- 1Department of Neurosurgery, Affiliated Hospital of Weifang Medical University, Weifang, China
- 2Department of Spinal Surgery, Provincial Hospital Affiliated to Shandong First Medical University, Jinan, China
- 3Department of Pediatrics, Jilin University, Changchun, China
- 4Preventive Medicine, Qingdao University, Qingdao, China
Objectives: Capsaicin is a specific agonist of TRPV1 (multimodal sensory receptor), which improves oropharyngeal dysphagia by increasing sensory input from the oropharynx and hypopharynx and by increasing repetitive stimulation of the cerebral cortex. The aim of this systematic review was to evaluate the therapeutic effect of capsaicin on swallowing disorders in stroke patients and the elderly.
Method: We searched Medline, Embase, PubMed, and Cochrane Library databases. We used the Mesh terms search database to screen all clinical trials that complied with the inclusion criteria. Studies were subjected to literature screening, quality assessment, and data extraction to remove studies that did not meet the inclusion criteria. After literature screening, quality assessment, and data extraction, a systematic review and meta-analysis of the included study were performed.
Results: This systematic review and meta-analysis were prospectively registered on PROSPERO under registration number CRD42022313958. Five high-quality randomized controlled trials were ultimately included. The results of our meta-analysis showed a more significant reduction in swallowing function score change in the capsaicin group compared to the control group [SMD = −1.30, 95% CI: (−2.35, −0.25), P = 0.01] and on the Water swallowing test the improvement was significantly higher in the capsaicin group [RR = 2.46, 95% CI: (1.73, 3.50), P < 0.0001].
Conclusions: Although the results of our meta-analysis showed that capsaicin improved swallowing function, most studies had an unclear bias and included few studies. More studies are needed to support this in the future.
Systematic review registration: www.crd.york.ac.uk/prospero/display_record.php?RecordID=304061, identifier: 304061.
Introduction
Oropharyngeal dysphagia (OD) is caused by a variety of diseases, the most common causes being stroke and aging (Baijens et al., 2016). As the population is rapidly aging, stroke has gradually become the leading cause of death in the elderly (Lui and Nguyen, 2018). OD is a common complication after acute stroke. It is estimated that 37–78% of stroke survivors suffer from dysphagia (Marin et al., 2018). Moreover, in a survey of older adults, 20–23% of them had swallowing disorders (Serra-Prat et al., 2011). Patients with mild dysphagia may experience choking on water, difficulty in eating, and recurrent fever (Cui et al., 2020). Severely patients are prone to aspiration pneumonia and asphyxia and malnutrition, which seriously impair the quality of life of patients and sometimes even endanger their lives (Goldsmith et al., 2021). Traditional treatments for dysphagia include dietary modification, postural modification, motor training, and swallowing motions (Speyer et al., 2010). Swallowing training is a cumbersome procedure with a long duration of treatment and negative impact on patient compliance (White et al., 2008). Therefore, there is a need for continued research into new treatment methods.
The pathological condition of swallowing can be divided into three stages: the oral stage, the pharyngeal stage, and the esophageal phase (Sasegbon and Hamdy, 2017). Except for the oral phase, swallowing movements are involuntary and are accomplished mainly by reflexes (Hashimoto et al., 2021). Studies have shown that sensory input is important for the initiation and regulation of swallowing (Steele and Miller, 2010), and the cause of the patient's swallowing dysfunction may be a disruption in the connection between cortical sensory afferents and the brainstem (Sasegbon and Hamdy, 2021). Those aging or stroke patients have lengthened latency and reduced amplitude of the characteristic peaks of pharyngeal sensory evoked potentials (PSEP) (Cabib et al., 2017), in addition to symmetric PSEP and its cortical representation loss in patients with chronic dysphagia after stroke (Cabib et al., 2020a). Eventually, impaired conduction and integration of sensory input may affect output pathways, leading to impaired oropharyngeal responses (Cabib et al., 2020b).
A multimodal sensory receptor TRPV1 was found to be expressed in epithelial cells and sensory neurons of the population pharynx and larynx (Alvarez-Berdugo et al., 2016), which induces peripheral sensory neurons, modulates sensory input from oropharyngeal and hypopharyngeal regions and improves swallowing function (Steele and Miller, 2010). TRPV1 has two effects on improving swallowing ability (Rofes et al., 2013). It increases sensory input from the oropharynx and hypopharynx and increases repetitive stimulation of the cerebral cortex (Cabib et al., 2016). In addition to oral pathway stimulation, there are also nasal and ear pathways that may stimulate the swallowing reflex through TRPV1 receptors (Ravindran et al., 2016). Capsaicin is a specific agonist of TRPV1 (Ferreira et al., 2020). Several studies have shown that TRPV1 stimulation by capsaicin increased the concentration of salivary substance P (SP) (Kondo et al., 2017; Cui et al., 2020). SP is a neuropeptide that induces centrally originated swallowing through afferent stimulation of the vagus and pharyngeal nerves and enhances swallowing and cough reflexes (Mazzone and Undem, 2016). In addition, capsaicin shortens laryngeal vestibule closure (LVC) and upper esophageal sphincter opening (UESO) to improves swallowing safety (Rofes et al., 2013). Therefore, this systematic review attempts to assess the therapeutic effect of capsaicin on dysphagia in stroke patients and the elderly, providing new evidence for the rehabilitation of patients with dysphagia.
