
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 26 August 2022
Sec. Alzheimer's Disease and Related Dementias
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.935652
This article is part of the Research TopicTranslational Advances in Alzheimer's, Parkinson's, and other Dementia: Molecular Mechanisms, Biomarkers, Diagnosis, and Therapies, Volume IIIView all 42 articles
Purpose: White matter hyperintensities (WMHs) are frequently found in elderly individuals with or without dementia. However, the association between WMHs and clinical presentations of dementia with Lewy bodies (DLB) has rarely been studied.
Methods: We conducted a retrospective analysis of patients with DLB registered in a dementia database. WMHs were rated visually using the Fazekas scale, and its associated factors including dementia severity, cognitive functions, neuropsychiatric symptoms, and core clinical features were compared among different Fazekas scores. Domains in the Clinical Dementia Rating (CDR), Cognitive abilities Screening Instruments (CASI), and Neuropsychiatric Inventory (NPI) were compared among different Fazekas groups after adjusting for age, sex, education, and disease duration.
Results: Among the 449 patients, 76, 207, 110, and 56 had Fazekas score of 0, 1, 2, and 3, respectively. There was a positive association between dementia severity and WMHs severity, and the mean sums of boxes of the Clinical Dementia Rating (CDR-SB) were 5.9, 7.8, 9.5, and 11.2 (f = 16.84, p < 0.001) for the Fazekas scale scores 0, 1, 2, and 3, respectively. There was a negative association between cognitive performance and WMHs severity, and the mean CASI were 57.7, 45.4, 4.06, and 33.4 (f = 14.22, p < 0.001) for the Fazekas scale scores 0, 1, 2, and 3, respectively. However, WMHs were not associated with the core clinical features of DLB. After adjustment, all cognitive domains in CDR increased as the Fazekas score increased. In addition, performance on all cognitive domains in CASI decreased as the Fazekas score increased (all p < 0.001). Among neuropsychiatric symptoms, delusions, euphoria, apathy, aberrant motor behavior, and sleep disorders were significantly worse in the higher Fazekas groups compared to those in the group with Fazekas score of 0 after adjustment.
Conclusion: WMHs in DLB might contribute to deterioration of cognitive function, neuropsychiatric symptoms, and dementia stages. However, core clinical features were not significantly influenced by WMHs in DLB.
White matter hyperintensities (WMHs) resulting from chronic ischemia associated with cerebral small vessel disease are frequently detected on magnetic resonance imaging (MRI) in elderly individuals (Prins et al., 2004). WMHs predict an increased risk of stroke, dementia, and death; therefore, detailed screening for risk factors of stroke and dementia should be performed if WMHs were identified (Debette and Markus, 2010). The histopathology of WMHs is various. The tissue damages range from slight disentanglement of the matrix to myelin and axonal loss (Prins and Scheltens, 2015; Wardlaw et al., 2015). In a previous study, WMHs were more frequent in patients with dementia than in patients with normal cognition (Sarro et al., 2016) and were strongly associated with cognitive dysfunction, especially in processing speed or executive functions (de Groot et al., 2000; Prins et al., 2004; Burton et al., 2006; Defrancesco et al., 2013). In patients with mild cognitive impairment (MCI), low scores in orientation and verbal delayed recall were predictors of progression from MCI to Alzheimer’s disease (AD) (Defrancesco et al., 2013). WMHs can be used to predict an increased risk of dementia (Wolf et al., 2000; van Straaten et al., 2008; Brickman et al., 2012; Kamagata et al., 2013) and a high WMHs burden may result in imminent progression from MCI to dementia (de Groot et al., 2000).
Previous studies have focused on the association between WMHs and cerebrovascular disease or dementia due to AD (Wolf et al., 2000; Defrancesco et al., 2013; Taylor et al., 2017). However, the association or influence of WMHs burden on the clinical manifestation or progression of dementia with Lewy bodies (DLB) has less been studied. DLB is the second most common degenerative dementia, accounting for 0–26.3% of hospital- or population-based dementia cases (Zaccai et al., 2005). In a previous study, patients with DLB presented with fewer WMHs than those with vascular dementia (Barber et al., 1999; Ihara et al., 2010), while those with DLB and AD showed more severe WMHs than patients with Parkinson’s disease (PD) with dementia (Joki et al., 2018). According to a previous study, WMHs in AD and DLB may be determined by similar processes and periventricular hyperintensities, but not deep WMHs, independently correlate with advancing age and increasing ventricular dilatation (Barber et al., 2000). However, studies investigating the contribution of WMHs or its associated factors in DLB have shown greater occipital WMHs in patients with DLB than in those with AD (Watson et al., 2012; Nedelska et al., 2015; Sarro et al., 2016). In addition, another study has reported an inverse relationship between small vessel disease scores and the severity of Lewy pathology in patients with Lewy body disease (LBD) (Ghebremedhin et al., 2010). There was an increased severity of WMHs on MRI, but not neuropathology, in DLB and Parkinson’s disease (PD) with dementia compared to PD without dementia and age-matched controls (Hijazi et al., 2022).
Some studies have reported an association between cognitive function and WMHs in patients with PD or DLB. One study showed a significant correlation between WMHs and Mini-Mental State Examination and verbal fluency scores in the AD group, but not in the DLB group (Oppedal et al., 2012). Another found a significant association between increased total WMHs volume and worse performance in executive function, memory, and language (Vesely and Rektor, 2016).
The Fazekas scale is a simple visual rating tool for clinical assessment of WMHs in normal aging and dementia (Fazekas et al., 1987). To investigate the factors associated with WMHs in patients with DLB, the Fazekas scale was used in a relatively large sample size of patients with DLB. The important clinical features, cognitive performance, motor dysfunction including gait disturbance, neuropsychiatric symptoms, medication, and vascular risk factors (VRFs) were analyzed and compared between the high and low Fazekas groups. Based on the current evidence, we hypothesized that higher WMHs should have higher dementia severity, WMHs in DLB might contribute to more cognitive and motor dysfunction, and worse neuropsychiatric symptoms.
