- 1Department of Neurology, The First Affiliated Hospital of Soochow University, Suzhou, China
- 2Department of Neurology, Xiangya Hospital, Central South University, Changsha, China
- 3Department of Pathology, Xiangya Hospital, Central South University, Changsha, China
- 4School of Information Science and Engineering, Central South University, Changsha, China
- 5Key Laboratory of Hunan Province in Neurodegenerative Disorders, Central South University, Changsha, China
- 6National Clinical Research Center for Geriatric Diseases, Central South University, Changsha, China
- 7Laboratory of Medical Genetics, Central South University, Changsha, China
- 8School of Basic Medical Science, Central South University, Changsha, China
- 9Hunan International Scientific and Technological Cooperation Base of Neurodegenerative and Neurogenetic Diseases, Changsha, China
Recently, NPY overexpression has been proposed to alleviate motor deficits and neuropathy in Machado-Joseph disease (MJD) mouse models, indicating its neuroprotective role in the pathogenesis of MJD. We aimed to evaluate the association between SNPs in NPY and its receptors and the susceptibility of MJD in the Chinese population. Moreover, we investigated whether these SNPs modulate the age at onset (AO) of MJD. In total, 527 MJD patients and 487 healthy controls were enrolled in the study, and four specific selected SNPs (rs16139, rs3037354, rs2234759, and rs11100494) in NPY and its receptor genes were genotyped. In this study, the genotypic frequency using the dominant model and the allelic distribution of rs11100494 in NPY5R revealed a significant difference between the MJD and control group during the first-stage analysis (P = 0.048 and P = 0.024, respectively). After we expanded the sample size, significant differences were observed between the two groups using the dominant model in genotypic and allelic distribution (P = 0.034, P = 0.046, and P = 0.016, respectively). No significant differences in genotypic and allelic distribution were found between the MJD and control groups for the other three SNPs. All selected SNPs had no significant effect on the AO of MJD. The association of rs11100494 in the NPY5R gene and susceptibility of MJD suggested that the NPY system might be implicated in the pathogenesis of MJD. Our study demonstrated the existence of other genetic modifiers in MJD, along with CAG expansion and known genetic modifier factors, which might lead to a better understanding of MJD pathogenesis.
Introduction
Machado-Joseph disease (MJD), or spinocerebellar ataxia type 3 (SCA3), is a fatal, autosomal dominantly inherited neurodegenerative disease without curable therapies (Paulson, 2007). It was caused by an abnormal expansion of the CAG repeats in exon 10 of the ATXN3 gene, leading to an expanded polyglutamine tract within the ataxin3 protein (Kawaguchi et al., 1994). The expanded polyglutamine tract induces insoluble ubiquitin-positive protein aggregation and accumulation in neurons, leading to progressive neurodegeneration in the cerebellum, spinal cord, and substantia nigra (Taroni and DiDonato, 2004). There is a well-established inverse correlation between abnormally expanded CAG repeats and the age at onset (AO) in MJD (Matos et al., 2019). However, the abnormally expanded CAG repeats could only explain 50—70% of AO variability, showing that MJD is modulated by factors other than the expanded CAG repeats only (Tezenas et al., 2014; Chen et al., 2016). Recent studies have reported that in addition to CAG repeats in polyglutamine (polyQ) genes (Franca et al., 2012; Leotti et al., 2021), single nucleotide polymorphisms (SNPs) in crucial genes could also modulate the AO of MJD (Wang et al., 2018; Ding et al., 2019; Mergener et al., 2020), indicating that they could be new important genetic factors affecting the pathogenesis of MJD and composing “missing heritability” in AO of MJD (Ding et al., 2016). To date, the genotypic and/or allelic status of SNPs at approximately 10 genes, including ATXN3 (Long et al., 2015), ATXN2 (Ding et al., 2016), APOE (Bettencourt et al., 2011; Peng et al., 2014), MT-ND3 (Chen et al., 2016), CAST (Martins et al., 2021), FAN1 (Mergener et al., 2020), DNMT3A, DNMT3L (Ding et al., 2019), and IL6 (Raposo et al., 2017), has been demonstrated to modulate the AO of MJD patients. These genes are involved in mitochondrial function, the calpain-cleavage pathway, DNA repair, DNA methylation, and neuroimmunity, suggesting that the mechanism of MJD pathogenesis is complicated.
Neuropeptide Y (NPY) is an abundantly distributed neuropeptide in the mammalian brain and has been implicated in neuroprotection through inhibiting neuron death and excitotoxicity (Smialowska et al., 2009; Santos-Carvalho et al., 2013), increasing neuronal trophic support (Croce et al., 2013), stimulating the process of autophagy (Aveleira et al., 2015), and regulating transmission between cerebellar interneurons (Dubois et al., 2012). To date, five NPY receptors (NPY1R, NPY2R, NPY4R, NPY5R, and NPY6R) have been found in the mammalian brain; however, NPY6R has not been reported to be functional in the human brain (Diaz-delCastillo et al., 2018). NPY produces neuroprotective effects through NPY2R and NPY5R by alleviating the excitatory neurotoxic effect, inhibiting glutamate receptor overactivity, regulating calcium homeostasis, and attenuating neuroinflammation (Wu and Li, 2005; Farzi et al., 2015). In the central nervous system, NPY has been proven to control and alleviate neurodegeneration in models of Parkinson’s disease (PD) (Decressac et al., 2012), Huntington disease (HD) (Decressac et al., 2010), and Alzheimer’s disease (AD) (Chen et al., 2018). Moreover, SNPs (rs16139, rs3037354, rs2234759, and rs11100494) in NPY, NPY2R, and NPY5R have been found to be associated with AO in HD patients, major depressive disorder, and dyslipidemia (Coletta et al., 2007; Wang et al., 2013; Kloster et al., 2014). Recently, NPY overexpression or intranasal delivery in MJD mouse models have been found to alleviate MJD-associated motor deficits and neuropathy, indicating that NPY has neuroprotective potential in the pathogenesis of MJD (Duarte-Neves et al., 2021, 2015). This study was designed to determine whether NPY and its receptors contribute to the susceptibility of MJD and the variability of AO of this disease. For this purpose, we conducted a case–control study that analyzed the association between selected SNPs in NPY, NPY2R, and NPY5R and MJD. We also investigated whether these SNPs could explain some of the variability of AO in MJD patients.