Materials and methods
The protocol for this review was registered PROSPERO (International prospective register of systematic reviews) and the registration number was CRD42022313958 (Supplementary material 1).
Search strategy
The databases searched for this systematic review and meta-analysis included Medline, Embase, PubMed, and the Cochrane Library. The search keywords used MeSH terms which were “swallowing” OR “swallowing disorders” OR “deglutition disorders” OR “dysphagia” AND “capsaicin” OR “TRP” OR “vanilloid receptor agonist” (the search strategy is presented in Supplementary material 2). Randomized controlled trials (RCTs) published up to July 2022 were included based on inclusion and exclusion criteria. Two researchers with medical specialties independently assessed the title and abstract of each item using predetermined inclusion and exclusion criteria. When judgment could not be made on the basis of titles and abstracts, the full text of the articles was obtained. There was no potential bias between the two researchers and agreement was reached through discussion.
Inclusion and exclusion criteria
Participants/population
Inclusion criteria: (1) patients with dysphagia associated with aging and stroke [Elderly or stroke patients were evaluated for Oropharyngeal dysphagia by Standardized Swallowing Assessment (SSA) scales or Volume-Viscosity Swallowing Test (V-VST) or Video Fluoroscopic Swallowing (VFS)]; (2) age > 18 years old; (3) patients were willing to participant the study and signed the informed consent.
Exclusion criteria: (1) patients with lung disease and heart disease who cannot receive stimulation; (2) patient suffers from other diseases that may affect swallowing function; (3) patients who were allergic to capsaicin.
Intervention(s), exposure(s)
Inclusion criteria: (1) patients received oral or topical capsaicin stimulation; (2) may be combined with other treatments (ice stimulation, swallowing training).
Comparator(s)/control
Patients in the control group were treated with placebo or combined with other drugs same as in the experimental group. No control group was set and studies comparing capsaicin with other treatments were excluded.
Types of study to be included
The inclusion criteria were (1) only published RCTs were included; (2) studies with a follow-up of at least 80% and at least one primary outcome and (3) those with complete treatment outcomes.
Exclusion criteria: review articles, animal studies, a case study, not relevant to the question and data was not extractable.
Outcomes
The primary outcomes included: swallowing function score and water swallowing test. Secondary outcomes: efficacy of capsaicin, substance P (SP) concentrations, individual latency time, the standardized low-resolution brain electromagnetic tomography software (sLORETA), and safety of the treatment.
Data extraction
Two researchers individually extracted and tabulated data on authors, date of publication, study site, sample size, sample characteristics, gender, age, experimental design, interventions, mode of administration, and outcomes. For incomplete data, the authors were contacted and any missing data were obtained. Controversial data were discussed or a third investigator was consulted to resolve any disagreements.
Risk of bias and quality assessment
The PRISMA guidelines (Moher et al., 2009) and Cochrane Handbook (Higgins et al., 2019) were used to evaluate the quality of the results of all included studies to make sure that the results of our meta-analysis were reliable and authentic (Moher et al., 2009). The PRISMA 2020 checklist (Page et al., 2021) was shown in Supplementary Table 1.
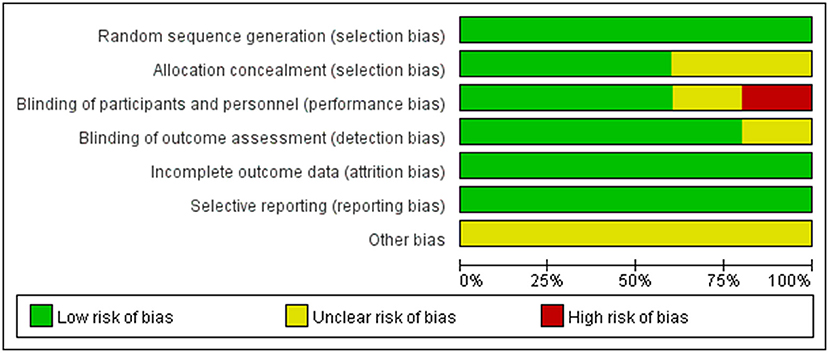
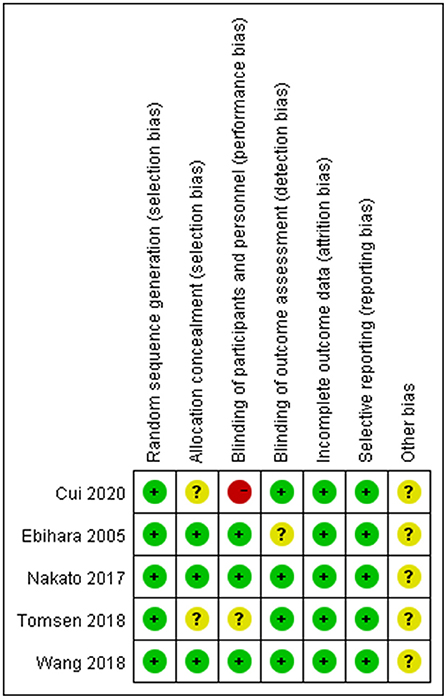
According to the Cochrane Handbook for Systematic Reviews of Interventions, the methodological quality and basis of the included RCTs were assessed as follows: randomization, allocation concealment, blind method, selective reporting, incomplete outcome data, and other bias (Faggion, 2015) (Figures 1, 2).
