- 1Department of Internal Medicine, Geriatrics Section, Amsterdam Cardiovascular Science, Amsterdam University Medical Centre, Amsterdam UMC, Amsterdam, Netherlands
- 2Department of Internal Medicine, Amsterdam Public Health Institute, Amsterdam UMC, Amsterdam, Netherlands
- 3Department of Neurology, Alzheimer Center Amsterdam, Amsterdam Neuroscience, VU University Amsterdam, Amsterdam UMC, Amsterdam, Netherlands
- 4Department of Neurology, UMC Utrecht Brain Center, University Medical Center Utrecht, Utrecht, Netherlands
- 5Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands
- 6The George Institute for Global Health, Imperial College London, London, United Kingdom
- 7The George Institute for Global Health, University of New South Wales, Sydney, NSW, Australia
- 8Department of Medical Psychology, Amsterdam University Medical Centers, Amsterdam, Netherlands
Introduction: Globally, women with dementia have a higher disease burden than men with dementia. In addition, women with diabetes especially are at higher risk for cognitive impairment and dementia compared to men with diabetes. Differences in the influence of diabetes on the cerebral vasculature and brain structure may contribute to these sex-specific differences. We examined sex-specific patterns in the relationship between diabetes and brain structure, as well as diabetes and cognitive function.
Methods: In total, 893 patients [age 79 ± 6.6 years, 446 (50%) women] from the Amsterdam Ageing Cohort with available data on brain structures (assessed by an MRI or CT scan) and cognitive function were included. All patients underwent a thorough standardized clinical and neuropsychological assessment (including tests on memory, executive functioning, processing speed, language). Brain structure abnormalities were quantified using visual scales.
Results: Cross-sectional multivariable regression analyses showed that diabetes was associated with increased incidence of cerebral lacunes and brain atrophy in women (OR 2.18 (1.00–4.72) but not in men. Furthermore, diabetes was associated with decreased executive function, processing speed and language in women [B −0.07 (0.00–0.13), −0.06 (0.02–0.10) and −0.07 (0.01–0.12) resp.] but not in men.
Conclusions: Diabetes is related to increased risk of having lacunes, brain atrophy and impaired cognitive function in women but not in men. Further research is required to understand the time trajectory leading up to these changes and to understand the mechanisms behind them in order to improve preventive health care for both sexes.
Introduction
The prevalence of diabetes is increasing worldwide, with an expected rise from 537 million adults in 2021 to 783 million in 2045 (Sun et al., 2021). This not only leads to high mortality – more than 6.7 million deaths in 2021 alone – but also to high morbidity, including an increased risk of cognitive impairment and dementia (Arvanitakis et al., 2004; Yaffe et al., 2004; Liu, J. et al., 2018). However, not all individuals are similarly affected by the complications of diabetes. As early as 1979, and as confirmed more recently by cohort studies, it was shown that type 2 diabetes is a stronger risk factor for ischemic heart disease and stroke in women than in men (Kannel and McGee, 1979; Peters et al., 2014, 2020, 2021). Women with type 2 diabetes also have a higher excess risk of cognitive decline and vascular dementia, than their male counterparts, although the extent of these differences are dependent of study populations and their characteristics (Verhagen et al., 2022).
To date, it is unclear why men and women with diabetes are dissimilarly impacted by dementia. Since sex-related differences in the incidence of dementia are only present in the group with vascular dementia – not in Alzheimer's dementia – sex-specific patterns of cerebral vascular pathology may play a role in mediating these differences (Hayden et al., 2006; Chatterjee et al., 2016; Liu et al., 2018). Cerebral small vessel disease (cSVD) – including the presence of microbleeds, white matter hyperintensities and lacunes – is more prevalent in individuals with type 2 diabetes than those without (Troncoso et al., 2008; Moran et al., 2013; Geijselaers et al., 2015; Ter Telgte et al., 2018; Wardlaw et al., 2019). Although little is known about sex-specific susceptibility for cSVD, it seems plausible that the increased susceptibility of women to cerebrovascular complications of diabetes is manifested as an increased susceptibility to disease of the smaller cerebral vessels (Jiménez-Sánchez et al., 2021). In addition, diabetes is associated with increased rates of atrophy (Moran et al., 2013). Again, it is not known whether there are sex-related differences in this association, but it is known that atrophy and cSVD are closely related, and they are sometimes even collectively referred to as “brain structure” or “markers for brain health” (Mahammedi et al., 2021). Our primary goal in the present analysis is to assess if there are sex-specific pattern in the relationship between diabetes and brain structure, including cSVD and atrophy, and cognitive function as their clinical correlates.
Methods
Study Population
The Amsterdam Aging Cohort is an ongoing longitudinal cohort study which includes patients from the outpatient geriatric clinic at the Amsterdam University Medical Center, location VUmc (Rhodius-Meester et al., 2021). We included 893 patients with brain imaging who attended the memory clinic seeking medical care between February 2016 and June 2021. During this period, almost a patients visiting the memory clinic (89%) were willing to participate in the study. All patients were given a complete standardized comprehensive geriatric assessment (CGA) by trained nurses and doctors. This included an assessment of multiple geriatric domains, including cognition, physical function, nutrition, revision of medication in use and detailed medical history. Cognitive diagnosis – such as dementia (McKhann et al., 1984; Román et al., 1993; Neary et al., 1998; McKeith et al., 2005; Dubois et al., 2007; Rascovsky et al., 2011), mild cognitive impairment (MCI) (Albert et al., 2013), or subjective cognitive decline (SCD) (Studart and Nitrini, 2016) – were evaluated in a multidisciplinary consensus meeting. Our analysis included only patients who underwent brain Magnetic Resonance Imaging (MRI) or Computed Tomography (CT) as part of the diagnostic work-up. All patients gave written informed consent for their data to be used and the study was approved by the local Medical Ethics Committee.
Cardiovascular Risk and Disease
Diabetes mellitus (DM) was defined either as having a history of diabetes or using antidiabetic medication. Other cardiovascular diseases – including coronary disease, heart failure, atrial fibrillation, and peripheral artery disease – were assessed on the basis of medical history and double checked with the patient and their family or carer. We dichotomized smoking status (never smoked v. ever smoked). Blood pressure and gait speed, as well as patient height and weight, were measured during the visit (Odden et al., 2012). Venous blood was drawn from all patients to measure cholesterol levels and non-fasting glucose. Medication as provided by the patient's pharmacy was reviewed with the patient, and with a partner, family member or carer if necessary.
