
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 27 September 2022
Sec. Neurocognitive Aging and Behavior
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.922530
This article is part of the Research TopicCerebral Small Vessel Disease: From the Pathogenesis to Therapeutic InterventionsView all 7 articles
Objectives: The present study was designed to evaluate the effects of total cerebral small vessel disease (CSVD) on early-onset depression after acute ischemic stroke (AIS), and to develop a new nomogram including total CSVD burden to predict early-onset post-stroke depression (PSD).
Methods: We continuously enrolled patients with AIS who were hospitalized at the First Affiliated Hospital of Soochow University between October 2017 and June 2019. All patients were assessed for depressive symptoms using the 17-item Hamilton Depression Scale (HAMD-17) at 14 ± 2 days after the onset of AIS. The diagnosis for depression was made according to the American Diagnostic and Statistical Manual of Mental Disorders Version 5 (DSM-5). The demographic and clinical data were collected including total CSVD burden. On the basis of a multivariate logistic model, the independent factors of early-onset PSD were identified and the predictive nomogram was generated. The performance of the nomogram was evaluated by Harrell's concordance index (C-index) and calibration plot.
Results: A total of 346 patients were enrolled. When contrasted to a 0 score of total CSVD burden, the score ≥2 (moderate to severe total CSVD burden) was an independent risk factor for early-onset PSD. Besides, gender, cognitive impairments, baseline Barthel Index (BI), and plasma fibrinogen were independently associated with early-onset PSD. The nomogram based on all these five independent risk factors was developed and validated with an Area Under Curve (AUC) of 0.780. In addition, the calibration plot revealed an adequate fit of the nomogram in predicting the risk of early-onset depression in patients with AIS.
Conclusions: Our study found the total CSVD burden score of 2–4 points was an independent risk factor of early-onset PSD. The proposed nomogram based on total CSVD burden, gender, cognitive impairments, baseline BI, and plasma fibrinogen concentration gave rise to a more accurate and more comprehensive prediction for early-onset PSD.
Post-stroke depression (PSD) is the most common emotional disorder after acute ischemic stroke (AIS) (Hackett and Pickles, 2014), which can increase the risk of stroke mortality (House et al., 2001; Williams et al., 2004), cause recurrence of stroke (Yuan et al., 2012) and lead to poor functional prognosis (Pohjasvaara et al., 2001; Ayerbe et al., 2014). About 31% of AIS patients may suffer from PSD during any period after stroke (Hackett and Pickles, 2014). Degree of physical disability, cognitive impairment, stroke severity, female gender and lack of social and family support are considered as the risk factors for PSD (Hackett and Anderson, 2005). Early-onset PSD refers to depression that occurs within 2 weeks after the onset of stroke (Altieri et al., 2012; He et al., 2017; Huang et al., 2018; Zhao et al., 2018). Compared to late-onset PSD, early-onset PSD possesses more depressive symptoms (Tateno et al., 2002) and may reduce patients' desire for drug and rehabilitation therapy (Willey et al., 2010). Thus, early detection and intervention of PSD are important to improve the prognosis of AIS patients (Gabaldón et al., 2007).
Cerebral small vessel disease (CSVD) refers to a group of pathological processes that affect the small arteries, arterioles, venules, and capillaries of the brain (Pantoni, 2010). CSVD may result in various clinical symptoms including cognitive disorders, dementia, balance disorders, gait disorders and emotional disorders (Wardlaw et al., 2013; van Agtmaal et al., 2017). Neuroimaging characteristics of CSVD include white matter hyperintensities (WMHs), silent lacunar infarction (sLI), enlarged perivascular spaces (EPVS), and cerebral microbleeds (CMBs) (Pantoni, 2010; Wardlaw et al., 2013). Nowadays, more and more existing evidence indicates that CSVD could be one of the crucial factors of PSD (Santos et al., 2009b). However, recent studies mostly focus on the relationship between PSD and a specific CSVD subtype. In fact, the coexistence of various subtypes of CSVD in one patient is common, thus the total CSVD burden may be a better indicator to reflect the severity of CSVD. Staals et al. developed a CSVD global burden rating scale including the WMHs, sLI, EPVS, and CMBs subtypes (Staals et al., 2014). The practicality and reliability of the rating scale have been verified in many studies (Staals et al., 2015; Lau et al., 2017; Jiang et al., 2019; Liu et al., 2019; Wu et al., 2020). However, the relationship between the total CSVD burden with PSD is poorly studied.
Nomogram, a graphical statistical instrument, has been generally used in medical decision-making. To date, there is no nomogram model to predict the risk of early-onset PSD. Therefore, the purposes of this study were to investigate the effects of CSVD on early-onset PSD and to build a nomogram as the prediction model of early-onset PSD. We assumed that total CSVD burden was a prominent risk factor for early-onset PSD, and the nomogram could be a promising prediction tool.