Statistical analysis and assessment of publication bias
Review Manager Software (Version 5.3) was used to perform the meta-analyses. For heterogeneity analysis, a fixed-effects model was used when I2 < 50% and P > 0.1 (Higgins et al., 2009). The meta-analysis was otherwise performed by applying a random-effects model (Friedrich et al., 2011). For dichotomous outcomes, the results were presented as relative risks (RR) with a 95% CI (Thakkinstian et al., 2005). The Chi-square test was used to evaluate the heterogeneity of studies based on the values of P and I2 (Shi et al., 2020). Mean difference (MD) or standardized mean difference (SMD) was used to assess continuous outcomes with 95% confidence intervals (CI) (Deeks et al., 2019). Subgroup analyses and random-effects models were performed to reduce heterogeneity (Langan, 2022), but potential heterogeneity remains unavoidable.
Some studies reported the median, first and third quartiles, and maximum and minimum values. To perform a valid meta-analysis of continuous variables, these data were then transformed into means and standard deviations using the Box-Cox transformation method (Langan, 2022). Change in swallowing scores before and after the intervention was calculated using a statistical formula (x̄= x̄1- x̄2; S= , x̄, Mean; S, Standard deviation).
Qualitative assessment of the funnel plot to determine publication bias, and visual inspection to determine whether there are any asymmetries (Sterne et al., 2011). Making funnel plots with Review Manager Software.
Results
Search results
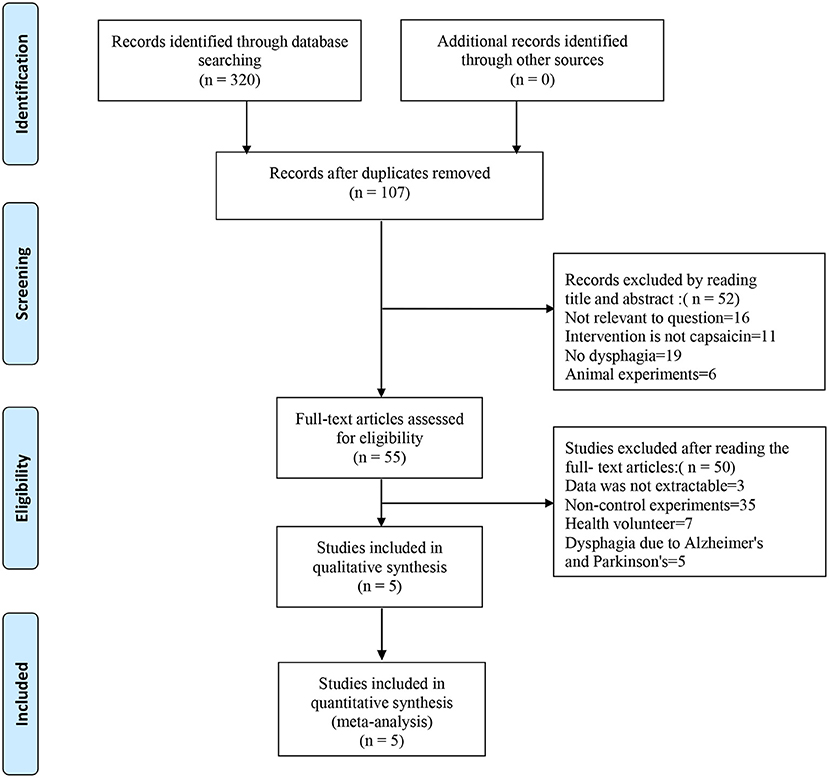
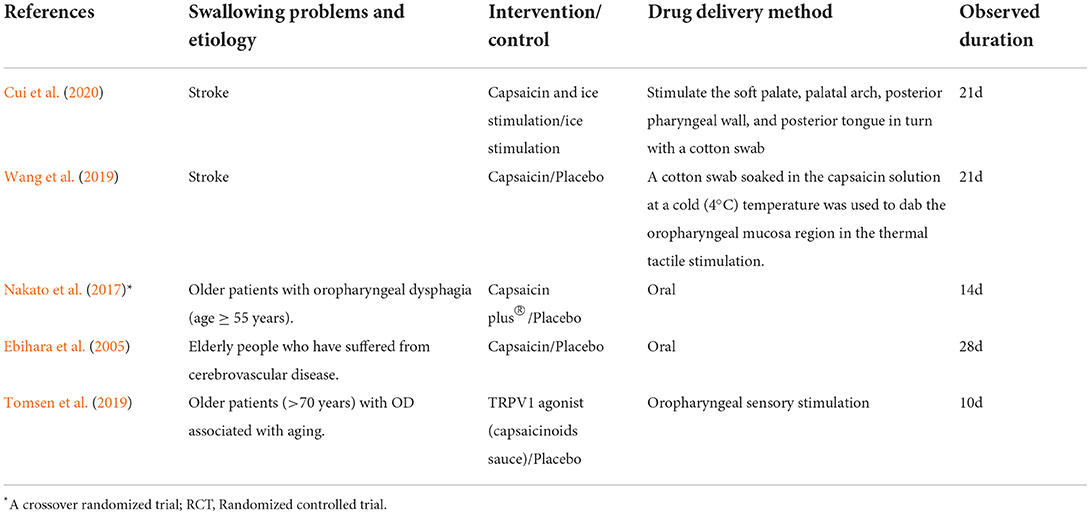
