
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
BRIEF RESEARCH REPORT article
Front. Aging Neurosci. , 03 August 2022
Sec. Neurocognitive Aging and Behavior
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.933958
This article is part of the Research Topic Early Detection of Neurodegenerative Disorders Using Behavioral Markers and New Technologies: New Methods and Perspectives View all 10 articles
Background: Apathy is attracting more and more attention in clinical practice. As one of the most common features of cerebral small vessel disease (CSVD), the assessment of apathy still mainly relies on observers. With the development of Information and Communication Technologies (ICTs), new objective tools take part in the early detection of apathy.
Objectives: To detect apathy in patients with CSVD and find out the relationship between apathy and actigraphic data sampled from the diurnal and nocturnal periods.
Methods: A total of 56 patients with CSVD were recruited for a cross-sectional observational study. Apathy was diagnosed by the diagnostic criteria for apathy in neurocognitive disorders. The presence of lacunes, white matter hyperintensities, cerebral microbleeds (CMBs), and perivascular spaces (PVS) in magnetic resonance imaging (MRI) images were rated independently. Actigraph devices were worn in the non-dominant hands of each subject for 7 consecutive days to collect samples of raw data, and diurnal vector magnitude (VM) and a series of sleep quality variables were obtained.
Results: We found that the frequency of apathy in Chinese patients with CSVD reached 37.50%. Patients in the Apathy+ group showed more lacunes and CMBs, and higher Fazekas scores in comparison to apathy-group individuals. Diurnal VM, instead of other sleep quality variables, was lower in CSVD patients with apathy relative to those without apathy. Lastly, we discovered that diurnal VM and total time in bed (TTB) correlated negatively with apathy severity in patients with CSVD.
Conclusion: Actigraphy is a promising choice to evaluate apathy in patients with CSVD.
Apathy, defined as a disorder of goal-directed behavior, has attracted more and more attention in aging neuroscience (Hachinski et al., 2022). It has been evidenced that apathy is be found in many neurodegenerative disorders, such as Alzheimer’s disease (AD), Parkinson’s disease (PD), and multiple sclerosis (MS) (Zhao et al., 2012; Niino et al., 2014; Brown et al., 2019), and apathy has been confirmed to be correlated with cognitive impairments in cross-sectional studies (Niino et al., 2014; Yu et al., 2020). Several longitudinal studies demonstrated that an obvious decline in cognitive function and daily living ability was accompanied by apathy (Robert et al., 2006; Lechowski et al., 2009). For example, a 4-year prospective longitudinal study recently discovered that a higher level of apathy at baseline enables the prediction of a progressive cognitive decline at 4-year follow-up in patients with MS (Raimo et al., 2022). In most previous research, apathy was diagnosed and assessed by scales such as the Neuropsychiatric Inventory (NPI), the Apathy Evaluation Scale (AES), the Apathy Inventory (AI), and the Geriatric Depression Scale (GDS) (David et al., 2012; Kuhlmei et al., 2013; Zhao et al., 2014; Tay et al., 2019). In Robert et al. (2018), Miller et al. (2021), the diagnostic criteria for apathy in neurocognitive disorders were discussed actively by experts from academia, industry, and regulatory bodies all around the world. In line with the evolvement of the diagnostic criteria of apathy, unreliable self-reporting has been proposed to be substituted and improved by informant-based observable traits and objective tools.
Cerebral small vessel disease (CSVD) refers to a group of pathological processes that affect the small arteries, arterioles, venules, and capillaries of the brain (Pantoni, 2010). CSVD is quite common in the older population and is featured as cognitive deficits, gait abnormalities, and affective disorders (Zhào et al., 2020). A recent systematic review revealed that CSVD affects all major domains of cognitive ability (Hamilton et al., 2021). Our previous study showed that the prevalence of apathy reached 37.3% in older Chinese patients with CSVD, with a lack of goal-directed behavior and diminished goal-directed activities in the dimension of behavior/cognition according to the diagnostic criteria for apathy revised in 2018 (Zhào et al., 2021).
Until now, various assessment scales for apathy have been developed, whereas, the detection of apathy is still debated. This phenomenon can be explained by the fact that the application of scales is rather limited, owing to its dependency on the human observer. Based on the development of novel technologies, apathy experts recommended objective ways such as Information and Communication Technologies (ICTs) on evaluating apathy in coordination with subjective inventories (Robert et al., 2013). For example, the ECOCAPTURE@HOME protocol was reported to assess apathy remotely in patients with AD and Frontotemporal Dementia (FTD) recently (Batrancourt et al., 2019; Godefroy et al., 2021).
