
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci., 09 March 2022
Sec. Neurocognitive Aging and Behavior
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.787552
This article is part of the Research TopicTo Know or Not to Know: Causes and Evolution of Lack of Awareness of Cognitive Decline in Neurodegenerative DiseasesView all 10 articles
 Silvia Chapman1,2†
Silvia Chapman1,2† Jillian L. Joyce1,3†
Jillian L. Joyce1,3† Megan S. Barker1,2
Megan S. Barker1,2 Preeti Sunderaraman1,2,3,4,5,6
Preeti Sunderaraman1,2,3,4,5,6 Sandra Rizer1,2
Sandra Rizer1,2 Edward D. Huey1,2,3,4,7
Edward D. Huey1,2,3,4,7 Jordan Dworkin1,7
Jordan Dworkin1,7 Yian Gu1,2,4
Yian Gu1,2,4 Stephanie Cosentino1,2,3,4*
Stephanie Cosentino1,2,3,4*
Objective: Subjective cognitive decline (SCD) has emerged as one of the first manifestations of Alzheimer’s disease (AD). However, discrepancies in its relationship with tests of memory and other cognitive abilities have hindered SCD’s diagnostic utility. Inter-individual heterogeneity in metamemory, or memory awareness, and the use of clinical measures of cognition lacking sensitivity to early cognitive dysfunction, may contribute to these discrepancies. We aimed to assess if the relationship between SCD and markers of early cognitive dysfunction is moderated by metamemory abilities.
Methods: The sample included 79 cognitively healthy older adults (77% female, 68% White, and 32% Black participants) with a mean age of 74.4 (SD = 6.1) and 15.9 (SD = 2.7) years of education. Metamemory was assessed using an episodic Feeling of Knowing test with four 5-item trials. Outcome measures included a resolution metric defined as a gamma correlation reflecting the accuracy of item-level predictions (“Will you know the correct answer?”). Early cognitive dysfunction was measured through the Loewenstein-Acevedo Scale for Semantic Interference and Learning (LASSI-L) and the Short-Term Memory Binding Test (STMB), measures sensitive to preclinical AD. SCD was assessed with a 20-item questionnaire that asked participants to compare themselves to others their age on a 7-point Likert scale. Regression analyses examined whether a potential relation between SCD and early cognitive dysfunction was moderated by metamemory.
Results: Subjective cognitive decline was associated with susceptibility to semantic proactive interference such that greater complaints were associated with increased susceptibility to semantic proactive interference (b = −0.30, p = 0.003) only. Metamemory moderated the association between SCD and susceptibility to and recovery of semantic proactive interference such that those with more accurate metamemory showed a stronger association between increased complaints and susceptibility to semantic proactive interference (b = −0.71, p = 0.005; b = −0.62, p = 0.034). Metamemory, however, did not moderate the association of SCD with retroactive semantic interference nor short term memory binding.
Discussion: The accuracy of an individual’s metamemory, specifically their ability to adjust moment to moment predictions in line with their performance, can influence the extent to which SCD maps onto objective cognition. Such self-referential assessment should be considered when interpreting SCD.
Researchers are mapping the earliest end of the Alzheimer’s disease (AD) continuum to identify patients in a critical window for therapeutic intervention (Dubois et al., 2016). While in vivo detection of AD pathologies using biomarkers is central to this process (Sperling et al., 2011), it is not sufficient given the imperfect association between neuropathology and clinical manifestation of disease (Negash et al., 2013). Indeed, at least a third of cognitively normal older adults have evidence of pathological AD on autopsy (Negash et al., 2013) or amyloid imaging (Chételat et al., 2013), and the pathological definition of AD continues to be debated (de la Torre, 2004; Castellani and Smith, 2011; Castellani and Perry, 2014). The ongoing questions and controversies surrounding clinical-pathological correlations in AD (Castellani and Smith, 2011; Castellani and Perry, 2014) emphasize the importance of identifying the earliest clinical manifestations of disease. Subjective cognitive decline (SCD), defined as the perception of cognitive decline despite normal performance on traditional neuropsychological testing, is likely to be one such early manifestation of illness with studies increasingly pointing to the potential relevance of SCD as an inexpensive and easily obtainable “pre-clinical” marker of AD (Geerlings et al., 1999; Reisberg et al., 2008; Sperling et al., 2011; Rabin et al., 2017; Jessen et al., 2020).
Research in AD as well as in aging generally supports an association between SCD and objective memory both cross-sectionally and longitudinally, and there is emerging evidence of the association between SCD and AD biomarkers (Gilewski et al., 1990; Hertzog et al., 1990; Pearman and Storandt, 2004; Beaudoin and Desrichard, 2011; Amariglio et al., 2012; Perrotin et al., 2012; Hülür et al., 2014; Snitz et al., 2015; Chen et al., 2019, 2021). However, the utility of SCD as a marker of cognitive functioning and biomarker status appears to vary as a function of multiple factors including task factors (e.g., measurement and operationalization issues) and person factors (e.g., individual characteristics) which together obscure its association with objective markers of disease (Schmidt et al., 2001; Jessen et al., 2010; Tandetnik et al., 2015; Ossenkoppele and Jagust, 2017). For example, the perceptions that memory is worse than others of the same age (i.e., age-anchored SCD) maps on more closely to AD biomarkers than perceptions of memory being bad in general, or worse than before, for example (Perrotin et al., 2012; Tandetnik et al., 2015; Chapman et al., 2021). With regard to person factors, there is recognition that personality and mood are likely important in the conceptualization of SCD; however, other factors remained to be explored (Pearman and Storandt, 2004; Slavin et al., 2010; Merema et al., 2013; Steinberg et al., 2013).
From a self-awareness perspective, SCD may be considered a hyperaware state (hypernosognosia) indicative of early dysfunction not yet detectable, or which does not reach a formal threshold for impairment, on clinical neuropsychological measures. As disease progresses, disordered awareness in the form or lack of awareness of deficits (anosognosia) likely follows SCD in a subset of individuals with mild cognitive impairment; this disordered awareness can be a prognostic indicator of disease progression as well as important clinical outcomes (Starkstein, 2014; Vannini et al., 2017; Munro et al., 2018). Knowledge of one’s own cognitive abilities (e.g., metacognition) has been examined extensively in healthy young and older adults (Nelson, 1990; Price et al., 2010; Hertzog and Dunlosky, 2011; Souchay and Isingrini, 2012; Cauvin et al., 2019; Siegel and Castel, 2019; Gagliardi et al., 2020) and has proven useful in understanding the clinical phenomenon of anosognosia, particularly disordered awareness of memory loss (Cosentino et al., 2007; Galeone et al., 2011; Rosen et al., 2014; DeLozier and Davalos, 2016).
