- 1Department of Geriatrics, Xiangya Hospital, Central South University, Changsha, China
- 2Aging Research Center, Xiangya Hospital, Central South University, Changsha, China
- 3National Clinical Research Center for Geriatric Disorders, Xiangya Hospital, Central South University, Changsha, China
- 4Department of Rheumatology and Immunology, Xiangya Hospital, Central South University, Changsha, China
- 5Provincial Clinical Research Center for Rheumatic and Immunologic Diseases, Xiangya Hospital, Central South University, Changsha, China
- 6Department of Neurology, Xiangya Hospital, Central South University, Changsha, China
- 7The Biobank of Xiangya Hospital, Central South University, Changsha, China
Leucine-rich repeat kinase 2 (LRRK2) is one of the most common causative genes in Parkinson’s disease (PD). The complex structure of this multiple domains’ protein determines its versatile functions in multiple physiological processes, including migration, autophagy, phagocytosis, and mitochondrial function, among others. Mounting studies have also demonstrated the role of LRRK2 in mediating neuroinflammation, the prominent hallmark of PD, and intricate functions in immune cells, such as microglia, macrophages, and astrocytes. Of those, microglia were extensively studied in PD, which serves as the resident immune cell of the central nervous system that is rapidly activated upon neuronal injury and pathogenic insult. Moreover, the activation and function of immune cells can be achieved by modulating their intracellular metabolic profiles, in which LRRK2 plays an emerging role. Here, we provide an updated review focusing on the double-faceted role of LRRK2 in regulating various cellular physiology and immune functions especially in microglia. Moreover, we will summarize the latest discovery of the three-dimensional structure of LRRK2, as well as the function and dysfunction of LRRK2 in immune cell-related pathways.
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease. PD affects at least 1% of people over the age of 65 and at least 4% of people over the age of 80 (Elbaz et al., 2016). The major pathological features of PD patients are the loss of dopaminergic (DA) neurons in the substantia nigra (SN) pars compacta, as well as the abnormal accumulation of α-synuclein that leads to the formation of Lewy bodies (Figure 1). Furthermore, PD is a progressive degenerative disease, a classic clinical motor syndrome characterized by bradykinesia, tremors, muscle rigidity, and postural instability. In addition to typical motor symptoms, PD patients also manifested several non-motor symptoms such as depression, anxiety, hallucinations, cognitive impairment, orthostatic hypotension, or sleep disturbance (Hussein et al., 2021; Figure 1). Clearly, the clinical diagnosis of PD is mainly based on the patient’s clinical symptoms, medical history, and responses to dopamine drug treatment. Of the approximately 145 PD treatments currently in clinical trials, 39% of the trials focus on long-term disease-modifying treatments, and the rest focus on short-term symptom relief treatments (McFarthing et al., 2020). What’s more, numerous clinical and basic research have linked autoimmune diseases, impaired cellular and humoral immune responses, inflammatory cell activation, and immune dysregulation to the pathogenesis of PD (Tan et al., 2020). Although the pathogenesis of PD has not been fully elucidated, there is increasing evidence indicating that PD is an immune-mediated inflammatory disorder.
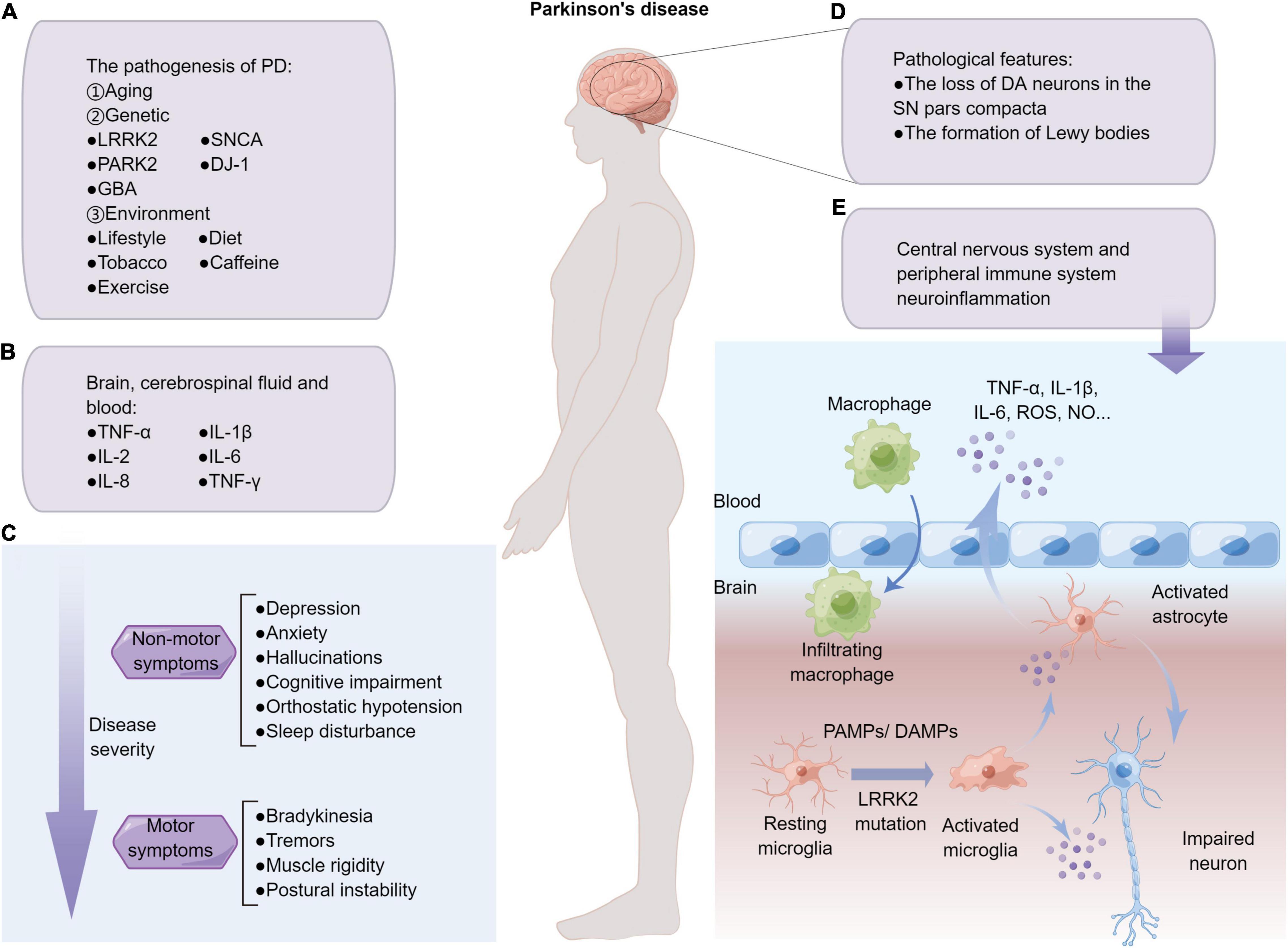
Figure 1. Overview of PD and neuroinflammation. (A) The pathogenesis factors of PD mainly include aging, genetic and environmental factors. (B) PD is a disease of systemic inflammation with pro-inflammatory factors that have been detected in the human brain slices, cerebrospinal, and blood. (C) Patients with PD often present with non-motor symptoms, which often precedes the development of motor symptoms. (D) Two major pathological features of Parkinson’s patients. (E) PAMPs/DAMPs stimulation, as well as PD-related gene mutation such as LRRK2G2019S, promote resting microglia skewing into pro-inflammatory phenotypes, which secrete excessive pro-inflammatory factors that may damage neurons and activate astrocytes in the milieu. The damaged blood-brain barrier of PD patients further causes neuroinflammation to diffuse in the CNS and peripheral system, and develop from acute inflammation to systemic chronic inflammation.
The pathogenesis of PD is yet to be fully elucidated. Apart from old age, other factors such as environmental factors and genetic deficiencies also contribute to the degeneration of DA neurons (Ascherio and Schwarzschild, 2016; Figure 1). Although the majority of PD patients have sporadic forms, with only 5–10% hereditary (Rocha et al., 2018), genome-wide association studies (GWAS) have reported a critical role for genetic variants that might contribute to idiopathic PD pathogenesis (Nalls et al., 2014). The pathology of PD not only exhibited on DA neurons, but also on non-neural cells, such as glia cells. Specifically, microglia-mediated neuroinflammation is a prominent hallmark of PD. Interestingly, most of the PD pathogenic genes identified so far, such as α-Synuclein, Parkin, DJ-1, LRRK2, and GBA, are expressed in microglia cells, albeit with complicated and even controversial effects (Martin et al., 2011; Dzamko et al., 2015). Studying the functions of PD-related genes, such as LRRK2, is of great significance for delving the onset and development of neurodegenerative diseases (Dachsel and Farrer, 2010). Currently, delivering LRRK2 kinase inhibitors such as DNL151 has become an important avenue for treating LRRK2 diseased patients (Ding and Ren, 2020). Mutations in LRRK2 are a relatively common cause of familial late-onset PD, and also linked to the more numerous sporadic PD, suggesting that understanding LRRK2-associated mechanisms might be a gateway to exploring sporadic PD (Rocha et al., 2022). Notably, the multi-domain structure of LRRK2 renders its versatile functions in multiple physiological processes, including migration, autophagy, phagocytosis, and mitochondrial function, among others. In this review, we therefore summarized the latest progress in the double-faceted role of LRRK2 in regulating various cellular physiology and particularly immune functions.
Microglial Activation and Systemic Inflammation
Microglia, as resident immune cells in the central nervous system (CNS), generally interact with other cells in the milieu and play a crucial role in maintaining brain homeostasis. In the normal brain, microglia are considered “resting” that constantly surveil the brain microenvironment and actively contact neuron synapses (Kettenmann et al., 2013). However, microglia could be activated by any type of pathological event or changes in brain homeostasis, including various PAMPs and DAMPs, and rapidly proliferate and accumulate at the injury site, where they engulf dead cells and secrete pro-inflammatory factors such as TNF-α, IL-1β, IL-6, as well as reactive oxygen species (ROS) and nitric oxide (NO) (Sanchez-Guajardo et al., 2013; Wolf et al., 2017). However, the persistent over-activated phenotypes of microglia are detrimental to resident neurons, so that the activation and subsequent resolution of microglia-mediated neuroinflammation are strictly controlled (Perry and Teeling, 2013; Marinelli et al., 2019). Specifically, under the diseased context, chronic pathological factors such as repeated exposure to environmental toxins/PAMPs/DAMPs, genetic susceptibility, as well as abnormal immune responses, might exist. Meanwhile, increased levels of pro-inflammatory cytokines were observed in the brain, cerebrospinal fluid, and blood of PD patients, especially tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-2, IL-6, IL-8, and interferon-γ (IFN-γ) (Dzamko et al., 2015; Marogianni et al., 2020; Figure 1). As such, the over-activation of microglia may be prolonged, which further triggers a vicious cycle of chronic neural degeneration and pro-inflammation (Newcombe et al., 2018). Furthermore, microglia have been identified as key regulators of synaptic remodeling and brain wiring. Studies have shown that GABA initiates a transcriptional synapse remodeling program within GABA-receptive microglia, promoting direct network connections between neurons and microglia (Favuzzi et al., 2021). Recent studies have shown that microglia are involved in regulating synaptic remodeling and pruning through different molecular mechanisms, such as the cytokine interleukin-33 (IL-33) (Nguyen et al., 2020), phosphatidylserine (Scott-Hewitt et al., 2020), and complement component 3 (C3) (Werneburg et al., 2020). Moreover, Xia et al. (2021) found that the interaction of α-synuclein and toll-like receptor 2 (TLR2) of microglia leads to the phagocytosis of α-synuclein and further activates microglia. During neuroinflammation and neurodegeneration, complex mechanisms of crosstalk between astrocytes and other cells also occurred in the CNS (Linnerbauer et al., 2020). Studies have shown that interaction of astrocytes and microglia favors the clearance of amyloid-β and α-synuclein aggregates, highlighting the important role of glial crosstalk in the progression of PD (Rostami et al., 2021). Therefore, targeting microglia-mediated neuroinflammation might produce promising therapeutic benefits (Prinz et al., 2019).