Actigraph devices, progressing from piezoelectric to captive sensors, from epoch-based count data to raw data sampled at high frequency, are a potential candidate for ICTs of that use (König et al., 2014). Actigraphy was confirmed to measure sleep quality and daytime activity in our recently published paper (Zhao et al., 2022). Thus, we sought to detect apathy in patients with CSVD and found out the relationship between apathy and actigraphic data sampled from the diurnal and nocturnal periods.
We launched a cross-sectional observational study from 1 November 2021 to 20 April 2022 and recruited 56 elderly patients with CSVD consecutively from the Department of Neurology at the Seventh Medical Center of PLA General Hospital (Beijing, China). Our study was approved by the Academic Ethics Committee of the Biological Sciences Division of PLA General Hospital in Beijing, China.
The exclusion criteria were as follows: patients with major stroke or cerebral bleeding episodes or tremor; other causes of leukoencephalopathy (e.g., immune, demyelination, genetic); use of psychotropic medications; multisystem diseases, such as polyarteritis nodosa, nervous system vasculitis associated with connective tissue disorders, and vasculitis secondary to infectious; arthritis; MRI contraindications; and other neurodegenerative disorders, such as AD, PD, and FTD.
A 3.0T MRI brain (Discovery MR750; GE Healthcare, United States) scan displayed white matter lesions reflecting the degree of CSVD. A brain MRI (slice and interslice thicknesses of 5 and 1.5 mm, respectively) was carried out as follows: longitdinal relaxation time (T1) fluid-attenuated inversion recovery [transverse relaxation time (TR), 1750 ms; time of echo (TE), 23 ms; T1, 780 ms; field of view (FOV), 24 cm] and T2-weighted imaging (TR, 7498 ms; TE, 105 ms; FOV, 24 cm) sequences. The assessors were blinded to imaging findings.
The total CSVD burden score was calculated according to our previous procedure (Zhao et al., 2022). One point was allocated to each of the following MRI parameters: moderate to severe white matter hyperintensities (WMH) (Fazekas score: 2–3), presence of lacunes, cerebral Microbleeds (CMBs), and moderate to severe basal ganglia-perivascular spaces (PVS) (semi-quantitative rating > 1), with total scores ranging from 0 to 4 points. To elucidate whether actigraphic data differed according to CSVD burden score, subjects were also divided by total CSVD burden score.
Each patient was diagnosed according to the diagnostic criteria for apathy in neurocognitive disorders (Miller et al., 2021). It is different from the diagnostic criteria proposed in 2018 (Robert et al). In fact, in criteria B (symptoms and duration), Dimensions B1, B2, and B3 were listed as diminished initiative, interest, and emotional expression/responsiveness, respectively. Dimension B4 (social interaction) was removed. The patient exhibits at least one symptom in at least two of the three dimensions. Details were listed in Supplementary material 1.
All participants completed both NPI and mini-mental State Evaluation (MMSE). The NPI (12 subscales) is a structured interview with a caregiver who is familiar with the subject. The overall frequency (1–4) and severity (1–3) are then rated. Scores on each NPI subscale range from 0 to 12, with higher scores indicating more severe symptoms (Zhao et al., 2014). MMSE is a widely used 30-point test that reflects the global cognitive function (Zhào et al., 2019).
According to the procedure, each participant was instructed to wear an ActiGraph GT3X+ device (ActiGraph, Pensacola, United States) on their non-dominant wrist for 24 h per day (except when bathing or swimming) for 7 days.
At the end of the wear period, data were downloaded using ActiLife software (ActiGraph, Pensacola, FL, United States). All data files were visually screened for sufficient wear time and then processed for analysis.
Data from the Actigraphic data were downloaded and analyzed using the software with a 60 s epoch. As the ActiGraph GT3X+ device is a triaxial accelerometer, the diurnal vector magnitude (VM) was sampled based on the equation listed as follows: VM = (X, Y, and Z are the VM counts at X-axis, Y-axis, and Z-axis, respectively) (Details given in Figure 1). Bedtime and wake time from the sleep diary was used to define sleep-wake variables. The sleep quality variables consisted of sleep efficiency (SE), total time in bed (TTB), total sleep time (TST), wake after sleep onset (WASO), times of awakenings (TA), and average duration of awakenings (ADA) (Zhao et al., 2022).
