- 1Department of Neurology, First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Department of Neurorehabilitation, Second Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 3Department of Psychiatry, First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 4Department of Anesthesiology, Beijing Friendship Hospital, Capital Medical University, Beijing, China
Objective: Accumulated studies have explored gene polymorphisms and circulating levels of tumor necrosis factor (TNF)-α and insulin-like growth factor (IGF)-1 in the etiology of ischemic stroke (IS). Of the numerous etiopathological factors for IS, a single-nucleotide polymorphism (SNP) rs1800629 located in the TNF-α gene promoter region and increased levels of TNF-α were found to be associated with IS in different ethnic backgrounds. However, the published results are inconsistent and inconclusive. The primary objective of this meta-analysis was to investigate the concordance between rs1800629 polymorphism and IS. A secondary aim was to explore circulating levels of TNF-α and IGF-1 with IS in different ethnic backgrounds and different sourced specimens.
Methods: In this study, we examined whether rs1800629 genetic variant and levels of TNF-α and IGF-1 were related to the etiology of IS by performing a meta-analysis. Relevant case-control studies were retrieved by database searching and systematically selected according to established inclusion criteria.
Results: A total of 47 articles were identified that explored the relationship between the rs1800629 polymorphism and levels of TNF-α and IGF-1 with IS risk susceptibility. Statistical analyses revealed a significant association between the rs1800629 polymorphism and levels of TNF-α and IGF-1 with IS pathogenesis.
Conclusion: Our findings demonstrated that the TNF-α rs1800629 polymorphism, the increased levels of TNF-α, and decreased levels of IGF-1 were involved in the etiology of IS.
Introduction
Ischemic stroke (IS) is the second leading cause of mortality and physical disability following the onset of neuropathological complications worldwide (Strong et al., 2007; Navis et al., 2019). Presently, it is crucial to identify the potential risk factors of IS that are associated with challenges in the early detection of stroke symptoms as well as poor survival outcomes.
Pro-inflammatory cytokines play a major role in the process of activation of various cellular mechanisms, including differentiation and proliferation processes. If the pro-inflammatory cytokine storm occurs in the cerebral tissue (King et al., 2013; Clausen et al., 2014; Arango-Dávila et al., 2015; Wu et al., 2016), it will contribute to additional brain injuries, such as IS (Jiang et al., 2020). Among the identified cytokines, the pathogenic roles of tumor necrosis factor α (TNF-α) have been extensively investigated. It has been demonstrated that an increased level of TNF-α is associated with greater neurological deficits and poorer treatment outcomes in IS patients (Pasarica et al., 2005; Cui et al., 2012; Bokhari et al., 2014; Boehme et al., 2016; Lasek-Bal et al., 2019). In preclinical studies, the application of TNF-α agonists or reduced expression of TNF-α has exhibited potential neuroprotective effects in the cerebral cortex of IS patients. Notably, Clausen et al. (2020) have demonstrated significantly increased risks of stroke in patients with elevated concentrations of TNFR1 and TNFR2 factors in plasma, suggesting that TNF-α level might serve as a risk factor for IS as well as the biomarker for the patient survival rate. Similarly, insulin-like growth factor-1 (IGF-1) that demonstrates cell proliferation, an inhibitor of cell apoptosis, exerts neuroprotective effects in both white and gray matter under different detrimental conditions. It is a key regulator of cell proliferation and an inhibitor of cell apoptosis and necrosis a key regulator in the development, cell differentiation, plasticity and survival of the nervous system (Juul, 2003; Benarroch, 2012; Shaheen et al., 2018). Based on this evidence, it can be speculated that IGF-1 might be involved in the etiology of IS.
Previous studies have also explored the association between the TNF-α gene polymorphism and peripheral blood levels of TNF-α and IGF-1 with IS onset in different ethnic-specific backgrounds. However, the findings from such studies have been inconsistent or inconclusive due to their highly diverse patients’ populations, differentially sourced specimens, small sample sizes, and most importantly, little influence of single-nucleotide polymorphisms (SNPs) on IS pathobiology. To overcome these roadblocks, an updated meta-analysis was performed to further testify whether (1) the TNF-α genetic SNPs could increase the susceptibility of different populations to IS and (2) the peripheral blood TNF-α and IGF-1 levels could be used as the biomarker for assessing the risks for IS in ethnic-specific background.
Methods
Inclusion Criteria
For this meta-analysis, full-length relevant articles and case studies were selected based on the following inclusion criteria: (1) The diagnosis of IS patients was based on the CT or MRI examinations; (2) selected studies were all case-control studies; (3) data on SNP frequency of circulating TNF-α and IGF-1 levels were clearly obtained; and (4) the frequency of each allele and genotype met the criteria for Hardy-Weinberg disequilibrium in both the case and control groups. In contrast, some studies were excluded based on the criteria that they were either case-only studies based on a family design or abstract-only reviews without the full case report.
Search Strategy
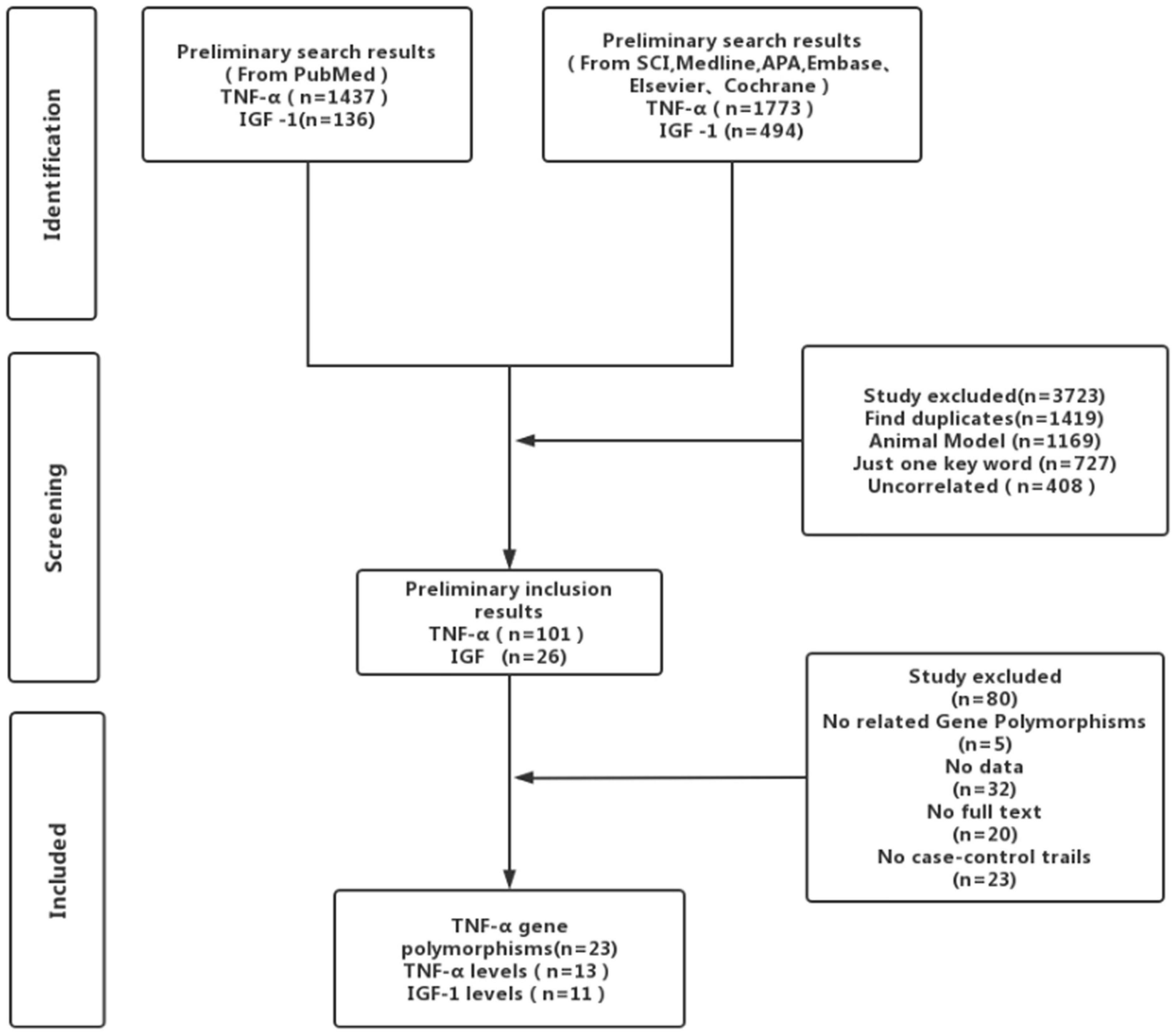
We used the keywords “tumor necrosis factor-alpha,” human recombinant tumor necrosis factor-alpha, “cachectin/tumor necrosis factor,” tumor necrosis factor ligand superfamily member 2, “TNF alpha,” “TNF-alpha insulin-like somatomedin peptide I,” “insulin-like somatomedin peptide I,” “IGF-I-SmC,” “IGF-1 insulin-like growth factor I,” “ischemic stroke,” “cryptogenic stroke,” and “cryptogenic embolism stroke” to search for the full-length case studies in the PubMed, Web of Science, Embase, Elsevier, Cochrane, Medline, and APA databases published until September 9, 2021. We thoroughly reviewed the retrieved literature and the corresponding reference lists. To avoid including duplicate articles, we selected studies from the larger sample size in the analysis.
Data Extraction
Three of the authors independently extracted the following data from each publication: Author, country of origin, racial descent of the study population, the number of eligible cases with proper controls, circulating levels of TNF-α, IGF-1 (mean and standard deviation), rs1800629 (G-308A) genotype, and allele frequencies.
Data Analysis and Statistical Methods
The data analysis using statistical methods was carried out according to the methods described in our previous study (Zhao et al., 2013).
