
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 18 July 2022
Sec. Cellular and Molecular Mechanisms of Brain-aging
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.931560
This article is part of the Research TopicMolecular Biomarkers in the Prediction, Diagnosis, and Prognosis of Neurodegenerative DiseasesView all 22 articles
 Huarong Zhou1†
Huarong Zhou1† Xiaomei Zhong1†
Xiaomei Zhong1† Ben Chen1
Ben Chen1 Qiang Wang1
Qiang Wang1 Min Zhang1
Min Zhang1 Naikeng Mai2
Naikeng Mai2 Zhangying Wu1
Zhangying Wu1 Xingxiao Huang1
Xingxiao Huang1 Xinru Chen3
Xinru Chen3 Qi Peng1
Qi Peng1 Yuping Ning1,3,4*
Yuping Ning1,3,4*Background: Cognitive impairment in late−life depression (LLD) is considered to be caused by neurodegenerative changes. Elevated homocysteine (Hcy) levels may be linked to cognitive abnormalities associated with LLD. The important role of white matter (WM) damage in cognitive impairment and pathogenesis in patients with LLD has been widely reported. However, no research has explored the interrelationships of these features in patients with LLD.
Objective: The goal of the study was to examine the interrelationship between Hcy levels, cognition, and variations in WM microstructure detected by diffusion tensor imaging (DTI) in patients with LLD.
Methods: We recruited 89 healthy controls (HCs) and 113 patients with LLD; then, we measured the plasma Hcy levels of participants in both groups. All individuals performed a battery of neuropsychological tests to measure cognitive ability. Seventy-four patients with LLD and 68 HCs experienced a DTI magnetic resonance imaging (MRI) scan.
Results: Patients with LLD showed significantly lower fractional anisotropy (FA) values in the bilateral inferior longitudinal fasciculus than those of healthy participants. Only in LLD patients was Hcy concentration inversely associated to FA values in the forceps minor. Finally, multiple regression analyses showed that an interaction between Hcy levels and FA values in the right cingulum of the cingulate cortex and right inferior longitudinal fasciculus were independent contributors to the executive function of patients with LLD.
Conclusion: Our results highlight the complex interplay between elevated homocysteine levels and WM abnormalities in the pathophysiology of LLD-related cognitive impairment, consistent with the neurodegeneration hypothesis.
Late-life depression (LLD) is related to a number of neurocognitive deficits, including impaired global cognition, executive functioning, memory, attention, and visuospatial perception (Reinlieb et al., 2014; Zhang et al., 2021). It was stated that cognitive impairment in LLD is thought to be related to neurodegenerative changes (Invernizzi et al., 2021; Rhodes et al., 2021). Cognitive impairments have been identified as essential features of LLD, as they persist even after depressive symptoms have subsided and are strongly linked to poor functional and therapeutic results (Bhalla et al., 2006). Furthermore, according to the findings of two large-sample prospective cohort studies, older people with indications of depression had an estimated twofold greater risk of developing dementia (Katon et al., 2015; Kaup et al., 2016). Therefore, knowing the pathogenic cause of cognitive deficits in patients with LLD is critical for dementia prevention and for effective therapy.
According to an increasing number of studies, elevated homocysteine (Hcy) levels are associated with cognitive impairments and dementia (Setien-Suero et al., 2016; Smith and Refsum, 2016; Kim et al., 2019). Furthermore, a prospective cohort study meta-analysis identified that each 5 μmol/L rise in homocysteine level increased the comparative Alzheimer’s disease risk by 15 percent (Zhou and Chen, 2019). Since both LLD and elevated Hcy levels make individuals susceptible to cognitive deficits, comorbidity between these two disorders may lead to an increased prevalence and extent of cognitive deficits. A recent community-based cohort study discovered that there was a significant inverse correlation between Hcy levels and cognitive capability in elderly people with depressive symptoms (Ford et al., 2013).
Our recent report demonstrated that LLD patients had higher plasma Hcy levels and worse cognitive performance than those of controls. Furthermore, in LLD patients, plasma Hcy concentrations were observed to be negatively related to global cognition, visual space, attention, and executive function. More interestingly, when compared to those with LLD or high Hcy concentrations alone, elderly people with both high Hcy concentrations and LLD had more severe cognitive impairment (Zhou et al., 2020). However, the mechanisms behind the additive cognitive deficits caused by LLD and increased Hcy levels are not fully understood.
Recently, numerous investigations have indicated that abnormal white matter (WM) microstructure detected by means of diffusion tensor imaging (DTI) is significantly associated with LLD (Wen et al., 2014) and cognitive impairment (Li et al., 2013). Fractional anisotropy (FA) values are susceptible to changes in WM structure, such as axonal injury, myelin loss, edema, and cell death (Tae et al., 2018). Many studies have demonstrated that the FA value of LLD patients decreases in a large variety of fiber bundles and brain areas, indicating that the WM network of patients with LLD may be damaged, potentially leading to poor connectivity with gray matter (Wen et al., 2014; Harada et al., 2018). Moreover, WM fiber bundles maintain high-speed signal connections across various areas of the brain, and diminished WM integrity may contribute to cognitive impairment and clinical symptoms in patients with LLD (Shen et al., 2019; Rashidi-Ranjbar et al., 2020). Recently, numerous DTI researches have shown that the integrity of the WM was positively correlated with cognitive function in patients with LLD (Shimony et al., 2009; Yuan et al., 2010; Alves et al., 2012; Mettenburg et al., 2012). For instance, researchers have identified that abnormal WM microstructure in patients with LLD is connected with impairments in cognitive functions, including cognitive processing speed (Shimony et al., 2009), memory (Mettenburg et al., 2012), language (Alves et al., 2012), and executive function (Mettenburg et al., 2012). These findings imply that unconnected WM bundles or tracts may be involved in cognitive abnormalities in patients with LLD.
