
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 06 May 2022
Sec. Neurocognitive Aging and Behavior
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.889930
This article is part of the Research TopicThe Many Faces of Brain AgingView all 8 articles
 Francesco Asci1†
Francesco Asci1† Simone Scardapane2†
Simone Scardapane2† Alessandro Zampogna3
Alessandro Zampogna3 Valentina D’Onofrio3
Valentina D’Onofrio3 Lucia Testa4
Lucia Testa4 Martina Patera3
Martina Patera3 Marco Falletti3
Marco Falletti3 Luca Marsili5
Luca Marsili5 Antonio Suppa1,3*
Antonio Suppa1,3*
Background: Handwriting is an acquired complex cognitive and motor skill resulting from the activation of a widespread brain network. Handwriting therefore may provide biologically relevant information on health status. Also, handwriting can be collected easily in an ecological scenario, through safe, cheap, and largely available tools. Hence, objective handwriting analysis through artificial intelligence would represent an innovative strategy for telemedicine purposes in healthy subjects and people affected by neurological disorders.
Materials and Methods: One-hundred and fifty-six healthy subjects (61 males; 49.6 ± 20.4 years) were enrolled and divided according to age into three subgroups: Younger adults (YA), middle-aged adults (MA), and older adults (OA). Participants performed an ecological handwriting task that was digitalized through smartphones. Data underwent the DBNet algorithm for measuring and comparing the average stroke sizes in the three groups. A convolutional neural network (CNN) was also used to classify handwriting samples. Lastly, receiver operating characteristic (ROC) curves and sensitivity, specificity, positive, negative predictive values (PPV, NPV), accuracy and area under the curve (AUC) were calculated to report the performance of the algorithm.
Results: Stroke sizes were significantly smaller in OA than in MA and YA. The CNN classifier objectively discriminated YA vs. OA (sensitivity = 82%, specificity = 80%, PPV = 78%, NPV = 79%, accuracy = 77%, and AUC = 0.84), MA vs. OA (sensitivity = 84%, specificity = 56%, PPV = 78%, NPV = 73%, accuracy = 74%, and AUC = 0.7), and YA vs. MA (sensitivity = 75%, specificity = 82%, PPV = 79%, NPV = 83%, accuracy = 79%, and AUC = 0.83).
Discussion: Handwriting progressively declines with human aging. The effect of physiological aging on handwriting abilities can be detected remotely and objectively by using machine learning algorithms.
Neurodegenerative disorders, including Parkinson’s disease (PD) and Alzheimer’s disease (AD), represent a relevant global health issue, and the number of cases is going to increase steeply in prevalence in the next few decades (Armstrong and Okun, 2020; Feigin et al., 2020). Currently, the lockdown restrictions due to the COVID-19 global pandemic have challenged the clinical management of patients affected by neurodegenerative diseases, thus requiring innovative telemedicine approaches (Valdovinos et al., 2022). To this aim, it would be relevant to identify novel telemedicine tools allowing early and objective diagnosis, tracking the severity of the disease and thus improving the overall remote clinical management of patients with neurodegenerative diseases (Chirra et al., 2019). Practically, an ideal telemedicine setting should imply a safe, costly affordable and largely available tool able to easily collect biologically relevant information, in an ecological scenario.
As a possible innovative telemedicine strategy, in this study, we propose the remote assessment of handwriting. Handwriting indeed only requires safe, cheap, and largely available tools and can be simply collected in an ecological scenario. Moreover, handwriting samples can be easily digitalized by using smartphone-based high-resolution cameras and transmitted directly to a central hub for subsequent analysis. From a biological perspective, handwriting represents an acquired complex cognitive and motor skill resulting from the activation of a widespread brain network (Menon and Desmond, 2001; Lubrano et al., 2004; Purcell et al., 2011; Planton et al., 2013; Baldo et al., 2018; Chen et al., 2019; Bartoň et al., 2020). Indeed, in the clinical setting, handwriting is yet largely used to assess motor abilities in patients with PD (i.e., evaluation of micrographia and slowness of hand movements) and it is typically included in standardized clinical scales designed to examine cognitive functions in patients with dementia such as AD (Folstein et al., 1975). However, the potential application of handwriting analysis as a new telemedicine tool for neurodegenerative disorders crucially requires the preliminary assessment of a large dataset of healthy controls and the investigation of the effect on the handwriting of relevant biological factors such as physiologic aging.
Seminal studies using perceptual as well as objective kinematic analysis (Hilton, 1977; Dixon et al., 1993; Slavin et al., 1996; Walton, 1997; Contreras-Vidal et al., 2002; Teulings et al., 2002; Rodríguez-Aranda, 2003; Rosenblum and Werner, 2006; Burger and McCluskey, 2011; Caligiuri et al., 2014), have demonstrated age-related changes in handwriting skills in healthy subjects. However, to analyze a large amount of data for telemedicine purposes, automatically and objectively, more robust methodological approaches based on artificial intelligence are required for the classification of variables obtained from large datasets (Deo, 2015). For this purpose, machine learning has already been demonstrated to be a reliable tool for the assessment of handwriting in healthy subjects (Pang and Yang, 2016; Guo et al., 2021; Pei and Ouyang, 2021). Moreover, handwriting analysis based on machine learning has allowed inferring several features, including gender (Illouz et al., 2018), left/right-handedness (Al-Maadeed et al., 2013), presence of dysgraphia (Asselborn et al., 2020), specific personality traits (Lemos et al., 2018), and finally individualized fingerprints (Srihari et al., 2001). Also, preliminary studies using handwriting analysis with machine learning have demonstrated the possibility to predict age in healthy subjects (Zouaoui et al., 2017; Basavaraja et al., 2019).
We here investigated possible age-related changes in handwriting, objectively, in a large cohort of healthy subjects. To this aim, we examined and compared a simple handwriting task, collected in a real-life setting in three independent sex-matched, age-based groups: younger adults (YA), middle-aged adults (MA), and finally older adults (OA). To verify the ability of machine learning analysis to automatically classify handwriting samples according to age, all data were submitted to a convolutional neural network (CNN) algorithm. Furthermore, the performance of the artificial classifier was assessed in detail in all comparisons (i.e., sensitivity, specificity, positive and negative predictive values, and accuracy). Lastly, we also calculated the area under the receiver operating characteristic (ROC) curves to verify the optimal threshold as reflected by the associated criterion (Ass. Crit.) and Youden Index (YI).
We consecutively and randomly recruited 156 healthy subjects (61 males; mean age ± SD 49.6 ± 20.4 years, range 18–90 years) from the IRCCS Neuromed, Pozzilli (IS), Italy. All subjects were right-handed and native Italian speakers. We further divided participants into three age-independent subgroups to conform with previous demographic definitions of younger adults (YA) (18–35 years), middle-aged adults (MA) (36–55 years), and older adults (OA) (>56 years) (Petry, 2002). Accordingly, we examined 51 YA (23 males; 25.7 ± 3.2 years, range 18–32 years), 40 MA (17 males; 48.9 ± 5.9 years, range 37–57 years), and finally 63 OA (21 males; 71.3 ± 6.6 years, range 62–90 years). Anthropometric features including weight, height, and body mass index (BMI) were collected. The cognitive functions of all participants were assessed through the Mini-Mental State Examination (MMSE) (Folstein et al., 1975). None of the participants manifested cognitive or mood disorders, and none reported osteoarticular disorders or visual deficits significantly affecting handwriting. Also, no subjects were taking any drug affecting the central nervous system. The demographic (i.e., age and gender), anthropometric (i.e., weight, height, and BMI) and clinical (i.e., MMSE) features of the participants are reported in Table 1. All participants gave written informed consent, and the study was approved by the institutional ethics committee.
