
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 10 March 2022
Sec. Neurocognitive Aging and Behavior
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.821537
This article is part of the Research Topic Impact of Hearing Loss on Aging Processes: Current Understanding, Mechanisms, and Treatment Strategies View all 23 articles
 Wen Ma1
Wen Ma1 Yue Zhang2
Yue Zhang2 Xiao Li3
Xiao Li3 Siqi Liu2
Siqi Liu2 Yuting Gao2
Yuting Gao2 Jing Yang3
Jing Yang3 Longji Xu3
Longji Xu3 Hudie Liang3
Hudie Liang3 Fuxin Ren3
Fuxin Ren3 Fei Gao3*
Fei Gao3* Yao Wang2,4*
Yao Wang2,4*
Age-related hearing loss (ARHL) is a kind of symmetrical and slow sensorineural hearing loss, which is a common condition in older adults. The characteristic of ARHL is hearing loss beginning in the high-frequency region and spreading toward low-frequency with age. Previous studies have linked it to anxiety, suggesting that brain structure may be involved in compensatory plasticity after partial hearing deprivation. However, the neural mechanisms of underlying ARHL-related anxiety remain unclear. The purpose of this cross-sectional study was to explore the interactions among high-frequency hearing loss and anxiety as well as brain structure in older adults. Sixty-seven ARHL patients and 68 normal hearing (NH) controls participated in this study, and the inclusion criterion of ARHL group was four-frequency (0.5, 1, 2, and 4 kHz) pure tone average (PTA) > 25 decibels hearing level of the better hearing ear. All participants performed three-dimensional T1-weighted magnetic resonance imaging (MRI), pure tone audiometry tests, anxiety and depression scales. Our results found gray matter volume (GMV) decreased in 20 brain regions in the ARHL group compared with the NH group, and a positive correlation existed between high-frequency pure tone audiometry (H-PT) and anxiety scores in the ARHL group. Among 20 brain regions, we also found the GMVs of the middle cingulate cortex (MCC), and the hippocampal/parahippocampal (H-P) regions were associated with H-PT and anxiety scores in all participants separately. However, the depressive symptoms indicated no relationship with hearing assessment or GMVs. Our findings revealed that the crucial role of MCC and H-P in a link of anxiety and hearing loss in older adults.
Age-related hearing loss (ARHL), also referred to as presbycusis, is a kind of symmetrical and slow sensorineural hearing loss that occurs with age, which is a common condition in older adults (Lin et al., 2011). In the world, ARHL affects more than half of adults under 75 years of age, and 80% of older adults over 80 years old suffer from ARHL (Wattamwar et al., 2017). With an increasing aging population, there will be 1.2 billion people over the age of 60 by 2025. Among them, more than 500 million older adults will be severely affected by ARHL (World Health Organization [WHO], 2021).
Age-related hearing loss has been reported to link a range of negative emotional outcomes, including depression based on the longitudinal studies (Brewster et al., 2018; Cosh et al., 2018), social isolation (Mick et al., 2014), and anxiety (Mehta et al., 2003b; Jayakody et al., 2018a,b) which are all cross-sectional studies. For example, compared with normal hearing controls, older adults with self-reported hearing loss were found to be more likely to have anxiety symptoms in a cross-sectional study (Mehta et al., 2003b). Compared with participants with no hearing impairment (HI), patients with moderate or severe HI had a 23% lower possibility of emotional vitality which contained low anxiety, low depressive symptomatology, a high sense of personal mastery, and happiness [odds ratio (OR) = 0.77] (Contrera et al., 2016). Moreover, ARHL patients with moderately severe hearing loss had a more significant anxiety symptom compared with mild hearing loss (Jayakody et al., 2018a). Furthermore, untreated hearing loss has been linked to the risk of anxiety, and the risk of anxiety may increase with pure tone audiometry thresholds elevated in the patients with ARHL (Jayakody et al., 2018b).
Magnetic resonance imaging (MRI) has become an acceptive technique that can study various neurological diseases’ pathogenic mechanisms (Feng et al., 2018). A structural MRI study revealed that hearing loss was related to decreased gray matter volume (GMV) in the right primary auditory cortex (Ren et al., 2018). Another study has found that high-frequency (>2 kHz) hearing loss was associated with auditory cortex atrophy in ARHL (Eckert et al., 2012). In a longitudinal study, compared to participants with normal hearing, patients with HI had an accelerated volume decline in the whole brain and regional volumes in the right temporal lobe (Lin et al., 2014). Compared with normal hearing controls, ARHL with cochlear amplifier dysfunction have shown reduced thickness of precentral and postcentral gyri (Belkhiria et al., 2019). Besides, a higher hearing threshold was significantly associated with a smaller brain volume in older adults (Rigters et al., 2017). Recently, one research has found that decreased GMV in the insula and amygdala wase associated with apathy symptoms in ARHL patients (Belkhiria et al., 2020). In addition, patients with ARHL have also shown reduced GMV in the superior and medial frontal gyrus, which were also associated with anxiety symptoms (Husain et al., 2011; Boyen et al., 2013). However, to date, the neural mechanisms of underlying ARHL-related anxiety remain unclear.
Given that ARHL begins in the high-frequency region of the auditory spectrum and spreads toward the low-frequency regions with age (Wang and Puel, 2020), the steep slope of the hearing threshold at high frequency is a very typical characteristic of patients with ARHL (Wolak et al., 2017). Accordingly, we used frequency division and the “steepness” of the audiogram to further refine the high-frequency region in this study. Meanwhile, MRI was used to explore the structural plasticity in the whole brain of patients with ARHL. We hypothesized that (1) high-frequency hearing loss was associated with anxiety in patients with ARHL, (2) there was an interaction between hearing loss, anxiety, and structural plasticity in older adults.
For this cross-sectional study, 135 participants aged 50 to 72 years old (mean age = 62.17 years, SD = 4.91 years), including 67 age-related hearing loss patients (ARHL group) and 68 normal-hearing controls (NH group). The inclusion criteria of ARHL group was four-frequency (0.5, 1, 2, and 4 kHz) pure tone average (PTA) > 25 decibels hearing level (dB HL) of the better hearing ear (Humes, 2019). Subjects with the following conditions were excluded: (1) suffering from diseases that affect the hearing threshold other than ARHL; (2) previous history of noise exposure and use of hearing aid; (3) conductive hearing impairment; (4) previous symptoms of tinnitus and head trauma; (5) history of psychiatric or neurological disease; (6) MRI contraindications. All the participants were native Mandarin speakers and right-handed (Hatta, 2007).
During the pure tone (PT) audiometry test, subjects were tested in a sound-attenuating booth and were told to keep awake during the test. The audiometer (GSI Audio Star Pro, United States) was used to test hearing thresholds at frequencies of 0.125, 0.25, 0.5, 1, 2, 4, and 8 kHz in each ear. By calculating the average hearing thresholds at 0.5, 1, 2, and 4 kHz in air conductance, the four-frequency PTA hearing threshold of both ears was obtained.
By performing factor analysis with principal components extraction and Varimax rotation (Eckert et al., 2012), the PT thresholds of each frequency point were divided into low- and high-frequency bands. According to the component score of the matrix after rotation (Supplementary Material), the PT thresholds of 0.125, 0.25, 0.5, and 1 kHz were loaded onto component 1 (low-frequency), while the PT thresholds of 2, 4, and 8 kHz were loaded onto component 2 (high-frequency).