Materials and Methods
Study Subjects
We enrolled 527 patients and 487 healthy controls (300 MJD patients and 300 controls from mainland China in the first stage (Supplementary Table 1), and an additional 227 MJD patients and 187 controls were added (Supplementary Table 2) to validate the obtained positive results) from Hunan, Hubei, Guizhou, Jiangxi, and Jiangsu provinces of China. The patients were consecutively recruited from the Department of Neurology of Xiangya Hospital, Central South University and the Department of Neurology of the First Affiliated Hospital of Soochow University during 2004–2020. A standard clinical neurological examination was performed by at least two experienced neurologists, and the clinical diagnoses were confirmed by molecular examinations. The healthy control subjects were matched to MJD patients by gender and did not have a positive family history of spinocerebellar ataxias (SCAs). Written informed consent was obtained from all subjects before they participated in this study, which was approved by the Ethics Committee of Xiangya Hospital, Central South University and the First Affiliated Hospital of Soochow University.
SNP Selection and Genotyping
Four SNPs (rs16139, rs3037354, rs2234759, and rs11100494) in NPY, NPY2R, and NPY5R were selected by giving priority to SNPs in the promoter or other regulatory regions and SNPs shown to be associated human diseases. Genomic DNA was extracted from peripheral blood leukocytes via standard phenol–chloroform extraction methods. CAG repeats in ATXN3 were determined by capillary electrophoresis and DNA sequencing with T-vector cloning. Genotypes of SNPs in the primary 600subjects were examined by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry via the MassARRAY system (Sequenom). To confirm the matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry results, 30 patients and 30 controls were randomly selected for Sanger sequencing. Genotypes of SNPs in an additional 414 subjects (227 MJD patients and 187 controls) were tested by Sanger sequencing. All primers (Supplementary Table 3) were designed using Primer31.
Statistical Analysis
For each SNP, the standard goodness-of-fit test was used to test Hardy–Weinberg equilibrium (HWE). The differences in the allele and genotype frequencies between cases and controls were determined using the standard chi-square (χ2) test or Fisher tests. The odds ratios (ORs) and associated 95% confidence intervals (95% CIs) were also calculated. Linear regression analysis was performed to determine the effect of each SNP on AO with the logarithmically (decimal) transformed AO as the dependent variable. When introducing each SNP in the linear regression analysis, dominant, codominant/genotypic, and recessive effects were assumed. The determinant coefficient (R2) was used to indicate the percentage of the explanation for AO variance via a given model. We analyzed covariance to adjust for the effect of the expanded CAG repeats on AO. Before that, a hypothesis test for regression coefficients was performed to examine the interaction between expanded CAG repeats and genotypes.
All analyses were performed using SPSS Ver23.0 (SPSS Inc., Chicago, IL, United States), and P < 0.05 was considered statistically significant.
Results
Subject Characteristics
The characteristics of MJD patients and healthy controls are summarized in Table 1. The study included 1,014 subjects, comprising 527 MJD patients [274 males and 253 females; mean age (±SD), 40.24 ± 11.65 years; Table 1] and 487 controls [250 males and 237 females; mean age (±SD), 41.83 ± 18.06 years; Table 1]. There was no significant difference in gnder distribution between MJD patients and controls (χ2 = 0.044, P = 0.834).
Association of NPY, NPY R2, and NPY R5 SNPs With MJD
The genotypic distribution and allelic frequency for the selected SNPs in the MJD patients and controls are described in Table 2. The genotypic distribution and allelic frequency did not deviate significantly from HWE (P > 0.05). In the primary cohort of 300 MJD patients and 300 controls, all genotypes of rs16139 in both MJD patients and controls were “TT” (not shown in Table 2). There were no significant differences in the genotypic distribution and allelic frequency of rs2234759 and rs3037354 between MJD patients and controls (Table 2). In the first stage of analysis, the frequencies of rs11100494 genotypes determined from MJD were 78.33% for CC, 20% for CA, and 1.67% for AA, and in the control group, the frequencies of CC, CA, and AA were 71.33, 25, and 3.67%, respectively (P = 0.086). The CC genotype of rs11100494 was significantly higher among the MJD patients than among the control group (78.33 and 71.33%, respectively, P = 0.048) and was associated with increased susceptibility of MJD (OR = 1.543, 95% CI: 1.002–2.107). The C allelic frequency showed a significant difference in MJD patients compared with the control group (P = 0.024, OR = 1.460, 95% CI: 1.049–2.032). After we expanded the sample size, CC, CA, and AA genotypic distributions were significantly different between the MJD patients and the control group (P = 0.046). Moreover, significant differences in CC genotypic and C allelic frequencies between MJD patients and the control group remained (P = 0.034, OR = 1.343, 95% CI: 1.022–1.767; P = 0.016, OR = 1.347, 95% CI: 1.058–1.715, respectively).
