000000248
Shi, H., Du, X., Wu, F., Hu, Y., Xv, Z., and Mi, W. (2020). Dexmedetomidine improves early postoperative neurocognitive disorder in elderly male patients undergoing thoracoscopic lobectomy. Exp. Ther. Med. 20, 3868–3877. doi: 10.3892/etm.2020.9113
Siddiqi, N., Harrison, J. K., Clegg, A., Teale, E. A., Young, J., Taylor, J., et al. (2016). Interventions for preventing delirium in hospitalised non-ICU patients. Cochrane Database Syst. Rev. 3, Cd005563. doi: 10.1002/14651858.CD005563.pub3
Steinmetz, J., Christensen, K. B., Lund, T., Lohse, N., and Rasmussen, L. S. (2009). Long-term consequences of postoperative cognitive dysfunction. Anesthesiology 110, 548–555. doi: 10.1097/ALN.0b013e318195b569
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi: 10.1136/bmj.l4898
Subramaniyan, S., and Terrando, N. (2019). Neuroinflammation and perioperative neurocognitive disorders. Anesth. Analg. 128, 781–788. doi: 10.1213/ane.0000000000004053
Vacas, S., Cole, D. J., and Cannesson, M. (2021). Cognitive decline associated with anesthesia and surgery in older patients. JAMA. doi: 10.1001/jama.2021.4773. [Epub ahead of print].
Valentin, L. S., Pereira, V. F., Pietrobon, R. S., Schmidt, A. P., Oses, J. P., Portela, L. V., et al. (2016). Effects of single low dose of dexamethasone before noncardiac and nonneurologic surgery and general anesthesia on postoperative cognitive dysfunction – a phase III double blind, randomized clinical trial. PLoS ONE 11:e0152308. doi: 10.1371/journal.pone.0152308
Vlisides, P. E., Das, A. R., Thompson, A. M., Kunkler, B., Zierau, M., Cantley, M. J., et al. (2019). Home-based cognitive prehabilitation in older surgical patients: a feasibility study. J. Neurosurg. Anesthesiol. 31, 212–217. doi: 10.1097/ana.0000000000000569
Wang, Y. Y., Yue, J. R., Xie, D. M., Carter, P., Li, Q. L., Gartaganis, S. L., et al. (2020). Effect of the tailored, family-involved hospital elder life program on postoperative delirium and function in older adults: a randomized clinical trial. JAMA Intern. Med. 180, 17–25. doi: 10.1001/jamainternmed.2019.4446
Wei, P., Yang, F., Zheng, Q., Tang, W., and Li, J. (2019). The potential role of the NLRP3 inflammasome activation as a link between mitochondria ROS generation and neuroinflammation in postoperative cognitive dysfunction. Front. Cell. Neurosci. 13:73. doi: 10.3389/fncel.2019.00073
Whitlock, R. P., Devereaux, P. J., Teoh, K. H., Lamy, A., Vincent, J., Pogue, J., et al. (2015). Methylprednisolone in patients undergoing cardiopulmonary bypass (SIRS): a randomised, double-blind, placebo-controlled trial. Lancet 386, 1243–1253. doi: 10.1016/s0140-6736(15)00273-1
Wu, M., Liang, Y., Dai, Z., and Wang, S. (2018). Perioperative dexmedetomidine reduces delirium after cardiac surgery: a meta-analysis of randomized controlled trials. J. Clin. Anesth. 50, 33–42. doi: 10.1016/j.jclinane.2018.06.045
Xiang, X. B., Chen, H., Wu, Y. L., Wang, K., Yue, X., and Cheng, X. Q. (2022). The effect of preoperative methylprednisolone on postoperative delirium in older patients undergoing gastrointestinal surgery: a randomized, double-blind, placebo-controlled trial. J. Gerontol. A Biol. Sci. Med. Sci. 77, 517–523. doi: 10.1093/gerona/glab248
Xiao, J. Y., Xiong, B. R., Zhang, W., Zhou, W. C., Yang, H., Gao, F., et al. (2018). PGE2-EP3 signaling exacerbates hippocampus-dependent cognitive impairment after laparotomy by reducing expression levels of hippocampal synaptic plasticity-related proteins in aged mice. CNS Neurosci. Ther. 24, 917–929. doi: 10.1111/cns.12832
Xie, Z., and Shen, Y. (2018). New biomarkers of postoperative neurocognitive disorders. Nat. Rev. Neurol. 14, 320–321. doi: 10.1038/s41582-018-0001-3
Yong, H. Y. F., Rawji, K. S., Ghorbani, S., Xue, M., and Yong, V. W. (2019). The benefits of neuroinflammation for the repair of the injured central nervous system. Cell. Mol. Immunol. 16, 540–546. doi: 10.1038/s41423-019-0223-3
Zhao, W., Hu, Y., Chen, H., Wang, X., Wang, L., Wang, Y., et al. (2020). The effect and optimal dosage of dexmedetomidine plus sufentanil for postoperative analgesia in elderly patients with postoperative delirium and early postoperative cognitive dysfunction: a single-center, prospective, randomized, double-blind, controlled trial. Front. Neurosci. 14:549516. doi: 10.3389/fnins.2020.549516
Keywords: glucocorticoids, postoperative neurocognitive disorders, adult patients, surgery, meta-analysis
Citation: Xie X, Gao R, Chen H, Zhang X, Cai X, Zhang C, Liu C, Zhu T and Chen C (2022) Effects of Glucocorticoids on Postoperative Neurocognitive Disorders in Adult Patients: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 14:939848. doi: 10.3389/fnagi.2022.939848
Received: 09 May 2022; Accepted: 13 June 2022;
Published: 30 June 2022.
Edited by:
Yingwei Wang, Fudan University, ChinaReviewed by:
Hao Wang, Shanghai Jiao Tong University, ChinaCopyright © 2022 Xie, Gao, Chen, Zhang, Cai, Zhang, Liu, Zhu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tao Zhu, eHd0YW8uemh1QGZveG1haWwuY29t; Chan Chen, eHljaGVuY2hhbkBnbWFpbC5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.