Cerebral Small Vessel Disease
Brain imaging was performed during a patient's first visit using CT (n = 238), 1.5T MRI (n = 162) and 3T MRI (n = 478) devices. The scans were reviewed and scored visually by two trained experts supervised by a clinical radiologist. Atrophy was scored on T1 sequence using visual rating scales ranging from 0 to 4 for medial temporal lobe atrophy (MTA), and from 0 to 3 for global cortical atrophy (GCA) (Harper et al., 2015). The average of the left and right side was used for MTA. White matter hyperintensities were scored on FLAIR/T2 sequence using the Fazekas scale (0–3) (Fazekas et al., 1987), and the number of microbleeds (on susceptibility-weighted imaging) and lacunes were counted. In this manuscript, we refer to either brain atrophy, white matter hyperintensities, lacunes or microbleeds collectively as “brain structure abnormalities.”
Cognitive Function
To assess whether the observed differences in brain structure also had a functional impact, cognition was included in the analysis. Cognitive performance was assessed in a standardized manner by trained neuropsychologists and divided into four domains: memory, language, executive function, and processing speed. All patients were assessed using the Mini Mental State Examination (MMSE) and the Geriatric Depression Scale (GDS). Memory was tested with the auditory verbal learning test (Van Der Elst et al., 2005) and Visual Association Test (VAT) (Lindeboom et al., 2002). Language was tested using the Category Fluency Animals Test (Van Der Elst et al., 2006) and the VAT naming test, a component of the VAT. Processing speed was examined with the Stroop Color-Word test (SCWT) (Van der Elst et al., 2006) and the Trail Making Test-A (TMT-A) (Reitan, 1955). Finally, executive function was assessed with the Behavioral Assessment of the Dysexecutive Rule-changing test (BADS) (Burrell and Piguet, 2015) while correcting for speed using the SCWT and the TMT. For the purposes of the analysis, all test results were converted to Z-scores or inverse Z-scores. A higher Z-score indicates poorer performance.
Statistical Analysis
Baseline characteristics for men, women, and the total population are reported as mean (SD), or median (interquartile range) for categorical variables. Differences between groups were analyzed using Student's T-test, the Mann-Whitney U-test, the Kruskal Wallis test, ANOVA and chi-square testing where appropriate. First, logistic regression analyses were performed to assess the association of diabetes with brain structures separately for men and women. For the logistic regression analysis, we dichotomized the scores of the visual rating scale and the values for microbleeds and lacunes. A cut-off value of two or more was used for the imaging scores of atrophy (MTA and/or CGA) and WMH (Rhodius-Meester et al., 2017). Microbleeds were dichotomized as present or not present, and a value of one or more was adopted a cut-off for lacunes (Henneman et al., 2009; Jokinen et al., 2011). Second, linear regression analyses were performed to assess the association of diabetes with cognitive functioning separately for men and women. All analyses were adjusted for age (model 1), and additionally for smoking and alcohol consumption (model 2), and hypertension and cardiovascular disease (coronary disease, heart failure, atrial fibrillation, stroke or TIA and peripheral arterial disease) (model 3). In addition, we adjusted for presence of subjective complaints, mild cognitive impairment, or dementia (data not shown). For the analyses of functional cognitive measures, furthermore, we corrected all models for level of education. A p-value < 0.05 was considered statistically significant. Data were analyzed with SPSS software, version 26 (IBM Corp, Armonk, NY, USA).
To determine whether male or female sex and CVD was associated with a higher risk of cSVD to a greater degree than these factors individually, we added an interaction term to the regression analysis, testing multiplicative interaction. To assess additive interaction, we calculated RERI (Relative Risk due to Interaction) (Knol et al., 2007; Knol and VanderWeele, 2012). For this analysis, when the combined risk of sex and CVD was higher than the sum of the risks associated with the individual factors, the interaction between sex and CVD was considered to constitute an additional risk factor. A RERI above zero indicated that interaction between female sex and cardiac disease had an additional effect on the outcome; a RERI below zero indicated that this was the case for the interaction between male sex and cardiac disease. In the RERI analysis, we also corrected for age, smoking, and alcohol consumption, analogous to the logistic regression analyses. These analyses were performed in R (R Core Team, 2020).
Results
A total of 893 patients were included in the analysis (Table 1). The mean (SD) age was 79.6 years (73–86.2) and 50% were women. The prevalence of diabetes was 23.3% in men and 15.7% in women (p = 0.004), and men with diabetes were more often insulin-dependent compared to women (6.3% for men, 3.1% for women, p = 0.03). Women lived alone more often than men and their level of education was lower. Further, women had a lower prevalence of cardiovascular disease, consumed less alcohol, smoked less, had a lower body mass index (BMI) and a slightly higher diastolic BP, and they used less statins and anticoagulation drugs (Table 1). Women had slightly lower Mini-Mental State Examination scores, GDS, and men had higher brain atrophy scores. No differences in cognitive diagnosis were observed between men and women. Stratified analyses for sex and diabetes showed that differences in cardiovascular risk between men and women were more pronounced in those with diabetes compared to the total population (Supplementary Table 1).
Sex Differences in the Relationship Between Diabetes and Brain Structure
The sex-specific logistic regression analyses of the relation between diabetes and brain structures showed that in women, the presence of diabetes was significantly associated with an increased risk of having brain atrophy and lacunes (Table 2). Age-adjusted odds ratios were 2.16 (95% CI 1.00–4.67) and 2.60 (95% CI 1.24–2.46). However, in men, diabetes was not associated with an increased risk of having brain structure abnormalities. Additional adjustments for cardiovascular risk factors and disease (model 2 and 3) did not change these effect estimates (Table 2). Adjusting for cognitive diagnosis (subjective complaints, mild cognitive impairment, or dementia) did not change the effect estimates (data not shown). Diabetes was not associated with an increased risk of WMH and microbleeds. When adding an interaction term to the regression, we did not find a significant interaction of sex with diabetes. When assessing additive interaction using RERI analysis, a trend was seen toward an increased risk for women with diabetes of atrophy and lacunes (RERI 0.45 for the presence of atrophy and female sex, and 0.48 for the presence of lacunes and female sex) (Supplementary Table 2).