Indeed, several groups have used metamemory testing to measure memory awareness in AD, and this type of assessment may offer a unique vantage point into the accuracy of SCD. As a direct measure of one’s memory awareness, metamemory is a critical person factor that should be considered in the interpretation of SCD. Specifically, individuals who demonstrate good metamemory (i.e., who have good awareness of their actual memory function), may be expected to have a more accurate subjective report of cognitive decline than those who have poor metamemory. Despite its clear relevance for understanding the prognostic relevance of SCD, metamemory has rarely been examined in relation to SCD (Buckley et al., 2016; Vannini et al., 2019; Chi et al., 2020; Gagliardi et al., 2020), perhaps because metamemory as a construct evolved primarily in the field of cognitive psychology and is not a formal component of clinical neuropsychological evaluations (Sunderaraman and Cosentino, 2017; Chapman et al., 2020).
The aim of this paper is to examine the extent to which metamemory moderates the relation between SCD and objective memory. As performance on traditional neuropsychological assessments of memory is by definition “normal” in individuals with SCD, we must utilize more challenging and sensitive neuropsychological tests to more rigorously examine the accuracy of SCD. The current study includes two memory measures shown to be sensitive to SCD as well as to AD biomarkers among clinically normal older adults. As stated above, our hypothesis postulates that those with better metamemory will have more accurate SCD; defined as a stronger association between SCD and objective memory testing on sensitive tasks.
Participants included in this study were selected from a larger cohort that comprises 157 participants recruited from the Columbia University Medical Center Aging and Dementia Neurology Clinic (n = 12) and ongoing aging studies at Taub Institute at Columbia University (n = 145). Two clinical cases were referred to the neurology clinic through a memory-concern screener administered in the Columbia University Department of Obstetrics and Gynecology. Referral studies included the Alzheimer’s Disease Research Center (n = 73), Washington Heights Inwood Columbia Aging Project (n = 35), Testing Olfaction in Primary care to detect Alzheimer’s disease and other Dementias (n = 11), and Cognitive Reserve and Reference Ability Neural Network studies (n = 22), Imaging inflammation in elders with different clinical and biomarker profiles of Alzheimer’s disease (n = 2) Concerns About Memory Problems (n = 2). To be included in the current study, participants were required to have performed within normal limits on standard neuropsychological testing (demographically adjusted z-scores above −1.5) within the last 12 months (see Supplementary Table 1 for neuropsychological screening measures). Exclusion criteria included past or current history of neurological conditions such as aneurysm, stroke, traumatic brain injury, epilepsy, etc. This study was reviewed and approved by Columbia University’s Institutional Review Board (Protocol AAAR5197). Participants provided written informed consent.
Subjective cognitive decline was measured using a 20-item, age-anchored scale previously shown to detect a range of self-reported cognitive problems among cognitively normal older adults (see Chapman et al., 2021 for full description). In brief, the scale comprises 10 items assessing aspects of episodic memory, and 10 non-memory items covering aspects of attention, language, spatial function, and executive abilities. Participants are asked to judge the extent to which they have difficulty with each item as compared to others their age. Responses are given ordinally (0 = no problem – 6 = major problem) with a total score ranging from 0 to 120. Higher scores represent more subjective cognitive problems.
The short-term memory binding task (STMB) assesses the integration of multi-modal information in short-term memory (Parra et al., 2010, 2011). Specifically, this task assesses the ability to integrate two features of a stimulus (shape and color) and hold this representation in short-term memory (Parra et al., 2010). The STMB has been shown to be robust against age effects (Parra et al., 2009) and is specific to AD dementia (Della Sala et al., 2012) showing high sensitivity and specificity for pre-clinical AD (Parra et al., 2010). The main outcome of the STMB task represents total stimuli correctly recognized, ranging from 0 to 16 with higher scores indicating better performance (see Parra et al., 2009 for full description). To ensure the validity of the STMB outcome measure, participants are required to pass a practice trial in which they need to integrate shape and color with no demands on short-term memory. The ability to integrate these two features has been associated primarily with posterior parietal-occipital regions implicated in the ventral visual stream, regions hypothesized to be affected during the sub-hippocampal stages of AD, which suggests the task can detect the earliest stages of AD development (Parra et al., 2014).
The Loewenstein-Acevedo Scales of Semantic Interference and Learning (LASSI-L) (Crocco et al., 2014) is a newly developed list-learning test that measures proactive semantic interference, retroactive interference, and the ability to recover from proactive semantic interference. Participants first read aloud a list of 15 words, List A, from three semantic categories: fruits, musical instruments, and articles of clothing. This is followed by a cued recall, with the three semantic categories as cues (“Can you tell me all the words on the list that were fruits?”). List A is then read again, followed by another cued recall. Then participants are presented with a new set of 15 words, List B, from the same semantic categories (fruits, musical instruments, and articles of clothing), followed by recall (B1, susceptibility to proactive semantic interference). The participants are presented with List B again, and recall (B2, recovery from semantic interference). Immediately following B2, participants are asked to recall all of the words from List A (A3, susceptibility to retroactive semantic interference). These three primary outcome measures (B1, B2, and A3) were included because they associate with biomarkers of AD such as amyloid load and volumetric loss. Specifically, this task has been shown to associate with amyloid accumulation in AD vulnerable regions such as the cingulate, precuneus, and frontal lobe in addition to volumetric and cortical reduction in the medial temporal lobe regions including the hippocampus (Loewenstein et al., 2016; Crocco et al., 2018).
Metamemory was assessed with a modified feeling of knowing (FOK) (Cosentino et al., 2007). This task is comprised of four trials with five fictional trivia items per trial (e.g., Cole Porter attended law school in Chicago). Participants are instructed as follows: “During this task, I am going to tell you about five people. I will tell you their name and something about their background. Your task is to try to remember this information as best you can. Please listen carefully”). Following the first learning trial of the five fictional trivia, participants are queried regarding each of the five items, one at a time in a random order (e.g., Who attended law school in Chicago?). For each item, the examiner asks participants to estimate the likelihood of knowing the right answer (FOK judgment; “There are eight possible answers on the next page). Will you know which one is right (“Yes, Maybe, or No?”). After each individual FOK judgment, participants are asked to identify the correct answer (e.g., Porter) from eight possible choices including the correct answer as well as seven distractors. Item level judgments are given ordinal values of 0 (No), 0.5 (Maybe), and 1 (Yes). Memory for each item is scored as 0 (incorrect) and 1 (correct). There are four learning trials yielding a total of 20 FOK judgments. This task has been utilized in both patients with AD and healthy older adults (Cosentino et al., 2007, 2011a,2011b).
The primary metamemory outcome derived from this task is a resolution score representing a person’s ability to adjust judgments of performance in line with actual memory performance from one item to the next. This score is calculated via the Goodman Kruskal gamma statistic; a rank order correlation assessing the total number of concordances (C) across the test (instances in which judgments and performance both increase from one item to another) versus the total number of discordances (D; judgments for performance decrease when performance increases and vice versa). Gamma is calculated as (C − D)/(C + D). Following this formula, tests characterized by relatively more concordances than discordances will result in a gamma value closer to 1 (perfect resolution), while the opposite will result in a gamma value closer to −1. This calculation does not take into account the number of “ties” across items, that is, any two items in which either the judgment or memory values are equal. Therefore, if someone “ties” across all items (e.g., always judges that they will know the answer), gamma is not calculated (Cosentino et al., 2007).