Results
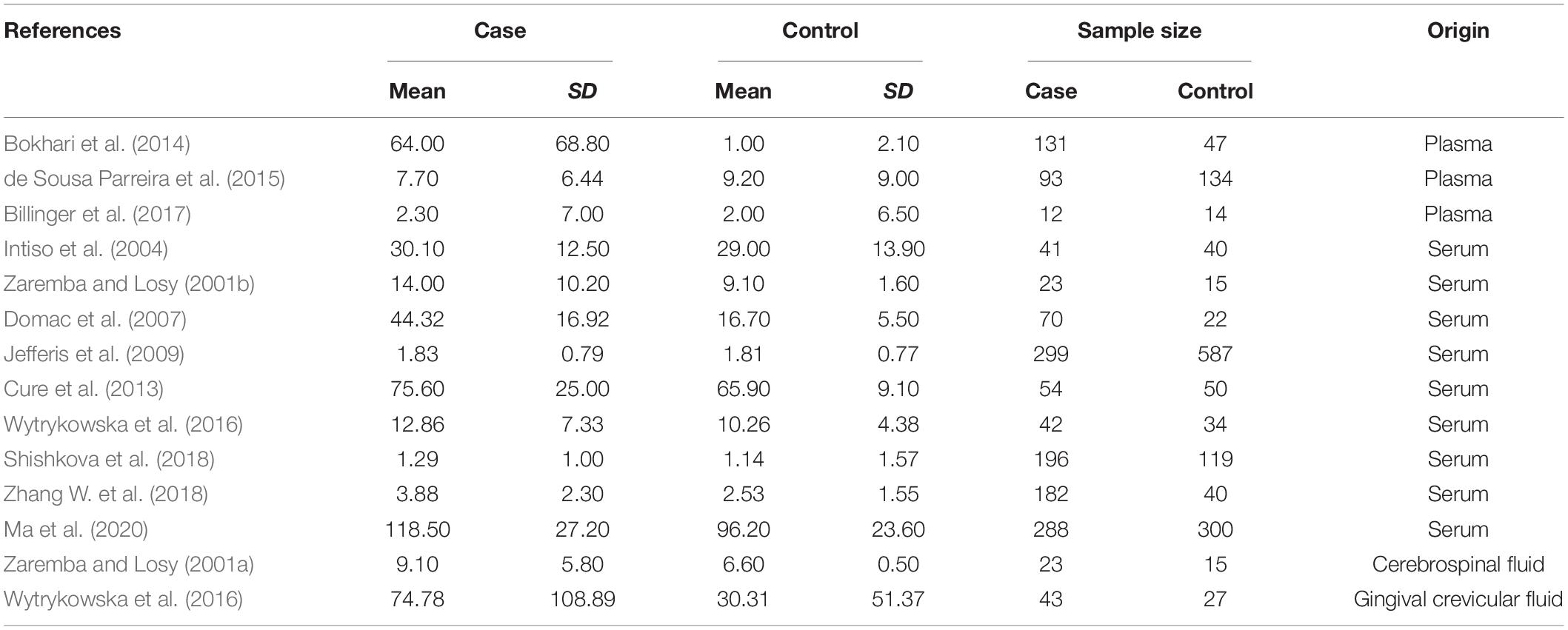
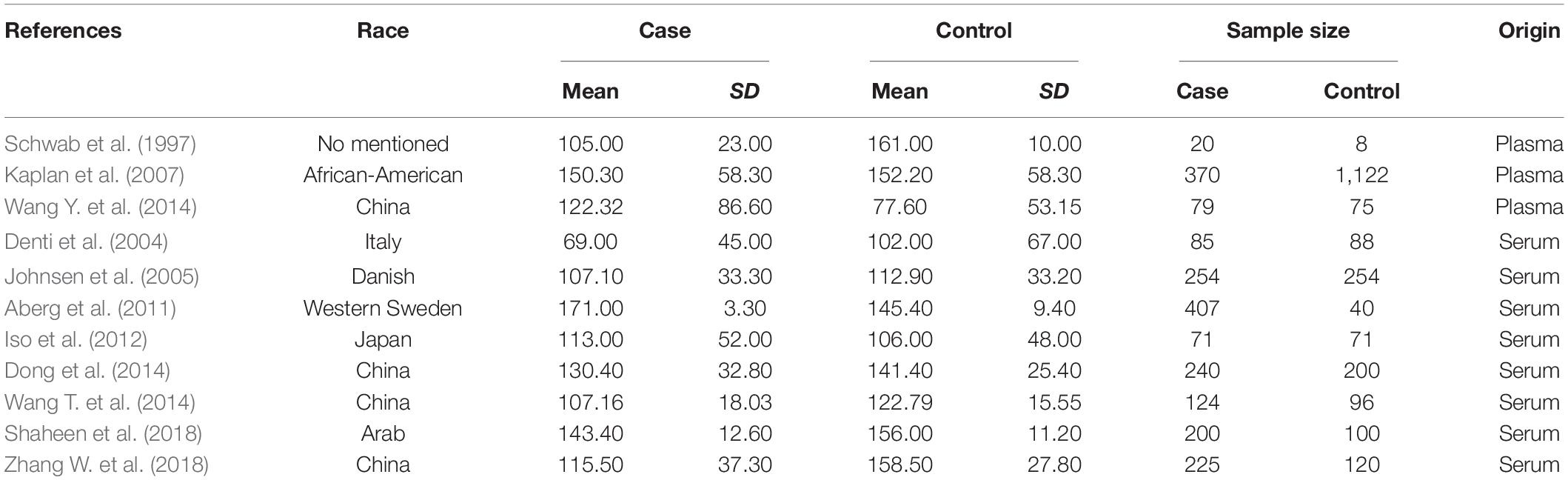
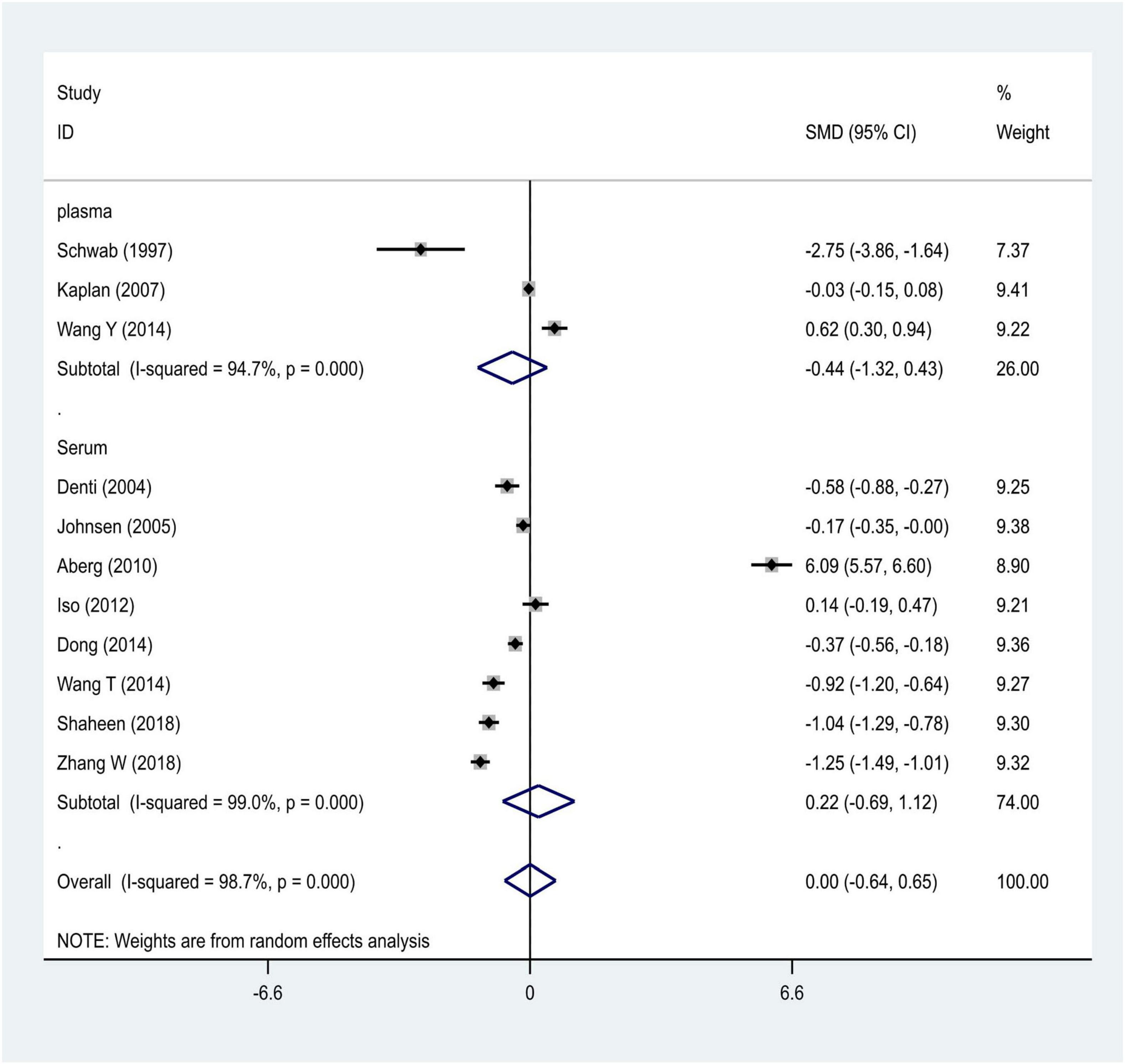
Strictly following the inclusion criteria, 47 properly controlled full-length studies, including 23 rs1800629 genotype-related studies (Um et al., 2003; Balding et al., 2004; Lee et al., 2004; Um and Kim, 2004; Karahan et al., 2005; Rubattu et al., 2005; Harcos et al., 2006; Lalouschek et al., 2006; Llamas Sillero et al., 2007; Banerjee et al., 2008; Shi et al., 2009; Kim et al., 2010; Tong et al., 2010; Sultana et al., 2011; Cui et al., 2012; Tuttolomondo et al., 2012; Zhao et al., 2012; Djordjevic et al., 2013; Wawrzynek et al., 2014; Gu et al., 2015; Kumar et al., 2016; Palm et al., 2020; Kamdee et al., 2021) and 13 case reports (Zaremba and Losy, 2001a,b; Intiso et al., 2004; Domac et al., 2007; Jefferis et al., 2009; Cure et al., 2013; Bokhari et al., 2014; de Sousa Parreira et al., 2015; Wytrykowska et al., 2016; Billinger et al., 2017; Shishkova et al., 2018; Zhang Y. Y. et al., 2018; Ma et al., 2020) on the circulating level of TNF-α were finally selected for the meta-analysis (Figure 1). Genotype-related literature included a total of 9,120 patients and 9,249 healthy controls (Caucasian, n = 10; Asian, n = 15). PCR screening was used to detect genotypes, and all genotypes met Hardy-Weinberg disequilibrium criteria. Similarly, the TNF-α circulation levels were measured in 1,497 cases and 1,444 control subjects. The distribution of articles based on the ethnic specificity was Caucasian (n = 6), Asian (n = 6), and American (n = 2) and that based on the laboratory findings was plasma data (n = 3), serum data (n = 9), cerebrospinal fluid (CSF) data (n = 1), and gingival fluid data (n = 1). We identified 11 articles concerning the relationship between TNF-α circulation levels and IS, three articles for plasma data (Schwab et al., 1997; Kaplan et al., 2007; Wang Y. et al., 2014), eight articles for serum data (Denti et al., 2004; Johnsen et al., 2005; Aberg et al., 2011; Iso et al., 2012; Dong et al., 2014; Wang T. et al., 2014; Shaheen et al., 2018; Zhang W. et al., 2018); 2,075 patients in case group; and 2,174 in the control group (Tables 1–3). All the selected articles were analyzed using the STATA15.0 software.

Table 1. Summary of studies exploring the relationship between rs1800629 of TNF-α gene polymorphism and IS.
We selected the nucleotide G to A modification as the risk factor among genotypes and established 2 models (GG vs. GA + AA and G vs. A). The heterogeneity test in each group indicated the presence of high heterogeneity among the samples. Heterogeneity models I–V were randomly selected for testing, which showed results for each model, namely, GG vs. GA + AA model [odds ratio (OR) = 1.22, 95% confidence interval (CI) = 1.05–1.42, Figure 2] and G vs. A model (OR = 1.19, 95% CI = 1.03–1.38, Figure 3). We found positive correlations for the GG vs. GA + AA and G vs. A models, suggesting that G-A mutation might be a risk factor for IS.