The present study was a retrospective analysis with collected data from the neurological department of First Hospital Affiliated of Soochow University between October 2017 and June 2019. All patients suffered from AIS within 3 days. Patients with the following conditions were excluded: depressive disorders, anxiety disorders, or other mental illnesses diagnosed by the professional psychiatrists before the stroke onset; accompanied by severe systemic diseases; medical histories of other central nervous system diseases; previous histories of alcohol or drug abuse; disturbance of consciousness, severe language disorders, Mini-Mental State Examination (MMSE) scale lower than 17 points or other situations that resulted in failure to complete the mental psychological assessment; not able to conduct an MRI examination. Ethical approval for this study was obtained from the institutional review board of the First Affiliated Hospital of Soochow University (2022310). The data were anonymous, and the requirement for informed consent was therefore waived.
Demographic and clinical baseline data were collected, including gender, age, smoking and alcohol consumption, past disease history, education level, baseline NIHSS scale, baseline Barthel Index (BI), TOAST type, baseline blood pressure, baseline blood glucose and lipid levels, homocysteinemia and other relevant laboratory data. TOAST type was adjusted according to further examination results before discharge.
17-item Hamilton Depression Scale (HAMD-17) was adopted at 14 ± 2 days after the onset of AIS to evaluate mental disorders of the patients. HAMD-17 points <7 means normal condition; mild depression for 7–17 points; moderate depression for 18–24 points; more than 24 points for severe depression (Zhu et al., 2016; He et al., 2017; Luan et al., 2020; Chi et al., 2021). The diagnosis was made according to the American Diagnostic and Statistical Manual of Mental Disorders Version 5 (DSM-5). MMSE scale was conducted to evaluate patients' cognitive function. Cognitive impairment was defined according to the MMSE score and education level referred to as standard. HAMD-17 and MMSE were conducted by two independent neuropsychological experts.
The magnetic resonance imaging (MRI) sequences involved in the study included diffusion-weighted imaging (DWI), 3d-TOF-MRA, FLAIR, T2-weighted, T1-weighted, and susceptibility weighted imaging (SWI).
The total CSVD burden score consisted of four imaging markers including WMHs, EPVS, CMBs, and sLI. When WMHs were graded according to Fazekas' scale, one point was awarded as either irregular periventricular WMH extending into the deep white matter (Fazekas score 3) or confluent deep WMH (Fazekas score 2 or 3). EPVS number was counted in the dominant side of basal ganglia and graded into 4 levels (grade 0 for no EPVS, grade 1 for <10 EPVS, grade 2 for 11 to 20 EPVS, grade 3 for 21–40 EPVS, grade 4 for more than 40 EPVS) according to Semiquantitative scale, one point was awarded if there were moderate to severe (Semiquantitative grade 2–4) EPVS. The number of deep CMBs in the cerebellum, brainstem, basal ganglia, and thalamus was involved in this study, one point was awarded if there was one or more CMBs. One point was awarded if the presence of any sLI. The severity of the total CSVD burden was graded into three levels: mild (0 or 1 point), moderate (2 points) and severe (3 or 4 points) (Arba et al., 2017a; Liu et al., 2019). Cerebral atrophy was assessed using the Global Cortical Atrophy Scale (GCA), Moderate to severe brain atrophy was recorded as positive (2 or 3 points) (Pasquier et al., 1996).
Continuous variables were compared by the Mann-Whitney U test for non-normally distributed variables and Student's t-test for normally distributed variables. Differences between proportions were assessed by Fisher's exact test or Chi-square test. Continuous variables were reported as the mean ± SD or median (interquartile range), and categorical variables were described by constituent ratio. The SPSS 20.0 was used for statistical analysis of baseline data. A univariable analysis was used to compare the baseline and clinical differences between the PSD and non-PSD groups. Variables with a P < 0.05 in univariable analysis were included in the multivariable logistic regression analysis. The nomogram is constructed by R version 4.0. Variables with a P < 0.05 in the multivariable logistic regression were entered into the nomogram. The nomogram was created by assigning a graphic preliminary score to each of the predictors with a point ranging from 0 to 100, which was then summed to generate a total score, finally converted to the logit, and then to an individual probability (from 0 to 100%) of early-onset PSD. The area under the receiver operating characteristic curve (AUC-ROC) was used to evaluate the differentiation of the prediction model. Calibration was tested using a calibration curve with bootstraps of 1,000 resamples, which reflected the agreement between nomogram and actual observation.