Interestingly, numerous investigations have shown an association between Hcy levels and WM abnormalities (Wright et al., 2005; Feng et al., 2013; Lee et al., 2017; Tan et al., 2018; Nam et al., 2019). For example, in people over 40 years old, Hcy level is a potential risk for WM injury (Wright et al., 2005). Another study showed that Alzheimer’s disease patients with high Hcy levels had lower FA values of WM bundles (Lee et al., 2017). Furthermore, a recent study illustrated that higher Hcy level was related to decreased WM volume and cognitive deterioration in healthy elderly individuals (Feng et al., 2013), implying that higher Hcy levels can affect cognitive performance and WM structure.
All of the above evidence indicates that either elevated Hcy levels or WM abnormalities may be correlated with cognitive impairment in LLD patients. However, to the best of our knowledge, the collaborative consequence of homocysteine and WM microstructure on cognitive performance in this specific population has not been investigated. Therefore, the current investigation was aimed to ascertain (1) whether FA values of WM fibers were altered in LLD patients and (2) the correlation between Hcy concentration and the FA value of WM bundles, as well as their combined effect on cognition in both patients with LLD and healthy controls.
Patients with LLD were enrolled at the Affiliated Brain Hospital of Guangzhou Medical University. Advertisements in the public were used to attract healthy elderly people. All participants signed a written informed consent form after getting a comprehensive overview of the study. The Ethics Committee of the Affiliated Brain Hospital of Guangzhou Medical University approved the study.
The inclusion and exclusion criteria have been described in previous studies (Zhou et al., 2020). Briefly, the inclusion criteria were the following: (1) A DSM-IV Structured Medical Interview-based diagnosis of major depressive disorder and (2) age ≥ 60 years. The following were the exclusion criteria: (1) A history of other serious mental illnesses; (3) a family history of schizophrenia and/or bipolar disorder; (4) transcranial magnetic stimulation and any electroconvulsive treatment in the last 6 months; (5) neurological disorders, for instance, brain tumor and stroke; and (6) physical disorders, such as hypothyroidism and anemia, that may cause emotional problems. Healthy control (HC) individuals who were not depressed, who were at least 60 years old and who had normal cognition were included. They were all found to be free of psychiatric disease and had 17-item Hamilton Depression Rating Scale (HAMD-17) scores of less than 7 (Zimmerman et al., 2013). Other criteria for exclusion were identical to those used for individuals in the LLD group.
A total of one hundred thirteen patients with LLD and eighty-nine HCs were recruited. The demographic information of the patients has been described in our previous studies (Zhou et al., 2020). Briefly, no significant differences were found in sex, age, or educational years between participants in the HC and LLD groups.
All of the participants completed a comprehensive battery of neuropsychological examinations as described in previous studies (Zhou et al., 2020). Briefly, the tests included the following six cognition domains: (1) Mini-Mental State Examination (MMSE) for global cognition; (2) Rey-Osterrieth Complex Figure (ROCF)-Delay Recall test and Auditory Verbal Learning Test (AVLT) for memory; (3) Trail Making Test (TMT)-B and Stroop Color and Word Test (SCWT)-C for executive function; (4) TMT-A and Symbol Digit Modalities Test (SDMT) for attention; (5) Verbal Fluency Test (VFT) and Boston Naming Test (BNT) for language ability; (6) Clock Drawing Test 4 (CDT4) and ROCF-Copy for visual space. The cognitive domain scores were determined by converting each test result to a standardized z score and taking the average of the total. Particularly, low scores implied high performance on exams that evaluate timing, such as SCWT-C, TMT-B, and TMT-A. As a result, before being transformed to the standard score, the scores were converted to the reciprocal (Shu et al., 2016).
The Hcy measurements have been described in previous studies (Zhou et al., 2020). Briefly, fasting plasma Hcy concentrations were assessed using the enzyme cycling assay by automatic testers (AU5800 testers, Beckman Coulter, Brea, CA). All of the samples were analyzed by a research assistant who was blinded to the status of the subjects.
Seventy-four patients with LLD and 68 HCs underwent MRI scans. MRI data were collected within 1 month of completing the neuropsychological evaluations. MRI data were obtained by means of a 3.0-Tesla Philips Achieva scanner (Philips, Best, Netherlands). Before DTI scanning, a T2weight image was taken to rule out major white matter lesions, tumors, and cerebral infarction. The participants were subjected to DTI with the following settings: Direction = 32, b0 = 1,000 s/mm2, echo time (TE) = 92 ms, repetition time (TR) = 10,015 ms, flip angle = 90°, field of view (FOV) = 256*256 mm2, imaging matrix = 128*128, voxel dimension of 2*2*2 mm3, and 75 contiguous slices.
All of the DTI images were obtained via the standard procedure PANDA software (a pipeline tool for analyzing brain diffusion images).1 PANDA is a MATLAB toolbox that incorporates FSL,2 Diffusion Toolkit3 and MRIcron4 (Cui et al., 2013). Each subject’s diffuse tensor data were skull-stripped and subjected to eddy current and head movement rectification. The directions of the diffusion gradients were adjusted. After that, each subject’s FA was calculated on a voxel-by-voxel basis. Individual FA pictures in native space were non-linearly registered to the FA template in Montreal Neurological Institute (MNI) space by means of the FNIRT command of FSL for normalization. After that, the mean of all aligned FA pictures was computed. We used an atlas-based segmentation strategy to examine diffusion changes in the major WM tracts. The FA maps of each subject were registered into the JHU WM Tractography Atlas (Hua et al., 2008). Twenty WM pathways were examined.
