
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Aging Neurosci., 22 July 2022
Sec. Neurocognitive Aging and Behavior
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.896218
This article is part of the Research TopicWomen in Aging Neuroscience 2021View all 10 articles
Parts of this article's content have been modified or rectified in:
Erratum: Feelings of loneliness and isolation: Social brain and social cognition in the elderly and Alzheimer's disease
 Rosalba Morese1,2*
Rosalba Morese1,2* Sara Palermo3,4
Sara Palermo3,4Interpersonal neurobiology presents an integrated view of the development of the human mind, investigating how this occurs from the mutual influence between human relationships and brain structure and function: the focus of this approach is to understand how the brain gives rise to mental processes and how it is directly shaped by interpersonal experiences (Siegel, 1999). Through this approach we can understand what processes are useful in facilitating cognitive-behavioral development, emotional and psychological wellbeing, and resilience certainly during early childhood but probably throughout life. Underlying the mentioned processes is indeed a fundamental mechanism of integration that can be examined at different levels, from interpersonal to neurological (Siegel, 2001). Interpersonal neurobiology proposes an interpretation of mental processes whose main characteristics are (1) being both embodied, in the body, and relational; (2) the smooth flow of metabolites and information; and (3) the flow of information in the percipient and between people (Siegel, 2001). Knowing how to control and knowing how to modify this flow of energy and information, the basis of healthy regulation, are skills that are acquired in families with secure attachment (Bowlby, 1969; Siegel, 2001; Bowlby and King, 2004).
The child comes into the world genetically programmed to establish attachment bonds with its caregivers who will become, thus, the child's attachment figures (Bowlby, 1969; Cassidy and Shaver, 1999; Bowlby and King, 2004). The attachment system is considered a motivational system: an innate, adaptive, biologically determined system that drives a child to create certain selective attachments in his or her life. Although the attachment system is programmed at the brain level, the experiences a child has throughout his or her childhood go a long way toward shaping that system (Bowlby, 1969; Cassidy and Shaver, 1999; Bowlby and King, 2004). The succession of relational experiences prompts the activation of brain neurons that respond to sensory events from the outside world or internally generated images from the brain itself (Gazzaniga, 1995; Kandel et al., 2012). The process is used to create a mental image, sensory image or linguistic representation of a concept or object (Siegel, 2001; Kandel et al., 2012). According to Siegel (2001), the neural substrate also allows the formation of an emergent self, a proto-self, determined largely by genetic and constitutional characteristics. This sense of self is embedded in the brain as well as in its interactions with the environment: internal working model (Siegel, 2001). On the other hand, the child's mind seems to develop a fundamental process in which the other's mental states (the so-called Theory of Mind, ToM) are also represented within the neuronal functioning of the brain (Stone et al., 1998). The sense of acting, coherence, affectivity and even the continuity of the self (memory) are therefore influenced by interaction with others. In this way, experience shapes the function of neural activity and can potentially shape the evolving structure of the brain throughout the lifespan. Developmental stages and aging change the concept of security from childhood's pursuit of physical proximity to sophisticated forms of relating and representing others. Through the process of creating new meanings from memories, internal working models reorganize themselves according to new relational experiences, thereby assisting in constructing a consistent self-model through adulthood (Bowlby, 1969; Cassidy and Shaver, 1999; Bowlby and King, 2004).
The attachment theory of John Bowlby is applicable to every age group, but researchers have been slow to study attachment in older adults (Bradley and Cafferty, 2001). Study findings suggest, however, that attachment issues could be relevant for the elderly, given that aging can be associated with separation, loss, and vulnerability (Bradley and Cafferty, 2001). As a matter of fact, the personal attachment style is associated with a range of outcomes in later life (such as reactions to the loss of a loved one, general wellbeing and adjustment to chronic illness and caregiver burden). The implications of attachment styles and the questions raised by interpersonal neurobiology regarding social isolation and loneliness on the directions of aging and the acceleration of any neurodegenerative process are discussed here.
Networks of relationships are important to Siegel (2001) since neural networks appear strongly influenced by relations with others, beyond genetic influences. In his words: “human connections shape neural connections, and each contributes to mind. Relationships and neural linkages together shape the mind. It is more than the sum of its parts; this is the essence of emergence” (Siegel, 2001, p. 3). The horizon of interpersonal neurobiology thus allows for a broad perspective that is reflected in neuroimaging investigations. Thus, social neuroscience hypothesizes brain evolution on the level of intersubjective actions. Due to the social environment in which primates live, specific selective pressures have led to the evolution of neurocognitive mechanisms capable of handling the challenges of social interaction (Adenzato and Enrici, 2005). Humans' social cognition consists of psychological processes that allow us to make inferences about what others are thinking and feeling (Adenzato and Enrici, 2005; Adolphs, 2009). The way social information is processed is divided into automatic and stimuli-driven processes, and those that are deliberate and controlled, but sensitive to context and strategy (Adolphs, 2009). In their proposal, the “social brain hypothesis,” Byrne and Whiten (1988) were among the first to argue that complex social environments serve as a dominant selective pressure for human brain size. By appealing to particular pressures that a species adapted to social interactions would have faced, the social brain hypothesis attempts to explain the extraordinary size and complexity of the human brain (Barrett and Henzi, 2005; Dunbar and Shultz, 2007a,b).
In everyday life, social interaction is one of the most complex mental activities in which humans engage. The high cognitive load is necessary to predict the behavior of people involved in social interaction. In particular, the functions involved in the social brain relate to social cognition, which is important for sociability. The term social cognition refers to the set of abilities that enables an individual to construct mental representations of his or her relationships with others and to use these representations to adapt behaviors to the context (Adolphs, 2001). Social groups are complex in nature, and it is their complexity that has led to the advancement of prefrontal brain functioning and specialization (Adenzato and Enrici, 2005). Not only the prefrontal areas but also other cortical and subcortical structures are involved in the processing of social stimuli. Social information activates complex neural circuits that connect cortical and subcortical regions, including those usually thought to be involved in the emotional processing of stimuli, such as the amygdala, as well as those usually thought to be involved in the cognitive processing of stimuli, such as the temporo-occipital junction and the medial prefrontal cortex (Van Overwalle, 2009). This widespread neural involvement reverberates the fact that social cognition is a high-order function. Indeed, social cognition is broad and varied; it refers to all mental processes useful in social interaction, among which the ToM and mentalizing play a significant role. Premack and Woodruff (1978) define ToM as the ability t to attribute to other individuals' mental states that are different from one's own. Mentalisation is an inherently imaginative activity involving the attribution of intentional mental states based on clues. In mentalisation, it is recognized that a person's actions are autonomous and can be explained by his or her internal state (McLaren and Sharp, 2020).