Differences between the groups’ clinical and demographic data were analyzed by using Student-t analysis or one-way analysis of variance. Correlations were determined using the Pearson correlation coefficient to assess the relationship between apathy severity and actigraphic data on the total sample. The significance threshold was set at p < 0.05 in all statistical tests. The analysis was carried out using SPSS 22.0 software.
The frequency of apathy among aged patients with CSVD was 37.50% in this study. Table 1 showed the demographic characteristics of all patients. The gender (66.67% men vs. 48.57% men; p = 0.268), age (69.24 ± 8.56 years vs. 66.11 ± 10.24 years; p = 0.246), height (167.14 ± 8.56 cm vs. 165.63 ± 6.83cm; p = 0.453), weight (72.52 ± 12.47 kg vs. 66.31 ± 11.98 kg; p = 0.074) and MMSE score (20.95 ± 4.26 vs. 23.40 ± 4.72; p = 0.057) did not reach significance. Individuals of Apathy+ group and Apathy- group did not show remarked differences in most NPI-subscale scores, except for the NPI-apathy subscale (6.00 ± 1.92 vs. 2.06 ± 1.02; p < 0.001) and total score (24.80 ± 9.14 vs. 16.69 ± 8.57; p = 0.002), details were shown in Supplementary material 2. Apathy+ group patients showed more lacunes (mean rank: 23.31 vs. 35.06; p = 0.004) and CMBs (mean rank: 34.12 vs. 24.33; p = 0.017), higher Fazekas score (2.09 ± 0.83 vs. 1.59 ± 0.74; p = 0.023), and higher CSVD burden score (3.24 ± 0.89 vs. 2.21 ± 1.27; p = 0.0001) compared with Apathy- group individuals. For actigraphic data, diurnal VM were lower in CSVD patients with apathy relative to those without apathy. On the contrary, all the sleep quality variables did not differ statistically between Apathy+ and Apathy- groups. Details are shown in Table 1.
Patients with a high CSVD burden (≥ 2 points) exhibited lower diurnal VM relative to those with a low CSVD burden (1 point). All the sleep quality variables, including SE, TTB, TST, WASO, TA, and ADA, were not statistically different between groups. Details are shown in Figure 2.
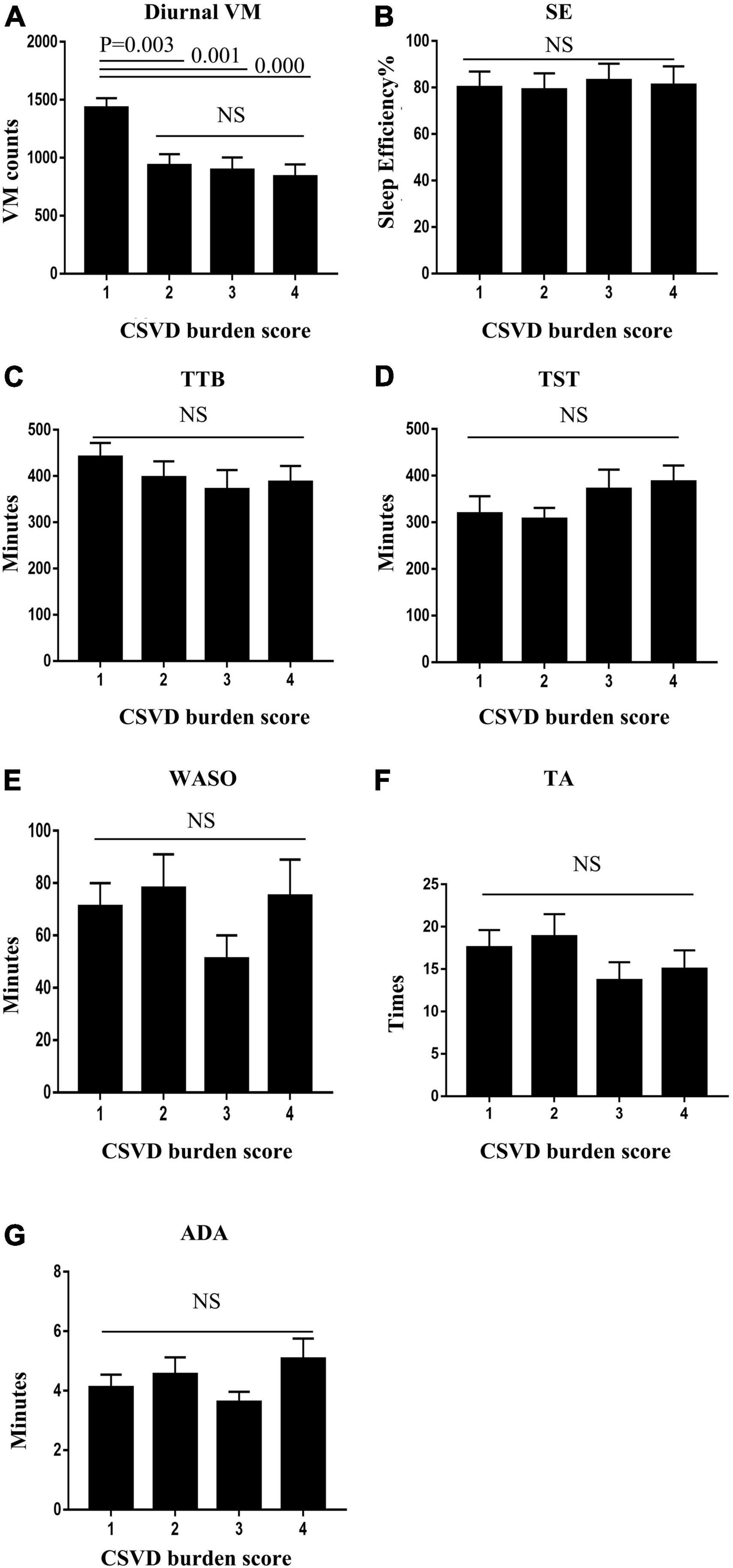