The 20 WM tracts’ mean FA values of the were compared using analysis of variance (ANOVA), with age, education, and sex included as covariates. Partial correlation was used to discover the interrelationships between Hcy levels, cognition and FA values of the WM tracts, and control variables included age, sex, and years of education. Furthermore, stepwise multiple regression analysis was performed to explore the associations between Hcy levels, FA values, their interaction (Hcy × FA values) and cognitive functioning after adjusting for education, sex, age, and HAMD-17 scores. All data were examined by means of SPSS version 23.0 (IBM, Chicago, Illinois, United States). The significance levels were set at 0.05, and two-tailed significance values were used. False discovery rate (FDR) corrections described by Benjamini and Hochberg (1995) were used for multiple test corrections. After utilizing the Benjamini and Hochberg (1995) procedure, FDR-corrected p-values (i.e., q-values) lower than 0.05 were considered statistically significant.
Participants in the LLD group had significantly lower FA values in the left inferior longitudinal fasciculus (F = 6.17, p = 0.014, q = 0.014) and right inferior longitudinal fasciculus (F = 5.75, p = 0.018, q = 0.019) after adjusting for age, sex, and education than those of controls (Figure 1). However, there were no significant differences in the bilateral anterior thalamic radiation, bilateral corticospinal tract, bilateral cingulum of the cingulate cortex, bilateral cingulum of the hippocampus, forceps major, forceps minor, bilateral inferior fronto-occipital fasciculus, bilateral superior longitudinal fasciculus, bilateral uncinate fasciculus, or bilateral superior longitudinal fasciculus (temporal part) between participants in the HC and LLD groups (all q > 0.05).
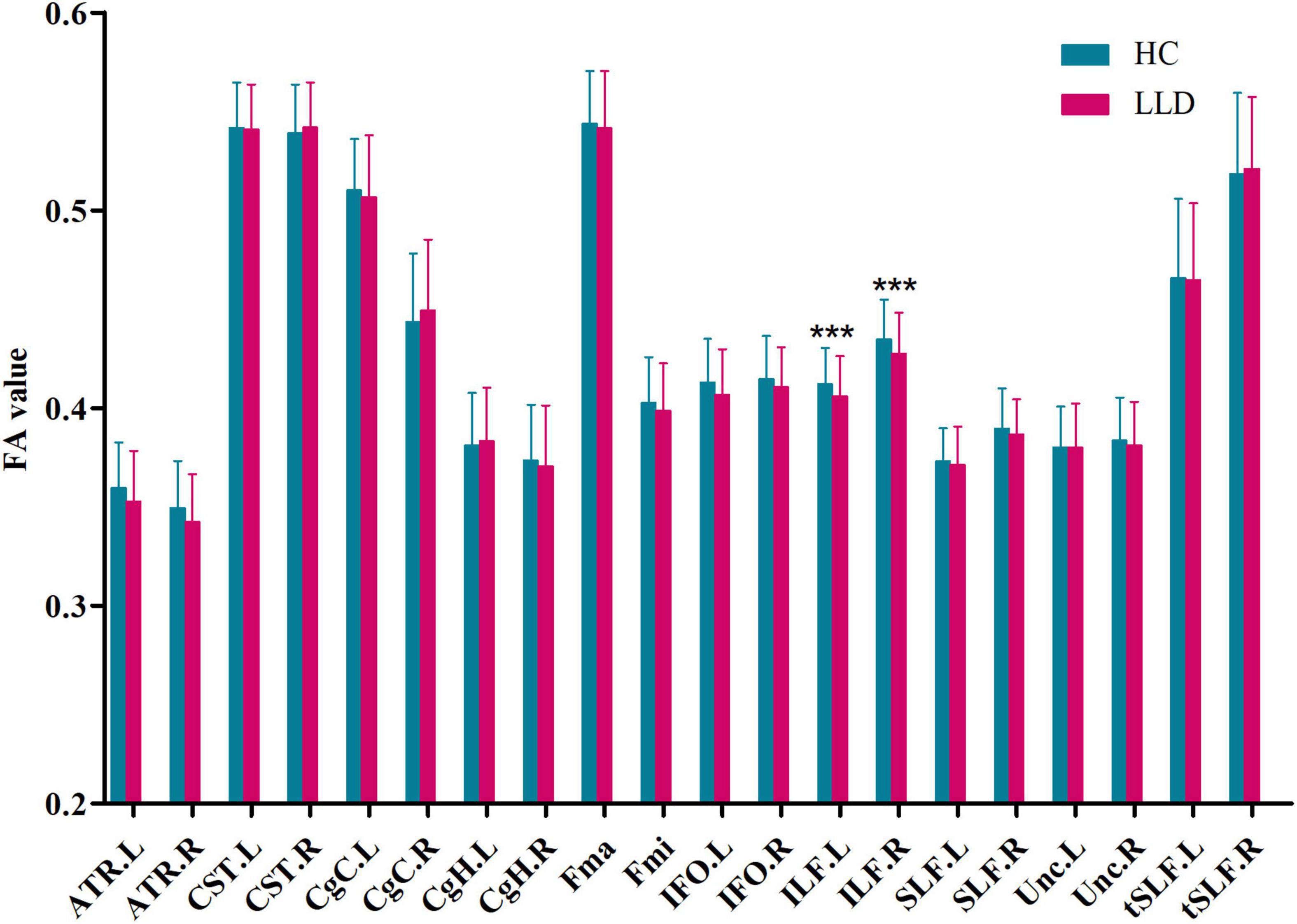
Figure 1. Group comparisons of the mean FA value of each tract in HCs and LLD patients. Each bar represents the mean ± SD. Analysis of covariance (ANCOVA) using age, sex, and education as covariates. *** indicates q< 0.05 after FDR correction. HC, healthy control; LLD, late-life depression; FA, fractional anisotropy; L, left, R, right; ATR, anterior thalamic radiation; CST, corticospinal tract; CgC, cingulum of the cingulate cortex; CgH, cingulum of the hippocampus; Fma, forceps major; Fmi, forceps minor; IFO, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; SLF, superior longitudinal fasciculus; Unc, uncinate fasciculus; tSLF, superior longitudinal fasciculus (temporal part).
