- 1The Fourth Hospital of Chengdu, Chengdu, China
- 2Qingdao Mental Health Center, Qingdao, China
- 3Department of Psychiatry and Behavioral Sciences, University of Texas Health Science Center at Houston, Houston, TX, United States
- 4Department of Psychiatry, First Hospital/First Clinical Medical College of Shanxi Medical University, Taiyuan, China
- 5Beijing HuiLongGuan Hospital, Peking University, Beijing, China
- 6Department of Psychiatry, The Affiliated Brain Hospital of Guangzhou Medical University, Guangzhou, China
- 7Guangdong Engineering Technology Research Center for Translational Medicine of Mental Disorders, Guangzhou, China
- 8Department of Psychology, University of Chinese Academy of Sciences, Beijing, China
FOXP2, cognitive deficits, and schizophrenia are associated with neurodegenerative pathophyisiology. Mounting evidence suggests that body mass index (BMI) and FOXP2 may contribute to cognitive deficits in schizophrenia. However, the sex difference in the contribution of FOXP2 and BMI, as well as their potential interaction with cognitive deficits in schizophrenia, have not been investigated. A total of 867 schizophrenia patients and 402 controls were recruited. Cognitive function was assessed using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS). The polymorphism rs10447760 of the FOXP2 gene was genotyped. Male schizophrenia patients had superior language performance compared to female patients (F = 17.83; pBonferroni < 0.0001). BMI was positively associated with language scores in male patients with schizophrenia (ß = 0.60, t = 3.30, p = 0.001), as well as in patients with schizophrenia who carried the FOXP2 rs10447760 CC genotype (ß = 0.53, t = 3.16, p = 0.002). Interestingly, this association was only found in male patients with schizophrenia who also carried the FOXP2 rs10447760 CC genotype (ß = 0.63, t = 3.44, p = 0.001). Our study reveals a sex difference in the language deficits of schizophrenia patients and shows sexual dimorphism in the contribution of FOXP2, BMI, and their interaction to cognitive deficits in patients with schizophrenia.
Introduction
Cognitive deficits, including in aspects of attention, memory, learning, language, and executive function, are an essential feature of schizophrenia—being displayed in 98% of patients (Keefe et al., 2005; Tripathi et al., 2018). These cognitive deficits can impinge on patient prognosis and quality of life (Harvey et al., 2009). Previous evidence has shown that cognitive deficits in schizophrenia may be an innate trait, as they can occur in the initial episode as well as in patients with chronic schizophrenia (Gerretsen et al., 2017). Cognitive deficits can also occur in the prodromal stages and throughout the illness course, despite the alleviation of symptoms with pharmacological therapy (Hughes et al., 2003; Kahn and Keefe, 2013).
Although it is recognized that certain patients are predisposed to cognitive deficits, the underlying multi-faceted cause of cognitive deficits in schizophrenia remains largely obscure (Toulopoulou et al., 2007). However, accumulating evidence suggests a genetic contribution to cognitive deficits in schizophrenia patients (Harvey et al., 2016). For instance, cognitive deficits have even been observed in the unaffected relatives of schizophrenia patients (Snitz et al., 2006). Currently, a primary hypothesis is that neurodevelopmental and neurodegenerative dysfunction underlies the etiology of both cognitive deficits and schizophrenia (Li et al., 2020). The forkhead-box P2 (FOXP2) gene, located on chromosome 7q31, which contributes to brain development and neuronal processes, was first identified as being involved in language and speech (Lai et al., 2001). It is well established that disturbances of speech and language processes are central components of both schizophrenia and cognitive deficits (Li et al., 2009). Furthermore, FOXP2 has been associated with schizophrenia in genome-wide association studies (Hagenaars et al., 2016). Our previous studies have reported that one of the FOXP2 polymorphisms, rs10447760 within the 5’ regulatory region, is associated with clinical outcomes and cognitive deficits in chronic schizophrenia (Rao et al., 2017; Lang et al., 2019). Additionally, FOXP2 has been associated with obesity in a genome-wide association study (Glessner et al., 2010; Clifton et al., 2018). Collectively, this evidence suggests that FOXP2 contributes to both cognitive deficits and obesity in patients with schizophrenia.
In addition, it is now well established that critical, non-genetic aspects, such as metabolic syndrome and obesity, also contribute to cognitive deficits in schizophrenia patients (Rashid et al., 2013; Bora et al., 2017). Prior studies have also suggested a relationship between body mass index (BMI) and cognitive deficits (Smith et al., 2011). The frequency of higher BMI and obesity are greater in schizophrenic patients overall (Li et al., 2017), and the association of BMI with cognitive deficits in schizophrenia has also been established (Bora et al., 2017). However, other studies have reported inconsistent findings (Friedman et al., 2010; Takayanagi et al., 2012).
The above-mentioned inconsistent results may be due to the possible confounding interactions of sex, BMI, and genetic background. Increasing evidence has indicated that there are sex differences in clinical characteristics and outcomes, especially cognitive deficits, among schizophrenia patients across (Leger and Neill, 2016). Sex differences in brain structure and function, as well as sexually dimorphic steroid hormones, may contribute to the sex-specific effects on cognitive deficits in schizophrenic patients (Mendrek and Mancini-Marie, 2016). Previous studies have demonstrated different cognitive deficits between male and female patients with schizophrenia during their first and/or chronic episodes that were not observed in healthy controls (Leger and Neill, 2016; Mendrek and Mancini-Marie, 2016). However, the results are still equivocal. For example, several studies did not find an association between sex and cognitive deficits in patients with schizophrenia (Mendrek and Mancini-Marie, 2016).
