
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 01 September 2022
Sec. Neurocognitive Aging and Behavior
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.895188
Background and aim: Previous studies on cardiovascular risk burden assessed by the Framingham General Cardiovascular Risk Score (FGCRS) and cognitive trajectories mainly focus on Western populations and most of them have used a single measure of cardiovascular risk. In this study, among middle-aged and older Chinese, we investigated (i) the association of baseline FGCRS with subsequent cognitive decline and (ii) the association of FGCRS change with concomitant cognitive decline.
Materials and methods: In wave 1 to wave 4 (2011–2018) of the China Health and Retirement Longitudinal Study, global cognition was assessed by orientation, memory, and executive function. FGCRS was assessed and categorized into tertiles (low, intermediate, and high) at baseline (2011) and 4 years after (2015). Furthermore, external validation was performed to check its generalizability using the English Longitudinal Study of Ageing (ELSA) 2008–2018.
Results: In total, 6,402 participants with a mean [standard deviation (SD) age of 57.8 (8.4) years, 49.0% women] with complete baseline data and at least one reassessment of cognitive function were included. A 10% increment in baseline FGCRS was associated with a faster decline in global cognition (−0.010 SD/year, 95% CI −0.013, −0.008). Among 4,336 participants [mean (SD) age of 57.8 (8.2) years, 50.0% women] with data on FGCRS changes, compared to individuals with the consistently low FGCRS (reference group), a faster global cognition decline rate was observed in the low to intermediate group (−0.026 SD/year, 95% CI −0.045, −0.007), the low to high group (−0.052 SD/year, 95% CI −0.102, −0.001), the consistently intermediate group (−0.019 SD/year, 95% CI −0.033, −0.005), the intermediate to high group (−0.040 SD/year, 95% CI −0.058, −0.022), the high to intermediate group (−0.024 SD/year, 95% CI −0.047, −0.002), and the consistently high group (−0.047 SD/year, 95% CI −0.060, −0.034). Similar trends were observed for individual cognitive domains. Results from the external validation using the ELSA remained consistent.
Conclusion: Higher baseline FGCRS was associated with faster cognitive decline. However, there was no consistent relationship between the direction of changes in FGCRS and cognitive decline.
Dementia is the leading cause of disability and dependency among the elderly. According to the Gauthier et al. (2021), over 55 million people live with dementia, which is projected to reach 78 million by 2030 (Gauthier et al., 2021). Due to the lack of effective treatment, identifying modifiable risk factors for cognitive decline, a prodromal feature of dementia, has become an important strategy to halt the dementia epidemic (Gorelick et al., 2017; World Health Organization, 2019; Livingston et al., 2020). The detrimental effect of traditional cardiovascular risk factors, including smoking, hypertension, and diabetes on cognitive function, has been well established (Baumgart et al., 2015). Still, these risk factors are interrelated, making it difficult to isolate their individual effects (Qiu and Fratiglioni, 2015; Armstrong et al., 2019). Also, given the multifactorial aetiology of cognitive decline and dementia, multidomain interventions that target several risk factors simultaneously might be necessary for an optimal preventive effect (Kivipelto et al., 2018).
The Framingham General Cardiovascular Risk Score (FGCRS), calculated using the information on age, total cholesterol, high-density lipoprotein (HDL) cholesterol, systolic blood pressure, use of blood pressure-lowering medication, smoking, and diabetes, was originally developed to assess general cardiovascular risk burden (D’Agostino et al., 2008). To date, many studies have investigated the association between FGCRS and cognitive decline, but mainly focusing on populations in Western countries (Harrison et al., 2014). Given the ethnic differences in the effects of these risk factors on cognitive function (Lipnicki et al., 2017; Volgman et al., 2018), this evidence may not be generalizable to the Chinese population. In addition, despite their prospective nature, former studies used a single assessment of FGCRS; whether and how the FGCRS changes over time are related to cognitive decline is largely unknown.
The China Health and Retirement Longitudinal Study (CHARLS) is a nationally representative aging cohort with large sample sizes and repeated cognitive assessments. Here we used it to investigate (i) the association of baseline FGCRS (measured at wave 1) with subsequent cognitive decline (wave 1 to wave 4); and (ii) the association of FGCRS change (measured between wave 1 and wave 3) with concomitant cognitive decline (wave 1 to wave 4).
In this study, we used data from wave 1 to wave 4 (2011 to 2018) of the CHARLS, a community-based longitudinal cohort conducted in China. The detailed study design has been described elsewhere (Zhao et al., 2014). The flow chart for participants’ selection of the present study is shown in Figure 1. Briefly, a total of 9,830 participants had physical and clinical data at baseline; 3,428 were excluded for the following reasons: younger than 45 years (n = 344), self-reported doctor-diagnosed mental disease (e.g., dementia, Alzheimer’s disease, or cognitive problem) (n = 184), unavailable information to assess FGCRS (n = 146), or cognitive function (n = 1990) at baseline, loss to follow-up (n = 689), or missing covariates at baseline (n = 75). Hence, 6,402 participants were included with complete measurements of FGCRS and cognitive function at baseline and at least one reassessment of cognitive function during 7 years of follow-up. Of these, 4336 participants also had data about FGCRS at wave 3 (4 years after baseline).