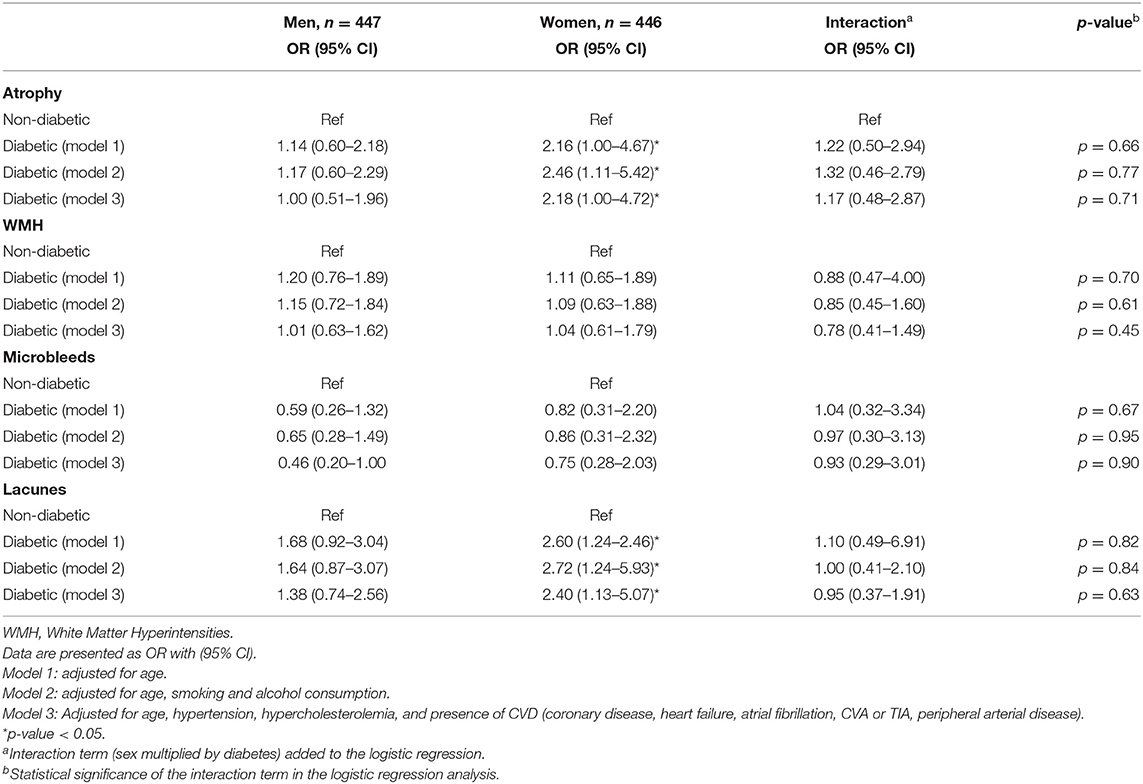
Table 2. The sex-specific relation of diabetes with changes in brain structure in older men and women (N = 893).
Sex Differences in the Relationship Between Diabetes and Cognitive Performance
The sex-specific linear regression analyses of the relation between diabetes and cognitive performance showed that diabetes in women was associated with a significantly lower score of executive function (beta z-score 0.07; 95% CI 0.00–0.14), processing speed (beta 0.06; 95% CI 0.90–0.95), and language (beta 0.07; 95% CI 0.01–0.12) (Table 3). In men, diabetes was not associated with cognitive performance. Additional adjustments for cardiovascular risk and disease, and cognitive diagnosis did not change the effect estimates (data for cognitive diagnosis not shown). We observed an interaction of sex and diabetes: women with diabetes were at increased risk for impaired processing speed (B 0.17 (0.03–0.33), p = 0.04). We observed no interaction between diabetes and sex in the other cognitive domains.
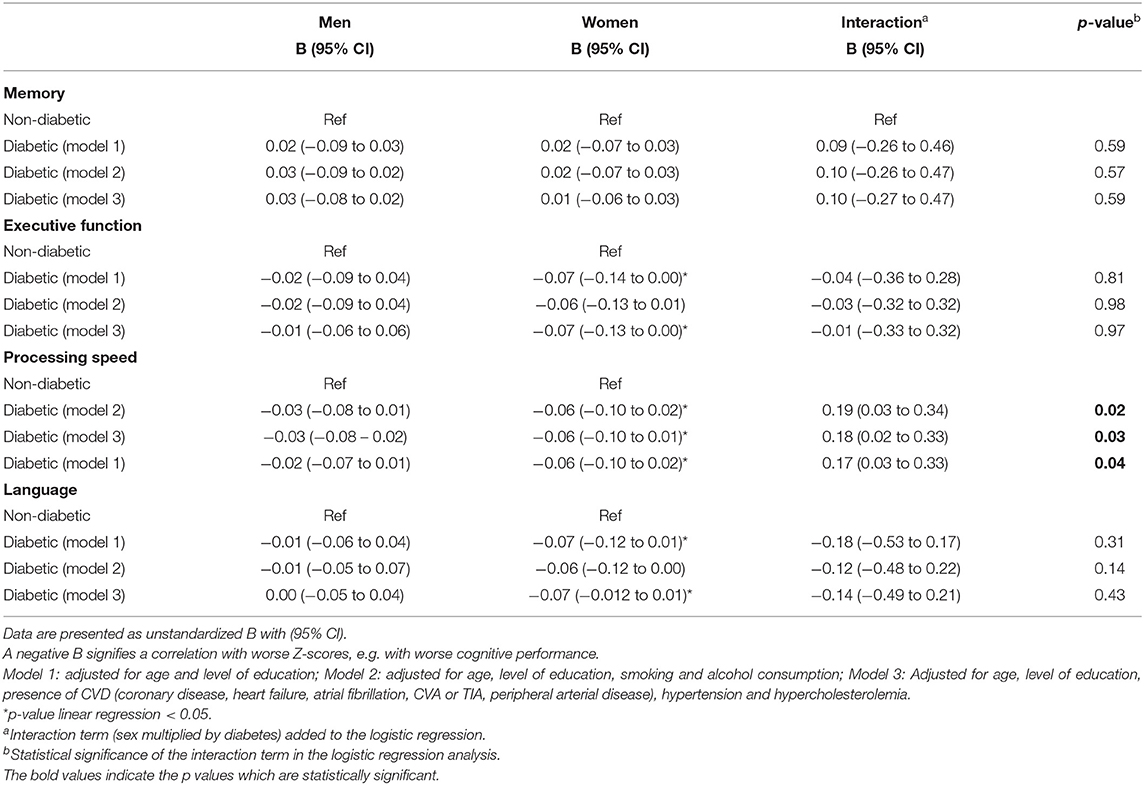
Table 3. The sex specific relation of diabetes with cognitive performance in older men and women (N = 893).