The search strategies are presented in Figure 3. We searched Medline, Embase, PubMed, and the Cochrane Library databases. Three hundred twenty studies were initially identified, and 107 studies were retained after excluding duplicate records. Reading the titles and abstracts, 52 studies were excluded that did not conform to the purpose of the study, such as excluding dysphagia and capsaicin. After careful reading of the full text of 55 studies, 50 studies that did not meet the inclusion criteria were excluded. Five studies were eventually included in this systematic review (Ebihara et al., 2005; Kondo et al., 2017; Nakato et al., 2017; Tomsen et al., 2019; Wang et al., 2019; Cui et al., 2020). Table 1 describes the study design.
Primary outcomes
Swallowing function score change
Studies Cui et al., Wang et al., and Tomsen et al. assessed swallowing function, using the SSA, V-VST and VFS scoring tools, respectively. Subgroup analysis was used to evaluate the effect of capsaicin on swallowing function scores. The meta-analysis results showed a significant difference between the capsaicin and control groups [SMD = −1.30, 95% CI: (−2.35, −0.25), P = 0.01; Figure 4A]. Therefore, the change in olfactory scores was more pronounced with capsaicin compared to the control group. It can be shown that capsaicin has an improving effect on swallowing function.
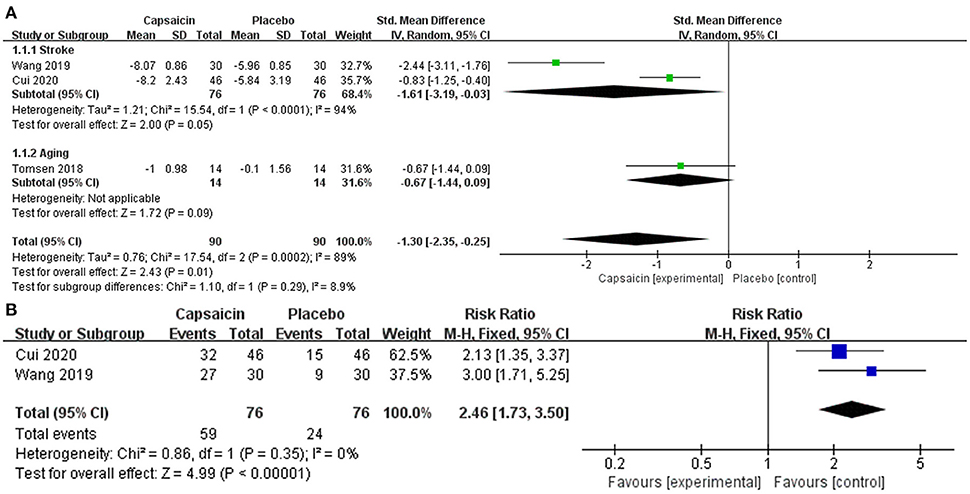
Figure 4. Forest plot demonstrates the effectiveness of capsaicin for oropharyngeal dysphagia: (A) swallowing function, (B) water swallowing test.
The funnel plot (Figure 5) is symmetrical showed a low risk of publication bias.