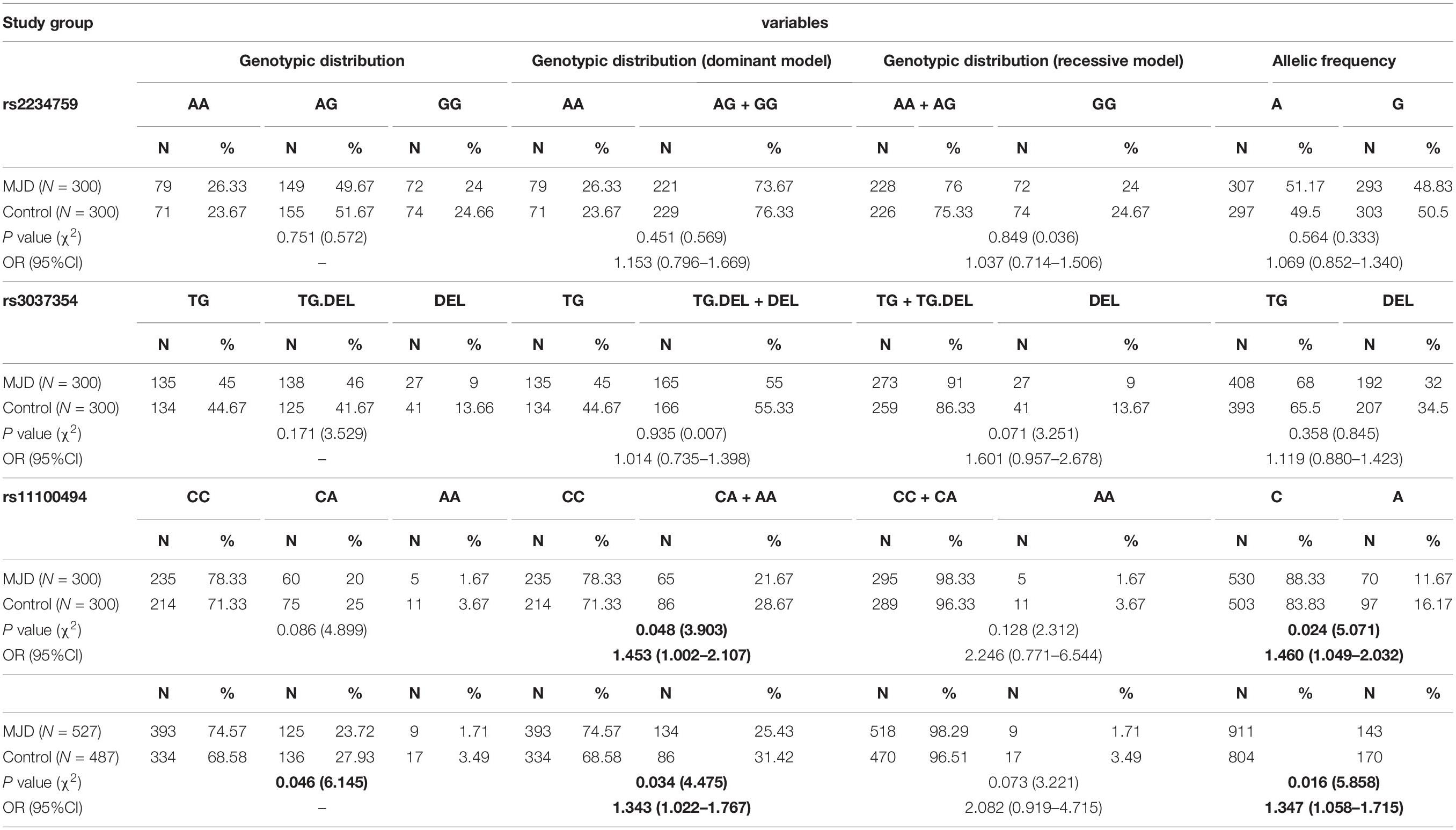
Table 2. Correlation analysis between genotypes and alleles of selected SNPs and susceptibility of MJD.
Association of NPY, NPY 2R, and NPY5R SNPs With the AO of MJD
We inspected the residuals to verify the validity of the linear regression model, and an extreme outlier with CAG repeats of 51 was eliminated in the primary 300-patient cohort. Analyzing the effect of the abnormally expanded CAG repeat in ATXN3 itself on ln(AO), R2 reaches values of 0.437 and 0.516 in linear and quadric models, respectively (Figure 1). Because of the additional increase of 7.9% in R2, the quadric model was used for the following analysis. As shown in Table 3, after inclusion of each of the four SNPs, the R2 value was raised to different degrees or not raised. However, after adjusting for the expanded CAG repeats in ATXN3, there were no significant differences among different genotypes of rs11100494 in the NPY5R gene (Table 4) in the analysis of covariance. In the replication stage, another 2 extreme outliers with CAG repeats of 55 and 86 were eliminated. The results of covariance analysis of rs11100494 showed no essential change after we enrolled the additional 227 MJD patients into the cohort (Table 4).
