
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci., 08 July 2022
Sec. Neurocognitive Aging and Behavior
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.834496
This article is part of the Research TopicCerebral Small Vessel Disease: From the Pathogenesis to Therapeutic InterventionsView all 7 articles
Cerebral small vessel disease (CSVD) is a common cerebrovascular disease and an important cause of gait and balance disorders. Gait and balance disorders can further lead to an increased risk of falls and a decreased quality of life. CSVD can damage gait and balance function by affecting cognitive function or directly disrupting motor pathways, and different CSVD imaging features have different characteristics of gait and balance impairment. In this article, the correlation between different imaging features of sporadic CSVD and gait and balance disorders has been reviewed as follows, which can provide beneficial help for standardized management of CSVD.
Cerebral small vessel disease (CSVD) is a pathological process of cerebral arterioles, capillaries, and venules caused by a variety of factors. CSVD appears on magnetic resonance imaging (MRI) as white matter hyperintensities (WMH), cerebral microbleeds (CMB), lacunar infarctions (LI), enlarged perivascular spaces (EPVS), and brain atrophy (Pantoni, 2010; Wardlaw et al., 2013a). Sporadic CSVD is closely correlated with age, with prevalence increasing from approximately 5% in people aged 50 to nearly 100% in people aged 90 (de Leeuw et al., 2001). CSVD is a major cause of cognitive impairment, dementia, and stroke, which is a huge burden on public health (Debette and Markus, 2010). Gait and balance disorders are the second most common problem in CSVD after cognitive impairment (Okroglic et al., 2013). Gait disorders are common in older adults and can lead to falls and functional dependence, increasing the risk of hospitalization and death (van der Holst et al., 2016; Bower et al., 2019). A study conducted by Wei et al. (2017) showed that gait and balance function were independent predictors of the risk of falls in patients 1 month after stroke, suggesting that appropriate gait training may be one of the key factors in preventing falls in patients with stroke. We aim to assist the standardized management of CSVD by discussing the pathogenesis, diagnosis, and treatment of gait and balance disorders associated with sporadic CSVD. The mechanism of the association between sporadic CSVD and gait and balance disorders is summarized as follows (Figure 1).
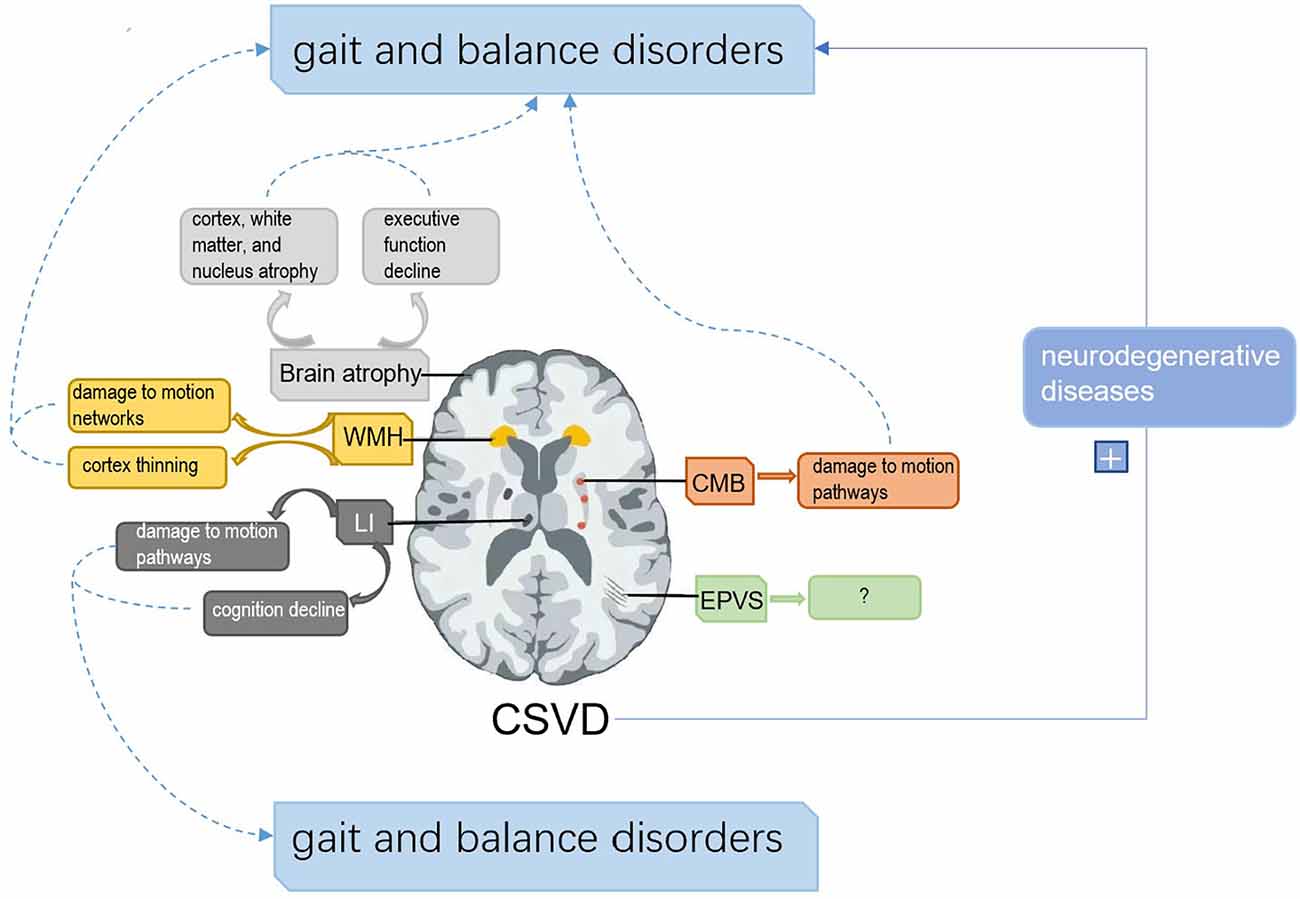
Figure 1. The framework of the associated mechanisms between cerebral small vascular disease (CSVD) and gait and balance disorders. (1) White matter hyperintensities (WMH) can disrupt gait and balance by interfering with motion pathways and cortical thickness. (2) Lacunar infarctions (LI) impair gait and balance by interfering with cognition and motion pathways. (3) Cerebral microbleeds (CMB) affect gait and balance by damaging motion pathways. (4) Brain atrophy can disrupt gait and balance by damage to cognition, nucleus atrophy related to motion control, and cortex and white matter atrophy. (5) Enlarged perivascular spaces (EPVS) have shown no direct evidence to disrupt gait and balance. (6) CSVD can aggravate the impairment of gait and balance by affecting neurodegenerative diseases.
Gait and balance are considered to be multisystem interactions, which suggests that clinical gait and balance problems may arise from different pathological mechanisms, such as attention, planning, visuospatial, and motor processes, and are closely related to executive function in the cognitive domain (Horak, 2006). The study of Cai et al. showed that gait disorders in elderly patients with CSVD are mediated by cognitive function impairment (Cai et al., 2021). Gait requires the supervision of cognitive function, especially executive function, and patients need the brain to allocate attention to control gait and balance. The decline in cognitive ability will damage their balance control ability (Yip et al., 2014; Cai et al., 2021). CSVD can cause extensive neural network damage by damaging important fiber bundles and loops, resulting in impaired visual, cognitive, sensory, and motor functions, especially in the frontal lobe, temporal lobe, basal ganglia, and other parts related to executive function, which will lead to a more obvious decline in executive function (Chao et al., 2009). Zhou et al. (2020) showed that gait and balance disorders in CSVD patients were related to the sensorimotor network and frontoparietal network as well as abnormalities in auxiliary motor areas. Other studies have shown that CSVD disrupts the cortex-striatum- globus pallidus-thalamic-cortical circuit, leading to depressive states, and poststroke depression also increases the risk of gait disorders (Wei et al., 2017; Rensma et al., 2018).
WMH are a type of sporadic small vascular disease, and axon loss and demyelination are the causes of WMH, which may be the result of chronic ischemia caused by CSVD (Staszewski et al., 2015). It is also believed that hypoperfusion is related to cerebral vascular autoregulation changes or blood–brain barrier dysfunction (Simpson et al., 2007), inflammation, and amyloid vascular disease (Gouw et al., 2011). 20% of WMH can be found in adults in their 60s and up to 94% in those in their 80s (Garde et al., 2000). On imaging, WMH show high signals on T2-weighted sequences and equal signals or low signals on T1-weighted sequences (Fujishima et al., 2014). WMH can be divided into periventricular white matter hyperintensities (PVWMH) and deep white matter hyperintensities (DWMH). Traditional imaging techniques or semiautomated techniques are used to measure the volume of white matter lesions. New methods, such as magnetic resonance diffusion tensor imaging (DTI) and voxel-based morphology analysis (VBM), can also be used to analyze the integrity of white matter. One study confirmed that WMH scores and total CSVD scores were associated with poorer gait speed and body balance in healthy older adults (Pinter et al., 2017). In patients with vascular cognitive impairment, gait and balance disorders were mainly characterized by reduced arm swing, stoop, reduced step length, and broad base gait, which were only associated with PVWMH volume and white matter integrity but not with other radiological markers of CSVD (Kim et al., 2016). The LADIS study confirmed that the severity of WMH in the deep frontal lobe and PVWMH significantly impaired balance function in the elderly population, which supported the hypothesis that the disruption of motor circuits in the subfrontal cortex caused balance disorders (Blahak et al., 2009). DTI provides an indicator of the internal microstructure integrity of neural networks and can reflect the loss of white matter integrity (Pasi et al., 2016). A study using DTI found that stride length decline was associated with white matter atrophy and an increase in mean white matter radial diffusivity and mean diffusivity and a decrease in mean fractional anisotropy (FA), with the strongest association found in the corpus callosum and radial crown fibers (van der Holst et al., 2017). The corpus callosum white matter connects the frontal, parietal, and occipital cortexes, and fibers from these areas reverse into the radiative crown, which contains projective fibers involved in motor pathways and thus plays a key role in motor function (Jang, 2009). In another study using DTI, total white matter lesions in CADASIL patients only affected stride length on a single task (Finsterwalder et al., 2019). PVWMH are more likely to cause cognitive impairment than DWMH and executive dysfunction due to the concentration of a large number of neurons and fibers related to learning, memory, and cognition, and the white matter bundle crossing periventricular white matter is denser (Onteddu et al., 2015). White matter injury, especially DWMH, also increased the likelihood of poststroke depression (Fujishima et al., 2014). However, no studies have directly shown that WMH affect gait independently by affecting cognition or depression. A study using the VBM technique showed that the bilateral frontal and periventricular white matter corresponding to the major anterior projection fibers (thalamic radiation, cortical motor tracts) and adjacent association fibers (corpus callosum, superior fronto-occipital fasciculus, short association fibers) showed the greatest correlation with poor gait (Srikanth et al., 2010). This suggested that white matter injury can lead to an age-related decline in gait and trunk balance stability by disconnecting the motor network composed of these fiber bundles. Another study using the VBM technique showed that fibers associated with gait and balance disorders crossed periventricular white matter more densely than deep white matter and that the cortical regions connected by these fibers overlapped with cortical thinning regions associated with gait performance. The mediation analysis showed that PVWMH mediated gait disorders through average FA or cortical thickness (Kim et al., 2016). We can determine that WMH affect gait and balance through the following mechanisms: (1) direct damage to motion-related neural networks; and (2) destruction of white matter integrity leading to thinning of the related cortex.