This retrospective study included patients aged more than 59 years old with probable DLB registered in the dementia database of the Show Chwan Health Care System. Registration is currently ongoing in three hospitals in Taiwan. The Committee for Medical Research Ethics of Show Chwan Memorial Hospital reviewed the project, and the Data Inspectorate approved it (SCMH_IRB No: IRB1081006). The following information was extracted from this database and used in this study:
(1) Diagnosis of dementia (major neurocognitive disorder) according to the criteria for primary degenerative dementia in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders. Diagnosis of DLB according to the revised consensus criteria for probable or possible DLB developed by the fourth report of the DLB consortium (McKeith et al., 2017).
(2) Age, sex, education, dementia severity according to the Clinical Dementia Rating Scale (CDR) and CDR-SB, such as memory, orientation, judgment, community affairs, home hobbies, and personal care (Morris, 1997), and motor severity assessed using the motor score (UPDRS-m) and gait sub-score (UPDRS-gait) of the Unified Parkinson’s Disease Rating Scale (UPDRS) (Ballard et al., 1997; Movement Disorder Society Task Force on Rating Scales for Parkinson’s, 2003) at the time of entry.
(3) Clinical features of DLB, including fluctuation in cognition, parkinsonism, visual hallucinations (VH), rapid eye movement sleep behavior disorder (RBD), and abnormal dopamine transporter imaging in partial participants (McKeith et al., 2017). To acquire a detailed clinical history, all participants were asked to complete a registration form, including a detailed history registration questionnaire, named the History-based Artificial Intelligent Clinical Dementia Diagnostic System (HAICDDS) (Lin et al., 2018; Chiu et al., 2019; Wang et al., 2020). Prior to starting the study, we trained 12 neuropsychologists in three centers, and reliability tests for HAICDDS and neuropsychological tests were performed after training. The HAICDDS is a structured questionnaire used for the diagnosis of core clinical features of DLB. It includes 6 domains on fluctuation, 7 domains on parkinsonism, 2 domains on VH, and 4 domains on RBD. Fluctuations were diagnosed when a mayo fluctuation composite score of > 2 and a clinical history of fluctuation in cognition were present; VH were diagnosed when a clinical history of recurrent well-formed, complex, and detailed VH was present. Parkinsonism was diagnosed when at least two of the following were present: bradykinesia, rigidity, tremor, and postural instability; RBD was diagnosed when the minimum criteria for RBD according to the International Classification of Sleep Disorders were met (American Academy of Sleep Medicine, 2005).
(4) Cognitive performance assessed using the Chinese version of the Cognitive Abilities Screening Instrument (CASI) with the following domains: remote memory, recent memory, attention, mental manipulation, orientation, abstract thinking, language, drawing, and verbal fluency (Teng et al., 1994). Montreal Cognitive Assessment (MoCA) includes the following domains: visuospatial/executive, naming, attention, language, abstraction, memory, and orientation (Nasreddine et al., 2005; Kasten et al., 2010). Activities of daily living (ADL) assessed using the ADL scale embedded in the HAICDDS questionnaire (HAIADL) (Hung et al., 2021).
(5) Composite scores of the neuropsychiatric symptoms in the 12-item version of the NPI, such as delusions, hallucinations, agitation, depression, anxiety, euphoria, apathy, disinhibition, irritation, aberrant motor behavior, night behavior, and eating/appetite behavior. They were monitored according to observations in the past month (Cummings et al., 1994).
(6) Information on clinically relevant vascular risk comorbidities including hypertension, diabetes, hyperlipidemia, coronary artery disease, arrhythmia, congestive heart failure, and cerebrovascular disease (history of stroke or the diagnosis of vascular encephalopathy in brain imaging).
(7). MRI and WMHs ratings: MRI was performed using one of the four MRI scans currently used in the three centers (one 1.5 T Ingenia, Philips Healthcare, Best, the Netherlands, one 1.5 T Avanto, Siemens Medical Solutions, Erlangen, Germany, one 1.5 T Optima 450W, GE Healthcare, Milwaukee, Wisconsin, United States; and one 1.5 T Signa EXCITE-HD, GE Healthcare, Milwaukee, Wisconsin, United States). They were obtained at first clinical workup. The Fazekas scale (Fazekas et al., 1987) was used to determine the severity of WMHs. A total Fazekas WMH score, usually ranging from 0 to 6, was obtained by summing the periventricular (PVWM) and deep white matter (DWM) scores. In this study, to simplify the categories, we combined the periventricular and DWM scores and reduced the categories to 0–3. If PVWM and DWM scores were different, higher ones were selected for categorizing. Protocols include axial DWI (b-value 0, 1,000 s/mm2) and ADC map, axial FLAIR (TE: 110 ms, TR: 9,000 ms, Flip angle (FA): 90, inversion time: 2,400 ms, FOV: 14 × 23 cm, slice thickness: 5 mm), axial T1WI (TE: 13 ms, TR: 450 ms, FA: 90, FOV: 14 × 23 cm, slice thickness: 5 mm), axial T2WI (TE: 85 ms, TR: 5,000 ms, FA: 90, FOV: 14 × 23 cm, slice thickness: 5 mm), axial SWI (3D-MRA TOF without contrast), sagittal 3D-T1 SPGR (fast spoiled gradient-recalled echo), and coronal T2WI (TE: 85 ms, TR: 3,500 ms, FA: 150, FOV: 20 × 20 cm, SL: 3 mm). The key sequence for evaluating the severity of WMHs is axial FLAIR sequence. Before the starting of the study, 36 patients were tested by one neuroradiologist (TY Chen) and one neurologist (PY Chiu) and the reproducibility was studied using the interrater reliability analysis with a high intra-class correlation coefficient of 0.927.
(8) Striatal background ratio and caudate putamen ratio (CPR) were assessed using Tc-99m Trodat-1 SPECT and analyzed semi-quantitatively (Huang et al., 2012). Dopamine transporter imaging is one of the indicative biomarkers; therefore, some participants received 99m Trodat-1 SPECT or cerebral perfusion SPECT to obtain more accurate diagnosis. The procedure of the dopamine transporter imaging is as the following: A dose of 25 mCi of [99 mTc] TRODAT-1 was injected intravenously into each patient. The binding to dopamine transporter was assessed 4 h after injection by SPECT. A rotating three-headed gamma camera with fan-beam collimator (Multi SPECT 3, Siemens, Germany) and a commercially available computer system were used for data acquisition and processing. Data were collected for 120 projections (360° rotation) in a 128 × 128 matrix. The acquisition time was 40 s per projection. Attenuation correction was performed in the selected transverse slices according to a modified Chang’s method. In-plane resolution of the reconstructed images was 8.5 mm FWHM, and slice thickness was approximately 6 mm.