Notably, neuroinflammation of the CNS and peripheral immune system are closely associated that act in synergy to the pathogenesis and development of PD (Tansey and Goldberg, 2010; Gelders et al., 2018; Figure 1). As one of the most sophisticated organs, the brain exists extensive bi-directional communication between resident immune cells with the peripheral immune system, the disruption of which could lead to cognitive and behavioral disorders (Filiano et al., 2015; Louveau et al., 2015; Kipnis, 2016). Systemic chronic inflammation is the pathological basis of neurodegenerative diseases including PD (Furman et al., 2019). When the risk factors are eliminated but the inflammatory response continues, it will lead to the occurrence of immune tolerance (Straub, 2017). It is known that macrophages and microglia play an important role in communication between the peripheral immune system and the CNS. Importantly, PD-related genes including LRRK2 have been confirmed to exist both in CNS immune cells and peripheral immune cells; the communication between the two immune systems carrying PD mutations might render the brain easily reaching the critical threshold of inflammation, thereby exacerbating disease development (Huang et al., 2021).
Leucine-Rich Repeat Kinase 2: Structure and Role in the Immune Cells
The Structure and Function of Leucine-Rich Repeat Kinase 2
Although the underlying mechanisms of pathogenic mutations contribute to PD are yet fully investigated, the involvement of LRRK2 in the pathogenesis of PD has been continuously established. LRRK2 is expressed at a comparatively low level in the brain, but is highly expressed in the lung, spleen, kidney, and peripheral immune cells such as neutrophils, monocytes, and dendritic cells (Biskup et al., 2007; Dzamko, 2017; Lee et al., 2017). Di Maio et al. (2018) have shown that, independent of mutations, wild-type (WT) LRRK2 plays a role in idiopathic PD and that LRRK2 kinase inhibitors can be used to treat idiopathic patients without LRRK2 mutations. LRRK2 mutations are the most frequent cause of autosomal dominant PD, accounting for approximately 3% of all cases (Paisan-Ruiz et al., 2004; Zimprich et al., 2004). The LRRK2 protein is a complex multi-domain protein with a size of 286 kDa, which contains seven domains, including armadillo repeat motif (ARM), ankyrin repeat (ANK), leucine-rich repeat (LRR), ras-of-complex (ROC), C-terminal of ROC (COR), kinase (KIN), and WD40 domains (Mills et al., 2018; Figure 2). The nature of large size and complex domains of LRRK2 protein thus might produce both great challenges and immense significance for medical research (Mata et al., 2006; Rui et al., 2018). Moreover, the structural differences between human and mouse LRRK2 determine the diverse characteristics of the LRRK2 protein. The mouse model is widely used as the major PD animal model for studying LRRK2 functions, which remains different from the actual clinical situation (Langston et al., 2019). Newly identified structures of LRRK2, as well as a better understanding of the interaction between LRRK2 and microtubules, canthus help elucidate the role of LRRK2 in the pathogenic mechanism of PD (Leschziner and Reck-Peterson, 2021).
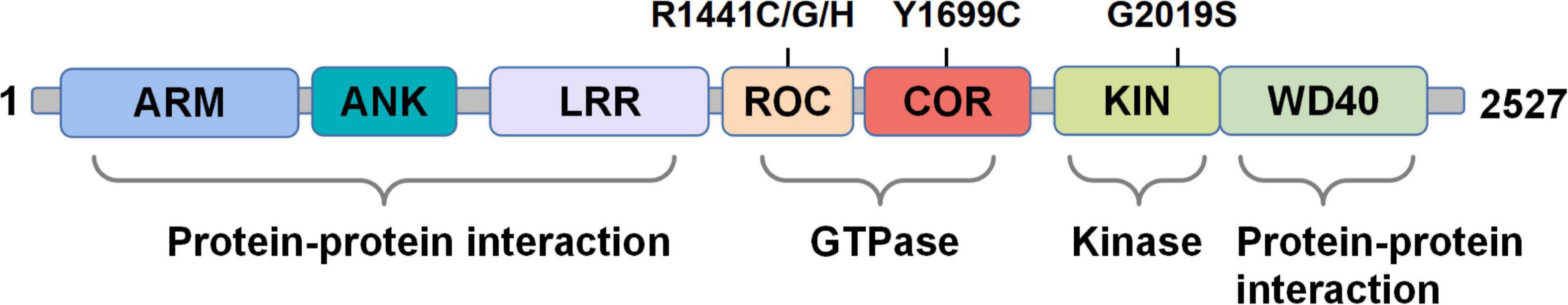
Figure 2. The domain structure of the LRRK2 protein. The domains are divided into protein-protein interaction, GTPase, kinase, and protein-protein interaction according to their functions. PD-associated mutations mentioned in the review are depicted on top.
Numerous studies have revealed that LRRK2 mutants related to the pathogenesis of PD could lead to the over-activation of LRRK2 protein, in which G2019S and R1441C/G are the most common ones (Li et al., 2014). Specifically, the G2019S mutation is located at the kinase domain, while the R1441C/G mutations are located at the ROC GTPase domain (Iannotta et al., 2020). Functional alterations associated with pathogenic LRRK2 mutations include, but are not limited to, alterations in vesicular trafficking and cytoskeleton dynamics, autophagy and lysosomal degradation, neurotransmission, mitochondrial function, and immune/microglial responses (Herzig et al., 2011; Chan and Tan, 2017; Cookson, 2017; West, 2017; Chen et al., 2018a; Shihabuddin et al., 2018; Cresto et al., 2019). The elaborated analysis of the direct relationship between the structure and function of LRRK2 would thus be helpful in the treatment of PD (Zhang et al., 2019; Deniston et al., 2020; Bolz et al., 2021). A recent study demonstrated that pathological α-synuclein activates LRRK2 expression and kinase activity in monocytes, inhibition of which may attenuate the pro-inflammatory monocyte responses in the brain (Xu et al., 2022). It is worth noting that the treatment of PD is not limited to modulating the LRRK2 kinase activity, other physiological processes mediated by LRRK2 such as autophagy could also be potential therapeutic targets (Wojewska and Kortholt, 2021). To determine how LRRK2 protein levels regulate inflammatory cytokine/chemokine levels in human immune cells, Ahmadi Rastegar et al. (2022) demonstrated that the LRRK2G2019S mutation may aggravate inflammation following TLR activation in the differentiated monocytes and macrophages from human induced pluripotent stem cells (iPSCs), whereas LRRK2 kinase inhibitors manifest limited effect on TLR-mediated inflammation. More importantly, unlike other PD-related genes, hereditary and sporadic PD carrying LRRK2 mutations have a high degree of consistency in clinical characteristics and treatment response (Kett and Dauer, 2012; Tolosa et al., 2020). Based on these, the LRRK2 gene has become an important target for studying both hereditary and sporadic PD.
The G2019S mutation in the KIN domain of LRRK2 leads to increased kinase activity plausibly explaining the abnormal LRRK2 kinase activity. However, current studies still have many puzzles in explaining how mutations in the ROC and COR domains alter the function of the LRRK2 protein. Therefore, there is an urgent need for high-resolution LRRK2 structures to facilitate a deep understanding of the functions of individual domains. In the past few years, the high-resolution structures of LRRK2 protein have been successfully revealed, by using a combination of in vitro and in situ techniques (Deniston et al., 2020; Watanabe et al., 2020; Myasnikov et al., 2021; Tokars et al., 2021; Ullrich, 2021). Specifically, Weng et al. (2022) generated a comprehensive dynamic allosteric map of the C-terminal domain of LRRK2 (LRRK2RCKW) using hydrogen-deuterium exchange mass spectrometry (HDX-MS) and molecular dynamics (MD) simulations, while confirming that the kinase domain is a central hub for conformational control. Another study has obtained the structure of the mutant LRRK2I2020T at the in situ level of the cell, which forms a double helix structure around microtubules (Watanabe et al., 2020). This study later constructed a microtubule atom model related to LRRK2, confirming that the closed conformation of the LRRK2 N-terminal catalytic structure can block the movement of the microtubule motor, whereas kinase inhibitors can interfere with the obstructive effect of LRRK2 (Deniston et al., 2020).
It is well-established that LRRK2 has three quaternary structures: monomer, dimer, and oligomer, which were found to display different biological activities. Tokars et al. (2021) cracked the structure of LRRK2 monomer, dimer and mutant G2019S based on the high-resolution structure of the full-length human LRRK2, and thus explained the internal structure, assembly and activity regulation mechanism of LRRK2. Myasnikov et al. (2021) further confirmed that COR-mediated single point mutations at the LRRK2 dimer interface can eliminate the pathogenic filaments formed by LRRK2. Specifically, the link between LRRK2 and microtubules have shed new insights into exploring the exact functions of LRRK2 and its inhibitors.
Leucine-Rich Repeat Kinase 2 Expression in Immune Cells
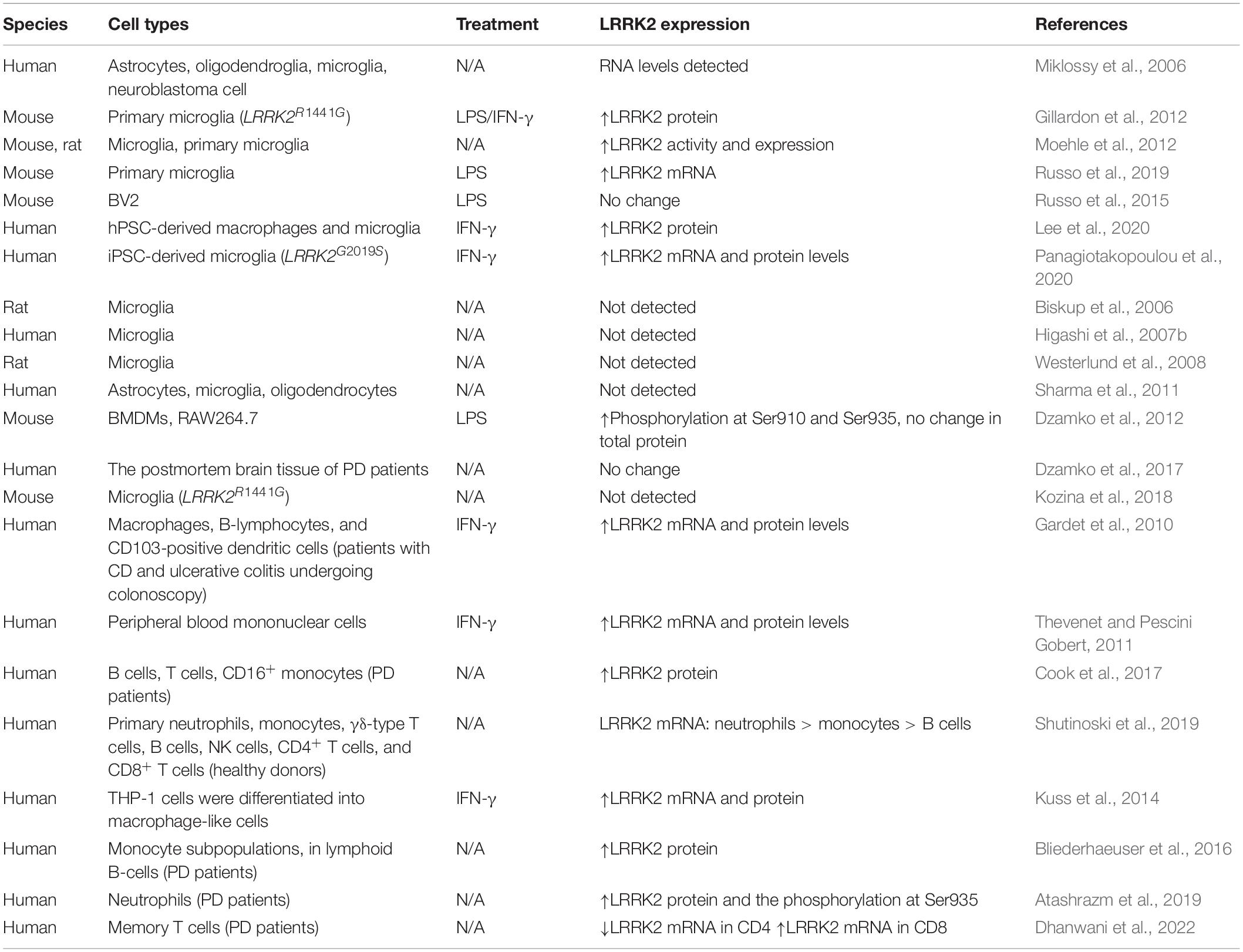
To understand the physiological and pathological functions of LRRK2, it is important to recognize the role of LRRK2 in different types of immune cells (Table 1). Most previous efforts to elucidate the LRRK2 functions during neuroinflammation have been focused on microglia, which is the first barrier of the brain’s innate immune system. A recent study revealed that LRRK2 mediated the neurotoxicity of microglia through phosphorylation, interaction and activation of the nuclear factor of NFATc2 in the mouse model of synucleinopathies (Kim et al., 2020; Figure 3). LRRK2-NFATc2 signaling axis may be a new therapeutic target for PD. Advances in the study of LRRK2 as a target for the treatment of PD bring hope to patients who are deeply troubled and harmed by the disease. Various studies have shown that LRRK2 mRNA and protein expression levels in human and rodent microglia were upregulated upon stimulation with lipopolysaccharide (LPS) in vitro (Miklossy et al., 2006; Gillardon et al., 2012; Moehle et al., 2012; Russo et al., 2015, 2019). Similarly, the LRRK2 expression in both human pluripotent stem cell (hPSC)-derived macrophages and microglia was enhanced in a time-dependent manner after IFN-γ treatment (Lee et al., 2020; Panagiotakopoulou et al., 2020). However, there also exhibits conflicting results. Several studies failed to determine the expression of LRRK2 in microglia from WT mice (Biskup et al., 2006; Higashi et al., 2007b; Westerlund et al., 2008), neither in brain slices of PD patients and healthy controls (Higashi et al., 2007a; Hakimi et al., 2011; Sharma et al., 2011; Dzamko et al., 2012, 2017). In another study, microglia isolated from rodent brains cannot induce the LRRK2 expression after LPS stimulation (Kozina et al., 2018).