Water swallowing test
A meta-analysis of the treatment efficacy of the water swallowing test after administration of capsaicin or placebo. Treatment is considered effective when the experimental grade is raised to level I or II. The heterogeneity was observed in forest plots (I2 = 0%, p = 0.35). There is no heterogeneity therefore a fixed-effects model is used. Relative risks (RR) with 95% confidence intervals (CI) were used to assess the results of the dichotomous method. This meta-analysis results showed a significant difference between the capsaicin and control groups [RR = 2.46, 95% CI: (1.73, 3.50), P < 0.0001; Figure 4B].
Second outcome
Efficacy of capsaicin
Treatment was effective in a significantly higher number of patients (38.8%) in the capsaicin group than those who took the placebo (6.1%, p < 0.001) in Nakato's study (Nakato et al., 2017).
Substance P concentrations
In Cui's study, the serum substance P level in the capsaicin group was significantly higher than in the control group (p = 0.007) (Cui et al., 2020). Furthermore, in Nakato's study, the administration of capsaicin caused a reduction in the cervical esophageal wall opening time along with an increase in SP in saliva (p = 0.001) (Nakato et al., 2017).
Individual latency time
In Ebihara's study, individual latency time of the swallowing reflex was significantly better in the capsaicin group than before the start of the study (p < 0.05) (Ebihara et al., 2005).
The standardized low-resolution brain electromagnetic tomography software (sLORETA)
In Tomsen's study, measurement results with sLORETA show that compared with basal activation, patients that received the capsaicin treatment showed a significant reduction in cortical activity at the N1, P1 and N2 peaks (p = 0.0002) (Tomsen et al., 2019). And patients in the capsaicin group had a significant reduction in laryngeal vestibule closure time compared to the control group. Studies show that impaired safety of deglutition and aspirations in older people are mainly caused by delayed LVC (Tomsen et al., 2019).
Safety of the treatment
In terms of treatment safety, only two adverse events for respiratory infections were reported in Wang's study (Wang et al., 2019), one in the capsaicin intervention group and one in the placebo control group. No treatment-related adverse events were identified in the other five studies.
Discussion
The six randomized controlled trials included in this study were high-quality studies by quality assessment. The overall risk of bias in this study was low level.
The results of this meta showed that swallowing scores improved more in the capsaicin group compared to the control group in stroke patients and elderly patients. In our meta-analysis of the water swallowing experiment, the increased grade of the patients was considered an improvement in swallowing function. The grade was significantly higher in the capsaicin group compared to the control group (RR = 2.46, P < 0.0001). Therefore, both meta-analyses demonstrated that capsaicin can improve swallowing function. The Swallowing Function Test describes basic aspects of dysphagia, but it is not the gold standard for evaluating dysphagia, and clinical outcomes may be influenced by other important parameters, such as consciousness, cognitive ability, and language impairment, which can have an impact on swallowing scores. Therefore, caution is needed when using swallowing function tests to describe swallowing function.
In addition, capsaicin can mediate the local release of substance P (SP). Two studies reported the concentration of substance p in patients' serum and saliva, respectively. The SP of the capsaicin group was significantly higher than that of the control group in both studies.
Stimulation of transient receptor potentials has been reported as a target for the treatment of swallowing disorders, and activation of transient receptor potentials may improve swallowing function (Legrand et al., 2020). A new study found that most oropharyngeal chemical stimuli are associated with polymodal receptors, particularly transient receptor potential vanilloid 1 (TRPV1) (Cui et al., 2016). Capsaicin is an agonist of TRPV1 and activates TRPV1 in peripheral sensory c-fibers. Studies have shown that TRPA1 agonist can improve swallowing by reducing laryngeal vestibular closure time, upper esophageal sphincter opening time, and penetration-aspiration score (Rofes et al., 2014).
In Tomsen's trial, patients in the capsaicin group showed a clinically relevant and statistically significant reduction in laryngeal vestibule closure (LVC) time (p = 0.042) (Tomsen et al., 2019). EEG revealed a highly significant correlation between the time of LVC reduction and the reduction in peak latency of pharyngeal event-related potentials. In addition, elderly patients with OD have impaired cortical responses to electrical stimulation of the pharynx compared to those without dysphagia. In elderly OD patients, the amplitudes of all peaks were significantly lower, and only the latencies of the N1 and N2 peaks were delayed (Tomsen et al., 2019). sLORETA measurement results show that patients who received the capsaicin treatment showed a significant reduction in cortical activity at the N1, P1, and N2 peaks compared with basal activation (Tomsen et al., 2019). In summary, Capsaicin can induce afferent neurons to transmit signals to the insular cortex activating feedback loops and brainstem swallowing centers through repeated repetitive stimulation of the cerebral cortex, which can restore the function of the insular cortex and induce cortical neuroplasticity, thus restoring swallowing function.