The Chinese version of SPSS 22.0 for Windows (IBM, SPSS Inc., Chicago) was used for statistical analyses. Background characteristics, clinical features, VRFs, current medication usage, performance on different cognitive domains, and manifestation of neuropsychiatric symptoms of patients with DLB in different Fazekas groups were compared. Comparison of characteristics included age, education level, global dementia severity according to the CDR and CDR-SB, results of cognitive tests including subscale scores of the CASI and MoCA, and subscale scores of the neuropsychiatric symptoms according to the NPI. One-way ANOVA with either Bonferroni or Dunnett T3 post hoc analysis was according to the homogeneity of variance. Sex, clinical features of DLB, VRFs, and current medication usage were analyzed using the chi-square test with Bonferroni correction. The motor scores of the UPDRS and findings of dopamine transporter imaging performed using Tc-99m Trodat-1 SPECT were analyzed among the partial participants (36, 86, 42, and 22 cases with Fazekas 0, 1, 2, and 3, respectively). Domains in the CDR including a supplementary domain of language (HAICDDS-Language, Lin et al., 2018), CASI, and NPI were compared using multivariate logistic regression among different Fazekas group after adjusting for age, sex, education, and disease duration. Odds ratio (OR) of the higher Fazekas groups were compared to that of the group with Fazekas score of 0.
The registration period for this study was from September 2015 to August 2020. During this period, we registered 9,607 individuals with either normal cognition (n = 2,131), MCI (n = 2,485), or dementia (n = 4,269) in a hospital-based cohort. In the dementia group, 1,841 (47.1%) patients had probable AD, 449 (10.5%) patients had DLB, 1,979 (43.4%) patients had other subtypes of dementia or undetermined dementia. Among patients with DLB, 76, 207, 110, and 56 patients had Fazekas scale scores of 0, 1, 2, and 3, respectively (Table 1). A comparison of ages among the four Fazekas subgroups revealed no significant association, and the mean age was 78.6, 79.5, 80.8, and 80.5 for the Fazekas scale scores of 0, 1, 2, and 3, respectively. The sex disparity, education, and disease duration were not significantly different among the four Fazekas subgroups, either. However, there was a positive association between dementia severity and WMH severity, and the mean
CDR-SB were 5.9, 7.8, 9.5, and 11.2 (f = 16.84, p < 0.001) for the Fazekas scale scores 0, 1, 2, and 3, respectively. There was a negative association between cognitive performance and WMH severity, and the mean CASI were 57.7, 45.4, 4.06, and 33.4 (f = 14.22, p < 0.001) for the Fazekas scale scores 0, 1, 2, and 3, respectively. In addition, there was a negative association between ADL function and WMH severity, and the mean HAIADL were 11.0, 14.7, 17.8, and 2.3 (f = 17.02, p < 0.001) for the Fazekas scale scores 0, 1, 2, and 3, respectively. Poorer motor and gait functions according to UPDRS-m (f = 4.81, p = 0.003) and UPDRS-gait (f = 4.50, p = 0.004) were found only in the Fazekas 3. The CPR according to Tc-99m Trodat-1 SPECT was significantly higher in the lower Fazekas subgroups (Fazekas 0 and 1) compared to those in the higher Fazekas groups (Fazekas 2 and 3).
Comparisons of core clinical features for the diagnosis of DLB, VRFs, and current medications among different Fazekas groups are summarized in Table 2. Among all participants, frequencies of core clinical features including fluctuation in cognition, VH, parkinsonism, and RBD were 67.3, 43.2, 87.5, and 45.9%, respectively. Frequency of abnormal dopamine transporter imaging was 82.3% (153 with abnormal finding within 186 participants that performed dopamine transporter imaging). Comparison of these important diagnostic features for DLB were not significantly different among the Fazekas groups. Comparisons of VRFs and current medications usage among different Fazekas groups demonstrated that no significant difference among groups after Bonferroni correction except for CVD was higher in the Fazekas 3 group compared to those in the Fazekas 0 or 1 groups (f = 14.40, p = 0.002).
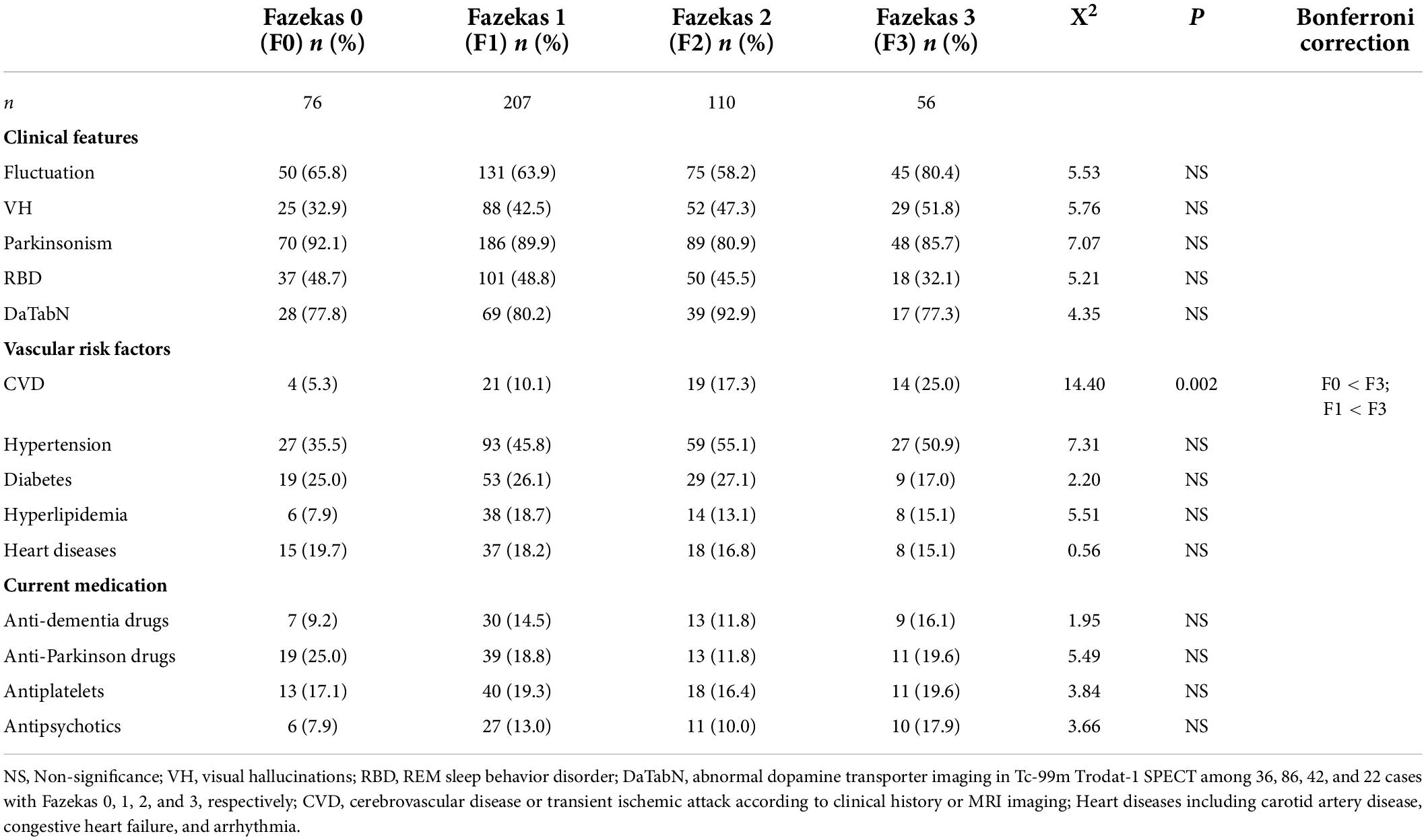
Table 2. Comparison of clinical features, vascular risk factors, and current medication usage among different Fazekas groups.