Structured questionnaires were administered by trained field workers using a computer-assisted personal interview system to collect demographic, lifestyle, and medical history data. Body mass index (BMI) was calculated as body weight divided by the square of height (kg/m2). A high level of education was defined as completing at least the senior level of high school. Smoking status was recorded and dichotomized into never/former vs. current. The 10-item CES-D Scale was used and participants scoring higher than 12 were defined as having depressive symptoms. Blood pressure level was measured three times at a sitting resting position. The average of the second and third blood pressure readings, or the average of the first and second blood pressure readings if the third reading was unavailable, was used for the analyses. Information about blood pressure- or blood glucose-lowering medications and self-reported doctor-diagnosed cardiovascular disease (heart disease and stroke) were also registered.
Blood samples were collected every two waves with details of the process described elsewhere (Chen et al., 2019). Participants were asked to fast overnight before collection. Blood glucose, total cholesterol and HDL cholesterol were assessed with routine clinical chemistry methods. Glycated hemoglobin (HbA1c) was measured using the high-performance liquid chromatography method. For the current study, we used the blood biomarker data measured at baseline (wave 1) and 4 years after (wave 3).
Type 2 diabetes was defined based on the 2020 American Diabetes Association criteria (American Diabetes, 2020) as fasting blood glucose ≥ 7.0 mmol/L (126 mg/dL), non-fasting blood glucose ≥ 11.1 mmol/L (200 mg/dL), HbA1c level ≥ 47.5 mmol/mol (6.5%), self-reported doctor-diagnosed diabetes, or current use of blood glucose-lowering medications. We calculated FGCRS based on age, total cholesterol, HDL cholesterol, systolic blood pressure, blood pressure-lowering medication, smoking, and diabetes for each participant at baseline and 4-years after. A higher FGCRS indicates a greater risk of future cardiovascular events.
Cognitive assessment was performed in all waves, including three domains: orientation, memory, and executive function, with higher scores indicating better cognitive function (Ma et al., 2020). Orientation was measured by asking four questions regarding the date (year, month, day of month, and day of week). One point was given for each correct answer, with the sum score ranging from 0 to 4. The memory assessment comprised immediate and delayed recall for ten unrelated words. The sum of words successfully recalled in these two tests was used and ranged from 0 to 20. For the assessment of executive function, the participant was asked to observe and draw a picture of two overlapping pentagons (three points were given for a successful drawing and 0 points for an unsuccessful drawing) and also to do the serial seven test, in which the participant was asked to serial subtraction of 7 from 100 (up to five times). The executive function score was the sum of these two tests, ranging from 0 to 8.
The z scores were calculated and used to allow direct comparisons across different cognitive tests. Specifically, we standardized the follow-up score by subtracting the mean of the baseline score and then dividing it by the baseline standard deviation (SD). The global cognitive z score was estimated by averaging the z scores from the three tests and then standardizing it to baseline using the mean and SD of the global cognitive z score. Therefore, a unit of z score would mean the one SD above the mean baseline score.
Baseline characteristics are presented as mean (SD) or median (interquartile range, IQR) for continuous variables and frequency (percentage) for categorical variables. Linear mixed models were used to investigate the difference in annual change of global cognitive function and specific cognitive domains per 10% increment in baseline FGCRS. We used the follow-up time (years since baseline) as a time scale. We fitted the models with the intercept and the time term as random effects accounting for inter-individual differences at baseline and changing rates in outcome variables during follow-up. For the fixed-effects part, we included baseline FGCRS, time, and baseline FGCRS × time interaction. The “baseline FGCRS × time” interaction term indicated a differential changing rate. We also categorized baseline FGCRS into tertiles and repeated the analysis using the low subgroup as the reference.
Change in FGCRS tertiles, assessed between baseline and 4 years after, yielded 9 possible combinations of FGCRS status: consistently low, low to intermediate, low to high, intermediate to low, consistently intermediate, intermediate to high, high to low, high to intermediate, and consistently high. Follow-up for cognitive decline still started from baseline. Then we investigated the difference in annual changes of cognitive decline among different FGCRS changing statuses using the consistently low group as the reference. The impact of continuous FGCRS change (per 10% increment in the difference) and its quintiles (using the middle quintile as the reference) on the cognitive decline was also estimated.
