
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 23 June 2022
Sec. Alzheimer's Disease and Related Dementias
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.912709
This article is part of the Research Topic Infection, Inflammation and Neurodegeneration: A Critical Path to Alzheimer’s disease, Volume II View all 5 articles
 Hongle Wu1,2,3†
Hongle Wu1,2,3† Wei Qiu4†
Wei Qiu4† Xiaofang Zhu3,5
Xiaofang Zhu3,5 Xiangfen Li2
Xiangfen Li2 Zhongcong Xie6
Zhongcong Xie6 Isabel Carreras7,8
Isabel Carreras7,8 Alpaslan Dedeoglu9,10
Alpaslan Dedeoglu9,10 Thomas Van Dyke11,12
Thomas Van Dyke11,12 Yiping W. Han13,14
Yiping W. Han13,14 Nadeem Karimbux5
Nadeem Karimbux5 Qisheng Tu3,5
Qisheng Tu3,5 Lei Cheng2*
Lei Cheng2* Jake Chen3,5,15,16*
Jake Chen3,5,15,16*Alzheimer’s Disease (AD) is the most common form of dementia in older adults and has a devastating impact on the patient’s quality of life, which creates a significant socio-economic burden for the affected individuals and their families. In recent years, studies have identified a relationship between periodontitis and AD. Periodontitis is an infectious/inflammatory disease that destroys the supporting periodontal structure leading to tooth loss. Dysbiosis of the oral microbiome plays a significant role in the onset and development of periodontitis exhibiting a shift to overgrowth of pathobionts in the normal microflora with increasing local inflammation. Fusobacterium nucleatum is a common pathogen that significantly overgrows in periodontitis and has also been linked to various systemic diseases. Earlier studies have reported that antibodies to F. nucleatum can be detected in the serum of patients with AD or cognitive impairment, but a causal relationship and a plausible mechanism linking the two diseases have not been identified. In this study, we conducted both in vivo and in vitro experiments and found that F. nucleatum activates microglial cells causing morphological changes, accelerated proliferation and enhanced expression of TNF-α and IL-1β in microglial cells. In our in vivo experiments, we found that F. nucleatum-induced periodontitis resulted in the exacerbation of Alzheimer’s symptoms in 5XFAD mice including increased cognitive impairment, beta-amyloid accumulation and Tau protein phosphorylation in the mouse cerebrum. This study may suggest a possible link between a periodontal pathogen and AD and F. nucleatum could be a risk factor in the pathogenesis of AD. We are currently further identifying the pathways through which F. nucleatum modulates molecular elements in enhancing AD symptoms and signs. Data are available via ProteomeXchange with identifier PXD033147.
Alzheimer’s Disease (AD) is the most common cause of dementia in older adults. Patients suffer from cognitive impairment and memory loss, and in later stages, language and visuospatial dysfunction seriously impact daily activities and quality of life (Lane et al., 2018; Weuve et al., 2018; Jia et al., 2020). Complications of AD include skin infections, organ failure, blood clots and other problems that can be fatal. AD is the main cause of dependency in the elderly over 65, which has created a huge public health burden (Jia et al., 2018, 2020). In United States, among people over 65, about 6.5 million suffer from AD in 2022 (Hebert et al., 2013; Alzheimer’s Association Report, 2022). The prevalence of this disease increases significantly with age; reaching nearly 33.2% in the elderly over the age of 85 (Alzheimer’s Association Report, 2022). With the increase in population age and life expectancy, the prevalence of AD will further increase, and it is expected to affect approximately 14 million people by 2060 in the United States (Abbayya et al., 2015; Alzheimer’s Association Report, 2022).
The main pathological manifestation of AD is senile plaque formation by abnormally folded beta-amyloid (Aβ) protein outside of neurons and neurofibrillary tangles. The proposed cause of Aβ plaques is hyperphosphorylated Tau protein inside neurons (Ando et al., 2020; Coomans et al., 2021), which leads to neurodegeneration in the brain (Karran et al., 2011; Yu et al., 2020). In addition, microbiota of host may also be a key factor that might influence the accumulation of beta-amyloid. Wang et al. (2015) found that intestinal microbiota GSPE metabolites could interfere the Aβ assembly by enter into the brain. “Microbiota-gut-brain axis” is a significant theory which tells us the close relationship between gut microbiota and brain function (Liu et al., 2020a; Doifode et al., 2021). Oral mictobiota as the indispensable part of host microbiome, more and more researchers put an eye on its impact on Alzheimer’s Disease and found the possible role that oral microbe could play on the onset and progress of Alzheimer’s Disease. However, the exact pathogenesis of AD is still unclear. Age, genetics and having a family history are the greatest risk factors for Alzheimer’s Disease (2020).
Inflammation is a key factor in the pathogenesis of AD. Pro-inflammatory cytokines produced by various immune-associated cells, including activated microglia, can further auto-stimulate through paracrine and/or autocrine pathways leading to increases in β-amyloid, p -Tau and pro-inflammatory molecules and finally, neurodegeneration (Akiyama et al., 2000; Cai et al., 2014). Here, we investigate microglia as they play a significant role in the progression of AD.
Periodontitis is the most common oral inflammatory disease and is the single greatest cause of adult tooth loss (Kinane et al., 2017). Periodontitis has a major impact on quality of life, but also poses a threat to the patient’s overall health (Eke et al., 2012). A number of studies describe a link between periodontitis and systemic diseases (Sampaio-Maia et al., 2016), including diabetes (Chapple et al., 2013), cardiovascular disease (Dietrich et al., 2013; Tonetti et al., 2013), respiratory disease, adverse pregnancy outcomes (He et al., 2015), cancer and nervous system diseases (Noble et al., 2009; Perera et al., 2016). In recent years, there have been an increasing number of studies focused on the association between periodontitis and AD suggesting that periodontitis can promote the initiation and progression of AD (Kantarci et al., 2020; Liccardo et al., 2020). Through a population-based retrospective matched-cohort study, Chen et al. found that having periodontitis for 10 or more years increased the risk of AD (Odds Ratio 1.707) (Chen et al., 2017).
The oral microbiota is the initiating factor of periodontitis and can bridge between periodontitis and other diseases (Kinane et al., 2017; Sanz et al., 2017). For example, Naoyuki found that periodontitis associated with Porphyromonas gingivalis can aggravate the pathological characteristics of AD, including promoting the accumulation of Aβ protein and aggravating cognitive impairment. The main mechanism suggested is that local periodontal inflammation can stimulate brain tissue inflammation (Ishida et al., 2017). In addition, studies have found that P. gingivalis can be detected in the brain tissue of patients with AD. The study also confirmed that the gingipain proteases of P. gingivalis are associated with AD lesions suggesting neurotoxicity. Gingipain inhibitor treatment was shown to effectively prevent bacterial infections in experimental mice, reduce inflammation in the nervous system, and reduce neuronal damage (Dominy et al., 2019).
Another important periodontal pathogen implicated in systemic diseases is Fusobacterium nucleatum (F. nucleatum) (Tefiku et al., 2020). Previous research has shown that F. nucleatum is one of the most common oral microbes associated with pregnancy complications (Han, 2011); F. nucleatum can be detected in patients with multiple systemic conditions, including head and neck infections (Brook, 2013; Fischer et al., 2015; Stergiopoulou and Walsh, 2016) and gastrointestinal diseases, such as appendicitis (Swidsinski et al., 2011, 2012; Zhong et al., 2014). Experimental evidence suggests that F. nucleatum increases host inflammation and promotes cancer metastasis, immune-evasion and drug resistance (Casasanta et al., 2020). However, only one study has reported a correlation between F. nucleatum and AD to date. The results of this study indicate that the concentration of antibodies to F. nucleatum in patients’ serum with mild cognitive impairment and/or AD is significantly higher than that in a healthy cohort (Sparks Stein et al., 2012). This cross-sectional clinical study provides evidence for the association, but no mechanism study was proposed.
In this study, we explore the impact of F. nucleatum on AD progression in both in vitro experiments and animal models providing the initial evidence of the possible association between a periodontal pathogen and AD and the insight into the underlying mechanisms (Figure 1A).
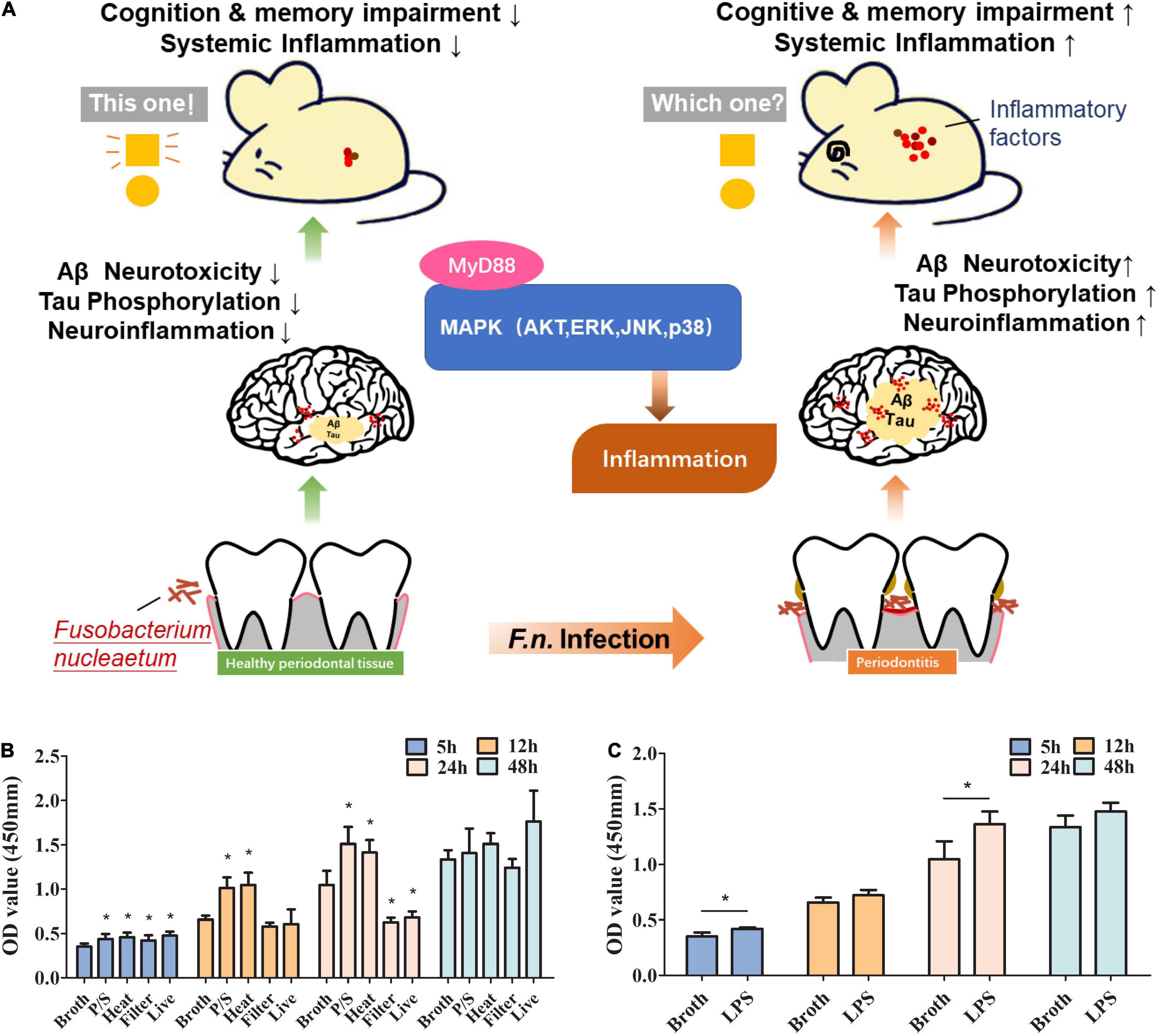
Figure 1. The roles of F. nucleatum in promoting proliferation of microglia in a study using an AD animal model. (A) Schematic diagram of the pathway that F. nucleatum exacerbates Alzheimer’s pathogenesis. (B) Evaluation of the effect of F. nucleatum has on the proliferation of SIM-A9 cells by CCK8 assay. Bacterial solution treated with the antibiotic, heating, or filtration, along with sterile bacteria broth and untreated bacterial solution were co-cultured with SIM-A9 for 5, 12, 24, and 48 h. (C) Evaluation of the effect of LPS of F. nucleatum on the proliferation of SIM-A9 cells by CCK8 assay. All data were expressed as mean ± SD.*P < 0.05 vs. Broth group.