Figure 2. Patients with high cerebral small vessel disease (CSVD) burden (≥ 2 points) exhibited higher diurnal VM relative to those with a low CSVD burden (1 point) (A). Sleep quality variables, including sleep efficiency (SE), total time in bed (TTB), total sleep time (TST), wake after sleep onset (WASO), times of awakenings (TA), and average duration of awakenings (ADA), were not statistically different between groups (B–G).
Furthermore, correlation was adopted to analyze the relationship between the NPI–apathy score and actigraphic variables; we found that apathy severity score was negatively associated with diurnal VM score (r = −0.811, p = 0.000) and TTB (r = −0.349, p = 0.030). Details are shown in Figure 3.
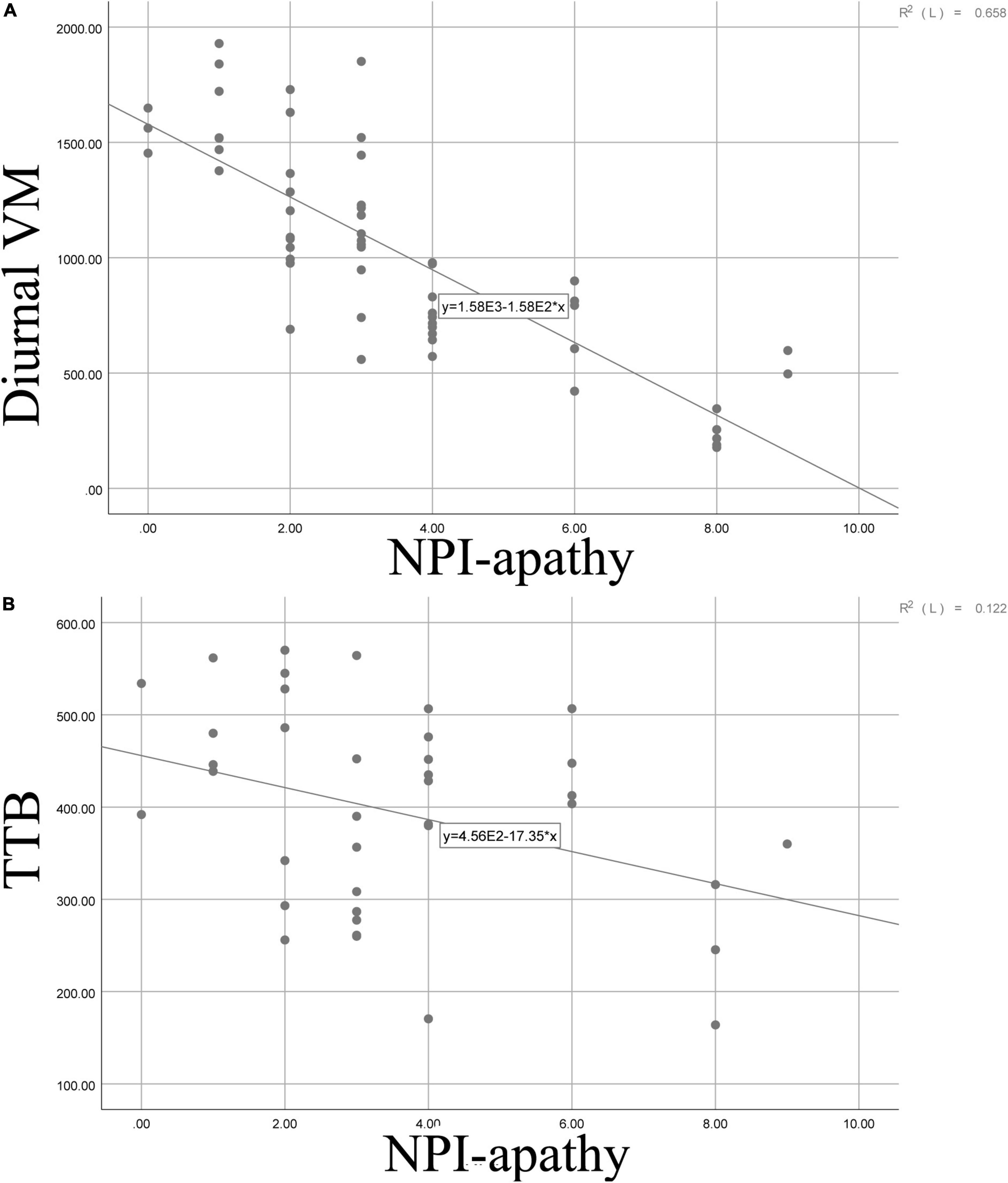
Figure 3. Scatter graphs demonstrating the relationship between apathy and diurnal VM (A) as well as between apathy and TTB (B). VM, vector magnitude; SE, sleep efficiency; TTB, total time in bed; TST, total sleep time; WASO, wake after sleep onset; TA, times of awakenings; ADA, average duration of awakenings.
Among patients with CSVD, the current study demonstrated that apathy showed more lacunes, CMBs, and higher Fazekas scores relative to individuals without apathy. Furthermore, diurnal VM rather than sleep quality variables were statistically lower in subjects with apathy in comparison with those without apathy. Meanwhile, patients with a high CSVD burden exhibited lower diurnal VM relative to individuals with a low CSVD burden. Lastly, diurnal VM and total time in bed (TTB) correlated negatively with apathy severity in the total sample.
Although the prevalence of apathy in kinds of neuropsychiatric disorders differs, it is no doubt that apathy is one of the most commonly encountered symptoms in the aged population with high frequency (Yao et al., 2015). In patients with CSVD, together with our previous reports, apathy can be found in more than one-third of hospitalized aged adults (Zhào et al., 2021). Systematic review and meta-analysis confirmed that apathy was associated independently with worse CSVD severity (Clancy et al., 2021), and a hypothesis of “vascular apathy” has been proposed