Although our previous study has shown that FOXP2 polymorphisms (rs10447760) is associated with cognitive deficits in chronic schizophrenia (Lang et al., 2019), it remains unclear whether there are sex differences in the degree of contribution of genetic factors to cognitive deficits in schizophrenia; whether there are sex differences in the influences of BMI on cognitive deficits in schizophrenia; and/or whether there are sex differences in the effects of genetic contributions and BMI on cognitive deficits in schizophrenia. These interesting questions prompt a need for further investigation.
Some previous evidence has demonstrated that sex differences in cognitive performance are associated with certain genetic backgrounds (Zhang et al., 2010; Jancke, 2018). Additionally, recent studies have shown that cognitive function and BMI may share a common genetic pathway (Davies et al., 2015; Marioni et al., 2016). Prior research has also suggested the existence of a shared genetic mutation that is associated with both BMI and cognitive performance (Marioni et al., 2016). Frazier-Wood et al. (2014) found that shared genetic factors contributed to cognitive function and BMI in a study of 1,312 twins. Additionally, Laitala et al. (2011) performed a study with a large sample of 2,606 twins which also showed a significant shared genetic influence on the correlation between cognitive decline and midlife BMI. The above evidence suggests that interactions between sex, genetic contribution, and BMI are involved in the pathophysiology of cognitive deficits.
Therefore, to the best of our knowledge, this study is the first to investigate: (1) whether the FOXP2 polymorphism rs10447760 affects cognition differently as a function of sex; (2) whether BMI was associated with cognitive deficits in schizophrenia patients; (3) and whether any relationship between BMI and cognitive deficits in schizophrenia was further altered by sex.
Materials and Methods
Subjects
The protocol was approved by the ethics committee of the Beijing Huilongguan Hospital (No. BJ-7072035; Date: July 10th, 2016). Each subject or guardian signed a written informed consent prior to being enrolled in the study. The inclusion criteria have been described in previous studies (Lang et al., 2019), but, briefly, include: (1) patients must be between 18 and 75 years old; (2) patients must be of Han Chinese descent; (3) patients met diagnostic criteria for schizophrenia, as defined by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV); (4) patients experienced disease courses of at least 5 years; (5) patients had been treated mainly with monotherapy of an antipsychotic drug for at least 12 months.
Healthy controls without a family history of psychotic disorders were recruited through local advertisements. Individuals were excluded if they had any other major Axis I disorders.
Any patients who were pregnant or experiencing severe physical diseases, such as cardiovascular disease, cerebrovascular disease, cancer, infection, or unstable diabetes, were excluded. Any patient who had alcohol or drug abuse/dependence, as determined by laboratory urine tests, was excluded.
A total of 867 patients and 402 healthy controls were recruited. The demographic information of patients has been described in our previous studies. Briefly, there were differences in sex, BMI, and education between patients and healthy controls (Lang et al., 2019).
The antipsychotics that patients received have been described in our previous study but included clozapine, risperidone, quetiapine, chlorpromazine, sulpiride, aripiprazole, perphenazine, olanzapine, haloperidol, and other antipsychotics. The antipsychotic doses were calculated by being equivalent to chlorpromazine (Lang et al., 2019).
Clinical interview assessments
All participants were interviewed independently by two psychiatrists using the Structured Clinical Interview for DSM-IV (SCID-I/P). We also used the Positive and Negative Symptoms Scale (PANSS) to assess clinical symptoms. Cognitive performance was evaluated using the Chinese version of the Assessment of Neuropsychological Status (RBANS, Form A).
DNA isolation and SNP genotyping
Peripheral venous blood was sampled, and DNA was extracted using a salting-out method followed by preservation at −80°C (Rao et al., 2017).
The polymorphism rs10447760 of FOXP2 was genotyped using the methods described before (Rao et al., 2017). 5% of the samples were randomly selected for repeated genotyping as a quality control measure.
Statistical analyses
The normality of variable distribution was assessed using the Kolmogorov-Smirnov test. Chi-square tests and student’s t-tests were used, for categorical variables and continuous variables, respectively. Hardy–Weinberg equilibrium was detected using a goodness of fit of χ2 test.
To investigate the effects of differing antipsychotics on cognitive scores in patients, we conducted a multiple analysis of covariance (MANCOVA). We chose this approach to address the overall p-value because we thought it would reduce type I error, while also adjusting for age, BMI, smoking, education, onset age, illness duration, hospitalization times, antipsychotic duration, and daily dose. Then, an analysis of covariance (ANCOVA) was performed to examine the effects of different antipsychotics on the cognitive score, while adjusting for the same parameters as in the primary MANCOVA.