This study was conducted in accordance with the policies of Tufts University. All mice used in this study were housed at the Tufts Medical Center Animal Facility (Boston, MA), which is fully accredited by the American Association for Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee (IACUC) approved all animal protocols for this study.
Fusobacterium nucleatum subsp. nucleatum (ATCC® 25586™) and SIM-A9 microglial cells (ATCC® CRL3265™) were obtained from ATCC (Manassas, VA, United States). Fusobacterium nucleatum was anaerobically cultured overnight in brain heart infusion broth (Difco, Sparks, MD, United States) containing hemin (0.5%) and vitamin K (0.1%) at 37°C (90% N2, 5% H2, 5% CO2) and adjusted to a concentration of 2 × 108CFU/ml. The experimental groups were designated as follow: P/S: F. nucleatum was incubated in broth containing 10% penicillin-streptomycin antibiotic mixture (PS, Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, United States) for 2 h at 37°C, the dead cells were centrifugated and resuspended in sterile broth; Heat: F. nucleatum was incubated in broth for 2 h at 80°C, and the dead cells were centrifugated and resuspended in sterile broth (Liu et al., 2020b); Filter: F. nucleatum culture supernatant was filtered with a 0.2 μm filter; Live: untreated F. nucleatum in culture medium. Sterile broth alone was used as a control (Broth). SIM-A9 cells were cultured in a 6-well plate or 96-well plate in 5% CO2 and 95% air at 37°C in complete growth medium: DMEF/12 medium containing 10% Fetal bovine serum and 5% Horse serum (Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, United States). One percent phosphatidyl serine (PS) was added. When the cells achieved 80% confluence, the medium was changed to complete growth medium without PS and bacteria were added (ratio of bacterial broth and cell medium was 1:10) and co-cultured for different periods for different experiments as indicated (Nagamoto-Combs et al., 2014).
The impact of F. nucleatum on SIM-A9 proliferation was assessed using the Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan) following the manufacturer’s instructions. After co-culture, cells were treated with 10 % CCK-8 for approximately 2 h. The optical density (OD) value was determined at 450 nm using a microplate reader to calculate cell proliferation.
The bacterial suspension (OD600nm = 0.8-1.2) was treated with proteinase K for 30 min at 50°C and LPS was extracted using an LPS extraction kit (iNtRON Biotechnology, United States) following the manufacturer’s instructions.
SIM A-9 cells were collected from plates and total RNA was extracted using a Quick-RNA Miniprep Kit (ZYMO Research, Irvine, CA, United States). 1 μg of total RNA was subjected to reverse transcription using M-MLV Reverse Transcriptase (Thermo Scientific, Waltham, MA, United States) according to the manufacturer’s protocol. For qRT-PCR, PowerUp SYBR Green Master Mix (Thermo Scientific) was used, and experiments conducted on a Bio-Rad iQ5 thermal cycler (Bio-Rad Laboratories, Hercules, CA, United States). Differences in expression were evaluated by the comparative cycle threshold method using GAPDH as a control. To extract total RNA from mouse tissues, TriZol reagent (Life Technologies) was added to homogenized tissue according to the manufacturer’s instructions followed by qRT-PCR assays as we described previously (Qiu et al., 2020).
B6 congenic 5XFAD transgenic (Tg) mice were obtained from Jackson Laboratories (MMRRC Stock No: 34848-JAX, Bar Harbor, ME, United States). 5XFAD transgenic mice overexpress both mutant human amyloid beta (A4) precursor protein 695 (APP) with the Swedish (K670N, M671L), Florida (I716V), and London (V717I) Familial AD (FAD) mutations and human PS1 harboring two FAD mutations, M146L and L286V. The expression of both transgenes is regulated by neural-specific elements of the mouse Thy1 promoter to drive overexpression in the brain. These mice exhibit plaque deposition at 2 months of age, along with increased microgliosis and astrocytosis (Oakley et al., 2006; Barrett et al., 2019). Wildtype B6 mice were also purchased from Jackson Laboratories (Stock No: 000664, Bar Harbor, ME, United States) as control.
To induce periodontitis, after ligature placement around maxillary second molars (left side) under anesthesia with ketamine and xylazine, 100 μL F. nucleatum (OD600nm = 1) suspension was applied topically to the buccal surface of the molars of 4-month-old mice (n = 5) every other day for 2 months under anesthesia as we previously described (Wu et al., 2019; Lian et al., 2020). The ligatures were checked every other day, replaced as necessary and kept in place until the end of F. nucleatum application. The uninfected group of mice was the same age, and 100 μL of sterile broth was applied to the molars without ligation at the same frequency.
Two months after induction of periodontitis, the animals were anesthetized with ketamine and xylazine and euthanized by transcranial perfusion with normal saline and 4% paraformaldehyde in phosphate-buffered saline (PBS, 0.1 M, pH 7.4). All the mice survived until the sample collection. The brain was dissected; one hemibrain was fixed in 4% formalin and dehydrated for histological analysis and the other was stored at −80°C for future analyses. Mouse whole blood was collected and immediately centrifuged at 4200 × g for 10 min to obtain the plasma for further use.
The novel object recognition task test was performed before, one and two months after induction of periodontitis. The apparatus consisted of a dark open field box (45 cm × 45 cm × 50 cm) according to the method of Lueptow (2017). Ethovision XT software (Noldus, Wageningen, Netherlands) was used to measure the patterns of movement, time spent on each item, velocity and distance traveled. These measurements were used as indicators of exploration.
Mouse brains were homogenized in liquid nitrogen and protein was extracted using RIPA Lysis and Extraction Buffer containing three protease inhibitors (Thermo Scientific, Waltham, MA, United States). Western blot analyses were performed as previously described (Qiu et al., 2020). Antibodies for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (cat# 2118,1:1,000), MyD88 (cat# 4283,1:1,000), p-P38 (cat# 9211,1:1,000), P38 (cat# 8690, 1:1,000) and p-Akt (cat# 4060, 1:500), were purchased from Cell Signaling Technology (Danvers, MA, United States), p-JNK (cat# sc-12882, 1:500), JNK (cat# sc-7345, 1:500), p-ERK (cat# sc-7383,1:500), ERK (cat# sc-514,302, 1:500) and Akt (cat# sc-81434, 1:500) were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, United States) and antibodies for beta-amyloid (clone 6E10, 1:1,000) were purchased from BioLegend (San Diego, CA, United States). Antibodies for AT8 (cat MN1020, 1:1000) were purchased from Thermo Scientific (Waltham, MA, United States). Blots were visualized using ECL chemiluminescence reagents from Thermo Fisher. All bands were quantitatively analyzed using Image J (ImageJ, RRID: SCR_003070).
Hemi-brains were paraffin embedded and 6-μM-thick sections were prepared for IHC. IHC was performed using a Histostain-SP Kit (Life Technologies, Waltham, MA, United States) following the manufacturer’s recommendations. Primary antibody for iba-1 (cat# 17198, 1:2,000) was purchased from Cell Signaling Technology (Danvers, MA, United States), p-tau (cat MN1020, 1:500) was from Thermo Scientific (Waltham, MA, United States)and antibody to beta-amyloid (clone 6E10, 1:5,000) was purchased from BioLegend (San Diego, CA, United States). Digital images of stained tissues were taken with an Olympus BX53 microscope.
Mouse plasma was collected and used for the measurement of TNF-α using ELISA kits (Abcam, Cambridge, United Kingdom) according to the manufacturer’s instructions.
Mouse brain samples from infected and uninfected 5XFAD mice (n = 3) were sent to Poochon Scientific LLC. (Frederick, Maryland, United States) for Quantitative Proteome analysis using a standardized mass spectrometry-based quantitative proteomic profiling workflow. In brief, the workflow includes the preparation of cell lysate, trypsin digestion, TMT-7plex labeling of tryptic peptides, fractionation of labeled peptides by reverse-phase UHPLC, LC-MS/MS analysis, database search, quantification analysis and data analysis.
Mouse samples were prepared for lysis and the protein concentration of the lysates was determined using a BCA™ Reducing Reagent compatible assay kit (Thermo Scientific; Rockford, IL, United States). One TMT-7plex set was used for labeling six samples (Supplementary Table 1) taking 100 μg of protein lysate from each sample for in-solution trypsin digestion. Pre-digestion samples were run along with digested samples on SDS-PAGE and stained to check digestion efficiency. Isobaric labeling was performed using the TMT-7plex kit for six samples (six samples plus one of master mix of equal amount of six digested peptide samples as the reference) according to the product manual (Supplementary Table 1). Each set of TMT labeled peptide mixtures was combined and the labeled peptide mixtures were dried in a vacuum concentrator and kept at −80°C.
