
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 13 July 2022
Sec. Alzheimer's Disease and Related Dementias
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.900581
This article is part of the Research TopicInsights in Alzheimer’s Disease and Related DementiasView all 20 articles
 Hwamee Oh1,2,3,4,5*
Hwamee Oh1,2,3,4,5*Emerging evidence suggests that some personality traits may link to the vulnerability to or protection for Alzheimer’s disease (AD). A causal mechanism underlying this relationship, however, remains largely unknown. Using 18F-Florbetaben positron emission tomography (PET) binding to beta-amyloid (Aβ) plaques, a pathological feature of AD, and functional magnetic resonance imaging (fMRI), we investigated pathological and functional correlates of extraversion and neuroticism in a group of healthy young and older subjects. We quantified the level of brain Aβ deposition in older individuals. Brain activity was measured in young adults using a task-switching fMRI paradigm. When we correlated personality scores of extraversion and neuroticism with these pathological and functional measures, higher extraversion, but not neuroticism, was significantly associated with lower global Aβ measures among older adults, accounting for age and sex. This association was present across widespread brain regions. Among young subjects, higher extraversion was associated with lower activity during task switching in the anterior cingulate cortex, left anterior insular cortex, left putamen, and middle frontal gyrus bilaterally, while higher neuroticism was associated with increased activity throughout the brain. The present results suggest that possibly via efficient neuronal activity, extraversion, one of the lifelong personality traits, may confer the protective mechanism against the development of Aβ pathology during aging.
Alzheimer’s disease (AD) is characterized by the pathological accumulation of beta-amyloid (Aβ) peptides and tau-protein neurofibrillary tangles, followed by neuronal injury and severe cognitive impairment (Hardy and Selkoe, 2002; Jack et al., 2013). Neuropathological and epidemiological studies suggest that lifestyle factors such as education, social network, diet, and cognitive and physical activities, may confer an increased risk or conversely a protective mechanism for the clinical manifestation of AD as well as the development of AD pathology (Bennett et al., 2006, 2014; Stern, 2009). The observed association between lifestyle factors and clinical and pathological manifestation of AD points to the possibility that the vulnerability to AD pathology and onset of AD symptoms may develop over the lifespan. Although relatively less attention has been given compared to these lifestyle factors, emerging evidence suggests that an individual’s personality, a trait that is found to be relatively stable across the lifespan, relates to the increased risk or protection of AD (Wilson et al., 2007; Wang et al., 2009; Terracciano et al., 2013, 2014). A neuro-mechanistic explanation linking the personality traits to AD, however, is largely unknown. Personality often interacts with the proposed lifestyle factors such as cognitive and physical activity and social network (Ackerman and Heggestad, 1997; Bennett et al., 2014). Therefore, the understanding of the neural mechanism linking personality to AD risk will be an important step in disentangling specific effects of complex lifestyle variables that may act simultaneously across the lifespan in the development of resilience or vulnerability to AD.
Personality traits are an individual’s styles and tendencies of interacting with external events. There is a general consensus that adult personality can be defined by five factors, known as the Big Five: neuroticism, extraversion, openness to experience, agreeableness, and conscientiousness (McCrae and Costa, 2008). Among personality traits, neuroticism has been mostly linked to an increased risk of dementia, while conscientiousness has been related to healthier lifestyle choices (Wilson et al., 2011; Low et al., 2013). Extraversion and openness have been associated positively with the intellectual ability which in turn moderates the severity of clinical symptoms (Ackerman and Heggestad, 1997; Valenzuela and Sachdev, 2006; Meng and D’Arcy, 2012). Over the course of AD, the level of neuroticism and extraversion has changed, showing a higher level of neuroticism and a lower level of extraversion in AD patients compared to premorbid status. Collectively, personality traits and AD risk have been linked; however, what neural mechanism underlies this relationship remains to be elucidated.
In the process of AD, Aβ accumulation, a pathological feature of AD, is considered as a relatively early event that antedates more than a decade the appearance of clinical symptoms (Bateman et al., 2012; Jack et al., 2013). Findings from both animal and human studies suggest that Aβ accumulation relates to neural activity and physiology early in life before plaque accumulation (Yan et al., 2009; Vlassenko et al., 2010; Bero et al., 2011; Oh et al., 2016a). For example, the regional concentration of interstitial fluid Aβ in young mice was associated with lactate production indicating neuronal activity, and was further related to amyloid-β plaque deposition in the same brain regions in APP transgenic aged mice (Bero et al., 2011). In humans, Aβ accumulation in older adults topographically overlaps with brain regions showing higher metabolic rates in young adults (Vlassenko et al., 2010; Oh et al., 2016a). Interestingly, neuroimaging studies examining personality trait-based phenotypes have shown differential brain activity patterns in relation to personality types. Among personality traits, introversion/extraversion and neuroticism have been the most influential and frequently studied dimensions of human personality. Across a range of emotional and cognitive tasks, neuroticism has been associated with increased brain activity in the medial frontoparietal network including the medial prefrontal cortex and precuneus (Hariri et al., 2002; Eisenberger et al., 2005; Haas et al., 2007). On the other hand, extraversion has been associated with decreased activity across the brain reflecting neural processing efficiency or reduced self-consciousness (Eisenberger et al., 2005; Gray et al., 2005). Considering the epidemiological, neuropathological, and neurocognitive findings, personality traits, possibly via differential regulation of regional neural activity across the lifespan, may contribute to an individual’s risk of the buildup of AD pathology during aging.
Based on the literature linking personality traits, neural activity, and Aβ deposition, we sought to directly test the relationship between personality and Aβ pathology and a potential neural mechanism underlying this relationship. Specifically, we tested whether extraversion is associated with lower Aβ pathology measured by 18F-Florbetaben PET in cognitively normal older adults and reduced neural activity in the medial frontoparietal cortex during executive control in young adults. To confirm the specificity of the effect of extraversion on Aβ deposition via neural activity, we also examined the relationship between neuroticism and brain activation patterns in young adults. We hypothesized that cognitively normal older adults with higher extraversion scores would show lower Aβ pathology and that higher extraversion in young adults would be associated with reduced activity in the medial frontoparietal cortices during executive control. With neuroticism, we hypothesized that higher neuroticism scores would be associated with greater Aβ pathology in cognitively normal elderly and increased task-related brain activity in young adults. Because Aβ deposition is highly regulated by neural activity, a differential pattern of brain activity in association with extraversion and neuroticism in young adulthood would provide a link between personality and Aβ pathology, which may form across the lifespan and would be observed among older adults.