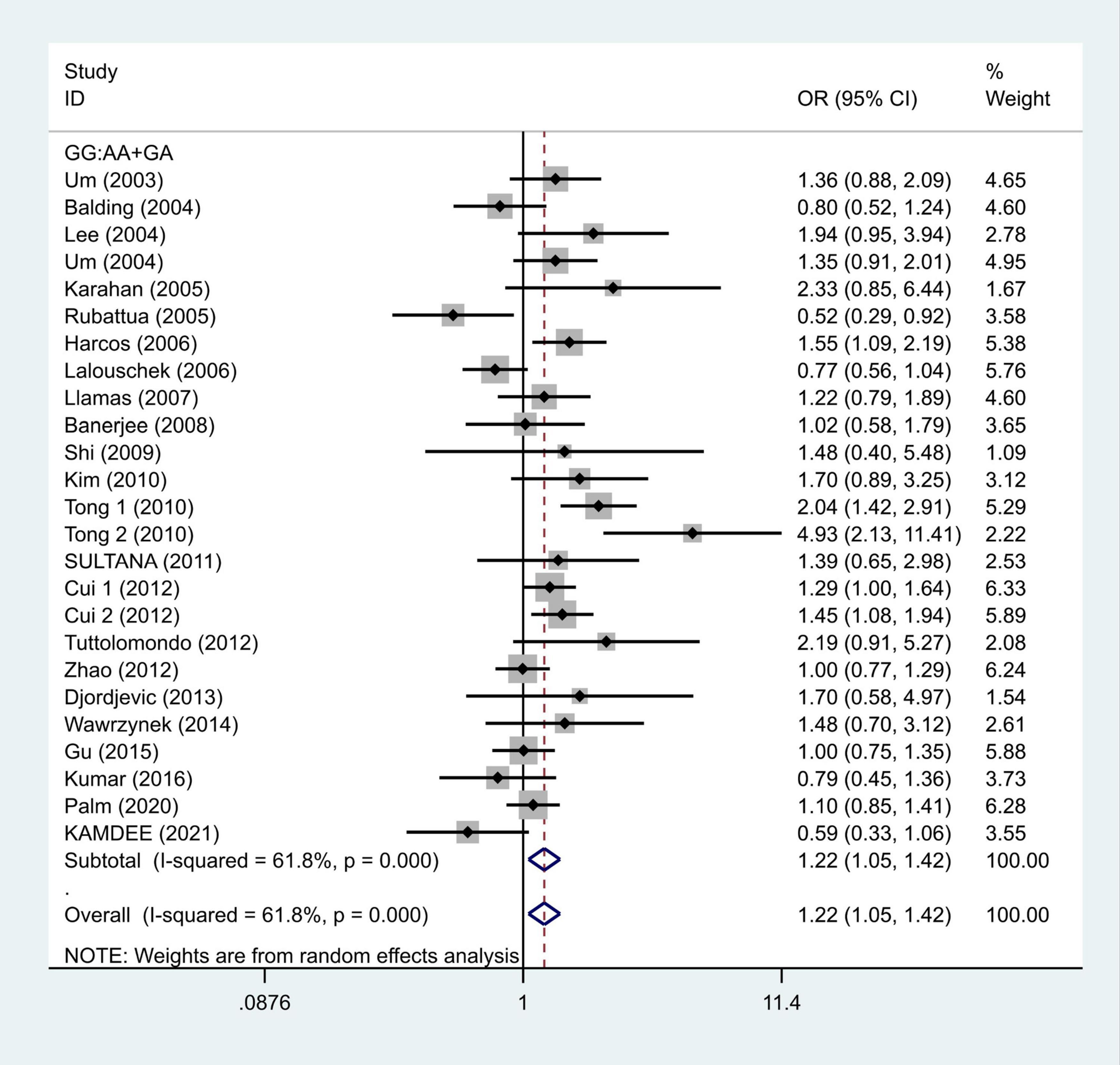
Figure 2. Results of the random-effects meta-analysis for the TNF-α G308A genotype (GG vs. AA + GA) in IS and control groups.
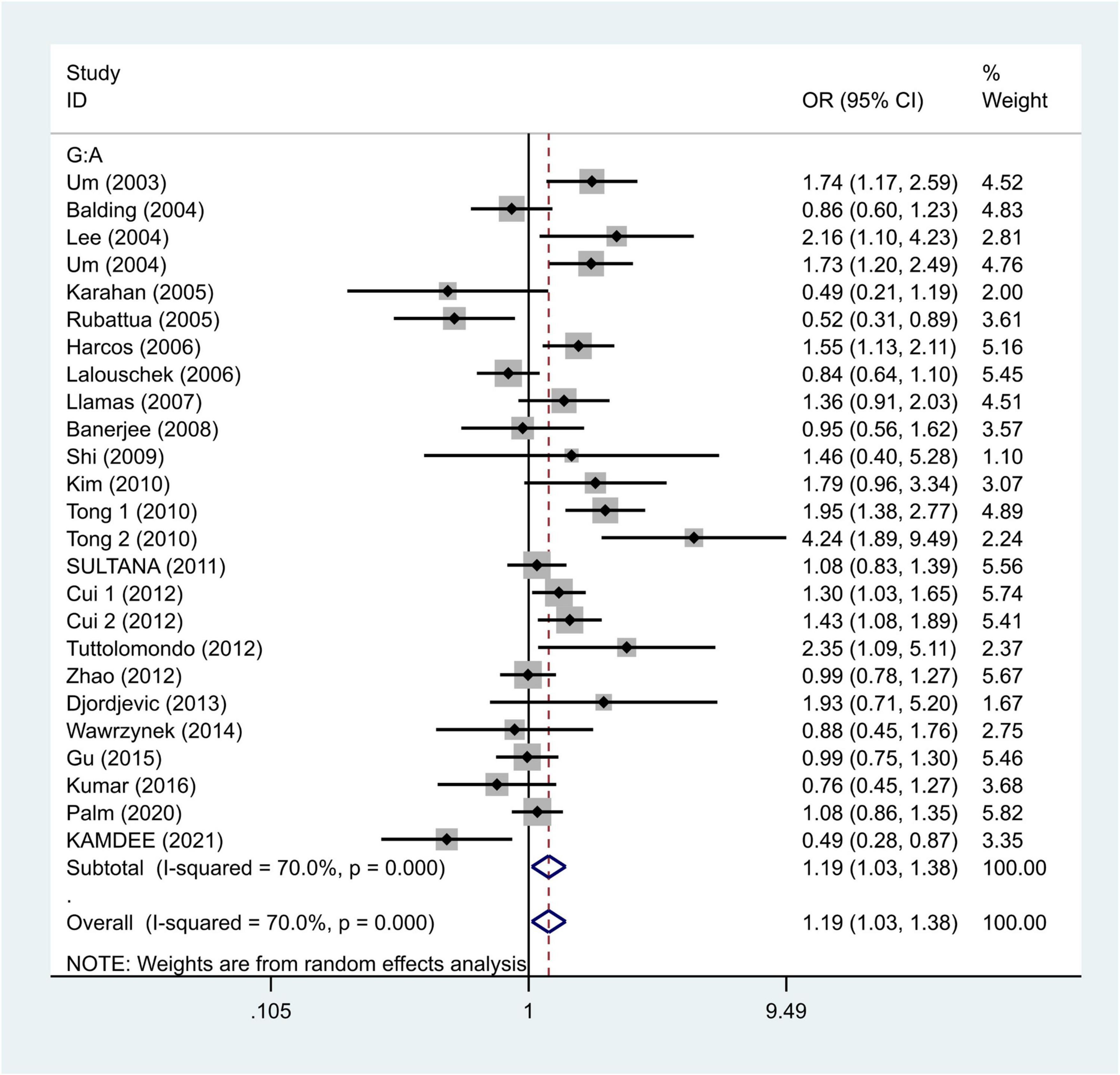
Figure 3. Results of the random-effects meta-analysis for the TNF-α G308A allele (G vs. A) in IS and control groups.
To further study the source of heterogeneity and whether there were differences among races, we conducted a racial stratification analysis on the same dataset. Among them, positive results were found in the Asian race with the models such as GG vs. GA + AA (OR = 1.33, 95% CI = 1.09–1.62, Figure 4) and G vs. A (OR = 1.30, 95% CI = 1.07–1.57, Figure 5), while no positive results were found in case of the Caucasian population. Together, these results indicate that the intensity of G modification-associated risk factors can be linked to racial differences, especially as a potential risk factor for the Asian population.
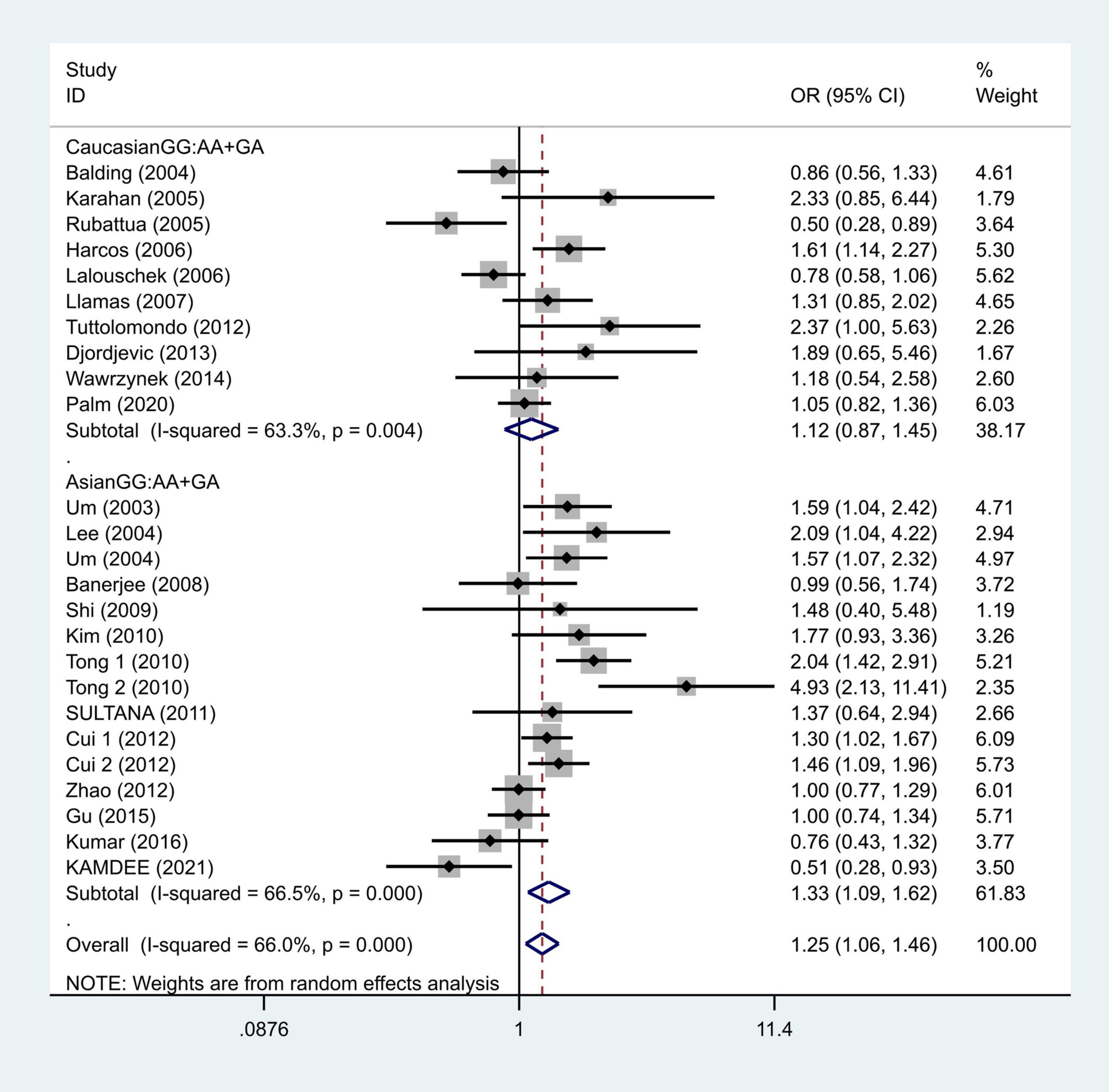
Figure 4. Results of the random-effects meta-analysis for the TNF-α G308A genotype (GG vs. AA + GA) in Caucasian, Asian, IS, and control groups, respectively.