A total of 346 patients were enrolled in the study, including 280 patients (80.9%) in the non-PSD group and 66 patients (19.1%) in the PSD group. The baseline characteristics of the patients in the two groups are shown in Table 1. In the non-PSD group, the median age was 63 (53–70) years old, and 67.5% were male. The median age was 64 (59–68) years old in the PSD group, with 53% being male. Univariate analysis showed that the differences between the two cohorts were gender, plasma fibrinogen, previous history of stroke, baseline NIHSS score, baseline BI, moderate to severe cerebral atrophy, and cognitive disorder (all P < 0.05).
In our study, moderate to severe WMHs were found in 36.1% of the subjects, sLI were in 17.1% of the subjects, CMBs were in 27.5% of the subjects and EPVS grades 2–4 were in 40.5%. In the PSD group, total CSVD burden assessment showed that 27.3% were 1 score, 18.2% were 2 score, 22.7% were 3 and 15.2% were 4, while in the non-PSD group, 31.8% were 1 score, 17.1% were 2, 8.9% were 3 and 2.9% were 4 (Figure 1). Univariate analysis showed that mild to severe WMHs, EPVS grades 2–4, the existence of sLI or CMBs, and total CSVD burden score were different between the non-PSD and PSD groups (Table 2).
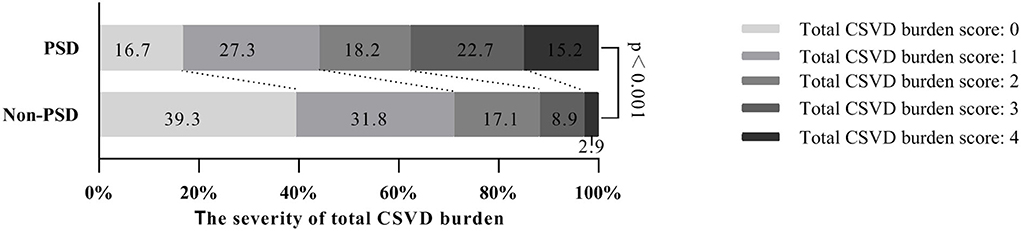
Figure 1. Total CSVD burden score distribution between the two cohorts. CSVD, cerebral small vessel disease.
Variables with a P < 0.05 were included in the multivariable logistic regression model. The results indicate that gender (OR = 0.440, 95%CI: 0.238–0.813), cognitive disorder (OR = 2.501, 95%CI: 1.283–4.876), baseline BI (OR = 0.986, 95%CI: 0.975–0.997), plasma fibrinogen concentration (OR = 1.425, 95%CI: 1.050–1.933) were independently related to early-onset PSD. In reference to the 0 score of the total CSVD burden, 2–4 points (moderate to severe total CSVD burden) was an independent risk factor of early-onset PSD and the higher the point was, the higher the OR value was, which meant a higher risk of the occurrence of early-onset PSD (Table 3).
The nomogram consisted of the variables that had P < 0.05 in the multivariable logistic regression (Figure 2). It was generated by assigning a graphic preliminary score to each of the 5 factors (gender, cognitive disorder, baseline BI, fibrinogen, and moderate to severe total CSVD burden score) with a point ranging from 0 to 100. Then all the scores were summed up to generate a total score and finally converted into an individual probability of the onset of early-onset PSD. The probability was from 0 to 100%.
The C-Statistics was used to assess the discriminative capacity of the nomogram as demonstrated in Figure 3. The AUC was 0.780 (95%CI 0.751–0.810). The calibration curves were demonstrated in the Figure 4. The x-axis exhibited the predicted possibility of PSD, and the y-axis represented the actual possibility of PSD. The calibration plot revealed a general fit of the nomogram predicting the risk of early-onset depression in patients with AIS.
PSD is a specific type of depression that can be classified into depressive disorders caused by other physical disorders according to the DSM-5 (Mittal and Walker, 2011). In 1975, a study conducted by Robinson et al. indicated that decreased cerebral blood flow could affect catecholamine concentration and induce “fight or flight” phenomenon (Robinson et al., 1975). Folstein et al. (1977) found that about 1/2 of the stroke patients suffered from depression, while only 1/5 of orthopedic patients suffered from depression to the same disability degree. These findings inspire neurologists to explore the relationship between depression and cerebral injury.
Early-onset PSD refers to the depression that occurs within 2 weeks after the onset of stroke. Compared to late-onset PSD, early-onset PSD possesses more depression symptoms and may affect patients' prognosis (Tateno et al., 2002; Willey et al., 2010). Large amounts of guidelines and expert consensus pointed out the importance of early diagnosis and treatment of PSD. For instance, the European Guidelines for stroke management, published in 2008 emphasizes that doctors must pay more attention to the early diagnosis of PSD (ESO, 2008). Canadian Guidelines for the best management of stroke delivered in 2009 also point out that depression screening is recommended before discharge for patients in the acute phase of stroke (Lanctôt et al., 2020).
