
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Aging Neurosci. , 29 March 2022
Sec. Cellular and Molecular Mechanisms of Brain-aging
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.888952
This article is part of the Research Topic Mitochondrial Dysfunction in Stroke View all 12 articles
Editorial on the Research Topic
Editorial: Mitochondrial Dysfunction in Stroke
Stroke is one of the main causes of mortality and remains the second leading cause of death worldwide (Chen et al., 2020). The current therapies of tissue plasminogen activator (tPA) thrombosis and mechanical thrombectomy for ischemic stroke are limited by the narrow therapeutic time window (Zhao et al., 2022). Effective pharmacologic treatments for hemorrhagic stroke are lacking. Emerging evidences demonstrate the importance of mitochondria hemostasis in cell survival and the critical role of mitochondrial dysfunction in the stroke pathogenesis (Kaur and Sharma, 2022). The calcium overload, opening of mitochondrial permeability transition pore (mPTP), and excessive generation of reactive oxygen species (ROS) are mitochondrial pathology contributing to neuronal death after stroke (Figure 1). A better understanding of mitochondrial self-regulation mechanisms and its interaction with other intracellular organelles may reveal novel molecular targets of neuroprotection against stroke (Jia et al., 2021).
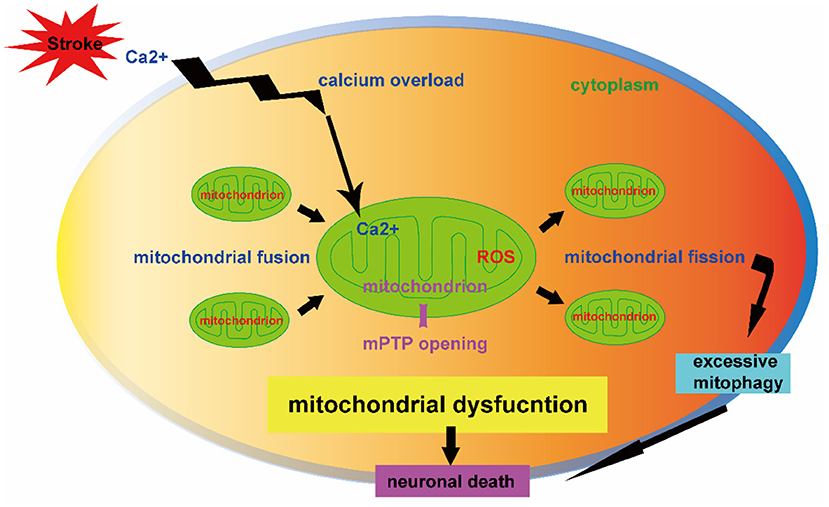
Figure 1. Mitochondrial dysfunction contributing to neuronal death after stroke. The mitochondrial dynamics (fission and fusion) and mitophagy-mediated mitochondrial quality control are key mechanisms in regulation of neuron fate after stroke. The mitochondrial calcium overload, opening of mitochondrial permeability transition pore (mPTP) and over production of reactive oxygen species (ROS) impair mitochondrial dynamics. The effect of mitophagy depends on its injury severity in which overactive mitophagy is detrimental to neuronal survival. The dysregulation of mitochondrial dynamics and excessive mitophagy result in neuronal death.
In this Research Topic of “mitochondrial dysfunction in stroke,” we totally collected 11 articles, majority of which are focusing on the pathophysiology and molecular mechanisms of mitochondrial dysfunction after stroke, as well as potential stroke therapeutics from the mitochondrial perspective.
Mitochondrial dynamics including fusion, fission, selective degradation, and transport processes are important for immunity, apoptosis, and the cell cycle (An et al., 2021; Carinci et al., 2021; Wu et al., 2021; Yang et al., 2022). Zhou et al. reviewed the molecular mechanisms of mitochondrial dynamics and its role in ischemic stroke. The inhibition of excessive mitochondrial fission and preservation of mitochondrial hemostasis are suggested to be potential strategies of neural repair after ischemic stroke.