Hcy levels were significantly inversely correlated with FA values in the forceps major (Fma) only in patients with LLD after controlling for age, sex, and education (r = –0.31, p = 0.009, q = 0.009) (Table 1).
As shown in Figure 2A, the left cingulum of the cingulate cortex (r = 0.32, p = 0.008, q = 0.008) displayed positive associations with global cognition in patients with LLD after adjusting for age, sex, education, and HAMD-17 scores. As shown in Figure 2B, the forceps major (r = 0.26, p = 0.033, q = 0.034) displayed positive associations with global cognition in patients with LLD after controlling for age, sex, education, and HAMD-17 scores. The left anterior thalamic radiation (r = 0.26, p = 0.029, q = 0.030) displayed positive associations with executive function in patients with LLD after adjusting for covariates (Figure 2C). The right anterior thalamic radiation (r = 0.27, p = 0.024, q = 0.025) displayed positive associations with executive function in patients with LLD after adjusting for covariates (Figure 2D). There was no correlation between FA values and other cognitive domains.
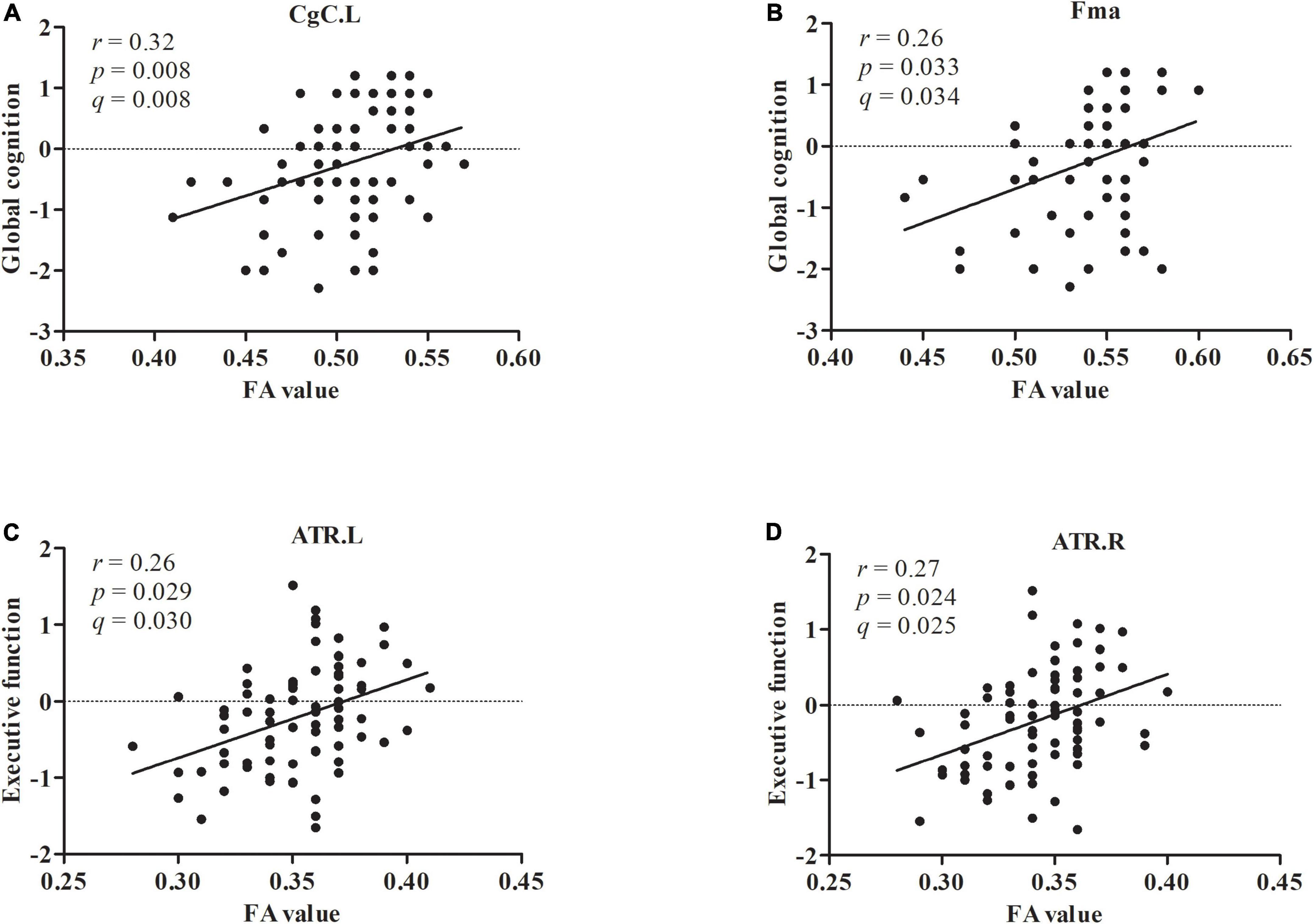
Figure 2. The FA value in CgC.L and Fma displayed positive associations with global cognition (A,B). The FA value in ATR.L and ATR.R displayed positive associations with executive function (C,D).