The dried labeled peptides were resuspended in 10 mM TEABC. The labeling efficiency was determined before fractionation by analysis of a small aliquot of the sample (1%). A minimum of 95% labeling efficiency was required. The fractionation of the TMT-11plex labeled peptide mixture was carried out using an Agilent AdvanceBio Column (2.7 μm, 2.1 × 250 mm) with Solvent A (10 mM TEABC, pH 8.0) and an Agilent UHPLC 1290 system. The separation was performed by running a gradient of Solvent B (10 mM TEABC, pH 8.0, 90% ACN) and Solvent A (10 mM TEABC, pH 8.0) at a flow rate of 250 μL/min. The eluted fractions were collected into a 96-well plate using a 1260 series auto-sample fraction collector. The 96 eluted fractions were further combined into 24 fractions according to collection time (e.g., Ax/Cx/Ex/Gx, and Bx/Dx/Fx/Hx; A/B/C/D/E/F/G/H represents the column, x represents row 1 through row 12) for LC/MS/MS analysis.
The LC/MS/MS analysis was carried out using a Thermo Scientific Q-Exactive hybrid Quadrupole-Orbitrap Mass Spectrometer and a Thermo Dionex UltiMate 3000 RSLCnano System. Each peptide fraction from a set of 24 fractions was loaded into a peptide trap cartridge at a flow rate of 5 μL/min. The trapped peptides were eluted onto a reverse-phase 20 cm C18 PicoFrit column (New Objective, Woburn, MA) using a linear gradient of acetonitrile (3-36%) in 0.1% formic acid. The elution duration was 110 min at a flow rate of 0.3 μL/min. Eluted peptides from the PicoFrit column are ionized and sprayed into the mass spectrometer, using a Nanospray Flex Ion Source ES071 (Thermo, San Jose, CA, United States) at spray voltage 1.8 kV and capillary temperature 250°C. Twenty four fractions were analyzed sequentially.
The Q Exactive instrument was operated in the data-dependent mode to automatically switch between full scan MS and MS/MS acquisition. Survey full scan MS spectra (m/z 350-1800) were acquired in the Orbitrap with 35,000 resolutions (m/z 200) after an accumulation of ions to a 3 × 106 target value based on predictive automatic gain control (AGC). The maximal injection time was set to 100 ms. The 15 most intense multiply charged ions (z ≥ 2) were sequentially isolated and fragmented in the octupole collision cell by higher-energy collisional dissociation (HCD) using a normalized HCD collision energy of 30 with an AGC target 1 × 105 and a maximal injection time of 400 ms at 17,500 resolutions. The isolation window was set to 2 and fixed first mass was 120 m/z. The dynamic exclusion was set to 20 s. Charge state screening was enabled to reject unassigned and 1 +, 7 +, 8 +, and > 8 + ions.
One set of 24 MS Raw data files acquired from the analysis of 24 fractions was searched against mouse protein sequence databases obtained from the UniProt KB website using Proteome Discoverer 2.4 software (Thermo, San Jose, CA, United States) based on the SEQUEST and percolator algorithms. The false positive discovery rates (FDR) were set at 1%. The resulting Proteome Discoverer Report contains all assembled proteins with peptide sequences and peptide spectrum match counts (PSM#) and TMT-tag based quantification ratio.
TMT-tag based quantification was used for determining the relative abundance of proteins identified from six samples using one TMT-7plex. The common reference tag 130C (a mix of six samples) was used for the calculation of the ratio (e.g., sample 1-TMT-tag-126 versus Mix-Tag-130C). The relative abundance of proteins in each TMT-set was normalized using the reference mix. The raw data has been uploaded to the ProteomeXchange Consortium via the PRIDE (Perez-Riverol et al., 2022) partner repository.
All the experiments were designed to generate groups of equal size, using randomization and blinded analysis. The initial sample sizes were estimated using the PASS statistical software package (Number Cruncher Statistical Systems, Kaysville, UT, United States). The significance level (alpha) was set as 0.05 and the power (1-beta) was set as 0.9. The experimental data obtained are expressed as mean ± standard deviation, and SPSS19.0 and GraphPad Prism5.0 software were used for statistical analysis and visualization. The comparison between groups was performed by two-tailed Student’s t-test, and the comparison between three or more groups was performed by one-way ANOVA in which Dunnett’s test was used for the pairwise comparison. P < 0.05 was considered as a significant difference.
Microglia account for about 10% of the total number of cells in neuronal tissues. Under normal circumstances, the number of microglia in the nervous system is relatively stable. To explore whether F. nucleatum affects the proliferation of microglia, SIM-A9 cells were co-cultured with the bacteria. CCK8 was used to detect changes in the number of microglial cells at 5h, 12h, 24h and 48h after co-cultivation. The results showed that compared with the control group, after 5 h of co-cultivation, all F. nucleatum preparations promoted the growth of SIM-A9 cells (P < 0.05) (Figure 1B), while after 12 h of co-cultivation, only the P/S treated and heat-treated F. nucleatum promoted cell proliferation (P < 0.05); filtered bacterial solution and live bacteria did not significantly promote cell growth. After 24 h, P/S treated, and heat-treated F. nucleatum still promoted proliferation of SIM-A9 cells (P < 0.05), but the filtered bacterial broth and the live bacteria inhibited the growth of SIM-A9. At 48 h, there was no significant difference between groups (P > 0.05).
Lipopolysaccharide is a heat resistant component of cell wall and an important virulence factor of gram-negative bacteria (Dominy et al., 2019). P/S treated, and heat-treated bacteria promoted the growth of SIM-A9 cells implicating LPS as a key factor. To determine the impact of F. nucleatum LPS on microglial proliferation, LPS was extracted and added to the SIM-A9 cell culture medium. The results reveal that LPS promoted the proliferation of brain microglia (Figure 1C), which was significant at 5h and 24h (P < 0.05). At 12h and 48h, there was non-significant promotion of proliferation.
When stimulated, microglia change morphology from ramified to polygon or amoeboid. To explore whether F. nucleatum influenced the morphology of SIM-A9 cells, pictures of the cell morphology were captured after co-cultivation for 5, 24, and 48 h. After cells were co-cultured with bacteria, cell morphology changed significantly. Cell volume increased and assumed a rod-shaped or amoeba-like appearance (Figures 2A–C). However, in the control group, no obvious morphological changes occurred.
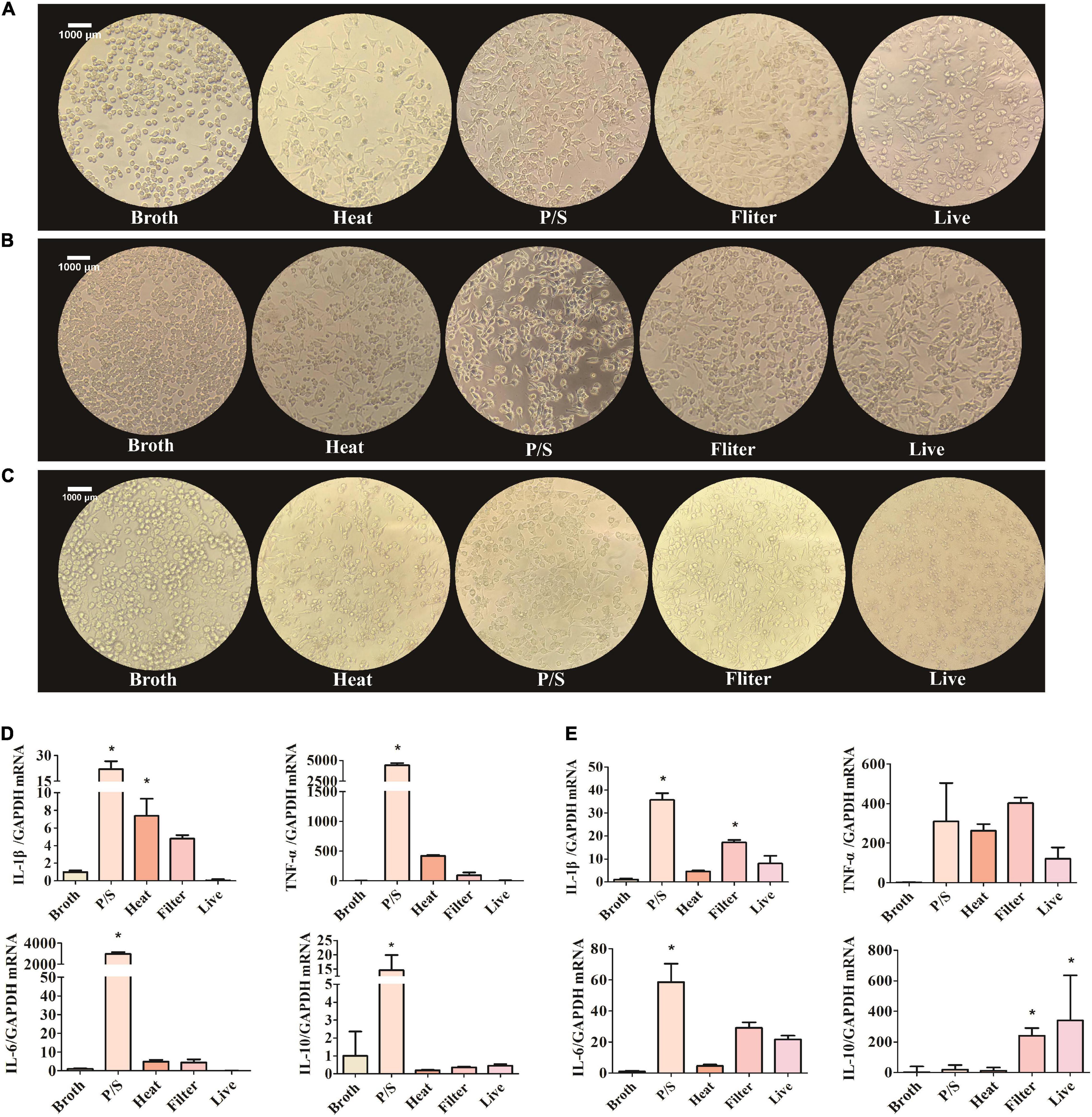
Figure 2. F. nucleatum could activate the SIM-A9 cells in vitro. (A–C) The morphological changes of SIM-A9 cells after co-culturing with F. nucleatum for (A) 5 h, (B) 24 h and (C) 48 h. (D,E) The expression level of genes of inflammatory factors from SIM-A9 after co-culturing with F. nucleatum for (D) 5 h and (E) 12 h. All data were expressed as mean ± SD. *P < 0.05 vs. Broth group.