LI, which account for 25% of all acute ischemic strokes, are radiographically characterized by round, oval, or tubular infarcts with a diameter <20 mm, hypersignal on diffusion weighted imaging (DWI), hypersignal on T2-weighted and fluid-attenuated inversion recovery (FLAIR), and hypersignal on T1. They usually occur in the basal ganglia, inner sac, thalamus, corona radialis, centrum semiovale, and brain stem (Wardlaw et al., 2013a; Li et al., 2018). LI are usually silent and found in 20%–50% of older adults (Vermeer et al., 2007). While many LI lack acute symptoms, their presence in large numbers has been associated with dementia, cognitive impairment, gait impairment, and an increased risk of stroke (Shi and Wardlaw, 2016). A study of healthy community populations showed that LI were associated with slower gait speed and balance disorders (Smith et al., 2015). LI located in the frontal lobe and thalamus were associated with lower gait speed, and stride length was more sensitive than stride speed as an outcome indicator (de Laat et al., 2010), which can be related to the involvement of the frontal-basal ganglia-thalamic-cortex circuit in gait motor function (Bazner et al., 2000).
LI are an important predictor of cognitive impairment in CSVD with significant spatial distribution characteristics, especially the anterior medial thalamus LI, which are associated with impaired information processing speed and may lead to cognitive impairment by disrupting connections to the prefrontal cortex (Benjamin et al., 2014, 2018). A study linked LI in the frontal lobe to motoric cognitive risk syndrome (MCR; Wang et al., 2016). MCR is a predementia syndrome characterized by cognitive complaints and slow gait in elderly people without dementia (Verghese et al., 2014). LI in the frontal lobe may cause MCR by disrupting neural networks based on the frontal lobe that assist memory and gait function (Takakusaki, 2013). Studies have shown that LI interact with WMH, and the presence of LI can aggravate the impairment of gait and postural stability in patients with WMH, which may be the result of a higher brain injury load leading to more easily impaired functions (Choi et al., 2012). In addition, deep white matter LI are also associated with the severity and fluctuation of depressive symptoms, which may be related to the disruption of the emotional circuit (Grool et al., 2013). However, whether LI can affect gait and balance by affecting WMH or depression is unclear.
In conclusion, LI can affect gait and balance function through the following two mechanisms: (1) direct damage to motor pathways, such as the frontal lobe and basal ganglia; and (2) MCR by influencing cognition.
CMB are circular or elliptical uniform low signal regions with a clear boundary and a diameter of 2–5 mm and a maximum of 10 mm on gradient recalled echo (GRE) and susceptibility weighted imaging (SWI). CMB have been shown to occur in 23.5% of healthy elderly populations (Pasi et al., 2017). CMB were found in 17.8% of patients aged 60–69 years and 38.3% of patients aged over 80 years, with the prevalence fluctuating by approximately 5% (Cordonnier et al., 2007; Ding et al., 2015). The CMB has the same spatial distribution characteristics as LI and is mostly located at the corticocortical subcortical junction and deep gray or white matter in the hemispheres, brainstem, and cerebellum (Wardlaw et al., 2013b). Currently, there are few studies on the association between CMB and gait and balance disorders. For instance, de Laat et al. (2011) demonstrated for the first time that CMB located in the basal ganglia, thalamus, and frontal lobe were associated with gait and balance disorders in elderly patients without dementia, independent of other coexisting CSVD markers, and these regions were associated with gait control. Gait and balance disorders were mainly represented by shorter stride length and decreased trunk balance (de Laat et al., 2011). This suggested that CMB can directly damage the site and thus disrupt motor pathways. However, most studies since then have shown no direct link between CMB and gait and balance. A cross-sectional study in Japan showed that short standing time on one leg was independently associated with the number of lacunes and CMB and was associated with decreased cognitive function (Tabara et al., 2015). The CMB is an important factor in the decline in cognitive function (Lei et al., 2013). The presence of CMB in the frontal and temporal lobes may be associated with decreased nonverbal memory, visual-spatial memory, and psychomotor speed (van Norden et al., 2011). The existence of the basal ganglia CMB may be associated with attention and computing ability, and the existence of the thalamus CMB may be associated with independent overall cognition (Yakushiji et al., 2008). Whether CMB can indirectly lead to gait and balance disorders by influencing cognition remains to be further studied. CMB interact with WMH, and similar to LI, the presence of CMB also increases the negative effects of WMH on gait and balance (Choi et al., 2012). However, whether CMB can affect gait and balance through WMH is unknown.
As discussed above, CMB can affect gait and balance through direct damage to motor pathways associated with gait and balance control.
Brain atrophy is characterized by a reduced brain volume on CT or MRI and is unrelated to local volume reduction caused by trauma or infarction. Atrophy is characterized by enlarged ventricles and widened sulci gyrus (Muller et al., 2011; Wardlaw et al., 2013b). Many imaging studies have reported the presence and severity of CSVD associated with brain atrophy, including global, cortical, subcortical, central (enlarged ventricles and basal ganglia), midbrain, and hippocampal atrophy (Appelman et al., 2009, 2010; Aribisala et al., 2013). A study of CSVD and gait disorders showed that gait scores were associated with thinning of the bilateral motor area, premotor area, dorsolateral prefrontal area, anterior cingulate area, and lateral temporoparietal occipital cortex. Thinning of the mean cortex was associated with gait severity, stooped posture, magnetic gait, small step, shuffling, and broad based gait (Kim et al., 2016). Brain white matter atrophy is associated with decreased gait speed, step length, and step frequency in community residents aged 60–86 years, hippocampal atrophy is associated with decreased gait speed and step length, and total gray matter atrophy is associated with decreased gait rhythm in patients with cerebral infarction (Callisaya et al., 2013). Basal ganglia LI, caudate nucleus atrophy, and global brain atrophy are associated with bradykinesia and balance disorders in healthy subjects aged 45–84 years (Camarda et al., 2018). Brain atrophy predicts cognitive decline, hippocampal volume correlates with changes in executive function performance, and frontal and temporal gray matter predicts changes in the verbal episodic memory performance (Aljondi et al., 2019). A study using PET technology found that reduced glucose metabolism in the posterior cingulate cortex and primary sensorimotor cortex was associated with lower gait performance (reduced step speed, reduced stride frequency) and executive function, suggesting that gait control and executive function may share the same neural substrate (Sakurai et al., 2017). Mediation analysis confirmed that thalamic atrophy mediated the effect of CSVD on walking speed in elderly people, and CSVD affected walking performance by destroying thalamic integrity (Su et al., 2018).
In summary, brain atrophy can affect gait and balance through the following two mechanisms: (1) atrophy of the cortex, white matter, and nucleus; and (2) reduced executive function.
Perivascular spaces (PVS) are formed when the arterioles and venules pass through the brain parenchyma from the subarachnoid space. PVS are important drainage channels for the interstitial fluid of the brain. When the PVS is enlarged, EPVS are seen as punctate or linear high intensity on T2-weighted MRI, and diameters are usually no more than 3 mm (Wardlaw et al., 2013b; Potter et al., 2015). The incidence of EPVS was 79.9%, and the incidence of EPVS in the basal ganglia was slightly higher than that in white matter (Zhang et al., 2014). EPVS are generally associated with other morphological features of CSVD, such as WMH and LI, but not with brain atrophy (Kwee and Kwee, 2007; Zhu et al., 2010). At present, there are few reports on EPVS related to gait and balance, and only a few studies have reported the relationship between EPVS and Parkinson’s disease and Alzheimer’s disease populations. Basal ganglia EPVS were related to the poor prognosis of Parkinson’s disease motor symptoms and the tremor score of Parkinson’s disease patients (Wan et al., 2019; Chung et al., 2021). Basal ganglia EPVS were also a biomarker of CSVD in patients with senile dementia and higher in patients with vascular dementia than in Alzheimer’s disease and healthy volunteers. Poor scores of nonverbal reasoning and global visuospatial tasks were significantly associated with EPVS load in healthy people (Hansen et al., 2015).
At present, the relationship between EPVS and gait and balance function is unclear and requires further study.
The above explores the relationship between individual imaging markers of CSVD and gait and balance. Total CSVD burden (0–4 points): ≥1 cavity is one point; DWMH score is 2 or 3 or PVWMH score is 3 points; moderate to severe EPVS (2–4 points) was scored as one point; CMB ≥1 score is 1 point (Wardlaw et al., 2013b; Staals et al., 2015). A growing body of research shows that summarizing the total CSVD load of individual markers of CDVD on the load scale may better reflect the overall effect of CSVD on the brain (Staals et al., 2015; Pinter et al., 2017). However, there have been few studies on the effect of the total burden of CSVD on gait and balance. A study of elderly subjects with vascular risk factors showed that concurrent gait function as measured according to the Parkinson’s disease assessment scale was associated with the overall CSVD burden. This is the first study to examine concurrent gait function and overall CSVD burden, although the CSVD burden score does not include EPVS (Hatate et al., 2016). A study of 678 elderly healthy subjects in the community found that the total WMH and CSVD scores were independently associated with slower gait speeds in the 6-meter walk test (Pinter et al., 2017). The results of this cross-sectional study need to be validated by large prospective studies. However, in a recent prospective study of total CSVD burden and minor stroke patients, CSVD scores at 3 years of follow-up were not associated with objective gait and balance disorders but only with subjective mobility disorders (Loos et al., 2018). The relationship between the overall burden of CSVD and gait and balance needs further study.
There is growing evidence of an interaction between CSVD and neurodegenerative diseases, and we explore the effects of both on gait and balance below.