To identify the effects of sex on cognitive scores in patients and healthy controls, a 2 × 2 MANCOVA (sex × diagnosis) was conducted. All of the RBANS subscores were dependent variables, and diagnosis and sex were fixed factors. We also adjusted for age, BMI, smoking, and education level. Then, an ANCOVA was performed with individual RBANS scores as the dependent variable. In this model, sex and diagnosis were the fixed factors, and we adjusted for age, BMI, smoking, and education in the control group, and for onset age, hospitalization time, antipsychotic types (atypical or typical antipsychotics), duration of treatment with antipsychotics and antipsychotics dose in the patient group. Stepwise multivariate regression analyses were conducted with each RBANS score as a dependent variable, where sex was an independent variable, and the same confounding covariates were controlled for patients.
To identify the effects of the FOXP2 genotype on the relationship between sex and cognitive scores in patients and healthy controls, we used 2 × 2 MANCOVAs (genotype × sex) separately in patient and control groups. All RBANS scores were set as dependent variables, with the genotype and sex as the independent variables, and adjustment for confounding covariates. We then used an ANCOVA with each RBANS score as a dependent variable, genotype, and sex as independent variables, and with separate adjustments for confounding covariates in the patient and control groups.
To explore the association between BMI and cognitive scores in the patients and controls separately, as well as in the different sex and genotypic groupings, we used partial correlations which adjusted for demographic and clinical covariates. We also carried out multivariate regression analyses, with each cognitive domain as a dependent variable, BMI as an independent variable, and controlling for confounding variables.
Bonferroni corrections were performed for multiple tests. The power of the sample in the present study was calculated using Quanto Software under dominant, log additive, and recessive models, and setting the prevalence of schizophrenia as 1% in the population. The statistical analyses were applied using SPSS version 25.0, and statistical significance was identified as a 2-tailed p-value that was less than 0.05.
Results
The effect of different antipsychotics on cognitive performance in patients
The MANCOVA demonstrated that there were no significant effects of differing antipsychotics on cognitive function (Wilks’ Lambda, F = 0.94, p = 0.61). Furthermore, the ANCOVA demonstrated that differing antipsychotics did not have different effects on immediate memory (F = 1.46, p = 0.16), visual space/structure (F = 0.85, p = 0.56), language (F = 0.51, p = 0.89), attention (F = 1.03, p = 0.42), delayed memory (F = 1.18, p = 0.31), or total RBANS scores (F = 1.13, p = 0.34).
Sex difference in cognitive scores of patients and controls
The 2 × 2 MANCOVA analysis (sex × diagnosis), which adjusted for age, BMI, and educational years, showed a main effect of sex on cognitive performance (Wilks’ lambda F = 8.01; p < 0.0001). Then, as shown in Table 1, after adjustments for age, BMI, and educational years, ANCOVA showed a main effect of sex on language performance (F = 25.13; corrected p < 0.0001). There was a significant effect of diagnosis × sex on language scores (F = 16.71, p < 0.001) which also survived Bonferroni correction. Further ANCOVA analysis of the patient group showed male patients with schizophrenia had better language performance than female patients (F = 17.83; p < 0.0001), after adjusting for confounding variables. This result also persisted after Bonferroni correction (p < 0.0001). Further multivariate regression analysis demonstrated that sex was correlated with language performance in patients (ß = −7.69, t = 4.22, p < 0.0001); that is, female patients performed worse than males did. In the control group, there were no significant differences in any of the RBANS subscale scores, or the total RBANS scores, between males and females (p > 0.05).
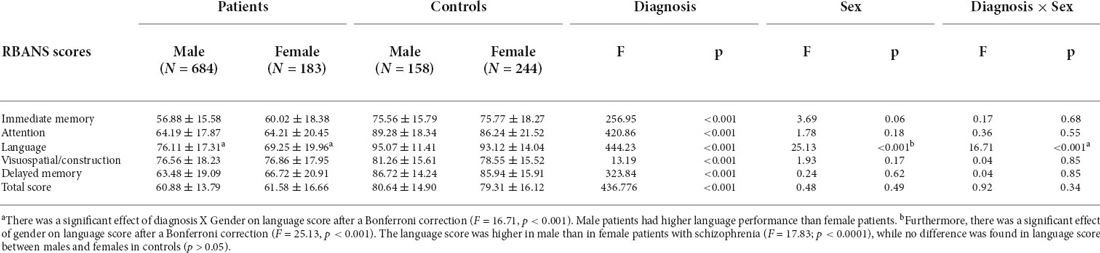
Table 1. Comparisons among the RBANS total and five subscale scores by diagnostic and sex groupings (Mean ± SD).
Sex differences in the effects of FOXP2 rs10447760 on cognitive scores in patients and controls
The 2 × 2 MANCOVA demonstrated no effects of genotype (Wilks’ lambda F = 1.41; p = 0.21), sex (Wilks’ lambda F = 1.71; p = 0.12), or genotype × sex (Wilks’ lambda F = 0.56; p = 0.77) on cognitive performance. ANCOVA analysis showed that there were genotype effects on immediate memory scores (F = 4.14, p = 0.04) and language scores (F = 4.89, p = 0.03; Table 2) in the patient group. However, the results did not persist after Bonferroni correction (p = 0.24, p = 0.18). No sex differences in the genotypic effects on BMI was found.