All analyses were conducted with IBM SPSS v.26. Descriptive statistics were conducted for demographic, SCD, metamemory, and memory measures. Spearman one-tailed correlations were conducted to examine the bivariate associations between SCD, gamma and memory. To examine the moderating effect of metamemory on the association between SCD and memory outcomes, linear regression models were conducted in complete case data. Influential univariate outliers (standardized residuals >3 or <−3) and multivariate outliers (determined through Mahalanobis distance) were examined for each model. To test for a specification error in the moderation models, namely that there is curvilinearity in the relation of each predictor to the dependent variable, quadratic effects of both SCD and gamma were included in separate models (Lubinski and Humphreys, 1990). Next, models were rerun without cases of gamma = 1 to examine if the frequency of these cases biased results. Finally, sensitivity analyses were conducted with imputed case data. A regression based multiple imputation approach was utilized for imputation. The pooled data from 25 imputations were utilized to obtain the estimates of variables in the model. All models were adjusted for demographic factors including age, self-reported gender, race, and education. In addition, a False Discovery Rate correction was implemented to complete cases that adjusted for the main comparisons of interest in the study which included demographical associations with main variables of interest, main effects of SCD and gamma on cognitive outcomes as well as their interactive effects.
Table 1 summarizes descriptives of demographics, cognitive, and metacognitive measures in the sample. All participants completed the SCD questionnaire (n = 157). A total of 156 participants completed the metamemory test, and 1 refused. Of the 156, 29 participants had ties across their pairs in the metamemory test and therefore gamma could not be calculated. The LASSI-L was available for 98 participants, as it was added to the study battery later. Finally, 9 participants failed to pass the validity trial for the STMB and one refused to complete due to color blindness leaving a total sample of 79 participants with all available measures. Descriptives are thus provided for these 79 participants with available data across all measures in Table 1. Demographics were found to be associated with gamma and cognitive outcomes. Specifically, age was negatively associated with gamma, susceptibility and ability to recover from proactive interference and retroactive interference (r range = −0.20, −0.29, p range = 0.004, 0.042). Greater levels of educational attainment were significantly associated with better performance in trials assessing susceptibility and ability to recover from proactive interference as well as retroactive interference (r range = 0.21, 0.36, p range = <0.001, 0.035). With regards to race, significant differences were observed with regards to performance in the STMB task only wherein White participants had higher performance (M = 10.61, SD = 9.56) than Black participants (M = 9.56, SD = 2.27) [t(77) = 2.24, p = 0.028], however, this difference did not withstand adjustment for educational attainment. No differences were observed in SCD, gamma nor cognitive outcomes regarding gender.
Table 2 summarizes bivariate association between SCD, metamemory and cognitive outcomes. Increased SCD was associated with worse recall on B1 and A3 indicating that individuals endorsing more complaints had increased susceptibility to semantic proactive and retroactive interference. For sensitivity analyses with imputed data please see Supplementary Table 2.
Table 3 summarizes main effect models without interaction terms and Table 4 summarizes results of the interactive effect of metamemory (gamma) with SCD on cognitive outcomes. Increased age, SCD, being male and having lower educational attainment was associated with increased susceptibility to proactive semantic interference reflected by lower recall on B1. In the second main effect model with B2 as the outcome, increased age was associated with reduced ability to recover from proactive interference. In the third main effect model examining A3 as an outcome, increased age was associated with increased susceptibility to retroactive semantic interference. Finally, in the main effect model of STMB, there were no variables that individually predicted STMB. With regard to moderation models, a significant interaction effect of metamemory and SCD was observed for B1 (susceptibility to proactive semantic interference) such that individuals with higher levels of metamemory had a stronger negative association between SCD and proactive interference. Metamemory’s also moderated the association SCD and B2 (ability to recover from proactive semantic interference).
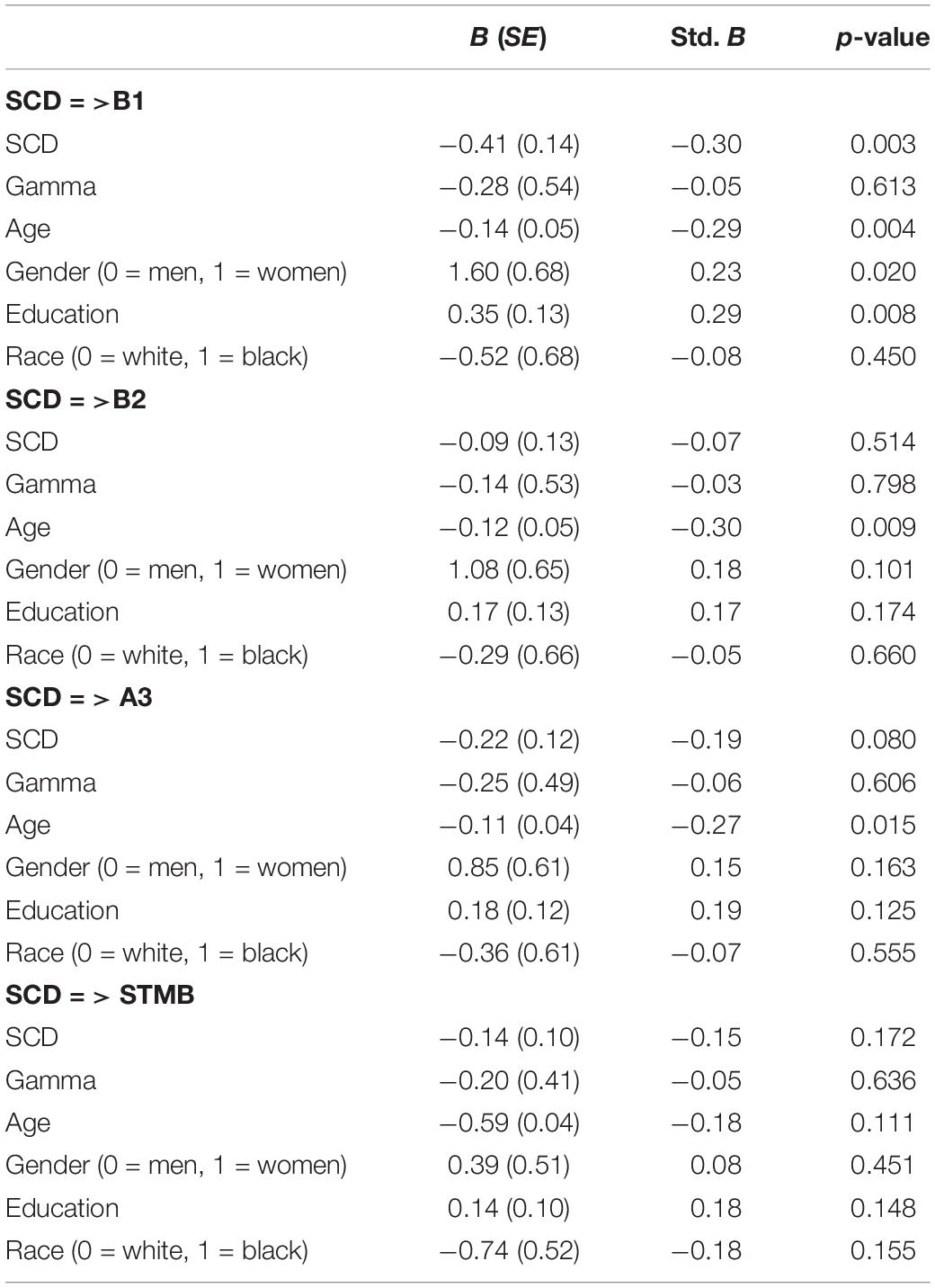
Table 3. Main effect models of SCD, gamma and demographic associations with LASSI-L and STMB outcomes.