According to Dodich et al. (2015), we deal with a multidimensional process in which different components are integrated. Among these, attribution of emotions and intentions is a very important component during the representation of mental states. Other authors use this term to refer to thinking or feeling about others' mental states (Saxe et al., 2006a,b; Van Overwalle, 2009). Some neural structures, such as the anterior cingulate cortex (ACC) (Palermo, 2017, 2020b), the medial prefrontal cortex (MPFC), the temporoparietal junction (TPJ), the posterior cingulate cortex (PCC), and the superior temporal sulcus (STS) are known to play a role (Adolphs, 2001; Saxe and Kanwisher, 2003). On the other hand, Sebastian et al. (2012) used functional magnetic resonance imaging (fMRI) on healthy subjects which showed that some brain areas involved with mentalization and perspective-taking, like the temporoparietal junction and the ventromedial prefrontal cortex, are recruited when affective stimuli are present.
In fact, it is only one behavioral domain, that of social cognition (Laird et al., 2011), that is strongly and exclusively associated with a neural network that closely resembles the default mode network (DMN), demonstrating bilateral activation of the inferior parietal/TPJ, posterior precuneus/cingulate, and medial frontal (Smith et al., 2009; Mars et al., 2012). Similar conclusions had previously been reached by Schilbach et al. (2008). When they examined the DMNs' responses to different types of cognitive stimulation some activations were quite similar to those observed in various aspects of social cognition: the left angular gyrus/TPJ in differentiating between self and others (Vogeley and Fink, 2003); the anterior cingulate in monitoring action in self and others (Amodio and Frith, 2006); the precuneus in social interactions (Schilbach et al., 2006). According to the authors, the biological “baseline” of the human brain corresponds to a psychological “baseline,” our predisposition to engage in social cognition by default (Schilbach et al., 2008; Mars et al., 2012). Cognitive processes geared toward self-reflection, such as introspection and autobiographical memory, have been also linked to the DMN, and its integrity is now considered crucial to mental health (Grieder et al., 2018).
Loneliness is a negative emotional state experienced when there is a discrepancy between the relationships one would like to have and that one perceives to have (Alberti, 2019). This condition does not so much concern the amount of time spent with other people as the quality of the relationships themselves.
In industrialized countries about one-third of people are affected by this condition, with one in severely affected, with these proportions constantly increasing (Cacioppo and Cacioppo, 2018). Loneliness is to such an extent a painful companion for many people that an editorial in the New York Times on the issue was entitled, “Is Loneliness a Health Epidemic?” (Klinenberg, 2018). Those who are most likely to report a significant feeling of loneliness tend to belong to the most vulnerable social groups, such as the young, the elderly, the poor, the chronically ill, and the mentally ill (Hawkins-Elder et al., 2018).
Importantly, loneliness has a profound impact on physical and psychological health, often leading to negative outcomes; loneliness and social isolation would appear to be associated with a reduction in lifespan like that caused by smoking 15 cigarettes a day, with a 27% increased risk of premature mortality (Holt-Lunstad et al., 2010). On the other hand, establishing strong relationships would lead to a reduced risk of mortality (Holt-Lunstad et al., 2017).
Several studies on the effects of loneliness on the health of the general population have been conducted over time. Loneliness is known to affect mental influence mental health, by leading to depression (Alpass and Neville, 2003; Cacioppo et al., 2006a; Hawkley and Cacioppo, 2010). Indeed, loneliness precedes mood disorder in time, proving to be a key factor in the onset of the disorder (Cacioppo et al., 2010): loneliness seems to mediate the anxiety-depression relationship, with loneliness potentially resulting from anxiety and subsequently being able to sequentially activate depressive symptoms (Ebesutani et al., 2015). The process behind this phenomenon is quite complex. It is believed that oxytocin and arginine vasopressin act as key mediators of social behavior in non-human mammals and human (Heinrichs and Domes, 2008). Oxytocin reduces behavioral and neuroendocrine responses to social stress and, as a result, may allow animals to overcome their natural aversion to close proximity and inhibit defensive behavior, thus facilitating approaches (Heinrichs and Domes, 2008). Seven primary emotional processes have been described by affective neuroscience: SEEKING, RAGE, FEAR, sexual LUST, maternal CARE, separation-distress PANIC/GRIEF and joyful PLAY (Panksepp, 1998; Zellner et al., 2011). Social loss, perhaps the biggest epidemiological determinant of depression, may promote deflection of mood through overactivity of separation-distress PANIC/GRIEF and hypoactivity of SEEKING networks (Panksepp, 1998, 2003; Zellner et al., 2011). Endogenous opioids, which may mediate attachment and separation distress via oxytocin pathways, contribute to initiating depressive cascades through decreased SEEKING (Gunnar and Quevedo, 2007; Heinrichs and Domes, 2008; Nolte et al., 2011). Thus, altered affective networks occurring in depression may explain psychological pain and dysphoria. Human health, including the need for social relationships, is largely driven by the endogenous opioid hormonal system (Johnson et al., 2014). Illness may result from disrupting this system.
Indeed, from a biomedical point of view, loneliness has been associated also with poor self-rated health (Stickley et al., 2013). Consistent with this, persistent loneliness has been associated with physical health problems (Newall et al., 2014) and sleep disorders (Cacioppo and Cacioppo, 2014). Moreover, it has also been linked with negative health habits, such as alcohol consumption (Stickley et al., 2013; Arpin et al., 2015) and smoking (Stickley et al., 2013).
In the neuropsychiatric field, loneliness has also been linked to obsessive-compulsive disorder (Timpano et al., 2014), social anxiety (Lim et al., 2016), and paranoia (Jaya et al., 2017). Loneliness has been associated with psychological distress (Stickley et al., 2013) but the most dramatic outcome of loneliness is suicide especially in populations at risk such as adolescents and old adults (Stravynski and Boyer, 2001; Morese et al., 2019a; Morese and Longobardi, 2020; Morese et al., 2020), an act almost constantly associated with the idea of being left alone and no longer able to receive help from anyone (De Leo and Diekstra, 1990). Indeed, strong associations among suicide ideation, parasuicide, and different ways of being lonely and alone were verified (Stravynski and Boyer, 2001). Importantly, the prevalence of suicide ideation and parasuicide increased with the degree of loneliness with differences between men and women (Stravynski and Boyer, 2001).
From a neuropsychological perspective, perceived social isolation (i.e., loneliness) is a risk factor for - and may contribute to - poorer overall cognitive performance, faster cognitive decline, poorer executive functioning, greater sensitivity to social threats, which is a confirmation bias in social cognition (Cacioppo and Hawkley, 2009). Therefore, social worlds tend to be perceived as threatening and punishing by lonely people (Cacioppo and Hawkley, 2009). Researchers have found that manipulating feelings of loneliness causes people to feel more anxious, fear negative evaluation, and act more coldly toward others (Cacioppo et al., 2006b), while also making them feel colder (Zhong and Leonardelli, 2008). Also, lonely people are more likely to form negative social impressions of others, and their expectations, attributional reasoning, and behavior toward others are less charitable than those of non-lonely individuals (Cacioppo and Hawkley, 2005). As a result of negative social expectations being validated by others, these expectations are reinforced and an individual is more likely to behave in ways that distance them from the very people they want to be close to better meet their social needs (Cacioppo and Hawkley, 2009). Hence, lonely individuals may perceive themselves as passive victims in their social world, yet they are active agents through their self-destructive interactions with others (Cacioppo and Hawkley, 2005).