To refine the high-frequency region and quantify the audiogram process, the audiogram “steepness” of each adjacent frequencies was calculated in decibels per octave (dB/octave), namely the hearing level difference divided by the frequency difference (Konig et al., 2006).
where HT (fi) is the HL threshold in dB at the frequency fi and fi ∈ [0.125, 0.25, 0.5, 1, 2, 4, 8] kHz. S (i) is the steepness of the audiogram in each adjacent two-frequency. For example, S (1) represents PT between 0.125 and 0.25 kHz. The steepness between two adjacent frequencies was regarded as an audiological marker of discontinuity in the shape of the audiogram (Schecklmann et al., 2012; Ponticorvo et al., 2019), reflecting the corresponding discontinuities in the inner hair cells.
All participants were scanned on a 3T MRI scanner (Philips, Achieva) using an eight-channel phased-array head coil as the receiver. T1-weighted three-dimensional TFE images were used as the localizer, acquired with the following parameters: TR = 8.1 ms; TE = 3.7 ms; slice thickness = 1 mm; field of view = 24 cm × 24 cm; and flip angle = 8°. Images were reconstructed with 1 mm × 1 mm × 1 mm isotropic voxels.
The MRI images were processed by using the cat toolbox for the SPM12 in Matlab R2020b. We used voxel-based morphological (VBM) measurements to identify statistically significant brain regions. VBM includes spatial normalization, organization and segmentation, and spatial smoothing. In short, each subject’s image is spatially normalized and segmented into gray matter, white matter, and cerebrospinal fluid. After data preprocessing, smoothing was performed on normalized GMV before second-level intergroup analysis. GMV differences between the two groups were calculated using a two-sample t-test model, and the total intracranial volume was regressive as a covariable. For multiple comparisons, the analyses were calibrated by using false discovery rate (FDR) criteria, with statistical significance set as p < 0.05 and cluster size > 20 voxels.
All subjects assessed the levels of anxiety and depression without knowing the self-assessment scale scores. The psychiatric evaluation of each subject lasted about 20 min. The questionnaire included two subscales of anxiety and depression, with seven questions for anxiety and depression separately. For anxiety, the questions were 1, 3, 5, 7, 9, 11, and 13, and for depression, the questions were 2, 4, 6, 8, 10, 12, and 14. Some of the mandatory questions that involve anxiety were: “Do you ever feel tensed up?” “Worry a lot?” “Have panic attacks?” “Feel something awful is about to happen?” Each test item was scored from 0 to 3. Scores for depression and anxiety were separately calculated by summing the scores. The scores of the scale were: 0–7 were asymptomatic; 8–10 were suspected; and 11–21 were certainly existed. The score ≥ 8 was considered test positive. Levels of anxiety and depression were assessed according to the hospital anxiety and depression scale (Zigmond and Snaith, 1983).
Participants’ gender (male or female), age (in years), hypertension (yes or no), diabetes (yes or no), hyperlipidemia (yes or no), smoking (yes or no), and education (in years) were self-reported. Alcohol abuse identification test was assessed by the World Health Organization as a self-report screening, which included three problems about alcohol consumption, three problems about dependence symptoms, and four problems related to alcohol use. Each problem was scored from 0 to 4, generally based on frequency of occurrence, resulting in a total score of 0–40. The score ≥ 8 was considered alcohol abuse (Boschloo et al., 2010).
All data were tested by the Kolmogorov–Smirnov test to verify normal distribution. The group differences in NH and ARHL group of age, education, anxiety, depression, and auditory test results were assessed by the two-tailed t test. The sex, hypertension, diabetes, hyperlipidemia, smoking, and alcohol abuse were examined by the χ2 test. Partial correlation analyses were performed to explore the relationships both between structural changes and hearing loss, and between hearing loss and anxiety in older adults (controlled for age, sex, education). Spearman or Pearson correlation was used to analyze the relationships between anxiety and brain structure. Data processing and analysis were performed using SPSS 25.0 (IBM Corp., Armonk, NY, United States). The significance level was examined at p < 0.05. A linear regression model was adopted to evaluate the hearing loss on anxiety symptoms. Then we designed a weighted linear regression to reduce autocorrelation and heteroskedasticity. First, the residuals square of the original linear model was extracted and made as logarithmic transform. Then, we constructed a linear regression, making logarithmic residuals square as the dependent variable and taking the fitted value as the weights for the final linear model between anxiety and each feature. The degree of prediction was indicated by R2 and β. Data processing and analysis were performed using R 3.6.0.
The demographics and clinical characteristics are listed in Table 1. A total of 135 subjects (59 males/76 females) were recruited for the study. The PTA of patients with ARHL was significantly higher than the NH group (p < 0.001). In psychological assessment, there was no significant difference in anxiety and depression scores between the ARHL and NH groups (p > 0.05). No significant differences in age, sex, education, hypertension, diabetes, hyperlipidemia, smoking, and alcohol abuse were identified between the NH and ARHL groups (p > 0.05).
Table 2 shows the difference of GMV between ARHL and NH groups, as seen in Figure 1. Compared with NH group, ARHL group showed significantly decreased GMV in the MCC, H-P regions, supplementary motor area, superior frontal gyrus, medial orbital, lingual gyrus, insula, inferior temporal gyrus, and so on.
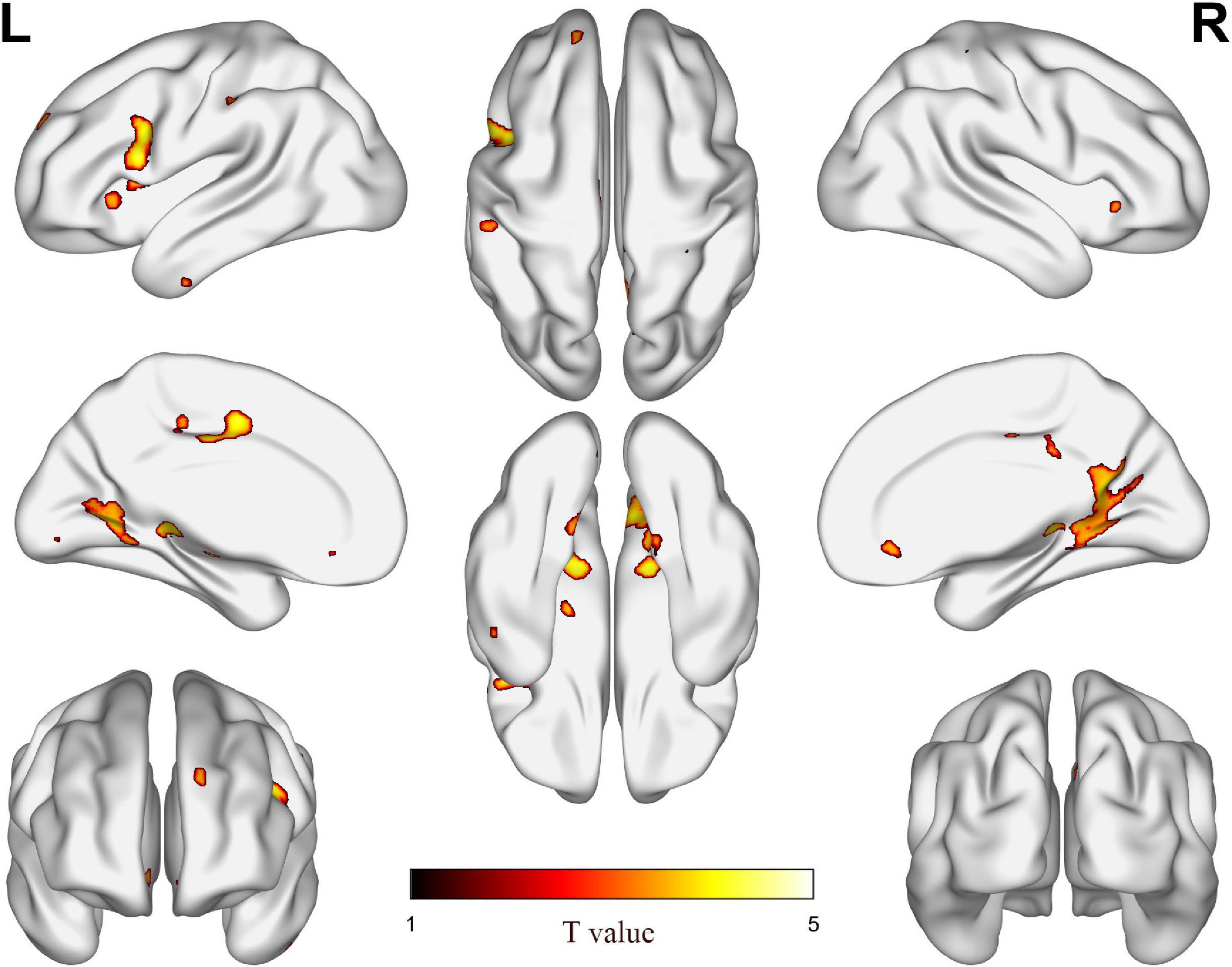
Figure 1. The difference of gray matter volume (GMV) between age-related hearing loss (ARHL) and normal hearing (NH) groups. Hot colors indicate significantly decreased GMV in the ARHL group compared to the NH group. FDR corrected p < 0.05, cluster size > 20 voxels. L, left; R, right.
