
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 08 April 2022
Sec. Neurocognitive Aging and Behavior
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.862856
This article is part of the Research Topic Insights in Neurocognitive Aging and Behavior: 2021 View all 16 articles
 Gizem Ayerdem1†
Gizem Ayerdem1† Matthijs J. Bosma1†
Matthijs J. Bosma1† Joanna Sophia J. Vinke1
Joanna Sophia J. Vinke1 Aaltje L. Ziengs2
Aaltje L. Ziengs2 Adriaan R. E. Potgieser3
Adriaan R. E. Potgieser3 Ron T. Gansevoort1
Ron T. Gansevoort1 Stephan J. L. Bakker1
Stephan J. L. Bakker1 Martin H. De Borst1
Martin H. De Borst1 Michele F. Eisenga1*
Michele F. Eisenga1*
Background: Emerging data suggest that erythropoietin (EPO) promotes neural plasticity and that iron homeostasis is needed to maintain normal physiological brain function. Cognitive functioning could therefore be influenced by endogenous EPO levels and disturbances in iron status.
Objective: To determine whether endogenous EPO levels and disturbances in iron status are associated with alterations in cognitive functioning in the general population.
Materials and Methods: Community-dwelling individuals from the Prevention of Renal and Vascular End-Stage Disease (PREVEND) study, a general population-based cohort in Groningen, Netherlands, were surveyed between 2003 and 2006. Additionally, endogenous EPO levels and iron status, consisting of serum iron, transferrin, ferritin, and transferrin saturation were analyzed. Cognitive function was assessed by scores on the Ruff Figural Fluency Test (RFFT), as a reflection of executive function, and the Visual Association Test (VAT), as a reflection of associative memory.
Results: Among 851 participants (57% males; mean age 60 ± 13 years), higher endogenous EPO levels were independently associated with an improved cognitive function, reflected by RFFT scores (ß = 0.09, P = 0.008). In multivariable backward linear regression analysis, EPO levels were among the most important modifiable determinants of RFFT scores (ß = 0.09, P = 0.002), but not of VAT scores. Of the iron status parameters, only serum ferritin levels were inversely associated with cognitive function, reflected by VAT scores, in multivariable logistic regression analysis (odds ratio, 0.77; 95% confidence interval 0.63–0.95; P = 0.02 for high performance on VAT, i.e., ≥11 points). No association between iron status parameters and RFFT scores was identified.
Conclusion: The findings suggest that endogenous EPO levels and serum ferritin levels are associated with specific cognitive functioning tests in the general population. Higher EPO levels are associated with better RFFT scores, implying better executive function. Serum ferritin levels, but not other iron status parameters, were inversely associated with high performance on the VAT score, implying a reduced ability to create new memories and recall recent past. Further research is warranted to unravel underlying mechanisms and possible benefits of therapeutic interventions.
Erythropoietin (EPO) and iron are the primary regulators of red blood cell production. Besides being the fuel for erythropoiesis, EPO, and iron are known to express a myriad of non-hematopoietic effects (Nekoui and Blaise, 2017). An important non-hematopoietic effect concerns the maintenance of a normal physiological brain function (Ehrenreich et al., 2004; Ji et al., 2017). As a consequence, disturbances in EPO levels and iron status might negatively affect cognitive functioning, which is pivotal to focus, process information, and adapt or maintain a healthy lifestyle (Gill et al., 2020).
EPO receptors (EPOR) are prominently expressed in the brain in glial cells, neurons, and endothelial cells (Konishi et al., 1993; Digicaylioglu et al., 1995; Marti et al., 1996). EPO can pass the blood-brain barrier to exert its effect on the EPOR in the brain (Brines et al., 2000; Ehrenreich et al., 2004; Xenocostas et al., 2005). Moreover, in experimental models, astrocytes have been shown to produce and secrete EPO (Masuda et al., 1994). EPO promotes neural plasticity and has anti-inflammatory, anti-apoptotic, anti-oxidative, angiogenic, and stemcell-modulatory effects (Sirén et al., 2001; Gorio et al., 2002; Springborg et al., 2002; Buemi et al., 2003; Villa et al., 2003; Lykissas et al., 2007; Sargin et al., 2010; Girolamo et al., 2014; Nekoui et al., 2015). Therefore, EPO appears to have neuroprotective and neurotrophic properties, which in turn might hypothetically affect cognitive functioning (Wakhloo et al., 2020). Various studies focusing on different cerebral disease models support such a hypothesis, with some authors reporting that administration of recombinant human EPO (rhEPO) has a positive effect on cognitive functioning (Ehrenreich et al., 2008; Miskowiak et al., 2008, 2012, 2016; Sargin et al., 2010; Nekoui and Blaise, 2017).
Similarly, several studies have shown a relationship between serum iron levels and cognitive functioning (Miskowiak et al., 2012; Ji et al., 2017). Iron is a crucial part of many proteins including heme, iron sulfur clusters, and other functional groups (Schiepers et al., 2010). These proteins are essential for the formation of myelin surrounding axons and adenosine triphosphate in mitochondria, cell signaling, host defense, and nucleic acid replication and repair (Todorich et al., 2009; Mills et al., 2010; Evstatiev and Gasche, 2012). Iron is also crucially involved in the synthesis of several neurotransmitters, including tyrosine hydroxylase (dopamine and norepinephrine) and tryptophan (serotonin) (Moos et al., 2007; Todorich et al., 2009). As iron homeostasis is normally tightly regulated, iron deficiency, and overload affect enzymatic and structural proteins. Indeed, both iron deficiency and iron overload have been implicated in impaired cognitive functioning (Casanova and Araque, 2003; Miskowiak et al., 2012; Ji et al., 2017).
To date, the relationship between EPO levels and iron status with cognitive functioning has only been assessed in experimental models and relatively small sample size populations, often involving specific patient populations, e.g., having mood disorders (Beard et al., 1997; Milward et al., 1999; Weiskopf et al., 2006) questioning the role of endogenous EPO levels and iron status with cognitive functioning in the general population. Hence, we aimed to assess in a large population-based cohort the association between endogenous EPO levels and iron status parameters with cognitive functioning as reflected by two cognitive tests, i.e., the Ruff Figural Fluency Test (RFFT) and the Visual Association Test (VAT).
For this study, we used the PREVEND (Prevention of REnal and Vascular ENd stage Disease) database, a general population-based cohort study. In 1997 and 1998, all inhabitants from the city of Groningen, between the age of 28–75 years (n = 85,421), were sent a short questionnaire and a vial to collect a first-morning void urine sample. 40,856 (48%) people responded. Individuals with insulin-dependent Diabetes Mellitus and pregnant women were excluded. Six thousand subjects with a urinary albumin excretion ≥ 10 mg/L and 2,592 randomly selected subjects (control group) with a urinary albumin excretion < 10 mg/L completed the screening protocol and formed the PREVEND cohort baseline (n = 8,592). We used the third survey of PREVEND, which took place between 2003 and 2006. Of these participants, multiple blood samples were collected in which, among others, EPO levels and iron status parameters were measured. Two tests reflecting certain cognitive domains (i.e., RFFT and VAT) were introduced during the same survey. For current analysis, we included 851 patients with data available on EPO levels, iron status, and both cognitive tests (as depicted in Figure 1). The PREVEND study protocol was approved by the institutional medical ethical review board of the University Medical Center Groningen and was conducted in accordance with the Helsinki declaration. All subjects provided written informed consent.
The procedures at each examination in the PREVEND study have been described in detail previously (Hillege et al., 2001). In short, upon entry into the study, all participants completed a questionnaire regarding demographics, current diagnoses, medical history, smoking habits, alcohol consumption, and medication use. Information on medication use was combined with information from a pharmacy-dispensing registry. Educational level was defined as low (primary education or intermediate vocational education), middle (higher secondary education), and high (higher vocational education and university). Antihypertensives included diuretics, β–blockers, calcium channel blockers, and renin-angiotensin system inhibitors.