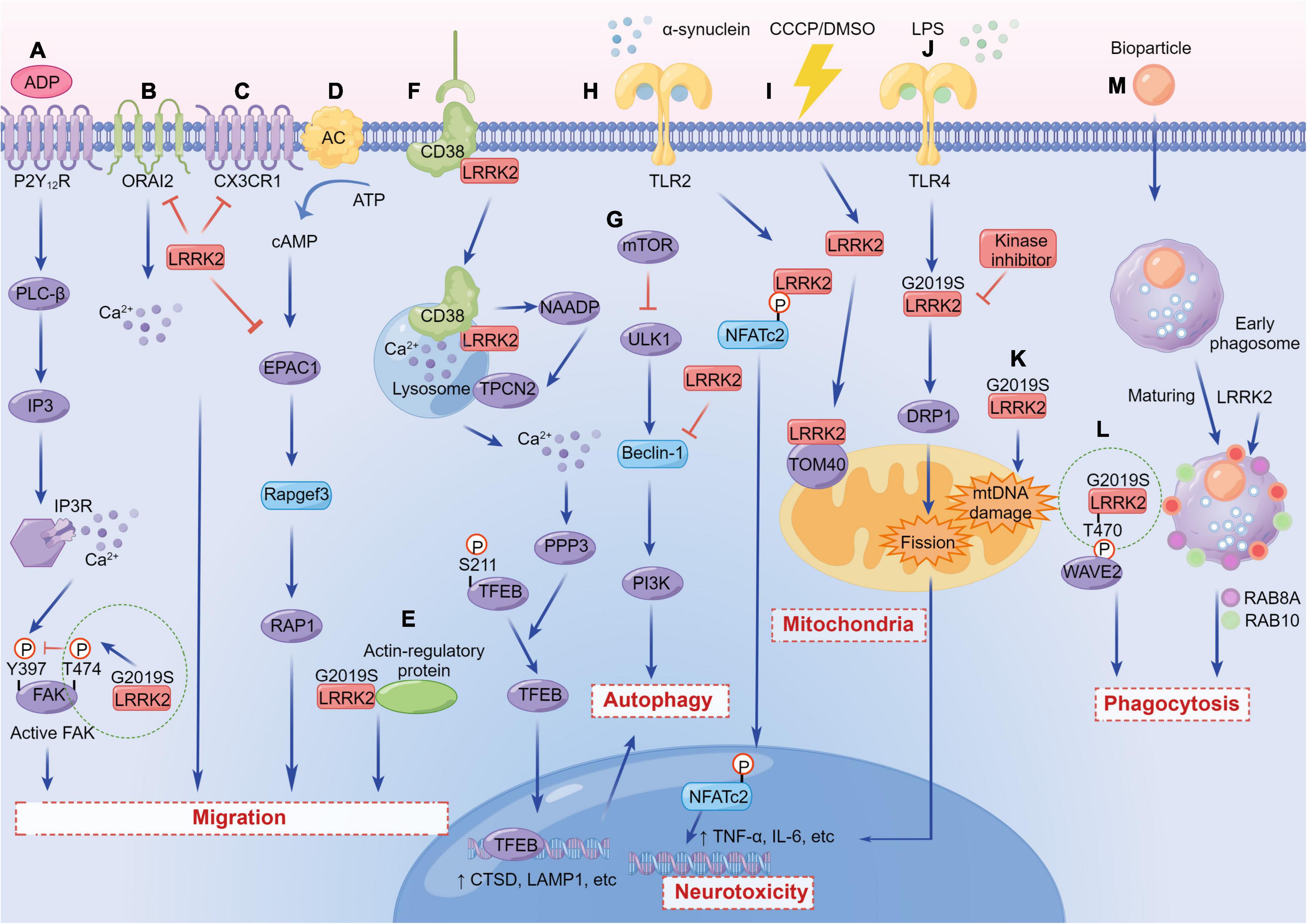
Figure 3. The versatile functions of LRRK2 in immune cells. (A) LRRK2G2019S regulates microglial motility through inhibition of FAK. (B) LRRK2 suppresses the migration of microglia and enhances microglial inflammation by inhibiting the activity of CX3CR1. (C) LRRK2 modulates dendritic cells migration by interfering with ORAI2. (D) LRRK2 suppresses EPAC-1 activity, further restricting motility in macrophages. (E) LRRK2G2019S enhances chemotaxis through enhanced interaction with actin-regulatory proteins in myeloid cells. (F) LRRK2 regulates autophagy by mediating the physiological CD38-LRRK2-TFEB signaling axis in B-lymphocytes and macrophages. (G) LRRK2 inhibits Beclin-1-induced macroautophagy independently of mammalian target of rapamycin (mTOR) and Unc-51-like kinase 1 (ULK1). (H) LRRK2 promotes a neuroinflammatory cascade by selectively phosphorylating and inducing nuclear translocation of the NFATc2. (I) WT and LRRK2G2019S associate and co-localize with subunits of the TOM complex, either under DMSO or carbonyl cyanide m-chlorophenyl hydrazine (CCCP) conditions. (J) LRRK2 promotes microglial mitochondrial alteration via DRP1 in a kinase-dependent manner, initiating pro-inflammatory responses. (K) LRRK2G2019S causes a significant increase in mtDNA damage in PD patient-derived immune cells. (L) LRRK2G2019S binds and phosphorylates WAVE2 at Thr470, stabilizes and prevents its proteasomal degradation, and increases WAVE2-mediated phagocytosis. (M) LRRK2 is required for RAB8A and RAB10 recruitment to phagosomes in macrophages.
Yet, LRRK2 was involved in the immune regulation of PD in both peripheral systems and CNS (Lee et al., 2017). LRRK2 is most highly expressed in bone marrow cells such as monocytes, dendritic cells, and neutrophils, whereas minimally expressed in B cells and T cells (Gardet et al., 2010; Hakimi et al., 2011; Thevenet and Pescini Gobert, 2011; Daher et al., 2015; Cook et al., 2017). Using fluorescence-activated cell sorting (FACS) of white blood cell subtypes isolated from the venous blood of healthy people, the LRRK2 expression is found highest in neutrophils, followed by monocytes, and then B cells (Shutinoski et al., 2019). LRRK2 mRNA and protein levels in both macrophages and leukocytes in vitro were up-regulated after exposure to pathogens and inflammatory mediators such as IFN-γ, IFN-β, TNF-α, and IL-6 (Hakimi et al., 2011; Thevenet and Pescini Gobert, 2011; Kuss et al., 2014). Similarly, the levels of LRRK2 protein in PD patients’ B cells, T cells (CD4+, CD8+, and T regulatory cells), CD14+, and CD16+ monocytes were increased compared with healthy controls (Bliederhaeuser et al., 2016; Cook et al., 2017). Moreover, this study revealed that the expression of LRRK2 protein and its phosphorylated level at Ser935 were significantly increased in neutrophils of PD patients (Atashrazm et al., 2019). Very recently, Dhanwani et al. (2022) first discovered that memory T cells expressed LRRK2 in the peripheral blood of diagnosed motor PD, which was differentially expressed in CD4 and CD8 memory T cells. LRRK2 was downregulated in CD4 and upregulated in CD8 memory T cells in PD patients, indicating that the two kinds of T cells play opposing roles in PD-related T-cell autoimmunity.
The Role of Leucine-Rich Repeat Kinase 2 in the Immunopathogenesis of Parkinson’s Disease
LRRK2 protein is a complex multi-domain protein with a size of 286 kDa and contains seven domains (Mills et al., 2018), which endows its sophisticated function during the immunopathogenesis of PD. For example, LRRK2 serves a variety of functions including protein translation, cytoskeleton remodeling, vesicle transport, autophagy, mitochondrial homeostasis, and so on. LRRK2-mediated neuroinflammation is tightly associated with those functions. It is well known that immune cells are indispensable for homeostasis, immune defense, and tissue repair, hence the migration of immune cells to the injury sites is a key factor of disease progression in PD. LRRK2 mutants display significant impairments of selective forms of autophagy (i.e., mitophagy and chaperone-mediated autophagy) and lysosomal function (Albanese et al., 2022). As the energy factories of cells, mitochondria are involved in the regulation of cell growth, apoptosis and other processes, and mitochondrial dysfunction is related to a variety of neurodegenerative diseases. Here, we will summarize the versatile functions of LRRK2 in immune cells that may contribute to more comprehensive of PD therapy.
Migration
Recent studies have shown that LRRK2 mediates the process of cell migration to sites of injury or infection. LRRK2 exerts a reducing effect on microglial migration. Microglia carrying LRRK2G2019S showed ADP-induced motor retardation and delayed injury isolation, and LRRK2-knockdown microglia are highly motile compared with control cells (Choi et al., 2015; Figure 3). Moreover, LRRK2-null microglia migrated faster and traveled a longer distance toward the regulation of chemokine (C-X3-C) receptor 1 (CX3CR1)-mediated signaling pathways (Ma et al., 2016; Figure 3). It has been shown that microglia carrying the LRRK2G2019S mutation show ADP-induced motor deficits. The underlying mechanism is that LRRK2 binds focal adhesion kinase (FAK) and phosphorylates its Thr-X-Arg/Lys (TXR/K) motif(s), resulting in a decrease in FAK’s pY397 phosphorylation (Choi et al., 2015; Figure 3).
Similarly, the phenotypic and functional characteristics for migration have been observed in immune cells. The migration ability of human monocytes differentiated from iPSCs carrying the G2019S mutation has a moderate defect compared with the control (Speidel et al., 2016). Conversely, LRRK2 deficiency in mouse dendritic cells mainly manifested the enhancement of migration by interfering with ORAI2 (Yan et al., 2019; Figure 3). In addition, RNA sequencing (RNA-seq) profiled transcriptomic changes of activated primary macrophages from both WT and LRRK2-knockout (KO) mice, and found that LRRK2 may inhibit cAMP/EPAC-1 activity, further restricting movement and effective migration of cells to the site of neuronal injury (Levy et al., 2020; Figure 3).