The hearing thresholds at different frequencies in both ears of NH and ARHL subjects are shown in Figure 2. The trend of hearing thresholds in the NH group was flat in the frequency range of 0.125–2 kHz and became steeper from 2 to 8 kHz. For ARHL group, hearing thresholds at different frequencies of both ears was higher than those in the NH group. In addition, the audiogram steepness of patients with ARHL was growing larger with the frequency increasing, except the steepness between 4 and 8 kHz was flatter than that between 2 and 4 kHz in the left ear. The pattern of audiogram in ARHL group indicated a steeply sloping high-frequency hearing loss.
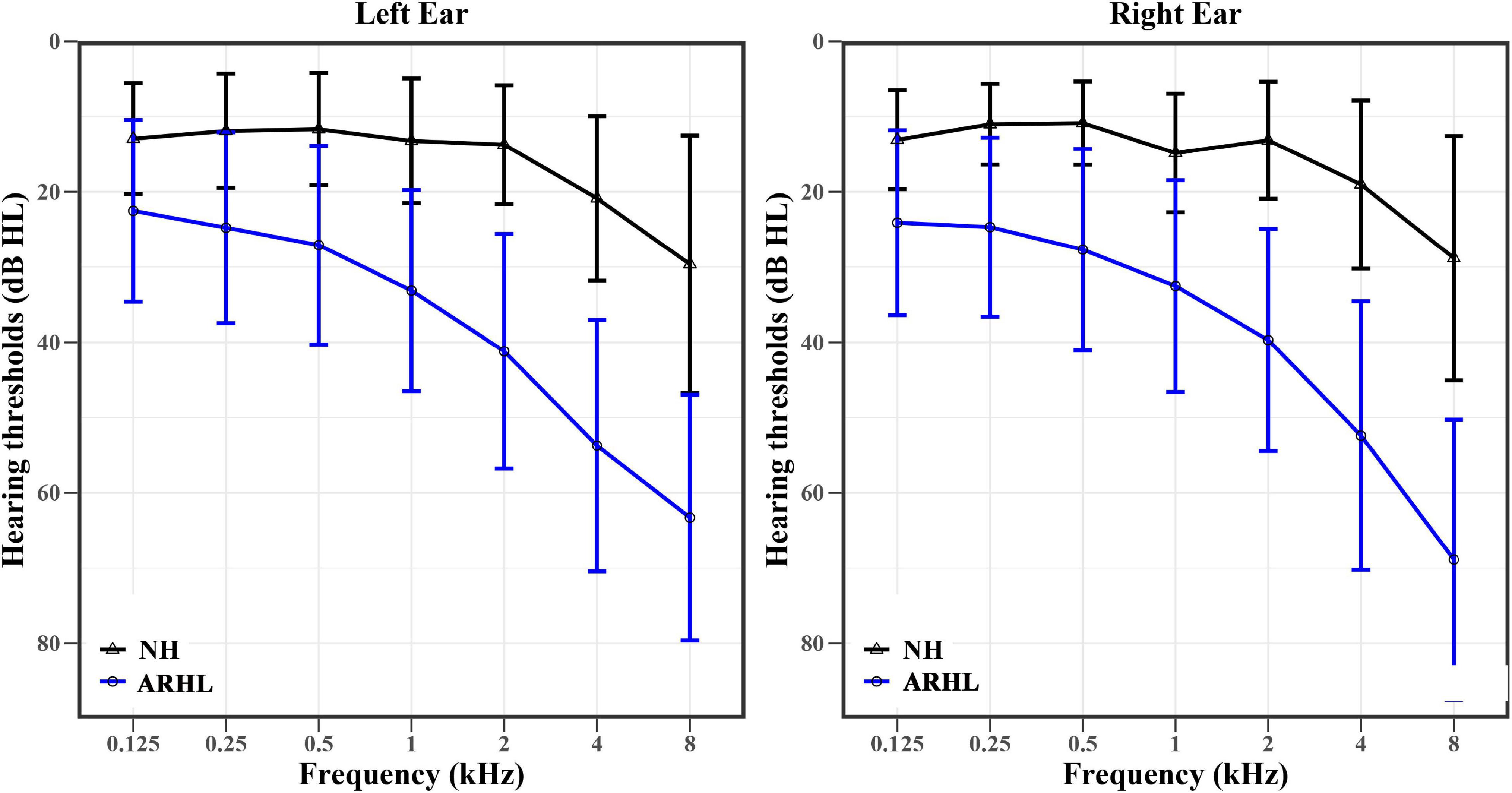
Figure 2. The hearing thresholds (means ± standard deviation) at different frequencies of the right and left ears in NH (black) and ARHL (blue) groups. NH: normal hearing; ARHL: Age-related hearing loss.
The relationship between hearing loss and anxiety scores in each group is listed in Table 3. Among them, high-frequency pure tone audiometry (H-PT) was positively correlated with anxiety scores in the ARHL group (r = 0.289, p = 0.021) (Figure 3). Besides, the correlation coefficients of the linear regression model showed that H-PT predicted anxiety symptoms in the ARHL group (R2 = 0.812, β = 1.976, 95% CI, 1.761 to 2.131, p = 0.003). The results indicated that higher anxiety symptoms were associated with high-frequency hearing loss.
