
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Aging Neurosci. , 09 December 2022
Sec. Alzheimer's Disease and Related Dementias
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.1077384
 Dan Shan1*†
Dan Shan1*† Shaoyang Li2†
Shaoyang Li2† Ruichen Xu3†
Ruichen Xu3† Glen Nie4
Glen Nie4 Yangyiran Xie5
Yangyiran Xie5 Junchu Han6
Junchu Han6 Xiaoyi Gao7
Xiaoyi Gao7 Yuandian Zheng1
Yuandian Zheng1 Zhen Xu8
Zhen Xu8 Zhihao Dai9
Zhihao Dai9Many people with coronavirus disease 2019 (COVID-19) report varying degrees of memory impairment. Neuroimaging techniques such as MRI and PET have been utilized to shed light on how COVID-19 affects brain function in humans, including memory dysfunction. In this PRISMA-based systematic review, we compared and summarized the current literature looking at the relationship between COVID-19-induced neuropathological changes by neuroimaging scans and memory symptoms experienced by patients who recovered from COVID-19. Overall, this review suggests a correlational trend between structural abnormalities (e.g., cortical atrophy and white matter hyperintensities) or functional abnormalities (e.g., hypometabolism) in a wide range of brain regions (particularly in the frontal, parietal and temporal regions) and memory impairments in COVID-19 survivors, although a causal relationship between them remains elusive in the absence of sufficient caution. Further longitudinal investigations, particularly controlled studies combined with correlational analyses, are needed to provide additional evidence.
It is not uncommon for patients who recovered from coronavirus disease-2019 (COVID-19) to experience memory impairment in conjunction with other systemic or respiratory symptoms (Alonso-Lana et al., 2020; Huang et al., 2020; Ahmed et al., 2022). Specifically, short-term and long-term neurological and neuropsychiatric changes were observed after the onset of COVID-19 in patients (Dasgupta et al., 2020; Beghi et al., 2021; Roy et al., 2021), possibly leading to difficulties with memory (Al-Ramadan et al., 2021; Llach and Vieta, 2021). Ahmed et al. found that approximately one in five patients with varying severity of COVID-19 experienced memory difficulties during their recovery period within 1 year (Ahmed et al., 2022). Søraas et al. noted that about 10 percent of mild and non-hospitalized patients still had memory complaints 8 months after recovery from COVID-19 (Søraas et al., 2021).
Recent studies have provided evidence for the etiology of memory deficit caused by COVID-19 (Cataldi et al., 2020; Iadecola et al., 2020; Koralnik and Tyler, 2020; Wu et al., 2020; Najt et al., 2021). For example, a reduction in the gray matter volume such as the frontal lobe, which is responsible for working memory capacity, has been reported in certain COVID-19 patients (Prabhakaran et al., 2000; Lu et al., 2020; Douaud et al., 2021). The hippocampus, important for especially episodic and spatial memory (Scoville and Milner, 1957; Parkin, 1996; Eichenbaum, 2001), has emerged as a particularly vulnerable region affected by the SARS-CoV-2 virus. The virus could travel along peripheral olfactory neurons (Han et al., 2020; Soler et al., 2020; Kay, 2022), and invade the hippocampus through rapid interneuronal trans-synaptic viral dissemination within brain cortical regions (Kumar et al., 2020; Lisianiy and Pedachenko, 2022), potentially resulting in impaired memory after infection (Ritchie et al., 2020). Furthermore, the damage in the hippocampus could be further amplified by the immune response against SARS-CoV-2 virus, which could affect the blood-brain barrier (BBB) (Achar and Ghosh, 2020; Morris and Zohrabian, 2020). In addition, COVID-19 has been reported to cause silent brain hypoxia (Somers et al., 2020; Garg et al., 2021; Rahman et al., 2021), which also contributes to the hippocampal damage (Ahmed et al., 2022).
These findings raise three questions. First, how do findings on the effects of SARS-CoV-2 virus on the brain at the tissue, cellular, and molecular levels relate to the clinical and structural brain characteristics of individuals infected with COVID-19? Second, could self-report symptoms or memory tests truly reflect the memory impairment of COVID-19 survivors? Third, what types of memory problems are unique to COVID-19 patients? Answering these questions is critical to interpreting the relationship between COVID-19 and memory impairment. To answer the first question, although brain-related pathologies regarding COVID-19 were well interpreted in previous studies, memory deficits associated with COVID-19 are still being explored. There is mixed evidence of hippocampal volume change post-COVID-19. Mahajan and Mason pointed out that the reduction in hippocampal volume could indicate cognitive deficits in memory (Mahajan and Mason, 2021), and this has been reported in COVID-19 patients (Liu et al., 2022). Similarly, decreased neurogeneration due to COVID-19-related neuroinflammation in the hippocampus could result in cognitive decline, including memory loss (Lynch, 2010; Bayat et al., 2022; Radhakrishnan and Kandasamy, 2022). In contrast, a follow-up study looking at both self-report and brain imaging data showed that COVID-19 survivors had significantly greater structural volume and functional activity in the hippocampus bilaterally as compared with healthy controls (Tu et al., 2021). Although memory function was not directly assessed in these COVID-19 survivors (Tu et al., 2021), a larger hippocampal volume is considered to be associated with better memory capability (Biegler et al., 2001; Pohlack et al., 2014). To answer the second question, participants' self-reported outcomes (i.e., subjective memory complaints assumed to arise due to COVID-19 infection) have limited reliability (e.g., Hampshire et al., 2021; Søraas et al., 2021). Memory test is more objective than self-report, but still cannot provide definitive evidence of neuropathological changes associated with memory impairment. To answer the third question, brain imaging data can help monitor structural and functional changes in brain regions implicated in memory (Weiner and Khachaturian, 2005; Health Quality Ontario, 2014; Morita et al., 2016). This can also be used in evaluating asymptomatic COVID-19 patients (Samkaria and Mandal, 2021). However, the diagnostic value of various brain scans regarding memory impairment in COVID-19 patients is unclear.
Memory plays an enormous role in a person's daily living (Cohen, 2008), therefore, access to the post-COVID-19 memory dysfunction is crucial. Direct evidence of memory dysfunction may come from brain imaging studies in COVID-19 survivors, but little review has been done in this field. In this systemic review, we intended to present the most up-to-date information on memory dysfunction in COVID-19 survivors by collecting previous neuroimaging evidence.
The current systematic review was carried out based on the guidelines and principles outlined by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement 2020 and checklist (Page et al., 2021).
We performed a search through the PubMed database for relevant studies published in English from Jan 1, 2020, to September 7, 2022. The reference lists of all original articles and reviews retrieved from the search were subjected to manually review to identify additional studies that may fit the systematic review objective in the current article but were not identified by the PubMed database. Following keywords were used in our search strategy: (“memory complaint” OR “memory deficit” OR “memory impairment” OR “memory loss”), AND (“COVID-19” OR “COVID pandemic”), AND (“brain imaging” OR “magnetic resonance imaging” OR “MRI” OR “functional magnetic resonance imaging” OR “fMRI” OR “positron emission tomography” OR “PET” OR “electroencephalography” OR “EEG” OR “magnetoencephalography” OR “MEG” OR “event related potential” OR “ERP” OR “diffusor tensor imaging” OR “DTI” OR “SPECT” OR “CT”).
We set inclusion criteria that primarily specified original peer-reviewed research articles, in which case, only original empirical articles were retained when any systematic or narrative review that also discussed the neuroimaging findings regarding memory in COVID-19 patients was identified. We screened all relevant studies that recruited COVID-19 patients and utilized in-vivo brain-imaging modalities. We retained studies that reported neuroimaging findings regarding memory in patients with COVID-19; studies that examined the memory function of participants only through memory tests or self-reported results were excluded; case series or case reports were also systematically reviewed and discussed, but separately from the original articles.
Apart from clinical imaging data that could be relevant to the brain memory function of COVID-19 participants, the data extracted from each article included brain-imaging modality. In addition, where possible, we calculated effect sizes (Cohen's d for differences between means) for the purpose of comparisons (Larner, 2014; Mouchlianitis et al., 2016).