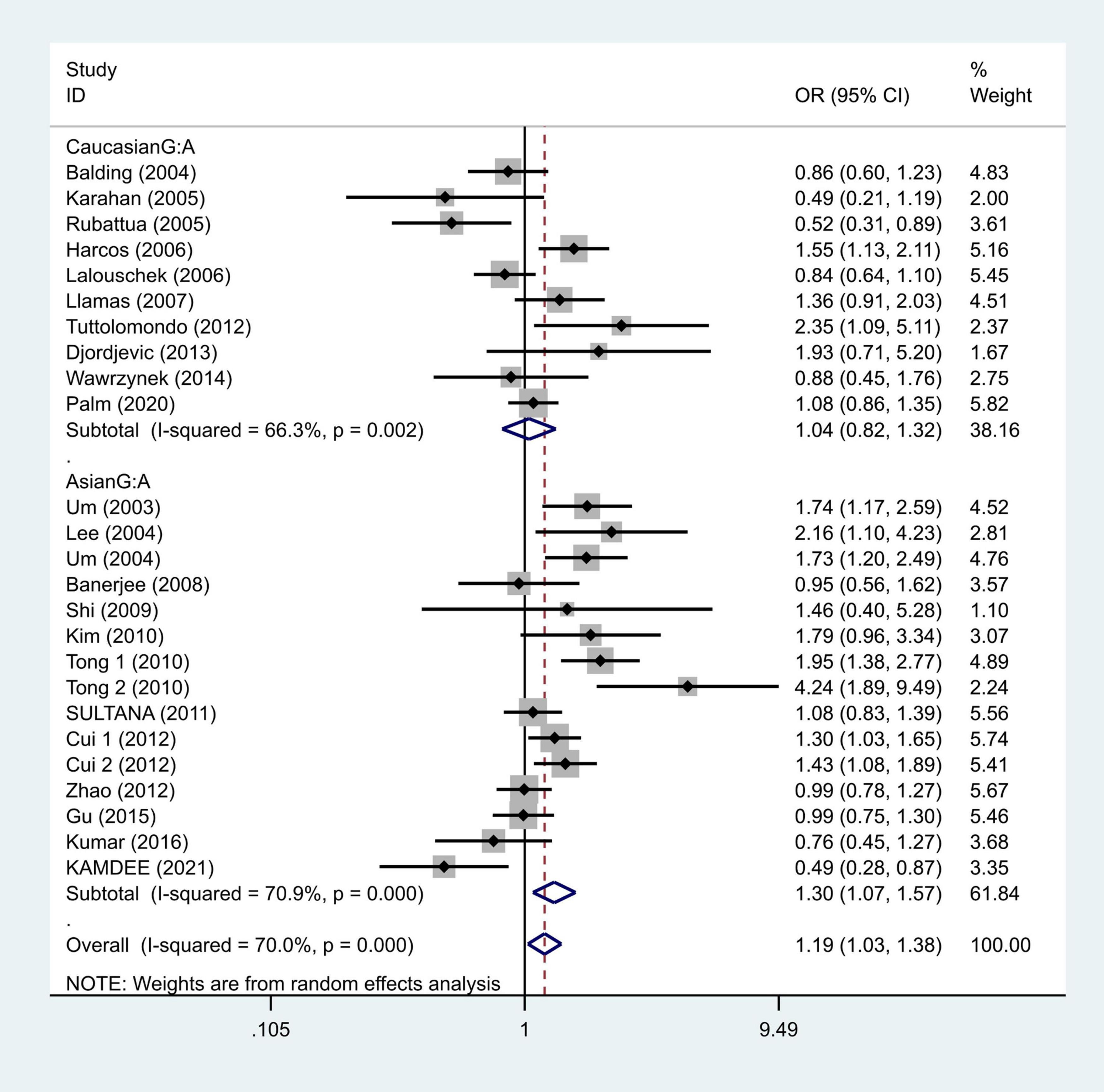
Figure 5. Results of the random-effects meta-analysis for the TNF-α G308A allele (G vs. A) in Caucasian, Asian, IS, and control groups, respectively.
In the TNF-α level detection, the data of Q25–Q75 in the original text were transformed into mean and SD. We conducted continuous variable analysis by recording mean, SD, and total number.
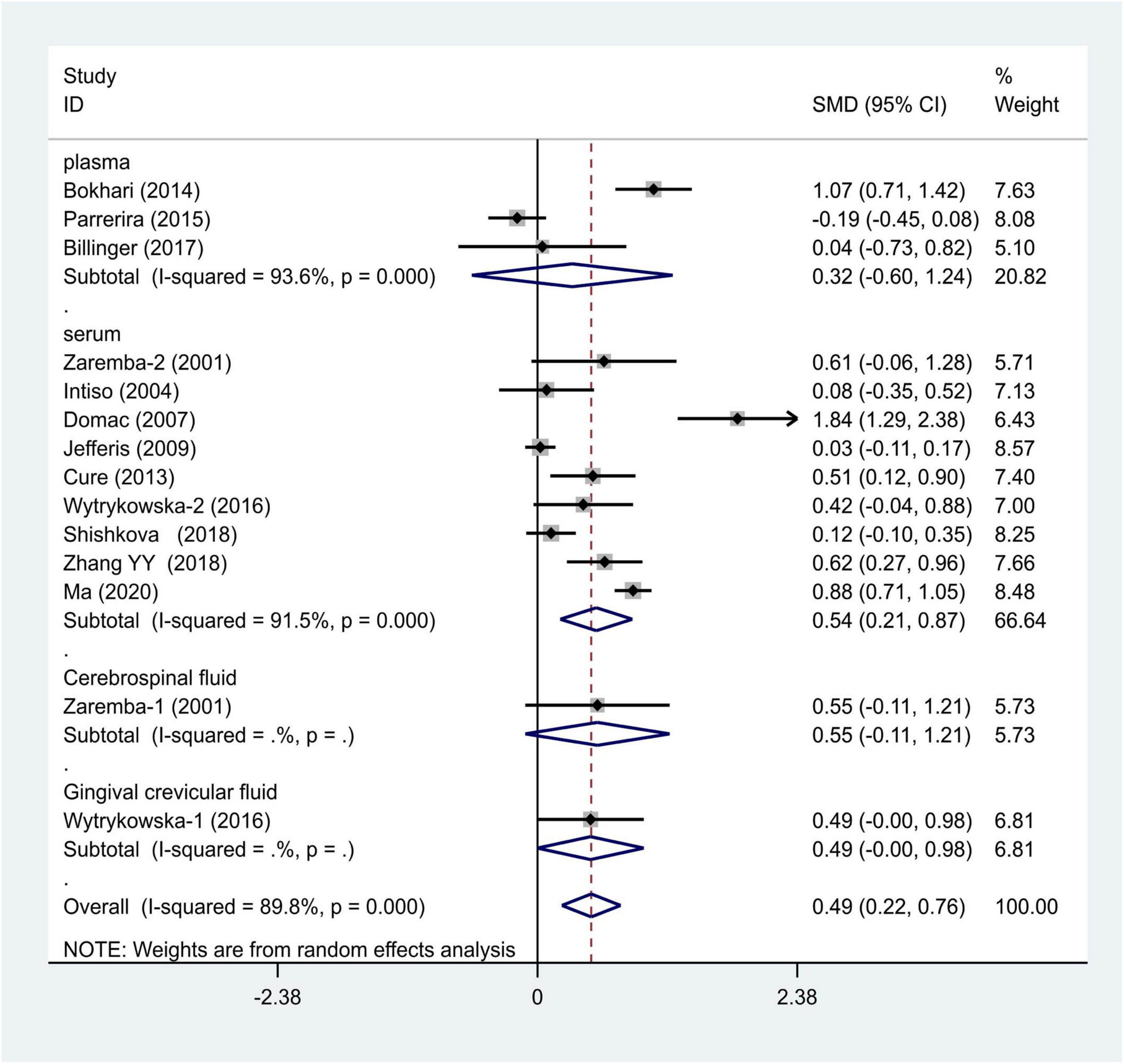
We distributed the cases into the particular group to establish the model based on the sample source. We found that the serum model had a positive result with the SMD value of 0.54 (95% CI = 0.21–0.87, Figure 6) and also had heterogeneity in sample quality. We need to conduct further studies to identify if the advent of heterogeneity can be caused by racial differences. Furthermore, non-positive results were found in the South American population, and positive results were found in the Asian (SMD = 0.75, 95% CI = 0.31–1.20, Figure 7) and Caucasian populations (SMD = 0.18, 95% CI = –0.07–0.42, Figure 7). To further explore the interactions between serological indicators and races, serum results were grouped according to ethnic specificity, which yielded positive results for the Asian population (SMD = 0.75, 95% CI = 0.31–1.20, Figure 7). Therefore, elevated serum TNF-α levels were considered to be a risk factor for IS in the Asian population. The same method was conducted to explore the connection of IGF-1 concentration with IS in different ethnic backgrounds. The decreased serum IGF-1 levels were considered to be a risk factor for IS in the Asian population not in the Caucasian population (SMD = –0.61, 95% CI = –1.16 to –0.50, Figures 8–10).
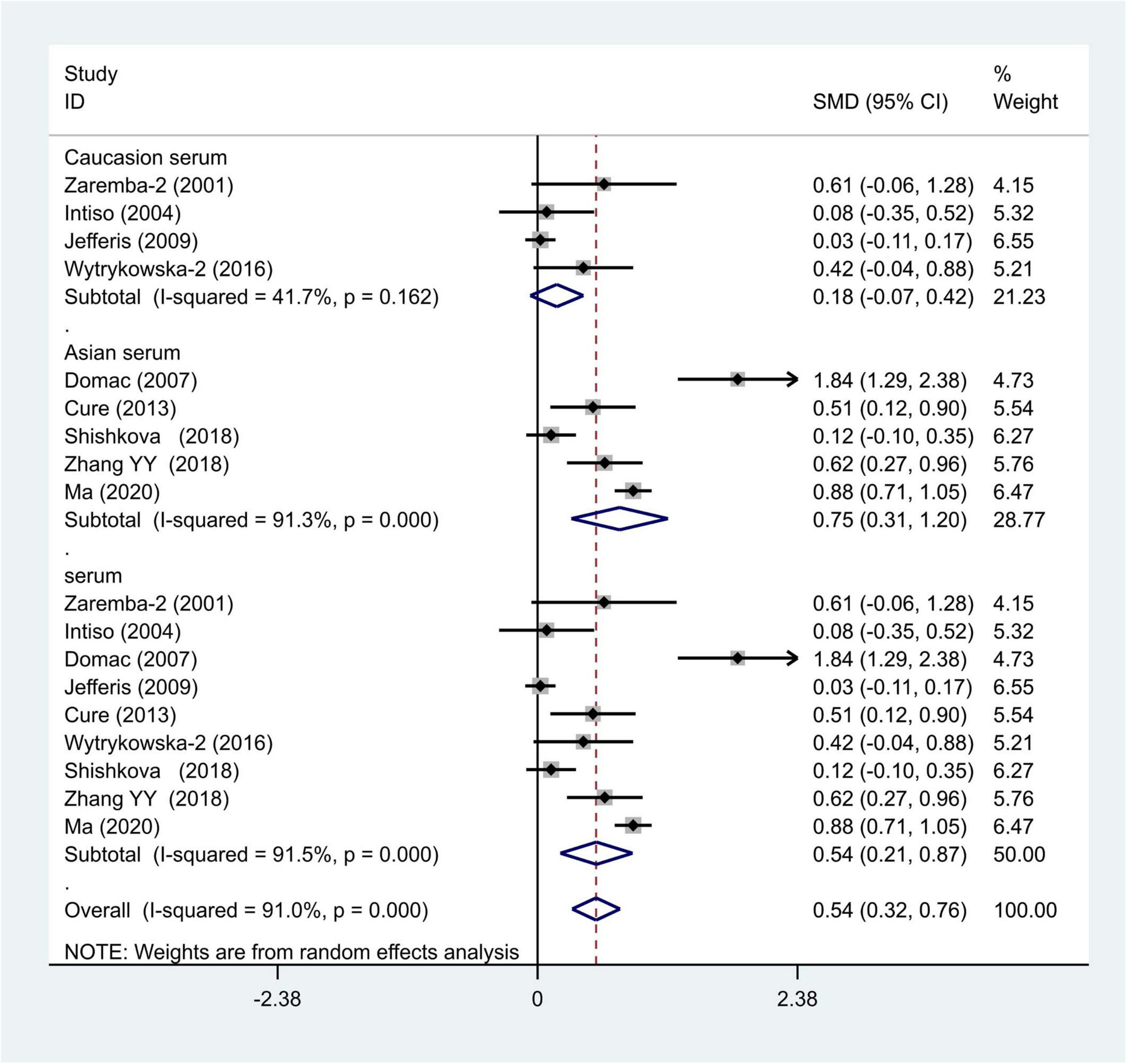
Figure 7. Results of meta-analysis for the level of TNF-α in the Asian and Caucasian populations, respectively.