Esophageal manometry studies confirm that capsaicin exerts a prokinetic effect on esophageal motility by increasing the amplitude, duration, and speed of contraction of esophageal body peristalsis (Suntrup-Krueger et al., 2021). In Tomsen's study, there was no significant difference in the upper esophageal sphincter (p < 0.539). In Nakato's study, only patients who responded to capsaicin were found to have a cervical esophageal wall opening time duration was significantly shorter (p = 0.0003).
In Kondo's study, reflex scores for glottal closure and cough reflex improved more significantly in the capsaicin group than in the control group. Glottal closure and cough reflex are important protective mechanisms against aspiration, so capsaicin can effectively prevent aspiration pneumonia.
Our systematic review and meta-analysis had the following limitations. (1) Our meta-analysis included only 6 studies; our analysis would have been more credible if we had included more RCTs. (2) The Water Swallow Test and Swallowing Score are reliable but subjective methods for evaluating dysphagia. (3) Only English-language publications were included in our meta-analysis. (4) Heterogeneity is inevitable due to differences in gender, age, mode of administration, differences in experimental design and different disease conditions of the patients included in the studies.
Conclusions
In summary, capsaicin may be a new perspective in treatment of oropharyngeal disorders in the elderly and stroke patients. There is still a lack of evidence for capsaicin in swallowing function, and more studies are needed to support this in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
CY proposed the study idea, searched the database, screened and evaluated the evidence, and wrote the manuscript. RC extracted the data and analyzed the data. MF revised the manuscript. MZ revised the English. DW, XL, and WL suggested revisions to the article. All authors read and approved the final manuscript.
Acknowledgments
The authors thank Dr. Ming-jie Kuang (Shandong Provincial Hospital Affiliated to Shandong University) for his valuable feedback and critical review of the draft manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.931016/full#supplementary-material
Supplementary material 1. The results of this systematic review and meta-analysis registered on PROSPERO.
Supplementary material 2. Search strategy.
Supplementary Table 1. PRISMA 2020 checklist.
Abbreviations
OD, oropharyngeal dysphagia; PSEP, pharyngeal sensory evoked potential; RCTs, Randomized controlled trials; SP, substance P; WST, water swallowing test.
References
Alvarez-Berdugo, D., Rofes, L., Casamitjana, J. F., Padrón, A., Quer, M., and Clavé, P. (2016). Oropharyngeal and laryngeal sensory innervation in the pathophysiology of swallowing disorders and sensory stimulation treatments. Ann. N. Y. Acad. Sci. 1380, 104–120. doi: 10.1111/nyas.13150
Baijens, L. W., Clavé, P., Cras, P., Ekberg, O., Forster, A., Kolb, G. F., et al. (2016). European Society for Swallowing Disorders–European Union Geriatric Medicine Society white paper: oropharyngeal dysphagia as a geriatric syndrome. Clin. Interv. Aging 11, 1403. doi: 10.2147/CIA.S107750
Cabib, C., Nascimento, W., Rofes, L., Arreola, V., Tomsen, N., Mundet, L., et al. (2020a). Neurophysiological and biomechanical evaluation of the mechanisms which impair safety of swallow in chronic post-stroke patients. Transl. Stroke Res. 11, 16–28. doi: 10.1007/s12975-019-00701-2
Cabib, C., Nascimento, W., Rofes, L., Arreola, V., Tomsen, N., Mundet, L., et al. (2020b). Short-term neurophysiological effects of sensory pathway neurorehabilitation strategies on chronic poststroke oropharyngeal dysphagia. Neurogastroenterol. Motility 32, e13887. doi: 10.1111/nmo.13887
Cabib, C., Ortega, O., Kumru, H., Palomeras, E., Vilardell, N., Alvarez-Berdugo, D., et al. (2016). Neurorehabilitation strategies for poststroke oropharyngeal dysphagia: from compensation to the recovery of swallowing function. Ann. N. Y. Acad. Sci. 1380, 121–138. doi: 10.1111/nyas.13135
Cabib, C., Ortega, O., Vilardell, N., Mundet, L., Clavé, P., and Rofes, L. (2017). Chronic post-stroke oropharyngeal dysphagia is associated with impaired cortical activation to pharyngeal sensory inputs. Eur. J. Neurol. 24, 1355–1362. doi: 10.1111/ene.13392