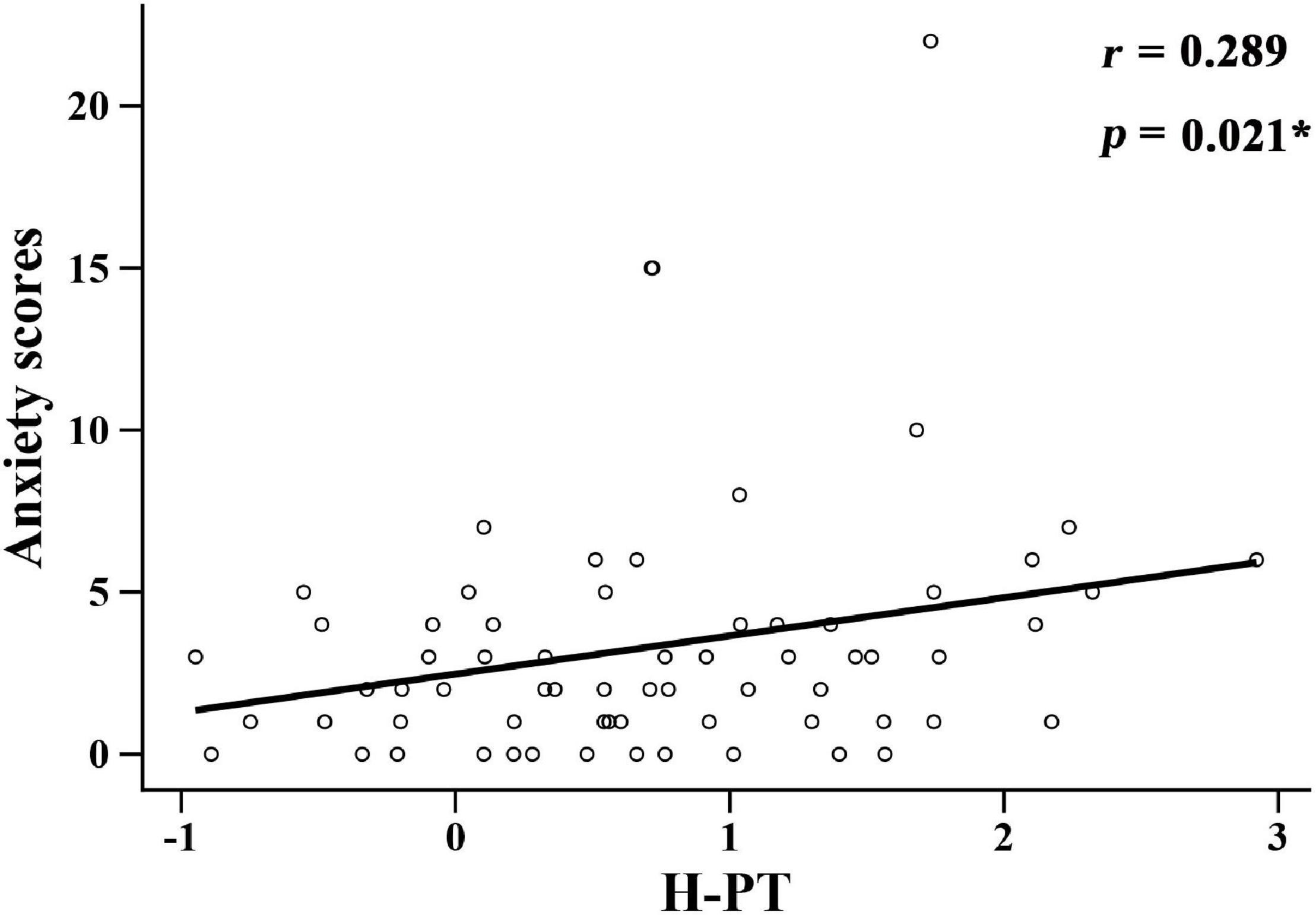
Figure 3. Correlation between high-frequency pure tone audiometry factor scores and anxiety scores in the ARHL group. H-PT: high-frequency pure tone audiometry factor scores.
In this study, the relationship between anxiety scores and the brain regions in group differences was analyzed. Figure 4 shows the significant correlations between anxiety scores and brain structure in each group. In the NH group, anxiety scores were positively related to the GMV of hippocampal/parahippocampal (H-P) regions (r = 0.331, p = 0.006) (Figure 4A), anxiety scores were positively correlated with the GMV of middle cingulate cortex (MCC) (r = 0.26, p = 0.032) (Figure 4B). In all participants, anxiety scores were positively correlated with the GMV of MCC (r = 0.191, p = 0.027) (Figure 4C). Figure 5 shows the locations of H-P regions and MCC.
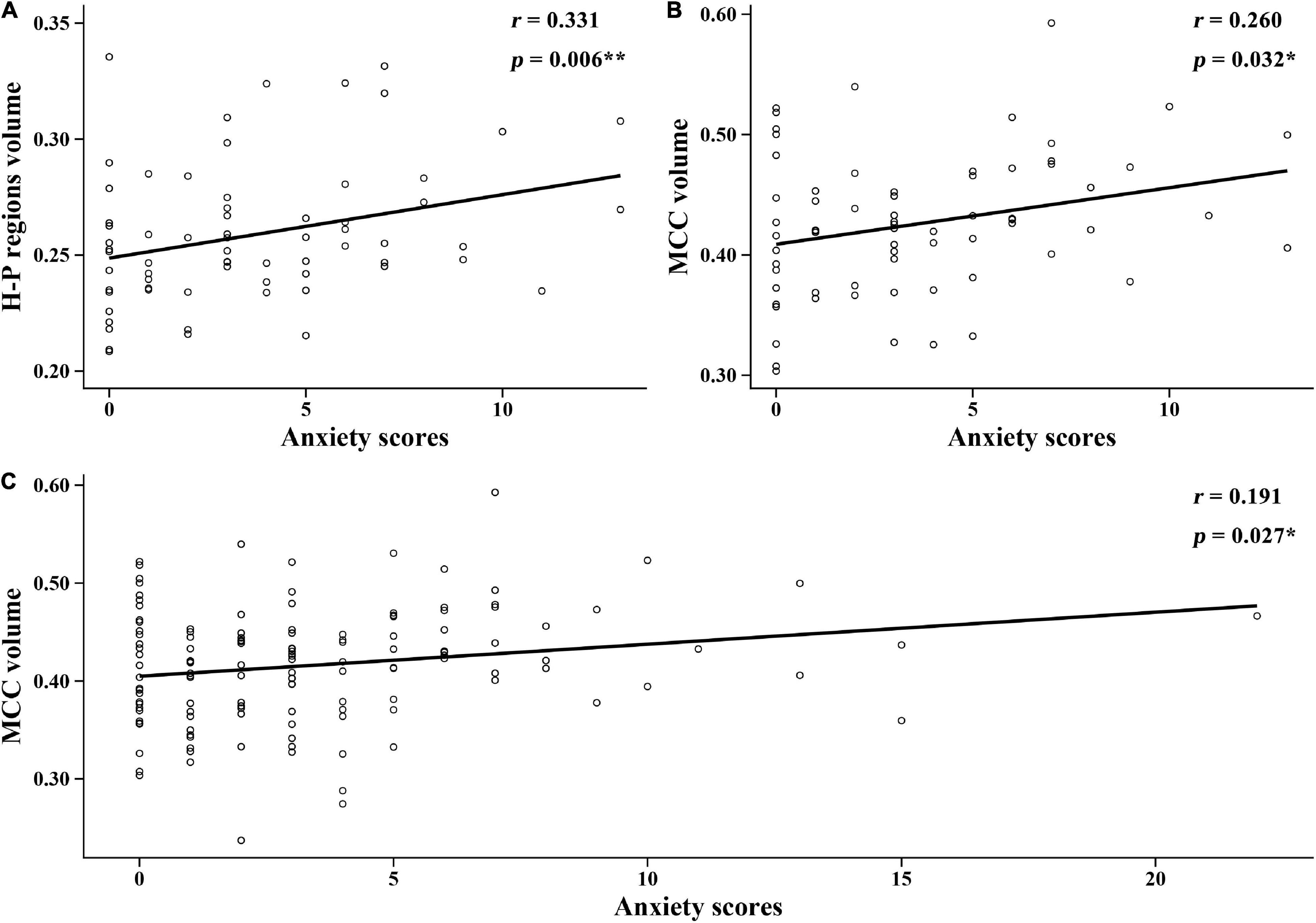
Figure 4. Correlations between the anxiety scores and brain structure gray matter volume (GMV) in each group. (A) In the normal hearing (NH) group, anxiety scores were positively correlated with the GMV of H-P regions. (B) In the NH group, anxiety scores were positively correlated with the GMV of MCC. (C) In all participants, anxiety scores were positively correlated with the GMV of MCC. H-P regions: hippocampal/parahippocampal regions; MCC, middle cingulate cortex.