Discussion
In this study of 893 patients attending a geriatric outpatient memory clinic, we found that the presence of diabetes was associated with an increased risk of having brain structure abnormalities, specifically lacunes and atrophy, in women but not in men. We also found an additive interaction between female sex and these brain structure abnormalities, as tested by a RERI analysis. This finding complements previous studies which showed that women with diabetes may be more at risk of multiple forms of vascular pathology than men with diabetes, including coronary heart disease, stroke, and vascular dementia (Huxley et al., 2006; Peters et al., 2014; Chatterjee et al., 2016). It could be argued that other age-mediated cardiovascular risk factors such as hypertension and cardiac disease play a role in mediating the relationship between diabetes and brain structure abnormalities. However, we show that this relationship was independent of age, lifestyle, cardiovascular risk factors, and cardiac disease.
Additionally, we found that diabetes was significantly associated with worse cognitive performance in terms of executive function, processing speed and language, in the women in our population but not in the men. An interaction between sex and diabetes was also observed for processing speed, further strengthening the hypothesis of a true sex difference. These findings are in line with a recent study showing that women with diabetes have a higher risk of accelerated cognitive decline than men with diabetes (Verhagen et al., 2022). Sex-dependent physiology, as well as socio-cultural differences between men and women, may be the cause of these differences. We postulate a number of hypotheses below which may explain the association between diabetes and brain structure abnormalities as it is seen in women but not in men.
Pathophysiological Differences
Mechanisms which may affect susceptibility to the vascular complications of diabetes include altered coagulation, oxidative stress, endothelial dysfunction and impaired vasodilation (Kautzky-Willer et al., 2016; de Ritter et al., 2020). Women with diabetes might be in a more pro-thrombotic state than men, which may lead to lacunes and atrophy, and a more general decline in brain health, even when the prevalence of diabetes is similar in both sexes (Smith et al., 2012; Neergaard-Petersen et al., 2014). They generally also have greater levels of systemic inflammation and more oxidative stress than men with diabetes, leading to impaired vascular reactivity, which is specifically associated with the occurrence of lacunes (Mrgan et al., 2018). Sex-dependent differences in vascular physiology may therefore render women more susceptible to the cerebrovascular complications of diabetes, and also lead to functional decline.
Levels of central adiposity in men and women with diabetes may also be of importance. There is evidence to suggest that women have a poorer cardiovascular risk profile than men when they are diagnosed with diabetes, especially when central adiposity is measured (Paul et al., 2012; Peters et al., 2016a). This may be the result of a longer period of development of diabetes in women: women are more insulin-sensitive in middle age, and their insulin sensitivity deteriorates more than in men before they reach the diagnosis of diabetes. A longer period of time before a formal diagnosis of diabetes can be made may also lead to an increased occurrence of other risk factors such as abdominal adiposity, and to higher levels of subclinical damage mediated by hyperglycemia (Woodward et al., 2015; Peters et al., 2016b). Abdominal adiposity is independently related to brain structure abnormalities, including silent lacunary infarcts, and therefore may mediate the increased prevalence in women with diabetes by comparison with men (Yamashiro et al., 2014).
Furthermore, there is little awareness in the field of geriatrics about the long-term cardiovascular effects of pregnancy-related complications, as well as other women-specific factors such as timing of menopause and gestational diabetes (Keskin et al., 2015; Kuh et al., 2018). These important cardiovascular parameters are therefore often not registered in medical files, as is the case in our dataset (Wilkins-Haug et al., 2015). It has long been known that menopausal status and the timing of menopause influence cardiovascular risk, abdominal obesity, occurrence of DM, and clinical course of dementia (Archer, 2009; Gong et al., 2021; Hickey and Mishra, 2021). They may therefore also have affected the clinical outcome in our study. Further studies investigating the long-term effects of DM should therefore include sex-specific cardiovascular risk factors to assess their impact on brain structure and cognitive function.
Sex-Related Differences in Current Care
As a consequence of ongoing lower inclusion rates of women in studies investigating the long-term effects of diabetes, at least in part, it is unclear which mechanisms lead to the poorer clinical outcome of women with diabetes (Norhammar and Schenck-Gustafsson, 2013). However, there are several scientific findings which may play a role. Social gender norms have a profound influence on patients' disease perception, moment of referral, interpretation of symptoms and the likelihood of receiving guideline-recommended treatment. In the case of diabetes, evidence shows that women diagnosed with diabetes attain glycemic targets less often, and are screened less for the complications of diabetes (Choe et al., 2018). Risk factor targets for co-morbid cardiovascular disease are also less often achieved in women (Ferrara et al., 2008; Wannamethee et al., 2012; Rossi et al., 2013; de Jong et al., 2020). Since early intervention in diabetes improves long-term outcome, this may also have implications for the incidence of (cerebral) complications (Group UPDS, 1998). The same truth holds for cognitive impairment: women with dementia are generally referred later than men (Howard et al., 1998; Sourial et al., 2020). Women might therefore experience delay in receiving adequate supporting care when experiencing cognitive impairment. Hence, inequities in the recognition and treatment of (cardiovascular) risk factors for dementia, as well as the recognition and treatment of dementia itself, may play a role in the occurrence of sex-related differences in the cerebral complications of diabetes.
