
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Aging Neurosci. , 28 November 2022
Sec. Parkinson’s Disease and Aging-related Movement Disorders
Volume 14 - 2022 | https://doi.org/10.3389/fnagi.2022.1069837
 Lingyu Zhang1,2†
Lingyu Zhang1,2† Ruicen Li1†
Ruicen Li1† Yanbing Hou2
Yanbing Hou2 Bei Cao2
Bei Cao2 Qianqian Wei2
Qianqian Wei2 Ruwei Ou2
Ruwei Ou2 Kuncheng Liu2
Kuncheng Liu2 Junyu Lin2
Junyu Lin2 Tianmi Yang2
Tianmi Yang2 Yi Xiao2
Yi Xiao2 Wenxia Huang1*
Wenxia Huang1* Huifang Shang2*
Huifang Shang2*Background: Accumulating evidence has suggested that cystatin C is associated with cognitive impairment in patients with neurodegenerative diseases. However, the association between cystatin C and cognitive decline in patients with multiple system atrophy (MSA) remains largely unknown.
Objectives: The objective was to determine whether cystatin C was independently associated with cognitive decline in patients with early-stage MSA.
Methods: Patients with MSA underwent evaluation at baseline and the 1-year follow-up. Cognitive function was evaluated with Montreal cognitive assessment (MoCA). Changes in the MoCA score and the absolute MoCA score at the 1-year assessment were considered the main cognitive outcome. The cystatin C concentrations in patients with MSA and age, sex, and body mass index matched-healthy controls (HCs) were measured. A multiple linear regression model was used to test the association between cystatin C and cognitive decline.
Results: A total of 117 patients with MSA and 416 HCs were enrolled in the study. The cystatin C levels were significantly higher in patients with MSA than in HCs (p < 0.001). Cystatin C levels were negatively correlated with MoCA score at baseline and at 1-year follow-up. Multiple linear regression model adjusted for potential confounders showed that baseline cystatin C levels were significantly associated with the MoCA score (p = 0.004) or change in the MoCA score (p = 0.008) at 1-year follow-up.
Conclusion: Our results suggested that cystatin C may serve as a potential biomarker of cognitive decline in patients with early-stage MSA.
Multiple system atrophy (MSA) is an adult-onset progressive neurodegenerative disease that manifests clinically with autonomic failure, parkinsonism, and a cerebellar syndrome in various combinations (Wenning et al., 2022). The cause of MSA is unknown, but it has been proven that glial cytoplasmic inclusions, neuronal loss, and astrogliosis predominantly involved in striatonigral and olivopontocerebellar structures (Koga and Dickson, 2018). However, the current treatment is not effective and cannot delay the progress of the disease. The prognosis is poor with a median survival time range from 6 to10 years (Low et al., 2015; Cao et al., 2018).
In recent years, an increasing number of studies have reported that non-motor symptoms in patients with MSA are prevalent (Schrag et al., 2010; Colosimo, 2011; Cao et al., 2015; Zhang et al., 2017, 2022b), such as depression, anxiety, fatigue, sleep problems, and cognition impairment, which seriously affected their quality of life and increased the burden on their families and society. Additionally, previous studies have reported that patients with MSA can suffer from mild single or multiple domains cognitive deficits (Stankovic et al., 2014; Koga et al., 2017). The cognitive function of patients with MSA declined with the progression of the disease (Zhang et al., 2022a). Therefore, to discover the potential factors that predict such cognitive decline in patients with MSA is of great significance.
Cystatin C is an endogenous cysteine proteinase inhibitor belonging to the type 2 cystatin superfamily and encoded by the CST3 gene, which is presented in all human body fluids (Mussap and Plebani, 2004). Accumulating evidence has suggested that cystatin C was significantly associated with cognitive impairment in older adults, as well as patients with neurodegenerative diseases (Cathcart et al., 2005; Chen et al., 2015; Cui et al., 2020; Nair et al., 2020; Yang et al., 2021). A large prospective cohort study indicated that higher level of cystatin C was associated with cognitive impairment in older Chinese adults (Cui et al., 2020). A meta-analysis suggested that cystatin C could be considered as a predictor for the risk of cognitive impairment (Nair et al., 2020). Additionally, higher level of serum cystatin C was associated with worse cognitive performance in patients with Alzheimer’s disease (AD; Chen et al., 2021). The level of cystatin C was higher in patients with Parkinson’s disease (PD) with mild cognitive impairment (Chen et al., 2015; Yang et al., 2021). Our recent study observed a significant cognitive decline in patients with MSA after a 1-year follow-up (Zhang et al., 2022a). Cystatin C was found to be associated with the disease severity in patients with MSA (Ye et al., 2021). However, the association between cystatin C and cognitive decline in patients with MSA remains largely unknown.