Figure 1 demonstrated a comparison of domains in CDR among different Fazekas groups and showed positive correlations of domains including memory, orientation, judgment, community affairs, home hobbies, personal care, and language with all p < 0.001. After adjustment for age, sex, education, and disease duration, all cognitive domains increased as the Fazekas score increased. After adjustment, compared to Fazekas 0 in memory, the ORs were 1.38, 1.81, and 1.99 in Fazekas 1, 2, and 3, respectively. In orientation, the ORs were 1.34, 1.88, and 2.55 in Fazekas 1, 2, and 3, respectively. In judgment, the ORs were 1.34, 1.99, and 2.52 in Fazekas 1, 2, and 3, respectively. In community affairs, the ORs were 1.63, 2.61, and 2.91 in Fazekas 1, 2, and 3, respectively. In home hobbies, the ORs were 1.65, 2.12, and 2.65 in Fazekas 1, 2, and 3, respectively. In personal care, the ORs were 1.34, 2.00, and 2.40 in Fazekas 1, 2, and 3, respectively. In language, the ORs were 1.38, 1.62, and 2.31 in Fazekas 1, 2, and 3, respectively.
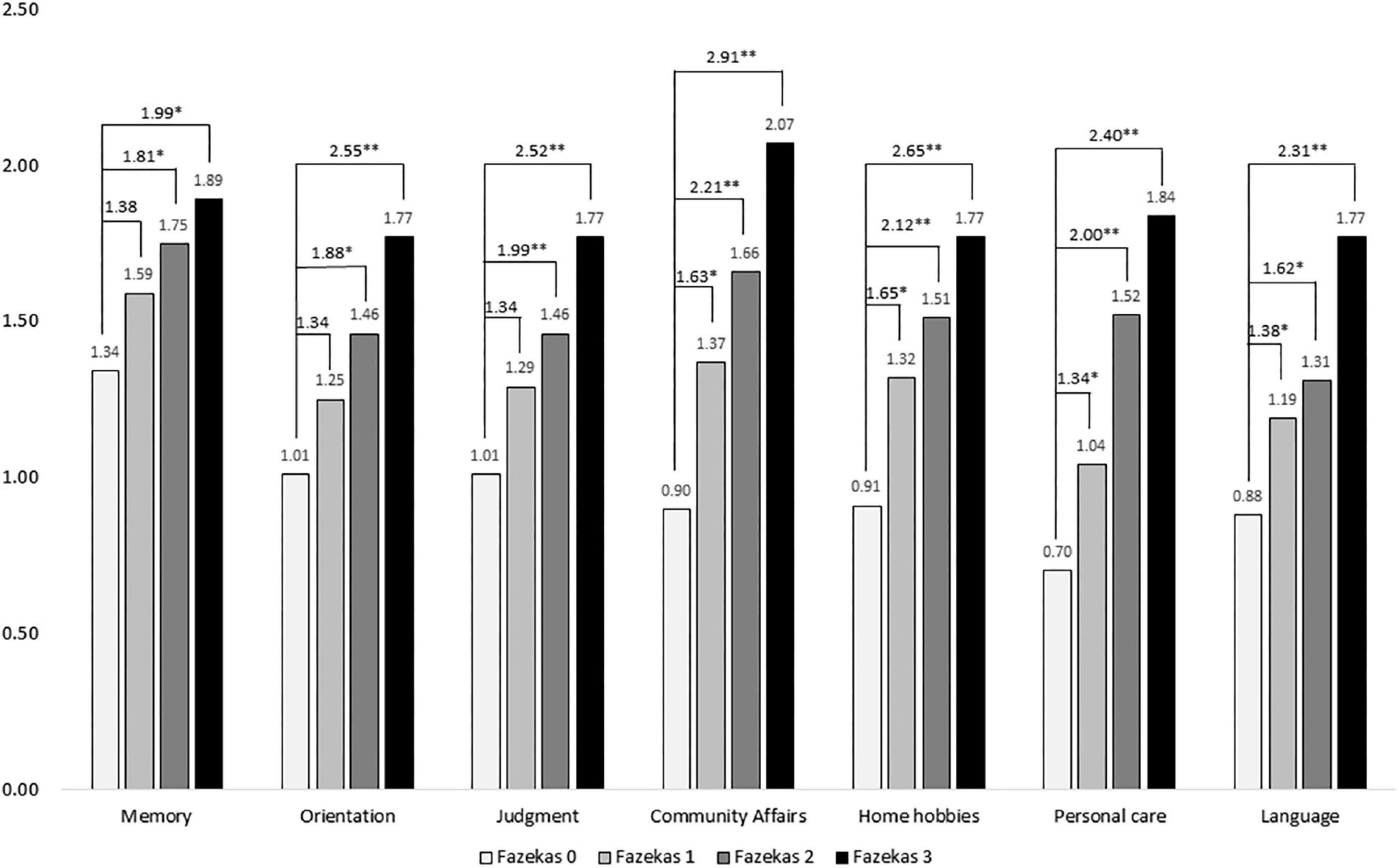
Figure 1. Comparison of scores of boxes of the Clinical Dementia Rating (CDR-SB) among different Fazekas groups adjusted for age, sex, education, and disease duration. ORs were compared to Fazekas score of 0. *p < 0.05; **p < 0.001.