A written protocol with instructions on how to perform the handwriting task was sent to participants by the authors using the institutional email address. Also, following the enrollment, subjects received a preliminary supervised training trial to familiarize themselves with the experimental procedures. Then, participants were asked to perform the handwriting task while sitting on a chair, with their arms lying on a table, at home and in the morning. Concerning the handwriting task used in this study, participants were asked to write their own first and last names ten consecutive times on a paper sheet. We selected this specific task to exclude the influence of cultural factors and the contribution of higher cognitive abilities related to the symbolic aspects of handwriting potentially affecting the assessment of basic writing features. The first and last names were written starting from the upper and left sides of the document and then proceeding downward, in a single column, with a self-paced and comfortable speed. We preliminary provided participants with a fold of white A4 (210 mm × 297 mm) paper sheet (Fabriano, PG, Italy), and a couple of black ballpoint pen types (Bic, Clichy, France). After collecting the handwriting task three consecutive times, participants were asked to scan the signed paper sheet using the camera included in their smartphone (required resolution of at least 5 Megapixels). After scanning the handwriting samples, participants were asked to convert photos into portable document format (PDF) files using dedicated apps available for free download. Finally, participants completed the requested procedures by sending the PDF files to the authors’ institutional email server, and the files were stored anonymously on a dedicated Drive, encrypted, and password-protected (Figure 1).
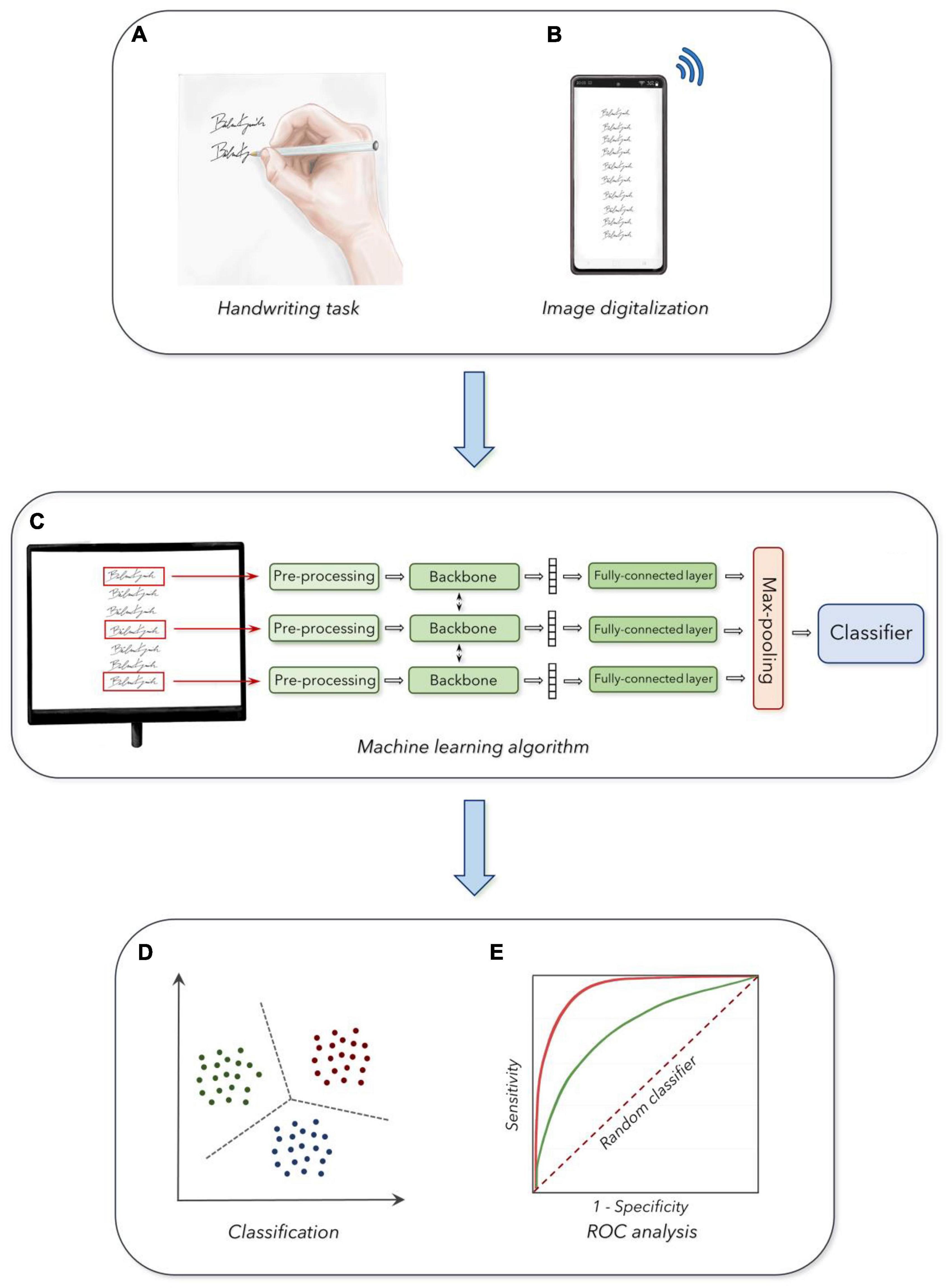
Figure 1. Experimental design: (A) Acquisition of handwriting samples. (B) Digitalization and collection of the handwriting task. (C) Machine learning analysis of handwriting samples. (D) Output of the classifier in the three age groups. (E) Receiver operating characteristic curves (ROC analysis) for the discrimination between the three groups (see section “Materials and Methods” for further details).
All handwriting samples were preliminarily assessed using a perceptive evaluation, to exclude corrupted files from subsequent analysis. Then, based on previous data reporting changes in stroke sizes in the elderly as a prominent age-related feature (Hilton, 1977; Dixon et al., 1993; Slavin et al., 1996; Walton, 1997; Contreras-Vidal et al., 2002; Teulings et al., 2002; Rodríguez-Aranda, 2003; Rosenblum and Werner, 2006; Burger and McCluskey, 2011; Caligiuri et al., 2014), we first automatically measured the average height of strokes in the handwriting samples in each participant. As a text detection method, we used the DBNet algorithm (i.e., ResNet-50 backbone), included in the docTR package, owing to its open-source availability and state-of-the-art performance for the task (Cao et al., 2020). The DBNet, was trained according to Cao et al. (2020) and was then applied to extract the precise bounding boxes corresponding to the handwriting samples1 (Cao et al., 2020). To limit the number of false-positive tests due to stylized strokes, we selected only bounding boxes with a height higher than 50 pixels (IC > 0.5) and relative to easily readable handwriting strokes. Finally, the DBNet algorithm calculated specific digital values as outcome measures.
The PDF files including the handwriting samples were further digitalized to fit with standard requirements for machine learning procedures, according to basic standards of artificial intelligence algorithms. A customized CNN model was built to perform classification in the age groups, following standard methodologies in the field of fine-tuning pre-trained models (Bengio et al., 2021). We classified pictures of single handwriting samples collected in the three age groups (i.e., YA, MA, and OA), according to standardized machine learning procedures, based on a fine-tuning of a pre-trained CNN with a randomly initialized fully connected layer at the end (Bengio et al., 2021). We developed a specialized multi-view CNN able to simultaneously process a set of k handwriting samples extracted from a single participant, to increase the robustness of the analysis (Figure 1). The size k of the input set was a hyper-parameter that was optimized with a dedicated grid search procedure. The architecture for further classification analysis considered the overall set as input. Accordingly, this architecture was composed of three blocks, a backbone which was used to independently process each sample in the set, a pooling block used to merge the outputs of the backbone and, finally, a classification block applied to provide a single prediction for the set (i.e., the age group of the handwriting sample). Then, after achieving a robust architecture, we organized the following steps consisting of the design of each component, the training procedure, and the hyper-parameters optimization procedures. First, the handwriting samples were resized to a single common dimension, to match the spatial dimension of the pre-trained backbone. Specifically, we resized all images to a 128 × 256-pixel proportion for all CNNs pre-trained on ImageNet (Bengio et al., 2021). To handle the handwriting samples characterized by images smaller than the optimal dimension, we applied white padding according to the respective size, matching the color of the underlying sheet of paper. We then converted the single out-of-scale image into a black and white negative image, thus obtaining large values for the corresponding output tensor. During training, we randomly selected subsets of k samples from the same PDF file as input to the network. We kept the amount of random rotation and translation to the inputs as lower as possible, to avoid image artifacts (Pang and Yang, 2016). All images were processed using a shared backbone, and we experimented with either (a) an AlexNet network trained from scratch, (b) a pre-trained ResNet-50 model, and (c) a customized Convolutional Recurrent Neural Network (CRNN), which was pre-trained on a task of optical character recognition (Wang et al., 2019). We included the choice of backbone as a second hyper-parameter to optimize the classifying procedure. We performed average pooling on the outputs of the backbone (across the spatial dimensions for the CNNs, and the temporal dimension for the CRNN), to associate each image in the input set with a fixed-dimensional embedding vector (i.e., size 1024 for the pre-trained ResNet-50 model). We applied a small fully connected layer with 100 hidden units and Gaussian Error Linear Units activation function (Hendrycks and Gimpel, 2016) on each embedding vector, and then we performed a max-pooling for all embeddings corresponding to the same input set, to obtain a fixed-dimensional vector for the entire set (Figure 1). The overall design was built to avoid the architecture being affected by the permutations of the samples in input (i.e., the output of the network does not change whether we shuffled the samples inside a set, and it could also potentially work for variable-sized sets). The final embedding vector was passed to a fully connected layer with three outputs (corresponding to the three age groups), trained with either a standard cross-entropy loss function or a weighted kappa loss function exploiting the fact that the three classes were naturally ordered (de la Torre et al., 2018; Figure 1).