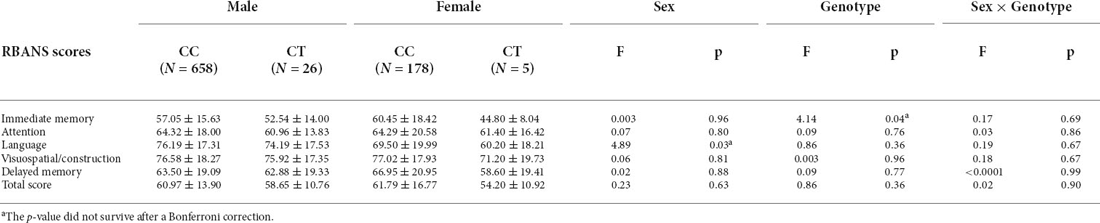
Table 2. Comparisons among the RBANS total and five subscale scores by gender and genotypic groupings in patient group (Mean ± SD).
In the control group, there were no effects of sex, genotype, or sex × genotype on any of the RBANS scores (all p > 0.05).
Sex difference in BMI of patients and healthy controls
There were no significant effects of sex or diagnosis × sex effects on BMI (F = 0.96, p = 0.33; F = 0.16, p = 0.69). Furthermore, there were no significant sex differences in BMI in any of the subjects, whether they were patients or healthy controls (all p’s > 0.05), which suggested that sex had no effects on BMI in patients or controls.
Sex difference in the association between BMI and cognitive scores in patients and controls
To further investigate whether BMI was associated with cognitive scores in different sexes, we further divided the patients into a male patient group and a female patient group. Additional multivariate regression analysis demonstrated a positive association of BMI with language performance in the male patient group (ß = 0.60, t = 3.30, p = 0.001), while other cognitive subscale scores and total scores showed no significant differences after adjusting for confounding variables and performing Bonferroni corrections (all p’s > 0.05).
In the healthy control group, BMI was negatively correlated with attention performance in the male control group (r = −0.26, p = 0.001), including after controlling confounding covariables (partial r = -0.21, partial p = 0.009). However, this did not persist after Bonferroni correction. Other cognitive subscale scores and total scores showed no significant associations with BMI after adjusting for confounding variables (all p’s > 0.05).
Sex differences in the association of BMI with cognitive performance: adjusted by FOXP2 rs10447760
To further explore the association between BMI and cognitive performance in different FOXP2 rs10447760 genotypes, we divided the patients into two genotypic groups: patients carrying CC and patients carrying CT. Multivariate regression analysis demonstrated a positive association between BMI and language scores in the patient group carrying the CC genotype (ß = 0.53, t = 3.16, p = 0.002). Interestingly, this positive association was found only in male patients (ß = 0.63, t = 3.44, p = 0.001), not in female patients (p > 0.05).
In the healthy control group, no association was found between BMI and any of the five RBANS subscale scores or total scores in either FOXP2 genotypic subgroup, after adjusting for confounding variables and performing Bonferroni corrections (all p’s > 0.05).
Discussion
The main findings of this study were: (1) male schizophrenia patients had better language performance than female patients; (2) the FOXP2 rs10447760 genotype did not show an interaction with sex on cognitive performance in schizophrenia patients; (3) there was a positive correlation between BMI and language performance only in male schizophrenia patients; (4) after stratification by FOXP2 rs10447760 genotype, there was a positive correlation between BMI and language performance only in schizophrenia patients carrying the CC genotype.
Considering differences in hormonal status, psychosocial stress, and brain structure and function between male and female patients with schizophrenia, previous evidence has documented sex-specific effects on cognitive deficits in schizophrenia (Han et al., 2012). However, these results are still controversial. Most prior studies have shown that male schizophrenia patients have relatively worse performances on various cognitive dimensions (Han et al., 2012; Leger and Neill, 2016), but some studies have shown opposite results (Gogos et al., 2010; Leger and Neill, 2016). Additionally, other studies have shown that sex has no effect on cognitive changes in schizophrenia (Karilampi et al., 2011; Kao et al., 2013). Our results demonstrated better language cognitive performance in males compared to female patients with chronic schizophrenia. Previously, Lewine et al. (1996) found greater deficits in verbal and spatial memory in female patients with schizophrenia. They also found the worse performance of the right hemisphere than that of the left hemisphere, consistent with previous evidence showing right-hemispheric brain activation contributes to language function (van Ettinger-Veenstra et al., 2010).
We have previously reported that FOXP2 rs10447760 affects immediate memory in chronic schizophrenia (Lang et al., 2019). However, we did not find any effects of FOXP2 rs10447760 on any cognitive scores across sexes, indicating that there were no interactive effects of FOXP2 rs10447760 and sex on cognitive scores in schizophrenia. Few prior studies have explored the interplay of sex and gene polymorphism on cognitive deficits in schizophrenia. One previous study found significant sex interactions when assessing the relationship between BDNF AL66Met polymorphism, another neurotrophic factor, and cognitive performance (Kim et al., 2016). This evidence suggests that different genes, or even different polymorphisms in the same gene, can lead to different sex-specific interactions on cognitive functioning.