However, in another study, both in vitro and in vivo experiments on the myeloid cells expressing LRRK2G2019S showed that LRRK2 mutant enhanced chemotaxis through the association between LRRK2 and actin-regulatory protein, which is blocked by the treatment of LRRK2 kinase inhibitors (Moehle et al., 2015; Figure 3). In conclusion, LRRK2 affects the migration of microglia and other immune cells, but the functions of elevated LRRK2 expression or carrying LRRK2 mutations in different cell types might have double-faceted roles.
Autophagy
Autophagy refers to the recycling of intracellular components by degrading dysfunctional or damaged proteins and organelles within the cell. Multiple studies have shown that LRRK2 protein is related to impaired autophagy (Schapansky et al., 2014; Cherubini and Wade-Martins, 2018; di Domenico et al., 2019). LRRK2 was first reported to regulate autophagy specifically in immune cells where monocytic cell lines (BV2 and RAW264.7) increased LRRK2 translocation to autophagosome membrane after LPS stimulation, whereas loss of LRRK2 lead to autophagic deficits (Schapansky et al., 2014). Inhibition of LRRK2 kinase activity also reduced autophagic degradation, reminiscent of the importance of the kinase domain in regulating autophagy. In consistent with this observation, toll-like receptor 4 (TLR4)-mediated microglial activation showed increased expression of endogenous LRRK2 and further increased autophagic flux (Moehle et al., 2012; Schapansky et al., 2014). In LRRK2-KO macrophages, autophagy was defective and not able to be converted to glycolytic metabolism after LPS treatment, whereas the pathogenic LRRK2G2019S mutant caused overwhelming autophagy (Nabar et al., 2021; Figure 3). Collectively, the above studies have demonstrated that LRRK2 is significantly beneficial for autophagy.
Conversely, some studies have gave rise to opposite results. Compared with the normal control, the iPSCs derived astrocytes from PD patients carrying the G2019S mutation showed a significant reduction in autophagic flux (di Domenico et al., 2019). PD-related LRRK2 mutations (G2019S, R1441C, or Y1699C) in astrocytes disrupted the function of lysosomes in a kinase-dependent manner. The autophagy flux could be restored upon LRRK2 knockdown or supplementation with LRRK2 kinase inhibitors (Henry et al., 2015). LRRK2 overexpression caused inactivation of Beclin-1 and inhibition of autophagy in mouse bone-marrow-derived dendritic cells (Manzoni et al., 2016; Figure 3). Moreover, Chen et al. (2018b) found that the Mn exposure up-regulated microglial LRRK2 expression both in vitro and in vivo, accompanied by autophagy dysfunction, which could be reversed by the inhibition of LRRK2. Also, the reduction of autophagy marker, LC3-II, has been demonstrated in cultured bone marrow-derived macrophages (BMDM) from mice carrying LRRK2R1441C mutation (Hakimi et al., 2011). In summary, the link between LRRK2 and autophagy has been widely studied, albeit with conflicting results. However, the specific mechanisms of how LRRK2 regulates immune autophagy and lysosomal degradation are yet completely understood, which requires more efforts and explorations.
Phagocytosis
Enhanced phagocytosis is associated with increased kinase activity in both macrophages and microglia from PD patients and mice. LRRK2 regulates the phagocytic responses by binding and phosphorylating the Thr470 site of actin-cytoskeletal regulator, WASP-family verprolin-homologous protein-2 (WAVE2) (Kim et al., 2018; Figure 3). Studies have shown that the RAB protein network was essential for the maturation of phagosomes by proteomics analysis (Gutierrez, 2013). Specifically, RAB5A co-localizes with the complex, formed during the phagosome-early endosome fusion of LRRK2 and WAVE2 in BMDM (Kim et al., 2018). A recent in vitro study indicates that LRRK2 and WAVE2 are important mediators of cytokine production and cytoskeletal rearrangement necessary for microglia-induced neurotoxicity (Fenner et al., 2022). LRRK2 is also required for the recruitment of RAB8A and RAB10 to phagosomes in macrophages derived from hPSCs (Lee et al., 2020; Figure 3). Meanwhile, endogenous LRRK2 is involved in the process of phagocytosis and bacterial killing in human immune cells; LRRK2 knockdown could dampen the production of ROS (Gardet et al., 2010). Multiple studies have also demonstrated that microglia from mice with LRRK2G2019S mutant showed increased cell activity and phagocytic responses in vitro (Choi et al., 2015; Kim et al., 2018; Dwyer et al., 2020). After trans activator of transcription (Tat) treatment, LRRK2 kinase inhibitors can inhibit the expression of key receptors for phagocytosis (brain-specific angiogenesis inhibitor 1, BAI1), thereby preventing phagocytosis in murine microglial line BV2 (Marker et al., 2012).
On the contrary, reducing LRRK2 kinase activity in both mouse and human macrophages could control the replication of Mycobacterium tuberculosis by enhancing the maturation of phagosomes, regardless of autophagy (Hartlova et al., 2018). Knockout of LRRK2 in microglia increased the number of RAB5-positive endosomes, which later increased levels of the uptake and clearance of α-synuclein aggregates (Maekawa et al., 2016). In summary, for different models and immune cells, dissimilar mutations of LRRK2 play disparate regulatory roles in phagocytosis.
Mitochondria
Mitochondrial dysfunction is one of the pathological manifestations of neurodegenerative diseases such as PD. LRRK2 in mainly located in the cytoplasm, while around 10% is present in the mitochondria of cells with LRRK2 overexpression (Biskup et al., 2006). The precipitation, super-resolution structured illumination microscopy (SR-SIM), and 3D virtual reality (VR) assisted colocalization analysis showed that the overexpression of LRRK2G2019S leads to the formation of large perinuclear aggregates colocalized with the TOM (the translocase of outer mitochondrial membrane) complex (Neethling et al., 2019; Figure 3). The research models of LRRK2 and mitochondria mainly focus on autopsy tissues from PD patients and animal models carrying LRRK2 mutants, as well as various cell models of LRRK2-related diseases. So far, multiple manifestations of mitochondrial dysfunction in PD have been revealed, including increased oxidative stress, decreased mitochondrial membrane potential, decreased ATP production, aggravated mitochondrial DNA (mtDNA) damage, mitochondrial elongation, mitochondrial fragmentation, and damaged mitochondrial phagocytosis (Singh et al., 2019).
Pathogenic LRRK2 mutants are associated with increased sensitivity to oxidative stress that lead to extensive cell death. The mutations could also impair the antioxidant defense of mitochondria through several different mechanisms. In BV2 cells and primary microglia cells, LRRK2G2019S revealed a decrease in mitochondrial area and shortage of microglial processes through DRP1 in a kinase-dependent manner, thereby initiating pro-inflammatory responses (Ho et al., 2018; Figure 3). The LRRK2 deficiency changed the expression of natural immune genes in macrophages driven by mitochondrial stress, suggesting that LRRK2-dependent mitochondrial defects may be involved in the regulation of innate immunity (Weindel et al., 2020).
The LRRK2G2019S mutation also causes mtDNA damage, which is dependent on LRRK2 kinase activity. Specifically, the mtDNA damage in immune cells derived from PD patients with the LRRK2G2019S mutation was significantly observed, and the mitochondrial mass and mtDNA copy number were also increased (Gonzalez-Hunt et al., 2020; Figure 3). Several studies have shown that the transport and movement of axon mitochondria are inhibited in mutants carrying LRRK2R1441C/G2019S (Godena et al., 2014). LRRK2 kinase inhibitors failed to alleviate those defects related to mitochondrial transport (Hsieh et al., 2016; Thomas et al., 2016; Schwab et al., 2017). A recent study confirmed that microglia transport mitochondria through tunnel nanotubes to co-degrade with neighboring microglia. However, the transfer strategy is compromised in the microglia carrying LRRK2G2019S (Scheiblich et al., 2021).
Moreover, the extra roles of LRRK2 in mitochondrial biology has yet studied exclusively in immune cells. For example, recent studies have shown that LRRK2 affected the autophagy pathway of mitochondria by direct and indirect effects. The LRRK2G2019S mutation played a direct role in delaying mitochondrial phagocytosis by disrupting the removal of MIRO (Hsieh et al., 2016). Conversely, LRRK2 caused mitochondrial autophagy defects by affecting mitochondrial membrane potential damage (Su et al., 2015) and susceptibility to mitochondrial toxins (Novello et al., 2021). LRRK2 directly participates in the calcium homeostasis of mitochondria. The loss, inhibition, and mutation of LRRK2 lead to impaired mitochondrial Ca2+ buffering capacity, which leads to damage and degradation of mitochondrial function (Cherra et al., 2013; Schwab and Ebert, 2015; Ludtmann et al., 2019). In addition, LRRK2 has also been shown to alter mitochondrial dynamics and quality control. Basically, the fission and fusion process of mitochondria is regulated by stringent molecular mechanisms of dynein-related GTPases and WD40 repetitive proteins. Interestingly, LRRK2 contains both GTPase and WD40 domains, which structurally hints the importance of LRRK2 in mitochondrial dynamics (Walter et al., 2019).
Mitochondria are the main sites of intracellular energy synthesis and play an important role in maintaining the normal physiological functions of cells. According to the above studies, LRRK2 has been shown to be involved in various mitochondrial pathways, however, there still exist several questions in studying the link between LRRK2 and mitochondrial dysfunctions specifically in immune cells including microglia. Furthermore, how mitochondrial dysfunction mediates the immunopathogenesis of PD remains to be fully elucidated.
Immunometabolism
Metabolism is the last step in the research pipeline, explaining the association of discovered metabolites with biological processes or biological states. In a sense, it may be considered that metabolism better reflects the real situation of the interaction between genes and the environment. Notably, as an important branch of systems biology, metabolomics has many unique advantages compared with other genomics, such as transcriptomics, and proteomics. For example, metabolites can be directly correlated to phenotypic changes in an organism while being more easily detected and metabolites’ functions clearer. Immunometabolism is an emerging field to study the interaction between immune cells and metabolic processes (Artyomov and Van den Bossche, 2020). With the latest advances, several findings highlighted multiple shared pathways between immune and metabolic processes, which are highly unified yet interdependent.
Basically, in resting cells, the major metabolic pathway is oxidative phosphorylation (OXPHOS), in which glucose is metabolized to pyruvic acid by glycolysis, and most pyruvic acid enters the tricarboxylic acid (TCA) cycle (Paolicelli and Angiari, 2019). However, in highly proliferative or tumor cells, the metabolic pathway switches from OXPHOS to aerobic glycolysis, which is known as the Warburg effect (Warburg et al., 1927). Importantly, this metabolic reprogramming mechanism also recapitulates in innate immune cells, including microglia, activated after LPS stimulation (Kelly and O’Neill, 2015). Moreover, LRRK2 regulates the glycolytic switch and cytokine production in response to stimulation by IFN-γ in microglia (Panagiotakopoulou et al., 2020).
So far, metabolomics in immune cells is substantially more challenging than transcriptomics and proteomics. Studies have shown that astrocytes derived from patient-specific iPSCs with LRRK2G2019S mutation have lower glycolysis levels than from healthy people, and the metabolic profile has occurred significant changes (Sonninen et al., 2020). To more truly and accurately reflect the role of LRRK2 in PD, studies have directly performed metabolite testing on patients with LRRK2 mutations. The metabolomics analysis of the cerebrospinal fluid showed that six metabolic pathways changed: fatty acid metabolism, beta-oxidation of short-chain fatty acids (SCFAs), bile-acid metabolism, spermidine, and spermine biosynthesis, methionine metabolism, mitochondrial beta-oxidation of long chain fatty acids (LCFAs), and methionine metabolism. The study again proved that bile acid metabolism is one of the major abnormal metabolic pathways in PD patients carrying LRRK2 mutations (Yilmaz et al., 2020). Other studies have tested the metabolomics of the plasma of patients with LRRK2 G2019S or R1441G mutations. Compared with the control, they did not combine bile acids (cholic acid, lithocholic acid, and deoxycholic acid), and intermediate metabolites of purine bases (especially hypoxanthine) levels were found elevated (Yakhine-Diop et al., 2020). Moreover, a recent study also demonstrated that LRRK2-KO macrophages were unable to switch to glycolytic metabolism after LPS treatment (Nabar et al., 2021).