on the ground of the empirical findings that supported the close relationship between apathy and CSVD (Wouts et al., 2020). The hypothesis was based on the inference that apathy symptoms and CSVD share the same functional brain area. In detail, the brain circuits such as the frontal regions with their projections to the prefrontal regions, the basal ganglia, the parietal regions, and the anterior cingulate, which play key roles in planning, motivation, and autoactivation, could be injured in CSVD (Wouts et al., 2020). More recently, apathy, combined with gait impairment and executive dysfunction, was conveyed as a new vascular triad in patients with CSVD by Hachinski et al. (2022). Hypertension, cerebral hypoperfusion, white matter tract disconnection, and other CSVD etiological factors were reported to cause apathy (Moretti et al., 2015; Tay et al., 2019; Hachinski et al., 2022).
Given that individuals with apathy often manifested in those with a lack of goal-directed behavior, there exists several studies that tried to elucidate the definite relationship between apathy and actigraphic data in patients with different neurological disorders. Müller et al. (2006) observed that patients with high apathy exhibited significantly reduced locomotor activity and more episodes of inactivity (naps) during the daytime in patients with traumatic brain injury. David et al. (2012) found that individuals with AD who had symptoms of apathy had significantly lower daytime mean motor activity than AD patients without apathy. In patients with mild cognitive impairment (MCI) and dementia, AES scores correlated negatively with actigraphic daytime activity (Kuhlmei et al., 2013). The present study also concluded that apathy severity was negatively associated with diurnal VM, which implied that lower daytime activity sampled by actigraph devices could be a representation of apathy in patients with CSVD. The difference observed with the other studies using actigraphy was mainly due to the population selection. In addition, the algorithm we used was different from other research, which might be another reason.