Higher BMI and obesity are common in schizophrenia patients. However, any effects of BMI on cognitive performance within schizophrenia patients are unknown (Bora et al., 2017). Previous findings have suggested that the influence of BMI on cognitive deficits in schizophrenia is complex (Friedman et al., 2010; Takayanagi et al., 2012; Rashid et al., 2013; Hidese et al., 2018). Other aspects, including sex and genetic background, may be involved in the association between BMI and cognitive deficits in schizophrenia patients (Rashid et al., 2013). A previous study by our group also showed that NRG3 polymorphism altered the effect of BMI on cognitive deficits of patients with schizophrenia (Zhou et al., 2020). Here, we showed that there was a positive association between BMI and language performance in schizophrenia patients, even after adjusting for demographic and clinical covariables. Interestingly, this positive association was found in male patients, but not in female patients. It seems likely that BMI affects cognitive deficits as a function of sex in schizophrenia. Previously, one study demonstrated that increased BMI correlated with worse executive function and attention in male patients with heart failure, but not in female patients, which also indicated an interacting sex effect on the relationship between BMI and cognitive function (Hawkins et al., 2014).
FOXP2 plays a critical role in the neuronal processes that contribute to language and speech performance (Liegeois et al., 2003; Vernes et al., 2007), and its polymorphisms have been reported to be involved in the pathophysiology of cognitive function and language phenotypes in schizophrenia (Tolosa et al., 2010; Mozzi et al., 2017). However, we did not find any associations between the FOXP2 rs10447760 genotype and language scores in our previous study (Lang et al., 2019), and a recent meta-analysis also showed no association between FOXP2 variations and language deficits in schizophrenia (McCarthy et al., 2019). These inconsistent findings might be the result of foregoing consideration of other crucial demographic or clinical characteristics, including BMI and sex. In this study, although we did not observe an interactive effect between sex and FOXP2 rs10447760 on cognitive deficits, we did find that FOXP2 rs10447760 interplayed with BMI to affect cognitive changes in schizophrenia patients: There was a positive correlation of BMI with language function only in schizophrenia patients with the FOXP2 rs10447760 CC genotype. This suggests that BMI might affect cognitive deficits in schizophrenia as a condition of specific FOXP2 genotypes. FOXP2 has also been associated with obesity in previous studies (Glessner et al., 2010; Xia and Grant, 2013; Clifton et al., 2018). Accumulating evidence indicates there is a shared genetic pathway or system for cognitive performance and BMI (Marioni et al., 2016). Marioni et al. (2016) demonstrated that individual genetic mutations correlating with BMI account for a critical component of the variance in cognitive performance, and vice versa. They also showed that genetic variants leading to higher cognitive performance correlated with lower BMI (Marioni et al., 2016). In addition, some studies have also suggested that genetic factors contribute highly to cognitive function and BMI phenotypes. For example, Frazier-Wood et al. (2014) reported between 20% and 30% shared genetic variance of cognitive function and BMI of 20%–30% (Laitala et al., 2011). Additionally, these shared genetic pathways or systems appear to display overlapping gene expression profiles in specific areas of the brain (Locke et al., 2015). Our present results provide further evidence of the shared genetic influences underlying BMI and cognitive function.
The present study has some limitations. First, we recruited patients with chronic schizophrenia and with different antipsychotic treatment regimens. However, several lines of evidence indicate that the style and severity of cognitive deficits are fundamentally stable. In addition, cognitive deficits were independent of illness duration and remained relatively stable without changes over time (Harvey et al., 1990; Bowie and Harvey, 2006). Furthermore, the different antipsychotics used all converted to chlorpromazine. Second, although we recruited relatively larger samples, unequal sample sizes between the patient and control groups, as we had here, may cause type II error. Furthermore, there were unequal sample sizes between males and females within patient or healthy controls, and unequal sample sizes between FOXP2 CC and FOXP2 CT genotypes within the patient group. Further studies in first episode drug naïve schizophrenia patients, and studies with equal sample sizes between patients and controls, should be conducted to validate our results. With regard to the unequal sample sizes between FOXP2 C and T alleles within subjects, FOXP2 rs10447760 has the rare variant allele T in the Asian population. Thus, a small number of subjects with CT genotype may dramatically change the significance of p-value. Last but not least, the differences in sex, BMI, and education between patients and healthy controls might influence the results, although these factors have been adjusted for.
In conclusion, the present study showed sex differences in language deficits in schizophrenia patients. Furthermore, sexual dimorphism and FOXP2 rs10447760 may impact the influence of BMI on language-based cognitive deficits in schizophrenia patients.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: https://ngdc.cncb.ac.cn/gsub/submit/bioproject/list,subSAM098688.
Ethics Statement
The studies involving human participants were reviewed and approved and the protocol was approved by the ethics committee of the Beijing Huilongguan Hospital (No. BJ-7072035). The patients/participants provided their written informed consent to participate in this study.
Authors Contributions
ZL, MXi, and XZ designed the study. ZL, MF, MXu, and YC wrote the article. MY and XL did the statistical analysis. All authors contributed to the article and approved the submitted version.
Funding
This research was funded by National Natural Science Foundation of China (81401127, 62073058), Chengdu Municipal Health Commission (2021057), Open Project Program of State Key Laboratory of Virtual Reality Technology and Systems, Beihang University (No. VRLAB2022 B02), and Shanghai Key Laboratory of Psychotic Disorders Open Grant (21-K03). Funding had no role in study design, data analysis, or the decision to submit the article for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all of the patients and healthy volunteers who participated in this research. We also thank Dr. Dachun Chen, Song Chen, and Gui Gang Yang for all of their hard work and significant contributions to this study.