Our study indicated that the four imaging subtypes of CSVD, including WMHs, sLI, CMBs, and EPVS, were all related to the early-onset PSD. Current studies also indicate that the CSVD subtypes are related to PSD. WMHs were the most representative imaging marker of CSVD. A case-control study conducted by Tang et al. illuminated that WMHs were related to PSD 3 months after a stroke attack (Tang et al., 2010). Another study that followed up 294 lacunar infarction patients with CSVD found that the severity of baseline WMHs was independently related to late-onset PSD (Pavlovic et al., 2016). An up-to-date research also concluded that deep WMHs were an independent risk factor of PSD in TIA or mild stroke patients (Carnes-Vendrell et al., 2019). sLI is also called asymptomatic lacunar infarcts or lacunes. Santos et al. revealed that the accumulative chronic lacunes in the thalamus, basal ganglia, and deep white matter were obviously related to PSD onset rather than single infarction by conducting an autopsy (Santos et al., 2009a). Another study on patients with acute lacunar infarction found that baseline sLI was independently related to PSD 3 months after stroke onset (Zhang et al., 2017). The study of the relationship between deep CMBs and PSD is rare. Tang et al. reported that lobular CMBs were the independent risk factor of PSD 3 months after stroke onset (Tang et al., 2014) and the number of lobular CMBs was positively related to PSD severity (Tang et al., 2011). The relationship between basal ganglia EPVS and PSD is not clear yet. Zhang et al. indicated that severe EPVS was not independently related to PSD (Zhang et al., 2017). Another 3-months prospective study targeting AIS patients indicated that severe EPVS in the basal ganglia region was not related to PSD, while centrum semiovale EPVS was remarkably related to PSD (Liang et al., 2018a). To sum up, the relationship between the imaging manifestations of CSVD subtypes and PSD is not better researched and heterogenicity is prominent, thus, further research is required in this field.
In our study, about 1/3 of patients possessed 2 or more characteristics, which was similar to the distribution of CSVD overall burden in other studies. As mentioned before, different CSVD imaging characteristics usually coexist in one patient, and CSVD overall scale is more clinically significant than a single CSVD type. Hence, a rating system that involves multiple CSVD types can better evaluate the overall burden of CSVD (Staals et al., 2014). However, so far, only a few studies have explored the relationship between total CSVD burden and PSD. Zhang et al. found that CSVD overall burden was an independent prediction factor of PSD while the research only focused on patients that first suffered from lacunar infarction (Zhang et al., 2017). Another study explored the relationship between baseline total CSVD burden and poststroke depressive symptoms (PDS) (Liang et al., 2018b). The study involved 563 mild AIS patients and followed them on the 3th, 9th and 15th month. The results showed that the baseline total CSVD burden was positively related to PSD risk at all these three periods of the follow-up visit. Another study reported that CSVD burden not only directly determined PSD symptoms but also worsened stroke severity, and post-stroke neurological deficits (Liang et al., 2019). To our knowledge, there has not been any research that focuses on the relationship between total CSVD burden and early-onset PSD. In this research, we found that 2–4 points of total CSVD burden were obviously related to early-onset PSD and the patients with higher total CSVD burden were more likely to suffer from early-onset PSD.
Several mechanisms could explain the relationship between CSVD and PSD. First of all, patients with pre-stroke CSVD often suffer from more vascular risk factors such as hypertension, diabetes, and so on, which are proven to be closely related to brain dysfunction (Muresanu et al., 2014). Besides, CSVD means the chronic cumulative ischemia of the brain, which may cause damage to hemodynamic neurovascular coupling (Monteiro et al., 2021; Yang et al., 2022). The hemodynamic neurovascular coupling ensures a strong increase in cerebral blood flow and a fast increase in neuronal glucose uptake in the case of acute incidents of the brain (Popa-Wagner et al., 2015). When patients with CSVD suffer from a sudden attack of acute ischemic stroke, the compensatory ability of the brain is weaker, thus, it is more likely to damage the depression-related network such as the frontal-subcortical circuit (Brookes et al., 2014; Arba et al., 2017b; van Agtmaal et al., 2017; Gu et al., 2022). In addition, local vascular inflammation contributes to the development of PSD (Spalletta et al., 2006). Pre-stroke CSVD may worsen the immune-inflammatory activation following AIS (Licata et al., 2006), which promotes depression.