Another typical phenotypic change of microglia in response to external stimuli is the secretion of inflammatory molecules. To detect the expression of inflammatory factors by SIM-A9 cells after co-cultivation with F. nucleatum, RNA was extracted from cells and RT-qPCR was used to detect the expression of mRNA of inflammatory genes. After 5 h of co-culture, the F. nucleatum treated with P/S group significantly promoted the expression of IL-1β, TNF-α, IL-6 and IL −10 mRNA by SIM-A9 cells (P < 0.05) (Figure 2D). Although heat-treated and filtered bacterial culture medium of F. nucleatum also increased the expression of inflammatory factor genes, the results were not statistically significant. Live bacteria had no obvious effect on the inflammatory response.
Similar results were obtained after 12 h of co-culture. The gene expression levels of IL-1β, TNF-α and IL-6 by SIM-A9 cells in the P/S-treated F. nucleatum group increased significantly (P < 0.05) (Figure 2E), but the level of IL-10 did not change significantly (P > 0.05). When microglia were cultured with filtered bacterial culture medium for 12 h, the gene expression of IL-1β, TNF-α, IL-6 and IL-10 were elevated; only IL-1β and IL-10 were statistically significant (P < 0.05).
To determine the clinical consequences of F. nucleatum infection, the novel object recognition task test was employed. Results revealed that the infected group of mice exhibited a lower discrimination index compared to controls, especially one month after the treatment. Mice infected with F. nucleatum failed to differentiate between a novel object and a familiar object, which suggests that the F. nucleatum treated mice suffered from a more serious memory impairment than the control 5XFAD mice (Figure 3A). No significant differences were seen between any conditions in control wild type B6 mice (Figure 3A). The results indicate that in 5XFAD mice, memory impairment was enhanced by F. nucleatum infection.
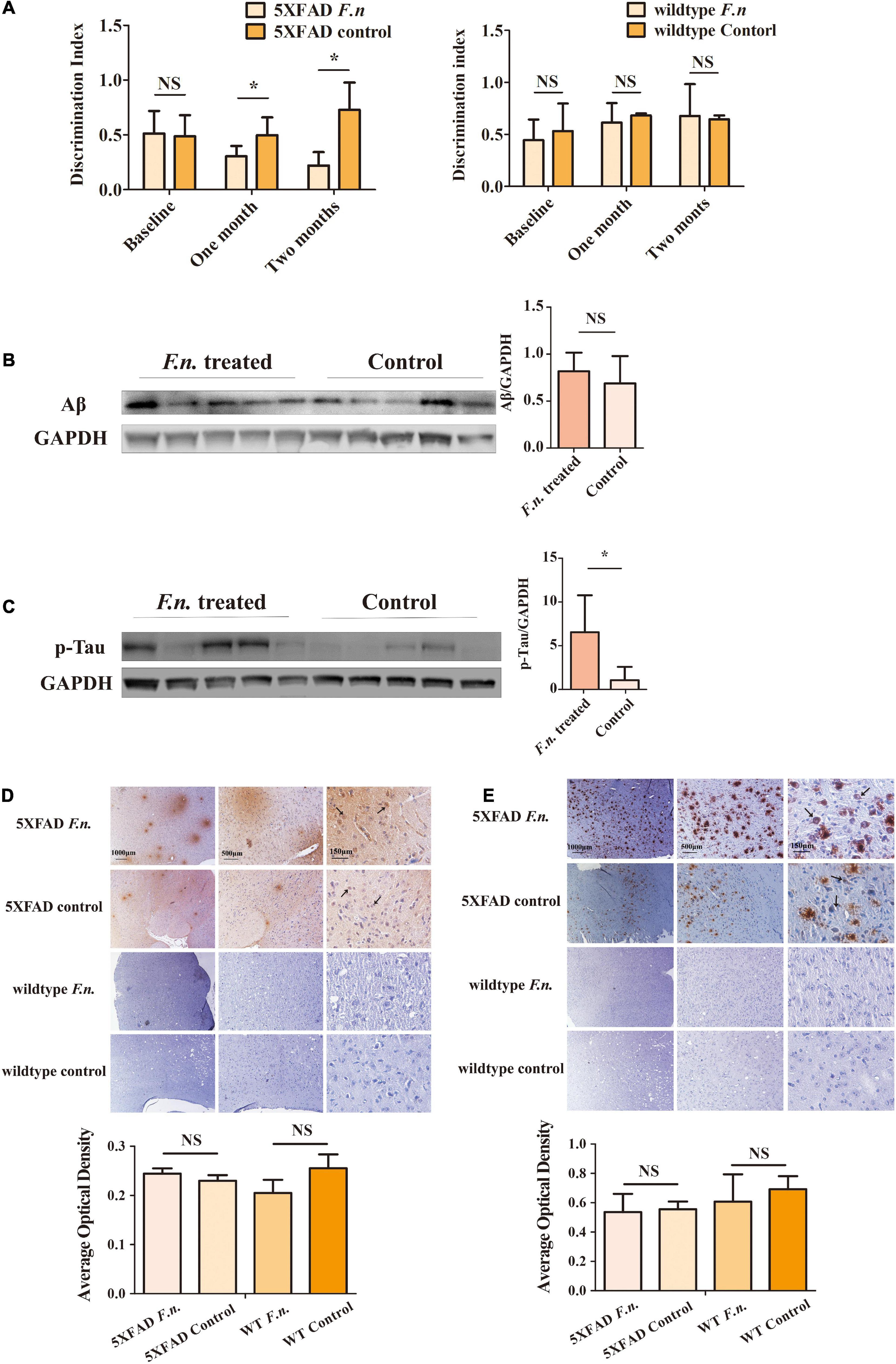
Figure 3. The behavioral and pathological manifestation of 6-month-old 5XFAD mice and 6-month-old wild-type mice with or without F. nucleatum infection. (A) Results of novel object recognition (NOR) test of mice at baseline, one month and two months after infection. (B,C) The expression levels of (B) Aβ and (C) Tau protein in mice brain after two months of infection with F. nucleatum. (D,E) The distribution of (D) Aβ and (E) Tau protein in mice cerebrum cortex after two months of infection. All data were expressed as mean ± SD, n = 5, *P < 0.05 vs. the control group. NA, not significantly different.
In addition to behavioral testing, biological manifestations of F. nucleatum treatment in the brain were also determined. After two-months of infection with F. nucleatum, the brain tissues of the mice were collected and protein was extracted. The amount of beta-amyloid and of p-Tau protein in the brain tissue were detected (Jeon et al., 2020; Delizannis et al., 2021). The results of western blot analysis showed there was a slightly higher expression of beta-amyloid in infected 5XFAD mice compared to non-treated 5XFAD mice, but the results were not statistically significant (P > 0.05) (Figure 3B). The amount of p-Tau protein in the brain tissue of the treated group was significantly higher than that of the control group (P < 0.05) (Figure 3C).
Immunohistochemical staining also showed a wider distribution of Tau protein and beta-amyloid plaques in F. nucleatum infected 5XFAD mice (Figures 3D,E, black arrows) than the non-treated group. Neither Tau protein nor beta-amyloid was detectable in control wild type B6 mice. The above results suggest that F. nucleatum might play a significant role in exacerbating the behavioral and biological manifestations of 5XFAD mice, promoting the development of the disease.
Inflammation is a driving factor for the development of AD. To determine whether F. nucleatum influences systemic and local inflammation in 5XFAD and wild-type mice, the expression of TNF-α and IL-β genes in brain tissue was assessed by RT qPCR (Figure 4A), and in plasma, TNF-α levels were determined by ELISA. The results revealed that in 5XFAD mice, the expression of TNF-α and IL-β genes in the brain was significantly increased in mice with F. nucleatum infection (P < 0.05). The expression of TNF-α and IL-β genes was approximately 3 times higher than in the uninfected group. In wild-type mice, no significant differences were seen between groups after 2 months of infection (P > 0.05). The concentration of plasma TNF-α in the F. nucleatum treated group was not significantly different from the uninfected group (Figure 4B).
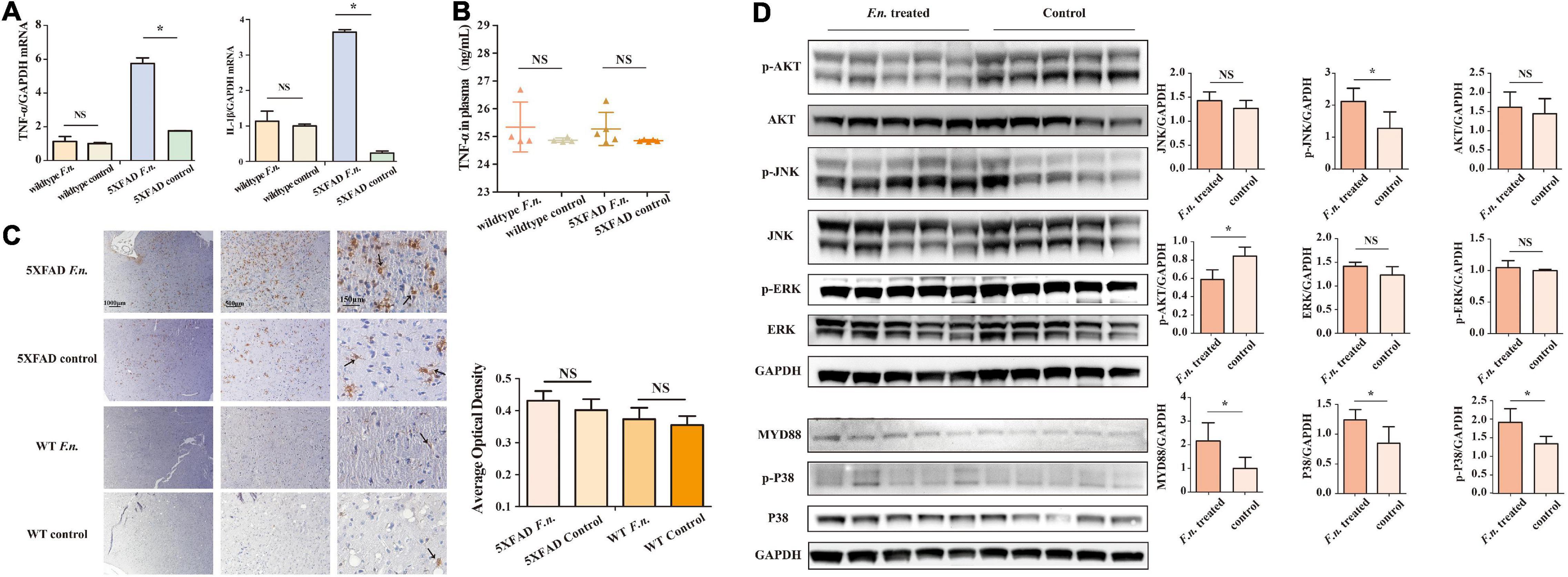
Figure 4. F. nucleatum could promote inflammatory processes in 6-month-old 5XFAD mice brain. (A) The expression levels of genes of TNF-α and IL-β in the brain tissue of 5XFAD mice and wild-type mice. (B) The concentration of TNF-α in mice plasma. (C) The distribution of microglia in mice cerebrum cortex. (D) The expression levels of proteins in inflammation-related pathways in 5XFAD mice brain. All data were expressed in mean ± SD, n = 5, *P < 0.05 vs. the control group. NA, not significantly different.