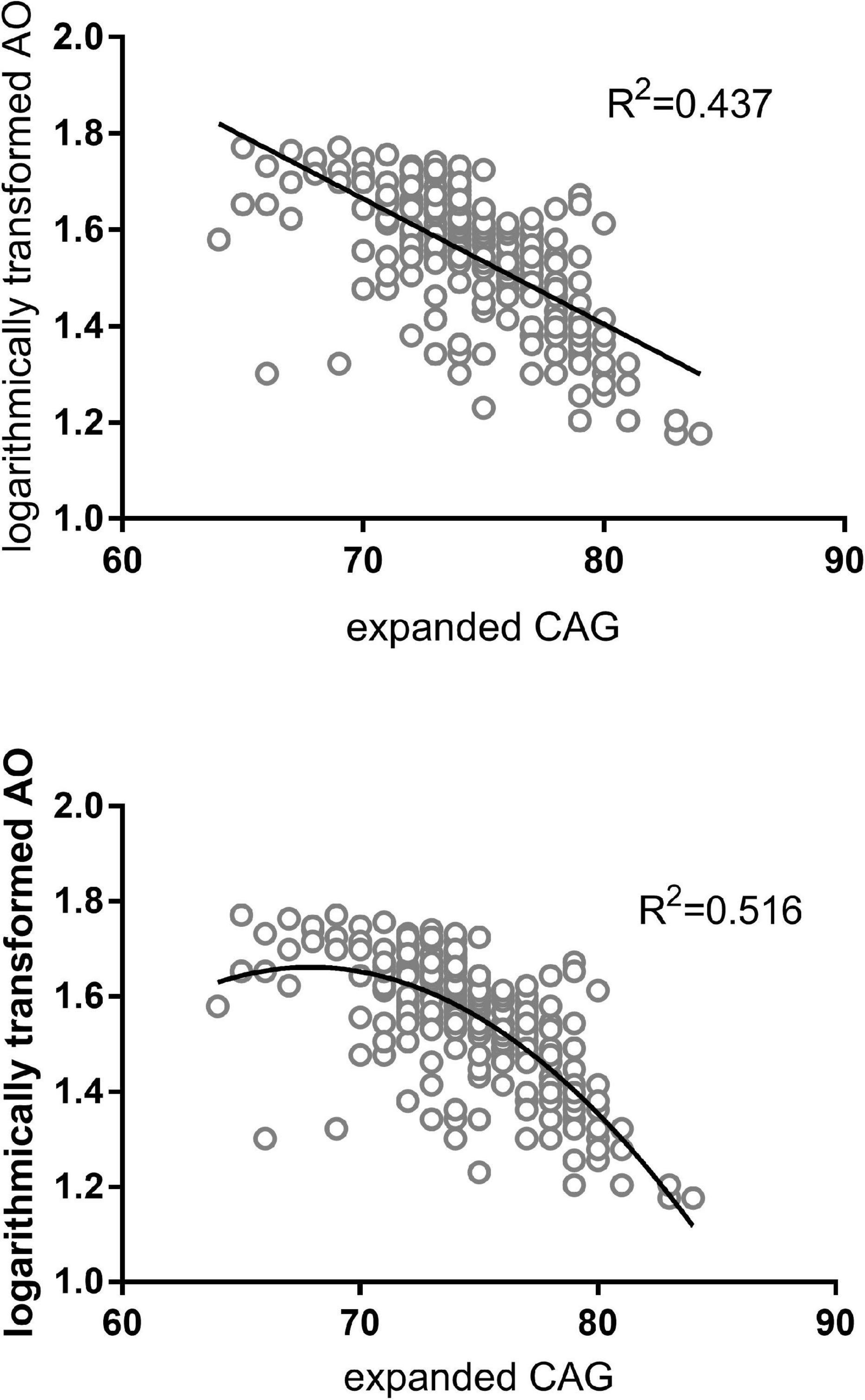
Figure 1. AO attributed to abnormal CAG repeats in ATXN3 of MJD patients. The figures on the above and below are regression analyses in linear and quadratic models, respectively. The X-axis denotes the expanded CAG repeat length, and the Y-axis indicates the logarithmically transformed AO. AO of MJD patients was inversely correlated with the length of expanded CAG repeats in the ATXN3 gene.
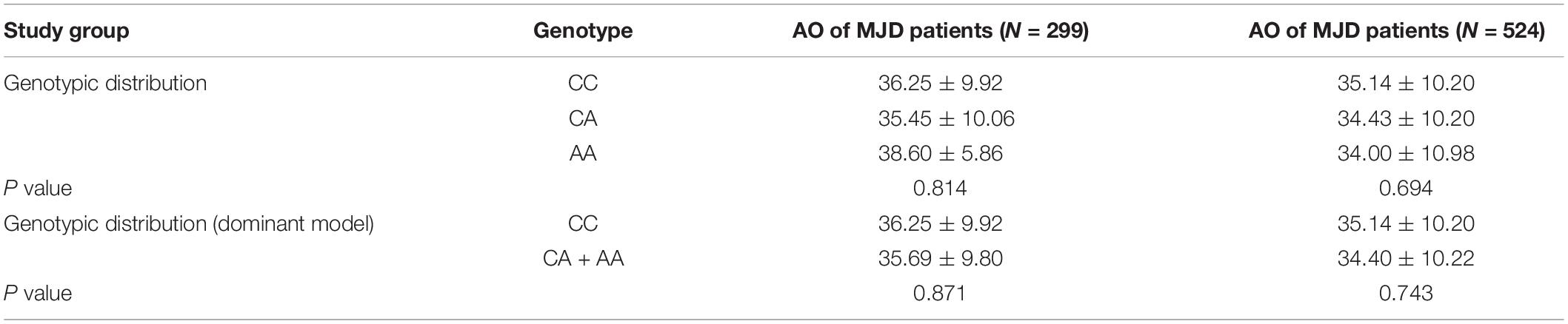
Table 4. AO differences among different genotypes of rs11100494 in the NPY5R gene after adjusting for the effect of expanded CAG repeats.