The RFFT measures a subject’s ability to produce novel figures utilizing five different dot configurations (Ruff et al., 1987). Participants were instructed to produce as many unique designs as possible using at least two of the dots in a 5-dot matrix. The lowest score is 0 points, the highest and best score is 175 points. The RFFT is a sensitive test for executive cognitive abilities such as non-verbal fluency, planning strategies, task shifting, selective attention, response evaluation, and response suppression, which are necessary to coordinate this process (Mulder et al., 2006). It has been shown to be sensitive to early changes in cognitive function in young as well as middle-aged people (Foster et al., 2005). A reduced ability to produce novel figures can indicate a disability in executive function in general and has been linked to processes in the frontal lobe, most prominently in the right frontal lobe (Ruff et al., 1994; Mulder et al., 2006).
The VAT is a brief episodic memory test presenting six paired pictures of two interacting objects where one has to name the missing object on a cue card which has been shown before. One point is given if the missing object is correctly identified. The minimum score is 0 points; the maximum score is 12 points. The test is used to detect anterograde amnesia and related syndromes, usually associated with atrophy of the (medial-temporal areas of the) limbic system. It is hypothesized that a low score on the VAT is related to impaired ability in coding new information or, less likely, in short-term memory (Lindeboom et al., 2002).
Fresh fasting blood samples were collected in the morning from all participants and stored at −80°C. EPO was measured using an immunoassay based on chemiluminescence (Immulite EPO assay, DPC, Los Angeles, CA, United States). Serum iron, ferritin, and transferrin were measured using colorimetrix assay, immunoassay, or immunoturbidimetric assay (Roche Diagnostics, Mannheim, Germany), respectively. Transferrin saturation (TSAT,%) was calculated as 100 × serum iron (μmol/L)÷(25 × transferrin[g/L]). A Coulter Counter STKS sum was used to measure hemoglobin (g/dL) (Coulter Corporation, Miami, FL, United States). Serum creatinine was measured using an enzymatic method on a Roche Modular analyzer (Roche Diagnostics). The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula was used to calculate the estimated glomerular filtration rate (eGFR) (Levey et al., 2009). Urinary albumin concentration was determined by nephelometry (BN II, Dade Behring Diagnostica, Marburg, Germany). Urinary albumin excretion (UAE) was calculated as the average UAE in the two consecutive 24-h urine collections. Body mass index (BMI) was calculated as the ratio of weight divided by height squared (kg/m2). High-sensitivity C-reactive protein (hs-CRP), cholesterol, high- density lipoprotein (HDL), and low-density lipoprotein (LDL) were measured using routine laboratory procedures.
Data were analyzed using IBM SPSS, version 23.0 (SPSS, Chicago, IL, United States) and R version 4.0.2 (Vienna, Austria). Baseline characteristics were described as means with standard deviation when normally distributed and medians with interquartile range when distributions were skewed. Categorical variables were reported using numbers (percentage). We described baseline characteristics both for the total population and across quartiles of EPO and ferritin levels. Differences between the quartiles were calculated using analysis of variance (ANOVA), Kruskal-Wallis test or χ2-test, as appropriate. In regression analysis, we determined if serum EPO levels and iron status parameters can be regarded as important determinants of cognitive capacity domains measured by the RFFT and VAT. Univariable linear regression analysis was performed of all included factors with RFFT as the dependent variable. Subsequently, we performed multivariable-adjusted models and multivariable backward linear regression analysis (entry and exit level set at P < 0.2 and P < 0.1, respectively). In all regression analyses, skewed variables were naturally log-transformed. In the multivariable model, we adjusted for age, sex, education, BMI, eGFR, and urinary albumin excretion (model 1); for systolic blood pressure, alcohol use, smoking, hemoglobin, hs-CRP, serum HDL, and serum LDL levels (model 2); and for history of a myocardial or cerebrovascular event, diabetes mellitus, and the use of antihypertensives, and lipid lowering drugs (model 3). Due to the skewed distribution, the VAT scores were dichotomized and divided at the median into high performance (≥11 points) and low performance (≤10 points), in line with previous investigations of the VAT score (Joosten et al., 2014). The association of EPO levels and iron status parameters were evaluated by logistic regression analysis in a similar way. In multiple regression analyses, iron status parameters were assessed separately due to multi-collinearity between the iron status parameters. The contribution of EPO levels was reported with ferritin levels as only iron status parameter in all models. Statistical significance was considered as a two-tailed p-value of <0.05.
We included 851 participants (57% males) with a mean age of 60.3 ± 13.0 years. Participants had a mean BMI of 27.1 ± 3.9 kg/m2 and an eGFR of 85.5 ± 19.5 ml/min/1.73m2. A majority of the participants [412 (48%)] registered a low educational level, whereas 226 (27%) and 213 (25%) had a middle and high educational level, respectively. The median EPO level in the total population was 7.8 (5.9–10.1) IU/L; median ferritin concentration was 117 (58–197) μg/L; mean iron level was 16.3 ± 5.2 μmol/L; mean transferrin level was 2.5 ± 0.4 g/L; and mean TSAT was 26.4 ± 9.2%. Further baseline demographics, clinical characteristics, and laboratory parameters according to quartiles of EPO and ferritin levels in the total population are depicted in Tables 1, 2, respectively.
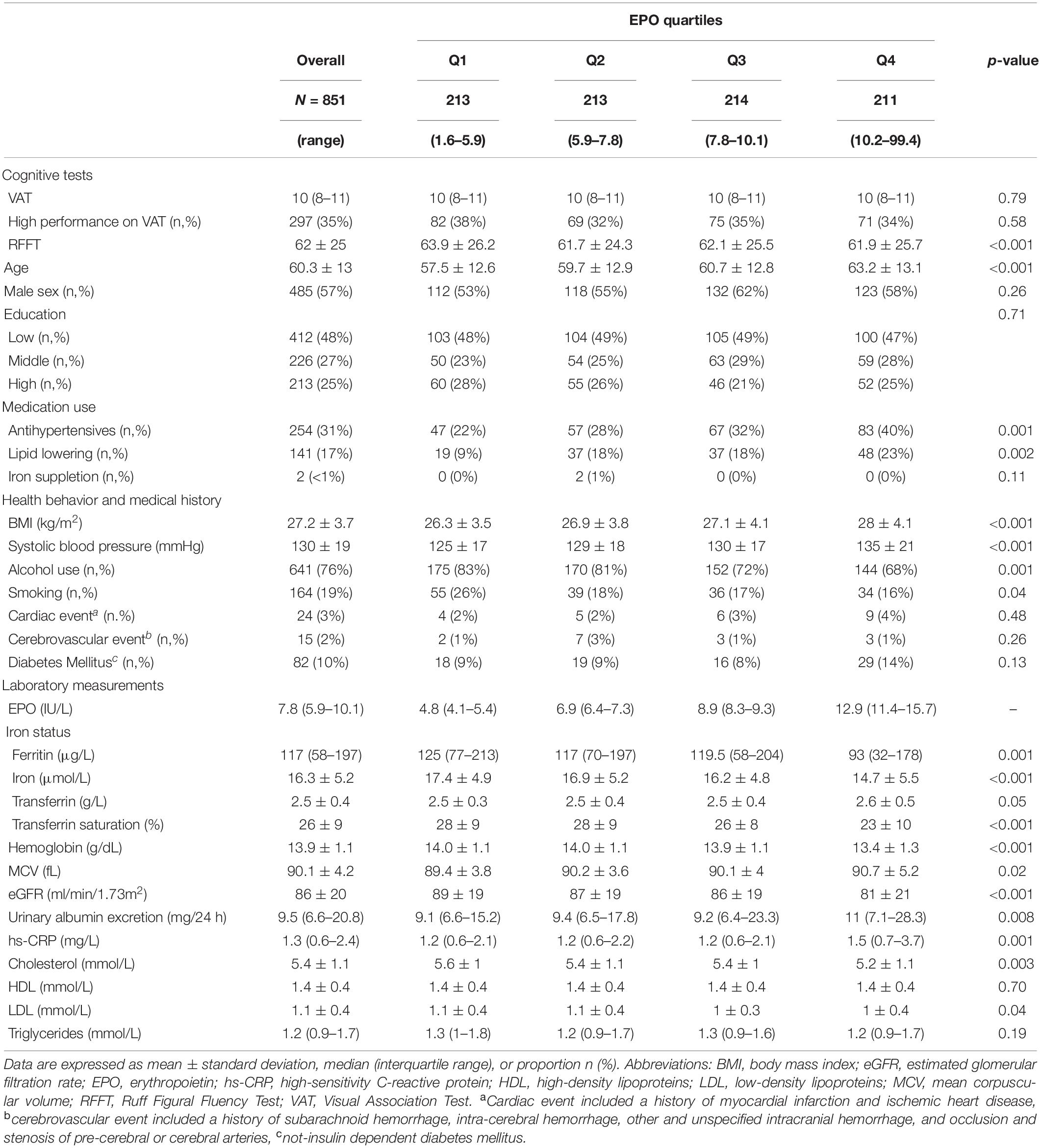
Table 1. Baseline characteristics of 851 community-dwelling subjects according to quartiles of EPO levels.