Figure 2 demonstrated a comparison of cognitive domains of the CASI among different Fazekas groups and showed positive correlations of domains including remote memory, recent memory, attention, mental manipulation, orientation, abstract thinking, language, drawing, and verbal fluency with all p < 0.001. Performance on all cognitive domains decreased as the Fazekas score increased. After adjustment, in remote memory, the ORs were 0.83, 0.82, and 0.78 in Fazekas 1, 2, and 3, respectively. In recent memory, the ORs were 0.93, 0.86, and 0.82 in Fazekas 1, 2, and 3, respectively. In attention, the ORs were 0.93, 0.82, and 0.92 in Fazekas 1, 2, and 3, respectively. In orientation, the ORs were 0.90, 0.85, and 0.82 in Fazekas 1, 2, and 3, respectively. In mental manipulation, the ORs were 0.83, 0.85, and 0.67 in Fazekas 1, 2, and 3, respectively. In abstract thinking, the ORs were 0.84, 0.79, and 0.75 in Fazekas 1, 2, and 3, respectively. In language, the ORs were 0.90, 0.85, and 0.82 in Fazekas 1, 2, and 3, respectively. In drawing, the ORs were 0.90, 0.91, and 0.81 in Fazekas 1, 2, and 3, respectively. In verbal fluency, the ORs were 0.95, 0.89, and 0.80 in Fazekas 1, 2, and 3, respectively.

Figure 2. Comparison of cognitive domains of the CASI among different Fazekas groups adjusted for age, sex, education, and disease duration. ORs were compared to Fazekas score of 0. *p < 0.05; **p < 0.001.
Figure 3 shows a comparison of composite scores of neuropsychiatric symptoms in the NPI among different Fazekas groups. Among them, delusions, euphoria, apathy, aberrant motor behavior, and sleep disorders were significantly worse in the higher Fazekas (2 or 3) groups compared to those in the group with Fazekas score of 0 after adjustment. In delusions, the ORs were 1.6, 2.0, and 2.0 in Fazekas 1, 2, and 3, respectively. In euphoria, the ORs were 0.8, 3.8, and 3.3 in Fazekas 1, 2, and 3, respectively. In apathy, the ORs were 1.5, 2.2, and 2.1 in Fazekas 1, 2, and 3, respectively. In sleep disorders, the ORs were 1.0, 1.1, and 1.1 in Fazekas 1, 2, and 3, respectively.
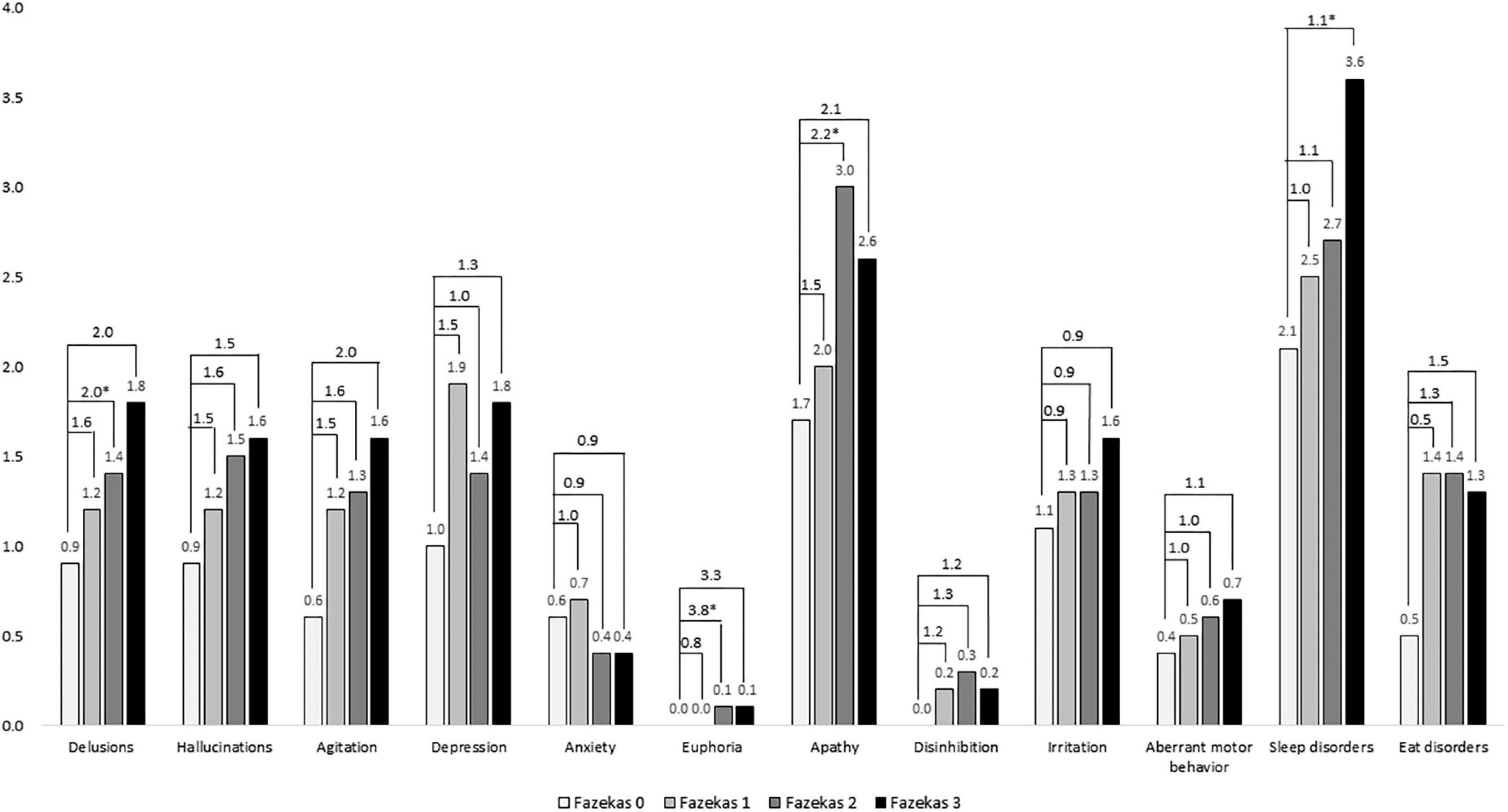
Figure 3. Comparison of composite scores of neuropsychiatric symptoms in the NPI among different Fazekas groups adjusted for age, sex, education, and disease duration. ORs were compared to Fazekas score of 0. *p < 0.05.