All models were adjusted for baseline covariates (age, sex, education, BMI, depression status, prevalent cardiovascular diseases) and baseline FGCRS score as appropriate. Given that the relationships of cardiovascular risk factors with cognition may vary with age (Armstrong et al., 2019), we tested the possible interaction by incorporating the “baseline FGCRS × time × baseline age” term into the model. Also, we repeated the main analyses after excluding those older than 75 years or with prevalent cardiovascular disease at baseline according to the FGCRS application recommendation (D’Agostino et al., 2008). In addition, to study the generalizability of our findings, external validation was performed using data from the English Longitudinal Study of Ageing (ELSA) (2008–2018), which is a nationally representative, biennial longitudinal survey of adults ≥ 50 years old residing in the United Kingdom (Steptoe et al., 2013), with similar statistical plan used. A more detailed description of the ELSA sample is provided in Supplementary material.
Data were handled and analyzed with SPSS Statistics version 25.0.0.1 (IBM Corp., Armonk, NY, United States) and R, CRAN version 4.1.2, with packages “lme4” and “lmerTest”. All analyses were performed at the significance level of 0.05 (2-tailed), unless specified.
Among the 6,402 participants with complete baseline data and at least one reassessment of cognitive function, the mean (SD) age was 57.8 (8.4) years, 49.0% were women, the median (IQR) follow-up duration was 6.9 (4.0–7.0) years, and the median (IQR) number of cognitive assessments was 3 (Gorelick et al., 2017; World Health Organization, 2019; Livingston et al., 2020). Of those, 4,336 participants also had repeated FGCRS measurements. The distributions of baseline characteristics are shown in Table 1.
Table 2 and Supplementary Figure 1 demonstrate the annual change in cognition z scores among continuous and categorized FGCRS at baseline. A 10% increment in baseline FGCRS was associated with a faster decline in global cognition (−0.010 SD/year, 95% CI −0.013, −0.008), orientation (−0.006 SD/year, 95% CI −0.008, −0.003), memory (−0.014 SD/year, 95% CI −0.017, −0.010), and executive function (−0.005 SD/year, 95% CI −0.008, −0.002). When FGCRS was used as tertiles, the higher FGCRS was still associated with faster cognitive decline than the low tertile. For example, compared to those within the low FGCRS tertile, those within the high FGCRS tertile had significantly worse performance in global cognition (−0.035 SD/year, 95% CI −0.045, −0.026), orientation (−0.014 SD/year, 95% CI −0.025, −0.004), memory (−0.048 SD/year, 95% CI −0.060, −0.036), and executive function (−0.021 SD/year, 95% CI −0.031, −0.011).
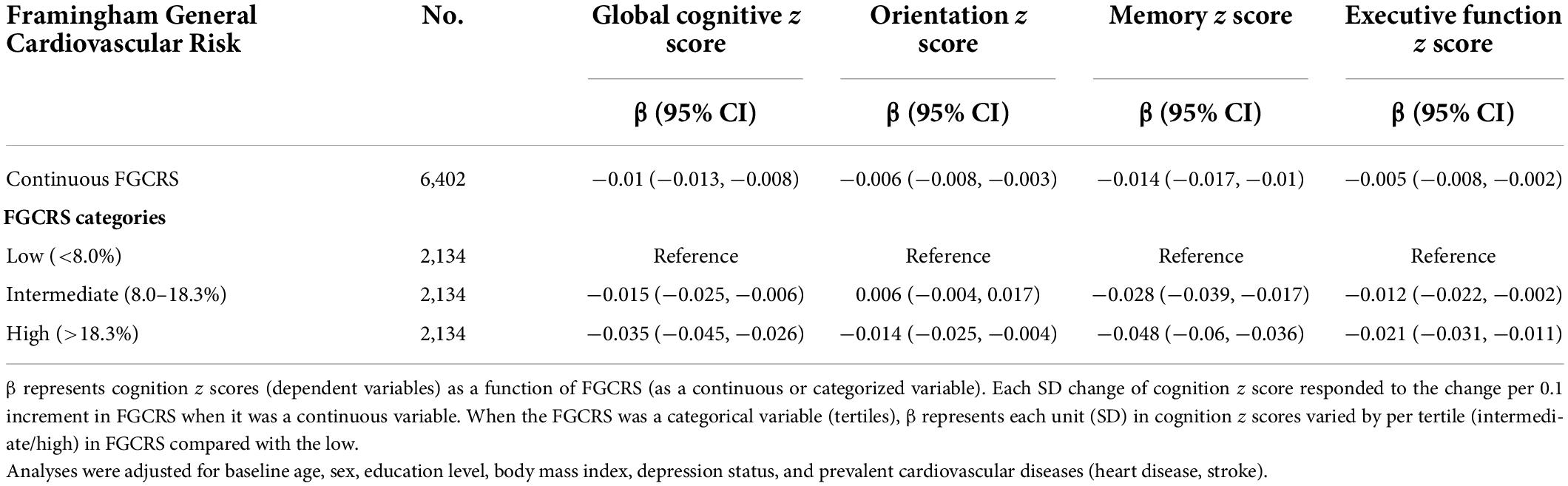
Table 2. The associations between baseline Framingham General Cardiovascular Risk Score (FGCRS) and annual changes in cognition z scores (SD/year), using linear mixed models.