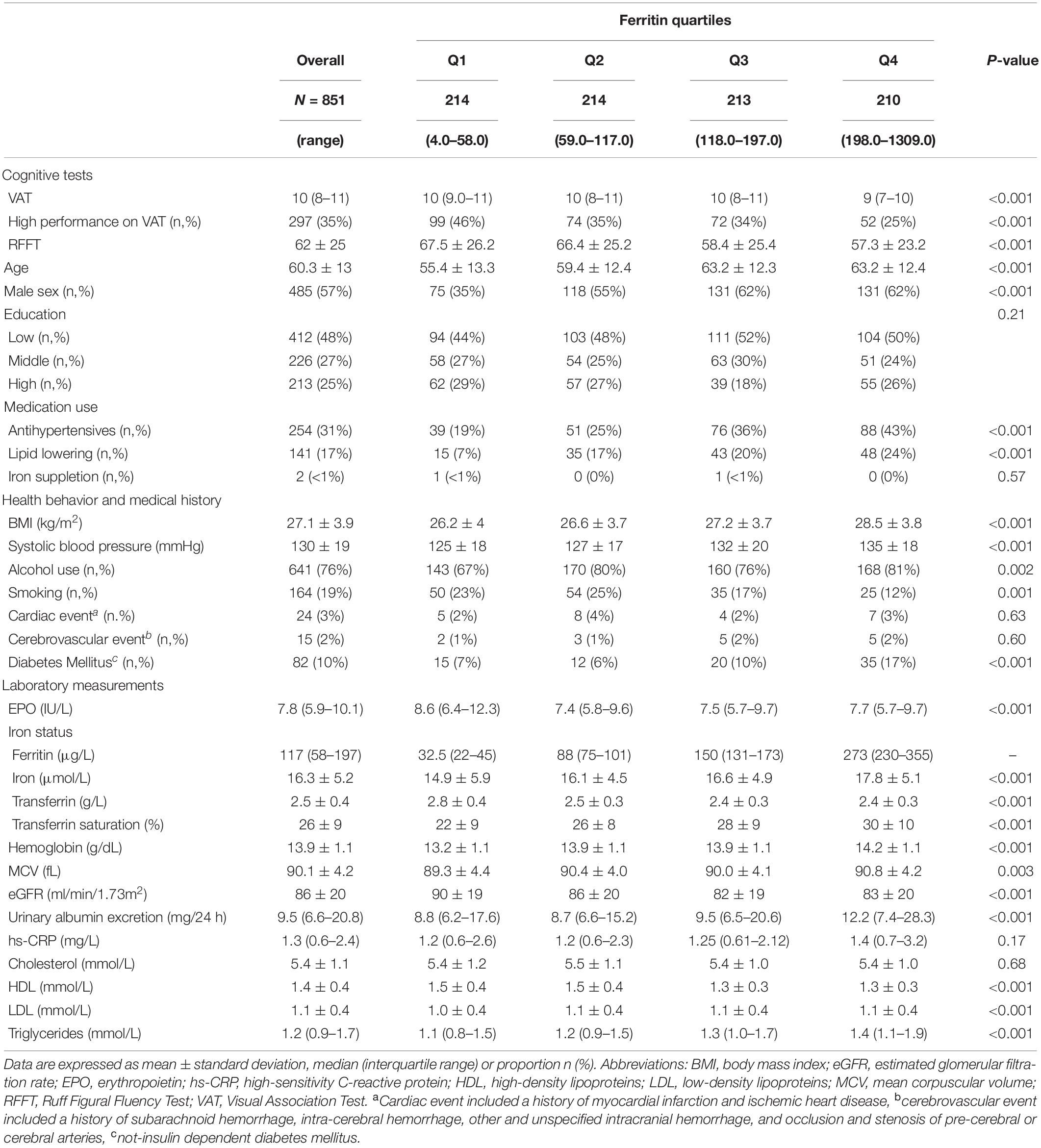
Table 2. Baseline characteristics of 851 community-dwelling subjects according to quartiles of ferritin levels.
Participant scored an average of 62 ± 25 points on the RFFT. If compared to the norm score, 672 (79%) scored average on the RFFT, 85 (10%) above average, 68 (8%) below average, and 26 (31%) participants had a deviant. Participants obtained a median VAT score of 10 points (IQR = 8–11). Considering the norm, 684 (80%) participants scored average on the VAT, 107 (12.6%) above average, 42 (5%) below average, and 18 (2%) people had a deviant.
Across quartiles of serum EPO levels, we noticed a significant inverse association with RFFT score. Individuals in the lowest quartile of EPO levels had 63.9 ± 26.2 points, whereas participants in the upper quartile of EPO scored 61.9 ± 25.7 points on the RFFT (P < 0.001). Similarly, we identified a significant inverse association, even more pronounced, across quartiles of ferritin levels. Participants within the lowest quartile of ferritin obtained 67.5 ± 26.2 points, whereas participants in the highest quartile of ferritin obtained 57.3 ± 23.2 points on the RFFT (P < 0.001).
In multivariable linear regression analysis, EPO levels were significantly associated with RFFT scores independent of potential confounders (fully adjusted ß = 0.09, P = 0.008 as depicted in model 3; Table 3). None of the iron status parameters, including serum ferritin, were significantly associated with RFFT scores.