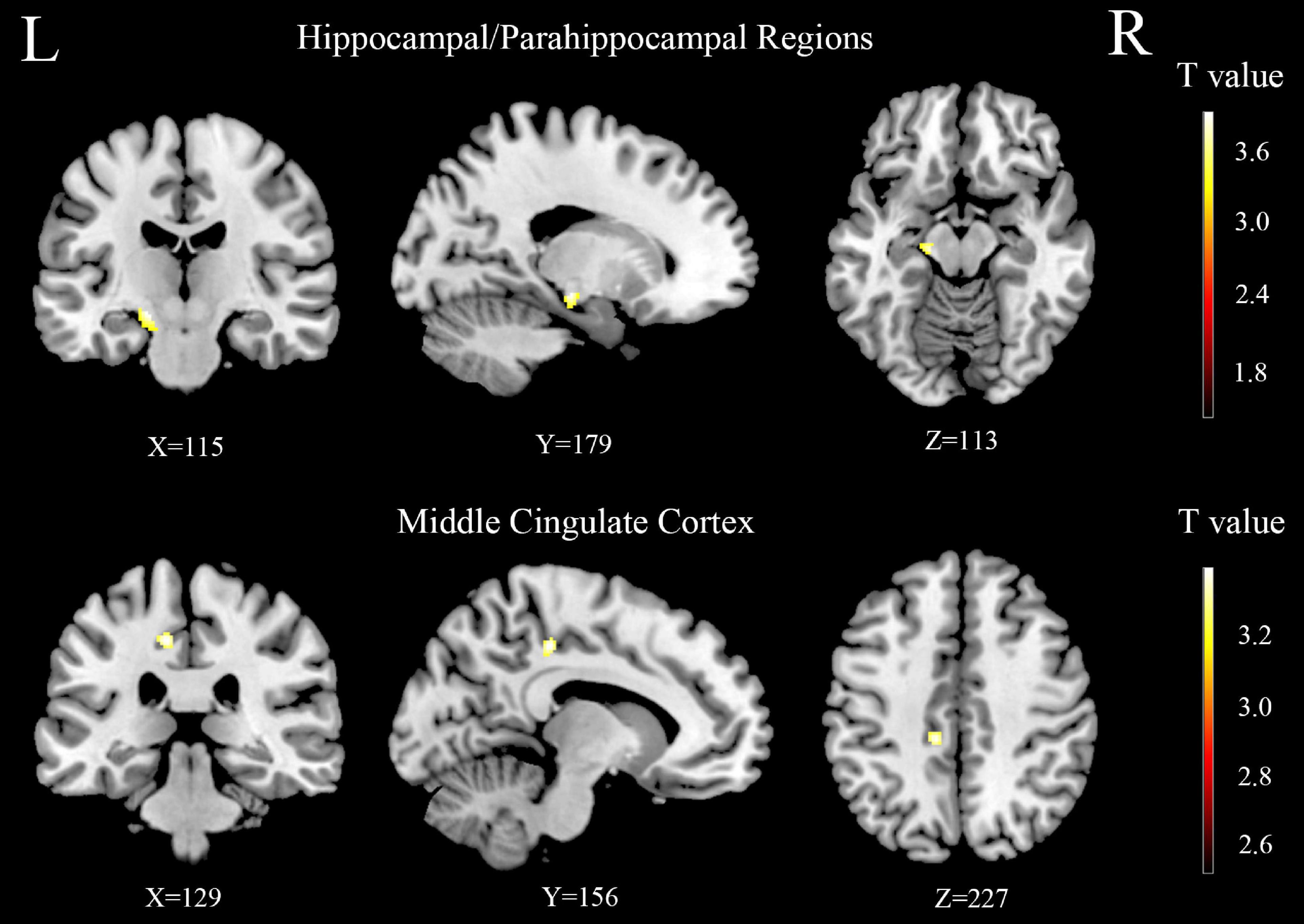
Figure 5. The difference of gray matter volume (GMV) between the age-related hearing loss (ARHL) and normal hearing controls (NH) groups in hippocampal/parahippocampal and middle cingulate cortex regions. Hot colors indicate significantly decreased GMV in the ARHL group compared to the NH group. FDR corrected p < 0.05, cluster size > 20 voxels. L, left; R, right.
Figure 6 shows the correlations between the hearing loss of both ears and brain structure GMV in each group. In the NH group, S (6) of the left ear was negatively correlated with the GMV of H-P regions (r = −0.263, p = 0.030), S (5) of the left ear was positively associated with the GMV of MCC (r = 0.286, p = 0.018), S (6) of the right ear was negatively correlated with the GMV of H-P regions (r = −0.210, p = 0.085), and S (5) of the right ear was positively associated with the GMV of MCC (r = 0.207, p = 0.091). In all participants, S (6) of the right ear was negatively correlated with the GMV of H-P regions (r = −0.209, p = 0.015), and S (5) of the left ear was positively associated with the GMV of MCC (r = 0.175, p = 0.042).