To optimize the architecture, we randomly divided the dataset into three subsets with 80% of the subjects for training, 10% for validation, and 10% for test. We performed a grid search on the size of the input set, the choice of the backbone, and the selection of the loss function using the validation set, with the macro-averaged AUC score as a metric. The best performing architecture after the grid search, with a validation AUC of 81%, exploited the ResNet-50 model as the backbone, a set of size 3, and the cross-entropy loss, and we used these hyper-parameters in the following. All results shown in the experimental section were computed on the test set, by training over 50 different initializations and using the concatenation of the previously defined training and validation sets as training data. The final hyper-parameters of the model used in the experimental section were the following: the size of the crops (128; 256; RGB), number of views (Valdovinos et al., 2022), backbone model (ResNet-50—pre-trained), loss function (cross-entropy—multi-class). For the implementation, we used TensorFlow for the design of the model, with the pre-trained ResNet-50 weights taken from the official Google implementation in TensorFlow Hub, while the pre-trained CRNN weights were taken from the Keras-ocr repository.
The normal distribution of demographic and anthropometric features of subjects included in the YA, MA, and OA groups was assessed using the Kolmogorov-Smirnov test. The Chi-Square Test was used to compare the frequency of males and females in the three groups. The Mann-Whitney U-test was used to compare demographic and anthropometric parameters, as well as clinical scores (i.e., MMSE) in YA, MA, and OA. The Mann-Whitney U-test was also used to compare the stroke dimensions (i.e., heights) in participants from YA, MA, and OA groups. ROC analyses were calculated to identify the optimal cut-off values to discriminate between YA and MA, YA, and OA, and finally MA and OA, according to standardized procedures. For each ROC curve, we analyzed the Youden Index and its associated criterion (i.e., optimal threshold), as well as the Sensitivity (Se.), Specificity (Sp.), Positive Predictive Value (PPV), Negative Predictive Value (NPV), Accuracy (Acc.), Area Under the Curve (AUC), standard error (SE). Also, we compared AUCs of independent ROC curves to verify possible differences in statistical analysis, according to standardized procedures (Figure 1).
A p-value < 0.05 was considered statistically significant. Statistical analyses were conducted using STATA v17.0 (StataCorp LLC, United States).
Demographic and anthropometric variables were normally distributed among participants. The Chi-square test showed a balanced distribution of female and male participants within the three subgroups (chi-square: 1.48, p = 0.48). The Mann-Whitney U-test showed decreased weight and BMI in YA compared with MA (p > 0.05) and OA (p > 0.05), as well as decreased height in OA compared with YA (p > 0.05) and MA (p > 0.05).
We discarded less than 5% of all samples in case of a poor-quality acquisition of handwriting as evaluated through our preliminary perceptual analysis, and accordingly, these participants were asked to repeat the acquisition of new handwriting samples.
When comparing the average stroke dimensions (i.e., heights) achieved by using the DBNet software to the handwriting samples collected from YA, MA, and OA, the Mann-Whitney U-test showed smaller stroke heights in OA than YA and MA, as well as in MA than in YA (p < 0.01 for all comparisons) (Figure 2).
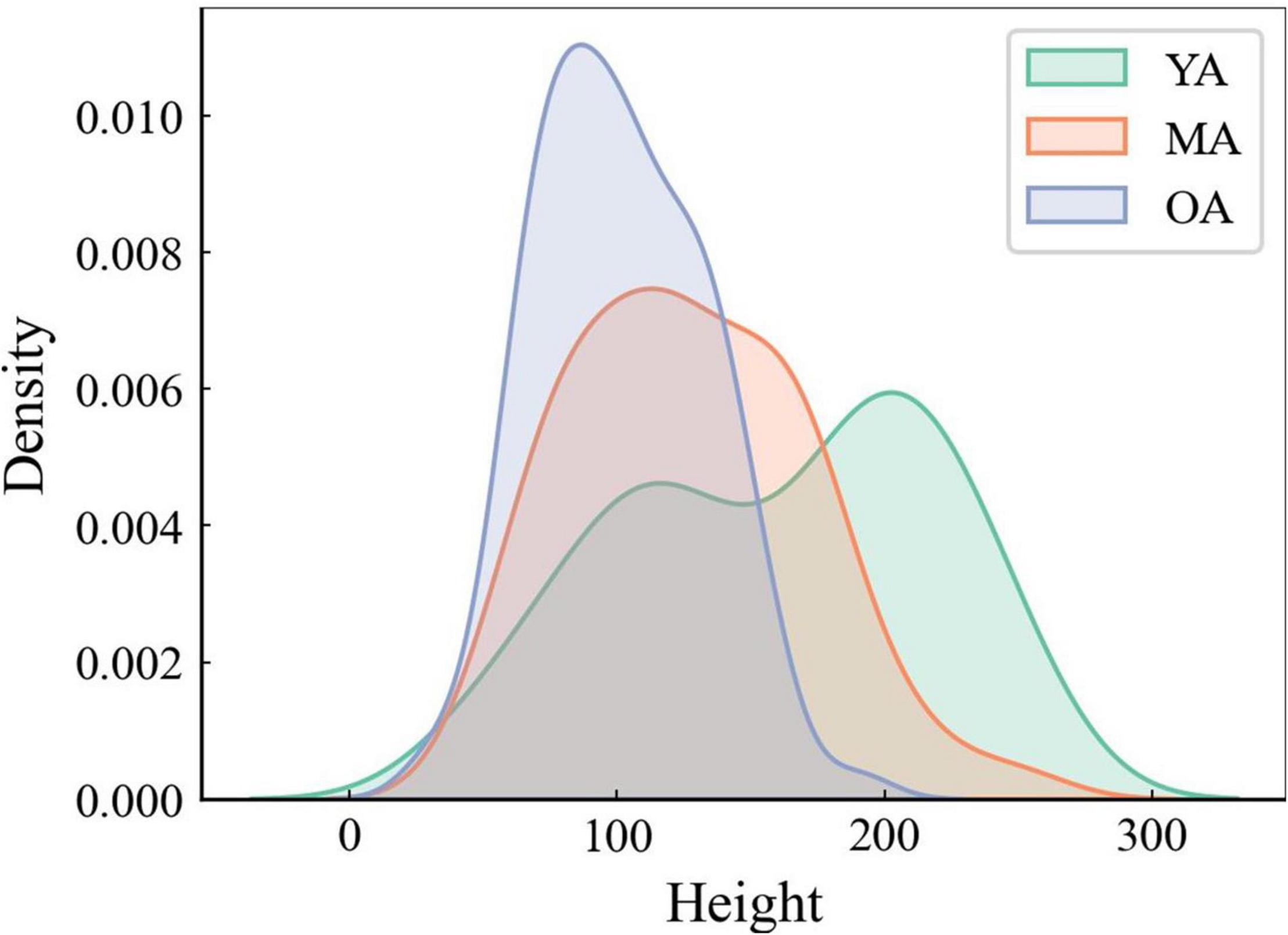
Figure 2. The average height of strokes analysis through DBNet algorithm. Note that the average height of strokes is smaller in OA than in MA and YA.