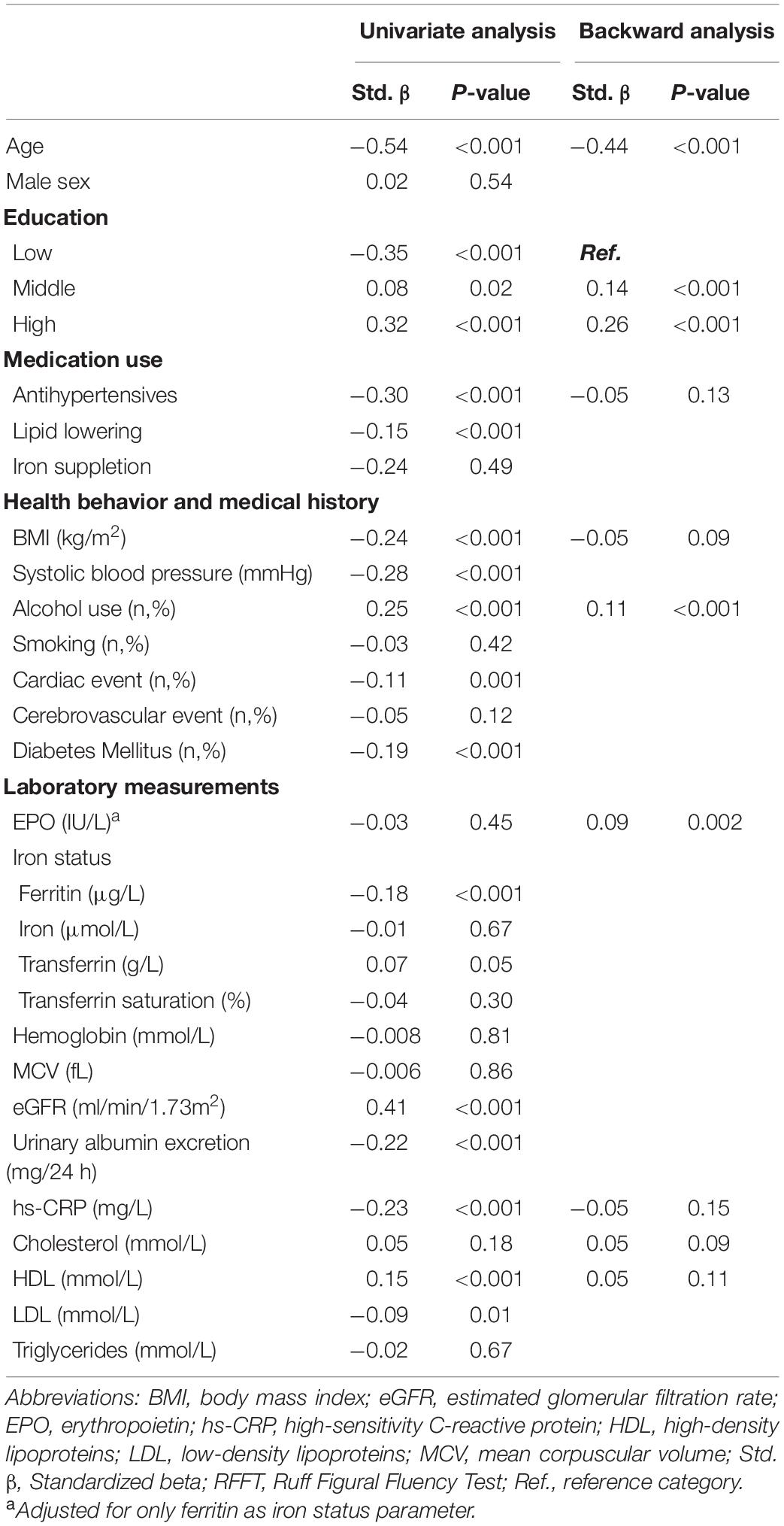
Table 3. Univariate and backward linear regression analyses of potential determinants of RFFT scores.
In multivariable linear backward regression analysis, EPO levels were among the main determinants of RFFT (β = 0.09, P = 0.002). In contrast, none of the iron status parameters was significantly associated with RFFT (Table 4).
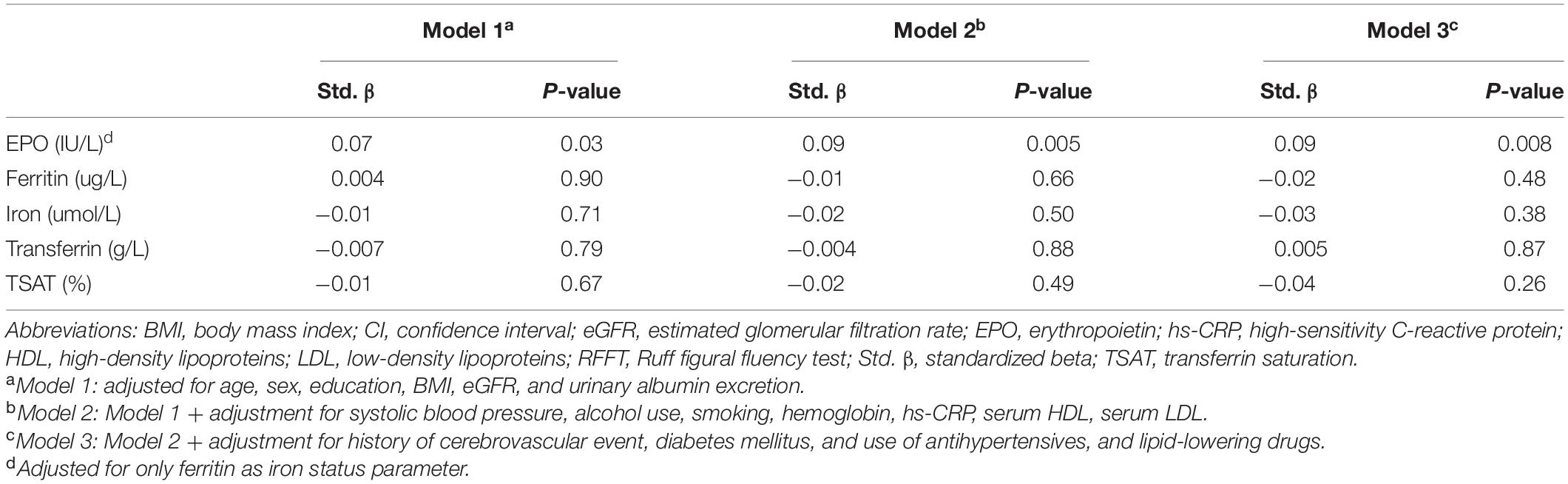
Table 4. Multivariate linear regression analyses of the association of individual iron status parameters and erythropoietin with RFFT score.
Across quartiles of EPO, we identified no significant differences in VAT score (P = 0.79). In contrast, we identified that the prevalence of high performance scores (i.e., ≥11 points) was significantly different across quartiles of ferritin levels. Of the participants within the lowest quartile of ferritin, 46% had a high performance score, whereas only 25% of the participants within the upper quartile of ferritin had a high performance score (P < 0.001).
In multivariable logistic regression analysis, EPO levels were not significantly associated with a high performance on the VAT. Of the iron status parameters, only serum ferritin was significantly inversely associated with a high performance on the VAT (fully adjusted OR, 0.77; 95% CI 0.63 – 0.95; P = 0.02 as depicted in model 3, Table 5). In contrast, serum iron, transferrin, and TSAT were not associated with a high performance on the VAT score (Table 5).