References
Bora, E., Akdede, B. B., and Alptekin, K. (2017). The relationship between cognitive impairment in schizophrenia and metabolic syndrome: a systematic review and meta-analysis. Psychol. Med. 47, 1030–1040. doi: 10.1017/S0033291716003366
Bowie, C. R., and Harvey, P. D. (2006). Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr. Dis. Treat. 2, 531–536. doi: 10.2147/nedt.2006.2.4.531
Clifton, E. A. D., Perry, J. R. B., Imamura, F., Lotta, L. A., Brage, S., Forouhi, N. G., et al. (2018). Genome-wide association study for risk taking propensity indicates shared pathways with body mass index. Commun. Biol. 1:36. doi: 10.1038/s42003-018-0042-6
Davies, G., Armstrong, N., Bis, J. C., Bressler, J., Chouraki, V., Giddaluru, S., et al. (2015). Genetic contributions to variation in general cognitive function: a meta-analysis of genome-wide association studies in the CHARGE consortium (N=53949). Mol. Psychiatry 20, 183–192. doi: 10.1038/mp.2014.188
Frazier-Wood, A. C., Carnell, S., Pena, O., Hughes, S. O., O’Connor, T. M., Asherson, P., et al. (2014). Cognitive performance and BMI in childhood: shared genetic influences between reaction time but not response inhibition. Obesity (Silver Spring) 22, 2312–2318. doi: 10.1002/oby.20862
Friedman, J. I., Wallenstein, S., Moshier, E., Parrella, M., White, L., Bowler, S., et al. (2010). The effects of hypertension and body mass index on cognition in schizophrenia. Am. J. Psychiatry 167, 1232–1239. doi: 10.1176/appi.ajp.2010.09091328
Gerretsen, P., Voineskos, A. N., Graff-Guerrero, A., Menon, M., Pollock, B. G., Mamo, D. C., et al. (2017). Insight into illness and cognition in schizophrenia in earlier and later life. J. Clin. Psychiatry 78, e390–e397. doi: 10.4088/JCP.16m10741
Glessner, J. T., Bradfield, J. P., Wang, K., Takahashi, N., Zhang, H., Sleiman, P. M., et al. (2010). A genome-wide study reveals copy number variants exclusive to childhood obesity cases. Am. J. Hum. Genet. 87, 661–666. doi: 10.1016/j.ajhg.2010.09.014
Gogos, A., Joshua, N., and Rossell, S. L. (2010). Use of the repeatable battery for the assessment of neuropsychological status (RBANS) to investigate group and gender differences in schizophrenia and bipolar disorder. Aust. N. Z. J. Psychiatry 44, 220–229. doi: 10.3109/00048670903446882
Hagenaars, S. P., Harris, S. E., Davies, G., Hill, W. D., Liewald, D. C., Ritchie, S. J., et al. (2016). Shared genetic aetiology between cognitive functions and physical and mental health in UK Biobank (N=112 151) and 24 GWAS consortia. Mol. Psychiatry 21, 1624–1632. doi: 10.1038/mp.2015.225
Han, M., Huang, X. F., Chen, D. C., Xiu, M. H., Hui, L., Liu, H., et al. (2012). Gender differences in cognitive function of patients with chronic schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 39, 358–363. doi: 10.1016/j.pnpbp.2012.07.010
Harvey, P. D., Aslan, M., Du, M., Zhao, H., Siever, L. J., Pulver, A., et al. (2016). Factor structure of cognition and functional capacity in two studies of schizophrenia and bipolar disorder: implications for genomic studies. Neuropsychology 30, 28–39. doi: 10.1037/neu0000245
Harvey, P. D., Docherty, N. M., Serper, M. R., and Rasmussen, M. (1990). Cognitive deficits and thought disorder: II. An 8-month followup study. Schizophr. Bull. 16, 147–156. doi: 10.1093/schbul/16.1.147
Harvey, P. D., Keefe, R. S., Patterson, T. L., Heaton, R. K., and Bowie, C. R. (2009). Abbreviated neuropsychological assessment in schizophrenia: prediction of different aspects of outcome. J. Clin. Exp. Neuropsychol. 31, 462–471. doi: 10.1080/13803390802251386
Hawkins, M. A., Gunstad, J., Dolansky, M. A., Redle, J. D., Josephson, R., Moore, S. M., et al. (2014). Greater body mass index is associated with poorer cognitive functioning in male heart failure patients. J. Card. Fail. 20, 199–206. doi: 10.1016/j.cardfail.2013.12.014
Hidese, S., Matsuo, J., Ishida, I., Hiraishi, M., Teraishi, T., Ota, M., et al. (2018). Relationship of handgrip strength and body mass index with cognitive function in patients with schizophrenia. Front. Psychiatry 9:156. doi: 10.3389/fpsyt.2018.00156
Hughes, C., Kumari, V., Soni, W., Das, M., Binneman, B., Drozd, S., et al. (2003). Longitudinal study of symptoms and cognitive function in chronic schizophrenia. Schizophr. Res. 59, 137–146. doi: 10.1016/s0920-9964(01)00393-0