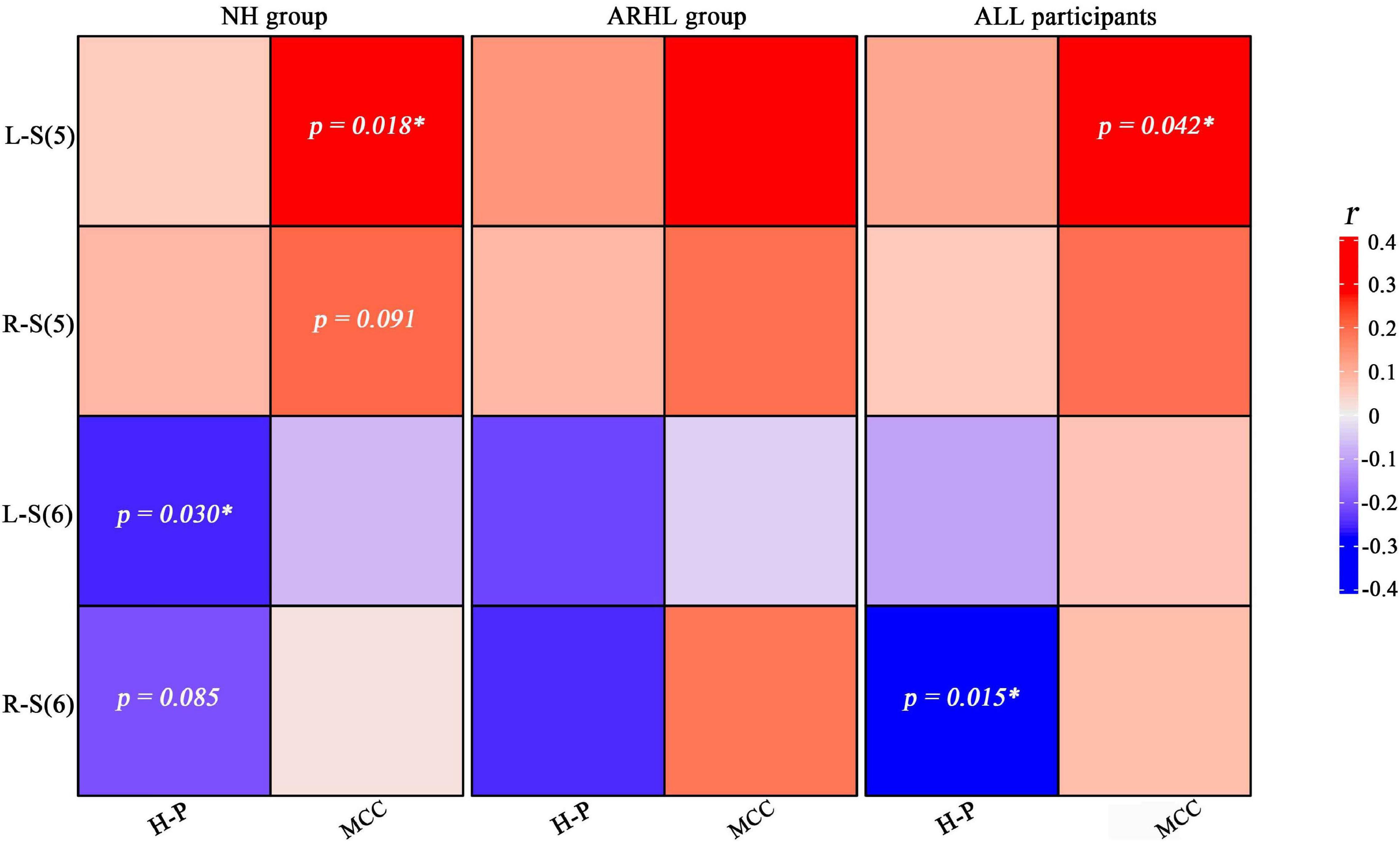
Figure 6. Heat map of the relationship between the brain structure gray matter volume and steepness of the audiogram in right and left ears. Blue and red colors indicate positive and negative correlations, respectively. Areas with significant correlations or trends have been marked with p values. H-P regions: hippocampal/parahippocampal regions; MCC: middle cingulate cortex; L-S (5): Steepness (5) between 2 and 4 kHz of left ear; R-S (6): Steepness (6) between 4 and 8 kHz of right ear; NH, normal hearing; ARHL, Age-related hearing loss.
In this study, we found gray matter (GM) atrophy in ARHL group compared with NH group. Our results also showed that in older adults, the GMV of MCC was positively associated with steepness between 2 and 4 kHz, but the GMV of H-P regions was negatively associated with steepness between 4 and 8 kHz. In addition, there was a significant association between high-frequency hearing loss and anxiety scores in patients with ARHL. Moreover, anxiety scores were positively associated with the GMV of H-P regions and MCC. Above all, we found an interaction between hearing loss, anxiety, and structural plasticity in older adults.
Our study found that high-frequency hearing loss was positively associated with anxiety scores in the ARHL group. Previous study has linked hearing loss to the increased risks of depression, anxiety, and stress (Ronnberg et al., 2008). In addition, increasing the hearing threshold of patients with ARHL may increase the risks of these psychological symptoms (Jayakody et al., 2018b; Maletic-Sekulic et al., 2019). Jayakody et al. found that the older adults with high-frequencies (6 and 8 kHz) of hearing loss in the existence of normal speech frequencies (0.5, 1, 2, and 4 kHz) still had the risk of anxiety (Odds ratios = 1.6), which further supported our findings (Jayakody et al., 2018a). The prevalence of hearing loss (>25 dB HL) in the better ear is about three-fifths when the PTA is at 0.5, 1, 2, and 4 kHz, and about nine-tenths when the PTA is at 3, 4, 6, and 8 kHz in older adults (Lin et al., 2011). In addition, ARHL is characterized by bilateral high-frequency hearing loss and spreads toward low-frequency regions with age (Arvin et al., 2013; Wang and Puel, 2020). The high-frequency hearing loss also means that it was difficult to distinguish consonants (>4 kHz), and consonants were a key factor in semantic understanding (Jayakody et al., 2018a). Therefore, older adults with high-frequency hearing loss had difficulty in understanding speech in noise and communicating with family and friends, which might further lead to anxiety. Meanwhile, another study suggested that the anxiety caused by hearing loss in the elderly was due to sensory deprivation (Mehta et al., 2003a). Therefore, it is necessary to conduct a longitudinal study of ARHL-related anxiety, which further clarifies whether anxiety was a predictor or consequence of high-frequency hearing loss in patients with ARHL. Besides, it is also important to screen the high-frequency hearing thresholds for older adults so that not to overlook their potential decline in psychosocial health.
Furthermore, the audiogram steepness was used to refine the frequency range in high-frequency hearing loss. Our study showed that steepness between 2 and 4 kHz was positively correlated with the GMV of MCC in older adults. In other words, our study showed that there was a same variation tendency between the GMV of MCC in the older adults and the variation of hearing thresholds in 2 and 4 kHz. Previous study has found that patients with sensorineural hearing loss showed significantly reduced functional connectivity in the cingulate gyrus (Xu et al., 2019). Meanwhile, it has also been found that the hearing threshold of patients with ARHL began to decline rapidly at 2–4 kHz (Wolak et al., 2017). In addition, we found a significant positive correlation between the GMV of MCC and anxiety scores. The possible reason was that the MCC was an important component of the limbic system, which was a deep structure of the entire brain involved in motivation, emotion, and memory functions (Powell et al., 2018). Besides, participants’ negative emotions might lead to an increased processing capacity in the cingulate cortex, which would activate MCC (DiMenichi et al., 2019). The present study revealed a strong relationship between MCC and both hearing thresholds and anxiety scores in older adults. Therefore, it is necessary to further investigate the intrinsic underlying neural mechanisms in the future.