Contrary to the findings which revealed that high CSVD burden individuals showed less diurnal VM, all the sleep quality variables did not differ between groups of patients with distinct CSVD burden. This phenomenon is not similar to the findings of Zhào et al. (2021). We considered that these dissimilarities resulted from the sleep evaluation methods we selected. Actigraphy is an objective tool to measure sleep quality, which is different from the subjective questionnaire chosen by Zhao. Pearson correlation demonstrated that apathy was negatively associated with TTB, which is in accordance with the results from patients with AD (Mulin et al., 2011). Taken together, the close relationship between apathy and diurnal activity, as well as TTB, might imply a potential alteration of the sleep/wake circadian rhythm in CSVD patients with apathy. A previous study indicated that abnormalities in sleep/wake circadian rhythm detected by actigraph devices were associated with a high risk of post-stroke apathy (Cosin et al., 2015). It is quite attractive in recent years that patients with CSVD were found to show disrupted 24-h activity rhythm (Zuurbier et al., 2015; Sommer et al., 2021).
Several limitations of this study need to be mentioned. First, apathy was assessed using NPI-apathy, instead of diagnostic criteria of apathy, due to a lack of consensus criteria for apathy diagnosis in CSVD these years. Second, the sample size was not large. In future studies, we need to collect more patients with CSVD.
In conclusion, patients with CSVD have a high incidence of apathy. Actigraphy is a promising choice to evaluate apathy in patients with CSVD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Academic Ethics Committee of the Biological Sciences Division of PLA General Hospital in Beijing, China. The patients/participants provided their written informed consent to participate in this study.
XC was responsible for statistical analysis. HZ was responsible for manuscript writing. ZL and YD were responsible for data collection. YH was responsible for studying concepts and design. All authors contributed to the article and approved the submitted version.
This study was supported by WU Jieping Foundation (Grant No.: 320.6750.18456) and the Second Medical Center open grant of Chinese PLA General Hospital (Grant No.: ZXBJ2004).
We thank Shuo Tian for the digital technology support.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.933958/full#supplementary-material
Batrancourt, B., Lecouturier, K., Ferrand-Verdejo, J., Guillemot, V., Azuar, C., Bendetowicz, D., et al. (2019). Exploration deficits under ecological conditions as a marker of apathy in frontotemporal dementia. Front. Neurol. 10:941. doi: 10.3389/fneur.2019.00941
Brown, D. S., Barrett, M. J., Flanigan, J. L., and Sperling, S. A. (2019). Clinical and demographic correlates of apathy in Parkinson’s disease. J. Neurol. 266, 507–514. doi: 10.1007/s00415-018-9166-3
Clancy, U., Gilmartin, D., Jochems, A. C. C., Knox, L., Doubal, F. N., and Wardlaw, J. M. (2021). Neuropsychiatric symptoms associated with cerebral small vessel disease: A systematic review and meta-analysis. Lancet. Psychiatry 8, 225–236. doi: 10.1016/S2215-0366(20)30431-4
Cosin, C., Sibon, I., Poli, M., Allard, M., Debruxelles, S., Renou, P., et al. (2015). Circadian sleep/wake rhythm abnormalities as a risk factor of a poststroke apathy. Int. J. Stroke 10, 710–715. doi: 10.1111/ijs.12433
David, R., Mulin, E., Friedman, L., Le Duff, F., Cygankiewicz, E., Deschaux, O., et al. (2012). Decreased daytime motor activity associated with apathy in Alzheimer disease: An actigraphic study. Am. J. Geriatr. Psychiatry 20, 806–814. doi: 10.1097/JGP.0b013e31823038af