Jancke, L. (2018). Sex/gender differences in cognition, neurophysiology and neuroanatomy. F1000Res 7:F1000 Faculty Rev-805. doi: 10.12688/f1000research.13917.1
Kahn, R. S., and Keefe, R. S. (2013). Schizophrenia is a cognitive illness: time for a change in focus. JAMA Psychiatry 70, 1107–1112. doi: 10.1001/jamapsychiatry.2013.155
Kao, Y. C., Liu, Y. P., Lien, Y. J., Lin, S. J., Lu, C. W., Wang, T. S., et al. (2013). The influence of sex on cognitive insight and neurocognitive functioning in schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 44, 193–200. doi: 10.1016/j.pnpbp.2013.02.006
Karilampi, U., Helldin, L., and Archer, T. (2011). Cognition and global assessment of functioning in male and female outpatients with schizophrenia spectrum disorders. J. Nerv. Ment. Dis. 199, 445–448. doi: 10.1097/NMD.0b013e318221413e
Keefe, R. S., Eesley, C. E., and Poe, M. P. (2005). Defining a cognitive function decrement in schizophrenia. Biol. Psychiatry 57, 688–691. doi: 10.1016/j.biopsych.2005.01.003
Kim, S. W., Lee, J. Y., Kang, H. J., Kim, S. Y., Bae, K. Y., Kim, J. M., et al. (2016). Gender-specific associations of the brain-derived neurotrophic factor Val66Met polymorphism with neurocognitive and clinical features in schizophrenia. Clin. Psychopharmacol. Neurosci. 14, 270–278. doi: 10.9758/cpn.2016.14.3.270
Lai, C. S. L., Fisher, S. E., Hurst, J. A., Vargha-Khadem, F., and Monaco, A. P. (2001). A forkhead-domain gene is mutated in a severe speech and language disorder. Nature 413, 519–523. doi: 10.1038/35097076
Laitala, V. S., Kaprio, J., Koskenvuo, M., Raiha, I., Rinne, J. O., and Silventoinen, K. (2011). Association and causal relationship of midlife obesity and related metabolic disorders with old age cognition. Curr. Alzheimer Res. 8, 699–706. doi: 10.2174/156720511796717186
Lang, X., Zhang, W., Song, X., Zhang, G., Du, X., Zhou, Y., et al. (2019). FOXP2 contributes to the cognitive impairment in chronic patients with schizophrenia. Aging (Albany NY) 11, 6440–6448. doi: 10.18632/aging.102198
Leger, M., and Neill, J. C. (2016). A systematic review comparing sex differences in cognitive function in schizophrenia and in rodent models for schizophrenia, implications for improved therapeutic strategies. Neurosci. Biobehav. Rev. 68, 979–1000. doi: 10.1016/j.neubiorev.2016.06.029
Lewine, R. R., Walker, E. F., Shurett, R., Caudle, J., and Haden, C. (1996). Sex differences in neuropsychological functioning among schizophrenic patients. Am. J. Psychiatry 153, 1178–1184. doi: 10.1176/ajp.153.9.1178
Li, Q., Du, X., Zhang, Y., Yin, G., Zhang, G., Walss-Bass, C., et al. (2017). The prevalence, risk factors and clinical correlates of obesity in Chinese patients with schizophrenia. Psychiatry Res. 251, 131–136. doi: 10.1016/j.psychres.2016.12.041
Li, X., Branch, C. A., and DeLisi, L. E. (2009). Language pathway abnormalities in schizophrenia: a review of fMRI and other imaging studies. Curr. Opin. Psychiatry 22, 131–139. doi: 10.1097/YCO.0b013e328324bc43
Li, Z., Liu, L., Lin, W., Zhou, Y., Zhang, G., Du, X., et al. (2020). NRG3 contributes to cognitive deficits in chronic patients with schizophrenia. Schizophr. Res. 215, 134–139. doi: 10.1016/j.schres.2019.10.060
Liegeois, F., Baldeweg, T., Connelly, A., Gadian, D. G., Mishkin, M., and Vargha-Khadem, F. (2003). Language fMRI abnormalities associated with FOXP2 gene mutation. Nat. Neurosci. 6, 1230–1237. doi: 10.1038/nn1138
Locke, A. E., Kahali, B., Berndt, S. I., Justice, A. E., Pers, T. H., Day, F. R., et al. (2015). Genetic studies of body mass index yield new insights for obesity biology. Nature 518, 197–206. doi: 10.1038/nature14177
Marioni, R. E., Yang, J., Dykiert, D., Mottus, R., Campbell, A., Group, C. C. W., et al. (2016). Assessing the genetic overlap between BMI and cognitive function. Mol. Psychiatry 21, 1477–1482. doi: 10.1038/mp.2015.205
McCarthy, N. S., Clark, M. L., Jablensky, A., and Badcock, J. C. (2019). No association between common genetic variation in FOXP2 and language impairment in schizophrenia. Psychiatry Res. 271, 590–597. doi: 10.1016/j.psychres.2018.12.016