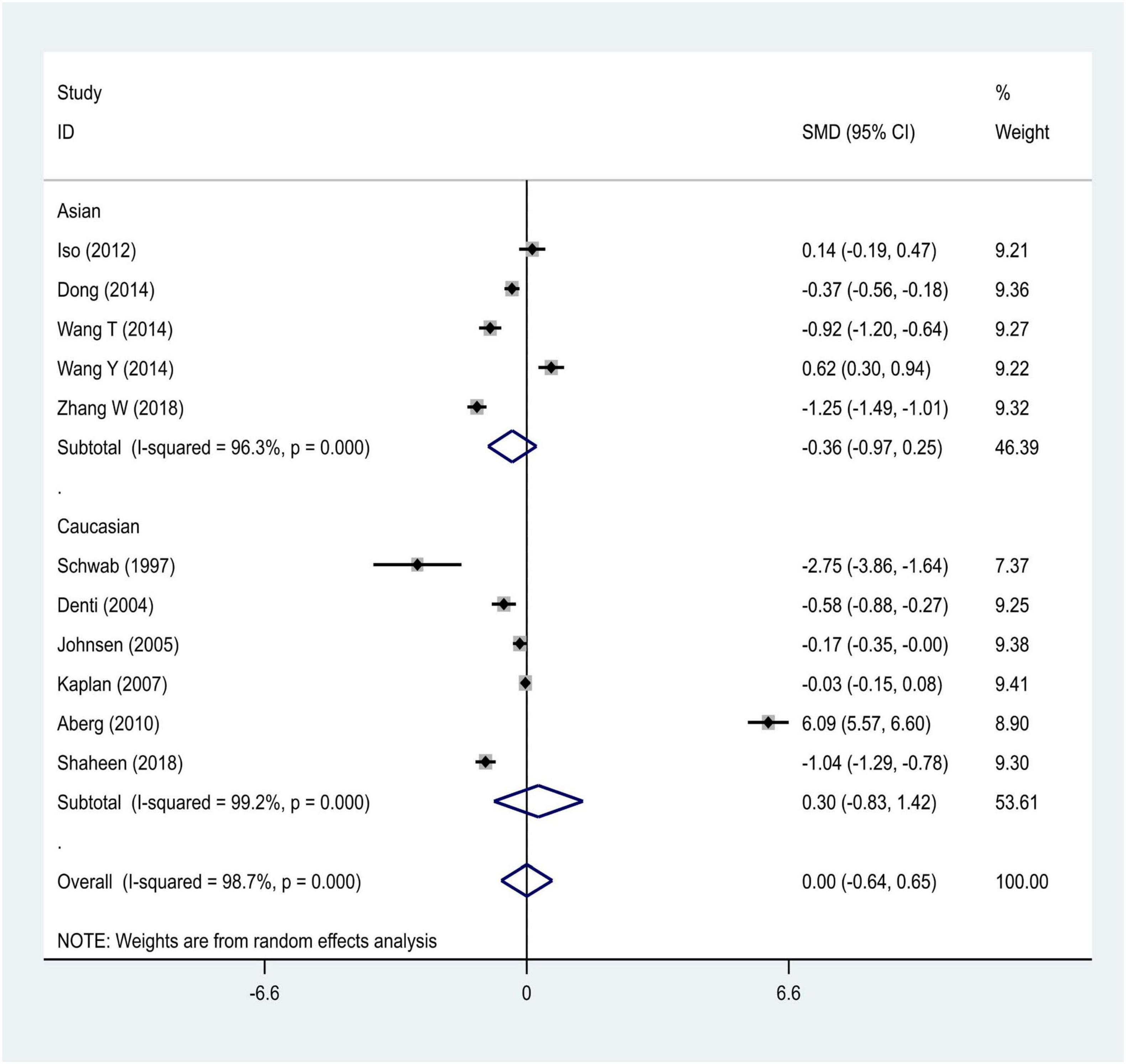
Figure 8. Results of meta-analysis for the level of IGF-1 in the Asian and Caucasian populations, respectively.
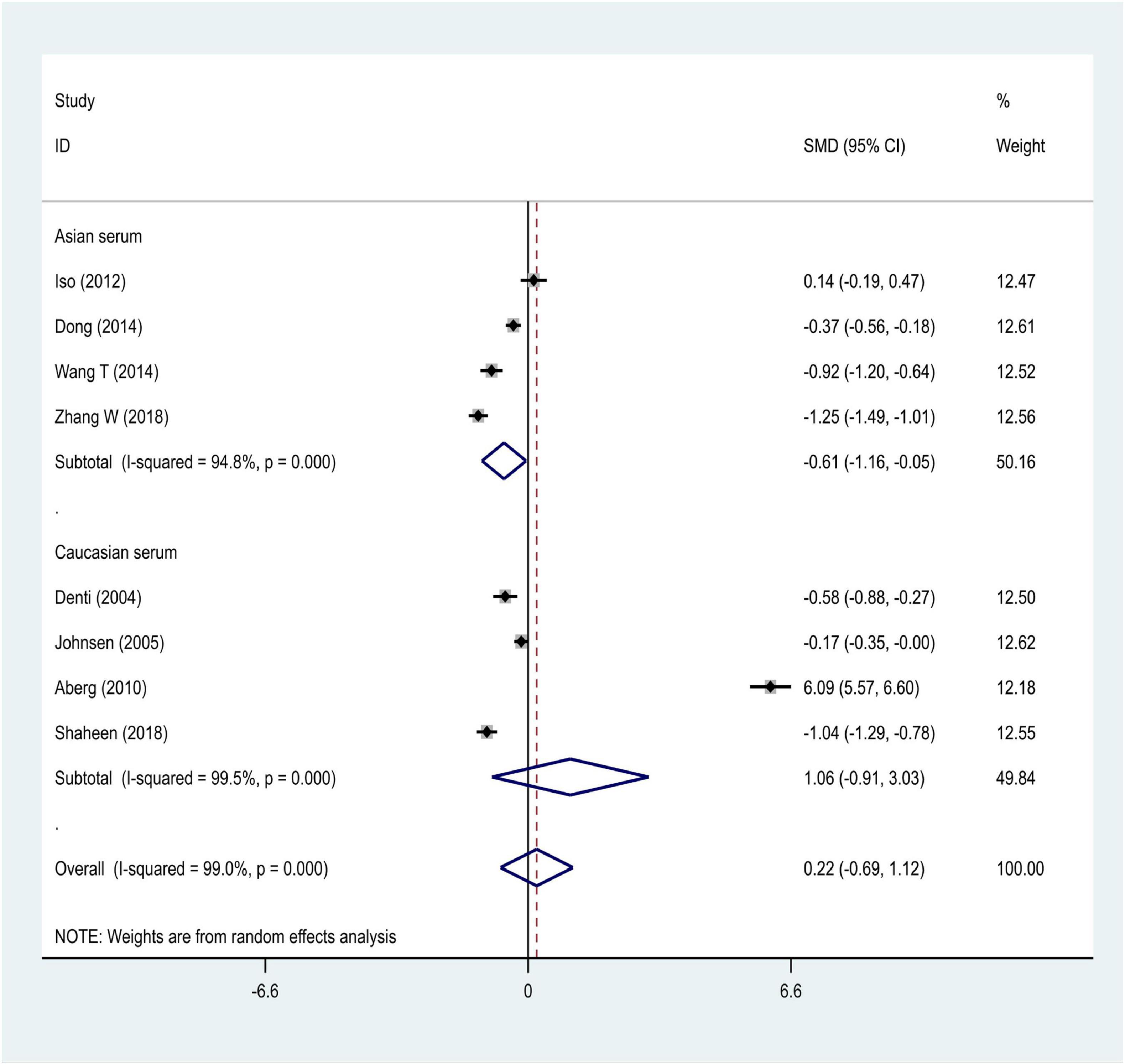
Figure 10. Results of meta-analysis for the IGF-1 serum levels in Caucasian, Asian, IS, and control groups, respectively.
Sensitivity Analysis
Sensitivity analysis of the data showed that it did not affect the results of meta-analysis (OR = 1.17, 95% CI = 1.09–1.26 for rs1800629; OR = 0.54, 95% CI = 0.21–0.87 for TNF-α; OR = –0.61, 95% CI = –1.16 to –0.05 for IGF-1).
Publication Bias
Begg’s test was used to verify publication bias, which showed that publication bias was negligible (z = 1.24, p = 0.22 for rs1800629; z = 0.73, p = 0.47 for TNF-α; z = 0.96, p = 0.34 for IGF-1).
Discussion
This updated meta-analysis study provided evidence that the rs1800629 SNP was associated with IS susceptibility. In addition, the level of TNF-α cytokine was elevated in IS patients compared with that in controls. Of particular interest, we first explored the relationship between levels of IGF-1 in the etiology of IS by performing a meta-analysis method. Especially, in the subgroup analysis, increased levels of TNF-α cytokine were found among the Asian and Caucasian populations, respectively. In contrast, no significant differences were found between IS patients and controls regarding the concentrations of IGF-1. Notably, we also first explored the interactions between differentially sourced specimens and specific ethnic backgrounds. Based on the stratification of the specimen and specific ethnic background, elevated concentrations of TNF-α and decreased levels of IGF-1 were noted in the serum samples of IS patients in the Asian population but not in the Caucasian population. These findings help us understand the role of gene polymorphisms and abnormal levels of cytokines such as TNF-α and IGF-1 in the pathogenesis of IS. In line with previous findings, our results also demonstrated that the rs1800629 polymorphisms were associated with the IS pathogenesis (Wu et al., 2019). The level of TNF-α was increased in IS patients as compared with that of the control subjects. Importantly, our updated meta-analysis, including 44 studies with 11,480 patents related to genetic SNPs and serum levels of cytokines in IS patients, was larger than the previous study (Wu et al., 2019). We speculated that the greater number of studies and larger sample sizes might obtain precise and valuable results.