One multivariate outlier was found in the moderation models with B1 and B2 as outcomes; exclusion of this outlier did not change results. In order to examine the influence of gamma = 1, moderation regression models were rerun without these cases (n = 60); the significant moderation effect remained. Specifically, the moderating effect of gamma was significant in models with B1 and B2 as outcomes (p = 0.006; p = 0.020). Third, in order to examine specification error, moderation models were rerun with quadratic terms of SCD and gamma. The moderation effect of gamma remained significant (p = 0.009) for the model with B1 as an outcome but not B2 where the effect lost significance at the margin (p = 0.055). Further, given that various measures had missing data, sensitivity analyses were conducted with all imputed data. Please see Supplementary Tables 3, 4. Whilst most results remained consistent, the moderating effect of gamma for models with B2 as an outcome lost significance (p = 0.085) consistent with our FDR correction.
This study examined the extent to which metamemory moderated the association between SCD and memory abilities in older adults. Consistent with previous work showing an association between SCD and rigorous measures of subtle cognitive dysfunction (Chapman et al., 2021), bivariate associations revealed that individuals with higher SCD had weaker performance on select list learning measures including greater susceptibility to both proactive interference and retroactive interference. With regard to the moderating role of metamemory, results from this study support the idea that in general, SCD is more strongly linked to memory abilities among individuals with better metamemory. Indeed, metamemory moderated the association between SCD and susceptibility to proactive interference Metamemory did not, however, moderate the association between SCD and retroactive interference or short-term memory binding. Below we offer potential interpretations for these findings and discuss current issues in the measurement and conceptualization of SCD more broadly, beginning with the variable associations between SCD and the memory outcomes selected for the current study.
The selective associations between SCD and only two of four memory outcomes, all previously shown to be sensitive to preclinical AD (Parra et al., 2010; Loewenstein et al., 2016; Crocco et al., 2018), was somewhat unexpected. For example, both proactive and retroactive interference on the LASSI-L have been linked to total cortical loading of amyloid and the precuneus specifically, among cognitively normal older adults (Loewenstein et al., 2016). In fact, the ability to recover from proactive interference has repeatedly been shown to be more sensitive to pre-clinical AD than other LASSI markers (Loewenstein et al., 2016, 2017). It is thus not immediately clear why SCD relates differently to each of these metrics. Susceptibility to proactive interference, associated with SCD in the current study, is assessed by measuring recall of List B after two study trials of List A. Recovery from proactive interference, not currently associated with SCD, is defined as recall of List B after its second presentation. It may be that in the current cognitively normal sample, there is little variability in performance after studying this list twice, limiting the degree to which it maps onto SCD. Indeed, average scores were higher (11.9) and the minimum score higher (6) than on the susceptibility metric (8.3 and 1, respectively). Nevertheless, the selective associations between SCD and increased susceptibility to proactive and retroactive interference may reflect specific early dysfunctions in cognitive control mechanisms. Previous research has shown that individuals with reduced working memory capacity (Rosen and Engle, 1998; Brewin and Smart, 2005) or inhibitory control (Anderson et al., 2000; Anderson, 2003; Anderson and Levy, 2007) tend to be more susceptible to interference effects and intrusive thoughts. Subtle changes in these cognitive control mechanisms could impact the use of specific and more effective retrieval mechanisms (Anderson and Levy, 2007; Unsworth, 2016, 2019).
Unexpectedly, SCD was also unrelated to short-term memory binding, the latter measure having previously been associated with SCD in a subset of this same cohort (Chapman et al., 2021). It is important to keep in mind, however, that while both the LASSI-L and STMB are sensitive to preclinical AD, their neural underpinnings are not synonymous. As highlighted earlier, LASSI-L measures have been associated with amyloid load in key AD regions such as cingulate, precuneus, frontal lobe as well as volumetric and cortical integrity of medial temporal lobe regions including the hippocampus. In contrast, the STMB has been associated primarily with posterior parietal-occipital regions implicated in the ventral visual stream, regions hypothesized to be affected during the sub-hippocampal stages of AD (Parra et al., 2014). As such, depending on the regional distribution of potential brain changes among individuals in a given sample, the extent to which SCD maps onto one or another cognitive measure will likely differ.
The inconsistency of metamemory as a moderator was also unexpected. While the size and direction of the moderation effect were generally comparable across different outcome measures, the moderating effect was only significant for SCD and measures of proactive interference (susceptibility to and recovery from), but not retroactive interference or short-term memory binding. There are several factors that could have led to this discrepancy. First, the link between SCD and memory itself is variable as discussed above. It may not be feasible to detect a significant moderation effect in situations where SCD is not even weakly associated with a specific memory outcome, as was the case for STMB in the current study. A second potential issue is that metamemory itself is heterogeneous, consisting of two broad categories: monitoring (i.e., what you know about your memory) and control (i.e., how you manage your memory). Monitoring, the focus of the current study, is itself multi-dimensional and can be operationalized in a number of ways that capture individuals’ confidence level (i.e., calibration) as well as their ability to adjust their expectations for performance as it varies over the course of a test (i.e., resolution). Furthermore, metamemory can be measured at different levels including an item-by-item basis (e.g., will you know the answer to this question?), or a summary level (e.g., how many answers will you know overall?) as well as at different points in time, including prior to or following memory performance (Nelson, 1984, 1990). Different studies have revealed nuances in the correlates of individual metamemory measures depending on a variety of factors including the score that is used (calibration versus resolution), the level at which it is measured (item versus summary), and the population in which it is measured (cognitively normal older adults versus AD) (Kikyo et al., 2002; Maril et al., 2003; Kikyo and Miyashita, 2004; Chua et al., 2006, 2009; Cosentino et al., 2007; Bertrand et al., 2018). From a cognitive perspective, aging studies have shown that confidence in retrieval judgments may be susceptible to variations in memory functioning (Hertzog et al., 2010, 2021). In line with this, reduced memory abilities in older adults may limit their access to diagnostic cues necessary to make accurate metacognitive judgments (Dunlosky and Metcalfe, 2008). Alternatively, older adults might have access to adequate cues but be unable to make valid inferences to reach accurate metacognitive judgments, possibly due to age-related changes in pre-frontal networks (Perrotin et al., 2008; Thomas et al., 2011; Fleming and Dolan, 2012). Given the seeming susceptibility in the current cohort to interference effects, and the moderating effects in this domain, we could also speculate that early vulnerability in frontal medial regions results in compromise to inferential judgments and resultingly to less accurate metacognitive judgments. Additional work is needed to tease apart the underlying cognitive as well as neuroanatomical substrates of both the susceptibility to interference and the moderating effects of metamemory ability.