Our study reports some important findings. First, the age, sex disparity, education, and disease duration in this study were not significantly different among the four Fazekas subgroups; however, WMHs were positively correlated with dementia severity and negatively correlated with cognitive performance and ADL function which are consistent with the results of most previous studies on WMHs and dementia (Prins et al., 2004; Sarro et al., 2016). Dopamine transporter imaging is one of the indicative biomarkers for the diagnosis of DLB. In this study, there is no difference in the SBR (striatal background ratio) among different Fazekas groups; however, the CPR is significantly lower in the higher Fazekas groups (Fazekas 2 and 3) indicating a worse cognitive function instead of motor function in the higher Fazekas groups compared to the lower Fazekas groups (Fazekas 0 and 1). This is also compatible with clinical findings of higher dementia severity and lower cognitive performance in the higher Fazekas groups.
Second, we found that there was no significant association between WMHs and core clinical features for the diagnosis of DLB including fluctuation, VH, parkinsonism, and RBD. However, significantly poorer motor and gait functions according to motor and gait sub-scores found in the Fazekas 3 indicated a potential contribution of severe WMHs to motor dysfunction in DLB. In addition, most of the VRFs and current medication usage were not different among Fazekas groups, either. These results suggest that cerebral or systemic
vascular factors contribute less to the emergence of the clinical presentation in DLB. However, in this study, we found a positive association between WMHs and CVD (5.3, 10.1, 17.3, and 25.3% for the Fazekas scale scores of 0, 1, 2, 3, respectively) after Bonferroni correction. This finding is also consistent with the evidence of an inverse relationship between small vessel disease and the severity of cerebral vascular pathology in patients with vascular dementia (Barber et al., 1999; Ihara et al., 2010).
Third, although cognitive screening tests (such as the CASI and MoCA) were used to study the association between cognitive function and WMHs. After adjustment for age, sex, education, and disease duration, patients with DLB and higher Fazekas scale scores had poorer cognitive functions in all domains of CASI including remote memory, recent memory, attention, mental manipulation, orientation, abstract thinking, language, drawing, and verbal fluency than those with lower Fazekas scale scores. Based on the consensus criteria, cognitive deficits in the domains of executive function, visuospatial function, and attention are considered essential for the diagnosis of DLB (McKeith et al., 2017). Therefore, in this study, most cognitive domains in which WMHs contributed to the clinical presentation of DLB were not only the essential cognitive features (visuospatial function, attention, mental manipulation, abstract thinking, and verbal fluency) for the diagnosis of DLB but were also some other cognitive features (orientation, language, remote memory, and recent memory). The poor performance of the higher Fazekas groups could be attributed to the additional effects of WMHs on cognitive dysfunction in the patients with DLB.
Finally, when studying the association between WMHs and neuropsychiatric features in DLB, we found that among neuropsychiatric symptoms, delusions, euphoria, apathy, aberrant motor behavior, and sleep disorders were significantly worse in the higher Fazekas groups compared to those in the group with the Fazekas score of 0 after adjustment. This finding suggests that WMHs might have partial contribution to the neuropsychiatric manifestation of DLB. For example, according to the previous studies and consensus criteria, sleep disorders are striking features of DLB (Yamane et al., 2011; Chiu et al., 2017; McKeith et al., 2017). These findings also support the previous findings that WMHs cannot be considered as mere incidental findings, at least in patients who show severe lesions (Pantoni et al., 2007).
This study has some limitations. First, it was conducted in three hospitals in Taiwan. Therefore, the study findings may not be generalizable to all patients with DLB. Second, the comparison of the associated factors between the high and low Fazekas groups was cross-sectional. Therefore, causal relationships between the factors and dementia could not be ascertained. Third, the current study did not compare DLB with non-synucleinopathy disease; therefore, both contribution of synucleinopathy to deterioration of cognition and interaction of synucleinopathy with WMH were not able to be well demonstrated. In addition, this study did not include the CHIPS score for analysis. According to the previous studies, cholinergic function decline was not only found in the patients with AD, but was also noticed in the patients with Lewy body dementia including DLB and PDD (Francis and Perry, 2007). Fourth, the identification of RBD or DLB type VH is relatively strict in our cohort. Therefore, the prevalence of DLB core clinical features in our cohort was relatively lower. We considered a relatively strict evaluation of core clinical features and neuropsychiatric symptoms to guarantee a better discrimination of DLB from AD. Lastly, only 41.4% study participants had undergone dopamine transporter uptake imaging; this may have resulted in a lower diagnostic rate of probable DLB in this study.
In conclusion, we evaluated WMHs using brain MRI in a relatively large sample of patients with DLB and performed multi-dimensional analysis of the associated factors of WMHs in DLB according to different Fazekas scale scores. Compared to the lower Fazekas group, the higher Fazekas groups had more severe stages of dementia, poorer cognitive function and ADL, and more severe sleep disorders. There was no significant association between WMHs and core clinical features for the diagnosis of DLB including fluctuation, VH, parkinsonism, and RBD. This cohort will be prospectively followed up for the influence of WMHs on the progression of clinical manifestations of DLB.
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
The studies involving human participants were reviewed and approved by the Show Chwan Memorial Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
P-YC: conception and study design. C-FT and P-YC: statistical analysis. T-YC: drafting the manuscript work. T-YC, P-CC, and P-YC: revising the manuscript. All authors: data collection and acquisition, interpretation of results, and approval of final version.