Furthermore, no correlation between FA values and Hcy levels or between FA values and cognitive performance was found in HCs (all q > 0.05).
Multiple regression analysis displayed that the Hcy × FA interactions in the right cingulum of the cingulate cortex (beta = –0.24, t = –2.06, p = 0.043, q = 0.044) and right inferior longitudinal fasciculus (beta = -0.24, t = -2.12, p = 0.038, q = 0.038) were independent predictors for executive function only in patients with LLD. No association between the Hcy level × FA interaction in any of the 20 WM fiber tracts and other cognitive domains was found in patients with LLD (all q > 0.05).
Furthermore, no association between the Hcy level × FA interaction in any of the 20 WM fiber tracts and cognition was found in HCs (all q > 0.05).
The findings from the current study showed (1) significantly lower FA values in the bilateral inferior longitudinal fasciculus in patients with LLD than those in HCs; (2) disruption of WM structure was linked to elevated Hcy concentrations and cognitive impairments in global cognition and executive function in LLD patients; and (3) the interaction between elevated Hcy concentrations and structural destruction of WM may affect executive deficits in patients with LLD.
Our previous report demonstrated that LLD patients had higher plasma Hcy levels and worse cognitive performance than those of healthy controls. Furthermore, higher plasma homocysteine levels were associated with poorer cognition in patients with LLD (Zhou et al., 2020). In the current study, we discovered lower FA values in the bilateral inferior longitudinal fasciculus in LLD patients, which is consistent with the majority of prior findings on FA values in patients with LLD (Alves et al., 2012; Mettenburg et al., 2012; Guo et al., 2014; Li et al., 2014, 2020; Wen et al., 2014; Emsell et al., 2017), confirming the LLD disconnection theory. Furthermore, we recognized a reverse relationship between plasma Hcy concentrations and FA values in the forceps minor in patients with LLD, suggesting that Hcy level is related to the WM structure. Because this is the first study to explore the increased homocysteine level and WM abnormalities of patients with LLD, any causal inferences are speculative.
Previous reports revealed that high Hcy concentrations inhibit mitochondrial activity in brain cells (Zhang et al., 2020), which might explain the link between hyperhomocysteinemia and cognitive impairment. In the developing brain, homocysteine induces cell cycle disruption and reactive gliosis (Cecchini et al., 2019). Data from animal studies demonstrate that even a slight increase in serum homocysteine levels upregulates matrix metalloproteinase-9 expression levels and disrupts blood–brain barrier integrity (Chu et al., 2021). In addition, endothelial dysfunction may result from an elevation in Hcy levels by enhancing oxidation and endothelial cell activation (Faverzani et al., 2017), increasing levels of adhesion molecules and proinflammatory cytokines (Barroso et al., 2016), and decreasing vascular wall integrity (Jakubowski, 2001). Endothelial cell damage causes a disruption in the tissue milieu, which leads to subsequent myelin injury and neurodegeneration. Moreover, a recent study demonstrated that increased Hcy levels were related to the imaging burden of cerebral small vessel disease (Cao et al., 2021). Taken together, animal and human research has suggested that high Hcy levels may affect adult neurogenesis as well as neuronal structure and function.
Our findings revealed a negative relationship between plasma Hcy levels and FA values in the forceps minor in patients with LLD, which reflects the interaction between elevated Hcy levels and abnormal WM structure as the pathogenic mechanism of LLD. Moreover, we found that the Hcy × FA interaction in the right cingulum of the cingulate cortex and right inferior longitudinal fasciculus contributed to executive dysfunction in patients with LLD. Executive deterioration is common in patients with LLD and predicts a worse response to antidepressant therapy as well as a greater recurrence risk (Alexopoulos et al., 2005). Growing evidence has shown that high levels of homocysteine and WM degeneration are associated with cerebrovascular disease (Kynast et al., 2018; Cao et al., 2021). Therefore, elevated Hcy levels and WM abnormalities support vascularity impairment in patients with LLD. Although these correlations do not provide proof of causality in pathophysiology, it is speculated that high Hcy levels may disrupt WM microstructural connections and induce cognitive impairment. Additionally, our findings showed that there was a positive association between global cognition and FA values in the left cingulum of the cingulate cortex or the forceps major, as well as between executive function and FA values in the bilateral anterior thalamic radiation in patients with LLD. Several investigations have described that neurocognitive impairment is related to WM abnormalities in LLD (Charlton et al., 2014; Respino et al., 2019; Wang et al., 2020). These findings support the hypothesis that damaged WM connections are related to cognitive abnormalities in patients with LLD.
This research investigation had several limitations that must be noted. First, this was a case-control study. Thus, the causal relationship between elevated Hcy levels, WM injury and cognitive deficits in LLD patients is still uncertain. Therefore, in the future, a longitudinal investigation with a larger sample size will be required to obtain more persuasive and accurate results. Second, our research only investigated changes in WM structure; however, whether the change in WM structure is related to a change in function must be further explored. Third, the study did not assess diet, physical activity or lifestyle. All of these factors may influence Hcy levels and WM, but they were not controlled for in the present analysis.
In conclusion, both increased Hcy levels and the disturbance of WM structure may be involved in the cognitive impairment observed in patients with LLD. Only in the patient group was a negative relation between plasma Hcy levels and WM disconnectivity identified, implying pathogenic processes underlying the interaction between increased Hcy levels and WM disconnection. Furthermore, because our current investigation used a case-control methodology, a larger longitudinal sample is needed to identify the causal relationship among aberrant Hcy metabolism, WM disconnection, and cognitive deficits in patients with LLD.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Affiliated Brain Hospital of Guangzhou Medical University. The patients/participants provided their written informed consent to participate in this study.