Thirty-three healthy young (age range: 20–30) and 57 cognitively normal older adults (age range: 60–70) participated in the study. Demographic details are provided in Table 1. Participants were a subset of subjects who participated in our previous study (Oh et al., 2016b) and whose personality test measures were available. A detailed description of subject characteristics can be found in our previous report (Oh et al., 2016b). Briefly, all subjects were recruited via a market mailing procedure and older subjects were classified as either “Amyloid-positive” (Aβ+O) or “Amyloid-negative” (Aβ−O) based on amyloid PET measurements as described below. All subjects provided informed consent in accordance with the Institutional Review Boards of the College of Physicians and Surgeons of Columbia University. Participants were paid for their participation in the study.
A comprehensive battery of neuropsychological tests was administered to all participants to assess cognition. Among cognitive domains, we computed a composite score for processing speed/attention using Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) Digit Symbol subtest (Wechsler, 1997), Trail Making Test Part A (Reitan, 1978; inversed value), and Stroop Color Naming test- Color naming in 90 s (Golden, 1978) and a composite score of episodic memory combining scores from Selective Reminding Test (SRT; Buschke and Fuld, 1974)—long term storage, SRT-continued recall, and SRT-recall at the last trial.
For extraversion and neuroticism scores, we used a subset of the international personality item pool (IPIP) Big-Five personality inventory (Goldberg, 1992), which additionally consists of items for openness, agreeableness, and conscientiousness. The IPIP inventory is a 50-item questionnaire, with 10 items for each personality type, on a 5-point Likert scale (1=“Very Inaccurate”, 2 = “Moderately Inaccurate”, 3 = “Neither Inaccurate nor Accurate”, 4 = “Moderately Accurate”, 5 = “Very Accurate”). An averaged score for each personality type represented each subject’s personality score.
As summarized in Table 1, older subjects were more educated, although at a trend level, than young subjects (T(88) = −1.8, p = 0.07). Aβ+O vs. Aβ−O groups did not differ in age, gender, education, dementia rating scale (DRS), or IQ estimated by AMNART. With cognitive composite scores, young subjects showed significantly higher scores in memory than Aβ−O subjects, while no other group differences were found for both memory and processing speed composite scores. For extraversion and neuroticism scores, young subjects showed significantly higher scores in neuroticism compared to Aβ+O subjects. Personality scores for five factor personality domains are summarized for all subjects and by age group in Supplementary Table 1.
18F-Florbetaben was donated by Piramal (Piramal Pharma Inc.). Detailed information on 18F-Florbetaben PET data acquisition and image processing can be found in our previous report (Oh et al., 2016b). Briefly, scans were performed on a Siemens MCT PET/CT scanner in dynamic, three-dimensional acquisition mode over 20 min (4 × 5 min frames) beginning 50 min following the bolus injection of 10 mCi of 18F-Florbetaben. Dynamic PET frames (four scans) were aligned and averaged to form a mean PET image that was coregistered to each individual’s structural T1 image in FreeSurfer space. The standardized uptake value ratio (SUVR) was calculated for selected cortical regions encompassing frontal, temporal, parietal, and anterior/posterior cingulate cortices with the cerebellar gray matter as a reference region (Landau et al., 2013, 2015). Mean SUVR values from these lobar ROIs constituted a global amyloid index for each subject. Amyloid positivity of elderly subjects was determined using a K-means clustering method as previously reported (Oh et al., 2015). Fourteen and 43 older subjects were classified as Aβ+ and Aβ− subjects, respectively. The proportion of Aβ+ subjects in our sample is comparable to the reports from other studies (Mormino, 2014).
In order to assess the relationship between personality traits and Aβ deposition, we performed two analyses: (Hardy and Selkoe, 2002) a multiple regression model using a mean cortical Aβ measure as a dependent variable and personality scores as an independent variable, and (Jack et al., 2013) the whole-brain voxel-wise analysis using general linear model treating voxel-wise Aβ level as a dependent measure and personality scores as an independent measure. The whole-brain family-wise error was cluster corrected to p < 0.05 (two-sided) using a cluster forming threshold of p < 0.05. Age and sex were controlled in all analyses.
To assess a brain activation pattern in association with extraversion and neuroticism, we used the executive contextual task adapted from Koechlin et al. (2003) which is described in detail in our previous report (Oh et al., 2016b). Briefly, the fMRI task was designed to assess executive control function in a block-design in which either single or dual-task condition was assigned (Supplementary Figure 1). During fMRI scans, a letter in either red or green appeared on the screen and subjects were asked to do vowel/consonant judgment for a green letter and lower/upper-case judgment for a red letter. For a single task condition, either a green or a red letter appeared throughout a block so that subjects had to do only one type of task throughout the task block. For a dual-task condition, color of the letters changed between green and red so that subjects had to switch between two tasks within a task block accordingly. Twelve letters were presented for a maximum of 2,400 ms each within a block that was 33.5 s in duration. One-third of letters in a block appeared in white, for which no response was required. Each functional run consisted of four tasks (two single-task and two dual-task) blocks and two resting condition blocks during which no stimuli were presented and no response was required; in total, the scan session was composed of six functional runs. The executive contextual task fMRI session lasted approximately 26 min.
Response time (RT) of correct trials and proportion correct for single and dual-task conditions were calculated for each individual and reported as behavioral measures.
Participants underwent MRI using a 3T Philips Achieva System equipped with a standard quadrature headcoil. High-resolution T1-weighted magnetization-prepared rapid gradient echo (MPPAGE) scans were collected axially for each subject (TR = 6.6 ms, TE = 3 ms, flip angle = 8°, field of view (FOV) = 256 × 256 mm, matrix size: 256 × 256 mm, slices: 165, voxel size = 1 × 1 × 1 mm3). For the executive context task scans, 111 volumes of functional images were acquired in each run (six runs in total) using a T2*-weighted gradient-echo echo planar images (EPI) sequence (TR = 2,000 ms; TE = 20 ms; flip angle = 72°; FOV = 224 × 224 mm; voxel size = 2 × 2 mm; slice thickness = 3 mm; duration = 3.5 min). Each functional volume consisted of 41 transverse slices per volume. Four dummy volumes acquired at the beginning of each functional run were discarded from the data set before image processing and analysis.
For each subject, a single structural T1 image was processed through FreeSurfer v5.1 to implement region of interest (ROI) labeling following the FreeSurfer processing pipeline1. Briefly, structural images were bias field corrected, intensity normalized, and skull stripped using a watershed algorithm, followed by a tissue-based segmentation, defining gray/white matter and pial surfaces, and topology correction (Dale et al., 1999). Subcortical and cortical ROIs spanning the entire brain were defined in each subject’s native space (Fischl et al., 2002).