Perspectives
As an essential pathogenic gene, LRRK2 plays versatile roles throughout development of PD. However, LRRK2 seems to have diverse effects in different immune cells and PD models (Figure 4), which should be explored in future studies. For a long time, neuroscientists have largely focused on the role of LRRK2 in neurons, where the endogenous LRRK2 expression is low (Schapansky et al., 2014). As such, numerous studies have relied on the overexpression of LRRK2 in cell lines or animal models, which might not recapitulate the genuine physiological interaction of LRRK2 (Takagawa et al., 2018; Liu et al., 2020). Furthermore, there remain several unanswered questions about LRRK2, due to the complex structure of LRRK2 protein and the diversity of models in LRRK2-related research. As is well known, the phosphorylation level of LRRK2 is one of the conditions that researchers are more concerned about, especially the two phosphorylation sites of G2019S and R1441C. However, whether LRRK2 phosphorylation is beneficial or harmful in humans is still controversial. Since most of the data of LRRK2 phosphorylation were derived from the condition of overexpressed LRRK2, the studies on the phosphorylation of endogenous LRRK2, at a lower level, probably with more appropriate models, are required.
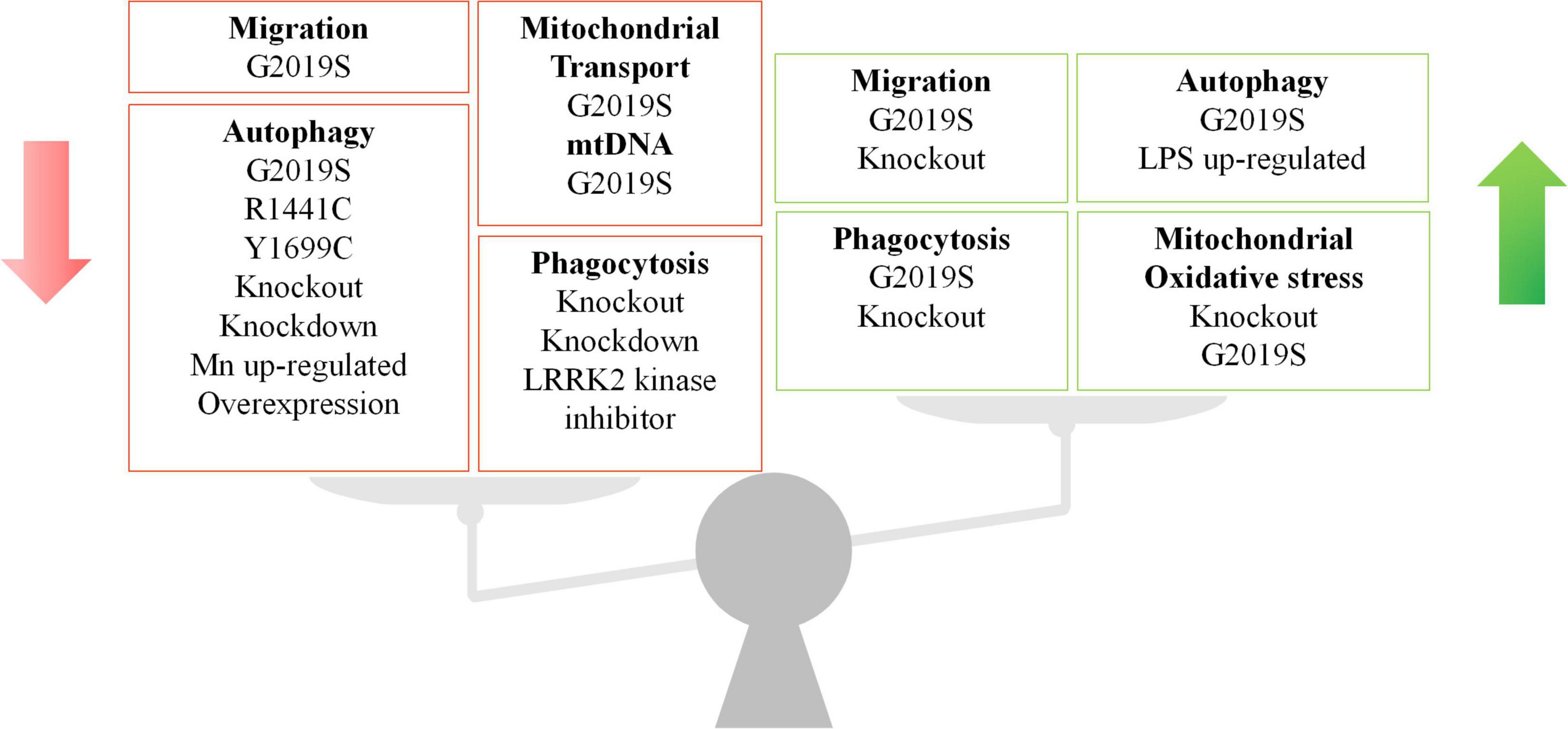
Figure 4. The double-faceted role of LRRK2 in different immune cell types and LRRK2 models. LRRK2 has been shown to regulate migration, autophagy, phagocytosis, and mitochondrial function in immune cells. The regulation role of LRRK2 expression is inconsistent across cell types and models. The green arrow indicates an increase and the red arrow indicates a decrease.
It is also highly desirable to identify novel signaling molecules/pathways that might regulate LRRK2 functions to provide theoretical benefits for PD treatment. Targeting LRRK2 pathways/axis is beneficial not only for patients with LRRK2 mutations, but also idiopathic patients. As increased LRRK2 kinase activity is associated with both familial and sporadic PD patients, a large number of small molecules that can specifically inhibit kinase activity have been developed and launched in various clinical trials. At present, the search for therapeutically effective LRRK2 kinase inhibitors has made relatively optimistic progress, with two molecules in clinical trials and multiple alternatives in the pipeline (Azeggagh and Berwick, 2022). Interestingly, LRRK2 expression in peripheral blood mononuclear cells may be related to type II interferon response, and is also induced by interferon in T cells. Thus, targeting LRRK2 may not only affect the nervous system, but also be involved in complex autoimmune processes (Dhanwani et al., 2022).
Author Contributions
YT conceived and designed the study. MZ and YT prepared the draft and figures. All authors have read, revised, and agreed to the published version of the manuscript.
Funding
This study was funded by the National Natural Sciences Foundation of China (Nos. 81801200 to YT; 81901223 to FY), the Hunan Provincial Natural Science Foundation of China (No. 2019JJ40476 to YT), Talents Startup Fund (No. 2209090550 to YT), and Youth Science Foundation (No. 2021Q04 to JW) of Xiangya Hospital, Central South University, Changsha, China.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank members of the Tang laboratory for discussions. We apologize to the authors whose work might have been unintentionally omitted due to space limitations. Figures were drawn by the help of Figdraw (www.figdraw.com).
References
Ahmadi Rastegar, D., Hughes, L. P., Perera, G., Keshiya, S., Zhong, S., Gao, J., et al. (2022). Effect of LRRK2 protein and activity on stimulated cytokines in human monocytes and macrophages. NPJ Park. Dis. 8:34. doi: 10.1038/s41531-022-00297-9
Albanese, F., Domenicale, C., Volta, M., and Morari, M. (2022). Modeling Parkinson’s disease in LRRK2 mice: focus on synaptic dysfunction and the autophagy-lysosomal pathway. Biochem. Soc. Trans. 50, 621–632. doi: 10.1042/BST20211288
Artyomov, M. N., and Van den Bossche, J. (2020). Immunometabolism in the Single-Cell Era. Cell Metab. 32, 710–725. doi: 10.1016/j.cmet.2020.09.013
Ascherio, A., and Schwarzschild, M. A. (2016). The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 15, 1257–1272. doi: 10.1016/S1474-4422(16)30230-7
Atashrazm, F., Hammond, D., Perera, G., Bolliger, M. F., Matar, E., Halliday, G. M., et al. (2019). LRRK2-mediated Rab10 phosphorylation in immune cells from Parkinson’s disease patients. Mov. Dis. 34, 406–415. doi: 10.1002/mds.27601
Azeggagh, S., and Berwick, D. C. (2022). The development of inhibitors of leucine-rich repeat kinase 2 (LRRK2) as a therapeutic strategy for Parkinson’s disease: the current state of play. Br. J. Pharmacol. 179, 1478–1495. doi: 10.1111/bph.15575
Biskup, S., Moore, D. J., Celsi, F., Higashi, S., West, A. B., Andrabi, S. A., et al. (2006). Localization of LRRK2 to membranous and vesicular structures in mammalian brain. Ann. Neurol. 60, 557–569. doi: 10.1002/ana.21019
Biskup, S., Moore, D. J., Rea, A., Lorenz-Deperieux, B., Coombes, C. E., Dawson, V. L., et al. (2007). Dynamic and redundant regulation of LRRK2 and LRRK1 expression. BMC Neurosci. 8:102. doi: 10.1186/1471-2202-8-102
Bliederhaeuser, C., Zondler, L., Grozdanov, V., Ruf, W. P., Brenner, D., Melrose, H. L., et al. (2016). LRRK2 contributes to monocyte dysregulation in Parkinson’s disease. Acta Neuropathol. Commun. 4:123. doi: 10.1186/s40478-016-0396-2
Bolz, S. N., Salentin, S., Jennings, G., Haupt, V. J., Sterneckert, J., and Schroeder, M. (2021). Structural binding site comparisons reveal Crizotinib as a novel LRRK2 inhibitor. Comput. Struct. Biotechnol. J. 19, 3674–3681. doi: 10.1016/j.csbj.2021.06.013
Chan, S. L., and Tan, E. K. (2017). Targeting LRRK2 in Parkinson’s disease: an update on recent developments. Expert Opin. Ther. Targets 21, 601–610. doi: 10.1080/14728222.2017.1323881
Chen, J., Chen, Y., and Pu, J. (2018a). Leucine-Rich Repeat Kinase 2 in Parkinson’s Disease: Updated from Pathogenesis to Potential Therapeutic Target. Eur. Neurol. 79, 256–265. doi: 10.1159/000488938
Chen, J., Su, P., Luo, W., and Chen, J. (2018b). Role of LRRK2 in manganese-induced neuroinflammation and microglial autophagy. Biochem. Biophys. Res. Commun. 498, 171–177. doi: 10.1016/j.bbrc.2018.02.007
Cherra, S. J. III, Steer, E., Gusdon, A. M., Kiselyov, K., and Chu, C. T. (2013). Mutant LRRK2 elicits calcium imbalance and depletion of dendritic mitochondria in neurons. Am. J. Pathol. 182, 474–484. doi: 10.1016/j.ajpath.2012.10.027
Cherubini, M., and Wade-Martins, R. (2018). Convergent pathways in Parkinson’s disease. Cell Tissue Res. 373, 79–90. doi: 10.1007/s00441-017-2700-2
Choi, I., Kim, B., Byun, J. W., Baik, S. H., Huh, Y. H., Kim, J. H., et al. (2015). LRRK2 G2019S mutation attenuates microglial motility by inhibiting focal adhesion kinase. Nat. Commun. 6:8255. doi: 10.1038/ncomms9255
Cook, D. A., Kannarkat, G. T., Cintron, A. F., Butkovich, L. M., Fraser, K. B., Chang, J., et al. (2017). LRRK2 levels in immune cells are increased in Parkinson’s disease. NPJ Park. Dis. 3:11. doi: 10.1038/s41531-017-0010-8
Cookson, M. R. (2017). Mechanisms of Mutant LRRK2 Neurodegeneration. Adv. Neurobiol. 14, 227–239. doi: 10.1007/978-3-319-49969-7_12
Cresto, N., Gardier, C., Gubinelli, F., Gaillard, M. C., Liot, G., West, A. B., et al. (2019). The unlikely partnership between LRRK2 and alpha-synuclein in Parkinson’s disease. Eur. J. Neurosci. 49, 339–363. doi: 10.1111/ejn.14182
Daher, J. P., Abdelmotilib, H. A., Hu, X., Volpicelli-Daley, L. A., Moehle, M. S., Fraser, K. B., et al. (2015). Leucine-rich Repeat Kinase 2 (LRRK2) Pharmacological Inhibition Abates alpha-Synuclein Gene-induced Neurodegeneration. J. Biol. Chem. 290, 19433–19444. doi: 10.1074/jbc.M115.660001
Deniston, C. K., Salogiannis, J., Mathea, S., Snead, D. M., Lahiri, I., Matyszewski, M., et al. (2020). Structure of LRRK2 in Parkinson’s disease and model for microtubule interaction. Nature 588, 344–349. doi: 10.1038/s41586-020-2673-2
Dhanwani, R., Lima-Junior, J. R., Sethi, A., Pham, J., Williams, G., Frazier, A., et al. (2022). Transcriptional analysis of peripheral memory T cells reveals Parkinson’s disease-specific gene signatures. NPJ Park. Dis. 8:30. doi: 10.1038/s41531-022-00282-2
di Domenico, A., Carola, G., Calatayud, C., Pons-Espinal, M., Munoz, J. P., Richaud-Patin, Y., et al. (2019). Patient-Specific iPSC-Derived Astrocytes Contribute to Non-Cell-Autonomous Neurodegeneration in Parkinson’s Disease. Stem Cell Rep. 12, 213–229. doi: 10.1016/j.stemcr.2018.12.011