Parkinson’s disease (PD) is a neurodegenerative disorder that causes decreased motor capacity and nonmotor symptoms (Pfeiffer, 2016). PD and CSVD are both age-related diseases (De Virgilio et al., 2016), and a large number of relevant studies in recent years have continued to confirm the prevalence of concomitant CSVD in patients with PD. The study found that 76% of patients with PD had concomitant CSVD (Song et al., 2017). PD and CSVD have a common pathological inflammatory response, and studies have confirmed that CSVD can participate in the development of PD through an inflammatory response (Lenart et al., 2016; La Vitola et al., 2018). Bohnen et al. (2011) found that the severity of WMH was positively correlated with the axial motor symptom score and bradycardia score of patients with PD. Recent studies have also confirmed that the presence of WMH is an independent risk factor for postural gait disorders in patients with PD (Wan et al., 2019). Another study found that in patients with PD, the postural instability disorder type with WMH was more severe than the tremor type, which further confirmed that WMH were associated with axial dyskinesia of PD (Malek et al., 2016). It has been suggested that WMH, especially frontal white matter lesions, may disrupt the integrity of the frontal-striatal neural circuit and the frontal parietal circuit, thereby aggravating the damage to axial motor function in patients with PD (Lee et al., 2009). There are few studies on the correlation between LI and CMB and motor symptoms in PD patients. A cross-sectional study conducted by Zhang et al. (2016) showed that patients with PD who had asymptomatic LI in the striatum had severe disruption of substantia nigra structures. The study suggests that striatal LI in patients with PD may disrupt fibrous bundles in the striatum-nigra loop, which in turn affects the severity of motor symptoms in patients with PD. A cross-sectional study was grouped according to whether the subjects had CMB, and the results suggested that CMB may have no effect on motor symptoms in patients with PD (Kim et al., 2015).
Alzheimer’s disease (AD) is a progressive neurodegenerative disease of the brain in which clinical symptoms gradually develop from cognitive impairment to dementia (Vinters, 2015). It is well known that adult patients with AD have balance and gait deficits compared to non-AD patients (Kato-Narita et al., 2011; Suttanon et al., 2012; Gras et al., 2015). As cognitive dysfunction progresses, physical function in patients with AD declines, and deficits in balance and gait become more pronounced. This is associated with impaired executive function in patients with AD (Scherder et al., 2007). Many studies have shown that CSVD increases the risk of developing AD, and pathological signs of AD can also be present in both vascular dementia and CSVD (Henry-Feugeas, 2008). Although the underlying mechanism of CSVD-induced AD pathology is unclear, it could be explained by myelin fibrosis degeneration and neuronal death due to chronic cerebral hypoperfusion or blood–brain barrier (BBB) disruption from CSVD (Pantoni, 2010; Kim H. W. et al., 2020). WMH has been widely accepted in imaging associations with aging and AD (Chen et al., 2011). For example, a longitudinal study showed that a high burden of WMH was associated with an increased risk of developing AD over a 5-year follow-up period (Ye et al., 2019). One cohort study showed that overall WMH load was associated with AD risk factors in subjects whose cognitive function was not impaired. The main drivers of this association are age and high blood pressure (Salvado et al., 2019). The role of CMB and LI in AD is unclear. A cross-sectional study found that CMB was associated with vascular burden and the diagnosis of AD (Caballero et al., 2020). A meta-analysis showed that CMB did not affect the course of AD (Liu et al., 2018). One cross-sectional study showed that elevated levels of LI were associated with increased levels of Aβ42 in vascular dementia and decreased levels of Tau in AD (Kester et al., 2014). Microinfarction is closely related to AD, and the number of microinfarcts is related to overall cognitive ability (van Rooden et al., 2014). Current studies do not support the role of EPVS in the early pathogenesis of AD (Gertje et al., 2021). Brain atrophy is the most important imaging morphological feature of AD (Guo et al., 2014), but the link between cerebral atrophy in the context of CSVD and AD remains unclear.
Multiple sclerosis (MS) is a chronic inflammatory demyelinating disorder of the central nervous system in which neurodegeneration leads to long-term disability (Lassmann et al., 2007). MS leads to gait and balance disturbances and falls (Chee et al., 2021). Age is a major determinant of MS progression, onset, and disability (Tutuncu et al., 2013). CSVD is a common age-related cerebrovascular disease (de Leeuw et al., 2001). An autopsy study showed severe small artery disease in the brain of MS patients, and MS patients had a high burden of CSVD (Geraldes et al., 2020). Multiple lines of evidence suggest that the interaction between MS and CSVD may affect MS-related neurodegeneration. The chronic inflammatory environment of MS can increase the vulnerability of the cerebral vasculature to the vascular risk factors, thereby exacerbating CSVD (Scalfari et al., 2014). At the same time, CSVD can cause oligodendrocyte damage, white matter damage due to demyelination, and neuronal loss through hypoperfusion and BBB dysfunction, and this “vasculo-neuronal inflammatory” model can be applied to MS (Wardlaw et al., 2013a; Geraldes et al., 2017). Tissue hypoxia is associated with neurological deficits (Davies et al., 2013), and the presence of tissue hypoxia associated with inflammation in MS (Desai et al., 2016). The presence of CSVD exacerbates the hypoxic environment, thereby exacerbating tissue damage (Martinez Sosa and Smith, 2017). However, a recent study of genes showed no association between WMH volume and genetic polymorphisms in MS. This study concluded that ischemic white matter damage and MS differ in the nature of their primary damage. Inflammation acts through different pathways and there are no common physiological mechanisms (Brown et al., 2019). However, the MS genetic data used in this study focused on disease risk rather than the degree of white matter lesions in the brain. The above suggests that CSVD can exacerbate MS. However, no trials have directly examined the relationship between CSVD and gait and balance deficits in MS patients.
The assessment of gait and balance function is crucial to evaluating the association between CSVD and gait and balance. The gait disorders caused by CSVD are different from the characteristics of Parkinson’s disease, so the traditional Parkinson’s disease motor assessment scale is unsuitable. The Short Physical Performance Battery (SPPB), Berg Balance Scale (BBS), Timed Up and Go Test (TUG), Tinetti Mobility Test (TMT), and other semiquantitative scales have been used more widely to measure gait and balance function in clinical studies. At present, the rise of some new measurement methods different from traditional scales, such as wearable electronic devices, can obtain more comprehensive and detailed gait parameters for more accurate evaluation.
The SPPB is a rapid, objective three-part physical function test that is a comprehensive indicator of walking speed, standing balance, and sitting performance. It has good predictive validity and broad clinical applicability. It includes a normal walking speed of more than 4 m, five sit-stand tests, and a balance test. The score of each task is four points, and the scores of the three tests are added to obtain a total, with the maximum value being 12 and the minimum value being 0. The higher the total score is, the higher the functional level is (Guralnik et al., 2000; Perera et al., 2006). The SPPB has been shown to be predictive of adverse outcomes, including all-cause mortality, disability, and hospitalization (Volpato et al., 2011; Minneci et al., 2015; Pavasini et al., 2016), and SPPB scores have been shown to be independently associated with falls among hospitalized patients and elderly community residents (Lauretani et al., 2019; Welch et al., 2021). The SPPB has been primarily used to test and measure activity levels in the recovered population, but it cannot effectively measure physical performance indicators in young people with good motor function (Treacy and Hassett, 2018).
The BBS consists of 14 items, each of which is scored from 0 (unable to perform) to 4 (normal execution) on a scale of 0–56, involving functional balance control, including transfer, steering, and walking, with a higher score indicating better balance (Berg et al., 1992). BBS is a reliable, effective, and widely used tool. It does not require people to be able to walk or stand independently. It has often been used to evaluate the body balance ability of people with various physical conditions and disabilities, such as poststroke patients (Moon et al., 2017; Ursin et al., 2019), and the BBS can also predict the loss of important activities in daily life (Wennie Huang et al., 2010). However, BBS also has limitations. The BBS cannot measure gait quality and walking speed and is not suitable for screening patients under 75 years old (Downs, 2015). The evidence supporting the use of BBS to predict falls is insufficient, and it should not be used solely to determine the risk of falls in the elderly (Lima et al., 2018).
The TUG test is a reliable and effective rapid measurement method for evaluating athletic ability and quantifying athletic performance. This test records the time it takes subjects to sit back down after completing a 3-m walk and returning to the armchair, involving the movement speed and trunk balance (Wu et al., 2019). The TUG test has been shown to have a good correlation with the BBS (Podsiadlo and Richardson, 1991), but it is more convenient and commonly used to assess senior functional activities after stroke (Chan et al., 2017). TUG can assess realistic mobility, including potential falls (Bischoff et al., 2003). However, the TUG test has some limitations. It does not address the problem of decreased motor performance when performing cognitive and motor tasks at the same time (Plummer et al., 2013). The latest meta-analysis suggests that TUG should not be used alone to predict fall risk (Park, 2018).
TMT consists of nine balance tests (0–16 points) and eight gait tests (0–12 points), with a higher total score indicating better balance and motor function. TMT is a reliable and effective method for assessing mobility, balance and gait, as well as predicting the risk of falling (Kopke and Meyer, 2006; Panzer et al., 2011). TMT has been widely used in studies of older people, as well as in other diseases, such as Parkinson’s disease and stroke (Alvarez et al., 2020; Perez-de la Cruz, 2020). However, recent studies have shown that TMT alone does not predict fall risk in older adults (Omana et al., 2021).
Gait and balance instability are important factors that cause falls in older adults (Wei et al., 2017; Bower et al., 2019), so screening out patients with CSVD with impaired gait and balance function and intervening with pharmacological and nonpharmacological interventions is important to improve the prognosis of CSVD.
Walking speed is a very meaningful parameter for assessing the health of older adults, and if a person’s spontaneous walking speed is below 0.6 m/s, it is considered problematic (Panzer et al., 2011; Studenski et al., 2011). A total TMT score <19, 3BBS scores < 40 (Muir et al., 2008), and TUG ≥ 13.5 s (Clemson et al., 2015) indicate a higher risk of falling. A simple balance test, such as recording the time spent standing on one leg or in tandem, can also be used to identify CSVD patients at risk of falling early and assess their balance function (Jahn et al., 2015). Gait degradation under dual-task requirements is typical of gait disorders with cortical and subcortical involvement. Setting up dual tasks can catch defects at an early stage and suggest preventive measures such as increasing physical activity by increasing daily walking distance (Montero-Odasso et al., 2012). Subjective visual vertical tests are helpful in patients with unstable gait and a tendency to fall and are simple to perform (“bucket test”; Jahn et al., 2015).
In patients with impaired gait and balance, early rehabilitation is necessary. Traditional gait training methods include one-legged weight-bearing, the center of gravity shifting, stepping and hip stretching training, using the affected limb to go up and down stairs, walking laterally, and walking in place (Brock et al., 2011). Balance training included standing on one leg, walking in a straight line, throwing and catching the ball, standing in front of the mirror and being pushed in different directions by the therapist, and moving the body’s center of gravity forward, backward, sideways, and obliquely when the eyes were open and closed (Chung et al., 2014). The pro-kin system is a new visual feedback balance training method. The combination of pro-Kin system and conventional rehabilitation therapy can improve the gait and balance function of CSVD patients without obvious cognitive impairment better than traditional rehabilitation training (You et al., 2017; Zhao et al., 2019). In addition to physical exercises such as walking, people can also try exercise cognitive training such as tai chi and Dalcroze music pedagogy to improve the executive function of the elderly (Jahn et al., 2015).