In conclusion, results partially support our hypothesis that metamemory would moderate the association between SCD and memory performance, and provide rationale for consideration of metamemory when evaluating the accuracy of SCD. However, this study was not without limitations. First, the current sample included only participants with all available measures which reduced the sample significantly. However, in order to address this limitation, a multiple imputation approach was conducted in sensitivity analyses which revealed no significant differences between the initial model and the imputed model with the exception of the interactive effect of gamma and SCD on B2, also indicated in the False Discovery Rate adjusted p-values applied to complete-case analyses. A second limitation was that in 24 participants, gamma was not computed due to ties (i.e., no variability in either their FOK judgments or performance accuracy, with the majority of these cases always indicating “yes” for the FOK judgment with accuracy scores = 1). These cases could be considered as having perfect metamemory, highlighting a possible limitation of our task which for some participants may have a ceiling effect. A greater number of items within each learning trial would increase the likelihood of calculable gamma scores and provide a more comprehensive measure of metamemory in older adults. Another possible limitation was the relatively low level of SCD reported within this sample, along with possible ceiling effects on some cognitive measures which also may have reduced the strength of associations between SCD and cognition, as well as the moderating role of metamemory. Finally, the cohort included in this sample primarily included individuals drawn from other ongoing research studies rather than individuals presenting to a memory disorders clinic, which could skew not only the distribution of SCD but the level of concern regarding SCD, a factor known to increase SCD’s utility as a maker of preclinical AD (Jessen et al., 2010). Ideally, this study would have included sensitivity analyses to explore the effects of community/research recruited versus clinically recruited. This analysis, however, was not possible given that only 13/157 individuals were clinic recruited. There are numerous ways in which we are currently tailoring our ongoing study of SCD, including increasing SCD screenings and referrals from the community and local clinical practices to enroll individuals with higher levels of SCD. Moreover, we are tracking participants longitudinally to examine the extent to which SCD predicts decline over time, as well as the extent to which change in SCD is more predictive than a single SCD assessment. The current literature is mixed; For example, while Drouin et al. (2021) found that subjective memory change predicted longitudinal memory change, Hertzog et al. (2018) found that subjective memory change was more related to current memory complaint rather than an indicator of actual memory change.
This study also had a number of considerable strengths including the prospective, rigorous assessment of SCD using an age-anchored framework shown to relate more closely than other measurement frameworks (e.g., comparing one’s memory to 5 years ago) to objective measures of cognition (Perrotin et al., 2012; Tandetnik et al., 2015; Chapman et al., 2021). Another notable strength was the inclusion of objective metamemory testing, as well as two novel memory tests sensitive to pre-clinical AD, all of which have rarely if ever been combined in a single cohort. Finally, all participants completed comprehensive neuropsychological testing to ensure that they did not meet criteria for Mild Cognitive Impairment. Ongoing work, in addition to enriching our sample with individuals who present to the clinic with complaints, is examining not only the relative contribution of metamemory as a moderator, but of other person factors such as mood, personality, and attitudes about aging (Chapman et al., in preperation). Together, these analyses will continue to inform the way in which SCD can be optimized as a marker of pre-clinical AD.
The datasets presented in this article are not readily available because due to IRB restrictions we cannot share the data. Requests to access the datasets should be directed to StC, c2MyNDYwQGN1bWMuY29sdW1iaWEuZWR1.
The studies involving human participants were reviewed and approved by IRB COLUMBIA UNIVERSITY. The patients/participants provided their written informed consent to participate in this study.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This study was funded by the National Institute on Aging (NIA) R01 award AG054525-01A1, P30 award AG066462 and the National Center for Advancing Translational Sciences, National Institutes of Health, through award UL1TR001873.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.787552/full#supplementary-material
Amariglio, R. E., Becker, J. A., Carmasin, J., Wadsworth, L. P., Lorius, N., Sullivan, C., et al. (2012). Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 50, 2880–2886. doi: 10.1016/j.neuropsychologia.2012.08.011
Anderson, M. C. (2003). Rethinking interference theory: executive control and the mechanisms of forgetting. J. Mem. Lang. 49, 415–445. doi: 10.1016/j.jml.2003.08.006
Anderson, M. C., Bjork, E. L., and Bjork, R. A. (2000). Retrieval-induced forgetting: evidence for a recall-specific mechanism. Psychon. Bull. Rev. 7, 522–530. doi: 10.3758/BF03214366
Anderson, M. C., and Levy, B. J. (2007). “Theoretical issues in inhibition: insights from research on human memory,” in Inhibition in Cognition, eds D. S. Gorfein and C. M. MacLeod (Washington: DC: American Psychological Association), 81–102.