Pandemic conditions have exacerbated these effects today as social distancing, fiduciary isolation, and quarantine have been imposed. The consequences on the elderly population are of particular interest (Morese et al., 2019b; Palermo, 2020a, 2021a; Amanzio et al., 2021).
Since the second half of the 20th century, advances in healthcare and nutrition have led to an increase in the number of older adults in Western societies. Research in developmental and health care is challenged by this rapid increase. Aging involves new definitions of both self and relational issues in many developmental models (Blatt, 2008), since it concurs to psycho-physical, cognitive, and social impairment (Maylor et al., 2002). Moreover, as older adults age, they become increasingly confronted with the loss of loved ones.
Loneliness and social isolation are growing public health concerns in our aging society (Akoya et al., 2020). The prevalence of loneliness among the population is high. Eighty per cent of those under 18 years of age and 40% of those over 65 years of age report feeling lonely at least occasionally (Giné-Garriga et al., 2021). A growing number of older people in the EU are living alone: they form a particularly vulnerable group in society, with an increased risk of social exclusion or poverty (Eurostat, 2020). Seniors who live alone are often facing complex dynamics that rarely have an easy explanation: loneliness can occur due to the natural events of life, age-related challenges, and changes in social life (Savikko et al., 2005). The most common causes include family crisis, physical and motor limitations, death of many peers, widowhood, limiting housing conditions, increased use of communication through electronic devices rather than face-to-face.
Women suffer from this condition more often than men. Indeed, women tend to live longer than men and are more likely to experience any of the above-listed situations. In 2018, the share of older women (aged 65 years or more) in the EU-27 living alone was 40 % (Eurostat, 2020). Older women living alone reported severe difficulties and are hence more likely to be frail. Loneliness, together with age, chronic pathologies, and non-self-sufficiency, must be considered a risk factor for the frailty process. A longitudinal study on a sample of 1,600 respondents, found that 43% of the elderly lived in a condition of loneliness and, 6 years after the first interview, the researchers discovered that those who were lonely had a 45% higher risk of mortality, with a worsening of the quality of life and personal autonomy (Perissinotto et al., 2012).
According to large-scale surveys, loneliness increases the risk of mortality because it increases the presence of diseases as well as the use of pharmaceuticals and health services, thereby increasing the costs of public health (Holwerda et al., 2012; Banks et al., 2016).
Just for an example, the 4-years long-term effects of loneliness on health include increased blood pressure, depression, weight gain, smoking alcohol/drug use, alone time and decreased physical activity, cognition, heart health, sleep, stroke, and coronary heart disease (Berg-Weger and Morley, 2020).
Loneliness also appears to be associated with cognitive decline and the onset of major neurocognitive disorder (Donovan et al., 2016; Rafnsson et al., 2017; Luchetti et al., 2020). It is precisely in neurocognitive diseases that the importance of social relations has been studied and how they can modulate and enhance the neural correlates of the circuits that create wellbeing in feeling that one belongs to a specific social group (Morese et al., 2018; Morese and Palermo, 2020) (Figure 1). Several studies have shown that the social brain network, associated with a positive feeling of wellbeing and pleasure (“warm glow”), is the one that is activated when people feel part of their communities and experience social support (Morese et al., 2016, 2019a; Lo Gerfo et al., 2019; Auriemma et al., 2020; Longobardi et al., 2020; Morese and Longobardi, 2022). This might suggest that older adults' ToM is driven by the retrieval of information relevant to isolation (Beadle et al., 2012).
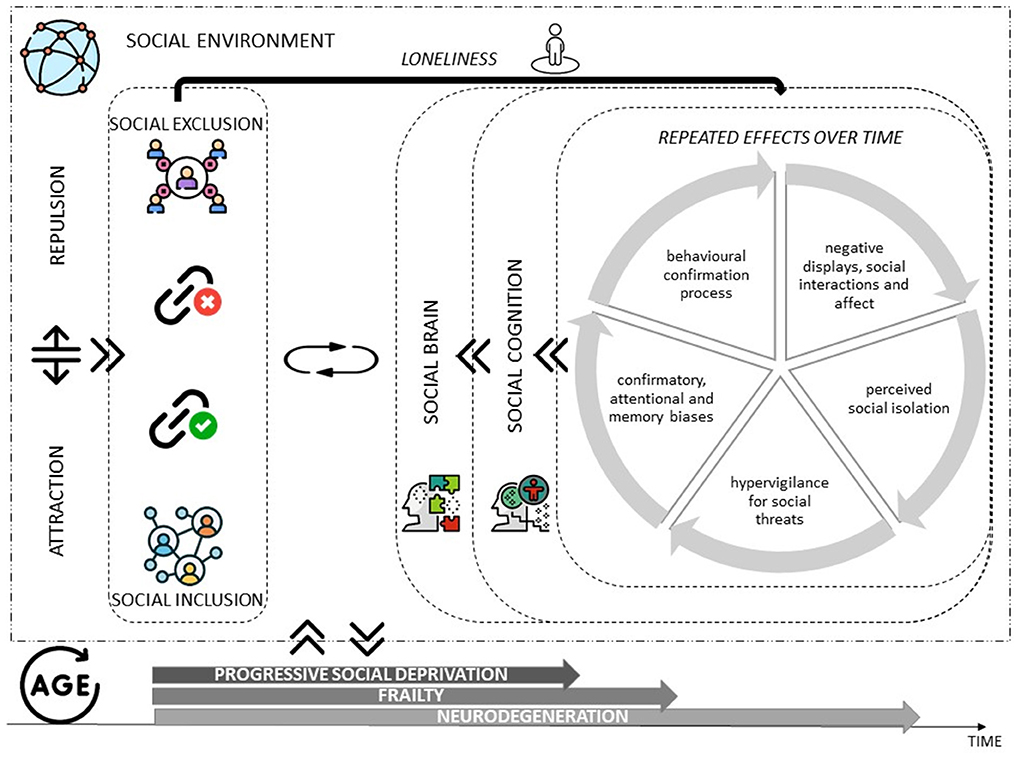
Figure 1. Theoretical model summarizing the relationships that exist between the dimensions of interest, the interactions between the individual and the social environment, and the dynamics of mutual interchange that affect the social cognition and social brain along the passage of time and the increasing age of the individual.
Given the above, loneliness is a factor that should not be taken lightly in the daily lives of older people, since this existential condition may have affected their health by initiating an iatrogenic cascade process on all the body's physiological systems (Morese et al., 2019b; Palermo, 2021b). Elderly people living alone are undoubtedly made more frailty by a possible deprivation of a social support network (family or friends) that they can rely on in times of need. In many western countries, this issue is becoming increasingly important: this is undoubtedly due to demographic concerns linked to an aging population and increased life expectancy (Palermo, 2020a).
As cognitive aging progresses, it is normal that there will be a progressive decline of social cognition (Moran et al., 2012). Performance during social-cognitive tasks was impaired and selectively accompanied by age-related decreases in the functional response of the dorsomedial prefrontal cortex. Based on these findings, age-related deficits in mentalizing are localized to circumscribed subregions of the default mode network, independent of tasks (Moran et al., 2012). The overlap between the social brain and the DMN have potential implications on neurodegenerative process. Indeed, several modifications of this network have been reported in in healthy older adults as well as in populations at risk for Alzheimer's disease (AD) (Mevel et al., 2011). In particular, decreased signal complexity in DMN nodes might contribute to cognitive decline in AD, which leads to even more dysfunctional DMN connectivity (Grieder et al., 2018) and potential effects on social cognition (Mars et al., 2012).