We identified 4,561 reports, 35 of which fulfilled the inclusion criteria after the full texts were assessed (Figure 1) (Garg et al., 2020; Kushwaha et al., 2020; Lu et al., 2020; Sun et al., 2020; Vandervorst et al., 2020; Woo et al., 2020; Blazhenets et al., 2021; Branco de Oliveira et al., 2021; Donegani et al., 2021; Ermis et al., 2021; Guedj et al., 2021; Hellgren et al., 2021; Hosp et al., 2021; Jacobs et al., 2021; Kas et al., 2021; Matias-Guiu et al., 2021; Polascik et al., 2021; Ravaglia et al., 2021; Silva et al., 2021; Sollini et al., 2021; Yesilkaya et al., 2021; Allen and Absar, 2022; Bungenberg et al., 2022; Cecchetti et al., 2022; Das et al., 2022; Douaud et al., 2022; Dressing et al., 2022; Hadad et al., 2022; Huang C. et al., 2022; Huang S. et al., 2022; Hugon et al., 2022a,b; Martini et al., 2022; Morand et al., 2022; Savino et al., 2022; Terruzzi et al., 2022). All the 35 studies assessing brain anatomical or functional changes in patients following SARS-CoV-2 infection discussed the impacts of COVID-19 on memory function. 20 studies were classified as original articles, 10 as case studies, and 5 as case series.
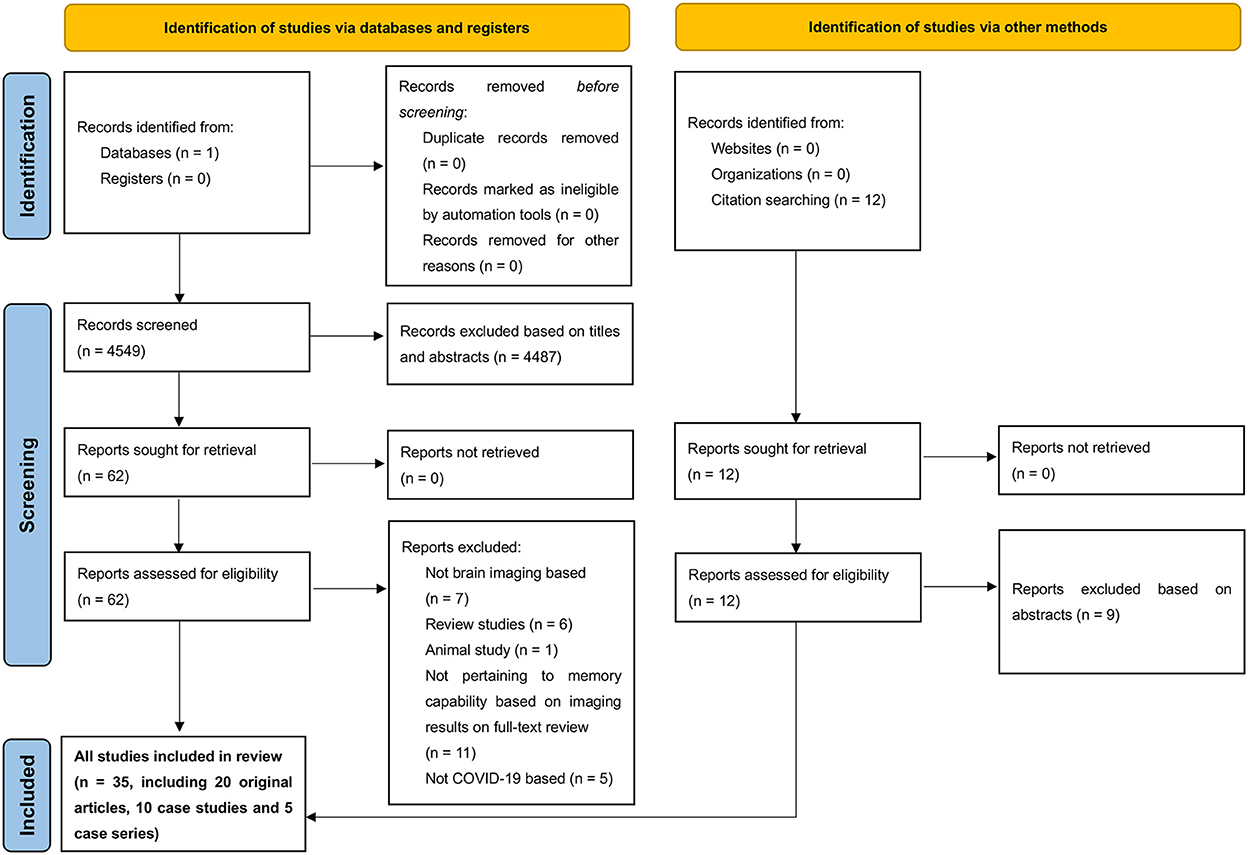
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram demonstrating search strategy.
These included studies were deemed of sufficient methodological qualities, in terms of the JBI Critical Appraisal Checklists (and the mean percentage scored points 97.3%) (Barker et al., 2022), though some were of relatively lower quality. The main missing quality components are as follows: The criteria of “consecutive inclusion of participants” was absent in two case series studies (Hugon et al., 2022b; Savino et al., 2022); of “control group” was absent in one prospective cohort study (Hellgren et al., 2021); of “identified confounding factors” was absent in three studies (Sun et al., 2020; Guedj et al., 2021; Hadad et al., 2022); And, of “strategies to deal with confounding factors” was absent in three studies (Ermis et al., 2021; Hellgren et al., 2021; Bungenberg et al., 2022). Refer to Table 1 for a summary of the quality of the included studies.
Brain scans were implemented through multiple neuroimaging techniques: 23 studies used MRI, 15 used PET, 9 used EEG, 8 used CT, 3 used DTI, and 1 used MRS (see Tables 2, 3).
Among the 20 original studies included, findings suggested that the frequency of memory impairment ranged from 0 to 100%, with a mean average of more than 30% across studies, subjected to the age of the participants, past medical history, the recovery time after COVID-19 infection, severity of COVID-19 symptoms, and type of memory impairment (e.g., short-term vs. long-term memory) (Kushwaha et al., 2020; Lu et al., 2020; Sun et al., 2020; Woo et al., 2020; Blazhenets et al., 2021; Donegani et al., 2021; Ermis et al., 2021; Guedj et al., 2021; Hellgren et al., 2021; Hosp et al., 2021; Kas et al., 2021; Silva et al., 2021; Sollini et al., 2021; Bungenberg et al., 2022; Cecchetti et al., 2022; Douaud et al., 2022; Dressing et al., 2022; Hadad et al., 2022; Huang C. et al., 2022; Huang S. et al., 2022; Martini et al., 2022). COVID-19 patients were much more likely to experience short-memory deficits compared to long-term memory. Memory impairment could be more severe in those with other neurological conditions such as cerebral ischemia. In terms of brain scan results, all 18F-FDG PET scans, with the exception of the study by Dressing et al. (2022), showed initially a markedly widespread hypometabolism after COVID-19 infection, which may be found in the prefrontal, frontoparietal and temporal regions (e.g., amygdala and hippocampus), thalamus, pons/medulla brainstem, and cerebellum. Over time, most of the affected brain regions of the patient's hypometabolism improved, in most cases accompanied by a substantial improvement in memory function. MRI results showed inconsistent findings: around 50% of MRI scans revealed that no significant brain pathological changes (e.g., brain atrophy or white matter hyperintensities) were associated with COVID-19 that caused patients' memory problems; whilst the other 50% of MRI scans showed white matter hyperintensities (particularly in the frontal and parietal lobes) and significant reductions in gray matter thickness bilaterally (especially in the parahippocampal gyrus, anterior cingulate cortex and temporal pole) in COVID-19 patients, as compared to healthy controls; although Lu et al. found that COVID-19 patients had higher global gray matter volume than healthy controls 3 months after infection with COVID-19 (Lu et al., 2020). EEG scans showed that diffuse pathological slowing, intermittent rhythmic delta-activity and low delta band at baseline were associated with memory impairment in COVID-19 patients. All three DTI studies indicated white matter abnormalities in COVID-19 patients, especially during the acute phase of infection (Lu et al., 2020; Silva et al., 2021; Huang C. et al., 2022; Huang S. et al., 2022). Although this might be biased due to the limited previous studies carried out utilizing DTI as references, the high sensitivity of DTI to brain white matter damage has been demonstrated (Kiely et al., 2022). Despite Lu et al. found increased white matter integrity in COVID-19 patients 3 months after infection, compared to healthy controls, Lu et al. attributed this to the underlying intrinsic reconstruction processes of brain white matter tracts (e.g., remyelination), which occurred following infection (Cauley and Cataltepe, 2014; Lu et al., 2020) (see Table 2 for details).