Strengths and Limitations
A major strength of our study is the standardized work-up of a large group of “real-life” patients. We included a multi-domain assessment which was part of medical routine care. Because of this integration in regular care practice, routinely used measurements and tools were used, facilitating the translation of our research to clinical practice. In addition, we combined these clinically used parameters with imaging markers as well as extensive neuropsychological testing, bridging the gap between etiological research and clinical practice. Our study has also several limitations. Firstly, because of our cross-sectional design, we cannot draw conclusions about the causality of our findings. We balanced this with logistic regression models in which we corrected for confounding factors. Related to this, we have no data showing possible differences between brain structure at the time of diagnosis of diabetes, and later life. It is possible that women with diabetes already have worse brain health at this time, and that it is not a direct consequence of diabetes, but merely coexists due to other pathological processes. Furthermore, HbA1c and time since diagnosis of DM were not included in our dataset. We were therefore unable to assess the influence of glycemic regulation. We did include non-fasted glucose, which was similar for men and women, in our baseline characteristics. Also, it is remarkable that the mean age in our sample is similar for men and women. Since the life expectancy in women exceeds the life expectancy in men, we would expect a higher mean age for women. The similar age for men and women may reflect underlying gender bias in referral to our clinic. However, it may also be explained by other forms of sampling bias not related sex or gender. Furthermore, as mentioned before, women-specific pathology was hardly registered at all in our patient files. A history of gestational diabetes, polycystic ovary syndrome and premature menopause contribute to the excess risk of diabetic complications and diabetes (Soedamah-Muthu et al., 2004; Huxley et al., 2006; Peters et al., 2014). Furthermore, preeclampsia is related to structural brain damage later in life (Siepmann et al., 2017). Lastly, premature menopause is additionally associated with poorer cognitive performance and a higher risk of dementia later in life (Ryan et al., 2014). However, the precise relationship between cerebrovascular disease and pregnancy-related cardiovascular disease remains unclear, since a recent review concluded that gestational hypertension is not related to cerebral stroke (Lo et al., 2020). Finally, other factors that affect the social position of patients and therefore their quality of care – such as class, cultural background, and variables associated with poorer referral and poorer health care provision in general – were not available in our study (Vaccarino et al., 2002; LaVeist et al., 2003).
In conclusion, this sex-specific analysis of the association between diabetes and its cerebrovascular complications shows that diabetes is significantly associated with brain structure abnormalities and function in women but not in men. We can only speculate on the nature of these differences, and whether they are dependent on gender or sex. Although we did not find a statistically significant interaction between female sex and diabetes, the differences in associations for men and women are striking and underline the importance of the sex-specific analysis of clinical data. Further research should at least include data on abdominal obesity and female-specific risk factors such as pregnancy-related complications and menopause, and more studies are needed to elucidate the mechanisms which contribute to the association between diabetes, brain structure and cognition in women. Elucidating the sex-specific relationships between diabetes and cSVD may help to understand the gap in burden of dementia and help to achieve more equity in the care for this group of patients.
Data Availability Statement
The data analyzed in this study is subject to the following licenses/restrictions: The data is not publicly available since it contains clinical privacy-sensitive patient information. Requests to access these datasets should be directed to bWFqb24ubXVsbGVyQGFtc3RlcmRhbXVtYy5ubA==.
Ethics Statement
The studies involving human participants were reviewed and approved by Medical Ethical Committee of the Amsterdam UMC, location VUmc. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Study conception and design and data collection: ET, MM, and HR-M. Analysis and interpretation of the results: ET, MM, HR-M, and SP. Draft manuscript preparation: ET, MM, HR-M, LE, SP, RP, and LB. All authors reviewed the results and approved the final version of the manuscript.
Funding
HR-M was recipient of the Memorabel Dementia Fellowship 2021 (ZonMw project number 10510022110004). Also, we acknowledge the support from the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation (CVON 2018-28 and 2012-06 Heart Brain Connection), Dutch Federation of University Medical Centres, the Netherlands Organisation for Health Research and Development, and the Royal Netherlands Academy of Sciences.
Conflict of Interest
HR-M performs contract research for Combinostics. All funding is paid to her institution.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Dr. S.P. Thomas for his proofreading of the manuscript and perpetual moral support.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.885787/full#supplementary-material
References
Albert, M. S., DeKosky, S. T., Dickson, D., Dubois, B., Feldman, H. H., Fox, N. C., et al. (2013). The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Focus 11, 96–106. doi: 10.1176/appi.focus.11.1.96
Archer, D. (2009). Premature menopause increases cardiovascular risk. Climacteric 12(sup1), 26–31. doi: 10.1080/13697130903013452