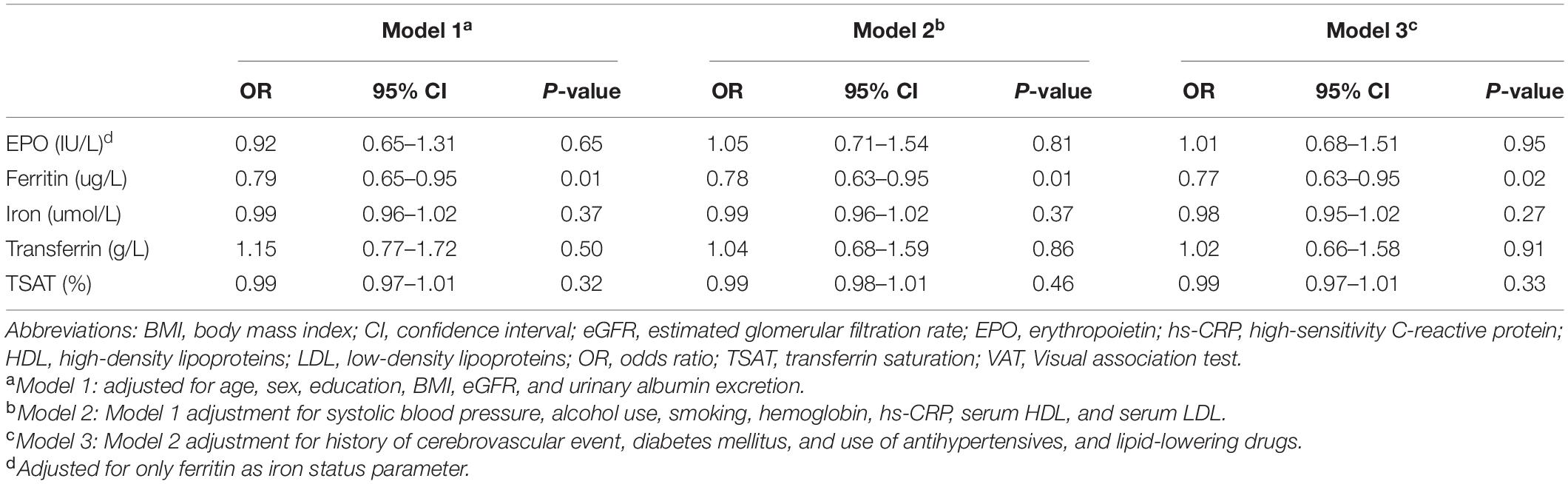
Table 5. Binomial logistic regression analyses of the association of individual iron status parameters and erythropoietin with a high performance on the VAT score.
In this study, we show that in the general population higher endogenous EPO levels are associated with better executive function, reflected by RFFT scores, whereas higher ferritin levels, but not other iron status parameters, are associated with a lower VAT score, reflecting associative memory. To the best of our knowledge, this is the first study to show associations between serum EPO and ferritin levels and specific domains of cognitive functioning in the general population.
It has been suggested that EPO exerts a protective effect on cognitive functioning due to its neuroprotective and neurotrophic potential (Sirén et al., 2001; Gorio et al., 2002; Springborg et al., 2002; Buemi et al., 2003; Villa et al., 2003; Lykissas et al., 2007; Sargin et al., 2010; Girolamo et al., 2014; Nekoui et al., 2015). The latter has mainly been concluded based on studies in which exogenous EPO was administered (Ehrenreich et al., 2008; Nekoui and Blaise, 2017). Specifically, these studies showed an increase in cognitive function test scores. Here, we demonstrate that higher endogenous EPO levels are associated with better RFFT scores, reflecting improved executive function with improved capabilities such as non-verbal fluency, planning strategies, task shifting, selective attention, response evaluation, and response suppression, which are necessary to coordinate this process (Mulder et al., 2006). This is in line with the hypothesis based on earlier findings of EPO and EPOR expression in (mammalian) brain areas related to executive functioning, and with studies by Ehrenreich et al. and Miskowiak et al. in which exogenous EPO increased several of these (or related) executive functions (Digicaylioglu et al., 1995; Marti et al., 1996; Ehrenreich et al., 2007a,b; Miskowiak et al., 2008, 2014; Nowrangi et al., 2014). Importantly, the effect of high endogenous EPO levels on RFFT scores seems to be independent of the effect of EPO on hematopoiesis, as adjustment for hemoglobin levels did not alter the association. The latter suggests a direct neurobiological effect of EPO on cognition, most likely because of its neuroprotective and neurotrophic potential as an underlying mechanism, which is in line with the long-term impact of EPO on cognition in several other studies (Ehrenreich et al., 2007a,b; Miskowiak et al., 2014).
The exact biological relevance of our endogenous serum EPO levels is difficult to interpret in the absence of a direct measurement of EPO in the brain. EPO is known to cross the blood-brain barrier by active translocation, most likely via EPOR expressed in the brain vasculature pattern (Brines et al., 2000). The studies who investigated the neuroprotective and neurotrophic potential of exogenous EPO administered high-dose EPO to induce significant elevations in cerebrospinal fluid and brain EPO levels to improve cognitive function. However, the importance of endogenous circulating EPO levels has also previously been shown in children with malaria where high EPO levels were associated with a reduced risk of neurological sequelae (Casals-Pascual et al., 2008). Similarly, in a recent study, Shim et al. (2021) showed the relationship between circulating EPO levels and attention deficit hyperactivity disorder (ADHD) rating scale in children with ADHD and healthy controls.
We did not find an association between endogenous EPO levels and performance on the VAT, suggesting that endogenous EPO levels did not affect the ability to create new memories and to recall the recent past. Aside from a few exceptions, this runs counter to reports that exogenous EPO improves certain memory-related abilities, as can be seen through upregulation of activity during memory tasks (Ehrenreich et al., 2007b; Miskowiak et al., 2008, 2009, 2014) and upregulation of memory-related brain areas during a memory task (Miskowiak et al., 2007, 2016). With evidence of EPO and EPOR being present in brain areas related to memory, e.g., the hippocampus and areas within the temporal lobe, we expected a positive association of endogenous EPO levels on the VAT score (Digicaylioglu et al., 1995; Marti et al., 1996; Rombouts et al., 1997). The discrepancy between our currently identified results and those from other studies might be related to the use of different populations in earlier studies, which focused on subjects with depression or schizophrenia.
Moreover, and more likely, the VAT is designed to detect anterograde amnesia and related syndromes. It is a relatively simple task with a small range in scores compared to the RFFT and less suitable to detect subtle differences in memory ability.
Regarding iron status, we did not identify a U-shaped association between iron status and cognitive function, as might have been expected, since previous studies related both a low and high serum iron to a decline in certain cognitive abilities (Lam et al., 2008; Schiepers et al., 2010; Ji et al., 2017). However, we did find that higher ferritin levels increased the risk of a low performance on the VAT. This suggests that increased ferritin levels in the general population are associated with a diminished ability to create new memories and recall the recent past. Since serum ferritin is not related to the iron content in brain regions involved in memory abilities, like the hippocampus and temporal cortex (Gao et al., 2017), the underlying mechanism is not clear. Our finding is contrary to the few previous studies on serum ferritin levels and cognition. Schiepers et al. (2010) found that higher serum ferritin was associated with decreased speed of cognitive functioning, but did not find serum ferritin to be related to memory processes. Milward et al. (2010) found that abnormal levels of ferritin were not associated with global cognitive performance or executive function. When considering serum ferritin as a proxy for body iron stores, our findings are in line with research by Lam et al. (2008), in which very high serum iron concentrations were associated with poorer outcomes on tests measuring short and long-term memory processes. However, caution is warranted to consider serum ferritin solely as surrogate for body iron stores. Serum ferritin is also an acute-phase reactant, which is upregulated by inflammation, excessive use of alcohol, metabolic syndrome, and tissue damage or turnover (e.g., hepatic or malignancy) (Cullis et al., 2018). Previous studies suggest an association with cognitive decline and (biomarkers of) inflammation (Yaffe et al., 2003; Schram et al., 2007; Sartori et al., 2012). Similar associations are seen with direct or indirect effects of alcohol use, metabolic syndrome, tissue damage, -turnover, or a combination (Brust, 2010; Yates et al., 2012; Janelsins et al., 2014; Nardelli et al., 2019). Notably, the association between serum ferritin and VAT remained independent of adjustment for alcohol use, BMI, hemoglobin, and hs-CRP. Although we tried to fully adjust for these potential confounders, we cannot exclude that these mechanisms, at least in part, might have contributed to the identified association between higher ferritin and lower performance on the VAT score. In the patient setting of neurodegenerative diseases, strong associations of cerebrospinal fluid ferritin have been identified with worse cognitive function in patients with Alzheimer’s disease, patients with Parkinson’s disease, and patients with dementia with Lewy bodies (Ayton et al., 2022). In fact, cerebrospinal fluid ferritin levels even predicted outcomes in patients with Alzheimer’s disease (Ayton et al., 2015, 2017), and could be used as a readout for the inflammatory response during the neurodegenerative phase of Alzheimer’s disease (Brosseron et al., 2021).