In the 15 case studies and case series included, findings suggested that COVID-19 patients in all age groups (from 10 to 79 years) in the absence of significant past medical or neuropsychiatric conditions could still suffer memory problems after the onset of COVID-19 (Garg et al., 2020; Vandervorst et al., 2020; Branco de Oliveira et al., 2021; Jacobs et al., 2021; Matias-Guiu et al., 2021; Polascik et al., 2021; Ravaglia et al., 2021; Yesilkaya et al., 2021; Allen and Absar, 2022; Das et al., 2022; Hugon et al., 2022a,b; Morand et al., 2022; Savino et al., 2022; Terruzzi et al., 2022). These patients' self-reported or cognitive function assessment-confirmed memory deficits may be in the form of short-term memory or long-term memory, or both. Memory symptoms could appear quickly after the initial onset of COVID-19, and may persist for more than 10 months, although they could be improved over time during recovery phases in most patients. The severity of memory symptoms could be worse in elderly patients, especially in the case that they were having other neurological conditions, such as stroke elicited by the consequences of COVID-19. In terms of brain imaging results, brain abnormalities in patients after the onset of COVID-19 might be reflected in the hypometabolism in bilateral medial temporal lobes (e.g., amygdala, parahippocampal gyrus), adjacent left frontal and parietal regions, brainstem (especially the pons) and cerebellum, as shown by PET scans; in the hyperintensities of white matter in various regions (e.g., left frontal lobe, paracallosal and periventricular regions), as well as hippocampal atrophy and biparietal lobe atrophy, as revealed by structural MRI; in the intermittent diffuse slow waves with atypical triphasic waves, as EEG showed; in the hypodensity in the left frontal lobe and periventricular area, as CT showed; and in the significantly decreased levels in N-acetylaspartate, glutamate and glutamate/glutamine ratio in bilateral dorsolateral prefrontal cortex, as MRS showed; although these abnormalities could be ameliorated in follow-up evaluations (see Table 3 for details).
This systematic review revealed that either structural abnormalities (e.g., cortical atrophy and white matter hyperintensities) or functional abnormalities (e.g., hypometabolism) in widespread brain regions (particularly in the frontal, parietal and temporal regions) may exist in COVID-19 patients with memory impairment compared to healthy controls. These brain abnormalities and memory dysfunction were likely to be reversible over time in most cases. The direction (i.e., increase vs. decrease) of the anatomical and metabolic alterations initially was in line with imaging findings in patients with comparable memory impairments such as dementia and Alzheimer's disease (Meyer et al., 2022).
To understand the association between COVID-19 and ensuing memory impairment in the human brain, three key questions need to be considered. The first question is, which certain brain regions are highly responsible for memory functions? The second question is that whether these regions are significantly affected by COVID-19? The third question is that whether there is a threshold for the severity of COVID-19 infection to elicit memory symptoms in patients? For the last question, Ahmed et al. found that COVID-19 severity was independent of patients' memory impairments (Ahmed et al., 2022), competing with the results of other studies (e.g., Sun et al., 2020; Méndez et al., 2021). All these studies shared the consensus that memory complaints may still occur in patients with mild COVID-19, suggesting that there was no obvious threshold of COVID-19 severity inducing memory complaints. For the first and second questions, from a neuroscience perspective, the fronto-parietal brain regions are important for short-term memory (e.g., working memory) (Chai et al., 2018; Assem et al., 2020), and the temporal region for long-term memory (Hershey et al., 1998; Simons and Spiers, 2003; Zokaei et al., 2019). All these regions are highly susceptible to COVID-19 infection in patients, especially during the early post-infection phase (Egbert et al., 2020; Douaud et al., 2022; Oumerzouk et al., 2022; Zalpoor et al., 2022). Thus, it is possible for COVID-19 infection to cause brain damage and subsequent memory dysfunction in patients.
To better understand this relationship between COVID-19 infection and memory dysfunction, another key point to concern is what the specific neurological anatomical or functional manifestations of COVID-19 infection are. Although impaired memory functions in COVID-19 patients are probably associated with the brain abnormalities as shown in the previous neuroimaging findings, a direct causal relationship cannot be established without sufficient caution.
Structural MRI and 18F-FDG PET were the most frequently utilized imaging scans in the previous studies investigating brain changes associated with memory impairment. Of note, these studies did not disclose fully consistent findings. For MRI research, not all studies using structural MRI/CT techniques found consistent structural brain abnormalities in COVID-19 patients with memory impairment. For example, Hosp et al. suggested that no sign of cerebral atrophy was seen in subacute COVID-19 patients with memory impairment (Hosp et al., 2021). Hospitalized COVID-19 patients were found to have worse verbal memory compared to non-hospitalized COVID-19 patients; however, no significant difference was found in terms of brain atrophy and white matter hyperintensities between the two groups (Bungenberg et al., 2022). Kas et al. suggested that no specific abnormalities in white matter in COVID-19 patients with memory deficits were found (Kas et al., 2021). Particularly, Lu et al. proposed that COVID-19 patients (including those with memory loss) had statistically significantly higher global gray matter volumes 3 months after COVID-19 infection, compared to non-COVID-19 controls (Lu et al., 2020), contrasting to many previous findings (Newhouse et al., 2022). In addition, 7 of 11 case studies or case series that utilized structural MRI scans also showed that very few abnormalities were seen in COVID-19 patients with memory dysfunction (Branco de Oliveira et al., 2021; Matias-Guiu et al., 2021; Yesilkaya et al., 2021; Allen and Absar, 2022; Das et al., 2022; Hugon et al., 2022a; Savino et al., 2022). In terms of PET research, although most previous studies using FDG-PET provided substantial evidence of hypometabolism in widespread brain regions (e.g., frontoparietal and temporal cortical regions) especially during the initial stage of COVID-19 infection, Dressing et al. found no significantly distinct changes of regional cerebral glucose metabolism in COVID-19 patients with memory complaints (Dressing et al., 2022).
There are several explanations for these inconsistent findings. First, differences in methods might yield conflicting findings, including the inclusion criteria for recruiting participants, follow-up duration of the studies, the approaches of determination of memory impairment (e.g., subjective self-report vs. objective memory assessment scores), the time points of memory symptoms reported and neuroimaging scans conducted, and data analysis methods. For instance, although Hosp et al. did not find cerebral structural abnormalities in COVID-19 patients using MRI, no correlational analysis between cerebral gray matter volume (or relevant) and memory performance was performed in those with memory symptoms; meanwhile, 18FDG PET imaging findings did show predominant frontoparietal hypometabolism, which was highly correlated with lower memory performance in COVID-19 patients (R2 = 0.62, p < 0.001) (Hosp et al., 2021). Similarly, although Kas et al. suggested that no specific abnormalities in white matter were seen on the MRI scan in COVID-19 patients with memory deficits, the PET scan showed widespread brain hypometabolism in these patients, especially during the acute phase of COVID-19 infection (Kas et al., 2021). Bungenberg et al. found hospitalized COVID-19 patients with worse memory capability had the similar brain atrophy and white matter hyperintensities as compared to non-hospitalized COVID-19 patients, which may indicate that brain pathoanatomical change was independent of COVID-19 severity in patients. Nevertheless, the correlational analysis of the relationship between memory performance and brain structural change was not conducted to better evaluate this association either in hospitalized patients or non-hospitalized ones (Bungenberg et al., 2022). Lu et al. found higher global gray matter volume in COVID-19 patients as compared to healthy controls. However, within the COVID-19 patients' cohort, global gray matter volume and reginal gray matter volume such as bilateral hippocampi were found to be negatively associated with memory loss in these patients (r = −0.341, −0.321, respectively; p-value = 0.008, 0.012, respectively) (Lu et al., 2020). Additionally, for the studies which did not conduct memory assessments via scales but relied solely on self-reported memory symptoms of patients to reflect their memory performance, there is a limitation regarding the quality (e.g., validity and generalizability of findings) of such research during the COVID-19 pandemic (Nieto et al., 2020). Second, Tian et al. pointed out that COVID-19 patients without the manifestations of memory deficits could still have brain pathological changes such as declined global cortical thickness. Hence, decreased cortical thickness (i.e., brain atrophy) might not be necessarily a direct cause of memory impairments in COVID-19 patients (Tian et al., 2022). In turn, it is possible that individuals with memory deficits had little apparent brain pathological changes in MRI scans as shown in the contrasting studies (Lu et al., 2020; Branco de Oliveira et al., 2021; Hosp et al., 2021; Kas et al., 2021; Matias-Guiu et al., 2021; Yesilkaya et al., 2021; Allen and Absar, 2022; Das et al., 2022; Hugon et al., 2022a; Savino et al., 2022). This may also partially explain the nuanced results in different studies. Finally, other confounding factors may also interfere with the relationship we are interested in exploring, including the types of memory symptoms (e.g., short-term vs. long-term memory, verbal vs. visual memory) manifested in the recruited participants, the recovery time (i.e., onset time of COVID-19) following infection, severity level of COVID-19, age of patients, past medical history, and etc.