Di Maio, R., Hoffman, E. K., Rocha, E. M., Keeney, M. T., Sanders, L. H., De Miranda, B. R., et al. (2018). LRRK2 activation in idiopathic Parkinson’s disease. Sci. Transl. Med. 10:eaar5429 doi: 10.1126/scitranslmed.aar5429
Ding, X., and Ren, F. (2020). Leucine-rich repeat kinase 2 inhibitors: a patent review (2014-present). Expert Opin. Ther. Pat. 30, 275–286. doi: 10.1080/13543776.2020.1729354
Dwyer, Z., Rudyk, C., Thompson, A., Farmer, K., Fenner, B., Fortin, T., et al. (2020). Leucine-rich repeat kinase-2 (LRRK2) modulates microglial phenotype and dopaminergic neurodegeneration. Neurobiol. Aging. 91, 45–55. doi: 10.1016/j.neurobiolaging.2020.02.017
Dzamko, N. L. (2017). LRRK2 and the Immune System. Adv. Neurobiol. 14, 123–143. doi: 10.1007/978-3-319-49969-7_7
Dzamko, N., Geczy, C. L., and Halliday, G. M. (2015). Inflammation Is Genetically Implicated in Parkinson’s Disease. Neuroscience 302, 89–102. doi: 10.1016/j.neuroscience.2014.10.028
Dzamko, N., Gysbers, A. M., Bandopadhyay, R., Bolliger, M. F., Uchino, A., Zhao, Y., et al. (2017). LRRK2 levels and phosphorylation in Parkinson’s disease brain and cases with restricted Lewy bodies. Mov. Dis. 32, 423–432. doi: 10.1002/mds.26892
Dzamko, N., Inesta-Vaquera, F., Zhang, J., Xie, C., Cai, H., Arthur, S., et al. (2012). The IkappaB kinase family phosphorylates the Parkinson’s disease kinase LRRK2 at Ser935 and Ser910 during Toll-like receptor signaling. PLoS One 7:e39132. doi: 10.1371/journal.pone.0039132
Elbaz, A., Carcaillon, L., Kab, S., and Moisan, F. (2016). Epidemiology of Parkinson’s disease. Rev. Neurol. 172, 14–26.
Favuzzi, E., Huang, S., Saldi, G. A., Binan, L., Ibrahim, L. A., Fernandez-Otero, M., et al. (2021). GABA-receptive microglia selectively sculpt developing inhibitory circuits. Cell 184:5686. doi: 10.1016/j.cell.2021.10.009
Fenner, B. M., Fenner, M. E., Prowse, N., and Hayley, S. P. (2022). LRRK2 and WAVE2 regulate microglial-transition through distinct morphological phenotypes to induce neurotoxicity in a novel two-hit in vitro model of neurodegeneration. J. Cell Physiol. 237, 1013–1032. doi: 10.1002/jcp.30588
Filiano, A. J., Gadani, S. P., and Kipnis, J. (2015). Interactions of innate and adaptive immunity in brain development and function. Brain Res. 1617, 18–27. doi: 10.1016/j.brainres.2014.07.050
Furman, D., Campisi, J., Verdin, E., Carrera-Bastos, P., Targ, S., Franceschi, C., et al. (2019). Chronic inflammation in the etiology of disease across the life span. Nat. Med. 25, 1822–1832. doi: 10.1038/s41591-019-0675-0
Gardet, A., Benita, Y., Li, C., Sands, B. E., Ballester, I., Stevens, C., et al. (2010). LRRK2 is involved in the IFN-gamma response and host response to pathogens. J. Immunol. 185, 5577–5585. doi: 10.4049/jimmunol.1000548
Gelders, G., Baekelandt, V., and Van der Perren, A. (2018). Linking Neuroinflammation and Neurodegeneration in Parkinson’s Disease. J. Immunol. Res. 2018:4784268. doi: 10.1155/2018/4784268
Gillardon, F., Schmid, R., and Draheim, H. (2012). Parkinson’s disease-linked leucine-rich repeat kinase 2(R1441G) mutation increases proinflammatory cytokine release from activated primary microglial cells and resultant neurotoxicity. Neuroscience 208, 41–48. doi: 10.1016/j.neuroscience.2012.02.001
Godena, V. K., Brookes-Hocking, N., Moller, A., Shaw, G., Oswald, M., Sancho, R. M., et al. (2014). Increasing microtubule acetylation rescues axonal transport and locomotor deficits caused by LRRK2 Roc-COR domain mutations. Nat. Commun. 5:5245. doi: 10.1038/ncomms6245
Gonzalez-Hunt, C. P., Thacker, E. A., Toste, C. M., Boularand, S., Deprets, S., Dubois, L., et al. (2020). Mitochondrial DNA damage as a potential biomarker of LRRK2 kinase activity in LRRK2 Parkinson’s disease. Sci. Rep. 10:17293. doi: 10.1038/s41598-020-74195-6
Gutierrez, M. G. (2013). Functional role(s) of phagosomal Rab GTPases. Small GTPases. 4, 148–158. doi: 10.4161/sgtp.25604
Hakimi, M., Selvanantham, T., Swinton, E., Padmore, R. F., Tong, Y., Kabbach, G., et al. (2011). Parkinson’s disease-linked LRRK2 is expressed in circulating and tissue immune cells and upregulated following recognition of microbial structures. J. Neural. Transm. 118, 795–808. doi: 10.1007/s00702-011-0653-2
Hartlova, A., Herbst, S., Peltier, J., Rodgers, A., Bilkei-Gorzo, O., Fearns, A., et al. (2018). LRRK2 is a negative regulator of Mycobacterium tuberculosis phagosome maturation in macrophages. EMBO J. 37:e98694 doi: 10.15252/embj.201798694
Henry, A. G., Aghamohammadzadeh, S., Samaroo, H., Chen, Y., Mou, K., Needle, E., et al. (2015). Pathogenic LRRK2 mutations, through increased kinase activity, produce enlarged lysosomes with reduced degradative capacity and increase ATP13A2 expression. Hum. Mol. Genet. 24, 6013–6028. doi: 10.1093/hmg/ddv314
Herzig, M. C., Kolly, C., Persohn, E., Theil, D., Schweizer, T., Hafner, T., et al. (2011). LRRK2 protein levels are determined by kinase function and are crucial for kidney and lung homeostasis in mice. Hum. Mol. Genet. 20, 4209–4223. doi: 10.1093/hmg/ddr348
Higashi, S., Biskup, S., West, A. B., Trinkaus, D., Dawson, V. L., Faull, R. L., et al. (2007a). Localization of Parkinson’s disease-associated LRRK2 in normal and pathological human brain. Brain Res. 1155, 208–219. doi: 10.1016/j.brainres.2007.04.034
Higashi, S., Moore, D. J., Colebrooke, R. E., Biskup, S., Dawson, V. L., Arai, H., et al. (2007b). Expression and localization of Parkinson’s disease-associated leucine-rich repeat kinase 2 in the mouse brain. J. Neurochem. 100, 368–381. doi: 10.1111/j.1471-4159.2006.04246.x
Ho, D. H., Je, A. R., Lee, H., Son, I., Kweon, H. S., Kim, H. G., et al. (2018). LRRK2 Kinase Activity Induces Mitochondrial Fission in Microglia via Drp1 and Modulates Neuroinflammation. Exp. Neurobiol. 27, 171–180. doi: 10.5607/en.2018.27.3.171
Hsieh, C. H., Shaltouki, A., Gonzalez, A. E., Bettencourt da Cruz, A., Burbulla, L. F., St Lawrence, E., et al. (2016). Functional Impairment in Miro Degradation and Mitophagy Is a Shared Feature in Familial and Sporadic Parkinson’s Disease. Cell Stem Cell. 19, 709–724. doi: 10.1016/j.stem.2016.08.002
Huang, Y., Liu, H., Hu, J., Han, C., Zhong, Z., Luo, W., et al. (2021). Significant Difference of Immune Cell Fractions and Their Correlations With Differential Expression Genes in Parkinson’s Disease. Front. Aging Neurosci. 13:686066. doi: 10.3389/fnagi.2021.686066
Hussein, A., Guevara, C. A., Del Valle, P., Gupta, S., Benson, D. L., and Huntley, G. W. (2021). Non-Motor Symptoms of Parkinson’s Disease: The Neurobiology of Early Psychiatric and Cognitive Dysfunction. Neuroscientist 8:10738584211011979. doi: 10.1177/10738584211011979
Iannotta, L., Biosa, A., Kluss, J. H., Tombesi, G., Kaganovich, A., Cogo, S., et al. (2020). Divergent Effects of G2019S and R1441C LRRK2 Mutations on LRRK2 and Rab10 Phosphorylations in Mouse Tissues. Cells 9:2344 doi: 10.3390/cells9112344
Kelly, B., and O’Neill, L. A. (2015). Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 25, 771–784. doi: 10.1038/cr.2015.68
Kett, L. R., and Dauer, W. T. (2012). Leucine-rich repeat kinase 2 for beginners: six key questions. Cold Spring Harb. Perspect. Med. 2:a009407. doi: 10.1101/cshperspect.a009407
Kettenmann, H., Kirchhoff, F., and Verkhratsky, A. (2013). Microglia: new roles for the synaptic stripper. Neuron 77, 10–18. doi: 10.1016/j.neuron.2012.12.023
Kim, C., Beilina, A., Smith, N., Li, Y., Kim, M., Kumaran, R., et al. (2020). LRRK2 mediates microglial neurotoxicity via NFATc2 in rodent models of synucleinopathies. Sci. Transl. Med. 12:eaay0399 doi: 10.1126/scitranslmed.aay0399
Kim, K. S., Marcogliese, P. C., Yang, J., Callaghan, S. M., Resende, V., Abdel-Messih, E., et al. (2018). Regulation of myeloid cell phagocytosis by LRRK2 via WAVE2 complex stabilization is altered in Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A. 115, E5164–E5173. doi: 10.1073/pnas.1718946115
Kipnis, J. (2016). Multifaceted interactions between adaptive immunity and the central nervous system. Science 353, 766–771. doi: 10.1126/science.aag2638
Kozina, E., Sadasivan, S., Jiao, Y., Dou, Y., Ma, Z., Tan, H., et al. (2018). Mutant LRRK2 mediates peripheral and central immune responses leading to neurodegeneration in vivo. Brain 141, 1753–1769. doi: 10.1093/brain/awy077
Kuss, M., Adamopoulou, E., and Kahle, P. J. (2014). Interferon-gamma induces leucine-rich repeat kinase LRRK2 via extracellular signal-regulated kinase ERK5 in macrophages. J. Neurochem. 129, 980–987. doi: 10.1111/jnc.12668
Langston, R. G., Rudenko, I. N., Kumaran, R., Hauser, D. N., Kaganovich, A., Ponce, L. B., et al. (2019). Differences in Stability, Activity and Mutation Effects Between Human and Mouse Leucine-Rich Repeat Kinase 2. Neurochem. Res. 44, 1446–1459. doi: 10.1007/s11064-018-2650-4