There were several studies that built nomograms to predict PSD. Lan et al. identified that HAMD-17 scores, APTT, serum direct bilirubin, and FT4 at admission were related to the onset of persistent PSD in the first year and developed a nomogram to predict the risk of persistent PSD, which was proved to have good clinical reliability (Lan et al., 2022). Li et al. developed and validated a nomogram to predict the risk of major post-stroke depression at 3 months after AIS onset, which could be used to promote the early screening of PSD and was clinically practical (Li et al., 2021). Another study constructed an effective nomogram to predict the probability of PSD based on magnetic resonance spectroscopy (Qiao et al., 2020). To our best knowledge, this study was the first attempt to establish a practical and visual prediction model of early-onset PSD. The nomogram prediction model based on total CSVD burden score, gender, cognitive disorder, baseline BI and fibrinogen concentration had a C statistical magnitude of 0.780 and could predict early-onset PSD onset well. Thus, our nomogram model could increase early-onset PSD diagnosis efficiency and help doctors develop management and prevention strategies for PSD.
However, there were still deficiencies in our study. First, the study was a monocentric and small sample research. Bias might exist due to a lack of samples. In addition, since it was difficult to distinguish whether depression symptoms were caused by stroke or previous depression, patients with a depression medical history were not included in the research, which might have influenced our results. Besides, several patients were excluded from our study for the reason of aphasia or disturbance of consciousness or dementia at admission, and yet these patients may have experienced more severe strokes. Thus, patients included in our study may have had a relatively mild stroke attack. This might explain why there was no significant association between NIHSS score and early-onset PSD in our study to some extent. Finally, the nomogram designed by the research was barely applied to the prediction of early-onset PSD in the acute phase. Whether the nomogram could be used to predict PSD risk in different phases was not verified. Therefore, we would conduct careful follow-ups on patients involved in further research.
We found that a total CSVD burden score of 2–4 points was an independent risk factor of early-onset PSD. We also proposed and validated a nomogram model that included total CSVD burden score, gender, cognition disorder, baseline BI and plasma fibrinogen concentration, which reliably assessed the probability of early-onset depression in AIS patients.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Hospital Affiliated to Soochow University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
TL and QF conceived and designed the research. LZ and LC analyzed the data and drafted the manuscript. SD and YQ collected the data and performed the research. LM collected and analyzed the data. All authors reviewed and edited the manuscript and approved the final version of the manuscript.
This study was supported by the National Natural Science Foundation of China (82071300), the Suzhou Science and Technology Development Plan (SYSD2020073), and the Stroke Team of Professor QF from the First Affiliated Hospital of Soochow University (SZYQTD202106).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
AIS, acute ischemic stroke; AUC-ROC, area under the receiver operating characteristic curve; BI, Barthel index; CMBs, cerebral microbleeds; CSVD, cerebral small vessel disease; DSM-5, Diagnostic and Statistical Manual of Mental Disorders Version 5; DWI, diffusion-weighted imaging; EPVS, enlarged perivascular spaces; HAMD-17, 17-item Hamilton Depression scale; MMSE, Mini-Mental State Examination; MRI, magnetic resonance imaging; PDS, poststroke depressive symptoms; PSD, post-stroke depression; sLI, silent lacunar infarction; SWI, susceptibility weighted imaging; WMHs, white matter hyperintensities.
Altieri, M., Maestrini, I., Mercurio, A., Troisi, P., Sgarlata, E., Rea, V., et al. (2012). Depression after minor stroke: prevalence and predictors. Eur. J. Neurol. 19, 517–521. doi: 10.1111/j.1468-1331.2011.03583.x
Arba, F., Inzitari, D., Ali, M., Warach, S., Luby, M., Lees, K., et al. (2017a). Small vessel disease and clinical outcomes after IV rt-PA treatment. Acta Neurol. Scand. 136, 72–77. doi: 10.1111/ane.12745