The distribution of microglia in the mouse brain was determined by immunohistochemical staining (Figure 4C), after brain sections were stained with iba1. The results show a wider distribution of microglia in the brain of 5XFAD mice with infection than their non-infected counterparts, indicating that the proliferation and migration of microglia in the brain was increased. However, in control wild type mice B6, few microglia were detected in the brain and F. nucleatum did not have any impact on the distribution of microglia.
The relevant signaling pathways of inflammation in 5XFAD mouse brains were determined by western blot (Figure 4D). The results revealed that MyD88 protein in the brains of infected mice was higher (P < 0.05) than non-infected group. Likewise, the expression of P38 protein was significantly increased as was phosphorylated P38 protein (P < 0.05). Moreover, the results also show that F. nucleatum stimulated the JNK pathway; the expression levels of p-JNK and JNK proteins were raised. Among them, phosphorylated JNK increased significantly (P < 0.05), while the level of JNK increased slightly (P > 0.05). The expression of Akt and ERK proteins was not influenced by F. nucleatum (P > 0.05).
Quantitative proteomic analyses were performed to detect proteins in the brains of two groups of 5XFAD mice. Total of 7,558 proteins were found in all samples (Figure 5A). Among them 31 proteins were significantly up- or down-regulated by at least 15% in treated groups compared to control group (P < 0.05). Specifically, 24 proteins were up-regulated (Ratio (treated/control) > 1.15, P < 0.05) in the treated groups and 7 were down-regulated (Ratio (treated/control) < 0.87, P < 0.05).
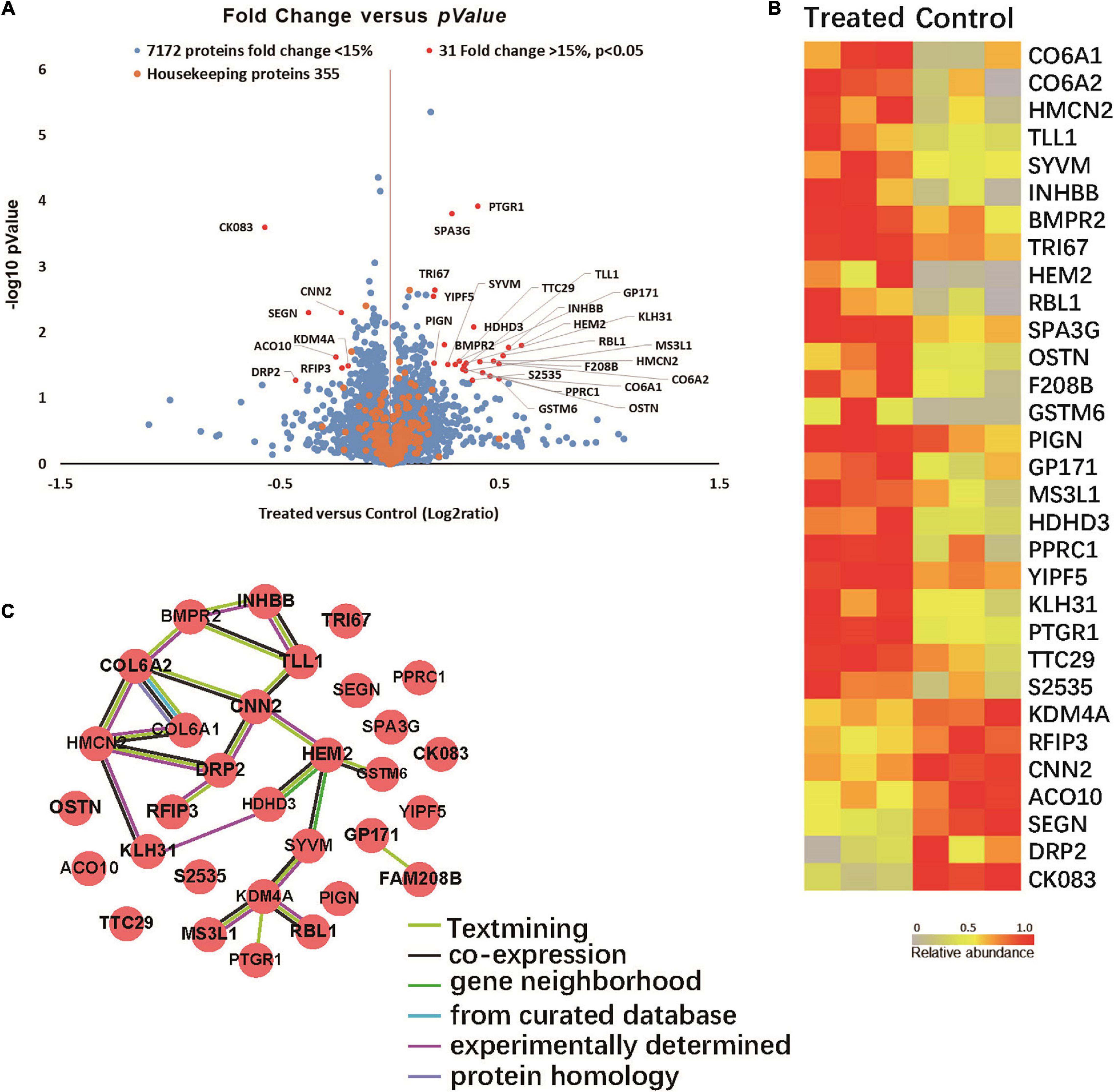
Figure 5. (A) Volcano plot demonstrating the fold change of 7558 protein abundance between treated group and control group (n = 3). Volcano plot demonstrating the fold change of 7558 protein abundance between treated group and control group (n = 3). The x-axis represents the log2 of fold changes (treated versus control), and the y-axis represents the statistically significant p-value (–log10 of p-value, n = 3). Blue dots represent 7172 protein fold change <1.15, Red dots are 31 proteins fold change >1.15, p > 0.05, and Orange dots represent 355 housekeeping proteins. (B) Proteomic characterization of the proteomes of 6 mice brain tissue samples by TMT-7plex labeling based quantitative proteomics. A heat map showing the relative abundance of 31 ranked proteins (Ratio of Treated to Control ≥ 1.15 or ≤ 0.87, p < 0.05, n = 3) was identified across two groups of 6 samples. The color key indicates the relative abundance of each protein (0 to 1.0) across 6 samples. (C) Protein-protein interaction networks. (31 items (mouse) - STRING interaction network (string-db.org)).
Among the proteins with significant changes in expression (Figure 5B), GSTM6, RBL1, SPA3G and PTGR1 were upregulated in the treated group. These changes correlated with glutathione transferase activity, p53 pathway feedback loop 2, the apoptotic process and response to toxic substance pathways. SEGN and DRP2 were downregulated in the infected group. SEGN and DRP2 are involved in central nervous system development and calcium ion binding pathways.
The protein-protein interactions of the 31 differentially expressed proteins were analyzed via the String website. Figure 5C shows that COLA1, COLA2, HMCN2, and HEM2 proteins interact with more protein nodes, which might suggest their central role in this interaction network that can play an important role in the progression of AD.
Microglia are the major immune cells in the nervous system (Bagheri-Mohammadi, 2020) that are activated by abnormal signals or exogenous stimuli, such as LPS and Aβ. It is well known that microglia could be categorized into two types: the M1 phenotype and M2 phenotype. The stimnuli like LPS, Aβ or IFN-γ would activate M1 phenotype, leading to the expression of pro-inflammatory cytokines and irreversible neuron loss. The M2 phenotype could be due to the exposure of IL-4 or IL-13, resulting in resolution of inflammation and tissue repair (Orihuela et al., 2016; Tang and Le, 2016).
Microglia reside in the brain parenchyma and do not circulate, but they proliferate in a large quantity when being activated to regulate the inflammatory response (Crotti and Ransohoff, 2016). In healthy brain, microglia could help maintain the stability of the nervous system. They can impact the function of astrocytes and neurons by expressing soluble molecules, help remove cell debris and polymerized proteins, and they also play an active role in synaptic pruning (Paolicelli et al., 2011). There is evidence that the activation of microglia is related to many neurological diseases or conditions, such as brain injury, ischemic stroke, and neuroinflammatory diseases. Due to the close relationship between activation of microglia and inflammation in the nervous system (Araki et al., 2020; Bourgognon and Cavanagh, 2020), it is generally accepted that the activation of microglia is closely related to the pathogenesis of neurodegenerative diseases, such as AD and Parkinson’s disease (Ashford et al., 2020; Stolero and Frenkel, 2020; Forloni et al., 2021).
This report demonstrates that F. nucleatum promotes the proliferation of microglia. The increase in cell mass is an important indicator of microglial activation (Crotti and Ransohoff, 2016). Promoting proliferation was observed in short-term co-culture; after 5, 12, and 24 h, but not in 48 h suggesting a rapid cellular response to challenge. As microglial cells proliferate, inflammatory immune function increases resulting in changes in cell morphology and the increased expression of inflammatory genes.