Beaudoin, M., and Desrichard, O. (2011). Are memory self-efficacy and memory performance related? A meta-analysis. Psychol. Bull. 137, 211–241. doi: 10.1037/a0022106
Bertrand, E., Azar, M., Rizvi, B., Brickman, A. M., Huey, E. D., Habeck, C., et al. (2018). Cortical thickness and metacognition in cognitively diverse older adults. Neuropsychology 32, 700–710. doi: 10.1037/neu0000458
Brandt, J. (1991). The hopkins verbal learning test: development of a new memory test with six equivalent forms. Clin. Neuropsychol. 5, 125–142. doi: 10.1080/13854049108403297
Brewin, C. R., and Smart, L. (2005). Working memory capacity and suppression of intrusive thoughts. J. Behav. Ther. Exp. Psychiatry 36, 61–68. doi: 10.1016/j.jbtep.2004.11.006
Buckley, R. F., Laming, G., Chen, L. P. E., Crole, A., and Hester, R. (2016). Assessing Error Awareness as a Mediator of the Relationship between Subjective Concerns and Cognitive Performance in Older Adults. PLoS One 11:e0166315. doi: 10.1371/journal.pone.0166315
Buschke, H. (1984). Cued recall in Amnesia. J. Clin. Neuropsychol. 6, 433–440. doi: 10.1080/01688638408401233
Castellani, R. J., and Perry, G. (2014). The complexities of the pathology-pathogenesis relationship in Alzheimer disease. Biochem. Pharmacol. 88, 671–676. doi: 10.1016/j.bcp.2014.01.009
Castellani, R. J., and Smith, M. A. (2011). Compounding artefacts with uncertainty, and an amyloid cascade hypothesis that is ‘too big to fail’. J. Pathol. 224, 147–152. doi: 10.1002/path.2885
Cauvin, S., Moulin, C. J. A., Souchay, C., Kliegel, M., and Schnitzspahn, K. M. (2019). Prospective Memory Predictions in Aging: increased Overconfidence in Older Adults. Exp. Aging Res. 45, 436–459. doi: 10.1080/0361073x.2019.1664471
Chapman, S., Colvin, L. E., and Cosentino, S. (2020). Translational Aspects of the Multidisciplinary Study of Metacognition. Transl. Issues Psychol. Sci. 6, 26–31. doi: 10.1037/tps0000224
Chapman, S., Sunderaraman, P., Joyce, J. L., Azar, M., Colvin, L. E., Barker, M. S., et al. (2021). Optimizing Subjective Cognitive Decline to Detect Early Cognitive Dysfunction. J. Alzheimers Dis. 80, 1185–1196. doi: 10.3233/jad-201322
Chen, X., Farrell, M. E., Moore, W., and Park, D. C. (2019). Actual memory as a mediator of the amyloid-subjective cognitive decline relationship. Alzheimers Dement. 11, 151–160. doi: 10.1016/j.dadm.2018.12.007
Chen, X., Farrell, M. E., Rundle, M. M., Chan, M. Y., Moore, W., Wig, G. S., et al. (2021). The relationship of functional hippocampal activity, amyloid deposition, and longitudinal memory decline to memory complaints in cognitively healthy older adults. Neurobiol. Aging 105, 318–326. doi: 10.1016/j.neurobiolaging.2021.04.020
Chételat, G., La Joie, R., Villain, N., Perrotin, A., de La Sayette, V., Eustache, F., et al. (2013). Amyloid imaging in cognitively normal individuals, at-risk populations and preclinical Alzheimer’s disease. NeuroImage Clin. 2, 356–365. doi: 10.1016/j.nicl.2013.02.006
Chi, S. Y., Chua, E. F., Kieschnick, D. W., and Rabin, L. A. (2020). Retrospective metamemory monitoring of semantic memory in community-dwelling older adults with subjective cognitive decline and mild cognitive impairment. Neuropsychol. Rehabil. [Epub Online ahead of print], doi: 10.1080/09602011.2020.1831552
Chua, E. F., Schacter, D. L., Rand-Giovannetti, E., and Sperling, R. A. (2006). Understanding metamemory: neural correlates of the cognitive process and subjective level of confidence in recognition memory. Neuroimage 29, 1150–1160. doi: 10.1016/j.neuroimage.2005.09.058
Chua, E. F., Schacter, D. L., and Sperling, R. A. (2009). Neural correlates of metamemory: a comparison of feeling-of-knowing and retrospective confidence judgments. J. Cogn. Neurosci. 21, 1751–1765. doi: 10.1162/jocn.2009.21123
Cosentino, S., Metcalfe, J., Butterfield, B., and Stern, Y. (2007). Objective metamemory testing captures awareness of deficit in Alzheimer’s disease. Cortex 43, 1004–1019.
Cosentino, S., Metcalfe, J., Cary, M. S., De Leon, J., and Karlawish, J. (2011a). Memory Awareness Influences Everyday Decision Making Capacity about Medication Management in Alzheimer’s Disease. Int. J. Alzheimers Dis. 2011:9. doi: 10.4061/2011/483897
Cosentino, S., Metcalfe, J., Holmes, B., Steffener, J., and Stern, Y. (2011b). Finding the self in metacognitive evaluations: metamemory and agency in nondemented elders. Neuropsychology 25, 602–612. doi: 10.1037/a0023972
Crocco, E., Curiel, R. E., Acevedo, A., Czaja, S. J., and Loewenstein, D. A. (2014). An Evaluation of Deficits in Semantic Cueing and Proactive and Retroactive Interference as Early Features of Alzheimer’s Disease. Am. J. Geriatr. Psychiatry 22, 889–897. doi: 10.1016/j.jagp.2013.01.066
Crocco, E. A., Loewenstein, D. A., Curiel, R. E., Alperin, N., Czaja, S. J., Harvey, P. D., et al. (2018). A novel cognitive assessment paradigm to detect Pre-mild cognitive impairment (PreMCI) and the relationship to biological markers of Alzheimer’s disease. J. Psychiatr. Res. 96, 33–38. doi: 10.1016/j.jpsychires.2017.08.015
de la Torre, J. C. (2004). Is Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 3, 184–190. doi: 10.1016/s1474-4422(04)00683-0
Della Sala, S., Parra, M. A., Fabi, K., Luzzi, S., and Abrahams, S. (2012). Short-term memory binding is impaired in AD but not in non-AD dementias. Neuropsychologia 50, 833–840. doi: 10.1016/j.neuropsychologia.2012.01.018
DeLozier, S. J., and Davalos, D. (2016). A Systematic Review of Metacognitive Differences Between Alzheimer’s Disease and Frontotemporal Dementia. Am. J. Alzheimers Dis. Other Demen. 31, 381–388. doi: 10.1177/1533317515618899
Drouin, S. M., McFall, G. P., and Dixon, R. A. (2021). Subjective memory concerns, poor vascular health, and male sex predict exacerbated memory decline trajectories: an integrative data-driven class and prediction analysis. Neuropsychology. doi: 10.1037/neu0000784