Lee, H., Flynn, R., Sharma, I., Haberman, E., Carling, P. J., Nicholls, F. J., et al. (2020). LRRK2 Is Recruited to Phagosomes and Co-recruits RAB8 and RAB10 in Human Pluripotent Stem Cell-Derived Macrophages. Stem Cell Rep. 14, 940–955. doi: 10.1016/j.stemcr.2020.04.001
Lee, H., James, W. S., and Cowley, S. A. (2017). LRRK2 in peripheral and central nervous system innate immunity: its link to Parkinson’s disease. Biochem. Soc. Trans. 45, 131–139. doi: 10.1042/BST20160262
Leschziner, A. E., and Reck-Peterson, S. L. (2021). Structural Biology of LRRK2 and its Interaction with Microtubules. Mov. Dis. 36, 2494–2504. doi: 10.1002/mds.28755
Levy, D. R., Udgata, A., Tourlomousis, P., Symmons, M. F., Hopkins, L. J., Bryant, C. E., et al. (2020). The Parkinson’s disease-associated kinase LRRK2 regulates genes required for cell adhesion, polarization, and chemotaxis in activated murine macrophages. J. Biol. Chem. 295, 10857–10867. doi: 10.1074/jbc.RA119.011842
Li, J. Q., Tan, L., and Yu, J. T. (2014). The role of the LRRK2 gene in Parkinsonism. Mol. Neurodegener. 9:47. doi: 10.1186/1750-1326-9-47
Linnerbauer, M., Wheeler, M. A., and Quintana, F. J. (2020). Astrocyte Crosstalk in CNS Inflammation. Neuron 108, 608–622. doi: 10.1016/j.neuron.2020.08.012
Liu, Z., Xu, E., Zhao, H. T., Cole, T., and West, A. B. (2020). LRRK2 and Rab10 coordinate macropinocytosis to mediate immunological responses in phagocytes. EMBO J. 39:e104862. doi: 10.15252/embj.2020104862
Louveau, A., Harris, T. H., and Kipnis, J. (2015). Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 36, 569–577. doi: 10.1016/j.it.2015.08.006
Ludtmann, M. H. R., Kostic, M., Horne, A., Gandhi, S., Sekler, I., and Abramov, A. Y. (2019). LRRK2 deficiency induced mitochondrial Ca(2+) efflux inhibition can be rescued by Na(+)/Ca(2+)/Li(+) exchanger upregulation. Cell Death Dis. 10:265. doi: 10.1038/s41419-019-1469-5
Ma, B., Xu, L., Pan, X., Sun, L., Ding, J., Xie, C., et al. (2016). LRRK2 modulates microglial activity through regulation of chemokine (C-X3-C) receptor 1 -mediated signalling pathways. Hum. Mol. Genet. 25, 3515–3523. doi: 10.1093/hmg/ddw194
Maekawa, T., Sasaoka, T., Azuma, S., Ichikawa, T., Melrose, H. L., Farrer, M. J., et al. (2016). Leucine-rich repeat kinase 2 (LRRK2) regulates alpha-synuclein clearance in microglia. BMC Neurosci. 17:77. doi: 10.1186/s12868-016-0315-2
Manzoni, C., Mamais, A., Roosen, D. A., Dihanich, S., Soutar, M. P., Plun-Favreau, H., et al. (2016). mTOR independent regulation of macroautophagy by Leucine Rich Repeat Kinase 2 via Beclin-1. Sci. Rep. 6:35106. doi: 10.1038/srep35106
Marinelli, S., Basilico, B., Marrone, M. C., and Ragozzino, D. (2019). Microglia-neuron crosstalk: Signaling mechanism and control of synaptic transmission. Semin Cell Dev. Biol. 94, 138–151. doi: 10.1016/j.semcdb.2019.05.017
Marker, D. F., Puccini, J. M., Mockus, T. E., Barbieri, J., Lu, S. M., and Gelbard, H. A. (2012). LRRK2 kinase inhibition prevents pathological microglial phagocytosis in response to HIV-1 Tat protein. J. Neuroinflam. 9:261. doi: 10.1186/1742-2094-9-261
Marogianni, C., Sokratous, M., Dardiotis, E., Hadjigeorgiou, G. M., Bogdanos, D., and Xiromerisiou, G. (2020). Neurodegeneration and Inflammation-An Interesting Interplay in Parkinson’s Disease. Int. J. Mol. Sci. 21:8421 doi: 10.3390/ijms21228421
Martin, I., Dawson, V. L., and Dawson, T. M. (2011). Recent advances in the genetics of Parkinson’s disease. Annu. Rev. Gen. Hum. Genet. 12, 301–325.
Mata, I. F., Wedemeyer, W. J., Farrer, M. J., Taylor, J. P., and Gallo, K. A. (2006). LRRK2 in Parkinson’s disease: protein domains and functional insights. Trends Neurosci. 29, 286–293. doi: 10.1016/j.tins.2006.03.006
McFarthing, K., Buff, S., Rafaloff, G., Dominey, T., Wyse, R. K., and Stott, S. R. W. (2020). Parkinson’s Disease Drug Therapies in the Clinical Trial Pipeline: 2020. J. Park. Dis. 10, 757–774. doi: 10.3233/JPD-202128
Miklossy, J., Arai, T., Guo, J. P., Klegeris, A., Yu, S., McGeer, E. G., et al. (2006). LRRK2 expression in normal and pathologic human brain and in human cell lines. J. Neuropathol. Exp. Neurol. 65, 953–963. doi: 10.1097/01.jnen.0000235121.98052.54
Mills, R. D., Liang, L. Y., Lio, D. S., Mok, Y. F., Mulhern, T. D., Cao, G., et al. (2018). The Roc-COR tandem domain of leucine-rich repeat kinase 2 forms dimers and exhibits conventional Ras-like GTPase properties. J. Neurochem. 147, 409–428. doi: 10.1111/jnc.14566
Moehle, M. S., Daher, J. P., Hull, T. D., Boddu, R., Abdelmotilib, H. A., Mobley, J., et al. (2015). The G2019S LRRK2 mutation increases myeloid cell chemotactic responses and enhances LRRK2 binding to actin-regulatory proteins. Hum. Mol. Genet. 24, 4250–4267. doi: 10.1093/hmg/ddv157
Moehle, M. S., Webber, P. J., Tse, T., Sukar, N., Standaert, D. G., DeSilva, T. M., et al. (2012). LRRK2 inhibition attenuates microglial inflammatory responses. J. Neurosci. 32, 1602–1611. doi: 10.1523/jneurosci.5601-11.2012
Myasnikov, A., Zhu, H., Hixson, P., Xie, B., Yu, K., Pitre, A., et al. (2021). Structural analysis of the full-length human LRRK2. Cell 184, 3519–27e10. doi: 10.1016/j.cell.2021.05.004
Nabar, N. R., Heijjer, C. N., Shi, C. S., Hwang, I. Y., Ganesan, S., Karlsson, M. C. I., et al. (2021). LRRK2 is required for CD38-mediated NAADP-Ca(2+) signaling and the downstream activation of TFEB (transcription factor EB) in immune cells. Autophagy 18, 204–222. doi: 10.1080/15548627.2021.1954779
Nalls, M. A., Pankratz, N., Lill, C. M., Do, C. B., Hernandez, D. G., Saad, M., et al. (2014). Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 46, 989–993. doi: 10.1038/ng.3043
Neethling, A., Engelbrecht, L., Loos, B., Kinnear, C., Theart, R., Abrahams, S., et al. (2019). Wild-type and mutant (G2019S) leucine-rich repeat kinase 2 (LRRK2) associate with subunits of the translocase of outer mitochondrial membrane (TOM) complex. Exp. Cell Res. 375, 72–79. doi: 10.1016/j.yexcr.2018.12.022
Newcombe, E. A., Camats-Perna, J., Silva, M. L., Valmas, N., Huat, T. J., and Medeiros, R. (2018). Inflammation: the link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflamm. 15:276. doi: 10.1186/s12974-018-1313-3
Nguyen, P. T., Dorman, L. C., Pan, S., Vainchtein, I. D., Han, R. T., Nakao-Inoue, H., et al. (2020). Microglial Remodeling of the Extracellular Matrix Promotes Synapse Plasticity. Cell 182, 388–403e15. doi: 10.1016/j.cell.2020.05.050
Novello, S., Mercatelli, D., Albanese, F., Domenicale, C., Brugnoli, A., D’Aversa, E., et al. (2021). In vivo susceptibility to energy failure parkinsonism and LRRK2 kinase activity. Neurobiol. Dis. 162:105579. doi: 10.1016/j.nbd.2021.105579
Paisan-Ruiz, C., Jain, S., Evans, E. W., Gilks, W. P., Simon, J., van der Brug, M., et al. (2004). Cloning of the gene containing mutations that cause PARK8-linked Parkinson’s disease. Neuron 44, 595–600. doi: 10.1016/j.neuron.2004.10.023
Panagiotakopoulou, V., Ivanyuk, D., De Cicco, S., Haq, W., Arsic, A., Yu, C., et al. (2020). Interferon-gamma signaling synergizes with LRRK2 in neurons and microglia derived from human induced pluripotent stem cells. Nat. Commun. 11:5163. doi: 10.1038/s41467-020-18755-4
Paolicelli, R. C., and Angiari, S. (2019). Microglia immunometabolism: From metabolic disorders to single cell metabolism. Semin Cell Dev. Biol. 94, 129–137. doi: 10.1016/j.semcdb.2019.03.012
Perry, V. H., and Teeling, J. (2013). Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol. 35, 601–612. doi: 10.1007/s00281-013-0382-8
Prinz, M., Jung, S., and Priller, J. (2019). Microglia Biology: One Century of Evolving Concepts. Cell 179, 292–311. doi: 10.1016/j.cell.2019.08.053
Rocha, E. M., De Miranda, B., and Sanders, L. H. (2018). Alpha-synuclein: Pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol. Dis. 109, 249–257. doi: 10.1016/j.nbd.2017.04.004
Rocha, E. M., Keeney, M. T., Di Maio, R., De Miranda, B. R., and Greenamyre, J. T. (2022). LRRK2 and idiopathic Parkinson’s disease. Trends Neurosci. 45, 224–236.
Rostami, J., Mothes, T., Kolahdouzan, M., Eriksson, O., Moslem, M., Bergstrom, J., et al. (2021). Crosstalk between astrocytes and microglia results in increased degradation of alpha-synuclein and amyloid-beta aggregates. J. Neuroinflamm. 18:124. doi: 10.1186/s12974-021-02158-3
Rui, Q., Ni, H., Li, D., Gao, R., and Chen, G. (2018). The Role of LRRK2 in Neurodegeneration of Parkinson Disease. Curr. Neuropharmacol. 16, 1348–1357. doi: 10.2174/1570159X16666180222165418
Russo, I., Berti, G., Plotegher, N., Bernardo, G., Filograna, R., Bubacco, L., et al. (2015). Leucine-rich repeat kinase 2 positively regulates inflammation and down-regulates NF-kappaB p50 signaling in cultured microglia cells. J. Neuroinflamm. 12:230.