The human TNF-α gene is located on chromosome 6p21.1–21.3 within the highly polymorphic region of the major histocompatibility complex (MHC). Among the several known TNF-α gene polymorphisms, studies have extensively focused on the G-308A (rs1800629) mutation in the etiology of IS. The polymorphisms are reportedly located in the TNF-α gene promoter region, which contains a number of regulatory elements that can influence the transcriptional activity of the gene (Wilson et al., 1997), and may also influence the DNA binding affinity of transcription factors leading to differential expressions of the TNF-α gene and the disease susceptibility (Wilson et al., 1997). Hence, it has been suggested that a more common regulatory variant may be a more likely risk factor for common disorders than rare variants within the gene coding region (Zill et al., 2002). Notably, the G308A (rs1800629) variant involved SNP mutation in the promoter region, which could upregulate the TNF-α gene’s transcriptional activity, resulting in the increased plasma concentrations of TNF-α in IS pathogenesis, and this finding was verified using genetic association analysis.
In IS, the activated microglia and astrocytes release high levels of TNF-α, which is considered toxic for negatively influencing synaptic transmission and plasticity in the learning and memory processes (Beattie et al., 2002), which is the core symptom of IS patients. In contrast, TNF-α combines with its receptors, leading to NF-κB activation that may play dual roles in inducing neurotoxicity as well as neuroprotective responses in brain cells depending on the stage of neuronal development, target cell type, and receptor subtypes (Shohami et al., 1999; Vila et al., 2000; Wang et al., 2000; Castillo and Leira, 2001; Cárdenas et al., 2002; Hallenbeck, 2002; Hurtado et al., 2002). Moreover, in clinical studies, patients with high TNF-α levels (Vila et al., 2000; Wang et al., 2000) have been shown to develop greater neurological deficits and poorer outcomes. In contrast, blocking TNF-α improves clinical outcomes (Tobinick et al., 2012). Similar phenomena have also been observed in several preclinical studies (King et al., 2013; Clausen et al., 2014; Arango-Dávila et al., 2015; Wu et al., 2016; Lin et al., 2020). Importantly, posttreatment with TNF-α neutralizing antibodies or TNF-α agonists alleviates poststroke brain injury in rodents (Vakili et al., 2011). Furthermore, at the molecular level, R-7050 acts as an anti-TNF-α receptor inhibitor demonstrating protection against stroke-induced brain injury (King et al., 2013; Clausen et al., 2014; Arango-Dávila et al., 2015; Wu et al., 2016) as well as enhancing the brain TNFRI and NF-κB signaling cascades along with increased levels of the Nrf2 protein in stroke rats, suggesting that R-7050 may enhance Nrf2 signaling, thus representing the involvement of another signal transduction to alleviate inflammatory responses in IS (Lin et al., 2021). Regarding the role of inflammation in stroke pathogenesis, Della Corte (Della Corte et al., 2016) has reviewed the role of immune-inflammatory variables in patients with IS, assessing the therapeutic perspectives that it offers. Patients with the cardioembolic (CEI) subtype of IS was reported to show significantly higher median plasma levels of TNF-α, compared with that of other subtypes. Multiple linear regression showed a significant association between the Scandinavian Stroke Scale (SSS) score at admission and diagnostic subtype infarct volume of cardioembolic strokes and some inflammatory variable. Tuttolomondo suggested that cardioembolic strokes have a worse clinical presentation and produce larger and more disabling strokes than other IS subtypes reporting a possible explanation of higher immuno-inflammatory activation of the acute phase (Albanese et al., 2005; Tuttolomondo et al., 2008).
Therefore, in stroke, the TNF-α signal transduction is activated during ischemic injuries, and this fact has been further verified in subsequent clinical studies. In our study, the presence of the TNF-α-308 GG genotype and a higher serum concentration of TNF-α increases the likelihood of a stroke pathology. Thus, the TNF-α signal transduction response may explain our results. The molecular mechanism of the association between IGF-1 and IS has not yet been fully elucidated. Previous studies have demonstrated that the IGF-1 couples with protein-3 primarily via the PI3-kinase pathway, which, on the one hand, mediates cell survival of neurons under oxidative stress (Gustafsson et al., 2004), and, on the other hand, is preceded by improvement in the blood-brain barrier and suppression of local inflammatory mediators, indicating a unique anti-inflammatory role for IGF-1 in the blood–brain barrier as a novel target for IGF-1-mediated neuroprotection response (Bake et al., 2014; De Geyter et al., 2016). This was verified in both preclinical and clinical studies and our results (Bake et al., 2014; De Geyter et al., 2016; Mehrpour et al., 2016; Lee et al., 2021). This meta-analysis results demonstrated the association between serum IGF-1 levels and ischemic stroke in the Asian population not in the Caucasian population. These differences can be explained by different genetic backgrounds.
One major limitation for this study is that except for different genetic backgrounds, we did not address the relationship between proinflammatory gene polymorphisms and stroke subtypes, because a more common, regulatory variant may be more likely to be involved in the etiology of IS (Hahn and Blakely, 2002). It has been suggested that etiologic factors for the development of the particular subtype may be different in stroke subtypes. As it has been mentioned except for other risk factors, age and sex may also involve in the etiology of different stroke subtypes (Moond et al., 2020; Tang et al., 2020). Thus, in future studies, we will consider stroke severity, subtype, hypertension, dyslipidemia, diabetes, and psychosocial stress in the etiology of IS (Sarfo et al., 2022).
Conclusion
This updated meta-analysis study demonstrated that the GG genotype might be considered as a risk factor for IS (especially in Asians), and the circulating levels of TNF-α were elevated in the Caucasian and Asian patients as compared with controls. At the same time, a positive association was found between serum IGF-1 levels and IS in the Asian population but not in the Caucasian population. Therefore, based on previous meta-analysis results and those combined with ours, we proposed that therapeutic strategies to decrease the circulating levels of TNF-α and increased levels of IGF-1 might be considered as a promising therapeutic target with potential neuroprotective effects for the treatment of IS.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
XZ and LL equally contributed to the study design of this review. RD, NW, and YS equally performed the literature search, interpreted the data, and wrote the manuscript. QL and HL profoundly enriched the manuscript by adding important intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.831910/full#supplementary-material
Supplementary Figure 1 | The result of publication bias between the allele of TNF-α (G:A) and IS.
Supplementary Figure 2 | The result of publication bias between IGF-1, TNF-α, and IS. (A) The result of publication bias between IGF-1 and IS and (B) the result of publication bias between TNF-α and IS.
References
Aberg, D., Jood, K., Blomstrand, C., Jern, C., Nilsson, M., Isgaard, J., et al. (2011). Serum IGF-I levels correlate to improvement of functional outcome after ischemic stroke. J. Clin. Endocrinol. Metab. 96, E1055–E1064. doi: 10.1210/jc.2010-2802
Albanese, A., Tuttolomondo, A., Anile, C., Sabatino, G., Pompucci, A., Pinto, A., et al. (2005). Spontaneous chronic subdural hematomas in young adults with a deficiency in coagulation factor XIII. Report of three cases. J. Neurosurg. 102, 1130–1132. doi: 10.3171/jns.2005.102.6.1130
Arango-Dávila, C. A., Vera, A., Londoño, A. C., Echeverri, A. F., Cañas, F., Cardozo, C. F., et al. (2015). Soluble or soluble/membrane TNF-α inhibitors protect the brain from focal ischemic injury in rats. Int. J. Neurosci. 125, 936–940. doi: 10.3109/00207454.2014.980906
Bake, S., Selvamani, A., Cherry, J., and Sohrabji, F. (2014). Blood brain barrier and neuroinflammation are critical targets of IGF-1-mediated neuroprotection in s troke for middle-aged female rats. PLoS One 9:e91427. doi: 10.1371/journal.pone.0091427
Balding, J., Livingstone, W. J., Pittock, S. J., Mynett-Johnson, L., Ahern, T., Hodgson, A., et al. (2004). The IL-6 G-174C polymorphism may be associated with ischaemic stroke in patients without a history of hypertension. Ir. J. Med. Sci. 173, 200–203. doi: 10.1007/bf02914551
Banerjee, I., Gupta, V., Ahmed, T., Faizaan, M., Agarwal, P., and Ganesh, S. (2008). Inflammatory system gene polymorphism and the risk of stroke: a case-control study in an Indian population. Brain Res. Bull. 75, 158–165. doi: 10.1016/j.brainresbull.2007.08.007
Beattie, E. C., Stellwagen, D., Morishita, W., Bresnahan, J. C., Ha, B. K., Von Zastrow, M., et al. (2002). Control of synaptic strength by glial TNFalpha. Science 295, 2282–2285. doi: 10.1126/science.1067859
Benarroch, E. E. (2012). Insulin-like growth factors in the brain and their potential clinical implications. Neurology 79, 2148–2153. doi: 10.1212/WNL.0b013e3182752eef
Billinger, S. A., Sisante, J. V., Mattlage, A. E., Alqahtani, A. S., Abraham, M. G., Rymer, M. M., et al. (2017). The relationship of pro-inflammatory markers to vascular endothelial function after acute stroke. Int. J. Neurosci. 127, 486–492. doi: 10.1080/00207454.2016.1198344
Boehme, A. K., Mcclure, L. A., Zhang, Y., Luna, J. M., Del Brutto, O. H., Benavente, O. R., et al. (2016). Inflammatory markers and outcomes after lacunar stroke: levels of inflammatory markers in treatment of stroke study. Stroke 47, 659–667. doi: 10.1161/STROKEAHA.115.012166
Bokhari, F. A., Shakoori, T. A., Butt, A., and Ghafoor, F. (2014). TNF-alpha: a risk factor for ischemic stroke. J. Ayub Med. Coll. Abbottabad 26, 111–114.