All fMRI analyses were performed with SPM8 (Wellcome Department of Imaging Neuroscience, London, UK). A detailed description of fMRI data preprocessing and subject-level analysis can be found elsewhere (Oh et al., 2016b). To identify brain regions that show task-related activity associated with extraversion and neuroticism in young adults, we focused on task-related activity from three comparisons/contrasts: single-task condition compared with baseline, dual-task condition compared with baseline, dual vs. single task contrast. Using general linear models, each comparison/contrast-related activity was regressed on extraversion and neuroticism scores, with gender as covariates of no interest. The whole-brain family-wise error was cluster corrected to p < 0.05 (two-sided) using a cluster forming threshold of p < 0.05.
All non-image data analysis was conducted using SPSS v.22. The relationship between personality traits and Aβ deposition was assessed using multiple regression with a mean cortical Aβ measure as a dependent measure and personality scores as an independent measure. The direct correlation across all older adults was further probed using analysis of covariance (ANCOVA) to assess whether the relationship between personality scores and global Aβ measures changes as a function of amyloid positivity status (i.e., an interaction effect of amyloid positivity status and personality scores on global Aβ measures). This analysis can help determine whether the association between levels of amyloid and extraversion is primarily driven by the Aβ+ group or if the effect is seen across all individuals (meaning sub-threshold amyloid levels showed similar associations). The association between personality scores and cognition was assessed using cognitive composite scores of memory and processing speed as well as executive control task performance (e.g., RT and proportion accuracy). Age and sex were controlled as covariates of no interest.
We assessed the relationship between personality traits and Aβ deposition using a mean cortical and whole-brain voxel-wise Aβ measures. Using multiple regression model with a mean cortical Aβ measure, we found that, among cognitively normal older adults, higher extraversion scores were significantly associated with lower global amyloid index (β = −0.31, p < 0.05; Figure 1B). In addition to a global Aβ measure, we found similar results using local amyloid deposition in the posterior cingulate cortex (β = −0.37, p < 0.05; Figure 1C). Neuroticism scores, however, were not significantly associated with amyloid burden (p > 0.1). A significant relationship between extraversion scores and amyloid burden was mainly driven by a significant association within the Aβ+ group, as revealed by a significant interaction between extraversion scores and amyloid positivity status on the global amyloid index (F = 4.2, p < 0.05). Next, we performed the whole-brain voxel-wise analysis to further probe regional specificity for Aβ deposition in relation to extraversion scores. The cortical regions wherein the level of Aβ deposition is significantly related to extraversion score are shown in Figure 1A (cluster p < 0.05), indicating that higher extraversion scores are associated with lower Aβ deposition across frontal, parietal, and temporal cortices. These brain regions highly overlap with regions that typically accumulate more Aβ than other brain areas.
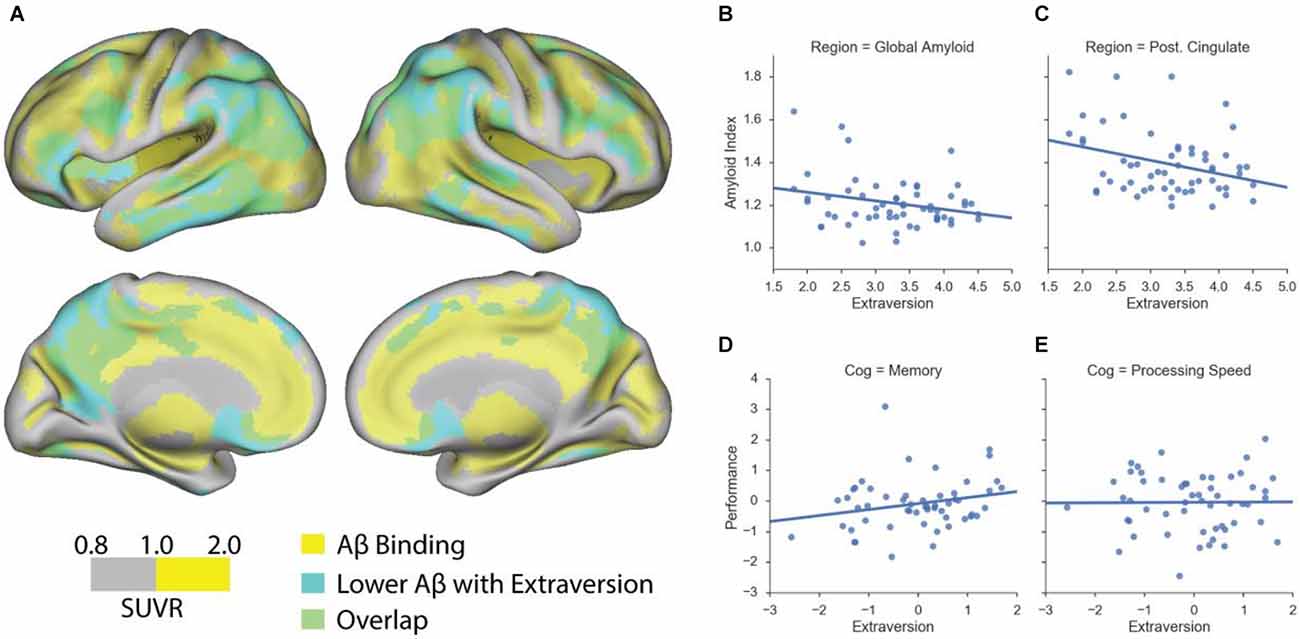
Figure 1. Extraversion is associated with lower amyloid deposition in cognitively normal older adults. (A) Association between voxel-wise standardized uptake value ratio (SUVR) measures and extraversion among cognitively normal older adults. Scale represents voxel-wise SUVR measures. Regions highlighted in yellow represent voxels whose SUVR values are equal to or are greater than 1. Regions highlighted in cyan represent suprathreshold voxels showing a significant negative association between extraversion and amyloid burden. Regions highlighted in green indicate an overlap between amyloid deposition and a significant negative association with extraversion scores. (B) Extraversion scores are associated with lower amyloid deposition as quantified by a global amyloid index that represents the mean cortical amyloid burden. (C) A significant negative association between extraversion and amyloid deposition is found using the mean amyloid accumulation in posterior cingulate cortex (Post. Cingulate). (D) Extraversion scores (residual values) are associated with better, although at a trend level, memory performance (residual values) among cognitively normal older adults. (E) Extraversion scores are not associated with processing speed. Age and sex were controlled in the analyses.
Using the two approaches, we further performed exploratory analyses to test an association between global Aβ deposition and other personality scores: conscientiousness, openness, and agreeableness. No significant association was found between global Aβ level and these personality measures (ps > 0.1). Age and sex were controlled in all analyses.