Russo, I., Kaganovich, A., Ding, J., Landeck, N., Mamais, A., Varanita, T., et al. (2019). Transcriptome analysis of LRRK2 knock-out microglia cells reveals alterations of inflammatory- and oxidative stress-related pathways upon treatment with alpha-synuclein fibrils. Neurobiol. Dis. 129, 67–78. doi: 10.1016/j.nbd.2019.05.012
Sanchez-Guajardo, V., Barnum, C. J., Tansey, M. G., and Romero-Ramos, M. (2013). Neuroimmunological processes in Parkinson’s disease and their relation to alpha-synuclein: microglia as the referee between neuronal processes and peripheral immunity. ASN Neuro. 5, 113–139. doi: 10.1042/AN20120066
Schapansky, J., Nardozzi, J. D., Felizia, F., and LaVoie, M. J. (2014). Membrane recruitment of endogenous LRRK2 precedes its potent regulation of autophagy. Hum. Mol. Genet. 23, 4201–4214. doi: 10.1093/hmg/ddu138
Scheiblich, H., Dansokho, C., Mercan, D., Schmidt, S. V., Bousset, L., Wischhof, L., et al. (2021). Microglia jointly degrade fibrillar alpha-synuclein cargo by distribution through tunneling nanotubes. Cell 184, 5089–106e21. doi: 10.1016/j.cell.2021.09.007
Schwab, A. J., and Ebert, A. D. (2015). Neurite Aggregation and Calcium Dysfunction in iPSC-Derived Sensory Neurons with Parkinson’s Disease-Related LRRK2 G2019S Mutation. Stem Cell Rep. 5, 1039–1052. doi: 10.1016/j.stemcr.2015.11.004
Schwab, A. J., Sison, S. L., Meade, M. R., Broniowska, K. A., Corbett, J. A., and Ebert, A. D. (2017). Decreased Sirtuin Deacetylase Activity in LRRK2 G2019S iPSC-Derived Dopaminergic Neurons. Stem Cell Rep. 9, 1839–1852. doi: 10.1016/j.stemcr.2017.10.010
Scott-Hewitt, N., Perrucci, F., Morini, R., Erreni, M., Mahoney, M., Witkowska, A., et al. (2020). Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 39:e105380. doi: 10.15252/embj.2020105380
Sharma, S., Bandopadhyay, R., Lashley, T., Renton, A. E., Kingsbury, A. E., Kumaran, R., et al. (2011). LRRK2 expression in idiopathic and G2019S positive Parkinson’s disease subjects: a morphological and quantitative study. Neuropathol. Appl. Neurobiol. 37, 777–790. doi: 10.1111/j.1365-2990.2011.01187.x
Shihabuddin, L. S., Brundin, P., Greenamyre, J. T., Stephenson, D., and Sardi, S. P. (2018). New Frontiers in Parkinson’s Disease: From Genetics to the Clinic. J. Neurosci. 38, 9375–9382. doi: 10.1523/JNEUROSCI.1666-18.2018
Shutinoski, B., Hakimi, M., Harmsen, I. E., Lunn, M., Rocha, J., Lengacher, N., et al. (2019). Lrrk2 alleles modulate inflammation during microbial infection of mice in a sex-dependent manner. Sci. Transl. Med. 11:eaas9292 doi: 10.1126/scitranslmed.aas9292
Singh, A., Zhi, L., and Zhang, H. (2019). LRRK2 and mitochondria: Recent advances and current views. Brain Res. 1702, 96–104. doi: 10.1016/j.brainres.2018.06.010
Sonninen, T. M., Hamalainen, R. H., Koskuvi, M., Oksanen, M., Shakirzyanova, A., Wojciechowski, S., et al. (2020). Metabolic alterations in Parkinson’s disease astrocytes. Sci. Rep. 10:14474. doi: 10.1038/s41598-020-71329-8
Speidel, A., Felk, S., Reinhardt, P., Sterneckert, J., and Gillardon, F. (2016). Leucine-Rich Repeat Kinase 2 Influences Fate Decision of Human Monocytes Differentiated from Induced Pluripotent Stem Cells. PLoS One 11:e0165949. doi: 10.1371/journal.pone.0165949
Straub, R. H. (2017). The brain and immune system prompt energy shortage in chronic inflammation and ageing. Nat. Rev. Rheumatol. 13, 743–751. doi: 10.1038/nrrheum.2017.172
Su, Y. C., Guo, X., and Qi, X. (2015). Threonine 56 phosphorylation of Bcl-2 is required for LRRK2 G2019S-induced mitochondrial depolarization and autophagy. Biochim. Biophys. Acta. 1852, 12–21.
Takagawa, T., Kitani, A., Fuss, I., Levine, B., Brant, S. R., Peter, I., et al. (2018). An increase in LRRK2 suppresses autophagy and enhances Dectin-1-induced immunity in a mouse model of colitis. Sci. Transl. Med. 10:eaan8162 doi: 10.1126/scitranslmed.aan8162
Tan, E. K., Chao, Y. X., West, A., Chan, L. L., Poewe, W., and Jankovic, J. (2020). Parkinson disease and the immune system - associations, mechanisms and therapeutics. Nat. Rev. Neurol. 16, 303–318. doi: 10.1038/s41582-020-0344-4
Tansey, M. G., and Goldberg, M. S. (2010). Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 37, 510–518. doi: 10.1016/j.nbd.2009.11.004
Thevenet, J., and Pescini Gobert, R. (2011). Hooft van Huijsduijnen R. Wiessner C, Sagot YJ. Regulation of LRRK2 expression points to a functional role in human monocyte maturation. PLoS One 6:e21519. doi: 10.1371/journal.pone.0021519
Thomas, J. M., Li, T., Yang, W., Xue, F., Fishman, P. S., and Smith, W. W. (2016). 68 and FX2149 Attenuate Mutant LRRK2-R1441C-Induced Neural Transport Impairment. Front. Aging Neurosci. 8:337. doi: 10.3389/fnagi.2016.00337
Tokars, V., Chen, C., and Parisiadou, L. (2021). Closing the structure-to-function gap for LRRK2. Trends Biochem. Sci. 47, 187–188.
Tolosa, E., Vila, M., Klein, C., and Rascol, O. (2020). LRRK2 in Parkinson disease: challenges of clinical trials. Nat. Rev. Neurol. 16, 97–107. doi: 10.1038/s41582-019-0301-2
Ullrich, F. (2021). Seeing the bigger picture: First full-length human LRRK2 structures. Nat. Struct. Mol. Biol. 28:546. doi: 10.1038/s41594-021-00628-z
Walter, J., Bolognin, S., Antony, P. M. A., Nickels, S. L., Poovathingal, S. K., Salamanca, L., et al. (2019). Neural Stem Cells of Parkinson’s Disease Patients Exhibit Aberrant Mitochondrial Morphology and Functionality. Stem Cell Rep. 12, 878–889. doi: 10.1016/j.stemcr.2019.03.004
Warburg, O., Wind, F., and Negelein, E. (1927). The Metabolism of Tumors in the Body. J. Gen. Physiol. 8, 519–530. doi: 10.1085/jgp.8.6.519
Watanabe, R., Buschauer, R., Bohning, J., Audagnotto, M., Lasker, K., Lu, T. W., et al. (2020). The In Situ Structure of Parkinson’s Disease-Linked LRRK2. Cell 182, 1508–18e16. doi: 10.1016/j.cell.2020.08.004
Weindel, C. G., Bell, S. L., Vail, K. J., West, K. O., Patrick, K. L., and Watson, R. O. (2020). LRRK2 maintains mitochondrial homeostasis and regulates innate immune responses to Mycobacterium tuberculosis. Elife 9:e51071 doi: 10.7554/eLife.51071
Weng, J. H., Aoto, P. C., Lorenz, R., Wu, J., Schmidt, S. H., Manschwetus, J. T., et al. (2022). LRRK2 dynamics analysis identifies allosteric control of the crosstalk between its catalytic domains. PLoS Biol. 20:e3001427. doi: 10.1371/journal.pbio.3001427
Werneburg, S., Jung, J., Kunjamma, R. B., Ha, S. K., Luciano, N. J., Willis, C. M., et al. (2020). Targeted Complement Inhibition at Synapses Prevents Microglial Synaptic Engulfment and Synapse Loss in Demyelinating Disease. Immunity 52, 167–82e7. doi: 10.1016/j.immuni.2019.12.004
West, A. B. (2017). Achieving neuroprotection with LRRK2 kinase inhibitors in Parkinson disease. Exp. Neurol. 298, 236–245. doi: 10.1016/j.expneurol.2017.07.019
Westerlund, M., Belin, A. C., Anvret, A., Bickford, P., Olson, L., and Galter, D. (2008). Developmental regulation of leucine-rich repeat kinase 1 and 2 expression in the brain and other rodent and human organs: Implications for Parkinson’s disease. Neuroscience 152, 429–436. doi: 10.1016/j.neuroscience.2007.10.062
Wojewska, D. N., and Kortholt, A. (2021). LRRK2 Targeting Strategies as Potential Treatment of Parkinson’s Disease. Biomolecules 11:1101 doi: 10.3390/biom11081101
Wolf, S. A., Boddeke, H. W., and Kettenmann, H. (2017). Microglia in Physiology and Disease. Annu. Rev. Physiol. 79, 619–643. doi: 10.1146/annurev-physiol-022516-034406
Xia, Y., Zhang, G., Kou, L., Yin, S., Han, C., Hu, J., et al. (2021). Reactive microglia enhance the transmission of exosomal alpha-synuclein via toll-like receptor 2. Brain 144, 2024–2037. doi: 10.1093/brain/awab122
Xu, E., Boddu, R., Abdelmotilib, H. A., Sokratian, A., Kelly, K., Liu, Z., et al. (2022). Pathological alpha-synuclein recruits LRRK2 expressing pro-inflammatory monocytes to the brain. Mol. Neurodegener. 17:7. doi: 10.1186/s13024-021-00509-5
Yakhine-Diop, S. M. S., Morales-Garcia, J. A., Niso-Santano, M., Gonzalez-Polo, R. A., Uribe-Carretero, E., Martinez-Chacon, G., et al. (2020). Metabolic alterations in plasma from patients with familial and idiopathic Parkinson’s disease. Aging 12, 16690–16708. doi: 10.18632/aging.103992
Yan, J., Zhao, W., Gao, C., Liu, X., Zhao, X., Wei, T., et al. (2019). Leucine-rich repeat kinase 2 regulates mouse dendritic cell migration by ORAI2. FASEB J. 33, 9775–9784. doi: 10.1096/fj.201802550R
Yilmaz, A., Ugur, Z., Ustun, I., Akyol, S., Bahado-Singh, R. O., Maddens, M., et al. (2020). Metabolic Profiling of CSF from People Suffering from Sporadic and LRRK2 Parkinson’s Disease: A Pilot Study. Cells 9:2394 doi: 10.3390/cells9112394
Zhang, P., Fan, Y., Ru, H., Wang, L., Magupalli, V. G., Taylor, S. S., et al. (2019). Crystal structure of the WD40 domain dimer of LRRK2. Proc. Natl. Acad. Sci. U.S.A. 116, 1579–1584. doi: 10.1073/pnas.1817889116
Keywords: Parkinson’s disease, LRRK2, neuroinflammation, microglia, immune function
Citation: Zhang M, Li C, Ren J, Wang H, Yi F, Wu J and Tang Y (2022) The Double-Faceted Role of Leucine-Rich Repeat Kinase 2 in the Immunopathogenesis of Parkinson’s Disease. Front. Aging Neurosci. 14:909303. doi: 10.3389/fnagi.2022.909303
Received: 31 March 2022; Accepted: 20 April 2022;
Published: 11 May 2022.
Edited by:
Weidong Le, Dalian Medical University, ChinaReviewed by:
Yi Fan, Nanjing Medical University, ChinaXi Chen, Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, China
Copyright © 2022 Zhang, Li, Ren, Wang, Yi, Wu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Tang, tangyusky@csu.edu.cn; tangyu-sky@163.com
 Mengfei Zhang
Mengfei Zhang