Cárdenas, A., Moro, M. A., Leza, J. C., O’shea, E., Dávalos, A., Castillo, J., et al. (2002). Upregulation of TACE/ADAM17 after ischemic preconditioning is involved in brain tolerance. J. Cereb. Blood Flow Metab. 22, 1297–1302. doi: 10.1097/01.WCB.0000033968.83623.D0
Castillo, J., and Leira, R. (2001). Predictors of deteriorating cerebral infarct: role of inflammatory mechanisms. Would its early treatm ent be useful? Cerebrovasc. Dis. 11(Suppl. 1), 40–48. doi: 10.1159/000049124
Clausen, B. H., Degn, M., Martin, N. A., Couch, Y., Karimi, L., Ormhøj, M., et al. (2014). Systemically administered anti-TNF therapy ameliorates functional outcomes after focal cerebral ische mia. J. Neuroinflammation 11:203. doi: 10.1186/s12974-014-0203-6
Clausen, B. H., Wirenfeldt, M., Høgedal, S. S., Frich, L. H., Nielsen, H. H., Schrøder, H. D., et al. (2020). Characterization of the TNF and IL-1 systems in human brain and blood after ischemic stroke. Acta Neuropathol. Commun. 8:81. doi: 10.1186/s40478-020-00957-y
Cui, G., Wang, H., Li, R., Zhang, L., Li, Z., Wang, Y., et al. (2012). Polymorphism of tumor necrosis factor alpha (TNF-alpha) gene promoter, circulating TNF-alpha level, a nd cardiovascular risk factor for ischemic stroke. J. Neuroinflammation 9:235. doi: 10.1186/1742-2094-9-235
Cure, M. C., Tufekci, A., Cure, E., Kirbas, S., Ogullar, S., Kirbas, A., et al. (2013). Low-density lipoprotein subfraction, carotid artery intima-media thickness, nitric oxide, and tumor n ecrosis factor alpha are associated with newly diagnosed ischemic stroke. Ann. Indian Acad. Neurol. 16, 498–503. doi: 10.4103/0972-2327.120438
De Geyter, D., De Smedt, A., Stoop, W., De Keyser, J., and Kooijman, R. (2016). Central IGF-I Receptors in the Brain are Instrumental to Neuroprotection by Systemically Injected IGF -I in a Rat Model for Ischemic Stroke. CNS Neurosci. Ther. 22, 611–616. doi: 10.1111/cns.12550
de Sousa Parreira, J., Kallaur, A. P., Lehmann, M. F., Oliveira, S. R., Frizon, D. A., Delongui, F., et al. (2015). Tumor necrosis factor beta NcoI polymorphism (rs909253) is associated with inflammatory and metabolic markers in acute ischemic stroke. Metab. Brain Dis. 30, 159–167. doi: 10.1007/s11011-014-9584-6
Della Corte, V., Tuttolomondo, A., Pecoraro, R., Di Raimondo, D., Vassallo, V., and Pinto, A. (2016). Inflammation, endothelial dysfunction and arterial stiffness as therapeutic targets in cardiovascular medicine. Curr. Pharm. Des. 22, 4658–4668. doi: 10.2174/1381612822666160510124801
Denti, L., Annoni, V., Cattadori, E., Salvagnini, M. A., Visioli, S., Merli, M. F., et al. (2004). Insulin-like growth factor 1 as a predictor of ischemic stroke outcome in the elderly. Am. J. Med. 117, 312–317. doi: 10.1016/j.amjmed.2004.02.049
Djordjevic, V., Divac-Rankov, A., Stankovic, M., Brankovic-Sreckovic, V., and Radojkovic, D. (2013). Tumor necrosis factor alpha and alpha-1 antitrypsin gene variants in Serbian pediatric arterial ischemic stroke patients. Genetika 45, 621–628. doi: 10.2298/GENSR1303621D
Domac, F. M., Somay, G., Misirli, H., and Erenoglu, N. Y. (2007). Tumor necrosis factor alpha serum levels and inflammatory response in acute ischemic stroke. Neurosciences 12:25. doi: 10.1177/1545968306290225
Dong, X., Chang, G., Ji, X. F., Tao, D. B., and Wang, Y. X. (2014). The relationship between serum insulin-like growth factor I levels and ischemic stroke risk. PLoS One 9:e94845. doi: 10.1371/journal.pone.0094845
Gu, L., Wu, G., Su, L., Yan, Y., and Wei, A. (2015). TNF-a (-238G/A and -308G/A) gene polymorphisms may not contribute to the risk of ischemic stroke. Int. J. Neurosci. 126, 219–226. doi: 10.3109/00207454.2015.1010200
Gustafsson, H., Tamm, C., and Forsby, A. (2004). Signalling pathways for insulin-like growth factor type 1-mediated expression of uncoupling protein 3. J. Neurochem. 88, 462–468. doi: 10.1046/j.1471-4159.2003.02162.x
Hahn, M. K., and Blakely, R. D. (2002). Monoamine transporter gene structure and polymorphisms in relation to psychiatric and other complex d isorders. Pharmacogenomics J. 2, 217–235. doi: 10.1038/sj.tpj.6500106
Hallenbeck, J. M. (2002). The many faces of tumor necrosis factor in stroke. Nat. Med. 8, 1363–1368. doi: 10.1038/nm1202-1363
Harcos, P., Laki, J., Kiszel, P., Széplaki, Z., Szolnoki, Z., Kovács, M., et al. (2006). Decreased frequency of the TNF2 allele of TNF-alpha -308 promoter polymorphism is associated with lac unar infarction. Cytokine 33, 100–105. doi: 10.1016/j.cyto.2005.12.006
Hurtado, O., Lizasoain, I., Fernández-Tomé, P., Alvarez-Barrientos, A., Leza, J. C., Lorenzo, P., et al. (2002). TACE/ADAM17-TNF-alpha pathway in rat cortical cultures after exposure to oxygen-glucose deprivation or glutamate. J. Cereb. Blood Flow Metab. 22, 576–585. doi: 10.1097/00004647-200205000-00009
Intiso, D., Zarrelli, M. M., Lagioia, G., Rienzo, F. D., and Dagger, R. J. (2004). Tumor necrosis factor alpha serum levels and inflammatory response in acute ischemic stroke patients. Neurol. Sci. 24, 390–396. doi: 10.1007/s10072-003-0194-z
Iso, H., Maruyama, K., Ikehara, S., Yamagishi, K., and Tamakoshi, A. (2012). Cellular growth factors in relation to mortality from cardiovascular disease in middle-aged Japanese: the JACC study. Atherosclerosis 224, 154–160. doi: 10.1016/j.atherosclerosis.2012.05.026
Jefferis, B. J., Whincup, P. H., Welsh, P., Wannamethee, S. G., Rumley, A., Lennon, L. T., et al. (2009). Circulating TNFα levels in older men and women do not show independent prospective relations with MI or stroke. Atherosclerosis 205, 302–308. doi: 10.1016/j.atherosclerosis.2008.12.001
Jiang, C. T., Wu, W. F., Deng, Y. H., and Ge, J. W. (2020). Modulators of microglia activation and polarization in ischemic stroke (Review). Mol. Med. Rep. 21, 2006–2018. doi: 10.3892/mmr.2020.11003
Johnsen, S. P., Hundborg, H. H., Sørensen, H. T., Orskov, H., Tjønneland, A., and Overvad, K. (2005). Insulin-like growth factor (IGF) I, -II, and IGF binding protein-3 and risk of ischemic stroke. J. Clin. Endocrinol. Metab. 90, 5937–5941. doi: 10.1210/jc.2004-2088
Juul, A. (2003). Serum levels of insulin-like growth factor I and its binding proteins in health and disease. Growth Horm. IGF Res. 13, 113–170. doi: 10.1016/s1096-6374(03)00038-8
Kamdee, K., Panadsako, N., Mueangson, O., Nuinoon, M., and Chunglok, W. (2021). Promoter polymorphism of TNFα (rs1800629) is associated with ischemic stroke susceptibility in a southern Thai population. Biomed. Rep. 15:78. doi: 10.3892/br.2021.1454
Kaplan, R. C., Mcginn, A. P., Pollak, M. N., Kuller, L. H., Strickler, H. D., Rohan, T. E., et al. (2007). Association of total insulin-like growth factor-I, insulin-like growth factor binding protein-1 (IGFB P-1), and IGFBP-3 levels with incident coronary events and ischemic stroke. J. Clin. Endocrinol. Metab. 92, 1319–1325. doi: 10.1210/jc.2006-1631
Karahan, Z. C., Deda, G., Sipahi, T., Elhan, A. H., and Akar, N. (2005). TNF-alpha -308G/A and IL-6 -174 G/C polymorphisms in the Turkish pediatric stroke patients. Thromb. Res. 115, 393–398. doi: 10.1016/j.thromres.2004.09.008
Kim, O. J., Lee, J. H., Choi, J. K., Oh, S. H., Hong, S. H., Oh, D., et al. (2010). Association between tumor necrosis factor-alpha (-308G-A and -238G-A) polymorphisms and homocysteine levels in patients with ischemic strokes and silent brain infarctions. Cerebrovasc. Dis. 30, 483–490. doi: 10.1159/000319023
King, M. D., Alleyne, C. H., and Dhandapani, K. M. (2013). TNF-alpha receptor antagonist, R-7050, improves neurological outcomes following intracerebral hemorrh age in mice. Neurosci. Lett. 542, 92–96. doi: 10.1016/j.neulet.2013.02.051
Kumar, P., Kumar, A., Misra, S., Sagar, R., Faruq, M., Suroliya, V., et al. (2016). Tumor necrosis factor-alpha (308G/A, +488G/A, 857C/T and -1031T/C) gene polymorphisms and risk of ischemic stroke in north Indian population: a hospital based case–control study. Meta Gene 7, 34–39. doi: 10.1016/j.mgene.2015.11.003
Lalouschek, W., Schillinger, M., Hsieh, K., Endler, G., Greisenegger, S., Marculescu, R., et al. (2006). Polymorphisms of the inflammatory system and risk of ischemic cerebrovascular events. Clin. Chem. Lab. Med. 44, 918–923. doi: 10.1515/CCLM.2006.165
Lasek-Bal, A., Jedrzejowska-Szypulka, H., Student, S., Warsz-Wianecka, A., Zareba, K., Puz, P., et al. (2019). The importance of selected markers of inflammation and blood-brain barrier damage for short-term isch emic stroke prognosis. J. Physiol. Pharmacol. 70, 209–217. doi: 10.26402/jpp.2019.2.04
Lee, B. C., Ahn, S. Y., Doo, H. K., Yim, S. V., Lee, H. J., Jin, S. Y., et al. (2004). Susceptibility for ischemic stroke in Korean population is associated with polymorphisms of the inter leukin-1 receptor antagonist and tumor necrosis factor-alpha genes, but not the interleukin-1beta gene. Neurosci. Lett. 357, 33–36. doi: 10.1016/j.neulet.2003.12.041
Lee, J., Lee, J., Lee, M., Lim, J. S., Kim, J. H., Yu, K. H., et al. (2021). Association between Serum Insulin-Like Growth Factor-1 and Neurological Severity in Acute Ischemic St roke. J. Clin. Neurol. 17, 206–212. doi: 10.3988/jcn.2021.17.2.206
Lin, S. Y., Wang, Y. Y., Chang, C. Y., Wu, C. C., Chen, W. Y., Kuan, Y. H., et al. (2020). Effects of β-adrenergic blockade on metabolic and inflammatory responses in a rat model of ischemic stroke. Cells 9:1373. doi: 10.3390/cells9061373
Lin, S. Y., Wang, Y. Y., Chang, C. Y., Wu, C. C., Chen, W. Y., Liao, S. L., et al. (2021). TNF-α receptor inhibitor alleviates metabolic and inflammatory changes in a rat model of ischemic stroke. Antioxidants 10:851. doi: 10.3390/antiox10060851
Ma, Z., Yue, Y., Luo, Y., Wang, W., Cao, Y., and Fang, Q. (2020). Clinical utility of the inflammatory factors combined with lipid markers in the diagnostic and progno stic assessment of ischemic stroke: based on logistic regression models. J. Stroke Cerebrovasc. Dis. 29:104653. doi: 10.1016/j.jstrokecerebrovasdis.2020.104653
Mehrpour, M., Rahatlou, H., Hamzehpur, N., Kia, S., and Safdarian, M. (2016). Association of insulin-like growth factor-I with the severity and outcomes of acute ischemic stroke. Iran. J. Neurol. 15, 214–218.
Moond, V., Bansal, K., and Jain, R. (2020). Risk factors and subtyping of ischemic stroke in young adults in the indian population. Cureus 12:e11388. doi: 10.7759/cureus.11388
Navis, A., Garcia-Santibanez, R., and Skliut, M. (2019). Epidemiology and outcomes of ischemic stroke and transient ischemic attack in the adult and geriatric population. J. Stroke Cerebrovasc. Dis. 28, 84–89. doi: 10.1016/j.jstrokecerebrovasdis.2018.09.013
Palm, F., Aigner, A., Pussinen, P. J., Urbanek, C., Buggle, F., Safer, A., et al. (2020). Association of a multigenetic pro-inflammatory profile with ischaemic stroke. Cerebrovasc. Dis. 49, 170–176. doi: 10.1159/000507042
Pasarica, D., Gheorghiu, M., Topârceanu, F., Bleotu, C., Ichim, L., and Trandafir, T. (2005). Neurotrophin-3, TNF-alpha and IL-6 relations in serum and cerebrospinal fluid of ischemic stroke patients. Roum. Arch. Microbiol. Immunol. 64, 27–33.