From the perspective of neuroinflammatory-related changes in brain white matter, which are often reflected through DTI (Ji et al., 2017; Soni et al., 2021; Ouellette, 2022), all the three DTI studies included in this review suggested the abnormal changes in white matter in COVID-19 patients with memory symptoms (Lu et al., 2020; Silva et al., 2021; Huang C. et al., 2022; Huang S. et al., 2022), indicating the involvement of neuroinflammatory factors in these symptoms. Fernández-Castañeda et al. found that even mild COVID-19 infections could result in significant brain inflammation followed by brain cell dysregulation (e.g., loss of oligodendrocytes in white matter), and this would be expected to predict cognitive problems (Fernández-Castañeda et al., 2022a). Fernández-Castañeda et al. also proposed many similarities in inflammatory white matter damages and the pathophysiological processes of brain fog after COVID-19 as those after cancer chemotherapy. Evidence from both animal and postmortem showed that elevated inflammatory chemokines, especially CCL11, found in long COVID-19 patients with cognitive symptoms, directly contributed to the increased white matter microglial reactivity particularly in the hippocampus, an area highly responsible for learning and memory (Fernández-Castañeda et al., 2022a,b). These neuroinflammatory-related changes might be directly associated with early and transient memory impairment in COVID-19 patients.
In addition to the anatomical and physiological associations that have been discussed above, COVID-19 may also contribute to memory dysfunction by affecting patients' mental health (Amerio et al., 2020; Essadek and Rabeyron, 2020; Hyland et al., 2020; Shan et al., 2022a,b). This could also help explain the persistent memory symptoms in Long-COVID patients (Walia et al., 2021).
Since younger people were much more resilient against COVID-19 compared to the elderly (Bajaj et al., 2021), it is more reasonable that younger people infected with COVID-19 who subsequently self-reported memory impairment but with minimal neuroimaging changes actually experienced psychogenic causes such as brain fog, which could be elicited due to a stressful life event and ultimately led to their mild and temporary memory dysfunction, rather than triggered by direct and pronounced neural pathological changes on the brain (Loewenstein, 1993; Jennings et al., 2022). For example, Savino et al. noted a case of a 15-year-old male who presented with memory impairment, but his brain CT, EEG and MRI did not show obviously significant abnormalities (Savino et al., 2022). Hence, subjective self-reported memory symptoms at this time are more reflective of negative changes in memory performance in younger people than neuroimaging results. In contrast, the aging population were found to be more prone to evident brain pathological changes, such as reported evidence of accelerated amyloid formation of neurodegenerative proteins (de Erausquin et al., 2021; Semerdzhiev et al., 2021; Silva et al., 2022; Wu et al., 2022), so the objective Montreal Cognitive Assessment (MoCA) and neuroimaging results could better reflect the changes in these patients' memory performance. Thus, in future studies, we believe that subjects in different age groups should not be studied based on the same memory evaluation method in order to reflect actual changes in memory performance.
No permanent memory impairment induced by COVID-19 was demonstrated yet, and most memory symptoms are reversible over time. Tian et al. noted that the decrease in cortical thickness in COVID-19 patients improved and returned to baseline 10 months after discharge (regardless of COVID-19 severity), with no significant difference from healthy controls (Tian et al., 2022). Rogers et al. pointed out that 34.1% of patients with severe COVID-19 experienced memory impairment during the acute stage, but only 18.9% in the post-illness stage showed memory impairment (Rogers et al., 2020). Therefore, to promote fast recovery of memory function in COVID-19 patients, treatment options were listed in Table 4. Potential therapeutic targets investigated by past studies were also summarized in Table 4 for future research in this field.
Neuroimaging scans help to elucidate the COVID-19-related neuropathological changes in human brains, therefore providing avenues to explore memory impairment. According to the past evidence-based findings, although clinicoimaging correlations cannot prove causality between COVID-19 infection and memory deficits, we still argue that hypometabolism in certain brain regions, increased white matter hyperintensities and decline in cerebral gray matter volume may be effective predictors of the memory symptoms in COVID-19 patients, especially in the early stage of COVID-19 infection and those with persistent memory complaints. To assess such association, many other factors are still needed to be taken into consideration. Figure 2 is a schematic representation of memory impairment in patients following COVID-19 infection.
The available imaging evidence in this review has provided substantial support for interpreting the association between COVID-19-induced pathological changes in brain anatomy and metabolism and impaired memory performance in patients, but the type of memory impairment in patients remains obscure and therefore warrants further investigation. Future research focusing on a specific type of memory (e.g., working memory, procedural memory and episodic memory) is strongly encouraged, so as to narrow down the most obvious type of memory deficits caused by COVID-19, and thus find specialized treatment strategies to counteract them. Also, most of the included studies were conducted in the context of early variants of COVID-19, so very little research has specifically examined the long-term effects of the latest and most prevalent one—Omicron and its subvariants on memory performance. Hence, the recruitment of patients with recent COVID-19 infection for additional studies would be valuable. In addition, the vaccination status of subjects in early studies varied widely, in contrast to the current global COVID-19 vaccination coverage, which has been much higher than previously (Huang C. et al., 2022; Huang S. et al., 2022; McLaughlin et al., 2022). Since Ku et al. noted the protective effects of COVID-19 vaccine on brain after COVID-19 infection (Ku et al., 2021), patients' vaccination status might be an important factor to be considered while exploring this association in future studies.
To the best of our knowledge, this is the first systematic review study to present impacts of COVID-19 on human memory functions via neuroimaging evidence. We compared and contrasted past studies using neuroimaging of COVID-19-related memory dysfunction (Tables 2, 3). Some prior studies investigating patients' memory deficits in the absence of brain imaging methods were not included in this review, but they also provided ample evidence of memory impairment associated with COVID-19 infection (Bertuccelli et al., 2022). For instance, Reiken et al. provided evidence linking COVID-19 infection, cognitive impairment, and Alzheimer's-like signals shown in brain lysates of COVID-19 patients (Reiken et al., 2022).
The major limitation was about methodologic limitations of the original studies included. Specifically, there is a lack of a correlational analysis to appraise the relationship between brain pathological changes and memory impairment in patients. Also, over 40% of the studies included were identified as case studies or case series, which limited the validity and reliability of the perspectives in this review study. Moreover, we were only able to provide qualitative syntheses of the data due to the limited number of quantitative controlled neuroimaging studies. Thus, comprehensive meta-analyses studies and longitudinal investigation with correlational analyses are needed in further investigation.
In conclusion, sufficient evidence shows that memory impairment is a prominent symptom of COVID-19, and is likely associated with COVID-19-induced brain dysfunction. We are beginning to understand the pathophysiology of COVID-19-related memory impairment. Hypometabolism, increased white matter hyperintensities, and decreased cerebral gray matter volume may be effective indicators of memory dysfunction in COVID-19 patients, but a causal relationship remains unproven. Further longitudinal investigations combined with correlational analyses are needed to better understand such correlation.