Our study has several strengths and limitations. We used a well-phenotyped large cohort of community-dwelling individuals, reflecting a large proportion of the general Dutch population. Moreover, we tried to account as fully as possible for confounders as cognitive functioning is known to be influenced by multiple factors. Limitations of the current study are that cognitive functioning was measured only with two tests, which cover a diverse set of cognitive capabilities but do not reflect performance on all cognitive domains. Although the RFFT is a more sensitive and reliable test for detecting subtle changes in cognitive functioning in both young and old people when compared to tests like the Mini Mental State Examination (MMSE), Trail-Making Test (TMT) or Modified Telephone Interview for Cognitive Status (TICS-M) (Foster et al., 2005; Izaks et al., 2011; Joosten et al., 2014), we are not able to extend our findings to cognitive functioning as a whole. Other tests, e.g., the Rey Auditory Verbal Learning Test (RAVLT) and the Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire (CPFQ) would have given important additional information on cognitive functioning. Another limitation is that we did not have data available on a broader range of iron status parameters, such as hepcidin and soluble transferrin receptor. Finally, a limitation of our study is that we did not identify with biomarkers participants with underlying Alzheimer’s disease and that we did not have availability of a direct measurement of EPO or ferritin in the brain.
In conclusion, this study demonstrates a relatively strong association between higher endogenous EPO levels and better performance on several executive cognitive abilities, as reflected by the RFFT, in the general population. Furthermore, we found that ferritin levels, but not other iron status parameters, were inversely associated with a high performance on VAT scores, reflecting associative memory. Future research should focus on a more comprehensive examination of cognitive functioning, time-dependent relationships, underlying mechanisms, use of brain imaging, identification of patients with Alzheimer’s disease, and opportunities and obstacles for therapeutic interventions.
The data analyzed in this study is subject to the following licenses/restrictions: Public sharing of individual participant data was not included in the informed consent form of the study, but data can be made available to interested researchers upon reasonable request. Requests to access these datasets should be directed to the data manager of the PREVEND study, Dr. Lyanne Kieneker, bC5tLmtpZW5la2VyQHVtY2cubmw=.
The study involving human participants was reviewed and approved by Institutional Medical Ethical Review Board of the University Medical Center Groningen. The participants provided their written informed consent to participate in this study.
ME realized the research idea and study design and provided supervision and mentorship. GA, MB, SB, and ME carried out data acquisition. GA, MB, and ME carried out statistical analysis. All authors performed the data analysis/interpretation, contributed to important intellectual content during manuscript drafting or revision, and agreed to be personally accountable for the individual’s own contributions and to ensured that questions pertaining to the accuracy or integrity of any portion of the work, even on in which the author was not directly involved, were appropriately investigated and resolved, including documentation in the literature if appropriate.
JV received consultancy fees from Vifor Pharma. MD has received consultancy fees from Astellas, Kyowa Kirin, Pharmacosmos, Sanofi Genzyme, and Vifor Pharma (all to employer), grant support from Sanofi Genzyme and Vifor Pharma. ME received speakers’ and consultancy fees from Vifor Pharma and served the Advisory Board of Cablon Medical.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ayton, S., Faux, N. G., and Bush, A. I. (2017). Association of Cerebrospinal Fluid Ferritin Level With Preclinical Cognitive Decline in APOE-ε4 Carriers. JAMA Neurol. 74, 122–125. doi: 10.1001/jamaneurol.2016.4406
Ayton, S., Faux, N. G., and Bush, A. I., Alzheimer’s Disease Neuroimaging Initiative. (2015). Ferritin levels in the cerebrospinal fluid predict Alzheimer’s disease outcomes and are regulated by APOE. Nat. Commun. 6:6760. doi: 10.1038/ncomms7760
Ayton, S., Hall, S., Janelidze, S., Kalinowski, P., Palmqvist, S., Belaidi, A. A., et al. (2022). The Neuroinflammatory Acute Phase Response in Parkinsonian-Related Disorders. Mov. Disord. [Epub online ahead of print] doi: 10.1002/mds.28958
Beard, C. M., Kokmen, E., O’Brien, P. C., Ania, B. J., and Melton, L. J. (1997). Risk of Alzheimer’s disease among elderly patients with anemia: population-based investigations in Olmsted County, Minnesota. Ann. Epidemiol. 7, 219–224. doi: 10.1016/s1047-2797(97)00015-x
Brines, M. L., Ghezzi, P., Keenan, S., Agnello, D., de Lanerolle, N. C., Cerami, C., et al. (2000). Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl. Acad. Sci. U.S.A. 97, 10526–10531. doi: 10.1073/pnas.97.19.10526
Brosseron, F., Kleemann, K., Kolbe, C. C., Santarelli, F., Castro-Gomez, S., Tacik, P., et al. (2021). Interrelations of Alzheimer’s disease candidate biomarkers neurogranin, fatty acid-binding protein 3 and ferritin to neurodegeneration and neuroinflammation. J. Neurochem. 157, 2210–2224. doi: 10.1111/jnc.15175
Brust, J. C. M. (2010). Ethanol and cognition: indirect effects, neurotoxicity and neuroprotection: a review. Int. J. Environ. Res. Public Health 7, 1540–1557. doi: 10.3390/ijerph7041540
Buemi, M., Cavallaro, E., Floccari, F., Sturiale, A., Aloisi, C., Trimarchi, M., et al. (2003). The pleiotropic effects of erythropoietin in the central nervous system. J. Neuropathol. Exp. Neurol. 62, 228–236. doi: 10.1093/jnen/62.3.228
Casals-Pascual, C., Idro, R., Gicheru, N., Gwer, S., Kitsao, B., Gitau, E., et al. (2008). High levels of erythropoietin are associated with protection against neurological sequelae in African children with cerebral malaria. Proc. Natl. Acad. Sci. U.S.A. 105, 2634–2639. doi: 10.1073/pnas.0709715105
Casanova, M. F., and Araque, J. M. (2003). Mineralization of the basal ganglia: implications for neuropsychiatry, pathology and neuroimaging. Psychiatry Res. 121, 59–87. doi: 10.1016/s0165-1781(03)00202-6
Cullis, J. O., Fitzsimons, E. J., Griffiths, W. J. H., Tsochatzis, E., and Thomas, D. W., British Society for Haematology. (2018). Investigation and management of a raised serum ferritin. Br. J. Haematol. 181, 331–340. doi: 10.1111/bjh.15166
Digicaylioglu, M., Bichet, S., Marti, H. H., Wenger, R. H., Rivas, L. A., Bauer, C., et al. (1995). Localization of specific erythropoietin binding sites in defined areas of the mouse brain. Proc. Natl. Acad. Sci. U.S.A. 92, 3717–3720. doi: 10.1073/pnas.92.9.3717
Ehrenreich, H., Bartels, C., Sargin, D., Stawicki, S., and Krampe, H. (2008). Recombinant human erythropoietin in the treatment of human brain disease: focus on cognition. J. Ren. Nutr. 18, 146–153. doi: 10.1053/j.jrn.2007.10.029
Ehrenreich, H., Degner, D., Meller, J., Brines, M., Béhé, M., Hasselblatt, M., et al. (2004). Erythropoietin: a candidate compound for neuroprotection in schizophrenia. Mol. Psychiatry 9, 42–54. doi: 10.1038/sj.mp.4001442