Thus, a 1-year prospective cohort study was performed to explore the association between cystatin C and cognitive function, as well as whether cystatin C could be a potential predictor of cognitive decline in patients with early-stage MSA.
This study recruited consecutive patients with MSA with a baseline disease duration of <3 years from the Department of Neurology, West China Hospital of Sichuan University, between January 2015 and May 2021. All patients underwent brain magnetic resonance imaging to exclude other neurological disorders. To exclude common forms of spinocerebellar ataxia (SCA), we performed screening for SCA genes including SCA1, SCA2, SCA3, SCA6, and SCA7. Patients underwent face-to-face interviews by neurologists at baseline and at 1-year follow-up. Among the 128 patients recruited at baseline, 8 patients refused to follow up and 120 patients completed the 1-year follow-up evaluation. Finally, 3 patients diagnosed with PD were excluded from the study, and 117 patients who met the criteria for probable diagnosis of MSA (Gilman et al., 2008) were included in the final analysis (Figure 1). A total of 416 healthy controls (HCs) were recruited from the Health Management Center, West China Hospital of Sichuan University, and their age, sex, and body mass index (BMI) were matched to the patients with MSA. HCs diagnosed with heart disease, hypertension, diabetes mellitus, or renal dysfunction were excluded. And none of them had any neurological diseases or psychiatric disorders.
All participants underwent hematological examinations at baseline. Blood samples were taken by venipuncture in the morning after fasting from midnight. The following hematological tests were considered for this study: cystatin C, creatinine, urea, and estimated glomerular filtration rate (eGFR).
Baseline demographic and clinical data including age, sex, age of onset, height, weight, educational year, and disease duration was recorded. Disease duration was defined as the time between disease onset and evaluation. Disease onset was defined as the initial presentation of any motor symptoms (Parkinsonism or cerebellar dysfunction) or autonomic features, except erectile dysfunction. BMI was calculated as body weight (kg) divided by height squared (m2).
Patients with MSA underwent evaluation for disease severity and cognitive function at baseline and the 1-year follow-up. Disease severity was assessed using the Unified Multiple System Atrophy Rating Scale (UMSARS; Wenning et al., 2004), and the total UMSARS score was calculated as the sum of UMSARS parts I and II. Orthostatic hypotension (OH) was defined as a reduction in the systolic blood pressure by at least 20 mmHg and/or diastolic blood pressure by at least 10 mmHg after 3 min of standing up from a previous recumbent position of 3 min. Cognitive function was evaluated using a comprehensive and standardized cognitive screening tool [Montreal cognitive assessment (MoCA); Nasreddine et al., 2005]. The optimal cutoff scores of MoCA for cognitive impairment (CI) screening were 19 for individuals with no more than 6 years of education, 22 for individuals with 7–12 years of education, and 24 for individuals with more than 12 years of education (Chen et al., 2016).
IBM SPSS software (version 26.0) was used for the statistical analysis. Continuous variables are exhibited as means and SD, and categorical variables as counts and percentages. Mann–Whitney U test was used to compare the continuous variables between different groups, since these data were non-normal distribution. Chi-squared test was used to compare the categorical variables between different groups. Spearman’s rank correlation coefficient was used to assess the correlation between baseline levels of cystatin C and clinical features at baseline and at 1-year follow-up. The change in the MoCA score at 1-year follow-up, represents cognitive decline. A multiple linear regression model was performed to investigate the association between baseline cystatin C and the cognitive decline after adjusting for confounding factors, including age, sex, educational years, disease duration, diagnosis subtype, OH, eGFR, and baseline MoCA score. The MoCA score at the 1-year follow-up and change in the MoCA score at 1-year follow-up were used as separate dependent variables. Two-tailed p values <0.05 were considered statistically significant.
Demographic and clinical information for MSA and HCs are shown in Table 1. A total of 117 patients with MSA and 416 HCs were enrolled in the study. The mean age of patients with MSA was 59.45 ± 7.50 years and the mean disease duration was 1.69 ± 0.74 years. There were no significant differences in the age, sex, or BMI between patients with MSA and HCs. The cystatin C levels were significantly higher in patients with MSA than that in HCs (0.92 ± 0.17 vs. 0.86 ± 0.12, p < 0.001; Figure 2A). There was no difference in cystatin C levels between MSA-P and MSA-C (0.90 ± 0.19 vs. 0.94 ± 0.16, p = 0.119; Figure 2B). The levels of cystatin C were significantly higher in MSA patients with CI than those without CI at baseline (0.98 ± 0.22 vs. 0.90 ± 0.14, p = 0.034; Figure 2C). A total of 29.9% (35/117) patients with MSA developed cognitive impairment (CI) at an early stage, while the frequency of CI was 50.4% (59/117) after 1-year follow-up. The levels of creatinine, urea, and eGFR exhibited that the renal function of patients with MSA was normal.