The associations between change in FGCRS and cognitive decline are reported in Table 3, Figure 2, and Supplementary Figure 2. Among the nine combinations of FGCRS changing status, two included very few subjects (36 for the low to high group; 13 for the high to low group). In multivariable linear mixed model analysis, the rate of annual global cognitive decline was not statistically different from that of the consistently low group (reference) in the intermediate to low group (−0.022 SD/year, 95% CI −0.050, 0.005) and the high to low group (−0.019 SD/year, 95% CI −0.103, 0.066). Instead, the annual global cognitive decline was faster in the low to intermediate group (−0.026 SD/year, 95% CI −0.045, −0.007), the low to high group (−0.052 SD/year, 95% CI −0.102, −0.001), the consistently intermediate group (−0.019 SD/year, 95% CI −0.033, −0.005), the intermediate to high group (−0.040 SD/year, 95% CI −0.058, −0.022), the high to intermediate group (−0.024 SD/year, 95% CI −0.047, −0.002), and the consistently high group (−0.047 SD/year, 95% CI −0.060, −0.034). In addition, there was no significant faster global cognitive decline in difference in continuous FGCRS (10% increment of difference: −0.001, 95% CI −0.006, 0.004). When a change in continuous FGCRS was used as a quantile, the lower and higher FGCRS quintiles were significantly associated with faster cognitive decline than the middle quintile. For example, compared to those with middle FGCRS, those within the lowest and highest FGCRS quintiles had significantly worse performance in global cognition (−0.028 SD/year, 95% CI −0.043, −0.013 for the lowest quintile; −0.032 SD/year, 95% CI −0.047, −0.017 for the highest quintile). The results were similar for specific cognitive domains.
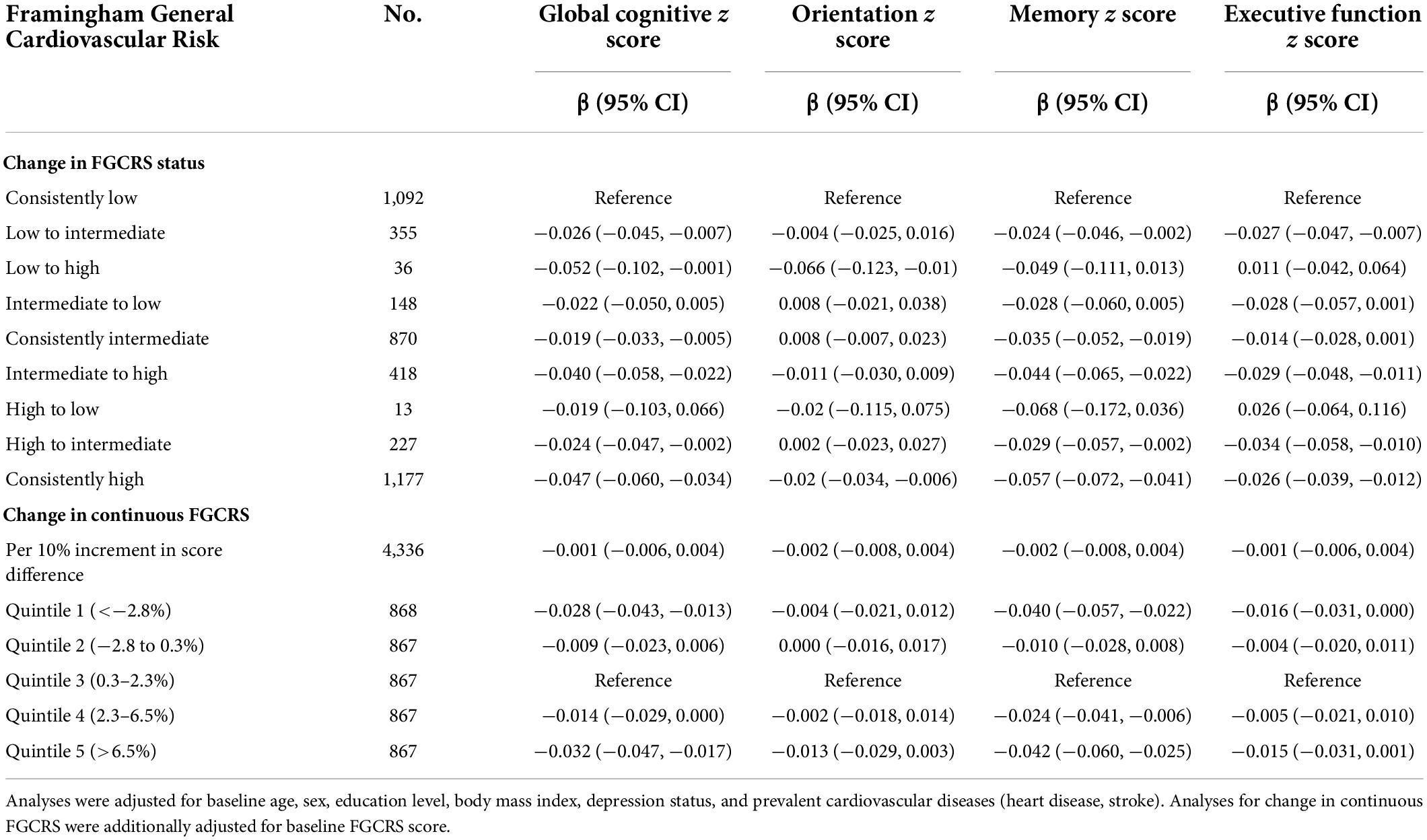
Table 3. The associations between change in Framingham General Cardiovascular Risk Score (FGCRS) and annual changes in cognition z scores (SD/year), using linear mixed models.