For fMRI task behavioral measures, we examined an association between extraversion/neuroticism scores and response time and proportion correct during single and dual-task conditions. Neither extraversion nor neuroticism was related to any behavioral performance measure (ps > 0.1).
For task-evoked brain activation, overall, higher extraversion scores in young adults were related to less brain activation in single task compared to baseline and dual-task compared to baseline (Figure 2A). Specifically, in the single task compared to baseline activity, higher extraversion was associated with reduced activation in the anterior cingulate, medial frontal cortex, and lateral middle frontal cortex. In the dual-task compared to baseline activity, a similar but more extended pattern of activity was found in association with extraversion scores. In the dual vs. single task contrast, there was no suprathreshold activity. With neuroticism, we found extensive increases in brain activity in association with higher neuroticism (Figure 2B). Across the activation maps, higher neuroticism was associated with increased activity in the anterior cingulate cortex, anterior frontal cortex, right insular cortex, lateral inferior and middle frontal cortex, posterior cingulate cortex, right superior frontal cortex, caudate, and thalamus, while more extensive regions showed a positive association between neuroticism and brain activity for the dual-task compared to baseline activity.
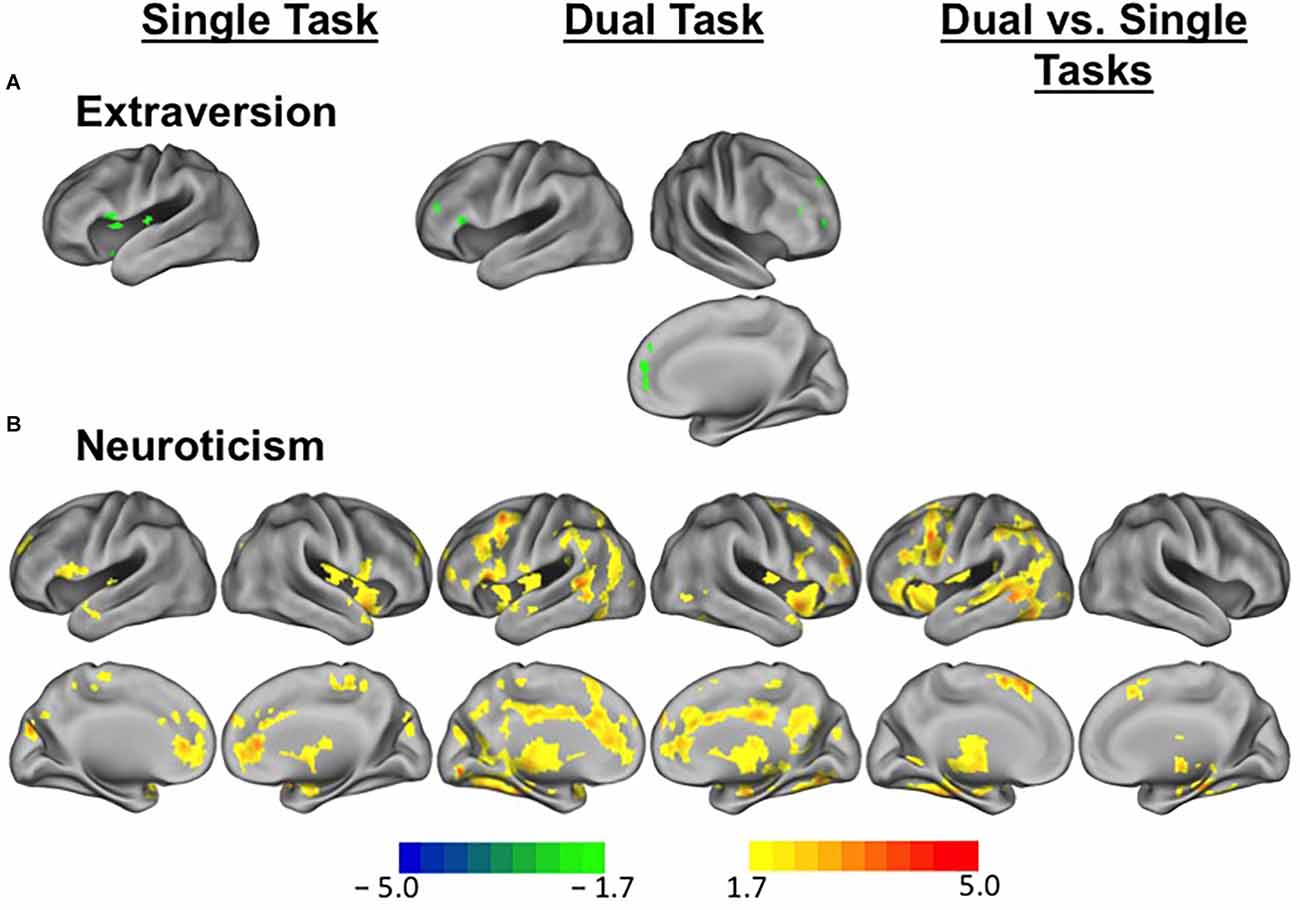
Figure 2. Differential brain activity patterns in association with extraversion and neuroticism among young adults. (A) Task-evoked brain activity during executive control tasks in relation to extraversion scores among young adults. Regions in green indicate suprathreshold voxels showing lower brain activity in relation to higher extraversion scores for single task vs. baseline and dual-task vs. baseline comparisons. (B) Task-evoked brain activity during executive control tasks in relation to neuroticism scores among young adults. Regions in warm colors indicate suprathreshold voxels showing increased brain activity in relation to higher neuroticism scores for single task vs. baseline and dual-task vs. baseline comparisons and dual vs. single task contrasts. Scale represents T values.
We conducted several exploratory analyses to better understand the demographic and psychosocial factors and cognition that relate to personality traits of extraversion and neuroticism in healthy young and cognitively normal older adults. Among cognitively normal older adults, we found no association between extraversion and neuroticism, controlling for age and sex. Extraversion was not correlated with age, but significantly correlated with sex, such that women showed significantly higher extraversion compared to men (β = 0.4, p = 0.003). Neuroticism was not correlated with sex, but significantly correlated with age, showing that the older the age is, the lower the neuroticism scores are (β = −0.33, p = 0.02). A significant relationship between extraversion and memory composite scores was found at a trend level (β = 0.28, p = 0.08; Figure 1D), but no significant relationship was found between extraversion and processing speed composite score (β = 0.03, p > 0.1; Figure 1E). Neuroticism was not related to any cognitive composite measure. In relation to other personality traits, extraversion was significantly associated with openness (β = 0.3, p = 0.02), controlling for age and sex. Neuroticism was significantly negatively associated with agreeableness (β = −0.27, p = 0.046) as well as conscientiousness (β = −0.35, p = 0.01), controlling for age and sex.