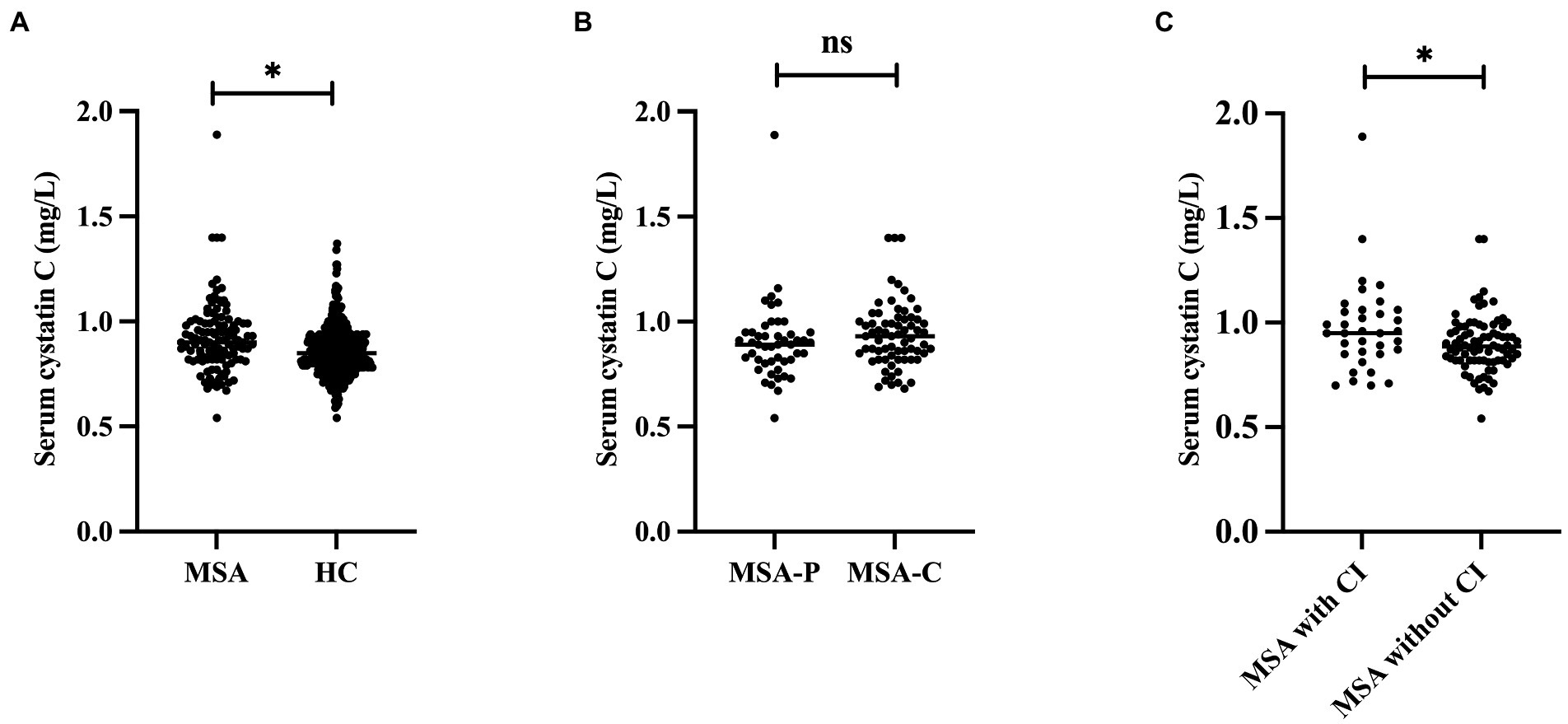
Figure 2. Comparison the cystatin C levels between different groups. (A) Comparison the cystatin C levels between MSA and HC. (B) Comparison the cystatin C levels between MSA-P and MSA-C. (C) Comparison the cystatin C levels between MSA with and without CI. *Significant difference.