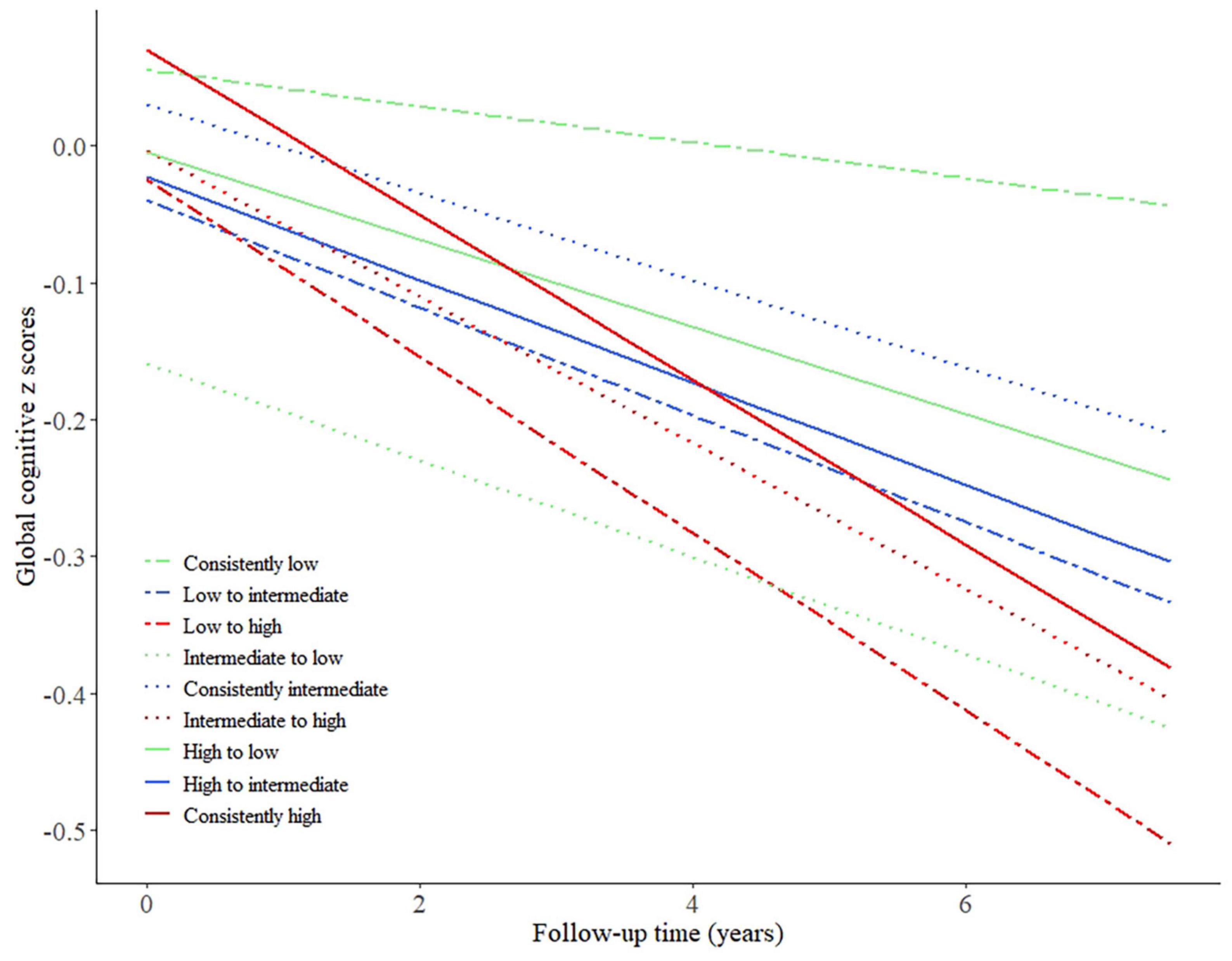
Figure 2. Predicted trajectories of global cognitive z scores by patterns of change in Framingham General Cardiovascular Risk Score between baseline and 4 years after, adjusted for baseline age, sex, education level, body mass index, depression status, prevalent cardiovascular diseases (heart disease, stroke).