YN conceived and designed the study. HZ and XZ performed testing and data collection and drafted the manuscript. BC, QW, MZ, NM, ZW, XH, XC, and QP performed the data analysis and interpretation. All authors contributed to the article and approved the submitted version.
This study was supported by the National Natural Science Foundation of China (Nos. 81701341, 82101508, and 82171533), the Key Laboratory for Innovation Platform Plan, Science and Technology Program of Guangzhou, China, Science and Technology Plan Project of Guangdong Province (No. 2019B030316001), the Guangzhou Municipal Psychiatric Diseases Clinical Transformation Laboratory (No. 201805010009), Medical Scientific Technology Research Foundation of Guangdong Province of China (No. A2020446), Key Medical Specialty Construction Project of Traditional Chinese Medical Science in the 13th 5-Year Plan of Guangdong Province, Key Medical Specialty Construction Project of Traditional Chinese Medical Science of Guangzhou (2020–2022), and the National Key Research and Development Program of China (No. 2016YFC0906300). The funders had no role in the study design, data collection and analysis, decision to publish or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Cong Ouyang, Weiru Zhang, Wanruan Liang, and Chunying Dai for their assistance in data collection.
Alexopoulos, G. S., Kiosses, D. N., Heo, M., Murphy, C. F., Shanmugham, B., and Gunning-Dixon, F. (2005). Executive dysfunction and the course of geriatric depression. Biol. Psychiatry 58, 204–210. doi: 10.1016/j.biopsych.2005.04.024
Alves, G. S., Karakaya, T., Fußer, F., Kordulla, M., O’Dwyer, L., Christl, J., et al. (2012). Association of microstructural white matter abnormalities with cognitive dysfunction in geriatric patients with major depression. Psychiatry Res. 203, 194–200. doi: 10.1016/j.pscychresns.2011.12.006
Barroso, M., Kao, D., Blom, H. J., Tavares de Almeida, I., Castro, R., Loscalzo, J., et al. (2016). S-adenosylhomocysteine induces inflammation through NFkB: a possible role for EZH2 in endothelial cell activation. Biochim. Biophys. Acta 1862, 82–92. doi: 10.1016/j.bbadis.2015.10.019
Benjamini, Y., and Hochberg, Y. (1995). Controlling the False Discovery Rate: a Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Series B 57, 289–300.
Bhalla, R. K., Butters, M. A., Mulsant, B. H., Begley, A. E., Zmuda, M. D., Schoderbek, B., et al. (2006). Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am. J. Geriatr. Psychiatry 14, 419–427. doi: 10.1097/01.jgp.0000203130.45421.69
Cao, Y., Su, N., Zhang, D., Zhou, L., and Yao, M. (2021). Correlation between total homocysteine and cerebral small vessel disease: a Mendelian randomization study. Eur. J. Neurol. 28, 1931–1938. doi: 10.1111/ene.14708
Cecchini, M. S., Bourckhardt, G. F., Jaramillo, M. L., Ammar, D., Müller, Y. M. R., and Nazari, E. M. (2019). Exposure to homocysteine leads to cell cycle damage and reactive gliosis in the developing brain. Reprod. Toxicol. 87, 60–69. doi: 10.1016/j.reprotox.2019.05.054
Charlton, R. A., Lamar, M., Zhang, A., Yang, S., Ajilore, O., and Kumar, A. (2014). White-matter tract integrity in late-life depression: associations with severity and cognition. Psychol. Med. 44, 1427–1437. doi: 10.1017/s0033291713001980
Chu, M., Teng, J., Guo, L., Wang, Y., Zhang, L., Gao, J., et al. (2021). Mild hyperhomocysteinemia induces blood-brain barrier dysfunction but not neuroinflammation in the cerebral cortex and hippocampus of wild-type mice. Can. J. Physiol. Pharmacol. 99, 847–856. doi: 10.1139/cjpp-2020-0507
Cui, Z., Zhong, S., Xu, P., He, Y., and Gong, G. (2013). PANDA: a pipeline toolbox for analyzing brain diffusion images. Front. Hum. Neurosci. 7:42. doi: 10.3389/fnhum.2013.00042
Emsell, L., Adamson, C., De Winter, F. L., Billiet, T., Christiaens, D., Bouckaert, F., et al. (2017). Corpus callosum macro and microstructure in late-life depression. J. Affect. Disord. 222, 63–70. doi: 10.1016/j.jad.2017.06.063
Faverzani, J. L., Hammerschmidt, T. G., Sitta, A., Deon, M., Wajner, M., and Vargas, C. R. (2017). Oxidative Stress in Homocystinuria Due to Cystathionine ß-Synthase Deficiency: findings in Patients and in Animal Models. Cell. Mol. Neurobiol. 37, 1477–1485. doi: 10.1007/s10571-017-0478-0
Feng, L., Isaac, V., Sim, S., Ng, T. P., Krishnan, K. R., and Chee, M. W. (2013). Associations between elevated homocysteine, cognitive impairment, and reduced white matter volume in healthy old adults. Am. J. Geriatr. Psychiatry 21, 164–172. doi: 10.1016/j.jagp.2012.10.017
Ford, A. H., Flicker, L., Singh, U., Hirani, V., and Almeida, O. P. (2013). Homocysteine, depression and cognitive function in older adults. J. Affect. Disord. 151, 646–651. doi: 10.1016/j.jad.2013.07.012