Concerning the perception of affiliation and isolation (i.e., loneliness), age-related similarities in the recruitment of brain regions involved in the ToM and self-referentiality (e.g., temporal pole, medial prefrontal cortex) are known (Beadle et al., 2012). Specifically, in response to isolation vs. affiliation imagery, older adults show greater recruitment than younger adults of the temporal pole, a region that is important for retrieving personally relevant memories used to understand the mental states of others (Beadle et al., 2012). A notable example of the effects of damage in that brain region is AD, which affects social functioning primarily through atrophy of the medial temporal lobe. Few studies have investigated ToM in AD patients using questionnaires, PET, and MRI/rs-fMRI. Yet no study has examined the neural correlates recruited during a social situation that involved the Theory of Mind. To measure the two components of the ToM, the attribution of emotions and intentions, Dodich et al. (2016) administered the Story-based Empathy task to patients with different neurodegenerative diseases. Both performances on the attribution of emotions and intentions were low in AD patients. Their poor performance may be due to a general cognitive decline, according to the authors (Dodich et al., 2016). The impairment of social skills in AD has a strong impact on interpersonal relationships, but to date, no neurophysiology studies deeply investigated the cognitive and emotional processes. Patients who were poorly involved in social activities and interactions tended to show more psychological and behavioral symptoms at baseline than socially involved patients (Arai et al., 2021). In addition, poor communication with family at baseline was associated with increased severity of psychiatric and behavioral disorders after 1 year (Arai et al., 2021). It, therefore, seems crucial to maximizing patients' involvement, as well as their opportunities for socialization and interaction, to prevent the exacerbation of symptoms over time (Arai et al., 2021).
An impairment of cognitive function may have an impact on loneliness for older people as it can hinder social interaction with family and friends, or interfere with judgements regarding relationship satisfaction (Burholt et al., 2017). The iatrogenic process tends to be self-sustaining. While alterations in social cognition occur with physiological aging, at the same time social isolation is itself inherently depressogenic and results in cascading effects on neurophysiological systems (Heinrichs and Domes, 2008; Nolte et al., 2011). Depressogenic mechanisms that emerge from loss of sociality include alterations in the stress axis, disinhibition of pro-inflammatory signals (Slavich and Irwin, 2014), particularly of the innate branch of the immune system (Cañas-González et al., 2020; Palermo, 2020a). The process contributes to the dysregulation of the stress axis, the decline in neurotrophins (also associated with increased inflammation), in opioids and oxytocin, which imay recursively contribute to a new reduction in neurotrophins (Heinrichs and Domes, 2008; Nolte et al., 2011).
The above fits into the context of the social safety theory (Slavich, 2020), which hypothesizes that the development and maintenance of friendly social ties is a fundamental organizing principle of human behavior and that threats to social security are a critical feature of psychological stressors that increase the risk of illness. It is likely that anticipatory neural-immune reactivity to social threat is highly conserved due to situations of social conflict, isolation, devaluation, rejection, and exclusion historically increasing risk of physical injury and infection (Slavich, 2020). Survival ultimately depends on humans' ability to elaborate symbolically and respond to a potential danger situation, which is a neurocognitive and immunological function (Slavich, 2020). Positive and negative social experiences can be explained by the social safety theory on a biological and evolutionary basis, allowing us to explain why certain stressors are particularly harmful. The framework also provides a multilevel approach to explore the biopsychosocial determinants of health and aging disparities, physical and cognitive frailty, and interpersonal behavior impairment (Slavich, 2022).
Social isolation – through the biological routes described by the social safety theory- may contribute, through the primary induction of depression, to the increased risk of AD (Kuo et al., 2020; Drinkwater et al., 2021). Indeed, socially isolated people had lower volume in the brain's gray matter in brain regions involved with learning and thinking. Social isolation counts for a 26 percent increase in the likelihood of dementia onset (Shen et al., 2022). Due to their complex relationship, the association between dementia and late-life depression is still unclear (Kuo et al., 2020). Indeed, it has been proposed that to understand the pathogenic mechanisms of AD, it is necessary to consider its multifactorial nature considering all together risk factors such as hypertension, social engagement, obesity, education level, or physical inactivity (Kuo et al., 2020). There could therefore be “softer” secondary pathways to neurodegeneration even for those who do not become formally depressed, but simply for those who were isolated and for dysphoric without being formally depressed. Just as an example, there is a well-known association between social isolation, loneliness, and cardiovascular disease (Golaszewski et al., 2022). Epidemiological studies report an independent association between dementia and cardiovascular disease, suggesting that stroke and dementia may share overlapping molecular mechanisms (Stakos et al., 2020; Leszek et al., 2021). Indeed, the pathogenesis of cardiovascular disease and AD is influenced by low-grade inflammation (Stakos et al., 2020). In particular, one of the major risk factors found to affect the cardiovascular system as well as the nervous system is ApoEε4 (Leszek et al., 2021).
Attachment theory highlights the existence in humans of an innate need to seek protective closeness and intimacy with significant figures during times of crisis, suffering, need or distress. However, this innate need is supplemented from an early age with experiences resulting from the environment in which the individual is immersed. Accordingly, the human tendency to desire and seek the closeness of attachment figures corresponds to an innate schema whose full operationalization is dependent on concrete experiences in relationships. In this sense, children's first relationships with caregivers influence the development of internal working models, expectations of themselves and others, and provide the basis for learning and social interactions. Being able to experience meaningful affective relationships and the quality of these relationships is essential both for maintaining self-confidence and emotional stability in the elderly and for coping with the traumas associated with neuropsychological and physical frailty. How the elderly person becomes available for the type of care they will receive depends on the relationship they have with their reference figures from the past. People who have a history of insecure attachments may be less likely to trust caregivers and medical personnel.
Often the lack of meaningful interpersonal relationships in old age results in loneliness, which some studies show is related to particular attachment styles (Cicirelli, 2010). It is not simply “being alone” that is an indicative or predictive factor of loneliness. Instead, the perception of loneliness is influenced by expectations about oneself and others. These expectations are the results of internal working models, which function in accordance with the person attachment style.
Social cognition, in turn, is associated with social inclusion; impairment of social cognition associated with social brain dysfunctioning is associated with loneliness (Figure 1). One might ask whether experiences of loneliness have increased and whether loneliness is a characteristic of modern societies, or it has always existed to a certain extent. It is not possible to answer this question because there is still no international standard for the definition of loneliness (and therefore the available data often do not distinguish between social isolation, living alone, and loneliness), and because past epidemiological investigations did not pay attention to this issue. However, the general feeling is that the phenomenon is increasing and has important consequences for the psychophysical health of individuals, especially the elderly. A modifiable factor, loneliness, can be ameliorated before the development of severe impairment or neurodegenerative disorders likely-due-to-AD. Indeed, inflammation-related diseases and viral infections are among the most prevalent forms of morbidity and mortality associated with this multilevel biological threat response due to social stress and according to the social safety theory (Slavich, 2022). Therefore, this theoretical paper's primary aim is to underline the importance of the social neuroscience perspective to study the social brain and social cognition in the older population, to draw useful indications for preventing the iatrogenic effects of isolation on psychophysical wellbeing and acceleration of neurodegeneration-related processes.