Blood pressure is the most important controllable risk factor for CSVD. Intensive antihypertensive therapy can reduce the progression of WMH but the effect on brain atrophy is unclear (van Middelaar et al., 2018; Su et al., 2021). Intensive blood pressure lowering reduced the recurrence of stroke in patients with LI through a multicenter clinical trial called SPS3 (SPS3 Study Group et al., 2013). A more aggressive antihypertensive regimen may be justified, but too low a blood pressure carries the risk of cognitive impairment (Webb et al., 2010). Blood pressure variability is associated with WMH, CMB, EPVS, and total CSVD burden and may also lead to a poor prognosis. Therefore, reducing ambulatory blood pressure variability is important for the control of CSVD (Chen et al., 2019; Zhang et al., 2019a).
Patients who received intravenous r-tPA had a better neurological prognosis than those who received a placebo in LI (Mok and Kim, 2015). The presence of CMB and WMH increases the risk of symptomatic cerebral hemorrhage and poor prognosis, but patients still have a net benefit (Charidimou et al., 2016). The benefits and risks of intravenous thrombolysis in patients with CSVD need to be assessed individually to minimize the occurrence of cerebral hemorrhage and poor prognosis after thrombolysis in ischemic stroke.
For antiplatelet therapy, refer to the 2018 American Heart Association/American Stroke Association published guidelines for the early management of patients with acute ischemic stroke (Powers et al., 2019). Given the bidirectional nature of CSVD with both ischemia and bleeding risk, an assessment of treatment benefits and bleeding risk should be performed. Antiplatelet agents should be used with caution in patients with severe WMH and a large number of CMB. In patients with severe WMH and multiple CMB, cilostazol may be a safer option compared to aspirin and clopidogrel (Kim et al., 2020).
Statins have been shown to lower lipids, reduce oxidative stress and protect the vascular endothelium (Sander et al., 2005; Erdos et al., 2006), which may reduce the risk of stroke recurrence including ischemic stroke due to CSVD (Amarenco et al., 2009). Studies have shown that the application of statins can reduce the progression of WMH and the decline in cognitive function (Xiong et al., 2014). However, in patients with severe CSVD, high doses of statin are associated with an increased risk of a cerebral hemorrhage (Amarenco et al., 2009).
Daily B vitamins can lower homocysteine and thus may reduce WMH volume progression in patients with severe CSVD (Cavalieri et al., 2012). In addition, vitamin E tocotrienols slow the progression of WMH in subjects with brain white matter injury (Gopalan et al., 2014). Prevention and treatment strategies for CMB should target cerebral microvascular endothelium and function, blood-brain barrier, and neuroinflammation, such as endothelin antagonists, peroxisome proliferator-activated receptor-agonists, and neurotrophic factors (Nagasawa et al., 2014).
CSVD-associated cognitive dysfunction is an important subtype of vascular cognitive dysfunction and is one of the common causes of vascular dementia. There is some degree of overlap in the neuropathology of AD and vascular dementia, and cholinergic insufficiency may lead to vascular cognitive impairment (Roman and Kalaria, 2006). Various drugs, including donepezil and galantamine, have shown modest cognitive benefits in patients with vascular dementia. However, the benefits of other drugs, such as rivastigmine, memantine, nimodipine, piracetam, and herbal remedies, remain unclear (Farooq et al., 2017). Large randomized controlled trials have shown benefits in improving cognitive function in patients with mild to moderate vascular cognitive impairment at 24 weeks, and patients tolerate donepezil well (Goldsmith and Scott, 2003; Malouf and Birks, 2004). Nevertheless, in patients with CADASIL alone, donepezil did not significantly improve overall cognitive function (Dichgans et al., 2008). After 6 months of galantamine use, patients with mild to moderate cognitive impairment with AD and vascular dementia had significant improvements in cognitive function, but the side effects were significant (Erkinjuntti et al., 2002; Auchus et al., 2007). There are also small sample studies that have found that antihypertensive drugs, statins, and nimodipine drugs may have some effect on CSVD-related cognitive impairment, but more large randomized controlled trials are needed to confirm this (Zhang et al., 2019b; Zhang J. et al., 2019). Patients with vascular depression responded poorly to common antidepressants, and the response rate of combination therapy with citalopram and methylphenidate was higher than that of the drug alone. This treatment also leads to improved function (Lavretsky et al., 2015; Smith et al., 2021). In elderly patients with depression and cognitive impairment, the combined use of antidepressants and donepezil had a temporary positive effect on cognitive function but increases the risk of recurrence (Reynolds et al., 2011).
In conclusion, exploring the correlation between CSVD and gait and balance disorders is of great clinical significance for the diagnosis and treatment of gait and balance disorders and the prevention of falls. CSVD is a disease in which multiple pathological changes can coexist, and its influence on gait and balance function is not caused by a single factor but may be the result of the joint action of multiple factors. In addition, imaging features of different CSVD and injury of different parts may lead to different characteristics and mechanisms of gait and balance injury but there may be overlap between them. And different small vascular diseases may also affect each other. In general, CSVD can affect gait and balance by affecting cognitive function, disrupting gait structures associated with balance control, influencing depressive states, and interactions. Rehabilitation and medication may play a role in gait and balance disorders.
Currently, most studies on CSVD and gait and balance disorders are cross-sectional studies or retrospective studies, and more prospective studies with large samples are needed in the future to further clarify the correlation between the two. In terms of the crowd, future studies should stratify patients with different causes and severity of the disease, such as the mild stroke population (Loos et al., 2018). The application of advanced imaging technology, such as VBM technology and DTI technology, can be a more accurate and comprehensive evaluation of the imaging appearances of CSVD, to clarify the role of CSVD in different brain diseases. As for the evaluation methods of gait and balance function, new measurement methods such as electronic sensors should be applied in gait and balance evaluation in the future, so as to obtain a more real and comprehensive evaluation and reduce the results bias caused by different test methods. Regarding the treatment of CSVD-related gait and balance disorders, future large-scale randomized controlled trials are needed to determine the direct effects of medication or rehabilitation on gait and balance function. The mechanisms of CSVD affecting gait and balance need to be further clarified, so as to provide more perspectives for the prevention and treatment of gait and balance disorders.
RZ planned the study. CS, XY, and SW analyzed the data and edited the manuscript. CS wrote the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Aljondi, R., Szoeke, C., Steward, C., Yates, P., and Desmond, P. (2019). A decade of changes in brain volume and cognition. Brain Imaging Behav. 13, 554–563. doi: 10.1007/s11682-018-9887-z
Alvarez, I., Latorre, J., Aguilar, M., Pastor, P., and Llorens, R. (2020). Validity and sensitivity of instrumented postural and gait assessment using low-cost devices in Parkinson’s disease. J. Neuroeng. Rehabil. 17:149. doi: 10.1186/s12984-020-00770-7
Amarenco, P., Benavente, O., Goldstein, L. B., Callahan, A., 3rd, Sillesen, H., Hennerici, M. G., et al. (2009). Results of the stroke prevention by aggressive reduction in cholesterol levels (SPARCL) trial by stroke subtypes. Stroke 40, 1405–1409. doi: 10.1161/STROKEAHA.108.534107
Appelman, A. P., Exalto, L. G., van der Graaf, Y., Biessels, G. J., Mali, W. P., and Geerlings, M. I. (2009). White matter lesions and brain atrophy: more than shared risk factors? A systematic review. Cerebrovasc. Dis. 28, 227–242. doi: 10.1159/000226774
Appelman, A. P., Vincken, K. L., van der Graaf, Y., Vlek, A. L., Witkamp, T. D., Mali, W. P., et al. (2010). White matter lesions and lacunar infarcts are independently and differently associated with brain atrophy: the SMART-MR study. Cerebrovasc. Dis. 29, 28–35. doi: 10.1159/000255971
Aribisala, B. S., Valdes Hernandez, M. C., Royle, N. A., Morris, Z., Munoz Maniega, S., Bastin, M. E., et al. (2013). Brain atrophy associations with white matter lesions in the ageing brain: the Lothian Birth Cohort 1936. Eur. Radiol. 23, 1084–1092. doi: 10.1007/s00330-012-2677-x
Auchus, A. P., Brashear, H. R., Salloway, S., Korczyn, A. D., De Deyn, P. P., Gassmann-Mayer, C., et al. (2007). Galantamine treatment of vascular dementia: a randomized trial. Neurology 69, 448–458. doi: 10.1212/01.wnl.0000266625.31615.f6
Bazner, H., Oster, M., Daffertshofer, M., and Hennerici, M. (2000). Assessment of gait in subcortical vascular encephalopathy by computerized analysis: a cross-sectional and longitudinal study. J. Neurol. 247, 841–849. doi: 10.1007/s004150070070
Benjamin, P., Lawrence, A. J., Lambert, C., Patel, B., Chung, A. W., MacKinnon, A. D., et al. (2014). Strategic lacunes and their relationship to cognitive impairment in cerebral small vessel disease. Neuroimage Clin. 4, 828–837. doi: 10.1016/j.nicl.2014.05.009
Benjamin, P., Trippier, S., Lawrence, A. J., Lambert, C., Zeestraten, E., Williams, O. A., et al. (2018). Lacunar infarcts, but not perivascular spaces, are predictors of cognitive decline in cerebral small-vessel disease. Stroke 49, 586–593. doi: 10.1161/STROKEAHA.117.017526
Berg, K. O., Wood-Dauphinee, S. L., Williams, J. I., and Maki, B. (1992). Measuring balance in the elderly: validation of an instrument. Can. J. Public Health 83, S7–S11.