Godefroy, V., Levy, R., Bouzigues, A., Rametti-Lacroux, A., Migliaccio, R., and Batrancourt, B. (2021). ECOCAPTURE@HOME: Protocol for the remote assessment of apathy and its everyday-life consequences. Int. J. Environ. Res. Public Health 18, 7824. doi: 10.3390/ijerph18157824
Hachinski, V., Finger, E., Pieruccini-Faria, F., and Montero-Odasso, M. (2022). The apathy, gait impairment, and executive dysfunction (AGED) triad vascular variant. Alzheimers Dement. [Epub ahead of print]. doi: 10.1002/alz.12637
Hamilton, O. K. L., Backhouse, E. V., Janssen, E., Jochems, A. C. C., Maher, C., Ritakari, T. E., et al. (2021). Cognitive impairment in sporadic cerebral small vessel disease: A systematic review and meta-analysis. Alzheimers Dement. 7, 665–685. doi: 10.1002/alz.12221
König, A., Aalten, P., Verhey, F., Bensadoun, G., Petit, P. D., et al. (2014). A review of current information and communication technologies: Can they be used to assess apathy? Int. J. Geriatr. Psychiatry 29, 345–358. doi: 10.1002/gps.4017
Kuhlmei, A., Walther, B., Becker, T., Müller, U., and Nikolaus, T. (2013). Actigraphic daytime activity is reduced in patients with cognitive impairment and apathy. Eur. Psychiatry 28, 94–97. doi: 10.1016/j.eurpsy.2011.04.006
Lechowski, L., Benoit, M., Chassagne, P., Vedel, I., Tortrat, D., Teillet, L., et al. (2009). Persistent apathy in Alzheimer’s disease as an independent factor of rapid functional decline: The REAL longitudinal cohort study. Int. J. Geriatr. Psychiatry 24, 341–346. doi: 10.1002/gps.2125
Miller, D. S., Robert, P., Ereshefsky, L., Adler, L., Bateman, D., Cummings, J., et al. (2021). Diagnostic criteria for apathy in neurocognitive disorders. Alzheimers Dement. 17, 1892–1904. doi: 10.1002/alz.12358
Moretti, R., Cavressi, M., and Tomietto, P. (2015). Gait and apathy as relevant symptoms of subcortical vascular dementia. Am. J. Alzheimers Dis. Other Demen. 30, 390–399. doi: 10.1177/1533317514550329
Mulin, E., Zeitzer, J. M., Friedman, L., Le Duff, F., Yesavage, J., et al. (2011). Relationship between apathy and sleep disturbance in mild and moderate Alzheimer’s disease: An actigraphic study. J. Alzheimers Dis. 25, 85–91. doi: 10.3233/JAD-2011-101701
Müller, U., Czymmek, J., Thöne-Otto, A., and Von Cramon, D. Y. (2006). Reduced daytime activity in patients with acquired brain damage and apathy: A study with ambulatory actigraphy. Brain Inj. 20, 157–160. doi: 10.1080/02699050500443467
Niino, M., Mifune, N., Kohriyama, T., Mori, M., Ohashi, T., Kawachi, I., et al. (2014). Apathy/depression, but not subjective fatigue, is related with cognitive dysfunction in patients with multiple sclerosis. BMC Neurol. 14:3. doi: 10.1186/1471-2377-14-3
Pantoni, L. (2010). Cerebral small vessel disease: From pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701. doi: 10.1016/S1474-4422(10)70104-6
Raimo, S., Trojano, L., Gaita, M., d’Onofrio, F., Spitaleri, D., and Santangelo, G. (2022). Relationship between apathy and cognitive dysfunctions in multiple sclerosis: A 4-year prospective longitudinal study. Mult. Scler. Relat. Disord. 63:103929. doi: 10.1016/j.msard.2022.103929
Robert, P. H., Berr, C., Volteau, M., Bertogliati, C., Benoit, M., Sarazin, M., et al. (2006). Apathy in patients with mild cognitive impairment and the risk of developing dementia of Alzheimer’s disease: A one-year follow-up study. Clin. Neurol. Neurosurg. 108, 733–736. doi: 10.1016/j.clineuro.2006.02.003
Robert, P. H., Konig, A., Andrieu, S., Bremond, F., Chemin, I., Chung, P. C., et al. (2013). Recommendations for ICT use in Alzheimer’s disease assessment: Monaco CTAD Expert Meeting. J. Nutr. Health Aging 17, 653–660. doi: 10.1007/s12603-013-0046-3