Arba, F., Mair, G., Carpenter, T., Sakka, E., Sandercock, P., Lindley, R., et al. (2017b). Cerebral white matter hypoperfusion increases with small-vessel disease burden. Data From the Third International Stroke Trial. J. Stroke Cerebrovasc. Dis. 26, 1506–1513. doi: 10.1016/j.jstrokecerebrovasdis.2017.03.002
Ayerbe, L., Ayis, S., Crichton, S., Wolfe, C., and Rudd, A. (2014). The long-term outcomes of depression up to 10 years after stroke; the South London Stroke Register. J. Neurol. Neurosurg. Psychiatry 85, 514–521. doi: 10.1136/jnnp-2013-306448
Brookes, R. L., Herbert, V., Lawrence, A. J., Morris, R. G., and Markus, H. S. (2014). Depression in small-vessel disease relates to white matter ultrastructural damage, not disability. Neurology 83, 1417–1423. doi: 10.1212/wnl.0000000000000882
Carnes-Vendrell, A., Deus, J., Molina-Seguin, J., Pifarré, J., and Purroy, F. (2019). Depression and apathy after transient ischemic attack or minor stroke: prevalence, evolution and predictors. Sci. Rep. 9, 16248. doi: 10.1038/s41598-019-52721-5
Chi, C., Huang, Y., Ye, S., Shao, M., Jiang, M., Yang, M., et al. (2021). Interleukin-10 level is associated with post-stroke depression in acute ischaemic stroke patients. J. Affect. Disord. 293, 254–260. doi: 10.1016/j.jad.2021.06.037
ESO (2008). Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc. Dis. 25, 457–507. doi: 10.1159/000131083
Folstein, M., Maiberger, R., and McHugh, P. (1977). Mood disorder as a specific complication of stroke. J. Neurol. Neurosurg. Psychiatry 40, 1018–1020. doi: 10.1136/jnnp.40.10.1018
Gabaldón, L., Fuentes, B., Frank-García, A., and Díez-Tejedor, E. (2007). Poststroke depression: importance of its detection and treatment. Cerebrovasc. Dis. 181–188. doi: 10.1159/000107394
Gu, Y., Zhao, P., Feng, W., Xia, X., Tian, X., Yan, Y., et al. (2022). Structural brain network measures in elderly patients with cerebral small vessel disease and depressive symptoms. BMC Geriatr. 22, 568. doi: 10.1186/s12877-022-03245-7
Hackett, M., and Anderson, C. (2005). Predictors of depression after stroke: a systematic review of observational studies. Stroke 36, 2296–2301. doi: 10.1161/01.STR.0000183622.75135.a4
Hackett, M., and Pickles, K. (2014). Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int. J. Stroke 9, 1017–1025. doi: 10.1111/ijs.12357
He, J., Zhang, Y., Lu, W., Liang, H., Tu, X., Ma, F., et al. (2017). Age-related frontal periventricular white matter hyperintensities and miR-92a-3p are associated with early-onset post-stroke depression. Front. Aging Neurosci. 9, 328. doi: 10.3389/fnagi.2017.00328
House, A., Knapp, P., Bamford, J., and Vail, A. (2001). Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke 32, 696–701. doi: 10.1161/01.str.32.3.696
Huang, J., Zhou, F., Guan, B., Zhang, N., Wang, A., Yu, P., et al. (2018). Predictors of remission of early-onset poststroke depression and the interaction between depression and cognition during follow-up. Front. Psychiatry 9, 738. doi: 10.3389/fpsyt.2018.00738
Jiang, J., Huang, X., Zhang, Y., Deng, W., Shen, F., Liu, J., et al. (2019). Total MRI burden of cerebral vessel disease correlates with the progression in patients with acute single small subcortical strokes. Brain Behav. 9, e01173. doi: 10.1002/brb3.1173
Lan, Y., Pan, C., Qiu, X., Miao, J., Sun, W., Li, G., et al. (2022). Nomogram for persistent post-stroke depression and decision curve analysis. Clin. Interv. Aging 17, 393–403. doi: 10.2147/cia.S357639
Lanctôt, K., Lindsay, M., Smith, E., Sahlas, D., Foley, N., Gubitz, G., et al. (2020). Canadian stroke best practice recommendations: mood, cognition and fatigue following stroke, 6th edition update 2019. Int. J. Stroke 15, 668–688. doi: 10.1177/1747493019847334
Lau, K., Li, L., Schulz, U., Simoni, M., Chan, K., Ho, S., et al. (2017). Total small vessel disease score and risk of recurrent stroke: validation in 2 large cohorts. Neurology 88, 2260–2267. doi: 10.1212/wnl.0000000000004042
Li, G., Jing, P., Chen, G., Mei, J., Miao, J., Sun, W., et al. (2021). Development and validation of 3-month major post-stroke depression prediction nomogram after acute ischemic stroke onset. Clin. Interv. Aging 16, 1439–1447. doi: 10.2147/cia.S318857