Bischoff, H. A., Stahelin, H. B., Monsch, A. U., Iversen, M. D., Weyh, A., von Dechend, M., et al. (2003). Identifying a cut-off point for normal mobility: a comparison of the timed ‘up and go’ test in community-dwelling and institutionalised elderly women. Age Ageing 32, 315–320. doi: 10.1093/ageing/32.3.315
Blahak, C., Baezner, H., Pantoni, L., Poggesi, A., Chabriat, H., Erkinjuntti, T., et al. (2009). Deep frontal and periventricular age related white matter changes but not basal ganglia and infratentorial hyperintensities are associated with falls: cross sectional results from the LADIS study. J. Neurol. Neurosurg. Psychiatry 80, 608–613. doi: 10.1136/jnnp.2008.154633
Bohnen, N. I., Muller, M. L., Zarzhevsky, N., Koeppe, R. A., Bogan, C. W., Kilbourn, M. R., et al. (2011). Leucoaraiosis, nigrostriatal denervation and motor symptoms in Parkinson’s disease. Brain 134, 2358–2365. doi: 10.1093/brain/awr139
Bower, K., Thilarajah, S., Pua, Y. H., Williams, G., Tan, D., Mentiplay, B., et al. (2019). Dynamic balance and instrumented gait variables are independent predictors of falls following stroke. J. Neuroeng. Rehabil. 16:3. doi: 10.1186/s12984-018-0478-4
Brock, K., Haase, G., Rothacher, G., and Cotton, S. (2011). Does physiotherapy based on the Bobath concept, in conjunction with a task practice, achieve greater improvement in walking ability in people with stroke compared to physiotherapy focused on structured task practice alone?: A pilot randomized controlled trial. Clin. Rehabil. 25, 903–912. doi: 10.1177/0269215511406557
Brown, R. B., Traylor, M., Burgess, S., Sawcer, S., and Markus, H. S. (2019). Do cerebral small vessel disease and multiple sclerosis share common mechanisms of white matter injury? Stroke 50, 1968–1972. doi: 10.1161/STROKEAHA.118.023649
Caballero, M. A. A., Song, Z., Rubinski, A., Duering, M., Dichgans, M., Park, D. C., et al. (2020). Age-dependent amyloid deposition is associated with white matter alterations in cognitively normal adults during the adult life span. Alzheimers Dement. 16, 651–661. doi: 10.1002/alz.12062
Cai, M., Jacob, M. A., Norris, D. G., Duering, M., de Leeuw, F. E., and Tuladhar, A. M. (2021). Cognition mediates the relation between structural network efficiency and gait in small vessel disease. Neuroimage Clin. 30:102667. doi: 10.1016/j.nicl.2021.102667
Callisaya, M. L., Beare, R., Phan, T. G., Blizzard, L., Thrift, A. G., Chen, J., et al. (2013). Brain structural change and gait decline: a longitudinal population-based study. J. Am. Geriatr. Soc. 61, 1074–1079. doi: 10.1111/jgs.12331
Camarda, C., Torelli, P., Pipia, C., Battaglini, I., Azzarello, D., Rosano, R., et al. (2018). Association between atrophy of the caudate nuclei, global brain atrophy, cerebral small vessel disease and mild Parkinsonian signs in neurologically and cognitively healthy subjects aged 45-84 years: a crosssectional study. Curr. Alzheimer Res. 15, 1013–1026. doi: 10.2174/1567205015666180702111110
Cavalieri, M., Schmidt, R., Chen, C., Mok, V., de Freitas, G. R., Song, S., et al. (2012). B vitamins and magnetic resonance imaging-detected ischemic brain lesions in patients with recent transient ischemic attack or stroke: the VITAmins TO Prevent Stroke (VITATOPS) MRI-substudy. Stroke 43, 3266–3270. doi: 10.1161/STROKEAHA.112.665703
Chan, P. P., Si Tou, J. I., Tse, M. M., and Ng, S. S. (2017). Reliability and validity of the timed up and go test with a motor task in people with chronic stroke. Arch. Phys. Med. Rehabil. 98, 2213–2220. doi: 10.1016/j.apmr.2017.03.008
Chao, Y. P., Cho, K. H., Yeh, C. H., Chou, K. H., Chen, J. H., and Lin, C. P. (2009). Probabilistic topography of human corpus callosum using cytoarchitectural parcellation and high angular resolution diffusion imaging tractography. Hum. Brain Mapp. 30, 3172–3187. doi: 10.1002/hbm.20739
Charidimou, A., Pasi, M., Fiorelli, M., Shams, S., von Kummer, R., Pantoni, L., et al. (2016). Leukoaraiosis, cerebral hemorrhage and outcome after intravenous thrombolysis for acute ischemic stroke: a meta-analysis (v1). Stroke 47, 2364–2372. doi: 10.1161/STROKEAHA.116.014096
Chee, J. N., Ye, B., Gregor, S., Berbrayer, D., Mihailidis, A., and Patterson, K. K. (2021). Influence of multiple sclerosis on spatiotemporal gait parameters: a systematic review and meta-regression. Arch. Phys. Med. Rehabil. 102, 1801–1815. doi: 10.1016/j.apmr.2020.12.013
Chen, W., Song, X., Zhang, Y., and Alzheimer’s Disease Neuroimaging Initiative (2011). Assessment of the virchow-robin spaces in Alzheimer disease, mild cognitive impairment and normal aging, using high-field MR imaging. Am. J. Neuroradiol. 32, 1490–1495. doi: 10.3174/ajnr.A2541
Chen, X., Zhu, Y., Geng, S., Li, Q., and Jiang, H. (2019). Association of blood pressure variability and intima-media thickness with white matter hyperintensities in hypertensive patients. Front. Aging Neurosci. 11:192. doi: 10.3389/fnagi.2019.00192
Choi, P., Ren, M., Phan, T. G., Callisaya, M., Ly, J. V., Beare, R., et al. (2012). Silent infarcts and cerebral microbleeds modify the associations of white matter lesions with gait and postural stability: population-based study. Stroke 43, 1505–1510. doi: 10.1161/STROKEAHA.111.647271
Chung, E., Lee, B. H., and Hwang, S. (2014). Core stabilization exercise with real-time feedback for chronic hemiparetic stroke: a pilot randomized controlled trials. Restor. Neurol. Neurosci. 32, 313–321. doi: 10.3233/RNN-130353
Chung, S. J., Yoo, H. S., Shin, N. Y., Park, Y. W., Lee, H. S., Hong, J. M., et al. (2021). Perivascular spaces in the basal ganglia and long-term motor prognosis in newly diagnosed Parkinson disease. Neurology 96, e2121–e2131. doi: 10.1212/WNL.0000000000011797
Clemson, L., Kendig, H., Mackenzie, L., and Browning, C. (2015). Predictors of injurious falls and fear of falling differ: an 11-year longitudinal study of incident events in older people. J. Aging Health 27, 239–256. doi: 10.1177/0898264314546716
Cordonnier, C., Al-Shahi Salman, R., and Wardlaw, J. (2007). Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain 130, 1988–2003. doi: 10.1093/brain/awl387
Davies, A. L., Desai, R. A., Bloomfield, P. S., McIntosh, P. R., Chapple, K. J., Linington, C., et al. (2013). Neurological deficits caused by tissue hypoxia in neuroinflammatory disease. Ann. Neurol. 74, 815–825. doi: 10.1002/ana.24006
de Laat, K. F., van den Berg, H. A., van Norden, A. G., Gons, R. A., Olde Rikkert, M. G., and de Leeuw, F. E. (2011). Microbleeds are independently related to gait disturbances in elderly individuals with cerebral small vessel disease. Stroke 42, 494–497. doi: 10.1161/STROKEAHA.110.596122
de Laat, K. F., van Norden, A. G., Gons, R. A., van Oudheusden, L. J., van Uden, I. W., Bloem, B. R., et al. (2010). Gait in elderly with cerebral small vessel disease. Stroke 41, 1652–1658. doi: 10.1161/STROKEAHA.110.583229
de Leeuw, F. E., de Groot, J. C., Achten, E., Oudkerk, M., Ramos, L. M., Heijboer, R., et al. (2001). Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The rotterdam scan study. J. Neurol. Neurosurg. Psychiatry 70, 9–14. doi: 10.1136/jnnp.70.1.9
De Virgilio, A., Greco, A., Fabbrini, G., Inghilleri, M., Rizzo, M. I., Gallo, A., et al. (2016). Parkinson’s disease: autoimmunity and neuroinflammation. Autoimmun. Rev. 15, 1005–1011. doi: 10.1016/j.autrev.2016.07.022
Debette, S., and Markus, H. S. (2010). The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 341:c3666. doi: 10.1136/bmj.c3666
Desai, R. A., Davies, A. L., Tachrount, M., Kasti, M., Laulund, F., Golay, X., et al. (2016). Cause and prevention of demyelination in a model multiple sclerosis lesion. Ann. Neurol. 79, 591–604. doi: 10.1002/ana.24607
Dichgans, M., Markus, H. S., Salloway, S., Verkkoniemi, A., Moline, M., Wang, Q., et al. (2008). Donepezil in patients with subcortical vascular cognitive impairment: a randomised double-blind trial in CADASIL. Lancet Neurol. 7, 310–318. doi: 10.1016/S1474-4422(08)70046-2
Ding, J., Sigurdsson, S., Garcia, M., Phillips, C. L., Eiriksdottir, G., Gudnason, V., et al. (2015). Risk factors associated with incident cerebral microbleeds according to location in older people: the age, gene/environment susceptibility (AGES)-reykjavik study. JAMA Neurol. 72, 682–688. doi: 10.1001/jamaneurol.2015.0174
Erdos, B., Snipes, J. A., Tulbert, C. D., Katakam, P., Miller, A. W., and Busija, D. W. (2006). Rosuvastatin improves cerebrovascular function in Zucker obese rats by inhibiting NAD(P)H oxidase-dependent superoxide production. Am. J. Physiol. Heart Circ. Physiol. 290, H1264–1270. doi: 10.1152/ajpheart.00804.2005
Erkinjuntti, T., Kurz, A., Gauthier, S., Bullock, R., Lilienfeld, S., and Damaraju, C. V. (2002). Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet 359, 1283–1290. doi: 10.1016/S0140-6736(02)08267-3
Farooq, M. U., Min, J., Goshgarian, C., and Gorelick, P. B. (2017). Pharmacotherapy for vascular cognitive impairment. CNS Drugs 31, 759–776. doi: 10.1007/s40263-017-0459-3
Finsterwalder, S., Wuehr, M., Gesierich, B., Dietze, A., Konieczny, M. J., Schmidt, R., et al. (2019). Minor gait impairment despite white matter damage in pure small vessel disease. Ann. Clin. Transl. Neurol. 6, 2026–2036. doi: 10.1002/acn3.50891
Fujishima, M., Maikusa, N., Nakamura, K., Nakatsuka, M., Matsuda, H., and Meguro, K. (2014). Mild cognitive impairment, poor episodic memory and late-life depression are associated with cerebral cortical thinning and increased white matter hyperintensities. Front. Aging Neurosci. 6:306. doi: 10.3389/fnagi.2014.00306