DS, SL, and RX contributed to conception and design of the study. DS and SL conducted the protocol and the searches. RX, GN, and JH performed the screening. GN, JH, and ZD performed the data extraction and rating. DS wrote the first draft of the manuscript. YZ, ZX, and XG revised the first version of the manuscript. YX made a significant contribution to the latest manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Achar, A., and Ghosh, C. (2020). COVID-19-associated neurological disorders: the potential route of CNS invasion and blood-brain barrier relevance. Cells 9, 2360. doi: 10.3390/cells9112360
Ahmed, M., Roy, S., Iktidar, M. A., Chowdhury, S., Akhter, S., Islam, A. K., et al. (2022). Post-COVID-19 memory complaints: prevalence and associated factors. Neurología. doi: 10.1016/j.nrl.2022.03.007
Allen, T. Y., and Absar, N. M. (2022). Long-term neuropsychiatric complications and 18F-FDG-PET hypometabolism in the brain from prolonged infection of COVID-19. Alzheimer Dis. Assoc. Disord. 36, 173–175. doi: 10.1097/WAD.0000000000000485
Alonso-Lana, S., Marqui,é, M., Ruiz, A., and Boada, M. (2020). Cognitive and neuropsychiatric manifestations of COVID-19 and effects on elderly individuals with dementia. Front. Aging Neurosci. 12, 588872. doi: 10.3389/fnagi.2020.588872
Al-Ramadan, A., Rabab'h, O., Shah, J., and Gharaibeh, A. (2021). Acute and post-acute neurological complications of COVID-19. Neurol. Int. 13, 102–119. doi: 10.3390/neurolint13010010
Amerio, A., Brambilla, A., Morganti, A., Aguglia, A., Bianchi, D., Santi, F., et al. (2020). Covid-19 lockdown: Housing built environment's effects on Mental Health. Int. J. Environ. Res. Public Health. 17, 5973. doi: 10.3390/ijerph17165973
Assem, M., Blank, I. A., Mineroff, Z., Ademoglu, A., and Fedorenko, E. (2020). Activity in the fronto-parietal multiple-demand network is robustly associated with individual differences in working memory and fluid intelligence. Cortex 131, 1–16. doi: 10.1016/j.cortex.2020.06.013
Bajaj, V., Gadi, N., Spihlman, A. P., Wu, S. C., Choi, C. H., and Moulton, V. R. (2021). Aging, immunity, and COVID-19: how age influences the host immune response to coronavirus infections? Front. Physiol. 11, 571416. doi: 10.3389/fphys.2020.571416
Barker, T. H., Stone, J. C., Sears, K., Klugar, M., Leonardi-Bee, J., Tufanaru, C., et al. (2022). Revising the JBI quantitative critical appraisal tools to improve their applicability: an overview of methods and the development process. JBI Evid. Synth. 10–11124. doi: 10.11124/JBIES-22-00125
Bayat, A. H., Azimi, H., Hassani Moghaddam, M., Ebrahimi, V., Fathi, M., Vakili, K., et al. (2022). COVID-19 causes neuronal degeneration and reduces neurogenesis in human hippocampus. Apoptosis 27, 1–17. doi: 10.1007/s10495-022-01754-9
Beghi, E., Michael, B. D., Solomon, T., Westenberg, E., Winkler, A. S., and Global COVID-19 Neuro Research Coalition, Schmutzhard, E. (2021). Approaches to understanding COVID-19 and its neurological associations. Ann. Neurol. 89, 1059–1067. doi: 10.1002/ana.26076
Bertuccelli, M., Ciringione, L., Rubega, M., Bisiacchi, P., Masiero, S., and Del Felice, A. (2022). Cognitive impairment in people with previous COVID-19 infection: a scoping review. Cortex. 154, 212–230. doi: 10.1016/j.cortex.2022.06.002
Biegler, R., McGregor, A., Krebs, J. R., and Healy, S. D. (2001). A larger hippocampus is associated with longer-lasting spatial memory. Proc. Nat. Acad. Sci. 98, 6941–6944. doi: 10.1073/pnas.121034798
Blazhenets, G., Schröter, N., Bormann, T., Thurow, J., Wagner, D., Frings, L., et al. (2021). Slow but evident recovery from neocortical dysfunction and cognitive impairment in a series of chronic COVID-19 patients. J. Nucl. Med. 62, 910–915. doi: 10.2967/jnumed.121.262128
Branco de Oliveira, M. V., Bernab,é, D. G., Irikura, S., Irikura, R. B., Fontanelli, A. M., and Gonçalves, M. V. M. (2021). Wernicke encephalopathy in COVID-19 patients: report of three cases. Front. Neurol. 12, 629273. doi: 10.3389/fneur.2021.629273
Bungenberg, J., Humkamp, K., Hohenfeld, C., Rust, M. I., Ermis, U., Dreher, M., et al. (2022). Long COVID-19: objectifying most self-reported neurological symptoms. Annal. Clin. Transl. Neurol. 9, 141–154. doi: 10.1002/acn3.51496
Cataldi, M., Pignataro, G., and Taglialatela, M. (2020). Neurobiology of coronaviruses: potential relevance for COVID-19. Neurobiol. Dis. 143, 105007. doi: 10.1016/j.nbd.2020.105007
Cauley, K. A., and Cataltepe, O. (2014). Axial diffusivity of the corona radiata correlated with ventricular size in adult hydrocephalus. Am. J. Roentgenol. 203, 170–179. doi: 10.2214/AJR.12.10009
Cecchetti, G., Agosta, F., Canu, E., Basaia, S., Barbieri, A., Cardamone, R., et al. (2022). Cognitive, EEG, and MRI features of COVID-19 survivors: a 10-month study. J. Neurol. 1–13. doi: 10.1007/s00415-022-11047-5
Chai, W. J., Abd Hamid, A. I., and Abdullah, J. M. (2018). Working memory from the psychological and neurosciences perspectives: a review. Front. Psychol. 9, 401. doi: 10.3389/fpsyg.2018.00401
Cooper, C., Mansour, H., Carter, C., Rapaport, P., Morgan-Trimmer, S., Marchant, N. L., et al. (2021). Social connectedness and dementia prevention: pilot of the APPLE-Tree video-call intervention during the Covid-19 pandemic. Dementia 20, 2779–2801. doi: 10.1177/14713012211014382
Cruz, G. P. D., Pereira, L. S., and Raymundo, T. M. (2022). Cognitive training for elderly people without cognitive impairment: an occupational therapy intervention during the COVID-19 pandemic. Cadernos Brasileiros de Terapia Ocupacional 30:e3030. doi: 10.1590/2526-8910.ctoao22963030
Cysique, L. A., Jakabek, D., Bracken, S. G., Allen-Davidian, Y., Heng, B., Chow, S., et al. (2022). Post-acute COVID-19 cognitive impairment and decline uniquely associate with kynurenine pathway activation: a longitudinal observational study. medRxiv. 2022.06.07.22276020. doi: 10.1101/2022.06.07.22276020
Das, M., Ali, A., and Menon, V. (2022). Hashimoto's encephalopathy presenting as acute mania following recovery from COVID-19. Asian J. Psychiatr. 73, 103115. doi: 10.1016/j.ajp.2022.103115
Dasgupta, A., Kalhan, A., and Kalra, S. (2020). Long term complications and rehabilitation of COVID-19 patients. J. Pak. Med. Assoc. 70, S131–S135. doi: 10.5455/JPMA.32
de Erausquin, G. A., Snyder, H., Carrillo, M., Hosseini, A. A., Brugha, T. S., and Seshadri, S. (2021). The chronic neuropsychiatric sequelae of COVID-19: the need for a prospective study of viral impact on brain functioning. Alzheimers Dement. 17, 1056–1065. doi: 10.1002/alz.12255
Donegani, M. I., Miceli, A., Pardini, M., Bauckneht, M., Chiola, S., Pennone, M., et al. (2021). Brain metabolic correlates of persistent olfactory dysfunction after SARS-Cov2 infection. Biomedicines 9, 287. doi: 10.3390/biomedicines9030287
Douaud, G., Lee, S., Alfaro-Almagro, F., Arthofer, C., Wang, C., Lange, F., et al. (2021). Brain imaging before and after COVID-19 in UK Biobank. medRxiv. 2021.06.11.21258690. doi: 10.1101/2021.06.11.21258690
Douaud, G., Lee, S., Alfaro-Almagro, F., Arthofer, C., Wang, C., McCarthy, P., et al. (2022). SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 604, 697–707. doi: 10.1038/s41586-022-04569-5
Dressing, A., Bormann, T., Blazhenets, G., Schroeter, N., Walter, L. I., Thurow, J., et al. (2022). Neuropsychologic profiles and cerebral glucose metabolism in neurocognitive long COVID syndrome. J. Nucl. Med. 63, 1058–1063. doi: 10.2967/jnumed.121.262677
Drissi, I., Deschamps, C., Fouquet, G., Alary, R., Peineau, S., Gosset, P., et al. (2020). Memory and plasticity impairment after binge drinking in adolescent rat hippocampus: GluN2A/GluN2B NMDA receptor subunits imbalance through HDAC2. Addict. Biol. 25, e12760. doi: 10.1111/adb.12760
Egbert, A. R., Cankurtaran, S., and Karpiak, S. (2020). Brain abnormalities in COVID-19 acute/subacute phase: a rapid systematic review. Brain Behav. Immun. 89, 543–554. doi: 10.1016/j.bbi.2020.07.014
Eichenbaum, H. (2001). The hippocampus and declarative memory: cognitive mechanisms and neural codes. Behav. Brain Res. 127, 199–207. doi: 10.1016/S0166-4328(01)00365-5
Ermis, U., Rust, M. I., Bungenberg, J., Costa, A., Dreher, M., Balfanz, P., et al. (2021). Neurological symptoms in COVID-19: a cross-sectional monocentric study of hospitalized patients. Neurol. Res. Pract. 3, 1–12. doi: 10.1186/s42466-021-00116-1
Essadek, A., and Rabeyron, T. (2020). Mental health of French students during the COVID-19 pandemic. J. Affect. Disord. 277, 392–393. doi: 10.1016/j.jad.2020.08.042
Fernández-Castañeda, A., Lu, P., Geraghty, A. C., Song, E., Lee, M. H., Wood, J., et al. (2022a). Mild respiratory COVID can cause multi-lineage neural cell and myelin dysregulation. Cell 185, 2452–2468. doi: 10.1016/j.cell.2022.06.008
Fernández-Castañeda, A., Lu, P., Geraghty, A. C., Song, E., Lee, M. H., Wood, J., et al. (2022b). Mild respiratory SARS-CoV-2 infection can cause multi-lineage cellular dysregulation and myelin loss in the brain. BioRxiv. 2022.01.07.475453. doi: 10.1101/2022.01.07.475453
Garg, A., Marji, A., Goyal, S., and Ismail, R. (2020). A case of COVID-19 with memory impairment and delayed presentation as stroke. Cureus 12, e10025. doi: 10.7759/cureus.10025
Garg, R. K., Paliwal, V. K., and Gupta, A. (2021). Encephalopathy in patients with COVID-19: a review. J. Med. Virol. 93, 206–222. doi: 10.1002/jmv.26207
Guedj, E., Campion, J. Y., Dudouet, P., Kaphan, E., Bregeon, F., Tissot-Dupont, H., et al. (2021). 18F-FDG brain PET hypometabolism in patients with long COVID. Eur. J. Nucl. Med. Mol. Imaging 48, 2823–2833. doi: 10.1007/s00259-021-05215-4
Hadad, R., Khoury, J., Stanger, C., Fisher, T., Schneer, S., Ben-Hayun, R., et al. (2022). Cognitive dysfunction following COVID-19 infection. J. Neurovirol. 1–8. doi: 10.1007/s13365-022-01079-y
Hampshire, A., Trender, W., Chamberlain, S. R., Jolly, A. E., Grant, J. E., Patrick, F., et al. (2021). Cognitive deficits in people who have recovered from COVID-19. Eclin. Med. 39, 101044. doi: 10.1016/j.eclinm.2021.101044
Han, A. Y., Mukdad, L., Long, J. L., and Lopez, I. A. (2020). Anosmia in COVID-19: mechanisms and significance. Chem. Senses 45, 423–428. doi: 10.1093/chemse/bjaa040
Hartmann, H., Pauli, L. K., Janssen, L. K., Huhn, S., Ceglarek, U., and Horstmann, A. (2020). Preliminary evidence for an association between intake of high-fat high-sugar diet, variations in peripheral dopamine precursor availability and dopamine-dependent cognition in humans. J. Neuroendocrinol. 32, e12917. doi: 10.1111/jne.12917
Hawkins, M. A., Keirns, N. G., and Helms, Z. (2018). Carbohydrates and cognitive function. Curr. Opin. Clin. Nutr. Metab. Care 21, 302–307. doi: 10.1097/MCO.0000000000000471
Health Quality Ontario (2014). The appropriate use of neuroimaging in the diagnostic work-up of dementia: an evidence-based analysis. Ont. Health Technol. Assess. Ser. 14, 1.