RM conceived the content of the article, participated in writing and supervised the manuscript for the parts falling within her competence. SP conceived the content of the article, participated in writing the first draft of the manuscript, wrote the last version, produced the infographics and supervised the entire manuscript.
Open access funding was provided by the Università Della Svizzera Italiana.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Adenzato, M., and Enrici, I. (2005). Comprendere le menti altrui: meccanismi neurocognitivi dell'interazione sociale. Quad. Psicoterapia Cognitiva 16, 14–28.
Adolphs, R. (2001). The neurobiology of social cognition. Curr. Opin. Neurobiol. 2, 231–239. doi: 10.1016/S0959-4388(00)00202-6
Adolphs, R. (2009). The social brain: neural basis of social knowledge. Annu. Rev. Psychol. 60, 693–716. doi: 10.1146/annurev.psych.60.110707.163514
Akoya, O. A., McCorry, N. K., and Donnelly, M. (2020). Loneliness and social isolation interventions for older adults: a scoping review of reviews. BMC Public Health. 20, f129. doi: 10.1186/s12889-020-8251-6
Alberti, F. B. (2019). A Biography of Loneliness: The History of an Emotion. New York: Oxford University Press, pp. 1–40, 61–83. ISBN 9780198811343.
Alpass, F. M., and Neville, S. (2003). Loneliness, health and depression in older males. Aging Ment. Health. 7, 212–216. doi: 10.1080/1360786031000101193
Amanzio, M., Canessa, N., Bartoli, M., Cipriani, G. E., Palermo, S., and Cappa, S. F. (2021). Lockdown effects on healthy cognitive aging during the COVID-19 pandemic: a longitudinal study. Front. Psychol. 12, 685180. doi: 10.3389/fpsyg.2021.685180
Amodio, D. M., and Frith, C. D. (2006). Meeting of minds: the medial frontal cortex and social cognition. Nat. Rev. Neurosci. 7, 268–277. doi: 10.1038/nrn1884
Arai, A., Khaltar, A., Ozaki, T., and Katsumata, Y. (2021). Influence of social interaction on behavioral and psychological symptoms of dementia over 1 year among long-term care facility residents. Geriatr. Nurs. 42, 509–516. doi: 10.1016/j.gerinurse.2020.09.008
Arpin, S. N., Mohr, C. D., and Brannan, D. (2015). Having friends and feeling lonely: a daily process examination of transient loneliness, socialization, and drinking behavior. Pers. Soc. Psychol. Bull. 41, 615–628. doi: 10.1177/0146167215569722
Auriemma, V., Iorio, G., Roberti, G., and Morese, R. (2020). Cyberbullying and empathy in the age of hyperconnection: an interdisciplinary approach. Fron. Sociol. 5, 551881. doi: 10.3389/fsoc.2020.551881
Banks, J., Batty, G. D., Nazroo, J., and Steptoe, A. (2016). The Dynamics of Ageing: Evidence from the English Longitudinal Study of Ageing 2002-15 (Wave 7). London: The Institute for Fiscal Studies. ISBN: 978-1-911102-21-22
Barrett, L., and Henzi, P. (2005). The social nature of primate cognition. Proc. Biol. Sci. 272, 1865–1875. doi: 10.1098/rspb.2005.3200
Beadle, J. N., Yoon, C., and Gutchess, A. H. (2012). Age-related neural differences in affiliation and isolation. Cogn. Affect. Behav. Neurosci. 12, 269–279. doi: 10.3758/s13415-012-0085-y
Berg-Weger, M., and Morley, J. E. (2020). Loneliness in old age: an unaddressed health problem. J. Nutr. Health Aging 24, 243–245. doi: 10.1007/s12603-020-1323-6
Blatt, S. J. (2008). Polarities of Experience: Relatedness and Self-Definition in Personality Development, Psychopathology, and the Therapeutic Process. American Psychological Association. doi: 10.1037/11749-000
Bowlby, J. (1969). Attachment and Loss, Vol. 1 Attachment. Attachment and Loss. New York, NY: Basic Books. ISBN 0-465-00543-8
Bowlby, R., and King, P. (2004). Fifty Years of Attachment Theory. London: Karnac on behalf of the Winnicott Clinic of Psychotherapy. ISBN 9781855753853
Bradley, J. M., and Cafferty, T. P. (2001). Attachment among older adults: current issues and directions for future research. Attach. Hum. Dev. 3, 200–221. doi: 10.1080/14616730110058016
Burholt, V., Windle, G., Morgan, D. J., C. F. A. S., and Wales team (2017). A social model of loneliness: the roles of disability, social resources, and cognitive impairment. Gerontologist 57, 1020–1030. doi: 10.1093/geront/gnw125
Byrne, R. W., and Whiten, A., eds. (1988). Machiavellian Intelligence: Social Expertise and the Evolution of Intellect in Monkeys, Apes, and Humans. Clarendon Press/Oxford University Press.
Cacioppo, J. T., and Cacioppo, S. (2014). Social relationships and health: the toxic effects of perceived social isolation. Soc. Personal. Psychol. Compass. 8, 58–72. doi: 10.1111/spc3.12087
Cacioppo, J. T., and Cacioppo, S. (2018). The growing problem of loneliness. Lancet. 391, 426. doi: 10.1016/S0140-6736(18)30142-9
Cacioppo, J. T., and Hawkley, L. C. (2005). “People thinking about people: The vicious cycle of being a social outcast in one's own mind,” in The social Outcast: Ostracism, Social Exclusion, Rejection, and Bullying, eds K. D. Williams, J. P. Forgas, and W. von Hippel (London: Psychology Press), 91–108. ISBN 9781138006133
Cacioppo, J. T., and Hawkley, L. C. (2009). Perceived social isolation and cognition. Trends Cogn. Sci. 13, 447–454. doi: 10.1016/j.tics.2009.06.005
Cacioppo, J. T., Hawkley, L. C., Ernst, J. M., Burleson, M., Berntson, G. G., Nouriani, B., et al. (2006b). Loneliness within a nomological net: an evolutionary perspective. J. Res. Pers. 40, 1054–1085 doi: 10.1016/j.jrp.2005.11.007
Cacioppo, J. T., Hawkley, L. C., and Thisted, R. A. (2010). Perceived social isolation makes me sad: 5-year cross-lagged analyses of loneliness and depressive symptomatology in the Chicago Health, Aging, and Social Relations Study. Psychol. Aging. 25, 453–463. doi: 10.1037/a0017216
Cacioppo, J. T., Hughes, M. E., Waite, L. J., Hawkley, L. C., and Thisted, R. A. (2006a). Loneliness as a specific risk factor for depressive symptoms: cross-sectional and longitudinal analyses. Psychol. Aging. 21, 140–151. doi: 10.1037/0882-7974.21.1.140
Cañas-González, B., Fernández-Nistal, A., Ramírez, J. M., and Martínez-Fernández, V. (2020). Influence of stress and depression on the immune system in patients evaluated in an anti-aging unit. Front. Psychol. 11, 844. doi: 10.3389/fpsyg.2020.01844
Cassidy, J., and Shaver, P. R. eds. (1999). Handbook of Attachment: Theory, Research, and Clinical Applications. New York, NY: Guilford Press.