We would like to thank Dr. Hsing-Ju Wu at the Research Assistant Center of the Show Chwan Memorial Hospital for editing the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
American Academy of Sleep Medicine (2005). International Classification of Sleep Disorders: Diagnostic and Coding Manual. Westchester, IL: American Academy of Sleep Medicine.
Ballard, C., McKeith, I., Burn, D., Harrison, R., O’Brien, J., Lowery, K., et al. (1997). The UPDRS scale as a means of identifying extrapyramidal signs in patients suffering from dementia with Lewy bodies. Acta Neurol. Scand. 96, 366–371. doi: 10.1111/j.1600-0404.1997.tb00299.x
Barber, R., Gholkar, A., Scheltens, P., Ballard, C., McKeith, I. G., and O’Brien, J. T. (2000). MRI volumetric correlates of white matter lesions in dementia with Lewy bodies and Alzheimer’s disease. Int. J. Geriatr. Psychiatry 15, 911–916.
Barber, R., Scheltens, P., Gholkar, A., Ballard, C., McKeith, I., Ince, P., et al. (1999). White matter lesions on magnetic resonance imaging in dementia with Lewy bodies, Alzheimer’s disease, vascular dementia, and normal aging. J. Neurol. Neurosurg. Psychiatry 67, 66–72. doi: 10.1136/jnnp.67.1.66
Brickman, A. M., Provenzano, F. A., Muraskin, J., Manly, J. J., Blum, S., Apa, Z., et al. (2012). Regional white matter hyperintensity volume, not hippocampal atrophy, predicts incident Alzheimer disease in the community. Arch. Neurol. 69, 1621–1627. doi: 10.1001/archneurol.2012.1527
Burton, E. J., McKeith, I. G., Burn, D. J., Firbank, M. J., and O’Brien, J. T. (2006). Progression of white matter hyperintensities in Alzheimer disease, dementia with lewy bodies, and Parkinson disease dementia: a comparison with normal aging. Am. J. Geriat.r Psychiatry 14, 842–849. doi: 10.1097/01.JGP.0000236596.56982.1c
Chiu, P. Y., Wang, C. W., Tsai, C. T., Li, S. H., Lin, C. L., and Lai, T. J. (2017). Depression in dementia with Lewy bodies: a comparison with Alzheimer’s disease. PLoS One 12:e0179399. doi: 10.1371/journal.pone.0179399
Chiu, P. Y., Wei, C. Y., and Hung, G. U. (2019). Preliminary study of the history-based artificial intelligent clinical dementia diagnostic system. Show Chwan Med. J. 18, 18–27.
Cummings, J. L., Mega, M., Gray, K., Rosenberg-Thompson, S., Carusi, D. A., and Gornbein, J. (1994). The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314. doi: 10.1212/wnl.44.12.2308
de Groot, J. C., de Leeuw, F. E., Oudkerk, M., van Gijn, J., Hofman, A., Jolles, J., et al. (2000). Cerebral white matter lesions and cognitive function: the rotterdam scan study. Ann. Neurol. 47, 145–151.
Debette, S., and Markus, H. S. (2010). The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 341:c3666. doi: 10.1136/bmj.c3666
Defrancesco, M., Marksteiner, J., Deisenhammer, E., Kemmler, G., Djurdjevic, T., and Schocke, M. (2013). Impact of white matter lesions and cognitive deficits on conversion from mild cognitive impairment to Alzheimer’s disease. J. Alzheimers Dis. 34, 665–672. doi: 10.3233/JAD-122095
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., and Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 149, 351–356. doi: 10.2214/ajr.149.2.351
Francis, P. T., and Perry, E. K. (2007). Cholinergic and other neurotransmitter mechanisms in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies. Move. Disord. 22, S351–S357. doi: 10.1002/mds.21683
Ghebremedhin, E., Rosenberger, A., Rub, U., Vuksic, M., Berhe, T., Bickeboller, H., et al. (2010). Inverse relationship between cerebrovascular lesions and severity of lewy body pathology in patients with lewy body diseases. J. Neuropathol. Exp. Neurol. 69, 442–448. doi: 10.1097/NEN.0b013e3181d88e63
Hijazi, Z., Yassi, N., O’Brien, J. T., and Watson, R. (2022). The influence of cerebrovascular disease in dementia with Lewy bodies and Parkinson’s disease dementia. Eur. J. Neurol. 29, 1254–1265. doi: 10.1111/ene.15211
Huang, C. C., Yen, T. C., and Lu, C. S. (2012). “Dopamine Transporter Imaging for Distinguishing Between Idiopathic Parkinson’s Disease and Secondary Parkinsonism,” in Neuroimaging - Clinical Applications, ed. P. Bright (London: IntechOpen), 401–422.
Hung, C. H., Hung, G. U., Wei, C. Y., Tzeng, R. C., and Chiu, P. Y. (2021). Function-based dementia severity assessment for vascular cognitive impairment. J. Formos. Med. Assoc. 120, 533–541. doi: 10.1016/j.jfma.2020.07.001
Ihara, M., Polvikoski, T. M., Hall, R., Slade, J. Y., Perry, R. H., Oakley, A. E., et al. (2010). Quantification of myelin loss in frontal lobe white matter in vascular dementia, Alzheimer’s disease, and dementia with Lewy bodies. Acta Neuropathol. 119, 579–589. doi: 10.1007/s00401-009-0635-8
Joki, H., Higashiyama, Y., Nakae, Y., Kugimoto, C., Doi, H., Kimura, K., et al. (2018). White matter hyperintensities on MRI in dementia with Lewy bodies, Parkinson’s disease with dementia, and Alzheimer’s disease. J. Neurol. Sci. 385, 99–104. doi: 10.1016/j.jns.2017.12.018
Kamagata, K., Motoi, Y., Tomiyama, H., Abe, O., Ito, K., Shimoji, K., et al. (2013). Relationship between cognitive impairment and white-matter alteration in Parkinson’s disease with dementia: tract-based spatial statistics and tract-specific analysis. Eur. Radiol. 23, 1946–1955. doi: 10.1007/s00330-013-2775-4
Kasten, M., Bruggemann, N., Schmidt, A., and Klein, C. (2010). Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology 75, 478–479. doi: 10.1212/WNL.0b013e3181e7948a
Lin, C. M., Hung, G. U., Wei, C. Y., Tzeng, R. C., and Chiu, P. Y. (2018). An Informant-based simple questionnaire for language assessment in neurodegenerative disorders. Dement. Geriatr. Cogn. Disord. 46, 207–216. doi: 10.1159/000493540
McKeith, I. G., Boeve, B. F., Dickson, D. W., Halliday, G., Taylor, J. P., Weintraub, D., et al. (2017). Diagnosis and management of dementia with lewy bodies: fourth consensus report of the DLB Consortium. Neurology 89, 88–100. doi: 10.1212/WNL.0000000000004058
Morris, J. C. (1997). Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int. Psychogeriatr. 9, 173–176.