The main known virulence factors of F. nucleatum include FadA, Fap2, and LPS (Han, 2015), which possess unique characteristics. We employed a series of pretreatments to F. nucleatum to systematically inactivate these various virulence factors and determined that LPS is a one of the candidates for microglial activation. For example, antibiotics kill bacteria or limit growth, but do not affect bacterial virulence factors on the cell surface; heating denatures bacterial surface proteins, but not LPS. Filtration removes bacterial cells leaving metabolites in the culture medium, which might contain exosomes, secreted products or bacterial fragments. Interestingly, live bacteria reduced SIM-A9 cell viability after 5 h in vitro, thus while we were able to assess the impact of various virulence factors in longer experiments, the differences in proliferation and inflammatory responses with live bacteria could not be assessed after 5 h.
The results obtained with these different treatments suggest that LPS is one of the possible candidates for microglial activation. There are previous reports supporting that LPS is an important factor leading to inflammation in the nervous system (Catorce and Gevorkian, 2016). We further explored this pathway using pure LPS extracted from F. nucleatum and demonstrate that LPS from F. nucleatum promotes the proliferation of SIM-A9, which was in line with expectations. However, LPS is just one of the many virulence factors of F. nucleatum. Whether other heat resistant virulence factors activate microglia remains to be explored. For example, previous study reported that the virulence factor FadA secreted by F. nucleatum promotes both periodontal bone loss and colorectal tumorigenesis (Meng et al., 2021). This occurs when FadA transforms into an amyloid-like structure converting F. nucleatum from a benign commensal to a virulent pathogen. Since amyloid proteins are also heat resistant, it could be possible that amyloid FadA may be involved in microglial activation. The effects of amyloid FadA on microglia cells and Alzheimer’s Disease warrant further examination.
Apart from in vitro experiments, the impact of F. nucleatum on mouse pathological manifestations was also determined in vivo. A mouse oral infection model was established by applying bacterial solution topically to the buccal surface of the maxillary mucosa and gingiva of ligatured teeth. A review of previous literature revealed that it could take 22 weeks of chronic oral topical bacteria application to induce brain inflammation (Ilievski et al., 2018). Thus, to ensure the attachment and colonization of F. nucleatum and establish a rapid infection model, the mouse maxillary left second molar was ligated with silk thread. Two months later, periodontitis was confirmed with the changes that height of the alveolar bone in infected mice was decreased by micro-CT scanning (Supplementary Figure 1). Previous studies also showed that even without ligature, F. nucleatum induced periodontal bone loss in an FadA-depedent manner after ten weeks of oral inoculation (Meng et al., 2021).
As shown in the results, F. nucleatum exacerbates pathological and behavioral manifestations in 5XFAD mice. Compared with uninfected 5XFAD mice, infected mice performed poorly in the novel object recognition task test (spent less time exploring novel items during the testing phase). In addition, the amount of Aβ and p-Tau protein in mouse brain tissue was significantly higher than that of the untreated group suggesting that F. nucleatum exacerbates pathological and behavioral manifestations in 5XFAD mice. We have conducted the PCR to identify the presence of F. nucleatum in brain tissue but the result was negative, which imply that F. nucleatum may induce inflammatory responses without actually colonizing the brain or the amount of bacteria in brain was beblow the limit of the detection. Furthermore, it is well-known that the gram-negative endotoxin, namely lipopolysaccharide (LPS), could induce blood-brain barrier (BBB) disruption (Banks et al., 2015), leading to altered permeability or breakdown of the BBB. Thus, the BBB injury could happen and affect the onset and progression of AD (Sweeney et al., 2019; Yang et al., 2021). In the present study, the BBB breakdown might happen due to the LPS of F. nucleatum and consequently result in exacerbation of pathological and behavioral manifestations of 5XFAD mice. This might explain the corresponding changes in symptoms even without the detection of F. nucleatum in the brain tissue.
Previous studies suggested that local inflammation in the central nervous system leads to cognitive impairment. For example, the inflammation in the brain of AD patients is increased (Teixeira et al., 2008). Inflammatory cytokines are released, including interleukin family proteins, TNF-α, TGF-β and chemokines, which may be used as serum and plasma markers in AD (Lee et al., 2009). The over-activation of microglia is an early feature of AD, and TNF-α is a pro-inflammatory factor mainly produced by activated microglia/macrophages, which could play a central role in the mechanism of the onset of AD (Perry et al., 2001). Many clinical and animal studies have shown that there is a link between excessive TNF-α in the brain and AD (Akiyama et al., 2000; Tarkowski et al., 2003). Excessive TNF-α in brain tissue disrupts Aβ clearance mediated by brain microglia (Koenigsknecht-Talboo and Landreth, 2005) resulting in increased Aβ protein accumulation (Liao et al., 2004; Yamamoto et al., 2007), causing synaptic dysfunction (Ralay Ranaivo et al., 2006), thereby speeding up disease development and cognitive impairment (McGeer and McGeer, 2003).
Another pro-inflammatory factor that is essential in AD is IL-1β. It is known that IL-1β is a key regulator of acute inflammation in the central nervous system. Neuroinflammation caused by IL-1β is also related to the pathophysiological processes of chronic neurodegenerative diseases, including AD. Previous studies have suggested that the deposition of Aβ in the brain causes microglia to secrete IL-1β, which leads to chronic neuroinflammation in AD, which causes neuronal dysfunction and ultimately accelerates the process of neurodegeneration (Teixeira et al., 2008; Lai et al., 2017). IL-1β also enhances the synthesis of Aβ protein precursor mRNA in human endothelial cells (Goldgaber et al., 1989) suggesting that IL-1β also influences the formation of senile plaques.
Several studies investigating AD-related inflammation have determined the levels of TNF-α and IL-1β in brain tissue (Yasutake et al., 2006; Li et al., 2017; Saffari et al., 2020) confirming the close connection between the two and AD. Here, we focused on the change in TNF-α and IL-1β message levels in the brain tissue of mice after infection with F. nucleatum. The results of qRT-PCR showed that, in 5XFAD mice, the expression levels of TNF-α and IL-1β mRNA are elevated after infection with F. nucleatum. Elevations of TNF-α and IL-1β were not detected in serum and plasma. Subsequently, the expression of proteins related to inflammation signaling pathways in mouse brain tissue was assessed. The adapter protein MyD88 plays a pivotal role in LPS stimulated pro-inflammatory signaling pathways and P38 is known to contribute to the LPS-stimulated inflammatory response (Qiu et al., 2020). Here, we showed that in the presence of F. nucleatum infection, the expression of P38 protein in 5XFAD mouse brain tissue was significantly upregulated, as was its phosphorylation level. MyD88 protein was upregulated. The results of immunohistochemical staining revealed a significant increase in the numbers of microglia in the brains of 5XFAD mice treated with F. nucleatum. The actions of these potent cytokines appear to be local as no increases in TNF-α or IL-1β were noted in plasma. Together the data suggest that local overgrowth of bacteria induced by periodontitis could be transported to the brain and lead to local inflammation, thereby aggravating AD.
Quantitative proteomics refers to the mass spectrometric detection of specific known proteins (Doerr, 2013). Proteomic studies were performed to compare the similarities and differences in protein expression by microglial cells under different physiological or pathological conditions, and to classify and identify related proteins. More importantly, proteomics can be used to analyze the interactions between proteins, and the function of individual proteins. We used quantitative proteomics technology to detect the proteins in the brain tissues of 5XFAD with or without F. nucleatum infection and found 31 proteins that were significantly differentially expressed by the two groups of mice. The results of quantitative proteomics will be further verified and explored to find key proteins that act in key roles in signaling pathways and to further elucidate the mechanism of F. nucleatum actions in AD in future studies.
In summary, F. nucleatum activates microglia cells in vitro promoting proliferation and increased inflammatory response. In vivo, in a mouse model of periodontitis, F. nucleatum accelerates the development of disease by promoting inflammatory responses in the brain, exacerbating the behavioral and pathological manifestations of the 5XFAD mice. As a periodontal pathogen, F. nucleatum accelerates the development of AD. These findings provide a theoretical basis for the study of the relationship between F. nucleatum and AD and lay a preliminary foundation for further exploring the mechanism of F. nucleatum impact on the onset and development of AD.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found below: The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Perez-Riverol et al., 2022) partner repository with the dataset identifier PXD033147.
The animal study was reviewed and approved by Tufts Institutional Animal Care and Use Committee (IACUC).
HW and WQ contributed to the conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. XZ and XL contributed to the design, data acquisition and analysis, and drafted and revised the manuscript. ZX, IC, AD, TV, YH, NK, and QT contributed to the design and critically revised the manuscript. LC and JC contributed to the conception, design, data analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work.
This work was supported by NIH grants DE26507 and DE30074 to JC, RF1AG056032, RF1 AG062496, and RF1 AG069228 to AD, DE25020 to TV, and R01 DE 029532 and R01 DE 029532-S2 to YH.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.912709/full#supplementary-material
Alzheimer’s Disease (2020). Alzheimer’s and Dementia: the Journal of the Alzheimer’s Association. Chicago, Il: Alzheimer’s Association
Abbayya, K., Puthanakar, N. Y., Naduwinmani, S., and Chidambar, Y. S. (2015). Association between periodontitis and Alzheimer’s Disease. N. Am. J. Med. Sci. 7, 241–246.
Akiyama, H., Barger, S., Barnum, S., Bradt, B., Bauer, J., Cole, G. M., et al. (2000). Inflammation and Alzheimer’s disease. Neurobiol. Aging 21, 383–421.