Concerning machine learning analysis, the CNN artificial classifier discriminated between YA and OA with high significant performance. The ROC curve analyses identified an optimal threshold value of 0.60 (associated criterion), when including 114 instances (Y.I. = 0.52). Using this cut-off value, the performance of our test was sensitivity = 82%, specificity = 80%, PPV = 78%, NPV = 79%, Acc. = 77%, and AUC = 0.840 (Figure 3A and Table 2).
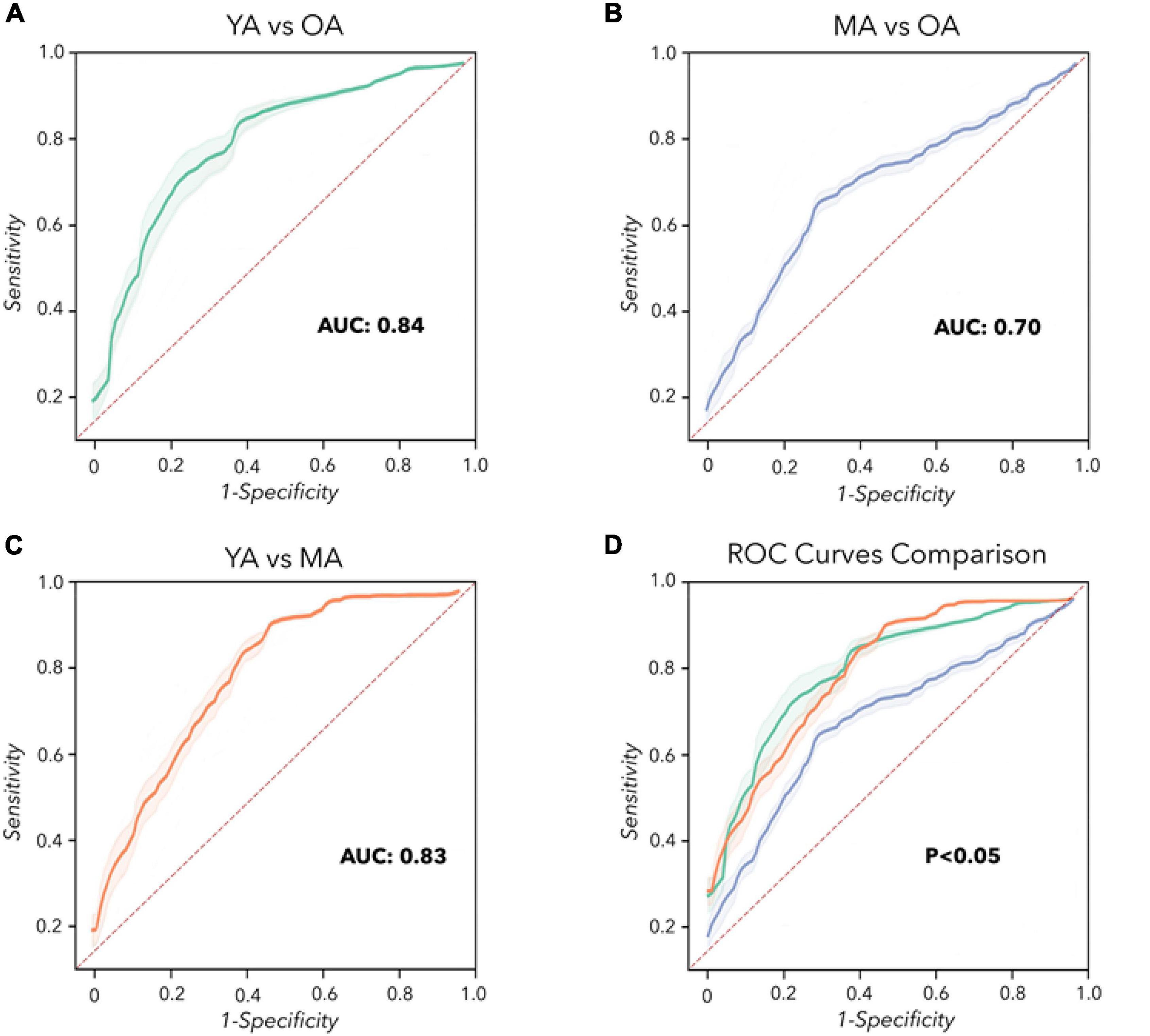
Figure 3. Convolutional Neural Network analysis. Receiver operating characteristic (ROC) curves were calculated to differentiate YA, MA, and OA. (A) YA vs. OA (green line). (B) MA vs. OA (blue line). (C) YA vs. MA (orange line). (D) Comparison of the ROC curves. The dashed red line represents the performance of a random classifier.
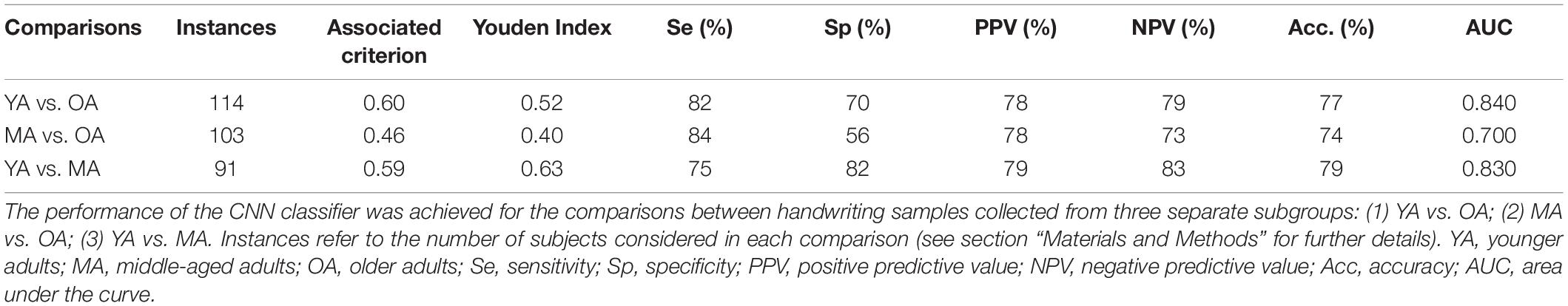
Table 2. Performance of the CNN algorithm in classifying handwriting samples collected from the whole group of healthy participants.
The differentiation between MA and OA achieved by the CNN algorithm also disclosed significant performances. The ROC curve analyses identified an optimal threshold value of 0.46 (associated criterion), when including 103 instances (Y.I. = 0.40). Using this cut-off value, the performance of our test was sensitivity = 84%, specificity = 56%, PPV = 78%, NPV = 73%, Acc. = 74%, and AUC = 0.700 (Figure 3B and Table 2).
When discriminating YA and MA, the artificial classifier achieved a significant performance of the test. ROC curve analyses identified an optimal threshold value of 0.59 (associated criterion) when including 91 instances (Y.I. = 0.63). Using this cut-off value, the performance of our test was sensitivity = 75%, specificity = 82%, PPV = 79%, NPV = 83%, Acc. = 79%, and AUC = 0.830 (Figure 3C and Table 2).