Discussion
Machado-Joseph disease is the most common type of autosomal dominant ataxia worldwide, and this disease accounts for approximately 62.64% of cases in China (Paulson, 2012; Chen et al., 2018). Although the genetic cause of MJD, abnormal CAG expansion in ATXN3, has been clearly defined for many years (Takiyama et al., 1993; Kawaguchi et al., 1994), the pathogenesis mechanisms of MJD have not been fully elucidated. To date, several different pathways have been identified to be involved in the pathogenesis of MJD: RNA toxicity (Nalavade et al., 2013), abnormal protein aggregation (Seidel et al., 2012), dysregulation of transcription (Raposo et al., 2015), proteolytic cleavage (Weber et al., 2017), post-translational modification (Wan et al., 2018), mitochondrial dysfunction (Ramos et al., 2015), calcium signaling dysregulation (Chen et al., 2008), and damage of neuronal homeostasis (Cunha-Santos et al., 2016).
NPY, widely expressed in the central nervous system (CNS), has been implicated in neurogenesis and neuroprotection, playing a crucial role in maintaining neuronal homeostasis (Vezzani et al., 1999). The functions in neurogenesis and neuroprotection are performed by binding to different G-coupled NPY receptors distributed in different organs (Pedrazzini et al., 2003). To date, the NPY system has been found to play a potential role and to be a therapeutic target in many neurodegenerative diseases, such as AD, PD, and HD (Decressac et al., 2010, 2012; Croce et al., 2013). Recently, Duarte-Neves et al. (2015) found that NPY overexpression alleviated motor coordination and balance disability, prevented mutant ataxin3-induced immune activity increase, increased BDNF levels, and reduced IL-6 levels in MJD mouse models. Their results indicate the neuroprotective role of NPY in the pathogenesis of MJD.
This study is the first extensive examination of the association between variations in NPY, NPY2R, NPY5R genes and the pathogenesis and AO of MJD. In the present study, we provide evidence that variation in the NPY5R gene is associated with susceptibility to MJD. The genotypic distribution and allelic frequency of rs11100494 were significantly different between MJD patients and the control group. The CC genotypic and C allelic frequencies were significantly higher in MJD patients than in the control group, indicating that they were genetic modifier factors of MJD. To date, this SNP has been demonstrated to be associated with plasma TG levels and HDL concentrations in a Mexican-American dyslipidemia cohort (Coletta et al., 2007) and the phenotype of alcohol withdrawal with seizures (Wetherill et al., 2008). It was proven for the first time to be associated with neurodegenerative disease. As mentioned above, NPY could alleviate the excitatory neurotoxic effect, inhibit glutamate receptor overactivity, regulate calcium homeostasis, and attenuate neuroinflammation by binding to NPY2R and NPY5R in the CNS (Wu and Li, 2005; Farzi et al., 2015). Moreover, IL-6 levels were reduced in an MJD mouse model with NPY overexpression (Duarte-Neves et al., 2015), and a variant in IL6 was associated with the AO of MJD patients (Raposo et al., 2017). We speculated that rs11100494 might modulate the susceptibility of MJD by influencing the levels of interleukins and the process of neuroinflammation. However, the mechanism remains to be fully elucidated in the future.
In our data, all the genotypes of rs16139 in both MJD patients and controls were “TT”, which is different from some previously reported results (MAF = 0.019 in major depression) (Wang et al., 2013; Kloster et al., 2014). Therefore, this SNP was not included in the next analysis. rs3037354 of the NPY gene and rs2234759 of the NPY2R gene were previously demonstrated to be associated with the pathogenesis of HD (Kloster et al., 2014). However, no significant differences were observed between MJD patients and controls in our data. This result may be explained by the different distribution of the variations in various racial groups and different diseases. For all SNPs analyzed in our study, no differences in AO were found among their different genotypes and alleles. Due to the incompleteness of the information provided by single SNPs, the genetic association conclusion drawn by individual SNP data analysis may not be definite. Negative results from a specific SNP cannot rule out the possible association of diseases with candidate genes. Given the neuroprotective role of NPY and its receptors in neurodegenerative disease (Decressac and Barker, 2012; Decressac et al., 2012; Geloso et al., 2015), exploring other functional SNPs or the whole exons of these genes in MJD patients is suggested before a definite conclusion is reached.