Cicirelli, V. G. (2010). Attachment relationships in old age. J. Soc. Pers. Relat. 27, 191–199. doi: 10.1177/0265407509360984
De Leo, D., and Diekstra, R. F. (1990). Depression and Suicide in Late Life. Toronto/Bern: Hogrefe and Huber.
Dodich, A., Cerami, C., Canessa, N., Crespi, C., Iannaccone, S., Marcone, A., et al. (2015). A novel task assessing intention and emotion attribution: Italian standardization and normative data of the Story-based Empathy Task. Neurol. Sci. 36, 1907–1912. doi: 10.1007/s10072-015-2281-3
Dodich, A., Cerami, C., Crespi, C., Canessa, N., Lettieri, G., Iannaccone, S., et al. (2016). Differential impairment of cognitive and affective mentalizing abilities in neurodegenerative dementias: evidence from behavioral variant of frontotemporal dementia, Alzheimer's disease, and mild cognitive impairment. J. Alzheimers Dis. 50, 1011–1022. doi: 10.3233/JAD-150605
Donovan, N. J., Okereke, O. I., Vannini, P., Amariglio, R. E., Rentz, D. M., Marshall, G. A., et al. (2016). Association of higher cortical amyloid burden with loneliness in cognitively normal older adults. JAMA Psychiatry. 73, 1230–1237. doi: 10.1001/jamapsychiatry.2016.2657
Drinkwater, E., Davies, C., and Spires-Jones, T. L. (2021). Potential neurobiological links between social isolation and Alzheimer's disease risk. Eur. J. Neurosci. 12:670959. doi: 10.1111/ejn.15373
Dunbar, R. I., and Shultz, S. (2007a). Evolution in the social brain. Science 317, 1344–1347. doi: 10.1126/science.1145463
Dunbar, R. I., and Shultz, S. (2007b). Understanding primate brain evolution. Philos. Trans. R Soc. Lond. B. Biol. Sci. 362, 649–658. doi: 10.1098/rstb.2006.2001
Ebesutani, C., Fierstein, M., Viana, A. G., Trent, L., Young, J., and Sprung, M. (2015). The role of loneliness in the relationship between anxiety and depression in clinical and school-based youth. Psychol. Sch. 52, 223–234. doi: 10.1002/pits.21818
Eurostat (2020). Ageing Europe - Looking at the Lives of Older People in the EU. Bruxelles: European Commission. doi: 10.2785/628105
Gazzaniga, M. S. (1995). Principles of human brain organization derived from split-brain studies. Neuron 14, 217–228. doi: 10.1016/0896-6273(95)90280-5
Giné-Garriga, M., Jerez-Roig, J., Coll-Planas, L., Skelton, D. A., Inzitari, M., Booth, J., et al. (2021). Is loneliness a predictor of the modern geriatric giants? Analysis from the survey of health, ageing, and retirement in Europe. Maturitas 144, 93–101. doi: 10.1016/j.maturitas.2020.11.010
Golaszewski, N. M., LaCroix, A. Z., Godino, J. G., Allison, M. A., Manson, J. E., King, J. J., et al. (2022). Evaluation of social isolation, loneliness, and cardiovascular disease among older women in the US. JAMA Netw. Open 5:e2146461. doi: 10.1001/jamanetworkopen.2021.46461
Grieder, M., Wang, D., Dierks, T., Wahlund, L. O., and Jann, K. (2018). Default mode network complexity and cognitive decline in mild Alzheimer's disease. Front. Neurosci. 12, 770. doi: 10.3389/fnins.2018.00770
Gunnar, M., and Quevedo, K. (2007). The neurobiology of stress and development. Annu. Rev. Psychol. 58, 145–173. doi: 10.1146/annurev.psych.58.110405.085605
Hawkins-Elder, H., Milfont, T. L., Hammond, M. D., and Sibley, C. G. (2018). Who are the lonely? A typology of loneliness in New Zealand. Aust. N. Z. J. Psychiatry. 52, 357–364. doi: 10.1177/0004867417718944
Hawkley, L. C., and Cacioppo, J. T. (2010). Loneliness matters: a theoretical and empirical review of consequences and mechanisms. Ann. Behav. Med. 40, 218–227. doi: 10.1007/s12160-010-9210-8
Heinrichs, M., and Domes, G. (2008). Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog. Brain Res. 170, 337–350. doi: 10.1016/S0079-6123(08)00428-7
Holt-Lunstad, J., Robles, T. F., and Sbarra, D. A. (2017). Advancing social connection as a public health priority in the United States. Am. Psychol. 72, 517–530. doi: 10.1037/amp0000103
Holt-Lunstad, J., Smith, T. B., and Layton, J. B. (2010). Social relationships and mortality risk: a meta-analytic review. PLoS Med. 7:e1000316. doi: 10.1371/journal.pmed.1000316
Holwerda, T. J., Beekman, A. T., Deeg, D. J., Stek, M. L., van Tilburg, T. G., Visser, P. J., et al. (2012). Increased risk of mortality associated with social isolation in older men: only when feeling lonely? Results from the Amsterdam Study of the Elderly (AMSTEL). Psychol. Med. 42, 843–853. doi: 10.1017/S0033291711001772
Jaya, E. S., Hillmann, T. E., Reininger, K. M., Gollwitzer, A., and Lincoln, T. M. (2017). Loneliness and psychotic symptoms: the mediating role of depression. Cognit. Ther. Res. 41, 106–116. doi: 10.1007/s10608-016-9799-4
Johnson, B., Ulberg, S., Shivale, S., Donaldson, J., Milczarski, B., and Faraone, S. V. (2014). Fibromyalgia, autism, and opioid addiction as natural and induced disorders of the endogenous opioid hormonal system. Discov. Med. 18, 209–220.
Kandel, E. R., Schwartz, J. H., Jessell, T. M., Siegelbaum, S. A., and Hudspeth, A. J. (2012). Principles of Neural Science, 5th ed. New York, NY: McGraw-Hill. ISBN 0-07-139011-1
Klinenberg, E. (2018). Is loneliness a health epidemic? The New York Times, 8th february 2018. Available online at: https://www.nytimes.com/2018/02/09/opinion/sunday/loneliness-health.html (accessed February 09, 2018).