No significant interaction between baseline FGCRS and age was observed in the changes in cognitive decline (p for “baseline FGCRS × time × baseline age” term: 0.692 for global cognition, 0.507 for orientation, 0.127 for memory, and 0.362 for executive function). Similar associations between baseline FGCRS and its change with cognitive decline were observed after excluding participants with prevalent cardiovascular disease or aged above 75 years at baseline (Supplementary Tables 1, 2). When the external validation was performed using data from the ELSA, the results remained consistent with our main findings (Supplementary Tables 3, 4).
As shown in Figure 1, among the 7,166 participants who had complete FGCRS and cognition measurements at baseline, 764 (10.7%) were excluded from the baseline FGCRS analysis because of missing information or loss of follow-up. Compared to the included participants, those excluded participants were older, more often women, less educated, and had worse cardiovascular risk profiles at baseline. 2,066 (32.3%) out of 6,402 participants were further excluded due to unavailable FGCRS reassessment. Those included and excluded from the FGCRS change analysis shared similar baseline characteristics (Supplementary Table 5).
In this longitudinal analysis of 6,402 community-dwelling middle-aged and older adults from the CHARLS, we found that increased cardiovascular risk burden measured by baseline FGCRS was associated with a faster decline in global cognition and specific cognitive domains during follow-up. However, there was no consistent relationship between the direction of changes in FGCRS and later cognitive decline.
The association of composite cardiovascular risk score with cognitive decline has been investigated from a life-course perspective (Kaffashian et al., 2011; Yaffe et al., 2014, 2021; George et al., 2021), but with inconsistent results, especially for the exposure during older age. The Framingham Offspring Study showed that cardiovascular risk burden during midlife, but not late-life, was associated with faster cognitive decline (Armstrong et al., 2019). Prior neuroimaging studies also found that only midlife, but not late-life, vascular risk factors were associated with elevated brain amyloid and lower gray matter perfusion at older ages (Gottesman et al., 2017; Suri et al., 2019). However, one recent study based on the Rush Memory and Aging Project reported that among participants with an average age of 80 years, a higher cardiovascular risk burden can still predict a decline in episodic memory, working memory, and perceptual speed and is associated with neurodegeneration and vascular lesions in the brain (Song et al., 2020). Here among the middle-aged and older Chinese participants, we found that increased cardiovascular risk burden was associated with faster cognitive decline. This association was not modified by baseline age. The differences between the findings may be explained by the heterogeneity in cohort characteristics (e.g., ethnicity, education level), and methodological discrepancies, such as duration of follow-up and the tools for cognitive assessment. Although the 95% CIs of global cognition decline would meet the clinical threshold of cognition change, a decline of ≥0.5 SD (Norman et al., 2003), in approximately 10 years since baseline, even minuscule cognitive function decline can result in a substantially increased risk of dementia over a long-term (Bozoki et al., 2001). Our findings support careful monitoring for cognitive impairment among patients with a higher cardiovascular risk burden.
The present study is the first prospective investigation of the cognitive trajectories among different patterns of change in composite cardiovascular risk scores. Previous studies mainly documented changes in specific cardiovascular risk factors such as BMI, blood pressure with cognitive decline, and dementia onset (Walker et al., 2019; Wu et al., 2021). The Atherosclerosis Risk in Communities study found that sustained hypertension in midlife to late-life and a pattern of midlife hypertension and late-life hypotension, compared with midlife and late-life normal blood pressure, were associated with increased risk for subsequent dementia. In contrast, no significant association was found between 24-year blood pressure patterns and cognitive change in late life (Walker et al., 2019). Here we calculated the changing pattern of FGCRS within 4 years among middle-aged and older Chinese participants and found no consistent relationship between the direction of changes in FGCRS and cognitive decline. These inconsistent relationship patterns indicate that the concept of FGCRS may not be useful for assessing the change in cardiovascular risk burden over time. A composite score cannot simply capture the complex relationship between cardiovascular risk factors and cognitive decline. The disease prognosis may be determined primarily by early exposure to a high cardiovascular risk burden, which may explain that participants who changed from high FGCRS to a lower category still had a relatively faster cognitive decline.
The current study has several strengths. The CHARLS is well designed to provide a nationally representative estimate for middle-aged and older people in China. The data on biomarkers were collected following standardized protocols with high quality. Additionally, with repeated data of FGCRS during follow-up, we explored any possible effect caused by different changing patterns of FGCRS. Furthermore, our findings can be generalized to Western populations by externally validating our results using the ELSA. Taken together, our study filled in a specific knowledge gap about the association between changes in FGCRS and cognitive decline. However, our study also has some limitations that should be acknowledged. First, limited by the available waves in the CHARLS, the follow-up interval was relatively short, and we could not investigate the association of changes in FGCRS with subsequent cognitive decline in our main analysis. However, since results from the external validation using the ELSA data with cognition measured from wave 6 onward remained consistent, it is unlikely that our main findings would be biased by reverse causation. Second, genetic data were unavailable, so we could not adjust for the APOE genotype; however, previous studies indicated no interaction between FGCRS and APOE status on cognitive decline (Armstrong et al., 2019; Song et al., 2020). Meanwhile, only those with complete FGCRS information and at least one repeated measurement were eligible for the current study, leading possibly to selection bias. The non-response analysis showed that the included participants were relatively healthier than those excluded, which may limit the internal validity. Moreover, our analysis of responders’ data may have underestimated complications by excluding non-responders’ potentially faster cognitive decline.