Garde, E., Mortensen, E. L., Krabbe, K., Rostrup, E., and Larsson, H. B. (2000). Relation between age-related decline in intelligence and cerebral white-matter hyperintensities in healthy octogenarians: a longitudinal study. Lancet 356, 628–634. doi: 10.1016/S0140-6736(00)02604-0
Geraldes, R., Esiri, M. M., DeLuca, G. C., and Palace, J. (2017). Age-related small vessel disease: a potential contributor to neurodegeneration in multiple sclerosis. Brain Pathol. 27, 707–722. doi: 10.1111/bpa.12460
Geraldes, R., Esiri, M. M., Perera, R., Yee, S. A., Jenkins, D., Palace, J., et al. (2020). Vascular disease and multiple sclerosis: a post-mortem study exploring their relationships. Brain 143, 2998–3012. doi: 10.1093/brain/awaa255
Gertje, E. C., van Westen, D., Panizo, C., Mattsson-Carlgren, N., and Hansson, O. (2021). Association of enlarged perivascular spaces and measures of small vessel and Alzheimer disease. Neurology 96, e193–e202. doi: 10.1212/WNL.0000000000011046
Goldsmith, D. R., and Scott, L. J. (2003). Donepezil: in vascular dementia. Drugs Aging 20, 1127–1136. doi: 10.2165/00002512-200320150-00005
Gopalan, Y., Shuaib, I. L., Magosso, E., Ansari, M. A., Abu Bakar, M. R., Wong, J. W., et al. (2014). Clinical investigation of the protective effects of palm vitamin E tocotrienols on brain white matter. Stroke 45, 1422–1428. doi: 10.1161/STROKEAHA.113.004449
Gouw, A. A., Seewann, A., van der Flier, W. M., Barkhof, F., Rozemuller, A. M., Scheltens, P., et al. (2011). Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J. Neurol. Neurosurg. Psychiatry 82, 126–135. doi: 10.1136/jnnp.2009.204685
Gras, L. Z., Kanaan, S. F., McDowd, J. M., Colgrove, Y. M., Burns, J., and Pohl, P. S. (2015). Balance and gait of adults with very mild Alzheimer disease. J. Geriatr. Phys. Ther. 38, 1–7. doi: 10.1519/JPT.0000000000000020
Grool, A. M., Gerritsen, L., Zuithoff, N. P., Mali, W. P., van der Graaf, Y., and Geerlings, M. I. (2013). Lacunar infarcts in deep white matter are associated with higher and more fluctuating depressive symptoms during three years follow-up. Biol. Psychiatry 73, 169–176. doi: 10.1016/j.biopsych.2012.08.024
Guo, H., Song, X., Vandorpe, R., Zhang, Y., Chen, W., Zhang, N., et al. (2014). Evaluation of common structural brain changes in aging and Alzheimer disease with the use of an MRI-based brain atrophy and lesion index: a comparison between T1WI and T2WI at 1.5T and 3T. Am. J. Neuroradiol. 35, 504–512. doi: 10.3174/ajnr.A3709
Guralnik, J. M., Ferrucci, L., Pieper, C. F., Leveille, S. G., Markides, K. S., Ostir, G. V., et al. (2000). Lower extremity function and subsequent disability: consistency across studies, predictive models and value of gait speed alone compared with the short physical performance battery. J. Gerontol. A Biol. Sci. Med. Sci. 55, M221–M231. doi: 10.1093/gerona/55.4.m221
Hansen, T. P., Cain, J., Thomas, O., and Jackson, A. (2015). Dilated perivascular spaces in the Basal Ganglia are a biomarker of small-vessel disease in a very elderly population with dementia. Am. J. Neuroradiol. 36, 893–898. doi: 10.3174/ajnr.A4237
Hatate, J., Miwa, K., Matsumoto, M., Sasaki, T., Yagita, Y., Sakaguchi, M., et al. (2016). Association between cerebral small vessel diseases and mild parkinsonian signs in the elderly with vascular risk factors. Parkinsonism Relat. Disord. 26, 29–34. doi: 10.1016/j.parkreldis.2016.02.011
Henry-Feugeas, M. C. (2008). Alzheimer’s disease in late-life dementia: a minor toxic consequence of devastating cerebrovascular dysfunction. Med. Hypotheses 70, 866–875. doi: 10.1016/j.mehy.2007.07.027
Horak, F. B. (2006). Postural orientation and equilibrium: what do we need to know about neural control of balance to prevent falls? Age Ageing 35, ii7–ii11. doi: 10.1093/ageing/afl077
Jahn, K., Kressig, R. W., Bridenbaugh, S. A., Brandt, T., and Schniepp, R. (2015). Dizziness and unstable gait in old age: etiology, diagnosis and treatment. Dtsch. Arztebl. Int. 112, 387–393. doi: 10.3238/arztebl.2015.0387
Jang, S. H. (2009). A review of corticospinal tract location at corona radiata and posterior limb of the internal capsule in human brain. NeuroRehabilitation 24, 279–283. doi: 10.3233/NRE-2009-0479
Kato-Narita, E. M., Nitrini, R., and Radanovic, M. (2011). Assessment of balance in mild and moderate stages of Alzheimer’s disease: implications on falls and functional capacity. Arq. Neuropsiquiatr. 69, 202–207. doi: 10.1590/s0004-282x2011000200012
Kester, M. I., Goos, J. D., Teunissen, C. E., Benedictus, M. R., Bouwman, F. H., Wattjes, M. P., et al. (2014). Associations between cerebral small-vessel disease and Alzheimer disease pathology as measured by cerebrospinal fluid biomarkers. JAMA Neurol. 71, 855–862. doi: 10.1001/jamaneurol.2014.754
Kim, H. W., Hong, J., and Jeon, J. C. (2020). Cerebral small vessel disease and Alzheimer’s disease: a review. Front. Neurol. 11:927. doi: 10.3389/fneur.2020.00927
Kim, Y. J., Kwon, H. K., Lee, J. M., Cho, H., Kim, H. J., Park, H. K., et al. (2016). Gray and white matter changes linking cerebral small vessel disease to gait disturbances. Neurology 86, 1199–1207. doi: 10.1212/WNL.0000000000002516
Kim, B. J., Kwon, S. U., Park, J. H., Kim, Y. J., Hong, K. S., Wong, L. K. S., et al. (2020). Cilostazol versus aspirin in ischemic stroke patients with high-risk cerebral hemorrhage: subgroup analysis of the PICASSO trial. Stroke 51, 931–937. doi: 10.1161/STROKEAHA.119.023855
Kim, J. H., Park, J., Kim, Y. H., Ma, H. I., and Kim, Y. J. (2015). Characterization of cerebral microbleeds in idiopathic Parkinson’s disease. Eur. J. Neurol. 22, 377–383. doi: 10.1111/ene.12584
Kopke, S., and Meyer, G. (2006). The Tinetti test: babylon in geriatric assessment. Z. Gerontol. Geriatr. 39, 288–291. doi: 10.1007/s00391-006-0398-y
Kwee, R. M., and Kwee, T. C. (2007). Virchow-Robin spaces at MR imaging. Radiographics 27, 1071–1086. doi: 10.1148/rg.274065722
La Vitola, P., Balducci, C., Cerovic, M., Santamaria, G., Brandi, E., Grandi, F., et al. (2018). Alpha-synuclein oligomers impair memory through glial cell activation and via Toll-like receptor 2. Brain Behav. Immun. 69, 591–602. doi: 10.1016/j.bbi.2018.02.012
Lassmann, H., Bruck, W., and Lucchinetti, C. F. (2007). The immunopathology of multiple sclerosis: an overview. Brain Pathol. 17, 210–218. doi: 10.1111/j.1750-3639.2007.00064.x
Lauretani, F., Ticinesi, A., Gionti, L., Prati, B., Nouvenne, A., Tana, C., et al. (2019). Short-physical performance battery (SPPB) score is associated with falls in older outpatients. Aging Clin. Exp. Res. 31, 1435–1442. doi: 10.1007/s40520-018-1082-y
Lavretsky, H., Reinlieb, M., St Cyr, N., Siddarth, P., Ercoli, L. M., and Senturk, D. (2015). Citalopram, methylphenidate, or their combination in geriatric depression: a randomized, double-blind, placebo-controlled trial. Am. J. Psychiatry 172, 561–569. doi: 10.1176/appi.ajp.2014.14070889
Lee, S. J., Kim, J. S., Lee, K. S., An, J. Y., Kim, W., Kim, Y. I., et al. (2009). The severity of leukoaraiosis correlates with the clinical phenotype of Parkinson’s disease. Arch. Gerontol. Geriatr. 49, 255–259. doi: 10.1016/j.archger.2008.09.005
Lei, C., Lin, S., Tao, W., Hao, Z., Liu, M., and Wu, B. (2013). Association between cerebral microbleeds and cognitive function: a systematic review. J. Neurol. Neurosurg. Psychiatry 84, 693–697. doi: 10.1136/jnnp-2012-303948
Lenart, N., Brough, D., and Denes, A. (2016). Inflammasomes link vascular disease with neuroinflammation and brain disorders. J. Cereb. Blood Flow Metab. 36, 1668–1685. doi: 10.1177/0271678X16662043
Li, Q., Yang, Y., Reis, C., Tao, T., Li, W., Li, X., et al. (2018). Cerebral small vessel disease. Cell Transplant. 27, 1711–1722. doi: 10.1177/0963689718795148
Lima, C. A., Ricci, N. A., Nogueira, E. C., and Perracini, M. R. (2018). The berg balance scale as a clinical screening tool to predict fall risk in older adults: a systematic review. Physiotherapy 104, 383–394. doi: 10.1016/j.physio.2018.02.002
Liu, Y., Braidy, N., Poljak, A., Chan, D. K. Y., and Sachdev, P. (2018). Cerebral small vessel disease and the risk of Alzheimer’s disease: a systematic review. Ageing Res. Rev. 47, 41–48. doi: 10.1016/j.arr.2018.06.002
Loos, C. M., McHutchison, C., Cvoro, V., Makin, S. D., Staals, J., Chappell, F., et al. (2018). The relation between total cerebral small vessel disease burden and gait impairment in patients with minor stroke. Int. J. Stroke 13, 518–524. doi: 10.1177/1747493017730780
Malek, N., Lawton, M. A., Swallow, D. M., Grosset, K. A., Marrinan, S. L., Bajaj, N., et al. (2016). Vascular disease and vascular risk factors in relation to motor features and cognition in early Parkinson’s disease. Mov. Disord. 31, 1518–1526. doi: 10.1002/mds.26698
Malouf, R., and Birks, J. (2004). Donepezil for vascular cognitive impairment. Cochrane Database Syst. Rev. CD004395. doi: 10.1002/14651858.CD004395.pub2
Martinez Sosa, S., and Smith, K. J. (2017). Understanding a role for hypoxia in lesion formation and location in the deep and periventricular white matter in small vessel disease and multiple sclerosis. Clin. Sci. (Lond) 131, 2503–2524. doi: 10.1042/CS20170981