Mendrek, A., and Mancini-Marie, A. (2016). Sex/gender differences in the brain and cognition in schizophrenia. Neurosci. Biobehav. Rev. 67, 57–78. doi: 10.1016/j.neubiorev.2015.10.013
Mozzi, A., Riva, V., Forni, D., Sironi, M., Marino, C., Molteni, M., et al. (2017). A common genetic variant in FOXP2 is associated with language-based learning (dis)abilities: evidence from two Italian independent samples. Am. J. Med. Genet. B Neuropsychiatr. Genet. 174, 578–586. doi: 10.1002/ajmg.b.32546
Rao, W., Du, X., Zhang, Y., Yu, Q., Hui, L., Yu, Y., et al. (2017). Association between forkhead-box P2 gene polymorphism and clinical symptoms in chronic schizophrenia in a Chinese population. J. Neural Transm. (Vienna) 124, 891–897. doi: 10.1007/s00702-017-1723-x
Rashid, N. A., Lim, J., Lam, M., Chong, S. A., Keefe, R. S., and Lee, J. (2013). Unraveling the relationship between obesity, schizophrenia and cognition. Schizophr. Res. 151, 107–112. doi: 10.1016/j.schres.2013.09.020
Smith, E., Hay, P., Campbell, L., and Trollor, J. N. (2011). A review of the association between obesity and cognitive function across the lifespan: implications for novel approaches to prevention and treatment. Obes. Rev. 12, 740–755. doi: 10.1111/j.1467-789X.2011.00920.x
Snitz, B. E., Macdonald, A. W., 3rd, and Carter, C. S. (2006). Cognitive deficits in unaffected first-degree relatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr. Bull. 32, 179–194. doi: 10.1093/schbul/sbi048
Takayanagi, Y., Cascella, N. G., Sawa, A., and Eaton, W. W. (2012). Diabetes is associated with lower global cognitive function in schizophrenia. Schizophr. Res. 142, 183–187. doi: 10.1016/j.schres.2012.08.034
Tolosa, A., Sanjuan, J., Dagnall, A. M., Molto, M. D., Herrero, N., and de Frutos, R. (2010). FOXP2 gene and language impairment in schizophrenia: association and epigenetic studies. BMC Med. Genet. 11:114. doi: 10.1186/1471-2350-11-114
Toulopoulou, T., Picchioni, M., Rijsdijk, F., Hua-Hall, M., Ettinger, U., Sham, P., et al. (2007). Substantial genetic overlap between neurocognition and schizophrenia: genetic modeling in twin samples. Arch. Gen. Psychiatry 64, 1348–1355. doi: 10.1001/archpsyc.64.12.1348
Tripathi, A., Kar, S. K., and Shukla, R. (2018). Cognitive deficits in schizophrenia: understanding the biological correlates and remediation strategies. Clin. Psychopharmacol. Neurosci. 16, 7–17. doi: 10.9758/cpn.2018.16.1.7
van Ettinger-Veenstra, H. M., Ragnehed, M., Hallgren, M., Karlsson, T., Landtblom, A. M., Lundberg, P., et al. (2010). Right-hemispheric brain activation correlates to language performance. Neuroimage 49, 3481–8. doi: 10.1016/j.neuroimage.2009.10.041
Vernes, S. C., Spiteri, E., Nicod, J., Groszer, M., Taylor, J. M., Davies, K. E., et al. (2007). High-throughput analysis of promoter occupancy reveals direct neural targets of FOXP2, a gene mutated in speech and language disorders. Am. J. Hum. Genet. 81, 1232–1250. doi: 10.1086/522238
Xia, Q., and Grant, S. F. (2013). The genetics of human obesity. Ann. N Y Acad. Sci. 1281, 178–190. doi: 10.1111/nyas.12020
Zhang, K., Gao, X., Qi, H., Li, J., Zheng, Z., and Zhang, F. (2010). Gender differences in cognitive ability associated with genetic variants of NLGN4. Neuropsychobiology 62, 221–228. doi: 10.1159/000319948
Keywords: sexual dimorphism, FOXP2, BMI, cognitive deficit, schizophrenia
Citation: Yang M, Cui Y, Xue M, Forster MT, Lang X, Xiu MH, Li Z and Zhang XY (2022) Sexual dimorphism in the relationship between Forkhead-box P2 and BMI with cognitive deficits in schizophrenia. Front. Aging Neurosci. 14:920352. doi: 10.3389/fnagi.2022.920352
Received: 14 April 2021; Accepted: 06 July 2022;
Published: 03 August 2022.
Edited by:
Yuzhen Xu, Shandong First Medical University, ChinaReviewed by:
Qin Xu, National Institute of Allergy and Infectious Diseases (NIH), United StatesReviewed by:
Yu Wang, Tianjin Medical University General Hospital, ChinaCopyright © 2022 Yang, Cui, Xue, Forster, Lang, Xiu, Li and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zezhi Li, YmlvbHBzeWNoaWF0cnlAMTI2LmNvbQ==; Meihong Xiu, eGl1bWVpaG9uZzk3QDE2My5jb20=; Xiangyang Zhang, emhhbmd4eUBwc3ljaC5hYy5jbg==
 Mi Yang
Mi Yang Ying Cui2
Ying Cui2 Meihong Xiu
Meihong Xiu Zezhi Li
Zezhi Li Xiangyang Zhang
Xiangyang Zhang