In summary, we found that a higher cardiovascular risk burden assessed by baseline FGCRS was associated with faster cognitive decline among the middle-aged and older Chinese population. However, there was no consistent relationship between the direction of change in FGCRS and cognitive decline. Further studies are needed to determine whether decreasing FGCRS would benefit cognitive decline.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Peking University Institutional Review Board (for the CHARLS) and the London Multicenter Research Ethics Committee (for the ELSA). The patients/participants provided their written informed consent to participate in this study.
XJ, HG, KW, and FA were responsible for the study concept and design. KW composed the statistical dataset, performed the statistical analyses, guarantor of this work and had full access to all data in the study, and took responsibility for the integrity of the data and the accuracy of the data analysis. XJ and HG wrote the manuscript. All authors contributed to the interpretation of the data and critical revision of the manuscript.
This work was supported by the Research Project of Changning District Health Committee of Shanghai Municipality, China (20214Y032) to HG and the Domestic Cooperation Project of Science and Technology Commission of Shanghai Municipality, China (20015800300) to DS. The funders had no role in the study design, data collection, analysis, decision to publish or preparation of the manuscript.
We thank the staff and participants of the CHARLS and ELSA study. We would also like to thank the China Scholarship Council for the scholarship to KW.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.895188/full#supplementary-material
American Diabetes, A. (2020). 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 43(Suppl 1), S14–S31. doi: 10.2337/dc20-S002
Armstrong, N. M., Bangen, K. J., Au, R., and Gross, A. L. (2019). Associations between midlife (but not late-life) elevated coronary heart disease risk and lower cognitive performance: results from the framingham offspring study. Am. J. Epidemiol. 188, 2175–2187. doi: 10.1093/aje/kwz210
Baumgart, M., Snyder, H. M., Carrillo, M. C., Fazio, S., Kim, H., and Johns, H. (2015). Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement 11, 718–726. doi: 10.1016/j.jalz.2015.05.016
Bozoki, A., Giordani, B., Heidebrink, J. L., Berent, S., and Foster, N. L. (2001). Mild cognitive impairments predict dementia in nondemented elderly patients with memory loss. Arch. Neurol. 58, 411–416. doi: 10.1001/archneur.58.3.411
Chen, X., Crimmins, E., Hu, P. P., Kim, J. K., Meng, Q., Strauss, J., et al. (2019). Venous blood-based biomarkers in the china health and retirement longitudinal study: rationale, design, and results from the 2015 wave. Am. J. Epidemiol. 188, 1871–1877. doi: 10.1093/aje/kwz170
D’Agostino, R. B., Vasan, R. S., Pencina, M. J., Wolf, P. A., Cobain, M., Massaro, J. M., et al. (2008). General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 117, 743–753. doi: 10.1161/CIRCULATIONAHA.107.699579
Gauthier, S. R.-N. P., Morais, J. A., and Webster, C. (2021). World Alzheimer Report 2021: Journey through the diagnosis of dementia. London: Alzheimer’s Disease International.