Our study showed that the steepness between 4 and 8 kHz was negatively correlated with the GMV of H-P regions in older adults. Other studies have found that the hippocampus received neural input from the central auditory system directly or indirectly via (a) the parahippocampal cortex or peripheral cortex, or (b) other brain pathways, including the medial frontal cortex, insula, or amygdala. Hippocampal microstructural analysis revealed that compared to normal hearing controls, the more severe the hearing loss in moderate/severe patients with ARHL, the lower the microstructural integrity and the higher of the mean diffusivity in the GM of the hippocampus (Croll et al., 2020). Furthermore, in an animal model of C57BL/6J mice, the increase in hearing threshold with age was found to be accompanied by synaptic losses in the hippocampus. The H-P regions were responsible for people’s cognition and comprehension (DiMenichi et al., 2019). Consonants and vowels were critical for speech understanding at 0.5–4 kHz, while frequencies > 4 kHz contained consonants that contribute to the understanding of speech intelligibility (Jayakody et al., 2018a). Our study also further focused on the frequency of hearing loss at 4–8 kHz. Meanwhile, we also found a significant positive correlation between the GMV of H-P regions and anxiety scores. H-P regions were an important limbic system in memory and learning, which were closely related to the amygdala encoding emotion (DiMenichi et al., 2019). Recently, a study has found that higher activation of the parahippocampal gyrus was associated with anxiety in patients with first-episode depressive disorder (Lin et al., 2021). In addition, a functional MRI study showed that hippocampal activation was sensitive to different emotional music. Activation of the right hippocampus and amygdala increases when listening to sad music; however, there is no change when listening to happy or neutral music.
There are some limitations of this study that need to be noted. Firstly, our sample size was relatively modest, and so we should further increase the sample size to conduct the study in the future. Secondly, in our study, the anxiety scale was selected broadly and further examination was not carried out. Thirdly, it was a cross-sectional study, so it was not possible to explore the causal relationship between high-frequency hearing loss and anxiety. Finally, this study used a clinical audiometer to measure hearing loss, and the ultra-high frequency audiometry could be used to study the high-frequency region in the future. In addition, we have assessed the pure tone thresholds at the frequencies of 0.125, 0.25, 0.5, 1, 2, 4, and 8 kHz which were well-set in the audiometer, but lacked the frequencies of 3 and 6 kHz that would contribute to the speech intelligibility of some phonemes. We will supplement these test frequencies in the future.
Our study showed that there was a close positive relationship between high-frequency hearing loss and anxiety scores in ARHL group. In addition, we found GM atrophy of MCC and H-P regions in ARHL group compared with the NH group. The GMV of MCC was positively associated with high-frequency hearing loss; however, the GMV of H-P regions was negatively correlated with high-frequency hearing loss. Our results also showed that the GMV of MCC and H-P regions had a significant positive relationship with ARHL-related anxiety in older adults. Taken together, we found an interaction between hearing loss, anxiety, and structural plasticity in older adults. Our findings revealed the crucial role of MCC and H-P in a link of anxiety-hearing loss link in older adults. In the future, it is necessary to conduct longitudinal studies to further clarify the causal relationship between hearing threshold changes and psychological status and structural plasticity in older adults.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Shandong First Medical University institutional review board. The patients/participants provided their written informed consent to participate in this study.
YW and FG had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis and concepted and designed the experiments. WM, XL, and FR carried out the experiments. YZ, SL, JY, LX, HL, and YG analyzed the experimental results. WM and YZ wrote the manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Nos. 61701342 and 81601479); Jinan Science and Technology Development Program of China (Nos. 201907097 and 202019098); Taishan Scholars Project of Shandong Province (No. tsqn201812147); Tianjin Natural Science Foundation (No. 19JCQNJC13100); Shandong Provincial Natural Science Foundation of China (Nos. ZR2021MH030, ZR2021MH355); and Academic promotion programme of Shandong First Medical University (No. 2019QL023).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.821537/full#supplementary-material
Arvin, B., Prepageran, N., and Raman, R. (2013). “High frequency presbycusis”-is there an earlier onset? Indian J. Otolaryngol. Head Neck Surg. 65(Suppl. 3), 480–484. doi: 10.1007/s12070-011-0356-x
Belkhiria, C., Vergara, R. C., San Martin, S., Leiva, A., Marcenaro, B., Martinez, M., et al. (2019). cingulate cortex atrophy is associated with hearing loss in presbycusis with cochlear amplifier dysfunction. Front. Aging Neurosci. 11:97. doi: 10.3389/fnagi.2019.00097
Belkhiria, C., Vergara, R. C., San Martin, S., Leiva, A., Martinez, M., Marcenaro, B., et al. (2020). Insula and amygdala atrophy are associated with functional impairment in subjects with presbycusis. Front. Aging Neurosci. 12:102. doi: 10.3389/fnagi.2020.00102
Boschloo, L., Vogelzangs, N., Smit, J. H., van den Brink, W., Veltman, D. J., Beekman, A. T., et al. (2010). The performance of the Alcohol Use Disorder Identification Test (AUDIT) in detecting alcohol abuse and dependence in a population of depressed or anxious persons. J. Affect. Disord. 126, 441–446. doi: 10.1016/j.jad.2010.04.019
Boyen, K., Langers, D. R., de Kleine, E., and van Dijk, P. (2013). Gray matter in the brain: differences associated with tinnitus and hearing loss. Hear. Res. 295, 67–78. doi: 10.1016/j.heares.2012.02.010
Brewster, K. K., Ciarleglio, A., Brown, P. J., Chen, C., Kim, H. O., Roose, S. P., et al. (2018). Age-Related hearing loss and its association with depression in later life. Am. J. Geriatr. Psychiatry 26, 788–796. doi: 10.1016/j.jagp.2018.04.003
Contrera, K. J., Betz, J., Deal, J. A., Choi, J. S., Ayonayon, H. N., Harris, T., et al. (2016). Association of hearing impairment and emotional vitality in older adults. J. Gerontol. B-Psychol. 71, 400–404. doi: 10.1093/geronb/gbw005
Cosh, S., Carriere, I., Daien, V., Amieva, H., Tzourio, C., Delcourt, C., et al. (2018). The relationship between hearing loss in older adults and depression over 12 years: findings from the three-city prospective cohort study. Int. J. Geriatr. Psychiatry 33, 1654–1661. doi: 10.1002/gps.4968
Croll, P. H., Vernooij, M. W., Reid, R. I., Goedegebure, A., Power, M. C., Rigters, S. C., et al. (2020). Hearing loss and microstructural integrity of the brain in a dementia-free older population. Alzheimers Dement. 16, 1515–1523. doi: 10.1002/alz.12151