Ando, K., Houben, S., Homa, M., De Fisenne, M. A., Potier, M. C., Erneux, C., et al. (2020). Alzheimer’s disease: tau pathology and dysfunction of endocytosis. Front. Mol. Neurosci. 13:583755. doi: 10.3389/fnmol.2020.583755
Araki, T., Ikegaya, Y., and Koyama, R. (2020). The effects of microglia- and astrocyte-derived factors on neurogenesis in health and disease. Eur. J. Neurosci. [Online ahead of print] doi: 10.1111/ejn.14969
Ashford, B. A., Boche, D., Cooper-Knock, J., Heath, P. R., Simpson, J. E., and Highley, J. R. (2020). Review: microglia in motor neuron disease. Neuropathol. Appl. Neurobiol. 47, 179–197. doi: 10.1111/nan.12640
Alzheimer’s Association Report (2022). 2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 18, 700–789. doi: 10.1002/alz.12638
Bagheri-Mohammadi, S. (2020). Microglia in Alzheimer’s Disease: the role of stem cell-microglia interaction in brain homeostasis. Neurochem. Res. 46, 141–148. doi: 10.1007/s11064-020-03162-4
Banks, W. A., Gray, A. M., Erickson, M. A., Salameh, T. S., Damodarasamy, M., Sheibani, N., et al. (2015). Lipopolysaccharide-induced blood-brain barrier disruption: roles of cyclooxygenase, oxidative stress, neuroinflammation, and elements of the neurovascular unit. J. Neuroinflammation 12:223. doi: 10.1186/s12974-015-0434-1
Barrett, T., Marchalant, Y., and Park, K. H. J. (2019). p35 hemizygous deletion in 5xFAD mice increases abeta plaque load in males but not in females. Neuroscience 417, 45–56. doi: 10.1016/j.neuroscience.2019.08.017
Bourgognon, J. M., and Cavanagh, J. (2020). The role of cytokines in modulating learning and memory and brain plasticity. Brain Neurosci. Adv. 4:2398212820979802. doi: 10.1177/2398212820979802
Cai, Z., Hussain, M. D., and Yan, L. J. (2014). Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 124, 307–321. doi: 10.3109/00207454.2013.833510
Casasanta, M. A., Yoo, C. C., Udayasuryan, B., Sanders, B. E., Umana, A., Zhang, Y., et al. (2020). Fusobacterium nucleatum host-cell binding and invasion induces IL-8 and CXCL1 secretion that drives colorectal cancer cell migration. Sci. Signal 13:eaba9157. doi: 10.1126/scisignal.aba9157
Catorce, M. N., and Gevorkian, G. (2016). LPS-induced murine neuroinflammation model: main features and suitability for pre-clinical assessment of nutraceuticals. Curr. Neuropharmacol. 14, 155–164. doi: 10.2174/1570159x14666151204122017
Chapple, I. L., and Genco, R. working group 2 of the joint Efp/Aap workshop. (2013). Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP workshop on periodontitis and systemic diseases. J. Clin. Periodontol. 40, (Suppl. 14), S106–S112.
Chen, C. K., Wu, Y. T., and Chang, Y. C. (2017). Association between chronic periodontitis and the risk of Alzheimer’s disease: a retrospective, population-based, matched-cohort study. Alzheimers Res. Ther. 9:56. doi: 10.1186/s13195-017-0282-6
Coomans, E. M., Schoonhoven, D. N., Tuncel, H., Verfaillie, S. C. J., Wolters, E. E., Boellaard, R., et al. (2021). In vivo tau pathology is associated with synaptic loss and altered synaptic function. Alzheimers Res. Ther. 13:35. doi: 10.1186/s13195-021-00772-0
Crotti, A., and Ransohoff, R. M. (2016). Microglial physiology and pathophysiology: insights from genome-wide transcriptional profiling. Immunity 44, 505–515. doi: 10.1016/j.immuni.2016.02.013
Delizannis, A. T., Nonneman, A., Tsering, W., De Bondt, A., Van Den Wyngaert, I., Zhang, B., et al. (2021). Effects of microglial depletion and TREM2 deficiency on Abeta plaque burden and neuritic plaque tau pathology in 5XFAD mice. Acta Neuropathol. Commun. 9:150. doi: 10.1186/s40478-021-01251-1
Dietrich, T., Sharma, P., Walter, C., Weston, P., and Beck, J. (2013). The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J. Periodontol. 84, S70–S84.
Doifode, T., Giridharan, V. V., Generoso, J. S., Bhatti, G., Collodel, A., Schulz, P. E., et al. (2021). The impact of the microbiota-gut-brain axis on Alzheimer’s disease pathophysiology. Pharmacol. Res. 164:105314. doi: 10.1016/j.phrs.2020.105314
Dominy, S. S., Lynch, C., Ermini, F., Benedyk, M., Marczyk, A., Konradi, A., et al. (2019). Porphyromonas gingivalis in Alzheimer’s disease brains: evidence for disease causation and treatment with small-molecule inhibitors. Sci. Adv. 5:eaau3333. doi: 10.1126/sciadv.aau3333
Eke, P. I., Dye, B. A., Wei, L., Thornton-Evans, G. O., Genco, R. J. CDC Periodontal Disease Surveillance workgroup., et al. (2012). Prevalence of periodontitis in adults in the United States: 2009 and 2010. J. Dent. Res. 91, 914–920. doi: 10.1177/0022034512457373
Fischer, J. B., Prout, A., Blackwood, R. A., and Warrier, K. (2015). Lemierre syndrome presenting as acute mastoiditis in a 2-year-old girl with congenital dwarfism. Infect Dis. Rep. 7:5922. doi: 10.4081/idr.2015.5922
Forloni, G., La Vitola, P., Cerovic, M., and Balducci, C. (2021). Inflammation and Parkinson’s disease pathogenesis: mechanisms and therapeutic insight. Prog. Mol. Biol. Transl. Sci. 177, 175–202. doi: 10.1016/bs.pmbts.2020.11.001
Goldgaber, D., Harris, H. W., Hla, T., Maciag, T., Donnelly, R. J., Jacobsen, J. S., et al. (1989). Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 86, 7606–7610. doi: 10.1073/pnas.86.19.7606
Han, Y. W. (2011). Oral health and adverse pregnancy outcomes - what’s next? J. Dent. Res. 90, 289–293. doi: 10.1177/0022034510381905
Han, Y. W. (2015). Fusobacterium nucleatum: a commensal-turned pathogen. Curr. Opin. Microbiol. 23, 141–147. doi: 10.1016/j.mib.2014.11.013
He, J., Li, Y., Cao, Y., Xue, J., and Zhou, X. (2015). The oral microbiome diversity and its relation to human diseases. Folia Microbiol. 60, 69–80. doi: 10.1007/s12223-014-0342-2
Hebert, L. E., Weuve, J., Scherr, P. A., and Evans, D. A. (2013). Alzheimer disease in the United States (2010-2050) estimated using the 2010 census. Neurology 80, 1778–1783. doi: 10.1212/WNL.0b013e31828726f5
Ilievski, V., Zuchowska, P. K., Green, S. J., Toth, P. T., Ragozzino, M. E., Le, K., et al. (2018). Chronic oral application of a periodontal pathogen results in brain inflammation, neurodegeneration and amyloid beta production in wild type mice. PLoS One 13:e0204941. doi: 10.1371/journal.pone.0204941
Ishida, N., Ishihara, Y., Ishida, K., Tada, H., Funaki-Kato, Y., Hagiwara, M., et al. (2017). Periodontitis induced by bacterial infection exacerbates features of Alzheimer’s disease in transgenic mice. NPJ Aging Mech. Dis. 3:15. doi: 10.1038/s41514-017-0015-x
Jeon, S. G., Lee, H. J., Park, H., Han, K. M., and Hoe, H. S. (2020). The VEGF inhibitor vatalanib regulates AD pathology in 5xFAD mice. Mol. Brain 13:131. doi: 10.1186/s13041-020-00673-7
Jia, J., Wei, C., Chen, S., Li, F., Tang, Y., Qin, W., et al. (2018). The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimers Dement. 14, 483–491. doi: 10.1016/j.jalz.2017.12.006
Jia, L., Quan, M., Fu, Y., Zhao, T., Li, Y., Wei, C., et al. (2020). Dementia in China: epidemiology, clinical management, and research advances. Lancet Neurol. 19, 81–92. doi: 10.1016/S1474-4422(19)30290-X
Kantarci, A., Tognoni, C. M., Yaghmoor, W., Marghalani, A., Stephens, D., Ahn, J. Y., et al. (2020). Microglial response to experimental periodontitis in a murine model of Alzheimer’s disease. Sci. Rep. 10:18561. doi: 10.1038/s41598-020-75517-4
Karran, E., Mercken, M., and De Strooper, B. (2011). The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 10, 698–712. doi: 10.1038/nrd3505
Kinane, D. F., Stathopoulou, P. G., and Papapanou, P. N. (2017). Periodontal diseases. Nat. Rev. Dis. Primers 3:17038.
Koenigsknecht-Talboo, J., and Landreth, G. E. (2005). Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J. Neurosci. 25, 8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005
Lai, K. S. P., Liu, C. S., Rau, A., Lanctot, K. L., Kohler, C. A., Pakosh, M., et al. (2017). Peripheral inflammatory markers in Alzheimer’s disease: a systematic review and meta-analysis of 175 studies. J. Neurol. Neurosurg. Psychiatry 88, 876–882. doi: 10.1136/jnnp-2017-316201
Lee, K. S., Chung, J. H., Choi, T. K., Suh, S. Y., Oh, B. H., and Hong, C. H. (2009). Peripheral cytokines and chemokines in Alzheimer’s disease. Dement. Geriatr. Cogn. Disord. 28, 281–287. doi: 10.1159/000245156
Li, L., Xu, S., Liu, L., Feng, R., Gong, Y., Zhao, X., et al. (2017). Multifunctional compound AD-35 improves cognitive impairment and attenuates the production of TNF-alpha and IL-1beta in an Abeta25-35-induced Rat Model of Alzheimer’s Disease. J. Alzheimers Dis. 56, 1403–1417. doi: 10.3233/JAD-160587
Lian, J., Wu, X., Liu, Y., Qiu, W., Zhu, X., Wang, X., et al. (2020). Potential roles of miR-335-5p on pathogenesis of experimental periodontitis. J. Periodontal. Res. 55, 191–198. doi: 10.1111/jre.12701
Liao, Y. F., Wang, B. J., Cheng, H. T., Kuo, L. H., and Wolfe, M. S. (2004). Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J. Biol. Chem. 279, 49523–49532. doi: 10.1074/jbc.M402034200
Liccardo, D., Marzano, F., Carraturo, F., Guida, M., Femminella, G. D., Bencivenga, L., et al. (2020). Potential bidirectional relationship between periodontitis and Alzheimer’s Disease. Front. Physiol. 11:683. doi: 10.3389/fphys.2020.00683
Liu, S., Gao, J., Zhu, M., Liu, K., and Zhang, H. L. (2020a). Gut microbiota and dysbiosis in Alzheimer’s Disease: implications for pathogenesis and treatment. Mol. Neurobiol. 57, 5026–5043. doi: 10.1007/s12035-020-02073-3
Liu, S., Zhou, X., Peng, X., Li, M., Ren, B., Cheng, G., et al. (2020b). Porphyromonas gingivalis promotes immunoevasion of oral cancer by protecting cancer from macrophage attack. J. Immunol. 205, 282–289. doi: 10.4049/jimmunol.1901138
Lueptow, L. M. (2017). Novel object recognition test for the investigation of learning and memory in mice. J. Vis. Exp. 126:55718. doi: 10.3791/55718
McGeer, E. G., and McGeer, P. L. (2003). Inflammatory processes in Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 741–749.