Hellgren, L., Thornberg, U. B., Samuelsson, K., Levi, R., Divanoglou, A., and Blystad, I. (2021). Brain MRI and neuropsychological findings at long-term follow-up after COVID-19 hospitalisation: an observational cohort study. BMJ Open 11, e055164. doi: 10.1136/bmjopen-2021-055164
Hershey, T., Craft, S., Glauser, T. A., and Hale, S. (1998). Short-term and long-term memory in early temporal lobe dysfunction. Neuropsychology 12, 52. doi: 10.1037/0894-4105.12.1.52
Hosp, J. A., Dressing, A., Blazhenets, G., Bormann, T., Rau, A., Schwabenland, M., et al. (2021). Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain 144, 1263–1276. doi: 10.1093/brain/awab009
Huang, C., Wang, Y., Li, X., Ren, L., Zhao, J., Hu, Y., et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506. doi: 10.1016/S0140-6736(20)30183-5
Huang, C., Yang, L., Pan, J., Xu, X., and Peng, R. (2022). Correlation between vaccine coverage and the COVID-19 pandemic throughout the world: based on real-world data. J. Med. Virol. 94, 2181–2187. doi: 10.1002/jmv.27609
Huang, S., Zhou, Z., Yang, D., Zhao, W., Zeng, M., Xie, X., et al. (2022). Persistent white matter changes in recovered COVID-19 patients at the 1-year follow-up. Brain 145, 1830–1838. doi: 10.1093/brain/awab435
Hugon, J., Msika, E. F., Queneau, M., Farid, K., and Paquet, C. (2022a). Long COVID: cognitive complaints (brain fog) and dysfunction of the cingulate cortex. J. Neurol. 269, 44–46. doi: 10.1007/s00415-021-10655-x
Hugon, J., Queneau, M., Sanchez Ortiz, M., Msika, E. F., Farid, K., and Paquet, C. (2022b). Cognitive decline and brainstem hypometabolism in long COVID: a case series. Brain Behav. 12, e2513. doi: 10.1002/brb3.2513
Hyland, P., Shevlin, M., McBride, O., Murphy, J., Karatzias, T., Bentall, R., et al. (2020). Anxiety and depression in the Republic of Ireland during the COVID-19 pandemic. PsyArXiv. doi: 10.31234/osf.io/8yqxr
Iadecola, C., Anrather, J., and Kamel, H. (2020). Effects of COVID-19 on the nervous system. Cell 183, 16–27. doi: 10.1016/j.cell.2020.08.028
Jacobs, L. M., Gazagnes, M. D., Sanoussi, S., Collart, F., and Mesquita, M. D. C. F. (2021). Cognitive impairment in a patient with COVID-19 on hemodialysis: Too dangerous to neglect!. Hemodial. Int. 25, E44–E47. doi: 10.1111/hdi.12955
Jennings, G., Monaghan, A., Xue, F., Duggan, E., and Romero-Ortuño, R. (2022). Comprehensive clinical characterisation of brain fog in adults reporting long COVID symptoms. J. Clin. Med. 11, 3440. doi: 10.3390/jcm11123440
Ji, F., Pasternak, O., Liu, S., Loke, Y. M., Choo, B. L., Hilal, S., et al. (2017). Distinct white matter microstructural abnormalities and extracellular water increases relate to cognitive impairment in Alzheimer's disease with and without cerebrovascular disease. Alzheimers Res. Ther. 9, 1–10. doi: 10.1186/s13195-017-0292-4
Jimeno-Almazán, A., Pallarés, J. G., Buendía-Romero, Á., Martínez-Cava, A., Franco-López, F., Sánchez-Alcaraz Martínez, B. J., et al. (2021). Post-COVID-19 syndrome and the potential benefits of exercise. Int. J. Environ. Res. Public Health 18, 5329. doi: 10.3390/ijerph18105329
Kas, A., Soret, M., Pyatigoskaya, N., Habert, M. O., Hesters, A., Le Guennec, L., et al. (2021). The cerebral network of COVID-19-related encephalopathy: a longitudinal voxel-based 18F-FDG-PET study. Eur. J. Nucl. Med. Mol. Imaging 48, 2543–2557. doi: 10.1007/s00259-020-05178-y
Kay, L. M. (2022). COVID-19 and olfactory dysfunction: a looming wave of dementia? J. Neurophysiol. 128, 436–444. doi: 10.1152/jn.00255.2022
Kiely, M., Triebswetter, C., Cortina, L. E., Gong, Z., Alsameen, M. H., Spencer, R. G., et al. (2022). Insights into human cerebral white matter maturation and degeneration across the adult lifespan. Neuroimage 247, 118727. doi: 10.1016/j.neuroimage.2021.118727
Koralnik, I. J., and Tyler, K. L. (2020). COVID-19: a global threat to the nervous system. Ann. Neurol. 88, 1–11. doi: 10.1002/ana.25807
Ku, M. W., Authi,é, P., Bourgine, M., Anna, F., Noirat, A., Moncoq, F., et al. (2021). Brain cross-protection against SARS-CoV-2 variants by a lentiviral vaccine in new transgenic mice. EMBO Mol. Med. 13, e14459. doi: 10.15252/emmm.202114459
Kuelzow, N., Witte, A. V., Kerti, L., Grittner, U., Schuchardt, J. P., Hahn, A., et al. (2016). Impact of omega-3 fatty acid supplementation on memory functions in healthy older adults. J. Alzheimers Dis. 51, 713–725. doi: 10.3233/JAD-150886
Kumar, A., Pareek, V., Prasoon, P., Faiq, M. A., Kumar, P., Kumari, C., et al. (2020). Possible routes of SARS-CoV-2 invasion in brain: in context of neurological symptoms in COVID-19 patients. J. Neurosci. Res. 98, 2376–2383. doi: 10.1002/jnr.24717
Kushwaha, S., Seth, V., Bapat, P., Chaturvedi, M., Gupta, R., Bhattar, S., et al. (2020). Neurological associations of COVID-19—do we know enough: a tertiary care hospital based study. Front. Neurol. 11, 588879. doi: 10.3389/fneur.2020.588879
Larner, A. J. (2014). Effect size (Cohen's d) of cognitive screening instruments examined in pragmatic diagnostic accuracy studies. Dement. Geriatr. Cogn. Dis. Extra 4, 236–241. doi: 10.1159/000363735
Lisianiy, M. I., and Pedachenko, E. G. (2022). COVID-19: infection and neurological complications. Ukrain. Neurosurg. J. 28, N1. doi: 10.25305/unj.243599
Liu, X., Yan, W., Lu, T., Han, Y., and Lu, L. (2022). Longitudinal abnormalities in brain structure in COVID-19 patients. Neurosci. Bull. 1–5. doi: 10.1007/s12264-022-00913-x
Llach, C. D., and Vieta, E. (2021). Mind long COVID: psychiatric sequelae of SARS-CoV-2 infection. Eur. Neuropsychopharmacol. 49, 119. doi: 10.1016/j.euroneuro.2021.04.019
Loewenstein, R. J. (1993). Psychogenic amnesia and psychogenic fugue: A comprehensive review. In: Dissociative disorders: A clinical review. ed. Spiegel, D. Lutherville, MD: The Sidran Press, 45–78.