Mitophagy is a kind of autophagy by specific wipe out of damaged or dysfunctional mitochondria, in order to prevent excessive generation of ROS and neural cell death (He et al., 2021). Mitochondrial fission and mitophagy are two cellular mechanisms that coordinately control mitochondrial quality. In another review article, Shen et al. discussed the involvement of mitochondria dynamics and mitophagy regulation in the pathophysiology of ischemic stroke and ischemic/reperfusion (I/R) injury in particular. The mitophagy-targeted interventions may be potentially applied as an adjunctive therapeutic to extend neuroprotective time window after ischemic stroke. Lei et al.
further reviewed the current research advances of mitophagy regulatory mechanisms after ischemic and hemorrhagic stroke. However, they stressed that the cytoprotective effects of mitophagy modulation in cerebral stroke need further validation by clarifying its possible side effects.
Mitochondrial dynamics can affect energy metabolism and post-stroke neuronal function by regulating the number, morphology, and function of mitochondria. Irisin, a cleaved version of fibronectin domain-containing protein 5 (FNDC5), has been shown to regulate mitochondrial homeostasis. In a mouse model of subarachnoid hemorrhage (SAH), Tu et al. explored the protective effects of irisin and the underlying mechanisms related to mitochondrial biogenesis. The administration of exogenous irisin conserved the mitochondrial morphology and promoted mitochondrial biogenesis, partly through mitochondrial uncoupling protein-2.
Given that early surgical clearance of hematomas does not improve the prognosis in intracerebral hemorrhage (ICH) patients, interventions that attenuate ICH-induced secondary brain injury (SBI) are critical. Chen W et al. summarized the mitochondrial mechanisms in ICH pathology. Abnormal regulation of mitochondrial dynamics that shifts to excessive fission is involved in the pathological process of SBI. Therefore, mitochondrial protection could be a therapeutic target for SBI following ICH.
Damaged cells can produce phosphatidylserine, inducing the tunneling nanotubes (TNTs) formation promoting mitochondrial transfer. Intercellular mitochondrial transfer between different cell types as a potential therapeutic approach has been widely studied (Norat et al., 2020; Gomzikova et al., 2021; Lu et al., 2021). Transient focal cerebral ischemia in mice induced astrocytic mitochondria entry to adjacent neurons that amplified cell survival signals (Hayakawa et al., 2016). Mitochondrial transfer improves functional neuron damage after stroke. Gao et al. evaluated the mitochondria transfer and underlying mechanism involved in the neuron-glia crosstalk in primary cultured mouse cortical neurons subjected to a variety of ischemic related insults. They found that the neuron-derived mitochondria may serve as a “help-me” signal and mediate the neuron-astrocyte crosstalk. Promoting the intercellular mitochondrial transfer by accelerating the neuronal releasing or astrocytic engulfing may serve as a potential therapeutic strategy for the treatment of ischemic stroke in the future.
Mitochondrial dynamics and mitophagy are of great importance in the mitochondrial quantity and quality control. This Research Topic discusses the role of mitochondria in the process of neuronal injury and protection in stroke, aiming to provide valuable insights in aspect of mitochondrial-targeted stroke therapy. Mitochondrial hemostasis preservation, and intracellular mitochondrial transport have a key function in the protection of neuronal injury after experimental stroke, which need future clinical validation in stroke patients. Basic science research is warranted regarding exact interaction mechanism of mitophagy and mitochondria quality control in contribution to pathological or/and protective effect in stroke. The detailed mechanisms of mitochondria release and receptor recognition in donor cells are also needs further investigation in the setting of stroke.
FY and HT wrote the editorial equally. LW draw the picture. LH and JZ revised the editorial. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The guest editors sincerely thank the journal of Frontiers in Aging Neuroscience for providing us the chance to organize the Research Topic. We would like to thank all the authors who contribute to the topic collection and all the reviewers who participate in the review process. Finally, we are very grateful to all the editors for assisting us in manuscripts handling.