Movement Disorder Society Task Force on Rating Scales for Parkinson’s (2003). The Unified Parkinson’s Disease Rating Scale (UPDRS): status and recommendations. Mov. Disord. 18, 738–750. doi: 10.1002/mds.10473
Nasreddine, Z. S., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Nedelska, Z., Schwarz, C. G., Boeve, B. F., Lowe, V. J., Reid, R. I., Przybelski, S. A., et al. (2015). White matter integrity in dementia with Lewy bodies: a voxel-based analysis of diffusion tensor imaging. Neurobiol. Aging 36, 2010–2017. doi: 10.1016/j.neurobiolaging.2015.03.007
Oppedal, K., Aarsland, D., Firbank, M. J., Sonnesyn, H., Tysnes, O. B., O’Brien, J. T., et al. (2012). White matter hyperintensities in mild lewy body dementia. Dement. Geriatr. Cogn. Dis. Extra 2, 481–495. doi: 10.1159/000343480
Pantoni, L., Poggesi, A., and Inzitari, D. (2007). The relation between white-matter lesions and cognition. Curr. Opin. Neurol. 20, 390–397. doi: 10.1097/WCO.0b013e328172d661
Prins, N. D., and Scheltens, P. (2015). White matter hyperintensities, cognitive impairment and dementia: an update. Nat. Rev. Neurol. 11, 157–165. doi: 10.1038/nrneurol.2015.10
Prins, N. D., van Dijk, E. J., den Heijer, T., Vermeer, S. E., Koudstaal, P. J., Oudkerk, M., et al. (2004). Cerebral white matter lesions and the risk of dementia. Arch. Neurol. 61, 1531–1534. doi: 10.1001/archneur.61.10.1531
Sarro, L., Schwarz, C., Graff-Radford, J., Tosakulwong, N., Reid, R. I., Przybelski, S., et al. (2016). An investigation of cerebrovascular lesions in dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimers Dement. 13:86.
Taylor, A. N. W., Kambeitz-Ilankovic, L., Gesierich, B., Simon-Vermot, L., Franzmeier, N., Araque Caballero, M. A., et al. (2017). Tract-specific white matter hyperintensities disrupt neural network function in Alzheimer’s disease. Alzheimers Dement 13, 225–235. doi: 10.1016/j.jalz.2016.06.2358
Teng, E. L., Hasegawa, K., Homma, A., Imai, Y., Larson, E., Graves, A., et al. (1994). The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int. Psychogeriatr. 6, 45–58. doi: 10.1017/s1041610294001602
van Straaten, E. C., Harvey, D., Scheltens, P., Barkhof, F., Petersen, R. C., Thal, L. J., et al. (2008). Periventricular white matter hyperintensities increase the likelihood of progression from amnestic mild cognitive impairment to dementia. J. Neurol. 255, 1302–1308. doi: 10.1007/s00415-008-0874-y
Vesely, B., and Rektor, I. (2016). The contribution of white matter lesions (WML) to Parkinson’s disease cognitive impairment symptoms: a critical review of the literature. Parkinsonism Relat. Disord. 22, S166–S170. doi: 10.1016/j.parkreldis.2015.09.019
Wang, C. T., Hung, G. U., Wei, C. Y., Tzeng, R. C., and Chiu, P. Y. (2020). An informant-based simple questionnaire for visuospatial dysfunction assessment in dementia. Front. Neurosci. 14:44. doi: 10.3389/fnins.2020.00044
Wardlaw, J. M., Valdes Hernandez, M. C., and Munoz-Maniega, S. (2015). What are white matter hyperintensities made of? Relevance to vascular cognitive impairment. J. Am. Heart Assoc. 4:001140. doi: 10.1161/JAHA.114.001140
Watson, R., Blamire, A. M., Colloby, S. J., Wood, J. S., Barber, R., He, J., et al. (2012). Characterizing dementia with Lewy bodies by means of diffusion tensor imaging. Neurology 79, 906–914. doi: 10.1212/WNL.0b013e318266fc51
Wolf, H., Ecke, G. M., Bettin, S., Dietrich, J., and Gertz, H. J. (2000). Do white matter changes contribute to the subsequent development of dementia in patients with mild cognitive impairment? A longitudinal study. Int. J. Geriatr. Psychiatry 15, 803–812. doi: 10.1002/1099-1166(200009)15:9<803::aid-gps190>3.0.co;2-w
Yamane, Y., Sakai, K., and Maeda, K. (2011). Dementia with lewy bodies is associated with higher scores on the geriatric depression scale than is Alzheimer’s disease. Psychogeriatrics 11, 157–165. doi: 10.1111/j.1479-8301.2011.00368.x
Keywords: white matter hyperintensities, dementia with Lewy bodies, Fazekas, cognitive, sums of boxes of the clinical dementia rating, cognitive abilities screening instruments
Citation: Chen T-Y, Chan P-C, Tsai C-F, Wei C-Y and Chiu P-Y (2022) White matter hyperintensities in dementia with Lewy bodies are associated with poorer cognitive function and higher dementia stages. Front. Aging Neurosci. 14:935652. doi: 10.3389/fnagi.2022.935652
Received: 04 May 2022; Accepted: 10 August 2022;
Published: 26 August 2022.
Edited by:
Jiehui Jiang, Shanghai University, ChinaReviewed by:
Jong-Min Kim, Seoul National University Bundang Hospital, South KoreaCopyright © 2022 Chen, Chan, Tsai, Wei and Chiu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pai-Yi Chiu, cGFpeWlib3hAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.