When comparing the two independent ROC curves relative to the analysis of YA vs. MA and YA vs. OA, we obtained similar results: the difference between AUCs = –0.02, z = –0.205, SE = 0.098, p = 0.84 (Table 3). Also, when comparing the two ROC curves relative to the classification between YA vs. MA and MA vs. OA, the statistical analysis showed overlapping results: the difference between AUCs = 0.10, z = 0.726, SE = 0.138, p = 0.47 (Table 3). Finally, when discriminating between the two ROC curves relative to YA vs. OA and MA vs. OA, we demonstrated similar results: the difference between AUCs = 0.12, z = 0.822, SE = 0.146, p = 0.41 (Table 3 and Figure 3D).
In the present study in a large cohort of healthy subjects, we demonstrated that physiologic aging deteriorates handwriting abilities. Specifically, we report, for the first time, that an advanced analysis based on machine learning algorithms, applied to a simple handwriting task collected in a real-life setting, objectively and automatically discriminated three independent sex-matched, age-based groups: YA, MA, and finally OA. The accuracy of the ROC curves here obtained pointed to a significant worsening of handwriting abilities led by physiological aging. Also, our study indicates that an ecologic machine learning-based analysis of handwriting in healthy subjects constitutes a reliable telemedicine approach, potentially useful for the future and remote recognition of neurological disorders.
Our first analysis demonstrated significant smaller stroke sizes in OA than in YA and MA samples, suggesting an age-related progressive decline of handwriting abilities in healthy subjects. Our findings fully agree with previous observations reporting decreased sizes along with reduced velocities and pressures of strokes during handwriting in elderly people (Hilton, 1977; Dixon et al., 1993; Slavin et al., 1996; Walton, 1997; Contreras-Vidal et al., 2002; Teulings et al., 2002; Rodríguez-Aranda, 2003; Kim et al., 2005; Rosenblum and Werner, 2006; Burger and McCluskey, 2011; Plamondon et al., 2013; Caligiuri et al., 2014; Zham et al., 2019; Kanno et al., 2020). By contrast, some reports suggested increased rather than decreased stroke size in elderly subjects (Rosenblum et al., 2013) raising the possibility of relevant heterogeneity in previous methodologies and experimental approaches. Abnormal stroke sizes observed in the elderly during handwriting would point to the effect of physiological aging on brain networks contributing to this high-level cognitive function (Purcell et al., 2011; Planton et al., 2013; Bartoň et al., 2020). Clinical, neuropsychological and neuroimaging studies have demonstrated that handwriting relies on the widespread synergic activity of several brain regions, the writing network. More in detail, the angular gyrus (Roeltgen and Heilman, 1984) and the precentral gyrus (Rapcsak et al., 1988) contribute to lexical processes of handwriting, the left perisylvian regions play a role in phonological aspects (Roeltgen and Heilman, 1984; Rapcsak et al., 1988, 2009; Alexander et al., 1992b), whereas the left superior parietal or premotor regions are responsible for handwriting execution (Auerbach and Alexander, 1981; Anderson et al., 1990; Alexander et al., 1992a). Indeed, selective brain lesions in the angular gyrus/precentral gyrus/left perisylvian regions and in the left superior parietal/premotor regions are known to lead to dysorthographias (i.e., lexical or phonological components) (Roeltgen and Heilman, 1984; Rapcsak et al., 1988, 2009; Alexander et al., 1992b) and apraxic agraphia (i.e., grapheme tracing) (Auerbach and Alexander, 1981; Anderson et al., 1990; Alexander et al., 1992a), respectively. Lastly, the writing network also reflects the activation of subcortical structures such as the basal ganglia (i.e., striatum) and the anterior cerebellum (Kim et al., 2005; Purcell et al., 2011; Planton et al., 2013, 2017; Letanneux et al., 2014; Zham et al., 2019; Bartoň et al., 2020; Kanno et al., 2020). In the elderly, the age-related progressive reduction in stroke sizes during handwriting would therefore reflect structural or functional changes in cortico-subcortical components of the writing network (Purcell et al., 2011; Planton et al., 2013; Bartoň et al., 2020). Several previous reports have demonstrated an overall reduction of default subnetwork connectivity in elderly subjects when performing experimental paradigms assessing executive functions, such as handwriting (Planton et al., 2017; Schulz et al., 2022). This would reflect several age-related biological factors including progressive white matter involvement in the frontal lobe attributable to a small vessel disease (de Leeuw et al., 2001; Kim and Melhem, 2008; Walhovd et al., 2011; Hirsiger et al., 2017; Zhuang et al., 2018). A further consideration concerns the possible link between the age-related progressive reduction in stroke sizes and the parkinsonian micrographia. Previous observations have demonstrated that micrographia in PD is related to the severity of bradykinesia (Wu et al., 2016; Wyss-Coray, 2016; Schott, 2017; Canevelli and Marsili, 2022), thus reflecting dopaminergic striatal denervation (Hilton, 1977; Dixon et al., 1993; Slavin et al., 1996; Walton, 1997; Contreras-Vidal et al., 2002; Teulings et al., 2002; Rodríguez-Aranda, 2003; Rosenblum and Werner, 2006; Burger and McCluskey, 2011; Plamondon et al., 2013; Rosenblum et al., 2013; Caligiuri et al., 2014; Wu et al., 2016; Kanno et al., 2020). Accordingly, a further hypothesis would attribute the age-related reduction in stroke sizes observed in healthy elderly to abnormal activity in cortico-subcortical components of the writing network which at least in part overlap with those responsible for micrographia in PD (Kim et al., 2005; Rosenblum et al., 2013; Wu et al., 2016; Zham et al., 2019; Kanno et al., 2020). Future studies will disclose possible similarities between age-related reduction of stroke sizes and parkinsonian “consistent” (i.e., overall small handwriting) and “progressive” micrographia (i.e., serial reduction in handwriting size) (Kim et al., 2005; Rosenblum et al., 2013; Wu et al., 2016; Zham et al., 2019; Kanno et al., 2020). Despite PD, converging clinical, neuropsychological and neuroimaging evidence point to abnormal handwriting abilities also in patients with other neurodegenerative disorders including AD (Forbes et al., 2004; Delazer et al., 2021). Patients with AD may manifest agraphia associated with a reduction in stroke sizes (Delazer et al., 2021). Overall, given that micrographia constitutes a reliable writing feature in PD (Letanneux et al., 2014) and possibly characterizes also other neurodegenerative diseases such as AD (Delazer et al., 2021), we speculate that future handwriting analysis would help to assess the phenoconversion from physiologic aging into neurodegenerative disorders (Letanneux et al., 2014).
Relevant new findings also came from our machine learning analysis which allowed us to achieve high accuracy obtained when discriminating handwriting samples collected from YA and OA. This finding, which has been achieved objectively and automatically, strongly supports the hypothesis of a relevant detrimental effect of human aging on handwriting samples. Further relevant information on age-related changes in handwriting came from the high accuracy obtained when discriminating between handwriting samples from MA and OA. Given that our cohort of MA aged from 37 to 57 years, whereas the group of OA ranged from 62 to 90 years, our results would raise the intriguing hypothesis that handwriting prominently declines at the age of about 60 years. These findings agree with a previous study based on standard handwriting analysis which has shown a consistent deterioration of handwriting skills starting from 60 years of age (Verwey et al., 2011). This would reflect a relevant cognitive workload associated with a global reduced sensory processing performance leading to a prominent deterioration of handwriting skills in the elderly of similar age ranges (Engel-Yeger et al., 2012).