An, H, Zhou, B, and Ji, X. Mitochondrial quality control in acute ischemic stroke. J Cereb Blood Flow Metab. (2021) 41:3157–70. doi: 10.1177/0271678X211046992.
Carinci, M, Vezzani, B, Patergnani, S, Ludewig, P, Lessmann, K, Magnus, T, et al Different roles of mitochondria in cell death and inflammation: focusing on mitochondrial quality control in ischemic stroke and reperfusion. Biomedicines. (2021) 9:169. doi: 10.3390/biomedicines9020169.
Chen, W, Huang, J, Hu, Y, Khoshnam, SE, and Sarkaki, A. Mitochondrial transfer as a therapeutic strategy against ischemic stroke. Transl Stroke Res. (2020) 11:1214–28. doi: 10.1007/s12975-020-00828-7.
Gomzikova, MO, James, V, and Rizvanov, AA. Mitochondria donation by mesenchymal stem cells: current understanding and mitochondria transplantation strategies. Front Cell Dev Biol. (2021) 9:653322. doi: 10.3389/fcell.2021.653322.
Hayakawa, K, Esposito, E, Wang, X, Terasaki, Y, Liu, Y, Xing, C, et al. Transfer of mitochondria from astrocytes to neurons after stroke. Nature. (2016) 535:551–5. doi: 10.1038/nature18928.
He, J, Liu, J, Huang, Y, Tang, X, Xiao, H, and Hu, Z. Oxidative stress, inflammation, and autophagy: potential targets of mesenchymal stem cells-based therapies in ischemic stroke. Front Neurosci. (2021) 15:641157. doi: 10.3389/fnins.2021.641157.
Jia, J, Jin, H, Nan, D, Yu, W, and Huang, Y. New insights into targeting mitochondria in ischemic injury. Apoptosis. (2021) 26:163–83. doi: 10.1007/s10495-021-01661-5.
Kaur, MM, and Sharma, DS. Mitochondrial repair as potential pharmacological target in cerebral ischemia. Mitochondrion. (2022) 63:23–31. doi: 10.1016/j.mito.2022.01.001.
Lu, M, Guo, J, Wu, B, Zhou, Y, Wu, M, Farzaneh, M, et al. Mesenchymal stem cell-mediated mitochondrial transfer: a therapeutic approach for ischemic stroke. Transl Stroke Res. (2021) 12:212–29. doi: 10.1007/s12975-020-00853-6.
Norat, P, Soldozy, S, Sokolowski, JD, Gorick, CM, Kumar, JS, Chae, Y, et al. Mitochondrial dysfunction in neurological disorders: Exploring mitochondrial transplantation. NPJ Regen Med. (2020) 5:22. doi: 10.1038/s41536-020-00107-x.
Wu, M, Gu, X, and Ma, Z. Mitochondrial quality control in cerebral ischemia-reperfusion injury. Mol Neurobiol. (2021) 58:5253–71. doi: 10.1007/s12035-021-02494-8.
Yang, M, He, Y, Deng, S, Xiao, L, Tian, M, Xin, Y, et al. Mitochondrial quality control: a pathophysiological mechanism and therapeutic target for stroke. Front Mol Neurosci. (2022) 14:786099. doi: 10.3389/fnmol.2021.786099.
Keywords: mitochondrial dysfunction, stroke, mitochondrial dynamics, mitophagy and apoptosis, mitochondrial transfer
Citation: Yan F, Tang H, Wang L, Huang L and Zhang J (2022) Editorial: Mitochondrial Dysfunction in Stroke. Front. Aging Neurosci. 14:888952. doi: 10.3389/fnagi.2022.888952
Received: 03 March 2022; Accepted: 07 March 2022;
Published: 29 March 2022.
Edited and reviewed by: Jorge Busciglio, University of California, Irvine, United States
Copyright © 2022 Yan, Tang, Wang, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Huang, bGh1YW5nQGxsdS5lZHU=; John Zhang, am9obnpoYW5nMzkxMEB5YWhvby5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.