Minneci, C., Mello, A. M., Mossello, E., Baldasseroni, S., Macchi, L., Cipolletti, S., et al. (2015). Comparative study of four physical performance measures as predictors of death, incident disability and falls in unselected older persons: the insufficienza cardiaca negli anziani residenti a dicomano study. J. Am. Geriatr. Soc. 63, 136–141. doi: 10.1111/jgs.13195
Mok, V., and Kim, J. S. (2015). Prevention and management of cerebral small vessel disease. J. Stroke 17, 111–122. doi: 10.5853/jos.2015.17.2.111
Montero-Odasso, M., Verghese, J., Beauchet, O., and Hausdorff, J. M. (2012). Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J. Am. Geriatr. Soc. 60, 2127–2136. doi: 10.1111/j.1532-5415.2012.04209.x
Moon, H. I., Lee, H. J., and Yoon, S. Y. (2017). Lesion location associated with balance recovery and gait velocity change after rehabilitation in stroke patients. Neuroradiology 59, 609–618. doi: 10.1007/s00234-017-1840-0
Muir, S. W., Berg, K., Chesworth, B., and Speechley, M. (2008). Use of the Berg Balance Scale for predicting multiple falls in community-dwelling elderly people: a prospective study. Phys. Ther. 88, 449–459. doi: 10.2522/ptj.20070251
Muller, M., Appelman, A. P., van der Graaf, Y., Vincken, K. L., Mali, W. P., and Geerlings, M. I. (2011). Brain atrophy and cognition: interaction with cerebrovascular pathology? Neurobiol. Aging 32, 885–893. doi: 10.1016/j.neurobiolaging.2009.05.005
Nagasawa, J., Kiyozaka, T., and Ikeda, K. (2014). Prevalence and clinicoradiological analyses of patients with Alzheimer disease coexisting multiple microbleeds. J. Stroke Cerebrovasc. Dis. 23, 2444–2449. doi: 10.1016/j.jstrokecerebrovasdis.2014.05.036
Okroglic, S., Widmann, C. N., Urbach, H., Scheltens, P., and Heneka, M. T. (2013). Clinical symptoms and risk factors in cerebral microangiopathy patients. PLoS One 8:e53455. doi: 10.1371/journal.pone.0053455
Omana, H., Bezaire, K., Brady, K., Davies, J., Louwagie, N., Power, S., et al. (2021). Functional reach test, single-leg stance test and tinetti performance-oriented mobility assessment for the prediction of falls in older adults: a systematic review. Phys. Ther. 101:pzab173. doi: 10.1093/ptj/pzab173
Onteddu, S. R., Goddeau, R. P., Jr., Minaeian, A., and Henninger, N. (2015). Clinical impact of leukoaraiosis burden and chronological age on neurological deficit recovery and 90-day outcome after minor ischemic stroke. J. Neurol. Sci. 359, 418–423. doi: 10.1016/j.jns.2015.10.005
Pantoni, L. (2010). Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 9, 689–701. doi: 10.1016/S1474-4422(10)70104-6
Panzer, V. P., Wakefield, D. B., Hall, C. B., and Wolfson, L. I. (2011). Mobility assessment: sensitivity and specificity of measurement sets in older adults. Arch. Phys. Med. Rehabil. 92, 905–912. doi: 10.1016/j.apmr.2011.01.004
Park, S. H. (2018). Tools for assessing fall risk in the elderly: a systematic review and meta-analysis. Aging Clin. Exp. Res. 30, 1–16. doi: 10.1007/s40520-017-0749-0
Pasi, M., Boulouis, G., Fotiadis, P., Auriel, E., Charidimou, A., Haley, K., et al. (2017). Distribution of lacunes in cerebral amyloid angiopathy and hypertensive small vessel disease. Neurology 88, 2162–2168. doi: 10.1212/WNL.0000000000004007
Pasi, M., van Uden, I. W., Tuladhar, A. M., de Leeuw, F. E., and Pantoni, L. (2016). White Matter microstructural damage on diffusion tensor imaging in cerebral small vessel disease: clinical consequences. Stroke 47, 1679–1684. doi: 10.1161/STROKEAHA.115.012065
Pavasini, R., Guralnik, J., Brown, J. C., di Bari, M., Cesari, M., Landi, F., et al. (2016). Short physical performance battery and all-cause mortality: systematic review and meta-analysis. BMC Med. 14:215. doi: 10.1186/s12916-016-0763-7
Perera, S., Mody, S. H., Woodman, R. C., and Studenski, S. A. (2006). Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 54, 743–749. doi: 10.1111/j.1532-5415.2006.00701.x
Perez-de la Cruz, S. (2020). Comparison of aquatic therapy vs. dry land therapy to improve mobility of chronic stroke patients. Int. J. Environ. Res. Public Health 17:4728. doi: 10.3390/ijerph17134728
Pfeiffer, R. F. (2016). Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat. Disord. 22, S119–122. doi: 10.1016/j.parkreldis.2015.09.004
Pinter, D., Ritchie, S. J., Doubal, F., Gattringer, T., Morris, Z., Bastin, M. E., et al. (2017). Impact of small vessel disease in the brain on gait and balance. Sci. Rep. 7:41637. doi: 10.1038/srep41637
Plummer, P., Eskes, G., Wallace, S., Giuffrida, C., Fraas, M., Campbell, G., et al. (2013). Cognitive-motor interference during functional mobility after stroke: state of the science and implications for future research. Arch. Phys. Med. Rehabil. 94, 2565–2574.e6. doi: 10.1016/j.apmr.2013.08.002
Podsiadlo, D., and Richardson, S. (1991). The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 39, 142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x
Potter, G. M., Doubal, F. N., Jackson, C. A., Chappell, F. M., Sudlow, C. L., Dennis, M. S., et al. (2015). Enlarged perivascular spaces and cerebral small vessel disease. Int. J. Stroke 10, 376–381. doi: 10.1111/ijs.12054
Powers, W. J., Rabinstein, A. A., Ackerson, T., Adeoye, O. M., Bambakidis, N. C., Becker, K., et al. (2019). Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the american heart association/american stroke association. Stroke 50, e344–e418. doi: 10.1161/STR.0000000000000211
Rensma, S. P., van Sloten, T. T., Launer, L. J., and Stehouwer, C. D. A. (2018). Cerebral small vessel disease and risk of incident stroke, dementia and depression and all-cause mortality: a systematic review and meta-analysis. Neurosci. Biobehav. Rev. 90, 164–173. doi: 10.1016/j.neubiorev.2018.04.003
Reynolds, C. F., 3rd, Butters, M. A., Lopez, O., Pollock, B. G., Dew, M. A., Mulsant, B. H., et al. (2011). Maintenance treatment of depression in old age: a randomized, double-blind, placebo-controlled evaluation of the efficacy and safety of donepezil combined with antidepressant pharmacotherapy. Arch. Gen. Psychiatry 68, 51–60. doi: 10.1001/archgenpsychiatry.2010.184
Roman, G. C., and Kalaria, R. N. (2006). Vascular determinants of cholinergic deficits in Alzheimer disease and vascular dementia. Neurobiol. Aging 27, 1769–1785. doi: 10.1016/j.neurobiolaging.2005.10.004
Sakurai, R., Ishii, K., Yasunaga, M., Takeuchi, R., Murayama, Y., Sakuma, N., et al. (2017). The neural substrate of gait and executive function relationship in elderly women: a PET study. Geriatr. Gerontol. Int. 17, 1873–1880. doi: 10.1111/ggi.12982
Salvado, G., Brugulat-Serrat, A., Sudre, C. H., Grau-Rivera, O., Suarez-Calvet, M., Falcon, C., et al. (2019). Spatial patterns of white matter hyperintensities associated with Alzheimer’s disease risk factors in a cognitively healthy middle-aged cohort. Alzheimers Res. Ther. 11:12. doi: 10.1186/s13195-018-0460-1
Sander, K., Hof, U., Poppert, H., Conrad, B., and Sander, D. (2005). Improved cerebral vasoreactivity after statin administration in healthy adults. J. Neuroimaging 15, 266–270. doi: 10.1177/1051228405277403
Scalfari, A., Neuhaus, A., Daumer, M., Muraro, P. A., and Ebers, G. C. (2014). Onset of secondary progressive phase and long-term evolution of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 85, 67–75. doi: 10.1136/jnnp-2012-304333
Scherder, E., Eggermont, L., Sergeant, J., and Boersma, F. (2007). Physical activity and cognition in Alzheimer’s disease: relationship to vascular risk factors, executive functions and gait. Rev. Neurosci. 18, 149–158. doi: 10.1515/revneuro.2007.18.2.149
Shi, Y., and Wardlaw, J. M. (2016). Update on cerebral small vessel disease: a dynamic whole-brain disease. Stroke Vasc. Neurol. 1, 83–92. doi: 10.1136/svn-2016-000035
Simpson, J. E., Fernando, M. S., Clark, L., Ince, P. G., Matthews, F., Forster, G., et al. (2007). White matter lesions in an unselected cohort of the elderly: astrocytic, microglial and oligodendrocyte precursor cell responses. Neuropathol. Appl. Neurobiol. 33, 410–419. doi: 10.1111/j.1365-2990.2007.00828.x
Smith, K. R., Kahlon, C. H., Brown, J. N., and Britt, R. B. (2021). Methylphenidate use in geriatric depression: a systematic review. Int. J. Geriatr. Psychiatry 36, 1304–1312. doi: 10.1002/gps.5536
Smith, E. E., O’Donnell, M., Dagenais, G., Lear, S. A., Wielgosz, A., Sharma, M., et al. (2015). Early cerebral small vessel disease and brain volume, cognition and gait. Ann. Neurol. 77, 251–261. doi: 10.1002/ana.24320
Song, I. U., Lee, J. E., Kwon, D. Y., Park, J. H., and Ma, H. I. (2017). Parkinson’s disease might increase the risk of cerebral ischemic lesions. Int. J. Med. Sci. 14, 319–322. doi: 10.7150/ijms.18025
SPS3 Study Group, Benavente, O. R., Coffey, C. S., Conwit, R., Hart, R. G., McClure, L. A., et al. (2013). Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 382, P507–515. doi: 10.1016/S0140-6736(13)60852-1
Srikanth, V., Phan, T. G., Chen, J., Beare, R., Stapleton, J. M., and Reutens, D. C. (2010). The location of white matter lesions and gait—a voxel-based study. Ann. Neurol. 67, 265–269. doi: 10.1002/ana.21826
Staals, J., Booth, T., Morris, Z., Bastin, M. E., Gow, A. J., Corley, J., et al. (2015). Total MRI load of cerebral small vessel disease and cognitive ability in older people. Neurobiol. Aging 36, 2806–2811. doi: 10.1016/j.neurobiolaging.2015.06.024
Staszewski, J., Piusinska-Macoch, R., Skrobowska, E., Pawlik, R., Brodacki, B., and Stepien, A. (2015). [Pathogenesis and clinical manifestations of sporadic cerebral small vessel disease]. Pol. Merkur. Lekarski 39, 398–404.