Arvanitakis, Z., Wilson, R. S., Bienias, J. L., Evans, D. A., and Bennett, D. A. (2004). Diabetes mellitus and risk of Alzheimer's disease and decline in cognitive function. Arch. Neurol. 61, 661–666. doi: 10.1001/archneur.61.5.661
Burrell, J. R., and Piguet, O. (2015). Lifting the veil: how to use clinical neuropsychology to assess dementia. J. Neurol. Neurosurg. Psychiatry 86, 1216–1224. doi: 10.1136/jnnp-2013-307483
Chatterjee, S., Peters, S. A., Woodward, M., Arango, S. M., Batty, G. D., Beckett, N., et al. (2016). Type 2 diabetes as a risk factor for dementia in women compared with men: a pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 39, 300–307. doi: 10.2337/dc15-1588
Choe, S.-A., Kim, J. Y., Ro, Y. S., and Cho, S.-I. (2018). Women are less likely than men to achieve optimal glycemic control after 1 year of treatment: a multi-level analysis of a Korean primary care cohort. PLoS One 13, e0196719. doi: 10.1371/journal.pone.0196719
de Jong, M., Oskam, M. J., Sep, S. J., Ozcan, B., Rutters, F., Sijbrands, E. J., et al. (2020). Sex differences in cardiometabolic risk factors, pharmacological treatment and risk factor control in type 2 diabetes: findings from the Dutch Diabetes Pearl cohort. BMJ Open Diabetes Res. Care 8, e001365. doi: 10.1136/bmjdrc-2020-001365
de Ritter, R., de Jong, M., Vos, R. C., van der Kallen, C. J., Sep, S. J., Woodward, M., et al. (2020). Sex differences in the risk of vascular disease associated with diabetes. Biol. Sex Differ. 11, 1–11. doi: 10.1186/s13293-019-0277-z
Dubois, B., Feldman, H. H., Jacova, C., DeKosky, S. T., Barberger-Gateau, P., Cummings, J., et al. (2007). Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS–ADRDA criteria. Lancet Neurol. 6, 734–746. doi: 10.1016/S1474-4422(07)70178-3
Fazekas, F., Chawluk, J. B., Alavi, A., Hurtig, H. I., and Zimmerman, R. A. (1987). MR signal abnormalities at 1.5 T in Alzheimer's dementia and normal aging. Am. J. Neuroradiol. 8, 421–426. doi: 10.2214/ajr.149.2.351
Ferrara, A., Mangione, C. M., Kim, C., Marrero, D. G., Curb, D., Stevens, M., et al. (2008). Sex disparities in control and treatment of modifiable cardiovascular disease risk factors among patients with diabetes: Translating Research Into Action for Diabetes (TRIAD) Study. Diabetes Care. 31, 69–74. doi: 10.2337/dc07-1244
Geijselaers, S. L., Sep, S. J., Stehouwer, C. D., and Biessels, G. J. (2015). Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol.. 3, 75–89. doi: 10.1016/S2213-8587(14)70148-2
Gong, J., Harris, K., Peters, S. A., and Woodward, M. (2021). Reproductive factors and the risk of incident dementia: results from the UK Biobank. doi: 10.21203/rs.3.rs-359787/v1
Group UPDS (1998). Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352, 837–853. doi: 10.1016/S0140-6736(98)07019-6
Harper, L., Barkhof, F., Fox, N. C., and Schott, J. M. (2015). Using visual rating to diagnose dementia: a critical evaluation of MRI atrophy scales. J. Neurol. Neurosurg. Psychiatry 86, 1225–1233. doi: 10.1136/jnnp-2014-310090
Hayden, K. M., Zandi, P. P., Lyketsos, C. G., Khachaturian, A. S., Bastian, L. A., Charoonruk, G., et al. (2006). Vascular risk factors for incident Alzheimer's disease and vascular dementia: the Cache County study. Alzheimer's Dis. Assoc. Disord. 20, 93–100. doi: 10.1097/01.wad.0000213814.43047.86
Henneman, W. J., Sluimer, J. D., Cordonnier, C., Baak, M. M., Scheltens, P., Barkhof, F., et al. (2009). MRI biomarkers of vascular damage and atrophy predicting mortality in a memory clinic population. Stroke 40, 492–498. doi: 10.1161/STROKEAHA.108.516286
Hickey, M., and Mishra, G. D. (2021). Timing and type of menopause and risk of cardiovascular disease. Menopause 28, 477–479. doi: 10.1097/GME.0000000000001747
Howard, B. V., Cowan, L. D., Go, O., Welty, T. K., Robbins, D. C., Lee, E. T., et al. (1998). Adverse effects of diabetes on multiple cardiovascular disease risk factors in women: the Strong Heart Study. Diabetes care. 21, 1258–1265. doi: 10.2337/diacare.21.8.1258
Huxley, R., Barzi, F., and Woodward, M. (2006). Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 332, 73–78. doi: 10.1136/bmj.38678.389583.7C
Jiménez-Sánchez, L., Hamilton, O. K., Clancy, U., Backhouse, E. V., Stewart, C. R., Stringer, M. S., et al. (2021). Sex differences in Cerebral Small Vessel Disease: a systematic review and meta-analysis. Front. Neurol. 12, 756887. doi: 10.3389/fneur.2021.756887
Jokinen, H., Gouw, A., Madureira, S., Ylikoski, R., Van Straaten, E., Van Der Flier, W., et al. (2011). Incident lacunes influence cognitive decline: the LADIS study. Neurology. 76, 1872–1878. doi: 10.1212/WNL.0b013e31821d752f
Kannel, W. B., and McGee, D. L. (1979). Diabetes and cardiovascular risk factors: the Framingham study. Circulation. 59, 8–13. doi: 10.1161/01.CIR.59.1.8
Kautzky-Willer, A., Harreiter, J., and Pacini, G. (2016). Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocr. Rev. 37, 278–316. doi: 10.1210/er.2015-1137
Keskin, F., Ozyazar, M., Pala, A., Elmali, A., Yilmaz, B., Uygunoglu, U., et al. (2015). Evaluation of cognitive functions in gestational diabetes mellitus. Exp. Clin. Endocrinol. Diabetes. 123, 246–251. doi: 10.1055/s-0034-1395634
Knol, M. J., van der Tweel, I., Grobbee, D. E., Numans, M. E., and Geerlings, M. I. (2007). Estimating interaction on an additive scale between continuous determinants in a logistic regression model. Int. J. Epidemiol. 36, 1111–1118. doi: 10.1093/ije/dym157
Knol, M. J., and VanderWeele, T. J. (2012). Recommendations for presenting analyses of effect modification and interaction. Int. J Epidemiol. 41, 514–520. doi: 10.1093/ije/dyr218
Kuh, D., Cooper, R., Moore, A., Richards, M., and Hardy, R. (2018). Age at menopause and lifetime cognition: findings from a British birth cohort study. Neurology. 90:e1673–e81. doi: 10.1212/WNL.0000000000005486
LaVeist, T. A., Nuru-Jeter, A., and Jones, K. E. (2003). The association of doctor-patient race concordance with health services utilization. J. Public Health Policy 24, 312–323. doi: 10.2307/3343378