Dubois, B., Hampel, H., Feldman, H. H., Scheltens, P., Aisen, P., Andrieu, S., et al. (2016). Preclinical Alzheimer’s disease: definition, natural history, and diagnostic criteria. Alzheimers Dement. 12, 292–323. doi: 10.1016/j.jalz.2016.02.002
Fleming, S. M., and Dolan, R. J. (2012). The neural basis of metacognitive ability. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 367, 1338–1349. doi: 10.1098/rstb.2011.0417
Gagliardi, G., Houot, M., Cacciamani, F., Habert, M. O., Dubois, B., and Epelbaum, S. (2020). The meta-memory ratio: a new cohort-independent way to measure cognitive awareness in asymptomatic individuals at risk for Alzheimer’s disease. Alzheimers Res. Ther. 12:57. doi: 10.1186/s13195-020-00626-1
Galeone, F., Pappalardo, S., Chieffi, S., Iavarone, A., and Carlomagno, S. (2011). Anosognosia for memory deficit in amnestic mild cognitive impairment and Alzheimer’s disease. Int. J. Geriatr. Psychiatry 26, 695–701. doi: 10.1002/gps.2583
Geerlings, M. I., Jonker, C., Bouter, L. M., Ader, H. J., and Schmand, B. (1999). Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am. J. Psychiatry 156, 531–537. doi: 10.1176/ajp.156.4.531
Gilewski, M. J., Zelinski, E. M., and Schaie, K. W. (1990). The Memory Functioning Questionnaire for assessment of memory complaints in adulthood and old age. Psychol. Aging 5, 482–490. doi: 10.1037/0882-7974.5.4.482
Hertzog, C., Curley, T., and Dunlosky, J. (2021). Are age differences in recognition-based retrieval monitoring an epiphenomenon of age differences in memory? Psychol. Aging 36, 186–199. doi: 10.1037/pag0000595
Hertzog, C., Dixon, R. A., and Hultsch, D. F. (1990). Relationships between metamemory, memory predictions, and memory task performance in adults. Psychol. Aging 5, 215–227. doi: 10.1037/0882-7974.5.2.215
Hertzog, C., and Dunlosky, J. (2011). Metacognition in Later Adulthood: spared Monitoring Can Benefit Older Adults’ Self-regulation. Curr. Dir. Psychol. Sci. 20, 167–173. doi: 10.1177/0963721411409026
Hertzog, C., Dunlosky, J., and Sinclair, S. M. (2010). Episodic Feeling-of-Knowing Resolution Derives from the Quality of Original Encoding. Mem. Cogn. 38, 771–784. doi: 10.3758/MC.38.6.771
Hertzog, C., Hülür, G., Gerstorf, D., and Pearman, A. M. (2018). Is subjective memory change in old age based on accurate monitoring of age-related memory change? Evidence from two longitudinal studies. Psychol. Aging 33, 273–287. doi: 10.1037/pag0000232
Hülür, G., Hertzog, C., Pearman, A., Ram, N., and Gerstorf, D. (2014). Longitudinal associations of subjective memory with memory performance and depressive symptoms: between-person and within-person perspectives. Psychol. Aging 29, 814–827. doi: 10.1037/a0037619
Jessen, F., Amariglio, R. E., Buckley, R. F., van der Flier, W. M., Han, Y., Molinuevo, J. L., et al. (2020). The characterisation of subjective cognitive decline. Lancet Neurol. 19, 271–278. doi: 10.1016/s1474-4422(19)30368-0
Jessen, F., Wiese, B., Bachmann, C., Eifflaender-Gorfer, S., Haller, F., Kölsch, H., et al. (2010). Prediction of dementia by subjective memory impairment: effects of severity and temporal association with cognitive impairment. Arch. Gen. Psychiatry 67, 414–422. doi: 10.1001/archgenpsychiatry.2010.30
Kikyo, H., and Miyashita, Y. (2004). Temporal lobe activations of “feeling-of-knowing” induced by face-name associations. Neuroimage 23, 1348–1357. doi: 10.1016/j.neuroimage.2004.08.013
Kikyo, H., Ohki, K., and Miyashita, Y. (2002). Neural Correlates for Feeling-of-Knowing: an fMRI Parametric Analysis. Neuron 36, 177–186. doi: 10.1016/S0896-6273(02)00939-X
Loewenstein, D. A., Curiel, R. E., Greig, M. T., Bauer, R. M., Rosado, M., Bowers, D., et al. (2016). A Novel Cognitive Stress Test for the Detection of Preclinical Alzheimer Disease: discriminative Properties and Relation to Amyloid Load. Am. J. Geriatr. Psychiatry 24, 804–813. doi: 10.1016/j.jagp.2016.02.056
Loewenstein, D. A., Curiel, R. E., Wright, C., Sun, X., Alperin, N., Crocco, E., et al. (2017). Recovery from Proactive Semantic Interference in Mild Cognitive Impairment and Normal Aging: relationship to Atrophy in Brain Regions Vulnerable to Alzheimer’s Disease. J. Alzheimers Dis. 56, 1119–1126. doi: 10.3233/JAD-160881
Lubinski, D., and Humphreys, L. G. (1990). Assessing spurious “moderator effects”: illustrated substantively with the hypothesized (”synergistic”) relation between spatial and mathematical ability. Psychol. Bull. 107, 385–393. doi: 10.1037/0033-2909.107.3.385
Maril, A., Simons, J. S., Mitchell, J. P., Schwartz, B. L., and Schacter, D. L. (2003). Feeling-of-knowing in episodic memory: an event-related fMRI study. Neuroimage 18, 827–836. doi: 10.1016/s1053-8119(03)00014-4
Merema, M. R., Speelman, C. P., Foster, J. K., and Kaczmarek, E. A. (2013). Neuroticism (not depressive symptoms) predicts memory complaints in some community-dwelling older adults. Am. J. Geriatr. Psychiatry 21, 729–736. doi: 10.1016/j.jagp.2013.01.059
Munro, C. E., Donovan, N. J., Amariglio, R. E., Papp, K. V., Marshall, G. A., Rentz, D. M., et al. (2018). The Impact of Awareness of and Concern About Memory Performance on the Prediction of Progression From Mild Cognitive Impairment to Alzheimer Disease Dementia. Am. J. Geriatr. Psychiatry 26, 896–904. doi: 10.1016/j.jagp.2018.04.008
Negash, S., Wilson, R. S., Leurgans, S. E., Wolk, D. A., Schneider, J. A., Buchman, A. S., et al. (2013). Resilient brain aging: characterization of discordance between Alzheimer’s disease pathology and cognition. Curr. Alzheimer Res. 10, 844–851. doi: 10.2174/15672050113109990157
Nelson, T. O. (1984). A comparison of current measures of the accuracy of feeling-of-knowing predictions. Psychol. Bull. 95, 109–133.