Robert, P., Lanctôt, K. L., Agüera-Ortiz, L., Aalten, P., Bremond, F., Defrancesco, M., et al. (2018). Is it time to revise the diagnostic criteria for apathy in brain disorders? The 2018 international consensus group. Eur. Psychiatry 54, 71–76. doi: 10.1016/j.eurpsy.2018.07.008
Sommer, R., Yu, L., Schneider, J. A., Bennett, D. A., Buchman, A. S., and Lim, A. S. P. (2021). Disrupted rest-activity rhythms and cerebral small vessel disease pathology in older adults. Stroke 52, 2427–2431. doi: 10.1161/STROKEAHA.120.030870
Tay, J., Tuladhar, A. M., Hollocks, M. J., Brookes, R. L., Tozer, D. J., Barrick, T. R., et al. (2019). Apathy is associated with large-scale white matter network disruption in small vessel disease. Neurology 92:e1157–e1167. doi: 10.1212/WNL.0000000000007095
Wouts, L., van Kessel, M., Beekman, A. T. F., Marijnissen, R. M., and Oude Voshaar, R. C. (2020). Empirical support for the vascular apathy hypothesis: A structured review. Int. J. Geriatr. Psychiatry 35, 3–11. doi: 10.1002/gps.5217
Yao, H., Takashima, Y., Araki, Y., Uchino, A., Yuzuriha, T., and Hashimoto, M. (2015). Leisure-time physical inactivity associated with vascular depression or apathy in community-dwelling elderly subjects: The Sefuri Study. J. Stroke Cerebrovasc. Dis. 24, 2625–2631. doi: 10.1016/j.jstrokecerebrovasdis.2015.07.018
Yu, S. Y., Lian, T. H., Guo, P., Li, L. X., Ding, D. Y., Li, D. N., et al. (2020). Correlations of apathy with clinical symptoms of Alzheimer’s disease and olfactory dysfunctions: A cross-sectional study. BMC Neurol. 20:416. doi: 10.1186/s12883-020-01978-9
Zhào, H., Liu, Y., Xia, Z., Xie, H., and Huang, Y. (2021). Diagnosis and assessment of apathy in elderly Chinese patients With cerebral small vessel disease. Front. Psychiatry 12:688685. doi: 10.3389/fpsyt.2021.688685
Zhao, H., Tang, W., Xu, X., Zhao, Z., and Huang, L. (2014). Functional magnetic resonance imaging study of apathy in Alzheimer’s disease. J. Neuropsychiatry Clin. Neurosci. 26, 134–141. doi: 10.1176/appi.neuropsych.12110261
Zhào, H., Wei, W., Do, E. Y., and Huang, Y. (2019). Assessing performance on digital clock drawing test in aged patients With cerebral small vessel disease. Front. Neurol. 10:1259. doi: 10.3389/fneur.2019.01259
Zhào, H., Wei, W., Liu, Y., Gao, J., and Huang, Y. (2020). Cognitive frailty among elderly Chinese patients with cerebral small vessel disease: A structural MRI study. Front. Med. 4:397. doi: 10.3389/fmed.2020.00397
Zhao, H., Zhao, Z., Huang, L., and Preter, M. (2012). Diagnosis and assessment of apathy in Chinese patients with Alzheimer’s disease. J. Psychosom. Res. 72, 405–407. doi: 10.1016/j.jpsychores.2012.01.003
Zhao, J., Qu, W., Zhou, X., Guo, Y., Zhang, Y., Wu, L., et al. (2021). Sleep quality mediates the association between cerebral small vessel disease burden and frailty: A community-based study. Front. Aging Neurosci. 13:751369. doi: 10.3389/fnagi.2021.751369
Zhao, X., Tang, Q., Feng, Z., and Zhào, H. (2022). Executive performance is associated with rest-activity rhythm in nurses working rotating shifts. Front. Neurosci. 16:805039. doi: 10.3389/fnins.2022.805039
Keywords: actigraphy, apathy, small vessel disease, sleep, neuropsychiatric disorders
Citation: Cai X, Zhào H, Li Z, Ding Y and Huang Y (2022) Detecting apathy in patients with cerebral small vessel disease. Front. Aging Neurosci. 14:933958. doi: 10.3389/fnagi.2022.933958
Received: 01 May 2022; Accepted: 13 July 2022;
Published: 03 August 2022.
Edited by:
Valeria Manera, Université Côte d’Azur, FranceReviewed by:
Philippe Robert, Université Côte d’Azur, FranceCopyright © 2022 Cai, Zhào, Li, Ding and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yonghua Huang, aHVhbmd5b25naHVhMjAxN0AxMjYuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.