Lindeboom, J., Schmand, B., Tulner, L., Walstra, G., and Jonker, C. (2002). Visual association test to detect early dementia of the Alzheimer's type. J. Neurol. Neurosurg. Psychiatry 73, 126–133. doi: 10.1136/jnnp.73.2.126
Liu, C.-L., Lin, M.-Y., Hwang, S.-J., Liu, C.-K., Lee, H.-L., and Wu, M.-T. (2018). Factors associated with type 2 diabetes in patients with vascular dementia: a population-based cross-sectional study. BMC Endocr. Disord. 18, 1–7. doi: 10.1186/s12902-018-0273-z
Liu, J., Rutten-Jacobs, L., Liu, M., Markus, H. S., and Traylor, M. (2018). Causal impact of type 2 diabetes mellitus on cerebral small vessel disease: a Mendelian randomization analysis. Stroke. 49, 1325–1331. doi: 10.1161/STROKEAHA.117.020536
Lo, C. C. W., Lo, A. C., Leow, S. H., Fisher, G., Corker, B., Batho, O., et al. (2020). Future cardiovascular disease risk for women with gestational hypertension: a systematic review and meta-analysis. J. Am. Heart Assoc. 9, e013991. doi: 10.1161/JAHA.119.013991
Mahammedi, A., Wang, L., Williamson, B., Khatri, P., Kissela, B., Sawyer, R., et al. (2021). Small vessel disease, a marker of brain health: what the radiologist needs to know. Am. J. Neuroradiol. 43, 650–660. doi: 10.3174/ajnr.A7302
McKeith, I. G., Dickson, D. W., Lowe, J., Emre, M., O'brien, J., Feldman, H., et al. (2005). Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65, 1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1
McKhann, G., Drachman, D., Folstein, M., Katzman, R., Price, D., and Stadlan, E. M. (1984). Clinical diagnosis of Alzheimer's disease: Report of the NINCDS-ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 34, 939-. doi: 10.1212/WNL.34.7.939
Moran, C., Phan, T. G., Chen, J., Blizzard, L., Beare, R., Venn, A., et al. (2013). Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 36, 4036–4042. doi: 10.2337/dc13-0143
Mrgan, M., Gram, J., Hecht Olsen, M., Dey, D., Linde Nørgaard, B., Gram, J., et al. (2018). Sex differences in coronary plaque composition evaluated by coronary computed tomography angiography in newly diagnosed Type 2 diabetes: association with low-grade inflammation. Diabetic Med. 35, 1588–1595. doi: 10.1111/dme.13768
Neary, D., Snowden, J. S., Gustafson, L., Passant, U., Stuss, D., Black, S., et al. (1998). Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51, 1546–1554. doi: 10.1212/WNL.51.6.1546
Neergaard-Petersen, S., Hvas, A.-M., Kristensen, S. D., Grove, E. L., Larsen, S. B., Phoenix, F., et al. (2014). The influence of type 2 diabetes on fibrin clot properties in patients with coronary artery disease. Thromb. Haemost. 112, 1142–1150. doi: 10.1160/th14-05-0468
Norhammar, A., and Schenck-Gustafsson, K. (2013). Type 2 diabetes and cardiovascular disease in women. Diabetologia 56, 1–9. doi: 10.1007/s00125-012-2694-y
Odden, M. C., Peralta, C. A., Haan, M. N., and Covinsky, K. E. (2012). Rethinking the association of high blood pressure with mortality in elderly adults: the impact of frailty. Arch. Intern. Med. 172, 1162–1168. doi: 10.1001/archinternmed.2012.2555
Paul, S., Thomas, G., Majeed, A., Khunti, K., and Klein, K. (2012). Women develop type 2 diabetes at a higher body mass index than men. Diabetologia 55, 1556–1557. doi: 10.1007/s00125-012-2496-2
Peters, S. A., Carcel, C., Millett, E. R., and Woodward, M. (2020). Sex differences in the association between major risk factors and the risk of stroke in the UK Biobank cohort study. Neurology 95, e2715–e2726. doi: 10.1212/WNL.0000000000010982
Peters, S. A., Huxley, R. R., and Woodward, M. (2014). Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775 385 individuals and 12 539 strokes. The Lancet. 383, 1973–1980. doi: 10.1016/S0140-6736(14)60040-4
Peters, S. A., Huxley, R. R., and Woodward, M. (2016a). Sex differences in body anthropometry and composition in individuals with and without diabetes in the UK Biobank. BMJ Open 6, e010007. doi: 10.1136/bmjopen-2015-010007
Peters, S. A., Singhateh, Y., Mackay, D., Huxley, R. R., and Woodward, M. (2016b). Total cholesterol as a risk factor for coronary heart disease and stroke in women compared with men: a systematic review and meta-analysis. Atherosclerosis 248, 123–131. doi: 10.1016/j.atherosclerosis.2016.03.016
Peters, T. M., Holmes, M. V., Richards, J. B., Palmer, T., Forgetta, V., Lindgren, C. M., et al. (2021). Sex differences in the risk of coronary heart disease associated with type 2 diabetes: a mendelian randomization analysis. Diabetes Care. 44, 556–562. doi: 10.2337/dc20-1137
R Core Team. (2020). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. Available online at: https://www.R-project.org/
Rascovsky, K., Hodges, J. R., Knopman, D., Mendez, M. F., Kramer, J. H., Neuhaus, J., et al. (2011). Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 134, 2456–2477. doi: 10.1093/brain/awr179
Reitan, R. M. (1955). The relation of the trail making test to organic brain damage. J. Consult. Psychol. 19, 393. doi: 10.1037/h0044509
Rhodius-Meester, H. F., Benedictus, M. R., Wattjes, M. P., Barkhof, F., Scheltens, P., Muller, M., et al. (2017). MRI visual ratings of brain atrophy and white matter hyperintensities across the spectrum of cognitive decline are differently affected by age and diagnosis. Front. Aging Neurosci. 9, 117. doi: 10.3389/fnagi.2017.00117
Rhodius-Meester, H. F. M., van de Schraaf, S. A., Peters, M. J., Kleipool, E. E., Trappenburg, M. C., and Muller, M. (2021). Mortality risk and its association with geriatric domain deficits in older outpatients: the amsterdam ageing cohort. Gerontology 67, 194–201. doi: 10.1159/000512048
Román, G. C., Tatemichi, T. K., Erkinjuntti, T., Cummings, J., Masdeu, J., Garcia, J., et al. (1993). Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. Neurology 43, 250. doi: 10.1212/WNL.43.2.250