Nelson, T. O. (1990). “Metamemory: a Theoretical Framework and New Findings,” in Psychology of Learning and Motivation, ed. G. H. Bower (Cambridge, MA: Academic Press), 125–173. doi: 10.1080/13546805.2016.1167031
Ossenkoppele, R., and Jagust, W. J. (2017). The Complexity of Subjective Cognitive Decline. JAMA Neurol. 74, 1400–1402. doi: 10.1001/jamaneurol.2017.2224
Parra, M. A., Abrahams, S., Fabi, K., Logie, R., Luzzi, S., and Sala, S. D. (2009). Short-term memory binding deficits in Alzheimer’s disease. Brain 132, 1057–1066. doi: 10.1093/brain/awp036
Parra, M. A., Abrahams, S., Logie, R. H., Méndez, L. G., Lopera, F., and Della Sala, S. (2010). Visual short-term memory binding deficits in familial Alzheimer’s disease. Brain 133, 2702–2713. doi: 10.1093/brain/awq148
Parra, M. A., Della Sala, S., Logie, R. H., and Morcom, A. M. (2014). Neural correlates of shape–color binding in visual working memory. Neuropsychologia 52, 27–36. doi: 10.1016/j.neuropsychologia.2013.09.036
Parra, M. A., Sala, S. D., Abrahams, S., Logie, R. H., Méndez, L. G., and Lopera, F. (2011). Specific deficit of colour–colour short-term memory binding in sporadic and familial Alzheimer’s disease. Neuropsychologia 49, 1943–1952. doi: 10.1016/j.neuropsychologia.2011.03.022
Pearman, A., and Storandt, M. (2004). Predictors of subjective memory in older adults. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 59, 4–6. doi: 10.1093/geronb/59.1.p4
Perrotin, A., Mormino, E. C., Madison, C. M., Hayenga, A. O., and Jagust, W. J. (2012). Subjective Cognition and Amyloid Deposition Imaging: a Pittsburgh Compound B Positron Emission Tomography Study in Normal Elderly Individuals. Arch. Neurol. 69, 223–229. doi: 10.1001/archneurol.2011.666
Perrotin, A., Tournelle, L., and Isingrini, M. (2008). Executive functioning and memory as potential mediators of the episodic feeling-of-knowing accuracy. Brain Cogn. 67, 76–87. doi: 10.1016/j.bandc.2007.11.006
Price, J., Hertzog, C., and Dunlosky, J. (2010). Self-regulated learning in younger and older adults: does aging affect metacognitive control? Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 17, 329–359. doi: 10.1080/13825580903287941
Rabin, L. A., Smart, C. M., and Amariglio, R. E. (2017). Subjective Cognitive Decline in Preclinical Alzheimer’s Disease. Annu. Rev. Clin. Psychol. 13, 369–396. doi: 10.1146/annurev-clinpsy-032816-045136
Reisberg, B., Prichep, L., Mosconi, L., John, E. R., Glodzik-Sobanska, L., Boksay, I., et al. (2008). The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement. 4, S98–S108. doi: 10.1016/j.jalz.2007.11.017
Reitan, R. M., and Wolfson, D. (1985). The Halstead–Reitan Neuropsycholgical Test Battery: Therapy and Clinical Interpretation. Tucson, AZ: Neuropsychological Press.
Rosen, H. J., Alcantar, O., Zakrzewski, J., Shimamura, A. P., Neuhaus, J., and Miller, B. L. (2014). Metacognition in the behavioral variant of frontotemporal dementia and Alzheimer’s disease. Neuropsychology 28, 436–447. doi: 10.1037/neu0000012
Rosen, V. M., and Engle, R. W. (1998). Working Memory Capacity and Suppression. J. Mem. Lang. 39, 418–436. doi: 10.1006/jmla.1998.2590
Schmidt, I. W., Berg, I. J., and Deelman, B. G. (2001). Relations between subjective evaluations of memory and objective memory performance. Percept. Mot. Skills 93, 761–776. doi: 10.2466/pms.2001.93.3.761
Siegel, A. L. M., and Castel, A. D. (2019). Age-related differences in metacognition for memory capacity and selectivity. Memory 27, 1236–1249. doi: 10.1080/09658211.2019.1645859
Slavin, M. J., Brodaty, H., Kochan, N. A., Crawford, J. D., Trollor, J. N., Draper, B., et al. (2010). Prevalence and Predictors of “Subjective Cognitive Complaints” in the Sydney Memory and Ageing Study. Am. J. Geriatr. Psychiatry 18, 701–710. doi: 10.1097/JGP.0b013e3181df49fb
Snitz, B. E., Small, B. J., Wang, T., Chang, C.-C. H., Hughes, T. F., and Ganguli, M. (2015). Do Subjective Memory Complaints Lead or Follow Objective Cognitive Change? A Five-Year Population Study of Temporal Influence. J. Int. Neuropsychol. Soc. JINS 21, 732–742. doi: 10.1017/S1355617715000922
Souchay, C., and Isingrini, M. (2012). Are feeling-of-knowing and judgment-of-learning different? Evidence from older adults. Acta Psychol. 139, 458–464. doi: 10.1016/j.actpsy.2012.01.007
Sperling, R. A., Aisen, P. S., Beckett, L. A., Bennett, D. A., Craft, S., Fagan, A. M., et al. (2011). Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7, 280–292. doi: 10.1016/j.jalz.2011.03.003
Starkstein, S. E. (2014). Anosognosia in Alzheimer’s disease: diagnosis, frequency, mechanism and clinical correlates. Cortex 61, 64–73. doi: 10.1016/j.cortex.2014.07.019
Steinberg, S. I., Negash, S., Sammel, M. D., Bogner, H., Harel, B. T., Livney, M. G., et al. (2013). Subjective memory complaints, cognitive performance, and psychological factors in healthy older adults. Am. J. Alzheimers Dis. Other Demen. 28, 776–783. doi: 10.1177/1533317513504817
Sunderaraman, P., and Cosentino, S. (2017). Integrating the Constructs of Anosognosia and Metacognition: a Review of Recent Findings in Dementia. Curr. Neurol. Neurosci. Rep. 17:27. doi: 10.1007/s11910-017-0734-1
Tandetnik, C., Farrell, M. T., Cary, M. S., Cines, S., Emrani, S., Karlawish, J., et al. (2015). Ascertaining Subjective Cognitive Decline: a Comparison of Approaches and Evidence for Using an Age-Anchored Reference Group. J. Alzheimers Dis. 48, S43–S55. doi: 10.3233/jad-150251
Thomas, A. K., Bulevich, J. B., and Dubois, S. J. (2011). Context affects feeling-of-knowing accuracy in younger and older adults. J. Exp. Psychol. Learn. Mem. Cogn. 37, 96–108. doi: 10.1037/a0021612
Unsworth, N. (2016). Working memory capacity and recall from long-term memory: examining the influences of encoding strategies, study time allocation, search efficiency, and monitoring abilities. J. Exp. Psychol. Learn. Mem. Cogn. 42, 50–61. doi: 10.1037/xlm0000148
Unsworth, N. (2019). Individual differences in long-term memory. Psychol. Bull. 145, 79–139. doi: 10.1037/bul0000176
Vannini, P., Amariglio, R., Hanseeuw, B., Johnson, K. A., McLaren, D. G., Chhatwal, J., et al. (2017). Memory self-awareness in the preclinical and prodromal stages of Alzheimer’s disease. Neuropsychologia 99, 343–349. doi: 10.1016/j.neuropsychologia.2017.04.002
Keywords: subjective cognitive decline, metamemory, preclinical Alzheimer’s disease, self awareness, early cognitive dysfunction
Citation: Chapman S, Joyce JL, Barker MS, Sunderaraman P, Rizer S, Huey ED, Dworkin J, Gu Y and Cosentino S (2022) Subjective Cognitive Decline Is More Accurate When Metamemory Is Better. Front. Aging Neurosci. 14:787552. doi: 10.3389/fnagi.2022.787552
Received: 30 September 2021; Accepted: 24 January 2022;
Published: 09 March 2022.
Edited by:
Bernard Jimmy Hanseeuw, Cliniques Universitaires Saint-Luc, BelgiumReviewed by:
Christopher Hertzog, Georgia Institute of Technology, United StatesCopyright © 2022 Chapman, Joyce, Barker, Sunderaraman, Rizer, Huey, Dworkin, Gu and Cosentino. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie Cosentino, c2MyNDYwQGN1bWMuY29sdW1iaWEuZWR1
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.