The UMSARS-I score, UMSARS-II score, UMSARS-IV score, and total UMSARS score increased significantly from baseline to 1-year follow-up in patients with MSA (p < 0.001). Meanwhile, the MoCA score of patients with MSA decreased significantly from baseline to 1-year follow-up (22.66 ± 3.86 vs. 20.13 ± 5.30, p < 0.001).
Consequently, we then examined the correlation between cystatin C levels and clinical features in patients with MSA. Cystatin C levels were significantly negatively correlated with MoCA score at baseline and at 1-year follow-up (r = −0.252, p = 0.006; r = −0.317, p < 0.001, respectively; Figures 3A,B), while cystatin C levels were not significantly correlated with UMSARS score at baseline and at 1-year follow-up (p > 0.05; Figures 3C,D). We found that baseline cystatin C levels were negatively correlated with visuospatial and executive function domain (r = −0.188, p = 0.043) and naming domain (r = −0.264, p = 0.004) at baseline. And baseline cystatin C levels were negatively correlated with all cognitive domains of MoCA except language at 1-year follow-up (Supplementary Table 1). Multiple linear regression model adjusted for age, sex, educational years, disease duration, diagnosis subtype, OH, eGFR, and baseline MoCA score showed that baseline cystatin C levels were significantly associated with the MoCA score (β = −5.416, 95% CI −9.040 to −1.792, SE = 1.829, p = 0.004) or change in the MoCA score (β = −4.434, 95% CI −7.682 to −1.186, SE = 1.640, p = 0.008) at 1-year follow-up (Table 2).
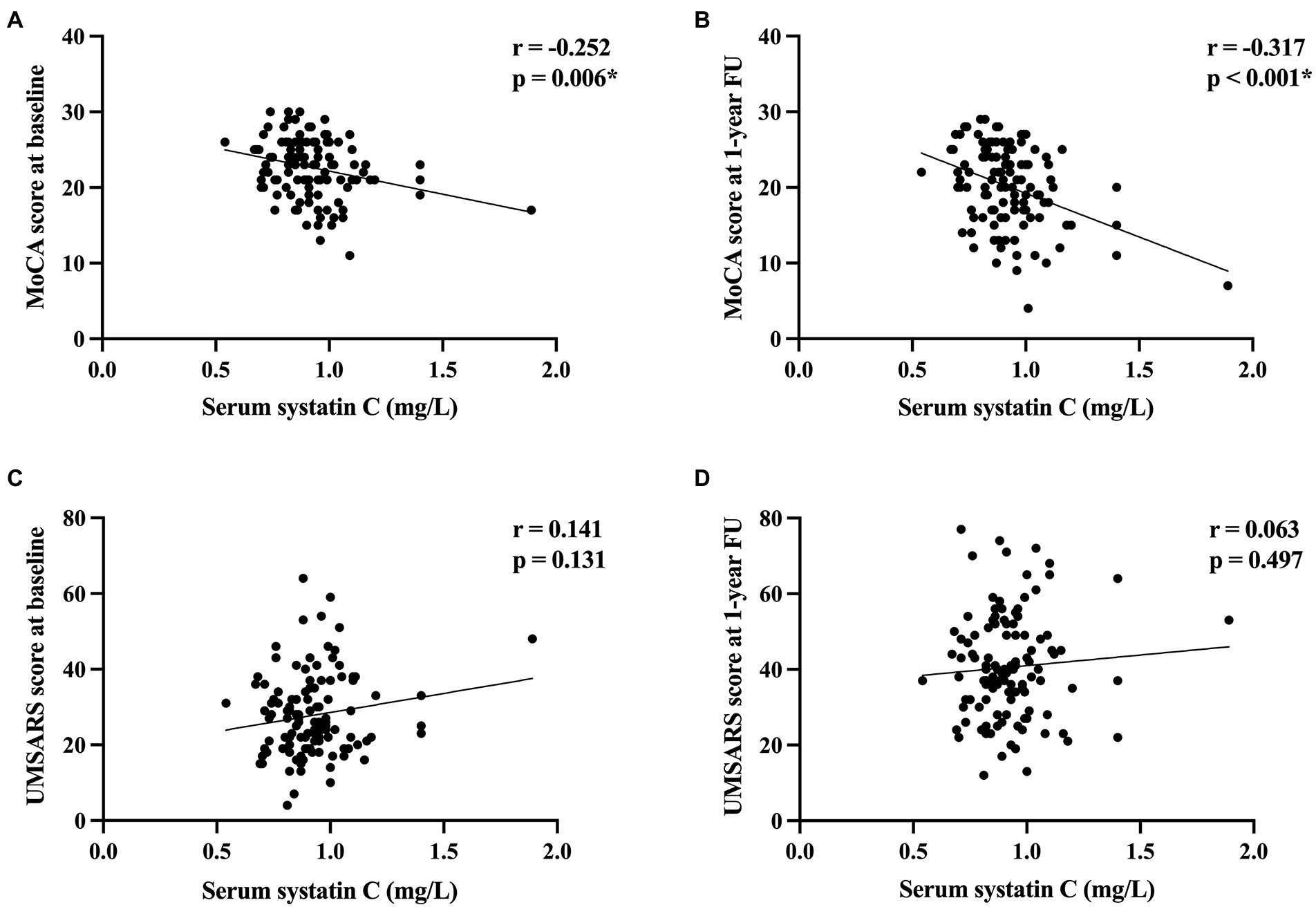
Figure 3. The correlation between cystatin C levels and cognitive function and disease severity in patients with MSA. (A) The correlation between cystatin C levels and MoCA score at baseline. (B) The correlation between cystatin C levels and MoCA score at 1-year follow-up. (C) The correlation between cystatin C levels and UMSARA score at baseline. (D) The correlation between cystatin C levels and UMSARS score at 1-year follow-up. *Significant difference.