Rubattu, S., Speranza, R., Ferrari, M., Evangelista, A., Beccia, M., Stanzione, R., et al. (2005). A role of TNF-alpha gene variant on juvenile ischemic stroke: a case-control study. Eur. J. Neurol. 12, 989–993. doi: 10.1111/j.1468-1331.2005.01136.x
Sarfo, F. S., Ovbiagele, B., Akpa, O., Akpalu, A., Wahab, K., Obiako, R., et al. (2022). Risk factor characterization of ischemic stroke subtypes among West Africans. Stroke 53, 134–144. doi: 10.1161/STROKEAHA.120.032072
Schwab, S., Spranger, M., Krempien, S., Hacke, W., and Bettendorf, M. (1997). Plasma insulin-like growth factor I and IGF binding protein 3 levels in patients with acute cerebral ischemic injury. Stroke 28, 1744–1748. doi: 10.1161/01.str.28.9.1744
Shaheen, H., Sobhy, S., El Mously, S., Niazi, M., and Gomaa, M. (2018). Insulin-like growth factor-1 in acute ischemic stroke. Egypt. J. Neurol. Psychiatr. Neurosurg. 54:42. doi: 10.1186/s41983-018-0042-y
Shi, K. L., He, B., Wang, J. J., and Zou, L. P. (2009). Role of TNF-alpha gene variation in idiopathic childhood ischemic stroke: a case-control study. J. Child Neurol. 24, 25–29. doi: 10.1177/0883073808321046
Shishkova, V. N., Adasheva, T. V., Remenik, A. Y., Valyaeva, V. N., and Shklovsky, V. M. (2018). Prognostic significance of clinical-anthropometric, biochemical, metabolic, vascular-inflammatory an d molecular-genetic markers in the development of the first ischemic stroke. Zh. Nevrol. Psikhiatr. Im. S S Korsakova 118, 4–11. doi: 10.17116/jnevro2018118214-11
Shohami, E., Ginis, I., and Hallenbeck, J. M. (1999). Dual role of tumor necrosis factor alpha in brain injury. Cytokine Growth Factor Rev. 10, 119–130. doi: 10.1016/s1359-6101(99)00008-8
Sillero, L. P., Fernández de Velasco Casarrubios, J., García-Raso, A., Meseguer Gancedo, E., Santos Montero, A. B., and Tomás Martínez, J. F. (2007). Polymorphism -238 G/A of tumor necrosis factor alpha gene promoter is a genetic risk factor for ischemic cerebrovascular disease. J. Mol. Neurosci. 32, 108–110. doi: 10.1007/s12031-007-0021-8
Strong, K., Mathers, C., and Bonita, R. (2007). Preventing stroke: saving lives around the world. Lancet Neurol. 6, 182–187. doi: 10.1016/S1474-4422(07)70031-5
Sultana, S., Kolla, V. K., Jeedigunta, Y., Penagaluru, P. K., Joshi, S., Rani, P. U., et al. (2011). Tumour necrosis factor alpha and interleukin 10 gene polymorphisms and the risk of ischemic stroke in south Indian population. J. Genet. 90, 361–364. doi: 10.1007/s12041-011-0079-5
Tang, M., Yao, M., Zhu, Y., Peng, B., Zhou, L., and Ni, J. (2020). Sex differences of ischemic stroke in young adults-A single-center Chinese cohort study. J. Stroke Cerebrovasc. Dis. 29:105087. doi: 10.1016/j.jstrokecerebrovasdis.2020.105087
Tobinick, E., Kim, N. M., Reyzin, G., Rodriguez-Romanacce, H., and Depuy, V. (2012). Selective TNF inhibition for chronic stroke and traumatic brain injury: an observational study involving 629 consecutive patients treated with perispinal etanercept. CNS Drugs 26, 1051–1070. doi: 10.1007/s40263-012-0013-2
Tong, Y., Geng, Y., Xu, J., Wang, Z., Zhang, Y., Lin, L., et al. (2010). The role of functional polymorphisms of the TNF-α gene promoter in the risk of ischemic stroke in Chinese Han and Uyghur populations: two case–control studies. Clin. Chim. Acta 411, 1291–1295. doi: 10.1016/j.cca.2010.05.007
Tuttolomondo, A., Pedone, C., Pinto, A., Di Raimondo, D., Fernandez, P., Di Sciacca, R., et al. (2008). Predictors of outcome in acute ischemic cerebrovascular syndromes: the GIFA study. Int. J. Cardiol. 125, 391–396. doi: 10.1016/j.ijcard.2007.03.109
Tuttolomondo, A., Raimondo, D. D., Forte, G. I., Casuccio, A., Vaccarino, L., Scola, L., et al. (2012). Single nucleotide polymorphisms (SNPs) of pro-inflammatory/anti-inflammatory and thrombotic/fibrinolytic genes in patients with acute ischemic stroke in relation to TOAST subtype. Cytokine 58, 398–405. doi: 10.1016/j.cyto.2012.02.012
Um, J. Y., An, N. H., and Kim, H. M. (2003). TNF-alpha and TNF-beta gene polymorphisms in cerebral infarction. J. Mol. Neurosci. 21, 167–171. doi: 10.1385/JMN:21:2:167
Um, J. Y., and Kim, H. M. (2004). Tumor necrosis factor alpha gene polymorphism is associated with cerebral infarction. Brain Res. Mol. Brain Res. 122, 99–102. doi: 10.1016/j.molbrainres.2003.11.019
Vakili, A., Mojarrad, S., Akhavan, M. M., and Rashidy-Pour, A. (2011). Pentoxifylline attenuates TNF-α protein levels and brain edema following temporary focal cerebral isc hemia in rats. Brain Res. 1377, 119–125. doi: 10.1016/j.brainres.2011.01.001
Vila, N., Castillo, J., Dávalos, A., and Chamorro, A. (2000). Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke 31, 2325–2329. doi: 10.1161/01.str.31.10.2325
Wang, Y., Zhang, Y., Huang, J., Chen, X., Gu, X., Wang, Y., et al. (2014). Increase of circulating miR-223 and insulin-like growth factor-1 is associated with the pathogenesis of acute ischemic stroke in patients. BMC Neurol. 14:77. doi: 10.1186/1471-2377-14-77
Wang, T., Bin, L. I., and Zhong, P. A. (2014). The relationship between the serum VILIP-1,IGF-1 concentration and cognitive impairment in patients with cerebral infarction. J. Apoplexy Nerv. Dis. 31, 121–124.
Wang, X., Li, X., Erhardt, J. A., Barone, F. C., and Feuerstein, G. Z. (2000). Detection of tumor necrosis factor-alpha mRNA induction in ischemic brain tolerance by means of real- time polymerase chain reaction. J. Cereb. Blood Flow Metab. 20, 15–20. doi: 10.1097/00004647-200001000-00004
Wawrzynek, A., Dobiała, J., Wender, M., Kozubski, W., and Michałowska-Wender, G. (2014). TNF-α gene G-308A polymorphism and the risk of ischemic stroke. Neurol. Neurochir. Pol. 48, 387–390. doi: 10.1016/j.pjnns.2014.09.007
Wilson, A. G., Symons, J. A., Mcdowell, T. L., Mcdevitt, H. O., and Duff, G. W. (1997). Effects of a polymorphism in the human tumor necrosis factor alpha promoter on transcriptional activa tion. Proc. Natl. Acad. Sci. U.S.A. 94, 3195–3199. doi: 10.1073/pnas.94.7.3195
Wu, J. C., Zhang, X., Wang, J. H., Liu, Q. W., Wang, X. Q., Wu, Z. Q., et al. (2019). Gene polymorphisms and circulating levels of the TNF-alpha are associated with ischemic stroke: a met a-analysis based on 19,873 individuals. Int. Immunopharmacol. 75:105827. doi: 10.1016/j.intimp.2019.105827
Wu, M. H., Huang, C. C., Chio, C. C., Tsai, K. J., Chang, C. P., Lin, N. K., et al. (2016). Inhibition of peripheral TNF-α and downregulation of microglial activation by Alpha-lipoic acid and e tanercept protect rat brain against ischemic stroke. Mol. Neurobiol. 53, 4961–4971. doi: 10.1007/s12035-015-9418-5
Wytrykowska, A., Prosba-Mackiewicz, M., and Nyka, W. M. (2016). IL-1β, TNF-α, and IL-6 levels in gingival fluid and serum of patients with ischemic stroke. J. Oral Sci. 58, 509–513. doi: 10.2334/josnusd.16-0278
Zaremba, J., and Losy, J. (2001a). Early TNF-alpha levels correlate with ischaemic stroke severity. Acta Neurol. Scand. 104, 288–295. doi: 10.1034/j.1600-0404.2001.00053.x
Zaremba, J., and Losy, J. (2001b). The levels of TNF-alpha in cerebrospinal fluid and serum do not correlate with the counts of the white blood cells in acute phase of ischaemic stroke. Folia Morphol. 60, 91–97.
Zhang, Y. Y., Huang, N. N., Zhao, Y. X., Li, Y. S., Wang, D., Fan, Y. C., et al. (2018). Elevated tumor necrosis factor-a-induced protein 8-like 2 mRNA from peripheral blood mononuclear cell s in patients with acute ischemic stroke. Int. J. Med. Sci. 15, 1713–1722. doi: 10.7150/ijms.27817
Zhang, W., Wang, W., and Kuang, L. (2018). The relation between insulin-like growth factor 1 levels and risk of depression in ischemic stroke. Int. J. Geriatr. Psychiatry 33, e228–e233. doi: 10.1002/gps.4774
Zhao, N., Liu, X., Wang, Y., Liu, X., Li, J., Yu, L., et al. (2012). Association of inflammatory gene polymorphisms with ischemic stroke in a Chinese Han population. J. Neuroinflammation 9:162. doi: 10.1186/1742-2094-9-162
Zhao, X., Huang, Y., Ma, H., Jin, Q., Wang, Y., and Zhu, G. (2013). Association between major depressive disorder and the norepinephrine transporter polymorphisms T-182C and G1287A: a meta-analysis. J. Affect. Disord. 150, 23–28. doi: 10.1016/j.jad.2013.03.016
Zill, P., Engel, R., Baghai, T. C., Juckel, G., Frodl, T., Muller-Siecheneder, F., et al. (2002). Identification of a naturally occurring polymorphism in the promoter region of the norepinephrine tra nsporter and analysis in major depression. Neuropsychopharmacology 26, 489–493. doi: 10.1016/S0893-133X(01)00386-4
Keywords: TNF-α, IGF-1, ischemic stroke, meta-analysis, gene polymorphism
Citation: Duan R, Wang N, Shang Y, Li H, Liu Q, Li L and Zhao X (2022) TNF-α (G-308A) Polymorphism, Circulating Levels of TNF-α and IGF-1: Risk Factors for Ischemic Stroke—An Updated Meta-Analysis. Front. Aging Neurosci. 14:831910. doi: 10.3389/fnagi.2022.831910
Received: 09 December 2021; Accepted: 27 January 2022;
Published: 16 March 2022.
Edited by:
Yu-Min Kuo, National Cheng Kung University, TaiwanReviewed by:
Wei Wu, Nanjing Medical University, ChinaAntonino Tuttolomondo, University of Palermo, Italy
Copyright © 2022 Duan, Wang, Shang, Li, Liu, Li and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Li, bGlfYW5ldGhlc2lhQDE2My5jb20=; Xiaofeng Zhao, enhmMDYwNTAwNEAxNjMuY29t
†These authors have contributed equally to this work
 Ranran Duan
Ranran Duan Na Wang
Na Wang Yanan Shang
Yanan Shang Hengfen Li
Hengfen Li Qian Liu
Qian Liu Li Li
Li Li Xiaofeng Zhao
Xiaofeng Zhao