Liang, Y., Chan, Y., Deng, M., Chen, Y., Mok, V., Wang, F., et al. (2018a). Enlarged perivascular spaces in the centrum semiovale are associated with poststroke depression: a 3-month prospective study. J. Affect. Disord. 228, 166–172. doi: 10.1016/j.jad.2017.11.080
Liang, Y., Chen, Y., Liu, Y., Mok, V., Ungvari, G., Chu, W., et al. (2019). Exploring causal pathways linking cerebral small vessel diseases burden to poststroke depressive symptoms with structural equation model analysis. J. Affect. Disord. 253, 218–223. doi: 10.1016/j.jad.2019.04.092
Liang, Y., Chen, Y., Mok, V., Wang, D., Ungvari, G., Chu, W., et al. (2018b). Cerebral small vessel disease burden is associated with poststroke depressive symptoms: a 15-month prospective study. Front. Aging Neurosci. 10, 46. doi: 10.3389/fnagi.2018.00046
Licata, G., Tuttolomondo, A., Corrao, S., Di Raimondo, D., Fernandez, P., Caruso, C., et al. (2006). Immunoinflammatory activation during the acute phase of lacunar and non-lacunar ischemic stroke: association with time of onset and diabetic state. Int. J. Immunopathol. Pharmacol. 19, 639–646. doi: 10.1177/039463200601900320
Liu, X., Li, T., Diao, S., Cai, X., Kong, Y., Zhang, L., et al. (2019). The global burden of cerebral small vessel disease related to neurological deficit severity and clinical outcomes of acute ischemic stroke after IV rt-PA treatment. Neurol. Sci. 40, 1157–1166. doi: 10.1007/s10072-019-03790-x
Luan, X., Cheng, H., Chen, Y., Cheng, L., Zhou, S., Song, J., et al. (2020). High levels of plasma fibrinogen and prothrombin time are related to post-stroke emotional impairment. Brain Res. 1748, 147017. doi: 10.1016/j.brainres.2020.147017
Mittal, V., and Walker, E. (2011). Diagnostic and statistical manual of mental disorders. J. Psychiatric Res. 189, 158–159. doi: 10.1016/j.psychres.2011.06.006
Monteiro, A., Castro, P., Pereira, G., Ferreira, C., Sorond, F., Milstead, A., et al. (2021). Neurovascular coupling is impaired in hypertensive and diabetic subjects without symptomatic cerebrovascular disease. Front. Aging Neurosci. 13, 728007. doi: 10.3389/fnagi.2021.728007
Muresanu, D. F., Popa-Wagner, A., Stan, A., Buga, A. M., and Popescu, B. O. (2014). The vascular component of Alzheimer's disease. Curr. Neurovasc. Res. 11, 168–176. doi: 10.2174/1567202611666140408105333
Pantoni, L. (2010). Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701. doi: 10.1016/s1474-4422(10)70104-6
Pasquier, F., Leys, D., Weerts, J., Mounier-Vehier, F., Barkhof, F., Scheltens, P., et al. (1996). Inter- and intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur. Neurol. 36, 268–272. doi: 10.1159/000117270
Pavlovic, A., Pekmezovic, T., Zidverc Trajkovic, J., Svabic Medjedovic, T., Veselinovic, N., Radojicic, A., et al. (2016). Baseline characteristic of patients presenting with lacunar stroke and cerebral small vessel disease may predict future development of depression. Int. J. Geriatr. Psychiatric 31, 58–65. doi: 10.1002/gps.4289
Pohjasvaara, T., Vataja, R., Leppävuori, A., Kaste, M., and Erkinjuntti, T. (2001). Depression is an independent predictor of poor long-term functional outcome post-stroke. Eur. J. Neurol. 8, 315–319. doi: 10.1046/j.1468-1331.2001.00182.x
Popa-Wagner, A., Buga, A. M., Popescu, B., and Muresanu, D. (2015). Vascular cognitive impairment, dementia, aging and energy demand. A vicious cycle. J. Neural Transm. (Vienna) 122 Suppl 1, S47–54. doi: 10.1007/s00702-013-1129-3
Qiao, J., Sui, R., Zhang, L., and Wang, J. (2020). Construction of a risk model associated with prognosis of post-stroke depression based on magnetic resonance spectroscopy. Neuropsychiatr. Dis. Treat 16, 1171–1180. doi: 10.2147/ndt.S245129
Robinson, R., Shoemaker, W., Schlumpf, M., Valk, T., and Bloom, F. E. (1975). Effect of experimental cerebral infarction in rat brain on catecholamines and behaviour. Nature 255, 332–334. doi: 10.1038/255332a0
Santos, M., Gold, G., Kövari, E., Herrmann, F., Bozikas, V., Bouras, C., et al. (2009a). Differential impact of lacunes and microvascular lesions on poststroke depression. Stroke 40, 3557–3562. doi: 10.1161/strokeaha.109.548545
Santos, M., Kövari, E., Gold, G., Bozikas, V., Hof, P., Bouras, C., et al. (2009b). The neuroanatomical model of post-stroke depression: towards a change of focus? J. Neurol. Sci. 283, 158–162. doi: 10.1016/j.jns.2009.02.334