Cui, F., Yin, Q., Wu, C., Shen, M., Zhang, Y., Ma, C., et al. (2020). Capsaicin combined with ice stimulation improves swallowing function in patients with dysphagia after stroke: a randomised controlled trial. J. Oral Rehabil. 47, 1297–1303. doi: 10.1111/joor.13068
Cui, M., Gosu, V., Basith, S., Hong, S., and Choi, S. (2016). Polymodal transient receptor potential vanilloid type 1 nocisensor: structure, modulators, and therapeutic applications. Sci. Rep. 104, 81–125. doi: 10.1016/bs.apcsb.2015.11.005
Deeks, J. J., Higgins, J. P. T., Altman, D. G., Higgins, J. P. T., Thomas, J., Chandler, J., et al. (2019). Analysing data and undertaking meta-analyses. Cochrane Handbook Syst Rev Intervent. 2019, 241–284. doi: 10.1002/9781119536604.ch10
Ebihara, T., Takahashi, H., Ebihara, S., Okazaki, T., Sasaki, T., Watando, A., et al. (2005). Capsaicin troche for swallowing dysfunction in older people. J. Am. Geriatr. Soc. 53, 824–828. doi: 10.1111/j.1532-5415.2005.53261.x
Faggion, C. M. Jr. (2015). Evaluating the risk of bias of a study. J. Evid. Based Dental Pract. 15, 164–170. doi: 10.1016/j.jebdp.2015.09.002
Ferreira, L. G. B., Faria, J. V., Dos Santos, J. P. S., and Faria, R. X. (2020). Capsaicin: TRPV1-independent mechanisms and novel therapeutic possibilities. Eur. J. Pharmacol. 887, 173356. doi: 10.1016/j.ejphar.2020.173356
Friedrich, J. O., Adhikari, N. K., and Beyene, J. (2011). Ratio of means for analyzing continuous outcomes in meta-analysis performed as well as mean difference methods. J. Clin. Epidemiol. 64, 556–564. doi: 10.1016/j.jclinepi.2010.09.016
Goldsmith, T., Cohen, A. K., Schmader, K. E., Deschler, D. G., Smith, T. J., Givens, J., et al (2021). Swallowing Disorders and Aspiration in Palliative Care: Assessment and Strategies for Management. Waltham, MA: UpToDate. Available online at: https://www.uptodate.com/contents/swallowing-disorders-and-aspiration-in-palliative-care-assessment-and-strategies-for-management/print
Hashimoto, H., Takahashi, K., Kameda, S., Yoshida, F., Maezawa, H., Oshino, S., et al. (2021). Swallowing-related neural oscillation: an intracranial EEG study. Ann. Clin. Trans. Neurol. 8, 1224–1238. doi: 10.1002/acn3.51344
Higgins, J. P., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2019). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons.
Higgins, J. P., Thompson, S. G., and Spiegelhalter, D. J. (2009). A re-evaluation of random-effects meta-analysis. J. R. Stat. Soc. Series A 172, 137–159. doi: 10.1111/j.1467-985X.2008.00552.x
Kondo, E., Jinnouchi, O., Nakano, S., Ohnishi, H., Kawata, I., Okamoto, H., et al. (2017). Aural stimulation with capsaicin ointment improved swallowing function in elderly patients with dysphagia: a randomized, placebo-controlled, double-blind, comparative study. Clin. Interv. Aging 12, 1921–1928. doi: 10.2147/CIA.S138357
Langan, D. (2022). Assessing heterogeneity in random-effects meta-analysis. Methods Mol. Biol. 2345, 67–89. doi: 10.1007/978-1-0716-1566-9_4
Legrand, C., Merlini, J. M., de Senarclens-Bezençon, C., and Michlig, S. (2020). New natural agonists of the transient receptor potential Ankyrin 1 (TRPA1) channel. Sci. Rep. 10, 1–10. doi: 10.1038/s41598-020-68013-2
Lui, S. K., and Nguyen, M. H. (2018). Elderly stroke rehabilitation: Overcoming the complications and its associated challenges. Curr. Gerontol. Geriatr. Res. 2018, 9853837. doi: 10.1155/2018/9853837
Marin, S., Serra-Prat, M., Ortega, O., and Clavé, P. (2018). Cost of oropharyngeal dysphagia after stroke: protocol for a systematic review. BMJ Open 8, e022775. doi: 10.1136/bmjopen-2018-022775
Mazzone, S. B., and Undem, B. J. (2016). Vagal afferent innervation of the airways in health and disease. Physiol. Rev. 96, 975–1024. doi: 10.1152/physrev.00039.2015
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and PRISMA Group (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann. Intern. Med. 151, 264–269. doi: 10.7326/0003-4819-151-4-200908180-00135
Nakato, R., Manabe, N., Shimizu, S., Hanayama, K., Shiotani, A., Hata, J., et al. (2017). Effects of capsaicin on older patients with oropharyngeal dysphagia: a double-blind, placebo-controlled, crossover study. Digestion 95, 210–220. doi: 10.1159/000463382
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 88, 105906. doi: 10.1186/s13643-021-01626-4
Ravindran, M., Merck, S. J., and Baraniuk, J. N. (2016). Functional Neuroanatomy of the Human Upper Airway. Toxicology of the Nose and Upper Airways. Boca Raton, FL: CRC Press. p. 79–95.