Studenski, S., Perera, S., Patel, K., Rosano, C., Faulkner, K., Inzitari, M., et al. (2011). Gait speed and survival in older adults. JAMA 305, 50–58. doi: 10.1001/jama.2010.1923
Su, N., Liang, X., Zhai, F. F., Zhou, L. X., Ni, J., Yao, M., et al. (2018). The consequence of cerebral small vessel disease: linking brain atrophy to motor impairment in the elderly. Hum. Brain Mapp. 39, 4452–4461. doi: 10.1002/hbm.24284
Su, C., Wu, H., Yang, X., Zhao, B., and Zhao, R. (2021). The relation between antihypertensive treatment and progression of cerebral small vessel disease: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 100:e26749. doi: 10.1097/MD.0000000000026749
Suttanon, P., Hill, K. D., Said, C. M., Logiudice, D., Lautenschlager, N. T., and Dodd, K. J. (2012). Balance and mobility dysfunction and falls risk in older people with mild to moderate Alzheimer disease. Am. J. Phys. Med. Rehabil. 91, 12–23. doi: 10.1097/PHM.0b013e31823caeea
Tabara, Y., Okada, Y., Ohara, M., Uetani, E., Kido, T., Ochi, N., et al. (2015). Association of postural instability with asymptomatic cerebrovascular damage and cognitive decline: the Japan Shimanami health promoting program study. Stroke 46, 16–22. doi: 10.1161/STROKEAHA.114.006704
Takakusaki, K. (2013). Neurophysiology of gait: from the spinal cord to the frontal lobe. Mov. Disord. 28, 1483–1491. doi: 10.1002/mds.25669
Treacy, D., and Hassett, L. (2018). The short physical performance battery. J. Physiother. 64:61. doi: 10.1016/j.jphys.2017.04.002
Tutuncu, M., Tang, J., Zeid, N. A., Kale, N., Crusan, D. J., Atkinson, E. J., et al. (2013). Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult. Scler. 19, 188–198. doi: 10.1177/1352458512451510
Ursin, M. H., Bergland, A., Fure, B., Thommessen, B., Hagberg, G., Oksengard, A. R., et al. (2019). Gait and balance one year after stroke; relationships with lesion side, subtypes of cognitive impairment and neuroimaging findings-a longitudinal, cohort study. Physiotherapy 105, 254–261. doi: 10.1016/j.physio.2018.07.007
van der Holst, H. M., Tuladhar, A. M., Zerbi, V., van Uden, I. W. M., de Laat, K. F., van Leijsen, E. M. C., et al. (2017). White matter changes and gait decline in cerebral small vessel disease. Neuroimage Clin. 17, 731–738. doi: 10.1016/j.nicl.2017.12.007
van der Holst, H. M., van Uden, I. W., Tuladhar, A. M., de Laat, K. F., van Norden, A. G., Norris, D. G., et al. (2016). Factors associated with 8-year mortality in older patients with cerebral small vessel disease: the radboud university nijmegen diffusion tensor and magnetic resonance cohort (RUN DMC) study. JAMA Neurol. 73, 402–409. doi: 10.1001/jamaneurol.2015.4560
van Middelaar, T., Argillander, T. E., Schreuder, F., Deinum, J., Richard, E., and Klijn, C. J. M. (2018). Effect of antihypertensive medication on cerebral small vessel disease: a systematic review and meta-analysis. Stroke 49, 1531–1533. doi: 10.1161/STROKEAHA.118.021160
van Norden, A. G., van den Berg, H. A., de Laat, K. F., Gons, R. A., van Dijk, E. J., and de Leeuw, F. E. (2011). Frontal and temporal microbleeds are related to cognitive function: the radboud university nijmegen diffusion tensor and magnetic resonance cohort (RUN DMC) study. Stroke 42, 3382–3386. doi: 10.1161/STROKEAHA.111.629634
van Rooden, S., Goos, J. D., van Opstal, A. M., Versluis, M. J., Webb, A. G., Blauw, G. J., et al. (2014). Increased number of microinfarcts in Alzheimer disease at 7-T MR imaging. Radiology 270, 205–211. doi: 10.1148/radiol.13130743
Verghese, J., Ayers, E., Barzilai, N., Bennett, D. A., Buchman, A. S., Holtzer, R., et al. (2014). Motoric cognitive risk syndrome: multicenter incidence study. Neurology 83, 2278–2284. doi: 10.1212/WNL.0000000000001084
Vermeer, S. E., Longstreth, W. T., Jr., and Koudstaal, P. J. (2007). Silent brain infarcts: a systematic review. Lancet Neurol. 6, 611–619. doi: 10.1016/S1474-4422(07)70170-9
Vinters, H. V. (2015). Emerging concepts in Alzheimer’s disease. Annu. Rev. Pathol. 10, 291–319. doi: 10.1146/annurev-pathol-020712-163927
Volpato, S., Cavalieri, M., Sioulis, F., Guerra, G., Maraldi, C., Zuliani, G., et al. (2011). Predictive value of the short physical performance battery following hospitalization in older patients. J. Gerontol. A Biol. Sci. Med. Sci. 66, 89–96. doi: 10.1093/gerona/glq167
Wan, Y., Hu, W., Gan, J., Song, L., Wu, N., Chen, Y., et al. (2019). Exploring the association between Cerebral small-vessel diseases and motor symptoms in Parkinson’s disease. Brain Behav. 9:e01219. doi: 10.1002/brb3.1219
Wang, N., Allali, G., Kesavadas, C., Noone, M. L., Pradeep, V. G., Blumen, H. M., et al. (2016). Cerebral small vessel disease and motoric cognitive risk syndrome: results from the kerala-einstein study. J. Alzheimers Dis. 50, 699–707. doi: 10.3233/JAD-150523
Wardlaw, J. M., Smith, C., and Dichgans, M. (2013a). Mechanisms of sporadic cerebral small vessel disease: insights from neuroimaging. Lancet Neurol. 12, 483–497. doi: 10.1016/S1474-4422(13)70060-7
Wardlaw, J. M., Smith, E. E., Biessels, G. J., Cordonnier, C., Fazekas, F., Frayne, R., et al. (2013b). Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 12, 822–838. doi: 10.1016/S1474-4422(13)70124-8
Webb, A. J., Fischer, U., Mehta, Z., and Rothwell, P. M. (2010). Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet 375, 906–915. doi: 10.1016/S0140-6736(10)60235-8
Wei, T. S., Liu, P. T., Chang, L. W., and Liu, S. Y. (2017). Gait asymmetry, ankle spasticity and depression as independent predictors of falls in ambulatory stroke patients. PLoS One 12:e0177136. doi: 10.1371/journal.pone.0177136
Welch, S. A., Ward, R. E., Beauchamp, M. K., Leveille, S. G., Travison, T., and Bean, J. F. (2021). The short physical performance battery (SPPB): a quick and useful tool for fall risk stratification among older primary care patients. J. Am. Med. Dir. Assoc. 22, 1646–1651. doi: 10.1016/j.jamda.2020.09.038
Wennie Huang, W. N., Perera, S., VanSwearingen, J., and Studenski, S. (2010). Performance measures predict onset of activity of daily living difficulty in community-dwelling older adults. J. Am. Geriatr. Soc. 58, 844–852. doi: 10.1111/j.1532-5415.2010.02820.x
Wu, Y. Z., Lin, J. Y., Wu, P. L., and Kuo, Y. F. (2019). Effects of a hybrid intervention combining exergaming and physical therapy among older adults in a long-term care facility. Geriatr. Gerontol. Int. 19, 147–152. doi: 10.1111/ggi.13575
Xiong, Y., Wong, A., Cavalieri, M., Schmidt, R., Chu, W. W., Liu, X., et al. (2014). Prestroke statins, progression of white matter hyperintensities and cognitive decline in stroke patients with confluent white matter hyperintensities. Neurotherapeutics 11, 606–611. doi: 10.1007/s13311-014-0270-5
Yakushiji, Y., Nishiyama, M., Yakushiji, S., Hirotsu, T., Uchino, A., Nakajima, J., et al. (2008). Brain microbleeds and global cognitive function in adults without neurological disorder. Stroke 39, 3323–3328. doi: 10.1161/STROKEAHA.108.516112
Ye, S., Dong, S., Tan, J., Chen, L., Yang, H., Chen, Y., et al. (2019). White-matter hyperintensities and lacunar infarcts are associated with an increased risk of Alzheimer’s disease in the elderly in China. J. Clin. Neurol. 15, 46–53. doi: 10.3988/jcn.2019.15.1.46
Yip, J. L., Khawaja, A. P., Broadway, D., Luben, R., Hayat, S., Dalzell, N., et al. (2014). Visual acuity, self-reported vision and falls in the EPIC-Norfolk eye study. Br. J. Ophthalmol. 98, 377–382. doi: 10.1136/bjophthalmol-2013-304179
You, H., Zhang, H., Liu, J., Han, T., Zhang, M., Zhao, W., et al. (2017). Effect of balance training with pro-kin system on balance in patients with white matter lesions. Medicine (Baltimore) 96:e9057. doi: 10.1097/MD.0000000000009057
Zhang, C., Chen, Q., Wang, Y., Zhao, X., Wang, C., Liu, L., et al. (2014). Risk factors of dilated virchow-robin spaces are different in various brain regions. PLoS One 9:e105505. doi: 10.1371/journal.pone.0105505
Zhang, H., Cui, Y., Zhao, Y., Dong, Y., Wang, J., Duan, D., et al. (2019a). Association of circadian rhythm of blood pressure and cerebral small vessel disease in community-based elderly population. J. Gerontol. A Biol. Sci. Med. Sci. 74, 1322–1330. doi: 10.1093/gerona/gly212
Zhang, H., Cui, Y., Zhao, Y., Dong, Y., Duan, D., Wang, J., et al. (2019b). Effects of sartans and low-dose statins on cerebral white matter hyperintensities and cognitive function in older patients with hypertension: a randomized, double-blind and placebo-controlled clinical trial. Hypertens. Res. 42, 717–729. doi: 10.1038/s41440-018-0165-7
Zhang, J., Liu, N., and Yang, C. (2019). Effects of rosuvastatin in combination with nimodipine in patients with mild cognitive impairment caused by cerebral small vessel disease. Panminerva Med. 61, 439–443. doi: 10.23736/S0031-0808.18.03475-4
Zhang, G., Zhang, Y., Zhang, C., Wang, Y., Ma, G., Nie, K., et al. (2016). Striatal silent lacunar infarction is associated with changes to the substantia nigra in patients with early-stage Parkinson’s disease: a diffusion kurtosis imaging study. J. Clin. Neurosci. 33, 138–141. doi: 10.1016/j.jocn.2016.03.032
Zhao, W., You, H., Jiang, S., Zhang, H., Yang, Y., and Zhang, M. (2019). Effect of Pro-kin visual feedback balance training system on gait stability in patients with cerebral small vessel disease. Medicine (Baltimore) 98:e14503. doi: 10.1097/MD.0000000000014503
Zhou, X., Zhang, C., Li, L., Zhang, Y., Zhang, W., Yin, W., et al. (2020). Altered brain function in cerebral small vessel disease patients with gait disorders: a resting-state functional MRI study. Front. Aging Neurosci. 12:234. doi: 10.3389/fnagi.2020.00234
Keywords: gait and balance disorders, cerebrovascular disease, white matter hyperintensity, lacunar infarction, cerebral microbleeds, enlarged perivascular space, brain atrophy
Citation: Su C, Yang X, Wei S and Zhao R (2022) Association of Cerebral Small Vessel Disease With Gait and Balance Disorders. Front. Aging Neurosci. 14:834496. doi: 10.3389/fnagi.2022.834496
Received: 13 December 2021; Accepted: 14 June 2022;
Published: 08 July 2022.
Edited by:
Hua Zhang, Shandong First Medical University, ChinaReviewed by:
Jacek Staszewski, Military Institute of Medicine (Poland), PolandCopyright © 2022 Su, Yang, Wei and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renliang Zhao, emhyZW5saWFuZ0AxNjMuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.