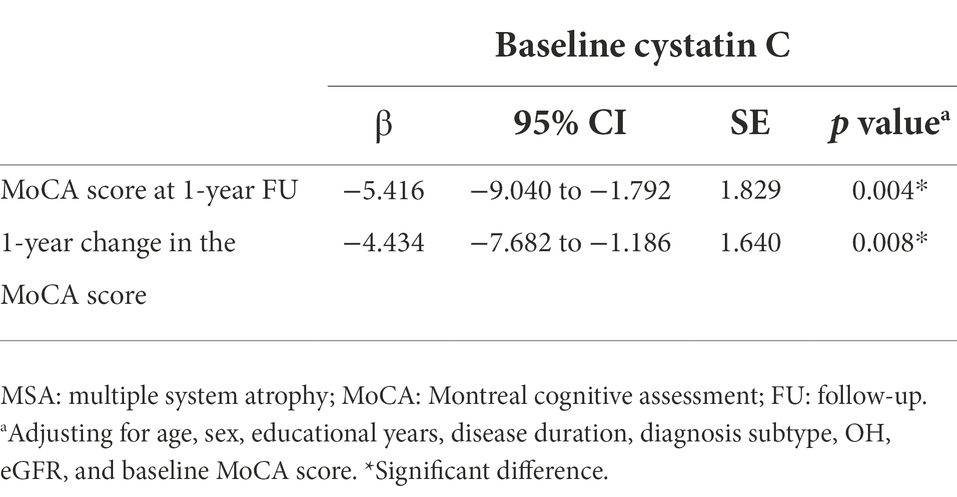
Table 2. The correlation between baseline cystatin C and cognition outcome in patients with MSA in multiple linear regression model.
To our knowledge, this is the first study that examined the association between cystatin C and cognitive outcome in patients with MSA after a 1-year follow-up. The main finding demonstrated that cystatin C levels reflected cognitive functions in patients with early-stage MSA. We found a significant cognitive decline in patients with MSA after a 1-year follow-up. Cystatin C levels correlated with the cognitive status of patients with MSA. Additionally, in a prospective follow-up of these patients with MSA, we found that higher baseline cystatin C levels could predict a higher risk of cognitive decline after accounting for potential confounders, including age, sex, educational years, disease duration, diagnosis subtype, OH, eGFR, and baseline MoCA score. Therefore, these findings indicated that cystatin C may serve as a potential biomarker of cognitive decline in MSA.
Our finding that cystatin C levels were significantly higher in patients with MSA than in HCs, which was consistent with a previous case–control study in Chinese population (Ye et al., 2021). We observed a significant cognitive decline in patients with MSA after a 1-year follow-up, which was supported by our previous study (Zhang et al., 2022a). There was a negative correlation between cystatin C and cognitive status in patients with MSA. Additionally, multiple linear regression model showed that baseline cystatin C levels were significantly associated with cognitive decline in early-stage MSA after adjusting for age, sex, educational years, disease duration, diagnosis subtype, OH, eGFR, and baseline MoCA score. A meta-analysis including 12 studies with a total of 2,433 mild cognitive impairment patients and 1,034 controls suggested that cystatin C could be considered as a predictor for the risk of cognitive impairment (Nair et al., 2020). It has been reported that higher levels of cystatin C were associated with cognitive impairment in neurodegenerative diseases, including AD and PD (Chen et al., 2015, 2021; Yang et al., 2021). These findings may partially support our results since AD, PD, and MSA belong to neurodegenerative diseases, as well as PD and MSA belong to α-synucleinopathy.