Rofes, L., Arreola, V., Martin, A., and Clavé, P. (2013). Natural capsaicinoids improve swallow response in older patients with oropharyngeal dysphagia. Gut 62, 1280–1287. doi: 10.1136/gutjnl-2011-300753
Rofes, L., Arreola, V., Martin, A., and Clavé, P. (2014). Effect of oral piperine on the swallow response of patients with oropharyngeal dysphagia. J. Gastroenterol. 49, 1517–1523. doi: 10.1007/s00535-013-0920-0
Sasegbon, A., and Hamdy, S. (2017). The anatomy and physiology of normal and abnormal swallowing in oropharyngeal dysphagia. Neurogastroenterol. Motility 29, e13100. doi: 10.1111/nmo.13100
Sasegbon, A., and Hamdy, S. (2021). The role of the cerebellum in swallowing. Dysphagia. doi: 10.1007/s00455-021-10271-x. [Epub ahead of print].
Serra-Prat, M., Hinojosa, G., López, D., Juan, M., Fabré, E., Voss, D. S., et al. (2011). Prevalence of oropharyngeal dysphagia and impaired safety and efficacy of swallow in independently living older persons. J. Am. Geriatr. Soc. 59, 186–187. doi: 10.1111/j.1532-5415.2010.03227.x
Shi, J., Luo, D., Wan, X., Liu, Y., Liu, J., Bian, Z., et al. (2020). Detecting the skewness of data from the sample size and the five-number summary. arXiv [Preprint]. arXiv: 2010.05749. doi: 10.48550/arXiv.2010.05749
Speyer, R., Baijens, L., Heijnen, M., and Zwijnenberg, I. (2010). Effects of therapy in oropharyngeal dysphagia by speech and language therapists: a systematic review. Dysphagia 25, 40–65. doi: 10.1007/s00455-009-9239-7
Steele, C. M., and Miller, A. J. (2010). Sensory input pathways and mechanisms in swallowing: a review. Dysphagia 25, 323–333. doi: 10.1007/s00455-010-9301-5
Sterne, J. A., Sutton, A. J., Ioannidis, J. P., Terrin, N., Jones, D. R., Lau, J., et al. (2011). Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343, d4002. doi: 10.1136/bmj.d4002
Suntrup-Krueger, S., Muhle, P., Kampe, I., Egidi, P., Ruck, T., Lenze, F., et al. (2021). Effect of capsaicinoids on neurophysiological, biochemical, and mechanical parameters of swallowing function. Neurotherapeutics 18, 1360–1370. doi: 10.1007/s13311-020-00996-2
Thakkinstian, A., McElduff, P., D'Este, C., Duffy, D., and Attia, J. (2005). A method for meta-analysis of molecular association studies. Stat. Med. 24, 1291–1306. doi: 10.1002/sim.2010
Tomsen, N., Ortega, O., Rofes, L., Arreola, V., Martin, A., Mundet, L., et al. (2019). Acute and subacute effects of oropharyngeal sensory stimulation with TRPV1 agonists in older patients with oropharyngeal dysphagia: a biomechanical and neurophysiological randomized pilot study. Therap. Adv. Gastroenterol. 12, 1756284819842043. doi: 10.1177/1756284819842043
Wang, Z., Wu, L., Fang, Q., Shen, M., Zhang, L., and Liu, X. (2019). Effects of capsaicin on swallowing function in stroke patients with dysphagia: a randomized controlled trial. J. Stroke Cerebrovasc. Dis. 28, 1744–1751. doi: 10.1016/j.jstrokecerebrovasdis.2019.02.008
Keywords: oropharyngeal dysphagia, capsaicin, TRPV1, randomized controlled trials, swallowing function score
Citation: Yang C-w, Chen R-d, Feng M-t, Zhang M-z, Liu W, Liu X-c and Wang D-c (2022) The therapeutic effect of capsaicin on oropharyngeal dysphagia: A systematic review and meta-analysis. Front. Aging Neurosci. 14:931016. doi: 10.3389/fnagi.2022.931016
Received: 28 April 2022; Accepted: 17 October 2022;
Published: 08 November 2022.
Edited by:
Fabio Pilato, Policlinico Universitario Campus Bio-Medico, ItalyReviewed by:
Annelise Ayres, Hospital de Clínicas de Porto Alegre, BrazilMaira Rozenfeld Olchik, Federal University of Rio Grande do Sul, Brazil
Copyright © 2022 Yang, Chen, Feng, Zhang, Liu, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Da-chuan Wang, d2FuZ2RhY2h1YW5zZEAxNjMuY29t; Xu-chang Liu, bGl1eHVjaGFuZ0BzZGZtdS5lZHUuY24=; Wei Liu, eWN3OTk1MDg5NDM1QDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work
 Cong-wen Yang
Cong-wen Yang Ru-dong Chen
Ru-dong Chen Meng-ting Feng3
Meng-ting Feng3 Wei Liu
Wei Liu