Guo, W., Liu, F., Xun, G., Hu, M., Guo, X., Xiao, C., et al. (2014). Disrupted white matter integrity in first-episode, drug-naive, late-onset depression. J. Affect. Disord. 163, 70–75. doi: 10.1016/j.jad.2014.03.044
Harada, K., Ikuta, T., Nakashima, M., Watanuki, T., Hirotsu, M., Matsubara, T., et al. (2018). Altered Connectivity of the Anterior Cingulate and the Posterior Superior Temporal Gyrus in a Longitudinal Study of Later-life Depression. Front. Aging Neurosci. 10:31. doi: 10.3389/fnagi.2018.00031
Hua, K., Zhang, J., Wakana, S., Jiang, H., Li, X., Reich, D. S., et al. (2008). Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 39, 336–347. doi: 10.1016/j.neuroimage.2007.07.053
Invernizzi, S., Simoes Loureiro, I., Kandana Arachchige, K. G., and Lefebvre, L. (2021). Late-Life Depression, Cognitive Impairment, and Relationship with Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. 50, 414–424. doi: 10.1159/000519453
Jakubowski, H. (2001). Protein N-homocysteinylation: implications for atherosclerosis. Biomed. Pharmacother. 55, 443–447. doi: 10.1016/s0753-3322(01)00085-3
Katon, W., Pedersen, H. S., Ribe, A. R., Fenger-Gron, M., Davydow, D., Waldorff, F. B., et al. (2015). Effect of depression and diabetes mellitus on the risk for dementia: a national population-based cohort study. JAMA Psychiatry 72, 612–619. doi: 10.1001/jamapsychiatry.2015.0082
Kaup, A. R., Byers, A. L., Falvey, C., Simonsick, E. M., Satterfield, S., Ayonayon, H. N., et al. (2016). Trajectories of Depressive Symptoms in Older Adults and Risk of Dementia. JAMA Psychiatry 73, 525–531. doi: 10.1001/jamapsychiatry.2016.0004
Kim, S., Choi, B. Y., Nam, J. H., Kim, M. K., Oh, D. H., and Yang, Y. J. (2019). Cognitive impairment is associated with elevated serum homocysteine levels among older adults. Eur. J. Nutr. 58, 399–408. doi: 10.1007/s00394-017-1604-y
Kynast, J., Lampe, L., Luck, T., Frisch, S., Arelin, K., Hoffmann, K. T., et al. (2018). White matter hyperintensities associated with small vessel disease impair social cognition beside attention and memory. J. Cereb. Blood Flow Metab. 38, 996–1009. doi: 10.1177/0271678x17719380
Lee, C. C., Hsu, S. W., Huang, C. W., Chang, W. N., Chen, S. F., Wu, M. K., et al. (2017). Effects of Homocysteine on white matter diffusion parameters in Alzheimer’s disease. BMC Neurol. 17:192. doi: 10.1186/s12883-017-0970-7
Li, H., Liang, Y., Chen, K., Li, X., Shu, N., Zhang, Z., et al. (2013). Different patterns of white matter disruption among amnestic mild cognitive impairment subtypes: relationship with neuropsychological performance. J. Alzheimers Dis. 36, 365–376. doi: 10.3233/jad-122023
Li, H., Lin, X., Liu, L., Su, S., Zhu, X., Zheng, Y., et al. (2020). Disruption of the structural and functional connectivity of the frontoparietal network underlies symptomatic anxiety in late-life depression. Neuroimage Clin. 28:102398. doi: 10.1016/j.nicl.2020.102398
Li, W., Muftuler, L. T., Chen, G., Ward, B. D., Budde, M. D., Jones, J. L., et al. (2014). Effects of the coexistence of late-life depression and mild cognitive impairment on white matter microstructure. J. Neurol. Sci. 338, 46–56. doi: 10.1016/j.jns.2013.12.016
Mettenburg, J. M., Benzinger, T. L., Shimony, J. S., Snyder, A. Z., and Sheline, Y. I. (2012). Diminished performance on neuropsychological testing in late life depression is correlated with microstructural white matter abnormalities. Neuroimage 60, 2182–2190. doi: 10.1016/j.neuroimage.2012.02.044
Nam, K. W., Kwon, H. M., Jeong, H. Y., Park, J. H., Kwon, H., and Jeong, S. M. (2019). Serum homocysteine level is related to cerebral small vessel disease in a healthy population. Neurology 92, e317–25. doi: 10.1212/wnl.0000000000006816
Rashidi-Ranjbar, N., Miranda, D., Butters, M. A., Mulsant, B. H., and Voineskos, A. N. (2020). Evidence for Structural and Functional Alterations of Frontal-Executive and Corticolimbic Circuits in Late-Life Depression and Relationship to Mild Cognitive Impairment and Dementia: a Systematic Review. Front. Neurosci. 14:253. doi: 10.3389/fnins.2020.00253
Reinlieb, M., Ercoli, L. M., Siddarth, P., St Cyr, N., and Lavretsky, H. (2014). The patterns of cognitive and functional impairment in amnestic and non-amnestic mild cognitive impairment in geriatric depression. Am. J. Geriatr. Psychiatry 22, 1487–1495. doi: 10.1016/j.jagp.2013.10.010
Respino, M., Jaywant, A., Kuceyeski, A., Victoria, L. W., Hoptman, M. J., Scult, M. A., et al. (2019). The impact of white matter hyperintensities on the structural connectome in late-life depression: relationship to executive functions. Neuroimage Clin. 23:101852. doi: 10.1016/j.nicl.2019.101852