Furthermore, chronic kidney disease is a recognized risk factor for cognitive impairment, which is usually judged by eGFR (Chu et al., 2022). The clearance of cystatin C through glomerular filtration can be affected by renal function. However, the increase of cystatin C levels in patients with MSA cannot be simply ascribed to the changes of renal function since the eGFR levels were not significantly different between patients with MSA and HCs. Additionally, cystatin C was associated with cognitive decline in patients with MSA at the 1-year follow-up independent of eGFR.
The pathogenesis of MSA is complex, but the propagation of misfolded α-synuclein from neurons to oligodendroglia and neuroinflammation caused by microglial activation play an important role (Jellinger, 2018). Cysteine cathepsins are essential in lysosomal degradation of α-synuclein (McGlinchey and Lee, 2015). Cystatin C is an endogenous cysteine proteinase inhibitor belonging to the type 2 cystatin superfamily, which is presented in all human body fluids (Mussap and Plebani, 2004). The high level of cystatin C can lead to accumulation of aggregated α-synuclein. And cystatin C also is a disease-associated protein involving in inflammatory and oxidative stress process (Xu et al., 2015). Additionally, cystatin C from neurotoxicant-injured rat dopaminergic neurons may be involved in the induction of microglial activation and exacerbation of neurotoxicity in a rat dopaminergic neuron-microglial cell-based in vitro model (Dutta et al., 2012). Cystatin C also can be released by mouse oligodendrocytes overexpressing human α-synuclein, triggering α-synuclein up-regulation and insoluble α-synuclein accumulation in a transgenic mouse mode of MSA (Suzuki et al., 2014). The neuropathological underpinnings of cognitive impairment in MSA are not clear. However, after a semiquantitative assessment of the burden of glial cytoplasmic inclusions and neuronal cytoplasmic inclusion (NCI) in autopsy-confirmed MSA, a greater burden of NCI in the limbic regions was found to be associated with cognitive impairment in MSA (Koga et al., 2017; Miki et al., 2020). Taken together, we hypothesized that cystatin C might be able to trigger NCI up-regulation accumulation in the limbic regions based on the above evidences. Further studies are needed to confirm our results.
The major advantages of the study include (1) we performed a prospective follow-up study; (2) we included patients with early-stage MSA; (3) we enrolled HCs. However, several limitations need to be addressed. First, we assessed cognitive function only with MoCA, a brief screening tool of global cognitive function. Detailed neuro- psychological tests can provide more comprehensive cognitive domains for assessing the correlation between cystatin C and cognitive domain declines in patients with MSA. And it will be considered in our further study. Second, the follow-up period was a relatively short since MSA is a rare and rapidly progressive condition. Third, all patients were diagnosed clinically without postmortem confirmation. Fourth, the correlation between cystatin C levels and disease severity was not significantly in our study, which may be because the patients enrolled in our study were at an early stage. Further large sample study with different disease duration and longer follow-up period are needed to clarify the issue.
We found a significant cognitive decline in patients with MSA after a 1-year follow-up. Our results suggested that higher baseline cystatin C levels could predict a higher risk of cognitive decline after accounting for potential confounders. Therefore, these findings indicated that cystatin C may serve as a potential biomarker of cognitive decline in patients with MSA.
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by Ethics Committee of West China Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study.
LZ and RL: research project: conception, organization, execution, design the statistical analysis, and wrote the first draft of the manuscript. YH, BC, QW, RO, KL, JL, TY, and YX: data collection. WH and HS: research project: conception, review and critique the statistical analysis and manuscript. All authors contributed to the article and approved the submitted version.
The present study was supported by the funding of 1.3.5 project for disciplines of excellence–Clinical Research Incubation Project, West China Hospital, Sichuan University (Grant No. 2022HXFH023), Sichuan Science and Technology Program (Grant No. 2022ZDZX0023), and Sichuan Postdoctoral Program (Grant No. TB2022043).