A rather unexpected finding of our study consists of the high accuracy achieved by machine learning when classifying between handwriting collected in YA and MA. Given that our cohort of YA ranged from 18 to 32 years, whereas MA aged from 37 to 57 years, our results would suggest early age-related changes in handwriting skills. The differences here observed when comparing handwriting between YA and MA would reflect several possible mechanisms. A first hypothesis concerns a putative early deterioration of brain networks responsible for complex motor tasks at about 35 years of age. Such a hypothesis would receive support from the observation that since the mid-twenties, complex motor, as well as cognitive functions progressively, decline (Berghuis et al., 2019; Stojan and Voelcker-Rehage, 2021). Given that brain networks regulating handwriting partially overlap with those controlling abstract thinking or complex motor planning, we believe that handwriting deteriorates in MA as a result of the relentless phenomenon of aging (Fraser et al., 2009). An alternative hypothesis would imply non-biological mechanisms, including social issues. Our group of YA consists of participants who belong to the so-called “millennial generation” and are possibly characterized by social- and cultural-driven changes in writing habits. The “millennial generation” indeed concerns teenagers and younger adults born since 1985 and refers to people that grew up in the internet age. These youngers largely use computers and other technological devices for writing their academic essays from the early school classes and for communicating on social media. Hence, instead of traditional writing, they prefer typing on a clipboard. Accordingly, it can be argued that this recently acquired social habit would impact significantly on handwriting skills in YA. Future studies could confirm our speculation on social-related changes in handwriting patterns in the “millennial generation” (Dressler et al., 2018).
A final consideration concerns the real-life setting of our experimental design. Indeed, we here report the first handwriting analysis based on machine learning to apply homemade recordings of handwriting samples. The high significance of the results achieved in such an ecologic scenario discloses plenty of future perspectives, including social distancing and disparities in the access to care (Chirra et al., 2019). Therefore, we suggest that future telemedicine studies on handwriting analysis in physiologic and pathologic aging should be based on the examination of their impact on daily activities, to improve daily performance and quality of life (Marsili et al., 2018; Marsili and Mahajan, 2022). Lastly, our study would also be useful to develop a methodology based on the automatic machine learning analysis of handwriting according to age. For instance, an automatic technique able to recognize age-related features of signatures would be applied by authorities to discriminate against authentic wills in heritage disputes or for dating documents and paintings.
We acknowledge that our study has potential limitations. Our sample size as well as the number of handwriting samples collected in healthy subjects would be considered relatively small. However, the accuracy achieved in the classification of handwriting in the three cohorts of healthy subjects was remarkable pointing to the reliability of our machine learning analysis. Also, given that we have not recorded handwriting samples serially in each participant, our study does not allow us to reach conclusions about the intrasubject variability in handwriting skills. Furthermore, we cannot fully exclude that the decreased weight and BMI observed in YA, and the decreased height observed in OA subjects would have contributed at least in part to our findings.
In this study, by using an advanced handwriting analysis based on machine learning algorithms, we have objectively and automatically demonstrated that handwriting undergoes significant age-related changes. This would reflect age-related changes in the activity of cortico-subcortical components of the writing network. The high accuracy of our ROC curves analysis suggests that handwriting is a simple task that can be reliably used in a real-life setting for telemedicine purposes (Engel-Yeger et al., 2012; Canevelli et al., 2014; Camicioli et al., 2015; Marzinotto et al., 2016). Hence, we believe that our study provides the background for future applications in the field of telemedicine in patients with neurodegenerative disorders, including PD and AD. Also, we speculate that future investigations would benefit from concurrent recording and analysis of handwriting and other motor tasks (i.e., finger tapping) to objectively assess bradykinesia of the upper limbs in the elders as well as in patients with neurodegenerative disorders.
All clinical and instrumental data are stored offline and are available on reasonable request to the corresponding author.
The studies involving human participants were reviewed and approved by the IRB of the IRCCS Neuromed Institute. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
FA, SS, and AS: research project (conception). FA, SS, and LT: research project (organization). FA, AZ, VDO, LT, MP, and MF: research project (execution). FA, SS, LT, and AS: statistical analysis (design and execution). SS and AS: statistical analysis (review and critique). FA, LT and LM: manuscript preparation (writing of the first draft). SS and AS: manuscript preparation (review and critique). All authors contributed to the article and approved the submitted version.
LM has received honoraria from the International Association of Parkinsonism and Related Disorders (IAPRD) Society for social media and web support.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Alexander, M. P., Fischer, R. S., and Friedman, R. (1992a). Lesion localization in apractic agraphia. Arch. Neurol. 49, 246–251. doi: 10.1001/archneur.1992.00530270060019
Alexander, M. P., Friedman, R. B., Loverso, F., and Fischer, R. S. (1992b). Lesion localization of phonological agraphia. Brain Lang. 43, 83–95. doi: 10.1016/0093-934x(92)90022-7
Al-Maadeed, S., Ferjani, F., Elloumi, S., Hassaine, A., and Jaoua, A. (2013). “Automatic handedness detection from off-line handwriting,” in Proceedings of the 2013 7th IEEE GCC Conference and Exhibition (GCC). (Doha), 119–124. doi: 10.1109/IEEEGCC.2013.6705761
Anderson, S. W., Damasio, A. R., and Damasio, H. (1990). Troubled letters but not numbers, Domain specific cognitive impairments following focal damage in frontal cortex. Brain 113(Pt 3), 749–766. doi: 10.1093/brain/113.3.749
Armstrong, M. J., and Okun, M. S. (2020). Diagnosis and treatment of Parkinson disease: a review. JAMA 323, 548–560. doi: 10.1001/jama.2019.22360
Asselborn, T., Chapatte, M., and Dillenbourg, P. (2020). Extending the spectrum of dysgraphia: a data driven strategy to estimate handwriting quality. Sci. Rep. 10:3140. doi: 10.1038/s41598-020-60011-8
Auerbach, S. H., and Alexander, M. P. (1981). Pure agraphia and unilateral optic ataxia associated with a left superior parietal lobule lesion. J. Neurol. Neurosurg. Psychiatry 44, 430–432. doi: 10.1136/jnnp.44.5.430
Baldo, J. V., Kacinik, N., Ludy, C., Paulraj, S., Moncrief, A., Piai, V., et al. (2018). Voxel-based lesion analysis of brain regions underlying reading and writing. Neuropsychologia 115, 51–59. doi: 10.1016/j.neuropsychologia.2018.03.021