Kuo, C. Y., Stachiv, I., and Nikolai, T. (2020). Association of late life depression, (Non-) modifiable risk and protective factors with dementia and Alzheimer's disease: literature review on current evidences, preventive interventions and possible future trends in prevention and treatment of dementia. Int. J. Environ. Res. Public Health 17, 7475. doi: 10.3390/ijerph17207475
Laird, A. R., Fox, P. M., Eickhoff, S. B., Turner, J. A., Ray, K. L., McKay, D. R., et al. (2011). Behavioral interpretations of intrinsic connectivity networks. J. Cogn. Neurosci. 23, 4022–4037. doi: 10.1162/jocn_a_00077
Leszek, J., Mikhaylenko, E. V., Belousov, D. M., Koutsouraki, E., Szczechowiak, K., Kobusiak-Prokopowicz, M., et al. (2021). The links between cardiovascular diseases and Alzheimer's disease. Curr. Neuropharmacol. 19, 152–169. doi: 10.2174/1570159X18666200729093724
Lim, M. H., Rodebaugh, T. L., Zyphur, M. J., and Gleeson, J. F. (2016). Loneliness over time: the crucial role of social anxiety. J. Abnorm. Psychol. 125, 620–630. doi: 10.1037/abn0000162
Lo Gerfo, E., Gallucci, A., Morese, R., Vergallito, A., Ottone, S., Ponzano, F., et al. (2019). The role of ventromedial prefrontal cortex and temporo-parietal junction in third-party punishment behavior. NeuroImage 200, 501–510. doi: 10.1016/j.neuroimage.2019.06.047
Longobardi, C., Morese, R., and Fabris, M. A. (2020). COVID-19 emergency: social distancing and social exclusion as risks for suicide ideation and attempts in adolescents. Front. Psychol. 11, 551113. doi: 10.3389/fpsyg.2020.551113
Luchetti, M., Terracciano, A., Aschwanden, D., Lee, J. H., Stephan, Y., and Sutin, A. R. (2020). Loneliness is associated with risk of cognitive impairment in the Survey of Health, Ageing and Retirement in Europe. Int. J. Geriatr. Psychiatry 35, 794–801. doi: 10.1002/gps.5304
Mars, R. B., Neubert, F. X., Noonan, M. P., Sallet, J., Toni, I., and Rushworth, M. F. (2012). On the relationship between the “default mode network” and the “social brain”. Front. Hum. Neurosci. 6, 189. doi: 10.3389/fnhum.2012.00189
Maylor, E. A., Moulson, J. M., Muncer, A. M., and Taylor, L. A. (2002). Does performance on theory of mind tasks decline in old age?. Br. J. Psychol. 93, 465–485. doi: 10.1348/000712602761381358
McLaren, V., and Sharp, C. (2020). “What is mentalizing?” in Adolescent Suicide and Self-Injury, eds L. Williams, and O. Muir (Cham: Springer). doi: 10.1007/978-3-030-42875-4_1
Mevel, K., Chételat, G., Eustache, F., and Desgranges, B. (2011). The default mode network in healthy aging and Alzheimer's disease. Int. J. Alzheimers Dis. 2011, 535816. doi: 10.4061/2011/535816
Moran, J. M., Jolly, E., and Mitchell, J. P. (2012). Social-cognitive deficits in normal aging. J. Neurosci. 32, 5553–5561. doi: 10.1523/JNEUROSCI.5511-11.2012
Morese, R., Lamm, C., Bosco, F. M., Valentini, M. C., and Silani, G. (2019b). Social support modulates the neural correlates underlying social exclusion. Soc. Cogn. Affect. Neurosci. 14:633–643. doi: 10.1093/scan/nsz033
Morese, R., and Longobardi, C. (2020). Suicidal ideation in adolescence: a perspective view on the role of the ventromedial prefrontal cortex. Front. Psychol. 11, 713. doi: 10.3389/fpsyg.2020.00713
Morese, R., and Longobardi, C. (2022). The impact of physical distancing in the pandemic situation: considering the role of loneliness and social brain. Front. Psychol. 13, 861329. doi: 10.3389/fpsyg.2022.861329
Morese, R., and Palermo, S. (2020). Altruistic punishment and impulsivity in parkinson's disease: a social neuroscience perspective. Front. Behav. Neurosci. 14, 102. doi: 10.3389/fnbeh.2020.00102
Morese, R., Palermo, S., Defedele, M., Nervo, J., and Borraccino, A. (2019a). “Vulnerability and social exclusion: risk in adolescence and old age,” in The New Forms of Social Exclusion, eds R. Morese, and S. Palermo (London: IntechOpen Limited), 1–16. doi: 10.5772/intechopen.85463
Morese, R., Palermo, S., and Raffaella, F. (2020). Social Isolation - An Interdisciplinary View. London: IntechOpen Limited. ISBN 9781789847598 doi: 10.5772/intechopen.77635
Morese, R., Rabellino, D., Sambataro, F., Perussia, F., Valentini, M. C., Bara, B. G., et al. (2016). Group membership modulates the neural circuitry underlying third party punishment. PLoS ONE 11:e0166357. doi: 10.1371/journal.pone.0166357
Morese, R., Stanziano, M., and Palermo, S. (2018). Commentary: metacognition and perspective-taking in Alzheimer's disease: a mini-review. Front. Psychol. 9, 2010. doi: 10.3389/fpsyg.2018.02010
Newall, N. E., Chipperfield, J. G., and Bailis, D. S. (2014). Predicting stability and change in loneliness in later life. J. Soc. Pers. Relat. 31, 335–351. doi: 10.1177/0265407513494951
Nolte, T., Guiney, J., Fonagy, P., Mayes, L. C., and Luyten, P. (2011). Interpersonal stress regulation and the development of anxiety disorders: an attachment-based developmental framework. Front. Behav. Neurosci. 5, 55. doi: 10.3389/fnbeh.2011.00055
Palermo, S. (2017). “Reduced self-awareness across pathologies: Involvement of the Anterior Cingulate Cortex,” in Horizons in Neuroscience Research, Vol. 28, eds A. Costa, and E. Villalba (Hauppauge, NY: Nova Science Publishers), 137–176.
Palermo, S. (2020a). Covid-19 pandemic: maximizing future vaccination treatments considering aging and frailty. Front. Med. 7, 558835. doi: 10.3389/fmed.2020.558835
Palermo, S. (2020b). “The emergence of consciousness: the contribution of the cingulate cortex,” in Horizons in Neuroscience Research, Vol. 41, eds A. Costa, and E. Villalba (Hauppauge, NY: Nova Science Publishers), 157–180.
Palermo, S. (2021a). “Neurocovid”: an analysis of the impact of Covid-19 on the older adults. Evolving psychological and neuropsychological understanding,” in Manal Mohammad Baddour. Fighting the COVID-19 Pandemic (London: IntechOpen Limited). doi: 10.5772/intechopen.99414
Palermo, S. (2021b). “Frailty, vulnerability, and plasticity: towards a new medicine of complexity,” in Frailty in the Elderly - Understanding and Managing Complexity, S. Palermo (London: IntechOpen Limited), 1–16. doi: 10.5772/intechopen.96244
Panksepp, J. (1998). Affective Neuroscience: The Foundations of Human and Animal Emotion. New York, NY: Oxford University Press.