Spalletta, G., Bossù, P., Ciaramella, A., Bria, P., Caltagirone, C., and Robinson, R. G. (2006). The etiology of poststroke depression: a review of the literature and a new hypothesis involving inflammatory cytokines. Mol. Psychiatry 11, 984–991. doi: 10.1038/sj.mp.4001879
Staals, J., Booth, T., Morris, Z., Bastin, M., Gow, A., Corley, J., et al. (2015). Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol. Aging 36, 2806–2811. doi: 10.1016/j.neurobiolaging.2015.06.024
Staals, J., Makin, S., Doubal, F., Dennis, M., and Wardlaw, J. (2014). Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 83, 1228–1234. doi: 10.1212/wnl.0000000000000837
Tang, W., Chen, Y., Lu, J., Chu, W., Mok, V., Ungvari, G., et al. (2010). White matter hyperintensities in post-stroke depression: a case control study. J. Neurol. Neurosurg. Psychiatry 81, 1312–1315. doi: 10.1136/jnnp.2009.203141
Tang, W., Chen, Y., Lu, J., Chu, W., Mok, V., Ungvari, G., et al. (2011). Cerebral microbleeds and symptom severity of post-stroke depression: a magnetic resonance imaging study. J. Affect. Disord. 129, 354–358. doi: 10.1016/j.jad.2010.08.007
Tang, W., Liu, X., Chen, Y., Abrigo, J., Chu, W., Mok, V., et al. (2014). Pontine microbleeds and depression in stroke. J. Geriatr. Psychiatry Neurol. 27, 159–164. doi: 10.1177/0891988714522699
Tateno, A., Kimura, M., and Robinson, R. G. (2002). Phenomenological characteristics of poststroke depression: early-versus late-onset. Am. J. Geriatr. Psychiatry 10, 575–582. doi: 10.1097/00019442-200209000-00011
van Agtmaal, M., Houben, A., Pouwer, F., Stehouwer, C., and Schram, M. (2017). Association of microvascular dysfunction with late-life depression: a systematic review and meta-analysis. JAMA 74, 729–739. doi: 10.1001/jamapsychiatry.2017.0984
Wardlaw, J., Smith, C., and Dichgans, M. (2013). Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 12, 483–497. doi: 10.1016/s1474-4422(13)70060-7
Willey, J. Z., Disla, N., Moon, Y. P., Paik, M. C., Sacco, R. L., Boden-Albala, B., et al. (2010). Early depressed mood after stroke predicts long-term disability: the Northern Manhattan Stroke Study (NOMASS). Stroke 41, 1896–1900. doi: 10.1161/strokeaha.110.583997
Williams, L., Ghose, S., and Swindle, R. (2004). Depression and other mental health diagnoses increase mortality risk after ischemic stroke. Am. J. Psychiatry 161, 1090–1095. doi: 10.1176/appi.ajp.161.6.1090
Wu, Y., Chen, H., Liu, X., Cai, X., Kong, Y., Wang, H., et al. (2020). A new nomogram for individualized prediction of the probability of hemorrhagic transformation after intravenous thrombolysis for ischemic stroke patients. BMC Neurol. 20, 426. doi: 10.1186/s12883-020-02002-w
Yang, Q., Wei, X., Deng, B., Chang, Z., Jin, D., Huang, Y., et al. (2022). Cerebral small vessel disease alters neurovascular unit regulation of microcirculation integrity involved in vascular cognitive impairment. Neurobiol. Dis. 170, 105750. doi: 10.1016/j.nbd.2022.105750
Yuan, H. W., Wang, C. X., Zhang, N., Bai, Y., Shi, Y. Z., Zhou, Y., et al. (2012). Poststroke depression and risk of recurrent stroke at 1 year in a Chinese cohort study. PLoS ONE 7, e46906. doi: 10.1371/journal.pone.0046906
Zhang, X., Tang, Y., Xie, Y., Ding, C., Xiao, J., Jiang, X., et al. (2017). Total magnetic resonance imaging burden of cerebral small-vessel disease is associated with post-stroke depression in patients with acute lacunar stroke. Eur. J. Neurol. 24, 374–380. doi: 10.1111/ene.13213
Zhao, F., Yue, Y., Li, L., Lang, S., Wang, M., Du, X., et al. (2018). Clinical practice guidelines for post-stroke depression in China. Braz. J. Psychiatry 40, 325–334. doi: 10.1590/1516-4446-2017-2343
Keywords: cerebral small vessel disease burden, early-onset depression, acute ischemic stroke, nomogram, prediction
Citation: Zhou L, Chen L, Ma L, Diao S, Qin Y, Fang Q and Li T (2022) A new nomogram including total cerebral small vessel disease burden for individualized prediction of early-onset depression in patients with acute ischemic stroke. Front. Aging Neurosci. 14:922530. doi: 10.3389/fnagi.2022.922530
Received: 18 April 2022; Accepted: 24 August 2022;
Published: 27 September 2022.
Edited by:
Hua Zhang, Shandong First Medical University, ChinaReviewed by:
Chaur-Jong Hu, Taipei Medical University, TaiwanCopyright © 2022 Zhou, Chen, Ma, Diao, Qin, Fang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qi Fang, ZmFuZ3FpXzAwOEAxMjYuY29t; Tan Li, NTA0NTM1NDczQHFxLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.