The authors thank all subjects for their participation in the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2022.1069837/full#supplementary-material
Cao, B., Zhang, L., Zou, Y., Wei, Q., Ou, R., Chen, Y., et al. (2018). Survival analysis and prognostic nomogram model for multiple system atrophy. Parkinsonism Relat. Disord. 54, 68–73. doi: 10.1016/j.parkreldis.2018.04.016
Cao, B., Zhao, B., Wei, Q. Q., Chen, K., Yang, J., Ou, R., et al. (2015). The global cognition, frontal lobe dysfunction and behavior changes in Chinese patients with multiple system atrophy. PLoS One 10:e0139773. doi: 10.1371/journal.pone.0139773
Cathcart, H. M., Huang, R., Lanham, I. S., Corder, E. H., and Poduslo, S. E. (2005). Cystatin C as a risk factor for Alzheimer disease. Neurology 64, 755–757. doi: 10.1212/01.Wnl.0000151980.42337.44
Chen, W. W., Cheng, X., Zhang, X., Zhang, Q. S., Sun, H. Q., Huang, W. J., et al. (2015). The expression features of serum Cystatin C and homocysteine of Parkinson's disease with mild cognitive dysfunction. Eur. Rev. Med. Pharmacol. Sci. 19, 2957–2963.
Chen, X., Huang, Y., Bao, T., Jia, F., Ou, R., Wei, Q., et al. (2021). Changes in serum Cystatin C levels and the associations with cognitive function in Alzheimer's disease patients. Front. Aging Neurosci. 13:790939. doi: 10.3389/fnagi.2021.790939
Chen, K. L., Xu, Y., Chu, A. Q., Ding, D., Liang, X. N., Nasreddine, Z. S., et al. (2016). Validation of the Chinese version of Montreal cognitive assessment basic for screening mild cognitive impairment. J. Am. Geriatr. Soc. 64, e285–e290. doi: 10.1111/jgs.14530
Chu, N. M., Hong, J., Harasemiw, O., Chen, X., Fowler, K. J., Dasgupta, I., et al. (2022). Chronic kidney disease, physical activity, and cognitive function in older adults- results from the National Health and nutrition examination survey (2011-2014). Nephrol. Dial. Transplant. 37, 2180–2189. doi: 10.1093/ndt/gfab338
Colosimo, C. (2011). Nonmotor presentations of multiple system atrophy. Nat. Rev. Neurol. 7, 295–298. doi: 10.1038/nrneurol.2011.5
Cui, Z., Cao, G., Wang, Y., Ma, Q., Wang, C., Xu, Y., et al. (2020). Effects of Cystatin C on cognitive impairment in older Chinese adults. Am. J. Alzheimers Dis. Other Dement. 35:1533317520965101. doi: 10.1177/1533317520965101
Dutta, G., Barber, D. S., Zhang, P., Doperalski, N. J., and Liu, B. (2012). Involvement of dopaminergic neuronal cystatin C in neuronal injury-induced microglial activation and neurotoxicity. J. Neurochem. 122, 752–763. doi: 10.1111/j.1471-4159.2012.07826.x
Gilman, S., Wenning, G. K., Low, P. A., Brooks, D. J., Mathias, C. J., Trojanowski, J. Q., et al. (2008). Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71, 670–676. doi: 10.1212/01.wnl.0000324625.00404.15
Jellinger, K. A. (2018). Multiple system atrophy: an Oligodendroglioneural Synucleinopathy1. J. Alzheimers Dis. 62, 1141–1179. doi: 10.3233/jad-170397
Koga, S., and Dickson, D. W. (2018). Recent advances in neuropathology, biomarkers and therapeutic approach of multiple system atrophy. J. Neurol. Neurosurg. Psychiatry 89, 175–184. doi: 10.1136/jnnp-2017-315813
Koga, S., Parks, A., Uitti, R. J., van Gerpen, J. A., Cheshire, W. P., Wszolek, Z. K., et al. (2017). Profile of cognitive impairment and underlying pathology in multiple system atrophy. Mov. Disord. 32, 405–413. doi: 10.1002/mds.26874
Low, P. A., Reich, S. G., Jankovic, J., Shults, C. W., Stern, M. B., Novak, P., et al. (2015). Natural history of multiple system atrophy in the USA: a prospective cohort study. Lancet Neurol. 14, 710–719. doi: 10.1016/s1474-4422(15)00058-7
McGlinchey, R. P., and Lee, J. C. (2015). Cysteine cathepsins are essential in lysosomal degradation of α-synuclein. Proc. Natl. Acad. Sci. U. S. A. 112, 9322–9327. doi: 10.1073/pnas.1500937112
Miki, Y., Foti, S. C., Hansen, D., Strand, K. M., Asi, Y. T., Tsushima, E., et al. (2020). Hippocampal α-synuclein pathology correlates with memory impairment in multiple system atrophy. Brain 143, 1798–1810. doi: 10.1093/brain/awaa126