Rhodes, E., Insel, P. S., Butters, M. A., Morin, R., Bickford, D., Tosun, D., et al. (2021). The Impact of Amyloid Burden and APOE on Rates of Cognitive Impairment in Late Life Depression. J. Alzheimers Dis. 80, 991–1002. doi: 10.3233/jad-201089
Setien-Suero, E., Suarez-Pinilla, M., Suarez-Pinilla, P., Crespo-Facorro, B., and Ayesa-Arriola, R. (2016). Homocysteine and cognition: a systematic review of 111 studies. Neurosci. Biobehav. Rev. 69, 280–298. doi: 10.1016/j.neubiorev.2016.08.014
Shen, X., Adams, M. J., Ritakari, T. E., Cox, S. R., McIntosh, A. M., and Whalley, H. C. (2019). White Matter Microstructure and Its Relation to Longitudinal Measures of Depressive Symptoms in Mid- and Late Life. Biol. Psychiatry 86, 759–768. doi: 10.1016/j.biopsych.2019.06.011
Shimony, J. S., Sheline, Y. I., D’Angelo, G., Epstein, A. A., Benzinger, T. L., Mintun, M. A., et al. (2009). Diffuse microstructural abnormalities of normal-appearing white matter in late life depression: a diffusion tensor imaging study. Biol. Psychiatry 66, 245–252. doi: 10.1016/j.biopsych.2009.02.032
Shu, H., Shi, Y., Chen, G., Wang, Z., Liu, D., Yue, C., et al. (2016). Opposite Neural Trajectories of Apolipoprotein E 4 and 2 Alleles with Aging Associated with Different Risks of Alzheimer’s Disease. Cereb. Cortex 26, 1421–1429. doi: 10.1093/cercor/bhu237
Smith, A. D., and Refsum, H. (2016). Homocysteine, B Vitamins, and Cognitive Impairment. Annu. Rev. Nutr. 36, 211–239. doi: 10.1146/annurev-nutr-071715-050947
Tae, W. S., Ham, B. J., Pyun, S. B., Kang, S. H., and Kim, B. J. (2018). Current Clinical Applications of Diffusion-Tensor Imaging in Neurological Disorders. J. Clin. Neurol. 14, 129–140. doi: 10.3988/jcn.2018.14.2.129
Tan, B., Venketasubramanian, N., Vrooman, H., Cheng, C. Y., Wong, T. Y., Ikram, M. K., et al. (2018). Homocysteine and Cerebral Atrophy: the Epidemiology of Dementia in Singapore Study. J. Alzheimers Dis. 62, 877–885. doi: 10.3233/jad-170796
Wang, Z., Yuan, Y., You, J., and Zhang, Z. (2020). Disrupted structural brain connectome underlying the cognitive deficits in remitted late-onset depression. Brain Imaging Behav. 14, 1600–1611. doi: 10.1007/s11682-019-00091-x
Wen, M. C., Steffens, D. C., Chen, M. K., and Zainal, N. H. (2014). Diffusion tensor imaging studies in late-life depression: systematic review and meta-analysis. Int. J. Geriatr. Psychiatry 29, 1173–1184. doi: 10.1002/gps.4129
Wright, C. B., Paik, M. C., Brown, T. R., Stabler, S. P., Allen, R. H., Sacco, R. L., et al. (2005). Total homocysteine is associated with white matter hyperintensity volume: the Northern Manhattan Study. Stroke 36, 1207–1211. doi: 10.1161/01.str.0000165923.02318.22
Yuan, Y., Hou, Z., Zhang, Z., Bai, F., Yu, H., You, J., et al. (2010). Abnormal integrity of long association fiber tracts is associated with cognitive deficits in patients with remitted geriatric depression: a cross-sectional, case-control study. J. Clin. Psychiatry 71, 1386–1390. doi: 10.4088/JCP.09m05313gry
Zhang, M., Chen, B., Zhong, X., Zhou, H., Wang, Q., Mai, N., et al. (2021). Neuropsychiatric Symptoms Exacerbate the Cognitive Impairments in Patients With Late-Life Depression. Front. Psychiatry 12:757003. doi: 10.3389/fpsyt.2021.757003
Zhang, T., Huang, D., Hou, J., Li, J., Zhang, Y., Tian, M., et al. (2020). High-concentration homocysteine inhibits mitochondrial respiration function and production of reactive oxygen species in neuron cells. J. Stroke Cerebrovasc. Dis. 29:105109. doi: 10.1016/j.jstrokecerebrovasdis.2020.105109
Zhou, F., and Chen, S. (2019). Hyperhomocysteinemia and risk of incident cognitive outcomes: an updated dose-response meta-analysis of prospective cohort studies. Ageing Res. Rev. 51, 55–66. doi: 10.1016/j.arr.2019.02.006
Zhou, H., Zhong, X., Chen, B., Wu, Z., Zhang, M., Mai, N., et al. (2020). Interactive effects of elevated homocysteine and late-life depression on cognitive impairment. J. Affect. Disord. 277, 212–217. doi: 10.1016/j.jad.2020.08.022
Keywords: late-life depression, cognitive impairment, elevated homocysteine levels, white matter abnormalities, interaction
Citation: Zhou H, Zhong X, Chen B, Wang Q, Zhang M, Mai N, Wu Z, Huang X, Chen X, Peng Q and Ning Y (2022) Elevated homocysteine levels, white matter abnormalities and cognitive impairment in patients with late-life depression. Front. Aging Neurosci. 14:931560. doi: 10.3389/fnagi.2022.931560
Received: 29 April 2022; Accepted: 30 June 2022;
Published: 18 July 2022.
Edited by:
Yuzhen Xu, Shandong First Medical University, ChinaReviewed by:
Shuwei Xie, University of Nebraska Medical Center, United StatesCopyright © 2022 Zhou, Zhong, Chen, Wang, Zhang, Mai, Wu, Huang, Chen, Peng and Ning. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuping Ning, bmluZ2plbnlAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.