Ehrenreich, H., Fischer, B., Norra, C., Schellenberger, F., Stender, N., Stiefel, M., et al. (2007a). Exploring recombinant human erythropoietin in chronic progressive multiple sclerosis. Brain 130, 2577–2588. doi: 10.1093/brain/awm203
Ehrenreich, H., Hinze-Selch, D., Stawicki, S., Aust, C., Knolle-Veentjer, S., Wilms, S., et al. (2007b). Improvement of cognitive functions in chronic schizophrenic patients by recombinant human erythropoietin. Mol. Psychiatry 12, 206–220. doi: 10.1038/sj.mp.4001907
Evstatiev, R., and Gasche, C. (2012). Iron sensing and signalling. Gut 61, 933–952. doi: 10.1136/gut.2010.214312
Foster, P. S., Williamson, J. B., and Harrison, D. W. (2005). The Ruff Figural Fluency Test: heightened right frontal lobe delta activity as a function of performance. Arch. Clin. Neuropsychol. 20, 427–434. doi: 10.1016/j.acn.2004.09.010
Gao, L., Jiang, Z., Cai, Z., Cai, M., Zhang, Q., Ma, Y., et al. (2017). Brain iron deposition analysis using susceptibility weighted imaging and its association with body iron level in patients with mild cognitive impairment. Mol. Med. Rep. 16, 8209–8215. doi: 10.3892/mmr.2017.7668
Gill, T. M., Han, L., Gahbauer, E. A., Leo-Summers, L., and Murphy, T. E. (2020). Risk factors and precipitants of severe disability among community-living older persons. JAMA Netw. Open 3:e206021. doi: 10.1001/jamanetworkopen.2020.6021
Girolamo, F., Coppola, C., Ribatti, D., and Trojano, M. (2014). Angiogenesis in multiple sclerosis and experimental autoimmune encephalomyelitis. Acta Neuropathol. Commun. 2:84. doi: 10.1186/s40478-014-0084-z
Gorio, A., Gokmen, N., Erbayraktar, S., Yilmaz, O., Madaschi, L., Cichetti, C., et al. (2002). Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc. Natl. Acad. Sci. U.S.A. 99, 9450–9455. doi: 10.1073/pnas.142287899
Hillege, H. L., Janssen, W. M., Bak, A. A., Diercks, G. F., Grobbee, D. E., Crijns, H. J., et al. (2001). Microalbuminuria is common, also in a nondiabetic, nonhypertensive population, and an independent indicator of cardiovascular risk factors and cardiovascular morbidity. J. Intern. Med. 249, 519–526. doi: 10.1046/j.1365-2796.2001.00833.x
Izaks, G. J., Joosten, H., Koerts, J., Gansevoort, R. T., and Slaets, J. P. (2011). Reference data for the Ruff Figural Fluency Test stratified by age and educational level. PLoS One 6:e17045. doi: 10.1371/journal.pone.0017045
Janelsins, M. C., Kesler, S. R., Ahles, T. A., and Morrow, G. R. (2014). Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry 26, 102–113. doi: 10.3109/09540261.2013.864260
Ji, X., Cui, N., and Liu, J. (2017). Neurocognitive function is associated with serum iron status in early adolescents. Biol. Res. Nurs. 19, 269–277. doi: 10.1177/1099800417690828
Joosten, H., Visser, S. T., van Eersel, M. E., Gansevoort, R. T., Bilo, H. J. G., Slaets, J. P., et al. (2014). Statin use and cognitive function: population-based observational study with long-term follow-up. PLoS One 9:e115755. doi: 10.1371/journal.pone.0115755
Konishi, Y., Chui, D. H., Hirose, H., Kunishita, T., and Tabira, T. (1993). Trophic effect of erythropoietin and other hematopoietic factors on central cholinergic neurons in vitro and in vivo. Brain Res. 609, 29–35. doi: 10.1016/0006-8993(93)90850-m
Lam, P. K., Kritz-Silverstein, D., Barrett-Connor, E., Milne, D., Nielsen, F., Gamst, A., et al. (2008). Plasma trace elements and cognitive function in older men and women: the Rancho Bernardo study. J. Nutr. Heal. Aging 12, 22–27. doi: 10.1007/BF02982160
Levey, A. S., Stevens, L. A., Schmid, C. H., Zhang, Y. L., Castro, A. F., Feldman, H. I., et al. (2009). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 150, 604–612. doi: 10.7326/0003-4819-150-9-200905050-00006
Lindeboom, J., Schmand, B., Tulner, L., Walstra, G., and Jonker, C. (2002). Visual association test to detect early dementia of the Alzheimer type. J. Neurol. Neurosurg. Psychiatry 73, 126–133. doi: 10.1136/jnnp.73.2.126
Lykissas, M. G., Korompilias, A. V., Vekris, M. D., Mitsionis, G. I., Sakellariou, E., and Beris, A. E. (2007). The role of erythropoietin in central and peripheral nerve injury. Clin. Neurol. Neurosurg. 109, 639–644. doi: 10.1016/j.clineuro.2007.05.013
Marti, H. H., Wenger, R. H., Rivas, L. A., Straumann, U., Oigicaylioglu, M., Henn, V., et al. (1996). Erythropoietin gene expression in human, monkey and murine brain. Eur. J. Neurosci. 8, 666–676. doi: 10.1111/j.1460-9568.1996.tb01252.x
Masuda, S., Okano, M., Yamagishi, K., Nagao, M., Ueda, M., and Sasaki, R. (1994). A novel site of erythropoietin production. Oxygen-dependent production in cultured rat astrocytes. J. Biol. Chem. 269, 19488–19493. doi: 10.1016/s0021-9258(17)32195-6
Mills, E., Dong, X., Wang, F., and Xu, H. (2010). Mechanisms of brain iron transport: insight into neurodegeneration and CNS disorders. Future Med. Chem. 2, 51–64. doi: 10.4155/fmc.09.140
Milward, E. A., Bruce, D. G., Knuiman, M. W., Divitini, M. L., Cole, M., Inderjeeth, C. A., et al. (2010). A cross-sectional community study of serum iron measures and cognitive status in older adults. J. Alzheimers Dis. 20, 617–623. doi: 10.3233/JAD-2010-1402
Milward, E. A., Grayson, D. A., Creasey, H., Janu, M. R., Brooks, W. S., and Broe, G. A. (1999). Evidence for association of anaemia with vascular dementia. Neuroreport 10, 2377–2381. doi: 10.1097/00001756-199908020-00029
Miskowiak, K., Inkster, B., O’Sullivan, U., Selvaraj, S., Goodwin, G. M., and Harmer, C. J. (2008). Differential effects of erythropoietin on neural and cognitive measures of executive function 3 and 7 days post-administration. Exp. Brain Res. 184, 313–321. doi: 10.1007/s00221-007-1102-1
Miskowiak, K., O’Sullivan, U., and Harmer, C. J. (2007). Erythropoietin enhances hippocampal response during memory retrieval in Humans. J. Neurosci. 27, 2788–2792. doi: 10.1523/JNEUROSCI.5013-06.2007
Miskowiak, K. W., Ehrenreich, H., Christensen, E. M., Kessing, L. V., and Vinberg, M. (2014). Recombinant human erythropoietin to target cognitive dysfunction in bipolar disorder. J. Clin. Psychiatry 75, 1347–1355. doi: 10.4088/JCP.13m08839
Miskowiak, K. W., Favaron, E., Hafizi, S., Inkster, B., Goodwin, G. M., Cowen, P. J., et al. (2009). Effects of erythropoietin on emotional processing biases in patients with major depression: an exploratory fMRI study. Psychopharmacology 207, 133–142. doi: 10.1007/s00213-009-1641-1
Miskowiak, K. W., Vinberg, M., Glerup, L., Paulson, O. B., Knudsen, G. M., Ehrenreich, H., et al. (2016). Neural correlates of improved executive function following erythropoietin treatment in mood disorders. Psychol. Med. 46, 1679–1691. doi: 10.1017/S0033291716000209
Miskowiak, K. W., Vinberg, M., Harmer, C. J., Ehrenreich, H., and Kessing, L. V. (2012). Erythropoietin: a candidate treatment for mood symptoms and memory dysfunction in depression. Psychopharmacology 219, 687–698. doi: 10.1007/s00213-011-2511-1
Moos, T., Nielsen, T. R., Skjørringe, T., and Morgan, E. H. (2007). Iron trafficking inside the brain. J. Neurochem. 103, 1730–1740. doi: 10.1111/j.1471-4159.2007.04976.x
Mulder, J. L., Dekker, P. H., and Dekker, R. (2006). Word-Fluency Test/Figure Fluency Test, Tutorial. Leiden: PITS.