In summary, using a case–control study and an extended analysis in MJD patients, we found that the genotypic distribution and allelic frequency of the rs11100494 polymorphism in the NPY5R gene were significantly different between MJD patients and the control group. This result indicates that sequence variations in the NPY system might be associated with the pathogenesis of MJD. Therefore, the NPY system could be of interest in MJD as a possible therapeutic agent, and detection of SNPs in a larger sample should be performed in the future to confirm the results and find other “missing heritability” in MJD.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Soochow University; the Ethics Committee of Xiangya Hospital, Central South University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
DD conceived the research, analyzed the data, and wrote the manuscript. CW and ZC helped in data acquisition, analysis, and interpretation of the data. XT and LZ were involved in data acquisition. QF was involved in the study design. RQ helped with funding and was involved in the study design. HJ helped with funding and was involved in the study design, conceptualization, data acquisition, technical and material support, and critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the National Key Research and Development Program of China (No. 2021YFA0805200 to HJ), the National Natural Science Foundation of China (Nos. 81771231, 81974176, and 82171254 to HJ; 82001219 to DD, 81901169 to ZC; 81901305 to CW), the National Science Foundation of Jiangsu Province (No. BK20190183 to DD), the Innovation Research Group Project of Natural Science Foundation of Hunan Province (No. 2020JJ1008 to HJ), the Science and Technology Innovation Group of Hunan Province (No. 2020RC4043 to HJ), the Scientific Research Foundation of Health Commission of Hunan Province (No. B2019183 to HJ), the Key Research and Development Program of Hunan Province (Nos. 2020SK2064 and 2018SK2092 to HJ), the Innovative Research and Development Program of Development and Reform Commission of Hunan Province to HJ, the Natural Science Foundation of Hunan Province (Nos. 2019JJ40363 to RQ, 2021JJ40974 to ZC, and 2020JJ5925 to CW), the Project Program of National Clinical Research Center for Geriatric Disorders (Xiangya Hospital, Nos. 2020LNJJ12 and XYYYJSTG-05 to HJ), and the Youth Foundation of Xiangya Hospital (No. 2018Q05 to CW).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful to all of the subjects for their participation in our study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.822657/full#supplementary-material
Footnotes
References
Aveleira, C. A., Botelho, M., Carmo-Silva, S., Pascoal, J. F., Ferreira-Marques, M., Nobrega, C., et al. (2015). Neuropeptide Y stimulates autophagy in hypothalamic neurons. Proc. Natl. Acad. Sci. U.S.A. 112, E1642–E1651. doi: 10.1073/pnas.1416609112
Bettencourt, C., Raposo, M., Kazachkova, N., Cymbron, T., Santos, C., Kay, T., et al. (2011). The APOE epsilon2 allele increases the risk of earlier age at onset in Machado-Joseph disease. Arch. Neurol. 68, 1580–1583. doi: 10.1001/archneurol.2011.636
Chen, S., Gan, S. R., Cai, P. P., Ni, W., Zhou, Q., Dong, Y., et al. (2016). Mitochondrial NADH dehydrogenase subunit 3 polymorphism associated with an earlier age at onset in male Machado-Joseph disease patients. CNS Neurosci. Ther. 22, 38–42. doi: 10.1111/cns.12443
Chen, X., Tang, T. S., Tu, H., Nelson, O., Pook, M., Hammer, R., et al. (2008). Deranged calcium signaling and neurodegeneration in spinocerebellar ataxia type 3. J. Neurosci. 28, 12713–12724. doi: 10.1523/JNEUROSCI.3909-08.2008
Chen, X. Y., Du, Y. F., and Chen, L. (2018). Neuropeptides exert neuroprotective effects in Alzheimer’s disease. Front. Mol. Neurosci. 11:493. doi: 10.3389/fnmol.2018.00493
Chen, Z., Wang, P., Wang, C., Peng, Y., Hou, X., Zhou, X., et al. (2018). Updated frequency analysis of spinocerebellar ataxia in China. Brain 141:e22. doi: 10.1093/brain/awy016
Chen, Z., Zheng, C., Long, Z., Cao, L., Li, X., Shang, H., et al. (2016). (CAG)n loci as genetic modifiers of age at onset in patients with Machado-Joseph disease from mainland China. Brain 139(Pt 8):e41. doi: 10.1093/brain/aww087
Coletta, D. K., Schneider, J., Stern, M. P., Blangero, J., DeFronzo, R. A., Duggirala, R., et al. (2007). Association of neuropeptide Y receptor Y5 polymorphisms with dyslipidemia in Mexican Americans. Obesity 15, 809–815. doi: 10.1038/oby.2007.610
Croce, N., Gelfo, F., Ciotti, M. T., Federici, G., Caltagirone, C., Bernardini, S., et al. (2013). NPY modulates miR-30a-5p and BDNF in opposite direction in an in vitro model of Alzheimer disease: a possible role in neuroprotection? Mol. Cell. Biochem. 376, 189–195. doi: 10.1007/s11010-013-1567-0
Cunha-Santos, J., Duarte-Neves, J., Carmona, V., Guarente, L., Pereira, D. A. L., and Cavadas, C. (2016). Caloric restriction blocks neuropathology and motor deficits in Machado-Joseph disease mouse models through SIRT1 pathway. Nat. Commun. 7:11445. doi: 10.1038/ncomms11445