DiMenichi, B. C., Ceceli, A. O., Bhanji, J. P., and Tricomi, E. (2019). Effects of expressive writing on neural processing during learning. Front. Hum. Neurosci. 13:389. doi: 10.3389/fnhum.2019.00389
Eckert, M. A., Cute, S. L., Vaden, K. I. Jr., Kuchinsky, S. E., and Dubno, J. R. (2012). Auditory cortex signs of age-related hearing loss. J. Assoc. Res. Otolaryngol. 13, 703–713. doi: 10.1007/s10162-012-0332-5
Feng, X. Y., Hamberger, M. J., Sigmon, H. C., Guo, J., Small, S. A., and Provenzano, F. A. (2018). Temporal lobe epilepsy lateralization using retrospective cerebral blood volume MRI. Neuroimage Clin. 19, 911–917. doi: 10.1016/j.nicl.2018.05.012
Hatta, T. (2007). Handedness and the brain: a review of brain-imaging techniques. Magn. Reson. Med. Sci. 6, 99–112. doi: 10.2463/mrms.6.99
Humes, L. E. (2019). The World Health Organization’s hearing-impairment grading system: an evaluation for unaided communication in age-related hearing loss. Int. J. Audiol. 58, 12–20. doi: 10.1080/14992027.2018.1518598
Husain, F. T., Medina, R. E., Davis, C. W., Szymko-Bennett, Y., Simonyan, K., Pajor, N. M., et al. (2011). Neuroanatomical changes due to hearing loss and chronic tinnitus: a combined VBM and DTI study. Brain Res. 1369, 74–88. doi: 10.1016/j.brainres.2010.10.095
Jayakody, D. M. P., Almeida, O. P., Speelman, C. P., Bennett, R. J., Moyle, T. C., Yiannos, J. M., et al. (2018a). Association between speech and high-frequency hearing loss and depression, anxiety and stress in older adults. Maturitas 110, 86–91. doi: 10.1016/j.maturitas.2018.02.002
Jayakody, D. M. P., Friedland, P. L., Eikelboom, R. H., Martins, R. N., and Sohrabi, H. R. (2018b). A novel study on association between untreated hearing loss and cognitive functions of older adults: baseline non-verbal cognitive assessment results. Clin. Otolaryngol. 43, 182–191. doi: 10.1111/coa.12937
Konig, O., Schaette, R., Kempter, R., and Gross, M. (2006). Course of hearing loss and occurrence of tinnitus. Hear. Res. 221, 59–64. doi: 10.1016/j.heares.2006.07.007
Lin, F. R., Ferrucci, L., An, Y., Goh, J. O., Doshi, J., Metter, E. J., et al. (2014). Association of hearing impairment with brain volume changes in older adults. Neuroimage 90, 84–92. doi: 10.1016/j.neuroimage.2013.12.059
Lin, F. R., Thorpe, R., Gordon-Salant, S., and Ferrucci, L. (2011). Hearing loss prevalence and risk factors among older adults in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 66, 582–590. doi: 10.1093/gerona/glr002
Lin, Y. H., Dhanaraj, V., Mackenzie, A. E., Young, I. M., Tanglay, O., Briggs, R. G., et al. (2021). Anatomy and white matter connections of the parahippocampal gyrus. World Neurosurg. 148, e218–e226. doi: 10.1016/j.wneu.2020.12.136
Maletic-Sekulic, I., Petkovic, S., Dragutinovic, N., Veselinovic, I., and Jelicic, L. (2019). Òhe effects of auditory amplification on subjective assessments of hearing impairment and anxiety in people with presbycusis. Srp. Arh. Celok. Lek. 147, 461–467. doi: 10.2298/sarh190123067m
Mehta, K. M., Simonsick, E. M., Penninx, B. W., Schulz, R., Rubin, S. M., Satterfield, S., et al. (2003a). Prevalence and correlates of anxiety symptoms in well-functioning older adults: findings from the health aging and body composition study. J. Am. Geriatr. Soc. 51, 499–504. doi: 10.1046/j.1532-5415.2003.51158.x
Mehta, K. M., Simonsick, E. M., Penninx, B. W. J. H., Schulz, R., Rubin, S. M., Satterfield, S., et al. (2003b). Prevalence and correlates of anxiety symptoms in well-functioning older adults: findings from the health aging and body composition study. J. Am. Geriatr. Soc. 51, 499–504. doi: 10.1046/j.1532-5415.2003.51158.x
Mick, P., Kawachi, I., and Lin, F. R. (2014). The association between hearing loss and social isolation in older adults. Otolaryngol. Head Neck Surg. 150, 378–384. doi: 10.1177/0194599813518021
Ponticorvo, S., Manara, R., Pfeuffer, J., Cappiello, A., Cuoco, S., Pellecchia, M. T., et al. (2019). Cortical pattern of reduced perfusion in hearing loss revealed by ASL-MRI. Hum. Brain. Mapp. 40, 2475–2487. doi: 10.1002/hbm.24538
Powell, R., Elwes, R., Hamandi, K., and Mullatti, N. (2018). Cingulate gyrus epilepsy. Pract. Neurol. 18, 447–454. doi: 10.1136/practneurol-2017-001812
Ren, F., Ma, W., Li, M., Sun, H., Xin, Q., Zong, W., et al. (2018). Gray matter atrophy is associated with cognitive impairment in patients with presbycusis: a comprehensive morphometric study. Front. Neurosci. 12:744. doi: 10.3389/fnins.2018.00744
Rigters, S. C., Bos, D., Metselaar, M., Roshchupkin, G. V., Baatenburg de Jong, R. J., Ikram, M. A., et al. (2017). Hearing impairment is associated with smaller brain volume in aging. Front. Aging Neurosci. 9:2. doi: 10.3389/fnagi.2017.00002
Ronnberg, J., Rudner, M., Foo, C., and Lunner, T. (2008). Cognition counts: a working memory system for ease of language understanding (ELU). Int. J. Audiol. 47(Suppl. 2), S99–S105. doi: 10.1080/14992020802301167
Schecklmann, M., Vielsmeier, V., Steffens, T., Landgrebe, M., Langguth, B., and Kleinjung, T. (2012). Relationship between audiometric slope and tinnitus pitch in tinnitus patients: insights into the mechanisms of tinnitus generation. PLoS One 7:e34878. doi: 10.1371/journal.pone.0034878
Wang, J., and Puel, J. L. (2020). Presbycusis: an update on cochlear mechanisms and therapies. J. Clin. Med. 9:218. doi: 10.3390/jcm9010218
Wattamwar, K., Qian, Z. J., Otter, J., Leskowitz, M. J., Caruana, F. F., Siedlecki, B., et al. (2017). Increases in the rate of age-related hearing loss in the older old. JAMA Otolaryngol. Head Neck Surg. 143, 41–45. doi: 10.1001/jamaoto.2016.2661
Wolak, T., Ciesla, K., Lorens, A., Kochanek, K., Lewandowska, M., Rusiniak, M., et al. (2017). Tonotopic organisation of the auditory cortex in sloping sensorineural hearing loss. Hear. Res. 355, 81–96. doi: 10.1016/j.heares.2017.09.012
Xu, X. M., Jiao, Y., Tang, T. Y., Zhang, J., Salvi, R., and Teng, G. J. (2019). Inefficient involvement of insula in sensorineural hearing loss. Front. Neurosci. 13:133. doi: 10.3389/fnins.2019.00133
Keywords: age-related hearing loss, anxiety, high-frequency pure tone audiometry, the hippocampal/parahippocampal regions, middle cingulate cortex, magnetic resonance imaging
Citation: Ma W, Zhang Y, Li X, Liu S, Gao Y, Yang J, Xu L, Liang H, Ren F, Gao F and Wang Y (2022) High-Frequency Hearing Loss Is Associated With Anxiety and Brain Structural Plasticity in Older Adults. Front. Aging Neurosci. 14:821537. doi: 10.3389/fnagi.2022.821537
Received: 24 November 2021; Accepted: 09 February 2022;
Published: 10 March 2022.
Edited by:
Jennifer A. Deal, Johns Hopkins University, United StatesReviewed by:
Nicole Armstrong, Brown University, United StatesCopyright © 2022 Ma, Zhang, Li, Liu, Gao, Yang, Xu, Liang, Ren, Gao and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fei Gao, ZmVpZ2FvNjI2MkAxNjMuY29t; Yao Wang, d2FuZ3lhb19zaG93QDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.