Nardelli, S., Gioia, S., Faccioli, J., Riggio, O., and Ridola, L. (2019). Sarcopenia and cognitive impairment in liver cirrhosis: a viewpoint on the clinical impact of minimal hepatic encephalopathy. World J. Gastroenterol. 25, 5257–5265. doi: 10.3748/wjg.v25.i35.5257
Nekoui, A., and Blaise, G. (2017). Erythropoietin and nonhematopoietic effects. Am. J. Med. Sci. 353, 76–81. doi: 10.1016/j.amjms.2016.10.009
Nekoui, A., Tressierra, V. D. C. E., Abdolmohammadi, S., Shedid, D., and Blaise, G. (2015). Neuroprotective effect of erythropoietin in postoperation cervical spinal cord injury: case report and review. Anesth. Pain Med. 28:e28849. doi: 10.5812/aapm.28849
Nowrangi, M. A., Lyketosos, C., Rao, V., and Munro, C. A. (2014). Systematic review of neuroimaging correlates of executive functioning: converging evidence from different clinical population. J. Neuropsychiatry Clin. Neurosci. 26, 114–125. doi: 10.1176/appi.neuropsych.1207176
Rombouts, S. A. R. B., Machielsen, W. C. M., Witter, M. P., Barkhof, F., Lindeboom, J., and Scheltens, P. (1997). Visual association encoding activates the medial temporal lobe: a functional magnetic resonance imaging study. Hippocampus 7, 594–601. doi: 10.1002/(SICI)1098-106319977:6<594::AID-HIPO2<3.0.CO;2-F
Ruff, R. M., Allen, C. C., Farrow, C. E., Niemann, H., and Wylie, T. (1994). Figural fluency: differential impairment in patients with left versus right frontal lobe lesions. Arch. Clin. Neuropsychol. 9, 41–55. doi: 10.1016/0887-6177(94)90013-2
Ruff, R. M., Light, R. H., and Evans, R. W. (1987). The Ruff Figural Fluency Test: a normative study with adults. Dev. Neuropsychol. 3, 37–51. doi: 10.1007/978-0-387-79948-3_2048
Sargin, D., Friedrichs, H., El-Kordi, A., and Ehrenreich, H. (2010). Erythropoetin as neuroprotective and neurogenerative treatment strategy: comprehensive overview of 12 years of preclinical and clinical research. Best Pract. Res. Clin. Anaesthesiol. 24, 573–594. doi: 10.1016/j.bpa.2010.10.005
Sartori, A. C., Vance, D. E., Slater, L. Z., and Crowe, M. (2012). The impact of inflammation on cognitive function in older adults: implications for healthcare practice and research. J. Neurosci. Nurs. 44, 206–217. doi: 10.1097/JNN.0b013e3182527690
Schiepers, O. J. G., van Boxtel, M. P. J., de Groot, R. H. M., Jolles, J., De Kort, W. L. A. M., Swinkels, D. W., et al. (2010). Serum iron parameters, HFE C282Y genotype, and cognitive performance in older adults: results from the FACIT study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 65, 1312–1321. doi: 10.1093/gerona/glq149
Schram, M. T., Euser, S. M., de Craen, J. M., Witteman, J. C., Frölich, M., Hofman, A., et al. (2007). Systemic markers of inflammation and cognitive decline in old age. J. Am. Geriatr. Soc. 55, 708–716. doi: 10.1111/j.1532-5415.2007.01159.x
Shim, S. H., Kim, Y. K., Hwangbo, Y., Yoon, H. J., Kim, J. S., Lee, Y. J., et al. (2021). The Relationship between Plasma Erythropoietin Levels and Symptoms of Attention Deficit Hyperactivity Disorder. Clin. Psychopharmacol. Neurosci. 19, 334–340. doi: 10.9758/cpn.2021.19.2.334
Sirén, A. L., Fratelli, M., Brines, M., Goemans, C., Casagrande, S., Lewczuk, P., et al. (2001). Erythropoietin Prevents Neuronal Apoptosis after Cerebral Ischemia and Metabolic Stress. Proc. Natl. Acad. Sci. U.S.A. 98, 4044–4049. doi: 10.1073/pnas.051606598
Springborg, J. B., Ma, X., Rochat, P., Knudsen, G. M., Amtrop, O., Paulson, O. B., et al. (2002). A Single Subcutaneous Bolus of Erythropoietin Normalizes Cerebral Bloodflow Autoregulation after Subarachnoid Haemorrhage in Rats. Br. J. Pharmacol. 135, 823–829. doi: 10.1038/sj.bjp.0704521
Todorich, B., Pasquini, J. M., Garcia, C. I., Paez, P. M., and Connor, J. R. (2009). Oligodendrocytes and myelination: the role of iron. Glia 57, 467–478. doi: 10.1002/glia.20784
Villa, P., Bigini, P., Mennini, T., Agnello, D., Laragione, T., Cagnotto, A., et al. (2003). Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J. Exp. Med. 198, 971–975. doi: 10.1084/jem.20021067
Wakhloo, D., Scharkowski, F., Curto, Y., Butt, U. J., Bansal, V., Steixner-Kumar, A. A., et al. (2020). Functional hypoxia drives neuroplasticity and neurogenesis via brain erythropoietin. Nat. Commun. 11:1313. doi: 10.1038/s41467-020-15041-1
Weiskopf, R. B., Feiner, J., Hopf, H., Lieberman, J., Finlay, H. E., Quah, C., et al. (2006). Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology 104, 911–920. doi: 10.1097/00000542-200605000-00005
Xenocostas, A., Cheung, W. K., Farrell, F., Zakszewski, C., Kelley, M., Lutynski, A., et al. (2005). The pharmacokinetics of erythropoietin in the cerebrospinal fluid after intravenous administration of recombinant human erythropoietin. Eur. J. Clin. Pharmacol. 61, 189–195. doi: 10.1007/s00228-005-0896-7
Yaffe, K. M., Lindquist, K., Penninx, B. W., Simonsick, E. M., Pahor, M., Kritchevsky, S., et al. (2003). Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology 61, 76–80. doi: 10.1212/01.wnl.0000073620.42047.d7
Keywords: erythropoietin (EPO), iron, cognitive functioning, general population, visual association test, ruff figural fluency test
Citation: Ayerdem G, Bosma MJ, Vinke JSJ, Ziengs AL, Potgieser ARE, Gansevoort RT, Bakker SJL, De Borst MH and Eisenga MF (2022) Association of Endogenous Erythropoietin Levels and Iron Status With Cognitive Functioning in the General Population. Front. Aging Neurosci. 14:862856. doi: 10.3389/fnagi.2022.862856
Received: 26 January 2022; Accepted: 15 March 2022;
Published: 08 April 2022.
Edited by:
Paul Gerson Unschuld, Université de Genève, SwitzerlandReviewed by:
Scott Ayton, University of Melbourne, AustraliaCopyright © 2022 Ayerdem, Bosma, Vinke, Ziengs, Potgieser, Gansevoort, Bakker, De Borst and Eisenga. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michele F. Eisenga, bS5mLmVpc2VuZ2FAdW1jZy5ubA==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.