Decressac, M., and Barker, R. A. (2012). Neuropeptide Y and its role in CNS disease and repair. Exp. Neurol. 238, 265–272. doi: 10.1016/j.expneurol.2012.09.004
Decressac, M., Pain, S., Chabeauti, P. Y., Frangeul, L., Thiriet, N., Herzog, H., et al. (2012). Neuroprotection by neuropeptide Y in cell and animal models of Parkinson’s disease. Neurobiol. Aging 33, 2125–2137. doi: 10.1016/j.neurobiolaging.2011.06.018
Decressac, M., Wright, B., Tyers, P., Gaillard, A., and Barker, R. A. (2010). Neuropeptide Y modifies the disease course in the R6/2 transgenic model of Huntington’s disease. Exp. Neurol. 226, 24–32. doi: 10.1016/j.expneurol.2010.07.022
Diaz-delCastillo, M., Woldbye, D., and Heegaard, A. M. (2018). Neuropeptide y and its involvement in chronic pain. Neuroscience 387, 162–169. doi: 10.1016/j.neuroscience.2017.08.050
Ding, D., Li, K., Wang, C., Chen, Z., Long, Z., Peng, Y., et al. (2016). ATXN2 polymorphism modulates age at onset in Machado-Joseph disease. Brain 139:e59. doi: 10.1093/brain/aww176
Ding, D., Wang, C., Chen, Z., Peng, H., Li, K., Zhou, X., et al. (2019). Polymorphisms in DNA methylation-related genes are linked to the phenotype of Machado-Joseph disease. Neurobiol. Aging 75, 221–225. doi: 10.1016/j.neurobiolaging.2018.11.002
Duarte-Neves, J., Cavadas, C., and Pereira, D. A. L. (2021). Neuropeptide Y (NPY) intranasal delivery alleviates Machado-Joseph disease. Sci. Rep. 11:3345. doi: 10.1038/s41598-021-82339-5
Duarte-Neves, J., Goncalves, N., Cunha-Santos, J., Simoes, A. T., den Dunnen, W. F., Hirai, H., et al. (2015). Neuropeptide Y mitigates neuropathology and motor deficits in mouse models of Machado-Joseph disease. Hum. Mol. Genet. 24, 5451–5463. doi: 10.1093/hmg/ddv271
Dubois, C. J., Ramamoorthy, P., Whim, M. D., and Liu, S. J. (2012). Activation of NPY type 5 receptors induces a long-lasting increase in spontaneous GABA release from cerebellar inhibitory interneurons. J. Neurophysiol. 107, 1655–1665. doi: 10.1152/jn.00755.2011
Farzi, A., Reichmann, F., and Holzer, P. (2015). The homeostatic role of neuropeptide Y in immune function and its impact on mood and behavior. Acta Physiol. 213, 603–627. doi: 10.1111/apha.12445
Franca, M. J., Emmel, V. E., D’Abreu, A., Maurer-Morelli, C. V., Secolin, R., Bonadia, L. C., et al. (2012). Normal ATXN3 allele but not CHIP polymorphisms modulates age at onset in Machado-Joseph disease. Front. Neurol. 3:164. doi: 10.3389/fneur.2012.00164
Geloso, M. C., Corvino, V., Di Maria, V., Marchese, E., and Michetti, F. (2015). Cellular targets for neuropeptide Y-mediated control of adult neurogenesis. Front. Cell. Neurosci. 9:85. doi: 10.3389/fncel.2015.00085
Kawaguchi, Y., Okamoto, T., Taniwaki, M., Aizawa, M., Inoue, M., Katayama, S., et al. (1994). CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat. Genet. 8, 221–228. doi: 10.1038/ng1194-221
Kloster, E., Saft, C., Akkad, D. A., Epplen, J. T., and Arning, L. (2014). Association of age at onset in Huntington disease with functional promoter variations in NPY and NPY2R. J. Mol. Med. 92, 177–184. doi: 10.1007/s00109-013-1092-3
Leotti, V. B., de Vries, J. J., Oliveira, C. M., de Mattos, E. P., Te, M. G., Brunt, E. R., et al. (2021). CAG repeat size influences the progression rate of spinocerebellar ataxia type 3. Ann. Neurol. 89, 66–73. doi: 10.1002/ana.25919
Long, Z., Chen, Z., Wang, C., Huang, F., Peng, H., Hou, X., et al. (2015). Two novel SNPs in ATXN3 3′ UTR may decrease age at onset of SCA3/MJD in Chinese patients. PLoS One 10:e117488. doi: 10.1371/journal.pone.0117488
Martins, A. C., Rieck, M., Leotti, V. B., Saraiva-Pereira, M. L., and Jardim, L. B. (2021). Variants in genes of calpain system as modifiers of spinocerebellar ataxia type 3 phenotype. J. Mol. Neurosci. 71, 1906–1913. doi: 10.1007/s12031-021-01877-9
Matos, C. A., de Almeida, L. P., and Nobrega, C. (2019). Machado-Joseph disease/spinocerebellar ataxia type 3: lessons from disease pathogenesis and clues into therapy. J. Neurochem. 148, 8–28. doi: 10.1111/jnc.14541
Mergener, R., Furtado, G. V., de Mattos, E. P., Leotti, V. B., Jardim, L. B., and Saraiva-Pereira, M. L. (2020). Variation in DNA repair system gene as an additional modifier of age at onset in spinocerebellar ataxia type 3/Machado-Joseph disease. Neuromolecular Med. 22, 133–138. doi: 10.1007/s12017-019-08572-4
Nalavade, R., Griesche, N., Ryan, D. P., Hildebrand, S., and Krauss, S. (2013). Mechanisms of RNA-induced toxicity in CAG repeat disorders. Cell Death Dis. 4:e752. doi: 10.1038/cddis.2013.276
Paulson, H. (2012). Machado-Joseph disease/spinocerebellar ataxia type 3. Handb. Clin. Neurol. 103, 437–449. doi: 10.1016/B978-0-444-51892-7.00027-9
Paulson, H. L. (2007). Dominantly inherited ataxias: lessons learned from Machado-Joseph disease/spinocerebellar ataxia type 3. Semin. Neurol. 27, 133–142. doi: 10.1055/s-2007-971172