Mussap, M., and Plebani, M. (2004). Biochemistry and clinical role of human cystatin C. Crit. Rev. Clin. Lab. Sci. 41, 467–550. doi: 10.1080/10408360490504934
Nair, P., Misra, S., Nath, M., Vibha, D., Srivastava, A. K., Prasad, K., et al. (2020). Cystatin C and risk of mild cognitive impairment: a systematic review and meta-analysis. Dement. Geriatr. Cogn. Disord. 49, 471–482. doi: 10.1159/000510219
Nasreddine, Z. S., Phillips, N. A., Bedirian, V., Charbonneau, S., Whitehead, V., Collin, I., et al. (2005). The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 53, 695–699. doi: 10.1111/j.1532-5415.2005.53221.x
Schrag, A., Sheikh, S., Quinn, N. P., Lees, A. J., Selai, C., Mathias, C., et al. (2010). A comparison of depression, anxiety, and health status in patients with progressive supranuclear palsy and multiple system atrophy. Mov. Disord. 25, 1077–1081. doi: 10.1002/mds.22794
Stankovic, I., Krismer, F., Jesic, A., Antonini, A., Benke, T., Brown, R. G., et al. (2014). Cognitive impairment in multiple system atrophy: a position statement by the neuropsychology task force of the MDS multiple system atrophy (MODIMSA) study group. Mov. Disord. 29, 857–867. doi: 10.1002/mds.25880
Suzuki, Y., Jin, C., and Yazawa, I. (2014). Cystatin C triggers neuronal degeneration in a model of multiple system atrophy. Am. J. Pathol. 184, 790–799. doi: 10.1016/j.ajpath.2013.11.018
Wenning, G. K., Stankovic, I., Vignatelli, L., Fanciulli, A., Calandra-Buonaura, G., Seppi, K., et al. (2022). The Movement Disorder Society criteria for the diagnosis of multiple system atrophy. Mov. Disord. 37, 1131–1148. doi: 10.1002/mds.29005
Wenning, G. K., Tison, F., Seppi, K., Sampaio, C., Diem, A., Yekhlef, F., et al. (2004). Development and validation of the unified multiple system atrophy rating scale (UMSARS). Mov. Disord. 19, 1391–1402. doi: 10.1002/mds.20255
Xu, Y., Ding, Y., Li, X., and Wu, X. (2015). Cystatin C is a disease-associated protein subject to multiple regulation. Immunol. Cell Biol. 93, 442–451. doi: 10.1038/icb.2014.121
Yang, C. G., Cai, S. M., Liu, C. Y., and Chen, C. (2021). On the correlation between serum Cystatin C and Parkinson's disease in the Chinese population: a promising biomarker? J. Integr. Neurosci. 20, 349–357. doi: 10.31083/j.jin2002034
Ye, F., Wang, T., Li, H., Liang, J., Wu, X., and Sheng, W. (2021). Serum Cystatin C as a potential predictor of the severity of multiple system atrophy with predominant cerebellar ataxia: a case-control study in Chinese population. Front. Neurosci. 15:663980. doi: 10.3389/fnins.2021.663980
Zhang, L., Cao, B., Hou, Y., Gu, X., Wei, Q., Ou, R., et al. (2022a). Neurofilament light chain predicts disease severity and progression in multiple system atrophy. Mov. Disord. 37, 421–426. doi: 10.1002/mds.28847
Zhang, L., Cao, B., Hou, Y., Gu, X., Wei, Q. Q., Ou, R., et al. (2022b). Fatigue in patients with multiple system atrophy: a prospective cohort study. Neurology 98, e73–e82. doi: 10.1212/wnl.0000000000012968
Keywords: multiple system atrophy, cystatin C, cognitive decline, prospective study, movement disorder
Citation: Zhang L, Li R, Hou Y, Cao B, Wei Q, Ou R, Liu K, Lin J, Yang T, Xiao Y, Huang W and Shang H (2022) Cystatin C predicts cognitive decline in multiple system atrophy: A 1-year prospective cohort study. Front. Aging Neurosci. 14:1069837. doi: 10.3389/fnagi.2022.1069837
Received: 14 October 2022; Accepted: 14 November 2022;
Published: 28 November 2022.
Edited by:
Shunsuke Koga, Mayo Clinic Florida, United StatesCopyright © 2022 Zhang, Li, Hou, Cao, Wei, Ou, Liu, Lin, Yang, Xiao, Huang and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenxia Huang, aHVhbmd3ZW54aWEzMzFAc2N1LmVkdS5jbg==; Huifang Shang, aGZzaGFuZzIwMDJAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.