Panksepp, J. (2003). Neuroscience. Feeling the pain of social loss. Science 302, 237–239. doi: 10.1126/science.1091062
Perissinotto, C. M., Stijacic Cenzer, I., and Covinsky, K. E. (2012). Loneliness in older persons: a predictor of functional decline and death. Arch. Intern. Med. 172, 1078–1083. doi: 10.1001/archinternmed.2012.1993
Premack, D., and Woodruff, G. (1978). Chimpanzee problem-solving: a test for comprehension. Science 202, 532–535. doi: 10.1126/science.705342
Rafnsson, S. B., Shankar, A., and Steptoe, A. (2017). Informal caregiving transitions, subjective well-being and depressed mood: Findings from the english longitudinal study of ageing. Aging Ment. Health. 21, 104–112. doi: 10.1080/13607863.2015.1088510
Savikko, M., Routasalo, P., Tilvis, R. S., Strandberg, T. E., and Pitkala, K. H. (2005). Predictors and subjective causes of loneliness in an aged population. Arch. Gerontol. Geriatr. 41, 223–233. doi: 10.1016/j.archger.2005.03.002
Saxe, R., and Kanwisher, N. (2003). People thinking about thinking people. the role of the temporo-parietal junction in “theory of mind”. NeuroImage 19, 1835–1842. doi: 10.1016/S1053-8119(03)00230-1
Saxe, R., Moran, J. M., Scholz, J., and Gabrieli, J. (2006a). Overlapping and non-overlapping brain regions for theory of mind and self-reflection in individual subjects. Soc. Cogn. Affect. Neurosci. 1, 229–234. doi: 10.1093/scan/nsl034
Saxe, R., Schulz, L. E., and Jiang, Y. V. (2006b). Reading minds versus following rules: dissociating theory of mind and executive control in the brain. Soc. Neurosci. 1, 284–298. doi: 10.1080/17470910601000446
Schilbach, L., Eickhoff, S. B., Rotarska-Jagiela, A., Fink, G. R., and Vogeley, K. (2008). Minds at rest? Social cognition as the default mode of cognizing and its putative relationship to the “default system” of the brain. Conscious. Cogn. 17, 457–467. doi: 10.1016/j.concog.2008.03.013
Schilbach, L., Wohlschlaeger, A. M., Kraemer, N. C., Newen, A., Shah, N. J., Fink, G. R., et al. (2006). Being with virtual others: neural correlates of social interaction. Neuropsychologia 44, 718–730. doi: 10.1016/j.neuropsychologia.2005.07.017
Sebastian, C. L., Fontaine, N. M., Bird, G., Blakemore, S. J., Brito, S. A., McCrory, E. J., et al. (2012). Neural processing associated with cognitive and affective theory of mind in adolescents and adults. Soc. Cogn. Affect. Neurosci. 7, 53–63. doi: 10.1093/scan/nsr023
Shen, C., Rolls, E., Cheng, W., Kang, J., Dong, G., Xie, C., et al. (2022). Associations of social isolation and loneliness with later dementia. Neurology. doi: 10.1212/WNL.0000000000200583. [Epub ahead of print].
Siegel, D. J. (1999). The Developing Mind: Toward a Neurobiology of Interpersonal Experience. New York, NY: Guilford Press. ISBN 978–1572304536
Siegel, D. J. (2001). Toward an interpersonal neurobiology of the developing mind: attachment relationships, “mindsight,” and neural integration. Infant Ment. Health J. 22, 67–94. doi: 10.1002/1097-0355(200101/04)22:1<67::AID-IMHJ3>3.0.CO;2-G
Slavich, G. M. (2020). Social safety theory: a biologically based evolutionary perspective on life stress, health, and behavior. Annu. Rev. Clin. Psychol. 16, 265–295. doi: 10.1146/annurev-clinpsy-032816-045159
Slavich, G. M. (2022). Social safety theory: understanding social stress, disease risk, resilience, and behavior during the COVID-19 pandemic and beyond. Curr. Opin. Psychol. 45, 101299. doi: 10.1016/j.copsyc.2022.101299
Slavich, G. M., and Irwin, M. R. (2014). From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull. 140, 774–815. doi: 10.1037/a0035302
Smith, S. M., Fox, P. T., Miller, K. L., Glahn, D. C., Fox, P. M., Mackay, C. E., et al. (2009). Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA. 106, 13040–13045. doi: 10.1073/pnas.0905267106
Stakos, D. A., Stamatelopoulos, K., Bampatsias, D., Sachse, M., Zormpas, E., Vlachogiannis, N. I., et al. (2020). The Alzheimer's disease amyloid-beta hypothesis in cardiovascular aging and disease: JACC focus seminar. J. Am. Coll. Cardiol. 75, 952–967. doi: 10.1016/j.jacc.2019.12.033
Stickley, A., Koyanagi, A., Roberts, B., Richardson, E., Abbott, P., Tumanov, S., et al. (2013). Loneliness: its correlates and association with health behaviours and outcomes in nine countries of the former Soviet Union. PLoS ONE. 8:e67978. doi: 10.1371/journal.pone.0067978
Stone, V. E., Baron-Cohen, S., and Knight, R. T. (1998). Frontal lobe contributions to theory of mind. J. Cogn. Neurosci. 10, 640–656. doi: 10.1162/089892998562942
Stravynski, A., and Boyer, R. (2001). Loneliness in relation to suicide ideation and parasuicide: a population-wide study. Suicide Life Threat. Behav. 31, 32–40. doi: 10.1521/suli.31.1.32.21312
Timpano, K. R., Çek, D., Rubenstein, L. M., Murphy, D., and Schmidt, N. B. (2014). Exploring the association between obsessive-compulsive symptoms and loneliness: consideration of specificity and gender. J. Cogn. Psychother. 28, 264–273. doi: 10.1891/0889-8391.28.4.264
Van Overwalle, F. (2009). Social cognition and the brain: a meta-analysis. Hum. Brain Mapp. 30, 829–858. doi: 10.1002/hbm.20547
Vogeley, K., and Fink, G. R. (2003). Neural correlates of the first-person-perspective. Trends Cogn. Sci. 7, 38–42. doi: 10.1016/S1364-6613(02)00003-7
Zellner, M. R., Watt, D. F., Solms, M., and Panksepp, J. (2011). Affective neuroscientific and neuropsychoanalytic approaches to two intractable psychiatric problems: why depression feels so bad and what addicts really want. Neurosci. Biobehav. Rev. 35, 2000–2008. doi: 10.1016/j.neubiorev.2011.01.003
Keywords: elderly, loneliness, social isolation, social brain, social cognition, social neuroscience, mental health, Alzheimer's disease
Citation: Morese R and Palermo S (2022) Feelings of loneliness and isolation: Social brain and social cognition in the elderly and Alzheimer's disease. Front. Aging Neurosci. 14:896218. doi: 10.3389/fnagi.2022.896218
Received: 14 March 2022; Accepted: 06 July 2022;
Published: 22 July 2022.
Edited by:
Ignacio Torres-Aleman, Achucarro Basque Center for Neuroscience, SpainReviewed by:
Douglas F. Watt, Lesley University, United StatesCopyright © 2022 Morese and Palermo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosalba Morese, cm9zYWxiYS5tb3Jlc2VAdXNpLmNo
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.