Pedrazzini, T., Pralong, F., and Grouzmann, E. (2003). Neuropeptide Y: the universal soldier. Cell. Mol. Life Sci. 60, 350–377. doi: 10.1007/s000180300029
Peng, H., Wang, C., Chen, Z., Sun, Z., Jiao, B., Li, K., et al. (2014). APOE epsilon2 allele may decrease the age at onset in patients with spinocerebellar ataxia type 3 or Machado-Joseph disease from the Chinese Han population. Neurobiol. Aging 35, 2115–2179. doi: 10.1016/j.neurobiolaging.2014.03.020
Ramos, A., Kazachkova, N., Silva, F., Maciel, P., Silva-Fernandes, A., Duarte-Silva, S., et al. (2015). Differential mtDNA damage patterns in a transgenic mouse model of Machado-Joseph disease (MJD/SCA3). J. Mol. Neurosci. 55, 449–453. doi: 10.1007/s12031-014-0360-1
Raposo, M., Bettencourt, C., Maciel, P., Gao, F., Ramos, A., Kazachkova, N., et al. (2015). Novel candidate blood-based transcriptional biomarkers of Machado-Joseph disease. Mov Disord. 30, 968–975. doi: 10.1002/mds.26238
Raposo, M., Bettencourt, C., Ramos, A., Kazachkova, N., Vasconcelos, J., Kay, T., et al. (2017). Promoter variation and expression levels of inflammatory genes IL1A, IL1B, IL6 and TNF in blood of spinocerebellar ataxia type 3 (SCA3) patients. Neuromolecular Med. 19, 41–45. doi: 10.1007/s12017-016-8416-8
Santos-Carvalho, A., Elvas, F., Alvaro, A. R., Ambrosio, A. F., and Cavadas, C. (2013). Neuropeptide Y receptors activation protects rat retinal neural cells against necrotic and apoptotic cell death induced by glutamate. Cell Death Dis. 4:e636. doi: 10.1038/cddis.2013.160
Seidel, K., Meister, M., Dugbartey, G. J., Zijlstra, M. P., Vinet, J., Brunt, E. R., et al. (2012). Cellular protein quality control and the evolution of aggregates in spinocerebellar ataxia type 3 (SCA3). Neuropathol. Appl. Neurobiol. 38, 548–558. doi: 10.1111/j.1365-2990.2011.01220.x
Smialowska, M., Domin, H., Zieba, B., Kozniewska, E., Michalik, R., Piotrowski, P., et al. (2009). Neuroprotective effects of neuropeptide Y-Y2 and Y5 receptor agonists in vitro and in vivo. Neuropeptides 43, 235–249. doi: 10.1016/j.npep.2009.02.002
Takiyama, Y., Nishizawa, M., Tanaka, H., Kawashima, S., Sakamoto, H., Karube, Y., et al. (1993). The gene for Machado-Joseph disease maps to human chromosome 14q. Nat. Genet. 4, 300–304. doi: 10.1038/ng0793-300
Taroni, F., and DiDonato, S. (2004). Pathways to motor incoordination: the inherited ataxias. Nat. Rev. Neurosci. 5, 641–655. doi: 10.1038/nrn1474
Tezenas, D. M. S., Durr, A., Bauer, P., Figueroa, K. P., Ichikawa, Y., Brussino, A., et al. (2014). Modulation of the age at onset in spinocerebellar ataxia by CAG tracts in various genes. Brain 137(Pt 9), 2444–2455. doi: 10.1093/brain/awu174
Vezzani, A., Sperk, G., and Colmers, W. F. (1999). Neuropeptide Y: emerging evidence for a functional role in seizure modulation. Trends Neurosci. 22, 25–30. doi: 10.1016/s0166-2236(98)01284-3
Wan, L., Xu, K., Chen, Z., Tang, B., and Jiang, H. (2018). Roles of post-translational modifications in spinocerebellar ataxias. Front. Cell. Neurosci. 12:290. doi: 10.3389/fncel.2018.00290
Wang, C., Chen, Z., Peng, H., Peng, Y., Zhou, X., Yang, H., et al. (2018). Investigation on modulation of DNA repair pathways in Chinese MJD patients. Neurobiol. Aging 71, 265–267. doi: 10.1016/j.neurobiolaging.2018.06.024
Wang, Y., Yang, Y., Hui, L., Tie, C., Li, F., Xu, Z. Q., et al. (2013). A neuropeptide Y variant (rs16139) associated with major depressive disorder in replicate samples from Chinese Han population. PLoS One 8:e57042. doi: 10.1371/journal.pone.0057042
Weber, J. J., Golla, M., Guaitoli, G., Wanichawan, P., Hayer, S. N., Hauser, S., et al. (2017). A combinatorial approach to identify calpain cleavage sites in the Machado-Joseph disease protein ataxin-3. Brain 140, 1280–1299. doi: 10.1093/brain/awx039
Wetherill, L., Schuckit, M. A., Hesselbrock, V., Xuei, X., Liang, T., Dick, D. M., et al. (2008). Neuropeptide Y receptor genes are associated with alcohol dependence, alcohol withdrawal phenotypes, and cocaine dependence. Alcohol. Clin. Exp. Res. 32, 2031–2040. doi: 10.1111/j.1530-0277.2008.00790.x
Keywords: Machado-Joseph disease, age at onset, NPY, NPY2R, NPY5R, SNPs
Citation: Ding D, Chen Z, Wang C, Tang X, Zhang L, Fang Q, Qiu R and Jiang H (2022) A Variant in Genes of the NPY System as Modifier Factor of Machado-Joseph Disease in the Chinese Population. Front. Aging Neurosci. 14:822657. doi: 10.3389/fnagi.2022.822657
Received: 26 November 2021; Accepted: 11 January 2022;
Published: 03 February 2022.
Edited by:
Weidong Le, Dalian Medical University, ChinaReviewed by:
Maria Luiza Saraiva-Pereira, Federal University of Rio Grande do Sul, BrazilShi-Rui Gan, First Affiliated Hospital of Fujian Medical University, China
Copyright © 2022 Ding, Chen, Wang, Tang, Zhang, Fang, Qiu and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Jiang, amlhbmdob25nNzM4NjhAMTI2LmNvbQ==
 Dongxue Ding
Dongxue Ding Zhao Chen2
Zhao Chen2 Qi Fang
Qi Fang Hong Jiang
Hong Jiang