George, K. M., Gilsanz, P., Peterson, R. L., Barnes, L. L., DeCarli, C. S., Mayeda, E. R., et al. (2021). Impact of cardiovascular risk factors in adolescence, young adulthood, and midlife on late-life cognition: study of healthy aging in african americans. J. Gerontol. A Biol. Sci. Med. Sci. 76, 1692–1698. doi: 10.1093/gerona/glab143
Gorelick, P. B., Furie, K. L., Iadecola, C., Smith, E. E., Waddy, S. P., Lloyd-Jones, D. M., et al. (2017). Defining optimal brain health in adults: a presidential advisory from the american heart association/american stroke association. Stroke 48, e284–e303. doi: 10.1161/STR.0000000000000148
Gottesman, R. F., Schneider, A. L., Zhou, Y., Coresh, J., Green, E., Gupta, N., et al. (2017). Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. JAMA 317, 1443–1450. doi: 10.1001/jama.2017.3090
Harrison, S. L., Ding, J., Tang, E. Y., Siervo, M., Robinson, L., Jagger, C., et al. (2014). Cardiovascular disease risk models and longitudinal changes in cognition: a systematic review. PLoS One. 9:e114431. doi: 10.1371/journal.pone.0114431
Kaffashian, S., Dugravot, A., Nabi, H., Batty, G. D., Brunner, E., Kivimaki, M., et al. (2011). Predictive utility of the Framingham general cardiovascular disease risk profile for cognitive function: evidence from the Whitehall II study. Eur. Heart J. 32, 2326–2332. doi: 10.1093/eurheartj/ehr133
Kivipelto, M., Mangialasche, F., and Ngandu, T. (2018). Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 14, 653–666. doi: 10.1038/s41582-018-0070-3
Lipnicki, D. M., Crawford, J. D., Dutta, R., Thalamuthu, A., Kochan, N. A., Andrews, G., et al. (2017). Age-related cognitive decline and associations with sex, education and apolipoprotein E genotype across ethnocultural groups and geographic regions: a collaborative cohort study. PLoS Med. 14:e1002261. doi: 10.1371/journal.pmed.1002261
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396, 413–446. doi: 10.1016/S0140-6736(20)30367-6
Ma, Y., Liang, L., Zheng, F., Shi, L., Zhong, B., and Xie, W. (2020). Association between sleep duration and cognitive decline. JAMA Netw. Open. 3:e2013573. doi: 10.1001/jamanetworkopen.2020.13573
Norman, G. R., Sloan, J. A., and Wyrwich, K. W. (2003). Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med. Care 41, 582–592. doi: 10.1097/01.MLR.0000062554.74615.4C
Qiu, C., and Fratiglioni, L. (2015). A major role for cardiovascular burden in age-related cognitive decline. Nat. Rev. Cardiol. 12, 267–277. doi: 10.1038/nrcardio.2014.223
Song, R., Xu, H., Dintica, C. S., Pan, K. Y., Qi, X., Buchman, A. S., et al. (2020). Associations between cardiovascular risk, structural brain changes, and cognitive decline. J. Am. Coll. Cardiol. 75, 2525–2534. doi: 10.1016/j.jacc.2020.03.053
Steptoe, A., Breeze, E., Banks, J., and Nazroo, J. (2013). Cohort profile: the English longitudinal study of ageing. Int. J. Epidemiol. 42, 1640–1648. doi: 10.1093/ije/dys168
Suri, S., Topiwala, A., Chappell, M. A., Okell, T. W., Zsoldos, E., Singh-Manoux, A., et al. (2019). Association of Midlife Cardiovascular Risk Profiles With Cerebral Perfusion at Older Ages. JAMA Netw. Open. 2:e195776. doi: 10.1001/jamanetworkopen.2019.5776
Volgman, A. S., Palaniappan, L. S., Aggarwal, N. T., Gupta, M., Khandelwal, A., Krishnan, A. V., et al. (2018). Atherosclerotic cardiovascular disease in south asians in the united states: epidemiology, risk factors, and treatments: a scientific statement from the american heart association. Circulation. 138, e1–e34. doi: 10.1161/CIR.0000000000000580
Walker, K. A., Sharrett, A. R., Wu, A., Schneider, A. L. C., Albert, M., Lutsey, P. L., et al. (2019). Association of Midlife to Late-Life Blood Pressure Patterns With Incident Dementia. JAMA 322, 535–545. doi: 10.1001/jama.2019.10575
World Health Organization (2019). Risk reduction of cognitive decline and dementia: who guidelines. who guidelines approved by the guidelines review committee. Geneva: WHO.
Wu, S., Lv, X., Shen, J., Chen, H., Ma, Y., Jin, X., et al. (2021). Association between body mass index, its change and cognitive impairment among Chinese older adults: a community-based, 9-year prospective cohort study. Eur. J. Epidemiol. 36, 1043–1054. doi: 10.1007/s10654-021-00792-y
Yaffe, K., Vittinghoff, E., Hoang, T., Matthews, K., Golden, S. H., and Zeki Al Hazzouri, A. (2021). Cardiovascular risk factors across the life course and cognitive decline: a pooled cohort study. Neurology. 96, e2212–e2219. doi: 10.1212/WNL.0000000000011747
Yaffe, K., Vittinghoff, E., Pletcher, M. J., Hoang, T. D., Launer, L. J., Whitmer, R., et al. (2014). Early adult to midlife cardiovascular risk factors and cognitive function. Circulation. 129, 1560–1567. doi: 10.1161/CIRCULATIONAHA.113.004798
Keywords: Framingham General Cardiovascular Risk Score, change, cognitive decline, older people, cohort
Citation: Ji X, Gao H, Sun D, Zhao W, Zhuang J, Wang K and Ahmadizar F (2022) Association of baseline level of cardiovascular risk burden and its temporal changes with cognitive decline. Front. Aging Neurosci. 14:895188. doi: 10.3389/fnagi.2022.895188
Received: 13 March 2022; Accepted: 18 August 2022;
Published: 01 September 2022.
Edited by:
Anja Soldan, Johns Hopkins University, United StatesReviewed by:
Mauro Tettamanti, Mario Negri Pharmacological Research Institute (IRCCS), ItalyCopyright © 2022 Ji, Gao, Sun, Zhao, Zhuang, Wang and Ahmadizar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kan Wang, ay53YW5nQGVyYXNtdXNtYy5ubA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.