Among young subjects, there was no association between extraversion and neuroticism (p > 0.1). Neither age nor sex was associated with extraversion or neuroticism (ps > 0.1). With neuropsychological composite scores, personality measures were not associated with any neuropsychological score (ps > 0.1). In relation to other personality traits, extraversion was significantly associated with agreeableness (β = 0.5, p = 0.01) as well as conscientiousness (β = 0.4, p = 0.035), controlling for age and sex. Neuroticism was not associated with any other personality trait scores (ps > 0.1).
In the present study, we report a novel finding showing that higher extraversion scores are associated with lower Aβ deposition across the widespread brain regions among cognitively normal older adults. When we examined personality-related differences in task-evoked brain activity among young adults, we found a significant dissociation in brain activity as a function of personality traits: extraversion was related to lower brain activity, in particular, in the medial and lateral frontal cortex and anterior cingulate cortex, while neuroticism was associated with increased activity throughout the brain. Our results are consistent with a metabolism hypothesis of Aβ pathology (Kamenetz et al., 2003), which may, in part, provide neural evidence linking personality traits to a source of risk and protective factors of AD that are formed throughout the lifespan.
There is great interest in understanding individual differences that influence the vulnerability of individuals to age-related neurodegenerative diseases such as AD. A large body of neuropathological and epidemiological studies suggests that lifestyle factors such as education, social network, diet, and cognitive and physical activities, may affect the clinical manifestation of AD as well as the development of AD pathology. The relationship between lifestyle factors and AD has been commonly investigated with at least three hypothesized pathways. The pathway that received the most attention is its modulatory pathway that acts on the connection between disease pathology and clinical symptoms, which has been termed as cognitive or neural reserve (Stern, 2009; Bennett et al., 2014). The other equally plausible pathways are to influence directly either the buildup of AD pathology or clinical symptoms independent of AD pathology (Landau et al., 2011; Nyberg et al., 2012; Vemuri et al., 2012; Farfel et al., 2013; Terracciano et al., 2013; Wilson et al., 2013; Bennett et al., 2014; Arenaza-Urquijo et al., 2015; Gidicsin et al., 2015). Epidemiological and neuropathological data from large cohort studies are collectively more supportive of the modulatory effect of lifestyle factors on the clinical manifestation of AD both in cognitively normal older adults (Rentz et al., 2010) and AD patients (Bennett et al., 2014; Snitz et al., 2015).
Compared with the role of education and cognitive aspects of lifestyle factors in the occurrence of AD, less attention has been given to the role of personality and related social engagement factors in AD neuropathology and dementia status. The most common model of personality organizes personality traits into five factors: neuroticism, extraversion, openness to experience, agreeableness, and conscientiousness (McCrae and Costa, 2008). Studies have consistently found that measures of these personality traits predict AD risk and important health outcomes of their lives. Neuroticism, an individual’s increased tendency to negative affectivity, anxiety, and distress proneness, has been shown to predict higher psychological distress, cognitive decline, mortality, and increased risk of AD (Wilson et al., 2006; Low et al., 2013). Loneliness, which is defined as perceived isolation, was robustly associated with cognitive decline and the development of AD (Wilson et al., 2007). Personality-related risk and beneficial effects on the onset of clinical symptoms and AD pathology were further supported once a possibility of premorbid personality changes has been accounted for (Terracciano et al., 2013). Using a quantitative measure at both global and voxel-wise levels, our results provide evidence showing a significant association between amyloid deposition and extraversion among older adults who are yet clinically intact.
Contrary to our prediction, we did not find any association between neuroticism and Aβ deposition. Interestingly, however, we found a significant age-related difference in neuroticism scores between older and young subjects, which is consistent with the literature (Terracciano et al., 2005; Donnellan and Lucas, 2008). Therefore, we speculate that the older adults who remain cognitively normal may be individuals whose neuroticism scores were relatively lower across their lifetime. In a similar manner, individuals with higher neuroticism scores during their lifetime might have already developed cognitive impairment, and thus, could not meet the selection criteria to be included in the present study. On the other hand, extraversion scores did not differ between young and older subjects.
A central motivation of the present study was to elucidate the neural basis underlying the impact of personality traits on AD pathology. Some morphological studies have shown larger gray matter (GM) volume in association with low neuroticism and higher extraversion, openness, agreeableness, and conscientiousness mostly in higher-level association cortices (Kapogiannis et al., 2013). Because these results remained identical across different time points, findings further suggest that distinct personality traits are associated with stable individual differences in GM volume and potentially act as a risk or protective factor by presenting neural reserve during brain aging.
A more direct link in relation to AD pathology can be drawn from brain activation studies showing that personality traits relate to unique patterns of brain activity in response to emotional or cognitive events or stimuli. Neuroticism is associated with negative mood states, sensitivity to negative information, negative appraisal, and vulnerability to psychopathology, which commonly relate to the sustained processing of negative information. By assessing sustained patterns of activity within a brain region implicated in emotional self-evaluation and appraisal, such as the medial frontal cortex, studies have found that higher scores of neuroticism are associated with greater sustained patterns of brain activity in the medial frontal cortex when responding to blocks of negative facial expressions (Haas et al., 2008). In the cognitive domain, controlled processing mechanisms have been linked to the dorsal anterior cingulate cortex (dACC) and the reactivity of dACC has been directly related to neuroticism, due to its role in discrepancy detection (Eisenberger et al., 2005). With regard to extraversion, studies have consistently shown lower baseline activity with higher extraversion and task-related decreases in the lateral and medial frontoparietal network, typically associated with task-focused controlled processing, reflecting neural-processing efficiency, and reduced self-consciousness (Kumari et al., 2004; Eisenberger et al., 2005; Gray et al., 2005). Using the same personality questionnaire that we used for our older subjects, the present findings of differential activity as a function of extraversion and neuroticism during task-switching in young adults are consistent with findings of other studies and extend its implication to AD pathology possibly via differential regulation of neural activity throughout the lifetime, as discussed below.