Bartoň, M., Fňašková, M., Rektorová, I., Mikl, M., Mareček, R., Rapcsak, S. Z., et al. (2020). The role of the striatum in visuomotor integration during handwriting: an fMRI study. J. Neural Transm. (Vienna) 127, 331–337. doi: 10.1007/s00702-019-02131-8
Basavaraja, V., Shivakumara, P., Guru, D. S., Pal, U., Lu, T., and Blumenstein, M. (2019). “Age estimation using disconnectedness features in handwriting,” in Proceedings of the 2019 International Conference on Document Analysis and Recognition (ICDAR). (Sydney, NSW), 1131–1136. doi: 10.1109/ICDAR.2019.00183
Bengio, Y., Lecun, Y., and Hinton, G. (2021). Deep learning for AI. Commun. ACM 64, 58–65. doi: 10.1145/3448250
Berghuis, K. M. M., Fagioli, S., Maurits, N. M., Zijdewind, I., Marsman, J. B. C., Hortobágyi, T., et al. (2019). Age-related changes in brain deactivation but not in activation after motor learning. Neuroimage 186, 358–368. doi: 10.1016/j.neuroimage.2018.11.010
Burger, D. K., and McCluskey, A. (2011). Australian norms for handwriting speed in healthy adults aged 60-99 years. Aust. Occup. Ther. J. 58, 355–363. doi: 10.1111/j.1440-1630.2011.00955.x
Caligiuri, M. P., Kim, C., and Landy, K. M. (2014). Kinematics of signature writing in healthy aging. J. Forensic Sci. 59, 1020–1024. doi: 10.1111/1556-4029.12437
Camicioli, R., Mizrahi, S., Spagnoli, J., Büla, C., Demonet, J.-F., Vingerhoets, F., et al. (2015). Handwriting and pre-frailty in the Lausanne cohort 65+ (Lc65+) study. Arch. Gerontol. Geriatr. 61, 8–13. doi: 10.1016/j.archger.2015.01.006
Canevelli, M., and Marsili, L. (2022). “Ageing of the brain,” in Pathy’s Principles and Practice of Geriatric Medicine, eds A. J. Sinclair, J. E. Morley, B. Vellas, M. Cesari, and M. Munshi (Hoboken, NJ: Wiley), 68–76. doi: 10.1002/9781119484288.ch6
Canevelli, M., Troili, F., and Bruno, G. (2014). Reasoning about frailty in neurology: neurobiological correlates and clinical perspectives. J. Frailty Aging 3, 18–20. doi: 10.14283/jfa.2014.4
Cao, D., Zhong, Y., Wang, L., He, Y., and Dang, J. (2020). Scene text detection in natural images: a review. Symmetry 12:1956. doi: 10.3390/sym12121956
Chen, H., Pan, X., Bickerton, W.-L., Lau, J. K., Zhou, J., Zhou, B., et al. (2019). Delineating the cognitive-neural substrates of writing: a large scale behavioral and voxel based morphometry study. Sci. Rep. 9:18881. doi: 10.1038/s41598-019-55129-3
Chirra, M., Marsili, L., Wattley, L., Sokol, L. L., Keeling, E., Maule, S., et al. (2019). Telemedicine in neurological disorders: opportunities and challenges. Telemed. J. E Health 25, 541–550. doi: 10.1089/tmj.2018.0101
Contreras-Vidal, J. L., Teulings, H. L., Stelmach, G. E., and Adler, C. H. (2002). Adaptation to changes in vertical display gain during handwriting in Parkinson’s disease patients, elderly and young controls. Parkinsonism Relat. Disord. 9, 77–84. doi: 10.1016/s1353-8020(02)00013-5
de la Torre, J., Puig, D., and Valls, A. (2018). Weighted kappa loss function for multi-class classification of ordinal data in deep learning. Pattern Recogn. Lett. 105, 144–154. doi: 10.1016/j.patrec.2017.05.018
de Leeuw, F. E., de Groot, J. C., Achten, E., Oudkerk, M., Ramos, L. M., Heijboer, R., et al. (2001). Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J. Neurol. Neurosurg. Psychiatry 70, 9–14. doi: 10.1136/jnnp.70.1.9
Delazer, M., Zamarian, L., and Djamshidian, A. (2021). Handwriting in Alzheimer’s disease. JAD 82, 727–735. doi: 10.3233/JAD-210279
Deo, R. C. (2015). Machine learning in medicine. Circulation 132, 1920–1930. doi: 10.1161/CIRCULATIONAHA.115.001593
Dixon, R. A., Kurzman, D., and Friesen, I. C. (1993). Handwriting performance in younger and older adults: age, familiarity, and practice effects. Psychol. Aging 8, 360–370. doi: 10.1037//0882-7974.8.3.360
Dressler, J. A., Ryder, B. A., Connolly, M., Blais, M. D., Miner, T. J., and Harrington, D. T. (2018). “Tweet”-format writing is an effective tool for medical student reflection. J. Surg. Educ. 75, 1206–1210. doi: 10.1016/j.jsurg.2018.03.002
Engel-Yeger, B., Hus, S., and Rosenblum, S. (2012). Age effects on sensory-processing abilities and their impact on handwriting. Can. J. Occup. Ther. 79, 264–274. doi: 10.2182/CJOT.2012.79.5.2
Feigin, V. L., Vos, T., Nichols, E., Owolabi, M. O., Carroll, W. M., Dichgans, M., et al. (2020). The global burden of neurological disorders: translating evidence into policy. Lancet Neurol. 19, 255–265. doi: 10.1016/S1474-4422(19)30411-9
Folstein, M. F., Folstein, S. E., and McHugh, P. R. (1975). Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 12, 189–198.
Forbes, K. E., Shanks, M. F., and Venneri, A. (2004). The evolution of dysgraphia in Alzheimer’s disease. Brain Res. Bull. 63, 19–24. doi: 10.1016/j.brainresbull.2003.11.005
Fraser, S. A., Li, K. Z. H., and Penhune, V. B. (2009). A comparison of motor skill learning and retention in younger and older adults. Exp. Brain Res. 195, 419–427. doi: 10.1007/s00221-009-1806-5
Guo, H., Wan, J., Wang, H., Wu, H., Xu, C., Miao, L., et al. (2021). Self-powered intelligent human-machine interaction for handwriting recognition. Research (Wash D C) 2021:4689869. doi: 10.34133/2021/4689869
Hendrycks, D., and Gimpel, K. (2016). “Bridging nonlinearities and stochastic regularizers with Gaussian error linear units,” in Proceedings of the 2017 International Conference on Learning Representations, April 24–26, Toulon.
Hilton, O. (1977). Influence of age and illness of handwriting: identification problems. Forensic Sci. 9, 616–672.
Hirsiger, S., Koppelmans, V., Mérillat, S., Erdin, C., Narkhede, A., Brickman, A. M., et al. (2017). Executive functions in healthy older adults are differentially related to macro- and microstructural white matter characteristics of the cerebral lobes. Front. Aging Neurosci. 9:373. doi: 10.3389/fnagi.2017.00373
Illouz, E., (Omid) David, E., and Netanyahu, N. S. (2018). “Handwriting-based gender classification using end-to-end deep neural networks,” in Artificial Neural Networks and Machine Learning – ICANN 2018. Lecture Notes in Computer Science, eds V. Kůrková, Y. Manolopoulos, B. Hammer, L. Iliadis, and I. Maglogiannis (Cham: Springer International Publishing), 613–621. doi: 10.1007/978-3-030-01424-7_60
Kanno, S., Shinohara, M., Kanno, K., Gomi, Y., Uchiyama, M., Nishio, Y., et al. (2020). Neural substrates underlying progressive micrographia in Parkinson’s disease. Brain Behav. 10:e01669. doi: 10.1002/brb3.1669
Kim, E.-J., Lee, B. H., Park, K. C., Lee, W. Y., and Na, D. L. (2005). Micrographia on free writing versus copying tasks in idiopathic Parkinson’s disease. Parkinsonism Relat. Disord. 11, 57–63. doi: 10.1016/j.parkreldis.2004.08.005
Kim, S., and Melhem, E. R. (2008). Does diffusion-tensor MR imaging provide accurate tracing of specific white matter tracts that correspond to actual anatomic and functional units in the central nervous system? Radiology 249, 725–727. doi: 10.1148/radiol.2493081531
Lemos, N., Shah, K., Rade, R., and Shah, D. (2018). “Personality prediction based on handwriting using machine learning,” in Proceedings of the 2018 International Conference on Computational Techniques, Electronics and Mechanical Systems (CTEMS). (Belgaum), 110–113. doi: 10.1109/CTEMS.2018.8769221
Letanneux, A., Danna, J., Velay, J.-L., Viallet, F., and Pinto, S. (2014). From micrographia to Parkinson’s disease dysgraphia: Parkinson’s disease dysgraphia. Mov. Disord. 29, 1467–1475. doi: 10.1002/mds.25990
Lubrano, V., Roux, F.-E., and Démonet, J.-F. (2004). Writing-specific sites in frontal areas: a cortical stimulation study. J. Neurosurg. 101, 787–798. doi: 10.3171/jns.2004.101.5.0787
Marsili, L., and Mahajan, A. (2022). Clinical milestones in Parkinson’s disease: past, present, and future. J. Neurol. Sci. 432:120082. doi: 10.1016/j.jns.2021.120082