Lu, Y., Li, X., Geng, D., Mei, N., Wu, P. Y., Huang, C. C., et al. (2020). Cerebral micro-structural changes in COVID-19 patients–an MRI-based 3-month follow-up study. EClinicalMedicine 25, 100484. doi: 10.1016/j.eclinm.2020.100484
Lynch, M. A. (2010). Age-related neuroinflammatory changes negatively impact on neuronal function. Front. Aging Neurosci. 6. doi: 10.3389/neuro.24.006.2009
Mahajan, A., and Mason, G. F. (2021). A sobering addition to the literature on COVID-19 and the brain. J. Clin. Invest. 131(8). doi: 10.1172/JCI148376
Martini, A. L., Carli, G., Kiferle, L., Piersanti, P., Palumbo, P., Morbelli, S., et al. (2022). Time-dependent recovery of brain hypometabolism in neuro-COVID-19 patients. Eur. J. Nucl. Med. Mol. Imaging 1–13. doi: 10.1007/s00259-022-05942-2
Matias-Guiu, J. A., Delgado-Alonso, C., Yus, M., Polidura, C., Gómez-Ruiz, N., Valles-Salgado, M., et al. (2021). “Brain Fog” by COVID-19 or Alzheimer's disease? A case report. Front. Psychol. 5116. doi: 10.3389/fpsyg.2021.724022
McLaughlin, J. M., Khan, F., Pugh, S., Swerdlow, D. L., and Jodar, L. (2022). County-level vaccination coverage and rates of COVID-19 cases and deaths in the United States: an ecological analysis. Lancet Reg. Health-Am. 9, 100191. doi: 10.1016/j.lana.2022.100191
Méndez, R., Balanzá-Martínez, V., Luperdi, S. C., Estrada, I., Latorre, A., González-Jiménez, P., et al. (2021). Short-term neuropsychiatric outcomes and quality of life in COVID-19 survivors. J. Intern. Med. 290, 621–631. doi: 10.1111/joim.13262
Meyer, P. T., Hellwig, S., Blazhenets, G., and Hosp, J. A. (2022). Molecular imaging findings on acute and long-term effects of COVID-19 on the brain: a systematic review. J. Nucl. Med. 63, 971–980. doi: 10.2967/jnumed.121.263085
Morand, A., Campion, J. Y., Lepine, A., Bosdure, E., Luciani, L., Cammilleri, S., et al. (2022). Similar patterns of [18F]-FDG brain PET hypometabolism in paediatric and adult patients with long COVID: a paediatric case series. Eur. J. Nucl. Med. Mol. Imaging 49, 913–920. doi: 10.1007/s00259-021-05528-4
Morita, T., Asada, M., and Naito, E. (2016). Contribution of neuroimaging studies to understanding development of human cognitive brain functions. Front. Hum. Neurosci. 10, 464. doi: 10.3389/fnhum.2016.00464
Morris, M., and Zohrabian, V. M. (2020). Neuroradiologists, be mindful of the neuroinvasive potential of COVID-19. Am. J. Neuroradiol. 41, E37–E39. doi: 10.3174/ajnr.A6551
Mouchlianitis, E., McCutcheon, R., and Howes, O. D. (2016). Brain-imaging studies of treatment-resistant schizophrenia: a systematic review. Lancet Psychiatry 3, 451–463. doi: 10.1016/S2215-0366(15)00540-4
Najt, P., Richards, H. L., and Fortune, D. G. (2021). Brain imaging in patients with COVID-19: a systematic review. Brain Behav. Immun. Health 16, 100290. doi: 10.1016/j.bbih.2021.100290
Newhouse, A., Kritzer, M. D., Eryilmaz, H., Praschan, N., Camprodon, J. A., Fricchione, G., et al. (2022). Neurocircuitry hypothesis and clinical experience treating neuropsychiatric symptoms of post-acute sequelae of SARS-CoV-2 (PASC). J. Acad. Consult. Liaison Psychiatry. S2667962960(22)00309963. doi: 10.1016/j.jaclp.2022.08.007
Nieto, I., Navas, J. F., and Vázquez, C. (2020). The quality of research on mental health related to the COVID-19 pandemic: a note of caution after a systematic review. Brain Behav. Immun. Health 7, 100123. doi: 10.1016/j.bbih.2020.100123
Ouellette, R. (2022). Advanced MRI quantification of neuroinflammatory disorders. J. Neurosci. Res. 100, 1389. doi: 10.1002/jnr.25054
Oumerzouk, J., Nabil, M., Klevor, R., Belasri, S., Chraa, M., Louhab, N., et al. (2022). Clinicoradiological and prognostic features of COVID-19-associated acute disseminated encephalomyelitis. Rev. Neurol. 178, 144–150. doi: 10.1016/j.neurol.2021.11.003
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Syst. Rev. 10, 1–11. doi: 10.1186/s13643-021-01626-4
Parkin, A. J. (1996). Human memory: the hippocampus is the key. Curr. Biol. 6, 1583–1585. doi: 10.1016/S0960-9822(02)70778-1
Pohlack, S. T., Meyer, P., Cacciaglia, R., Liebscher, C., Ridder, S., and Flor, H. (2014). Bigger is better! Hippocampal volume and declarative memory performance in healthy young men. Brain Struct. Funct. 219, 255–267. doi: 10.1007/s00429-012-0497-z
Polascik, B., Browndyke, J., Davis, S., and Liu, A. (2021). Cognitive consequences of COVID-19 in older adults with cognitive impairment. Am. J. Geriat. Psychiatry 29, S75–S76. doi: 10.1016/j.jagp.2021.01.066
Prabhakaran, V., Narayanan, K., Zhao, Z., and Gabrieli, J. D. E. (2000). Integration of diverse information in working memory within the frontal lobe. Nat. Neurosci. 3, 85–90. doi: 10.1038/71156
Radhakrishnan, R. K., and Kandasamy, M. (2022). SARS-CoV-2-mediated neuropathogenesis, deterioration of hippocampal neurogenesis and dementia. Am. J. Alzheimers Dis. Other Dement. 37, 15333175221078418. doi: 10.1177/15333175221078418
Rahman, A., Tabassum, T., Araf, Y., Al Nahid, A., Ullah, M., and Hosen, M. J. (2021). Silent hypoxia in COVID-19: pathomechanism and possible management strategy. Mol. Biol. Rep. 48, 3863–3869. doi: 10.1007/s11033-021-06358-1
Ravaglia, S., Zito, A., Ahmad, L., and Canavero, I. (2021). How to forget a “traumatic” experience: a case report of transient global amnesia after nasopharyngeal swab for Coronavirus disease 19. BMC Neurol. 21, 1–3. doi: 10.1186/s12883-021-02295-5
Reiken, S., Sittenfeld, L., Dridi, H., Liu, Y., Liu, X., and Marks, A. R. (2022). Alzheimer's-like signaling in brains of COVID-19 patients. Alzheimer Dement. 18, 955–965. doi: 10.1002/alz.12558
Ritchie, K., Chan, D., and Watermeyer, T. (2020). The cognitive consequences of the COVID-19 epidemic: collateral damage? Brain Commun, 2, fcaa069. doi: 10.1093/braincomms/fcaa069
Rogers, J. P., Chesney, E., Oliver, D., Pollak, T. A., McGuire, P., Fusar-Poli, P., et al. (2020). Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 7, 611–627. doi: 10.1016/S2215-0366(20)30203-0
Roy, D., Ghosh, R., Dubey, S., Dubey, M. J., Benito-Leon, J., and Ray, B. K. (2021). Neurological and neuropsychiatric impacts of COVID-19 pandemic. Can. J. Neurol. Sci. 48, 9–24. doi: 10.1017/cjn.2020.173
Samkaria, A., and Mandal, P. K. (2021). Brain imaging in COVID-19. ACS Chem. Neurosci. 12, 2953–2955. doi: 10.1021/acschemneuro.1c00467
Savino, R., Polito, A. N., Arcidiacono, G., Poliseno, M., and Lo Caputo, S. (2022). Neuropsychiatric disorders in pediatric long COVID-19: a case series. Brain Sci. 12, 514. doi: 10.3390/brainsci12050514
Scoville, W. B., and Milner, B. (1957). Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatr. 20, 11. doi: 10.1136/jnnp.20.1.11
Semerdzhiev, S. A., Fakhree, M. A., Segers-Nolten, I., Blum, C., and Claessens, M. M. (2021). Interactions between SARS-CoV-2 N-protein and α-synuclein accelerate amyloid formation. ACS Chem. Neurosci. 13, 143–150. doi: 10.1021/acschemneuro.1c00666
Shan, D., Liu, C., Li, S., and Zheng, Y. (2022a). Corrigendum: increased anxiety from fear of Omicron in China as compared to North America and Western Europe: a cross-sectional Kendall's tau-b analysis using the generalized anxiety disorder 7-item questionnaire. Front. Psychiatry 2258.