Accumulation of Aβ peptides is considered an initiating event that precipitates downstream neuro/pathological changes in the process of AD (Hardy and Selkoe, 2002; Gandy, 2005). Because of its significant impact on the pathogenesis of AD, there has been great interest in understanding the causal factors of Aβ accumulation during aging. One of the proposed mechanisms is increased neural activity and physiology early in life before plaque formation during senescence (Cirrito et al., 2005; Jagust and Mormino, 2011). High topographic correspondence between increased metabolism in young people and increased Aβ deposition in older people has been consistently supported by both animal and human studies (Vlassenko et al., 2010; Bero et al., 2011; Oh et al., 2016a). Incipient amyloid accumulation via neural activity may also lead to amyloidosis secondarily in other regions through the prion-like spreading of the Aβ seed (Gandy, 2005). Therefore, an overall regulation of neural activity across the brain may be protective against brain Aβ deposition. Brain regions that accumulate brain Aβ include medial frontal and parietal cortices and anterior cingulate cortex that play a critical role in both cognitive and affective processes (Buckner et al., 2005; Haas et al., 2008). Dysfunction of these brain regions has been implicated in affective and psychiatric disorders such as depression and anxiety that are core personality characteristics associated with neuroticism (Sinha et al., 2016). Abnormal changes in these brain regions may relate to a dysfunction of the serotonergic system, which has been also suggested for amyloid precursor protein metabolism, or serotonin transporter polymorphisms (Pezawas et al., 2005; Fisher et al., 2016). It is important to note that the association of extraversion with lower activity in our executive contextual fMRI task was fairly circumscribed and showed both overlap but also differences compared to regions where extraversion was associated with amyloid. Thus, rather than complete topographic overlapping between lower brain activity in young adulthood and Aβ accumulation during aging, the results indicate a directional link between regional neural activity and Aβ pathology. Taken together, our findings provide evidence linking personality traits to Aβ pathology by showing differential lifelong regulations of brain activity in association with extraversion and neuroticism.
Although we aimed to examine the impact of personality in AD risk via the hypothesized relationship across the two personality traits, neural activity, and Aβ pathology, other psychosocial mechanisms could be considered as a mediator of the observed relationship between personality traits and Aβ pathology. As personality traits such as extraversion have been significantly related to other personality traits such as openness to experience among older adults and conscientiousness in young subjects, it is possible that other lifelong variables may mediate the observed relationship between extraversion and lower Aβ pathology. Some genetic factors associated with personality may also explain its relationship with AD pathology via prevalent comorbidity associated with the genes (Deuschle et al., 2010). More generally, personality may impact dementia risk and pathology by influencing health habits, number and quality of social relationships, cognitive activity, and reactions to stress over the lifespan, which could collectively have contributed to the observed relationship. Another limitation of the study is that the association of extraversion with each of the other measures of interest come from different groups, so can only provide indirect (yet converging) evidence that brain activity can explain the link between extraversion and amyloid deposition. Longitudinal fMRI and amyloid data from younger age to older age do not yet exist, so by necessity, this is a cross-sectional study. Therefore, the causal and temporal relationships between these measures cannot be determined definitively. In addition, because of the cross-sectional investigation of the current study, the observed association between extraversion and Aβ pathology in cognitively normal older adults may have reflected premorbid changes as other studies have suggested, although our prediction of Aβ pathology based on brain activation in young adulthood is against this possibility. Future studies are warranted to address these questions using a larger and longitudinal cohort and multivariate analysis approaches that allow assessing unique contributions of multiple lifetime variables to Aβ pathology.
The present study is the first to show that extraversion is associated with lower Aβ pathology among cognitively normal older adults and is associated with lower brain activity in regions including the medial frontal cortex and anterior cingulate cortex in response to a controlled processing task in young adulthood. Our results provide evidence showing the neural basis underlying the link between extraversion and Aβ pathology that may develop throughout the lifespan. It is important to note that, although personality can be seen as a trait vs. state, it is potentially modifiable. In addition, while it is possible that life events, especially early stressful life events, may influence the formation of the personality type of individuals, one’s personality type also can modify the impact of stressful life events on health outcomes, affect lifestyle choices, and, more directly, impact AD pathology. Our results suggest that personality is an important factor that should be considered for conceptual and biological models of dementia risk and designing clinical trials. Considering its modifiability, personality can be considered for dementia risk reduction strategies that can start early in the lifespan.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Institutional Review Boards of the College of Physicians and Surgeons of Columbia University. All methods were carried out in accordance with the approved guidelines. The patients/participants provided their written informed consent to participate in this study.
HO conceptualized the study, contributed to the acquisition of the data, analyzed and interpreted the data, wrote the manuscript, and approved the submitted version.
This research was supported by the National Institute on Aging (grant numbers R01AG026158, R01AG068990).
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.900581/full#supplementary-material.
Ackerman, P. L., and Heggestad, E. D. (1997). Intelligence, personality and interests: evidence for overlapping traits. Psychol. Bull. 121, 219–245. doi: 10.1037/0033-2909.121.2.219
Arenaza-Urquijo, E. M., Wirth, M., and Chetelat, G. (2015). Cognitive reserve and lifestyle: moving towards preclinical Alzheimer’s disease. Front. Aging Neurosci. 7:134. doi: 10.3389/fnagi.2015.00134
Bateman, R. J., Xiong, C., Benzinger, T. L., Fagan, A. M., Goate, A., Fox, N. C., et al. (2012). Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 367, 795–804. doi: 10.1056/NEJMoa1202753