Marsili, L., Espay, A. J., and Merola, A. (2018). Future of neurologic examination in clinical practice. JAMA Neurol. 75:383. doi: 10.1001/jamaneurol.2017.4998
Marzinotto, G., Rosales, J. C., El-Yacoubi, M. A., Garcia-Salicetti, S., Kahindo, C., Kerhervé, H., et al. (2016). Age-related evolution patterns in online handwriting. Comput. Math. Methods Med. 2016:3246595. doi: 10.1155/2016/3246595
Menon, V., and Desmond, J. E. (2001). Left superior parietal cortex involvement in writing: integrating fMRI with lesion evidence. Brain Res. Cogn. Brain Res. 12, 337–340. doi: 10.1016/s0926-6410(01)00063-5
Pang, S., and Yang, X. (2016). Deep convolutional extreme learning machine and its application in handwritten digit classification. Comput. Intell. Neurosci. 2016:3049632. doi: 10.1155/2016/3049632
Pei, L., and Ouyang, G. (2021). Online recognition of handwritten characters from scalp-recorded brain activities during handwriting. J. Neural Eng. 18. doi: 10.1088/1741-2552/ac01a0
Petry, N. M. (2002). A comparison of young, middle-aged, and older adult treatment-seeking pathological gamblers. Gerontologist 42, 92–99. doi: 10.1093/geront/42.1.92
Plamondon, R., O’Reilly, C., Rémi, C., and Duval, T. (2013). The lognormal handwriter: learning, performing, and declining. Front. Psychol. 4:945. doi: 10.3389/fpsyg.2013.00945
Planton, S., Jucla, M., Roux, F.-E., and Démonet, J.-F. (2013). The “handwriting brain”: a meta-analysis of neuroimaging studies of motor versus orthographic processes. Cortex 49, 2772–2787. doi: 10.1016/j.cortex.2013.05.011
Planton, S., Longcamp, M., Péran, P., Démonet, J.-F., and Jucla, M. (2017). How specialized are writing-specific brain regions? An fMRI study of writing, drawing and oral spelling. Cortex 88, 66–80. doi: 10.1016/j.cortex.2016.11.018
Purcell, J. J., Turkeltaub, P. E., Eden, G. F., and Rapp, B. (2011). Examining the central and peripheral processes of written word production through meta-analysis. Front. Psychol. 2:239. doi: 10.3389/fpsyg.2011.00239
Rapcsak, S. Z., Arthur, S. A., and Rubens, A. B. (1988). Lexical agraphia from focal lesion of the left precentral gyrus. Neurology 38, 1119–1123. doi: 10.1212/wnl.38.7.1119
Rapcsak, S. Z., Beeson, P. M., Henry, M. L., Leyden, A., Kim, E., Rising, K., et al. (2009). Phonological dyslexia and dysgraphia: cognitive mechanisms and neural substrates. Cortex 45, 575–591. doi: 10.1016/j.cortex.2008.04.006
Rodríguez-Aranda, C. (2003). Reduced writing and reading speed and age-related changes in verbal fluency tasks. Clin. Neuropsychol. 17, 203–215. doi: 10.1076/clin.17.2.203.16508
Roeltgen, D. P., and Heilman, K. M. (1984). Lexical agraphia. Further support for the two-system hypothesis of linguistic agraphia. Brain 107(Pt 3), 811–827. doi: 10.1093/brain/107.3.811
Rosenblum, S., and Werner, P. (2006). Assessing the handwriting process in healthy elderly persons using a computerized system. Aging Clin. Exp. Res. 18, 433–439. doi: 10.1007/BF03324840
Rosenblum, S., Engel-Yeger, B., and Fogel, Y. (2013). Reprint of “Age-related changes in executive control and their relationships with activity performance in handwriting.”. Hum. Mov. Sci. 32, 1056–1069. doi: 10.1016/j.humov.2013.08.001
Schott, J. M. (2017). The neurology of ageing: what is normal? Pract. Neurol. 17, 172–182. doi: 10.1136/practneurol-2016-001566
Schulz, M., Mayer, C., Schlemm, E., Frey, B. M., Malherbe, C., Petersen, M., et al. (2022). Association of age and structural brain changes with functional connectivity and executive function in a middle-aged to older population-based cohort. Front. Aging Neurosci. 14:782738. doi: 10.3389/fnagi.2022.782738
Slavin, M. J., Phillips, J. G., and Bradshaw, J. L. (1996). Visual cues and the handwriting of older adults: a kinematic analysis. Psychol. Aging 11, 521–526. doi: 10.1037//0882-7974.11.3.521
Srihari, S. N., Cha, S.-H, Arora, H., and Lee, S. (2001). “Individuality of handwriting: a validation study,” in Proceedings of the 6th International Conference on Document Analysis and Recognition, (Seattle, WA), 106–109. doi: 10.1109/ICDAR.2001.953764
Stojan, R., and Voelcker-Rehage, C. (2021). Neurophysiological correlates of age differences in driving behavior during concurrent subtask performance. Neuroimage 225:117492. doi: 10.1016/j.neuroimage.2020.117492
Teulings, H. L., Contreras-Vidal, J. L., Stelmach, G. E., and Adler, C. H. (2002). Adaptation of handwriting size under distorted visual feedback in patients with Parkinson’s disease and elderly and young controls. J. Neurol. Neurosurg. Psychiatry 72, 315–324. doi: 10.1136/jnnp.72.3.315
Valdovinos, B. Y., Modica, J. S., and Schneider, R. B. (2022). Moving forward from the COVID-19 pandemic: needed changes in movement disorders care and research. Curr. Neurol. Neurosci. Rep. 22, 113–122. doi: 10.1007/s11910-022-01178-7
Verwey, W. B., Abrahamse, E. L., Ruitenberg, M. F. L., Jiménez, L., and de Kleine, E. (2011). Motor skill learning in the middle-aged: limited development of motor chunks and explicit sequence knowledge. Psychol. Res. 75, 406–422. doi: 10.1007/s00426-011-0320-0
Walhovd, K. B., Westlye, L. T., Amlien, I., Espeseth, T., Reinvang, I., Raz, N., et al. (2011). Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol. Aging 32, 916–932. doi: 10.1016/j.neurobiolaging.2009.05.013
Walton, J. (1997). Handwriting changes due to aging and Parkinson’s syndrome. Forensic Sci. Int. 88, 197–214. doi: 10.1016/s0379-0738(97)00105-9
Wang, R., Li, Z., Cao, J., Chen, T., and Wang, L. (2019). “Convolutional recurrent neural networks for text classification,” in Proceedings of the 2019 International Joint Conference on Neural Networks (IJCNN). (Budapest), 1–6. doi: 10.1109/IJCNN.2019.8852406
Wu, T., Zhang, J., Hallett, M., Feng, T., Hou, Y., and Chan, P. (2016). Neural correlates underlying micrographia in Parkinson’s disease. Brain 139, 144–160. doi: 10.1093/brain/awv319
Wyss-Coray, T. (2016). Ageing, neurodegeneration and brain rejuvenation. Nature 539, 180–186. doi: 10.1038/nature20411
Zham, P., Raghav, S., Kempster, P., Poosapadi Arjunan, S., Wong, K., Nagao, K. J., et al. (2019). Kinematic study of progressive Micrographia in Parkinson’s disease. Front. Neurol. 10:403. doi: 10.3389/fneur.2019.00403
Zhuang, F.-J., Chen, Y., He, W.-B., and Cai, Z.-Y. (2018). Prevalence of white matter hyperintensities increases with age. Neural Regen. Res. 13, 2141–2146. doi: 10.4103/1673-5374.241465
Zouaoui, F., Bouadjenek, N., Nemmour, H., and Chibani, Y. (2017). “Co-training approach for improving age range prediction from handwritten text,” in Proceedings of the 2017 5th International Conference on Electrical Engineering - Boumerdes (ICEE-B), (Boumerdes), 1–5. doi: 10.1109/ICEE-B.2017.8192233
Keywords: handwriting, aging, machine learning, convolutional neural network, telemedicine, smartphone
Citation: Asci F, Scardapane S, Zampogna A, D’Onofrio V, Testa L, Patera M, Falletti M, Marsili L and Suppa A (2022) Handwriting Declines With Human Aging: A Machine Learning Study. Front. Aging Neurosci. 14:889930. doi: 10.3389/fnagi.2022.889930
Received: 04 March 2022; Accepted: 19 April 2022;
Published: 06 May 2022.
Edited by:
Fabio Pilato, Policlinico Universitario Campus Bio-Medico, ItalyReviewed by:
Anna Latorre, University College London, United KingdomCopyright © 2022 Asci, Scardapane, Zampogna, D’Onofrio, Testa, Patera, Falletti, Marsili and Suppa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Antonio Suppa, YW50b25pby5zdXBwYUB1bmlyb21hMS5pdA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.