Meng, Q., Gao, Q., Mehrazarin, S., Tangwanichgapong, K., Wang, Y., Huang, Y., et al. (2021). Fusobacterium nucleatum secretes amyloid-like FadA to enhance pathogenicity. EMBO Rep. 22:e52891. doi: 10.15252/embr.202152891
Nagamoto-Combs, K., Kulas, J., and Combs, C. K. (2014). A novel cell line from spontaneously immortalized murine microglia. J. Neurosci. Methods 233, 187–198. doi: 10.1016/j.jneumeth.2014.05.021
Noble, J. M., Borrell, L. N., Papapanou, P. N., Elkind, M. S., Scarmeas, N., and Wright, C. B. (2009). Periodontitis is associated with cognitive impairment among older adults: analysis of NHANES-III. J. Neurol. Neurosurg. Psychiatry 80, 1206–1211. doi: 10.1136/jnnp.2009.174029
Oakley, H., Cole, S. L., Logan, S., Maus, E., Shao, P., Craft, J., et al. (2006). Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 26, 10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006
Orihuela, R., Mcpherson, C. A., and Harry, G. J. (2016). Microglial M1/M2 polarization and metabolic states. Br. J. Pharmacol. 173, 649–665. doi: 10.1111/bph.13139
Paolicelli, R. C., Bolasco, G., Pagani, F., Maggi, L., Scianni, M., Panzanelli, P., et al. (2011). Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458. doi: 10.1126/science.1202529
Perera, M., Al-Hebshi, N. N., Speicher, D. J., Perera, I., and Johnson, N. W. (2016). Emerging role of bacteria in oral carcinogenesis: a review with special reference to perio-pathogenic bacteria. J. Oral Microbiol. 8:32762. doi: 10.3402/jom.v8.32762
Perez-Riverol, Y., Bai, J., Bandla, C., Garcia-Seisdedos, D., Hewapathirana, S., Kamatchinathan, S., et al. (2022). The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 50, D543–D552. doi: 10.1093/nar/gkab1038
Perry, R. T., Collins, J. S., Wiener, H., Acton, R., and Go, R. C. (2001). The role of TNF and its receptors in Alzheimer’s disease. Neurobiol. Aging 22, 873–883. doi: 10.1016/s0197-4580(01)00291-3
Qiu, W., Wu, H., Hu, Z., Wu, X., Tu, M., Fang, F., et al. (2020). Identification and characterization of a novel adiponectin receptor agonist adipo anti-inflammation agonist and its anti-inflammatory effects in vitro and in vivo. Br. J. Pharmacol. 178, 280–297. doi: 10.1111/bph.15277
Ralay Ranaivo, H., Craft, J. M., Hu, W., Guo, L., Wing, L. K., Van Eldik, L. J., et al. (2006). Glia as a therapeutic target: selective suppression of human amyloid-beta-induced upregulation of brain proinflammatory cytokine production attenuates neurodegeneration. J. Neurosci. 26, 662–670. doi: 10.1523/JNEUROSCI.4652-05.2006
Saffari, P. M., Alijanpour, S., Takzaree, N., Sahebgharani, M., Etemad-Moghadam, S., Noorbakhsh, F., et al. (2020). Metformin loaded phosphatidylserine nanoliposomes improve memory deficit and reduce neuroinflammation in streptozotocin-induced Alzheimer’s disease model. Life Sci. 255:117861. doi: 10.1016/j.lfs.2020.117861
Sampaio-Maia, B., Caldas, I. M., Pereira, M. L., Perez-Mongiovi, D., and Araujo, R. (2016). The oral microbiome in health and its implication in oral and systemic diseases. Adv. Appl. Microbiol. 97, 171–210. doi: 10.1016/bs.aambs.2016.08.002
Sanz, M., Beighton, D., Curtis, M. A., Cury, J. A., Dige, I., Dommisch, H., et al. (2017). Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the Joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Periodontol. 44, (Suppl. 18), S5–S11. doi: 10.1111/jcpe.12682
Sparks Stein, P., Steffen, M. J., Smith, C., Jicha, G., Ebersole, J. L., Abner, E., et al. (2012). Serum antibodies to periodontal pathogens are a risk factor for Alzheimer’s disease. Alzheimers Dement. 8, 196–203. doi: 10.1016/j.jalz.2011.04.006
Stergiopoulou, T., and Walsh, T. J. (2016). Fusobacterium necrophorum otitis and mastoiditis in infants and young toddlers. Eur. J. Clin. Microbiol. Infect Dis. 35, 735–740. doi: 10.1007/s10096-016-2612-1
Stolero, N., and Frenkel, D. (2020). The dialog between neurons and microglia in Alzheimer’s disease: the neurotransmitters view. J. Neurochem. [Epub ahead of print] doi: 10.1111/jnc.15262
Sweeney, M. D., Zhao, Z., Montagne, A., Nelson, A. R., and Zlokovic, B. V. (2019). Blood-brain barrier: from physiology to disease and back. Physiol. Rev. 99, 21–78. doi: 10.1152/physrev.00050.2017
Swidsinski, A., Dorffel, Y., Loening-Baucke, V., Tertychnyy, A., Biche-Ool, S., Stonogin, S., et al. (2012). Mucosal invasion by fusobacteria is a common feature of acute appendicitis in Germany, Russia, and China. Saudi J. Gastroenterol. 18, 55–58. doi: 10.4103/1319-3767.91734
Swidsinski, A., Dorffel, Y., Loening-Baucke, V., Theissig, F., Ruckert, J. C., Ismail, M., et al. (2011). Acute appendicitis is characterised by local invasion with Fusobacterium nucleatum/necrophorum. Gut 60, 34–40. doi: 10.1136/gut.2009.191320
Tang, Y., and Le, W. (2016). Differential Roles of M1 and M2 microglia in neurodegenerative diseases. Mol. Neurobiol. 53, 1181–1194. doi: 10.1007/s12035-014-9070-5
Tarkowski, E., Andreasen, N., Tarkowski, A., and Blennow, K. (2003). Intrathecal inflammation precedes development of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 74, 1200–1205. doi: 10.1136/jnnp.74.9.1200
Tefiku, U., Popovska, M., Cana, A., Zendeli-Bedxeti, L., Recica, B., Spasovska-Gjorgovska, A., et al. (2020). Determination of the role of Fusobacterium Nucleatum in the pathogenesis in and out the mouth. Pril 41, 87–99.
Teixeira, A. L., Reis, H. J., Coelho, F. M., Carneiro, D. S., Teixeira, M. M., Vieira, L. B., et al. (2008). All-or-nothing type biphasic cytokine production of human lymphocytes after exposure to Alzheimer’s beta-amyloid peptide. Biol. Psychiatry 64, 891–895. doi: 10.1016/j.biopsych.2008.07.019
Tonetti, M. S., and Van Dyke, T. E. Working group 1 of the joint Efp/Aap workshop (2013). Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP workshop on periodontitis and systemic diseases. J. Periodontol. 84, S24–S29.
Wang, D., Ho, L., Faith, J., Ono, K., Janle, E. M., Lachcik, P. J., et al. (2015). Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease beta-amyloid oligomerization. Mol. Nutr. Food Res. 59, 1025–1040. doi: 10.1002/mnfr.201400544
Weuve, J., Barnes, L. L., Mendes, De Leon, C. F., Rajan, K. B., Beck, T., et al. (2018). Cognitive aging in black and white americans: cognition, cognitive decline, and incidence of Alzheimer Disease dementia. Epidemiology 29, 151–159. doi: 10.1097/EDE.0000000000000747
Wu, X., Qiu, W., Hu, Z., Lian, J., Liu, Y., Zhu, X., et al. (2019). An adiponectin receptor agonist reduces type 2 diabetic periodontitis. J. Dent. Res. 98, 313–321. doi: 10.1177/0022034518818449
Yamamoto, M., Kiyota, T., Horiba, M., Buescher, J. L., Walsh, S. M., Gendelman, H. E., et al. (2007). Interferon-gamma and tumor necrosis factor-alpha regulate amyloid-beta plaque deposition and beta-secretase expression in Swedish mutant APP transgenic mice. Am. J. Pathol. 170, 680–692. doi: 10.2353/ajpath.2007.060378
Yang, Q., Yu, J., Qin, H., Liu, L., Di, C., Zhuang, Q., et al. (2021). Irbesartan suppresses lipopolysaccharide (LPS)-induced blood-brain barrier (BBB) dysfunction by inhibiting the activation of MLCK/MLC. Int. Immunopharmacol. 98:107834. doi: 10.1016/j.intimp.2021.107834
Yasutake, C., Kuroda, K., Yanagawa, T., Okamura, T., and Yoneda, H. (2006). Serum BDNF, TNF-alpha and IL-1beta levels in dementia patients: comparison between Alzheimer’s disease and vascular dementia. Eur. Arch. Psychiatry Clin. Neurosci. 256, 402–406. doi: 10.1007/s00406-006-0652-8
Yu, Y., Yang, Y., Tan, H., Boukhali, M., Khatri, A., Yu, Y., et al. (2020). Tau contributes to sevoflurane-induced neurocognitive impairment in neonatal mice. Anesthesiology 133, 595–610. doi: 10.1097/ALN.0000000000003452
Keywords: F. nucleatum, inflammation, periodontitis, Alzheimer’s Disease, mouse model
Citation: Wu H, Qiu W, Zhu X, Li X, Xie Z, Carreras I, Dedeoglu A, Van Dyke T, Han YW, Karimbux N, Tu Q, Cheng L and Chen J (2022) The Periodontal Pathogen Fusobacterium nucleatum Exacerbates Alzheimer’s Pathogenesis via Specific Pathways. Front. Aging Neurosci. 14:912709. doi: 10.3389/fnagi.2022.912709
Received: 04 April 2022; Accepted: 07 June 2022;
Published: 23 June 2022.
Edited by:
Denah Marie Appelt, Philadelphia College of Osteopathic Medicine (PCOM), United StatesReviewed by:
Amy R. Nelson, University of South Alabama, United StatesCopyright © 2022 Wu, Qiu, Zhu, Li, Xie, Carreras, Dedeoglu, Van Dyke, Han, Karimbux, Tu, Cheng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Cheng, Y2hlbmdsZWlAc2N1LmVkdS5jbg==; Jake Chen, amsuY2hlbkB0dWZ0cy5lZHU=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.