Bennett, D. A., Arnold, S. E., Valenzuela, M. J., Brayne, C., and Schneider, J. A. (2014). Cognitive and social lifestyle: links with neuropathology and cognition in late life. Acta Neuropathol. 127, 137–150. doi: 10.1007/s00401-013-1226-2
Bennett, D. A., Schneider, J. A., Tang, Y., Arnold, S. E., and Wilson, R. S. (2006). The effect of social networks on the relation between Alzheimer’s disease pathology and level of cognitive function in old people: a longitudinal cohort study. Lancet Neurol. 5, 406–412. doi: 10.1016/S1474-4422(06)70417-3
Bero, A. W., Yan, P., Roh, J. H., Cirrito, J. R., Stewart, F. R., Raichle, M. E., et al. (2011). Neuronal activity regulates the regional vulnerability to amyloid-β deposition. Nat. Neurosci. 14, 750–756. doi: 10.1038/nn.2801
Buckner, R. L., Snyder, A. Z., Shannon, B. J., LaRossa, G., Sachs, R., Fotenos, A. F., et al. (2005). Molecular, structural and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid and memory. J. Neurosci. 25, 7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005
Buschke, H., and Fuld, P. A. (1974). Evaluating storage, retention and retrieval in disordered memory and learning. Neurology 24, 1019–1025. doi: 10.1212/wnl.24.11.1019
Cirrito, J. R., Yamada, K. A., Finn, M. B., Sloviter, R. S., Bales, K. R., May, P. C., et al. (2005). Synaptic activity regulates interstitial fluid amyloid-β levels in vivo. Neuron 48, 913–922. doi: 10.1016/j.neuron.2005.10.028
Dale, A. M., Fischl, B., and Sereno, M. I. (1999). Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. doi: 10.1006/nimg.1998.0395
Deuschle, M., Schredl, M., Schilling, C., Wust, S., Frank, J., Witt, S. H., et al. (2010). Association between a serotonin transporter length polymorphism and primary insomnia. Sleep 33, 343–347. doi: 10.1093/sleep/33.3.343
Donnellan, M. B., and Lucas, R. E. (2008). Age differences in the Big Five across the life span: evidence from two national samples. Psychol. Aging 23, 558–566. doi: 10.1037/a0012897
Eisenberger, N. I., Lieberman, M. D., and Satpute, A. B. (2005). Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion and self-consciousness. Cogn. Affect. Behav. Neurosci. 5, 169–181. doi: 10.3758/cabn.5.2.169
Farfel, J. M., Nitrini, R., Suemoto, C. K., Grinberg, L. T., Ferretti, R. E., Leite, R. E., et al. (2013). Very low levels of education and cognitive reserve: a clinicopathologic study. Neurology 81, 650–657. doi: 10.1212/WNL.0b013e3182a08f1b
Fischl, B., Salat, D. H., Busa, E., Albert, M., Dieterich, M., Haselgrove, C., et al. (2002). Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. doi: 10.1016/s0896-6273(02)00569-x
Fisher, J. R., Wallace, C. E., Tripoli, D. L., Sheline, Y. I., and Cirrito, J. R. (2016). Redundant Gs-coupled serotonin receptors regulate amyloid-beta metabolism in vivo. Mol. Neurodegener. 11:45. doi: 10.1186/s13024-016-0112-5
Gandy, S. (2005). The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. J. Clin. Invest. 115, 1121–1129. doi: 10.1172/JCI25100
Gidicsin, C. M., Maye, J. E., Locascio, J. J., Pepin, L. C., Philiossaint, M., Becker, J. A., et al. (2015). Cognitive activity relates to cognitive performance but not to Alzheimer disease biomarkers. Neurology 85, 48–55. doi: 10.1212/WNL.0000000000001704
Goldberg, L. R. (1992). The development of markers for the big-five factor structure. Psychol. Assess. 4, 26–42. doi: 10.1037/1040-3590.4.1.26
Gray, J. R., Burgess, G. C., Schaefer, A., Yarkoni, T., Larsen, R. J., Braver, T. S., et al. (2005). Affective personality differences in neural processing efficiency confirmed using fMRI. Cogn. Affect. Behav. Neurosci. 5, 182–190. doi: 10.3758/cabn.5.2.182
Haas, B. W., Constable, R. T., and Canli, T. (2008). Stop the sadness: neuroticism is associated with sustained medial prefrontal cortex response to emotional facial expressions. Neuroimage 42, 385–392. doi: 10.1016/j.neuroimage.2008.04.027
Haas, B. W., Omura, K., Constable, R. T., and Canli, T. (2007). Emotional conflict and neuroticism: personality-dependent activation in the amygdala and subgenual anterior cingulate. Behav. Neurosci. 121, 249–256. doi: 10.1037/0735-7044.121.2.249
Hardy, J., and Selkoe, D. J. (2002). The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science 297, 353–356. doi: 10.1126/science.1072994
Hariri, A. R., Mattay, V. S., Tessitore, A., Kolachana, B., Fera, F., Goldman, D., et al. (2002). Serotonin transporter genetic variation and the response of the human amygdala. Science 297, 400–403. doi: 10.1126/science.1071829
Jack, C. R., Knopman, D. S. Jr., Jagust, W. J., Petersen, R. C., Weiner, M. W., Aisen, P. S., et al. (2013). Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216. doi: 10.1016/S1474-4422(12)70291-0
Jagust, W. J., and Mormino, E. C. (2011). Lifespan brain activity, beta-amyloid and Alzheimer’s disease. Trends Cogn. Sci. 15, 520–526. doi: 10.1016/j.tics.2011.09.004
Kamenetz, F., Tomita, T., Hsieh, H., Seabrook, G., Borchelt, D., Iwatsubo, T., et al. (2003). APP processing and synaptic function. Neuron 37, 925–937. doi: 10.1016/s0896-6273(03)00124-7
Kapogiannis, D., Sutin, A., Davatzikos, C., Costa, P. Jr., and Resnick, S. (2013). The five factors of personality and regional cortical variability in the Baltimore longitudinal study of aging. Hum. Brain Mapp. 34, 2829–2840. doi: 10.1002/hbm.22108
Koechlin, E., Ody, C., and Kouneiher, F. (2003). The architecture of cognitive control in the human prefrontal cortex. Science 302, 1181–1185. doi: 10.1126/science.1088545
Kumari, V., ffytche, D. H., Williams, S. C., and Gray, J. A. (2004). Personality predicts brain responses to cognitive demands. J. Neurosci. 24, 10636–10641. doi: 10.1523/JNEUROSCI.3206-04.2004
Landau, S. M., Breault, C., Joshi, A. D., Pontecorvo, M., Mathis, C. A., Jagust, W. J., et al. (2013). Amyloid-beta imaging with Pittsburgh compound B and florbetapir: comparing radiotracers and quantification methods. J. Nucl. Med. 54, 70–77. doi: 10.2967/jnumed.112.109009
Landau, S. M., Fero, A., Baker, S. L., Koeppe, R., Mintun, M., Chen, K., et al. (2015). Measurement of longitudinal beta-amyloid change with 18F-florbetapir PET and standardized uptake value ratios. J. Nucl. Med. 56, 567–574. doi: 10.2967/jnumed.114.148981
Landau, S. M., Harvey, D., Madison, C. M., Koeppe, R. A., Reiman, E. M., Foster, N. L., et al. (2011). Associations between cognitive, functional and FDG-PET measures of decline in AD and MCI. Neurobiol. Aging 32, 1207–1218. doi: 10.1016/j.neurobiolaging.2009.07.002
Low, L. F., Harrison, F., and Lackersteen, S. M. (2013). Does personality affect risk for dementia? A systematic review and meta-analysis. Am. J. Geriatr. Psychiatry 21, 713–728. doi: 10.1016/j.jagp.2012.08.004
McCrae, R. R., and Costa, P. T. Jr. (2008). “The five-factor theory of personality,” in Handbook of Personality: Theory and Research, eds O. P. John, R. W. Robins, and L. A. Pervin (New York, NY: Guilford Press), 159–181.