Rossi, M. C., Cristofaro, M. R., Gentile, S., Lucisano, G., Manicardi, V., Mulas, M. F., et al. (2013). Sex disparities in the quality of diabetes care: biological and cultural factors may play a different role for different outcomes: a cross-sectional observational study from the AMD Annals initiative. Diabetes Care 36, 3162–3168. doi: 10.2337/dc13-0184
Ryan, J., Scali, J., Carriere, I., Amieva, H., Rouaud, O., Berr, C., et al. (2014). Impact of a premature menopause on cognitive function in later life. BJOG 121, 1729–1739. doi: 10.1111/1471-0528.12828
Siepmann, T., Boardman, H., Bilderbeck, A., Griffanti, L., Kenworthy, Y., Zwager, C., et al. (2017). Long-term cerebral white and gray matter changes after preeclampsia. Neurology. 88, 1256–1264. doi: 10.1212/WNL.0000000000003765
Smith, E. E., Schneider, J. A., Wardlaw, J. M., and Greenberg, S. M. (2012). Cerebral microinfarcts: the invisible lesions. Lancet Neurol. 11, 272–282. doi: 10.1016/S1474-4422(11)70307-6
Soedamah-Muthu, S. S., Chaturvedi, N., Toeller, M., Ferriss, B., Reboldi, P., Michel, G., et al. (2004). Risk factors for coronary heart disease in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study. Diabetes Care. 27, 530–537. doi: 10.2337/diacare.27.2.530
Sourial, N., Arsenault-Lapierre, G., Margo-Dermer, E., Henein, M., and Vedel, I. (2020). Sex differences in the management of persons with dementia following a subnational primary care policy intervention. Int. J. Equity Health 19, 1–6. doi: 10.1186/s12939-020-01285-2
Studart, A. N., and Nitrini, R. (2016). Subjective cognitive decline: the first clinical manifestation of Alzheimer's disease? Dement Neuropsychol. 10, 170–177. doi: 10.1590/S1980-5764-2016DN1003002
Sun, H., Saeedi, P., Karuranga, S., Pinkepank, M., Ogurtsova, K., Duncan, B. B., et al. (2021). IDF diabetes Atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 183, 109119. doi: 10.1016/j.diabres.2021.109119
Ter Telgte, A., van Leijsen, E. M., Wiegertjes, K., Klijn, C. J., Tuladhar, A. M., and de Leeuw, F.-E. (2018). Cerebral small vessel disease: from a focal to a global perspective. Nat. Rev. Neurol. 14, 387–398. doi: 10.1038/s41582-018-0014-y
Troncoso, J. C., Zonderman, A. B., Resnick, S. M., Crain, B., Pletnikova, O., and O'Brien, R. J. (2008). Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann. Neurol. 64, 168–176. doi: 10.1002/ana.21413
Vaccarino, V., Abramson, J. L., Veledar, E., and Weintraub, W. S. (2002). Sex differences in hospital mortality after coronary artery bypass surgery: evidence for a higher mortality in younger women. Circulation 105, 1176–1181. doi: 10.1161/hc1002.105133
Van Der Elst, W., Van Boxtel, M. P., Van Breukelen, G. J., and Jolles, J. (2005). Rey's verbal learning test: normative data for 1855 healthy participants aged 24–81 years and the influence of age, sex, education, and mode of presentation. J. Int. Neuropsychol. Soc. 11, 290–302. doi: 10.1017/S1355617705050344
Van der Elst, W., Van Boxtel, M. P., Van Breukelen, G. J., and Jolles, J. (2006). The Stroop color-word test: influence of age, sex, and education; and normative data for a large sample across the adult age range. Assessment. 13, 62–79. doi: 10.1177/1073191105283427
Van Der Elst, W., Van Boxtel, M. P., Van Breukelen, G. J., and Jolles, J. (2006). Normative data for the Animal, Profession and Letter M Naming verbal fluency tests for Dutch speaking participants and the effects of age, education, and sex. J. Int. Neuropsychol. Soc. 12, 80–89. doi: 10.1017/S1355617706060115
Verhagen, C., Janssen, J., Biessels, G. J., Johansen, O. E., and Exalto, L. G. (2022). Females with type 2 diabetes are at higher risk for accelerated cognitive decline than males: CAROLINA-COGNITION study. Nutr. Metab. Cardiovasc. Dis. 32, 355–364. Available online at: https://doi.org/10.1016/j.numecd.2021.10.013
Wannamethee, S., Papacosta, O., Lawlor, D., Whincup, P., Lowe, G., Ebrahim, S., et al. (2012). Do women exhibit greater differences in established and novel risk factors between diabetes and non-diabetes than men? The British Regional Heart Study and British Women's Heart Health Study. Diabetologia 55, 80–87. doi: 10.1007/s00125-011-2284-4
Wardlaw, J. M., Smith, C., and Dichgans, M. (2019). Small vessel disease: mechanisms and clinical implications. Lancet Neurol. 18, 684–696. doi: 10.1016/S1474-4422(19)30079-1
Wilkins-Haug, L., Celi, A., Thomas, A., Frolkis, J., and Seely, E. W. (2015). Recognition by women's health care providers of long-term cardiovascular disease risk after preeclampsia. Obstet. Gynecol. 125, 1287–1292. doi: 10.1097/AOG.0000000000000856
Woodward, M., Peters, S. A., and Huxley, R. R. (2015). Diabetes and the female disadvantage. Women's Health 11, 833–839. doi: 10.2217/whe.15.67
Yaffe, K., Blackwell, T., Kanaya, A., Davidowitz, N., Barrett-Connor, E., and Krueger, K. (2004). Diabetes, impaired fasting glucose, and development of cognitive impairment in older women. Neurology. 63, 658–663. doi: 10.1212/01.WNL.0000134666.64593.BA
Keywords: sex-specific analysis, brain structure, diabetes, cognitive function, vascular aging, vascular cognitive impairment and dementia
Citation: Thomas EG, Rhodius-Meester H, Exalto L, Peters SAE, van Bloemendaal L, Ponds R and Muller M (2022) Sex-Specific Associations of Diabetes With Brain Structure and Function in a Geriatric Population. Front. Aging Neurosci. 14:885787. doi: 10.3389/fnagi.2022.885787
Received: 28 February 2022; Accepted: 20 May 2022;
Published: 28 June 2022.
Edited by:
Knut Engedal, Vestfold Hospital Trust, NorwayReviewed by:
Marie-Pierre Moisan, INRAE Nouvelle-Aquitaine Bordeaux, FranceHenrik Schirmer, Akershus University Hospital, Norway
Copyright © 2022 Thomas, Rhodius-Meester, Exalto, Peters, van Bloemendaal, Ponds and Muller. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elias G. Thomas, Zy50aG9tYXNAYW1zdGVyZGFtdW1jLm5s
 Elias G. Thomas
Elias G. Thomas Hanneke Rhodius-Meester
Hanneke Rhodius-Meester Lieza Exalto
Lieza Exalto Sanne A. E. Peters
Sanne A. E. Peters Liselotte van Bloemendaal
Liselotte van Bloemendaal Rudolf Ponds8
Rudolf Ponds8 Majon Muller
Majon Muller