Shan, D., Liu, C., Li, S., and Zheng, Y. (2022b). Increased anxiety from fear of Omicron in China as compared to North America and Western Europe: a cross-sectional Kendall's tau-b analysis using the generalized anxiety disorder 7-item questionnaire. Front. Psychiatry 2028. doi: 10.3389/fpsyt.2022.1038966
Silva, J., Patricio, F., Patricio-Martínez, A., Santos-López, G., Cedillo, L., Tizabi, Y., et al. (2022). Neuropathological aspects of SARS-CoV-2 infection: significance for both Alzheimer's and Parkinson's Disease. Front. Neurosci. 16:2021.03.20.21253414. doi: 10.3389/fnins.2022.867825
Silva, L. S., Joao, R. B., Nogueira, M. H., Aventurato, I. K., de Campos, B. M., de Brito, M. R., et al. (2021). Functional and microstructural brain abnormalities, fatigue, and cognitive dysfunction after mild COVID-19. Medrxiv. 2021.03.20.21253414. doi: 10.1101/2021.03.20.21253414
Simons, J. S., and Spiers, H. J. (2003). Prefrontal and medial temporal lobe interactions in long-term memory. Nat. Rev. Neurosci. 4, 637–648. doi: 10.1038/nrn1178
Sohel, M., Hossain, M., Hasan, M., Haque, A., Islam, M. S., Hossain, M. S., et al. (2021). Management of mental health during COVID 19 pandemic: possible strategies. J. Adv. Biotechnol. Exp. Therap. 4, 276–289. doi: 10.5455/jabet.2021.d128
Soler, Z. M., Patel, Z. M., Turner, J. H., and Holbrook, E. H. (2020). A primer on viral-associated olfactory loss in the era of COVID-19. Int. Forum Allergy Rhinol. 10, 814-820. doi: 10.1002/alr.22578
Sollini, M., Morbelli, S., Ciccarelli, M., Cecconi, M., Aghemo, A., Morelli, P., et al. (2021). Long COVID hallmarks on [18F] FDG-PET/CT: a case-control study. Eur. J. Nucl. Med. Mol. Imaging 48, 3187–3197. doi: 10.1007/s00259-021-05294-3
Somers, V. K., Kara, T., and Xie, J. (2020). Progressive hypoxia: a pivotal pathophysiologic mechanism of COVID-19 pneumonia. Mayo Clin. Proc. 95, 2339-2342. doi: 10.1016/j.mayocp.2020.09.015
Soni, N., Medeiros, R., Alateeq, K., To, X. V., and Nasrallah, F. A. (2021). Diffusion tensor imaging detects acute pathology-specific changes in the P301L tauopathy mouse model following traumatic brain injury. Front. Neurosci. 15, 611451. doi: 10.3389/fnins.2021.611451
Søraas, A., Bø, R., Kalleberg, K. T., Støer, N. C., Ellingjord-Dale, M., and Landr,ø, N. I. (2021). Self-reported memory problems 8 months after COVID-19 infection. JAMA Netw. Open 4, e2118717–e2118717. doi: 10.1001/jamanetworkopen.2021.18717
Street, M. E. (2020). HMGB1: a possible crucial therapeutic target for COVID-19? Horm. Res. Paediatr. 93, 73–75. doi: 10.1159/000508291
Sun F, Chen X, Ren H, Liu J, Jin W. (2020). Psychopathology analysis of 23 cases with COVID-19 pneumonia accompanied by abnormal brain imagine. Res Sq. doi: 10.21203/rs.3.rs-119056/v1
Terruzzi, S., Chiusole, M., Ottaviani, D., Rozzanigo, U., and Papagno, C. (2022). A case of anterograde amnesia in an MS-like demyelination after COVID-19. Neurol. Sci. 43, 89–94. doi: 10.1007/s10072-021-05665-6
Tian, T., Wu, J., Chen, T., Li, J., Yan, S., Zhou, Y., et al. (2022). Long-term follow-up of dynamic brain changes in patients recovered from COVID-19 without neurological manifestations. JCI Insight 7, e155827. doi: 10.1172/jci.insight.155827
Tu, Y., Zhang, Y., Li, Y., Zhao, Q., Bi, Y., Lu, X., et al. (2021). Post-traumatic stress symptoms in COVID-19 survivors: a self-report and brain imaging follow-up study. Mol. Psychiatry 26, 7475–7480. doi: 10.1038/s41380-021-01223-w
Vandervorst, F., Guldolf, K., Peeters, I., Vanderhasselt, T., Michiels, K., Berends, K. J., et al. (2020). Encephalitis associated with the SARS-CoV-2 virus: a case report. Interdiscip. Neurosurg. 22, 100821. doi: 10.1016/j.inat.2020.100821
Walia, N., Lat, J. O., Tariq, R., Tyagi, S., Qazi, A. M., Salari, S. W., et al. (2021). Post-acute sequelae of COVID-19 and the mental health implications. Discoveries 9, e140. doi: 10.15190/d.2021.19
Weiner, M., and Khachaturian, Z. (2005). The use of MRI and PET for clinical diagnosis of dementia and investigation of cognitive impairment: a consensus report. Alzheimers Assoc Chicago, IL 1, 1–15.
Wen, D., Xu, J., Wu, Z., Liu, Y., Zhou, Y., Li, J., et al. (2021). The effective cognitive assessment and training methods for COVID-19 patients with cognitive impairment. Front. Aging Neurosci. 13, 827273. doi: 10.3389/fnagi.2021.827273
Woo, M. S., Malsy, J., Pöttgen, J., Seddiq Zai, S., Ufer, F., Hadjilaou, A., et al. (2020). Frequent neurocognitive deficits after recovery from mild COVID-19. Brain Commun. 2, fcaa205. doi: 10.1093/braincomms/fcaa205
Wu, Y., Xu, X., Chen, Z., Duan, J., Hashimoto, K., Yang, L., et al. (2020). Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav. Immun. 87, 18–22. doi: 10.1016/j.bbi.2020.03.031
Wu, Z., Zhang, X., Huang, Z., and Ma, K. (2022). SARS-CoV-2 proteins interact with alpha synuclein and induce lewy body-like pathology in vitro. Int. J. Mol. Sci. 23, 3394. doi: 10.3390/ijms23063394
Yesilkaya, U. H., Sen, M., and Balcioglu, Y. H. (2021). COVID-19-related cognitive dysfunction may be associated with transient disruption in the DLPFC glutamatergic pathway. J. Clin. Neurosci. 87, 153–155. doi: 10.1016/j.jocn.2021.03.007
Zalpoor, H., Shapourian, H., Akbari, A., Shahveh, S., and Haghshenas, L. (2022). Increased neuropilin-1 expression by COVID-19: a possible cause of long-term neurological complications and progression of primary brain tumors. Hum. Cell 1–3. doi: 10.1007/s13577-022-00716-2
Keywords: brain, COVID-19, memory impairment, neuroimaging, PET, MRI
Citation: Shan D, Li S, Xu R, Nie G, Xie Y, Han J, Gao X, Zheng Y, Xu Z and Dai Z (2022) Post-COVID-19 human memory impairment: A PRISMA-based systematic review of evidence from brain imaging studies. Front. Aging Neurosci. 14:1077384. doi: 10.3389/fnagi.2022.1077384
Received: 22 October 2022; Accepted: 30 November 2022;
Published: 09 December 2022.
Edited by:
Xiaobo Mao, School of Medicine, Johns Hopkins Medicine, United StatesReviewed by:
Kundlik Gadhave, Johns Hopkins University, United StatesCopyright © 2022 Shan, Li, Xu, Nie, Xie, Han, Gao, Zheng, Xu and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dan Shan, ZHMzODA2QHRjLmNvbHVtYmlhLmVkdQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.