Meng, X., and D’Arcy, C. (2012). Education and dementia in the context of the cognitive reserve hypothesis: a systematic review with meta-analyses and qualitative analyses. PLoS One 7:e38268. doi: 10.1371/journal.pone.0038268
Mormino, E. C. (2014). The relevance of beta-amyloid on markers of Alzheimer’s disease in clinically normal individuals and factors that influence these associations. Neuropsychol. Rev. 24, 300–312. doi: 10.1007/s11065-014-9267-4
Nyberg, L., Lovden, M., Riklund, K., Lindenberger, U., and Backman, L. (2012). Memory aging and brain maintenance. Trends Cogn. Sci. 16, 292–305. doi: 10.1016/j.tics.2012.04.005
Oh, H., Madison, C., Baker, S., Rabinovici, G., and Jagust, W. (2016a). Dynamic relationships between age, amyloid-beta deposition and glucose metabolism link to the regional vulnerability to Alzheimer’s disease. Brain 139, 2275–2289. doi: 10.1093/brain/aww108
Oh, H., Steffener, J., Razlighi, Q. R., Habeck, C., and Stern, Y. (2016b). beta-Amyloid deposition is associated with decreased right prefrontal activation during task switching among cognitively normal elderly. J. Neurosci. 36, 1962–1970. doi: 10.1523/JNEUROSCI.3266-15.2016
Oh, H., Steffener, J., Razlighi, Q. R., Habeck, C., Liu, D., Gazes, Y., et al. (2015). Abeta-related hyperactivation in frontoparietal control regions in cognitively normal elderly. Neurobiol. Aging 36, 3247–3254. doi: 10.1016/j.neurobiolaging.2015.08.016
Pezawas, L., Meyer-Lindenberg, A., Drabant, E. M., Verchinski, B. A., Munoz, K. E., Kolachana, B. S., et al. (2005). 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci. 8, 828–834. doi: 10.1038/nn1463
Reitan, R. (1978). Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Tucson, AZ: Reitan Neuropsychology Laboratories, Inc.
Rentz, D. M., Locascio, J. J., Becker, J. A., Moran, E. K., Eng, E., Buckner, R. L., et al. (2010). Cognition, reserve and amyloid deposition in normal aging. Ann. Neurol. 67, 353–364. doi: 10.1002/ana.21904
Sinha, R., Lacadie, C. M., Constable, R. T., and Seo, D. (2016). Dynamic neural activity during stress signals resilient coping. Proc. Natl. Acad. Sci. U S A 113, 8837–8842. doi: 10.1073/pnas.1600965113
Snitz, B. E., Weissfeld, L. A., Cohen, A. D., Lopez, O. L., Nebes, R. D., Aizenstein, H. J., et al. (2015). Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am. J. Geriatr. Psychiatry 23, 985–993. doi: 10.1016/j.jagp.2015.01.008
Stern, Y. (2009). Cognitive reserve. Neuropsychologia 47, 2015–2028. doi: 10.1016/j.neuropsychologia.2009.03.004
Terracciano, A., Iacono, D., O’Brien, R. J., Troncoso, J. C., An, Y., Sutin, A. R., et al. (2013). Personality and resilience to Alzheimer’s disease neuropathology: a prospective autopsy study. Neurobiol. Aging 34, 1045–1050. doi: 10.1016/j.neurobiolaging.2012.08.008
Terracciano, A., McCrae, R. R., Brant, L. J., and Costa, P. T. Jr. (2005). Hierarchical linear modeling analyses of the NEO-PI-R scales in the baltimore longitudinal study of aging. Psychol. Aging 20, 493–506. doi: 10.1037/0882-7974.20.3.493
Terracciano, A., Sutin, A. R., An, Y., O’Brien, R. J., Ferrucci, L., Zonderman, A. B., et al. (2014). Personality and risk of Alzheimer’s disease: new data and meta-analysis. Alzheimers Dement. 10, 179–186. doi: 10.1016/j.jalz.2013.03.002
Valenzuela, M. J., and Sachdev, P. (2006). Brain reserve and dementia: a systematic review. Psychol. Med. 36, 441–454. doi: 10.1017/S0033291705006264
Vemuri, P., Lesnick, T. G., Przybelski, S. A., Knopman, D. S., Roberts, R. O., Lowe, V. J., et al. (2012). Effect of lifestyle activities on Alzheimer disease biomarkers and cognition. Ann. Neurol. 72, 730–738. doi: 10.1002/ana.23665
Vlassenko, A. G., Vaishnavi, S. N., Couture, L., Sacco, D., Shannon, B. J., Mach, R. H., et al. (2010). Spatial correlation between brain aerobic glycolysis and amyloid-beta (Aβ ) deposition. Proc. Natl. Acad. Sci. U S A 107, 17763–17777. doi: 10.1073/pnas.1010461107
Wang, H. X., Karp, A., Herlitz, A., Crowe, M., Kareholt, I., Winblad, B., et al. (2009). Personality and lifestyle in relation to dementia incidence. Neurology 72, 253–259. doi: 10.1212/01.wnl.0000339485.39246.87
Wechsler, D. (1997). Wechsler Adult Intelligence Scale - III. San Antonio, TX: Psychological Corporation.
Wilson, R. S., Arnold, S. E., Schneider, J. A., Kelly, J. F., Tang, Y., Bennett, D. A., et al. (2006). Chronic psychological distress and risk of Alzheimer’s disease in old age. Neuroepidemiology 27, 143–153. doi: 10.1159/000095761
Wilson, R. S., Begeny, C. T., Boyle, P. A., Schneider, J. A., and Bennett, D. A. (2011). Vulnerability to stress, anxiety and development of dementia in old age. Am. J. Geriatr. Psychiatry 19, 327–334. doi: 10.1097/JGP.0b013e31820119da
Wilson, R. S., Boyle, P. A., Yu, L., Barnes, L. L., Schneider, J. A., Bennett, D. A., et al. (2013). Life-span cognitive activity, neuropathologic burden and cognitive aging. Neurology 81, 314–321. doi: 10.1212/WNL.0b013e31829c5e8a
Wilson, R. S., Krueger, K. R., Arnold, S. E., Schneider, J. A., Kelly, J. F., Barnes, L. L., et al. (2007). Loneliness and risk of Alzheimer disease. Arch. Gen. Psychiatry 64, 234–240. doi: 10.1001/archpsyc.64.2.234
Keywords: beta amyloid deposition, extraversion, neuroticism, personality, Alzheimer’s disease risk factor
Citation: Oh H (2022) Extraversion Is Associated With Lower Brain Beta-Amyloid Deposition in Cognitively Normal Older Adults. Front. Aging Neurosci. 14:900581. doi: 10.3389/fnagi.2022.900581
Received: 20 March 2022; Accepted: 08 June 2022;
Published: 13 July 2022.
Edited by:
Agustin Ibanez, Latin American Brain Health Institute (BrainLat), ChileReviewed by:
Jeremy Andrew Elman, University of California, San Diego, United StatesCopyright © 2022 Oh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hwamee Oh, SHdhbWVlX09oQGJyb3duLmVkdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.